Graphical abstract
Abbreviations: ED, Endocrine disruptors; ENDs, Endocrine and nervous disruptors; ND, Nervous disruptors; WHO, World Health Organization
Keywords: Endocrine disruptors, Nervous disruptors, Neurotoxicity, Cognitive, Behaviour, Pollutants
Highlights
-
•
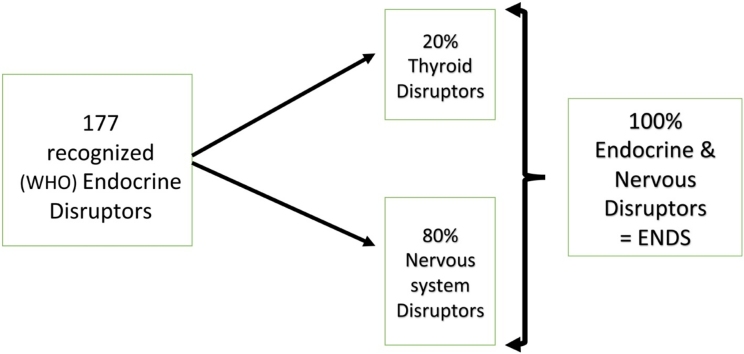
One hundred and seventy-seven compounds have been recognised as endocrine disruptors (EDs) by the World Health Organization.
-
•
Twenty percent disrupt the nervous system through the thyroid.
-
•
Eighty percent disrupt the nervous system via various other mechanisms and can be likened to internet spam.
-
•
It is proposed that these compounds should thus be renamed endocrine and nervous disruptors (ENDs).
Abstract
Endocrine disruption (ED) and endocrine disruptors (EDs) emerged as scientific concepts in 1995, after numerous chemical pollutants were found to be responsible for reproductive dysfunction. The World Health Organization established in the United Nations Environment Programme a list of materials, plasticizers, pesticides, and various pollutants synthesized from petrochemistry that impact not only reproduction, but also hormonal functions, directly or indirectly. Cells communicate via either chemical or electrical signals transmitted within the endocrine or nervous systems. To investigate whether hormone disruptors may also interfere directly or indirectly with the development or functioning of the nervous system through either a neuroendocrine or a more general mechanism, we examined the scientific literature to ascertain the effects of EDs on the nervous system, specifically in the categories of neurotoxicity, cognition, and behaviour. To date, we demonstrated that all of the 177 EDs identified internationally by WHO are known to have an impact on the nervous system. Furthermore, the precise mechanisms underlying this neurodisruption have also been established. It was previously believed that EDs primarily function via the thyroid. However, this study presents substantial evidence that approximately 80 % of EDs operate via other mechanisms. It thus outlines a novel concept: EDs are also neurodisruptors (NDs) and can be collectively termed endocrine and nervous disruptors (ENDs). Most of ENDs are derived from petroleum residues, and their various mechanisms of action are similar to those of “spam” in electronic communications technologies. Therefore, ENDs can be considered as an instance of spam in a biological context.
1. Introduction
Endocrine disruption (ED) or endocrine disruptors (EDs) emerged as scientific concepts in 1995 [Colborn [1]; Lindström et al. [2]; Ginsburg [3]] after numerous chemical pollutants were found to be responsible for reproductive dysfunction. This was first posited three decades prior [Carson [4]]. EDs were reviewed more recently in a book [Seralini [5]], which advanced the understanding of the molecular bioaccumulation of identified xenobiotics, as well as their combined and long-term effects on the whole physiology of one or several generations. They have been identified in organisms at all levels of the ecosystem and are also ubiquitously found in the food chain.
The World Health Organization established in the United Nations Environment Programme [WHO [6]], a list of 176 compounds comprising materials, pesticides, and various pollutants (Table 1, columns 1–3) impacting not only reproduction, but also hormonal functions—directly or indirectly—primarily in mammals, including humans. This has enabled numerous countries to establish regulatory policies for the production and use of these chemicals or to manage contamination in food, air and water. Numerous political debates have been raised around regulatory thresholds, based on the effects of ENDs demonstrated on a population or a subpopulation of animals or humans, and published at epidemiological and molecular levels. The herbicide Roundup has been added as the 177th compound due to its widespread usage as a pesticide, combined with the relatively recent demonstration of its ED effects [Richard et al. [7]].
Table 1.
Endocrine disruptors (according to WHO, 2013) also function as nervous system disruptors, and may thus be collectively termed as Endocrine and Nervous Disruptors (ENDs).
| Nb | Endocrine disruptor | Class or use | Mechanisms of nervous disruption |
|---|---|---|---|
| 1 | Acetochlor | Herbicide | Roman [28]: antithyroid agents |
| Helbing et al. [29]: thyroid hormone receptor gene expression in the brain | |||
| Zafeiridou et al. [30]: compound action potential of the sciatic nerve | |||
| 2 | Alachlor | Herbicide | Goldner et al. [31]: hypothyroidism |
| Seok et al. [32]: central nervous system symptoms | |||
| Lo et al. [33]: severe neurological and cardiovascular outcomes after acute poisoning | |||
| Doïcheva [34]: higher irritability, lack of coordination and orientation | |||
| 3 | Amitrole | Herbicide | Sirohi et al. [35]: specific binding to lactoperoxidase |
| Chilumuri et al. [36]: inhibit neuroprotection against amyloid peptides | |||
| Pan et al. [37]: reduction of thyroid-stimulating hormone receptors | |||
| Roman [28]: hypothyroxinemia | |||
| Brucker-Davis [38]: thyroid disruption in utero or direct neurotoxicity | |||
| 4 | Anthracene | PAH | Palanikumar et al. [39]: neurotoxicity by inhibition of acetylcholinesterase |
| Vieira et al. [40]: increase catalase activity and superoxide dismutase, glutathione reductase and peroxidase. | |||
| 5 | Aroclor 1254 | PCB mixture | Mucio-Ramírez et al. [41]: decrease somatodendritic vasopressin release |
| Wei et al. [42]: oxidative stress in the brain | |||
| Coburn et al. [43]: Inhibition of vasopressin release from magnocellular neuroendocrine cells | |||
| Majumdar et al. [44]: induces oxidative stress in the brain | |||
| Tilson et al. [45]: increases translocation of protein kinase C and decreases Ca2+-buffering in the brain | |||
| 6 | Arsenic (As) | Heavy metal, pesticide | Liu et al. [46]: cognitive impairment |
| Webb et al. [47]: chronic neurological disease | |||
| Preciados et al. [48]: influence nuclear respiratory factor 1 by genomic and epigenomic networks, this contributes in the development of complex chronic human brain disorders | |||
| Karri et al. [49]: pro-oxidant elements dominate antioxidants factors and leads to cognitive dysfunction | |||
| Tyler at al. [50]: alters epigenetics and hippocampal function, glutamatergic, cholinergic and monoaminergic signaling; altered adult neurogenesis; and increases Alzheimer's-associated pathologies | |||
| 7 | Atrazine | Herbicide | Ma et al. [51]: reduces dopamine levels in the substantia nigra and corpus striatum in the midbrain |
| Zhang et al. [52]: severe dopamine neuron degeneration | |||
| Lin et al. [53]: increases in striatal dopamine; decreases in perirhinal cortex serotonin | |||
| Ottinger et al. [54]: impact on arginine vasotocin, catecholamines and gonadotropin releasing hormone. | |||
| 8 | Benzo(a)anthracene | PAH | Dayal et al. [55]: most notable symptoms are neurologic |
| 9 | Benzo(a)pyrene | PAH | Slotkin et al. [56]: impairs neurodifferentiation and neurite formation, development of dopamine and acetylcholine phenotypes |
| Sarma et al. [57]: induces neuronal cell damage involving oxidative stress through mitochondria-mediated apoptosis pathway | |||
|
Yang et al. [58]: cause disruption of glutamate (Glu) neurotransmitter transmission by decreasing the level of Glu, reducing the expression of Glu receptors Chepelev et al. [59]: binds to the aryl hydrocarbon receptor, modulate the transcription of glutamate receptor subunits, decrease long-term potentiation, learning and memory | |||
| Niu et al. [60]: reduces neurobehavioral function and monoamine, amino acid and choline neurotransmitter levels | |||
| 10 | BB-153 | PBB | Jacobson et al. [61]: associations of PBBs and PCBs with thyroid disease and thyroid hormone levels. |
| Tilson et al. [62]: chronic exposure produces behavioral or neurological toxicity | |||
| 11 | Benzyl butyl phthalate | Phthalate | Morgenstern et al. [63]: impairs thyroid function in preschool children |
| Min et al. [64]: attenuation of the effects of cAMP Response Element-Binding Protein downstream, oxidative damage and impaired behavioral performance | |||
| Betz et al. [65]: changes in amygdala protein s related on synaptic plasticity | |||
| Kasuya M [66]: inhibited the outgrowth of nerve fibers and glial cells from cerebellar explants | |||
| 12 | BDE-209 | PBDE | Chen et al. [67]: activation of NMDA receptors inhibits the expression of phosphodiesterases, favoring apoptosis induction and induces neurotoxicity. |
| Li et al. [68]: the mRNA expressions of synaptobrevin 2, syntaxin 1A, SNAP-25, and synaptophysin are significantly decreases in the hippocampi of rat exposed to it | |||
| Sun et al. [69]: during rat pregnancy increases hippocampal autophagy, decreases neuron viability | |||
| Vuong et al. [70]: large prospective human cohorts demonstrates that prenatal and postnatal exposure adversely impacts externalizing behavior (e.g., hyperactivity and conduct problems) | |||
| 13 | BDE-47 | PBDE | Zhuang et al. [71]: induces neurotoxicity and cognitive impairment by upregulation of nuclear TAR DNA-binding protein 43 in the hippocampus provoking neuronal apoptosis. |
| Chen et al. [67]: inhibits axonal growth via ryanodine receptor-dependent mechanisms | |||
| Zhai et al. [72]: prenatal exposure inhibits neurodevelopmental function and behavior with an increase of luteinizing hormone levels. | |||
| 14 | BDE‐99 | PBDE | Dach et al. [73]: reduces expression of myelin associated genes like HMBP due to olligodendrocyte reduction. |
| Ding et al. [74]: prenatal exposure is associated with lower developmental quotients in young children. | |||
| Roze et al. [75]: transplacental transfer is associated with worse fine manipulative abilities, and worse attention of children at school age. | |||
| 15 | Benzene | Aromatic solvent | Bahadar et al. [76]: exposure can lead to aberration of vital systems in the body like nervous, cardiovascular, and respiratory. |
| Manto M. [77]: in humans, cerebellum is a main target of environmental toxins such as toluene/benzene derivatives. | |||
| Ritchie et al. [78]: a number of published studies report acute or persisting neurotoxic effects of hydrocarbon fuels. | |||
| 16 | Benzylidene camphor | UV filter | Ruszkiewicz et al. [79]: potential neurotoxicity |
| Faass et al. [80]: reduces proceptive and receptive behaviors and specific gene expression in ventromedial hypothalamic nucleus and medial preoptic area. | |||
| 17 | Bisphenol A | Plastics monomer | Ejaredar et al. [81]: exposure in childhood is associated with higher levels of anxiety, depression, hyperactivity, inattention, and conduct problems. |
| Zhou et al. [82]: affects neuron numbers in different regions of the hypocampus altering learning and memory ability of adolescent mice. | |||
| Inadera H. [83]: in a review, it has detrimental effects on neurological development. | |||
| Masuo et al. [11]: locus ceruleus is enlarged in treated male rats and neurodegenerative disorders. | |||
| 18 | Bisphenol A diglycid ether | Plastics monomer | Hutler et al. [84]: the main abnormalities in amphibians larvae related to neurotoxicity. |
| 19 | Bisphenol F | Plastics monomer | Ohtani et al. [85]: exposure alters offspring behavior, resulting in increases in anxiety and depressive state in mice. |
| Rosenfeld [86]: induces neurobehavioral disruptions. | |||
| Castro et al. [87]: affects genes in the juvenile female rats prefrontal cortex. | |||
| 20 | Bisphenol S | Plastics monomer | Wu et al. [88]: triggers oxidative stress in the nervous system in vivo and in vitro in humans. |
| Castro et al. [87]: affects genes expression and dopamin serotonin system in the prefrontal cortex of juvenile female rats. | |||
| 21 | Bromacil | Herbicide | Lombardi et al [89]: exposure through residential proximity to agricultural applications during pregnancy may increase the risk of childhood central nervous system tumors. |
| 22 | Butylate | Herbicide | Lulla et al. [90]: for Ziram: selectively toxic to dopaminergic neurons in vivo, and this toxicity is synuclein-dependent. |
| 23 | Butylated hydroxyanisole | Antioxidant for long preservation of food products | Miyazaki et al. [91]: quinone reductase inducer which significantly and dose dependently blocked methamphetamine-induced elevation of quinoprotein, and ameliorated methamphetamine-induced cell death. |
| Katsuki et al. [92]: abolishes neurotoxic action of arachidonic acid. | |||
| 24 | Cadmium (Cd) | Heavy metal | Raciti et al. [93]: affects epigenetics with negative consequences on the development of the nervous system. |
| Jacobo-Estrada et al. [94]: induces toxicity in fetus on the central nervous system. | |||
| Zhang et al. [95]: induces autophagy in neurons promoting neurodegenerative disorders. | |||
| Sanders et al [96]: exposure may be associated with poorer cognition. | |||
| Bo et al. [97]: neurotoxic on the long-term. | |||
| 25 | Carbamazepine | Pharmaceutical anti-epileptic | Al-Rubai et al. [98]: reduces neurosphere size and cell migration at high doses. Reduction in glial fibrillary protein and tubulin III. |
| Hansen et al. [99]: induces encephalopathy with hyperammonemia, intrinsic effects on cerebral receptors. | |||
| Gualtieri et al. [100]: affects tests of memory, psychomotor speed, cognitive flexibility, and attention. | |||
| 26 | Carbaryl | Insecticide | Lee et al. [101]: inhibits classically acetylcholinesterase in the nervous system; induces cognitive impairments by disturbed neurodevelopment. |
| Freeborn et al. [102]: affects electroencephalogram by decreasing theta area and delta frequency, increases beta frequency. | |||
| Wang et al. [103]: acetylcholinesterase is inhibited by high dose and damages the sciatic nerve. | |||
| 27 | CB‐15 | PCB | Cocco et al [104]: chronic exposure to PCBs affects the development and function of the nervous system. |
| Lovato et al [105]: a mixture of PCB can induce functional deficits and altered behavioral threat in zebrafish. | |||
| 28 | CB-77 | Coplanar PCB | Ozcan et al. [106]: dioxin-like and non-dioxin-like PCB congeners are equally potent in causing cognitive decrements seen in children exposed prenatally to PCBs. |
| Howard et al. [107]: binds the aryl hydrocarbon receptors with high affinity. | |||
| 29 | CB-118 | PCB | Brucker-Davis et al. [108]: negative impact on neurocognitive development, negatively correlated on motor and expressive language in children. |
| Doi et al. [109]: four-month-olds children with a low-level of prenatal exposure exhibits a preference for the upright biological motion, impairs the development functioning and brain development. | |||
| 30 | CB-126 | Planar PCB | Cauli et al. [110]: impairs motor coordination at 2 months in males but not in female rats, reduces locomotor activity in females. |
| 31 | CB-132 | PCB | Uwinana et al. [111]: does not appear to affect dopaminergic cells in cultures or levels of dopamine. To be further studied. |
| 32 | CB-138 | PCB | Boix et al. [112]: exposition activates metabotropic glutamate receptors and that increases dopamine in females and reduces it in males. The opposite changes are observed for glutamate, in rat nucleus accumbens. |
| Campagna et al. [113]: Ca2+ homeostasis and androgen receptor signaling pathways are primarily disrupted in cerebellum proteome, contributing toward a premature ageing and neurotoxicity. | |||
| Naert et al. [114]: birds bioaccumulate in brain and the central nervous system. | |||
| 33 | CB-153 | PCB | Enayah et al. [115]: neurotoxic, and affects dopamine turnover in vitro. |
| Cauli et al. [110]: many motor alterations and induces hyperactivity at adulthood in rats. | |||
| Gascon et al. [116]: deleterious effects on neuropsychological development which are mainly attributable to prenatal exposure. | |||
| 34 | CB-169 | Planar PCB | Morse et al. [117]: local hypothyroidism occurs in the brains of fetal and neonatal rats exposed by increase in type II thyroxine 5'-deiodinase in the brain. |
| 35 | CB-180 | PCB | Boix et al. [112]: affects motor activity in rats; increased glutamate release in nucleus accumbens following activation of metabotropic glutamate receptors would be involved in reduced dopamine release. |
| Naert et al. [114]: birds bioaccumulate in brain and the central nervous system. | |||
| 36 | Chlordane | Organo-chlorine insecticide | Kilburn [118]: it is suggested that it causes protracted neurotoxicity in patients. |
| Kilburn and Thornton. [119]: exposure is associated with protracted impairment of neurophysiological and psychological functions. The central nervous system is the most important target. | |||
| Grutsch et al. [120]: the characteristic signs of acute toxicity are hypothermia, hyperexcitability, tremors and convulsions. In human, signs of acute toxicity are tremors and convulsions. | |||
| 37 | Chlordibromo-methane | Trihalo-methane | Villanueva et al. [121]: Minor associations observed between exposure during gestation and child neuropsychological development. |
| Balster et al. [122]: effect on operant behavior in mice. | |||
| 38 | Chlorinated Paraffins | Flame retardants, lubricants, plasticizers | Liu et al. [123]: exposure could alter gene expression in the hypothalamic-pituitary-thyroid axis. |
| Mariussen et al. [124]: neurobehavioral effects, indicating adverse effects on the central nervous system: alteration of neurotransmitter functions, Ca2+ homeostasis processes, induction of protein kinase C and phospholipase A2 mobilization, and oxidative stress. | |||
| Eriksson et al. [125]: significant decrease of presynaptically sodium-dependent choline uptake in mice. | |||
| 39 | Chlorpyrifos | Insecticide | Burke et al. [126]: acute exposure of humans irreversibly inhibit acetylcholinesterase, and chronic exposure induces neurological deficits that range from cognitive impairments to tremors in childhood |
| Yamada et al. [127]: inhibit neural induction via mitochondrial fusion protein mitofusin 1-mediated mitochondrial dysfunction in human stem cells. | |||
| Sogorb et al. [128]: seems able to induce neurodevelopmental alterations in animals | |||
| Lee et al. [101]: affects protein levels in the mice developing brain and induces persistent adult behavior and cognitive impairments; neurotoxic effects. | |||
| 40 | Citalopram | Antidepressant | Yeo et al. [129]: disability is improved. |
| Gaanderse et al. [130]: induces dyskinesia of the tongue. | |||
| Sprowles et al. [131]: selective serotonin reuptake inhibitor, alters spatial learning and memory, anxiety, depression in rats. | |||
| 41 | Clofentezine | Pesticide | Hurley [132]: induces thyroid follicular cell tumors in rodents; disrupts thyroid-pituitary homeostasis. |
| Acaracide | |||
| 42 | Coumaphos | Pharmaceutical | Abdelsalam [133]: inhibition of brain of acetylcholinesterase, inhibition of brain neurotoxic esterase, plus delayed neurotoxicity. |
| Abou-Donia et al. [134]: degeneration of axons and myelin in the spinal cord. | |||
| 43 | Coumestrol | Phytoestrogen | Jantaratnotai et al. [135]: suppression of interferon regulatory factor-1 and phosphorylated STAT1 expression in lipopolysaccharide-activated microglia. |
| 44 | D4 Cyclic siloxane | Material | Andreou et al. [136]: unusual constriction of the isolated sciatic nerve, death of nerve fibers. |
| 45 | D5 Cyclic siloxane | ||
| Fuzzard et al. [137]: due to silicone implants, myalgias, chronic fatigue, cognitive impairment. | |||
| 46 | D6 Cyclic siloxane | ||
| 47 | Daidzein | Isoflavones | Yu et al. [138]: perinatal exposure enhances estrogen receptor alpha expression in several brain regions such as stria terminalis, arcuate hypothalamic nucleus, and central amygdaloid nucleus. |
| Zeng et al. [139]: significant effects on locomotor activity, mood and social behavior after long-term consumption. | |||
| Jin et al. [140]: the neurotoxic effect of daidzein could be due to the inhibition of the GABA(A) receptor resulting in further enhancement of excitation by glutamate and leading to cellular damage in primary rat neuronal cultures. | |||
| Phytoestrogen | |||
| Román GC [28]: inhibits thyroperoxidase that catalyzes iodination and thyroid hormone biosynthesis | |||
| 48 | Dibromochloropropane | Pesticides | Teitelbaum [141]: the product is central nervous system depressant. |
| 49 | Desethylatrazine | Herbicide | Liu et al. [46]: neuroendocrine disruptor that impacts the expression of neurotoxicity-related genes such as Ache, Gap43, Gfap, Syn2a, Shha, Mbp, Elavl3, Nestin and Ngn1 in early developmental stages of zebrafish. |
| Metabolite | Gunderson et al. [142]: Strong positive correlation between CYP19 and SF-1 transcript abundance in exposed tadpoles during brain development. | ||
| Hossain et al. [143]: decreases striatal dopamine levels and in synaptosomes in rat. | |||
| 50 | 2,4-D | Herbicide agent orange | Yi et al. [144]: Increases various neurologic diseases; systemic atrophies affecting the nervous system, including spinal muscular atrophy, Alzheimer disease, and peripheral polyneuropathies. |
| Bortolozzi et al [145]: changes in various neurotransmitter systems, such as serotonin (5-HT) and dopamine (DA), were proposed to mediate some of the behavioral effects in rats | |||
| Evangelista de Duffard et al. [146]: Increases sensitivity in dopamine D2-like brain receptor from 2,4-dichlorophenoxyacetic acid (2,4-D)-exposed and amphetamine challenged rats. | |||
| 51 | 2,4-Dichlorophenol | Chlorophenol | Krieg [147]: At low concentrations, it may act at acetylcholine and γ-aminobutyric acid synapses in the central nervous system to modify neurobehavioral test performance. |
| 52 | 3-Diltiazem | Pharmaceutical | Stevens et al. [148]: Calcium channel blocker. |
| 53 | 2,4′-DDD (o,p’-DDD) | Organo-chlorine | Heilmann et al. [149]: Some central nervous disorders were observed. |
| Insecticide and pharmaceutical mitotane | Lanser et al. [150]: Neuropsychologic and neurologic side effects. | ||
| Du Rostu et al. [151]: neurological symptoms and neurotoxicity both central and peripheral. | |||
| 54 | 2,4′-DDT (o,p’-DDT) | Organo-chlorine | Kajta et al. [152]: depressive-like symptoms after prenatal exposure possibly by DNA hypomethylation and estrogen receptor signaling pathway. |
| Zhang et al. [153]: effects on glucocorticoid-receptors on the nervous system. | |||
| Eskenazi et al. [154]: Prenatal exposure to DDT, and to a lesser extent DDE, was associated with neurodevelopmental delays during early childhood. | |||
| Insecticide | |||
| Halldin [155]: altered the development of the neural system and resulted in demasculinization of male quail. | |||
| Fry [156]: impaired differentiation of the nervous system through mechanisms of hormonal mimicking of estrogens. | |||
| 55 | 4,4′-DDD (p,p’-DDD) | Organo-chlorine | Al-Saleh et al. [157]: low parent's evaluation of developmental status of infants was significantly associated with DDD in breast milk. |
| Insecticide | |||
| 56 | 4,4′-DDE (p,p’-DDE) | Organo-chlorine | Wnuk et al. [158]: stimulation of retinoid X receptor α and retinoid X receptor β-mediated intracellular signaling plays an important role in the propagation of DDE-induced apoptosis during early stages of neural development. |
| Insecticide | Cartier et al. [159]: p,p'-DDE exposure, both pre- and postnatally, during early childhood is associated with visual processing impairment later in life. | ||
| Eskenazi et al. [154]: Prenatal exposure to DDT, and to a lesser extent DDE, was associated with neurodevelopmental delays during early childhood. | |||
| 57 | 4,4′-DDT (p,p’-DDT) | Organo-chlorine | Kajta et al. [152]: depressive-like symptoms after prenatal exposure possibly by DNA hypomethylation and estrogen receptor signaling pathway. |
| Zhang et al. [153]: effects on glucocorticoid-receptors on the nervous system. | |||
| Eskenazi et al. [154]: prenatal exposure to DDT, and to a lesser extent DDE, was associated with neurodevelopmental delays during early childhood | |||
| Insecticide | |||
| Parent et al. [160]: DDT is involved in neuroendocrine disruption of the reproductive axis. | |||
| 58 | Di-(2-ethylhexyl) adipate | Plasticizer | Lee et al. [161]: inappropriate expression of granulin and/or p130 genes in the brains of male and female neonatal rats by perinatal exposure may exert permanent effects on the hypothalamus, thereby decreasing sexual behavior after maturation. |
| 59 | Dehydroepi-androsterone | Natural hormone | Arbo et al. [162]: neuroactive steroid that modulate neuronal and astroglial function and have neuroprotective effects. |
| Woda et al. [163]: synthetized in nervous cells, neuroactive local factor in the central nervous system and the periphery. | |||
| Yu et al. [164]: induced depression-like behavior in polycystic ovary syndrome mice, possibly through down-regulation of brain monoamines and/or their metabolites. | |||
| Li et al. [165]: provides robust ischemic neuroprotection but also exerts neurotoxicity when administered during ischemia and early reperfusion. | |||
