Abstract
The plant antioxidant system plays important roles in response to diverse abiotic and biotic stresses. However, the effects of virus infection on host redox homeostasis and how antioxidant defense pathway is manipulated by viruses remain poorly understood. We previously demonstrated that the Barley stripe mosaic virus (BSMV) γb protein is recruited to the chloroplast by the viral αa replicase to enhance viral replication. Here, we show that BSMV infection induces chloroplast oxidative stress. The versatile γb protein interacts directly with NADPH‐dependent thioredoxin reductase C (NTRC), a core component of chloroplast antioxidant systems. Overexpression of NbNTRC significantly impairs BSMV replication in Nicotiana benthamiana plants, whereas disruption of NbNTRC expression leads to increased viral accumulation and infection severity. To counter NTRC‐mediated defenses, BSMV employs the γb protein to competitively interfere with NbNTRC binding to 2‐Cys Prx. Altogether, this study indicates that beyond acting as a helicase enhancer, γb also subverts NTRC‐mediated chloroplast antioxidant defenses to create an oxidative microenvironment conducive to viral replication.
Keywords: 2‐Cys Prx, chloroplast antioxidant defenses, NTRC, Viral replication, γb protein
Subject Categories: Metabolism; Microbiology, Virology & Host Pathogen Interaction; Plant Biology
Inhibition of the chloroplast‐localized NADPH‐dependent thioredoxin reductase C to create an oxidative microenvironment promotes plant infection by a chloroplast‐replicating virus.

Introduction
In the evolutionary arms race between invading viruses and their hosts, multilayered host defenses have arisen against virus infections, and viruses have also developed multiple counter‐defense strategies. Plant host defenses include cellular RNA silencing pathways (Baulcombe, 2004; Pumplin & Voinnet, 2013), R gene‐mediated resistance responses (Palukaitis & Yoon, 2020), autophagy‐ and ubiquitination‐mediated protein degradation (Vierstra, 2009; Alcaide‐Loridan & Jupin, 2012; Yang et al, 2020), and RNA decay (Li & Wang, 2019) mechanisms. Host factors such as eIF4G and eIF4E, which are necessary for viral infection in some plants, can also confer recessive resistance against plant viruses (Hashimoto et al, 2021). Antioxidant defenses also have roles in plant–virus interactions, but the mechanisms whereby viruses interfere with these defenses remain to be fully characterized.
Reactive oxygen species (ROS), such as superoxide (O2 •−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hydroxyl radicals (OH•), are produced in various cellular organelles including chloroplasts, peroxisomes, and mitochondria. The generation of ROS is one of the earliest cellular events in response to a variety of pathogen infections, and ROS has dual functions in plant cells. Low ROS levels serve as a secondary messenger to regulate the stromule biogenesis (Caplan et al, 2015) or plant growth and development (Zhao et al, 2021), but excessive ROS causes oxidative stress and damages intracellular macromolecular substances and other cellular processes (Choudhury et al, 2017; Waszczak et al, 2018). To avoid toxic effects, plants have evolved specific ROS‐generating and ROS‐scavenging systems to maintain cellular homeostasis under various oxidative stresses (Noctor et al, 2018; Qi et al, 2018; Fichman & Mittler, 2020; Hyodo & Okuno, 2020). The ROS‐scavenging enzymes in plant antioxidant systems include superoxide dismutase (SOD), catalase (CAT), peroxidase (Prx), ascorbate peroxidase (APX), glutathione S‐transferase (GST), and glutathione peroxidase (GPX; Waszczak et al, 2018).
Chloroplast antioxidant defenses have important roles in response to diverse plant abiotic and biotic stresses. To avoid intracellular oxidative damages, chloroplasts have evolved a series of antioxidant defense mechanisms to maintain equilibrium between ROS production and scavenging (Gill & Tuteja, 2010). Recent studies have suggested that NADPH‐dependent thioredoxin reductase C (NTRC) functions in chloroplast antioxidant defense responses (Mihara et al, 2017). NTRC contains an N‐terminal chloroplast transit peptide (cTP), a middle NADPH‐thioredoxin reductase domain (NTR‐D), and a thioredoxin domain (Trx‐D). NTRC regulates different chloroplast targeting proteins via thiol‐disulfide modulation (Serrato et al, 2004; Geigenberger et al, 2017) and participates in a variety of chloroplast processes, including starch biosynthesis (Michalska et al, 2009; Lepistö et al, 2013), ATP synthesis (Carrillo et al, 2016), carbon storage, chlorophyll biosynthesis (Richter et al, 2013; Perez‐Ruiz et al, 2014), and plastid gene expression (Yoshida & Hisabori, 2016; Geigenberger et al, 2017). NTRC also has a vital role in chloroplast protections against oxidative stresses through the reduction of 2‐Cys peroxiredoxin (2‐Cys Prx) (Da et al, 2017; Yoshida & Hisabori, 2019). In chloroplasts, the redox status of 2‐Cys Prx is highly dependent on NTRC binding, as verified by bimolecular fluorescent complementation (BiFC) assays and isothermal titration calorimetry (ITC) experiments (Bernal‐Bayard et al, 2014), and tandem affinity purification (TAP)‐Tag methods (Gonzalez et al, 2019).
Barley stripe mosaic virus (BSMV), the type member of the genus Hordeivirus, consists of three positive‐sense RNAs designated RNAα, RNAβ, and RNAγ. RNAα and RNAγ encode the replicase protein αa and γa subunits, respectively; RNAβ encodes the coat protein (CP) and triple gene block proteins (TGB1, TGB2, and TGB3). RNAα and RNAγ are required exclusively for BSMV replication in both protoplasts and host leaves (Jackson et al, 2009; Jiang et al, 2021). The γb protein encoded by RNAγ plays a versatile role in multiple steps of the BSMV infection cycle and interferes with several basal host defenses during BSMV–host interactions (Jiang et al, 2021). In addition to functioning as a viral suppressor of RNA silencing (VSR; Yelina et al, 2002; Bragg & Jackson, 2004), γb is a determinant of viral systemic movement (Petty et al, 1990; Yelina et al, 2002), viral pathogenesis (Donald & Jackson, 1994; Bragg et al, 2004), and seed transmission (Edwards, 1995). Moreover, γb is also phosphorylated by PKA‐like kinase to sustain virus infection and evade the host necrotic antiviral responses (Zhang et al, 2018b). In addition, γb interacts with autophagy‐related gene 7 (ATG7) and glycolate oxidase (GOX) to subvert autophagy pathways (Yang et al, 2018b) and inhibit peroxisomal ROS bursts (Yang et al, 2018a), respectively. The γb protein also takes part in viral movement by interacting with the TGB1 movement protein (Jiang et al, 2020). As a potential counter‐counter‐defense strategy, the cytosolic kinase STY46 negatively regulates BSMV infection by phosphorylating and interacting with the γb protein (Zhang et al, 2021).
Previously, we found that the γb protein is recruited to the chloroplast outer membrane by interacting with the αa replicase and enhances αa helicase activity at the membrane to promote BSMV replication (Zhang et al, 2017). In this study, we have extended these investigations to demonstrate that BSMV infection disturbs chloroplast redox homeostasis and reveal that γb subverts NTRC‐mediated antiviral defenses by interfering with interactions between NTRC and 2‐Cys Prx in a competitive manner. These results enhance our understanding of the versatility of the γb protein and reveal a novel role of chloroplast antioxidant defenses during virus–host interactions.
Results
BSMV infection disrupts chloroplast antioxidant defenses
Given that the rapid generation of ROS is one of the universal plant responses to various stresses (Sewelam et al, 2016), and that chloroplasts are the major sites where BSMV replication occurs (Zhang et al, 2017; Jin et al, 2018), we posited that BSMV infection might interfere with the chloroplast redox homeostasis. The HyPer2 protein, a genetically encoded fluorescent H2O2 sensor (Exposito‐Rodriguez et al, 2017), was fused with a transit peptide (aa 1–80) derived from the Rubisco small subunit (RbcS) to elicit chloroplast targeting (Nelson et al, 2007). We then used the Chl‐HyPer2 construct to monitor chloroplast redox states during virus infection. We found that in the context of BSMV infection, approximately 18% of the chloroplasts showed detectable H2O2 signals at 2 days post‐inoculation (dpi) of BSMV and the ROS‐positive chloroplasts increased to 40% at 3 dpi and 4 dpi. The rates gradually decreased at 5 dpi and 6 dpi, suggesting that active BSMV replication induces transient chloroplast oxidative stresses (Fig 1A and B).
Figure 1. BSMV infection disrupts chloroplast antioxidant defenses.

- Chl‐HyPer2 response to chloroplast hydrogen peroxide elicited by BSMV infection in N. benthamiana epidermal cells. Ratiometric images (F488/405 nm) of fluorescence excitation at 488 and 405 nm show the oxidized state of chloroplast‐targeted HyPer2, respectively. The false‐blue color indicates the chloroplasts. Scale bars, 30 μm.
- Quantification of the BSMV‐dependent changes in Chl‐HyPer2 fluorescence at 0–6 dpi. The percentage of ROS‐positive chloroplasts (white) among all chloroplasts (blue and white) per visual field.
- Western blot analysis of 2‐Cys Prx redox status in empty vector (EV) and BSMV inoculated leaves under non‐reducing conditions at 3 dpi. Western blots were analyzed with anti‐2‐Cys Prx or anti‐CP antibodies. The Rubisco large subunit (RbcL) served as a loading control.
- Relative mRNA levels of the sAPX, 2‐Cys Prx, GPXL, and PrxQ genes in response to BSMV infections at 1, 3, and 5 dpi. The empty vector (EV) was used as a negative control, and NbEF1α was used as an internal control.
- BSMV accumulation in N. benthamiana leaves transiently expressing chloroplast ROS‐scavenging proteins. Agrobacterium harboring plasmids expressing various proteins are indicated above the panels. BSMV was agroinfiltrated into the same leaves at 1.5 dpi. Three days later, Western blot analyses were conducted with anti‐TGB1 or anti‐Flag antibodies. RbcL served as the loading control. 2‐CP, 2‐Cys Prx.
- BSMV accumulation in 2‐Cys Prx or GPXL‐silenced N. benthamiana plants using anti‐TGB1 antibody. Empty vector TRV: 00 was used as negative control.
Data information: In (A), data are representative of at least three independent experiments. In (B), error bars indicate mean ± SEM (n = 5). a, b, and c indicate statistically significant differences among different groups (ANOVA, P < 0.05). In (C, E, and F), representative data are shown, and at least three independent replicates were performed with similar results. In (D), error bars indicate mean ± SEM from three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 from Student’s t‐test.
Source data are available online for this figure.
The 2‐Cys Prx protein is a peroxide scavenger and peroxide sensor that plays an important role in chloroplast redox homeostasis and is widely used to indicate the oxidative status of plant cells (Muthuramalingam et al, 2009; Perkins et al, 2015). To analyze the phenomenon shown in Fig 1A, variability in the redox forms of 2‐Cys Prx was tested by using non‐reducing SDS–PAGE (Cerveau et al, 2019). These results showed that BSMV infection increases 2‐Cys Prx monomers that contain both reductive and overoxidized forms (Puerto‐Galán et al, 2015; Cerveau et al, 2016) (Fig 1C), indicating that BSMV infection induces chloroplast oxidative stresses. Furthermore, we measured the transcript levels of several chloroplast ROS‐scavenging‐related genes, including 2‐Cys Prx, sAPX (stroma ascorbate peroxidase) (Exposito‐Rodriguez et al, 2017), GPXL (glutathione peroxidase‐like) (Waszczak et al, 2018), and PrxQ (peroxiredoxin Q) (Yoshida & Hisabori, 2016). These results showed remarkably downregulation of the mRNA levels of all these genes at 3 dpi of leaves infiltrated with Agrobacterium harboring wild‐type BSMV constructs (Fig 1D). These results collectively suggest that BSMV infection disturbs chloroplast redox homeostasis and induces chloroplast oxidative stresses.
