Abstract
Background
Most reports of influenza vaccine effectiveness consider current-season vaccination only.
Aim
We evaluated a method to estimate the effect of influenza vaccinations (EIV) considering vaccination history.
Methods
We used a test-negative design with well-documented vaccination history to evaluate the average EIV over eight influenza seasons (2011/12–2018/19; n = 10,356). Modifying effect was considered as difference in effects of vaccination in current and previous seasons and current-season vaccination only. We also explored differences between current-season estimates excluding from the reference category people vaccinated in any of the five previous seasons and estimates without this exclusion or only for one or three previous seasons.
Results
The EIV was 50%, 45% and 38% in people vaccinated in the current season who had previously received none, one to two and three to five doses, respectively, and it was 30% and 43% for one to two and three to five prior doses only. Vaccination in at least three previous seasons reduced the effect of current-season vaccination by 12 percentage points overall, 31 among outpatients, 22 in 9–65 year-olds, and 23 against influenza B. Including people vaccinated in previous seasons only in the unvaccinated category underestimated EIV by 9 percentage points on average (31% vs 40%). Estimates considering vaccination of three or five previous seasons were similar.
Conclusions
Vaccine effectiveness studies should consider influenza vaccination in previous seasons, as it can retain effect and is often an effect modifier. Vaccination status in three categories (current season, previous seasons only, unvaccinated) reflects the whole EIV.
Keywords: influenza vaccine effectiveness, influenza vaccination effect, prior vaccination, repeated vaccination, modifying effect, influenza vaccine
Introduction
The World Health Organization recommends annual influenza vaccination in the target population [1]. People vaccinated against influenza in one season are more likely to be vaccinated in the next one [2]; therefore those in the target population frequently accumulate influenza vaccines over the years.
Influenza vaccines received in previous seasons may retain a notable protective effect [3-5]. In contrast with that, frequent prior vaccination has been related with a reduced effect (i.e. effect modifier) of the current-season vaccine in some studies [6-8]. Since influenza vaccination history is associated with a higher probability of current season vaccination and a lower risk of influenza, it is also a potential confounding factor of the current-season influenza vaccine effectiveness (IVE) [9-11].
IVE studies usually aim at assessing how each seasonal vaccine composition has worked against the circulating influenza viruses in real-life conditions, when frequently repeated vaccination is the most common scenario. However, most of these studies consider only the current-season vaccination [12], and those that consider previous vaccines usually do so as a secondary analysis [3,13].
The estimates from IVE studies are also used to inform population and health professionals about how much influenza vaccination reduces the risk of influenza outcomes; they are also used to estimate the impact of influenza vaccination in the population. For these objectives, it seems more adequate to estimate the effect of influenza vaccinations (EIV) considering both the current-season vaccination and the vaccination history. The inclusion of people only vaccinated in prior seasons in the reference category of unvaccinated may underestimate the EIV.
Influenza epidemics and IVE show important variability among seasons because vaccine composition and circulating virus strains change. The pooled analysis of multiple seasons provides average patterns that can guide public health recommendations.
Given the annual recommendation for influenza vaccination, this study’s aim was to evaluate how vaccination history affects the estimates of current-season IVE and to determine the simplest method to obtain valid estimates of the EIV for people according to their influenza vaccination status in the current and previous seasons.
Methods
Design and setting
This methodological proposal is illustrated with data from patients attended in primary healthcare centres and hospitals in the region of Navarre, Spain, during influenza seasons 2011/12 to 2018/19. Annual IVE studies have been conducted since 2009 using the test-negative case–control study design [14], nested in the cohort of the population covered by the Regional Health Service [8,15-21]. This Health Service provides healthcare, free at point of service, to 97% of the population. The trivalent inactivated influenza vaccine was recommended annually and offered free of charge to people 60 years or older (although the uptake was higher from age 65 years upwards) and to those with risk factors or major chronic conditions. Other people could also be vaccinated if they paid for the vaccine.
Case definition and information sources
Influenza surveillance was based on automatic reporting of cases of medically attended influenza-like illness (ILI) from all primary healthcare centres and hospitals [18]. ILI was defined as the sudden onset of any general symptom (fever, malaise, headache or myalgia) in addition to any respiratory symptom (cough, sore throat or dyspnoea). A sentinel network composed of a representative sample of primary healthcare physicians took double swabs, nasopharyngeal and pharyngeal, after obtaining verbal informed consent, from all their patients diagnosed with ILI whose symptoms had begun fewer than 5 days before the patient consultation. The protocol for influenza cases in hospitals establishes early detection and nasopharyngeal and pharyngeal swabbing at admission of all hospitalised patients with ILI. Swabs were tested for influenza viruses by reverse transcription PCR (RT-PCR).
For the present study, we used the test-negative case–control design over eight influenza seasons, from 2011/12 to 2018/19. We included only patients with continued residence in the region during the previous 5 years. Children younger than 9 years, healthcare workers and nursing home residents were excluded. Cases were ILI patients of primary healthcare centres or hospitals, who were confirmed positive for influenza virus by RT-PCR, and controls were similar patients who tested negative for any influenza virus. The influenza vaccination status in the current season and vaccination history were obtained from the regional vaccination register, and only registered doses were considered [2]. Patients who had received an influenza vaccine fewer than 14 days before symptom onset were excluded.
Statistical analysis
Characteristics of study participants by confirmed influenza status and current-season influenza vaccination were compared by chi-squared test. Logistic regression was used to calculate the odds ratios (OR) with their 95% confidence intervals (CI). All models were adjusted for age group (9–44, 45–64, 65–84 and ≥ 85 years), major chronic conditions, calendar month and season of sample collection, and for healthcare setting (primary healthcare or hospital). The IVE and EIV were estimated as (1 − adjusted OR) × 100%.
