Abstract
Background
Clinical remission is an attainable goal for Rheumatoid Arthritis (RA). However, data on RA remission rates from multinational studies in the Asia-Pacific region are limited. We conducted a cross-sectional multicentric study to evaluate the clinical remission status and the related factors in RA patients in the Asia-Pacific region.
Methods
RA patients receiving standard care were enrolled consecutively from 17 sites in 11 countries from APLAR RA SIG group. Data were collected on-site by rheumatologists with a standardized case-report form. Remission was analyzed by different definitions including disease activity score using 28 joints (DAS28) based on ESR and CRP, clinical disease activity index (CDAI), simplified disease activity index (SDAI), Boolean remission definition, and clinical deep remission (CliDR). Logistic regression was used to determine related factors of remission.
Findings
A total of 2010 RA patients was included in the study, the overall remission rates were 62•3% (DAS28-CRP), 35•5% (DAS28-ESR), 30•8% (CDAI), 26•5% (SDAI), 24•7% (Boolean), and 17•1% (CliDR), respectively, and varied from countries to countries in the Asia-Pacific region. Biological and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) prescription rate was low (17•9%). Compared to patients in non-remission, patients in remission had higher rates of b/tsDMARDs usage and lower rates of GC usage. The favorable related factors were male sex, younger age, fewer comorbidities, fewer extra-articular manifestations (EAM), and use of b/tsDMARDs, while treatment with GC was negatively related to remission.
Interpretation
Remission rates were low and varied in the Asia-Pacific region. Treatment with b/tsDMARDs and less GC usage were related to higher remission rate. There is an unmet need for RA remission in the Asia-Pacific region.
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by symmetrical polyarthritis and results in dysfunction, work disability, reduced quality of life, and shortened life expectancy. Current treatment guidelines recommend the early use of disease-modifying antirheumatic drugs (DMARDs) in a 'treat-to-target' (T2T) strategy that aims to achieve disease remission or at least low disease activity (LDA). Clinical remission in RA patients is defined as the absence of signs and symptoms of significant inflammation, which can be variously defined by several instruments including DAS28, CDAI, SDAI, Boolean remission criteria, CliDR and other clinical definitions (such as RAPID3, MD, Clin28, etc.) .[1], [2], [3], [4], [5], [6], [7], [8]
Recently, rapid diagnosis and response to treatment modifications with tight monitoring and control have facilitated the induction and maintenance of remission in RA patients. Many well-designed registries and studies have documented the remission rate and status in RA patients, indicating that RA remission rate varied in different countries and centers. A study including 24 countries in the Euro-American region showed that 19•6% and 13•8% of RA patients achieved remission according to DAS28-ESR and CDAI. [9] Other single-country studies have been conducted showing that clinical remission rate was low in the Asia-Pacific region, such as 14•6% in Thailand, 20•0% in India, and 29•5% in China for DAS28-ESR. [6,10,11] However, there is no data about RA remission rate of multinational study in the Asia-Pacific region. Herein, this study was performed to assess the remission rates in a large-scale multicentric study in the Asia-Pacific region, and analyze the factors related to remission according to various remission definitions.
2. Methods
2.1. Study design and population
This is a multinational real-life cross-sectional study. Adult patients (≥18 years of age at entry) who fulfilled the American College of Rheumatology (ACR) 1987 criteria or the 2010 ACR/EULAR classification criteria of RA were collected from 17 centers in 11 countries in the Asia-Pacific region (including China (Mainland and Hong Kong), Singapore, Bangladesh, Indonesia, Thailand, Japan, Malaysia, Vietnam, India, Kuwait and Nepal).
2.2. Data Collection
Data collection was from December 2017 to February 2019. The study was approved by the ethics committee of each center. Physicians completed a standardized case report form for each patient. Demographic features collected included age at the data collection, sex, weight, height, ethnic group, education level, work status, smoking, and family history. Clinical characteristics collected included the locations and numbers of affected joints, disease duration, morning stiffness, extra-articular manifestations (EAM), and comorbidities. Laboratory variables included erythrocyte sedimentation rate (ESR), C-reactive protein level (CRP), the level of anti-cyclic citrullinated protein (CCP) antibody, and rheumatoid factor (RF) were also collected. Information on current therapeutic regimes was also included: therapy with csDMARDs, biologic or targeted synthetic DMARDs (b/tsDMARDs), and GC. DMARDs that patients received for fewer than 3 months prior to inclusion were not included in this report.
Clinical assessment included pain visual analog scale (pVAS, 0-10cm scale), fatigue visual analog scale (fVAS, 0-10cm scale), VAS scores for the physician's global (Ph Global score) and patient's global (Pt Global score) (both on a 0-10cm scale), and health assessment questionnaire disability index (HAQ-DI).
