Abstract
Purpose
To provide an overview of all published randomized controlled trials (RCTs) in anterior cruciate ligament reconstruction (ACLR) summarizing the available evidence.
Methods
Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we searched the Cochrane FIGCentral Register of Controlled Trials, Ovid MEDLINE, and Embase for RCTs of ACLR from their inception to August 26, 2020. Outcome measure was whether RCTs reported statistically significant findings. RCTs were then classified according to their intervention groups in a narrative synthesis of the evidence.
Results
In total, 299 RCTs met the inclusion criteria and were included with a total number of 25,186 patients. Only 30 RCTs (10%) reported significant differences between the intervention and the control groups. These included 101 RCTs on grafts, 20 RCTs on tunnel placements, 48 RCTs on graft fixation, 42 RCTs on single-bundle compared with double-bundle reconstructions, 11 RCTs on additional procedures, 11 RCTs on graft tensioning, 5 RCTs on timing of surgery, 25 RCTs on technical variations from standard techniques, 6 RCTs on ACL repair, 5 RCTs on navigation, 16 RCTs on perioperative management, and 9 RCTs on other aspects of ACLR. Only 14 RCTs (4.7%) reported outcomes beyond 10 years with greater allograft failures compared with autografts, high incidence of osteoarthritic changes in reconstructed knees (22%-100%), with no significant differences in outcomes between bioabsorbable or metal screws for graft fixation, patellar versus hamstrings or single- versus double-bundle reconstructions.
Conclusions
The evidence indicates that a standard arthroscopic single- or double-bundle ACLR with hamstrings/patella autografts, transportal technique, and fixation techniques familiar to the surgeon leads to comparable results. This evidence offers surgeons the flexibility to use standard and cost-effective techniques and achieve comparable outcomes.
Level of Evidence
Level II; systematic review of Level I-II randomized controlled trials.
The anterior cruciate ligament (ACL) is the most commonly injured ligament in the knee, with ∼200,000 anterior cruciate ligament reconstructions (ACLR) per year in the United States alone and an estimated 400,000 ACLRs per year worldwide.1, 2, 3 There is a substantial variation in ACLR surgery worldwide. Prentice et al.4 reported on a combined cohort of 101,125 ACLRs across 6 national, regional, and hospital-based registries (Denmark, Luxembourg, Norway, Sweden, UK, and the U.S.-based Kaiser Permanente registry). European countries mostly used autografts whereas allografts were more commonly used in the United States. Interference screw fixation was the most frequent femoral fixation technique in Luxembourg and the United States, whereas suspensory fixation was more frequently used in other countries. Interference screw was the most frequent tibial fixation type in all 6 cohorts, with overall 3-year cumulative revision ACLR rate of 2.8% to 3.7%.4
The modern intra-articular ACLR is largely based on the Hey Groves’ operation, which he described in 1917 using a strip of fascia lata passed through a tibial tunnel.5,6 Almost a century later, the debate continues in the literature on the timing of surgery, the best choice of graft to use, and single- versus double-bundle reconstruction and tunnel placements, to name but a few. ACLR is one of the most-studied procedures in sports medicine. with more than 22,000 publications on PubMed and more than 1,800 published in 2019 alone. In recognition of this challenge, the Panther Symposium ACL Injury Clinical Outcomes Consensus Group has recently published their consensus paper providing practical guidelines on preferred tools for reliable and valid assessment of outcomes after ACL treatment.7
High-quality randomized controlled trials (RCTs) provide strong evidence for the efficacy of health care interventions and help to inform evidence-based practice.8,9 This is especially true of RCTs, which show a statistically significant difference in the results of 2 treatments, or the absence of a significant difference but a narrow confidence interval indicating a positive effect of a treatment.10,11
Numerous systematic reviews have been published looking at specific aspects of ACLR.12, 13, 14 Notably, in their Level IV evidence systematic review of systematic reviews on ACLR, Anderson et al.15 provided a summary of 240 studies in an attempt to synthesize the literature, whereas Kay et al.16 focused on quality of reporting RCTs in ACLR and found that reporting of a methodologically sound randomization process and prospective calculation of sample size have significantly improved in recent years. The first of these 2 examples included all reviews of different study designs and the second focused on quality of reporting RCTs rather than the contents or results of those trials. The purpose of this systematic review is to provide an overview of all published RCTs in ACLR summarizing the available evidence. We hypothesized that the majority of RCTs in ACLR would find no significant differences.
Methods
Following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines,17 we searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2020, Issue 2), Ovid MEDLINE (including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid MEDLINE and Versions) (1946 to August 26, 2020), and Embase (1980 toAugust 26 , 2020). We limited our searches to the English-language literature. In MEDLINE, we combined the subject-specific search strategy with the sensitivity maximizing version of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials.18
The following search strategy was used [(rct OR randomised OR randomized OR "clinical trial" OR blinded OR "controlled trial").ti,ab∗ ”anterior cruciate ligament reconstruction"/ OR ∗"bone-patellar tendon-bone grafting"/ ("anterior cruciate ligament reconstruction" OR "anterior cruciate ligament reconstructive surger∗" OR "ACL reconstruction" OR "ACL reconstructive surger∗").ti,ab∗ OR ∗"anterior cruciate ligament repair"/.
We examined the titles and abstracts of articles identified in the search as potentially relevant trials. We obtained the full texts of trials that fulfilled our inclusion criteria (i.e., randomized controlled trials of ACLR; Levels I-II) and those that were unclear from perusal of the abstracts. We excluded nonrandomized trials, trials on revision ACLR, biomechanical or cadaver studies, systematic reviews and meta-analyses. Multiple publications of the same trial were counted as 1 RCT and counted if included new outcomes or longer follow-up. The reference lists of included studies were also searched. Trials that met our inclusion criteria were assessed by 2 authors (H.E.M., S.R.P.) using a binary outcome measure of whether they reported statistically significant findings. Any disagreements were resolved by discussions and consulting with a third author (B.V.B.).
These were then classified according to intervention groups in a narrative review summarizing the evidence. Results were expressed descriptively in numbers and percentages. SPSS 16.0 software (SPSS Inc., Chicago, Illinois, IL) was used for descriptive statistical analysis.
Results
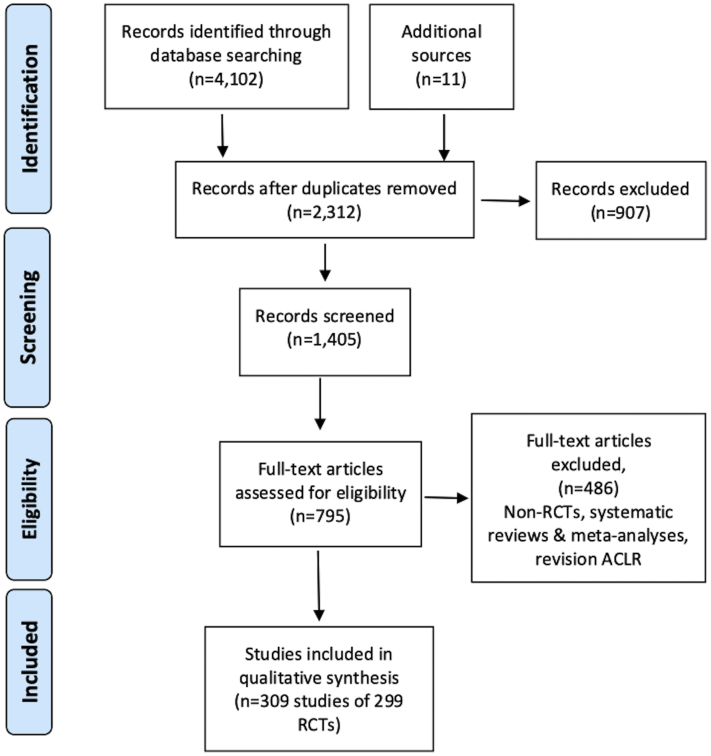
The electronic searches produced 4,102 records, with a further 11 records identified from reference lists of some included studies. After removing duplicates and screening abstracts 1,405 studies were assessed for eligibility and 299 RCTs (309 publications) met the inclusion criteria and were included (Fig 1).
Fig 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of electronic searches results and included studies. (ACL, anterior cruciate ligament; RCT, randomized controlled trial.)
