Abstract
Mechanical mitral valve replacement in infants and young children is associated with substantial morbidity and mortality. Lifelong anticoagulation is required, with all the accompanying challenges of maintaining levels in infants and children whose dietary input continually changes. Even with careful control of all aspects that can perturb the coagulation cascade, these patients have a substantial lifelong risk of thrombotic and hemorrhagic complications that can also affect the durability of the valve. Anticoagulation is usually achieved utilizing warfarin with the degree of anticoagulation measured via the international normalized ratio (INR). Unfortunately, in some cases, the INR can be falsely elevated and lead to inappropriate reassurance. We describe a 4-year-old patient with complex congenital heart disease palliated via a single ventricular pathway with a mechanical atrioventricular valve replacement. The patient experienced acute valvular thrombosis while receiving warfarin with INR at target levels. Chromogenic factor X (CFX) levels were discordant with INR measurements, suggesting a subtherapeutic level of anticoagulation despite maintaining the standard INR target. Therefore, CFX levels were used to interpret INR measurements and guide an individualized approach to anticoagulation. We propose a new role of CFX: to verify and guide warfarin anticoagulation in high-risk pediatric patients including those undergoing mechanical mitral valve replacement.
Abbreviations and Acronyms: AVV, atrioventricular valve; CFX, chromogenic factor X; INR, international normalized ratio
Children with congenital or acquired mitral valve abnormalities may require valve replacement if the native valve is unrepairable or fails after a surgical or catheterization-based intervention.1, 2, 3, 4, 5 In the mitral position, a mechanical prosthesis has improved durability when compared with a bioprosthetic valve but requires a higher degree of anticoagulation to prevent valve-associated thrombotic complications.6, 7, 8 The low-pressure gradient across the mitral valve, potential nonanatomic position with abnormal flow pattern, and associated comorbidities like atrial arrhythmias can predispose the patient to stasis and inefficient washing of the valve disks—thereby elevating the risk of thrombus formation. Thrombosis of the mechanical valve can result in valve dysfunction, obstruction, and thromboembolic complications, with potentially devastating results. Warfarin is the standard means for maintaining adequate anticoagulation in the setting of a mechanical mitral valve with a goal international normalized ratio (INR) range of 2.5 to 3.5. This is a standard recommendation adopted from the valve guidelines and is not individualized to the patient.9
Warfarin is a vitamin K epoxide reductase antagonist. The anticoagulant effect is the result of a reduction of hepatic synthesis of factors II, VII, IX, and X. Prothrombin time/INR is traditionally utilized to monitor warfarin-induced anticoagulation by measuring the extrinsic pathway of hemostasis. There are rare reports of falsely reassuring INRs that overpredict in situ anticoagulation.10
We describe a pediatric patient receiving warfarin who despite a presumed therapeutic INR experienced obstructive thrombosis of a mechanical mitral valve requiring emergency intervention. We discuss the need for a more individualized approach to high-risk patient populations.
Report of Case
Our patient was a 4-year-old girl with heterotaxy syndrome (asplenia subtype) with total anomalous pulmonary venous return to the right atrium, unbalanced complete atrioventricular canal defect to a morphological right ventricle, double-outlet right ventricle, and malposed great arteries. Her staged surgical single-ventricle palliation included a Damus-Kaye-Stansel procedure, Blalock-Taussig shunt, and atrial septectomy followed by shunt takedown and completion of a bidirectional cavopulmonary anastomosis. She also required replacement of her common atrioventricular valve (AVV) with a 23-mm mechanical prosthesis after 2 attempted repairs of the AVV. Warfarin was initiated for anticoagulation with a target INR goal of 2.5 to 3.5. She was admitted to the general pediatric hospital service 2.5 months after prosthesis placement with abdominal pain and distention, emesis, oliguria, and a symptomatic upper respiratory infection (laboratory testing positive for rhinovirus/enterovirus). Blood cultures were performed and yielded negative results. For 2 months leading up to admission, laboratory monitoring revealed predominately therapeutic and supratherapeutic INRs between 2.0 and 4.7 (Table).