To further investigate the effects of antioxidant defenses on BSMV infection, a reduction state of chloroplasts was created by transiently expressing several chloroplast ROS‐scavenging proteins including sAPX, 2‐Cys Prx, GPXL, and PrxQ in N. benthamiana leaves. Western blot analysis revealed that BSMV accumulation was approximately 5–10 times lower in sAPX, 2‐Cys Prx, GPXL, and PrxQ‐expressing plants than in leaves expressing the control Chl‐GFP protein, which the cTP of RbcS was fused to the N terminus of GFP (Fig 1E). In contrast, when the mRNAs of 2‐Cys Prx and GPXL in N. benthamiana were knocked down by Tobacco rattle virus (TRV)‐induced gene silencing (Liu et al, 2002), neither 2‐Cys Prx‐ nor GPXL‐silenced plants showed chlorotic and stunted phenotypes (Appendix Fig S1), and BSMV accumulated 2‐ to 4‐fold more in 2‐Cys Prx‐ or GPXL‐silenced plants than in the non‐silenced control (TRV:00) (Fig 1F). Altogether, these results indicate that BSMV infection disturbs chloroplast antioxidant defenses and creates an oxidative chloroplast microenvironment that is required for virus infection.
γb interacts with NTRC in vivo and in vitro
In order to identify host factors bound by the γb protein, we previously used γb as a bait protein to screen mixed yeast cDNA libraries of three tobacco species (N. tabacum, N. benthamiana, and N. glutinosa) in yeast two‐hybrid (Y2H) assays (Zhang et al, 2021). Interestingly, the chloroplast redox protein NTRC was identified as a γb interacting partner (Appendix Fig S2). To verify the interaction of NbNTRC with γb, full‐length cDNA of NTRC of N. benthamiana (NbNTRC, https://solgenomics.net, Niben101Scf06738g00002.1) was cloned and fused to the activation domain (AD) and paired with γb fused to the DNA‐binding domain (BD). This Y2H analysis confirmed the interaction between the NbNTRC and γb proteins (Fig 2A). To investigate the γb‐NbNTRC interactions in planta, we performed a BSMV‐based BiFC assay (Jiang et al, 2020; Zhang et al, 2021), which can reflect the native subcellular localization of γb‐NbNTRC interactions in BSMV‐infected N. benthamiana cells. Co‐expression of NbNTRC‐YFPc or NbNTRC‐YFPn with γb‐YFPn or γb‐YFPc from recombinant BSMV resulted in the reconstitution of YFP signals at the chloroplast periphery (Fig 2B, Appendix Fig S3).
Figure 2. BSMV γb interacts directly with NbNTRC in vivo and in vitro .

- Y2H analyses of interactions between γb and NbNTRC. Various Y2H combinations are indicated on the images. Self‐interactions of γb served as a positive control, and combinations containing either empty AD or BD constructs served as negative controls.
- BiFC analyses of γb and NbNTRC interactions during BSMV infections. Various BiFC combinations are indicated on the left. YFP signals were visualized by confocal microscopy at 3 dpi and depicted as a false‐green color, and chloroplasts were visualized by chloroplast autofluorescence as a false‐red color. Scale bars, 20 μm.
- Co‐IP analyses of interactions between γb and NbNTRC in N. benthamiana. Leaf tissues were agroinfiltrated with various combinations indicated above the panels were harvested at 3 dpi. Total proteins were immunoprecipitated with anti‐Flag beads. Total and immunoprecipitated (IP) proteins were analyzed by Western blotting with anti‐GFP or anti‐Flag antibodies.
- GST pull‐down assays to verify interactions between γb and NbNTRC in vitro. GST‐tagged NTRC or GFP was incubated with γb‐His or GFP‐His. Input and pull‐down products were analyzed by Western blot analyses with anti‐GST or anti‐His antibodies. GFP‐His and GST‐GFP served as negative controls.
- Co‐localization of γb and NbNTRC during BSMV infection. NbNTRC‐mCherry was co‐expressed with BSMVγb‐GFP (Jiang et al, 2020) in N. benthamiana, confocal analyses were conducted at 3 dpi, and chloroplast autofluorescence is displayed as a false‐blue color. Scale bars, 20 μm.
Data information: In (C and D), representative data are shown, and three replicates had similar results.
Source data are available online for this figure.
To further validate γb‐NbNTRC interactions, we carried out a co‐immunoprecipitation assay (co‐IP). A. tumefaciens harboring plasmids expressing NbNTRC‐GFP or γb‐3xFlag were co‐infiltrated into N. benthamiana epidermal cells. Leaves were harvested at 3 dpi, and total proteins were precipitated by anti‐Flag affinity beads. These results showed that γb‐3xFlag specifically co‐precipitated with NbNTRC‐GFP, but not with the untagged GFP control (Fig 2C). To further examine whether NbNTRC physically interacts with γb, GST‐NbNTRC was purified from Escherichia coli to test its interactions with γb‐His. The resulting GST pull‐down assays showed that GST‐NbNTRC directly binds to the γb‐His in vitro, but not to the GFP‐His control (Fig 2D).
We also conducted cytological assays to determine whether NbNTRC colocalizes with γb during BSMV infection, and these results showed that NbNTRC‐mCherry colocalizes with γb‐GFP at the chloroplast periphery (Fig 2E), consistent with these results shown in Fig 2B. Considering that chloroplast localization of NTRC has not been well characterized so far, we performed BiFC assays to determine whether NTRC locates to chloroplast membranes. Surprisingly, these results showed that self‐interacting NTRC (Bernal‐Bayard et al, 2012) appeared at the chloroplast membrane and in the chloroplasts stroma (Fig EV1A). To further confirm this result, we carried out protease protection assays as previously described (Breuers et al, 2012; Jin et al, 2018), and the results verified the localization of NTRC to the chloroplast surface and the chloroplast interior (Fig EV1B). These data, together with the recruitment of γb to chloroplast outer membrane‐invaginated vesicles during BSMV replication (Zhang et al, 2017; Jin et al, 2018), support the hypothesis that γb interacts with NTRC at the chloroplast outer membrane.
Figure EV1. NTRC localizes to chloroplast membranes.
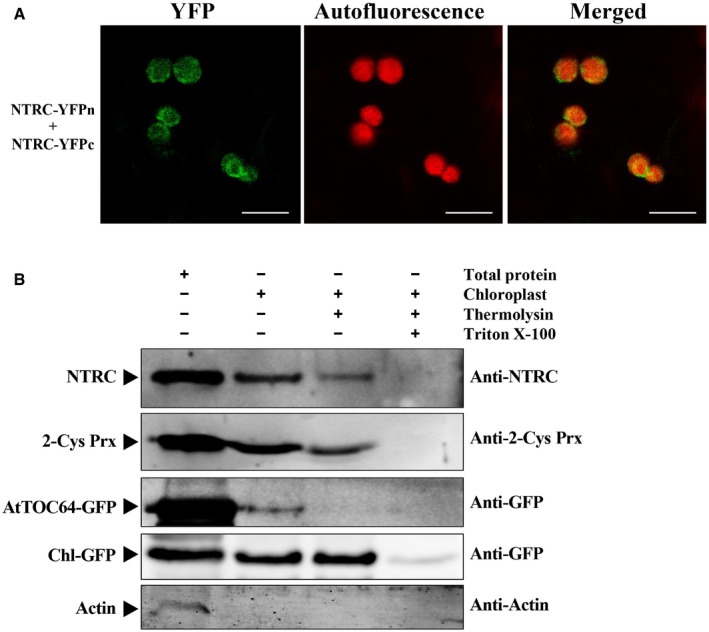
- BiFC assays of self‐interactions of NbNTRC in N. benthamiana. A. tumefaciens harboring NTRC‐YFPn, NTRC‐YFPc, and TBSV P19 were co‐infiltrated into N. benthamiana leaves. YFP signals were visualized by confocal microscopy at 2.5 dpi and depicted as a false‐green color, and chloroplasts were visualized by chloroplast autofluorescence as a false‐red color. Scale bars, 10 μm.
- Protease protection assay to determine chloroplast localization of NTRC and 2‐Cys Prx. Agrobacterium mixtures harboring BSMV, AtTOC64‐GFP, and Chl‐GFP plasmids were co‐infiltrated into N. benthamiana leaves. Intact chloroplasts were isolated and subjected to different treatments as indicated above the panels. Protein samples were prepared from 5‐μg chlorophyll chloroplast equivalents and subjected to Western blot analyses using antibodies indicated on the right. Actin served as a control to assess cytosolic contaminations of isolated chloroplasts. Arrowheads indicate the specific target protein band.
Data information: In (A), data are representative of at least three independent experiments. In (B), representative data are shown, and three replicates had similar results.
His‐85 of γb is required for NbNTRC interactions
To determine the key amino acids responsible for γb binding to NbNTRC, a series of γb mutants were constructed, and the interactions of these mutant proteins with NbNTRC were examined by Y2H assays. Among the assays, the H85A mutant, with a single Ala substitution for γb His‐85, failed to interact with NbNTRC, whereas a Cys substitution at His‐85 (H85C) had little effect on γb‐NbNTRC binding (Fig 3A). The BiFC assays consistently showed that reconstituted YFP fluorescence was not generated by BSMVH85A‐YFPc/NbNTRC‐YFPn pairs, whereas the fluorescence patterns of BSMVH85C‐YFPc/NbNTRC‐YFPn pairs were similar to those of the BSMVγb‐YFPc/NbNTRC‐YFPn positive control (Fig 3B, Appendix Fig S5). Co‐IP and GST pull‐down assays substantiated the interactions of both γb and γbH85C with NbNTRC‐3xFlag, in contrast to the dramatically reduced binding of the γbH85A mutant to NbNTRC (Fig 3C and D). These results demonstrate that His‐85 of γb is a key amino acid that determines γb binding to NbNTRC.
Figure 3. His‐85 of γb is required for NbNTRC interactions.

- Y2H assay to analyze interactions between NbNTRC and γb mutants. Various Y2H combinations are indicated on the images. Interactions of γb and NbNTRC were confirmed earlier and served as a positive control, combinations containing an empty BD construct served as a negative control.
- BiFC analysis of interactions between NbNTRC and γb mutants during BSMV infection of N. benthamiana. Various BiFC combinations are indicated at the top. YFP signals were visualized by confocal microscopy at 3 dpi and depicted as a false‐green color, and chloroplasts were visualized by chloroplast autofluorescence as a false‐red color. RbcL‐fused YFPc served as a negative control. Scale bars, 20 μm.
- Co‐IP analysis of interactions between NbNTRC and γb mutants. NbNTRC‐3xFlag proteins together with γb‐GFP, γbH85A‐GFP, or γbH85C‐GFP were co‐expressed in N. benthamiana leaves and harvested at 3 dpi. Total proteins were IP with anti‐Flag beads. Total and IP products were detected in Western blots with anti‐GFP or anti‐Flag antibodies. Uncleaved NTRC with cTP and cleaved NTRC without cTP are marked with black and gray arrows (see Appendix Fig S4).
- GST pull‐down assays to evaluate in vitro interactions between NbNTRC and γb mutants. Purified γb‐His, γbH85A‐His, γbH85C‐His, or GFP‐His proteins were incubated with GST‐tagged NbNTRC. Input and pull‐down products were analyzed by Western blotting with anti‐GST or anti‐His antibodies. GFP‐His served as a negative control. Arrowhead indicates the specific band of the target protein.
- Analysis of VSR activities of γb mutants. Infiltrated N. benthamiana leaves co‐expressing sGFP plus γbH85A‐3xFlag, γbH85C‐3xFlag, or the pGD empty vector (EV) were observed at 5 dpi under long‐wave UV illumination for GFP fluorescence. The γb‐3xFlag protein served as a positive control. The white dashed circles indicate the infiltrated zone.
- Western blot analysis of the GFP protein in co‐infiltrated leaves at 5 dpi with anti‐GFP antibody. RbcL was used as loading control.
Data information: In (C–F), representative data were shown, and three independent experiments showed a similar result.
Source data are available online for this figure.
Considering that γb is a classic VSR (Yelina et al, 2002; Bragg & Jackson, 2004), we also tested whether the His‐85 mutation affected VSR activity. The GFP protein was co‐expressed with either γb‐3xFlag or its mutants (γbH85A and γbH85C) in N. benthamiana leaves (Zhang et al, 2018b). These results revealed that both γbH85A and γbH85C mutants were unable to enhance GFP expression in comparison with the γb positive control (Fig 3E and F), indicating that both mutations abolish γb VSR activity.
