Abstract
Background
Pulmonary endarterectomy (PEA), pulmonary arterial hypertension (PAH) therapy and balloon pulmonary angioplasty (BPA) are currently accepted therapies for chronic thromboembolic pulmonary hypertension (CTEPH). This international CTEPH Registry identifies clinical characteristics of patients, diagnostic algorithms and treatment decisions in a global context.
Methods
1010 newly diagnosed consecutive patients were included in the registry between February 2015 and September 2016. Diagnosis was confirmed by right heart catheterisation, ventilation–perfusion lung scan, computerised pulmonary angiography and/or invasive pulmonary angiography after at least 3 months on anticoagulation.
Results
Overall, 649 patients (64.3%) were considered for PEA, 193 (19.1%) for BPA, 20 (2.0%) for both PEA and BPA, and 148 (14.7%) for PAH therapy only. Reasons for PEA inoperability were technical inaccessibility (n=235), comorbidities (n=63) and patient refusal (n=44). In Europe and America and other countries (AAO), 72% of patients were deemed suitable for PEA, whereas in Japan, 70% of patients were offered BPA as first choice. Sex was evenly balanced, except in Japan where 75% of patients were female. A history of acute pulmonary embolism was reported for 65.6% of patients. At least one PAH therapy was initiated in 35.8% of patients (26.2% of PEA candidates, 54.5% of BPA candidates and 54.1% of those not eligible for either PEA or BPA). At the time of analysis, 39 patients (3.9%) had died of pulmonary hypertension-related causes (3.5% after PEA and 1.8% after BPA).
Conclusions
The registry revealed noticeable differences in patient characteristics (rates of pulmonary embolism and sex) and therapeutic approaches in Japan compared with Europe and AAO.
Short abstract
There are distinct regional differences in the management of CTEPH patients but globally, the proportion of patients managed by PEA remains stable, independently of the new established treatment options of PAH therapies and BPA https://bit.ly/3zEXxkv
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) results from pulmonary artery obstruction by fibrotic organisation of thromboemboli [1]. Secondary microvasculopathy contributes to an increased pulmonary vascular resistance (PVR) leading to right heart dysfunction and failure. Two-thirds of the patients with CTEPH have a history of venous thromboembolism [2]. Pulmonary endarterectomy (PEA) is currently the international guideline-recommended treatment for operable patients with excellent results regarding symptomatic relief and long-term survival [3–7].
At the beginning of this millennium international data regarding the treatment of CTEPH were still scarce. At that time, patients were classified as operable or not. Some patients have received off-label pulmonary arterial hypertension (PAH) therapies. This was a focus of the first European prospective international registry recruiting CTEPH patients from February 2007 to January 2009 [8].
Since then, two additional treatment options emerged. Riociguat, a soluble guanylate cyclase stimulator, was approved in 2013 for treatment of inoperable CTEPH patients and residual pulmonary hypertension (PH) after PEA [9]. At almost the same time, several case series of successful refined balloon pulmonary angioplasty (BPA) were published by Japanese centres, and BPA intervention has been adopted across the world [10–12]. These additional therapeutic modalities have challenged decision-making that depends on the expertise of the centres regarding PEA surgery and BPA procedures and promotes a multidisciplinary approach [1, 2]. According to recent guidelines [13], medical treatment with or without BPA is recommended for inoperable patients or for patients with persistent or recurrent PH after PEA.
Given these significant changes in CTEPH management, this new prospective international CTEPH Registry was designed to focus on current worldwide patient characteristics, diagnosis and treatment strategies.
Methods
Study design
The worldwide CTEPH Registry is a prospective disease-specific observational registry conducted by the International CTEPH Association (ICA) collecting data on CTEPH patients from 34 centres in 19 countries. 10 centres performed both PEA and BPA, 11 centres only PEA and six centres only BPA. Seven centres did not perform either procedure themselves but referred patients for interventions. Supplementary table S1 shows details of patient inclusion by centres. Consecutive patients with CTEPH diagnosed within 12 months of study inclusion were recruited into the registry. The first patient was enrolled on February 16, 2015 and the last (n=1010) on September 21, 2016. Thereafter, follow-up data were collected for an additional 3 years, and the registry was closed at the end of September 2019. Local institutional review boards or independent ethics committees approved the protocol, and written informed consent was obtained from all patients, as required by local ethical committees. The study was conducted in accordance with the principles of the Declaration of Helsinki and registered in the clinicaltrials.gov database under the identifier NCT02656238.
Inclusion criteria
At all participating institutions, the diagnosis of CTEPH was established according to clinical guidelines valid at study initiation [13]. Patients had to be newly diagnosed (incident patients), fulfilling the following criteria: 1) be willing to provide informed consent, 2) have been treated with anticoagulation for at least 3 months before diagnosis of CTEPH established at the right heart catheterisation with mean pulmonary artery pressure (mPAP) ≥25 mmHg at rest, abnormal ventilation–perfusion (VQ) scan, pulmonary angiogram, computed tomography pulmonary angiography (CTPA) or magnetic resonance pulmonary angiography (MRPA) confirming chronic thromboembolic obstructions as described previously [13]. PAH therapy or BPA before diagnostic right heart catheterisation was not allowed. To accurately capture the target population and to minimise the potential for selection bias, all sites were encouraged to screen all consecutive CTEPH patients for potential inclusion.
Patient cohorts
This baseline analysis consists of descriptive statistics of the whole population further divided into four cohorts based on clinical judgement and management decision made by local CTEPH teams: 1) operable patients (PEA); 2) patients suitable for BPA; 3) patients suitable for either PEA or BPA; and 4) patients not suitable for PEA or BPA.