We evaluated different categorisations of the vaccination status. We considered the reference values to be the EIV estimates obtained from the ‘full model’ with the vaccination status in six categories: current-season vaccination and three to five prior doses, current-season vaccination and one to two prior doses, current-season vaccination and no prior doses, three to five prior doses and no current-season vaccination, one to two prior doses and no current-season vaccination, and unvaccinated in the current and five previous seasons as the reference category [6-8].
The modifying effect of previous seasons’ vaccines on current-season IVE was evaluated in the full model by the absolute difference in effectiveness of each vaccination status in comparison with the category of current-season vaccination and no prior doses as reference. When this difference was statistically significant (p < 0.05), modifying effect of vaccination history on the current-season vaccine effectiveness was concluded, and the final results were those from combining vaccination categories of the current and previous seasons. Interaction terms between current-season vaccination and prior doses received were tested and these results are presented in the Supplement.
As the full model requires high sample size, estimates from alternate models were compared with those from the full model to determine the analysis with the fewest requirements of vaccination history data and successful management of the remaining and modifying effects of prior vaccines. We also tested models combining vaccination status in the current and either one or three immediately preceding seasons, as well as the model considering only the current-season influenza vaccination (only-current-season model). When the three estimates of the current-season IVE provided by the full model were not statistically different, the analysis was summarised in a ‘summarised model’ with only three categories: current-season vaccination regardless of prior doses, no current-season vaccination but any prior doses, and neither current-season vaccination nor any prior doses as the reference category [22].
The inclusion in the reference category of individuals vaccinated only in previous seasons may bias the EIV estimates. This bias was evaluated as the absolute difference in estimates as ∆IVE = IVE0 – IVE1; where IVE1 was the current-season EIV estimate in the summarised model considering vaccination in the five preceding seasons, and IVE0 was the current-season IVE estimate in either the only-current-season model or the summarised models considering vaccination in either one or three previous seasons. A ∆IVE of five or more percentage points was considered as a bias and of 10 or more as relevant bias [23,24].
We tested the proposal in separated analyses by healthcare setting, age group, virus (sub-)type and influenza season. Seasons with minimal circulation of a given (sub-)type were excluded from the pooled analysis for that outcome. In sensitivity analyses, we included the diagnoses of ILI in the immediately preceding season as a covariable.
Ethical statement
The Navarra Ethical Committee for Medical Research approved the study protocol (Pyto 85/11, Pyto 2015/95 and Pyto 2017/88).
Results
Characteristics of participants
During the eight influenza seasons studied, 10,356 swabbed patients were enrolled: 4,412 patients attended in primary healthcare and 5,944 were hospitalised patients, of whom respectively 2,872 (65%) and 2,069 (35%) were confirmed for influenza virus infection.
Table 1 describes the participants’ characteristics by influenza case status and current vaccination status. Among the total number of patients, 46% were 65 years or older and 38% had received the influenza vaccine in the current season. Current season vaccination was less frequent in cases than in controls (29% vs 44%; p < 0.001). Influenza A(H3N2) accounted for almost half of the cases (47%), while influenza A(H1N1) and B were equally frequent (26% and 27%, respectively) (Supplementary Table S1).
Table 1. Participant profile, by influenza case status and current vaccination status, Navarre, Spain, pooled analysis of 2011/12–2018/19 seasons (n = 10,356).
| Laboratory-confirmed cases | Negative controls | p value | Vaccinated in current season | Unvaccinated in current season | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Total | 4,941 | 100 | 5,415 | 100 | NA | 3,952 | 100 | 6,404 | 100 | NA |
| Age groups (years) | ||||||||||
| 9–44 | 1,798 | 36 | 1,279 | 24 | < 0.001 | 265 | 7 | 2,812 | 44 | < 0.001 |
| 45–64 | 1,328 | 27 | 1,194 | 22 | 516 | 13 | 2,006 | 31 | ||
| 65–84 | 1,342 | 27 | 2,076 | 38 | 2,189 | 55 | 1,229 | 19 | ||
| ≥ 85 | 473 | 10 | 866 | 16 | 982 | 25 | 357 | 6 | ||
| Sex | ||||||||||
| Male | 2,510 | 51 | 2,837 | 52 | 0.105 | 2,089 | 53 | 3,258 | 51 | 0.050 |
| Female | 2,431 | 49 | 2,578 | 48 | 1,863 | 47 | 3,146 | 49 | ||
| Major chronic conditions | ||||||||||
| No | 2,437 | 49 | 1,794 | 33 | < 0.001 | 636 | 16 | 3,595 | 56 | < 0.001 |
| Yes | 2,504 | 51 | 3,621 | 67 | 3,316 | 84 | 2,809 | 44 | ||
| Healthcare setting | ||||||||||
| Primary care | 2,872 | 58 | 1,540 | 28 | < 0.001 | 693 | 18 | 3,719 | 58 | < 0.001 |
| Hospital | 2,069 | 42 | 3,875 | 72 | 3,259 | 82 | 2,685 | 42 | ||
| Influenza-like illness diagnosis in the immediately preceding season | ||||||||||
| No | 4,806 | 97 | 5,248 | 97 | 0.288 | 3,887 | 98 | 6,167 | 96 | < 0.001 |
| Yes | 135 | 3 | 167 | 3 | 65 | 2 | 237 | 4 | ||
| Vaccination in the current season | ||||||||||
| No | 3,500 | 71 | 2,904 | 54 | < 0.001 | NA | ||||
| Yes | 1,441 | 29 | 2,511 | 46 | ||||||
| Vaccination in the previous season | ||||||||||
| No | 3,561 | 72 | 2,974 | 55 | < 0.001 | 627 | 16 | 5,908 | 92 | < 0.001 |
| Yes | 1,380 | 28 | 2,441 | 45 | 3,325 | 84 | 496 | 8 | ||
| Vaccines in the five previous seasons | ||||||||||
| 0 | 3,191 | 65 | 2,410 | 45 | < 0.001 | 283 | 7 | 5,318 | 83 | < 0.001 |
| 1–2 | 402 | 8 | 625 | 12 | 454 | 11 | 573 | 9 | ||
| 3–5 | 1,348 | 27 | 2,380 | 44 | 3,215 | 81 | 513 | 8 | ||
| Influenza season | ||||||||||
| 2011/12 | 351 | 7 | 235 | 4 | < 0.001 | 96 | 2 | 490 | 8 | < 0.001 |
| 2012/13 | 315 | 6 | 258 | 5 | 92 | 2 | 481 | 8 | ||
| 2013/14 | 527 | 11 | 476 | 9 | 331 | 8 | 672 | 10 | ||
| 2014/15 | 573 | 12 | 482 | 9 | 325 | 8 | 730 | 11 | ||
| 2015/16 | 734 | 15 | 703 | 13 | 457 | 12 | 980 | 15 | ||
| 2016/17 | 662 | 13 | 878 | 16 | 711 | 18 | 829 | 13 | ||
| 2017/18 | 1,044 | 21 | 1,167 | 22 | 1,029 | 26 | 1,182 | 18 | ||
| 2018/19 | 735 | 15 | 1,216 | 22 | 911 | 23 | 1,040 | 16 | ||
NA: not applicable.
