Summary
Soil-borne diseases cause serious economic losses in agriculture. Managing diseases with microbial preparations is an excellent approach to soil-borne disease prevention. However, microbial preparations often exhibit unstable effects, limiting their large-scale application. This review introduces and summarizes disease-suppressive soils, the relationship between carbon sources and the microbial community, and the application of human microbial preparation concepts to plant microbial preparations. We also propose an innovative synthetic microbial community assembly strategy with synergistic prebiotics to promote healthy plant growth and resistance to disease. In this review, a new approach is proposed to improve traditional microbial preparations; provide a better understanding of the relationships among carbon sources, beneficial microorganisms, and plants; and lay a theoretical foundation for developing new microbial preparations.
Subject areas: Earth sciences, Biogeochemistry, Microbiology, Plant biology
Graphical abstract
Earth sciences; Biogeochemistry; Microbiology; Plant biology
Introduction
The interactions of pathogens, hosts, and the environment determine the occurrence of plant diseases. The environment, especially soil characteristics, determines the source of microorganisms recruited by plant roots, which mainly affects disease outbreaks (Chiaramonte et al., 2021; Zheng et al., 2021). Diseases that spread through soil are called soil-borne plant diseases, and they adversely affect crop production worldwide, whereas some other types of organic matter might exert beneficial effects that reduce the presence of pathogens such as Fusarium, Phytophthora, Pythium, and Rhizoctonia (Jambhulkar et al., 2015). If this organic matter is composed of recalcitrant carbon resources, then the ability of the soil to suppress soil pathogens is enhanced, while microbial activities in the soil are stimulated by the addition of labile carbon resources as a result of priming effects. Owing to the decomposition of microbial communities, which in turn promotes plant productivity, studies exploring a method for modifying microbial communities to improve the environment of plant root growth, which ultimately enhances plant resistance and yields, are important. Disease-suppressive soils depend on the uniformity, composition and abundance of the core microbial community (Mendes et al., 2011). Therefore, the intensified competition between microbial communities and plant pathogens in disease-suppressive soils may be a disadvantage in niche allocation in the soil. These microbial communities have the ability to adapt to the various environmental conditions established for the purpose of sustainable crop production (Keswani et al., 2019). Therefore, an understanding of and the targeted modification of soil microorganisms may provide new strategies for sustainable agricultural system management (Wang and Li, 2019).
The diversity and richness of soil bacteria and fungi determine many important aspects of ecosystem function to some extent (Singh and Gupta, 2018; Sintim et al., 2019; Wagg et al., 2014). However, the imbalance of nutrients and the presence of a large number of plant pathogens in the soil might exert a negative effect on plant health (Steffan et al., 2018). Soil management practices involving farming, crop rotation, and burning affect the quantity and quality of organic matter, which in turn affects soil health (Noble and Coventry, 2005). Microbiome engineering technology for core microorganism inoculation directly adjusts the relationship between microorganisms, inhibits harmful microorganisms, and recruits functional microorganisms, thereby improving soil health (Morais et al., 2019). Although the effects of chemical control are stable, this method often produces environmental pollution and promotes resistance in plant pathogens. In recent years, researchers have achieved outstanding progress in biological agent research and development (Compant et al., 2019; Fitzpatrick et al., 2020). Rhizosphere growth-promoting bacteria are also important biological control resources (Martins et al., 2013; Santiago et al., 2015). Therefore, the research and development of control methods that are harmless to the environment and exert stable effects have become increasingly important.
The effects of biological control are often unstable; however, the synthetic microbial community represents a new approach to address common problems associated with current microbial fertilizers (Qin et al., 2016). At the same time, disease-suppressive soil is a natural biological control agent (bca) that potentially affects the survival, infection or reproduction of pathogens. The core microbial community and its structural characteristics and the nutrients in the soil are closely related to the disease-suppressing ability of disease-suppressive soils (Carrion et al., 2018; Jaiswal et al., 2017; Xiong et al., 2017). Moreover, the disease-suppressing ability of disease-suppressive soils is also related to the mode of the nutrient-microbiome interaction (Mendes et al., 2011; Thakur and Geisen, 2019). In this review, we will discuss disease-suppressive soils, the application of human microbial preparation concepts to plant microbial preparations, and the relationship between carbon sources and the microbial community. This review aims to understand the relationship between carbon sources and microbial communities in this process.
Disease-suppressive soils
Soil with a specific microbial community structure is postulated to promote plant growth and defense by inhibiting pathogens, providing a strategy for preventing soil-borne diseases (Alabouvette, 1999; Haas and Défago, 2005; Singh et al., 2011). Since the idea of disease-suppressive soils was proposed, it has been a research hotspot (Alabouvette, 1999; Broadbent and Baker, 1974; Cook and Rovira, 1976; Schlatter et al., 2017). Disease-suppressive soils prevent and control soil-borne diseases through specific microbial communities (Latz et al., 2016). Specific microbial communities suppress the occurrence of soil-borne diseases mainly through nutrient competition, antagonism, hyperparasitism and plant resistance induction (Lu et al., 2019; Peng et al., 2020). Microbial function, diversity, and activity in rhizospheric soil are also involved in inhibiting the occurrence of soil-borne diseases and promoting plant growth (Jaiswal et al., 2017). The rhizosphere soil microbiome is the first line of defense against pathogens invading plant roots (Weller et al., 2002). Based on an analysis of the inhibitory soil microbial community associated with Fusarium wilt in a long-term continuous cropping system of vanilla, the fungal community was shown to play an important role in inhibiting the development of Fusarium wilt (Xiong et al., 2017). The addition of disease-suppressing compost to the soil, if combined with organic amendments and selected bca, will trigger various plant disease control mechanisms (Kuzyakov and Domanski, 2000).