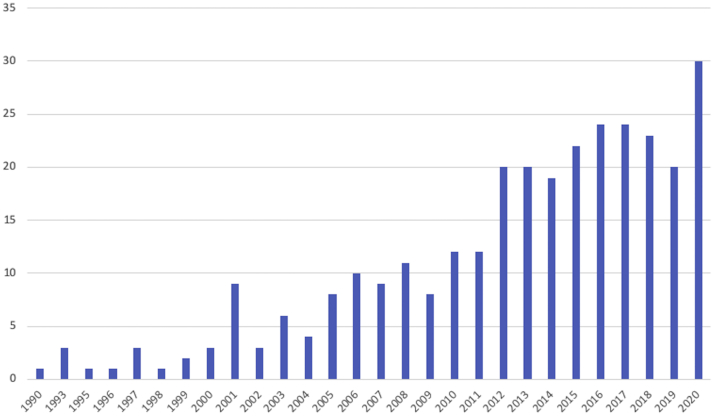
The total number of patients in those 299 RCTs was 25,186 patients. Only 30 RCTs (10%) reported significant differences between the intervention and the control groups (Table 1). The number of RCTs published per year has increased steadily since the early 1990s to an annual average of 25 RCTs over the last few years (Fig 2).
Table 1.
Summary of RCTs and Total Number of Patients Included
| Category | No. RCTs | No. Patients | No. RCTs (%) With Significant Findings |
|---|---|---|---|
| Grafts | |||
| Patella vs hamstrings | 45 | 4,582 | 2 (4.4) |
| Allografts | 28 | 2,698 | 3 (10.7) |
| Hamstrings | 12 | 953 | 2 (16.6) |
| Quads | 6 | 394 | – |
| Different autografts | 3 | 159 | – |
| Synthetic | 7 | 560 | 1 (14.3) |
| Tunnels | |||
| TP (AM) vs TT | 13 | 1,169 | 4 (30.7) |
| TP vs OI | 7 | 501 | 1 (14.3) |
| Fixation | |||
| Biofixation | 29 | 1,784 | – |
| Femoral fixation | 16 | 1,483 | – |
| Press-fit fixation | 3 | 225 | – |
| Additional procedures | |||
| Extra-articular tenodesis | 8 | 1,733 | 2 (25.0) |
| ALLR | 3 | 441 | 1 (33.3) |
| Single vs double | 42 | 2,976 | 2 (4.76) |
| Tensioning | 11 | 855 | 3 (27.3) |
| Navigation | 5 | 293 | – |
| ACL repair | 6 | 572 | 1 (16.6) |
| Timing of surgery | 5 | 419 | – |
| Techniques∗ | 25 | 1,970 | 3 (12.5) |
| Perioperative management | 16 | 1,610 | 2 (12.5) |
| Others | 9 | 439 | 3 (33.3) |
| Total | 299 | 25,816 | 30 (10%) |
ACL, anterior cruciate ligament; ALLR, anterolateral ligament reconstruction; AM, anteromedial; OI, outside-in; PRP, platelet-rich plasma; RCT, randomized controlled trial; TP, transportal; TT, transtibial; TXA, tranexamic acid.
Variations on standard techniques: ALLR, TP (AM) vs TT, TP vs OI, TXA.
Fig 2.
Number of RCTs per year of publication. (RCT, randomized controlled trial.)
Graft Choice
In total, 101 RCTs evaluated grafts in ACLR with a total of 9,346 patients; 45 RCTs compared hamstrings autografts with bone–patellar tendon–bone (BPTB), 28 RCTs evaluated allografts (compared with autografts or other allografts), 12 RCTs compared different hamstrings techniques (including different number of strands, preserved tibial attachments, etc.), 6 RCTs evaluated quadriceps grafts, 3 evaluated other autografts such as iliotibial band or anterior half of peroneus longus, and finally 7 RCTs evaluated synthetic grafts (Table 1).
Only 8 RCTs (7.9%) reported significant differences (Table 2). Tian et al.19 compared irradiated versus nonirradiated allografts at 5.7 years’ follow-up and reported better stability and less radiographic progression of arthritis with nonirradiated allografts, although there were no significant differences in functional scores. Niu et al.20 compared double-layer BPTB with 4-strand hamstrings allografts at 3 years with significantly lower rate of graft failure and better functional scores with BPTB allografts. Bottoni et al.21 compared hamstring autografts with tibialis posterior allografts at 10 years’ follow-up and reported a greater failure rate in the allograft group requiring revision reconstruction (26.5% vs 8.3%). Zhao et al.22 reported significant differences in stability and functional scores in favor of 8-strand hamstrings compared with 4-strand autografts at 2 years’ follow-up. Ferretti et al.23 reported better internal rotation strength using their modified technique in preserving the distal insertion of the hamstrings autografts compared with standard harvesting technique. Both Mohtadi et al.24 and Zaffagnini et al.25 compared BPTB with hamstrings autografts at 5 years’ follow-up and reported no significant differences in functional scores. Both studies reported more anterior knee pain with BPTB but more traumatic reinjuries with hamstrings grafts. Finally, in one of the first RCTs in ACLR (1990), Dahlstedt et al.26 reported high complications and worse outcomes with Gore-Tex prosthetic ligament reconstruction compared with the Kennedy ligament augmentation device at 3 years’ follow-up.
Table 2.
Summary of Grafts RCTs With Significant Findings
| RCT | Level of Evidence | Subgroup | Interventions | Outcome Measures | Results |
|---|---|---|---|---|---|
| Tian et al., 201719 | I | Allograft | Irradiated vs nonirradiated hamstrings allograft double bundle at 5.7 years’ FU (n = 83/112) | Lachman test, pivot shift test, KT-2000 arthrometer, IKDC, functional, subjective evaluations, activity level testing, and radiologic assessment. | Significant increase in laxity and arthritic progression found in irradiated grafts; KT-2000: 86.4% Non-ir-Allo vs 35.9% Ir-Allo had a side-to-side difference of <3 mm (P < .05). Arthritic progression: 30.8% Ir-Allo group vs 11.4% Non-ir-Allo group (P < .05). No significant differences in activity level or functional scores. |
| Niu et al., 201620 | II | Allograft | Double-layer BPTB allografts vs 4-strand hamstrings allograft at 3 years’ FU (n = 101) | Graft failure, KT-1000 arthrometer, Lachman tests, pivot-shift tests, IKDC, and Lysholm scores. | Graft failure: 2 (4%) BPTB vs 9 (17.6%) 4-SH (P = .028). Significantly better Lachman test, IKDC knee score, and Lysholm score in favor of BPTB (P < .05) although below the threshold for clinical significance. |
| Bottoni et al., 201521 | I | Allograft | Hamstring autograft vs tibialis posterior allograft at minimum 10 years’ FU (n = 96/99) | Graft failure, subjective knee stability, and functional status SANE, Tegner, and IKDC scores. | 4 (8.3%) autograft vs 13 (26.5%) allograft failures that required revision reconstruction. In the remaining patients whose graft was intact, there was no difference in functional scores. |
| Zhao et al., 200722 |
II | Hamstrings | 4- vs 8-strands hamstrings double bundle at minimum 2 years’ FU (n = 68/76) | KT-1000 arthrometer, IKCD, and Lysholm scores. | 8-SHG had significantly better results with: mean side-to-side difference in anterior knee laxity: 1.3 vs 2.8 mm (P = .0003). IKDC subjective: 96.3 vs 86.4 (P = .0007) Lysholm score: 96.5 vs 89.6 (P = .0006) |
| Ferretti et al., 200823 | I | Hamstrings | Hamstrings/preserved insertion vs standard harvesting at 25 months’ FU (n = 35) | Clinical examination, isokinetic tests, and MRI | Better internal rotation with modified technique: Isokinetic tests: internal rotation strength deficit at 60° 84.60% vs 97.37% MRI: greater percentage of regenerated semitendinosus. |
| Mohtadi et al., 201924 | I | Patella vs hamstrings | Patellar tendon, single-bundle 4-stranded hamstrings, or double-bundle hamstrings reconstruction at 5 years’ FU (n = 315/330). | ACL-QoL, IKDC, kneeling pain, Tegner activity scale, Cincinnati Occupational Rating Scale, re-ruptures, partial traumatic tears, total traumatic reinjuries, and atraumatic graft failures. | No difference in primary outcome ACL-QOL scores between groups (P = .548). No differences in IKDC, ROM, Cincinnati or Tegner scores. Kneeling pain: 10% vs 4% vs 2% (P = .029). Significantly more patients in the hamstring and double-bundle groups experienced traumatic graft reinjury compared with the patellar tendon group. Combined traumatic reinjuries: 4 vs 16 vs 17 (P = .01) |
| Zaffagnini et al., 200625 | II | Patella vs hamstrings | BPTB, 4-strand hamstrings or single hamstrings with extra-articular plasty at 5 years’ FU (n = 75) | IKDC, IKDC subjective, Tegner, muscle circumference, anterior knee pain, kneeling pain | Anterior knee pain: 36% vs 12% vs 8% (P = .03) Kneeling pain: 72% vs 44% vs 12% (P = .0001) IKDC subjective: 82 vs 76 vs 89 (P = .04) No significant differences in functional scores. |
| Dahlstedt et al., 199026 |
II | Synthetic graft | Gore-Tex prosthetic ligament vs Kennedy ligament augmentation device at 3 years’ FU (n = 41) | Lysholm scores, activity scores, and arthrometry | Better outcomes with augmentation device and more complication with Gore-Tex group. |
ACL-QoL, anterior cruciate ligament quality of life; BPTB, bone–patella tendon–bone; FU, follow-up; IKDC, International Knee Documentation Committee; MRI, magnetic resonance imaging; RCT, randomized controlled trial; ROM, range of motion.