Patients assigned to PEA
PEA surgery has been described previously, with variations between some centres [4, 7, 14, 15]. Predefined criteria for inoperability were assessed by local multidisciplinary CTEPH teams according to published data [16].
Patients assigned to BPA
BPA was performed as a staged procedure, with a limited number of pulmonary segments treated during each session. The different techniques and strategies have been reported previously [10, 17].
Statistical analysis
Results are expressed as medians with first and third quartiles (Q1–Q3) or absolute numbers and percentages of patients. Subgroups (by region or by patient disposition to treatment) were compared using the Kruskal–Wallis test for continuous variables and the chi-square test for categorical variables. The reported p-values are to be interpreted in the exploratory sense. Data were analysed with IBM SPSS software version 22 (IBM, Chicago, IL, USA). Figures were processed with GraphPad Prism version 7.03 for Windows (GraphPad Software, La Jolla, CA, USA; www.graphpad.com).
Results
Study population
The characteristics of the patient population at inclusion are summarised in table 1. Among the 1010 patients, 779 patients (77%) originated from 22 sites in Europe, 115 (11%) from four sites in Japan and 116 (11%) from eight sites in America and other countries (AAO). At the time of censoring (October 13, 2017), all patients had been observed for a minimum period of 12 months. The distribution by sex was generally equally distributed, except for Japan where there was a greater proportion of female patients (75%). The median age for the entire population was 63 years, similar for Europe and Japan, but lower for AAO. A history of acute pulmonary embolism was present in 65.6% of patients overall but was much less frequent in Japanese patients (supplementary figure S1). A history of deep venous thrombosis (DVT) was noted in 38.8% of patients with higher numbers in Japan and AAO. The need for hospitalisation due to right heart failure prior to diagnosis of CTEPH was recorded in 25% of patients but was more frequent in Japanese patients.
TABLE 1.
Baseline characteristics by region (n=1010)
| Europe | Japan | AAO | Total | p-value (exploratory) | |
| Subjects n | 779 | 115 | 116 | 1010 | |
| Sex male | 400 (51.3) | 29 (25.2) | 52 (44.8) | 481 (47.6) | <0.001 |
| Age at diagnosis years | 64.0 (52.0–73.0) | 65.0 (56.0–75.0) | 54.0 (40.3–66.0) | 63.0 (51.0–73.0) | <0.001 |
| Age category at diagnosis | |||||
| <50 years | 161 (20.7) | 20 (17.4) | 47 (40.5) | 228 (22.6) | <0.001 |
| ≥50 years and <70 years | 352 (45.2) | 46 (40.0) | 47 (40.5) | 445 (44.1) | |
| ≥70 years | 265 (34.1) | 49 (42.6) | 22 (19.0) | 336 (33.3) | |
| Not available | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | |
| Ethnicity # | |||||
| Caucasian/white | 749 (96.1) | 2 (1.7) | 69 (59.5) | 820 (81.2) | n/a |
| Black | 9 (1.2) | 0 (0.0) | 21 (18.1) | 30 (3.0) | |
| Asian | 6 (0.8) | 115 (100.0) | 21 (18.1) | 142 (14.1) | |
| Other | 8 (1.0) | 0 (0.0) | 3 (2.6) | 11 (1.1) | |
| Not available | 8 (1.0) | 0 (0.0) | 3 (2.6) | 11 (1.1) | |
Values are expressed as median with first and third quartiles (Q1–Q3) or n (%) unless otherwise indicated. #: totals exceed 100% since it is possible to record more than one ethnicity. “Caucasian/white” and “Asian” was recorded for one patient in Europe, two patients in Japan and one patient in America and others (AAO). Exploratory p-values are calculated for regions.
CTEPH diagnosis
Clinical status, medical history and haemodynamics according to region are presented in table 2. Median time from onset of PH symptoms to diagnosis by right heart catheterisation was 15 (7–32) months. Most patients were in New York Heart Association (NYHA) functional class III, but Japan had a higher proportion of patients in functional class I or II compared with Europe and AAO. Haemodynamic data showed marked differences among Europe, Japan and AAO. For the entire cohort, the median value for mPAP was 44 (35–51) mmHg, pulmonary capillary wedge pressure (PCWP) was 10 (7–13) mmHg, cardiac output was 4.1 (3.4–5.0) L·min−1and PVR was 641( 445–908) dyn·s·cm−5 (table 2).
TABLE 2.