| 60 | Dexamethasone | Synthetic steroid | Coplan et al. [166]: glucocorticoid-induced neurotoxicity. |
| Yu et al. [164]: protracted disruption of mental functions. | |||
| Lopes et al. [167]: affects the phosphorylation state of glutamate AMPA receptors in the human limbic system. | |||
| Feng et al. [168]: decreased both body and brain weight gain. Tapering and repeated doses increased caspase-3 activity. Impaired learning and memory capability at juvenile age. | |||
| Uno et al. [169]: induced degeneration and depletion of the hippocampal pyramidal and dentate granular neurons in the brains of primate fetuses. | |||
| 61 | Dibutyl phthalate | Phthalate | Wójtowicz et al. [158]: Aryl hydrocarbon receptor is involved in dibutyl phthalate induced apoptosis and neurotoxicity, while the estrogen receptors and peroxysome proliferator-activated receptor gamma signaling pathways are impaired by the phthalate. |
| Farzanehfar et al. [170]: could reduce total distance movement, impair memory function an induce anxiety in mice. Significant nuclei size reduction and condensation in dentate gyrus cells | |||
| Yan et al. [171]: link between oxidative stress and anxiety-like behavior produced by dibutyl phthalate at high doses. | |||
| 62 | Dibutyltin | Plastics stabilizer | Chantong et al. [172]: potentiation of oxidative stress and pro-inflammatory cytokine expression in microglia cells |
| Tsuji et al. [173]: Dibutyltin is neurotoxic and poly-L-lactides toxicity increases with the increase in tin concentration. | |||
| Jenkins et al. [174]: developmental neurotoxicant; the incidence of apoptotic cell death, was increased in the neocortex and hippocampus. | |||
| Kobayashi et al. [175]: synaptic parameters modulations; tributyltin metabolites inhibit various parameters of cholinergic activity with a potency ranking of tributyltin> dibutyltin> monobutyltin. | |||
| 63 | Dicofol | Organo-chlorine | Evangelista de Duffard et al. [146]: has effects on motor, sensory, or cognitive functions. |
| Insecticide | |||
| Lessenger et al. [176]: case report, neurological injury, cognitive and emotional difficulties persisted over an 18-mo period. | |||
| 64 | Dieldrin | Organochlorine | Cowie et al. [177]: disrupts proteins related to oxidative respiration and mitochondrial stress in the central nervous system. |
| Schmidt et al. [178]: induced neurotoxicity by impaired mitochondrial bioenergetics and endoplasmic reticulum stress in rat dopaminergic cells. | |||
| Babot et al. [179]: Long term exposure reduces gamma-aminobutyric acid type A and N -methyl—aspartate receptor function in primary culture of mouse cerebellar granule cells. | |||
| Insecticide | |||
| Evangelista de Duffard et al. [146]: motor sensory or cognitive function effects. | |||
| 65 | Diethyl hexyl phthalate | Phthalate | Luu et al. [180]: regulate microRNAs in a sex-specific manner which may interfere with proper hippocampal development in males and preserve hippocampal development in females. |
| Preciados et al. [48]: induced brain health deficits by NRF1 regulated gene networks. | |||
| Park et al. [181]:sex-dependent effect on anxiety proneness in childhood. | |||
| Quinnies et al. [182]: transgenerational modifications in the expression of several pituitary hormones involved in the hypothalamic-pituitary-adrenal axis and in stress hormones. | |||
| 66 | Mono‐2‐ethyl-hexyl phthalate | DEHP Hydrolysis product | Huang et al. [183]: prenatal exposure was associated with decreased cognitive development in the young children. |
| Téllez-Rojo et al. [184]: prenatal exposure creates sex specific neurodevelopmental effects. | |||
| 67 | Mono‐n‐butyl phthalate | DBP Hydrolysis product | Doherty et al. [185]: prenatal associations between urinary phtalates in aged mothers and brain performances in young children. |
| Mao et al. [186]: induce spatial cognitive deficits through altering the expression of apoptosis-related protein. | |||
| Won et al. [187]: increased exposure exhibited supralinear associations with social, thought and attention problems in children. | |||
| 68 | Diethylstilbestrol | Synthetic estrogen | Tomihara et al. [188]: developmental deficits may stem from both in utero toxicity and aberrant maternal care. |
| Frye et al. [189]: effects on the aryl hydrocarbon receptor, the peroxisome proliferator-activated receptor and the retinoid X receptor, signal transduction pathways, and on calcium influx and/or neurotransmitter receptors. | |||
| Sato et al. [190]: marked influence on synaptogenesis and neuronal vulnerability through mechanisms other than through estrogen receptors. | |||
| 69 | Diisononyl phthalate | Plasticizer | Ma et al. [191]: cause cognitive deficits and anxiety. |
| Peng L [192]: oral exposure of mice induced brain damage, and oxidative stress, inflammation, and apoptosis. | |||
| Boberg et al. [193]: behavioral effects, spatial learning effects in perinatally exposed rats. | |||
| 70 | Diphenhydramine | Antihistamine | Kim et al. [194]: Inhibitory effects on proton currents in microglial cells. |
| Mansfeild et al. [195]:reduced attention and increased self-reported drowsiness. | |||
| Wilken et al. [196]: caused significant decrements in vigilance and cognitive functioning. | |||
| 71 | Dimethyl-benz(a)anthracene | PAH | Vaswani et al. [197]: alterations of opioid neuropeptides such as beta endorphin, meth-enkephalin and dynorphin levels |
| 72 | Endosulfan (alpha/beta) | Organo-chlorine | Jang et al. [198]:induced acute neurotoxicity via induction of oxidative stress and pro-inflammatory responses. |
| Caudle WM [199]: can alter the normal development and potential function of neurotransmission in the frontal cortex | |||
| Insecticide | Silva et al. [200]: neurotoxicity and developmental effects in the zebrafish. | ||
| Silva et al. [201]:effects on brain biogenic amine levels Developmental reproductive toxicity or endocrine disruption occurs only at doses causing neurotoxicity. | |||
| 73 | Endrin | Organo-chlorine | Bagchi et al. [202]: induced lipid peroxidation and DNA damage in brain and regional distribution of catalase activity in rat brain. |
| Insecticide | Gray et al. [203]: alteration of central nervous system function in rats and hamsters even though endrin produces gross morphological defects only in hamsters. | ||
| 74 | Estradiol | Natural hormone | Li et al. [204]: anxiety disorders, augmentation of vulnerability factors associated with anxiety disorder development; and facilitation of the maintenance of anxious symptoms post-development. |
| Preciados et al. [48]: influences NRF1 regulated gene networks in the development of complex human brain diseases. | |||
| Perez-Alvarez et al. [205]: neuroprotective role after ischemic injury. | |||
| Rossetti et al. [206]: neurosteroid bind specific receptors to promote essential brain functions. | |||
| 75 | Estrone | Natural hormone | Mahmoud et al. [207]: may influence adult hippocampal neurogenesis, with a focus on cognitive function and mood regulation. |
| Grimm et al. [208]: may act upon neuronal bioenergetics in a delicate balance with an age-related effect that might be involved in mitochondrial dysfunction underlying neurodegenerative disorders. | |||
| 76 | Ethinylestradiol | Synthetic hormone | Porseryd et al. [209]: alteration in expression of genes involved in synaptogenesis and synaptic function. In female brains, produced significant effects on pathways connected to the circadian rhythm, cytoskeleton and motor proteins and synaptic proteins. In male brains effects on pathways related to cholesterol biosynthesis and synaptic proteins. |
| Preciados et al. [48]: influences NRF1 signaling pathways, and epigenomic multiple networks. | |||
| Zaccaroni et al. [210]: very low doses during development can affect key behavioral traits that are modulated by anxiety. | |||
| 77 | Ethylene thiourea | Herbicide | Wang et al. [211]: induced abnormal innervation patterns in the anorectum of fetal rats |
| Debbarh et al. [212]: neurotoxic in utero, increases sensitivity to genetic and environmental risk factors for cell death and apoptosis. | |||
| 78 | Ethylparaben | Antifungal | Merola et al. [213]: provoked behavioral changes including trembling of head, pectoral fins and spinal cord of zebrafish. |
| Preservative | |||
| 79 | Fadrozole | Pharmaceutical | Lynch et al. [214]: displayed significant fear generalization in rats. |
| Alward et al. [215]: this aromatase inhibitor reduced the motivation to sing as well as song acoustic stereotypy. | |||
| Xing et al. [216]:dopamine neuron degeneration and aromatase activity inhibition could be respectively achieved in vivo with treatments with the product in female goldfish. | |||
| Langlois et al. [217]: induced female- and male-biased sexual development on Silurana tropicalis brain mRNA levels, and reduced brain aromatase activity in frogs. | |||
| 80 | Fenbuconazole | Fungicide | Hurley et al. [132]: disrupts thyroid hormone excretion. |
| 81 | Fenitrothion | Organophosphate | Geraldi et al. [218]: affected the acquisition and, mainly, the retention of instrumental conditioning in rats. |
| Groszek et al. [219]: High concentration of the pesticides was found in adipose tissue and also in the brain. Respiratory failure was the syndrome; and inhibition of acetylcholinesterase activity persisted even for 30 days from poisoning. | |||
| Insecticide | |||
| Ram et al. [220]: Neurobehavioral changes in freshwater fish exposed | |||
| 82 | Fenoxycarb | Insecticide | Lenkic et al. [221]: allatostatin may be one of the effectors in the brain by which the pesticides inhibits juvenile hormone biosynthesis.in cockroach. |
| 83 | Finasteride | Pharmaceutical | Fertig et al. [222]: permanent sexual dysfunction and mood changes (fatigue, anxiety, depression and suicidal ideation) during treatment with this 5-alpha-reductase inhibitor. |
| Traish et al. [223]: Also non-sexual adverse effects such as diabetes, psychosis, depression, and cognitive function. | |||
| Ganzer et al. [224]: sexual libido, ejaculatory disorders, disorders of the penis and testes, cognitive symptoms, and psychological symptoms | |||
| 84 | Fipronil | Insecticide | Godinho et al. [225]: toxic interactions with the central nervous system of mammals and lead to memory impairment by modulating the GABAergic system. |
| Park et al. [181]: Progressive loss of nigrostriatal dopaminergic neurons induced by inflammatory responses to the pesticide. | |||
| Magalhães et al. [226]: acts on maternal aggressive behavior through GABA(A) receptors. | |||
| Simon-Delso et al. [227]: disrupting neural transmission in the central nervous system of invertebrates, inhibits neuronal receptors. | |||
| Marrs et al. [228]: 4-Aminobutyric acid (GABA) and glycine are inhibitory neurotransmitters and their antagonist, fipronil, is excitatory. | |||
| 85 | Fluoxetine | Pharmaceutical | Golub et al. [229]: provoked greater dendritic spine synapse density in prefrontal cortex of monkeys. |
| Hong et al. [230]: induced predominant sympatho-excitation and depressed parasympathetic activity leading to mild hypertension, tachycardia, and impairment of baroreflex function. | |||
| Sprowles et al. [131]: Differential effects of perinatal exposure to antidepressants on learning and memory, acoustic startle, anxiety, and open-field activity in rats. | |||
| 86 | Flutamide | Pharmaceutical | Yamada et al. [127]: the effects of postnatal treatment on brain masculinization were observed by analysis of male sexual behavior. |
| Svensson [231]: induced anxiolytic-like behavior in castrated rats | |||
| Zhang et al. [232]: Effects of neonatal treatment on hippocampal neurogenesis and synaptogenesis correlate with depression-like behaviors in preadolescent male rats. | |||
| Ahmadiani et al. [233]: Anticonvulsant effects on seizures involvement of benzodiazepine receptors. | |||
| 87 | Fonofos | Organo-phosphate | GK Sidhu, [234]: known to inhibit acetylcholinesterase activity, not only in insect, but in aquatic and terrestrial organisms leading to nervous abnormalities among others. |
| Insecticide | |||
| 88 | Formaldehyde | Solvent | Liu et al. (2018) [235]: Acute formaldehyde exposure induced early Alzheimer-like changes in mouse brain. Provoked the permeability of the blood-brain barrier, activation of astrocyte and microglia, oxidative stress and inflammation. |
| Li et al. [236]: effects on anxiety, depression-like behavior and cognition ability which may be associated with alterations in hippocampal glucocorticoid receptors and brain tyrosine hydroxylase levels. | |||
| Zendehdel et al. [237]: Its neurotoxic effect depend on acetylcholinesterase activity; provoked cholinergic signal reduction in cases of cognitive dysfunction. | |||
| Tulpule et al. [238]: contribute to the impaired cognitive performance and neurodegeneration in diseases. | |||
| Songur et al. [239]: neurotoxic characteristics; neurological diseases. | |||
| 89 | Furan | Solvent | Johnston et al [240]: exhibits a peculiar mode of attack on the central nervous system |
| 90 | Galaxolide | Synthetic musk | Ayuk-Takem et al. [241]: neurotoxicity may be associated with the inhibition of cellular; polyisoprenylated methylated protein methyl esterase activity; significant risk to individuals predisposed to developing degenerative disorders. |
| 91 | Genistein | Isoflavone | Patisaul HB [242]: involve action at nuclear estrogen receptors, effects on vasopressin, innervation of the lateral septum and other brain regions. |
| Luo et al. [243]: pretreatment significantly increased cell viability and protein kinase C activity, decreased the levels of intracellular calcium, and blocked caspase-3 activity in induced cells. | |||
| Román GC [28]: Transient in utero hypothyroxinemia is related to maternal flavonoid ingestion during pregnancy and may provoke autism. | |||
| Phyto-estrogen | |||
| Lee et al. [244]: mimic the actions and functions of estrogens on brain, two putative pathways; an estrogen receptor-mediated pathway and via the inhibition of tyrosine kinase. | |||
| Lephart et al. [245]: The specific influence of dietary soy phytoestrogens is identified on consumptive, learning and memory, and anxiety-related behaviors. | |||
| 92 | Hexabromocyclodo- decane | Flame retardant | Pham-Lake et al. [246]: Impairment in the mesohippocampal dopamine circuit following exposure. |
| Wang et al. [247]: main metabolic pathways perturbed, nervous system damage, and developmental disorders. | |||
| Maurice et al. [248]: Short-term effects of a perinatal exposure in rats provoked impairments of early locomotor activity and sensory development. | |||
| Al Mousa et al. [249]: inhibiting reticulum Ca(2+)ATPase in human neuroblastoma cells and induced cells death possibly causing neurological disorders. | |||
| Lilienthal et al. [250]: Effects on dopamine-dependent behavior and brainstem auditory evoked potentials in rats. | |||
| 93 | Hexachlorobenzene | Chlorinated | Fu et al. [251]: exhibits through its metabolite a neurotoxic effect by inducing oxidative stress-mediated inflammatory responses. |
| aromatic | |||
| Kyriklaki et al. [252]: High exposure during pregnancy reduction in working memory score and reduced cognitive development at preschool age. | |||
| Reed et al. [253]: exposure involved systemic impairment, as well as on nervous system. | |||
| Li et al. [254]: can induce enhanced lipid peroxidation on rats, and the oxidative stress plays an important role in the mechanism of neurotoxicity. | |||
| Goldey et al. [255]: behavioral teratogen, and suggests that human fetuses and suckling infants may be at risk because of the neurotoxic effects of the chemical. | |||
| 94 | Heptachlor | Organo-chlorine | Nyffeler et al. [256]: neural crest cell migration was inhibited by this toxicant disturbing a key neurodevelopmental process. |
| Christen et al. [257]: Strong and dose- dependent inhibition of neurite outgrowth was induced developmental neurotoxicity. | |||
| Hong et al. [258]: induced nigral dopaminergic neuronal loss and Parkinsonism-like movement deficits in mice. | |||
| Insecticide | |||
| Moser et al. [259]: perinatal exposure produced neurochemical and persistent neurobehavioral changes, including alterations in spatial learning and memory. | |||
| 95 | Heptachlor epoxide | Organo-chlorinei | Kirby et al. [260]: toxic effects of heptachlor epoxide may be responsible for loss of maximal dopamine uptake |
| Insecticide Metabolite | Yamaguchi et al. [261]: effects on calcium mediated transmitter release from brain synaptosomes of rats. | ||
| 96 | Hexachlorobutadiene | Solvent | Badaeva et al. [262,263]: neurotoxic effects in the postnatal period of ontogeny in the rats. |
| Murzakaev [264]: small doses affected central nervous activity. | |||
| 97 | Heptachloro-dibenzodioxin | Dioxin | Chen et al. [265]: synaptic plasticity and neuro-immune system may be two principal affected areas. |
| Kimura et al. [266]: over-activation of aryl hydrocarbon receptor following perinatal dioxin exposure, perturbs neuronal migration and morphological development in mammalian cortex, supporting previous observations of impaired dendritic structure, cortical dysgenesis, and behavioral abnormalities | |||
| 98 | HPTE | Methoxychlor | Not specifically studied (see Methoxyclor): |
| Metabolite | |||
| 99 | Iodine (I) | Halogen; Essential element | Román [28]: Iodine deficiency as a cause of autism. |
| 100 | Kepone | Organo-chlorine | Evangelista de Duffard et al. [146]: effects on motor, sensory, or cognitive function; developmental neurotoxicant. |
| Mactutus et al. [267]: Neonatal exposure impairs early learning and retention of active avoidance in the rat. | |||
| Insecticide | Mactutus et al. [268]: neurotoxic profile of tremor. | ||
| Mactutus et al. [269]: effect on the development of behavioral and/or neural function. | |||
| 101 | Lead | Heavy metal | Andrade et al. [270]: can induce dyshomeostasis, potentially triggering neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease Additionally, changes in heme synthesis have been associated with neurodegeneration. |
| Chen et al. [271]: exposure in the early stages of neurodevelopment results in long-lasting alterations that ultimately cognitive function and behavior. The prime targets of lead toxicity are the multipotent neural stem cells | |||
| Assi et al. [272]: wide spectrum of toxic effects, a real threat to the public health, including on the central nervous system | |||
| Karri et al. [49]: lead to imbalance between the pro-oxidant elements and the antioxidants, and induced cognitive dysfunction. | |||
| Caito et al. [273]: The central nervous system is particularly vulnerable. The brain accumulates metals. | |||
| 102 | Levonorgestrel | Synthetic Estrogen | Aleknaviciute et al. [274]: induces a centrally-mediated sensitization of both autonomic and hypothalamic-pituitary-adrenal (HPA) axis. |
| Simone et al. [275]: in combination with ethinyl estradiol reduced brain-derived neurotrophic factor mRNA in the hippocampus resulting in a decline in learning and memory. | |||
| Porcu et al. [276]: Long-term administration decreased allopregnanolone levels and altered GABA(A) receptor subunit expression and anxiety-like behavior. | |||
| 103 | Lindane | Organo-chlorine | Costa [277]: block the chloride channels of the GABA-A receptor. |
| Mariussen et al. [124]: has neurotoxic potentials after both acute and chronic exposure. | |||
| Insecticide | Evangelista de Duffard et al. [146]: has effects on motor, sensory, or cognitive function modifying behavior. | ||
| 104 | Linuron | Herbicide | Quintaneiro et al. [278]: adverse effects on neurotransmission and energy production, and interference with hypothalamic-pituitary-thyroid and -adrenal-axis. |
| Lichtensteiger et al. [279]: in antiandrogenic mixtures impacted genes encoding for components of excitatory glutamatergic synapses and genes controlling migration and pathfinding of glutamatergic and GABAergic neurons, as well as genes linked with increased risk of autism spectrum disorders. | |||
| Schinn et al. [280]: in mixture inhibited swimming activity of juvenile rainbow trout. | |||
| 105 | Malathion | Organophosphate | Richendrfer & Creton [281]: cause abnormalities in behavior and brain size during development, zebrafish larvae had significantly smaller forebrain and hindbrain regions. |
| Salama et al. [282]: affect proliferation, differentiation and viability of cultured neurospheres, | |||
| Hashjin et al. [283]: induced chronic toxicity and anxiety-like behavior in the male adult mouse. | |||
| Insecticide | |||
| Rastogi et al. [284]: In mixture provoked neurologic self-reported symptoms, headache, watering in eyes, and burning sensation in eye/face, cholinergic symptoms, such as insomnia, headache, muscle cramps, weakness, and anorexia, in children. High frequency of neurologic symptoms may be due to parasympathetic hyperactivity. | |||
| Valvassori et al. [285]: affects the central nervous system by inhibiting acetylcholinesterase, leading to an increase of acetylcholine in the synaptic cleft, and subsequent activation of cholinergic muscarinic and nicotinic receptors, and impairs aversive-memory retention but not non-associative memory, without affecting anxiety-related behaviors. | |||
| 106 | Mancozeb | herbicide | de Joode et al. [286]: poorer verbal learning outcomes in children, may affect their neurodevelopment. |
| Brody et al. [287]: behavioral dysfunction, notably serotonin-mediated egg-laying behavior in Caenorhabditis elegans. | |||
| Li et al. [288]: potentiation on KCNQ2 potassium channels might be the possible mechanism of this product toxicity in the nervous system. | |||
| Domico et al. [289]: acute exposure to high doses produces equipotent toxic effects in both dopamine and GABA neurons. | |||
| Kimura et al. [290]: nerve conduction velocities and postural sway seem to be sensitive indicators of the effects on the central and peripheral nervous system. | |||
| 107 | Manganese | Heavy metal | Lucchini et al. [291]: essential metal that plays a fundamental role for brain development and functioning. Environmental exposure may lead to accumulation in the basal ganglia and development of Parkinson-like disorders. |
| Peres et al. [292]: Various neurotransmitter systems may be impaired, especially dopaminergic, but also cholinergic and GABAergic. | |||
| Tarale et al. [293]: epigenetic mechanism in product-induced neurotoxicity, development of Parkinson’s disease. | |||
| Zhang et al. [293]: overexposure amplified the role of autophagy in the mechanisms of common neurodegenerative disorders. | |||
| 108 | Methylsulfonyl‐DDE | DDE Metabolite | Wnuk et al. [158]: apoptotic action during early stages of neural development with crucial involvement of retinoid X receptors. |
| Torres-Sanchez et al. [294]: prenatal exposure impaired early child neurodevelopment. | |||
| 109 | Methoxychlor | Organo-chlorine | Zhang et al. [153]: showed remarkable GR antagonistic properties, disruption of glucocorticoid-responsive genes. |
| Martini et al. [295]: perinatal exposure has an organizational effect on hippocampus-dependent memory and emotional behaviors. | |||
| Insecticide | |||
| Schuh et al. [296]: inhibited brain mitochondrial respiration and increases hydrogen peroxide production and CREB phosphorylation. | |||
| 110 | Methyl bromide | Fumigant, Pesticide | De Souza et al. [297]: acute and chronic progressive neurologic injury: seizures, myoclonus, ataxia or cerebral oedema, defective neurotransmitter function and abnormal oxidative phosphorylation. |
| Kim & Kang [298]: chronic toxic encephalopathy. | |||
| Yang et al. [299]: Sub chronically and chronically, principal target PAHsite appears to be the central nervous system. | |||
| Anger et al. [300]: produce slight neurotoxic effects in fumigation, reduced performance on all cognitive tests. | |||
| 111 | Methyl farnesoate | Juvenile Hormone | Moshitzky et al. [301]: neural inhibition from the brain (drosophila) act before farnesoic acid, a precursor of the product. |
| Prestwich et al. [302]: is secreted by the mandibular organs of crustaceans, role partially known. | |||
| 112 | Methyl triclosan | Triclosan metabolite Product | DeLeo et al. [303]: Effect on thyroid hormone action and stress in frog and mammalian culture systems. |
| 113 | Methylbenzylidene camphor | UV filter | Ruszkiewicz et al. [79]: potential neurotoxicity |
| Broniowska et al. [304]: affected the viability of nerve cells, most likely by enhancing the process of apoptosis. | |||
| Li et al. [305]: reduction of neuronal and muscular development in zebrafish embryos | |||
| Faass et al. [80]: effect on female sexual behavior and gene expression in sexually dimorphic brain regions after pre- and postnatal exposure in rats. | |||
| Maerkel et al. [306]:Sex- and region-specific alterations of progesterone receptor mRNA levels and estrogen sensitivity in rat brain | |||
| 114 | Methylcholanthrene | PAH | Singh et al. [307]: induces neurotoxicity in developing neurons derived from human stem cells by activation of aryl hydrocarbon receptor. |