2.3. Definitions of remission and therapeutic regimes
Remission rates were analyzed according to the following definitions: (1) DAS28-ESR<2•6; (2) DAS28-CRP<2•6; (3) CDAI≤2•8; (4) SDAI≤3•3; (5) Boolean-based definition: patients satisfy all of the following: tender joint count (TJC) ≤1; swollen joint count (SJC) ≤1; CRP≤1 mg/dl; patient global assessment≤1[range 0–10]; (6) CliDR: patients have no swollen or tender joint with normal ESR and CRP level (<0.8mg/dl).
We divided the therapeutic regimes into 5 categories, including csDMARD monotherapy, csDMARDs combination (two or more csDMARDs), GC co-prescribed with csDMARDs (csDMARDs not specified as single or combination), GC monotherapy, and b/tsDMARDs with or without csDMARDs.
2.4. Statistical analysis
The study was planned to collect a conservative estimate of 100 participants in each country or region. Demographic and disease characteristics were described as median (interquartile range, IQR) or proportions (%). The t test, chi-square, one-way analysis of variance and Kruskal-Wallis test were used to compare differences between groups, respectively. Remission rates were presented as percentages. Relationship between the remission and clinical features was analyzed by univariable logistic regression, and the results were expressed the odds ratio (OR) with 95% confidence interval (CI). P-value <0•1 was considered as the cut-off for inclusion of the variables in a multivariable model. Related factors of remission were identified using backward stepwise multivariable logistic regression and P-values < 0•05 were considered as significant. Missing data were omitted and the remaining data were analyzed in the study. Analysis was performed with the IBM SPSS, V24, PASS (Version 15) and GraphPad Prism V8.0.
2.5. Role of the funding source
The funders and sponsors of the study did not participate in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study. The corresponding author was responsible for the decision to submit for publication.
3. Results
3.1. Demographic and disease characteristics of the patients
Patients' characteristics are presented in Table 1. Eighty-three percent of patients were female, and the female: male ratio was 5:1. The median age was 54•7 (46•0, 62•0) years, and the median disease duration was 70•0 (28•7,141•3) months. Rates of Patients with positive RF or anti-CCP were 76•6% (1167/1523) and 71•7% (999/1394). Details of demographic and disease features of patients from different countries are shown in Supplementary Table 1.
Table 1.
Demographic characteristics of patients.
| All patients (n=2010) | |
|---|---|
| Female, n (%) | 1664/2001 (83•2) |
| Age, yrs, median (IQR) | 54•7 (46•0, 62•0) |
| Disease duration, months, median (IQR) | 70•0 (28•7, 141•3) |
| TJC28, median (IQR) | 1 (0, 3) |
| SJC28, median (IQR) | 0 (0, 2) |
| DJC28, median (IQR) | 0 (0, 0) |
| Morning Stiffness>30min, n (%) | 333/667 (49•9) |
| HAQ-DI, median (IQR) | 0•19 (0•00, 0•75) |
| Pain VAS, median (IQR) | 2•00 (0•10, 4•50) |
| Fatigue VAS, median (IQR) | 1•50 (0•00, 4•00) |
| Patient global VAS, median (IQR) | 2•00 (0•60, 4•13) |
| Physician global VAS, median (IQR) | 2•00 (0•10, 3•00) |
| High education, n (%) | 502 /1532(32•8) |
| Full-time work, n (%) | 563 /1390(40•5) |
| BMI, kg/m2, median (IQR) | 23•4 (20•7, 27•3) |
| Family history, n (%) | 285 /1751(16•3) |
| EAM, n (%) | 333 /1749(19•0) |
| Smoking | |
| Never, n (%) | 1652 /1900(87•0) |
| Former, n (%) | 156 /1900(8•21) |
| Current, n (%) | 92 /1900(4•84) |
| RF, n (%) | 1167/1523 (76•6) |
| Anti-CCP, n (%) | 999/1394(71•7) |
| ESR, mm/h, median (IQR) | 25•0 (13•0, 45•0) |
| CRP, mg/dL, median (IQR) | 0•57 (0•22, 1•90) |
3.2. Comparison of remission rates with different definitions
As illustrated in Table 2, there was wide variation of remission rates according to different definitions. Remission rates calculated using various definitions were 62•3% for DAS28-CRP, followed by 35•5% for DAS28-ESR, 30•8% for CDAI, 26•5% for SDAI, 24•7% for Boolean, and 17•1% (CliDR), respectively.
Table 2.