The microbiome is regulated by root exudates in disease-suppressive soil (Bakker et al., 2018). Plants, microbiomes, and the environment form a complex chemical network that harmonizes the plant microbiome. The rhizosphere is the soil plant root interface and in practice consists of the soil adhering to the root and the loose soil surrounding the root. Plant growth-promoting rhizobacteria are potential agents for the biological control of plant pathogens (Babalola, 2010). When plants growing in disease-suppressive soil are attacked by plant pathogens, the root system secretes metabolites to recruit beneficial microorganisms that protect the plants (Rosenzweig et al., 2012; Yang et al., 2016). Recently, the root system of Arabidopsis was shown to secrete amino acids, nucleotides, and long-chain organic acids that recruit beneficial microorganisms and make its progeny resistant to plant pathogens (Berendsen et al., 2018; Liu et al., 2014; Yuan et al., 2018). Plant-derived nutrients attract probiotics in a specific manner, and this mechanism may be used by pathogens. When the environment or plant-derived nutrition changes, plant pathogens in the soil may experience good growth conditions and ultimately increase in number (Bulgarelli et al., 2013; Fitzpatrick et al., 2020). When the plant pathogen density reaches a certain level in the soil, the defense function of beneficial microorganisms may be lost, and plant pathogens will move from the soil to inside the plant roots and stem. In cases of severe infection, plant pathogens are dispersed through the soil to neighboring plants and animals and into the air (Bai et al., 2015). However, when plant pathogens spread into disease-suppressive soil, the plants recruit beneficial microorganisms by secreting root metabolites to resist the attacks of plant pathogens. Root exudates or secondary metabolites of plants in disease-suppressive soils can be used as a signal for plants to reshape the root microbiome. They can be used as chemical attractants or nutrient sources to reorganize the microbiome and help alleviate the abiotic stress or biological stress of host plants (Figure 1A) (Cadot et al., 2020; Zhalnina et al., 2018). Under the selective pressure formed by a single carbon source, the synthetic microbial community tends to stabilize and simplify the composition of the microbial community (Goldford et al., 2018). Beneficial microbial strains may be combined with specific substrates to selectively stimulate their growth, colonization and beneficial activities (Cordovez et al., 2019; Pollak and Cordero, 2020). In the future, the development of methods to build a controllable and stable microbial interaction network and to achieve precise regulation of microbial community functions will help us understand the plant-beneficial microbe-pathogen relationship and prevent the occurrence of plant diseases.
Figure 1.
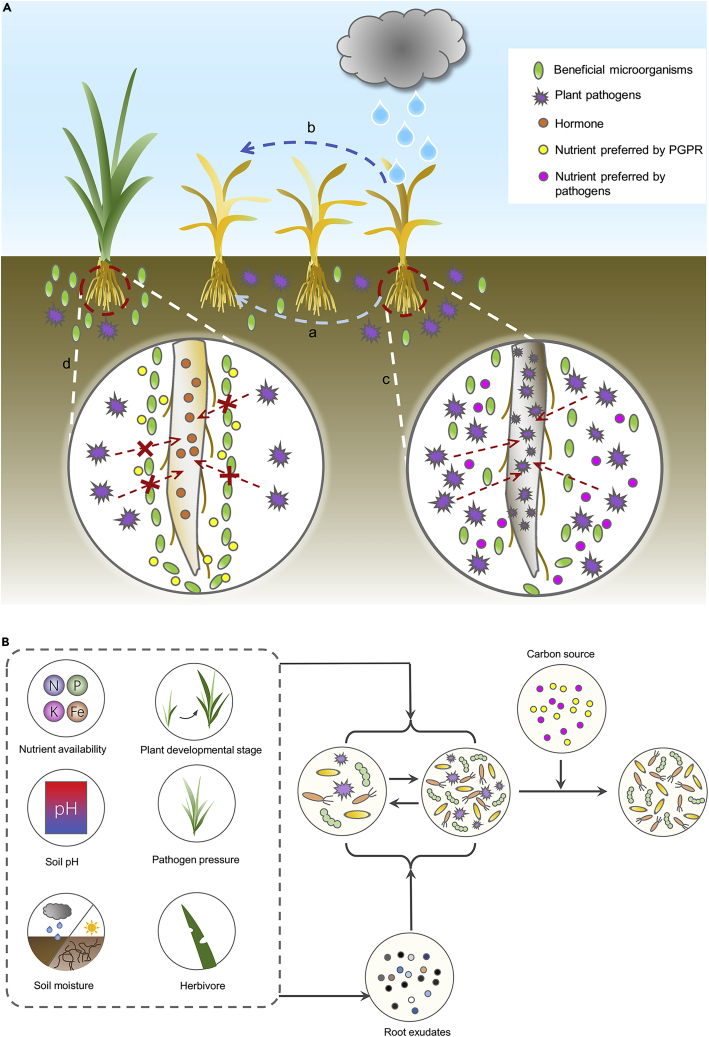
The relationship between the carbon source and the microbial community and the environment-plant-carbon source network affect the assembly of synthetic microbial communities and indigenous microbial communities in rhizosphere soil
Figure 1A presents the relationship between the carbon source and the microbial community. A red “X” indicates that plant pathogens are unable to infect the plant roots; (A) indicates that the plant pathogens can be spread through the soil; (B) indicates that the plant pathogens can also be spread by rain or wind; (C) indicates that when plant pathogens spread into this environment, where plant pathogens have more nutrients and beneficial microorganisms have fewer nutrients, beneficial microorganisms are unable to resist the attack of plant pathogens; and (D) indicates that when the plant pathogens spread to this environment, where plant pathogens have fewer nutrients and beneficial microorganisms have more nutrients, beneficial microorganisms are able to resist the attack of plant pathogens. Figure 1B shows that the environment-plant-carbon source network affects the assembly of synthetic microbial communities and indigenous microbial communities in rhizosphere soil. The figure shows the process of assembling synthetic microbial communities and indigenous microbial communities (the effects of root exudates, carbon sources, host plants, and environmental conditions on the assembly of synthetic microbial communities and indigenous microbial communities).
The relationship between carbon sources and the microbial community
Beneficial and harmful soil microorganisms can live in the same environment (Rosenzweig et al., 2012). Among the beneficial flora, endosymbionts colonized in cells can promote plant growth and enhance plant resistance. Growth-promoting or rhizosphere growth-promoting bacteria are defined as independent living beneficial bacteria, which can promote health and growth in plants (Fahad et al., 2015). However, beneficial and disease-causing microorganisms share common physiological characteristics and evolutionary similarities. To a certain extent, the performance of pathogenic phenotypes may depend on small differences in microorganisms and sometimes even on the host (Berendsen et al., 2018; Molina-Romero et al., 2017). The secretions of microorganisms and the number and nature of secretion effectors may constitute an important factor distinguishing between beneficial microorganisms and pathogens. Furthermore, multiple omics and mathematical models can also be used to analyze the interaction mode between pathogenic bacteria, beneficial microorganisms and host (Fiorilli et al., 2018)
One study reported that plants adjust their root microbiome upon pathogen infection and specifically recruit a group of disease resistance-inducing and growth-promoting beneficial microbes, thereby potentially maximizing the chances for the survival of their offspring that will grow in the same soil (Berendsen et al., 2018). The number of beneficial microorganisms in the soil determines whether the soil inhibits the occurrence of soil-borne diseases (Pereg and Mcmillan, 2015). Meanwhile, nutrient sources that affect the soil microbial community, such as carbon sources, may influence the growth and reproduction of beneficial microorganisms and harmful microorganisms (Bais et al., 2006; Kessler et al., 2012). Soil nutrients, soil organic carbon and soil organic matter affect the microbial activity of the growth and development of the microbial community, providing the most suitable habitat for the microbial community (Hadar and Papadopoulou, 2012).