Femoral Tunnel Techniques
Twenty RCTs with a total of 1,670 patients evaluated portal techniques. Thirteen RCTs compared “anteromedial/transportal” versus “transtibial” techniques to prepare the femoral tunnels, and 7 RCTs compared “transportal” versus “outside-in” techniques. Five RCTs reported significant findings. Femoral tunnel positions were compared using computed tomography between transtibial and transportal techniques by Takeda et al.,27 Venosa et al.,28 and Mirzatolooei,29 and all reported significant differences with more anatomical positioning achieved by the transportal technique. Similarly, transportal technique showed similar results when compared with outside-in technique by Kim et al.30 and Nakamura et al.,31 who compared the 3 techniques (transtibial, transportal and outside-in) (Table 3).
Table 3.
Summary of Femoral Tunnel Techniques RCTs With Significant Findings
| RCT | Level of Evidence | Subgroup | Interventions | Outcome Measures | Results |
|---|---|---|---|---|---|
| Takeda et al., 201327 | II | TP vs TT | Anteromedial vs Transtibial portals double-bundle hamstrings (n = 50) | Volume-rendering CT, 3D-CT tunnel placements on 7th postoperative day. | With AM technique, femoral tunnels were placed significantly deeper, lower, and closer to the femoral footprint and the overall femoral tunnel length was significantly shorter. |
| Venosa et al., 201728 | I | TP vs TT | Anteromedial vs Transtibial portals hamstrings (n = 52) | Femoral tunnel positioning 3D-CT | AM portal technique provided more anatomical graft placement than TT techniques. |
| Mirzatolooei, 201229 |
II | TP vs TT | Transportal TransFix femoral fixation vs Transtibial using hamstrings at minimum 18 months’ FU (n = 168/223) | IKDC, Lysholm, Tegner scores and rolimeter, tunnel positioning | Better reported outcomes for TP group: Laxity (mean difference between normal / affected side): TT 2.2 ± 1.13 vs TP 1.73 ± 0.85 mm (P = .002). Mean Lysholm score 81.41 TP vs 78.32 TT (P = .037). More anatomic tunnel placement with TP |
| Kim et al., 201330 | I | TP vs OI | Transportal vs Outside-in double bundle (n = 80) | CT analysis of the femoral tunnel position | TP technique had significantly more ellipsoidal AM femoral tunnel aperture than the OI technique. |
| Nakamura et al., 202031 | I | TP vs TT vs OI | Transportal vs Transtibial vs Outside-in techniques double-bundle (n = 86/98) | Femoral and tibial tunnel angles and positions 3D-CT | Femoral tunnel positions created by the TT technique were significantly higher, with larger variance, than the TP and OI technique. |
3D, 3-dimensional; AM, anteromedial; CT, computed tomography; FU, follow-up; IKDC, International Knee Documentation Committee; OI, outside-in; RCT, randomized controlled trial; TP, transportal; TT, transtibial.
Graft Fixation
Forty-eight RCTs with a total of 3,492 patients compared different methods of graft fixation (Appendix Table 1, available at www.arthroscopyjournal.org). None have reported significant differences. A total of 29 RCTs evaluated bioabsorbable fixation techniques, particularly bioabsorbable screws versus metal screws, and 16 RCTs compared different techniques of femoral graft fixation including screws versus suspensory button or screws versus cross pin fixation. The remaining 3 RCTs compared the press-fit fixation technique with conventional or interference screw technique with no significant differences reported.
Additional Procedures
Eleven RCTs compared the added value of additional procedures with the conventional ACLR including 8 RCTs with 1,733 patients that evaluated lateral extra-articular tenodesis. Two of these RCTs (25%) reported significant differences. In their STABILITY trial, Getgood et al.32 randomized 618 young patients (<25 years) to single-bundle ACLR with or without lateral extra-articular tenodesis and reported a statistically significant reduction in graft rupture and persistent rotatory laxity at 2 years after surgery, although there were no statistically significant differences in patient-reported or functional outcome scores. Porter and Shadbolt33 compared a modified iliotibial band tenodesis with standard ACLR at 2 years in 55 patients. They also reported reduced graft failure and better scores on some reported outcome measures; Knee injury and Osteoarthritis Outcome Score subscale of sport/recreation, Lysholm score, and Tegner activity scale. It is worth noting, however, that participants in both trials were at high risk of failure.
A further 3 RCTs compared the combined anterolateral ligament reconstruction with ACLR compared with ACLR alone, with 1 trial reporting significant differences. Hamido et al.34 randomized 107 male athletes and reported reduced instrumented knee laxity and a lower rate of graft failure with the added anterolateral ligament reconstruction at 60 months’ follow-up.
Single- Versus Double-Bundle Reconstruction
A total of 42 RCTs compared single- versus double-bundle reconstruction with a total of 2,976 patients (Appendix Table 1, available at www.arthroscopyjournal.org); only 2 RCTs (4.76%) reported significant differences. Siebold et al.35 reported significantly greater objective but not subjective International Knee Documentation Committee (IKDC) scores as well as improved rotational stability with double-bundle reconstructions in 70 patients at 19 months’ follow-up. There were no differences in other outcome measures (Cincinnati knee score, Lysholm score, and subjective IKDC 2000).
Zaffagnini et al.36 also reported on their 79 patients at 8-10 years’ follow-up. They showed no significant differences in subjective or objective IKDC scores, although the double-bundle group showed significantly greater Tegner level, passive range of motion recovery, faster sport resumption, lower glide pivot-shift, and lower reintervention rates. Their radiographic evaluation also showed significant lower objective degenerative changes in double-bundle group at final follow-up.
Graft Tensioning
Eleven RCTs with 855 patients compared different tensioning forces or techniques to apply the required tension on the grafts with 3 (27.3%) RCTs reporting significant differences. Yasuda et al.37 compared side-to-side knee laxity at 2 years with initial graft tension at 20 N, 40 N, or 80 N in 70 patients and reported that 80 N group reduced laxity the most. Khare et al.38 compared manual cyclical loading with the use of a tensioner at 1-year follow-up in 50 patients and reported no difference in laxity but better Lysholm score at short-term follow-up. Finally, DeFroda et al.39 also compared low-tension with high-tension (overconstrained by 2 mm) in 90 patients and reported on 72 patients at 7 years’ follow-up using hamstrings or BPTB autografts. There were no differences in the BPTB group but statistically significant differences were reported favoring the high-tension group when the hamstrings grafts were used.
Navigation
Five small RCTs with 293 patients compared navigation/computer assisted with conventional techniques with no significant differences reported in clinical outcomes although there was an overall trend toward more accurate tunnel placements on radiographic outcomes (Appendix Table 1, available at www.arthroscopyjournal.org).
ACL Repair Versus Reconstruction
Six RCTs with 572 patients compared a dynamic intraligamentary stabilization technique or bridge-enhanced repair with one trial reporting significant differences. In their trial, Drogset et al.40 reported long-term follow-up at 16 years comparing acute primary repair, acute primary repair augmented with a synthetic ligament-augmentation device, or acute repair augmented with BPTB autograft in 129 out of their original 147 patients. Revision rate at 16 years was 24%, 10%, and 2%, respectively. However, comparing the outcomes of those who did not require revision showed no significant differences in Lysholm score or radiographic arthritic changes but significantly better knee stability with the BPTB group on Lachman testing.
Timing of Surgery
Early versus delayed ACLR was compared across 5 RCTs. The main trial in this group is the landmark KANON trial (Knee ACL NON-operative vs operative treatment), which has produced multiple publications at various follow-up points and secondary analyses. Frobell et al.41 published the 2-year follow-up of the KANON trial comparing “structured rehabilitation plus early ACL reconstruction” with “structured rehabilitation with the option of later ACLR if needed” in 121 patients. Of 59 patients assigned to rehabilitation plus optional delayed ACLR, 23 underwent delayed ACLR; the other 36 underwent rehabilitation alone. There were no differences in either primary or secondary outcomes at 2 years. At 5 years’ follow-up, 30 of 59 patients had ACLR and outcomes remained similar between the 2 groups including radiographic arthritic changes or cost-effectiveness.42,43 Interestingly, patients who had early ACLR were found to have significantly greater patellofemoral cartilage loss measured on magnetic resonance imaging at 5 years.44
Eriksson et al.45 also compared early versus delayed and reported their outcomes at 6 months, 1 year,46 and 2 years47 and reported no significant differences except for more sick days leave taken by patients in the delayed group. The remining 3 RCTs also reported no significant differences (Table 4).41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51
Table 4.