Table.
Coagulation Factor Testing and Corresponding Chromogenic Factor X Levels in a Pediatric Patient With a Prosthetic Mitral Valve
| Date | INR | PT (s) | APTT (s) | Chromogenic factor X (%) |
|---|---|---|---|---|
| 10/1 | 2.2 | 24.7 | NA | 47 |
| 10/2 | 1.8 | 20.3 | 48, 52, 180, 47 | NA |
| 10/3 | 2.1 | 23.3 | 41, 60, 61, 87 | NA |
| 10/4 | 2.3 | 26.2 | 88, 92, 86 | NA |
| 10/5 | 2.4 | 27.2 | 95 | NA |
| 10/6 | 2.3 | 25.4 | NA | 49 |
| 10/7 | 2.5 | 27.5 | 77 | NA |
| 10/8 | 2.8 | 31.2 | 44 | 42 |
| 10/9 | 3.5 | NA | NA | 35 |
APTT, activated partial thromboplastin time; INR, international normalized ratio; PT, prothrombin time.
Echocardiography on the day of admission revealed severe mechanical AVV stenosis with a mean diastolic Doppler gradient of 17 mm Hg. Her systemic right ventricular function was preserved at 40% to 45%. Computed tomographic angiography of the chest revealed minimal excursion of disks, confirming severe obstruction. Fluoroscopy of the valve also revealed severe prosthesis dysfunction with no movement of the superior disk and limited excursion of inferior disk, consistent with acute thrombosis of her mechanical AVV. Her symptoms were presumed to be secondary to systemic and pulmonary venous hypertension with resultant development of abdominal ascites and pleural effusion.
Bivalirudin, a direct thrombin inhibitor that inhibits circulating and clot-associated thrombin, was initiated for stable anticoagulation. Despite this effort, the patient’s AVV gradient continued to increase with worsening symptoms, ultimately necessitating rereplacement of her AVV with a bioprosthetic valve. A laboratory evaluation was pursued to evaluate for an underlying etiology given valve thrombosis in the setting of therapeutic INRs. Factor V was found to be normal (82%), indicating normal liver synthetic function. The chromogenic factor X (CFX) level was 47%, corresponding to an INR of 2.2 and indicating suboptimal anticoagulation (Table). The hematology service recommended anticoagulation to an INR that corresponded to a goal CFX level of 20% to 30%. Progressive escalation of warfarin therapy was performed, documenting that a personalized INR goal of 3.0 to 4.0 was required to achieve this goal CFX level.
The patient was followed up for 12 months with this higher INR goal, and echocardiography has revealed excellent AVV function, preserved systemic ventricle function, and no evidence of rethrombosis. She also remains free of bleeding complications.
Discussion
This case outlines the need for an individualized approach to anticoagulation for high-risk patients. Traditional goal INR levels may not correspond to adequate degrees of anticoagulation, leaving the patient at risk for a potentially devastating complication.