NTRC negatively regulates BSMV infection
To investigate the function of NbNTRC during BSMV infection, we infiltrated Agrobacterium containing NbNTRC‐3xFlag or its inactive mutant NbNTRCC236/476S‐3xFlag derivatives into N. benthamiana leaves. Notably, the NbNTRCC236/476S mutation, which disrupts the redox activity of NTRC (Chae et al, 2013; Yoshida & Hisabori, 2016), continued to interact with γb (Appendix Fig S6). Overexpression of NbNTRC reduced TGB1 accumulation to approximately 5% of control leaves expressing the Chl‐GFP control, whereas overexpression of NbNTRCC236/476S‐3xFlag failed to impact BSMV infection and resulted in a TGB1 accumulation comparable to that of the Chl‐GFP control (Fig 4A), indicating that NTRC redox activity can inhibit BSMV infection.
Figure 4. NTRC negatively regulates BSMV infection.

- Effects of NbNTRC overexpression on BSMV protein accumulation. N. benthamiana leaves were first infiltrated with Agrobacterium containing various constructs indicated above the panel. After 1.5 dpi, BSMV was inoculated onto the leaves by agroinfiltration. Three days later, total protein was extracted for Western blotting with anti‐TGB1 and anti‐Flag antibodies. The uncleaved NTRC with cTP and cleaved NTRC without cTP are marked with black and gray arrows (see Appendix Fig S4). RbcL was a loading control.
- Effects of NbNTRC overexpression on BSMV replication. Movement‐deficient BSMV (RNAα + RNAγ) was infiltrated into leaves that had been agroinfiltrated previously with various constructs indicated above the panel. NbNTRC and viral γb proteins were detected by Western blotting with anti‐Flag and anti‐γb antibodies. Uncleaved NTRC with cTP and cleaved NTRC without cTP are marked with black and gray arrows (see Appendix Fig S4).
- RT–qPCR analyses of viral RNA accumulation in 4B. NbPP2A was used as an internal control.
- The symptoms of infection after BSMV inoculation of a different N. benthamiana plant at 8 dpi. Magnified images of leaves (in white boxes) are shown in the lower panels. Scale bars, 2 cm.
- Statistical analysis of symptom appearance in different N. benthamiana plants after inoculation with BSMV.
- Western blot analysis of the virus accumulation in Fig 4D with anti‐TGB1 antibody. RbcL served as loading control.
Data information: In (A, B and F), representative data are shown and three replicates had similar results. In (C), error bars indicate mean ± SEM from three independent experiments. a and b indicate statistically significant differences among different groups (ANOVA, P < 0.05). In (E), error bars indicate mean ± SD from three independent experiments.
Source data are available online for this figure.
To examine the role of NbNTRC in virus replication per se, BSMV movement‐deficient mutant (RNAα and RNAγ) was agroinfiltrated into N. benthamiana leaves (Zhang et al, 2017). The results showed that NbNTRC also suppressed the movement‐deficient virus infection (Fig 4B and C). Notably, BSMV replication alone is sufficient to elicit the antioxidant defenses in chloroplasts (Fig EV2), suggesting that NbNTRC can target the BSMV replication phase directly to inhibit viral infection.
Figure EV2. BSMV replication induces chloroplast oxidative stress.

- Confocal microscopy analysis of Chl‐HyPer2 in infected N. benthamiana epidermal cells. N. benthamiana leaves were infiltrated with A. tumefaciens harboring pCB301‐α and pCB301‐γ, and confocal observations were conducted at 3 dpi. The pCB301 empty vector (EV) was used as a negative control. Scale bars, 30 μm.
- Quantification of the changes in Chl‐HyPer2 fluorescence shown in A. ROS‐positive chloroplasts (white) were counted for all chloroplasts (blue and white), respectively.
- Western blot analysis of 2‐Cys Prx redox status under BSMV replication. Total proteins were extracted from infiltrated leaves at 3 dpi, and Western blots were performed with anti‐2‐Cys Prx under non‐reducing conditions. RbcL was used as a loading control.
Data information: In (A), data are representative of at least three independent experiments. In (B), error bars represent mean ± SEM (n = 10); ***P < 0.001 from Student’s t‐test. In (C), representative data are shown, and three replicates had similar results.
We then downregulated NbNTRC mRNA levels in N. benthamiana by using TRV‐based VIGS (Liu et al, 2002) and hairpin RNA‐mediated RNA interference (RNAi) (Macrae et al, 2006) approaches. RT–qPCR analysis confirmed the reductions in the mRNA levels of NbNTRC (Appendix Fig S7A), and BSMV was inoculated onto the NbNTRC downregulated plants. The accumulation of TGB1 was significantly higher in NbNTRC‐silenced plants than in non‐silenced plants (Appendix Fig S7B and C). We also generated the NbNTRC knock‐out N. benthamiana lines (NbNTRC‐KO) by CRISPR/Cas9‐mediated genome editing or transgenic N. benthamiana plants overexpressing NTRC (NbNTRC‐OE), and Western blot analyses confirmed the mutation of endogenous NbNTRC in the NbNTRC‐KO plants and the overexpression of NbNTRC in the NbNTRC‐OE plants (Appendix Fig S8B). Compared with non‐transgenic N. benthamiana, NbNTRC‐OE plants had normal developmental phenotypes (Appendix Fig S8A), whereas NbNTRC‐KO plants displayed dwarfism at the seedling stages, after which normal growth gradually resumed. After agroinfiltration of BSMV to the NbNTRC‐OE and NbNTRC‐KO leaves, BSMV induced mild symptoms in the NbNTRC‐OE plants (Fig 4D) with delayed symptom appearance in the upper uninoculated leaves compared to the non‐trangenic control (Fig 4E). In contrast, BSMV elicited more severe symptoms in NbNTRC‐KO plants concurrently with early symptom appearance (Fig 4D and E). Western blot analysis of systemically infected leaves consistently revealed reduced viral accumulation in NbNTRC‐OE plants, with enhanced accumulation in NbNTRC‐KO plants (Fig 4F). Altogether, these results demonstrate that NbNTRC has an antiviral role in BSMV infection.
γb protein subverts NTRC‐mediated antioxidant defenses
To investigate the mechanisms underlying γb‐NbNTRC interactions, we generated two γb His‐85 mutants (BSMVH85A and BSMVH85C) in the infectious BSMV clone (Fig 5A). It should be mentioned that γbH85A eliminates the VSR activity and has a dramatically reduced binding capacity with NbNTRC, whereas γbH85C loses VSR activity while maintaining NbNTRC binding (Fig 3). The results showed that BSMVH85A‐infected plants had lower BSMV RNA and protein accumulations (Figs 5B and EV3A) with minor chloroplast oxidative stress in inoculated leaves at 3 dpi, compared with BSMVH85C (Fig EV3B and C). At 7 dpi, BSMVH85C induced obvious symptoms in the first to fifth systemically infected leaves (older SL), and the BSMV CP accumulation was higher than that of wild‐type BSMV. In contrast, BSMVH85A mutant only induced symptoms in a relatively confined area close to the petiole of the systemically infected leaves and had lower virus accumulations (Fig 5A and C), indicating that NbNTRC‐γb interactions are required for viral systemic infection. However, at the late stage of infection (28 dpi), BSMV CP was not detected in newly emerging systemic leaves (younger SL) infected with either BSMVH85A or BSMVH85C mutant, suggesting that both BSMVH85C and BSMVH85A were unable to develop systemic infections (Fig 5C), which is consistent with previous studies that γb VSR activity is important for long‐distance movement and pathogenicity of BSMV (Yelina et al, 2002; Bragg & Jackson, 2004). Together, these results indicate that the interactions of γb with NbNTRC are essential for suppressing plant antioxidant defenses and support of viral infection.
Figure 5. The γb protein subverts NTRC‐mediated antioxidant defenses.

- Left panel: systemic symptoms of N. benthamiana plants inoculated with BSMV and BSMV γb mutants. Photographs were taken at 7 dpi. Right panel: schematic representation of inoculated (ino.), older systemic leaves (o‐SL), and younger systemic leaves (y‐SL) in N. benthamiana plants. Scale bars, 2 cm.
- Western blot analyses of TGB1 and CP accumulation in BSMV and BSMV γb mutant inoculated N. benthamiana leaves. Anti‐TGB1 and anti‐CP were used for protein detection.
- Western blot detection of BSMV with anti‐CP antibodies in upper N. benthamiana leaves at 7 days (o‐SL) or 28 days (y‐SL) after infiltration. o‐SL, older systemic leaves and y‐SL, younger systemic leaves.
- Effects of NbNTRC overexpression on infection of BSMV and BSMV γb mutants. A. tumefaciens harboring the indicated plasmids were infiltrated into N. benthamiana leaves. Note: Agrobacterium containing NbNTRC constructs were diluted 5‐fold compared to normal use (OD600 = 0.3). BSMV and the γb mutants were then agroinfiltrated into the same leaves at 1.5 dpi. Three days later, Western blots were conducted with anti‐TGB1 and anti‐Flag antibodies. Uncleaved NTRC with cTP and cleaved NTRC without cTP are marked with black and gray arrows (see Appendix Fig S4).
- Left panel: Western blot detection of BSMV CP accumulation in N. benthamiana leaves inoculated with BSMV and γb movement‐deficient mutants. Leaf samples were collected from different regions of the same leaf (right panel).
- Effects of γb and γb mutants on 2‐Cys Prx in N. benthamiana. Different combinations of the pGD empty vector (EV) or γb and γb mutants are indicated above the panel. NbNTRC and γb proteins were detected by Western blotting with anti‐Flag and anti‐γb antibodies under reducing conditions, Western blot analyses of 2‐Cys Prx abundance with anti‐2‐Cys Prx antibody under non‐reducing conditions. The uncleaved NTRC with cTP and cleaved NTRC without cTP are marked with black and gray arrows, respectively.
Data information: In (B–F), representative data are shown, and three replicates had similar results. (B–E) RbcL served as loading control.
Source data are available online for this figure.
Figure EV3. BSMVH85A infection is much milder than BSMVH85C .

- RT–qPCR analysis of wild‐type BSMV or γb mutant replication. RNAα was analyzed by RT–qPCR to evaluate virus replication. NbPP2A was used as an internal control.
- Chl‐HyPer2 showed chloroplast oxidative stress in BSMV‐, BSMVH85A‐, and BSMVH85C‐infected N. benthamiana leaves at 3 dpi. The empty vector pCB301 was used as a negative control. Scale bars, 30 μm.
- Quantification of the changes in Chl‐HyPer2 shown in B. ROS‐positive chloroplasts (white) were counted for all chloroplasts (blue and white), respectively.
Data information: In (A), error bars indicate mean ± SEM from three independent experiments. a, b, and c indicate statistically significant differences among different groups (ANOVA, P < 0.05). In (B), data are representative of at least three independent experiments. In (C), error bars indicate mean ± SEM (n = 10); a and b indicate statistically significant differences among different groups (ANOVA, P < 0.05).
To further investigate the effects of the γb protein on NTRC‐mediated antioxidant defenses, we infiltrated the Agrobacterium cultures containing wild‐type BSMV or the two His‐85 derivatives into N. benthamiana leaves transiently expressing NbNTRC. The results showed that overexpression of NbNTRC reduced both BSMV and BSMVH85C accumulation to approximately 74% and 47%, respectively, whereas BSMVH85A accumulation declined to approximately 8% (Fig 5D). We then separately introduced the H85A or H85C mutations into BSMVmTGB2 harboring a premature TGB2 translation mutation (Jiang et al, 2020) to generate two double virus mutants (BSMVmTGB2‐H85A and BSMVmTGB2‐H85C) deficient in VSR and movement. The two mutants were then inoculated into non‐transgenic N. benthamiana plants via agroinfiltration. In line with the results shown in Fig 5B and D, the BSMVmTGB2‐H85A infections, in which γb largely loses its ability to interact with NbNTRC, showed about 74% reduction in CP accumulation, and about 36% CP reduction was exhibited by BSMVmTGB2‐H85C, in which the expressed γb protein retains the ability to interact with NbNTRC (Fig 5E). In contrast, when the two double virus mutants were inoculated to the NbNTRC‐KO plants, the CP accumulation of BSMVmTGB2‐H85A and BSMVmTGB2‐H85C was comparable albeit lower than that of unmodified BSMVmTGB2. These results indicate that NbNTRC‐mediated antioxidant defenses against BSMV replication depend on its interactions with the γb protein (Fig 5E).