Summary of Timing of Surgery RCTs
| RCT | Level of Evidence | Interventions | Outcome Measures | Results | |
|---|---|---|---|---|---|
| KANON trial | Frobell et al., 201041 | I | Structured rehabilitation plus early ACLR vs structured rehabilitation with optional delayed ACLR at 2 years’ FU (n = 121) | KOOS, SF-36, Tegner Scale. | No statistically significant differences between the 2 groups. |
| Frobell et al., 201342 | at 5 years’ FU | KOOS, SF-36, Tegner activity scale, meniscal surgery, and radiographic osteoarthritis | No statistically significant differences between the 2 groups. | ||
| Kiadaliri et al., 201643 | Economic evaluation at 5 years | Cost-effectiveness QALYs | No significant differences between the groups. | ||
| Flosadottir et al., 201848 | at 6 years’ FU | Knee Self-Efficacy Scale | No significant differences between the groups | ||
| Culvenor et al., 201944 | Secondary analysis at 5 years’ FU | PFJ cartilage loss MRI based | Early ACLR group had significantly greater loss of patellar cartilage thickness compared with optional delayed ACLR. | ||
| Same trial | Eriksson et al., 201845 |
II | ACLR within 8 days of injury vs delayed after normalized ROM 6-10 weeks after injury at 6 months’ FU (n = 70) | Visual analog scale, ROM, IKDC, Stability | No significant differences between the 2 groups, although less muscle atrophy in the early group compared with their contralateral side. |
| Von Essen et al., 202046 | At 1-year FU | IKDC, stability, number of sick-leave days | No significant differences between the groups in clinical outcomes, significantly more sick days taken in the delayed group. | ||
| Von Essen et al., 202047 | At 2 years’ FU | IKDC, KOOS, and manual stability measurements | No significant differences between the groups. | ||
| Bottoni et al.,200849 | I | Early (<21 days) vs delayed (> 6 weeks) hamstring autograft ACLR at 1-year FU (n = 69) | KT-1000, SANE, Lysholm, and Tegner Activity Score | No statistically significant differences between the groups. | |
| Chen et al., 201550 | II | Acute (3-7 weeks) vs chronic (6-11 months) ACLR using ligament advanced reinforcement system (LARS) artificial ligament in young adults (n = 55) | Lysholm scale, Tegner rating, a KT-1000, IKDC, Isokinetic strength quadriceps and hamstring | No significant differences between the groups. | |
| Manandhar et al., 201851 | II | Early (3 weeks) vs delayed (6 weeks) ACLR (n = 104) | ROM, IKDC and Tegner scores | No significant differences between the groups | |
ACLR, anterior cruciate ligament reconstruction; FU, follow-up; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; MRI, magnetic resonance imaging; PFJ, patellofemoral joint; QALYs, quality-adjusted life years; ROM, range of motion; SANE, Single Assessment Numerical Evaluation; SF-36, Short Form-36.
Surgical Techniques
This is a heterogenous group of trials that evaluated modified or variations on standard surgical techniques. For example, RCTs examine the use of OSTEOSET pellets to fill the tibial defect compared with those left empty, water versus saline irrigation, use of a plasma ablation device versus standard ablation device, or standard versus minimal debridement of the ACL remnant; to name but a few (Appendix Table 1, available at www.arthroscopyjournal.org).
There were 25 RCTs with 1,970 patients; only 3 RCTs (12%) reported significant findings. Jepsen et al.52 compared femoral graft insertion site between the 1-o’clock (high) versus 2-o’clock (low) positions in 60 patients using hamstrings grafts and found no significant differences in laxity or objective IKDC scores. However, they did report a significant difference in the subjective IKDC scores favoring low position group (82.8 vs 70.4; P < .002; n = 51).
Mutsuzaki et al.53 compared calcium phosphate-hybridized- versus unhybridized hamstrings autografts in single-bundle ACLR and their effects on the morphological changes to bone tunnels at 1-year follow-up using 3-dimensional computed tomography images in 73 patients. There were no significant differences on the tibial tunnels, but hybridized grafts significantly reduced femoral tunnel enlargement; there were no differences in clinical outcomes or scores. Finally, Funchal et al.,54 in a recent RCT, compared the outcomes of patients with an arthroscopic floating meniscus sign at 2 years when treated with or without medial compartment reconstruction surgery in 112 patients. Unsurprisingly, patients with combined injuries had a significantly greater frequency of ACLR failure and worse outcomes when treated with ACLR alone.
Perioperative Management
Sixteen RCTs with 1,610 patients were identified including 2 RCTs on analgesia, 6 RCTs on the use of platelet-rich-plasma (PRP), 4 RCTs on tourniquet use, 3 RCTs on the use of tranexamic acid, and 1 RCT on the use of human growth hormone (Appendix Table 1, available at www.arthroscopyjournal.org). Only 2 RCTs (12.5%) reported significant findings; Vogrin et al.55 compared the effects of platelet gel produced from autologous PRP and applied locally on the grafts during ACLR using MRI at 4-6 weeks to measure revascularization in the osteoligamentous interface zone in bone tunnels and in the intra-articular part of the graft. They reported significantly greater level of vascularization with PRP (0.33 ± 0.09 vs 0.16 ± 0.09, P < .001; n = 50) in the tunnels but no difference on the intra-articular graft. Reda et al.56 compared the use of tourniquet versus no tourniquet during ACLR in 84 patients. Only 58 of 84 patients were included at 2 weeks’ follow-up and reported significantly more pain and hemarthrosis in the tourniquet group.
Others
In this group there were 9 RCTs with 439 patients (Appendix Table 1, available at www.arthroscopyjournal.org). These included 3 RCTs on different rehabilitation regimes with none reporting significant differences. A further 3 RCTs compared open versus arthroscopic ACLR; one trial reported statistically significant differences favoring arthroscopic ACLR at 6 months.57 Two RCTs compared outpatients versus inpatients ACLR with one trial reported better patients’ satisfaction at 1-week with outpatient procedures.58 Finally, in their interesting long-term results, Meunier et al.59 reported the outcomes of operative (primary repair augmented or nonaugmented) versus nonoperative management of ACL ruptures in 100 patients at 15 years. Subjectively, there were no differences in activity level or Knee injury and Osteoarthritis Outcome Score score but with a slightly lower Lysholm score for the nonsurgically treated group. This difference was attributed to more instability symptoms. However, there were significantly more meniscus injuries in patients initially treated non-surgically with the status of the menisci being the most important predictor of developing arthritic changes. Further, one-third of the nonsurgically treated patients later required ACLR for instability.
Long-Term Follow Up RCTs (≥10 Years)
The vast majority of RCTs have only reported short- to medium-term follow-up. Fourteen RCTs (4.7%) have reported long-term outcomes beyond 10 years’ follow-up; some have been listed in previous relevant group interventions. Some of the important long-term findings include greater allograft failures compared with autografts21 and high incidence of osteoarthritic changes in reconstructed knees ranging from 22% to 100%.60, 61, 62, 63, 64, 65 There were no significant differences in outcomes reported between bioabsorbable or metal screws for graft fixation,66 patellar versus hamstrings,62,63,67, 68, 69 open versus arthroscopic,61 or single- versus double-bundle reconstructions65 (Table 5).21,40,59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71
Table 5.