Comparison of remission rates in different countries.
| Total | Mainland | Hong Kong | Bangladesh | Indonesia | Thailand | Japan | Malaysia | Singapore | Vietnam | India | Kuwait | Nepal | Difference between lowest to highest | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAS28-ESR (%) | 35•5 | 30•8 | 39•8 | 9•00* | 18•8 | 33•1 | 48•2 | 33.0 | 34•9 | 6•25* | 23•8 | 58•5* | 17•2 | 52•2 |
| DAS28-CRP (%) | 62•3 | 52•9 | 72•0 | 13•9* | 64•7 | 63•6 | 74•6 | 44•4 | 70•2 | 22•3* | 76•8 | 77•1* | 37•9 | 63•3 |
| CDAI (%) | 30•8 | 13•9 | 26•5 | 9•00 | 35•0 | 36•9 | 42•7 | 14•0 | 32•9 | 3•57* | 9•47 | 54•3* | 19•0 | 50•7 |
| SDAI (%) | 26•5 | 10•2 | 26•5 | 2•27* | 20•1 | 16•7 | 42•6 | 2•53 | 25•4 | 4•46 | 8•99 | 54•9* | 19•0 | 52•6 |
| Boolean (%) | 24•7 | 22•1 | 25•3 | 2•25* | 12•4 | 14•7 | 36•4 | 3•41* | 18•7 | 2•68* | 10•1 | 51•9* | 10•3 | 49•6 |
| CliDR (%) | 17•1 | 14•5 | 20•5 | 3•23 | 5•08 | 1•72* | 26•3 | 5•13 | 7•88 | 2•68 | 2•56 | 38•4* | 10•3 | 36•7 |
Comparing to other countries and regions, P<0•0001.
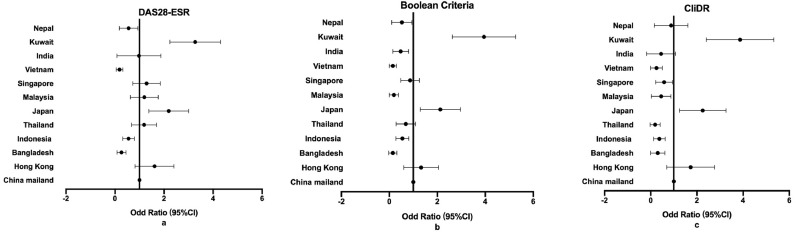
Among 11 countries, the remission rates ranged from 13•9%-77•1% for DAS28-CRP, 6•25%-58•5% for DAS28-ESR, 3•57%-54•3% for CDAI, 2•27%-54•9% for SDAI, 2•25%-51•9% for Boolean criteria, and 1•72%-38•4% for CliDR, which was consistent with the overall tendency. Remission rates varied greatly in different countries. The variance between the highest and lowest remission rate in different countries was from 36•7% to 63•3% (Table 2). Patients from Kuwait and Japan had significantly higher remission rates compared to Mainland China, while the remission rates of Bangladesh, Indonesia, and Vietnam were lower according to DAS28-ESR, Boolean and CliDR definition (Figure 1) (p <0•001). Comparing to RA patients from other countries, patients from Kuwait and Japan had higher b/tsDMARDs prescription, while patients from Bangladesh, Indonesia, and Vietnam had higher TJC counts, higher Patient Global score, and higher ESR levels. Moreover, b/tsDMARDs usage rate of patients from Bangladesh was only 4.0%, and patients from Vietnam had higher usage of GC co-prescribed with csDMARDs (Supplementary table 1).
Figure 1.
Multivariable model of remission rate in different countries in the Asia-Pacific region: a DAS28-ESR; b Boolean Criteria; c CliDR
3.3. Therapeutic regimes and remission
There were 80.7% (1598/1980) of patients using csDMARD. GC combined with csDMARDs were used in 23•7% (470/1980), while 23•2% (459/1980) patients received csDMARDs combination therapy, and 21•4% (424/1980) patients received csDMARD monotherapy. B/tsDMARDs was used in 17•9% (354/1980) of patients, while 1•6% (32/1980) RA patients was on GC monotherapy. The most commonly used csDMARDs were methotrexate (63•7%), hydroxychloroquine (27•2%), leflunomide (16•8%), and sulfasalazine (15•9%) in the Asia-Pacific region. Other csDMARDs, including Iguratimod (40/1980 (2.0%)), Tacrolimus (19/1980 (1.0%)), Mycophenolate mofetil (3/1980 (0.2%)), and cyclophosphamide (1/1980 (0.1%)), were also used by patients. There were 18•9% (238/1262) of patients with methotrexate <10mg, while 3•3% (18/538) of patients received GC>10mg. TNF inhibitors (TNFi) as the most commonly prescribed bDMARDs, were used in 7•7% (153/1980) of patients. In addition, 4•8% (95/1980), 2•6% (51/1980), 1•8% (36/1980), and 1•0% (19/1980) of patients received IL-6 inhibitor (tocilizumab), anti-CD20 mAb (rituximab), CTLA4-Ig (abatacept), and JAK inhibitor (Tofacitinib and Baricitinib), respectively. Therapeutic regimes in different countries are shown in Supplementary table 2.
We compared therapeutic regimes in remission or non-remission patients based on different definitions. Compared to patients not in remission, patients in remission had significantly higher rates of b/tsDMARDs, and lower rates of GC usage (Table 3) (p<0.0001).
