Abstract
Background and Purpose:
Endovascular thrombectomy (EVT) has revolutionized large vessel occlusion stroke care. However, not all patients with good endovascular results achieve good outcomes. We sought to understand the clinical significance of MRI-defined infarct growth despite adequate reperfusion and identify associated clinical and radiographic variables.
Methods:
History, presentation, treatments, and outcomes for consecutive EVT patients at a referral center were collected. Adequate reperfusion was defined as thrombolysis in cerebral infarction (TICI) score 2b-3. Region-specific infarct volumes in white matter, cortex, and basal ganglia were determined on diffusion-weighted imaging (DWI). Infarct growth was defined as post-EVT minus pre-EVT volume. Good outcome was defined as 90-day modified Rankin scale ≤2.
Results:
44 patients with adequate reperfusion were identified with median age 72 years; 64% were women. Each region showed infarct growth: white matter (median pre-EVT 7cc, post-EVT 16cc), cortex (4cc, 15cc), basal ganglia (2cc, 4cc), total (20cc, 39cc). In multivariable regression, total infarct growth independently decreased the odds of good outcome (aOR=0.946, 95%CI=0.897,0.998). Further multivariable analyses for determinants of infarct growth identified female sex was associated with less total growth (β=−0.294, P=0.042), TICI 3 was associated with less white matter growth (β=−0.277, P=0.048) and cortical growth (β=−0.335, p=0.017), and both female sex (β=−0.332, P=0.015) and coronary disease (β=−0.337, P=0.015) were associated with less cortical growth.
Conclusions:
Infarct growth occurred despite adequate reperfusion, disproportionately in the cortex, and independently decreased the odds of good outcome. Infarct growth occurred while patients were hospitalized and may represent a therapeutic target. Potential determinants of region-specific infarct growth were identified that require confirmation in larger studies.
Keywords: ischemic stroke, magnetic resonance imaging, cerebral infarction, endovascular thrombectomy, large vessel occlusion
Introduction
Ischemic strokes due to large vessel occlusion (LVO) account for the largest proportion of stroke-related disability and death.1,2 Endovascular thrombectomy (EVT) has revolutionized the acute care of these patients,3-5 but over half are functionally disabled or dead at 90 days despite treatment.3 Understanding the determinants of long-term outcomes after EVT therefore represents a highly clinically relevant issue with potential to directly impact patient care.6 One hypothesis that may explain poor outcomes is infarct growth despite EVT. Rates of reperfusion,7 distal embolization, and re-occlusion8 are variables that may be mitigated with new devices and techniques. However, there is growing data to suggest infarct growth may occur to some extent despite adequate reperfusion in many patients.9
The science of LVO stroke in the current EVT era is limited by our understanding of the effects of EVT at the level of the brain parenchyma beyond removal of the vessel occlusion. A ‘no-reflow’ phenomenon has been long described in the cardiology literature in which there is inadequate capillary reperfusion despite large vessel recanalization.10 Indeed there is growing interest in ‘futile recanalization’ during ischemic stroke, where there is substantial final infarct despite LVO removal.11,12 Infarct growth despite adequate reperfusion may be related to microvascular compromise, impaired autoregulation, and reperfusion injury, among other factors.13 Furthermore, preclinical studies suggest there may be differences in ischemia tolerance comparing gray versus white matter.14 We sought to quantify the degree of region-specific MRI-defined infarct growth in the white matter, cortex, and basal ganglia, despite adequate reperfusion, understand the relationship between infarct growth and outcomes, and identify determinants of region-specific infarct growth.
Methods
Study Population and Variables of Interest
Patients who underwent anterior circulation EVT were identified retrospectively from a prospectively maintained database at a single referral center.15 This database includes demographic information, medical history, clinical presentation, treatments, and outcomes for consecutive patients treated with EVT. Stroke severity (National Institutes of Health Stroke Scale, NIHSS) was determined as described.4 Alteplase treatment decisions were guideline-based and at the discretion of a vascular neurologist. EVT treatment decisions were at the discretion of the treating vascular neurologist and neurointerventionalist. Collateral grade was determined as previously described;16 the same scale was also used to assess MR angiograms when CT angiograms were not available.. Thrombolysis in cerebral infarction (TICI) scores were determined by a neurointerventionalist using the modified scale: 2a partial filling <50%, 2b partial filling ≥50%, 3 complete perfusion.4 Adequate reperfusion was defined as TICI 2b-3. Those with TICI 2b-3 were chosen for study because they represent EVT treatment success clinically.3 TICI 3 was included in multivariable models to control for the extent of adequate reperfusion. Cervical ICA stenosis was defined as >70% by NASCET criteria.17 ICH was defined as any symptomatic or asymptomatic PH1 or PH2 by ECASS criteria during the hospitalization.18 90-day modified Rankin Scale (mRS)19,20 was obtained by telephone call and available for 87% of the cohort. Good functional outcome was defined as 90-day mRS ≤2.
Image Analysis
Patients with available pre- and post-EVT MRIs were identified. Clinical MRIs were downloaded with OsiriX (Pixmeo). Diffusion-weighted imaging (DWI) sequences were obtained on Siemens 3T or a GE 1.5T MRI with echo time 60-120 ms, repetition time 5300-5600 ms, and slice thickness 5 mm with 1mm gap. Infarct lesion masks were manually traced by a vascular neurologist using Slicer 4.8.1 (Brigham and Women’s Hospital),21 blinded to clinical data. RegLSM (University of Calgary) was used to register these to MNI-152 space.22 The registration procedure consisted of linear registration followed by nonlinear registration using the elastix toolbox. Registered infarct masks were overlaid with the Harvard-Oxford subcortical structural atlas to determine in structure-specific infarct volumes using FSL (FMRIB Analysis Group).23 Regions of interest were white matter, cortex, and basal ganglia (Figure 1). All DWI sequences and DWI infarct registrations were inspected manually, blinded to clinical data, and those with poor registration were excluded. As an additional quality control, repeat manual inspection was performed if pre-EVT infarct volume was >70cc (local treatment guidelines) or post-EVT infarct volume was >300cc (average MCA territory volume24). Infarct growth was defined as post-EVT infarct volume minus pre-EVT infarct volume. Percent infarct growth was calculated as infarct growth divided by pre-EVT infarct volume then multiplied by 100.