| 115 | Mirex | Organo-chlorine | Puertas et al. [308]: showed a decrease in working memory in children. The deficit found in intellectual function during early childhood suggests that prenatal exposure may have a significant impact on school performance. |
| Insecticide | Shankland [309]: enhanced the release of neurotransmitters. Direct evidence is available on cholinergic and glutaminergic junctions, but other kinds of junctions may be affected. | ||
| 116 | Monosodium glutamate | Food Additive | Foran et al. [310]: Auditory hindbrain atrophy and anomalous calcium binding protein expression after neonatal exposure. |
| Sadek et al. [311]: induced neurotoxicity by cholinergic dysfunction, Bcl-2/Bax balance, and antioxidant enzymes gene transcripts in rats | |||
| Sasaki-Hamada et al. [312]: Changes in hippocampal synaptic functions and protein expression in obese mice. | |||
| Lau et al. [313]: glutamate excitotoxicity has also been linked to chronic neurodegenerative disorders such as amyotrophic lateral sclerosis; evidence for the product excitotoxicity in acute neurologic diseases | |||
| 117 | n-Butylbenzene | Chemical Synthesis | Chalansonnet et al. [314]: a decrease in the concentrations of free malondialdehyde in brain structures was observed after acute administration of this product. |
| Intermediate | |||
| 118 | Nicotine | Alkald | England et al. [315]: exposure during pregnancy and adolescence may contribute to cognitive and behavioral deficits in later life. Exposure during adolescence is associated with deficits in working memory, attention, and auditory processing, as well as increased impulsivity and anxiety. |
| Ferrea et al. [316]: Neuroprotective and neurotoxic effects, modifications of cholinergic transmission. | |||
| Ciani et al. [317]: neurotoxicity on rat habenulo-interpeduncular cholinergic neurons. | |||
| 119 | Nonachlor | Organochlorine | Lee et al. [318]: chronic exposure to low doses linked to the risk of developing cognitive impairment in elderly. |
| Insecticide | Kim et al. [319]: key role in the development of hypertension-related cognitive impairment. | ||
| 120 | Nonylphenol | Formulant | Litwa et al. [320]: RXRα, PXR and CAR xenobiotic receptors mediate the apoptotic and neurotoxic actions of the product in mouse hippocampal cells. |
| Tabassum et al. [321]: potential risk of cognitive, neurochemical and histopathological perturbations; induced toxicity in frontal cortex and hippocampus of rat brain. | |||
| Jie et al. [322]: inhibited neuronal development and differentiation as indicated by the reduction of the neurotrophic factor GAP-43. | |||
| Couderc et al. [323]: perinatal exposure induced behavioral and neuro-developmental impairments. | |||
| 121 | Norfluoxetine | Pharmaceutical | Pinna et al. [324]: selective brain steroidogenic stimulant, reduced post-traumatic stress disorder -like behavior in mice |
| Pinna et al. [325]: facilitated GABA(A) receptor neurotransmission and effectively ameliorate emotional and anxiety disorders and depression. | |||
| Matsumoto et al. [326]: non-serotonergic mechanism of action in mood and anxiety disorders. | |||
| Pinna et al. [327]: stereo specifically and selectively increase brain neurosteroid content. | |||
| 122 | Octachlorodibenzo- p- dioxin | Dioxin | Tawara et al [328]: fetal growth may be influenced by maternal total exposure to dioxins |
| 123 | Octachlorostyrene | Chlorinated | Chu et al. [329]: 90-day toxicity in the rat: effects on thyroid. |
| Aromatic | Chu et al. [330]: long-term toxicity in the rat: effects on thyroid. | ||
| 124 | Octyl-methoxycinnamate | UV filter | Ruszkiewicz et al. [79]: neurotoxic effect of active ingredients in sunscreen products. |
| Axelstad et al. [331]: effects on auditory and neurological development of rat offspring. | |||
| 125 | Octylphenol | Formulant | Bianco et al. [332]: greater accumulation in the cerebral cortex, more accumulation in the cerebellum compared to the mesencephalus and thalamus, with consequences to neural behaviour. |
| Ghisari et al. [333]: negative impact on fetal brain development, resulting in cognitive dysfunctions. | |||
| Shikimi et al. [334]: promote Purkinje dendritic growth during neonatal life, may be mediated by estrogen receptor in the Purkinje cell. | |||
| 126 | Oxychlordane | Chloridane | Kim et al. [335]: role of background exposure in the development of dementia should be explored |
| Kim et al. [336]: greater cognitive decline with aging among elders with high serum concentrations | |||
| Metabolite | |||
| Jain [337]: total serum thyroxine levels had an inverse association with the product. | |||
| 127 | Parathion | Organo-phosphate |
Slotkin et al. [56]: produced a net increase in norepinephrine emerged over the course of development in brain region. Liu et al. [338]: effects on endocannabinoid and endocannabinoid-like lipid metabolites in rat striatum. |
| Beard et al. [339]: positively associated with depression in. male private pesticide applicators in the agricultural health study. | |||
| Insecticide | |||
| 128 | Pendimethalin | Herbicide | Lerro et al. [340]: long-term exposure may alter thyroid function among male pesticide applicators. |
| Campillo et al. [341]: Biomarkers indicative of neurotoxicity and physiological stress in caged clams exposed to a contaminated water containing the product. | |||
| Pan et al. [342]: thyroglobulin decreased in rats thyroid cells after exposure. | |||
| 129 | Pentachlorobenzene | Chlorinated | Den Besten et al. [343]: severe effects on rats thyroid. |
| Aromatic | |||
| 130 | Pentachloro-nitrobenzene | Herbicide | Hurley PM [132]: disrupted thyroid-pituitary homeostasis |
| 131 | Pentachlorophenol | Herbicide, fungicide | Cheng et al. [344]: affected the timing and coordination of development in the central nervous system. |
| Krieg [147]: may act at acetylcholine and γ-aminobutyric acid synapses in the central nervous system. | |||
| Roze et al. [75]: worse coordination, less sensory integrity, worse attention, and worse visuomotor integration. | |||
| Jorens etal. [345]: increased risk for nasal carcinoma | |||
| 132 | Perchlorate | Oxidizer | Steinmaus et al. [346]: affected thyroid hormone production during pregnancy and fetal neurodevelopment. |
| Brent GA [347]: exposure in pregnancy impacted cognitive outcomes in children | |||
| Gilbert et al. [348]: developmental exposure altered synaptic transmission in hippocampus of the adult rat. | |||
| 133 | Permethrin | Insecticide | Hossain et al. [349]: may directly activate microglial cells and may contribute to neurodegeneration. |
| Nasuti et al. [350]: decreased levels of dopamine in the striatum, loss of dopaminergic neurons in the substantia nigra pars compacta and cognitive impairments. Motor coordination defects appeared at adult age after early life exposure. | |||
| Zakirova et al. [351]: persistent neuroinflammation, neurobehavioral and neuropathological cognitive impairment in mouse. | |||
| Yang et al. [352]: significant effects on the central nervous system. | |||
| 134 | Perfluorodecane sulfonic acid | Perfluoroalkyl substance | Ren et al. (2016) [353]: Binding interactions with thyroid hormone transport proteins and potential toxicological implications. |
| 135 | Perfluorohexane sulfonic acid | Perfluoroalkyl substance | Oulhote et al. [354]: High serum concentrations at ages 5- and 7-years, but not prenatally, were associated with parent-reported behavioral problems at age 7. |
| Ren et al. [353]: Binding interactions with thyroid hormone transport proteins and potential neurotoxicological implications. | |||
| 136 | Perfluorononanoic acid | Perfluoroalkyl substance | Jantzen et al. [355]: males exposed showed a reduction in total distance traveled and time of immobility, and an increase in thigmotaxis behavior, aggressive attacks, and preference for the bright. Acute, embryonic exposure resulted in significant biochemical and behavioral changes in young adult zebrafish. |
| Lien et al. [356]: prenatal exposure was found to associate with neurobehavioral symptoms related to attention deficit hyperactivity disorder among Asian seven-year-old children. | |||
| Oulhote et al. [354]: sex-dimorphic associations between concentrations and strengths and difficulties. | |||
| 137 | Perfluorooctanoic acid | Perfluoroalkyl substance | Jantzen et al. [355]: embryonic exposure resulted in significant biochemical and behavioral changes in young adult zebrafish. |
| Oulhote et al. [354]: significant associations were found in regard to hyperactivity, peer relationship, and conduct problems, as well as internalizing and externalizing problems and autism. | |||
| 138 | Perfluorooctane sulfonate | Perfluoroalkyl substance | Ge et al. [357]: could significantly reduce the cell viability and mediate cell apoptosis in HAPI microglia cells of rat. |
| Jantzen et al. [355]: embryonic exposure resulted in significant biochemical and behavioral changes in young adult zebrafish. | |||
| Oulhote et al. [354]: significant associations were found in regard to hyperactivity, peer relationship, and conduct problems, as well as internalizing and externalizing problems and autism. | |||
| 139 | Perfluorooctanesulfonyl fluoride | Perfluoroalkyl substance | Ren et al. [353]: Binding interactions with thyroid hormone transport proteins and potential neurotoxicological implications. |
| 140 | Phorate | Organo-phosphate | Starks et al. [358]: associated with better verbal learning and memory |
| Vandana et al. [359]: obvious effect on cholinesterase enzyme profile of olfactory bulb of mice after systemic administration of low doses for long terms. | |||
| Insecticide | |||
| 141 | Picloram | Herbicide | Reddy et al. [360]: decreased neuronal branching and degenerating neurons, probably through a mitochondrial pathway. |
| 142 | Polyvinylchloride | Polymer; PVC | Meshchakova et al. [361]: provoked functional disorders risk connected with cardiovascular and nervous diseases. |
| Podoll et al. [362]: acute intoxication resulted in vertigo, nausea and headache up to a narcotic effect. In patients with chronic occupational exposure, neurological disturbances included sensory-motor polyneuropathy, trigeminal sensory neuropathy, slight pyramidal signs and cerebellar and extrapyramidal motor disorders. Psychiatric disturbances present as neurasthenic or depressive syndromes. Sleep disorders and disorders of sexual functions are frequently encountered. | |||
| 143 | 8-Prenylnaringenin | Prenylflavonoid | Urmann et al. [363]: neurodifferentiating potential of the product. |
| Bagatin et al. [364]: panicolytic effects in rats with generalized anxiety and panic disorders. | |||
| Oberbauer et al. [365]: promote neuronal differentiation and neurite outgrowth and are neuroprotective. | |||
| 144 | Procloraz | Fungicide | Vinggaard et al. [366]: agonizes the aryl hydrocarbon receptor and inhibits aromatase activity. |
| Ghisari et al. [333]: inhibitory effect on rat pituitary cell growth increasing the risk or a negative impact on fetal brain development, resulting in cognitive dysfunctions. | |||
| 145 | Procymidone | Fungicide | Xiang et al. [367]: potential to disrupt thyroid homeostasis, agonistic effects. |
| 146 | Prodiamine | Herbicide | Radio et al [368]: selectively increased neurite outgrowth. |
| 147 | Propylthiouracil | Thyroid inhibitor | Gilbert et al. [369]: an impaired capacity for hippocampal neurogenesis may contribute to impairments in synaptic plasticity and cognitive deficits |
| Koromilas et al. [370]: inhibition of hypothalamic, pontine and cerebellar NaK-ATPase; a major marker of neuronal excitability and metabolic energy production as well as a regulator of important systems of neurotransmission. | |||
| Koromilas et al. [371]: impairs neurochemical mechanisms that could be involved in the way clinical hypothyroidism could affect optimal neurodevelopment and, ultimately, cognitive function. | |||
| Vanek et al. [372]: induced central nervous system vasculitis presenting as confusion. | |||
| 148 | Pyrene | Polycyclic Aromatic Hydrocarbon | Yang et al. [58]: Benzo[a]Pyrene (BaP) exposure caused the disruption of glutamate (Glu) neurotransmitter transmission by decreasing the level of Glu, reducing the expression of Glu receptors, enhancing the level of SNAP-25, and neurotoxicity. |
| Chepelev et al. [59]: BaP correlates with impaired learning and memory in adults, and poor neurodevelopment in children. Neurotoxic endpoints and DNA damages are more sensitive than cancer endpoints. | |||
| Chen et al. [373]: behavioral impairments resulting from postnatal BaP exposure are potentially long-lasting in rats. | |||
| Wormley et al. [374]: neurobehavioral deficits; gestational exposure to BaP and dioxin reduced specific indices of learning and memory, including hippocampal-based synaptic plasticity mechanisms. | |||
| Takeda et al. [375]: the fetal exposure of mice to diesel exhaust affected the emotional behaviors associated with the serotonergic and dopaminergic systems in the brain | |||
| 149 | Pyrimethanil | Fungicide | Hurley PM [132]: disrupt thyroid-pituitary homeostasis only |
| 150 | Pyriproxyfen | Juvenile hormone analog | Truong et al. [376]: induced craniofacial defects in zebrafish, and adverse behavioral effects. |
| Fourrier et al. [377]: changes in social integration, acceptance by nestmates and social behaviors performance in bees. | |||
| 151 | Resorcinol | Disinfectant, Chemical intermediate | Motonaga et al. [378]: inhibit thyroid peroxidase to cause developmental toxicity and neurotoxicity. |
| Román [28]: transient maternal hypothyroxinemia resulting from dietary and/or environmental exposure to this antithyroid agent. | |||
| 152 | Roundup | Main herbicide worldwide | Defarge et al. [20]: its formulants decrease aromatase activity below toxic levels. |
| Gress et al. [379]: the product altered locomotor activity in rats. | |||
| Modesto et al [380]: it inhibits acetylcholinesterase in fish brain. | |||
| 153 | Sertraline | Psychotropic | Lee et al. [381]: the product is used for trauma-focused psychotherapies |
| Frölich et al. [382]: selective serotonin reuptake inhibitor, which has demonstrated efficacy on neuropsychiatric behavioral symptoms in general. | |||
| 154 | Short chain chlorinated paraffins | Flame retardant; plasticizer | Liu et al. [123]: exposure could alter gene expression in the hypothalamic-pituitary-thyroid axis and thyroid hormone levels. |
| Wyatt et al. [383]: potent peroxisome proliferators; high dose shows a depressed plasma thyroxine level, with increase in thyroid stimulating hormone | |||
| 155 | 2,4,5‐T (in Agent Orange) | Herbicide | Gunderson and Daroff [384]: epilepsy and later on all effects of brain injury and post-traumatic stress disorders. |
| St Omer et al. [385]: together with 2.4D, increased significantly the concentration of norepinephrine in whole developing brain and increased dopamine. | |||
| Yi et al. [144]: increased the prevalence of endocrine disorders, especially in the thyroid and pituitary gland; and increased various neurologic diseases. | |||
| 156 | Tamoxifen | Pharmaceutical | Denk et al. [386]: granular neurons of the olfactory bulb and dentate gyrus, vascular cells and ependymal cells throughout the brain, and peripheral sensory neurons are modified by this treatment. |
| Boele et al. [387]: Cognitive domains that rely on verbal abilities (verbal memory and fluency) seem to be at risk for deterioration after treatment. | |||
| 157 | Tetrabromo- bisphenol A |
Flame retardant | Park et al. [388]: induced the loss of both zebrafish neuromasts and hair cells in the rat cochlea in a dose-dependent manner. |
| Chen et al. [389]: induced apoptotic cell death, delayed cranial motor neuron development, inhibited primary motor neuron development and loosed muscle fiber during the early development in zebra fish. | |||
| Jarema et al. [390]: may have developmental or pharmacological effects on the vertebrate nervous system. | |||
| Wojtowicz et al. [391]: decreased the expression of PPAR-γ protein in neocortical neurons; and the mechanism of action also induced apoptotic and neurotoxic effects. v | |||
| 158 | testosterone | Natural hormone | Holmes et al. [392]: testosterone increased the expression of COX2 and apoptosis in dopamine neurons, increased incidence of Parkinson's disease in men compared with women. |
| Cunningham et al. [393]: induces dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase C-delta. | |||
| 159 | Tetrachloro-dibenzofuran | Chlorinated dioxin | Xu et al. [394]: this dioxin-like compound suppresses acetylcholinesterase activity via transcriptional downregulations in vitro. |
| 160 | Tetrachloro-dibenzo-p- dioxin | Chlorinated dioxin | Pelclova et al. [395]: neurological and neurophysiological findings in workers with chronic intoxication 50 years after exposure. |
| Xu et al. [394]: this dioxin suppresses acetylcholinesterase activity via transcriptional downregulations in vitro. | |||
| Sánchez-Martín et al. [396]: aryl hydrocarbon receptor-dependent induction of apoptosis by the product in cerebellar granule cells from mouse. | |||
| 161 | PCB methyl sulfones | PCB metabolite | Kato et al. [397]: reduction of thyroid hormone levels by different mechanisms. |
| 162 | Tetraiodothyronine | Natural hormone * | Chen et al. [398]: inhibition of UDP-glucuronosyltransferases. |
| Dos Reis-Lunardelli et al. [399]: can alter animal behavior and learning and memory in rats. | |||
| Zamoner et al. [400]: reorganizes the cytoskeleton of glial cells through Gfap phosphorylation and Rhoa-dependent mechanisms. | |||
| 163 | Thiazopyr | Herbicide | Hurley PM [132]: disrupts thyroid-pituitary homeostasis only. |
| 164 | Toxaphene | Organo-chlorine | Calciu et al. [401]: congeners products showed a strong inhibitory effect on the otic system development. |
| Waritz et al. [402]: increases the occurrence of two thyroid tumors and increased excretion of thyroid hormones. | |||
| Insecticide | Kodavanti et al. [403]: inhibits calmodulin activated adenylate cyclase in rat brain. | ||
| Brunström. [404]: affected the growth of the chicks and had neurotoxic effects. | |||
| 165 | 2,4,6-Tribromophenol | BFR, Natural product | Leonetti et al. [405]: accumulate in the placenta and potentially alter thyroid hormone function in a sex-specific manner. |
| Lee et al. [406]: thyroid gland activity decreased, disrupted homeostasis and interfered with thyroid hormone system. | |||
| Lyubimov et al. [407]: developmental neurotoxicity and immunotoxicity in rats. | |||
| 166 | Trenbolone | Anabolic steroid | Quinn et al. [408]: disrupted development of either the central nervous system or the hypothalamic-pituitary-gonadal axis. |
| 167 | Tributyltin | Fungicide | Ishihara et al. [409]: induces oxidative neuronal injury. |
| Frye et al. [189]: effects through the aryl hydrocarbon receptor, the peroxisome proliferator-activated receptor and the retinoid X receptor, signal transduction pathways, calcium influx and/or neurotransmitter receptor. | |||
| Kotake [410]: neurotoxic, induces behavioral abnormalities and toxic to the developing central nervous system through AMPA receptor subunit. | |||
| 168 | Trichloroethylene | Chlorinated solvent | Yeung [411]: neurotoxicity inducing anxiety in man. |
| Da Broi et al. [412]: produces pleasant inebriating effects with rapid dissipation, followed by central nervous system depression, coma. | |||
| Kang et al. [413]: provokes chronic central nervous system disorders and peripheral neuropathy. | |||
| Chiu WA et al. [414]: carcinogenic to humans by all routes of exposure and toxic to the central nervous system. | |||
| Bale et al. [415]: interacts directly with several different classes of neuronal receptors by generally inhibiting excitatory ions receptors/channels and potentiating the function of inhibitory receptors/channels. | |||
| 169 | Trichlorophenol | Fungicide | Xu et al [416]: urinary levels increased risk of attention deficit hyperactivity disorder among school-aged children. |
| 170 | Triclocarban | Antibacterial agent | Dong et al. [417]: altered expression of proteins involved in nervous system development. |
| Barros et al. [418]: modified chronically female amphipod Gammarus behavior. | |||
| Wu et al. [419]: inhibited iodide uptake, but had differential effects on the expression of thyroid hormone synthesis-related genes and the activity of thyroid peroxidase. | |||
| 171 | Triclosan | Antibacterial agent | Ruszkiewicz et al. [79]: few neurotoxic effects. |
| Szychowski et al. [420]: activates aryl hydrocarbon receptor-dependent apoptosis and affects Cyp1a1 and Cyp1b1 expression in mouse neocortical neurons. | |||
| Wu et al. [419]: inhibited iodide uptake, but had differential effects on the expression of thyroid hormone synthesis-related genes and the activity of thyroid peroxidase. | |||
| 172 | Tri‐iodothyronine | Natural thyroid hormone | Noda [421]: impairs glial function as well as neuronal function and thus disturb the brain, which may cause mental disorders. |
| Zhang et al. [422]: affects feeding behavior. | |||
| Ortiga-Carvalho et al. [423]: regulates by negative feedback the hypothalamus pituitary-thyroid axis. | |||
| 173 | Triphenyl phosphate | Flame retardant | Sun et al. [424]: Developmental neurotoxicity in early life stages of Japanese medaka. |
| Kim et al. [425]: up-regulated the expression of the genes related to the metabolism, transport, and elimination of thyroid hormones. | |||
| Jarema et al. [390]: produced behavioral effects; may have developmental or pharmacological effects on the vertebrate nervous system. | |||
| Tanaka et al. [426]: induced shrinkage and increased packing densities, axonal and terminal degenerations in the developing visual system of the ferret. | |||
| 174 | Triphenyltin | Fungicide | Kotake [410]: potent inhibitor of mitochondrial ATP synthase, neurotoxic and induces behavioral abnormality. |
| Karpiak et al. [427]: disrupts components of glutamate homeostasis in rat astrocyte cultures. | |||
| Lin et al. [428]: triphenyltin acetate may cause cellular dysfunction of brain without structural damage and provoked demyelinated neuropathy. | |||
| Attahiru et al. [429]: produced significant central nervous system and respiratory depressions and produced brain congestion. | |||
| Lehotzky et al. [430]: crosses the blood-brain barrier, and induces diminished plasticity of the central nervous system. | |||
| 175 | Venlafaxine | Psychotropic | Belovicova et al. [431]: stressed rat dams had lowered hippocampal neurogenesis, while the product treatment reversed this lowering. |
| Singh et al. [432]: induced ROS-mediated apoptotic neurodegeneration in fetal neocortex, and neurobehavioral sequelae in rat offspring. | |||
| Pinzani et al. [433]: it is an antidepressant that selectively inhibits serotonin reuptake and is a norepinephrine inhibitor. | |||
| 176 | Vinclozolin | Fungicide | Gillette et al. [434]: Debilitating effects were seen at all levels of the phenotype, including physiology, behavior, brain metabolism, gene expression, and genome-wide transcriptome modifications in specific brain nuclei, and transgenerational effects. |
| León-Olea et al. [13]: can lead to severe and widespread neuroendocrine disruptions in discrete brain regions, including the hippocampus, amygdala, and hypothalamus, resulting in behavioral changes in a wide range of species. | |||
| Skinner et al. [435]: Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior are modified by the product. | |||
| André et al. [436]: learning deficits are expressed following perinatal exposure. | |||
| 177 | Zearalenone | Mycotoxin | Makowska et al. [437]: affects calcitonin gene related peptide-like immunoreactive neurons in the pig enteric nervous system. |
| Obremski et al. [438]: induced changes in the lymphoid tissue and mucosal nerve fibers in the porcine ileum. | |||
| Venkataramana et al. [439]: showed a marked suppressive effect on the neuronal gene expression; oxidative stress is the main upstream signal leading to increased neurotoxicity due to the product. |
Each chemical compound or pollutant has been numbered (Nb) out of 177 known endocrine disruptors; its name was associated with the key word “nervous” or “neurotoxicity” or “cognitive” or “behavio(u)r” on PubMed data bank, or eventually on Google Scholar. When the number of references per compound were too numerous, “or” was excluded in order to directly associate the keywords. If more than 20 references were found to be published, “review” was added to the keywords and cited as a reference. Finally, a maximum of five references were indicated, focusing on the most recent research in humans or mammals, without excluding other models. The mechanisms of nervous disruption could be direct, on the neurons or the nervous system, or indirect, through endocrine disruption interfering with neurodevelopment or nervous system functioning, including thyroid regulation. PAH, polycyclic aromatic hydrocarbon; PCB, polychlorobiphenyl; PBB, polybromobiphenyl; PBDE, polybrominated diphenyl ether; PFAS, perfluoroalkyl substances.