Long-Term Follow-Up RCTs
| RCT | Level of Evidence | Subgroup | Interventions | Outcome measures | Results |
|---|---|---|---|---|---|
| Bottoni et al., 201521 | I | Allograft | Hamstring autograft vs tibialis posterior allograft at minimum 10 years (n = 96/99) | Graft failure, subjective knee stability and functional status SANE, Tegner, and IKDC scores. | 4 (8.3%) autograft vs 13 (26.5%) allograft failures required revision. In remaining patients whose graft was intact, there was no difference in functional scores. |
| Sundaraj et al., 202066 | I | Biofixation | Bioabsorbable vs titanium screws (hamstring autograft) at 13 years (n = 40) | IKDC, KT-1000, MRI-tunnel volumes, ossification around screw, graft integration, and cyst formation. | No significant differences between the groups. |
| Stensbirk et al., 201470 |
I | Different autografts | Iliotibial band vs BPTB at 15 years (n = 49/60) | Failure rate, KOOS, Tegner, anterior knee pain, Lysholm score, Rolimeter laxity, extension deficit. | No significant differences between the groups. |
| Castoldi et al., 202060 | II | EAT | BPTB +/– lateral extra-articular tenodesis at 19.4 years (n = 79/121) | Clinical outcomes, IKDC, radiographs | No significant differences between the groups although more lateral OA in EAT group (59% vs 22%; P = .02; n = 45/121). |
| Meunier et al., 200759 |
II | Others | Operative vs non-op at 15 years’ FU (n = 100) | KOOS, Lysholm, OA | No significant differences between the groups, ACLR neither reduced risk of OA nor increased subjective outcome scores. However, there were significantly more meniscus injuries in patients initially treated nonoperatively; 1/3 nonoperative patients later had ACLR for instability. |
| Holm et al., 201261 | II | Others | Open vs arthroscopic at 12 years (n = 53/67) | Prevalence of OA on radiographs, Cincinnati score, clinical assessments | No significant differences between the groups (OA: 79% vs 80%) |
| Sajovic et al., 201862 | II | Patella vs hamstrings | Patellar vs hamstring autografts at 17 years (n = 48/64) | IKDC, KT-1000 arthrometer, and radiography, SF -36, graft failure | No significant differences between the groups although more OA with patella (100% vs 71%; (P = .004). |
| Björnsson et al., 201667 | II | Patella vs hamstrings | Patellar vs hamstrings autografts at 16 years (n = 147/193) | Laxity measurements, functional outcomes, PROMS, bilateral standing radiographs | No significant differences between the groups, significantly more signs of OA in the reconstructed knee vs contralateral knee. |
| Webster et al., 201669 | I | Patella vs hamstrings | Patellar vs hamstrings at 15.3 years (n = 47/65) | Clinical assessment, anterior pain, laxity, ROM, radiographic outcomes | No significant difference between the groups. |
| Barenius et al., 201463 | I | Patella vs hamstrings | Patella vs hamstrings at 14 years (n = 135/164) | Radiological examination, Tegner, KOOS | No significant difference between the groups. (OA 49% vs 65%; P = .073). |
| Konrads et al., 201668 | II | Patella vs hamstrings | Patella vs hamstrings at 10 years (n = 47/62) | KT-1000, VAS, IKDC, Lysholm score, Tegner scale, and standard radiographs | No significant difference between the groups. |
| Sporsheim et al., 201964 | I | Repair | Open repair methods: acute primary repair, acute repair with a ligament augmentation device or BPTB ACLR at 30 years (n = 113/150) | Tegner and Lysholm questionnaires, radiographic examination, revisions and knee arthroplasties. | Prevalence of OA 42%, BPTB had significantly less rate of revision. No significant differences between the groups (remaining patients) |
| Järvelä et al., 201765 |
II | Single- vs double- bundle | Single- (bio-screw) vs single- (metal-screw) vs double-bundle (bioscrew) at 10 years’ FU (n = 81/90) | KT-1000, IKDC, Lysholm scores, radiographic examination | Revision: 1 DB vs 7 SB-Bio vs 3 SB-metal (P = .043). No significant differences between the groups in clinical outcomes or OA (38% vs 28% contralateral knee). |
| Annear et al., 201971 | II | Technique | Remnant ACL preservation vs debridement graft hamstring autograft at 10 years (n = 44/49) | Graft failure rates, subjective outcomes | No significant differences between the groups. |
| Drogset et al., 200640 | II | Technique | Acute primary repair, acute repair augmented with a synthetic ligament-augmentation device or acute repair with autologous BPTB graft at 16 years (n = 129/147) | Tegner activity score and Lysholm functional score. Stability (clinical examination and KT-1000 arthrometer). | Revision rate: 24%, 10%, and 2% respectively. The rate of revision was 10 times greater in the group that had primary repair than in the group that had repair with BPTB (P = .003) and the latter had significantly better stability (Lachman). OA changes noted in 11% in the reconstructed knee vs 3.5% in the contralateral knee (P = .001); no differences between groups. |
ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; BPTB, bone–patella tendon–bone; DB, double bundle; FU, follow-up; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; MRI, magnetic resonance imaging; OA, osteoarthritis; PROM, patient-reported outcome measures; RCT, randomized controlled trial; ROM, range of motion; SANE, Single Assessment Numerical Evaluation; SB, single bundle; SF-36, Short Form-36; VAS, visual analog scale.
Discussion
The most important finding is that only 10% of trials reported any significant differences between the intervention and the control groups for the outcome measures used by those trials. While debates continue on choice of grafts, single- or double-bundle reconstruction, tunnel placements, and fixation techniques through to the use of navigation technology, a number of conclusions can be drawn from this study. For the vast majority of patients, using standard arthroscopic techniques, a single- or double-bundle ACLR with hamstrings/patella autografts, with transportal technique, and graft fixation techniques familiar to the surgeon achieve satisfactory clinical outcomes as reported by the included RCTs.
Timing of surgery is an important consideration for surgeons and patients alike and, overall, the RCT evidence showed that delayed surgery did not compromise outcome. More than one third of trials (101/299) looked at different graft choices for ACLR from the most commonly used autografts to synthetic ligaments with only 8 RCTs (7.9%) reporting significant differences. This demonstrates a lack of clinical superiority of any one graft type over another; each has its own advantages and disadvantages that should be taken into consideration. Similarly, single- versus double-bundle reconstruction has been examined by 42 RCTs and comparable outcomes are demonstrated in all but 2 RCTs. Another 48 RCTs compared graft fixation techniques with no reported significant differences between bioabsorbable and metal screws or the use of suspensory fixation techniques, cross-pin fixation, or interference screws. Other more recent and contemporary techniques also did not show any significant differences using navigation technology or PRP. Finally, almost 50 RCTs studied variations of standard techniques or perioperative interventions, of which only 16% reported some measurable differences at short-term but no long-term influence on patients’ outcomes. The majority of RCTs on ACLR have reported short- to medium-term follow-up. Only 4.7% included trials reported beyond 10 years outcomes. These trials provide valuable data on the sequalae of ACLR particularly on the development of degenerative changes.
In recent years, a number of systematic reviews and meta-analyses have evaluated different aspects of ACLR surgery as well as instructional reviews72 and practice guidelines.73 In their review of graft options, Mo et al.74 evaluated 45 RCTs and found that patellar tendon autograft was most appropriate in terms of IKDC and Lachman test results. Wang et al.75 evaluated 11 RCTs comparing clinical outcomes and adverse events associated with irradiated and nonirradiated allografts in ACLR and found no significant differences between autograft and nonirradiated allograft, although autograft offered greater advantages in functional outcomes and adverse events. Zeng et al.12 also compared autograft with allograft ACLR across 9 RCTs and 10 systematic reviews and found that autografts had greater advantages than irradiated allografts with respect to function and stability, whereas there were no significant differences between autografts and nonirradiated allografts. In their review of 8 RCTs, Belk et al.14 found that BPTB or hamstring autograft had a similar incidence of postoperative knee OA at long-term follow-up. Similarly, Chee et al. in their review of 19 RCTs comparing contemporary 4-strand hamstrings with patellar tendon autografts found comparable results in clinical stability and postoperative functional status across most parameters studied. Although, hamstrings autografts carried lower risk of postoperative complications such as anterior knee pain, kneeling discomfort, and extension deficit.13
Limitations
This study is not without limitations. We did not calculate the treatment effect of individual trials with significant statistical findings and whether this correlated with clinically measurable effects. Further, the quality of those RCTs or of reporting was not addressed as this aspect falls outside the scope of this study. Although debate may continue on certain variations of ACLR surgery, sufficient RCT evidence is available for some points of contention such as timing of surgery, choice of graft, graft fixation techniques and navigation. However, there is a need in the published literature for further long-term studies of high-quality RCTs particularly existing ones.
Conclusions
The evidence indicates that a standard arthroscopic single- or double-bundle ACLR with hamstrings/patella autografts, transportal technique, and fixation techniques familiar to the surgeon leads to comparable results. This evidence offers surgeons the flexibility to use standard and cost-effective techniques and achieve comparable outcomes.