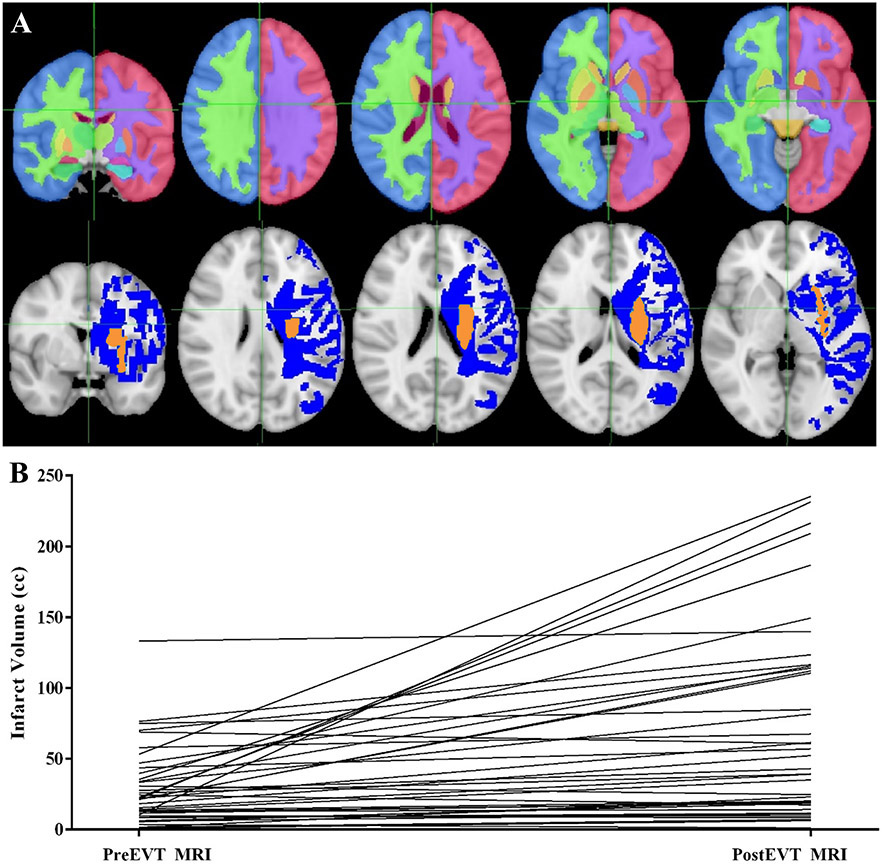
Figure 1.
Region specific infarct growth after endovascular thrombectomy (EVT) despite adequate reperfusion. Panel A: Top row shows sections through Harvard-Oxford subcortical atlas, demonstrating white matter, cortex, and basal ganglia regions, overlaid on MNI 152 standard brain. Bottom row shows sections through a single as a patient representative example of an infarct that grew from pre-EVT (orange) to post-EVT (blue) overlaid on MNI 152 standard brain. Panel B: Change in infarct volume for individual patients from pre-EVT to post-EVT.
Statistical Analysis and Ethics Review
Median and interquartile range (IQR) were reported for continuous variables. Percent and count were reported for categorical variables. Differences were assessed using nonparametric Wilcoxon rank-sum for continuous variables and Fisher’s Exact tests for categorical variables. Logistic regressions were performed for associations with good outcome. Linear regressions were performed for associations infarct volume growth. While we aimed to limit the transformation of data when not required for a statistical test, infarct volumes were natural log-transformed for use in linear regressions given they were not normally distributed. Negative changes in region-specific infarct volume were considered the smallest positive change for transformation as positive values were required (all <0.25cc). Variables with pre-specified significance of p<0.10 in univariable analyses were subsequently included in multivariable models. Two-tailed P values <0.05 were interpreted as statistically significant. Analyses were performed with SPSS Statistics, version 23.0 (IBM Corp). This study was approved by the local institutional review board. Informed consent was waived based on minimal patient risk and practical inability to perform the study without the waiver.
Results
381 consecutive patients underwent EVT for anterior LVO from January 2011 to September 2019. Both pre- and post-EVT MRIs were available for 81 patients. 58 patients (72%) had available DWI imaging for infarct outlining that registered adequately for both scans. 44 patients (76%) achieved TICI 2b-3 reperfusion after EVT. Of these patients, the median age was 72 years (IQR 62-79) and 64% were women. Risk factors included hypertension (66%), diabetes (14%), atrial fibrillation (27%), and prior stroke/transient ischemic attack (18%). Clinical presentation and treatment variables of the cohort included: median NIHSS 16 (IQR 13-19), alteplase treatment (48%), good collaterals (55%), median last known well (LKW)-to-groin time 342 min (IQR 214-528), and complete TICI 3 reperfusion (21%, Table 1).
Table 1.
Demographics, medical history, clinical presentations, treatments, infarct volumes, and outcomes of large vessel occlusion stroke patients treated with endovascular thrombectomy (EVT).