Epidemiology is not technically adapted to solve the questions on combined and long-term effects of molecules or mixtures on mammalian or human health [Mesnage et al. [8]]; this becomes further complicated when epigenetic and transgenerational impacts are studied [Skinner and Anway [9]]. For instance, pesticide accumulation is rarely measured in organs after death in order to ascertain whether they can be used as markers to correlate their levels with pathologies. Instead, the understanding of endocrine disruption may be aided by advances in the combined knowledge of biochemical, cellular, organic and environmental effects in experimental animal models, farm animals, wildlife observations in contaminated areas, and occupational medicine in factories producing the chemicals in question.
The endocrine system is not limited to the control of sexual reproduction and development. Endocrine disruptors may affect the thyroid as well as the glucocorticoid axis, adrenal and pancreatic systems, adipose tissue and immune or neuroendocrine targets [Laessig et al. [10]; Masuo and Ishido [11]; Weiss [12]; Leon-Olea et al. [13]]. They even possess cognitive effects [Schantz and Widholm [14]], particularly via various neuromediator interferences.
Cells communicate via either chemical or electrical signals that are transmitted within the endocrine or nervous systems. Generally, in endocrinology, hormones may have a biphasic action dependent on the receptors’ availability and concentration, resulting in time-, dose- and sex-dependent effects that vary according to the targeted tissue and the specific organism. It is thus reductionist to say, for instance, that a hormone such as estradiol “stimulates ovulation”, since it can inhibit this function when used at pharmacological doses as a pill or during embryonic or foetal life. Endocrine disruptors may thus possess the potential of exerting similar biphasic ambivalent effects.
Thus, to determine whether hormonal disruptors may also interfere directly or indirectly with neural development [Grandjean and Landrigan [15]] or functioning even in adults, either through a neuroendocrine or a more general mechanism, this study examined the scientific literature to ascertain the effects of ENDs on the nervous system —including neurotoxicity, cognition, and behaviour— of the major internationally identified (according to WHO) endocrine disruptors.
2. Materials and methods
Each compound was numbered (Nb, Table 1) out of 176 known endocrine disruptors [WHO [6]], plus Roundup. Its name was associated with the keyword “nervous” or “neurotoxicity” or “cognitive” or “behavio(u)r” on the PubMed data bank, and eventually on Google Scholar. When the references were too numerous, “or” was excluded in order to directly associate the keywords. If more than 20 references were found to be published, “review” was added to the keywords and cited. Finally, a maximum of five references were indicated, focusing on the most recent research in humans or mammals, without excluding other models. The mechanisms were documented (Table 1, column 4) as direct effects on the neurons or the nervous system, or as indirect effects, including thyroid regulation.
3. Results and discussion
All of the 176 internationally identified EDs (as per WHO guidelines) were studied in the international bibliography published between 1931 and 2021, in order to assess their effects on the nervous system (Table 1). It was observed that every single one of the EDs induced neurodisruption or clear neuromodulation, while even stimulation occurred in certain rare cases. Thus, 100 % of the EDs studied were known neuromodulators, either directly or indirectly. Previously, it was generally believed that endocrine disruptors with a neuronal impact functioned via the thyroid gland, which is classically known to control nervous system development. However, this study presents substantive evidence that at least 79.1 % of the EDs are, in fact, also NDs through various other mechanisms (Table 1). 37 compounds out of the total were implicated to possess a thyroid-dependent mechanism. Therefore, the emergence of this overarching concept compelled us to propose the introduction and use of a collective abbreviation “ENDs” for endocrine and nervous disruptors.
Therapeutic hormones or pharmaceutical products (see column 3 in Table 1) that may exist as pollutants in rivers [Arya et al. [16]; Saussereau et al. [17]; Goulle et al. [18]] often comprise ENDs—such as certain natural phytoestrogens that are more biodegradable than stable petrochemistry-based xenobiotics—often resulting in bioaccumulation within organisms. Mixtures of chemicals such as Agent Orange or dioxins have also been cited as ENDs. In addition, formulants of pesticides—such as POEA (polyoxyethylene tallow amine)—have been discovered to be EDs more recently [Gasnier et al. [19]; Defarge et al. [20]] and may also possess nervous effects [Malhotra et al. [21]; Sato et al. [22]]. In some models, glyphosate alone [Martinez et al. [23]; Coullery et al. [24]], known as the declared active ingredient of a major pesticide formulation used across the world, has been shown to be less toxic or disruptive to the nervous system than its equivalent formulants present in Roundup [Mesnage et al. [8]; Aitbali et al. [25]; Gallegos et al. [26]]. This also proves to be true for non-glyphosate-based herbicides containing non-declared polycyclic aromatic hydrocarbons and heavy metals that are individually identified as EDs [Seralini and Jungers [27]].
The impacts of ENDs on physiological functions and pathologies appear to be less specific than expected, especially since they interact with several intracellular and intercellular communications simultaneously. A majority of them are derived from petroleum residues, and their mechanisms of action can be likened to those of “spam” in electronic communications technologies. Thus, ENDs can be considered as a widespread instance of spam in a biological context.
Declaration of Competing Interest
The authors Seralini & Jungers declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Nicolas Defarge for the initiation of this work and Frederique Hilary for secretarial assistance in the early stages. We would also like to extend our gratitude to Joël Spiroux de Vendômois for his work on this topic. Funds for the bibliographic study were provided by Léa Nature and Biocoop Foundations. This work was supported by the University of Caen Normandy, Network on Risks, Quality and Sustainable Development, as well as Spark-Vie, France.
Handling Editor: DR. Aristidis Tsatsakis
References
- 1.Colborn T. Pesticides--how research has succeeded and failed to translate science into policy: endocrinological effects on wildlife. Environ. Health Perspect. 1995;103(suppl 6):81–85. doi: 10.1289/ehp.95103s681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindström G. Workshop on perinatal exposure to dioxin-like compounds. I. Summary. Environ. Health Perspect. 1995;103(suppl 2):135–142. doi: 10.1289/ehp.103-1518837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsburg J. Tackling environmental endocrine disrupters. Lancet (London, England) 1996;347(9014):1501–1502. doi: 10.1016/s0140-6736(96)90667-4. [DOI] [PubMed] [Google Scholar]
- 4.Carson R. 1962. Silent Spring Houghton Mifflin. Boston, MA, USA. [Google Scholar]
- 5.Seralini G.E. Flammarion; Paris: 2003. Génétiquement Incorrect. [Google Scholar]
- 6.Bergman Å. World Health Organization; 2013. State of the Science of Endocrine Disrupting Chemicals 2012. [Google Scholar]
- 7.Richard S. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ. Health Perspect. 2005;113(6):716–720. doi: 10.1289/ehp.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesnage R. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015;84:133–153. doi: 10.1016/j.fct.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Skinner M.K., Anway M.D. Seminiferous cord formation and germ-cell programming: epigenetic transgenerational actions of endocrine disruptors. Ann. N. Y. Acad. Sci. 2005;1061:18. doi: 10.1196/annals.1336.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laessig S.A., McCarthy M.M., Silbergeld E.K. Neurotoxic effects of endocrine disruptors. Curr. Opin. Neurol. 1999;12(6):745–751. doi: 10.1097/00019052-199912000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Masuo Y., Ishido M. Neurotoxicity of endocrine disruptors: possible involvement in brain development and neurodegeneration. J. Toxicol. Environ. Health Part B. 2011;14(5–7):346–369. doi: 10.1080/10937404.2011.578557. [DOI] [PubMed] [Google Scholar]
- 12.Weiss B. The intersection of neurotoxicology and endocrine disruption. Neurotoxicology. 2012;33(6):1410–1419. doi: 10.1016/j.neuro.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.León-Olea M. Current concepts in neuroendocrine disruption. Gen. Comp. Endocrinol. 2014;203:158–173. doi: 10.1016/j.ygcen.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schantz S.L., Widholm J.J. Cognitive effects of endocrine-disrupting chemicals in animals. Environ. Health Perspect. 2001;109(12):1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arya G. Pharmaceutical chemicals, steroids and xenoestrogens in water, sediments and fish from the tidal freshwater Potomac River (Virginia, USA) J. Environ. Sci. Health, Part A. 2017;52(7):686–696. doi: 10.1080/10934529.2017.1312975. [DOI] [PubMed] [Google Scholar]
- 17.Saussereau E. Determination of levels of current drugs in hospital and urban wastewater. Bull. Environ. Contam. Toxicol. 2013;91(2):171–176. doi: 10.1007/s00128-013-1030-7. [DOI] [PubMed] [Google Scholar]
- 18.Goullé J.-P. Importance of anthropogenic metals in hospital and urban wastewater: its significance for the environment. Bull. Environ. Contam. Toxicol. 2012;89(6):1220–1224. doi: 10.1007/s00128-012-0829-y. [DOI] [PubMed] [Google Scholar]
- 19.Gasnier C. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology. 2009;262(3):184–191. doi: 10.1016/j.tox.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Defarge N. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int. J. Environ. Res. Public Health. 2016;13(3):264. doi: 10.3390/ijerph13030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra R. Glyphosate–surfactant herbicide-induced reversible encephalopathy. J. Clin. Neurosci. 2010;17(11):1472–1473. doi: 10.1016/j.jocn.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Sato C. Aseptic meningitis in association with glyphosate-surfactant herbicide poisoning. Clin. Toxicol. 2011;49(2):118–120. doi: 10.3109/15563650.2011.552065. [DOI] [PubMed] [Google Scholar]
- 23.Martínez M.-A. Neurotransmitter changes in rat brain regions following glyphosate exposure. Environ. Res. 2018;161:212–219. doi: 10.1016/j.envres.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 24.Coullery R.P., Ferrari M.E., Rosso S.B. Neuronal development and axon growth are altered by glyphosate through a WNT non-canonical signaling pathway. Neurotoxicology. 2016;52:150–161. doi: 10.1016/j.neuro.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Aitbali Y. Glyphosate based-herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol. Teratol. 2018;67:44–49. doi: 10.1016/j.ntt.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Gallegos C.E. Exposure to a glyphosate-based herbicide during pregnancy and lactation induces neurobehavioral alterations in rat offspring. Neurotoxicology. 2016;53:20–28. doi: 10.1016/j.neuro.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Seralini G.-E., Jungers G. Toxic compounds in herbicides without glyphosate. Food Chem. Toxicol. 2020;146:111770. doi: 10.1016/j.fct.2020.111770. [DOI] [PubMed] [Google Scholar]
- 28.Román G.C. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J. Neurol. Sci. 2007;262(1–2):15–26. doi: 10.1016/j.jns.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Helbing C.C., Ovaska K., Ji L. Evaluation of the effect of acetochlor on thyroid hormone receptor gene expression in the brain and behavior of Rana catesbeiana tadpoles. Aquat. Toxicol. 2006;80(1):42–51. doi: 10.1016/j.aquatox.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Zafeiridou G. Assessing the effects of the three herbicides acetochlor, 2, 4, 5-trichlorophenoxyacetic acid (2, 4, 5-T) and 2, 4-dichlorophenoxyacetic acid on the compound action potential of the sciatic nerve of the frog (Rana ridibunda) Chemosphere. 2006;65(6):1040–1048. doi: 10.1016/j.chemosphere.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 31.Goldner W.S. Hypothyroidism and pesticide use among male private pesticide applicators in the agricultural health study. J. Occup. Environ. Med. Am. Coll. Occup. Environ. Med. 2013;55(10):1171. doi: 10.1097/JOM.0b013e31829b290b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seok S.-J. Acute oral poisoning due to chloracetanilide herbicides. J. Korean Med. Sci. 2012;27(2):111. doi: 10.3346/jkms.2012.27.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo Y.-C., Yang C.-C., Deng J.-F. Acute alachlor and butachlor herbicide poisoning. Clin. Toxicol. 2008;46(8):716–721. doi: 10.1080/15563650701704834. [DOI] [PubMed] [Google Scholar]
- 34.Doĭcheva L. Experimental poisoning of carp fingerlings (Cyprinus carpio L.) with the herbicidal preparation, lasagrin (alachlor) Vet. Nauki. 1978;15(4):108–113. [PubMed] [Google Scholar]
- 35.Sirohi H.V. Design of anti‐thyroid drugs: binding studies and structure determination of the complex of lactoperoxidase with 2‐mercaptoimidazole at 2.30 Å resolution. Proteins Struct. Funct. Bioinform. 2017;85(10):1882–1890. doi: 10.1002/prot.25342. [DOI] [PubMed] [Google Scholar]
- 36.Chilumuri A., Odell M., Milton N.G. Benzothiazole aniline tetra (ethylene glycol) and 3-amino-1, 2, 4-triazole inhibit neuroprotection against amyloid peptides by catalase overexpression in vitro. ACS Chem. Neurosci. 2013;4(11):1501–1512. doi: 10.1021/cn400146a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan H., Zhang L. Mechanism of the effects of amitrole on the thyroglobulin in Fischer rat thyroid follicle-5 cell. Wei sheng yan jiu= J. hygiene Res. 2011;40(4):434–436. 440. [PubMed] [Google Scholar]
- 38.Brucker-Davis F. Effects of environmental synthetic chemicals on thyroid function. Thyroid. 1998;8(9):827–856. doi: 10.1089/thy.1998.8.827. [DOI] [PubMed] [Google Scholar]
- 39.Palanikumar L. Biochemical response of anthracene and benzo [a] pyrene in milkfish Chanos chanos. Ecotoxicol. Environ. Saf. 2012;75:187–197. doi: 10.1016/j.ecoenv.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 40.Vieira L. Acute effects of Benzo [a] pyrene, anthracene and a fuel oil on biomarkers of the common goby Pomatoschistus microps (Teleostei, Gobiidae) Sci. Total Environ. 2008;395(2–3):87–100. doi: 10.1016/j.scitotenv.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 41.Mucio-Ramírez S. Perinatal exposure to organohalogen pollutants decreases vasopressin content and its mRNA expression in magnocellular neuroendocrine cells activated by osmotic stress in adult rats. Toxicol. Appl. Pharmacol. 2017;329:173–189. doi: 10.1016/j.taap.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei T. Assessing adverse effects of Aroclor 1254 on perinatally exposed rat offspring. Biomed. Environ. Sci. 2015;28(9):687–690. doi: 10.3967/bes2015.097. [DOI] [PubMed] [Google Scholar]
- 43.Coburn C.G. Permanently compromised NADPH-diaphorase activity within the osmotically activated supraoptic nucleus after in utero but not adult exposure to Aroclor 1254. Neurotoxicology. 2015;47:37–46. doi: 10.1016/j.neuro.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Majumdar A., Nirwane A., Kamble R. New evidences of neurotoxicity of aroclor 1254 in mice brain: potential of coenzyme q10 in abating the detrimental outcomes. Environ. Health Toxicol. 2014;29 doi: 10.5620/eht.2014.29.e2014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilson H., Kodavanti P. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology. 1998;19:517–526. Find this article online. [PubMed] [Google Scholar]
- 46.Liu Z. Atrazine and its main metabolites alter the locomotor activity of larval zebrafish (Danio rerio) Chemosphere. 2016;148:163–170. doi: 10.1016/j.chemosphere.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Webb E. Neurodevelopmental and neurological effects of chemicals associated with unconventional oil and natural gas operations and their potential effects on infants and children. Rev. Environ. Health. 2018;33(1):3–29. doi: 10.1515/reveh-2017-0008. [DOI] [PubMed] [Google Scholar]
- 48.Preciados M., Yoo C., Roy D. Estrogenic endocrine disrupting chemicals influencing NRF1 regulated gene networks in the development of complex human brain diseases. Int. J. Mol. Sci. 2016;17(12):2086. doi: 10.3390/ijms17122086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karri V., Schuhmacher M., Kumar V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016;48:203–213. doi: 10.1016/j.etap.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Tyler C.R., Allan A.M. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr. Environ. Health Rep. 2014;1(2):132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma K. Neurotoxicity effects of atrazine-induced SH-SY5Y human dopaminergic neuroblastoma cells via microglial activation. Mol. Biosyst. 2015;11(11):2915–2924. doi: 10.1039/c5mb00432b. [DOI] [PubMed] [Google Scholar]
- 52.Zhang B., Ma K., Li B. Inflammatory reaction regulated by microglia plays a role in atrazine-induced dopaminergic neuron degeneration in the substantia nigra. J. Toxicol. Sci. 2015;40(4):437–450. doi: 10.2131/jts.40.437. [DOI] [PubMed] [Google Scholar]
- 53.Lin Z. Gestational and lactational exposure to atrazine via the drinking water causes specific behavioral deficits and selectively alters monoaminergic systems in C57BL/6 mouse dams, juvenile and adult offspring. Toxicol. Sci. 2014;141(1):90–102. doi: 10.1093/toxsci/kfu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ottinger M.A. Neuroendocrine and behavioral effects of embryonic exposure to endocrine disrupting chemicals in birds. Brain Res. Rev. 2008;57(2):376–385. doi: 10.1016/j.brainresrev.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Dayal H. Symptom clusters in a community with chronic exposure to chemicals in two superfund sites. Arch. Environ. Health Int. J. 1995;50(2):108–111. doi: 10.1080/00039896.1995.9940887. [DOI] [PubMed] [Google Scholar]
- 56.Slotkin T.A. In vitro models reveal differences in the developmental neurotoxicity of an environmental polycylic aromatic hydrocarbon mixture compared to benzo [a] pyrene: neuronotypic PC12 Cells and embryonic neural stem cells. Toxicology. 2017;377:49–56. doi: 10.1016/j.tox.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarma S.N., Blais J.M., Chan H.M. Neurotoxicity of alkylated polycyclic aromatic compounds in human neuroblastoma cells. J. Toxicol. Environ. Health Part A. 2017;80(5):285–300. doi: 10.1080/15287394.2017.1314840. [DOI] [PubMed] [Google Scholar]
- 58.Yang K. Disruption of glutamate neurotransmitter transmission is modulated by SNAP-25 in benzo [a] pyrene-induced neurotoxic effects. Toxicology. 2017;384:11–22. doi: 10.1016/j.tox.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Chepelev N.L. Neurotoxicity may be an overlooked consequence of benzo [a] pyrene exposure that is relevant to human health risk assessment. Mutat. Res. Mutat. Res. 2015;764:64–89. doi: 10.1016/j.mrrev.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Niu Q., Zhang H., Li X. 2009. B [A] P-induced Neurobehavioral Function and. [Google Scholar]
- 61.Jacobson M.H. Serum polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) and thyroid function among Michigan adults several decades after the 1973–1974 PBB contamination of livestock feed. Environ. Health Perspect. 2017;125(9):097020. doi: 10.1289/EHP1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tilson H.A., Cabe P.A., Mitchell C.L. Behavioral and neurological toxicity of polybrominated biphenyls in rats and mice. Environ. Health Perspect. 1978;23:257–263. doi: 10.1289/ehp.7823257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgenstern R. Phthalates and thyroid function in preschool age children: sex specific associations. Environ. Int. 2017;106:11–18. doi: 10.1016/j.envint.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Min A. Benzyl butyl phthalate exposure impairs learning and memory and attenuates neurotransmission and CREB phosphorylation in mice. Food Chem. Toxicol. 2014;71:81–89. doi: 10.1016/j.fct.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 65.Betz A. Chronic exposure to benzyl butyl phthalate (BBP) alters social interaction and fear conditioning in male adult rats: alterations in amygdalar MeCP2, ERK1/2 and ERα. Neuroendocrinol Lett. 2013;34(5):347–358. [PubMed] [Google Scholar]
- 66.Kasuya M. Toxicity of butylbenzyl phthalate (BBP) and other phthalate esters to nervous tissue in culture. Toxicol. Lett. 1980;6(6):373–378. doi: 10.1016/0378-4274(80)90109-5. [DOI] [PubMed] [Google Scholar]