To investigate whether γb interferes with NTRC functions, we measured the redox states of 2‐Cys Prx, the direct downstream target of NTRC (Kirchsteiger et al, 2009; Pulido et al, 2010). To mimic the chloroplast localization of γb during BSMV infection, BSMV replicase αa together with γb or its mutant derivatives were co‐expressed in N. benthamiana leaves by agroinfitration. Leaves expressing either γb or γbH85C mutant, which retains NbNTRC binding activity, showed a 2‐fold lower accumulation of 2‐Cys Prx monomers than those expressing the empty vector (EV) or γbH85A (Fig 5F). These results suggest that γb interferes with NTRC/2‐Cys Prx redox functions by direct binding to the NbNTRC protein.
Altogether, the results shown above demonstrate that γb promotes chloroplast‐targeted replication of BSMV by disrupting NTRC‐mediated antioxidant defenses, which are functionally distinct from the γb helicase enhancer activities shown in our previous studies (Zhang et al, 2017).
γb competitively interferes with the binding of NTRC to 2‐Cys Prx
Based on the fact that NTRC interacts with 2‐Cys Prx in vivo and in vitro (Bernal‐Bayard et al, 2014; Gonzalez et al, 2019) and NTRC assists in reducing activity of 2‐Cys Prx in the chloroplast (Perez‐Ruiz et al, 2006; Alkhalfioui et al, 2007; Kirchsteiger et al, 2009), we hypothesized that γb protein may counteract NTRC‐mediated antioxidant defenses by disrupting the NTRC/2‐Cys Prx functional module (Fig 5). To test this notion, we first examined the effects of γb on NbNTRC‐2‐Cys Prx interactions using a BiFC assay, so the NbNTRC‐YFPn and 2‐Cys Prx‐YFPc proteins were co‐expressed with BSMV or its derivatives in N. benthamiana leaves (Appendix Fig S9). These results showed that the reconstituted YFP signals in BSMVH85A‐infected or EV‐infiltrated leaves were stronger than leaves infected with BSMV or BSMVH85C, which express γb proteins that can interact with NbNTRC (Fig 6A and B). Moreover, we also evaluated the NbNTRC‐2‐Cys Prx interaction intensity in the movement‐deficient virus BSMVmTGB2 or its derivatives in infected N. benthamiana leaves by using a split‐luciferase complementation assay (Zhao & Zhou, 2020). These results showed that the presence of BSMVmTGB2 and BSMVmTGB2‐H85C significantly inhibited interactions between NbNTRC and 2‐Cys Prx as evidenced by reduced luciferase activity compared to leaf regions inoculated with BSMVmTGB2‐H85A or the EV control (Fig 6C and D).
Figure 6. γb competitively interferes with interactions between NTRC and 2‐Cys Prx.

- BiFC assays to identify interactions between NbNTRC and 2‐Cys Prx in N. benthamiana during BSMV infection. NbNTRC‐YFPc and 2‐Cys Prx‐YFPn were co‐expressed with BSMV and γb mutant derivatives as indicated on the left. The EV was used as negative control. Scale bars, 30 μm.
- Quantification of the BiFC intensities shown in Fig 6A with ImageJ software. The NbNTRC‐2‐Cys Prx interaction was normalized to that of the EV control, which was set to 1.0.
- Split‐LUC assays to determine NbNTRC‐2‐Cys Prx interactions in N. benthamiana during BSMV infection. NbNTRC‐cLUC and 2‐Cys Prx‐nLUC were co‐expressed in N. benthamiana leaves infected with the indicated BSMV derivatives, Bioluminescence was observed at 3 dpi. The white dashed circles indicate the infiltrated regions.
- Quantification of the luciferase signals shown in Fig 6C as assessed by ImageJ software.
- Competitive GST pull‐down assays showing the effects of γb on interactions between NbNTRC and 2‐Cys Prx. GST‐NbNTRC and 2‐Cys Prx‐His were incubated with increasing amounts (5, 10, 20, and 40 μg) of γb and its mutant derivatives. Glutathione‐Sepharose beads were washed, and Western blot analysis was performed using anti‐GST and anti‐His antibodies.
- Analysis of NbNTRC‐2‐Cys Prx interactions in the presence of the γb protein. NbNTRC‐3xFlag and 2‐Cys Prx‐GFP were co‐expressed in N. benthamiana leaves. Total proteins were extracted at 4 dpi and immunoprecipitated with anti‐Flag beads, co‐incubated with increasing amounts (20, 40, and 60 μg) of γb‐His and γb‐His mutant derivatives. Input and IP proteins were analyzed by Western blotting with anti‐His, anti‐GFP, or anti‐Flag antibodies. The uncleaved 2‐Cys Prx with cTP and cleaved 2‐Cys Prx without cTP are marked with black and gray arrows, respectively.
Data information: Representative data were shown in (A and C), and three replicates had similar results. In B, error bars indicate the mean ± SEM, and a and b indicate statistically significant differences among different groups (n = 5, ANOVA, P < 0.05). In (D), error bars indicate mean ± SEM (n = 6). a, b, and c indicate statistically significant differences among different groups (ANOVA, P < 0.05). In (E, F), representative data are shown, and three independent experiments had similar results.
Source data are available online for this figure.
Furthermore, we also performed an in vitro competitive GST pull‐down assay. Different amounts of γb‐His, γbH85A‐His, or γbH85C‐His proteins were added to the GST‐NbNTRC and 2‐Cys Prx‐His protein mixture, followed by incubation and detection by Western blots. With the addition of increasing amounts of either γb‐His or γbH85C‐His protein, the interaction intensity between NbNTRC and 2‐Cys Prx gradually decreased. However, the NbNTRC‐2‐Cys Prx interaction was largely unaffected by increasing amounts of the γbH85A‐His protein (Fig 6E). Consistent results were observed when performing competitive co‐IP assays (Fig 6F). A. tumefaciens containing plasmids expressing NbNTRC‐3xFlag and 2‐Cys Prx‐GFP were co‐infiltrated into leaves, and the total proteins were extracted and immunoprecipitated with anti‐Flag beads. The precipitates were then incubated with increasing amounts of γb‐His, γbH85A‐His, or γbH85C‐His. Western blot analysis revealed that less 2‐Cys Prx‐GFP was detected with increasing amounts of either γb‐His or γbH85C‐His; in contrast, γbH85A‐His had no obvious effect on the interactions between NbNTRC and 2‐Cys Prx (Fig 6F).
In summary, these results indicate that γb competitively interferes with interactions between NbNTRC and 2‐Cys Prx by binding to NbNTRC to subvert NTRC‐mediated chloroplast antioxidant defenses and facilitate BSMV replication.
Interference with antioxidant defenses may be a general strategy employed by chloroplast‐replicating viruses
Our results indicate that BSMV infection and expression of the γb protein disturb chloroplast antioxidant defenses and facilitate viral replication. To investigate whether this is a general strategy employed by other viruses whose replication also occurs on chloroplast membranes, we selected Lychnis ringspot virus (LRSV, genus Hordeivirus) (Jiang et al, 2018) and Turnip yellow mosaic virus (TYMV, the genus Tymovirus in the family Tymoviridae) (Prod'homme et al, 2003; Shin et al, 2009) to test the effects of these virus infections on chloroplast antioxidant defense. These results showed that both LRSV and TYMV infection induced oxidative stresses at chloroplasts as evidenced by Chl‐HyPer2 imaging and 2‐Cys Prx monomer detection (Fig 7A–C and Fig 7E–G). To test the effects of the antioxidant defenses on LRSV and TYMV infection, we inoculated either LRSV or TYMV to NbNTRC‐OE and NbNTRC‐KO N. benthamiana plants by agroinfiltration. Both viruses showed reduced symptoms and viral accumulation in NbNTRC‐OE plants, but had enhanced infections in NbNTRC‐KO plants compared with the non‐transgenic plants (Figs 7D and H and Fig EV4). These results suggest that induction of chloroplast antioxidant defenses may commonly occur during chloroplast‐replicating virus infections.
Figure 7. Interfering with antioxidant defenses may be a general strategy employed by chloroplast‐replicating viruses.

- Chl‐HyPer2 responds to chloroplast hydrogen peroxide caused by LRSV infection at 3 dpi in N. benthamiana epidermal cells. The pCB301 empty vector (EV) was used as a negative control. Scale bars, 30 μm. The false‐blue color indicates the chloroplasts.
- Quantification of LRSV‐dependent changes in Chl‐HyPer2 fluorescence at 3 dpi.
- Western blot analyses of 2‐Cys Prx abundance in EV and LRSV inoculated leaves under non‐reducing conditions at 3 dpi. RbcL served as a loading control.
- Western blot analyses to detect LRSV γb in NbNTRC‐OE and NbNTRC‐KO N. benthamiana plants challenged with LRSV at 8 dpi. RbcL was used as the loading control.
- Chl‐HyPer2 responds to chloroplast hydrogen peroxide caused by TYMV infection at 5 dpi in N. benthamiana epidermal cells. Scale bars, 30 μm. The false‐blue color indicates the chloroplasts.
- Quantification of the TYMV‐dependent changes in Chl‐HyPer2 fluorescence at 5 dpi.
- Western blot analysis of 2‐Cys Prx abundance in EV or TYMV inoculated leaves under non‐reducing conditions at 5 dpi. RbcL was used as the loading control.
- RT–qPCR analysis of TYMV RNA accumulation at 8 dpi. NbEF1α served as a internal control.
Data information: In (A and E), data are representative of at least three independent experiments. In (B and F), error bars represent mean ± SEM (n = 5); **P < 0.01 from Student’s t‐test. In (C, D, G, H), representative data are shown, and three replicates had similar results.
Source data are available online for this figure.
Figure EV4. NbNTRC negatively regulates LRSV and TYMV infection.

-
A, BSystemic symptoms of non‐transgenic, NbNTRC‐OE and NbNTRC‐KO transgenic N. benthamiana plants inoculated with LRSV (A) and TYMV (B). The photographs were taken at 8 dpi. Magnified images of leaves (in white boxes) are shown in the lower panels. Three independent replicates were performed, and a representative leaves are shown. Scale bars, 2 cm.
Discussion
During evolution, plants have developed various biochemical and physiological processes to respond to different biotic stresses. Pathogen infection usually triggers ROS bursts to produce major signal molecules for activation of plant immunity (Dickinson & Chang, 2011), including the antioxidant defense responses (Zhang et al, 2018a). For example, Pseudomonas syringae invasion activates NADPH oxidase RBOHD for ROS production and positively regulates immune responses (Zhang et al, 2018a) and Tomato bushy stunt virus (TBSV) infection results in increased activities of CAT and SOD in N. benthamiana (Yergaliyev et al, 2016). However, manipulation of antioxidant pathways by plant viruses to counteract host defenses remains to be fully explored (Budziszewska & Obrepalska‐Steplowska, 2018). In this study, we showed that BSMV infection induces chloroplast oxidative stress and that the γb protein disrupts chloroplast antioxidant defenses by direct interactions with NTRC, a core component functioning in chloroplast redox pathway. Furthermore, we demonstrated that the γb protein interferes competitively with NbNTRC binding to 2‐Cys Prx, thereby promoting chloroplast‐targeted replication of BSMV. Our data thus unveil an important mechanism whereby a plant virus manipulates antioxidant‐mediated antiviral defense responses to support viral infection.