Table 3.
Treatment comparison of patients among different remission criteria.
| Variable | DAS28-ESR |
P Value | DAS28-CRP |
P Value | CDAI |
P Value | SDAI |
P Value | Boolean |
P Value | CliDR |
P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Remission | Non-remission | Remission | Non-remission | Remission | Non-remission | Remission | Non-remission | Remission | Non-remission | Remission | Non-remission | |||||||
| MTX, n(%) | 405(63•4) | 731(63•4) | 0•99 | 688(65•6) | 369(58•2) | 0•0022 | 397(66•4) | 843(62•7) | 0•12 | 320(64•8) | 855(63•3) | 0•57 | 300(63•2) | 908(63•7) | 0•82 | 199(63•2) | 950(63•0) | 0•95 |
| LEF, n(%) | 85(13•3) | 210(18•2) | 0•0072 | 154(14•7) | 126(19•9) | 0•0057 | 65(10•9) | 256(19•0) | <0•0001 | 54(10•9) | 256(19•0) | <0•0001 | 64(13•5) | 260(18•2) | 0•011 | 39(12•4) | 272(18•0) | 0•015 |
| SASP, n(%) | 83(13•0) | 191(16•6) | 0•044 | 158(15•1) | 93(14•7) | 0•82 | 84(14•0) | 226(16•8) | 0•12 | 63(12•8) | 228(16•9) | 0•031 | 60(12•6) | 239(16•8) | 0•032 | 29(9•2) | 263(17•4) | <0•0001 |
| HCQ, n(%) | 165(25•8) | 283(24•5) | 0•55 | 275(26•2) | 175(27•6) | 0•54 | 144(24•1) | 382(28•4) | 0•047 | 124(25•1) | 375(27•8) | 0•25 | 134(28•2) | 383(26•9) | 0•57 | 93(29•5) | 378(25•1) | 0•10 |
| CsA, n(%) | 4(0•6) | 18(1•6) | 0•085 | 17(1•6) | 7(1•1) | 0•39 | 6(1•0) | 21(1•6) | 0•33 | 4(0•8) | 22(1•6) | 0•19 | 5(1•1) | 21(1•5) | 0•49 | 1(0•3) | 25(1•7) | 0•068 |
| AZA, n(%) | 7(1•1) | 11(1•0) | 0•77 | 11(1•0) | 4(0•6) | 0•38 | 6(1•0) | 13(1•0) | 0•94 | 5(1•0) | 13(1•0) | 0•92 | 6(1•3) | 12(0•8) | 0•41 | 4(1•3) | 14(0•9) | 0•58 |
| TNF inhibitor, n(%) | 71(11•1) | 80(6•9) | 0•0023 | 115(11•0) | 36(5•7) | <0•0001 | 73(12•2) | 76(5•7) | <0•0001 | 70(14•2) | 78(5•8) | <0•0001 | 74(15•6) | 78(5•5) | <0•0001 | 45(14•3) | 108(7•2) | <0•0001 |
| IL-6 inhibitor, n(%) | 59(9•2) | 35(3•0) | <0•0001 | 73(7•0) | 20(3•2) | 0•00090 | 39(6•5) | 55(4•1) | 0•021 | 39(17•3) | 53(3•0) | 0•00053 | 34(7•2) | 60(4•2) | 0•010 | 31(9•8) | 63(4•2) | <0•0001 |
| Anti-CD20 mAb, n(%) | 29(4•5) | 22(1•9) | 0•0013 | 32(3•1) | 16(2•5) | 0•53 | 27(4•5) | 24(1•8) | 0•0052 | 24(4•9) | 24(1•8) | <0•0001 | 23(4•8) | 25(1•8) | <0•0001 | 20(6•3) | 30(2•0) | <0•0001 |
| Abatacept, n(%) | 17(2•7) | 19(1•6) | 0•14 | 28(2•7) | 8(1•3) | 0•053 | 20(3•3) | 16(1•2) | 0•0012 | 20(4•0) | 16(1•2) | <0•0001 | 19(4•0) | 17(1•2) | <0•0001 | 10(3•2) | 26(1•7) | 0•092 |
| JAK inhibitor, n(%) | 9(1•4) | 10(0•9) | 0•28 | 11(1•0) | 6(0•1) | 0•84 | 6(1•0) | 12(0•9) | 0•82 | 6(1•2) | 12(0•9) | 0•53 | 7(1•5) | 12(0•8) | 0•23 | 4(1•3) | 15(1•0) | 0•66 |
| Drug modes, n (%) | <0•0001 | <0•0001 | <0•0001 | <0•0001 | <0•0001 | <0•0001 | ||||||||||||
| csDMARD monotherapy | 171(29•1) | 222(22•6) | 236(24•6) | 93(18•5) | 155(28•1) | 263(22•7) | 128(28•3) | 253(21•7) | 111(25•5) | 284(23•1) | 68(23•9) | 315(24•0) | ||||||