Influenza vaccination in previous seasons met the conditions for being a potential confounding factor in the analysis of IVE, since it was more frequent among controls than in cases (55% vs 35%; p < 0.001) and among those patients vaccinated in the current season than in the rest (93% vs 17%; p < 0.001).
Effect of vaccination in the current and previous seasons
Compared with persons unvaccinated in the current and the five previous seasons, those vaccinated in the current season had an average protective effect of 50% (95% CI: 35–62), 45% (95% CI: 31–56) and 38% (95% CI: 30–45) if they had received none, one to two and three to five prior doses of vaccine, respectively. Persons unvaccinated in the current season also experienced a protective effect of 30% (95% CI: 16–42) and 43% (95% CI: 29–53) if they had received one to two or three to five prior doses, respectively. The EIV estimates from the full models considering combination of vaccination status in the current and previous seasons did not show relevant differences when three or five previous seasons were considered, but the differences increased when only one previous season was considered (Table 2).
Table 2. Effect of influenza vaccination in the current and previous seasons for all patients and by healthcare setting, Navarre, Spain, pooled analysis of the 2011/12–2018/19 seasons (n = 10,356).
| All patients | Primary healthcare patients | Hospital patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Vaccination effect | Cases | Controls | Vaccination effect | Cases | Controls | Vaccination effect | ||||
| % | 95% CI a | % | 95% CI a | % | 95% CI a | |||||||
| Only-current-season model | ||||||||||||
| Unvaccinated | 3,500 | 2,904 | 0 (ref) | 2,474 | 1,245 | 0 (ref) | 1,026 | 1,659 | 0 (ref) | |||
| Vaccinated | 1,441 | 2,511 | 31 | 23–38 | 398 | 295 | 35 | 21–47 | 1,043 | 2,216 | 29 | 19–37 |
| Summarised model with one previous season | ||||||||||||
| Never vaccinated | 3,333 | 2,575 | 0 (ref) | 2,433 | 1,210 | 0 (ref) | 900 | 1,365 | 0 (ref) | |||
| Prior and no current | 167 | 329 | 41 | 27–52 | 41 | 35 | 42 | 7–64 | 126 | 294 | 40 | 24–53 |
| Current regardless prior | 1,441 | 2,511 | 36 | 29–43 | 398 | 295 | 37 | 23–48 | 1,043 | 2,216 | 36 | 27–44 |
| Full model with one previous season | ||||||||||||
| Never vaccinated | 3,333 | 2,575 | 0 (ref) | 2,433 | 1,210 | 0 (ref) | 900 | 1,365 | 0 (ref) | |||
| Prior and no current | 167 | 329 | 41 | 27–52 | 41 | 35 | 41 | 6–64 | 126 | 294 | 40 | 24–53 |
| Current and no prior | 228 | 399 | 40 | 28–51 | 85 | 84 | 53 | 35–66 | 143 | 315 | 33 | 16–47 |
| Current and prior | 1,213 | 2,112 | 35 | 27–42 | 313 | 211 | 28 | 10–43b | 900 | 1,901 | 36 | 27–44 |
| Summarised model with three previous seasons | ||||||||||||
| Never vaccinated | 3,179 | 2,351 | 0 (ref) | 2,385 | 1,168 | 0 (ref) | 794 | 1,183 | 0 (ref) | |||
| Any prior and no current | 321 | 553 | 37 | 26–47 | 89 | 77 | 46 | 25–61 | 232 | 476 | 35 | 21–46 |
| Current regardless prior | 1,441 | 2,511 | 39 | 32–45 | 398 | 295 | 39 | 25–50 | 1,043 | 2,216 | 38 | 29–46 |
| Full model with three previous seasons | ||||||||||||
| Never vaccinated | 3,179 | 2,351 | 0 (ref) | 2,385 | 1,168 | 0 (ref) | 794 | 1,183 | 0 (ref) | |||
| 1–2 prior and no current | 235 | 362 | 30 | 16–42 | 79 | 61 | 40 | 14–58 | 156 | 301 | 27 | 9–42 |