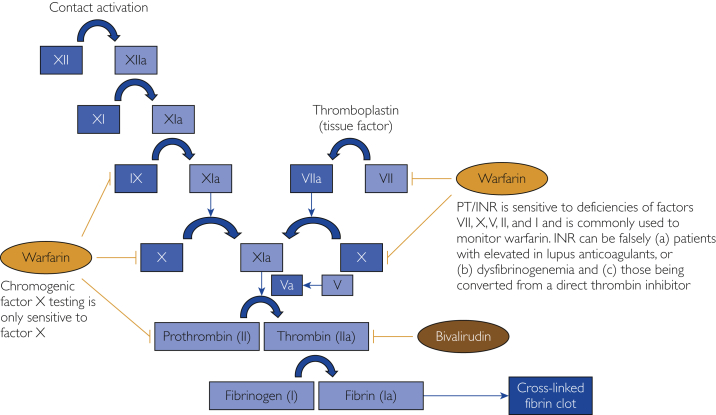
International normalized ratios are the conventional method of monitoring warfarin-mediated anticoagulation (Figure 1). However, the INR can be falsely elevated in certain cases. Falsely elevated INRs have been reported in patients with (1) lupus anticoagulant antibodies or (2) dysfibrinogenemia and (3) in those undergoing conversion from direct thrombin inhibitors.11, 12, 13, 14, 15, 16 First, an interfering substance can alter a clotable assay (such as INR), thereby leading to an inaccurate INR. Lupus anticoagulants, for example, are thought to erroneously elevate INR by reacting with the thromboplastin that is used to measure prothrombin time.16 Second, dysfibrinogenemias (eg, fibrinogen Longmont) can cause missed fibrin end points when laboratory instruments rely on photo-optical detection.14 Third, direct thrombin inhibitors prolong the INR through interactions with thromboplastins.17,18 An alternative to INR is necessary in these cases to evaluate the coagulation profile. Levels of CFX have been used in these scenarios to monitor adults with lupus anticoagulants or dysfibrinogenemia and those receiving direct thrombin inhibitors because CFX is resistant to these confounding variables.19, 20, 21
Figure 1.
Clotting cascade, anticoagulant effects, and points of monitoring. INR, international normalized ratio; PT, prothrombin time.
Chromogenic factor X is a laboratory test that measures the percentage of factor X activity. Because testing is not dependent on thromboplastin or fibrinogen, CFX testing bypasses in vitro interactions that could lead to a falsely elevated INR. Warfarin inhibits hepatic synthesis of factor X. Thus, CFX can be used to indirectly measure warfarin-associated anticoagulation in circumstances in which the interaction with thromboplastin or fibrinogen would render INR results unreliable.22 Chromogenic factor X can thereby be used to validate INR in high-risk patients from the onset of anticoagulation.
There are no unanimously accepted therapeutic levels for CFX, but previous studies have investigated the relationship between CFX values and INR.23, 24, 25, 26 In 2005, Arpino et al24 attempted to determine the use of CFX to predict the INR in 62 patients transitioning from argatroban to warfarin. A CFX level of 45% or less was used as a cutoff for predicting an INR of greater than 2 with a sensitivity of 93%, a specificity of 78%, accuracy of 89%, and an area under the receiver operating characteristic curve of 0.91.24 In 2008, McGlasson et al26 conducted a large study comprising of 339 specimens to assess comparison of CFX with INR for monitoring oral anticoagulation therapy. Predictive modeling suggested the following equation for expected CFX values given an INR: CFX = 13.2 + (5.3/INR) + (81.6/INR2). The authors concluded that the therapeutic INR range of 2.0 to 3.0 was equivalent to a CFX level of 23.5% to 35.5%. A CFX level of 35.5% and less yielded a sensitivity of 91.7% and a specificity of 91.9% for discriminating an INR of at least greater than 2.0. The supratherapeutic INR category of greater than 3.0 correlated with a mean CFX of 20.8% (range, 9% to 46%) (n=104). In 2009, Rosborough et al27 identified that the relationship between CFX and INR varies among patients with initiation of warfarin vs those receiving long-term warfarin therapy. They reported a 7% to 18% higher level of CFX activity for a given INR value during warfarin initiation compared with patients receiving long-term warfarin therapy and suggested predictive formulas for each phase of therapy. In our patient, an INR of 2.2 correlated to a CFX level of 47%, which is near the highest value in the previously mentioned therapeutic range. Our patient required an INR of 3.5 to achieve a CFX level of 35% (Table).
As exemplified by this case, there can be catastrophic consequences of a falsely reassuring INR in pediatric patients with mechanical mitral valves. This case documents the need for a more individualized approach to anticoagulation. We therefore propose that CFX levels should be obtained alongside initial INR values to verify anticoagulated status in high-risk patients. Chromogenic factor X levels can be used proactively rather than reactively to confirm or modify therapeutic INR goals. The primary limitation of this case report is that it represents an observation. Next steps include a prospective assessment of both INR and CFX levels in high-risk patients to evaluate the anticoagulation effects and CFX level correlation to INR for these patients.