Clinical status, medical history and haemodynamics at diagnosis by region (n=1010)
| Europe | Japan | AAO | Total | p-va lue (exploratory) | |
| Subjects n | 779 | 115 | 116 | 1010 | |
| Time from onset of PH symptoms to diagnosis months | 14.0 (7.0–28.0) | 12.0 (5.3–32.0) | 23.5 (12.0–46.8) | 15.0 (7.0–32.0) | <0.001 |
| NYHA functional class % I/II/III/IV | 1.7/22.5/61.3/14.5 | 0.9/36.5/53.9/8.7 | 0.0/25.2/66.1/8.7 | 1.4/24.4/61.0/13.2 | 0.012 |
| History of acute PE# | 544 (69.8) | 45 (39.1) | 74 (63.8) | 663 (65.6) | <0.001 |
| History of DVT# | 286 (36.7) | 50 (43.5) | 56 (48.3) | 392 (38.8) | 0.059 |
| Previous need for hospitalisation due to right heart failure# | 158 (20.3) | 68 (59.1) | 24 (20.7) | 250 (24.8) | <0.001 |
| Right heart catheterisation at diagnosis | |||||
| MRAP mmHg | 8.0 (5.0–12.0) | 5.0 (4.0–8.0) | 9.0 (6.0–13.5) | 8.0 (5.0–12.0) | <0.001 |
| n (missing) | 736 (43) | 110 (5) | 116 (0) | 962 (48) | |
| mPAP mmHg | 44.0 (35.0–51.0) | 40.0 (32.0–48.0) | 45.0 (37.0–55.0) | 44.0 (35.0–51.0) | 0.002 |
| n (missing) | 779 (0) | 115 (0) | 116 (0) | 1010 (0) | |
| PCWP mmHg | 10.0 (8.0–13.0) | 7.0 (5.0–10.0) | 12.0 (9.0–14.0) | 10.0 (7.0–13.0) | <0.001 |
| n (missing) | 750 (29) | 115 (0) | 115 (1) | 980 (30) | |
| Cardiac output L·min−1 | 4.20 (3.40–5.04) | 3.64 (2.90–4.56) | 4.10 (3.44–5.27) | 4.10 (3.40–5.00) | <0.001 |
| n (missing) | 762 (17) | 115 (0) | 115 (1) | 992 (18) | |
| CI L·min−1·m−2 | 2.20 (1.81–2.67) | 2.30 (1.90–3.04) | 2.20 (1.80–2.87) | 2.20 (1.82–2.70) | 0.230 |
| n (missing) | 756 (23) | 115 (0) | 116 (0) | 987 (23) | |
| PVR dyn·s·cm−5 | 626 (428–893) | 730 (517–938) | 693 (410–1000) | 641 (445–908) | 0.046 |
| n (missing) | 744 (35) | 115 (0) | 115 (1) | 974 (36) | |
| PVRI dyn·s·cm−5·m2 | 1216 (840–1658) | 1226 (778–1472) | 1225 (779–1846) | 1198 (834–1654) | 0.226 |
| n (missing) | 731 (48) | 115 (0) | 115 (0) | 961 (49) | |
Values are expressed as median with first and third quartiles (Q1–Q3) or n (%) unless otherwise indicated. AAO: America and others; PH: pulmonary hypertension; NYHA: New York Heart Association; PE: pulmonary embolism (see also supplementary figure 1); DVT: deep vein thrombosis; MRAP: mean right atrial pressure; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; CI: cardiac index; PVR: pulmonary vascular resistance; PVRI: pulmonary vascular resistance index. Exploratory p-values are calculated for regions. #: unknown cases included in the exploratory analysis of significance.
The radiological imaging evaluations are presented in table 3. VQ scans were performed in 740 of all patients. In Japan and AAO, nearly all patients had a VQ scan; in Europe, the proportion was considerably lower. The rate of pulmonary angiography was high in all regions, but the modality differed significantly (p<0.001). In all treatment groups, single (50% to 55%) or dual (40% to 47%) modalities of pulmonary angiography imaging were present in similar distribution. No pulmonary angiography imaging was found in up to 3%.
TABLE 3.
Patient disposition to intervention and diagnostic imaging by region (see also supplementary figure S2)
| Europe | Japan | AAO | Total | |
| Subjects n | 779 | 115 | 116 | 1010 |
| Candidates to intervention | ||||
| PEA operable | 562 (72.1) | 27 (23.5) | 80 (69.0) | 669 (66.2) |
| BPA, not operable | 107 (13.7) | 79 (68.7) | 7 (6.0) | 193 (19.1) |
| Neither | 110 (14.1) | 9 (7.8) | 29 (25.0) | 148 (14.7) |
| VQ scan | 512 (65.7) | 114 (99.1) | 114 (98.3) | 740 (73.3) |
| PA | 754 (96.8) | 114 (99.1) | 112 (96.6) | 980 (97.0) |
| Number of pulmonary angiograms performed n | 1156 | 174 | 121 | 1451 |
| Conventional PA | 459 (39.7) | 112 (64.4) | 60 (49.6) | 631 (43.5) |
| CT PA | 595 (51.5) | 62 (35.6) | 61 (50.4) | 718 (49.5) |
| MR PA | 102 (8.8) | 0 (0.0) | 0 (0.0) | 102 (7.0) |
Values are expressed as n (%) unless otherwise indicated. AAO: America and others; PEA: pulmonary endarterectomy; BPA: balloon pulmonary angioplasty; VQ: ventilation/perfusion; PA: pulmonary angiogram; CT: computed tomography; MR: magnetic resonance.
CTEPH therapeutic algorithms
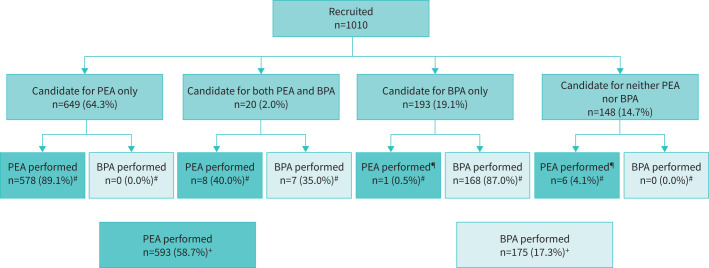
Patient haemodynamic data suitable for PEA or BPA, or neither intervention are presented in table 4. Figure 1 shows the different treatment algorithms. Of the entire cohort of 1010 patients, 669 were considered as candidates for PEA and 593 PEA procedures had been performed. The proportion of PEA candidates was considerably lower in Japan than in Europe or AAO (table 3). Out of 1010 patients, 213 were considered candidates for BPA; within this group, 20 patients were also assessed suitable for PEA. The proportion of BPA candidates (table 3, supplementary figure S2) was considerably higher in Japan than in Europe or AAO. 175 patients had undergone at least one BPA. Finally, 148 patients were originally not considered as candidates for either PEA or BPA.