| 3 prior and no current | 86 | 191 | 49 | 33–62 | 10 | 16 | 69 | 29–86 | 76 | 175 | 47 | 28–61 |
| Current and no prior | 118 | 215 | 50 | 36–61 | 58 | 65 | 59 | 41–72 | 60 | 150 | 41 | 18–57 |
| 1–2 prior and current | 352 | 631 | 40 | 29–49 | 108 | 82 | 38 | 14–55 | 244 | 549 | 41 | 28–51 |
| 3 prior and current | 971 | 1,665 | 36 | 28–44 | 232 | 148 | 27 | 4–44b | 739 | 1,517 | 37 | 27–46 |
| Summarised model with five previous seasons | ||||||||||||
| Never vaccinated | 3,089 | 2,229 | 0 (ref) | 2,345 | 1,135 | 0 (ref) | 744 | 1,094 | 0 (ref) | |||
| Any prior and no current | 411 | 675 | 37 | 26–46 | 129 | 110 | 46 | 28–59 | 282 | 565 | 33 | 20–4 |
| Current regardless prior | 1,441 | 2,511 | 40 | 33–47 | 398 | 295 | 40 | 27–51 | 1,043 | 2,216 | 39 | 30–47 |
| Full model with five previous seasons | ||||||||||||
| Never vaccinated | 3,089 | 2,229 | 0 (ref) | 2,345 | 1,135 | 0 (ref) | 744 | 1,094 | 0 (ref) | |||
| 1–2 prior and no current | 237 | 336 | 30 | 16–42 | 103 | 79 | 37 | 14–54 | 134 | 257 | 25 | 5–41 |
| 3–5 prior and no current | 174 | 339 | 43 | 29–53 | 26 | 31 | 63 | 36–79 | 148 | 308 | 39 | 23–52 |
| Current and no prior | 102 | 181 | 50 | 35–62 | 53 | 59 | 59 | 39–72 | 49 | 122 | 40 | 14–58 |
| 1–2 prior and current | 165 | 289 | 45 | 31–56 | 67 | 58 | 47 | 23–64 | 98 | 231 | 42 | 24–56 |
| 3–5 prior and current | 1,174 | 2,041 | 38 | 30–45 | 278 | 178 | 28 | 7–44b | 896 | 1,863 | 39 | 29–47 |
CI: confidence interval; ref: reference.
a Vaccination effect adjusted by age groups (9–44, 45–64, 65–84 and ≥ 85 years), major chronic conditions and month and season of sample collection.
b p value < 0.05 for comparison with the category of current season vaccination and no prior doses.
In the sensitivity analysis including the ILI diagnosis during the immediately previous season, the EIV estimates were not subject to relevant changes (Supplementary Table S2).
Modifying effect of prior doses on the current-season influenza vaccine effectiveness
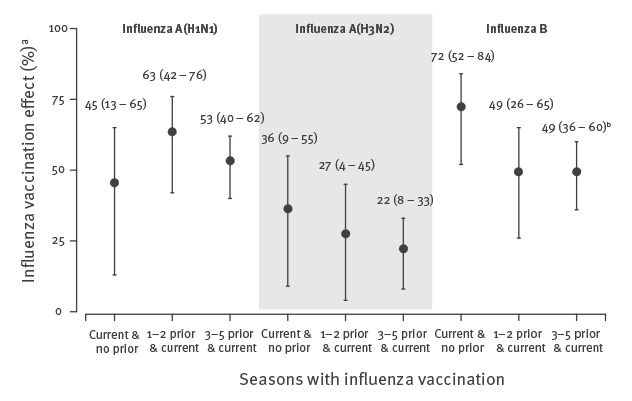
In the overall analysis, the absolute difference of IVE of patients vaccinated in the current and in three or more previous seasons minus IVE of patients vaccinated in the current season and unvaccinated in the five previous seasons was −12 percentage points (38% vs 50%; p = 0.118). This difference reached −31 percentage points in primary healthcare patients (28% vs 59%; p = 0.014), −22 points among patients aged 9–64 years (36% vs 58%; p = 0.046), and −23 points in the analysis of influenza B cases (49% vs 72%; p = 0.037) (Figures 1 and 2).
Figure 1.
Evaluation of the modifying effect of vaccination history on the effect of influenza vaccination in the current season for all patients and by healthcare setting, Navarre, Spain, pooled analysis of the 2011/12–2018/19 influenza seasons (n = 10,356)
a Influenza vaccination effect and 95% confidence interval (in brackets), adjusted by age groups (9–44, 45–64, 65–84 and ≥ 85 years), major chronic conditions, healthcare setting (primary healthcare and hospital) and month and season of sample collection.
b p value < 0.05 for comparison with the category of current season vaccination and no prior doses.
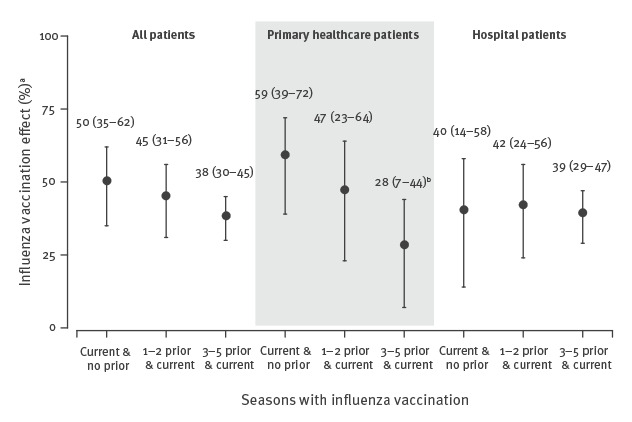
Figure 2.