Chloroplasts organelles are responsible for photosynthesis and have central roles in response to various biotic and abiotic stresses (Chan et al, 2016; Ding et al, 2019; Gan et al, 2019; Zhao et al, 2020). Chloroplasts produce an array of pro‐defense signals, including cytoplasmic calcium and ROS bursts, and phytohormones like salicylic acid (SA) and jasmonic acid (JA) upon perception of external stresses (de Torres Zabala et al, 2015; Sowden et al, 2018; Medina‐Puche et al, 2020). In addition, chloroplasts can generate stromules to mediate the transfer of H2O2 to the nucleus (Natesan et al, 2005; Caplan et al, 2015; Exposito‐Rodriguez et al, 2017). Recently Medina‐Puche et al (2020) reported that some plant virus‐encoded proteins re‐localize from plasma membrane (PM) to chloroplasts to interfere with the chloroplast‐dependent SA synthesis. Although the chloroplast antioxidant system has been described before, little is known about the role of antioxidant defenses in response to biotic stresses. In this work, we found that the BSMV γb protein interacts with NTRC to subvert the pathway via hampering reduction of 2‐Cys Prx, thereby shifting chloroplasts reductive states to create oxidative microenvironments beneficial for BSMV replication. Our study, for the first time, establishes a direct link between chloroplast antioxidant pathway and antiviral defenses and also provides evidence that NTRC is a novel target of chloroplast‐replicating viruses and that γb affects defense and counter‐defense strategies during virus–host interplay.
In contrast to previous studies showing that antioxidant genes are usually upregulated when the cells undergo oxidative stresses (Farooq et al, 2019), we found that some antioxidant genes such as 2‐Cys Prx and GPXL (Fig 1D) were slightly upregulated in response to BSMV infection at the early stage of virus infection, but then are significantly downregulated at 3–5 dpi following the active stages of RNA replication even though BSMV provokes oxidative stress in the cells. Because chloroplast‐derived ROS molecules are well characterized as mediators of retrograde signaling directed from the chloroplast to the nucleus to regulate gene expression (Caplan et al, 2015; Exposito‐Rodriguez et al, 2017), we speculate that BSMV may hijack the retrograde signaling pathway to manipulate transcription of antioxidant genes, which, together with the interference of the NTRC/2‐Cys Prx functional module described here, would be conducive to the formation of an oxidative microenvironment for optimal viral replication.
Previous studies have shown that chloroplast redox regulation is very complicated (Yoshida & Hisabori, 2016). The FTR/Trx pathway is exclusively dependent on the light‐driven photosynthetic electron transport chain, whereas NTRC also acts in darkness (Cejudo et al, 2019). The FTR/Trx and NTRC pathways cooperatively regulate a series of chloroplast functions to sustain plant viability (Buchanan & Balmer, 2005; Yoshida & Hisabori, 2016). Crosstalk between the FTR/Trx and NTRC pathways is important for the maintenance of chloroplast redox homeostasis, and it has been reported that the FTR‐Trx and NTRC redox systems that are crucial for chloroplast functions are linked by the redox balance of 2‐Cys Prxs (Perez‐Ruiz et al, 2017). The antioxidant protein peroxiredoxin Q (PrxQ) is mainly regulated by the FTR/Trx pathway (Yoshida & Hisabori, 2016). Our results show that the PrxQ gene is significantly downregulated in BSMV‐infected N. benthamiana leaves (Fig 1D) and that overexpression of PrxQ dramatically inhibited BSMV replication (Fig 1E). These results imply that FTR/Trx‐mediated antioxidant pathways may also be involved in plant antiviral defenses in addition to the NTRC pathways investigated in this study. How the two pathways coordinately regulate the chloroplast antioxidant defenses against virus replication remains to be further explored.
Our data demonstrate that NbNTRC inhibits the infection of several chloroplast‐replicating viruses including BSMV, LRSV, and TYMV. To investigate whether antioxidant defenses also function during infections of other viruses whose replication sites are not chloroplasts, we inoculated Beet black scorch virus (BBSV, the genus Betanecrovirus in the family Tombusviridae) that replicates on the endoplasmic reticulum (Cao et al, 2015) and Cucumber mosaic virus (CMV, the genus Cucumovirus in the family Bromoviridae) that replicates on the vacuolar membrane (Wang et al, 2021) onto NbNTRC‐OE transgenic N. benthamiana plants. The results showed that NbNTRC has no discernable effect on BBSV or CMV infection (Appendix Fig S10A and B). Interestingly, transient expression of NbNTRC failed to inhibit the infection of another chloroplast‐replicating virus, Bamboo mosaic virus (BaMV, the genus Potexvirus in the family Alphaflexiviridae) (Appendix Fig S11). Recent studies have suggested that BaMV hijacks NbGSTU4 to deliver GSH to the chloroplasts to generate a reduced cellular microenvironment for BaMV minus‐strand RNA synthesis (Chen et al, 2013). These results suggest that there may be considerable discrepancy in the strategies co‐opted by different viruses to manipulate the redox of the chloroplast to permit optimal viral replication.
To investigate whether the NbNTRC‐mediated antioxidant defense plays a general role among different hordeiviruses, we carried out BiFC assays to determine interactions between NbNTRC and γb proteins of LRSV and those of the peroxisome‐replicating Poa semilatent virus (PSLV). The results showed that NbNTRC could interact only with chloroplast‐replicating LRSV γb protein (Jiang et al, 2018), but not with the peroxisome‐replicating PSLV γb (Li et al, 2020) (Appendix Fig S12). Although chloroplast antioxidant defenses and γb interference with NTRC binding appear not to function during PSLV replication, other defense pathways may be targeted by γb or other virus‐encoded proteins. Taken together, our results imply that chloroplast‐replicating viruses have evolved different strategies than related viruses that replicate at alternative subcellular sites.
Reactive oxygen species are usually considered to be positive regulators of plant antiviral defenses (Deng et al, 2016). However, accumulating evidence indicates that antioxidant systems can be suppressed under pathological conditions to result in accumulation of ROS, which can positively regulate animal and plant virus infections. For example, influenza virus induces oxidative stress through production of ROS and NADPH oxidase 4 (NOX4)‐derived ROS plays an essential role in activating redox‐regulated virus replication (Amatore et al, 2015). Moreover, oxidative stress induction can benefit hepatitis C virus replication (Anticoli et al, 2019), blocking mitochondrial ROS levels inhibits respiratory syncytial virus multiplication (Hu et al, 2019b). In plant, red clover necrotic mosaic virus (the genus Dianthovirus in the family Tombusviridae) replication requires RBOHB‐mediated ROS production, the replication protein p27 hijacking ROS‐generating machinery for efficient viral RNA replication (Hyodo et al, 2017; Hyodo & Okuno, 2020). Collectively, these studies, together with our results, indicate that viruses utilize distinct strategies to create an oxidative microenvironment for viral replication.
It has been reported that oxidizing agents enhanced the RNA capping enzyme guanylyltransferase activity of flavivirus NS5 and alphavirus nsP1 in vitro (Gullberg et al, 2015). However, there is no direct in vivo evidence to demonstrate a positive role of ROS‐mediated oxidation in replication of these viruses. Ribonucleotide reductase (RNR) catalyzes reduction of ribonucleotides (NTP) to deoxyribonucleotides (dNTP) for DNA virus replication (Cohen et al, 2010; Ricardo‐Lax et al, 2015; Kitab et al, 2019), but whether it also functions in RNA virus genome synthesis remains to be tested. A previous study has shown that RNR enzyme activity requires a tyrosyl radical; however, superoxide quenches protein tyrosyl radicals in vitro. In yeast cells, lacking either manganese SOD or copper–zinc SOD had decreased RNR activity (Das et al, 2018). Based on these results, it is possible that oxidized microenvironments may reduce RNR catalytic activity to permit NTPs to accumulate sufficiently to support RNA virus replication. In addition, we also found that RNR mRNA levels were downregulated in BSMV‐infected N. benthamiana leaves (Fig EV5), suggesting that BSMV may also regulate RNR expression to benefit replication.
Figure EV5. RT–qPCR analysis of RNR gene expression in response to BSMV infection.

Total RNA was extracted from BSMV‐infected leaves at 3 dpi. NbPP2A was used as an internal control. The empty vector (EV) was used as a negative control. Error bars indicate mean ± SEM from at least three independent experiments; ***P < 0.001 from Student’s t‐test.
Our previous study verified that the γb protein is recruited to chloroplasts at early stages of BSMV infection by interactions with the αa replicase subunit (Zhang et al, 2017). The extended study presented here proves that in addition to acting as a helicase enhancer in the virus replication complex, the γb protein can subvert NTRC‐mediated antioxidant defenses at chloroplast replication sites to promote BSMV replication. We therefore propose a model illustrating the novel role of γb in disrupting chloroplast antioxidant defenses by NTRC binding (Fig 8). In summary, our results expand our understanding of the multifunctional roles played by virus‐encoded proteins. Considering that the replication of most positive‐strand RNA viruses occurs at specific organelles, our studies have broad implications for understanding how viruses manipulate organelle homeostasis to achieve efficient infection.
Figure 8. Model illustrating the role of the γb protein in disrupting chloroplast antioxidant defenses by targeting NTRC.

NTRC functions as an efficient reductant of 2‐Cys Prx to protect the chloroplast from oxidative stress and the redox status of 2‐Cys Prx depends on binding interactions with NTRC. The balance between NTRC and 2‐Cys Prx is important to maintain normal plant growth and development (left panel). In BSMV‐infected leaves, γb proteins are recruited to chloroplast replication sites at the early stage of infection by interactions with the αa replicase (Zhang et al, 2017). In addition to acting as a helicase enhancer, the γb protein also disrupts NTRC‐mediated antioxidant defenses by competitively interfering with the interaction of NTRC with 2‐Cys Prx through binding to NTRC. During this process, H2O2 accumulation moderately increases in chloroplasts, which shifts the balance toward oxidative stresses and facilitates BSMV chloroplast‐targeted replication (right panel). Under these circumstances, the increased oxidative stresses reduce RNR catalytic activity and lead to accumulation of NTPs, which benefits viral RNA synthesis (dotted circle on the right). Abbreviations: NTRC, NADPH‐dependent thioredoxin reductase C; HF, host factors; NTPs, ribonucleotides; and dNTPs, deoxyribonucleotides.
Materials and Methods
Plant materials and growth conditions
Nicotiana benthamiana plants were grown in an environment‐controlled growth chamber with 16‐h light and 8‐h dark photoperiods at 23–24°C as described previously (Hu et al, 2015).
Plasmid construction
The BSMV strain ND18 infectious clones used in this study were described previously (Hu et al, 2019a). A QuikChange Site‐Directed Mutagenesis Kit (Agilent Technologies, CA) was used to generate site‐specific mutagenesis using the corresponding primers listed in Appendix Table S1. All mutants used in this study were verified by DNA sequencing.
For the chloroplast H2O2 monitor Chl‐HyPer2, the full‐length HyPer2 (Exposito‐Rodriguez et al, 2017) from pUB‐cHyPer2 (www.addgene.org, codes 84728) was cloned into the pMDC32‐3xFlag vector (Gao et al, 2021), and the chloroplast transit peptide (cTP, aa 1–80) of the small Rubisco subunit (Nelson et al, 2007) was fused to the N terminus of HyPer2.
For yeast two‐hybrid assays, the coding sequence of NbNTRC was amplified from N. benthamiana cDNA and cloned into the pGADT7 vector at the NdeI site. The pGBKT7‐γb and pGADT7‐γb plasmids were described previously (Zhang et al, 2017).
For BiFC assays, the NbNTRC and 2‐Cys Prx were inserted into pSPYNE‐35S or pSPYCE‐35S vectors (Walter et al, 2004) at BamHI site. The pSPYNE‐35S‐γb and pSPYCE‐35S‐γb plasmids were described previously (Zhang et al, 2017).
For split‐luciferase assays, the NbNTRC and 2‐Cys Prx were engineered into BamHI and SalI‐digested pCAMBIA1300‐CCLuc (pCCL) and pCAMBIA1300‐NLuc (pNL) vectors (Zhao & Zhou, 2020), respectively, to generate the NbNTRC‐cLUC and 2‐Cys Prx‐nLUC constructs.