TABLE 4.
Sex, history of pulmonary embolism and haemodynamics by patient disposition to intervention, all regions (n=1010)
| PEA operable candidates | BPA candidates not PEA operable | Neither PEA nor BPA | p-value (exploratory) | |
| Subjects n | 669 | 193 | 148 | |
| Age at diagnosis years | 61.0 (49.0–71.0) | 65.0 (52.0–74.0) | 70.0 (57.0–77.0) | <0.001 |
| Sex male | 358 (53.5) | 65 (33.7) | 58 (39.2) | <0.001 |
| NYHA functional class % I/II/III/IV | 1.6/25.0/58.2/15.1 | 1.0/27.5/63.2/8.3 | 0.7/17.6/70.9/10.8 | 0.027 |
| History of acute PE# | 466 (69.7) | 100 (51.8) | 97 (65.5) | <0.001 |
| Right heart catheterisation at diagnosis | ||||
| MRAP mmHg | 8.0 (5.0–12.0) | 7.0 (4.0–10.0) | 8.0 (5.0–11.8) | <0.001 |
| n (missing) | 640 (29) | 182 (11) | 140 (8) | |
| mPAP mmHg | 45.0 (36.0–51.0) | 42.0 (35.5–51.0) | 40.0 (34.0–52.0) | 0.116 |
| n (missing) | 669 (0) | 193 (0) | 148 (0) | |
| PCWP mmHg | 10.0 (8.0–13.0) | 8.0 (6.0–11.0) | 10.5 (9.0–13.0) | <0.001 |
| n (missing) | 643 (26) | 193 (0) | 148 (0) | |
| Cardiac output L·min−1 | 4.20 (3.40–5.09) | 4.00 (3.15–4.90) | 4.18 (3.33–5.00) | 0.406 |
| n (missing) | 656 (13) | 192 (1) | 144 (4) | |
| CI L·min−1·m−2 | 2.17 (1.80–2.61) | 2.28 (1.86–2.85) | 2.30 (1.86–2.70) | 0.050 |
| n (missing) | 654 (15) | 192 (1) | 141 (7) | |
| PVR dyn·s·cm−5 | 642 (449–901) | 656 (467–912) | 592 (399–962) | 0.544 |
| n (missing) | 642 (27) | 189 (4) | 142 (6) | |
| PVRI dyn·s·cm−5·m2 | 1244 (869–1670) | 1137 (811–1600) | 1085 (740–1749) | 0.172 |
| n (missing) | 631 (38) | 192 (1) | 138 (10) | |
| Numbers operated by PEA or BPA at time of baseline analysis | 593 | 175 | ||
| PVR at the end of ICU¶ dyn·s·cm−5 | 262 (200–351) | 298 (228–394) | 0.004 | |
| n (missing), preliminary data | 507 (86) | 125 (50) | ||
| Reduction in PVR since diagnosis dyn·s·cm−5 | 372 (190–619) | 343 (154–527) | 0.185 | |
| n (missing), preliminary data | 496 (97) | 123 (52) | ||
Values are expressed as median with first and third quartiles (Q1–Q3) or n (%) unless otherwise indicated. PEA: pulmonary endarterectomy; BPA: balloon pulmonary angioplasty; NYHA: New York Heart Association; PE: pulmonary embolism; MRAP: mean right atrial pressure; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; CI: cardiac index; PVR: pulmonary vascular resistance; PVRI: pulmonary vascular resistance index; ICU: intensive care unit. #: unknown cases included in the exploratory analysis of significance. ¶: after last BPA for BPA patients.
FIGURE 1.
Patient disposition. Candidates for surgery (PEA) and/or angioplasty (BPA) or inoperable/non-interventional patients were assessed as such at time of diagnosis by the multidisciplinary CTEPH team with an experienced PEA surgeon, according to predefined criteria (see Methods). Operated patients are those who actually underwent surgery. BPA: balloon pulmonary angioplasty; CTEPH: chronic thromboembolic pulmonary hypertension; PEA: pulmonary endarterectomy. #: Percentage value corresponds to the disposition to intervention of recruited patients. ¶: Patients initially deemed inoperable but who were subsequently operated on. +: Percentage value corresponds to the total number of recruited patients.
Reasons for inoperability
Reasons for PEA inoperability are presented in table 5. The distinction between technical inoperability (68.1%), inoperable patients due to comorbidity conditions (18.5%) and patient's choice (12.9%) was made by local CTEPH teams. Inoperable patients were predominantly females; the median age of inoperable patients was 67 years. The proportion of patients who declined surgery was higher in Asians (21.2%) than Caucasians (9.1%). In Japan, 77.8% of operable CTEPH patients refused surgery compared to Europe with 3.7% and AAO with 2.5%.
TABLE 5.