The disease-suppressing ability of disease-suppressive soil is closely related to competition for carbon sources (Table 1). Moreover, Neeno-Eckwall et al. (2001) found that pathogenic Streptomyces scabies shows weaker competition for carbon than nonpathogenic Streptomyces sp. (Neeno-Eckwall et al., 2001). Soil-borne diseases can be managed by exploiting the competition for carbon sources between the pathogenic bacteria and the original microorganisms in the soil; this idea is inspired by the concept of prebiotics (Alabouvette, 1986; Duan et al., 2015; Xin et al., 2015; Yang et al., 2019). Moreover, carbon source competition is an important factor that affects the assembly, diversity, coexistence, operation, and evolution of microbial communities (Yang et al., 2018).
Table 1.
Combination of organic matter and root exudates with beneficial microorganisms to promote plant health
| Typology | Nutrition | Beneficial microorganisms | References |
|---|---|---|---|
| Organic amendments | Espresso spent coffee ground | Trichoderma. atroviride, Trichoderma citrinoviride, Aspergillus sp. | (Chilosi et al., 2020) |
| Green composts from defatted/nondefatted olive marc, spent coffee grounds and tea bags mixed with green wastes of various horticultural crops |
Alternaria sp. Fusarium oxysporum Fusarium solani Trichoderma atroviride T. harzianum T. asperellum B. subtilis Bacillus licheniformis Bacillus pumilus |
(De Corato et al., 2019) | |
| Biochar | Pseudomonas sp. NT-2 | (Tu et al., 2020) | |
| Bio-organic fertilizers | Pseudomonas | (Tao et al., 2020) | |
| Root exudates | Benzoxazinoids | Pseudomonas | (Neal et al., 2012) |
| Coumarin | Pseudomonas WCS417 | (Stringlis et al., 2018) (Harbort et al., 2020) |
|
| Long-chain fatty acids and amino acids | Pseudomonas | (Wen et al., 2021) | |
| Long-chain organic acids and amino acids | Fictibacillus Sphingomonas | (Yuan et al., 2018) | |
| Organic volatiles |
Streptomyces Bacillus |
(Kong et al., 2021) | |
| Sucrose | Bacillus subtilis | (Tian et al., 2021b) | |
| Flavonoids | Arbuscular mycorrhizal fungi | (Tian et al., 2021a) |
Similar to a prebiotic, the addition of an exogenous carbon source can stimulate the growth or activity of beneficial microorganisms around the rhizosphere of plants, thereby affecting the phenotype of the plants (Hacquard et al., 2015). For example, straw as an additional carbon source can benefit rhizosphere microorganisms, as well as reduces the absorption of root exudates by rhizosphere microorganisms (Maarastawi et al., 2019; Yang et al., 2014). Similarly, the application of biochar as a microbial carbon source changes the microbial community and increases rice yield (Nan et al., 2019). Biochar also changes the structure, function, and abundance of rhizosphere microbial communities that prevent soil-borne diseases (Jaiswal et al., 2017). Similarly, Compost fortified with bioinoculants ensures a supply of humus, minerals, and beneficial microbes associated with soil and plants (Zaccardelli et al., 2011). After determining the differences in the use of microbial carbon sources in disease-suppressive soils and disease-susceptible soils, specific carbon sources can be supplied to soils to regulate the population structure, abundance, and function of beneficial microorganisms.
Application of human microbial preparation concepts to plant microbial preparations
The human intestinal microbiota is a complex network composed of a variety of microorganisms that is inextricably linked to the physiological functions of the host. In addition, changes in the composition of nutrients in the intestine directly or indirectly affect the composition of the intestinal microbial community, and changes in the intestinal microbial community also affect host metabolism, which in turn affects the health of the host (Agus et al., 2018).
Similar to the role of the gut microbiome, the plant microbiome plays an important role in the process of plant disease resistance. Clarifying the relationship between community interaction characteristics and functions is very important for directly regulating the plant microbiome and promoting the healthy growth of plants (Kwak et al., 2018). Through studies of the microbiome, we have obtained a deeper understanding of the relationship between the microbial community and the host. In the medical field, live microorganisms such as probiotics, prebiotics, synbiotics, and epibiotics have been used as therapeutic agents or carriers to change the function or composition of the microbiota, reducing the colonization of harmful species and improving microbial community homeostasis (Plichta et al., 2019). Prebiotics are not decomposed and utilized by the digestive tract in organisms, but in the rectum, probiotics are absorbed and utilized to improve the colonization rate, viability and proliferation of probiotics in the gastrointestinal tract (Roberfroid, 2007). Synbiotics are microecological preparations generated by combining probiotics and prebiotics. Use of probiotics can help to proliferate, better physiological activities, and improvement in microecological and enzyme balance in the organism (Rastall and Maitin, 2002). Similarly, the composition and content of nutrients in the soil may affect the composition, activity and function of the soil microbial community (Harbort et al., 2020; Ladau and Eloe-Fadrosh, 2019). Nutrition derived from plant sources or other sources recruits beneficial microorganisms to preserve plant growth (Wen et al., 2021).
Currently, in the field of agriculture, beneficial microorganisms and microbial source metabolites are the only microbial preparations used to protect or promote plant growth (Trivedi et al., 2020). Microbial preparations refer to live beneficial microorganism preparations from fermentation broth that use porous substances as adsorbents to adsorb beneficial microorganism cells after the target microorganisms are multiplied through industrial production (Kaminsky et al., 2019). The intestines of vertebrates and plant roots have evolved independently in the animal and plant kingdoms, but they have similar basic physiological functions in terms of nutrient absorption (Hacquard et al., 2015). Currently, limited microbial preparations provide the beneficial microorganisms and the nutrients on which beneficial microorganisms rely on. Inspired by the concepts of prebiotics and synbiotics along with literature support, we propose a new useful synthetic microbial community assembly process with an intention to studying the relationship between microbial communities and hosts for the development of new microbial agents.
A new synthetic microbial community assembly process
The number of unconsumed (restricted) resources of the indigenous microbial community and the rate of resource consumption by the members of the synthetic microbial community determine the fate of the synthetic microbial community (Thakur and Geisen, 2019). Therefore, the addition of a specific carbon source may increase the probability that the synthetic microbial community will gradually reach a stable state through learning and adaptability. The screening of environmental factors eventually produces a microbial community with different ecological niches, different pressures, tolerance capabilities and nutrient acquisition capabilities (Anthony et al., 2020). The composition of the soil microbial community also depends on plant litter input and root deposition, as well as biological factors such as top-down regulation by predators of bacteria and fungi (Figure 1B) (Thakur and Geisen, 2019). Plants may promote the growth of rhizosphere microorganisms by regulating the rhizosphere environment to improve the adaptability of plants in specific ecosystems (Zhalnina et al., 2018).