Evaluation of the modifying effect of vaccination history on the effect of influenza vaccination in the current season by influenza virus (sub)type, Navarre, Spain, pooled analysis of the 2011/12–2018/19 influenza seasons (n = 10,356)
a Influenza vaccination effect and 95% confidence interval (in brackets), adjusted by age groups (9–44, 45–64, 65–84 and ≥ 85 years), major chronic conditions, healthcare setting (primary healthcare and hospital) and month and season of sample collection. Influenza A(H1N1) analysis includes 2012/13, 2013/14, 2015/16, 2017/18 and 2018/19 seasons. Influenza A(H3N2) analysis includes 2011/12, 2013/14, 2014/15, 2015/16, 2016/17, 2017/18 and 2018/19 seasons. Influenza B analysis includes 2011/12, 2012/13, 2014/15, 2015/16 and 2017/18 seasons (excluding seasons with minimal circulation of a given (sub-)type from the pooled analysis for that outcome).
b p value < 0.05 for comparison with the category of current season vaccination and no prior doses.
Therefore, all these analyses should maintain separate estimates by vaccination history. Such negative interference was not observed in hospitalised patients, in patients 65 years or older, or in analyses of influenza A(H1N1) and A(H3N2); therefore, the vaccination categories might be simplified in those analyses. The full models considering the vaccination history in three or five previous seasons provided similar results, supporting the use of the first option (Table 2 and Supplementary Tables S3–S8).
In separate analyses by influenza season, the absolute difference in IVE ranged from −3 to +14 percentage points, but the sample sizes were limited and none of these differences had p < 0.05 (Supplementary Table S9).
Estimating the bias from prior vaccinations in the reference category
We reduced the number of vaccination categories by combining all individuals vaccinated in the current season regardless of their vaccination history, and these were compared with subjects unvaccinated in the current and five previous seasons. Compared with this summarised model, the only-current-season model underestimated the EIV by an average of 9 percentage points (IVE = 31% vs EIV = 40%; absolute difference −9%), as did the summarised model considering vaccination in only one previous season (−4%) (Table 3). The only-current-season model was affected by a bias of at least 5 percentage points in the analysis of primary healthcare patients (−5%), hospitalised patients (−10%), patients 65 years and older (−11%), and in the analyses of influenza A(H1N1) (−6%), A(H3N2) (−10%) and influenza B (−9%). The bias also reached at least 5 percentage points in the summarised model with vaccination history in only one previous season in the analyses of influenza B (−6%; Table 3). No bias was observed in the summarised models including vaccination history in three previous seasons. The bias in the only-current-season model ranged from −1 to −19 percentage points among influenza seasons, and was at least 5 percentage points in five of the eight seasons studied (Supplementary Table S10).
Table 3. Comparison of the effect estimates of influenza vaccination from different models with the estimate that compares people vaccinated in the current season with those unvaccinated in the current and five previous seasons, Navarre, Spain, pooled analysis of 2011/12–2018/19 influenza seasons (n = 10,356).
| Vaccination effect | Absolute difference of vaccination effect in % | ||
|---|---|---|---|
| % | 95% CI a | ||
| All patients | |||
| Summarised model with five previous seasons | 40 | 33–47 | Reference |
| Summarised model with three previous seasons | 39 | 32–45 | −1 |
| Summarised model with one previous season | 36 | 29–43 | −4 |
| Only-current-season model | 31 | 23–38 | −9b |
| Primary healthcare patientsc | |||
| Summarised model with five previous seasons | 40 | 27–51 | Reference |
| Summarised model with three previous seasons | 39 | 25–50 | −1 |
| Summarised model with one previous season | 37 | 23–48 | −3 |
| Only-current-season model | 35 | 21–47 | −5b |
| Hospitalised patients | |||
| Summarised model with five previous seasons | 39 | 30–47 | Reference |
| Summarised model with three previous seasons | 38 | 29–46 | −1 |
| Summarised model with one previous season | 36 | 27–44 | −3 |
| Only-current-season model | 29 | 19–37 | −10b,d |
| Age 9–64 yearsc | |||
| Summarised model with five previous seasons | 45 | 35–54 | Reference |
| Summarised model with three previous seasons | 46 | 34–53 | 1 |
| Summarised model with one previous season | 43 | 32–52 | −2 |
| Only-current-season model | 42 | 31–51 | −3 |
| Age ≥ 65 years | |||
| Summarised model with five previous seasons | 35 | 24–44 | Reference |
| Summarised model with three previous seasons | 34 | 23–44 | −1 |
| Summarised model with one previous season | 32 | 21–41 | −3 |
| Only-current-season model | 24 | 13–33 | −11b,d |
| Influenza A(H1N1) | |||
| Summarised model with five previous seasons | 53 | 42–61 | Reference |
| Summarised model with three previous seasons | 50 | 39–60 | −3 |
| Summarised model with one previous season | 51 | 40–59 | −2 |
| Only-current-season model | 47 | 36–56 | −6b |
| Influenza A(H3N2) | |||
| Summarised model with five previous seasons | 24 | 11–34 | Reference |
| Summarised model with three previous seasons | 24 | 13–35 | 0 |
| Summarised model with one previous season | 21 | 9–32 | −3 |
| Only-current-season model | 14 | 2–25 | −10b,d |
| Influenza Bc | |||
| Summarised model with five previous seasons | 53 | 42–61 | Reference |
| Summarised model with three previous seasons | 50 | 39–59 | −3 |
| Summarised model with one previous season | 47 | 35–56 | −6b |
| Only-current-season model | 44 | 32–53 | −9b |
CI: confidence interval.
a Vaccination effect adjusted by age groups (9–44, 45–64, 65–84 and ≥ 85 years), major chronic conditions, healthcare setting (primary healthcare and hospital) and month and season of sample collection.
b Absolute difference of vaccine effect of at least ± 5% was considered as a bias.
c The results of this model should be considered with caution since there was a negative interference between vaccination in the current and previous seasons.
d Absolute difference of vaccine effect of at least ± 10% was considered as a relevant bias.