| csDMARDs combination | 148(25•2) | 249(25•4) | 254(26•5) | 120(23•8) | 134(24•3) | 316(27•2) | 113(24•9) | 312(26•1) | 116(26•7) | 322(26•1) | 74(26•0) | 331(25•2) | ||||||
| csDMARDs with GC | 125(13•6) | 317(32•3) | 202(21•0) | 186(36•9) | 92(16•7) | 372(32•1) | 51(11•3) | 393(33•6) | 49(11•3) | 405(32•9) | 427(10•9) | 396(30•0) | ||||||
| GC monotherapy | 4(0•7) | 37(2•8) | 9(0•9) | 19(3•8) | 5(0•9) | 26(2•2) | 2(0•4) | 27(2•3) | 2(0•5) | 29(2•4) | 2(0•7) | 28(2•1) | ||||||
| b/tsDMARDs with or without csDMARDs | 176(31•5) | 156(16•9) | 249(27•0) | 80(17•1) | 159(29•9) | 171(15•8) | 153(35•1) | 171(15•7) | 150(36•1) | 180(15•6) | 106(38•6) | 227(18•4) | ||||||
MTX: methotrexate; LEF: leflunomide; SASP: sulfasalazine; HCQ: hydroxychloroquine; CsA: Cyclosporine; AZA: azathioprine; TNF: tumor necrosis factor; JAK: Janus kinase
We further compared the remission rates among groups using different bDMARDs or tsDMARDs (Table 4). The results showed that there was no significant difference between bDMARDs or tsDMARDs for remission rates.
Table 4.
Comparison of the remission rate in different b/tsDMARDs
| Remission criteria | TNFi n=153 | IL-6i n=95 | Anti-CD20 mAb n=51 | CTLA4-Ig n=36 | JAKi n=19 | P value |
|---|---|---|---|---|---|---|
| DAS28-ESR, n (%) | 71 (47•0) | 59 (62•8) | 29 (56•9) | 17 (47•2) | 9 (47•4) | 0•15 |
| DAS28-CRP, n (%) | 115 (76•2) | 73 (78•5) | 32 (66•7) | 28 (77•8) | 11 (64•7) | 0•46 |
| CDAI, n (%) | 73 (49•0) | 39 (41•5) | 27 (52•9) | 20 (55•6) | 6 (33•3) | 0•34 |
| SDAI, n (%) | 70 (47•3) | 39 (42•4) | 24 (50•0) | 20 (55•6) | 6 (33•3) | 0•50 |
| Boolean, n (%) | 74 (48•7) | 34 (36•2) | 23 (47•9) | 19 (52•8) | 7 (36•8) | 0•25 |
| CliDR, n (%) | 45 (29•4) | 31 (33•0) | 20 (40•0) | 10 (27•8) | 4 (21•1) | 0•51 |
3.3. Factors related to remission
Univariable logistic regression analysis was performed to analyze the relationship between variables and treatment outcome based on various remission definitions (Supplementary table 4). Male sex, fewer comorbidities and EAM, and high education level were positively related to remission. Comparing to csDMARD monotherapy, treatment with GC monotherapy or co-prescribed with csDMARDs were related to non-remission, while b/tsDMARDs usage was related to remission according to different criteria (all P < 0•05).
The results of multivariable logistic regression analysis were represented in Table 5. The likelihood of being in remission was higher in patients with male sex, younger age, fewer EAM and comorbidities, while csDMARDs combination, GC monotherapy and co-prescribed with csDMARDs were negatively related to remission rates according to DAS28-ESR criteria. Based on Boolean criteria, csDMARD co-prescribed with GC was related to non-remission of RA, while b/tsDMARDs prescription was related to RA remission. Factors related to RA remission according to all criteria was presented in Table 5.
Table 5.
