Abstract
Hemifacial spasm (HFS) patients occasionally present with preoperative facial weakness (PFW) or develop delayed facial palsy (DFP) after microvascular decompression (MVD). This study is aimed to evaluate the neurophysiology underlying facial nerve motor dysfunction in HFS patients preoperatively and postoperatively. In all, 54 HFS patients without prior botulinum toxin injection who underwent MVD were retrospectively reviewed. The compound muscle action potential (CMAP) amplitude ratios of the affected and unaffected facial nerves, measured at 4 time points from preoperation to 1 year post-surgery, were aggregated. Clinical outcomes and the CMAP amplitude ratios were evaluated. Six patients (11.1%) presented with PFW, which correlated with advanced age (p = 0.007) and symptom duration (p = 0.001). The average duration to achieve PFW relief was 2.67 months postoperatively. The preoperative CMAP amplitude ratios of PFW patients were lower than those of patients without PFW (85.3% vs 95.7%). The ratios showed the lowest value at 1-week post-surgery in both groups (70.3% vs 90.9%), had a tendency toward improvement at 1 month, and finally recovered to almost the same level as that before the surgery at 1 year. Three patients (5.6%), whose CMAP ratios showed a persistent decrease from 1 week (56.5%) to 1 month (31%) after MVD, developed DFP. This study illustrates PFW in HFS patients reflects facial nerve axonal stress. MVD is effective in resolving spasm and PFW, without long-term damage to the facial nerve in most patients. In DFP patients, the direct and subsequent secondary axonal disorder develops on the postoperative facial nerve.
Keywords: compound muscle action potential, delayed facial palsy, facial weakness, hemifacial spasm, microvascular decompression
Introduction
Hemifacial spasm (HFS) is a motor disorder characterized by involuntary tonic and clonic activity of the muscles innervated by the facial nerve on the unilateral side of the face.1,2) Most cases are attributed to compression of the cerebral arteries on the facial nerve at its root exit zone (REZ). Microvascular decompression (MVD) of the facial nerve is a well-established surgical treatment for HFS.3–8)
Preoperative facial weakness (PFW) occasionally accompanies HFS,5,9) and has been reported to improve after MVD.1,3) Additionally, several studies have reported delayed facial palsy (DFP), occurring ipsilaterally, more than 24 hours post-surgery as one of the complications of MVD for HFS.1,10–13) These facts support the hypothesis that MVD for HFS patients, while resolving the spasm, can also affect the motor function of the ipsilateral facial nerve. However, only a few neurophysiological investigations have assessed the extent of the influence of MVD on the facial nerve motor function.
The purpose of this study was to illustrate the neurophysiology underlying facial nerve motor dysfunction preoperatively and postoperatively in HFS patients. With long-term clinical observations and repeated nerve conduction studies (NCS) of the facial nerve, we identified the characteristics of the patients with PFW or DFP, their clinical outcomes, and the electrophysiological features of the motor function of the facial nerve before and after MVD.
Materials and Methods
Patient population and evaluation protocol
We retrospectively investigated the patients who underwent MVD for HFS at our hospital between January 2013 and October 2017. To evaluate the unaffected motor function of the facial nerve, patients with past medical history of botulinum neurotoxin injection and previous MVD surgery were excluded.
First, the patients were divided into two groups based on the presence or absence of PFW, and their preoperative characteristics and postoperative outcomes were compared. Second, to identify the long-term influence of MVD on motor function of the facial nerve, we performed repeated measurements of the compound muscle action potential (CMAP) of the facial nerve, a total of four times before MVD, and 1 week, 1 month, and 1 year after the surgery. Finally, we investigated the clinical outcomes and CMAP amplitude of the patients who had developed DFP after MVD.