For protein purification, genes of γb, γb mutants, and 2‐Cys Prx were individually cloned into the NdeI and XhoI sites of pET30a (+) vector to express His‐tagged fusion proteins. NbNTRC was inserted into the pGEX‐KG vector (Guan & Dixon, 1991) to express GST‐NbNTRC fusion protein.
For subcellular localization and co‐IP assays, NbNTRC was cloned into the pGD3G‐mCherry and pGDGm vectors (Goodin et al, 2002; Fan et al, 2014), and γb and γb derivatives were cloned into the pMDC32‐3xFlag vector.
For VSR detection in N. benthamiana, the fragments of the γb and γb mutants were cloned into the pGD‐3xFlag vector (Goodin et al, 2002).
For transient expression in N. benthamiana, the full length of NbNTRC, 2‐Cys Prx, sAPX, GPXL, and PrxQ sequences was amplified from N. benthamiana cDNA and inserted into the BamHI and SpeI sites of the pMDC32‐3xFlag vector (Gao et al, 2021).
For genome editing vector constructions, a suitable CRISPR sgRNA targeting the NbNTRC locus was identified by the online server CRISPR‐P 2.0 (http://cbi.hzau.edu.cn/crispr/). The gRNA was amplified by PCR using the primers NTRC‐KO‐F (5′‐TGATT GGAGCATCACCAATTTTCAA‐3′) and NTRC‐KO‐R (5′‐AAACTTGAAAATTG GTGATGCTCCA‐3′) and annealed into double strands followed by insertion into the pBGK01 vector (Biogle, Hangzhou, China). To confirm target gene mutation, the DNA sequence surrounding the target site was amplified with the primer pairs: geNTRC‐F (5′‐ GAACATAAGGTAAAGTGTCATAG −3′) and geNTRC‐R (5′‐ CTTGCGGACAAGTAAATGGACAT −3′). The amplified DNA fragments were purified and analyzed by DNA sequencing to confirm editing of the target gene.
Yeast two‐hybrid assays
Yeast two‐hybrid (Y2H) assays were performed as described previously (Zhang et al, 2017). Various NbNTRC and γb derivatives were cloned into the pGADT7‐Rec or pGBKT7 vectors, followed by transformation into the AH109 or Y187 yeast strains by a lithium acetate method. The two yeast strains were mated and plated on SD/‐Leu/‐Trp or SD/‐Leu/‐Trp/‐His/‐Ade dropout medium.
Agroinfiltration of Nicotiana benthamiana leaves
Agrobacterium tumefaciens EHA105 containing different expression vectors and TBSV P19 (for enhancing protein expression; Scholthof, 2006) were adjusted to OD600 = 0.3 and 0.2, respectively. Different cultures were mixed and infiltrated into N. benthamiana leaves as described previously (Jiang et al, 2020), and the infiltrated leaves were harvested at 3–5 dpi for subsequent analysis.
Viral inoculation and detection
For agroinfiltration, equal amounts of A. tumefaciens strain EHA105 harboring pCB301‐α, pCB301‐β, and pCB301‐γ or its derivatives were mixed (Hu et al, 2019a) and infiltrated into the leaves of 3‐ to 4‐week‐old N. benthamiana plants as described previously (Yuan et al, 2011). Total proteins and RNAs were extracted from infiltrated leaves at 3 or 4 dpi for Western blot and RT–qPCR assays.
Bimolecular fluorescence complementation (BiFC) assays
BiFC assays were performed as described previously (Walter et al, 2004; Jiang et al, 2020). The infiltrated N. benthamiana leaves were observed at 3 dpi using Leica SP8 or Olympus FV1000 confocal microscopes. YFP and chlorophyll autofluorescence were excited at 514 nm and 633 nm, respectively.
Co‐immunoprecipitation (co‐IP) assays
The co‐IP assays were performed as described previously (Zhang et al, 2017). Various expression plasmids were co‐infiltrated into N. benthamiana leaves. The leaves were harvested at 3 dpi. Total proteins from infiltrated N. benthamiana leaf tissue were ground in extraction buffer [10% glycerol, 25 mM Tris–HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, 1% NP‐40, 1% PVPP, 1 mM DTT, and Protease Inhibitor Cocktail (Roche, Cat. #11697498001)]. The supernatant was incubated with Flag beads (Sigma‐Aldrich) for 4 h at 4°C. The precipitates were washed three times with IP buffer (10% glycerol, 25 mM Tris–HCl, pH 7.5, 1 mM EDTA, 150 mM NaCl, and 1% NP‐40) at 4°C and analyzed by Western blotting using anti‐Flag (at 1:5,000 dilution; Sigma‐Aldrich, Cat. #A2220) or anti‐GFP antibodies.
GST Pull‐down assays
GST pull‐down assays were performed as described previously (Yang et al, 2018b). Approximately 10 μg of purified His fusion γb or its mutants and GFP‐His proteins were incubated with GST‐NbNTRC fusion protein in 500 μl of incubation buffer (50 mM Tris–HCl, pH 9.0, 300 mM NaCl, 1.5% glycerol, 0.6% Triton X‐100, and 0.1% Tween) for 3 h at 4°C. The beads were collected and washed three times for 10 min per wash with incubation buffer. The washed beads were boiled in 2× SDS loading buffer, and proteins were separated by SDS–PAGE for Western blot analysis with anti‐GST (at 1:5,000 dilution; GenScript, Cat. #A00866) or anti‐His (at 1:5,000 dilution; Abmart, Cat. #M30111) antibodies.
Electrophoresis and immunoblot analysis
Agroinfiltrated N. benthamiana leaves were harvested and homogenized in liquid nitrogen. Proteins were extracted in loading buffer (200 mM β‐mercaptoethanol (β‐ME),100 mM Tris–HCl, pH 6.8, 20% glycerol, 4% SDS, 0.2% bromophenol blue) or loading buffer without β‐ME in the loading buffer for non‐reducing conditions (Perez‐Ruiz et al, 2017). Separations were carried out in 12.5% SDS–PAGE.
His‐tagged 2‐Cys Prx and NbNTRC proteins were expressed in E. coli strain BL21 (DE3) and recombinant proteins were purified by Ni‐affinity chromatography as described previously (Hu et al, 2015). Specific antisera against 2‐Cys Prx‐His and NbNTRC‐His were prepared by Beijing Protein Innovation (BPI) and diluted 1:1000 for protein detection.
Generation of NbNTRC transgenic N. benthamiana plants
The pMDC32‐NbNTRC‐3xFlag or pBGK01‐NbNTRC plasmids were transformed into A. tumefaciens strain EHA105, respectively. Leaf disk transformation was used to generate the transgenic N. benthamiana plants (McCormick et al, 1986). Western blots with anti‐NbNTRC antibody were performed to screen the transgenic plants.
RT–qPCR
For RT–qPCR analysis, cDNA was synthesized from 6 μg total RNA (DNase‐treated) using an oligo(dT) primer and M‐MLV reverse transcriptase (Promega). Gene fragments were amplified with 2X SsoFast EvaGreen Supermix (Bio‐Rad), and the primers are listed in Appendix Table S1. Data were analyzed with CFX Manger software (Bio‐Rad; Liu et al, 2012).
Viral suppressor of RNA silencing (VSR) assays
Positive‐sense GFP (sGFP)‐induced RNA silencing suppression experiments were performed as described previously (Zhang et al, 2018b). Briefly, equal volumes of A. tumefaciens cultures harboring plasmids expressing positive‐sense GFP (OD600 = 0.5) (Bragg & Jackson, 2004) and plasmids expressing γb‐3xFlag, γbH85A‐3xFlag, and γbH85C‐3xFlag (OD600 = 0.3) were infiltrated into N. benthamiana leaves. The agroinfiltrated leaves were illuminated under a long‐wavelength UV lamp (B‐100AP/R, UVP), and photographs were taken with a yellow filter at 5 dpi.
Chloroplast ROS assays
Chloroplast ROS assays were performed as described previously (Exposito‐Rodriguez et al, 2017) with minor modifications. Briefly, Agrobacterium harboring the Chl‐HyPer2 or TBSV P19 expression constructs were mixed at a density (OD600) of 0.3 and 0.05, followed by infiltration into N. benthamiana leaves. Next, Agrobacterium mixtures harboring BSMV or mutant derivatives were infiltrated into the same leaves two days later. At 3–4 dpi, 1‐cm‐diameter leaf disks were excised and observed with an Olympus FV1000 confocal microscope with X20 or X40 lens, and the 488/405‐nm laser power ratio for all fields was kept constant at about 1:3 depending on signal strength. The percentage of ROS‐positive chloroplasts was calculated using the number of 405 nm excitation chloroplasts as the numerator and number of all 488 nm excitation chloroplasts as the denominator.
Split‐luciferase assays
Split‐luciferase assays were performed as described previously (Zhao & Zhou, 2020) with minor modifications. Briefly, A. tumefaciens strains EHA105 containing the desired constructs were infiltrated into fully expanded N. benthamiana leaves. Three days after inoculation, 1 mM luciferin was sprayed onto the inoculated leaves and chemiluminescence images were photographed with a CCD camera after a 5‐min dark treatment. The fluorescence intensity was quantified using ImageJ software.
Protease protection assays
Protease protection assays were performed as described previously (Jin et al, 2018) with minor modifications. Briefly, intact chloroplasts were isolated from N. benthamiana leaves infiltrated with Agrobacterium harboring BSMV, AtTOC64‐GFP, or Chl‐GFP plasmids (Ling & Jarvis, 2015; Jin et al, 2018) and chloroplast yields were calculated using a unit chlorophyll basis as described previously (Jin et al, 2018). Chloroplast suspensions were then diluted to 1 mg/ml chlorophyll with 1 mM CaCl2, 300 mM sorbitol, and 50 mM HEPES‐KOH (pH 8.0). Purified chloroplast containing 1 mg chlorophyll was added 200 mg thermolysin, followed by incubation at 25°C for 1 h in the presence or absence of 0.5% Triton X‐100. After thermolysin treatments, protease inhibitor cocktail (Sigma) and 5 mM EDTA were added to terminate the reactions. The chloroplast suspensions were boiled in 2× SDS loading buffer, and proteins were separated by SDS–PAGE for Western blot analysis with anti‐NTRC, anti‐2‐Cys Prx, anti‐GFP, or anti‐actin antibodies.
Statistical analyses
The data shown in this study were expressed as mean ± SEM from three independent experiments. Protein band signals were quantified with ImageJ software (Bio‐Rad), and statistical analyses were performed with the Student’s t‐test or one‐way ANOVA. YFP fluorescence intensities in the BiFC assays were quantified with ImageJ software. Chlorophyll autofluorescence served as the internal control for normalization.
Accession numbers
Sequence information used in this article is available at Sol Genomics Network (https://solgenomics.net) and Nicotiana benthamiana Genome & Transcriptome database (http://benthgenome.qut.edu.au/) with the following accession numbers: NbNTRC (Niben101Scf06738g00002.1), 2‐Cys Prx (Nbv6.1trP21425), sAPX (Nbv6.1trP55008), PrxQ (Nbv5.1tr6241919), GPXL (Nbv6.1trP4770), and RNR (Nbv6.1trP4682).
Author contributions
DL and XW conceived and designed the experiments. XW performed the experiments with the help of NY, XJ, XZ, and ZL. YZ, X‐BW, CH, and JY discussed and interpreted the data. XW, ZJ, DL, and YZ wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Appendix
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Review Process File
Acknowledgments
We would like to thank Prof. Andrew O. Jackson (University of California‐Berkeley) for editing the manuscript. We thank Dr. Huiqiang Lou (China Agricultural University), Dr. Yule Liu (Tsinghua University), Dr. Kai Xu (Nanjing Normal University), and members of the Li laboratory for their helpful discussions and suggestions. We would like to thank Dr. Yau‐Heiu Hsu (National Chung Hsing University) for providing BaMV and its antibody and Dr. Tae‐Ju Cho (Chungbuk National University) for providing the TYMV infectious cDNA clone. This work was supported by the National Natural Science Foundation of China (31830106 and 31872637), the National Science & Technology Specific Projects of China (2016ZX08003001), and Beijing Outstanding University Discipline Program.
The EMBO Journal (2021) 40: e107660.