Multivariable analysis of different remission criteria
| DAS28-ESR | DAS28-CRP | CDAI | SDAI | Boolean | CliDR | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| male | ||||||
| female | 0•28 (0•17, 0•47) | 0•50 (0•29, 0•88) | ns | ns | ns | 0•46 (0•24, 0•88) |
| Age (years) | 0•98 (0•97, 1•00) | 0•986 (0•97,0•99) | ns | ns | ns | ns |
| Duration (months) | ns | 1•003 (1•001, 1•005) | ns | ns | ns | ns |
| Comorbidities | 0•62 (0•42, 0•92) | ns | ns | ns | ns | 0•61 (0•44, 0•85) |
| EAM | 0•58 (0•36, 0•95) | ns | ns | ns | ns | ns |
| Drug mode | ||||||
| csDMARD monotherapy | ||||||
| csDMARDs combination | 0•51 (0•30, 0•88) | 0•57 (0•33, 0•93) | ns | 0•52 (0•30, 0•92) | ns | ns |
| csDMARDs with GC | 0•40 (0•25, 0•64) | 0•35 (0•22, 0•56) | 0•54 (0•32, 0•91) | 0•40 (0•24, 0•67) | 0.47 (0.26, 0.85) | ns |
| GC monotherapy | 0•19 (0•04, 0•91) | 0•14 (0•05, 0•44) | ns | ns | ns | ns |
| b/tsDMARDs with or without csDMARDs | ns | ns | ns | 2•00 (1•2, 3•4) | 2.69 (1.50, 4.81) | 2•4 (1•2, 4•8) |
ns: no significance; All P < 0•05
4. Discussion
In this large-scale multinational study in the Asia-Pacific region, the pooled remission rates were varied from 17•1% to 62•3% according to different remission criteria. The highest remission rate was for DAS28-CRP, followed by DAS28-ESR, CDAI, SDAI, Boolean, and CliDR. We identified that several factors were related to RA remission based on different criteria in this dataset, including demographic data (age and sex), clinical features (EAM and comorbidities) and therapeutic regimes (csDMARDs, GC and b/tsDMARDs usage). The results showed that remission using stringent criteria including CliDR, Boolean and SDAI definition, was an achievable goal in selected patients in the Asia-Pacific region.
There are already some registries illustrating that RA remission rate has increased over the years, which may have resulted from early diagnosis and T2T strategy of RA. [12], [13], [14] In this study, RA patients achieved the highest remission rate according to DAS28-CRP (62•3%), higher than DAS28-ESR, which was consistent with other studies. [6,9,15,16] Remission rate based on CDAI was 30•8%, higher than SDAI (26•5%), which might be owing to elevated CRP levels in this cohort. Several studies suggested that Boolean remission is one of the most stringent criteria and more likely to prevent the progression of functional disability. [17] However, a substantial proportion of patients with one tender or swollen joint are categorized as being in Boolean remission while they were still having residual disease activity. Presenting as clinical deep remission criteria, CliDR might be feasible in daily practice, which was shown in other studies. [6,18] There was significant variance of remission rates among different countries, which could be owing to discrepancy in TJC, Patient and Physician Global score, ESR levels, and usage of b/tsDMARDs in these countries. Remission rates have been shown that differed in the range of 36•2%-61•6% for DAS-ESR, 17•3-34•0% for Boolean criteria, and 26•2%-39•0% for SDAI in different countries. [19], [20], [21] However, related factors including ethnicity, economy and education could also influence the remission status in different countries, which are not the concerns of this study but could be further analyzed.
There was high prescription rate of csDMARDs in this study. Comparing to cohorts from other regions, b/tsDMARDs prescription rate was low and dosage of MTX was insufficient from this dataset in the Asia-Pacific region, which might be related to low rates of remission. [22,23] In addition, different kinds of b/tsDMARDs showed no significant difference in remission rates. In other head-to-head studies, similar treatment efficacy has been described. [24], [25], [26]
In this real-world study, male sex was related to higher remission rates in DAS28-ESR and CliDR criteria, as female may appear to be more susceptible to pain, have higher PGA and ESR levels, and consequently reduce the remission rate. [27,28] To analyze the relationship between current therapeutic regimes and remission, we collected current-taking drugs for more than 3 months. The results showed that the use of b/tsDMARDs was positively related to RA remission, which was in line with the results of several previous studies. However, csDMARDs combination and GC co-prescribed with csDMARDs were related to non-remission according to all definitions except CliDR. As recommended, GC should be used as bridging therapy until csDMARDs exhibit their efficacy and tapered rapidly. GC co-prescribed with csDMARDs and combinations of csDMARDs are more intensive than csDMARD monotherapy, so the negative relationship between these therapies and remission might reflect the choice of treatment based on disease severity according to clinical practice and prospective randomized trials. [29,30]
There are several limitations to this study. First, our study is a cross-sectional study rather than a prospective cohort study. Patients in our study were not followed up regularly. Second, our results demonstrated differences in the participated countries or regions, and the population analyzed here only involved part of the Asia-Pacific region, which may not be applied for other RA populations in these countries. However, we believe that the relatively large sample size and multinationality mitigated this to some extent. Furthermore, remission rates varied across different countries, which might due to variation in evaluation of physician global scores of different investigators. Further analysis is required, including exploration of discrepancies among the regions in ethnicity, access to medical care and treatment options.
In summary, remission rates were low and varied in different countries, while b/tsDMARDs and less GC usage were related to higher remission rates. Low b/tsDMARDs prescription rate and insufficient dose of MTX might be related to unsatisfactory RA remission in the Asia-Pacific region.