| Median/Count | IQR/Percent | |
|---|---|---|
| Age, Years | 72 | 62-79 |
| Female | 28 | 64% |
| Race/Ethnicity | ||
| White | 37 | 84% |
| Hispanic | 3 | 7% |
| Asian | 3 | 7% |
| Black | 0 | 0 |
| Past Medical History | ||
| Hypertension | 29 | 66% |
| Diabetes | 6 | 14% |
| Atrial Fibrillation | 12 | 27% |
| Stroke/TIA History | 8 | 18% |
| Coronary Artery Disease | 6 | 14% |
| Presenting NIHSS | 16 | 13-19 |
| Cervical ICA Stenosis/Occlusion | 9 | 21% |
| Intracranial Occlusion Site | ||
| ICA Terminus | 10 | 23% |
| M1 | 27 | 61% |
| M2 | 7 | 16% |
| Collateral Grade | 2 | 1,3 |
| 0 | 8 | 18% |
| 1 | 12 | 27% |
| 2 | 7 | 16% |
| 3 | 17 | 39% |
| Intravenous Alteplase | 21 | 48% |
| LKW-Alteplase Time, Min | 135 | 80-176 |
| LKW-Groin Time, Min | 342 | 214-528 |
| Groin-Recanalization Time, Min | 46 | 29-66 |
| Anesthesia Type | ||
| General | 10 | 23% |
| Conscious Sedation | 34 | 77% |
| Number Passes, Total | 1.5 | 1.0-3.0 |
| Any Aspiration Only Pass | 28 | 64% |
| Any Stentriever Pass | 23 | 52% |
| Any Merci Pass | 2 | 5% |
| TICI 3 | 9 | 21% |
| Pre-EVT-Post-EVT MRI Time, Hours | 24 | 19-43 |
| Left Side Infarct | 24 | 55% |
| Total Infarct | ||
| Pre-EVT Volume, cc | 20 | 10-35 |
| Post-EVT Volume, cc | 39 | 15-114 |
| Volume Growth, cc | 13 | 5-47 |
| Percent Change | 76% | 19-322% |
| White Matter Infarct | ||
| Pre-EVT Volume, cc | 7 | 4-17 |
| Post-EVT Volume, cc | 16 | 5-43 |
| Volume Growth, cc | 4 | 1-17 |
| Percent Change | 61% | 9-260% |
| Cortex Infarct | ||
| Pre-EVT Volume, cc | 4 | 1-14 |
| Post-EVT Volume, cc | 15 | 5-45 |
| Volume Growth, cc | 6 | 1-23 |
| Percent Change | 177% | 40-889% |
| Basal Ganglia Infarct | ||
| Pre-EVT Volume, cc | 2 | 0-5 |
| Post-EVT Volume, cc | 4 | 1-8 |
| Volume Growth, cc | 1 | 0-2 |
| Percent Change | 54% | 7-269% |
| ICH | 3 | 7% |
| 90-day Good Outcome (mRS 0-2) | 18 | 46% |
Abbreviations: TIA: transient ischemic attack, NIHSS: NIH Stroke Scale, ICA: internal carotid artery, M1: first segment of middle cerebral artery, M2: second segment, LKW: last known well, TICI: thrombolysis in cerebral infarction, MRI: magnetic resonance imaging, cc: cubic centimeters, ICH: intracerebral hemorrhage, mRS: modified Rankin Scale, IQR: interquartile range.
The median time between MRI studies was 24 hours (IQR 19-42). 55% of the cohort had left-sided infarcts. Infarct growth from pre-EVT to post-EVT MRI was observed for total infarct volume and for each region of interest (white matter, cortex, and basal ganglia). Median pre-EVT total infarct volume was 20 cc (IQR 10-35), post-EVT was 39 cc (IQR 15-114), and median percent growth was 76% (IQR 19-322%). Median pre-EVT white matter infarct volume was 7cc (IQR 4-17), post-EVT was 16cc (IQR 5-43), and median percent growth was 61% (IQR 9-260%). Median pre-EVT cortical infarct volume was 4 cc (IQR 1-14), post-EVT was 15 cc (IQR 5-45), and median percent growth was 177% (IQR 40-889%). Median pre-EVT basal ganglia infarct volume was 2 cc (IQR 0-5), post-EVT was 4 cc (IQR 1-8), and median percent growth was 54% (IQR 7-269%).A minority of DWI lesions had little change, with 6 patients experiencing less than 5% total lesion volume growth and 3 experiencing total lesion volume reversal by >5 cc (Figures 1, Table 1).
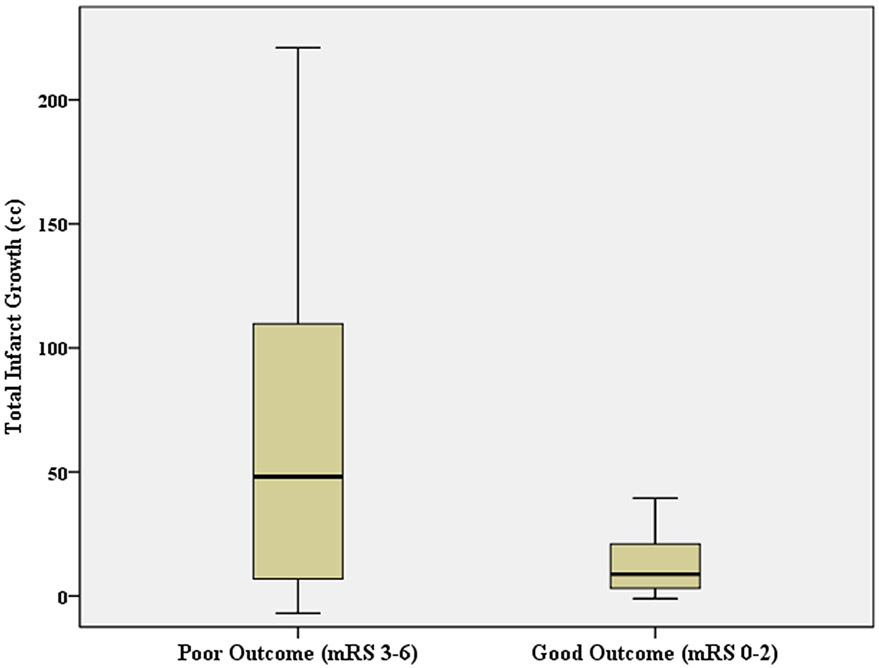
Total infarct growth independently decreased the odds of 90-day good functional outcome (OR=0.946, 95%CI=0.897-0.998) in a multivariable model including age (OR=0.796, 95%CI=0.639-0.993), TICI 3 reperfusion (OR=2.364, 95%CI=0.122-45.83), pre-EVT infarct volume (OR=0.907, 95%CI=0.798-1.030), and left-sided infarction (OR=3.622, 95%CI=0.332-39.55) as additional determinants (Table 2). Figure 2 shows total infarct growth for those with good versus poor 90-day outcomes. Individual brain regions were also assessed. Infarct growth in white matter and cortex decreased the odds of good outcome in univariable analyses but did not reach significance when both were combined in a multivariable model (white matter infarct growth: OR=0.970, 95%CI=0.800-1.175; cortex infarct growth: OR=0.912, 95%CI=0.768-1.082) that included age (OR=0.776, 95%CI=0.607-0.991), pre-EVT white matter infarct volume (OR=0.704, 95%CI=0.489-1.015), TICI 3 (OR=1.733, 95%CI=0.069-43.28), and left side infarct (OR=3.558, 95%CI=0.219-57.69). There were no associations found with other variables (Table 2).