Data availability
This study includes no newly generated data deposited in external repositories.
References
- Alcaide‐Loridan C, Jupin I (2012) Ubiquitin and plant viruses, let's play together!. Plant Physiol 160: 72–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhalfioui F, Renard M, Montrichard F (2007) Unique properties of NADP‐thioredoxin reductase C in legumes. J Exp Bot 58: 969–978 [DOI] [PubMed] [Google Scholar]
- Amatore D, Sgarbanti R, Aquilano K, Baldelli S, Limongi D, Civitelli L, Nencioni L, Garaci E, Ciriolo MR, Palamara AT (2015) Influenza virus replication in lung epithelial cells depends on redox‐sensitive pathways activated by NOX4‐derived ROS. Cell Microbiol 17: 131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticoli S, Amatore D, Matarrese P, De Angelis M, Palamara AT, Nencioni L, Ruggieri A (2019) Counteraction of HCV‐induced oxidative stress concurs to establish chronic infection in liver cell cultures. Oxid Med Cell Longev 2019: 6452390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D (2004) RNA silencing in plants. Nature 431: 356–363 [DOI] [PubMed] [Google Scholar]
- Bernal‐Bayard P, Hervas M, Cejudo FJ, Navarro JA (2012) Electron transfer pathways and dynamics of chloroplast NADPH‐dependent thioredoxin reductase C (NTRC). J Biol Chem 287: 33865–33872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal‐Bayard P, Ojeda V, Hervas M, Cejudo FJ, Navarro JA, Velazquez‐Campoy A, Perez‐Ruiz JM (2014) Molecular recognition in the interaction of chloroplast 2‐Cys peroxiredoxin with NADPH‐thioredoxin reductase C (NTRC) and thioredoxin x . FEBS Lett 588: 4342–4347 [DOI] [PubMed] [Google Scholar]
- Bragg JN, Jackson AO (2004) The C‐terminal region of the Barley stripe mosaic virus γb protein participates in homologous interactions and is required for suppression of RNA silencing. Mol Plant Pathol 5: 465–481 [DOI] [PubMed] [Google Scholar]
- Bragg JN, Lawrence DM, Jackson AO (2004) The N‐terminal 85 amino acids of the Barley stripe mosaic virus γb pathogenesis protein contain three zinc‐binding motifs. J Virol 78: 7379–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuers FK, Bräutigam A, Geimer S, Welzel UY, Stefano G, Renna L, Brandizzi F, Weber AP (2012) Dynamic remodeling of the plastid envelope membranes ‐ a tool for chloroplast envelope in vivo localizations. Front Plant Sci 3: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan BB, Balmer Y (2005) Redox regulation: a broadening horizon. Annu Rev Plant Biol 56: 187–220 [DOI] [PubMed] [Google Scholar]
- Budziszewska M, Obrepalska‐Steplowska A (2018) The role of the chloroplast in the replication of positive‐sense single‐stranded plant RNA viruses. Front Plant Sci 9: 1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Jin X, Zhang X, Li Y, Wang C, Wang X, Hong J, Wang X, Li D, Zhang Y (2015) Morphogenesis of endoplasmic reticulum membrane‐invaginated vesicles during Beet black scorch virus infection: role of auxiliary replication protein and new implications of three‐dimensional architecture. J Virol 89: 6184–6195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JL, Kumar AS, Park E, Padmanabhan MS, Hoban K, Modla S, Czymmek K, Dinesh‐Kumar SP (2015) Chloroplast stromules function during innate immunity. Dev Cell 34: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo LR, Froehlich JE, Cruz JA, Savage LJ, Kramer DM (2016) Multi‐level regulation of the chloroplast ATP synthase: the chloroplast NADPH thioredoxin reductase C (NTRC) is required for redox modulation specifically under low irradiance. Plant J 87: 654–663 [DOI] [PubMed] [Google Scholar]
- Cejudo FJ, Ojeda V, Delgado‐Requerey V, Gonzalez M, Perez‐Ruiz JM (2019) Chloroplast redox regulatory mechanisms in plant adaptation to light and darkness. Front Plant Sci 10: 380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveau D, Henri P, Blanchard L, Rey P (2019) Variability in the redox status of plant 2‐Cys peroxiredoxins in relation to species and light cycle. J Exp Bot 70: 5003–5016 [DOI] [PubMed] [Google Scholar]
- Cerveau D, Ouahrani D, Marok MA, Blanchard L, Rey P (2016) Physiological relevance of plant 2‐Cys peroxiredoxin overoxidation level and oligomerization status. Plant, Cell Environ 39: 103–119 [DOI] [PubMed] [Google Scholar]
- Chae HB, Moon JC, Shin MR, Chi YH, Jung YJ, Lee SY, Nawkar GM, Jung HS, Hyun JK, Kim WYet al (2013) Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox‐dependent holdase chaperone function. Mol Plant 6: 323–336 [DOI] [PubMed] [Google Scholar]
- Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ (2016) Learning the languages of the chloroplast: retrograde signaling and beyond. Annu Rev Plant Biol 67: 25–53 [DOI] [PubMed] [Google Scholar]
- Chen IH, Chiu MH, Cheng SF, Hsu YH, Tsai CH (2013) The glutathione transferase of Nicotiana benthamiana NbGSTU4 plays a role in regulating the early replication of Bamboo mosaic virus . New Phytol 199: 749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90: 856–867 [DOI] [PubMed] [Google Scholar]
- Cohen D, Adamovich Y, Reuven N, Shaul Y (2010) Hepatitis B virus activates deoxynucleotide synthesis in nondividing hepatocytes by targeting the R2 gene. Hepatology 51: 1538–1546 [DOI] [PubMed] [Google Scholar]
- Da Q, Wang P, Wang M, Sun T, Jin H, Liu B, Wang J, Grimm B, Wang HB (2017) Thioredoxin and NADPH‐dependent thioredoxin reductase C regulation of tetrapyrrole biosynthesis. Plant Physiol 175: 652–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AB, Sadowska‐Bartosz I, Konigstorfer A, Kettle AJ, Winterbourn CC (2018) Superoxide dismutase protects ribonucleotide reductase from inactivation in yeast. Free Radic Biol Med 116: 114–122 [DOI] [PubMed] [Google Scholar]
- de Torres Zabala M, Littlejohn G, Jayaraman S, Studholme D, Bailey T, Lawson T, Tillich M, Licht D, Bölter B, Delfino Let al (2015) Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat Plants 1: 15074 [DOI] [PubMed] [Google Scholar]
- Deng XG, Zhu T, Peng XJ, Xi DH, Guo H, Yin Y, Zhang DW, Lin HH (2016) Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana . Sci Rep 6: 20579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BC, Chang CJ (2011) Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 7: 504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Jimenez‐Gongora T, Krenz B, Lozano‐Duran R (2019) Chloroplast clustering around the nucleus is a general response to pathogen perception in Nicotiana benthamiana . Mol Plant Pathol 20: 1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RG, Jackson AO (1994) The Barley stripe mosaic virus γb gene encodes a multifunctional cysteine‐rich protein that affects pathogenesis. Plant Cell 6: 1593–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MC (1995) Mapping of the seed transmission determinants of Barley stripe mosaic virus . Mol Plant‐Microbe Interact 8: 906–915 [DOI] [PubMed] [Google Scholar]
- Exposito‐Rodriguez M, Laissue PP, Yvon‐Durocher G, Smirnoff N, Mullineaux PM (2017) Photosynthesis‐dependent H2O2 transfer from chloroplasts to nuclei provides a high‐light signalling mechanism. Nat Commun 8: 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Sun H, Wang Y, Zhang Y, Wang X, Li D, Yu J, Han C (2014) Deep sequencing‐based transcriptome profiling reveals comprehensive insights into the responses of Nicotiana benthamiana to Beet necrotic yellow vein virus infections containing or lacking RNA4. PLoS One 9: e85284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq MA, Niazi AK, Akhtar J, Saifullah, Farooq M, Souri Z, Karimi N, Rengel Z (2019) Acquiring control: the evolution of ROS‐induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem 141: 353–369 [DOI] [PubMed] [Google Scholar]
- Fichman Y, Mittler R (2020) Rapid systemic signaling during abiotic and biotic stresses: is the ROS wave master of all trades? Plant J 102: 887–896 [DOI] [PubMed] [Google Scholar]
- Gan P, Liu F, Li R, Wang S, Luo J (2019) Chloroplasts‐ beyond energy capture and carbon fixation: tuning of photosynthesis in response to chilling stress. Int J Mol Sci 20: 5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Pu H, Liu J, Wang X, Zhong C, Yue N, Zhang Z, Wang XB, Han C, Yu Jet al (2021) Tobacco necrosis virus‐AC single coat protein amino acid substitutions determine host‐specific systemic infections of Nicotiana benthamiana and soybean. Mol Plant‐Microbe Interact 34: 49–61 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Thormahlen I, Daloso DM, Fernie AR (2017) The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci 22: 249–262 [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48: 909–930 [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Delgado‐Requerey V, Ferrandez J, Serna A, Cejudo FJ (2019) Insights into the function of NADPH thioredoxin reductase C (NTRC) based on identification of NTRC‐interacting proteins in vivo . J Exp Bot 70: 5787–5798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin MM, Dietzgen RG, Schichnes D, Ruzin S, Jackson AO (2002) pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J 31: 375–383 [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE (1991) Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S‐transferase. Anal Biochem 192: 262–267 [DOI] [PubMed] [Google Scholar]
- Gullberg RC, Jordan Steel J, Moon SL, Soltani E, Geiss BJ (2015) Oxidative stress influences positive strand RNA virus genome synthesis and capping. Virology 475: 219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Maejima K, Yamaji Y, Namba S (2021) Plant resistance to viruses: natural resistance associated with recessive genes, In Encyclopedia of Virology, Bamford DH, Zuckerman M (eds), 4 th edn, pp 69–80. Oxford: Academic Press; [Google Scholar]
- Hu J, Li S, Li Z, Li H, Song W, Zhao H, Lai J, Xia L, Li D, Zhang Y (2019a) A barley stripe mosaic virus‐based guide RNA delivery system for targeted mutagenesis in wheat and maize. Mol Plant Pathol 20: 1463–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Bogoyevitch MA, Jans DA (2019b) Subversion of host cell mitochondria by RSV to favor virus production is dependent on inhibition of mitochondrial complex I and ROS generation. Cells 8: 1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li Z, Yuan C, Jin X, Yan L, Zhao X, Zhang Y, Jackson AO, Wang X, Han Cet al (2015) Phosphorylation of TGB1 by protein kinase CK2 promotes Barley stripe mosaic virus movement in monocots and dicots. J Exp Bot 66: 4733–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo K, Hashimoto K, Kuchitsu K, Suzuki N, Okuno T (2017) Harnessing host ROS‐generating machinery for the robust genome replication of a plant RNA virus. Proc Natl Acad Sci USA 114: E1282–E1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo K, Okuno T (2020) Hijacking of host cellular components as proviral factors by plant‐infecting viruses. Adv Virus Res 107: 37–86 [DOI] [PubMed] [Google Scholar]
- Jackson AO, Lim HS, Bragg J, Ganesan U, Lee MY (2009) Hordeivirus replication, movement, and pathogenesis. Annu Rev Phytopathol 47: 385–422 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Li Z, Yue N, Zhang K, Li D, Zhang Y (2018) Construction of infectious clones of Lychnis ringspot virus and evaluation of its relationship with Barley stripe mosaic virus by reassortment of genomic RNA segments. Virus Res 243: 106–109 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Yang M, Zhang Y, Jackson AO, Li D (2021) Hordeiviruses (Virgaviridae), In Encyclopedia of Virology, Bamford DH, Zuckerman M (eds), 4 th edn, pp 420–429., Oxford: Academic Press; [Google Scholar]
- Jiang Z, Zhang K, Li Z, Li Z, Yang M, Jin X, Cao Q, Wang X, Yue N, Li Det al (2020) The Barley stripe mosaic virus γb protein promotes viral cell‐to‐cell movement by enhancing ATPase‐mediated assembly of ribonucleoprotein movement complexes. PLoS Pathog 16: e1008709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Jiang Z, Zhang K, Wang P, Cao X, Yue N, Wang X, Zhang X, Li Y, Li Det al (2018) Three‐dimensional analysis of chloroplast structures associated with virus infection. Plant Physiol 176: 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchsteiger K, Pulido P, Gonzalez M, Cejudo FJ (2009) NADPH thioredoxin reductase C controls the redox status of chloroplast 2‐Cys peroxiredoxins in Arabidopsis thaliana . Mol Plant 2: 298–307 [DOI] [PubMed] [Google Scholar]