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Appendix
Appendix Table 1. References to RCTs per category with no significant findings
| Category | References to RCTs |
|---|---|
| Grafts | |
| Patella vs hamstrings | Laoruengthana 2009, de Souza 2015, Röpke 2001, Gupta 2019, Mohammadi 2013, Konrads 2016, Webster 2016, Sadeghpour 2017, Wipfler 2011, Holm 2010, Beard 2001, Aune 2001, Sajovic 2011, Smith 2020, Sajovic 2018, Sajovic 2006, Feller 2003, Feller 2001, Webster 2001, Heijne 2010, Lidén 2007, Razi 2014, Ejerhed 2003, Matsumoto 2006, Covey 2018, Jansson 2003, Eriksson 2001, Stańczak 2017, Maletis 2007, Stanczak 2018, Gifstad 2013, Drogset 2010, Aglietti 2004, Laxdal 2005, Barenius 2014, Björnsson 2016, Kautzner 2015, Barenius 2010, Gupta 2019, Mohtadi 2016, Mohtadi 2015, Shaieb 2002 |
| Allografts | Rose 2017, Lubowitz 2015, Moghtadaei 2013, Yoo 2017, Rose 2016, Noh 2013, Mutsuzaki 2012, Noh 2011, Sun 2011, Indelicato 2013, Sun 2012, Noh 2016, Noh 2013, Hong 2012, Tian 2016, Li 2015, Kang 2015, Bottoni 2015, Rose 2015, Lawhorn 2012, Dai 2016, Tian 2016, Sun 2009, Sun 2011, Sun 2015 |
| Hamstrings | Krishna 2020, Ibrahim 2005, Tashiro 2003, Gobbi 2005, Franz 2016, McRae 2013, Karimi-Mobarakeh 2015, Ruffilli 2016, Liu 2018, Gupta 2017 |
| Quads | Vilchez-Cavazos 2020, Martin-Alguacil 2018, Lind 2020, Sinding 2020, Lund 2014, Barié 2020 |
| Different autografts | Mei 2016, Stensbirk 2014, Bi 2018 |
| Synthetic | Muren 2003, Ghalayini 2010, Engstrom 1993, Drogset 2002, Elveos 2018, Peterson 2014 |
| Tunnels | |
| TP (AM) vs TT | Yanasse 2016, Zhang 2012, Guglielmetti 2014, MacDonald 2017, MacDonald 2018, Geng 2018, Minguell 2019, Godente 2018, Youm 2014 |
| TP vs OI | Reat 1997, Gerich 1997, Lee 2015, Kyung 2013, Lee 2016, Kim 2018 |
| Fixation | |
| Biofixation | Jagodzinski 2010, Hegde 2014, Capuano 2008, Buhren 200, Marks 2008, Arneja 2004, Sundaraj 2020, Arama 2015, Fink 2000, Hackl 2000, Drogset 2011, Drogset 2006, Robert 2004, Stengel 2009, Moisala 2008, Chiang 2019, Bourke 2013, Suomalainen 2012, Kaeding 2005, Roger 2020, Laxdal 2006, Noh 2012, Järvelä 2008, Stener 2010, Carulli 2017, Myers 2008, Harilainen 2009, Benedetto 2000, McGuire 1999 |
| Femoral fixation | Price 2010, Ibrahim 2015, Harilainen 2005, Björkman 2015, Sabat 2011, Mousavi 2017, da Silva Guarilha 2012, Hill 2005, Harilainen 2006, Fauno 2005, Mayr 2017, Mayr 2020, Kouloumentas 2019, Sharifzadeh 2017, DeWall 2011, Shumborski 2019 |
| Press-fit fixation | Geiges 2013, Hwang 2013, Sarzaeem 2014 |
| Additional procedures | |
| Extra-articular tenodesis | Acquitter 2003, Anderson 2001, Trichine 2014, Castoldi 2020, Getgood 2020, McCormack 2019 |
| ALLR | Ibrahim 2017, Sonnery-Cottet 2020 |
| Single vs double | Kalawadia 2015, Debieux 2012, Araki 2011, Kanaya 2009, Beyaz 2017, Ikuta 2020, Beyaz 2012, Abdelrazek 2019, Sastre 2010, Irrgang 2012, Bohn 2015, Núñez 2012, Koga 2015, Järvelä 2008, Taylor 2009, Xiang 2019, Järvelä 2007, Adravanti 2017, Yang 2017, Mayr 2018, Mayr 2016, Wang 2009, Koken 2014, Liu 2016, Muneta 2007, Aglietti 2010, Karikis 2016, Järvelä 2017, Zhang 2014, Zeman 2014, Ahldén 2013, Sernert 2017, Song 2013, Aga 2018, Sasaki 2017, Suomalainen 2011, Hussein 2012, Zhang 2014, Lui 2012, Streich 2008 |
| Tensioning | van Kampen1998, Kim 2006, Nicholas 2004, DeFroda 2018, Akelman 2016, Fleming 2013, Grunau 2016, Fleming 2020 |
| Navigation | Hart 2008, Endele 2009, Mauch 2007, Plaweski 2006, Meuffels 2012 |
| ACL repair | Hoogeslag 2019, Schliemann 2018, Kösters 2020, Murray 2020, Sporsheim 2019 |
| Techniques | Petruskevicius 2002, Amendola 1999, Sørensen 2011, Matthews 2017, Demirağ 2012, Silva 2014, Gohil 2007, Annear 2019, Kosy 2020, Pujol 2012, Navali 2014, Lu 2015, Liu 2017, Koga 2015, Zhu 2018, Sharaby 2019, Mutsuzaki 2018, Zhang 2018, McCormack 2006, Lubowitz 2013, Yazdi 2014, Ahn 2019 |
| Perioperative management | Mendias 2020, Tobias 2020, Johnston 2020, Mahdi 2019, Zeman 2018, Walters 2018, Mirzatolooei 2013, Valentí Azcárate 2014, Lee 2020, Felli 2019, Chiang 2019, Arciero 1996, Nicholas 2001, Nakayama 2013 |
| Others | McCarthy 1993, Maddison 2012, Curran 2020, Valkering 2015, Holm 2012, Raab 1993 |
ACL, anterior cruciate ligament; ALLR, anterolateral ligament reconstruction; AM, anteromedial; OI, outside-in; RCT, randomized controlled trial; TP, transportal; TT, transtibial.
Supplementary Data
References
- 1.Mall N.A., Chalmers P.N., Moric M. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42:2363–2370. doi: 10.1177/0363546514542796. [DOI] [PubMed] [Google Scholar]
- 2.Rayan F., Nanjayan S.K., Quah C., Ramoutar D., Konan S., Haddad F.S. Review of evolution of tunnel position in anterior cruciate ligament reconstruction. World J Orthop. 2015;6:252–262. doi: 10.5312/wjo.v6.i2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granan L.P., Forssblad M., Lind M., Engebretsen L. The Scandinavian ACL registries 2004-2007: Baseline epidemiology. Acta Orthop. 2009;80:563–567. doi: 10.3109/17453670903350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prentice H.A., Lind M., Mouton C. Patient demographic and surgical characteristics in anterior cruciate ligament reconstruction: A description of registries from six countries. Br J Sports Med. 2018;52:716–722. doi: 10.1136/bjsports-2017-098674. [DOI] [PubMed] [Google Scholar]
- 5.Snook G.A. A short history of the anterior cruciate ligament and the treatment of tears. Clin Orthop Relat Res. 1983;(172):11–13. [PubMed] [Google Scholar]
- 6.The classic. Operation for repair of the crucial ligaments Ernest W. Hey Groves, MD., F.R.C.S. Clin Orthop Relat Res. 1980;147:4–6. [PubMed] [Google Scholar]
- 7.Svantesson E., Hamrin Senorski E., Webster K.E. Clinical outcomes after anterior cruciate ligament injury: Panther symposium ACL injury clinical outcomes consensus group. Knee Surg Sports Traumatol Arthrosc. 2020;28:2415–2434. doi: 10.1007/s00167-020-06061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altman D.G., Schulz K.F., Moher D. The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 9.Evidence-Based-Medicine-Working-Group Evidence-based medicine: A new approach to teaching the practice of medicine. JAMA. 1992;268:2420–2425. doi: 10.1001/jama.1992.03490170092032. [DOI] [PubMed] [Google Scholar]
- 10.Prescott R.J., Counsell C.E., Gillespie W.J. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess. 1999;3:1–143. [PubMed] [Google Scholar]
- 11.McCulloch P., Taylor I., Sasako M., Lovett B., Griffin D. Randomised trials in surgery: Problems and possible solutions. BMJ. 2002;324:1448–1451. doi: 10.1136/bmj.324.7351.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng C., Gao S.G., Li H. Autograft versus allograft in anterior cruciate ligament reconstruction: A meta-analysis of randomized controlled trials and systematic review of overlapping systematic reviews. Arthroscopy. 2016;32:153–163.e118. doi: 10.1016/j.arthro.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Chee M.Y., Chen Y., Pearce C.J. Outcome of patellar tendon versus 4-strand hamstring tendon autografts for anterior cruciate ligament reconstruction: A systematic review and meta-analysis of prospective randomized trials. Arthroscopy. 2017;33:450–463. doi: 10.1016/j.arthro.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Belk J.W., Kraeutler M.J., Carver T.J., McCarty E.C. Knee osteoarthritis after anterior cruciate ligament reconstruction with bone-patellar tendon-bone versus hamstring tendon autograft: A systematic review of randomized controlled trials. Arthroscopy. 2018;34:1358–1365. doi: 10.1016/j.arthro.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Anderson M.J., Browning W.M., 3rd, Urband C.E., Kluczynski M.A., Bisson L.J. A systematic summary of systematic reviews on the topic of the anterior cruciate ligament. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967116634074. 2325967116634074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kay J., Memon M., Sa D. A historical analysis of randomized controlled trials in anterior cruciate ligament surgery. J Bone Joint Surg Am. 2017;99:2062–2068. doi: 10.2106/JBJS.16.01408. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 18.Lefebvre C., Manheimer E., Glanville J. Chapter 6: Searching for studies. In: Higgins J.P.T., Green S., editors. Cochrane Handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; 2011. www.handbook.cochrane.org [updated March 2011]. [Google Scholar]