- 67.Chen H. From the cover: BDE-47 and BDE-49 inhibit axonal growth in primary rat hippocampal neuron-glia co-cultures via ryanodine receptor-dependent mechanisms. Toxicol. Sci. 2017;156(2):375–386. doi: 10.1093/toxsci/kfw259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X. Neonatal exposure to BDE 209 impaired learning and memory, decreased expression of hippocampal core SNAREs and synaptophysin in adult rats. Neurotoxicology. 2017;59:40–48. doi: 10.1016/j.neuro.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 69.Sun W. PBDE-209 exposure damages learning and memory ability in rats potentially through increased autophagy and apoptosis in the hippocampus neuron. Environ. Toxicol. Pharmacol. 2017;50:151–158. doi: 10.1016/j.etap.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Vuong A.M. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: current findings and future directions. Horm. Behav. 2018;101:94–104. doi: 10.1016/j.yhbeh.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Zhuang J. TDP-43 upregulation mediated by the NLRP3 inflammasome induces cognitive impairment in 2 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47)-treated mice. Brain Behav. Immun. 2017;65:99–110. doi: 10.1016/j.bbi.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Zhai J., Tong S. Research progress of health effect of polybrominated diphenyl ethers. Zhonghua Yu Fang Yi Xue Za Zhi[Chin. J. Preventive Med.] 2016;50(6):559–562. doi: 10.3760/cma.j.issn.0253-9624.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 73.Dach K. BDE-99 impairs differentiation of human and mouse NPCs into the oligodendroglial lineage by species-specific modes of action. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/srep44861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding G. Association between prenatal exposure to polybrominated diphenyl ethers and young children’s neurodevelopment in China. Environ. Res. 2015;142:104–111. doi: 10.1016/j.envres.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 75.Roze E. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 2009;117(12):1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bahadar H., Mostafalou S., Abdollahi M. Current understandings and perspectives on non-cancer health effects of benzene: a global concern. Toxicol. Appl. Pharmacol. 2014;276(2):83–94. doi: 10.1016/j.taap.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 77.Manto M. Toxic agents causing cerebellar ataxias. Handb. Clin. Neurol. 2012;103:201–213. doi: 10.1016/B978-0-444-51892-7.00012-7. [DOI] [PubMed] [Google Scholar]
- 78.Ritchie G.D. A review of the neurotoxicity risk of selected hydrocarbon fuels. J. Toxicol. Environ. Health B Crit. Rev. 2001;4(3):223–312. doi: 10.1080/109374001301419728. [DOI] [PubMed] [Google Scholar]
- 79.Ruszkiewicz J.A. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017;4:245–259. doi: 10.1016/j.toxrep.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faass O. Female sexual behavior, estrous cycle and gene expression in sexually dimorphic brain regions after pre-and postnatal exposure to endocrine active UV filters. Neurotoxicology. 2009;30(2):249–260. doi: 10.1016/j.neuro.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Ejaredar M. Phthalate exposure and childrens neurodevelopment: a systematic review. Environ. Res. 2015;142:51–60. doi: 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 82.Zhou Y. Neurotoxicity of low bisphenol A (BPA) exposure for young male mice: implications for children exposed to environmental levels of BPA. Environ. Pollut. 2017;229:40–48. doi: 10.1016/j.envpol.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 83.Inadera H. Neurological effects of bisphenol A and its analogues. Int. J. Med. Sci. 2015;12(12):926. doi: 10.7150/ijms.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hutler Wolkowicz I. Developmental toxicity of bisphenol A diglycidyl ether (epoxide resin badge) during the early life cycle of a native amphibian species. Environ. Toxicol. Chem. 2016;35(12):3031–3038. doi: 10.1002/etc.3491. [DOI] [PubMed] [Google Scholar]
- 85.Ohtani N. Adverse effects of maternal exposure to bisphenol F on the anxiety-and depression-like behavior of offspring. J. Vet. Med. Sci. 2016:16–0502. doi: 10.1292/jvms.16-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenfeld C.S. Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front. Neuroendocrinol. 2017;47:123–133. doi: 10.1016/j.yfrne.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castro B. Bisphenol A, bisphenol F and bisphenol S affect differently 5α-reductase expression and dopamine–serotonin systems in the prefrontal cortex of juvenile female rats. Environ. Res. 2015;142:281–287. doi: 10.1016/j.envres.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Wu L.-H. Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci. Total Environ. 2018;615:87–98. doi: 10.1016/j.scitotenv.2017.09.194. [DOI] [PubMed] [Google Scholar]
- 89.Lombardi C. Residential proximity to pesticide application as a risk factor for childhood central nervous system tumors. Environ. Res. 2021:111078. doi: 10.1016/j.envres.2021.111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lulla A. Neurotoxicity of the Parkinson disease-associated pesticide ziram is synuclein-dependent in zebrafish embryos. Environ. Health Perspect. 2016;124(11):1766–1775. doi: 10.1289/EHP141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyazaki I. Methamphetamine‐induced dopaminergic neurotoxicity is regulated by quinone formation‐related molecules. Faseb J. 2006;20(3):571–573. doi: 10.1096/fj.05-4996fje. [DOI] [PubMed] [Google Scholar]
- 92.Katsuki H. Antioxidants, but not cAMP or high K+;, prevent arachidonic acid toxicity on neuronal cultures. Neuroreport. 1995;6(8):1101–1104. doi: 10.1097/00001756-199505300-00007. [DOI] [PubMed] [Google Scholar]
- 93.Raciti M., Ceccatelli S. Epigenetic mechanisms in developmental neurotoxicity. Neurotoxicol. Teratol. 2018;66:94–101. doi: 10.1016/j.ntt.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Jacobo-Estrada T. Cadmium handling, toxicity and molecular targets involved during pregnancy: lessons from experimental models. Int. J. Mol. Sci. 2017;18(7):1590. doi: 10.3390/ijms18071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z. Autophagy in neurodegenerative diseases and metal neurotoxicity. Neurochem. Res. 2016;41(1–2):409–422. doi: 10.1007/s11064-016-1844-x. [DOI] [PubMed] [Google Scholar]
- 96.Sanders A.P., Henn B.C., Wright R.O. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: a review of recent literature. Curr. Environ. Health Rep. 2015;2(3):284–294. doi: 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bo W., Yanli D. Cadmium and its neurotoxic effects. Oxidative Med Cell Longev. 2013;898034:12. doi: 10.1155/2013/898034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Rubai A.-j., Wigmore P., Pratten M.K. Evaluation of a human neural stem cell culture method for prediction of the neurotoxicity of anti-epileptics. Altern. Lab. Anim. 2017;45(2):67–81. doi: 10.1177/026119291704500202. [DOI] [PubMed] [Google Scholar]
- 99.Hansen N., Finzel M., Block F. Antiepileptic drug-induced encephalopathy. Fortschr. Neurol. 2010;78(10):590–598. doi: 10.1055/s-0029-1245632. [DOI] [PubMed] [Google Scholar]
- 100.Gualtieri C.T., Johnson L.G. Comparative neurocognitive effects of 5 psychotropic anticonvulsants and lithium. Medscape General Med. 2006;8(3):46. [PMC free article] [PubMed] [Google Scholar]
- 101.Lee I. Developmental neurotoxic effects of two pesticides: behavior and biomolecular studies on chlorpyrifos and carbaryl. Toxicol. Appl. Pharmacol. 2015;288(3):429–438. doi: 10.1016/j.taap.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 102.Freeborn D.L. Use of electroencephalography (EEG) to assess CNS changes produced by pesticides with different modes of action: effects of permethrin, deltamethrin, fipronil, imidacloprid, carbaryl, and triadimefon. Toxicol. Appl. Pharmacol. 2015;282(2):184–194. doi: 10.1016/j.taap.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 103.Wang H.P. Subchronic neurotoxicity of chlorpyrifos, carbaryl, and their combination in rats. Environ. Toxicol. 2014;29(10):1193–1200. doi: 10.1002/tox.21851. [DOI] [PubMed] [Google Scholar]
- 104.Cocco S. Polychlorinated biphenyls induce mitochondrial dysfunction in SH-SY5Y neuroblastoma cells. PLoS One. 2015;10(6):e0129481. doi: 10.1371/journal.pone.0129481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lovato A.K., Creton R., Colwill R.M. Effects of embryonic exposure to polychlorinated biphenyls (PCBs) on larval zebrafish behavior. Neurotoxicol. Teratol. 2016;53:1–10. doi: 10.1016/j.ntt.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ozcan M. Hippocampal long-term potentiation (LTP) is reduced by a coplanar PCB congener. Neurotoxicology. 2004;25(6):981–988. doi: 10.1016/j.neuro.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 107.Howard A.S. Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol. Appl. Pharmacol. 2003;190(1):72–86. doi: 10.1016/s0041-008x(03)00156-x. [DOI] [PubMed] [Google Scholar]
- 108.Brucker-Davis F. Neurotoxicant exposure during pregnancy is a confounder for assessment of iodine supplementation on neurodevelopment outcome. Neurotoxicol. Teratol. 2015;51:45–51. doi: 10.1016/j.ntt.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 109.Doi H. Prenatal exposure to a polychlorinated biphenyl (PCB) congener influences fixation duration on biological motion at 4-months-old: a preliminary study. PLoS One. 2013;8(3):e59196. doi: 10.1371/journal.pone.0059196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cauli O. Gender differential effects of developmental exposure to methyl-mercury, polychlorinated biphenyls 126 or 153, or its combinations on motor activity and coordination. Toxicology. 2013;311(1-2):61–68. doi: 10.1016/j.tox.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 111.Uwimana E. Atropselective Oxidation of 2, 2′, 3, 3′, 4, 6′-Hexachlorobiphenyl (PCB 132) to hydroxylated Metabolites by Human Liver Microsomes and its Implications for PCB 132 Neurotoxicity. Toxicol. Sci. 2019;171(2):406–420. doi: 10.1093/toxsci/kfz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boix J. Differential long-term effects of developmental exposure to polychlorinated biphenyls 52, 138 or 180 on motor activity and neurotransmission. Gender dependence and mechanisms involved. Neurochem. Int. 2011;58(1):69–77. doi: 10.1016/j.neuint.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 113.Campagna R. Cerebellum proteomics addressing the cognitive deficit of rats perinatally exposed to the food-relevant polychlorinated biphenyl 138. Toxicol. Sci. 2011;123(1):170–179. doi: 10.1093/toxsci/kfr156. [DOI] [PubMed] [Google Scholar]
- 114.Naert C. Distribution of polychlorinated biphenyls and polybrominated diphenyl ethers in birds of prey from Switzerland. Chemosphere. 2007;68(5):977–987. doi: 10.1016/j.chemosphere.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 115.Enayah S.H. PCB95 and PCB153 change dopamine levels and turn-over in PC12 cells. Toxicology. 2018;394:93–101. doi: 10.1016/j.tox.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gascon M. Evaluating the neurotoxic effects of lactational exposure to persistent organic pollutants (POPs) in Spanish children. Neurotoxicology. 2013;34:9–15. doi: 10.1016/j.neuro.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 117.Morse D. Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicol. Appl. Pharmacol. 1993;122(1):27–33. doi: 10.1006/taap.1993.1168. [DOI] [PubMed] [Google Scholar]
- 118.Kilburn K.H. Chlordane as a neurotoxin in humans. South. Med. J. 1997;90(3):299–304. doi: 10.1097/00007611-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 119.Kilburn K.H., Thornton J.C. Protracted neurotoxicity from chlordane sprayed to kill termites. Environ. Health Perspect. 1995;103(7–8):690–694. doi: 10.1289/ehp.95103690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grutsch J., Khasawinah A. Signs and mechanisms of chlordane intoxication. Biomed. Environ. Sci.: BES. 1991;4(3):317–326. [PubMed] [Google Scholar]
- 121.Villanueva C.M. Drinking water disinfection by-products during pregnancy and child neuropsychological development in the INMA Spanish cohort study. Environ. Int. 2018;110:113–122. doi: 10.1016/j.envint.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 122.Balster R.L., Borzelleca J.F. Behavioral toxicity of trihalomethane contaminants of drinking water in mice. Environ. Health Perspect. 1982;46:127–136. doi: 10.1289/ehp.8246127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu L. Relative developmental toxicity of short-chain chlorinated paraffins in Zebrafish (Danio rerio) embryos. Environ. Pollut. 2016;219:1122–1130. doi: 10.1016/j.envpol.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 124.Mariussen E., Fonnum F. Neurochemical targets and behavioral effects of organohalogen compounds: an update. Crit. Rev. Toxicol. 2006;36(3):253–289. doi: 10.1080/10408440500534164. [DOI] [PubMed] [Google Scholar]
- 125.Eriksson P., Nordberg A. The effects of DDT, DDOH-palmitic acid, and a chlorinated paraffin on muscarinic receptors and the sodium-dependent choline uptake in the central nervous system of immature mice. Toxicol. Appl. Pharmacol. 1986;85(2):121–127. doi: 10.1016/0041-008x(86)90105-5. [DOI] [PubMed] [Google Scholar]
- 126.Burke R.D. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: from clinical findings to preclinical models and potential mechanisms. J. Neurochem. 2017;142:162–177. doi: 10.1111/jnc.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamada S. Chlorpyrifos inhibits neural induction via Mfn1-mediated mitochondrial dysfunction in human induced pluripotent stem cells. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/srep40925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sogorb M.A. Roles of NTE protein and encoding gene in development and neurodevelopmental toxicity. Chem. Biol. Interact. 2016;259:352–357. doi: 10.1016/j.cbi.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 129.Yeo S.-H. Effects of central nervous system drugs on recovery after stroke: a systematic review and meta-analysis of randomized controlled trials. Clin. Drug Investig. 2017;37(10):901–928. doi: 10.1007/s40261-017-0558-4. [DOI] [PubMed] [Google Scholar]
- 130.Gaanderse M., Kliffen J., Linssen W. Citalopram-induced dyskinesia of the tongue: a video presentation. Case Rep. 2016;2016 doi: 10.1136/bcr-2016-216126. p. bcr2016216126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sprowles J.L. Perinatal exposure to the selective serotonin reuptake inhibitor citalopram alters spatial learning and memory, anxiety, depression, and startle in Sprague-Dawley rats. Int. J. Dev. Neurosci. 2016;54:39–52. doi: 10.1016/j.ijdevneu.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 132.Hurley P.M. Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environ. Health Perspect. 1998;106(8):437–445. doi: 10.1289/ehp.98106437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abdelsalam E. Neurotoxic potential of six organophosphorus compounds in adult hens. Vet. Hum. Toxicol. 1999;41(5):290–292. [PubMed] [Google Scholar]
- 134.Abou‐Donia M.B., Makkawy H.A., Graham D.G. Coumaphos: delayed neurotoxic effect following dermal administration in hens. J. Toxicol. Environ. Health, Part A Current Issues. 1982;10(1):87–99. doi: 10.1080/15287398209530233. [DOI] [PubMed] [Google Scholar]
- 135.Jantaratnotai N. Phytoestrogens mediated anti-inflammatory effect through suppression of IRF-1 and pSTAT1 expressions in lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2013;17(2):483–488. doi: 10.1016/j.intimp.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 136.Andreou A. Assessing the effects of three dental impression materials on the isolated sciatic nerve of rat and frog. Toxicol. Vitr. 2007;21(1):103–108. doi: 10.1016/j.tiv.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 137.Fuzzard S.K., Teixeira R., Zinn R. A review of the literature on the management of silicone implant incompatibility syndrome. Aesthetic Plast. Surg. 2019;43(5):1145–1149. doi: 10.1007/s00266-019-01407-4. [DOI] [PubMed] [Google Scholar]
- 138.Yu C. Effects of perinatal daidzein exposure on subsequent behavior and central estrogen receptor α expression in the adult male mouse. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;43:157–167. doi: 10.1016/j.pnpbp.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 139.Zeng S. Effect of daidzein on anxiety, social behavior and spatial learning in male Balb/cJ mice. Pharmacol. Biochem. Behav. 2010;96(1):16–23. doi: 10.1016/j.pbb.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 140.Jin Y. Genistein and daidzein induce neurotoxicity at high concentrations in primary rat neuronal cultures. J. Biomed. Sci. 2007;14(2):275–284. doi: 10.1007/s11373-006-9142-2. [DOI] [PubMed] [Google Scholar]
- 141.Teitelbaum D.T. The toxicology of 1, 2-dibromo-3-chloropropane (DBCP): a brief review. Int. J. Occup. Environ. Health. 1999;5(2):122–126. doi: 10.1179/oeh.1999.5.2.122. [DOI] [PubMed] [Google Scholar]
- 142.Gunderson M.P. Effect of low dose exposure to the herbicide atrazine and its metabolite on cytochrome P450 aromatase and steroidogenic factor-1 mRNA levels in the brain of premetamorphic bullfrog tadpoles (Rana catesbeiana) Aquat. Toxicol. 2011;102(1–2):31–38. doi: 10.1016/j.aquatox.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hossain M.M., Filipov N.M. Alteration of dopamine uptake into rat striatal vesicles and synaptosomes caused by an in vitro exposure to atrazine and some of its metabolites. Toxicology. 2008;248(1):52–58. doi: 10.1016/j.tox.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yi S.-W. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ. Res. 2014;133:56–65. doi: 10.1016/j.envres.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 145.Bortolozzi Aa.A. Effects of 2, 4-dichlorophenoxyacetic acid exposure on dopamine D2-like receptors in rat brain. Neurotoxicol. Teratol. 2004;26(4):599–605. doi: 10.1016/j.ntt.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 146.Evangelista de Duffard A., Duffard R. Behavioral toxicology, risk assessment, and chlorinated hydrocarbons. Environ. Health Perspect. 1996;104(suppl 2):353–360. doi: 10.1289/ehp.96104s2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krieg E.F., Jr. The relationships between pesticide metabolites and neurobehavioral test performance in the Third National Health and Nutrition Examination Survey. Arch. Environ. Occup. Health. 2013;68(1):39–46. doi: 10.1080/19338244.2011.633125. [DOI] [PubMed] [Google Scholar]
- 148.Stevens R.D., Burri H., Tramèr M.R. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesth. Analg. 2003;97(3):623–633. doi: 10.1213/01.ANE.0000074795.68061.16. [DOI] [PubMed] [Google Scholar]
- 149.Heilmann P. Therapy of the adrenocortical carcinoma with Lysodren (o, p’-DDD). Therapeutic management by monitoring o, p’-DDD blood levels. Med. Klin. Suppl. 2001;96(7):371–377. doi: 10.1007/pl00002218. [DOI] [PubMed] [Google Scholar]
- 150.Lanser J. Neuropsychologic and neurologic side effects of mitotane and reversibility of symptoms. J. Clin. Oncol. 1992;10(9) doi: 10.1200/JCO.1992.10.9.1504. p. 1504-1504. [DOI] [PubMed] [Google Scholar]
- 151.Du Rostu H. Neurotoxicity of mitotane therapy of adrenocortical carcinoma (5 cases) and Cushing’s syndrome (7 cases) Presse Med.(Paris, France: 1983) 1987;16(19):951–954. [PubMed] [Google Scholar]
- 152.Kajta M. Depressive-like effect of prenatal exposure to DDT involves global DNA hypomethylation and impairment of GPER1/ESR1 protein levels but not ESR2 and AHR/ARNT signaling. J. Steroid Biochem. Mol. Biol. 2017;171:94–109. doi: 10.1016/j.jsbmb.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 153.Zhang J. Endocrine-disrupting effects of pesticides through interference with human glucocorticoid receptor. Environ. Sci. Technol. 2016;50(1):435–443. doi: 10.1021/acs.est.5b03731. [DOI] [PubMed] [Google Scholar]
- 154.Eskenazi B. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics. 2006;118(1):233–241. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- 155.Halldin K. Impact of endocrine disrupting chemicals on reproduction in Japanese quail. Domest. Anim. Endocrinol. 2005;29(2):420–429. doi: 10.1016/j.domaniend.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 156.Fry D.M. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ. Health Perspect. 1995;103(suppl 7):165–171. doi: 10.1289/ehp.95103s7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Al-Saleh I. Selenium status in lactating mothers-infants and its potential protective role against the neurotoxicity of methylmercury, lead, manganese, and DDT. Environ. Res. 2019;176:108562. doi: 10.1016/j.envres.2019.108562. [DOI] [PubMed] [Google Scholar]
- 158.Wnuk A. The crucial involvement of retinoid X receptors in DDE neurotoxicity. Neurotox. Res. 2016;29(1):155–172. doi: 10.1007/s12640-015-9572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cartier C. Prenatal and 5-year p, p′-DDE exposures are associated with altered sensory processing in school-aged children in Nunavik: a visual evoked potential study. Neurotoxicology. 2014;44:8–16. doi: 10.1016/j.neuro.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 160.Parent A.-S. Early developmental actions of endocrine disruptors on the hypothalamus, hippocampus, and cerebral cortex. J. Toxicol. Environ. Health Part B. 2011;14(5–7):328–345. doi: 10.1080/10937404.2011.578556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee H.-C., Yamanouchi K., Nishihara M. Effects of perinatal exposure to phthalate/adipate esters on hypothalamic gene expression and sexual behavior in rats. J. Reprod. Dev. 2006 doi: 10.1262/jrd.17096. p. 0602200033-0602200033. [DOI] [PubMed] [Google Scholar]