Contributors
XS and RL contributed to the study conduct, data analysis, and writing of the manuscript. XS, RL, YMC, AA, ML, MRC, RH, BPPS, YK, KF, NVH, SP, LKP, WK, KRS, BP, PN, PC, CH and SSY were responsible for data collection. XS, RL, FS and YL did the statistical data analysis. ZGL contributed to the design and supervision of the study. All authors approved the final submitted version and agreed to publication.
Funding/support
This work was supported in part by grants from Macao science and technology development fund (0094/2018/A3); Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis (BZ0135); Beijing Municipal Science and Technology Project (Z191100006619109 and Z191100006619110); Peking-Tsinghua Center for Life Sciences.
Data sharing
The data supporting the analysis of this study are available from the corresponding author, Zhanguo Li, upon request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations
Funding
Macao science and technology development fund (0094/2018/A3) (partial).
Research in context
Evidence before this study
We searched PubMed for studies published on Feb 18, 2021, using the terms "rheumatoid arthritis" AND "remission" AND "Asia" with no language or time restrictions. Of the 197 articles identified, there were 8 articles on the RA remission rate and the influencing factors. However, there is no data about RA remission status of multinational studies in the Asia-Pacific region.
Added value of this study
This study is the first large-scale multinational study performed to assess the remission rates in the Asia-Pacific region. RA patients from 17 centers in 11 countries were involved, and the related factors of remission were analyzed.
Implications of all the available evidence
Our study fills the gaps in RA remission status data in the Asia-Pacific region. It was shown that the remission rates were low in the Asia-Pacific region and varied greatly in different countries. It urges the efforts to improve the treatment and remission of RA patients in the Asia-Pacific region.
Declaration of Competing Interest
ML reports speaker fees from Abbvie and Johnson & Johnson, conference sponsorship from Johnson & Johnson, Pfizer, and Sanofi, and has provided advisory services for Gilead, Eli Lilly. KF has received grants or contracts from Chugai, Bristol Myers, Abbvie, Otsuka Pharmaceutical, Eli Lilly, Pfizer, Tsumura, Asahi Kasei, Mitsubishi Tanabe, Esai, Japan Blood Products Organization, Novartis, Sanofi, and Astellas, reports payments or honoraria from Astellas, Abbvie, Amgen, Ayumi, MSD, Esai, Ono, Gilead, Kowa, Sanofi, Japan Blood Products Organization, Novartis, Pfizer, Bristol Myers, Mylan EPD, Janssen, Asahi Kasei, Daiichi Sankyo, Chugai, Mitsubishi Tanabe, Eli Lilly, and Boehringer Ingelheim and has participated in the Data Safety Monitoring Board or Advisory Board for Asahi Kasei, Astellas, Abbvie, Amgen, Ono, Gilead, Chugai, Eli Lilly, Bristol Myers, and Mylan EPD. PC reports honoraria from Novartis and Johnson and Johnson, is a member of the advisory board for Johnson & Johnson, and reports samples of Amgevita, Baricitinib, and Ixekizumab. All other authors declare no competing interests.
Acknowledgment
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100240.
Contributor Information
Ru Li, Email: doctorliru123@163.com.
Zhanguo Li, Email: li99@bjmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Smolen JS, Landewe RBM, Bijlsma JWJ. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 2.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 3.Aletaha D, Nell VP, Stamm T. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–R806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolen JS, Breedveld FC, Schiff MH. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42(2):244–257. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- 5.Dave B, Desai S, Ramadwar M. Kimura disease with proliferative squamous metaplasia: an unusual finding and a potential diagnostic pitfall. Indian J Pathol Microbiol. 2007;50(4):771–773. [PubMed] [Google Scholar]
- 6.Liu JJ, Li R, Gan YZ. Clinical deep remission and related factors in a large cohort of patients with rheumatoid arthritis. Chin Med J (Engl) 2019;132(9):1009–1014. doi: 10.1097/CM9.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol. 2008;35(11):2136–2147. doi: 10.3899/jrheum.080182. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe F, Rasker JJ, Boers M, Wells GA, Michaud K. Minimal disease activity, remission, and the long-term outcomes of rheumatoid arthritis. Arthritis Rheum. 2007;57(6):935–942. doi: 10.1002/art.22895. [DOI] [PubMed] [Google Scholar]
- 9.Sokka T, Hetland ML, Makinen H. Remission and rheumatoid arthritis - Data on patients receiving usual care in twenty-four countries. Arthritis and Rheumatism. 2008;58(9):2642–2651. doi: 10.1002/art.23794. [DOI] [PubMed] [Google Scholar]