Table 2.
Total infarct growth independently decreased the odds of 90-day good functional outcome (modified Rankin Scale 0-2) among large vessel occlusion stroke patients treated with endovascular thrombectomy (EVT).
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age, Years | 0.956 (0.911,1.004) | 0.071 | 0.796 (0.639,0.993) | 0.043 |
| Female | 2.364 (0.619,9.032) | 0.209 | ||
| Hypertension | 1.000 (0.263,3.802) | 1.000 | ||
| Diabetes | 1.187 (0.150,9.408) | 0.871 | ||
| Atrial Fibrillation | 1.600 (0.393,6.509) | 0.511 | ||
| Stroke/TIA History | 0.640 (0.130,3.155) | 0.583 | ||
| Coronary Artery Disease | 0.188 (0.020,1.791) | 0.146 | ||
| Cervical ICA Stenosis/Occlusion | 0.500 (0.105,2.379) | 0.384 | ||
| Collateral Grade | 1.011 (0.595,1.719) | 0.967 | ||
| Intravenous Alteplase | 1.375 (0.388,4.867) | 0.621 | ||
| LKW-Alteplase Time, Min | 1.003 (0.990,1.017) | 0.641 | ||
| Pre-EVT Total Infarct Volume, cc | 0.953 (0.913,0.995) | 0.029 | 0.907 (0.798,1.030) | 0.133 |
| Pre-EVT White Matter Infarct Volume, cc | 0.844 (0.748,0.953) | 0.006 | ||
| Pre-EVT Cortex Infarct Volume, cc | 0.952 (0.894,1.014) | 0.125 | ||
| Pre-EVT Basal Ganglia Infarct Volume, cc | 0.895 (0.730,1.099) | 0.290 | ||
| LKW-Groin Time, Min | 1.000 (0.998,1.002) | 0.953 | ||
| Groin-Recanalization Time, Min | 0.996 (0.976,1.016) | 0.675 | ||
| General Anesthesia | 0.400 (0.086,1.859) | 0.242 | ||
| Total Number Passes | 0.987 (0.558,1.746) | 0.964 | ||
| TICI 3 Reperfusion | 6.045 (1.063,34.38) | 0.042 | 2.364 (0.122,45.83) | 0.570 |
| Left Side Infarct | 5.687 (1.378,23.48) | 0.016 | 3.622 (0.332,39.55) | 0.291 |
| Total Infarct Growth, cc | 0.967 (0.939,0.995) | 0.023 | 0.946 (0.897,0.998) | 0.042 |
| White Matter Infarct Growth, cc | 0.938 (0.884,0.994) | 0.031 | ||
| Cortex Infarct Growth, cc | 0.931 (0.875,0.992) | 0.027 | ||
| Basal Ganglia Infarct Growth, cc | 0.845 (0.599,1.191) | 0.335 | ||
In a multivariable model (Nagelkerke R2=0.767) including total infarct growth and other variables associated with outcome in univariable analyses, total infarct growth and older age independently decreased the odds of 90-day good outcome. The odds of good outcome are decreased to 94.6% per cc or 57.6% per 10cc of total infarct growth. Abbreviations: TIA: transient ischemic attack, ICA: internal carotid artery, last known well, cc: cubic centimeters, TICI: thrombolysis in cerebral infarction, OR: odds ratio, CI: confidence interval.
Figure 2.
Total infarct growth despite endovascular thrombectomy (EVT) adequate reperfusion for large vessel occlusion stroke patients with good (modified Rankin Scale, mRS 0-2) versus poor 90-day outcomes. Box and whiskers plot showing median, interquartile range, minimum, and maximum.
To begin to identify the influential factors for infarct growth, regression analyses for determinants of total infarct growth were performed. Univariable analyses suggested age, female sex, hypertension, atrial fibrillation, alteplase, and complete TICI 3 reperfusion may have associations with total infarct growth. However, when all were included in a multivariable model, only female sex remained associated with less total infarct growth (β=−0.294, P=0.042; Table 3). Women showed a trend toward better median collateral grades (2.5 vs 1, p=0.055) and smaller pre-EVT infarct volumes (14cc vs 25cc, p=0.075) compared to men. Besides differences in total and cortical infarct growth, no other differences were identified (Table 4).
Table 3.
Associations with natural logarithm-transformed total, white matter, cortex, and basal ganglia infarct growth among large vessel occlusion stroke patients treated with endovascular thrombectomy (EVT).