- 19.Tian S., Ha C., Wang B. Arthroscopic anatomic double-bundle ACL reconstruction using irradiated versus non-irradiated hamstring tendon allograft. Knee Surg Sports Traumatol Arthrosc. 2017;25:251–259. doi: 10.1007/s00167-016-4154-6. [DOI] [PubMed] [Google Scholar]
- 20.Niu Y., Niu C., Wang X. Improved ACL reconstruction outcome using double-layer BPTB allograft compared to that using four-strand hamstring tendon allograft. Knee. 2016;23:1093–1097. doi: 10.1016/j.knee.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Bottoni C.R., Smith E.L., Shaha J. Autograft versus allograft anterior cruciate ligament reconstruction: A prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43:2501–2509. doi: 10.1177/0363546515596406. [DOI] [PubMed] [Google Scholar]
- 22.Zhao J., He Y., Wang J. Double-bundle anterior cruciate ligament reconstruction: Four versus eight strands of hamstring tendon graft. Arthroscopy. 2007;23:766–770. doi: 10.1016/j.arthro.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti A., Vadalà A., De Carli A., Argento G., Conteduca F., Severini G. Minimizing internal rotation strength deficit after use of semitendinosus for anterior cruciate ligament reconstruction: A modified harvesting technique. Arthroscopy. 2008;24:786–795. doi: 10.1016/j.arthro.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Mohtadi N.G., Chan D.S. A randomized clinical trial comparing patellar tendon, hamstring tendon, and double-bundle acl reconstructions: Patient-reported and clinical outcomes at 5-year follow-up. J Bone Joint Surg Am. 2019;101:949–960. doi: 10.2106/JBJS.18.01322. [DOI] [PubMed] [Google Scholar]
- 25.Zaffagnini S., Marcacci M., Lo Presti M., Giordano G., Iacono F., Neri M.P. Prospective and randomized evaluation of ACL reconstruction with three techniques: A clinical and radiographic evaluation at 5 years follow-up. Knee Surg Sports Traumatol Arthrosc. 2006;14:1060–1069. doi: 10.1007/s00167-006-0130-x. [DOI] [PubMed] [Google Scholar]
- 26.Dahlstedt L., Dalén N., Jonsson U. Goretex prosthetic ligament vs Kennedy ligament augmentation device in anterior cruciate ligament reconstruction. A prospective randomized 3-year follow-up of 41 cases. Acta Orthop Scand. 1990;61:217–224. doi: 10.3109/17453679008993504. [DOI] [PubMed] [Google Scholar]
- 27.Takeda Y., Iwame T., Takasago T. Comparison of tunnel orientation between transtibial and anteromedial portal techniques for anatomic double-bundle anterior cruciate ligament reconstruction using 3-dimensional computed tomography. Arthroscopy. 2013;29:195–204. doi: 10.1016/j.arthro.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Venosa M., Delcogliano M., Padua R., Alviti F., Delcogliano A. Femoral tunnel positioning in anterior cruciate ligament reconstruction: Anteromedial portal versus transtibial technique-a randomized clinical trial. Joints. 2017;5:34–38. doi: 10.1055/s-0037-1601413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirzatolooei F. Comparison of short term clinical outcomes between transtibial and transportal TransFix® femoral fixation in hamstring ACL reconstruction. Acta Orthop Traumatol Turc. 2012;46:361–366. doi: 10.3944/aott.2012.2679. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.G., Chang M.H., Lim H.C., Bae J.H., Ahn J.H., Wang J.H. Computed tomography analysis of the femoral tunnel position and aperture shape of transportal and outside-in ACL reconstruction: Do different anatomic reconstruction techniques create similar femoral tunnels? Am J Sports Med. 2013;41:2512–2520. doi: 10.1177/0363546513500626. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura K., Nakamura T., Horie M. Anatomic femoral tunnel placement is difficult by the transtibial technique: Comparison of three different femoral tunnel drilling techniques in double-bundle anterior cruciate ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2020;28:584–593. doi: 10.1007/s00167-019-05740-8. [DOI] [PubMed] [Google Scholar]
- 32.Getgood A.M.J., Bryant D.M., Litchfield R. Lateral extra-articular tenodesis reduces failure of hamstring tendon autograft anterior cruciate ligament reconstruction: 2-year outcomes from the STABILITY study randomized clinical trial. Am J Sports Med. 2020;48:285–297. doi: 10.1177/0363546519896333. [DOI] [PubMed] [Google Scholar]
- 33.Porter M., Shadbolt B. Modified iliotibial band tenodesis is indicated to correct intraoperative residual pivot shift after anterior cruciate ligament reconstruction using an autologous hamstring tendon graft: A prospective randomized controlled trial. Am J Sports Med. 2020;48:1069–1077. doi: 10.1177/0363546520910148. [DOI] [PubMed] [Google Scholar]
- 34.Hamido F., Habiba A.A., Marwan Y. Anterolateral ligament reconstruction improves the clinical and functional outcomes of anterior cruciate ligament reconstruction in athletes. Knee Surg Sports Traumatol Arthrosc. 2021;29:1173–1180. doi: 10.1007/s00167-020-06119-w. [DOI] [PubMed] [Google Scholar]
- 35.Siebold R., Dehler C., Ellert T. Prospective randomized comparison of double-bundle versus single-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2008;24:137–145. doi: 10.1016/j.arthro.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Zaffagnini S., Bruni D., Marcheggiani Muccioli G.M. Single-bundle patellar tendon versus non-anatomical double-bundle hamstrings ACL reconstruction: A prospective randomized study at 8-year minimum follow-up. Knee Surg Sports Traumatol Arthrosc. 2011;19:390–397. doi: 10.1007/s00167-010-1225-y. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda K., Tsujino J., Tanabe Y., Kaneda K. Effects of initial graft tension on clinical outcome after anterior cruciate ligament reconstruction. Autogenous doubled hamstring tendons connected in series with polyester tapes. Am J Sports Med. 1997;25:99–106. doi: 10.1177/036354659702500120. [DOI] [PubMed] [Google Scholar]
- 38.Khare R., Lal H., Vidyarthi K., Jangira V., Mittal D. Randomised comparison of pretensioning using cyclical loading and on tendon board for arthroscopic anterior cruciate ligament reconstruction using hamstring autograft. J Clin Orthop Trauma. 2017;8:259–264. doi: 10.1016/j.jcot.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeFroda SF, Karamchedu NP, Budacki R, et al. Evaluation of graft tensioning effects in anterior cruciate ligament reconstruction between hamstring and bone-patellar tendon bone autografts [published online January 21, 2020]. J Knee Surg. 10.1055/s-0039-3402046. [DOI] [PMC free article] [PubMed]
- 40.Drogset J.O., Grøntvedt T., Robak O.R., Mølster A., Viset A.T., Engebretsen L. A sixteen-year follow-up of three operative techniques for the treatment of acute ruptures of the anterior cruciate ligament. J Bone Joint Surg Am. 2006;88:944–952. doi: 10.2106/JBJS.D.02876. [DOI] [PubMed] [Google Scholar]
- 41.Frobell R.B., Roos E.M., Roos H.P., Ranstam J., Lohmander L.S. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363:331–342. doi: 10.1056/NEJMoa0907797. [DOI] [PubMed] [Google Scholar]
- 42.Frobell R.B., Roos H.P., Roos E.M., Roemer F.W., Ranstam J., Lohmander L.S. Treatment for acute anterior cruciate ligament tear: Five year outcome of randomised trial. BMJ. 2013;346:f232. doi: 10.1136/bmj.f232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiadaliri A.A., Englund M., Lohmander L.S., Carlsson K.S., Frobell R.B. No economic benefit of early knee reconstruction over optional delayed reconstruction for ACL tears: Registry enriched randomised controlled trial data. Br J Sports Med. 2016;50:558–563. doi: 10.1136/bjsports-2015-095308. [DOI] [PubMed] [Google Scholar]
- 44.Culvenor A.G., Eckstein F., Wirth W., Lohmander L.S., Frobell R. Loss of patellofemoral cartilage thickness over 5 years following ACL injury depends on the initial treatment strategy: results from the KANON trial. Br J Sports Med. 2019;53:1168–1173. doi: 10.1136/bjsports-2018-100167. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson K., von Essen C., Jönhagen S., Barenius B. No risk of arthrofibrosis after acute anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26:2875–2882. doi: 10.1007/s00167-017-4814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Essen C., McCallum S., Barenius B., Eriksson K. Acute reconstruction results in less sick-leave days and as such fewer indirect costs to the individual and society compared to delayed reconstruction for ACL injuries. Knee Surg Sports Traumatol Arthrosc. 2020;28:2044–2052. doi: 10.1007/s00167-019-05397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Essen C., Eriksson K., Barenius B. Acute ACL reconstruction shows superior clinical results and can be performed safely without an increased risk of developing arthrofibrosis. Knee Surg Sports Traumatol Arthrosc. 2020;28:2036–2043. doi: 10.1007/s00167-019-05722-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flosadottir V., Frobell R., Roos E.M., Ageberg E. Impact of treatment strategy and physical performance on future knee-related self-efficacy in individuals with ACL injury. BMC Musculoskelet Disord. 2018;19:50. doi: 10.1186/s12891-018-1973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bottoni C.R., Liddell T.R., Trainor T.J., Freccero D.M., Lindell K.K. Postoperative range of motion following anterior cruciate ligament reconstruction using autograft hamstrings: A prospective, randomized clinical trial of early versus delayed reconstructions. Am J Sports Med. 2008;36:656–662. doi: 10.1177/0363546507312164. [DOI] [PubMed] [Google Scholar]