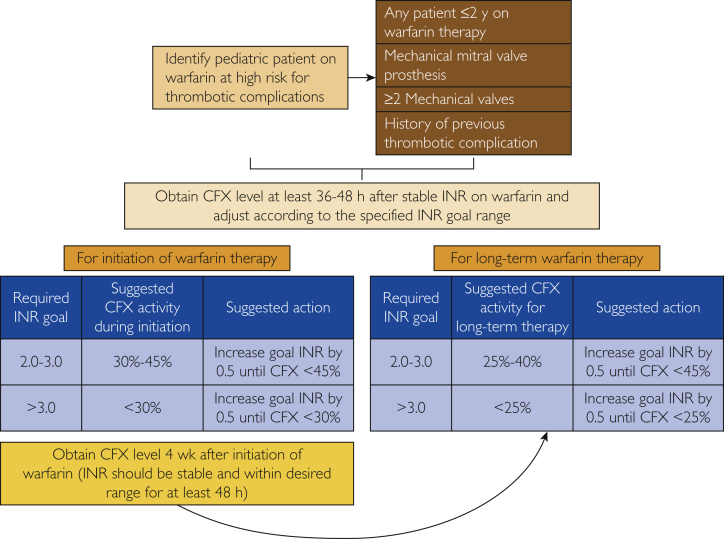
Therefore, we have designed a prospective protocol for assessment and validation of INR among pediatric patients receiving warfarin therapy who are at a high risk for thrombotic complications (Figure 2). We have selected cutoff ranges for INR and CFX correlation based on the data from studies by McGlasson et al26 and Arpino et al,24 while keeping in consideration the changes in the CFX activity based on the phases of initiation or long-term therapy as suggested by Rosborough et al.16 Considering that the evidence is only available for INR ranges of less than 2, 2 to 3, and greater than 3, we have also utilized the same ranges for the purpose of this protocol. We suggest increasing warfarin to achieve a corresponding escalation in INR by 0.5 until the satisfactory corresponding cutoff level of CFX is achieved, thus identifying the INR target for the patient. Currently, we are only utilizing this protocol for pediatric patients taking warfarin who are thought to be at a higher risk from thrombotic complications, including patients with mechanical mitral valve prosthesis, any patient 2 years old or younger receiving warfarin therapy, patients with 2 or more mechanical prosthetic valves, and those with a history of previous thrombotic complications.
Figure 2.
Validation of international normalized ratio (INR) among pediatric patients receiving warfarin therapy at high risk for thrombotic complications. CFX, chromogenic factor X.
Conclusion
Chromogenic factor X levels should be obtained alongside initial INR values to verify anticoagulated status in high-risk patients, such as those with mechanical mitral valves. We have designed a prospective protocol for assessment and validation of INR among pediatric patients receiving warfarin therapy who are at a high risk for thrombotic complications.
Footnotes
Potential Competing Interests: The authors report no competing interests.