| Total Infarct Growth | White Matter Infarct Growth | Cortex Infarct Growth | Basal Ganglia Infarct Growth | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | Univariable | Multivariable | |||||||||
| β | P | β | P | β | P | β | P | β | P | β | P | β | P | β | P | |
| Age, Years | −0.263 | 0.002 | −0.041 | 0.841 | −0.198 | 0.197 | −0.142 | 0.358 | −0.347 | 0.021 | −0.180 | 0.195 | ||||
| Female | −0.358 | 0.017 | −0.294 | 0.042 | −0.277 | 0.069 | −0.130 | 0.332 | −0.391 | 0.009 | −0.33 | 0.015 | −0.048 | 0.756 | ||
| Hypertension | −0.267 | 0.080 | −0.161 | 0.388 | −0.247 | 0.107 | −0.130 | 0.402 | −0.251 | 0.100 | ||||||
| Diabetes | −0.178 | 0.247 | −0.020 | 0.896 | −0.204 | 0.185 | 0.048 | 0.759 | ||||||||
| Atrial Fibrillation | −0.289 | 0.057 | −0.077 | 0.625 | −0.201 | 0.192 | −0.215 | 0.161 | −0.153 | 0.323 | ||||||
| Stroke/TIA History | −0.191 | 0.215 | −0.133 | 0.391 | −0.233 | 0.128 | −0.228 | 0.137 | ||||||||
| Coronary Artery Disease | −0.165 | 0.284 | −0.079 | 0.609 | −0.278 | 0.068 | −0.34 | 0.015 | 0.208 | 0.176 | ||||||
| Cervical ICA Disease | 0.243 | 0.111 | 0.262 | 0.086 | 0.209 | 0.143 | 0.142 | 0.358 | 0.393 | 0.008 | 0.272 | 0.047 | ||||
| ICA Terminus Occlusion | 0.011 | 0.943 | −0.020 | 0.897 | −0.026 | 0.868 | 0.089 | 0.564 | ||||||||
| M1 Occlusion | −0.202 | 0.188 | −0.185 | 0.230 | −0.102 | 0.509 | −0.046 | 0.767 | ||||||||
| M2 Occlusion | 0.256 | 0.093 | 0.190 | 0.191 | 0.269 | 0.077 | 0.206 | 0.131 | 0.166 | 0.282 | −0.041 | 0.791 | ||||
| Collateral Grade | 0.158 | 0.305 | 0.243 | 0.112 | 0.148 | 0.337 | 0.152 | 0.325 | ||||||||
| Intravenous Alteplase | 0.271 | 0.075 | 0.215 | 0.182 | 0.284 | 0.062 | 0.261 | 0.057 | 0.125 | 0.418 | 0.242 | 0.114 | ||||
| LKW-Alteplase Time, Min |
0.237 | 0.301 | 0.289 | 0.204 | 0.263 | 0.249 | −0.249 | 0.277 | ||||||||
| LKW-Pre-EVT MRI Time, Min |
−0.158 | 0.307 | −0.173 | 0.261 | −0.068 | 0.662 | −0.431 | 0.003 | −0.254 | 0.114 | ||||||
| Pre-EVT Total Infarct Volume |
0.189 | 0.218 | 0.195 | 0.205 | 0.065 | 0.677 | 0.262 | 0.086 | ||||||||
| Pre-EVT White Matter Infarct Volume |
0.155 | 0.315 | 0.167 | 0.279 | 0.001 | 0.994 | 0.262 | 0.085 | 0.190 | 0.138 | ||||||
| Pre-EVT Cortex Infarct Volume |
0.179 | 0.244 | 0.175 | 0.256 | 0.056 | 0.717 | 0.215 | 0.160 | ||||||||
| Pre-EVT Basal Ganglia Infarct Volume |
0.165 | 0.285 | 0.19 | 0.218 | 0.161 | 0.296 | 0.137 | 0.377 | ||||||||
| LKW-Groin Time, Min | −0.005 | 0.973 | 0.017 | 0.911 | 0.133 | 0.388 | −0.489 | 0.001 | −0.195 | 0.244 | ||||||
| Groin-Recanalization Time, Min |
0.129 | 0.414 | 0.118 | 0.456 | 0.073 | 0.646 | 0.238 | 0.129 | ||||||||
| Pre-EVT MRI- Reperfusion Time, Min |
0.215 | 0.171 | 0.252 | 0.108 | 0.189 | 0.230 | 0.235 | 0.134 | ||||||||
| General Anesthesia | 0.083 | 0.592 | 0.176 | 0.254 | 0.027 | 0.861 | −0.036 | 0.817 | ||||||||
| Total Number Passes | 0.140 | 0.365 | 0.110 | 0.478 | 0.182 | 0.236 | 0.240 | 0.116 | ||||||||
| TICI 3 Complete Reperfusion |
−0.268 | 0.079 | −0.171 | 0.262 | −0.354 | 0.019 | −0.280 | 0.048 | −0.316 | 0.036 | −0.34 | 0.017 | −0.007 | 0.964 | ||
| Pre-EVT-Post-EVT MRI Time, Hours |
0.196 | 0.202 | 0.259 | 0.090 | 0.240 | 0.083 | 0.125 | 0.420 | 0.067 | 0.666 | ||||||
Several variables were identified as being associated with region-specific infarct growth in univariable analyses. In a multivariable model for determinants of total infarct growth (R2=0.334, p=0.029), female sex was independently associated with less growth. In a multivariable model for determinants of white matter infarct growth (R2=0.381, p=0.005), TICI 3 was independently associated with less growth. In a multivariable model for determinants of cortical infarct growth (R2=0.329, p=0.001), female sex, coronary artery disease, and TICI 3 were independently associated with less growth. In a multivariable model for determinants of basal ganglia infarct growth (R2=0.348, p=0.001), cervical ICA disease was associated with more growth. Abbreviations: TIA: transient ischemic attack, ICA: internal carotid artery, M1: first segment of middle cerebral artery, M2: second segment, LKW: last known well, cc: cubic centimeters, TICI: thrombolysis in cerebral infarction, β: parameter estimate.
Table 4.