All patients routinely underwent preoperative and postoperative magnetic resonance (MR) imaging, MR angiography, computed tomography scanning, and pure tone audiometry. Clinical outcomes were evaluated immediately after MVD to 1 week after the surgery during hospital stay; at 1, 3, 6, and 9 months after the surgery; and at a 1-year interval in the outpatient clinic. We evaluated facial weakness using the House–Brackmann (HB) scale. We defined PFW as a case with HB grade II or higher before MVD surgery, and DFP as a case with HB grade II or higher, more than 24 hours after surgery. The facial weakness grading was performed by the same observers, two neurosurgeons. All participants provided informed consent, and the study design was approved by the appropriate ethics review board.
Surgical procedure
The surgery was performed using a lateral suboccipital retrosigmoid approach with continuous intraoperative monitoring of the brain auditory evoked potential and lateral spread response. A C-shaped skin incision was made behind the ear within the hairline. A 4-cm bone flap was made in the inferolateral portion of the suboccipital region to expose the inferior part of the sigmoid sinus. After dural opening, the cerebrospinal fluid was mildly drained from the lateral cerebellomedullary cistern. The arachnoid membrane dissection was initiated from the lower cranial nerves with gentle cerebellar retraction. Following exposure of the REZ of the facial nerve and the facial and vestibulocochlear nerves complex, a transposition of the offending vessels was performed using Teflon felts and fibrin glue. After ruling out the involvement of other vessels compressing the REZ and the proximal facial nerve, the dural mater was closed.
Stimulation and recording
The NCS measurement was performed according to a past report.2) The patients were laid in the supine position on a bed in a warm room. The facial nerves were stimulated using a bipolar surface electrode with the cathode positioned below the ear lobe and the anode on the mastoid tip. The recording electrodes were disks of 5 mm diameter, placed on the inferior part of orbicularis oculi muscle. The ground electrode was placed on the forehead. A square wave stimulation of 0.2 ms at 1 Hz frequency was used to generate the highest level of muscle action potential. We measured the CMAP amplitude on both sides of the face, and subsequently recorded the amplitude ratio of the affected and unaffected side. A CareFusion Nicolet EDX with Viking Software system (Natus Neurology, Middleton, WI, USA) was used for the stimulations and measurements.
Statistical analyses
All statistical calculations were performed using SPSS version 23.0 (IBM, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables as percentage (%). The group differences were analyzed using Mann–Whitney U test for continuous variables and Fisher’s exact probability test for binary variables. Additionally, a two-way repeated measures analysis of variance (ANOVA) was used to assess the changes in postoperative CMAP amplitude ratios between each group. A p value <0.05 was considered statistically significant.
Results
We analyzed the data from 54 of the 137 consecutive patients who underwent MVD for HFS at our hospital during the study period. In total, 66 patients with a past history of botulinum neurotoxin injection, 2 patients with prior MVD surgery, and 15 patients who were lost to follow-up were excluded. None of the patients had a history of Bell’s palsy, trauma, or other surgical treatment around their facial nerve area. The mean patient age was 52.2 ± 11.6 years (range: 24–73 years). The female:male ratio was 1.3:1, and the ratio of the left-:right-affected sides was 1.7:1. The mean follow-up period was 26.96 ± 15.12 months (range: 12–64 months).
Preoperative patient characteristics are summarized in Table 1. PFW was found in six patients (11.1%); all were classified as HB grade II facial weakness. The patients with PFW were significantly older than those without PFW (mean ± SD, 64 ± 7.3 vs 50.7 ± 11.2; p = 0.007). The average duration of HFS until surgery in patients with PFW was longer than that in patients without PFW (mean ± SD 142.8 ± 108.2 vs 45.7 ± 31.3; p <0.001). There was no significant difference between the two groups with respect to sex, affected side, past medical history of hypertension, and incidence of dyslipidemia, and diabetes mellitus. The surgical findings did not indicate any association between the severity of neurovascular compression and the occurrence of PFW.