Synthetic microbial communities have been used to harness the ability of microbial community members to stably suppress soil-borne diseases, despite complex environmental changes (Biliouris et al., 2012). Unlike natural microbial communities, the species in the synthetic functional flora are known; the community composition is relatively simple, and the community is highly controllable. These factors enable a better understanding of the mechanisms of interaction between the microbial community and the plant. Studying the composition of microbial communities in various environments has substantially improved our basic understanding of microorganisms and ecosystems; compared with these communities, those in synthetic microbial ecology have reduced complexity and increased controllability (Cira et al., 2018). Moreover, compared with single microbial strains, synthetic microbial communities achieve ecological functions in more variable environments, and mathematical models of artificially synthesized microbial communities can be developed to construct and study more complex ecosystems. Moreover, soil microorganisms use saponins in root exudates as a carbon source in Panax notoginseng fields, and these saponins specifically regulate the rhizosphere microbial community of P. notoginseng (Luo et al., 2020).
The Fabrication Ecosystems (EcoFABs) is the most mature strategy established in the microbial ecosystem model. The EcoFAB system uses 3D printing, mass spectrometry and other methods to simulate indoor habitats consisting of the existing microbial community structure, cultivation methods, and time-space analysis (Zengler et al., 2019). Since EcoFAB system data can also be shared, the EcoFAB strategy also realizes multilaboratory cooperation-adjustment-mechanism research, providing support for the establishment of theoretical doctrines and predictive models. At present, the EcoFAB system is trying to add single-cell imaging capabilities for a spatial analysis of the microbiome (Marx, 2019). We can also shape the microbial community in a targeted manner based on the establishment of a stable microbiome model. The current method of shaping the microbial community is mainly to shape the microbial community at the genetic level and the nutritional level. The method of using genes to shape the microbial community is the mobile genome MAGIC method and the bottom-up modification of the microbial community in the Design-Build-Test-Learn cycle (DBTL) (Du et al., 2020; Lawson et al., 2019; Ronda et al., 2019). The mobile genome MAGIC method is mainly applied to intestinal microorganisms. However, the mobile genome MAGIC method has recently been applied to the targeted modification of the microbiome in other environments. The mobile genome MAGIC method enables the transformation of the microbial community from the bottom-up in the DBTL cycle. Shaping the microbial community (sculpting microbiomes) can also be directed toward the microbial community by changing the metabolites or other chemical substances secreted by the root system. For example, salicylic acid secreted by roots shapes microbial communities in a targeted manner (Eichmann et al., 2021). Different advanced and traditional approaches have been introduced to assess the diversity and functions of soil microbiota. These approaches have been categorized as molecular, microbiological, and biochemical methods. Extraordinary functional and taxonomic diversity has been identified using molecular techniques. These molecular methods include quantitative real-time PCR (qPCR), terminal restriction fragment polymorphism analysis, temperature gradient agarose gel electrophoresis, and PCR alone or in combination with DNA sequencing (Chen et al., 2021). Among microbiological methods, traditional culture methods based on plate counts have been very productive when integrated with biochemical DNA-based approaches (Xu et al., 2020). Different growth media are useful for the isolation of a wide range of filamentous fungi, culturable bacteria, actinomycetes, yeasts, and oomycetes (Haas and Défago, 2005). Soil amendment with organic and inorganic degradable compounds is another useful biochemical method to stimulate the bioactivity of soil microbes (Mastan et al., 2019; Pang et al., 2021). These compounds also indicate the suppressive properties of soils containing microbial communities (Pane et al., 2015).
Synthetic microbial communities are abstractions of natural systems that allow the detailed study and analysis of the fundamental building blocks and processes that compose a microbial community (Groβkopf and Soyer, 2014). One of the basic principles of the interaction between microorganisms and hosts is metabolic exchange. Plants provide 40% of the compound carbon produced by photosynthesis through the roots to enter the rhizosphere to nourish microorganisms (Rodriguez et al., 2019). For soil-borne disease prevention and control mediated by adding synthetic microbial communities and specific carbon sources, several studies have used synthetic microbial communities or specific carbon sources to study the relationship between microorganisms and plants (Zhuang et al., 2020).
Concluding remarks
The soil microbiome plays a crucial role in plant protection. Through the development of synthetic microbial communities, a simple microbial community can be used to efficiently promote ecological functions. However, the mechanism of the nutrient-plant-microbial community interaction network and the effects of nutrition on regulating the microbial community remain unclear. Thus, an understanding of the regulation of the soil microbiome by soil microbial carbon sources will help us build better, more durable synthetic microbial communities. At present, microbial preparations must overcome many issues, such as unstable effects and poor rhizosphere competitiveness. Synthetic microbial communities represent a feasible approach to solve these problems. Although the mechanisms of disease suppression in soils are complex and diverse, studies have shown that the ability of disease-suppressive soils to inhibit disease is related to the enrichment of specific beneficial microbial communities. Microbial separation and culture techniques have been used to design a synthetic microbial community that inhibits the occurrence of soil-borne diseases. Simultaneously, we can also directionally shape the microbiome to serve agriculture through nutritional restriction or microbiome genetic modification. This synthetic microbial community assembly process not only provides useful insights into the development of composite microbial preparations but also helps us better understand the interactions among microbial communities, the mechanisms of microbial community and plant interactions, and soil microbial regulation by organic matter and root exudates in microbial communities. Synthetic microbial communities can help us understand the mechanisms of action of disease-suppressive soils. The composition of carbon sources in the soil plays an important role in regulating the microbiome in the soil. The stability of synthetic microbial communities can be improved by adding specific carbon sources, enabling to clarify the causal relationship between root microbiota and plant phenotypes and analyze the interactions between microbiota members under natural soil conditions.
Inspired by synbiotics and prebiotics, we propose that the Biolog-ECO plate method, high-throughput sequencing, and microbial isolation and culture technology will be useful to synthesize a more stable microbial community or directionally manipulate the microbial community based on the mode of action of the microbiome in the soil. Figure 2 roughly describes our idea of the process for assembling this synthetic microbial community. This idea will allow us to better understand the mechanism of action between the microbiome and the carbon source. Meanwhile, sequencing technology, synthetic community analysis and modeling, and functional joint analysis have important reference value for future research on rhizosphere interactions. The mechanism of plant-beneficial microorganism-harmful microorganism interactions is mostly unknown. More knowledge of the nutritional needs of the members of the microbiome, including pathogens and their social networks, may achieve the development of reasonable interventions and may produce new tailor-made disease prevention strategies.
Figure 2.