Demographics, medical history, clinical presentations, treatments, and infarct volumes of men versus women with large vessel occlusion stroke treated with endovascular thrombectomy (EVT).
| Men | Women | ||||
|---|---|---|---|---|---|
| Median/Count | IQR/Percent | Median/Count | IQR/Percent | P | |
| Age | 73 | 58-78 | 71 | 64-78 | 0.903 |
| Hypertension | 10 | 63 | 19 | 68 | 0.751 |
| Diabetes | 1 | 6 | 5 | 18 | 0.392 |
| Atrial Fibrillation | 3 | 19 | 9 | 32 | 0.487 |
| Stroke/TIA History | 2 | 13 | 6 | 21 | 0.689 |
| Coronary Artery Disease | 2 | 13 | 4 | 14 | 1.000 |
| Presenting NIHSS | 17 | 15-18 | 16 | 13-19 | 0.835 |
| Cervical ICA Stenosis/Occlusion | 5 | 31 | 4 | 14 | 0.250 |
| ICA Terminus Occlusion | 3 | 19 | 7 | 24 | 0.724 |
| M1 Occlusion | 10 | 63 | 17 | 61 | 1.000 |
| M2 Occlusion | 3 | 19 | 4 | 14 | 0.692 |
| Collateral Grade | 1 | 1,2 | 2.5 | 1-3 | 0.055 |
| Intravenous Alteplase | 8 | 50 | 13 | 46 | 1.000 |
| LKW-Alteplase Time, Min | 112 | 90-131 | 155 | 120-176 | 0.232 |
| Pre-EVT Total Volume, cc | 25 | 21-41 | 14 | 10-31 | 0.075 |
| Pre-EVT White Matter Volume, cc | 14 | 5-18 | 5 | 4-16 | 0.107 |
| Pre-EVT Cortical Volume, cc | 8 | 2-19 | 3 | 0-13 | 0.107 |
| Pre-EVT Basal Ganglia Volume, cc | 3 | 1-6 | 2 | 0-5 | 0.317 |
| Left Side Infarct | 8 | 50 | 16 | 57 | 0.757 |
| LKW-Groin Time, Min | 372 | 286-739 | 313 | 211-504 | 0.421 |
| Groin-Recanalization Time, Min | 42 | 32-59 | 51 | 30-66 | 0.753 |
| General Anesthesia | 4 | 25 | 6 | 22 | 1.000 |
| Number Passes, Total | 2 | 1,3 | 1 | 1-3 | 0.426 |
| Any Aspiration Only Pass | 13 | 81 | 15 | 54 | 0.104 |
| Any Stentriever Pass | 7 | 44 | 16 | 57 | 0.533 |
| Any Merci Pass | 0 | 0 | 2 | 7 | 0.526 |
| TICI 3 | 2 | 13 | 7 | 25 | 0.450 |
| Pre-EVT-Post-EVT MRI Time, Hours | 31.5 | 17-46 | 24 | 19-33 | 0.380 |
| Total Infarct Growth, cc | 40 | 13-94 | 7 | 1-22 | 0.015 |
| White Matter Infarct Growth, cc | 12 | 2-44 | 2 | 0-12 | 0.071 |
| Cortex Infarct Growth, cc | 18 | 6-45 | 2 | 0-12 | 0.010 |
| Basal Ganglia Infarct Growth, cc | 1.5 | 0-2 | 1 | 0-2 | 0.696 |
Compared to men, women had significantly less total and cortical infarct growth and non-significantly better median collateral grades and smaller pre-EVT infarct volumes. No other differences were identified. Abbreviations: TIA: transient ischemic attack, NIHSS: NIH Stroke Scale, ICA: internal carotid artery, M1: first segment of middle cerebral artery, M2: second segment, LKW: last known well, TICI: thrombolysis in cerebral infarction, MRI: magnetic resonance imaging, cc: cubic centimeters, ICH: intracerebral hemorrhage, mRS: modified Rankin Scale, IQR: interquartile range.
Determinants of region-specific infarct growth were also explored. Complete TICI 3 reperfusion (β=−0.277, P=0.048) was independently associated with less white matter infarct growth, while alteplase (β=0.261, P=0.057) and pre-EVT-to-post-EVT MRI time (β=0.240, P=0.083) trended toward association in a multivariable model (Table 3). Female sex (β=−0.332, P=0.015), coronary artery disease (β=−0.337, P=0.015), and complete TICI 3 reperfusion (β=−0.335, p=0.017) were independently associated with less cortex infarct growth (Table 3). Only cervical ICA stenosis/occlusion (β=0.272, P=0.047) was independently associated with basal ganglia infarct growth (Table 3).
Discussion
In this retrospective analysis of anterior circulation LVO stroke patients who achieved adequate reperfusion with EVT, we show that there is significant infarct growth despite reperfusion, to a greater extent in the cortex. Importantly, total infarct growth is independently associated with poor outcomes when included in a model with other deterministic variables. Lastly, we show that female sex is independently associated with reduced total infarct growth and other distinct associations with region-specific infarct growth.
We observed median pre-EVT infarct volume was 20 cc, post-EVT was 39 cc, and median percent growth was 76% over a median of 24 hours between scans in the present cohort of patients achieving TICI 2b-3 reperfusion. In comparison, one other small study of patients who achieved TICI 2c-3 (2c defined as near-complete reperfusion) showed mean pre-EVT infarct volume was 17 cc and post-EVT infarct volume was 48 cc. While insightful, this study included patients treated from 2008-2013, often with first generation devices, and CT was used instead of MRI to assess infarct volumes in over half.25
In the present analysis, total infarct growth despite adequate reperfusion after EVT was associated with decreased odds of good 90-day functional outcomes in a model adjusting for age, TICI 3 reperfusion, pre-EVT infarct volume, and left-sided infarction. In line with our observation, one study of 66 patients who underwent EVT showed that individuals with the most infarct growth were least likely to have good outcomes at 30 days.26 Another study of 60 patients treated with EVT who achieved TICI 2c-3 reperfusion showed that infarct growth >12cc, predominantly assessed by CT, was associated with lower rates of good functional outcome (23% vs 48%).25 This supports that total infarct growth despite good reperfusion is clinically relevant, especially as it occurs while patients are hospitalized and may represent a feasible therapeutic target to improve stroke outcomes. We also assessed the importance of infarct growth in particular brain regions. While white matter infarct growth and cortical infarct growth were associated with decreased odds of good outcome in univariable regressions, they did not reach significance in a multivariable model. This observation may be related to collinearity between these variables and smaller infarct volumes perhaps having less clinical significance when considering the sub-regions individually.