Selection of the simplest valid model to estimate the effect of influenza vaccinations
The EIV estimates in primary healthcare patients, in patients younger than 65 years and against influenza B were modified by the vaccination history; therefore, the analysis recommended in these situations is the full model which considers the vaccination history in at least three previous seasons. EIV estimates in the overall analysis and the specific analyses of hospitalised patients, of patients 65 years and older and against influenza A(H1N1) and A(H3N2) were not affected by a modifying effect, but were biased (± 5% or more) when at least one previous season vaccination was not considered; therefore the summarised model considering one prior dose may be sufficient (Table 4). When only relevant bias was considered (± 10% or more), the only-current-season model may be sufficient in the overall and in the specific analysis of influenza A(H1N1), but the summarised model would be the recommended analysis for hospitalised patients, patients 65 years and older and influenza A(H3N2).
Table 4. Simplest recommended model for estimating the influenza vaccination effect according to the modifying effect and bias from influenza vaccination history.
| Modifying effect | Bias ± 5% or more | Relevant bias ± 10% or more | Simplest model recommended | |
|---|---|---|---|---|
| All patients | No | Yes | No | Summarised model with one previous seasona,b |
| Primary healthcare patients | Yes | Yes | No | Full model with three previous seasonsc |
| Hospitalised patients | No | Yes | Yes | Summarised model with one previous seasona |
| Age 9–64 years | Yes | No | No | Full model with three previous seasonsc |
| Age ≥ 65 years | No | Yes | Yes | Summarised model with one previous seasona |
| Influenza A(H1N1) | No | Yes | No | Summarised model with one previous seasona,b |
| Influenza A(H3N2) | No | Yes | Yes | Summarised model with one previous seasona |
| Influenza B | Yes | Yes | No | Full model with three previous seasonsc |
a Summarised model categories: current-season vaccination regardless of prior doses, no current-season vaccination but any prior doses and no current-season vaccination or prior doses as reference category.
b If only relevant bias is considered (± 10% or more), the only-current-season model would be sufficient.
c Full model categories: current-season vaccination and three prior doses, current-season vaccination and one to two prior doses, current-season vaccination and no prior doses, no current-season vaccination and three prior doses, no current-season vaccination and one to two prior doses, and no current-season vaccination and no prior doses as reference category.
Discussion
Influenza vaccination in previous seasons retained a considerable protective effect and in some cases modified the effect of current-season vaccination. Most analyses that did not consider vaccination in the previous seasons underestimated the EIV. Thus, evaluations of EIV should incorporate previous vaccination history.
In the analyses of patients in primary healthcare, of persons younger than 65 years, and of influenza B, people who were first vaccinated in the current season had an IVE more than 20 percentage points higher than those who had received the vaccine in the current season and in three or more previous seasons. A new dose of influenza vaccine in a person vaccinated in previous seasons could act by adding its effect to the remaining effect, or by maintaining the greater effect of both. On average over eight influenza seasons, the results observed do not support the sum of effects; some were consistent with maintaining the greater effect; and in various situations, persons with repeated vaccinations had lower protection than those vaccinated for the first time, suggesting there is negative interference (modification of the effect) between influenza vaccines, as described in other studies [7,19,25-29]. In these situations, the final analysis should estimate the effect separately for different combinations of vaccination in the current and previous seasons (full model).
Regardless of the presence of an effect modification, the analyses that did not remove from the reference category individuals vaccinated in only previous seasons underestimated by an average of 9 percentage points (IVE = 31% vs EIV = 40%) the preventive effect enjoyed by people vaccinated in the current season. This was a relevant bias (± 10% or more) in hospitalised patients, in those 65 years and older, and in cases of A(H3N2) influenza. Incorporating vaccination in the immediately previous season into the model largely reduced the bias, and the estimate was even improved by incorporating information from the three previous seasons. Although vaccines received 4–5 years ago may retain some residual effect [5], they did not substantially modify the current-season vaccine effectiveness [10].
Once the existence of an effect modification had been ruled out, the summarised model was a good option for evaluation of EIV, since it controlled the bias, estimated the protective effect in persons vaccinated in the current season, provided information about the remaining effect of previous vaccinations and did not cause excessive fragmentation of the results. While the analysis that considers only vaccination in the current season attempted unsuccessfully to isolate the effect of this vaccine, the summarised model assumed the reality of repeated vaccination and estimated the average protective effect in vaccinated individuals.
Although this study aimed to manage the bias and modifying effect in IVE estimates that inform population and health professionals about the risk reduction of influenza outcomes, similar modifying effect and bias may also affect studies that evaluate the effectiveness of a specific vaccine composition against the circulating influenza virus. Most studies published to date have not taken into account the history of previous vaccination [12], which suggests that the actual protection in vaccinated persons would have been higher than reported in the literature. Since the level of IVE is often low or moderate [12], it is essential to correct for this bias to strengthen the confidence of health professionals and the general population in vaccination, and thus improve vaccination coverage [30]. For many studies, it may be challenging to obtain reliable data on prior vaccination.
Natural immunity due to prior influenza exposure could introduce a bias in IVE and EIV studies [31]. The sensitivity analysis including the diagnoses of ILI during the immediately preceding season showed that the IVE estimates did not suffer relevant changes; therefore, this adjustment in the analysis does not seem necessary, as had been reported [10,18].
Among the strengths of this study is that it analysed an average of eight seasons with circulation of different (sub-)types of influenza virus. Including general practice and hospital settings provides complementary views of the EIV in the same population [20]. Cases were compared with controls recruited in the same healthcare settings before either patient or physician knew the laboratory result, a fact that reduced selection bias [14,32]. The vaccination history was obtained from the regional vaccination registry [2], and the study was limited to the population with stable residence in the region to avoid biases due to vaccination information [33]. The full model has been used in other studies and allows analysis of the effect modification and control for bias due to vaccination in previous seasons [6-8].