- Kitab B, Satoh M, Ohmori Y, Munakata T, Sudoh M, Kohara M, Tsukiyama‐Kohara K (2019) Ribonucleotide reductase M2 promotes RNA replication of Hepatitis C virus by protecting NS5B protein from hPLIC1‐dependent proteasomal degradation. J Biol Chem 294: 5759–5773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepistö A, Pakula E, Toivola J, Krieger‐Liszkay A, Vignols F, Rintamäki E (2013) Deletion of chloroplast NADPH‐dependent thioredoxin reductase results in inability to regulate starch synthesis and causes stunted growth under short‐day photoperiods. J Exp Bot 64: 3843–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang A (2019) RNA‐targeted antiviral immunity: more than just RNA silencing. Trends Microbiol 27: 792–805 [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang Z, Yang X, Yue N, Wang X, Zhang K, Jackson AO, Li D, Zhang Y (2020) Construction of an infectious Poa semilatent virus cDNA clone and comparisons of Hordeivirus cytopathology and pathogenicity. Phytopathology 110: 215–227 [DOI] [PubMed] [Google Scholar]
- Ling Q, Jarvis P (2015) Regulation of chloroplast protein import by the ubiquitin E3 ligase SP1 is important for stress tolerance in plants. Curr Biol 25: 2527–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Shi L, Han C, Yu J, Li D, Zhang Y (2012) Validation of reference genes for gene expression studies in virus‐infected Nicotiana benthamiana using quantitative real‐time PCR. PLoS One 7: e46451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Dinesh‐Kumar SP (2002) Virus‐induced gene silencing in tomato. Plant J 31: 777–786 [DOI] [PubMed] [Google Scholar]
- Macrae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Doudna JA (2006) Structural basis for double‐stranded RNA processing by Dicer. Science 311: 195–198 [DOI] [PubMed] [Google Scholar]
- McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R (1986) Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens . Plant Cell Rep 5: 81–84 [DOI] [PubMed] [Google Scholar]
- Medina‐Puche L, Tan H, Dogra V, Wu M, Rosas‐Diaz T, Wang L, Ding X, Zhang D, Fu X, Kim Cet al (2020) A defense pathway linking plasma membrane and chloroplasts and co‐opted by pathogens. Cell 182: 1109–1124 [DOI] [PubMed] [Google Scholar]
- Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P (2009) NTRC links built‐in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA 106: 9908–9913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S, Yoshida K, Higo A, Hisabori T (2017) Functional significance of NADPH‐thioredoxin reductase C in the antioxidant defense system of cyanobacterium Anabaena sp. PCC 7120. Plant Cell Physiol 58: 86–94 [DOI] [PubMed] [Google Scholar]
- Muthuramalingam M, Seidel T, Laxa M, Nunes de Miranda SM, Gartner F, Stroher E, Kandlbinder A, Dietz KJ (2009) Multiple redox and non‐redox interactions define 2‐Cys peroxiredoxin as a regulatory hub in the chloroplast. Mol Plant 2: 1273–1288 [DOI] [PubMed] [Google Scholar]
- Natesan SK, Sullivan JA, Gray JC (2005) Stromules: a characteristic cell‐specific feature of plastid morphology. J Exp Bot 56: 787–797 [DOI] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenfuhr A (2007) A multicolored set of in vivo organelle markers for co‐localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Noctor G, Reichheld JP, Foyer CH (2018) ROS‐related redox regulation and signaling in plants. Semin Cell Dev Biol 80: 3–12 [DOI] [PubMed] [Google Scholar]
- Palukaitis P, Yoon JY (2020) R gene mediated defense against viruses. Curr Opin Virol 45: 1–7 [DOI] [PubMed] [Google Scholar]
- Perez‐Ruiz JM, Guinea M, Puerto‐Galán L, Cejudo FJ (2014) NADPH thioredoxin reductase C is involved in redox regulation of the Mg‐chelatase I subunit in Arabidopsis thaliana chloroplasts. Mol Plant 7: 1252–1255 [DOI] [PubMed] [Google Scholar]
- Perez‐Ruiz JM, Naranjo B, Ojeda V, Guinea M, Cejudo FJ (2017) NTRC‐dependent redox balance of 2‐Cys peroxiredoxins is needed for optimal function of the photosynthetic apparatus. Proc Natl Acad Sci USA 114: 12069–12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Ruiz JM, Spinola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ (2006) Rice NTRC is a high‐efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 18: 2356–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA (2015) Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci 40: 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty IT, French R, Jones RW, Jackson AO (1990) Identification of Barley stripe mosaic virus genes involved in viral RNA replication and systemic movement. EMBO J 9: 3453–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'homme D, Jakubiec A, Tournier V, Drugeon G, Jupin I (2003) Targeting of the Turnip yellow mosaic virus 66K replication protein to the chloroplast envelope is mediated by the 140K protein. J Virol 77: 9124–9135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerto‐Galán L, Pérez‐Ruiz JM, Guinea M, Cejudo FJ (2015) The contribution of NADPH thioredoxin reductase C (NTRC) and sulfiredoxin to 2‐Cys peroxiredoxin overoxidation in Arabidopsis thaliana chloroplasts. J Exp Bot 66: 2957–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido P, Spinola MC, Kirchsteiger K, Guinea M, Pascual MB, Sahrawy M, Sandalio LM, Dietz KJ, Gonzalez M, Cejudo FJ (2010) Functional analysis of the pathways for 2‐Cys peroxiredoxin reduction in Arabidopsis thaliana chloroplasts. J Exp Bot 61: 4043–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Voinnet O (2013) RNA silencing suppression by plant pathogens: defence, counter‐defence and counter‐counter‐defence. Nat Rev Microbiol 11: 745–760 [DOI] [PubMed] [Google Scholar]
- Qi J, Song CP, Wang B, Zhou J, Kangasjarvi J, Zhu JK, Gong Z (2018) Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol 60: 805–826 [DOI] [PubMed] [Google Scholar]
- Ricardo‐Lax I, Ramanan V, Michailidis E, Shamia T, Reuven N, Rice CM, Shlomai A, Shaul Y (2015) Hepatitis B virus induces RNR‐R2 expression via DNA damage response activation. J Hepatol 63: 789–796 [DOI] [PubMed] [Google Scholar]
- Richter AS, Peter E, Rothbart M, Schlicke H, Toivola J, Rintamäki E, Grimm B (2013) Posttranslational influence of NADPH‐dependent thioredoxin reductase C on enzymes in tetrapyrrole synthesis. Plant Physiol 162: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof HB (2006) The Tombusvirus‐encoded P19: from irrelevance to elegance. Nat Rev Microbiol 4: 405–411 [DOI] [PubMed] [Google Scholar]
- Serrato AJ, Perez‐Ruiz JM, Spinola MC, Cejudo FJ (2004) A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana . J Biol Chem 279: 43821–43827 [DOI] [PubMed] [Google Scholar]
- Sewelam N, Kazan K, Schenk PM (2016) Global plant stress signaling: reactive oxygen species at the cross‐road. Front Plant Sci 7: 187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HI, Tzanetakis IE, Dreher TW, Cho TJ (2009) The 5'‐UTR of Turnip yellow mosaic virus does not include a critical encapsidation signal. Virology 387: 427–435 [DOI] [PubMed] [Google Scholar]
- Sowden RG, Watson SJ, Jarvis P (2018) The role of chloroplasts in plant pathology. Essays Biochem 62: 21–39 [DOI] [PubMed] [Google Scholar]
- Vierstra RD (2009) The ubiquitin‐26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol 10: 385–397 [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, Batistic O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking Cet al (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang X, Ma J, Jin X, Yue N, Gao P, Mai KKK, Wang X‐B, Li D, Kang BH, Zhang Y (2021) Three‐dimensional reconstruction and comparison of vacuolar membranes in response to viral infection. J Integr Plant Biol 63: 353–364 [DOI] [PubMed] [Google Scholar]
- Waszczak C, Carmody M, Kangasjarvi J (2018) Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69: 209–236 [DOI] [PubMed] [Google Scholar]
- Yang M, Ismayil A, Liu Y (2020) Autophagy in plant‐virus interactions. Annu Rev Virol 7: 403–419 [DOI] [PubMed] [Google Scholar]
- Yang M, Li Z, Zhang K, Zhang X, Zhang Y, Wang X, Han C, Yu J, Xu K, Li D (2018a) Barley stripe mosaic virus γb interacts with glycolate oxidase and inhibits peroxisomal ROS production to facilitate virus infection. Mol Plant 11: 338–341 [DOI] [PubMed] [Google Scholar]
- Yang M, Zhang Y, Xie X, Yue N, Li J, Wang XB, Han C, Yu J, Liu Y, Li D (2018b) Barley stripe mosaic virus γb protein subverts autophagy to promote viral infection by disrupting the ATG7‐ATG8 interaction. Plant Cell 30: 1582–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelina NE, Savenkov EI, Solovyev AG, Morozov SY, Valkonen JP (2002) Long‐distance movement, virulence, and RNA silencing suppression controlled by a single protein in hordei‐ and potyviruses: complementary functions between virus families. J Virol 76: 12981–12991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yergaliyev TM, Nurbekova Z, Mukiyanova G, Akbassova A, Sutula M, Zhangazin S, Bari A, Tleukulova Z, Shamekova M, Masalimov ZKet al (2016) The involvement of ROS producing aldehyde oxidase in plant response to Tombusvirus infection. Plant Physiol Biochem 109: 36–44 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Hisabori T (2016) Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci USA 113: E3967–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Hisabori T (2019) Thiol‐based redox regulation in plant chloroplasts, In Redox homeostasis in plants: from signalling to stress tolerance, Panda SK, Yamamoto YY (eds), pp 1–17. Cham: Springer International Publishing; [Google Scholar]
- Yuan C, Li C, Yan L, Jackson AO, Liu Z, Han C, Yu J, Li D (2011) A high throughput Barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS One 6: e26468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Zhang Y, Yang M, Liu S, Li Z, Wang X, Han C, Yu J, Li D (2017) The Barley stripe mosaic virus γb protein promotes chloroplast‐targeted replication by enhancing unwinding of RNA duplexes. PLoS Pathog 13: e1006319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chiang Y‐H, Toruño TY, Lee DongHyuk, Ma M, Liang X, Lal NK, Lemos M, Lu Y‐J, Ma Set al (2018a) The MAP4 kinase SIK1 ensures robust extracellular ROS burst and antibacterial immunity in plants. Cell Host Microbe 24: 379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dong K, Xu K, Zhang K, Jin X, Yang M, Zhang Y, Wang X, Han C, Yu Jet al (2018b) Barley stripe mosaic virus infection requires PKA‐mediated phosphorylation of γb for suppression of both RNA silencing and the host cell death response. New Phytol 218: 1570–1585 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang X, Xu K, Jiang Z, Dong K, Xie X, Zhang He, Yue N, Zhang Y, Wang X‐Bet al (2021) The serine/threonine/tyrosine kinase STY46 defends against hordeivirus infection by phosphorylating γb protein. Plant Physiol 186: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Wang H, Chen S, Yu D, Reiter RJ (2021) Phytomelatonin: an emerging regulator of plant biotic stress resistance. Trends Plant Sci 26: 70–82 [DOI] [PubMed] [Google Scholar]
- Zhao X, Huang J, Chory J (2020) Unraveling the linkage between retrograde signaling and RNA metabolism in plants. Trends Plant Sci 25: 141–147 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhou J (2020) Luciferase complementation assay for detecting protein interactions. Chin Bull Bot 55: 69–75 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Appendix
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Review Process File
Data Availability Statement
This study includes no newly generated data deposited in external repositories.