To begin to understand mechanisms, we sought to identify determinants of infarct growth despite adequate perfusion. In a multivariable regression analysis, only female sex was independently associated with decreased total infarct growth. Interestingly, women with LVO among a DEFUSE 3 cohort had better collaterals, smaller pre-EVT core volumes, and slower infarct growth.27 While neither pre-EVT infarct volumes nor collaterals were associated with infarct growth in multivariable analyses of our cohort, women trended toward better median collateral grades and smaller pre-EVT infarct volumes compared to men. Other studies of infarct growth after EVT that were not restricted to patients with adequate reperfusion did not report sex-specific associations, but did show associations with larger pre-EVT infarct volume, poor collaterals, alteplase treatment, longer LKW-imaging time, and failure to achieve TICI 2b-3 reperfusion, among others.9,26,28 The aforementioned study that evaluated a small cohort with TICI 2c-3 reperfusion showed that infarct growth >12cc, evaluated by CT in most cases (mean 6 days apart), was associated with the use of first generation devices (as opposed to stentrievers), not receiving alteplase, and Hispanic ethnicity. There was no association with complete TICI 3 or with collateral grade.25
The present study is the first, to our knowledge, to examine region-specific MRI-defined infarct growth in the setting of EVT. Region-specific analyses showed TICI 3 complete reperfusion was independently associated with less white matter infarct growth, while alteplase and time between MRIs trended toward association with more white matter infarct growth. TICI 3 complete reperfusion has been associated with improved outcomes,29 and one study of 281 patients who underwent EVT (63% with post-EVT MRI at 24 hours) showed one of the strongest associations with reduced infarct growth was TICI 2b-3 reperfusion,9 suggesting TICI 3 may have an even greater effect. We also observed disproportionate infarct growth in the cortex compared to other regions on post-EVT MRI. This greater percentage infarct growth in the cortex may reflect varying hypoxemia tolerance of gray versus white matter in setting of hypoperfusion,14 likely related to different metabolic demands and different chemical cascades triggered by ischemia.30,31 Regional selective vulnerability to ischemia has been described for several structures, including the striatum.32 Female sex, coronary artery disease, and complete TICI 3 reperfusion were all independently associated with less cortical infarct growth. The association of female sex and less infarct growth in the cortex but not white matter may be explained, at least in part, by the observed trend toward increased collaterals in women compared to men. There may be other reasons for sex differences in infarct growth, such as differences in apoptosis pathways and inflammatory responses during ischemia and estrogen-mediated endothelial nitric oxide synthase-dependent vasodilation, that require further study.33 Possible mechanisms underlying the association of coronary artery disease and less cortical infarct growth are speculative but include ischemic preconditioning34 and prior medications; detailed coronary artery disease data were unavailable, precluding further analyses.
There are several limitations with the present study. Given its retrospective design, there is risk of selection bias in this analysis of patients with both pre- and post-EVT MRIs. However, patient demographics, clinical presentation, and outcomes, including adequate reperfusion and 90-day disability, were similar to randomized EVT trials3-5 underscoring the generalizability of this study. These variables were also controlled for in multivariable models when univariable associations were found. Another limitation is the study’s sample size given our intent to include only those with pre- and post-EVT MRIs for accurate infarct assessments and only those with adequate reperfusion. Adequate reperfusion was defined as TICI 2b-3 because this represents EVT treatment success clinically,3 but this may be a heterogenous group in terms of reperfusion extent. To control for the this, TICI 3 complete reperfusion was included in multivariable models. In addition, there was no screening or re-imaging for vessel re-occlusion after EVT. Pre-EVT CTA was also unavailable to assess collaterals in 22% of patients so MRA was used for this minority. Sensitivity analyses were performed excluding those with MRA-determined collaterals, which showed similar results (Table 5). There is also inherent error in the registration process of clinical scans to standard MNI-152 space. However, our successful registration rate was high despite our stringent a priori protocol for excluding poor registrations. Further, most of the infarct volumes are relatively large so registration error is unlikely to affect the interpretation of the results. The exception is basal ganglia infarct volumes, which should be interpreted with caution. We maintain that the analysis of clinical scans has a higher potential for immediate clinical relevance and translatability. Collinearity is a concern when investigating region-specific infarct growth given likely shared mechanisms across regions, such as degree of reperfusion. However, we show different percent changes in growth and different determinants of growth based on region suggesting there may also exist unique underlying mechanisms. Still, the determinants of infarct growth are exploratory, should be interpreted as hypothesis-generating, and require further study. Another potential limitation is the inclusion of patients over a time frame that spans several landmark trials that changed the standard of care for LVO treatment. Despite this, only a minority were treated with first generation devices (5%), we selected for only those achieving adequate reperfusion, and we included TICI score as a covariate in analyses.
Table 5.
Sensitivity analyses comparing results of outcome associations with collateral grades determined by CT angiography or MR angiography for the entire cohort (CTA+MRA) versus the cohort excluding those with MRA-determined collaterals (CTA).
| CTA+MRA | CTA | |||
|---|---|---|---|---|
| OR (95% CI)/β | P | OR (95% CI)/β | P | |
| 90 Day Good Outcome | 1.011 (0.595,1.719) | 0.967 | 1.148 (0.536,2.458) | 0.722 |
| Total Infarct Growth | 0.158 | 0.305 | 0.120 | 0.498 |
| White Matter Infarct Growth | 0.243 | 0.112 | 0.194 | 0.271 |
| Cortex Infarct Growth | 0.148 | 0.337 | 0.088 | 0.621 |
| Basal Ganglia Infarct Growth | 0.152 | 0.325 | 0.224 | 0.202 |
There were no meaningful differences in data interpretation of outcome associations with collaterals based on imaging modality used. Good outcome was defined as modified Rankin Scale 0-2. Infarct growth was natural logarithm-transformed for linear regressions. Abbreviations: OR: odds ratio, CI: confidence interval, β: parameter estimate
In conclusion, these data support MRI-defined infarct growth occurs despite adequate reperfusion after EVT and show, for the first time, that infarct growth occurs to a greater extent in the cortex. Infarct growth was independently associated with worse 90-day disability. Female sex was independently associated with reduced total infarct growth, and other distinct associations with region-specific infarct growth were identified that require confirmation in larger studies.