References
- 1.Ackermann K., Balling G., Eicken A., Günther T., Schreiber C., Hess J. Replacement of the systemic atrioventricular valve with a mechanical prosthesis in children aged less than 6 years: late clinical results of survival and subsequent replacement. J Thorac Cardiovasc Surg. 2007;134(3):750–756. doi: 10.1016/j.jtcvs.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Beierlein W., Becker V., Yates R. Long-term follow-up after mitral valve replacement in childhood: poor event-free survival in the young child. Eur J Cardiothorac Surg. 2007;31(5):860–865. doi: 10.1016/j.ejcts.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Henaine R., Nloga J., Wautot F. Long-term outcome after annular mechanical mitral valve replacement in children aged less than five years. Ann Thorac Surg. 2010;90(5):1570–1576. doi: 10.1016/j.athoracsur.2010.06.121. [DOI] [PubMed] [Google Scholar]
- 4.Caldarone C.A., Raghuveer G., Hills C.B. Long-term survival after mitral valve replacement in children aged <5 years: a multi-institutional study. Circulation. 2001;104(12, suppl 1):I143–I147. doi: 10.1161/hc37t1.094840. [DOI] [PubMed] [Google Scholar]
- 5.Elsisy M.F., Dearani J.A., Ashikhmina E. What factors should be considered to improve outcome of mechanical mitral valve replacement in children? World J Pediatr Congenit Heart Surg. 2021;12(3):367–374. doi: 10.1177/2150135121994084. [DOI] [PubMed] [Google Scholar]
- 6.Brown J.W., Fiore A.C., Ruzmetov M., Eltayeb O., Rodefeld M.D., Turrentine M.W. Evolution of mitral valve replacement in children: a 40-year experience. Ann Thorac Surg. 2012;93(2):626–633. doi: 10.1016/j.athoracsur.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 7.Masuda M., Kado H., Matsumoto T. Mitral valve replacement using bileaflet mechanical prosthetic valve in the first year of life. Jpn J Thorac Cardiovasc Surg. 2000;48(10):643–647. doi: 10.1007/BF03218220. [DOI] [PubMed] [Google Scholar]
- 8.Ibezim C., Sarvestani A.L., Knight J.H. Outcomes of mechanical mitral valve replacement in children. Ann Thorac Surg. 2019;107(1):143–150. doi: 10.1016/j.athoracsur.2018.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura R.A., Otto C.M., Bonow R.O., American College of Cardiology; American College of Cardiology/American Heart Association; American Heart Association 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Thorac Cardiovasc Surg. 2014;64(16):1763] J Thorac Cardiovas Surg. 2014;148(1):e1–e132. doi: 10.1016/j.jtcvs.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Delate T., Witt D.M., Jones J.R., Bhardwaja B., Senser M. Falsely elevated international normalized ratio values in patients undergoing anticoagulation therapy: a descriptive evaluation. Chest. 2007;131(3):816–822. doi: 10.1378/chest.06-2200. [DOI] [PubMed] [Google Scholar]
- 11.Ferrazzi P., Colombo A., Di Micco P. Differences in the INR evaluation of two different thromboplastins in patients with positivity to lupus anticoagulant in ongoing oral anticoagulation. J Blood Med. 2010;1:57–60. doi: 10.2147/JBM.S8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dush A., Erdeljac H.P. INR management of an antiphospholipid syndrome patient with point-of-care INR testing. J Pharm Pract. 2020;33(3):390–391. doi: 10.1177/0897190019838192. [DOI] [PubMed] [Google Scholar]
- 13.Lawrie A.S., Purdy G., Mackie I.J., Machin S.J. Monitoring of oral anticoagulant therapy in lupus anticoagulant positive patients with the anti-phospholipid syndrome. Br J Haematol. 1997;98(4):887–892. doi: 10.1046/j.1365-2141.1997.3283145.x. [DOI] [PubMed] [Google Scholar]