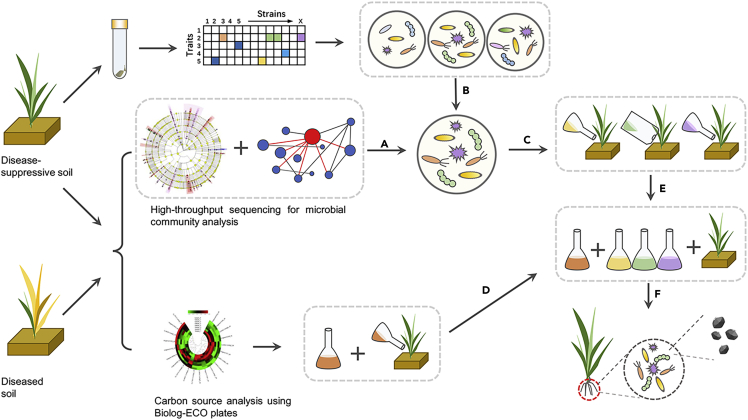
Process of synthetic microbial community assembly and community regulation by carbon sources
The letter (A) represents the use of high-throughput sequencing and bioinformatics analyses such as multilevel species linear discriminant analysis effect size (Lefse) analysis and cooccurrence network analysis to analyze the microbial communities corresponding to each particular function in the susceptible soil and the disease-suppressive soil; (B) represents the use of microorganism separation and culture techniques, in which microorganism strains are separated and cultivated and functional strains are screened using screening tests such as antagonism tests, etc.; (C) represents the use of the results of a microbiome analysis to select and test synthetic microbial community members; (D) represents the use of differential Biolog-ECO plate carbon sources in disease-sensitive and disease-suppressive soils and analyzing the results; (E) represents the addition of synthetic microbial communities and specific carbon sources to the susceptible soil to test whether the ability of the susceptible soil to suppress soil-borne diseases is further enhanced; and (F) represents that synthetic microbial communities are abstractions of natural systems that allow the detailed study and analysis of the fundamental building blocks and processes that compose a microbial community.
Acknowledgments
The work was supported by the Shandong Province Key Research and Development Plan (2019JZZY020608, 2020CXGC010803, 2019GNC106152), National Natural Science Foundation of China (32072500, 31872925), Natural Science Outstanding Youth Fund of Shandong Province (JQ201807), Science and Technology Support Plan for Youth Innovation of Colleges and Universities of Shandong Province (2019KJF023). The authors declare no conflict of interest.
Author contributions
X.D., J.D., and Y.L. designed and wrote the manuscript. Z.Y., H.W., X.Z., Y.J., Z.G., X.X., H.D., X.Z., and S.-U.-R. gave suggestions on the writing of manuscripts. All authors approved the manuscript.
Declaration of interests
The authors declare no competing interests.
References
- Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Alabouvette C. Fusarium-wilt suppressive soils from the Châteaurenard region : review of a 10-year study. Agronomie. 1986;6:273–284. [Google Scholar]
- Alabouvette C. Fusarium wilt suppressive soils: an example of disease-suppressive soils. Australas. Plant Pathol. 1999;28:57–64. [Google Scholar]
- Anthony M.A., Crowther T.W., Maynard D.S., van den Hoogen J., Averill C. Distinct assembly processes and microbial communities constrain soil organic carbon formation. One Earth. 2020;2:349–360. [Google Scholar]
- Babalola O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010;32:1559–1570. doi: 10.1007/s10529-010-0347-0. [DOI] [PubMed] [Google Scholar]
- Bai Y., Müller D.B., Srinivas G., Garrido-Oter R., Potthoff E., Rott M., Dombrowski N., Münch P.C., Spaepen S., Remus-Emsermann M. Functional overlap of the Arabidopsis leaf and root microbiota. Nature. 2015;528:364–369. doi: 10.1038/nature16192. [DOI] [PubMed] [Google Scholar]
- Bais H.P., Weir T.L., Perry L.G., Gilroy S., Vivanco J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Bakker P.A.H.M., Pieterse C.M.J., De Jonge R., Berendsen R.L. The soil-borne legacy. Cell. 2018;172:1178–1180. doi: 10.1016/j.cell.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Berendsen R.L., Vismans G., Yu K., Song Y., De Jonge R., Burgman W.P., Burmolle M., Herschend J., Bakker P.A.H.M., Pieterse C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018;12:1496–1507. doi: 10.1038/s41396-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biliouris K., Babson D., Schmidtdannert C., Kaznessis Y.N. Stochastic simulations of a synthetic bacteria-yeast ecosystem. BMC Syst. Biol. 2012;6:58. doi: 10.1186/1752-0509-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent P., Baker K.F. Behaviour of Phytophthora cinnamomi in soils suppressive and conducive to root rot. Crop Pasture Sci. 1974;25:121–137. [Google Scholar]
- Bulgarelli D., Schlaeppi K., Spaepen S., Ver Loren van Themaat E., Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013;64:807–838. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- Cadot S., Guan H., Bigalke M., Walser J.C., Schlaeppi K. Specific and conserved patterns of microbiota-structuring by maize benzoxazinoids in the field. Microbiome. 2020;9:103. doi: 10.1186/s40168-021-01049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion V.J., Cordovez V., Tyc O., Etalo D.W., De Bruijn I., De Jager V., Medema M.H., Eberl L., Raaijmakers J.M. Involvement of Burkholderiaceae and sulfurous volatiles in disease-suppressive soils. ISME J. 2018;12:2307–2321. doi: 10.1038/s41396-018-0186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.-L., Hu H.-W., He Z.-Y., Cui L., Zhu Y.-G., He J.-Z. Potential of indigenous crop microbiomes for sustainable agriculture. Nat. Food. 2021;2:233–240. doi: 10.1038/s43016-021-00253-5. [DOI] [PubMed] [Google Scholar]
- Chiaramonte J.B., Mendes L.W., Mendes R. Rhizosphere microbiome and soil-borne diseases. Rhizosphere Biol. Interactions Between Microbes Plants. 2021:155–168. [Google Scholar]
- Chilosi G., Aleandri M.P., Luccioli E., Stazi S.R., Marabottini R., Morales-Rodríguez C., Vettraino A.M., Vannini A. Suppression of soil-borne plant pathogens in growing media amended with espresso spent coffee grounds as a carrier of Trichoderma spp. Scientia Horticulturae. 2020;259:108666. [Google Scholar]
- Cira N., Pearce M., Quake S. Neutral and selective dynamics in a synthetic microbial community. Proc. Natl. Acad. Sci. 2018;115:201808118. doi: 10.1073/pnas.1808118115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compant S., Samad A., Faist H., Sessitsch A. A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019;19:29–37. doi: 10.1016/j.jare.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R.J., Rovira A.D. The role of bacteria in the biological control of Gaeumannomyces graminis by suppressive soils. Soil Biol. Biochem. 1976;8:269–273. [Google Scholar]
- Cordovez V., Dini-Andreote F., Carrión V., Raaijmakers J.M. Ecology and evolution of plant microbiomes. Annu. Rev. Microbiol. 2019;73:69–88. doi: 10.1146/annurev-micro-090817-062524. [DOI] [PubMed] [Google Scholar]