Baseline characteristics of inoperable patients, by reason for inoperability, all regions
| Technically inoperable | Comorbidities | Patient refusal | Total inoperable# | |
| Subjects n | 235 | 63 | 44 | 341 |
| Sex male | 80 (34.0) | 25 (39.7) | 16 (36.4) | 123 (36.1) |
| Age at diagnosis years | 66.0 (54.0–74.0) | 71.0 (59.3–76.8) | 71.0 (59.3–76.8) | 67.0 (56.0–76.0) |
| Age category at diagnosis | ||||
| <50 years | 42 (17.9) | 7 (11.1) | 9 (20.5) | 57 (16.8) |
| ≥50 years and <70 years | 99 (42.3) | 24 (38.1) | 11 (25.0) | 135 (39.7) |
| ≥70 years | 93 (39.7) | 32 (50.8) | 24 (54.5) | 148 (43.5) |
| Ethnicity¶ | ||||
| Caucasian/white | 167 (71.1) | 47 (74.6) | 21 (47.7) | 232 (68.0) |
| Black | 5 (2.1) | 2 (3.2) | 3 (6.8) | 11 (3.2) |
| Asian | 63 (26.8) | 14 (22.2) | 21 (47.7) | 99 (29.0) |
| Other | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| Time from onset of PH symptoms to diagnosis months | 13.0 (5.0–25.0) | 12.0 (5.5–44.5) | 16.0 (10.0–28.0) | 13.0 (6.0–28.0) |
| NYHA functional class % I/II/III/IV | 1.3/20.9/68.9/8.9 | 0.0/15.9/69.8/14.3 | 0.0/43.2/52.3/4.5 | 0.9/23.2/66.6/9.4 |
Values are expressed as median with first and third quartiles (Q1–Q3) or n (%) unless otherwise indicated. PH: pulmonary hypertension; NYHA: New York Heart Association. #: more than one reason for inoperability is possible. ¶: totals exceed 100% since it is possible to record more than one ethnicity. “Caucasian/white” and “Asian” was recorded for one patient in Europe, two patients in Japan and one patient in America and others.
Medical treatment at diagnosis
In 362 patients (35.8%), at least one PAH therapy was initiated at CTEPH diagnosis, including phosphodiesterase Type V (PDE5) inhibitors, a soluble guanylate cyclase stimulator, endothelin receptor antagonists, prostacyclin analogues or other drugs (supplementary table S2). The operable group received fewer PAH therapies than the BPA group or the non-PEA/BPA group. For the PEA group, a PDE5 inhibitor was the most frequently prescribed class of drug, whereas a soluble guanylate cyclase stimulator was prescribed preferentially for the BPA group and the non-interventional group. Most patients received a single-drug therapy.
Balloon pulmonary angioplasty
In the whole population, 175 patients (17.3%) were treated by BPA (supplementary table S3) and underwent a total of 815 BPA sessions with a mean of 4.7 procedures (median: 4, range: 1 to 14) per patient. The proportion of females differed among regions and was considerably higher in Japan compared to Europe/AAO. Further differences were seen across regions for time from diagnosis to first BPA, time between BPA sessions, reduction in PVR, proportion of patients treated with PH-targeted drugs at discharge and proportion of patients on oxygen therapy at discharge.
Mortality
During the 32 months of observation, 39 patients (3.9%) died due to PH-related causes. Overall, 21 patients (3.5%) died after PEA, three patients (1.8%) died after BPA and 15 patients (6.0%) died without intervention. Supplementary table S4 contains further details.
Discussion
This prospective observational registry represents the largest contemporary population of patients with CTEPH worldwide, with 1010 incident patients with prospective follow-up capturing all current treatment modalities [18].
There are three main findings of the study. First, we found distinct regional differences in age of the patients, their baseline haemodynamic data and management decisions concerning the three main treatment modalities of PEA, BPA and PAH therapy; CTEPH patients in Japan were more frequently treated with BPA than PEA, whereas PEA treatment was much higher than BPA in Europe and AAO. Second, sex was evenly distributed in all regions except for Japan where females were predominant. Third, there was a significantly lower rate of acute pulmonary embolism in Japan.
The median age at diagnosis in Europe and Japan was ∼10 years higher than in AAO, which may reflect the regional population profile and is consistent with previous reports [8, 10, 15]. Another explanation could be a selection bias and only selected patients were referred for CTEPH management by primary or secondary practitioners.
In >95% of patients, haemodynamic data were complete, showing comparable results for PEA-, BPA- and/or medically treated CTEPH patients, resembling previous reports [6, 10, 17]. However, haemodynamic data varied significantly across regions. Despite lower mPAP values, PVR was significantly higher in Japan due to lower cardiac output and PCWP. Lower cardiac output in Japan was explained by the smaller body size and female predominance of the Japanese cohort. Thus, for the sake of better comparability in future international trials it would be suggested to use values that take account of body surface area. Additionally, despite higher PVR at presentation, we observed more pronounced PVR reduction in Japan compared with Europe or AAO, which might be explained by the differences in patient selection and the greater BPA experience. We must emphasise that patients in Japan had a much shorter interval between time from diagnosis to first BPA, which reflects a higher proportion of patients from Europe and AAO with a higher degree of microvasculopathy. A difference in the approach to BPA procedures between the regions might also be of importance, but this registry cannot elucidate this topic.
Japan had the highest number of patients per country that were treated by BPA, which might reflect the longest experience with the BPA procedure compared to any other geographical region [10, 19, 20]. In Japan, BPA was the predominant treatment option for female patients. This contrasts with the European experience with almost balanced sex proportions [17, 21]. The reason for the difference remains speculative. In addition to experience related to centres with PEA operability, there might be a cultural reluctance regarding major cardiac surgery. We compared the proportions between coronary artery bypass graft (CABG) operations and percutaneous coronary interventions (PCI) for Japan (CABG:PCI=1:13) [22], Great Britain (1:11) [23] and Germany (1:10); [24] this revealed a trend for slightly more PCIs in Japan, but these numbers do not reflect the larger differences observed between BPA and PEA.