- 14.Lefkowitz J.B., DeBoom T., Weller A., Clarke S., Lavrinets D. Fibrinogen Longmont: a dysfibrinogenemia that causes prolonged clot-based test results only when using an optical detection method. Am J Hematol. 2000;63(3):149–155. doi: 10.1002/(sici)1096-8652(200003)63:3<149::aid-ajh8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Robert A., Le Querrec A., Delahousse B. Control of oral anticoagulation in patients with the antiphospholipid syndrome—influence of the lupus anticoagulant on International Normalized Ratio. Groupe Méthodologie en Hémostase du Groupe d'Etudes sur l'Hémostases et la Thrombose. Thromb Haemost. 1998;80(1):99–103. [PubMed] [Google Scholar]
- 16.Rosborough T.K., Shepherd M.F. Unreliability of international normalized ratio for monitoring warfarin therapy in patients with lupus anticoagulant. Pharmacotherapy. 2004;24(7):838–842. doi: 10.1592/phco.24.9.838.36102. [DOI] [PubMed] [Google Scholar]
- 17.Gosselin R.C., Dager W.E., King J.H. Effect of direct thrombin inhibitors, bivalirudin, lepirudin, and argatroban, on prothrombin time and INR values. Am J Clin Pathol. 2004;121(4):593–599. doi: 10.1309/D79K-4YG7-8NTN-YY38. [DOI] [PubMed] [Google Scholar]
- 18.Hursting M.J., Zehnder J.L., Joffrion J.L., Becker J.C., Knappenberger G.D., Schwarz R.P., Jr. The International Normalized Ratio during concurrent warfarin and argatroban anticoagulation: differential contributions of each agent and effects of the choice of thromboplastin used. Clin Chem. 1999;45(3):409–412. [PubMed] [Google Scholar]
- 19.Sucker C., Wenzel F., Zotz R.B., Hetzel G.R., Scharf R.E. Monitoring oral anticoagulation may require determination of single coagulation factor activities in patients with antiphospholipid syndrome. J Rheumatol. 2006;33(9):1881–1882. [PubMed] [Google Scholar]
- 20.Tripodi A., Chantarangkul V., Clerici M., Negri B., Galli M., Mannucci P.M. Laboratory control of oral anticoagulant treatment by the INR system in patients with the antiphospholipid syndrome and lupus anticoagulant: results of a collaborative study involving nine commercial thromboplastins. Br J Haematol. 2001;115(3):672–678. doi: 10.1046/j.1365-2141.2001.03178.x. [DOI] [PubMed] [Google Scholar]
- 21.Austin J.H., Stearns C.R., Winkler A.M., Paciullo C.A. Use of the chromogenic factor X assay in patients transitioning from argatroban to warfarin therapy. Pharmacotherapy. 2012;32(6):493–501. doi: 10.1002/j.1875-9114.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanfelippo M.J., Zinsmaster W., Scherr D.L., Shaw G.R. Use of chromogenic assay of factor X to accept or reject INR results in warfarin treated patients. Clin Med Res. 2009;7(3):103–105. doi: 10.3121/cmr.2009.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thom J., Ivey L., Gilmore G., Eikelboom J.W. Evaluation of the phospholipid-rich dilute Russell's viper venom assay to monitor oral anticoagulation in patients with lupus anticoagulant. Blood Coagul Fibrinolysis. 2004;15(4):353–357. doi: 10.1097/00001721-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Arpino P.A., Demirjian Z., Van Cott E.M. Use of the chromogenic factor X assay to predict the international normalized ratio in patients transitioning from argatroban to warfarin. Pharmacotherapy. 2005;25(2):157–164. doi: 10.1592/phco.25.2.157.56950. [DOI] [PubMed] [Google Scholar]
- 25.Trask A.S., Gosselin R.C., Diaz J.A., Dager W.E. Warfarin initiation and monitoring with clotting factors II, VII, and X. Ann Pharmacother. 2004;38(2):251–256. doi: 10.1345/aph.1D266. [DOI] [PubMed] [Google Scholar]
- 26.McGlasson D.L., Romick B.G., Rubal B.J. Comparison of a chromogenic factor X assay with international normalized ratio for monitoring oral anticoagulation therapy. Blood Coagul Fibrinolysis. 2008;19(6):513–517. doi: 10.1097/MBC.0b013e328304e066. [DOI] [PubMed] [Google Scholar]
- 27.Rosborough T.K., Jacobsen J.M., Shepherd M.F. Relationship between chromogenic factor X and international normalized ratio differs during early warfarin initiation compared with chornic warfarin administration. Blood Coagul Fibrinolysis. 2009;20(6):433–435. doi: 10.1097/MBC.0b013e32832ca31f. [DOI] [PubMed] [Google Scholar]