- De Corato U., Patruno L., Avella N., Lacolla G., Cucci G. Composts from green sources show an increased suppressiveness to soilborne plant pathogenic fungi: relationships between physicochemical properties, disease suppression, and the microbiome. Crop Prot. 2019;124:104870. [Google Scholar]
- Du J., Li Y., Yin Z., Wang H., Zhang X., Ding X. High-Throughput customization of plant microbiomes for sustainable agriculture. Front. Plant Sci. 2020;11:1365. doi: 10.3389/fpls.2020.569742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W., Shi Y., Zhao J., Zhang Y., Yu Z. Depth of nitrogen fertiliser placement affects nitrogen accumulation, translocation and nitrate-nitrogen content in soil of rainfed wheat. Int. J. Plant Prod. 2015;9:237–256. [Google Scholar]
- Eichmann R., Richards L., Schäfer P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021;105:518–541. doi: 10.1111/tpj.15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahad S., Hussain S., Bano A., Saud S., Hassan S., Shan D., Khan F.A., Khan F., Chen Y., Wu C. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ. Sci. Pollut. Res. 2015;22:4907–4921. doi: 10.1007/s11356-014-3754-2. [DOI] [PubMed] [Google Scholar]
- Fiorilli V., Vannini C., Ortolani F., Garcia-Seco D., Chiapello M., Novero M., Domingo G., Terzi V., Morcia C., Bagnaresi P. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018;8:9625. doi: 10.1038/s41598-018-27622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick C.R., Salas-González I., Conway J.M., Finkel O.M., Gilbert S., Russ D., Teixeira P.J.P.L., Dangl J.L. The plant microbiome: from ecology to reductionism and beyond. Annu. Rev. Microbiol. 2020;74:81–100. doi: 10.1146/annurev-micro-022620-014327. [DOI] [PubMed] [Google Scholar]
- Goldford J.E., Lu N., Bajic D., Estrela S., Tikhonov M., Sanchez-Gorostiaga A., Segre D., Mehta P., Sanchez A. Emergent simplicity in microbial community assembly. Science. 2018;361:469–474. doi: 10.1126/science.aat1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Großkopf T., Soyer O.S. Synthetic microbial communities. Curr. Opin. Microbiol. 2014;18:72–77. doi: 10.1016/j.mib.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- Hacquard S., Garrido-Oter R., González A., Spaepen S., Ackermann G., Lebeis S., McHardy Alice C., Dangl Jeffrey L., Knight R., Ley R., Schulze-Lefert P. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe. 2015;17:603–616. doi: 10.1016/j.chom.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Hadar Y., Papadopoulou K.K. Suppressive composts: microbial ecology links between abiotic environments and healthy plants. Annu. Rev. Phytopathol. 2012;50:133–153. doi: 10.1146/annurev-phyto-081211-172914. [DOI] [PubMed] [Google Scholar]
- Harbort C.J., Hashimoto M., Inoue H., Niu Y., Guan R., Rombolà A.D., Kopriva S., Voges M.J.E.E.E., Sattely E.S., Garrido-Oter R., Schulze-Lefert P. Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis. Cell Host Microbe. 2020;28:825–837. doi: 10.1016/j.chom.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A., Elad Y., Paudel I., Graber E., Cytryn E., Frenkel O. Linking the belowground microbial composition, diversity and activity to soilborne disease suppression and growth promotion of tomato amended with biochar. Sci. Rep. 2017;7:44382. doi: 10.1038/srep44382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambhulkar P.P., Sharma M., Lakshman D., Sharma P. Natural mechanisms of soil suppressiveness against diseases caused by Fusarium, Rhizoctonia, Pythium, and Phytophthora. In: Meghvansi M.K., Varma A., editors. Organic Amendments and Soil Suppressiveness in Plant Disease Management. Springer; 2015. pp. 95–124. [Google Scholar]
- Kaminsky L.M., Trexler R.V., Malik R.J., Hockett K.L., Bell T.H. The inherent conflicts in developing soil microbial inoculants. Trends Biotechnol. 2019;37:140–151. doi: 10.1016/j.tibtech.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Kessler D., Bhattacharya S., Diezel C., Rothe E., Gase K., Schottner M., Baldwin I.T. Unpredictability of nectar nicotine promotes outcrossing by hummingbirds in Nicotiana attenuata. Plant J. 2012;71:529–538. doi: 10.1111/j.1365-313X.2012.05008.x. [DOI] [PubMed] [Google Scholar]
- Keswani C., Dilnashin H., Birla H., Singh S. Unravelling efficient applications of agriculturally important microorganisms for alleviation of induced inter-cellular oxidative stress in crops. Acta agriculturae Slovenica. 2019;114:121–130. [Google Scholar]
- Kong H.G., Song G.C., Sim H.-J., Ryu C.-M. Achieving similar root microbiota composition in neighbouring plants through airborne signalling. ISME J. 2021;15:397–408. doi: 10.1038/s41396-020-00759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzyakov Y., Domanski G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000;163:421–431. [Google Scholar]
- Kwak M.-J., Kong H.G., Choi K., Kwon S.-K., Song J.Y., Lee J., Lee P.A., Choi S.Y., Seo M., Lee H.J. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018;36:1100–1109. doi: 10.1038/nbt.4232. [DOI] [PubMed] [Google Scholar]
- Ladau J., Eloe-Fadrosh E.A. Spatial, temporal, and phylogenetic scales of microbial ecology. Trends Microbiol. 2019;27:662–669. doi: 10.1016/j.tim.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Latz E., Eisenhauer N., Rall B.C., Scheu S., Jousset A. Unravelling linkages between plant community composition and the pathogen-suppressive potential of soils. Sci. Rep. 2016;6:23584. doi: 10.1038/srep23584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C.E., Harcombe W.R., Hatzenpichler R., Lindemann S.R., Löffler F.E., O’Malley M.A., García Martín H., Pfleger B.F., Raskin L., Venturelli O.S. Common principles and best practices for engineering microbiomes. Nat. Rev. Microbiol. 2019;17:725–741. doi: 10.1038/s41579-019-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Chang Q., Feng W., Zhang B., Wu T., Li N., Yao F., Ding X., Chu Z. Domain dissection of AvrRxo1 for suppressor, Avirulence and cytotoxicity functions. PLoS One. 2014;9:e113875. doi: 10.1371/journal.pone.0113875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Liu H., Jiang D., Wang L., Jiang Y., Tang S., Hou X., Han X., Liu Z., Zhang M. Paecilomyces variotii extracts (ZNC) enhance plant immunity and promote plant growth. Plant and Soil. 2019;441:383–397. [Google Scholar]