This new registry confirms that PEA remains the treatment of choice for CTEPH patients in Europe and AAO. Expert surgical centres (>50 PEAs per year) demonstrate an important advance in approach to technical operability specifically extending the distal limits of endarterectomy [14, 25]. The main reasons for ineligibility for PEA were technical inoperability, comorbidities, patient refusal to undergo surgery and probable limited access to BPA.
PAH therapy was used in around a quarter of operable patients and in around half of BPA patients or of patients without any intervention. These data are similar to those for operable and inoperable patients of the previous European registry [8]. The reason why only half of BPA patients received targeted drug treatment is unclear, but the availability and reimbursement of the targeted therapy for CTEPH patients varies among the countries, affecting prescription patterns. Riociguat was effectively available in more than half of the participating countries at registry start and became available in all but two of the remaining countries during the enrolment period. It remained the only approved drug for treatment of inoperable CTEPH patients (at the time of the registry) [9, 26] and was prescribed predominantly for BPA candidates, while PDE5 inhibitors were mainly used to bridge patients to the PEA surgery. In general, the use of PAH therapy has not substantially increased compared with a previous publication [8]. The benefit of preoperative drug treatment is still a matter of debate and is not recommended by the guidelines.
Female sex dominance was also present in a recently published multicentre study of seven BPA centres in Japan presenting a cohort of 308 patients [10–12, 27]. In a retrospective venous thromboembolism registry in Japan, women were over-represented with a proportion of 61% [28]. However, in the small prospective Japanese J-EINSTEIN DVT and pulmonary embolism trial comparing rivaroxaban with enoxaparin/vitamin K antagonist therapy, only 49 out of 100 patients were female [29]. We have not found an explanation in sex unbalance in the current registry or previous studies. The predominantly female sex distribution, and the higher rate of diagnosis of DVT and the lower rate of diagnosis of acute pulmonary embolism in Japan remains speculative and requires further prospective studies.
In contrast to the original European registry [8], the current registry is global including sites from America and Asia (34 centres overall) for worldwide assessment of CTEPH patients’ management in the current era with increased treatment options. Despite the additional treatment modality of BPA, the overall proportion of patients treated with PEA remained similar. In addition to the similar number of patients receiving intervention with PEA, 19.3% of patients underwent BPA in the current registry, leading to a smaller number of patients not suitable for any interventions and left only on PAH therapy. Regarding short-term mortality, the proportion of deaths after PEA is slightly higher compared to BPA and highest for patients without intervention.
Regarding diagnostic imaging in CTEPH patients, there was a significantly lower number of VQ scans performed in Europe than in Japan and AAO. The reason for the low rate of VQ scans in Europe could be driven by the fact that 520 patients (66.8% of all European patients) were included by four high-volume centres where the evaluation seeks to address suitability for surgery, with pulmonary angiography for final decision-making. In all regions, the rate of performing any kind of pulmonary angiography (i.e. conventional PA, CTPA and magnetic resonance PA) was almost 100%.
Finally, the number of patients declining surgery was considerable (12.9%), especially in Asian ethnicity (table 5). In this context, it should be highlighted that 43% of these patients were in NYHA functional class I/II and had a low surgical risk. Retrospective, single centre data analysis from the ASPIRE registry demonstrates increased mortality of operable patients who declined surgery compared with operated patients; the latter group had a far better 5-year survival (83% versus 53%) [30]. This highlights the importance of thorough and individualised patient information about all treatment modalities so that the risk of surgery can be weighed against the estimated benefits in quality of life and life expectancy without missing a potentially curative treatment option [1, 5].
Strength and limitations of the study
This is the first multicentre, worldwide prospective registry, where data from diverse international populations were collected providing information on the multimodality management of CTEPH patients. An additional strength of the registry is the investigators’ high compliance to the protocol; therefore, we demonstrated a low number of missing values indicating a robust database for analysis. The limitations of the study are inherent to an observational registry design: some assessments were not collected systematically, leading to underreporting (e.g. post-procedural right heart catheterisation). Right heart catheterisation was only performed in the intensive care unit or after the BPA procedure, which does not reflect final haemodynamic improvements. Not all three treatment options were available in all participating countries. Most of the participating centres were referral centres for PEA and/or BPA, which may have led to an overestimation in the proportion of eligible patients for PEA or BPA. In addition, there may have been a geographical bias in that 69.9% of the European patients were included by four high-volume centres (>50 patients per centre).