- 50.Chen J., Gu A., Jiang H., Zhang W., Yu X. A comparison of acute and chronic anterior cruciate ligament reconstruction using LARS artificial ligaments: A randomized prospective study with a 5-year follow-up. Arch Orthop Trauma Surg. 2015;135:95–102. doi: 10.1007/s00402-014-2108-3. [DOI] [PubMed] [Google Scholar]
- 51.Manandhar R.R., Chandrashekhar K., Kumaraswamy V., Sahanand S., Rajan D. Functional outcome of an early anterior cruciate ligament reconstruction in comparison to delayed: Are we waiting in vain? J Clin Orthop Trauma. 2018;9:163–166. doi: 10.1016/j.jcot.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jepsen C.F., Lundberg-Jensen A.K., Faunoe P. Does the position of the femoral tunnel affect the laxity or clinical outcome of the anterior cruciate ligament-reconstructed knee? A clinical, prospective, randomized, double-blind study. Arthroscopy. 2007;23:1326–1333. doi: 10.1016/j.arthro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Mutsuzaki H., Kinugasa T., Ikeda K., Sakane M. Calcium phosphate-hybridized tendon grafts reduce femoral bone tunnel enlargement in anatomic single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26:500–507. doi: 10.1007/s00167-017-4657-9. [DOI] [PubMed] [Google Scholar]
- 54.Funchal L.F.Z., Astur D.C., Ortiz R., Cohen M. The presence of the arthroscopic "floating meniscus" sign as an indicator for surgical intervention in patients with combined anterior cruciate ligament and grade II medial collateral ligament injury. Arthroscopy. 2019;35:930–937. doi: 10.1016/j.arthro.2018.10.114. [DOI] [PubMed] [Google Scholar]
- 55.Vogrin M., Rupreht M., Dinevski D. Effects of a platelet gel on early graft revascularization after anterior cruciate ligament reconstruction: A prospective, randomized, double-blind, clinical trial. Eur Surg Res. 2010;45:77–85. doi: 10.1159/000318597. [DOI] [PubMed] [Google Scholar]
- 56.Reda W., ElGuindy A.M.F., Zahry G., Faggal M.S., Karim M.A. Anterior cruciate ligament reconstruction; is a tourniquet necessary? A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2016;24:2948–2952. doi: 10.1007/s00167-015-3582-z. [DOI] [PubMed] [Google Scholar]
- 57.Cameron S.E., Wilson W., St Pierre P. A prospective, randomized comparison of open vs arthroscopically assisted ACL reconstruction. Orthopedics. 1995;18:249–252. doi: 10.3928/0147-7447-19950301-06. [DOI] [PubMed] [Google Scholar]
- 58.Krywulak S.A., Mohtadi N.G., Russell M.L., Sasyniuk T.M. Patient satisfaction with inpatient versus outpatient reconstruction of the anterior cruciate ligament: A randomized clinical trial. Can J Surg. 2005;48:201–206. [PMC free article] [PubMed] [Google Scholar]
- 59.Meunier A., Odensten M., Good L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: a randomized study with a 15-year follow-up. Scand J Med Sci Sports. 2007;17:230–237. doi: 10.1111/j.1600-0838.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 60.Castoldi M., Magnussen R.A., Gunst S. A Randomized controlled trial of bone-patellar tendon-bone anterior cruciate ligament reconstruction with and without lateral extra-articular tenodesis: 19-year clinical and radiological follow-up. Am J Sports Med. 2020;48:1665–1672. doi: 10.1177/0363546520914936. [DOI] [PubMed] [Google Scholar]
- 61.Holm I., Oiestad B.E., Risberg M.A., Gunderson R., Aune A.K. No differences in prevalence of osteoarthritis or function after open versus endoscopic technique for anterior cruciate ligament reconstruction: 12-year follow-up report of a randomized controlled trial. Am J Sports Med. 2012;40:2492–2498. doi: 10.1177/0363546512458766. [DOI] [PubMed] [Google Scholar]
- 62.Sajovic M., Stropnik D., Skaza K. Long-term comparison of semitendinosus and gracilis tendon versus patellar tendon autografts for anterior cruciate ligament reconstruction: A 17-year follow-up of a randomized controlled trial. Am J Sports Med. 2018;46:1800–1808. doi: 10.1177/0363546518768768. [DOI] [PubMed] [Google Scholar]
- 63.Barenius B., Ponzer S., Shalabi A., Bujak R., Norlén L., Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: A 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42:1049–1057. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 64.Sporsheim A.N., Gifstad T., Lundemo T.O. Autologous BPTB ACL reconstruction results in lower failure rates than ACL repair with and without synthetic augmentation at 30 years of follow-up: A prospective randomized study. J Bone Joint Surg Am. 2019;101:2074–2081. doi: 10.2106/JBJS.19.00098. [DOI] [PubMed] [Google Scholar]
- 65.Järvelä S., Kiekara T., Suomalainen P., Järvelä T. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 10-year results. Am J Sports Med. 2017;45:2578–2585. doi: 10.1177/0363546517712231. [DOI] [PubMed] [Google Scholar]
- 66.Sundaraj K., Salmon L.J., Heath E.L. Bioabsorbable versus titanium screws in anterior cruciate ligament reconstruction using hamstring autograft: A prospective, randomized controlled trial with 13-year follow-up. Am J Sports Med. 2020;48:1316–1326. doi: 10.1177/0363546520911024. [DOI] [PubMed] [Google Scholar]
- 67.Björnsson H., Samuelsson K., Sundemo D. A randomized controlled trial with mean 16-year follow-up comparing hamstring and patellar tendon autografts in anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44:2304–2313. doi: 10.1177/0363546516646378. [DOI] [PubMed] [Google Scholar]
- 68.Konrads C., Reppenhagen S., Plumhoff P., Hoberg M., Rudert M., Barthel T. No significant difference in clinical outcome and knee stability between patellar tendon and semitendinosus tendon in anterior cruciate ligament reconstruction. Arch Orthop Trauma Surg. 2016;136:521–525. doi: 10.1007/s00402-015-2386-4. [DOI] [PubMed] [Google Scholar]
- 69.Webster K.E., Feller J.A., Hartnett N., Leigh W.B., Richmond A.K. Comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction: A 15-year follow-up of a randomized controlled trial. Am J Sports Med. 2016;44:83–90. doi: 10.1177/0363546515611886. [DOI] [PubMed] [Google Scholar]
- 70.Stensbirk F., Thorborg K., Konradsen L., Jørgensen U., Hölmich P. Iliotibial band autograft versus bone-patella-tendon-bone autograft, a possible alternative for ACL reconstruction: A 15-year prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22:2094–2101. doi: 10.1007/s00167-013-2630-9. [DOI] [PubMed] [Google Scholar]
- 71.Annear P.T., Rohr E.J., Hille D.M., Gohil S., Ebert J.R. No clinical difference in 10-year outcomes between standard and minimal graft debridement techniques in patients undergoing anterior cruciate ligament reconstruction using autologous hamstrings: A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2019;27:516–523. doi: 10.1007/s00167-018-5146-5. [DOI] [PubMed] [Google Scholar]
- 72.Paschos N.K., Howell S.M. Anterior cruciate ligament reconstruction: Principles of treatment. EFORT Open Rev. 2016;1:398–408. doi: 10.1302/2058-5241.1.160032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shea K.G., Carey J.L., Richmond J. The American Academy of Orthopaedic Surgeons evidence-based guideline on management of anterior cruciate ligament injuries. J Bone Joint Surg Am. 2015;97:672–674. doi: 10.2106/jbjs.n.01257. [DOI] [PubMed] [Google Scholar]
- 74.Mo Z., Li D., Yang B., Tang S. Comparative efficacy of graft options in anterior cruciate ligament reconstruction: A systematic review and network meta-analysis. Arthrosc Sports Med Rehabil. 2020;2:e645–e654. doi: 10.1016/j.asmr.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S., Zhang C., Cai Y., Lin X. Autograft or allograft? Irradiated or not? A contrast between autograft and allograft in anterior cruciate ligament reconstruction: A meta-analysis. Arthroscopy. 2018;34:3258–3265. doi: 10.1016/j.arthro.2018.06.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.