- Luo L.F., Yang L., Yan Z.X., Jiang B.B., Li S., Huang H.C., Liu Y.X., Zhu S.S., Yang M. Ginsenosides in root exudates of Panax notoginseng drive the change of soil microbiota through carbon source different utilization. Plant and Soil. 2020;455:139–153. [Google Scholar]
- Maarastawi S.A., Frindte K., Bodelier P.L.E., Knief C. Rice straw serves as additional carbon source for rhizosphere microorganisms and reduces root exudate consumption. Soil Biol. Biochem. 2019;135:235–238. [Google Scholar]
- Martins S., De Medeiros F.H.V., De Souza R.M., De Resende M.L.V., Ribeiro P.M. Biological control of bacterial wilt of common bean by plant growth-promoting rhizobacteria. Biol. Control. 2013;66:65–71. [Google Scholar]
- Marx V. Engineers embrace microbiome messiness. Nat. Methods. 2019;16:581–584. doi: 10.1038/s41592-019-0460-5. [DOI] [PubMed] [Google Scholar]
- Mastan A., Bharadwaj R.K.B., Kushwaha R.K., Vivek Babu C.S. Functional fungal endophytes in coleus forskohlii regulate labdane diterpene biosynthesis for elevated forskolin accumulation in roots. Microb. Ecol. 2019;78:914–926. doi: 10.1007/s00248-019-01376-w. [DOI] [PubMed] [Google Scholar]
- Mendes R., Kruijt M., De Bruijn I., Dekkers E., Der Voort M.V., Schneider J., Piceno Y.M., Desantis T.Z., Andersen G.L., Bakker P.A.H.M. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332:1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- Molina-Romero D., Baez A., Quintero-Hernandez V., Castañeda M., Fuentes-Ramirez L., Bustillos M., Rodríguez-Andrade O., Morales-García Y., Munive A., Muñoz-Rojas J. Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS One. 2017;12:e0187913. doi: 10.1371/journal.pone.0187913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais C.L.M., Paraskevaidi M., Cui L., Fullwood N.J., Isabelle M., Lima K.M.G., Martin-Hirsch P.L., Sreedhar H., Trevisan J., Walsh M.J. Standardization of complex biologically derived spectrochemical datasets. Nat. Protoc. 2019;14:1546–1577. doi: 10.1038/s41596-019-0150-x. [DOI] [PubMed] [Google Scholar]
- Nan Q., Wang C., Wang H., Yi Q., Liang B., Xu J., Wu W. Biochar drives microbially-mediated rice production by increasing soil carbon. J. Hazard. Mater. 2019;387:121680. doi: 10.1016/j.jhazmat.2019.121680. [DOI] [PubMed] [Google Scholar]
- Neal A.L., Ahmad S., Gordon-Weeks R., Ton J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS One. 2012;7:e35498. doi: 10.1371/journal.pone.0035498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeno-Eckwall E., Kinkel L., Schottel J. Competition and antibiosis in the biological control of potato scab. Can. J. Microbiol. 2001;47:332–340. doi: 10.1139/w01-010. [DOI] [PubMed] [Google Scholar]
- Noble R., Coventry E. Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci. 2005;15:3–20. [Google Scholar]
- Pane C., Celano G., Piccolo A., Villecco D., Spaccini R., Palese A., Zaccardelli M. Effects of on-farm composted tomato residues on soil biological activity and yields in a tomato cropping system. Chem. Biol. Tech. Agric. 2015;24:2–13. [Google Scholar]
- Pang Z., Chen J., Wang T., Gao C., Li Z., Guo L., Xu J., Cheng Y. Linking plant secondary metabolites and plant microbiomes: a review. Front. Plant Sci. 2021;12:621276. doi: 10.3389/fpls.2021.621276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C., Zhang A., Wang Q., Song Y., Zhang M., Ding X., li Y., Geng Q., Zhu C. Ultrahigh-activity immune inducer from Endophytic Fungi induces tobacco resistance to virus by SA pathway and RNA silencing. BMC Plant Biol. 2020;20:169. doi: 10.1186/s12870-020-02386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereg L., Mcmillan M. Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biol. Biochem. 2015;80:349–358. [Google Scholar]
- Plichta D.R., Graham D.B., Subramanian S., Xavier R.J. Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell. 2019;178:1041–1056. doi: 10.1016/j.cell.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S., Cordero O.X. Rhizobiome shields plants from infection. Nat. Microbiol. 2020;5:978–979. doi: 10.1038/s41564-020-0766-1. [DOI] [PubMed] [Google Scholar]
- Qin Y., Druzhinina I.S., Pan X., Yuan Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016;34:1245–1259. doi: 10.1016/j.biotechadv.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Rastall R.A., Maitin V. Prebiotics and synbiotics: towards the next generation. Curr. Opin. Biotechnol. 2002;13:490–496. doi: 10.1016/s0958-1669(02)00365-8. [DOI] [PubMed] [Google Scholar]
- Roberfroid M. Prebiotics: the concept revisited. J. Nutr. 2007;137:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- Rodriguez P.A., Rothballer M., Chowdhury S.P., Nussbaumer T., Gutjahr C., Falter-Braun P. Systems biology of plant-microbiome interactions. Mol. Plant. 2019;12:804–821. doi: 10.1016/j.molp.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Ronda C., Chen S.P., Cabral V., Yaung S.J., Wang H.H. Metagenomic engineering of the mammalian gut microbiome in situ. Nat. Methods. 2019;16:167–170. doi: 10.1038/s41592-018-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig N., Tiedje J.M., Quensen J.F., Meng Q., Hao J. Microbial communities associated with potato common scab-suppressive soil determined by pyrosequencing analyses. Plant Dis. 2012;96:718–725. doi: 10.1094/PDIS-07-11-0571. [DOI] [PubMed] [Google Scholar]
- Santiago T.R., Grabowski C., Rossato M., Romeiro R.D.S., Mizubuti E.S.G. Biological control of eucalyptus bacterial wilt with rhizobacteria. Biol. Control. 2015;80:14–22. [Google Scholar]
- Schlatter D.C., Kinkel L.L., Thomashow L.S., Weller D.M., Paulitz T.C. Disease suppressive soils: new insights from the soil microbiome. Phytopathology. 2017;107:1284–1297. doi: 10.1094/PHYTO-03-17-0111-RVW. [DOI] [PubMed] [Google Scholar]
- Singh J.S., Gupta V.K. Soil microbial biomass: a key soil driver in management of ecosystem functioning. Sci. Total Environ. 2018;634:497–500. doi: 10.1016/j.scitotenv.2018.03.373. [DOI] [PubMed] [Google Scholar]
- Singh J.S., Pandey V.C., Singh D.P. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011;140:339–353. [Google Scholar]