Table 1. Clinical characteristics of patients.
| Variables | PFW | |||
|---|---|---|---|---|
| Total | Yes | No | p value | |
| No. of patients | 54 | 6 | 48 | |
| Mean age ± SD (years) | 52.2 ± 11.6 | 64 ± 7.3 | 50.7 ± 11.2 | 0.007 |
| Sex, women | 31 (57.4%) | 3 (50%) | 28 (58.3%) | 1.000 |
| Side, left | 34 (63%) | 2 (33.3%) | 32 (66.7%) | 0.179 |
| Mean duration of symptoms ± SD (months) | 56.5± 54.3 | 142.8 ± 108.2 | 45.7 ± 31.7 | <0.001 |
| Hypertension | 13 (24.1%) | 3 (50%) | 10 (20.8%) | 0.143 |
| Dyslipidemia | 11 (20.4%) | 2 (33.3%) | 9 (18.8%) | 0.590 |
| Diabetes mellitus | 4 (7.4%) | 2 (33.3%) | 2 (4.2%) | 0.057 |
| HB grade | ||||
| I | 48 | 0 | 48 | |
| II | 6 | 6 | 0 | |
| Offending vessels | ||||
| AICA | 27 | 5 (83.3%) | 22 (45.8%) | |
| PICA | 10 | 0 | 10 (20.8%) | |
| VA | 2 | 0 | 2 (4.2%) | |
| Complex | 15 | 1 (16.7%) | 14 (29.2%) | |
AICA: anterior inferior cerebellar artery, HB grade: House–Brackmann grade, PFW: preoperative facial weakness, PICA: posterior inferior cerebellar artery, SD: standard deviation, VA: vertebral artery.
Postoperative outcomes in the two groups are described in Table 2. Considerable spasm improvement was observed in both groups. A total of three patients (5.6%) underwent additional MVD surgery for HFS recurrence. All patients completely recovered from PFW within 1 year after MVD surgery. The average duration to achieve PFW relief was 2.67 months. Of the patients with PFW, three patients (50%) showed immediate recovery after surgery.
Table 2. Clinical outcomes of MVD in HFS patients.
| Variables | PFW | ||
|---|---|---|---|
| Total | Yes | No | |
| No. of patients | 54 | 6 | 48 |
| Spasm resolution | |||
| Postoperation | 31 | 2 (33.3%) | 29 (60.4%) |
| Within 1 month | 6 | 1 (16.7%) | 5 (10.4%) |
| Within follow-up | 14 | 2 (33.3%) | 12 (25%) |
| Spasm recurrence | 3 | 1 (16.7%) | 2 (4.2%) |
| PFW resolution | |||
| Within 1 month | 4 (66.7%) | ||
| Within 1 year | 2 (33.3%) | ||
| Postoperative DFP | 3 | 0 | 3 (6.3%) |
| Other complications | |||
| Subdural hemorrhage | 1 | 0 | 1 (2.1%) |
| Hoarseness | 1 | 0 | 1 (2.1%) |
| Dysphagia | 1 | 0 | 1 (2.1%) |
DFP: delayed facial palsy, HFS: hemifacial spasm, MVD: microvascular decompression, PFW: preoperative facial weakness.
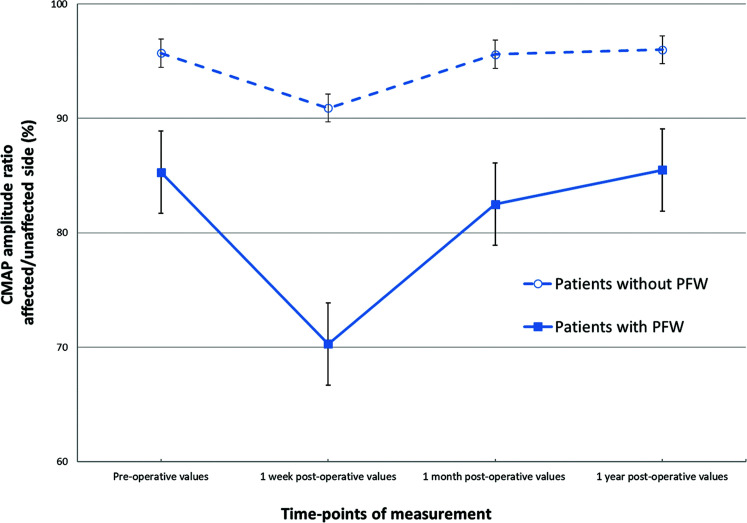
The mean ratios of CMAP amplitude of the affected and unaffected facial nerves in both the groups measured in each period are shown in Fig. 1. The preoperative CMAP amplitude ratios were 85.3% in the patients with PFW and 95.7% in those without PFW. The CMAP amplitude ratios were the lowest at 1 week after surgery in both the groups: 70.3% in the PFW and 90.9% in the non-PFW group. These showed a tendency toward improvement 1 month after surgery, and finally, slightly higher ratios were obtained at 1 year after surgery compared with those at preoperation. Although the actual CMAP amplitude ratios of the PFW group were lower than that of non-PFW group at each observation point, the lines of CMAP amplitude ratios of both groups showed a similar transition from preoperation to 1 year post-surgery and the values were not significantly different (p = 0.56, ANOVA).