- 162.Arbo B., Benetti F., Ribeiro M.F. 2016. Astrocytes As a Target for Neuroprotection: Modulation by Progesterone and Dehydroepiandrosterone. [DOI] [PubMed] [Google Scholar]
- 163.Woda A., Picard P., Dutheil F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology. 2016;71:127–135. doi: 10.1016/j.psyneuen.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 164.Yu Q. Depression-like behavior in a dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Biol. Reprod. 2016;95(4):79. doi: 10.1095/biolreprod.116.142117. 1-10. [DOI] [PubMed] [Google Scholar]
- 165.Li Z. DHEA-neuroprotection and-neurotoxicity after transient cerebral ischemia in rats. J. Cereb. Blood Flow & Metab. 2009;29(2):287–296. doi: 10.1038/jcbfm.2008.118. [DOI] [PubMed] [Google Scholar]
- 166.Coplan J.D. Effects of acute confinement stress-induced hypothalamic-pituitary adrenal Axis activation and concomitant peripheral and central transforming growth Factor-β1 measures in nonhuman Primates. Chronic Stress. 2017;1 doi: 10.1177/2470547016688693. p. 2470547016688693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lopes M. A single high dose of dexamethasone affects the phosphorylation state of glutamate AMPA receptors in the human limbic system. Translational psychiatry. 2016;6(12) doi: 10.1038/tp.2016.251. p. e986-e986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feng Y. Dexamethasone but not the equivalent doses of hydrocortisone induces neurotoxicity in neonatal rat brain. Pediatric Res. 2015;77(5):618–624. doi: 10.1038/pr.2015.19. [DOI] [PubMed] [Google Scholar]
- 169.Uno H., Eisele S., Sakai A., Shelton S., Baker E. Dejesus 0. Holden J. 1994:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 170.Farzanehfar V. Determination of dibutyl phthalate neurobehavioral toxicity in mice. Food and Chem. Toxicol. 2016;94:221–226. doi: 10.1016/j.fct.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 171.Yan B. Oxidative stress mediates dibutyl phthalateinduced anxiety-like behavior in Kunming mice. Environ. Toxicol. Pharmacol. 2016;45:45–51. doi: 10.1016/j.etap.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 172.Chantong B. Dibutyltin promotes oxidative stress and increases inflammatory mediators in BV-2 microglia cells. Toxicology letters. 2014;230(2):177–187. doi: 10.1016/j.toxlet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 173.Tsuji M. Higher toxicity of dibutyltin and poly-L-lactide with a large amount of tin but lower toxicity of poly-L-lactide of synthetic artificial dura mater exhibited on murine astrocyte cell line. Yakugaku Zasshi. 2010;130(6):847–855. doi: 10.1248/yakushi.130.847. [DOI] [PubMed] [Google Scholar]
- 174.Jenkins S.M., Ehman K., Barone S., Jr. Structure–activity comparison of organotin species: dibutyltin is a developmental neurotoxicant in vitro and in vivo. Dev. Brain Res. 2004;151(1–2):1–12. doi: 10.1016/j.devbrainres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 175.Kobayashi H. Effects of tri-, di and Monobutyltin on synaptic parameters of the cholinergic system in the cerebral cortex of mice. Jpn. J. Pharmacol. 1996;72(4):317–324. doi: 10.1254/jjp.72.317. [DOI] [PubMed] [Google Scholar]
- 176.Lessenger J.E., Riley N. Neurotoxicities and behavioral changes in a 12‐year‐old male exposed to dicofol, an organochloride pesticide. J. Toxicol. Environ. Health, Part A Current Issues. 1991;33(3):255–261. doi: 10.1080/15287399109531524. [DOI] [PubMed] [Google Scholar]
- 177.Cowie A.M. The pesticide dieldrin disrupts proteins related to oxidative respiration and mitochondrial stress in the central nervous system. Data in brief. 2017;11:628–633. doi: 10.1016/j.dib.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schmidt J.T. Dieldrin-induced neurotoxicity involves impaired mitochondrial bioenergetics and an endoplasmic reticulum stress response in rat dopaminergic cells. Neurotoxicology. 2017;63:1–12. doi: 10.1016/j.neuro.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 179.Babot Z., Vilaró M.T., Suñol C. Long‐term exposure to dieldrin reduces γ‐aminobutyric acid type A and N‐methyl‐D‐aspartate receptor function in primary cultures of mouse cerebellar granule cells. J. Neurosci. Res. 2007;85(16):3687–3695. doi: 10.1002/jnr.21433. [DOI] [PubMed] [Google Scholar]
- 180.Luu B.E. The roles of hippocampal microRNAs in response to acute postnatal exposure to di (2-ethylhexyl) phthalate in female and male rats. Neurotoxicology. 2017;59:98–104. doi: 10.1016/j.neuro.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 181.Park S. Di-(2-ethylhexyl) phthalate exposure is negatively correlated with trait anxiety in girls but not with trait anxiety in boys or anxiety-like behavior in male mice. J. Child Neurol. 2015;30(1):48–52. doi: 10.1177/0883073814532544. [DOI] [PubMed] [Google Scholar]
- 182.Quinnies K.M. Transgenerational effects of di-(2-ethylhexyl) phthalate (DEHP) on stress hormones and behavior. Endocrinology. 2015;156(9):3077–3083. doi: 10.1210/EN.2015-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huang H.-B. Fetal and childhood exposure to phthalate diesters and cognitive function in children up to 12 years of age: taiwanese maternal and infant cohort study. PloS one. 2015;10(6):e0131910. doi: 10.1371/journal.pone.0131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Téllez-Rojo M.M. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci. Total Environ. 2013;461:386–390. doi: 10.1016/j.scitotenv.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Doherty B.T. Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant Development-II in a population of young urban children. Environ. Res. 2017;152:51–58. doi: 10.1016/j.envres.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mao G. Neurological toxicity of individual and mixtures of low dose arsenic, mono and di (n-butyl) phthalates on sub-chronic exposure to mice. Biol. Trace Element Res. 2016;170(1):183–193. doi: 10.1007/s12011-015-0457-6. [DOI] [PubMed] [Google Scholar]
- 187.Won E.-K. Association of current phthalate exposure with neurobehavioral development in a national sample. Int. J. Hyg. Environ. Health. 2016;219(4–5):364–371. doi: 10.1016/j.ijheh.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 188.Tomihara K. Effects of diethylstilbestrol exposure during gestation on both maternal and offspring behavior. Frontiers in Neurosci. 2015;9:79. doi: 10.3389/fnins.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Frye C. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 2012;24(1):144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sato K. Effects of 17β-estradiol and xenoestrogens on the neuronal survival in an organotypic hippocampal culture. Neuroendocrinology. 2002;76(4):223–234. doi: 10.1159/000065948. [DOI] [PubMed] [Google Scholar]
- 191.Ma P. Cognitive deficits and anxiety induced by diisononyl phthalate in mice and the neuroprotective effects of melatonin. Sci. Rep. 2015;5(1):1–14. doi: 10.1038/srep14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Peng L. Mice brain tissue injury induced by diisononyl phthalate exposure and the protective application of vitamin E. J. Biochem. Mol. Toxicol. 2015;29(7):311–320. doi: 10.1002/jbt.21700. [DOI] [PubMed] [Google Scholar]
- 193.Boberg J. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Rep. Toxicol. 2011;31(2):200–209. doi: 10.1016/j.reprotox.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 194.Kim J., Song J.-H. Inhibitory effects of antihistamines, diphenhydramine and chlorpheniramine, on proton currents in BV2 microglial cells. Eur. J. Pharmacol. 2017;798:122–128. doi: 10.1016/j.ejphar.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 195.Mansfield L. Effects of fexofenadine, diphenhydramine, and placebo on performance of the test of variables of attention (TOVA) Annals of Allergy, Asthma & Immunol. 2003;90(5):554–559. doi: 10.1016/S1081-1206(10)61850-9. [DOI] [PubMed] [Google Scholar]
- 196.Wilken J.A. A comparison of the effect of diphenhydramine and desloratadine on vigilance and cognitive function during treatment of ragweed-induced allergic rhinitis. Annals of Allergy, Asthma & Immunol. 2003;91(4):375–385. doi: 10.1016/S1081-1206(10)61685-7. [DOI] [PubMed] [Google Scholar]
- 197.Vaswani K.K., Tejwani G.A., Abou-Issa H.M. Effect of 7, 12-dimethylbenz [a] anthracene-induced mammary carcinogenesis on the opioid peptide levels in the rat central nervous system. Cancer letters. 1986;31(2):115–122. doi: 10.1016/0304-3835(86)90001-7. [DOI] [PubMed] [Google Scholar]
- 198.Jang T.-c., Jang J.-h., Lee K.-w. Mehanizam akutne neurotoksičnosti u Sprague-Dawley štakora izazvane trovanjem endosulfanom. Arhiv za higijenu rada i toksikologiju. 2016;67(1):9–17. doi: 10.1515/aiht-2016-67-2702. [DOI] [PubMed] [Google Scholar]
- 199.Caudle W.M. Vulnerability of synapses in the frontal cortex of mice developmentally exposed to an insecticide: potential contribution to neuropsychiatric disease. Neurotransmitter (Houston, Tex.) 2015;2(1) doi: 10.14800/nt.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Silva M. A comparison of ToxCast test results with in vivo and other in vitro endpoints for neuro, endocrine, and developmental toxicities: a case study using endosulfan and methidathion. Birth Defects Res. Part B: Dev. Rep. Toxicol. 2015;104(2):71–89. doi: 10.1002/bdrb.21140. [DOI] [PubMed] [Google Scholar]
- 201.Silva M.H., Gammon D. An assessment of the developmental, reproductive, and neurotoxicity of endosulfan. Birth Defects Res. Part B: Dev. Rep. Toxicol. 2009;86(1):1–28. doi: 10.1002/bdrb.20183. [DOI] [PubMed] [Google Scholar]
- 202.Bagchi M. Protective effects of lazaroid U74389F (16-desmethyl tirilazad) on endrin-induced lipid peroxidation and DNA damage in brain and liver and regional distribution of catalase activity in rat brain. Free Radical Biol. Med. 1995;19(6):867–872. doi: 10.1016/0891-5849(95)00088-f. [DOI] [PubMed] [Google Scholar]
- 203.Gray L.E., Jr Perinatal toxicity of endrin in rodents. III. Alterations of behavioral ontogeny. Toxicology. 1981;21(3):187–202. doi: 10.1016/0300-483x(81)90155-4. [DOI] [PubMed] [Google Scholar]
- 204.Li S.H., Graham B.M. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. The Lancet Psychiatry. 2017;4(1):73–82. doi: 10.1016/S2215-0366(16)30358-3. [DOI] [PubMed] [Google Scholar]
- 205.Perez-Alvarez M., Wandosell F. Stroke and neuroinflamation: role of sexual hormones. Current Pharm. Design. 2016;22(10):1334–1349. doi: 10.2174/138161282210160304112834. [DOI] [PubMed] [Google Scholar]
- 206.Rossetti M.F. Oestrogens and progestagens: synthesis and action in the brain. J. Neuroendocrinol. 2016;28(7) doi: 10.1111/jne.12402. [DOI] [PubMed] [Google Scholar]
- 207.Mahmoud R., Wainwright S.R., Galea L.A. Sex hormones and adult hippocampal neurogenesis: regulation, implications, and potential mechanisms. Frontiers in Neuroendocrinol. 2016;41:129–152. doi: 10.1016/j.yfrne.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 208.Grimm A. Improvement of neuronal bioenergetics by neurosteroids: implications for age-related neurodegenerative disorders. Biochimica et Biophysica Acta (BBA)-Mol. Basis of Dis. 2014;1842(12):2427–2438. doi: 10.1016/j.bbadis.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 209.Porseryd T. Persistent effects of developmental exposure to 17α-ethinylestradiol on the zebrafish (Danio rerio) brain transcriptome and behavior. Frontiers in Behav. Neurosci. 2017;11:69. doi: 10.3389/fnbeh.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zaccaroni M. Developmental exposure to very low levels of ethynilestradiol affects anxiety in a novelty place preference test of juvenile rats. Neurotoxicity Res. 2016;30(4):553–562. doi: 10.1007/s12640-016-9645-1. [DOI] [PubMed] [Google Scholar]
- 211.Wang W. Abnormal innervation patterns in the anorectum of ETU-induced fetal rats with anorectal malformations. Neurosci. Lett. 2011;495(2):88–92. doi: 10.1016/j.neulet.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 212.Debbarh I. Human neurotoxicity of ethylene-bis-dithiocarbamates (EBDC) Revue neurologique. 2002;158(12 Pt 1):1175–1180. [PubMed] [Google Scholar]
- 213.Merola C. Toxicological assessment and developmental abnormalities induced by butylparaben and ethylparaben exposure in zebrafish early-life stages. Environ. Toxicol. Pharmacol. 2020;80:103504. doi: 10.1016/j.etap.2020.103504. [DOI] [PubMed] [Google Scholar]
- 214.Lynch J.F., I.I.I. Aromatized testosterone attenuates contextual generalization of fear in male rats. Hormones and Behav. 2016;84:127–135. doi: 10.1016/j.yhbeh.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 215.Alward B.A. Aromatase inhibition rapidly affects in a reversible manner distinct features of birdsong. Sci. Rep. 2016;6(1):1–9. doi: 10.1038/srep32344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Xing L., Venables M.J., Trudeau V.L. Role of aromatase and radial glial cells in neurotoxin-induced dopamine neuron degeneration and regeneration. General and Comp. Endocrinol. 2017;241:69–79. doi: 10.1016/j.ygcen.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 217.Langlois V.S., Duarte-Guterman P., Trudeau V.L. Expression profiles of reproduction-and thyroid hormone-related transcripts in the brains of chemically-induced intersex frogs. Sexual Dev. 2011;5(1):26–32. doi: 10.1159/000322875. [DOI] [PubMed] [Google Scholar]
- 218.Geraldi P.A., Delgado-GarcÍA J.M., Gruart A. Acute and repeated effects of three organophosphorus pesticides on the acquisition and retention of an instrumental learning task in rats. Neurotoxic. Res. 2008;13(3):253–263. doi: 10.1007/BF03033509. [DOI] [PubMed] [Google Scholar]
- 219.Groszek B., Pach J., Kłys M. Intermediate syndrome in acute fenitrothion poisoning. Przeglad lekarski. 1995;52(5):271–274. [PubMed] [Google Scholar]
- 220.Ram M.D., Gopal K. Neurobehavioral changes in freshwater fishChanna punctatus exposed to fenitrothion. Bull. Environ. Contam. Toxicol. 1991;47(3):455–458. doi: 10.1007/BF01702210. [DOI] [PubMed] [Google Scholar]
- 221.Lenkic L.E., Tiu S.H., Tobe S.S. Suppression of JH biosynthesis by JH analog treatment: mechanism of suppression and roles of allatostatins and nervous connections in the cockroach Diploptera punctata. J. Insect Physiol. 2009;55(11):967–975. doi: 10.1016/j.jinsphys.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 222.Fertig R. Investigation of the plausibility of 5-alpha-reductase inhibitor syndrome. Skin appendage Dis. 2016;2(3–4):120–129. doi: 10.1159/000450617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Traish A.M. Adverse effects of 5α-reductase inhibitors: what do we know, don’t know, and need to know? Rev. Endocrine and Metab. Dis. 2015;16(3):177–198. doi: 10.1007/s11154-015-9319-y. [DOI] [PubMed] [Google Scholar]
- 224.Ganzer C.A., Jacobs A.R., Iqbal F. Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. Am. J. men’s Health. 2015;9(3):222–228. doi: 10.1177/1557988314538445. [DOI] [PubMed] [Google Scholar]
- 225.Godinho A.F. Memory impairment due to fipronil pesticide exposure occurs at the GABAA receptor level, in rats. Physiol. Behave. 2016;165:28–34. doi: 10.1016/j.physbeh.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 226.Magalhães J.Z. Prenatal exposure to fipronil disturbs maternal aggressive behavior in rats. Neurotoxicol. Teratol. 2015;52:11–16. doi: 10.1016/j.ntt.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 227.Simon-Delso N. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015;22(1):5–34. doi: 10.1007/s11356-014-3470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marrs T.C., Maynard R. Neurotranmission systems as targets for toxicants: a review. Cell Boil.Toxicol. 2013;29(6):381–396. doi: 10.1007/s10565-013-9259-9. [DOI] [PubMed] [Google Scholar]
- 229.Golub M.S. Cognitive performance of juvenile monkeys after chronic fluoxetine treatment. Dev. Cognitive Neurosci. 2017;26:52–61. doi: 10.1016/j.dcn.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hong L.-Z. Chronic fluoxetine treatment enhances sympathetic activities associated with abnormality of baroreflex function in conscious normal rats. Eur. J. Pharmacol. 2017;811:164–170. doi: 10.1016/j.ejphar.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 231.Svensson A.I. Flutamide treatment induces anxiolytic-like behavior in adult castrated rats. Pharmacol. Rep. 2012;64(2):275–281. doi: 10.1016/s1734-1140(12)70765-x. [DOI] [PubMed] [Google Scholar]
- 232.Zhang J.-M. Effects of neonatal flutamide treatment on hippocampal neurogenesis and synaptogenesis correlate with depression-like behaviors in preadolescent male rats. Neuroscience. 2010;169(1):544–554. doi: 10.1016/j.neuroscience.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ahmadiani A., Mandgary A., Sayyah M. Anticonvulsant effect of flutamide on seizures induced by pentylenetetrazole: involvement of benzodiazepine receptors. Epilepsia. 2003;44(5):629–635. doi: 10.1046/j.1528-1157.2003.36402.x. [DOI] [PubMed] [Google Scholar]
- 234.Sidhu G.K. Toxicity, monitoring and biodegradation of organophosphate pesticides: a review. Crit. Rev. Environ. Sci. Technol. 2019;49(13):1135–1187. [Google Scholar]
- 235.Liu X. Acute formaldehyde exposure induced early Alzheimer-like changes in mouse brain. Toxicol. Mech. Methods. 2018;28(2):95–104. doi: 10.1080/15376516.2017.1368053. [DOI] [PubMed] [Google Scholar]
- 236.Li Y. Effects of formaldehyde exposure on anxiety-like and depression-like behavior, cognition, central levels of glucocorticoid receptor and tyrosine hydroxylase in mice. Chemosphere. 2016;144:2004–2012. doi: 10.1016/j.chemosphere.2015.10.102. [DOI] [PubMed] [Google Scholar]
- 237.Zendehdel R., Fazli Z., Mazinani M. Neurotoxicity effect of formaldehyde on occupational exposure and influence of individual susceptibility to some metabolism parameters. Environ. Monitoring Assess. 2016;188(11):1–7. doi: 10.1007/s10661-016-5662-z. [DOI] [PubMed] [Google Scholar]
- 238.Tulpule K., Dringen R. Formaldehyde in brain: an overlooked player in neurodegeneration? J. Neurochem. 2013;127(1):7–21. doi: 10.1111/jnc.12356. [DOI] [PubMed] [Google Scholar]
- 239.Songur A., Ozen O.A., Sarsilmaz M. The toxic effects of formaldehyde on the nervous system. Rev. Environ. Contam. Toxicol. 2010:105–118. doi: 10.1007/978-1-4419-1352-4_3. [DOI] [PubMed] [Google Scholar]
- 240.Johnston J. On the anesthetic action of furan. J. Pharmacol. Exp. Ther. 1931;43(1):85–88. [Google Scholar]
- 241.Ayuk‐Takem L. Inhibition of polyisoprenylated methylated protein methyl esterase by synthetic musks induces cell degeneration. Environ. Toxicol. 2014;29(4):466–477. doi: 10.1002/tox.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Patisaul H.B. Endocrine disruption of vasopressin systems and related behaviors. Frontiers in Endocrinol. 2017;8:134. doi: 10.3389/fendo.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Luo S. Genistein inhibits Aβ 25–35–induced neurotoxicity in PC12 cells via PKC signaling pathway. Neurochem. Res. 2012;37(12):2787–2794. doi: 10.1007/s11064-012-0872-4. [DOI] [PubMed] [Google Scholar]
- 244.Lee Y.-B., Lee H.J., Sohn H.S. Soy isoflavones and cognitive function. J. Nutr. Biochem. 2005;16(11):641–649. doi: 10.1016/j.jnutbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 245.Lephart E.D. Behavioral effects of endocrine-disrupting substances: phytoestrogens. ILAR J. 2004;45(4):443–454. doi: 10.1093/ilar.45.4.443. [DOI] [PubMed] [Google Scholar]
- 246.Pham-Lake C. Impairment in the mesohippocampal dopamine circuit following exposure to the brominated flame retardant, HBCDD. Environ. Toxicol. Pharmacol. 2017;50:167–174. doi: 10.1016/j.etap.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wang F. New insights into the cytotoxic mechanism of hexabromocyclododecane from a metabolomic approach. Environ. Sci. Technol. 2016;50(6):3145–3153. doi: 10.1021/acs.est.5b03678. [DOI] [PubMed] [Google Scholar]
- 248.Maurice N. Short-term effects of a perinatal exposure to the HBCDD α-isomer in rats: assessment of early motor and sensory development, spontaneous locomotor activity and anxiety in pups. Neurotoxicol. Teratol. 2015;52:170–180. doi: 10.1016/j.ntt.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 249.Al-Mousa F., Michelangeli F. The sarcoplasmic–endoplasmic reticulum Ca2+-ATPase (SERCA) is the likely molecular target for the acute toxicity of the brominated flame retardant hexabromocyclododecane (HBCD) Chem. –Biol. Interactions. 2014;207:1–6. doi: 10.1016/j.cbi.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 250.Lilienthal H. Effects of the brominated flame retardant hexabromocyclododecane (HBCD) on dopamine-dependent behavior and brainstem auditory evoked potentials in a one-generation reproduction study in Wistar rats. Toxicol. Lett. 2009;185(1):63–72. doi: 10.1016/j.toxlet.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 251.Fu J. Tetrachlorobenzoquinone exhibits neurotoxicity by inducing inflammatory responses through ROS-mediated IKK/IκB/NF-κB signaling. Environ. Toxicol. Pharmacol. 2016;41:241–250. doi: 10.1016/j.etap.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 252.Kyriklaki A. Prenatal exposure to persistent organic pollutants in association with offspring neuropsychological development at 4 years of age: the Rhea mother-child cohort, Crete. Greece. Environ. Int. 2016;97:204–211. doi: 10.1016/j.envint.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 253.Reed L., Büchner V., Tchounwou P.B. Environmental toxicology and health effects associated with hexachlorobenzene exposure. Rev. Environ. Health. 2007;22(3):213–244. doi: 10.1515/reveh.2007.22.3.213. [DOI] [PubMed] [Google Scholar]