- Sintim H.Y., Bandopadhyay S., English M.E., Bary A.I., DeBruyn J.M., Schaeffer S.M., Miles C.A., Reganold J.P., Flury M. Impacts of biodegradable plastic mulches on soil health. Agric. Ecosyst. Environ. 2019;273:36–49. [Google Scholar]
- Steffan J.J., Brevik E.C., Burgess L.C., Cerdà A. The effect of soil on human health: an overview. Eur. J. Soil Sci. 2018;69:159–171. doi: 10.1111/ejss.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringlis I.A., Yu K., Feussner K., de Jonge R., Van Bentum S., Van Verk M.C., Berendsen R.L., Bakker P.A.H.M. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. U S A. 2018;115:E5213–E5222. doi: 10.1073/pnas.1722335115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao C., Rong L., Xiong W., Shen Z., Liu S., Wang B., Ruan Y., Geisen S., Shen Q., Kowalchuk G. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome. 2020;8:137. doi: 10.1186/s40168-020-00892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M.P., Geisen S. Trophic regulations of the soil microbiome. Trends Microbiol. 2019;27:771–780. doi: 10.1016/j.tim.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Tian B., Pei Y., Huang W., Ding J., Siemann E. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J. 2021;15:1919–1930. doi: 10.1038/s41396-021-00894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Sun B., Shi H., Gao T., He Y., Li Y., Liu Y., Li X., Zhang L., Li S. Sucrose triggers a novel signaling cascade promoting Bacillus subtilis rhizosphere colonization. ISME J. 2021 doi: 10.1038/s41396-021-00966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P., Leach J., Tringe S., Sa T., Singh B. Plant–microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 2020;18:607–621. doi: 10.1038/s41579-020-0412-1. [DOI] [PubMed] [Google Scholar]
- Tu C., Wei J., Guan F., Liu Y., Sun Y., Luo Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020;137:105576. doi: 10.1016/j.envint.2020.105576. [DOI] [PubMed] [Google Scholar]
- Wagg C., Bender S.F., Widmer F., van der Heijden M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. U S A. 2014;111:5266–5270. doi: 10.1073/pnas.1320054111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li X. Steering soil microbiome to enhance soil system resilience. Crit. Rev. Microbiol. 2019;45:743–753. doi: 10.1080/1040841X.2019.1700906. [DOI] [PubMed] [Google Scholar]
- Weller D., Raaijmakers J., Gardener B., Thomashow L. Weller DM, Raaijmakers JM, McSpadden Gardener BB, Thomashow LS.. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- Wen T., Zhao M., Yuan J., Kowalchuk G.A., Shen Q. Root exudates mediate plant defense against foliar pathogens by recruiting beneficial microbes. Soil Ecol. Lett. 2021;3:42–51. [Google Scholar]
- Xin X.F., Nomura K., Ding X., Chen X., Wang K., Aung K., Uribe F., Rosa B., Yao J., Chen J., He S. Pseudomonas syringae effector avirulence protein E localizes to the host plasma membrane and down-regulates the expression of the nonrace-specific disease resistance1/harpin-like3 gene required for antibacterial immunity in Arabidopsis. Plant Physiol. 2015;169:793–802. doi: 10.1104/pp.15.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Rong L., Ren Y., Liu C., Zhao Q., Wu H., Jousset A., Shen Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 2017;107:198–207. [Google Scholar]
- Xu X., Wu B., Zhao W., Lao F., Wu J. Shifts in autochthonous microbial diversity and volatile metabolites during the fermentation of chili pepper (Capsicum frutescens L.) Food Chem. 2020;335:127512. doi: 10.1016/j.foodchem.2020.127512. [DOI] [PubMed] [Google Scholar]
- Yang C., Dong Y., Friman V.-P., Jousset A., Wei Z., Xu Y., Shen Q. Carbon resource richness shapes bacterial competitive interactions by alleviating growth-antibiosis trade-off. Funct. Ecol. 2018;33:868–875. [Google Scholar]
- Yang C., Dong Y., Friman V.-P., Jousset A., Wei Z., Xu Y., Shen Q. Carbon resource richness shapes bacterial competitive interactions by alleviating growth-antibiosis trade-off. Functional Ecology. 2019;33:868–875. doi: 10.1111/1365-2435.13292. [DOI] [Google Scholar]
- Yang W., Dong R., Liu L., Hu Z., Li J., Wang Y., Ding X., Chu Z. A novel mutant allele of SSI2 confers a better balance between disease resistance and plant growth inhibition on Arabidopsis thaliana. BMC Plant Biol. 2016;16:208. doi: 10.1186/s12870-016-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Yin Y., Li Y., Cai T., Ni Y., Peng D., Wang Z. Interactions between polyamines and ethylene during grain filling in wheat grown under water deficit conditions. Plant Growth Regul. 2014;72:189–201. [Google Scholar]
- Yuan J., Zhao J., Wen T., Zhao M., Li R., Goossens P., Huang Q., Bai Y., Vivanco J.M., Kowalchuk G.A. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome. 2018;6:156. doi: 10.1186/s40168-018-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccardelli M., Perrone D., Pane C., Pucci N., Infantino A. Control of corky root of tomato with compost and role of spore-forming bacteria to inhibit Pyrenochaeta lycopersici. Acta Hortic. 2011;914:393–396. [Google Scholar]
- Zengler K., Hofmockel K., Baliga N.S., Behie S.W., Bernstein H.C., Brown J.B., Dinneny J.R., Floge S.A., Forry S.P., Hess M. EcoFABs: advancing microbiome science through standardized fabricated ecosystems. Nat. Methods. 2019;16:567–571. doi: 10.1038/s41592-019-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhalnina K., Louie K.B., Hao Z., Mansoori N., da Rocha U.N., Shi S., Cho H., Karaoz U., Loqué D., Bowen B.P. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018;3:470–480. doi: 10.1038/s41564-018-0129-3. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Han X., Zhao D., Wei K., Yuan Y., Li Y., Liu M., Zhang C.S. Exploring biocontrol agents from microbial keystone taxa associated to suppressive soil: a new attempt for a biocontrol strategy. Front. Plant Sci. 2021;12:655673. doi: 10.3389/fpls.2021.655673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L., Li Y., Wang Z., Yu Y., Zhang N., Yang C., Zeng Q., Wang Q. Synthetic community with six Pseudomonas strains screened from garlic rhizosphere microbiome promotes plant growth. Microb. Biotechnol. 2020;14:488–502. doi: 10.1111/1751-7915.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]