Fig. 1.
The CMAP amplitude ratios of the affected and unaffected facial nerves in patients with HFS were measured four times, pre- and post-MVD surgery. The CMAP amplitude ratios were 95.7% at preoperation and 90.9% at 1 week, 95.6% at 1 month, and 96.0% at 1 year after MVD in the HFS patients without PFW; 85.3% at preoperation and 70.3% at 1 week, 82.5% at 1 month, and 85.5% at 1 year after surgery in the patients with PFW. The error bars represent standard error. CMAP: compound muscle action potential, HFS: hemifacial spasm, MVD: microvascular decompression, PFW: preoperative facial weakness.
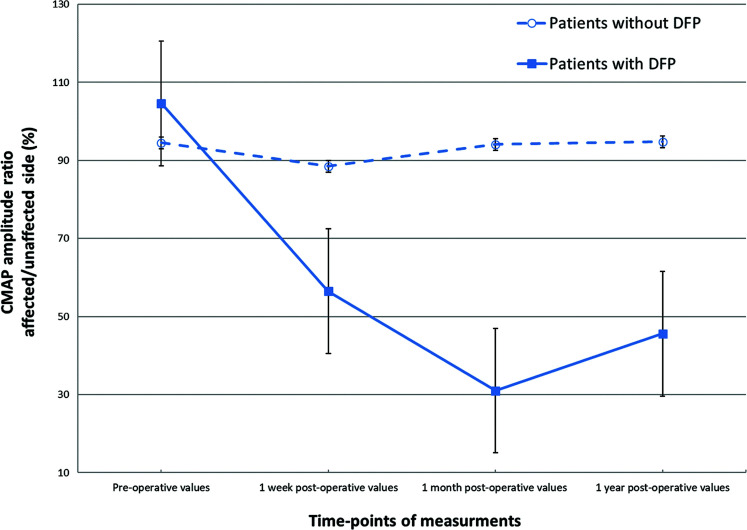
Three patients (5.6%) developed DFP after MVD. None of these patients had facial weakness before surgery (Table 2) and radiological abnormalities after surgery. DFP onset was observed on postoperative days 1, 8, and 11 among the patients. Two patients who had HB grade II facial weakness, recovered completely within 3 months after surgery. One patient with HB grade V facial weakness had residual HB grade II facial weakness at the final examination. The mean CMAP amplitude ratios between the affected and unaffected side in the patients with and without DFP are shown in Fig. 2. The preoperative CMAP amplitude ratio did not decrease in DFP group. A persistent and strong CMAP amplitude ratio decrease was observed from 1 week (56.5% in the DFP and 88.5% in the non-DFP group, p = 0.077) to 1 month (31% in the DFP and 94.1% in the non-DFP group, p = 0.002) after surgery and showed partial improvement at 1 year after surgery (45.6%). The changes over time of the CMAP amplitude ratios of the two groups showed significant difference (p = 0.020, ANOVA).
Fig. 2.