This study may also be subject to limitations. It was carried out in a single place where vaccination is indicated in persons 60 years and older and in those with risk factors, and only using the inactivated trivalent vaccine. Consequently, care must be taken when generalising the EIV results to other places with different indications for vaccination, different vaccination coverage, or where other types of vaccines are used. This study included individuals with different chances for repeated vaccination based on their age and influenza vaccination recommendation; therefore, all analyses were adjusted by age, comorbidities and season to control for potential confounding. The test-negative design in inpatient settings may be affected by bias, but the adjustment for cardiorespiratory conditions, as we have done, prevents this bias [34]. As the statistical power in the analysis of a single season was reduced, caution should be exercised when explaining its results. The results should be understood as an average of eight influenza seasons.
Conclusions
This study showed that influenza vaccination in previous seasons may retain an important protective effect and is often a relevant effect modifier of the current-season IVE estimates. Moreover, most analyses that did not consider vaccination in previous seasons underestimated the EIV. Combinations of influenza vaccinations in the current and at least three previous seasons should be analysed to evaluate the existence of a modifying effect. When this is ruled out, a summarised analysis including vaccination status in three categories (current season, previous seasons only and unvaccinated) is a simple option to obtain adjusted estimates and to detect the effect of previous season vaccination. As most previous reports of IVE have not considered vaccination history, the benefit of influenza vaccination may be higher than reported in the literature.
Acknowledgements
We thank Primary Health Care Sentinel Network and Network for Influenza Surveillance in Hospitals of Navarre for the recruitment of patients to the study.
Funding: This study was supported by the I-MOVE Network supported by the European Centre for Disease Prevention and Control, by the Horizon 2020 program of the European Commission (I-MOVE-plus, agreement 634446), by the Carlos III Institute of Health with the European Regional Development Fund (PI17/00868, PI12/00087 and INT19/00028) and by the Health Department of the Navarre Government (85-18). The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Data
Conflict of interest: None declared.
Authors’ contributions: IMB, IC and JC designed the study. IMB, IC and JC participated in the literature search. IMB, AN, AA, CE and JC participated in the data collection and interpreted the results. IMB and JC were responsible for the data analysis. IMB and JC wrote the manuscript, with editorial contributions from AN, IC, AA and CE. All authors reviewed the manuscript for accuracy and scientific content.
References
- 1.World Health Organization (WHO). Influenza (seasonal). Geneva: WHO; 2018. Available from: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
- 2.Aguilar I, Reyes M, Martínez-Baz I, Guevara M, Albeniz E, Belza M, et al. Use of the vaccination register to evaluate influenza vaccine coverage in seniors in the 2010/11 influenza season, Navarre, Spain. Euro Surveill. 2012;17(17):20154. 10.2807/ese.17.17.20154-en [DOI] [PubMed] [Google Scholar]
- 3.Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017;16(7):1-14. 10.1080/14760584.2017.1334554 [DOI] [PubMed] [Google Scholar]
- 4.Kwong JC, Chung H, Jung JK, Buchan SA, Campigotto A, Campitelli MA, et al. The impact of repeated vaccination using 10-year vaccination history on protection against influenza in older adults: a test-negative design study across the 2010/11 to 2015/16 influenza seasons in Ontario, Canada. Euro Surveill. 2020;25(1):1900245. 10.2807/1560-7917.ES.2020.25.1.1900245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Baz I, Navascués A, Casado I, Aguinaga A, Ezpeleta C, Castilla J. Remaining effect of influenza vaccines received in prior seasons. J Infect Dis. 2019;220(7):1136-40. 10.1093/infdis/jiz266 [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Baz I, Casado I, Navascués A, Díaz-González J, Aguinaga A, Barrado L, et al. Effect of repeated vaccination with the same vaccine component against 2009 pandemic influenza A(H1N1) virus. J Infect Dis. 2017;215(6):847-55. 10.1093/infdis/jix055 [DOI] [PubMed] [Google Scholar]
- 7.McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014;59(10):1375-85. 10.1093/cid/ciu680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castilla J, Navascués A, Casado I, Díaz-González J, Pérez-García A, Fernandino L, et al. Combined effectiveness of prior and current season influenza vaccination in northern Spain: 2016/17 mid-season analysis. Euro Surveill. 2017;22(7):30465. 10.2807/1560-7917.ES.2017.22.7.30465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol. 2016;184(5):345-53. 10.1093/aje/kww064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foppa IM, Ferdinands JM, Chung J, Flannery B, Fry AM. Vaccination history as a confounder of studies of influenza vaccine effectiveness. Vaccine X. 2019;1:100008. 10.1016/j.jvacx.2019.100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ainslie KEC, Haber M, Orenstein WA. Challenges in estimating influenza vaccine effectiveness. Expert Rev Vaccines. 2019;18(6):615-28. 10.1080/14760584.2019.1622419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942-51. 10.1016/S1473-3099(16)00129-8 [DOI] [PubMed] [Google Scholar]
- 13.Ramsay LC, Buchan SA, Stirling RG, Cowling BJ, Feng S, Kwong JC, et al. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med. 2019;17(1):9. 10.1186/s12916-018-1239-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). Evaluation of influenza vaccine effectiveness: a guide to the design and interpretation of observational studies. Geneva: WHO; 2017. Available from: https://apps.who.int/iris/bitstream/handle/10665/255203/9789241512121-eng.pdf;jsessionid=BF8E8B943503324D3B7CF47325EABD46?sequence=1