- 254.Li Y. Toxicity and oxidative stress on rats by hexachlorobenzene. Zhonghua lao dong wei sheng zhi ye bing za zhi= Zhonghua laodong weisheng zhiyebing zazhi= Chin. J. Ind. Hygiene and Occup. Dis. 2006;24(10):601–604. [PubMed] [Google Scholar]
- 255.Goldey E.S., Taylor D.H. Developmental neurotoxicity following premating maternal exposure to hexachlorobenzene in rats. Neurotoxicol. Teratol. 1992;14(1):15–21. doi: 10.1016/0892-0362(92)90024-5. [DOI] [PubMed] [Google Scholar]
- 256.Nyffeler J. Combination of multiple neural crest migration assays to identify environmental toxicants from a proof-of-concept chemical library. Arch. Toxicol. 2017;91(11):3613–3632. doi: 10.1007/s00204-017-1977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Christen V. Developmental neurotoxicity of different pesticides in PC-12 cells in vitro. Toxicol. Appl. Pharmacol. 2017;325:25–36. doi: 10.1016/j.taap.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 258.Hong S. Heptachlor induced nigral dopaminergic neuronal loss and Parkinsonism-like movement deficits in mice. Exp. Mol. Med. 2014;46(2) doi: 10.1038/emm.2014.12. p. e80-e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Moser V.C. Neurotoxicological outcomes of perinatal heptachlor exposure in the rat. Toxicol. Sci. 2001;60(2):315–326. doi: 10.1093/toxsci/60.2.315. [DOI] [PubMed] [Google Scholar]
- 260.Kirby M.L., Barlow R.L., Bloomquist J.R. Neurotoxicity of the organochlorine insecticide heptachlor to murine striatal dopaminergic pathways. Toxicol. Sci. 2001;61(1):100–106. doi: 10.1093/toxsci/61.1.100. [DOI] [PubMed] [Google Scholar]
- 261.Yamaguchi I., Matsumura F., Kadous A.A. Heptachlor epoxide: effects on calcium-mediated transmitter release from brain synaptosomes in rat. Biochem. Pharmacol. 1980;29(12):1815–1823. doi: 10.1016/0006-2952(80)90144-6. [DOI] [PubMed] [Google Scholar]
- 262.Badaeva L., Ovsiannikova L., Kiseleva N. Manifestation of the neurotoxic effect of the organochlorine pesticide hexachlorobutadiene in the postnatal period of ontogeny in the rat. Arkhiv Anatomii, Gistologii i Embriologii. 1985;89(8):44–49. [PubMed] [Google Scholar]
- 263.Badaeva L. Characteristics of the neuro-and immunotoxic effects of transplacental hexachlorobutadiene. Gigiena i sanitariia. 1990;(9):75–78. [PubMed] [Google Scholar]
- 264.Murzakaev F. The effect of small doses of hexachlorobutadiene on central nervous activity and morphological changes in the animal organism in hexachlorbutadiene poisoning. Gigiena truda i professional’nye zabolevaniia. 1967;11(3):23–28. [PubMed] [Google Scholar]
- 265.Chen Y. Identification of differentially expressed genes response to TCDD in rat brain after long-term low-dose exposure. J. Environ. Sci. 2017;62:92–99. doi: 10.1016/j.jes.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 266.Kimura E. Impaired dendritic growth and positioning of cortical pyramidal neurons by activation of aryl hydrocarbon receptor signaling in the developing mouse. PLoS One. 2017;12(8):e0183497. doi: 10.1371/journal.pone.0183497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mactutus C., Tilson H. Neonatal chlordecone exposure impairs early learning and retention of active avoidance in the rat. Neurobehav. Toxicol. Teratol. 1984;6(1):75–83. [PubMed] [Google Scholar]
- 268.Mactutus C., Unger K., Tilson H. Evaluation of neonatal chlordecone neurotoxicity during early development: initial characterization. Neurobehav. Toxicol. Teratol. 1984;6(1):67–73. [PubMed] [Google Scholar]
- 269.Mactutus C.F., Tilson H.A. Evaluation of long‐term consequences in behavioral and/or neural function following neonatal chlordecone exposure. Teratology. 1985;31(2):177–186. doi: 10.1002/tera.1420310202. [DOI] [PubMed] [Google Scholar]
- 270.Andrade V. Marreilha Dos Santos AP. Neurotoxicity of metal mixtures. Adv. Neurobiol. 2017;18:227–265. doi: 10.1007/978-3-319-60189-2_12. [DOI] [PubMed] [Google Scholar]
- 271.Chen W., Zhang X., Huang W. Neural stem cells in lead toxicity. Eur. Rev. Med. Pharm. Sci. 2016;20(24):5174–5177. [PubMed] [Google Scholar]
- 272.Assi M., Hezmee M., Haron A., Sabri M., Rajion M. The detrimental effects of lead on human and animal health. Veterinary World. 2016;9(6):660–671. doi: 10.14202/vetworld.2016.660-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Caito S., Aschner M. Neurotoxicity of metals. Handbook of Clin. Neurol. 2015;131:169–189. doi: 10.1016/B978-0-444-62627-1.00011-1. [DOI] [PubMed] [Google Scholar]
- 274.Aleknaviciute J. The levonorgestrel-releasing intrauterine device potentiates stress reactivity. Psychoneuroendocrinology. 2017;80:39–45. doi: 10.1016/j.psyneuen.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 275.Simone J. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology. 2015;62:265–278. doi: 10.1016/j.psyneuen.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 276.Porcu P. Long-term administration with levonorgestrel decreases allopregnanolone levels and alters GABAA receptor subunit expression and anxiety-like behavior. Pharm. Biochem. Behav. 2012;102(2):366–372. doi: 10.1016/j.pbb.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 277.Costa L.G. The neurotoxicity of organochlorine and pyrethroid pesticides. Handbook of Clin. Neurol. 2015;131:135–148. doi: 10.1016/B978-0-444-62627-1.00009-3. [DOI] [PubMed] [Google Scholar]
- 278.Quintaneiro C. Endocrine and physiological effects of linuron and S-metolachlor in zebrafish developing embryos. Sci. Total Environ. 2017;586:390–400. doi: 10.1016/j.scitotenv.2016.11.153. [DOI] [PubMed] [Google Scholar]
- 279.Lichtensteiger W. Differential gene expression patterns in developing sexually dimorphic rat brain regions exposed to antiandrogenic, estrogenic, or complex endocrine disruptor mixtures: glutamatergic synapses as target. Endocrinology. 2015;156(4):1477–1493. doi: 10.1210/en.2014-1504. [DOI] [PubMed] [Google Scholar]
- 280.Shinn C. Behavioral response of juvenile rainbow trout exposed to an herbicide mixture. Ecotoxicol. Environ. S. 2015;112:15–21. doi: 10.1016/j.ecoenv.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 281.Richendrfer H., Creton R. Chlorpyrifos and malathion have opposite effects on behaviors and brain size that are not correlated to changes in AChE activity. Neurotoxicology. 2015;49:50–58. doi: 10.1016/j.neuro.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Salama M. Developmental neurotoxic effects of Malathion on 3D neurosphere system. Appl. Trans. Genomics. 2015;7:13–18. doi: 10.1016/j.atg.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Hashjin G.S. Malathion induces anxiety in the male adult mouse. Arch. Med. Sci.: AMS. 2013;9(2):368. doi: 10.5114/aoms.2013.33174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rastogi S., Tripathi S., Ravishanker D. A study of neurologic symptoms on exposure to organophosphate pesticides in the children of agricultural workers. Indian J. Occup. Environ. Med. 2010;14(2):54. doi: 10.4103/0019-5278.72242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Valvassori S.S. Acute and subacute exposure to malathion impairs aversive but not non-associative memory in rats. Neurotoxicity Res. 2007;12(1):71–79. doi: 10.1007/BF03033902. [DOI] [PubMed] [Google Scholar]
- 286.de Joode Bv.W. Pesticide exposure and neurodevelopment in children aged 6–9 years from Talamanca, Costa rica. Cortex. 2016;85:137–150. doi: 10.1016/j.cortex.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 287.Brody A.H. Mancozeb-induced behavioral deficits precede structural neural degeneration. Neurotoxicology. 2013;34:74–81. doi: 10.1016/j.neuro.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 288.Li P. The ethylene bis-dithiocarbamate fungicide Mancozeb activates voltage-gated KCNQ2 potassium channel. Toxicol. Lett. 2013;219(3):211–217. doi: 10.1016/j.toxlet.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 289.Domico L.M. Acute neurotoxic effects of mancozeb and maneb in mesencephalic neuronal cultures are associated with mitochondrial dysfunction. Neurotoxicology. 2006;27(5):816–825. doi: 10.1016/j.neuro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 290.Kimura K. Effects of pesticides on the peripheral and central nervous system in tobacco farmers in Malaysia: studies on peripheral nerve conduction, brain-evoked potentials and computerized posturography. Industrial Health. 2005;43(2):285–294. doi: 10.2486/indhealth.43.285. [DOI] [PubMed] [Google Scholar]
- 291.Lucchini R. Manganese and developmental neurotoxicity. Neurotoxic. Metals. 2017:13–34. doi: 10.1007/978-3-319-60189-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Peres T.V. Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharm. Toxicol. 2016;17(1):1–20. doi: 10.1186/s40360-016-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tarale P. Potential role of epigenetic mechanism in manganese induced neurotoxicity. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/2548792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Torres-Sánchez L. Prenatal p, p-DDE exposure and neurodevelopment among children 3.5–5 years of age. Environ. Health Perspect. 2013;121(2):263–268. doi: 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Martini M. Perinatal exposure to methoxychlor enhances adult cognitive responses and hippocampal neurogenesis in mice. Frontiers in Behave. Neurosci. 2014;8:202. doi: 10.3389/fnbeh.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Schuh R.A. Methoxychlor inhibits brain mitochondrial respiration and increases hydrogen peroxide production and CREB phosphorylation. Toxicol. Sci. 2005;88(2):495–504. doi: 10.1093/toxsci/kfi334. [DOI] [PubMed] [Google Scholar]
- 297.de Souza A., Narvencar K.P., Sindhoora K. The neurological effects of methyl bromide intoxication. J. Neurol. Sci. 2013;335(1–2):36–41. doi: 10.1016/j.jns.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 298.Kim E.-A., Kang S.-K. Occupational neurological disorders in Korea. J. Korean Med. Sci. 2010;25(Suppl):S26. doi: 10.3346/jkms.2010.25.S.S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yang R.S. Toxicology of methyl bromide. Rev. Environ. Contam. Toxicol. 1995:65–85. doi: 10.1007/978-1-4612-4252-9_3. [DOI] [PubMed] [Google Scholar]
- 300.Anger W. Neurobehavioral evaluation of soil and structural fumigators using methyl bromide and sulfuryl fluoride. Neurotoxicology. 1986;7(3):137–156. [PubMed] [Google Scholar]
- 301.Moshitzky P., Applebaum S.W. Pathway and regulation of JHIII‐bisepoxide biosynthesis in adult Drosophila melanogaster corpus allatum. Arch. Insect Biochem. Physiol. 1995;30(2–3):225–237. doi: 10.1002/arch.940300211. [DOI] [PubMed] [Google Scholar]
- 302.Prestwich G.D. Binding proteins for methyl farnesoate in lobster tissues: detection by photoaffinity labeling. General and Comp. Endocrinol. 1990;80(2):232–237. doi: 10.1016/0016-6480(90)90168-l. [DOI] [PubMed] [Google Scholar]
- 303.DeLeo P. Comment on “Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems”. Environ. Sci. Technol. 2011;45(23):10283–10284. doi: 10.1021/es202937q. [DOI] [PubMed] [Google Scholar]
- 304.Broniowska Ż., Pomierny B., Smaga I., Filip M., Budziszewska B. The effect of UV-filters on the viability of neuroblastoma (SH-SY5Y) cell line. NeuroToxicol. 2016;54:44–52. doi: 10.1016/j.neuro.2016.03.003. Epub 2016 Mar 7. PMID: 26965011. [DOI] [PubMed] [Google Scholar]
- 305.Li V.W.T. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. 2016;23(9):8275–8285. doi: 10.1007/s11356-016-6180-9. [DOI] [PubMed] [Google Scholar]
- 306.Maerkel K. Sex-and region-specific alterations of progesterone receptor mRNA levels and estrogen sensitivity in rat brain following developmental exposure to the estrogenic UV filter 4-methylbenzylidene camphor. Environ. Toxicol. Pharm. 2005;19(3):761–765. doi: 10.1016/j.etap.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 307.Singh A.K. 3-Methylcholanthrene induces neurotoxicity in developing neurons derived from human CD34+ Thy1+ stem cells by activation of aryl hydrocarbon receptor. Neuromol. Med. 2013;15(3):570–592. doi: 10.1007/s12017-013-8243-0. [DOI] [PubMed] [Google Scholar]
- 308.Puertas R. Prenatal exposure to mirex impairs neurodevelopment at age of 4 years. Neurotoxicology. 2010;31(1):154–160. doi: 10.1016/j.neuro.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 309.Shankland D. Neurotoxic action of chlorinated hydrocarbon insecticides. Neurobehav. Toxicol. Teratol. 1982;4(6):805–811. [PubMed] [Google Scholar]
- 310.Foran L., Blackburn K., Kulesza R.J. Auditory hindbrain atrophy and anomalous calcium binding protein expression after neonatal exposure to monosodium glutamate. Neuroscience. 2017;344:406–417. doi: 10.1016/j.neuroscience.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 311.Sadek K., Abouzed T., Nasr S. Lycopene modulates cholinergic dysfunction, Bcl-2/Bax balance, and antioxidant enzymes gene transcripts in monosodium glutamate (E621) induced neurotoxicity in a rat model. Canadian J. Physiol. Pharmacol. 2016;94(4):394–401. doi: 10.1139/cjpp-2015-0388. [DOI] [PubMed] [Google Scholar]
- 312.Sasaki‐Hamada S. Changes in hippocampal synaptic functions and protein expression in monosodium glutamate‐treated obese mice during development of glucose intolerance. Eur. J. Neurosci. 2015;41(11):1393–1401. doi: 10.1111/ejn.12891. [DOI] [PubMed] [Google Scholar]
- 313.Lau A., Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Archiv-Eur. J. Physiol. 2010;460(2):525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 314.Chalansonnet M. Study of the potential oxidative stress induced by six solvents in the rat brain. Neurotoxicology. 2013;35:71–83. doi: 10.1016/j.neuro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 315.England L.J. Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neurosc. Biobehav. Rev. 2017;72:176–189. doi: 10.1016/j.neubiorev.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ferrea S., Winterer G. Neuroprotective and neurotoxic effects of nicotine. Pharmacopsychiatry. 2009;42(06):255–265. doi: 10.1055/s-0029-1224138. [DOI] [PubMed] [Google Scholar]
- 317.Ciani E. Neurochemical correlates of nicotine neurotoxicity on rat habenulo-interpeduncular cholinergic neurons. Neurotoxicology. 2005;26(3):467–474. doi: 10.1016/j.neuro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 318.Lee D.-H. Association between background exposure to organochlorine pesticides and the risk of cognitive impairment: a prospective study that accounts for weight change. Environment international. 2016;89:179–184. doi: 10.1016/j.envint.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 319.Kim S.-A. Can inconsistent association between hypertension and cognition in elders be explained by levels of organochlorine pesticides? PloS one. 2015;10(12):e0144205. doi: 10.1371/journal.pone.0144205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Litwa E. RXRα, PXR and CAR xenobiotic receptors mediate the apoptotic and neurotoxic actions of nonylphenol in mouse hippocampal cells. J. Steroid Biochem. Mol. Boil. 2016;156:43–52. doi: 10.1016/j.jsbmb.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 321.Tabassum H. Role of melatonin in mitigating nonylphenol-induced toxicity in frontal cortex and hippocampus of rat brain. Neurochem. Int. 2017;104:11–26. doi: 10.1016/j.neuint.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 322.Jie Y. Mechanism of nonylphenol-induced neurotoxicity in F 1 rats during sexual maturity. Wiener klinische Wochenschrift. 2016;128(11):426–434. doi: 10.1007/s00508-016-0960-6. [DOI] [PubMed] [Google Scholar]
- 323.Couderc M. Neurodevelopmental and behavioral effects of nonylphenol exposure during gestational and breastfeeding period on F1 rats. Neurotoxicology. 2014;44:237–249. doi: 10.1016/j.neuro.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 324.Pinna G., Rasmusson A.M. Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder. Frontiers in Cell. Neurosci. 2014;8:256. doi: 10.3389/fncel.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Pinna G., Costa E., Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr. Opinion Pharmacol. 2009;9(1):24–30. doi: 10.1016/j.coph.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Matsumoto K. GABAA receptor neurotransmission dysfunction in a mouse model of social isolation-induced stress: possible insights into a non-serotonergic mechanism of action of SSRIs in mood and anxiety disorders. Stress. 2007;10(1):3–12. doi: 10.1080/10253890701200997. [DOI] [PubMed] [Google Scholar]
- 327.Pinna G., Costa E., Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology. 2006;186(3):362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- 328.Tawara K. Effects of maternal dioxin exposure on newborn size at birth among Japanese mother–infant pairs. Environ. Health and Preventive Med. 2009;14(2):88–95. doi: 10.1007/s12199-008-0061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Chu I. Octachlorostyrene: a 90-day toxicity study in the rat. Fundam. Appl. Toxicol. 1984;4(4):547–557. doi: 10.1016/0272-0590(84)90044-7. [DOI] [PubMed] [Google Scholar]
- 330.Chu I. Long-term toxicity of octachlorostyrene in the rat. Toxicol. Sci. 1986;6(1):69–77. doi: 10.1016/0272-0590(86)90265-4. [DOI] [PubMed] [Google Scholar]
- 331.Axelstad M. Effects of pre-and postnatal exposure to the UV-filter octyl methoxycinnamate (OMC) on the reproductive, auditory and neurological development of rat offspring. Toxicol. Appl. Pharmacol. 2011;250(3):278–290. doi: 10.1016/j.taap.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 332.Bianco M. Differential accumulation levels in the brain of rats exposed to the endocrine disruptor 4-tert-octylphenol (OP) Environ. Toxicol. Pharm. 2011;31(1):198–204. doi: 10.1016/j.etap.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 333.Ghisari M., Bonefeld-Jorgensen E.C. Impact of environmental chemicals on the thyroid hormone function in pituitary rat GH3 cells. Mol. Cell. Endocrinol. 2005;244(1–2):31–41. doi: 10.1016/j.mce.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 334.Shikimi H. Dendritic growth in response to environmental estrogens in the developing Purkinje cell in rats. Neurosci. Lett. 2004;364(2):114–118. doi: 10.1016/j.neulet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 335.Kim K.-S. Associations between organochlorine pesticides and cognition in US elders: national Health and Nutrition Examination Survey 1999–2002. Environ. Int. 2015;75:87–92. doi: 10.1016/j.envint.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 336.Kim S.-A. Greater cognitive decline with aging among elders with high serum concentrations of organochlorine pesticides. PloS one. 2015;10(6):e0130623. doi: 10.1371/journal.pone.0130623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Jain R.B. Association between thyroid function and selected organochlorine pesticides: data from NHANES 2001–2002. Sci. Total Environ. 2014;466:706–715. doi: 10.1016/j.scitotenv.2013.07.087. [DOI] [PubMed] [Google Scholar]
- 338.Liu J., Parsons L., Pope C. Comparative effects of parathion and chlorpyrifos on extracellular endocannabinoid levels in rat hippocampus: influence on cholinergic toxicity. Toxicol. Appl. Pharmacol. 2013;272(3):608–615. doi: 10.1016/j.taap.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Beard J.D. Pesticide exposure and depression among male private pesticide applicators in the agricultural health study. Environ. Health Perspectives. 2014;122(9):984–991. doi: 10.1289/ehp.1307450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lerro C.C. Occupational pesticide exposure and subclinical hypothyroidism among male pesticide applicators. Occup. Environ. Med. 2018;75(2):79–89. doi: 10.1136/oemed-2017-104431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Campillo J.A. Impact assessment of agricultural inputs into a Mediterranean coastal lagoon (Mar Menor, SE Spain) on transplanted clams (Ruditapes decussatus) by biochemical and physiological responses. Aquatic Toxicol. 2013;142:365–379. doi: 10.1016/j.aquatox.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 342.Pan H. Using the FRTL-5 cell to screen the thyroxine disrupting effects of the two pesticides-ethylenethiourea and pendimethalin. Wei sheng yan jiu= J. Hygiene Res. 2004;33(3):267–269. [PubMed] [Google Scholar]
- 343.den Besten C. The liver, kidney, and thyroid toxicity of chlorinated benzenes. Toxicol. Appl. Pharmacol. 1991;111(1):69–81. doi: 10.1016/0041-008x(91)90135-2. [DOI] [PubMed] [Google Scholar]
- 344.Cheng Y., Ekker M., Chan H.M. Relative developmental toxicities of pentachloroanisole and pentachlorophenol in a zebrafish model (Danio rerio) Ecotoxicol. Environ. Safety. 2015;112:7–14. doi: 10.1016/j.ecoenv.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 345.Jorens P.G., Schepens P.J. Human pentachlorophenol poisoning. Human & Exp. Toxicol. 1993;12(6):479–495. doi: 10.1177/096032719301200605. [DOI] [PubMed] [Google Scholar]