The CMAP amplitude ratios between the affected and unaffected facial nerves in patients who developed DFP after MVD. The CMAP amplitude ratio did not show preoperative reduction. However, a persistent and steep decrease was observed starting 1 week (56.5%) till 1 month (31%) after MVD. It showed partial improvement at 1 year after surgery (45.6%). The error bars represent standard error. CMAP: compound muscle action potential, DFP: delayed facial palsy, MVD: microvascular decompression.
Discussion
Patients with HFS occasionally present with concurrent PFW or develop DFP after MVD.1,10–13) The presence of the preoperative and postoperative facial weakness suggests that MVD not only improves facial spasm but also affects facial nerve motor function in HFS patients. However, to the best of our knowledge, few studies have objectively investigated the motor function of the facial nerve in HFS patients and assessed the long-term influence of surgery on the facial motor nerve. This is the first study to identify the characteristics and clinical outcomes of PFW and DFP in HFS patients and to examine the long-term sequential electrophysiological change of the facial nerve motor function, pre- and post-MVD. The periodic CMAP amplitude measurements helped to elucidate the neurophysiological mechanisms of facial nerve motor dysfunction in HFS patients preoperatively and postoperatively.
Facial weakness in patients with HFS has been described in previous studies. The incidence of PFW was reported to be 10%–56% in HFS patients.1,3,5,9) Previous botulinum toxin treatment and symptom duration were thought to have a significant association with PFW.1,9) In our study, facial weakness was found in 11.1% of the primary HFS patients without a history of prior botulinum toxin injection therapy, and had a significant correlation with advanced age and the preoperative symptom duration (Table 1). Although detailed information has not been published, few reports with a long-term follow-up describe good outcomes for PFW after MVD surgery.1,3) Our study showed that facial weakness (5 of 6 cases, 83.3%) in most of the cases resolved completely within 3 months, and in all cases, within 1 year after surgery (Table 2).
We assessed the neurophysiological effects of MVD on facial nerve motor function with periodic, long-term, postoperative CMAP amplitude measurements. Regardless of the presence or absence of PFW, the facial nerve CMAP amplitude ratio was the lowest at 1 week after surgery and tended to improve at 1 month after surgery, reaching a value that was similar to that observed before surgery (Fig. 1). The low CMAP amplitude ratio at 1 week after surgery may reflect axonal stress due to surgical invasion. In the PFW group, the degree of amplitude decrease at 1 week was slightly larger than those without PFW. Improvement in the CMAP amplitude ratio at 1 month after surgery may be due to recovery from axonal stress due to surgery and relief from vascular compression. Although the CMAP amplitude ratios at each observation point are lower in the PFW group, the lines running through the CMAP amplitude ratios of both the groups did not show a significant difference in the statistical test. This fact assumes that MVD for HFS patients produces similar electrophysiological changes in the ipsilateral facial nerve for both the groups.
Several studies have discussed whether the improvement in spasms observed after MVD is due to chronic trauma of the facial nerve owing to the surgery.1,14,15) Our study results indicate that although transient and slight electrophysiological reduction in facial nerve motor function may occur at 1 week after MVD, in most cases with good spasm relief, long-term adverse effects on the facial nerve are not observed after surgery.