In conclusion, this is the first international multicentre, prospective observational registry to provide data regarding diagnosis, treatment decisions and management of CTEPH patients across the world. PEA remains the treatment of choice for CTEPH patients. However, the registry data highlight regional differences regarding patient characteristics (rates of pulmonary embolism and sex) and therapeutic approaches in Japan compared with Europe and AAO, diagnostic imaging and treatment allocations between operable and inoperable patients. One-year mortality was highest in patients without interventional treatment. The ongoing 3-year follow-up will give further insights into the impact of current management practices on survival, functional status and quality of life of CTEPH patients.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00850-2020.SUPPLEMENT (308.3KB, pdf)
Acknowledgements
The authors acknowledge the contribution of the following investigators: Joan A. Barberà, Hospital Clínic–IDIBAPS, CIBER Enfermedades Respiratorias, University of Barcelona, Barcelona, Spain; Gerry J. Coghlan, Dept of Cardiology, Royal Free London National Health Services Foundation Trust, London, UK; Gustavo Heresi, Dept of Pulmonary and Critical Care Medicine, Cleveland Clinic, Cleveland, OH, USA; Hsao-Hsun Hsu, Dept of Surgery, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan; Kim Kerr, Division of Pulmonary and Critical Care Medicine, University of California, San Diego, La Jolla, CA, USA; Jerzy Lewczuk, Regional Specialist Hospital and Medical University, Wroclaw, Poland; Trevor Williams, Dept of Cardiothoracic Surgery, Lung Transplantation, Anaesthesia and Intensive Care, The Alfred Hospital, Melbourne, Victoria, Australia; Kirill V. Mershin, Russian Cardiology Research and Production Complex, Ministry of Health of Russia, Moscow, Russia; Toru Satoh, Division of Cardiology, Second Dept of Internal Medicine, Kyorin University School of Medicine, Tokyo, Japan; Nobuhiro Tanabe, Dept of Respirology, Graduate School of Medicine, Chiba University, Chiba City, Japan; Anton Vonk Noordegraaf, Amsterdam UMC, Vrije Universiteit Amsterdam, Pulmonary Medicine, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
The authors also thank David Bowers for statistical analyses and Simone Lerch and Sonja Mariotti (International CTEPH Association, Switzerland) for project management. The CTEPH Registry is owned and managed by the International CTEPH Association. The association is headed by an Executive Board, composed of CTEPH experts. The Executive Board of the association was responsible for the design of the registry, provided input into the analyses, decided on medical interpretation, and oversaw the development of the manuscript.
Submitted article, peer reviewed.
This article has supplementary material available from openres.ersjournals.com.
This study is registered at www.clinicaltrials.gov with identifier number NCT02656238. The data underlying this article are the property of the International CTEPH Association (ICA). Data will be shared on reasonable request to the corresponding author with permission of the ICA.
Conflict of interest: S. Guth reports personal fees from Actelion, Bayer, GSK, MSD and Pfizer outside the submitted work.
Conflict of interest: A.M. D'Armini reports personal fees from Actelion, Bayer and MSD outside the submitted work.
Conflict of interest: M. Delcroix reports grants and personal fees from Actelion, and personal fees from Bayer, MSD, Reata and Bellarophon, outside the submitted work.
Conflict of interest: K. Nakayama has nothing to disclose.
Conflict of interest: E. Fadel has nothing to disclose.
Conflict of interest: S.P. Hoole has nothing to disclose.
Conflict of interest: D.P. Jenkins reports personal fees from Actelion, and grants and personal fees from Bayer, outside the submitted work.
Conflict of interest: D.G. Kiely reports grants, personal fees and nonfinancial support from Actelion, Bayer and GSK, and personal fees and nonfinancial support from MSD, outside the submitted work.
Conflict of interest: N.H. Kim reports personal fees from Actelion, Bayer and Merck, and grants from United Therapeutics and SoniVie, outside the submitted work.
Conflict of interest: I.M. Lang reports grants and personal fees from Actelion and AOPOrphan Pharma, personal fees from MSD, and nonfinancial support from Medtronic, during the conduct of the study; and grants and personal fees from Actelion and AOPOrphan, and personal fees from MSD and Ferrer, outside the submitted work.
Conflict of interest: M.M. Madani is a consultant for Actelion.
Conflict of interest: H. Matsubara reports personal fees from Actelion, AOP orphan Pharmaceuticals AG, Bayer, GlaxoSmithKline, Pfizer Japan, Inc., United Therapeutics, Nippon Shinyaku, Co., Ltd, and Kaneka Medix Corporation, outside the submitted work.
Conflict of interest: A. Ogawa reports personal fees from Nippon Shinyaku Co., Ltd., outside the submitted work.
Conflict of interest: J. Ota-Arakaki has nothing to disclose.
Conflict of interest: R. Quarck has nothing to disclose.
Conflict of interest: R. Sadushi-Kolici has nothing to disclose.
Conflict of interest: G. Simonneau has nothing to disclose.
Conflict of interest: C.B. Wiedenroth reports personal fees from Actelion, AOP, Bayer, MSD and Pfizer outside the submitted work.
Conflict of interest: B. Yildizeli has nothing to disclose.
Conflict of interest: E. Mayer reports personal fees from Actelion, Bayer, MSD and BMS outside the submitted work.
Conflict of interest: J. Pepke-Zaba reports personal fees and nonfinancial support from Actelion and Merck, and nonfinancial support from GSK, outside the submitted work.