- 346.Steinmaus C. Thyroid hormones and moderate exposure to perchlorate during pregnancy in women in southern California. Environ. health Perspectives. 2016;124(6):861–867. doi: 10.1289/ehp.1409614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Brent G.A. Oxford University Press; 2014. Perchlorate Exposure in Pregnancy and Cognitive Outcomes in Children: It’s Not Your Mother’s Thyroid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Gilbert M.E., Sui L. Developmental exposure to perchlorate alters synaptic transmission in hippocampus of the adult rat. Environ. Health Perspectives. 2008;116(6):752–760. doi: 10.1289/ehp.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hossain M.M., Liu J., Richardson J.R. Pyrethroid insecticides directly activate microglia through interaction with voltage-gated sodium channels. Toxicol. Sci. 2017;155(1):112–123. doi: 10.1093/toxsci/kfw187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Nasuti C. Early life exposure to permethrin: a progressive animal model of Parkinson’s disease. J. Pharm. Toxicol. Methods. 2017;83:80–86. doi: 10.1016/j.vascn.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 351.Zakirova Z. A chronic longitudinal characterization of neurobehavioral and neuropathological cognitive impairment in a mouse model of Gulf War agent exposure. Frontiers in Integrative Neurosci. 2016;9:71. doi: 10.3389/fnint.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yang P.-Y. Acute ingestion poisoning with insecticide formulations containing the pyrethroid permethrin, xylene, and surfactant: a review of 48 cases. J. Toxicol.: Clin. Toxicol. 2002;40(2):107–113. doi: 10.1081/clt-120004397. [DOI] [PubMed] [Google Scholar]
- 353.Ren X.-M. Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology. 2016;366:32–42. doi: 10.1016/j.tox.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 354.Oulhote Y. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environ. Int. 2016;97:237–245. doi: 10.1016/j.envint.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Jantzen C.E., Annunziato K.M., Cooper K.R. Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS, and PFNA. Aquatic Toxicol. 2016;180:123–130. doi: 10.1016/j.aquatox.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lien G.-W. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven-year-old children. Chemosphere. 2016;156:118–127. doi: 10.1016/j.chemosphere.2016.04.102. [DOI] [PubMed] [Google Scholar]
- 357.Ge J. ROS-mediated apoptosis of HAPI microglia through p53 signaling following PFOS exposure. Environ. Toxicol. Pharm. 2016;46:9–16. doi: 10.1016/j.etap.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 358.Starks S.E. Neurobehavioral function and organophosphate insecticide use among pesticide applicators in the Agricultural Health Study. Neurotoxicol. Teratol. 2012;34(1):168–176. doi: 10.1016/j.ntt.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Vandana S., Zzaman S. Phorate-induced enzymological alterations in mouse olfactory bulb. Brain Res. Bull. 1997;44(3):247–252. doi: 10.1016/s0361-9230(97)00116-0. [DOI] [PubMed] [Google Scholar]
- 360.Reddy T.P. Toxicity of neurons treated with herbicides and neuroprotection by mitochondria-targeted antioxidant SS31. Int. J. Environ. Res. Public Health. 2011;8(1):203–221. doi: 10.3390/ijerph8010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Meshchakova N. Risk assessment of health disorders and quality of life in employees of modern polyvinyl chloride production. Meditsina truda i promyshlennaia ekologiia. 2014;(4):24–29. [PubMed] [Google Scholar]
- 362.Podoll K., Berg-Dammer E., Noth J. Neurologic and psychiatric disorders in vinyl chloride disease. Fortschritte der Neurologie-psychiatrie. 1990;58(11):439–443. doi: 10.1055/s-2007-1001207. [DOI] [PubMed] [Google Scholar]
- 363.Urmann C. Neurodifferentiating potential of 8-prenylnaringenin and related compounds in neural precursor cells and correlation with estrogen-like activity. Planta Med. 2015;81:305–311. doi: 10.1055/s-0034-1396243. [DOI] [PubMed] [Google Scholar]
- 364.Bagatin M.C. Molecular docking and panicolytic effect of 8-Prenylnaringenin in the elevated T-Maze. Chem. Pharm.Bull. 2014;62(12):1231–1237. doi: 10.1248/cpb.c14-00569. [DOI] [PubMed] [Google Scholar]
- 365.Oberbauer E. Chroman-like cyclic prenylflavonoids promote neuronal differentiation and neurite outgrowth and are neuroprotective. J. Nutr. Biochem. 2013;24(11):1953–1962. doi: 10.1016/j.jnutbio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 366.Vinggaard A.M. Prochloraz: an imidazole fungicide with multiple mechanisms of action. Int. J. Androl. 2006;29(1):186–192. doi: 10.1111/j.1365-2605.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- 367.Xiang D. Editor’s highlight: structure-based investigation on the binding and activation of typical pesticides with thyroid receptor. Toxicol. Sci. 2017;160(2):205–216. doi: 10.1093/toxsci/kfx177. [DOI] [PubMed] [Google Scholar]
- 368.Radio N.M. Use of neural models of proliferation and neurite outgrowth to screen environmental chemicals in the ToxCast phase I library. Appl. In vitro Toxicol. 2015;1(2):131–139. [Google Scholar]
- 369.Gilbert M. Adult hippocampal neurogenesis is impaired by transient and moderate developmental thyroid hormone disruption. Neurotoxicology. 2017;59:9–21. doi: 10.1016/j.neuro.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Koromilas C. Inhibition of Na+, K+-ATPase in the hypothalamus, pons and cerebellum of the offspring rat due to experimentally-induced maternal hypothyroidism. J. Maternal-Fetal & Neonatal Med. 2015;28(12):1438–1444. doi: 10.3109/14767058.2014.955003. [DOI] [PubMed] [Google Scholar]
- 371.Koromilas C. Experimentally-induced maternal hypothyroidism alters crucial enzyme activities in the frontal cortex and hippocampus of the offspring rat. Metab. Brain Dis. 2015;30(1):241–246. doi: 10.1007/s11011-014-9581-9. [DOI] [PubMed] [Google Scholar]
- 372.Vanek C., Samuels M. Central nervous system vasculitis caused by propylthiouracil therapy: a case report and literature review. Thyroid. 2005;15(1):80–84. doi: 10.1089/thy.2005.15.80. [DOI] [PubMed] [Google Scholar]
- 373.Chen C. Early postnatal benzo (a) pyrene exposure in Sprague-Dawley rats causes persistent neurobehavioral impairments that emerge postnatally and continue into adolescence and adulthood. Toxicol. Sci. 2012;125(1):248–261. doi: 10.1093/toxsci/kfr265. [DOI] [PubMed] [Google Scholar]
- 374.Wormley D.D., Ramesh A., Hood D.B. Environmental contaminant–mixture effects on CNS development, plasticity, and behavior. Toxicol. Appl. Pharmacol. 2004;197(1):49–65. doi: 10.1016/j.taap.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 375.Takeda K., Tsukue N., Yoshida S. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ. Sci.: Int. J. Environ. Physiol. Toxicol. 2004;11(1):33–45. [PubMed] [Google Scholar]
- 376.Truong L. Assessment of the developmental and neurotoxicity of the mosquito control larvicide, pyriproxyfen, using embryonic zebrafish. Environ. Pollut. 2016;218:1089–1093. doi: 10.1016/j.envpol.2016.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Fourrier J. Larval exposure to the juvenile hormone analog pyriproxyfen disrupts acceptance of and social behavior performance in adult honeybees. PloS one. 2015;10(7):e0132985. doi: 10.1371/journal.pone.0132985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Motonaga K. A comparison of potency differences among thyroid peroxidase (TPO) inhibitors to induce developmental toxicity and other thyroid gland-linked toxicities in humans and rats. Regulatory Toxicol. Pharmacol. 2016;80:283–290. doi: 10.1016/j.yrtph.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 379.Gress S. Dig1 protects against locomotor and biochemical dysfunctions provoked by Roundup. BMC Compl.Altern. Med. 2016;16(1):1–8. doi: 10.1186/s12906-016-1226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Modesto K.A., Martinez C.B. Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere. 2010;78(3):294–299. doi: 10.1016/j.chemosphere.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 381.Lee D.J. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: systemic review and meta‐analyses to determine first‐line treatments. Depression and anxiety. 2016;33(9):792–806. doi: 10.1002/da.22511. [DOI] [PubMed] [Google Scholar]
- 382.Frölich L., Hausner L. Therapie mit Antipsychotika und Antidepressiva Bei Demenzen. Der Nervenarzt. 2015;86(4):461–467. doi: 10.1007/s00115-014-4178-4. [DOI] [PubMed] [Google Scholar]
- 383.Wyatt I., Coutss C., Elcombe C. The effect of chlorinated paraffins on hepatic enzymes and thyroid hormones. Toxicology. 1993;77(1-2):81–90. doi: 10.1016/0300-483x(93)90139-j. [DOI] [PubMed] [Google Scholar]
- 384.Gunderson C.H., Daroff R.B. War Neurology. Karger Publishers; 2016. Neurology in the Vietnam war; pp. 201–213. [DOI] [PubMed] [Google Scholar]
- 385.Omer V.S., Mohammad F. Ontogeny of swimming behavior and brain catecholamine turnover in rats prenatally exposed to a mixture of 2, 4-dichlorophenoxyacetic and 2, 4, 5-trichlorophenoxyacetic acids. Neuropharmacology. 1987;26(9):1351–1358. doi: 10.1016/0028-3908(87)90098-0. [DOI] [PubMed] [Google Scholar]
- 386.Denk F. Tamoxifen induces cellular stress in the nervous system by inhibiting cholesterol synthesis. Acta Neuropathol. Commun. 2015;3(1):1–15. doi: 10.1186/s40478-015-0255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Boele F.W. Cognitive functioning during long-term tamoxifen treatment in postmenopausal women with breast cancer. Menopause. 2015;22(1):17–25. doi: 10.1097/GME.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 388.Park C. Tetrabromobisphenol-A induces apoptotic death of auditory cells and hearing loss. Biochem. Biophys. Res. Commun. 2016;478(4):1667–1673. doi: 10.1016/j.bbrc.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 389.Chen J. TBBPA exposure during a sensitive developmental window produces neurobehavioral changes in larval zebrafish. Environ. Pollut. 2016;216:53–63. doi: 10.1016/j.envpol.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 390.Jarema K.A. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 2015;52:194–209. doi: 10.1016/j.ntt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Wojtowicz A.K., Szychowski K.A., Kajta M. PPAR-γ agonist GW1929 but not antagonist GW9662 reduces TBBPA-induced neurotoxicity in primary neocortical cells. Neurotoxicit. Res. 2014;25(3):311–322. doi: 10.1007/s12640-013-9434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Holmes S. Effects of oxidative stress and testosterone on pro-inflammatory signaling in a female rat dopaminergic neuronal cell line. Endocrinology. 2016;157(7):2824–2835. doi: 10.1210/en.2015-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Cunningham R.L., Giuffrida A., Roberts J.L. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cδ. Endocrinology. 2009;150(12):5539–5548. doi: 10.1210/en.2009-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Xu H.-M. Dioxin and dioxin-like compounds suppress acetylcholinesterase activity via transcriptional downregulations in vitro. J. Mol. Neurosci. 2014;53(3):417–423. doi: 10.1007/s12031-013-0167-5. [DOI] [PubMed] [Google Scholar]
- 395.Pelclova D. Neurological and neurophysiological findings in workers with chronic 2, 3, 7, 8‐Tetrachlorodibenzo‐p‐Dioxin intoxication 50 years after exposure. Basic & Clin. Pharmacol. Toxicol. 2018;122(2):271–277. doi: 10.1111/bcpt.12899. [DOI] [PubMed] [Google Scholar]
- 396.Sánchez‐Martín F.J., Fernández‐Salguero P.M., Merino J.M. Aryl hydrocarbon receptor‐dependent induction of apoptosis by 2, 3, 7, 8‐tetrachlorodibenzo‐p‐dioxin in cerebellar granule cells from mouse. J. Neurochem. 2011;118(1):153–162. doi: 10.1111/j.1471-4159.2011.07291.x. [DOI] [PubMed] [Google Scholar]
- 397.Kato Y. Reduction of thyroid hormone levels by methylsulfonyl metabolites of polychlorinated biphenyl congeners in rats. Arch. Toxicol. 1998;72(8):541–544. doi: 10.1007/s002040050540. [DOI] [PubMed] [Google Scholar]
- 398.Chen D.-W. The inhibition of UDP-glucuronosyltransferases (UGTs) by tetraiodothyronine (T4) and triiodothyronine (T3) Xenobiotica. 2018;48(3):250–257. doi: 10.1080/00498254.2017.1304593. [DOI] [PubMed] [Google Scholar]
- 399.dos Reis-Lunardelli E.A. Effects of thyroid hormones on memory and on Na+, K+-ATPase activity in rat brain. Curr. Neurovascular Res. 2007;4(3):184–193. doi: 10.2174/156720207781387204. [DOI] [PubMed] [Google Scholar]
- 400.Zamoner A. Thyroid hormones reorganize the cytoskeleton of glial cells through Gfap phosphorylation and Rhoa-dependent mechanisms. Cell. Mol. Neurobiol. 2007;27(7):845–865. doi: 10.1007/s10571-006-9084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Calciu C., Chan H.M., Kubow S. Toxaphene congeners differ from toxaphene mixtures in their dysmorphogenic effects on cultured rat embryos. Toxicology. 1997;124(2):153–162. doi: 10.1016/s0300-483x(97)00145-5. [DOI] [PubMed] [Google Scholar]
- 402.Waritz R.S. Thyroid function and thyroid tumors in toxaphene-treated rats. Regul. Toxicol. Pharmacol. 1996;24(2):184–192. doi: 10.1006/rtph.1996.0124. [DOI] [PubMed] [Google Scholar]
- 403.Kodavanti P. Inhibition of calmodulin activated adenylate cyclase in rat brain by selected insecticides. Neurotoxicology. 1989;10(2):219–228. [PubMed] [Google Scholar]
- 404.Brunström B. Toxicity in chick embryos of three commercial mixtures of chlorinated paraffins and of toxaphene injected into eggs. Arch. Toxicol. 1983;54(4):353–357. doi: 10.1007/BF01234488. [DOI] [PubMed] [Google Scholar]
- 405.Leonetti C. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ. Health. 2016;15(1):1–10. doi: 10.1186/s12940-016-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Lee D. 2, 4, 6-tribromophenol interferes with the thyroid hormone system by regulating thyroid hormones and the responsible genes in mice. Int. J. Environ. Res. Public Health. 2016;13(7):697. doi: 10.3390/ijerph13070697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Lyubimov A.V., Babin V.V., Kartashov A. Developmental neurotoxicity and immunotoxicity of 2, 4, 6-tribromophenol in Wistar rats. Neurotoxicology. 1998;19(2):303–312. [PubMed] [Google Scholar]
- 408.Quinn M.J., Jr, Lavoie E.T., Ottinger M.A. Reproductive toxicity of trenbolone acetate in embryonically exposed Japanese quail. Chemosphere. 2007;66(7):1191–1196. doi: 10.1016/j.chemosphere.2006.07.085. [DOI] [PubMed] [Google Scholar]
- 409.Ishihara Y. Protective actions of 17β-estradiol and progesterone on oxidative neuronal injury induced by organometallic compounds. Oxidative Med. Cell. Longevity. 2015;2015 doi: 10.1155/2015/343706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Kotake Y. Molecular mechanisms of environmental organotin toxicity in mammals. Biol. Pharm. Bull. 2012;35(11):1876–1880. doi: 10.1248/bpb.b212017. [DOI] [PubMed] [Google Scholar]
- 411.Yeung J.A. Unusual case of anxiety: trichloroethylene neurotoxicity. Case Rep. 2017;2017 doi: 10.1136/bcr-2017-223074. p. bcr-2017-223074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Da Broi U. Medico legal investigations into sudden sniffing deaths linked with trichloroethylene. J. Forensic and Legal Med. 2015;34:81–87. doi: 10.1016/j.jflm.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 413.Kang D.-M., Kim I. Compensation for occupational neurological and mental disorders. J. Korean Med. Sci. 2014;29(Suppl):S59. doi: 10.3346/jkms.2014.29.S.S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Chiu W.A. Human health effects of trichloroethylene: key findings and scientific issues. Environ. Health Perspectives. 2013;121(3):303–311. doi: 10.1289/ehp.1205879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Bale A.S. A review of potential neurotoxic mechanisms among three chlorinated organic solvents. Toxicol. Appl. Pharm. 2011;255(1):113–126. doi: 10.1016/j.taap.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 416.Xu X. Urinary trichlorophenol levels and increased risk of attention deficit hyperactivity disorder among US school-aged children. Occupat. Environ. Med. 2011;68(8):557–561. doi: 10.1136/oem.2010.063859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Dong X. Multiple bioanalytical method to reveal developmental biological responses in zebrafish embryos exposed to triclocarban. Chemosphere. 2018;193:251–258. doi: 10.1016/j.chemosphere.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 418.Barros S. Chronic effects of triclocarban in the amphipod Gammarus locusta: behavioural and biochemical impairment. Ecotoxicol. Environ. Safety. 2017;135:276–283. doi: 10.1016/j.ecoenv.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 419.Wu Y., Beland F.A., Fang J.-L. Effect of triclosan, triclocarban, 2, 2′, 4, 4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol. Vitro. 2016;32:310–319. doi: 10.1016/j.tiv.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 420.Szychowski K.A. Triclosan activates aryl hydrocarbon receptor (AhR)-dependent apoptosis and affects Cyp1a1 and Cyp1b1 expression in mouse neocortical neurons. Environ. Res. 2016;151:106–114. doi: 10.1016/j.envres.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 421.Noda M. Thyroid hormone in the CNS: contribution of neuron–glia interaction. Vitamins and hormones. 2018;106:313–331. doi: 10.1016/bs.vh.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 422.Zhang Z. Hypothalamic effects of thyroid hormone. Mol. Cell. Endocrinol. 2017;458:143–148. doi: 10.1016/j.mce.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 423.Ortiga‐Carvalho T.M. Hypothalamus‐pituitary‐thyroid axis. Compr. Physiol. 2011;6(3):1387–1428. doi: 10.1002/cphy.c150027. [DOI] [PubMed] [Google Scholar]
- 424.Sun L. Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka (Oryzias latipes) Environ. Toxicol. Chem. 2016;35(12):2931–2940. doi: 10.1002/etc.3477. [DOI] [PubMed] [Google Scholar]
- 425.Kim S. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquatic Toxicol. 2015;160:188–196. doi: 10.1016/j.aquatox.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 426.Tanaka D. Effects of dark-rearing on triphenyl phosphite-induced neuropathy in the visual system of the developing european ferret (Mustela putorius furo) J. Toxicol. Environ. Health Part A. 1999;58(4):215–231. doi: 10.1080/009841099157304. [DOI] [PubMed] [Google Scholar]
- 427.Karpiak V.C., Bridges R.J., Eyer C.L. Organotins disrupt components of glutamate homeostasis in rat astrocyte cultures. J. Toxicol. Environ. Health Part A. 2001;63(4):273–287. doi: 10.1080/15287390151143668. [DOI] [PubMed] [Google Scholar]
- 428.Lin T.-J. Unique cerebral dysfunction following triphenyltin acetate poisoning. Human & Exp. Toxicol. 1998;17(7):403–405. doi: 10.1177/096032719801700707. [DOI] [PubMed] [Google Scholar]
- 429.Attahiru U. Acute toxicity studies of tri-n-butyltin and triphenyltin acetates in rats. Vet. Human Toxicol. 1991;33(6):554–556. [PubMed] [Google Scholar]
- 430.Lehotzky K. The neurotoxicity of organotin: behavioural changes in rats. Acta Biologica Academiae Scientiarum Hungaricae. 1982;33(1):15–22. [PubMed] [Google Scholar]
- 431.Belovicova K. Effects of venlafaxine and chronic unpredictable stress on behavior and hippocampal neurogenesis of rat dams. Neuroendocrinol. Lett. 2017;38(1) [PubMed] [Google Scholar]
- 432.Singh M. Assessment of in-utero venlafaxine induced, ROS-mediated, apoptotic neurodegeneration in fetal neocortex and neurobehavioral sequelae in rat offspring. Int. J. Dev. Neurosci. 2015;40:60–69. doi: 10.1016/j.ijdevneu.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 433.Pinzani V. Venlafaxine withdrawal syndrome: report of six cases and review of the literature. La Revue de Med. Interne. 2000;21(3):282–284. doi: 10.1016/s0248-8663(00)80048-x. [DOI] [PubMed] [Google Scholar]
- 434.Gillette R. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology. 2014;155(10):3853–3866. doi: 10.1210/en.2014-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Skinner M.K. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PloS one. 2008;3(11):e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.André S.M., Markowski V.P. Learning deficits expressed as delayed extinction of a conditioned running response following perinatal exposure to vinclozolin. Neurotoxicol. Teratol. 2006;28(4):482–488. doi: 10.1016/j.ntt.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 437.Makowska K. The influence of low doses of zearalenone and T-2 toxin on calcitonin gene related peptide-like immunoreactive (CGRP-LI) neurons in the ENS of the porcine descending colon. Toxins. 2017;9(3):98. doi: 10.3390/toxins9030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Obremski K., Gonkowski S., Wojtacha P. Zearalenone-induced changes in the lymphoid tissue and mucosal nerve fibers in the porcine ileum. Polish J. Vet. Sci. 2015 doi: 10.1515/pjvs-2015-0046. [DOI] [PubMed] [Google Scholar]
- 439.Venkataramana M. Zearalenone induced toxicity in SHSY-5Y cells: the role of oxidative stress evidenced by N-acetyl cysteine. Food Chem. Toxicol. 2014;65:335–342. doi: 10.1016/j.fct.2013.12.042. [DOI] [PubMed] [Google Scholar]