DFP after MVD for HFS has been described in several studies, with an incidence of 2.7%–14.5% in patients. The average time to onset was 9.7–13.1 days (range, 1–44 postoperative days).1,3–6,11–13) Patient characteristics, such as age, sex, affected side, symptom duration, offending vessels, and previous botulinum toxin injection were not predictive factors.11,13) Most of the patients with DFP were reported to recover completely within a few weeks.11–13) In our study, DFP was observed in 5.6% patients after surgery (Table 2). Patients with DFP did not have facial weakness and a decrease in CMAP amplitude ratio preoperatively (Fig. 2), thus suggesting that preoperative facial nerve function does not affect DFP occurrence. A steep decrease in the CMAP amplitude ratio at 1 week after surgery in DFP patients (Fig. 2) may reflect intense axonal injury of the facial nerve owing to surgical manipulation. In addition, the most remarkable feature is that the strong decrease in CMAP amplitude ratio in DFP patients continued until 1 month after surgery. This observation is in contrast with that observed in the patients without DFP, whose CMAP amplitude ratios tended to recover at 1 month after surgery. This result shows that surgery not only causes direct damage, such as retraction or coagulation, but also result in secondary damages that are generated over time in the facial nerve of the DFP patients because the CMAP amplitude ratio in DFP patients persistently decreased even 1 week after the surgery. Several authors have hypothesized that DFP after MVD may be related to secondary disorders, such as nerve edema, vasospasm, virus reactivation, or chemical meningitis,1,10–13) but objective data which can prove the existence of the secondary damage of the facial nerve after surgery are few. Our present study is the first to illustrate the development of secondary damage of the facial nerve in DFP patients, using the periodic electrophysiological studies.
We need to mention a discrepancy between clinical symptoms and CMAP amplitude ratio measured at 1 year after surgery. In our study, all patients completely recovered from PFW within 1 year after surgery, but their CMAP amplitude ratio remained at the same level as that before surgery (85.3%). In addition, although postoperative DFP had recovered in two of three patients, their CMAP amplitude ratio recovered to only 45.6% 1 year after surgery. Ruby and Jannetta16) investigated histopathological changes about the facial nerve in HFS patients. Many of the nerves appeared normal; however, in some cases, the severe shrinking of the axis cylinder and disrupted myelin sheath were observed in the electron microscope examination. They thought these degenerative changes were a result from neurovascular compression-distortion. Taking this previous pathological investigation into consideration, we hypothesize that the facial nerves in patients with PFW are more degenerated due to long-term neurovascular compression and are more likely to susceptible to vascular compression. Although PFW can be clinically improved by resolving vascular compression, because of the irreversible degeneration in the facial nerve, the decrease in CMAP amplitude ratio remains at 1 year after surgery. For the same reason, while most postoperative DFP can be clinically improved to almost normal levels with time, the decreasing state of CMAP amplitude ratio at 1 year after surgery sensitively reflects the neurodegeneration caused by the surgery.
This study has some limitations. This is a single-institution retrospective study and the number of cases included was limited. Additionally, NCS were not conducted during or immediately after surgery. Because NCS were conducted at only four time points, it is possible that the changes in postoperative CMAP amplitude ratio may not have been captured accurately. For example, although the difference may not be very large, the time point at which the CMAP amplitude ratio may have been the lowest, may deviate from the time point of 1 week after surgery. Moreover, in CMAP measurements, we used a skin recording disk, which might be affected by the skin surface temperature and humidity. Therefore, ideally, the electromyogram should also have been conducted, especially in the patients with PFW or DFP, which would provide a more complete picture of the physiology. Finally, our study demonstrated the existence of secondary axonal damage of the facial nerve in DFP patients, but the specific cause has not been elucidated yet. The CMAP amplitude ratio at 1week after surgery was tend to be lower in the DFP group (56.5% vs 88.5%), but the trend did not reach statistical significance (p = 0.077). Further research is needed to determine whether the CMAP amplitude ratio measurement can be a predictive factor for DFP.
Facial weakness accompanying HFS correlates with advanced age and preoperative symptom duration, which may reflect degenerative change of facial nerve and axonal stress due to long-term vascular compression, and can be clinically resolved shortly after MVD. Periodic CMAP amplitude measurements until 1 year after surgery revealed that although transient and small electrophysiological reduction in the facial motor nerve occurs at 1 week after surgery, MVD can resolve the facial spasm and weakness without causing long-term damage to the facial motor nerve in most HFS patients. Meanwhile, further investigation is needed to illustrate the pathogenesis of the secondary axonal disorder of the facial nerve in DFP patients.
Acknowledgment
The authors express their deepest appreciation to the individuals who contributed to the study and manuscript preparation.
Footnotes
Conflicts of Interest Disclosure
None of the authors has any conflict of interest to disclosure.