- 15.Castilla J, Morán J, Martínez-Artola V, Fernández-Alonso M, Guevara M, Cenoz MG, et al. Effectiveness of the monovalent influenza A(H1N1)2009 vaccine in Navarre, Spain, 2009-2010: cohort and case-control study. Vaccine. 2011;29(35):5919-24. 10.1016/j.vaccine.2011.06.063 [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Baz I, Martínez-Artola V, Reina G, Guevara M, Cenoz MG, Morán J, et al. Effectiveness of the trivalent influenza vaccine in Navarre, Spain, 2010-2011: a population-based test-negative case-control study. BMC Public Health. 2013;13(1):191. 10.1186/1471-2458-13-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Baz I, Navascués A, Pozo F, Chamorro J, Albeniz E, Casado I, et al. Influenza vaccine effectiveness in preventing inpatient and outpatient cases in a season dominated by vaccine-matched influenza B virus. Hum Vaccin Immunother. 2015;11(7):1626-33. 10.1080/21645515.2015.1038002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castilla J, Navascués A, Fernández-Alonso M, Reina G, Albéniz E, Pozo F, et al. Effects of previous episodes of influenza and vaccination in preventing laboratory-confirmed influenza in Navarre, Spain, 2013/14 season. Euro Surveill. 2016;21(22):30243. 10.2807/1560-7917.ES.2016.21.22.30243 [DOI] [PubMed] [Google Scholar]
- 19.Castilla J, Navascués A, Fernández-Alonso M, Reina G, Pozo F, Casado I, et al. Effectiveness of subunit influenza vaccination in the 2014-2015 season and residual effect of split vaccination in previous seasons. Vaccine. 2016;34(11):1350-7. 10.1016/j.vaccine.2016.01.054 [DOI] [PubMed] [Google Scholar]
- 20.Castilla J, Martínez-Baz I, Navascués A, Casado I, Aguinaga A, Díaz-González J, et al. Primary Health Care Sentinel Network Of Navarre. Network For Influenza Surveillance In Hospitals Of Navarre . Comparison of influenza vaccine effectiveness in preventing outpatient and inpatient influenza cases in older adults, northern Spain, 2010/11 to 2015/16. Euro Surveill. 2018;23(2):16-00780. 10.2807/1560-7917.ES.2018.23.2.16-00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castilla J, Navascués A, Casado I, Pérez-García A, Aguinaga A, Ezpeleta G, et al. Primary Health Care Sentinel Network. Network For Influenza Surveillance In Hospitals Of Navarre . Interim effectiveness of trivalent influenza vaccine in a season dominated by lineage mismatched influenza B, northern Spain, 2017/18. Euro Surveill. 2018;23(7):18-00057. 10.2807/1560-7917.ES.2018.23.7.18-00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Baz I, Navascués A, Portillo ME, Casado I, Fresán U, Ezpeleta C, et al. Effect of influenza vaccination in preventing laboratory-confirmed influenza hospitalization in patients with diabetes mellitus. Clin Infect Dis. 2021;73(1):107-14. 10.1093/cid/ciaa564 [DOI] [PubMed] [Google Scholar]
- 23.Greenland S, Pearce N. Statistical foundations for model-based adjustments. Annu Rev Public Health. 2015;36(1):89-108. 10.1146/annurev-publhealth-031914-122559 [DOI] [PubMed] [Google Scholar]
- 24.Valenciano M, Kissling E, Larrauri A, Nunes B, Pitigoi D, O’Donnell J, et al. Exploring the effect of previous inactivated influenza vaccination on seasonal influenza vaccine effectiveness against medically attended influenza: Results of the European I-MOVE multicentre test-negative case-control study, 2011/2012-2016/2017. Influenza Other Respir Viruses. 2018;12(5):567-81. 10.1111/irv.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, et al. Influenza vaccine effectiveness in the 2011-2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319-27. 10.1093/cid/cit736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skowronski DM, Janjua NZ, Sabaiduc S, De Serres G, Winter AL, Gubbay JB, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011-2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis. 2014;210(1):126-37. 10.1093/infdis/jiu048 [DOI] [PubMed] [Google Scholar]
- 27.Ohmit SE, Petrie JG, Malosh RE, Fry AM, Thompson MG, Monto AS. Influenza vaccine effectiveness in households with children during the 2012-2013 season: assessments of prior vaccination and serologic susceptibility. J Infect Dis. 2015;211(10):1519-28. 10.1093/infdis/jiu650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gherasim A, Martínez-Baz I, Castilla J, Pozo F, Larrauri A, cycEVA working group . Effect of previous and current vaccination against influenza A(H1N1)pdm09, A(H3N2), and B during the post-pandemic period 2010-2016 in Spain. PLoS One. 2017;12(6):e0179160. 10.1371/journal.pone.0179160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols MK, Andrew MK, Ye L, Hatchette TF, Ambrose A, Boivin G, et al. The impact of prior season vaccination on subsequent influenza vaccine effectiveness to prevent influenza-related hospitalizations over 4 influenza seasons in Canada. Clin Infect Dis. 2019;69(6):970-9. 10.1093/cid/ciy1009 [DOI] [PubMed] [Google Scholar]
- 30.Castilla J, Martínez-Baz I, Godoy P, Toledo D, Astray J, García S, et al. Trends in influenza vaccine coverage among primary healthcare workers in Spain, 2008-2011. Prev Med. 2013;57(3):206-11. 10.1016/j.ypmed.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 31.Petrie JG, Ohmit SE, Johnson E, Truscon R, Monto AS. Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J Infect Dis. 2015;212(12):1914-22. 10.1093/infdis/jiv313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenciano M, Kissling E, Ciancio BC, Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine. 2010;28(46):7381-8. 10.1016/j.vaccine.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 33.Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis. 2013;57(3):474-6. 10.1093/cid/cit255 [DOI] [PubMed] [Google Scholar]
- 34.Foppa IM, Ferdinands JM, Chaves SS, Haber MJ, Reynolds SB, Flannery B, et al. The case test-negative design for studies of the effectiveness of influenza vaccine in inpatient settings. Int J Epidemiol. 2016;45(6):2052-9. 10.1093/ije/dyw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