Acknowledgments and Disclosures:
Joyce A. McIntyre maintained the prospective Massachusetts General Hospital stroke database. The National Institutes of Health, National Institute of Neurological Disorders and Stroke supported this work (RWR by R25 NS065743; NSR by R01 NS082285, R01 NS086905, U19 NS115388). There are no other relevant competing interests.
References
- 1.Regenhardt RW, Das AS, Stapleton CJ, et al. Blood pressure and penumbral sustenance in stroke from large vessel occlusion. Front Neurol 2017;8:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regenhardt RW, Biseko MR, Shayo AF, et al. Opportunities for intervention: stroke treatments, disability and mortality in urban Tanzania. Int J Qual Heal Care 2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387(10029):1723–31. [DOI] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2017;378(1):11–21. [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378(8):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regenhardt RW, Takase H, Lo EH, Lin DJ. Translating concepts of neural repair after stroke: Structural and functional targets for recovery. Restor Neurol Neurosci 2020;38(1):67–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivot J-M, Mlynash M, Thijs VN, et al. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE). Stroke 2008;39(8):2257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qureshi AI, Hussein HM, Abdelmoula M, Georgiadis AL, Janjua N. Subacute recanalization and reocclusion in patients with acute ischemic stroke following endovascular treatment. Neurocrit Care 2009;10(2):195–203. [DOI] [PubMed] [Google Scholar]
- 9.Simonsen CZ, Mikkelsen IK, Karabegovic S, Kristensen PK, Yoo AJ, Andersen G. Predictors of infarct growth in patients with large vessel occlusion treated with endovascular therapy. Front Neurol 2017;8:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morishima I, Sone T, Okumura K, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000;36(4):1202–9. [DOI] [PubMed] [Google Scholar]
- 11.Nie X, Pu Y, Zhang Z, Liu X, Duan W, Liu L. Futile recanalization after endovascular therapy in acute ischemic stroke. Biomed Res Int 2018:5879548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, Kim BJ, Han MK, et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol 2019;19(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol 2009;54(4):281–92. [DOI] [PubMed] [Google Scholar]
- 14.Marcoux FW, Morawetz RB, Crowell RM, DeGirolami U, Halsey JH, Halsey JH Jr. Differential regional vulnerability in transient focal cerebral ischemia. Stroke 1982;13(3):339–46. [DOI] [PubMed] [Google Scholar]
- 15.Regenhardt RW, Mecca AP, Flavin SA, et al. Delays in the air or ground transfer of patients for endovascular thrombectomy. Stroke 2018;49(6):1419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol 2007;61(6):533–43. [DOI] [PubMed] [Google Scholar]
- 17.Collaborators NASCET, Barnett HJM, Taylor DW, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325(7):445–53. [DOI] [PubMed] [Google Scholar]
- 18.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 19.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19(5):604–7. [DOI] [PubMed] [Google Scholar]
- 20.Young MJ, Regenhardt RW, Leslie-Mazwi TM, Stein MA. Disabling stroke in persons already with a disability: Ethical dimensions and directives. Neurology 2020;94(7):306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slicer. https://www.slicer.org/.Accessed May 5, 2020. [Google Scholar]
- 22.Aben HP, Biessels GJ, Weaver NA, et al. Extent to which network hubs are affected by ischemic stroke predicts cognitive recovery. Stroke 2019;50(10):2768–74. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62(2):782–90. [DOI] [PubMed] [Google Scholar]
- 24.van der Zwan A, Hillen B, Tulleken CA, Dujovny M. A quantitative investigation of the variability of the major cerebral arterial territories. Stroke 1993;24(12):1951–9. [DOI] [PubMed] [Google Scholar]
- 25.Haussen DC, Nogueira RG, Elhammady MS, et al. Infarct growth despite full reperfusion in endovascular therapy for acute ischemic stroke. J Neurointerv Surg 2016;8(2):117–21. [DOI] [PubMed] [Google Scholar]
- 26.Man S, Aoki J, Hussain MS, et al. Predictors of infarct growth after endovascular therapy for acute ischemic stroke. J Stroke Cerebrovasc Dis 2015;24(2):401–7. [DOI] [PubMed] [Google Scholar]
- 27.Dula AN, Mlynash M, Zuck ND, Albers GW, Warach SJ. Neuroimaging in ischemic stroke is different between men and women in the DEFUSE 3 cohort. Stroke 2020;51(2):481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raychev R, Liebeskind DS, Yoo AJ, et al. Physiologic predictors of collateral circulation and infarct growth during anesthesia – Detailed analyses of the GOLIATH trial. J Cereb Blood Flow Metab 2020;40(6):1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyal N, Tsivgoulis G, Frei D, et al. Comparative safety and efficacy of modified TICI 2b and TICI 3 reperfusion in acute ischemic strokes treated with mechanical thrombectomy. Clin Neurosurg 2019;84(3):680–6. [DOI] [PubMed] [Google Scholar]
- 30.Omata N, Murata T, Maruoka N, Fujibayashi Y, Yonekura Y, Wada Y. Different mechanisms of hypoxic injury on white matter and gray matter as revealed by dynamic changes in glucose metabolism in rats. Neurosci Lett 2003;353(2):148–52. [DOI] [PubMed] [Google Scholar]
- 31.Nishizaki T, Yamauchi R, Tanimoto M, Okada Y. Effects of temperature on the oxygen consumption in thin slices from different brain regions. Neurosci Lett 1988;86(3):301–5. [DOI] [PubMed] [Google Scholar]
- 32.Weinberger J, Thompson L, Samii M. The significance of basal ganglia infarction. J Stroke Cerebrovasc Dis 1995;5(1):6–11. [DOI] [PubMed] [Google Scholar]
- 33.Faber JE, Moore SM, Lucitti JL, Aghajanian A, Zhang H. Sex differences in the cerebral collateral circulation. Transl Stroke Res 2017;8(3):273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 2009;8(4):398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]