References
- 1).Barker FG, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD: Microvascular decompression for hemifacial spasm. J Neurosurg 82: 201–210, 1995 [DOI] [PubMed] [Google Scholar]
- 2).Auger RG: Hemifacial spasm: clinical and electrophysiologic observations. Neurology 29: 1261–1272, 1979 [DOI] [PubMed] [Google Scholar]
- 3).Huang CI, Chen IH, Lee LS: Microvascular decompression for hemifacial spasm: analyses of operative findings and results in 310 patients. Neurosurgery 30: 53–56; discussion 56–57, 1992 [DOI] [PubMed] [Google Scholar]
- 4).Huh R, Han IB, Moon JY, Chang JW, Chung SS: Microvascular decompression for hemifacial spasm: analyses of operative complications in 1582 consecutive patients. Surg Neurol 69: 153–157; discussion 157, 2008 [DOI] [PubMed] [Google Scholar]
- 5).Illingworth RD, Porter DG, Jakubowski J: Hemifacial spasm: a prospective long-term follow up of 83 cases treated by microvascular decompression at two neurosurgical centres in the United Kingdom. J Neurol Neurosurg Psychiatry 60: 72–77, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Samii M, Günther T, Iaconetta G, Muehling M, Vorkapic P, Samii A: Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery 50: 712–718; discussion 718–719, 2002 [DOI] [PubMed] [Google Scholar]
- 7).Shibahashi K, Morita A, Kimura T: Surgical results of microvascular decompression procedures and patient's postoperative quality of life: review of 139 cases. Neurol Med Chir (Tokyo) 53: 360–364, 2013 [DOI] [PubMed] [Google Scholar]
- 8).Mizobuchi Y, Muramatsu K, Ohtani M, et al. : The current status of microvascular decompression for the treatment of hemifacial spasm in Japan: an analysis of 2907 patients using the japanese diagnosis procedure combination database. Neurol Med Chir (Tokyo) 57: 184–190, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Batla A, Goyal C, Shukla G, Goyal V, Srivastava A, Behari M: Hemifacial spasm: clinical characteristics of 321 Indian patients. J Neurol 259: 1561–1565, 2012 [DOI] [PubMed] [Google Scholar]
- 10).Furukawa K, Sakoh M, Kumon Y, et al. : Delayed facial palsy after microvascular decompression for hemifacial spasm due to reactivation of varicella-zoster virus. No Shinkei Geka 31: 899–902, 2003. (Japanese) [PubMed] [Google Scholar]
- 11).Lee JM, Park HR, Choi YD, et al. : Delayed facial palsy after microvascular decompression for hemifacial spasm: friend or foe?. J Neurosurg 129: 299–307, 2018 [DOI] [PubMed] [Google Scholar]
- 12).Lovely TJ, Getch CC, Jannetta PJ: Delayed facial weakness after microvascular decompression of cranial nerve VII. Surg Neurol 50: 449–452, 1998 [DOI] [PubMed] [Google Scholar]
- 13).Rhee DJ, Kong DS, Park K, Lee JA: Frequency and prognosis of delayed facial palsy after microvascular decompression for hemifacial spasm. Acta Neurochir (Wien) 148: 839–843; discussion 843, 2006 [DOI] [PubMed] [Google Scholar]
- 14).Adams CB: Microvascular compression: an alternative view and hypothesis. J Neurosurg 70: 1–12, 1989 [DOI] [PubMed] [Google Scholar]
- 15).Wilkinson MF, Kaufmann AM: Monitoring of facial muscle motor evoked potentials during microvascular decompression for hemifacial spasm: evidence of changes in motor neuron excitability. J Neurosurg 103: 64–69, 2005 [DOI] [PubMed] [Google Scholar]
- 16).Ruby JR, Jannetta PJ: Hemifacial spasm: ultrastructural changes in the facial nerve induced by neurovascular compression. Surg Neurol 4: 369–370, 1975 [PubMed] [Google Scholar]