Support statement: The study was funded by a grant from Bayer AG, Merck Sharp & Dohme Corp (a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) and Actelion Pharmaceuticals Ltd (Allschwil, Switzerland). These companies had no influence on data interpretation and reporting of the registry. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Kim NH, Delcroix M, Jais X, et al. . Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. doi: 10.1183/13993003.01915-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmud E, Madani MM, Kim NH, et al. . Chronic thromboembolic pulmonary hypertension: evolving therapeutic approaches for operable and inoperable disease. J Am Coll Cardiol 2018; 71: 2468–2486. doi: 10.1016/j.jacc.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 3.Amsallem M, Guihaire J, Arthur Ataam J, et al. . Impact of the initiation of balloon pulmonary angioplasty program on referral of patients with chronic thromboembolic pulmonary hypertension to surgery. J Heart Lung Transplant 2018; 37: 1102–1110. doi: 10.1016/j.healun.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 4.Lankeit M, Krieg V, Hobohm L, et al. . Pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2018; 37: 250–258. doi: 10.1016/j.healun.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 5.Jenkins D, Madani M, Fadel E, et al. . Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26:160111. doi: 10.1183/16000617.0111-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delcroix M, Lang I, Pepke-Zaba J, et al. . Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation 2016; 133: 859–871. doi: 10.1161/CIRCULATIONAHA.115.016522 [DOI] [PubMed] [Google Scholar]
- 7.Cannon JE, Su L, Kiely DG, et al. . Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom National Cohort. Circulation 2016; 133: 1761–1771. doi: 10.1161/CIRCULATIONAHA.115.019470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepke-Zaba J, Delcroix M, Lang I, et al. . Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008 [DOI] [PubMed] [Google Scholar]
- 9.Halank M, Hoeper MM, Ghofrani HA, et al. . Riociguat for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: results from a phase II long-term extension study. Respir Med 2017; 128: 50–56. doi: 10.1016/j.rmed.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 10.Mizoguchi H, Ogawa A, Munemasa M, et al. . Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077 [DOI] [PubMed] [Google Scholar]
- 11.Sugimura K, Fukumoto Y, Satoh K, et al. . Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488. doi: 10.1253/circj.CJ-11-1217 [DOI] [PubMed] [Google Scholar]
- 12.Kataoka M, Inami T, Hayashida K, et al. . Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762. doi: 10.1161/CIRCINTERVENTIONS.112.971390 [DOI] [PubMed] [Google Scholar]
- 13.Galie N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 14.D'Armini AM, Morsolini M, Mattiucci G, et al. . Pulmonary endarterectomy for distal chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2014; 148: 1005–1011. doi: 10.1016/j.jtcvs.2014.06.052 [DOI] [PubMed] [Google Scholar]
- 15.Madani MM, Auger WR, Pretorius V, et al. . Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012; 94: 97–103. doi: 10.1016/j.athoracsur.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Mayer E, Jenkins D, Lindner J, et al. . Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. doi: 10.1016/j.jtcvs.2010.11.024 [DOI] [PubMed] [Google Scholar]
- 17.Olsson KM, Wiedenroth CB, Kamp JC, et al. . Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J 2017; 49:1602409. doi: 10.1183/13993003.02409-2016 [DOI] [PubMed] [Google Scholar]
- 18.Tanabe N, Kawakami T, Satoh T, et al. . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: a systematic review. Respir Investig 2018; 56: 332–341. doi: 10.1016/j.resinv.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 19.Fukui S, Ogo T, Morita Y, et al. . Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J 2014; 43: 1394–1402. doi: 10.1183/09031936.00012914 [DOI] [PubMed] [Google Scholar]
- 20.Inami T, Kataoka M, Shimura N, et al. . Pulmonary edema predictive scoring index (PEPSI), a new index to predict risk of reperfusion pulmonary edema and improvement of hemodynamics in percutaneous transluminal pulmonary angioplasty. JACC Cardiovasc Interv 2013; 6: 725–736. doi: 10.1016/j.jcin.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 21.Andreassen AK, Ragnarsson A, Gude E, et al. . Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013; 99: 1415–1420. doi: 10.1136/heartjnl-2012-303549 [DOI] [PubMed] [Google Scholar]
- 22.Yasuda S, Nakao K, Nishimura K, et al. T he Japanese Registry of all Cardiac and Vascular Diseases . 2016.http://j-circ.or.jp/jittai_chosa/jittai_chosa2015web.pdf Date last accessed: 21 July 2021.
- 23.National Institute for Cardiovascular Outcomes Research (NICOR) . National Cardiac Audit Programme (NCAP). Annual report 2020. 2020. https://www.nicor.org.uk/wp-content/uploads/2020/12/NCAP-Patient-and-Public-Report-2020-FINAL.pdf Date last accessed: 21 July 2021.
- 24.Deutscher Herzbericht 2020 . 2020. https://www.herzstiftung.de/system/files/2021-06/Deutscher-Herzbericht-2020.pdf Date last accessed: 21 July 2021.
- 25.Madani M, Mayer E, Fadel E, et al. . Pulmonary endarterectomy: patient selection, technical challenges, and outcomes. Ann Am Thorac Soc 2016; 13: Suppl. 3, S240–S247. doi: 10.1513/AnnalsATS.201601-014AS [DOI] [PubMed] [Google Scholar]
- 26.Ghofrani HA, D'Armini AM, Grimminger F, et al. . Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. doi: 10.1056/NEJMoa1209657 [DOI] [PubMed] [Google Scholar]
- 27.Ogawa A, Satoh T, Fukuda T, et al. . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes 2017; 10:e004029. doi: 10.1161/CIRCOUTCOMES.117.004029 [DOI] [PubMed] [Google Scholar]
- 28.Yamashita Y, Morimoto T, Amano H, et al. . Anticoagulation therapy for venous thromboembolism in the real world: from the COMMAND VTE registry. Circ J 2018; 82: 1262–1270. doi: 10.1253/circj.CJ-17-1128 [DOI] [PubMed] [Google Scholar]
- 29.Yamada N, Hirayama A, Maeda H, et al. . Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism – the J-EINSTEIN DVT and PE program. Thromb J 2015; 13: 2. doi: 10.1186/s12959-015-0035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quadery SR, Swift AJ, Billings CG, et al. . The impact of patient choice on survival in chronic thromboembolic pulmonary hypertension. Eur Respir J 2018; 52:1800589. doi: 10.1183/13993003.00589-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00850-2020.SUPPLEMENT (308.3KB, pdf)