- 10.Chandrashekara S, Shobha V, Dharmanand BG. Factors influencing remission in rheumatoid arthritis patients: results from Karnataka rheumatoid arthritis comorbidity (KRAC) study. International Journal of Rheumatic Diseases. 2018;21(11):1977–1985. doi: 10.1111/1756-185X.12908. [DOI] [PubMed] [Google Scholar]
- 11.Darawankul B, Chaiamnuay S, Pakchotanon R, Asavatanabodee P, Narongroeknawin P. The good EULAR response at the first year is strongly predictive of clinical remission in rheumatoid arthritis: results from the TARAC cohort. Clin Rheumatol. 2015;34(1):43–49. doi: 10.1007/s10067-014-2749-1. [DOI] [PubMed] [Google Scholar]
- 12.Hyrich KL, Watson KD, Lunt M, Symmons DPM. Bsrbr. Changes in disease characteristics and response rates among patients in the United Kingdom starting anti-tumour necrosis factor therapy for rheumatoid arthritis between 2001 and 2008. Rheumatology. 2011;50(1):117–123. doi: 10.1093/rheumatology/keq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Littlejohn G, Roberts L, Bird P. Patients with Rheumatoid Arthritis in the Australian OPAL Cohort Show Significant Improvement in Disease Activity over 5 Years: A Multicenter Observational Study. Journal of Rheumatology. 2015;42(9):1603–1609. doi: 10.3899/jrheum.141575. [DOI] [PubMed] [Google Scholar]
- 14.Aga A-B, Lie E, Uhlig T. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000-2010. Annals of the Rheumatic Diseases. 2015;74(2):381–388. doi: 10.1136/annrheumdis-2013-204020. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Li R, Da Z. Remission assessment of rheumatoid arthritis in daily practice in China: a cross-sectional observational study. Clin Rheumatol. 2018;37(3):597–605. doi: 10.1007/s10067-017-3850-z. [DOI] [PubMed] [Google Scholar]
- 16.Greenmyer JR, Stacy JM, Sahmoun AE, Beal JR, Diri E. DAS28-CRP Cutoffs for High Disease Activity and Remission Are Lower Than DAS28-ESR in Rheumatoid Arthritis. ACR Open Rheumatology. 2020;2(9):507–511. doi: 10.1002/acr2.11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aletaha D, Wang X, Zhong S, Florentinus S, Monastiriakos K, Smolen JS. Differences in disease activity measures in patients with rheumatoid arthritis who achieved DAS, SDAI, or CDAI remission but not Boolean remission. Semin Arthritis Rheum. 2020;50(2):276–284. doi: 10.1016/j.semarthrit.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Cai Y-M, Li R, Ye H. Effect of sustained intensive therapy with disease modifying anti-rheumatic drugs in rheumatoid arthritis: a 5-year real-world consecutive study. Chinese Medical Journal. 2020;133(12):1397–1403. doi: 10.1097/CM9.0000000000000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriya B, Xiong J, Boire G. Earlier time to remission predicts sustained clinical remission in early rheumatoid arthritis–results from the Canadian Early Arthritis Cohort (CATCH) J Rheumatol. 2014;41(11):2161–2166. doi: 10.3899/jrheum.140137. [DOI] [PubMed] [Google Scholar]
- 20.Svensson B, Andersson ML, Bala SV, Forslind K, Hafstrom I, group Bs. Long-term sustained remission in a cohort study of patients with rheumatoid arthritis: choice of remission criteria. BMJ Open. 2013;3(9) doi: 10.1136/bmjopen-2013-003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahouri SH, Michaud K, Mikuls TR. Remission of rheumatoid arthritis in clinical practice: application of the American College of Rheumatology/European League Against Rheumatism 2011 remission criteria. Arthritis Rheum. 2011;63(11):3204–3215. doi: 10.1002/art.30524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hetland ML, Jensen DV, Krogh NS. Monitoring patients with rheumatoid arthritis in routine care: experiences from a treat-to-target strategy using the DANBIO registry. Clin Exp Rheumatol. 2014;32 (5 Suppl 85): S-141-6. [PubMed] [Google Scholar]
- 23.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009;68(7):1100–1104. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam JL, Takase-Minegishi K, Ramiro S. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Annals of the Rheumatic Diseases. 2017;76(6):1113–1136. doi: 10.1136/annrheumdis-2016-210713. [DOI] [PubMed] [Google Scholar]
- 25.Weinblatt ME, Schiff M, Valente R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65(1):28–38. doi: 10.1002/art.37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burmester GR, Lin Y, Patel R. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2017;76(5):840–847. doi: 10.1136/annrheumdis-2016-210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maynard C, Mikuls TR, Cannon GW. Sex Differences in the Achievement of Remission and Low Disease Activity in Rheumatoid Arthritis. Arthritis Care & Research. 2020;72(3):326–333. doi: 10.1002/acr.23873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moller-Bisgaard S, Georgiadis S, Horslev-Petersen K. Predictors of joint damage progression and stringent remission in patients with established rheumatoid arthritis in clinical remission. Rheumatology (Oxford, England) 2020 doi: 10.1093/rheumatology/keaa496. [DOI] [PubMed] [Google Scholar]
- 29.Black RJ, Lester S, Buchbinder R. Factors associated with oral glucocorticoid use in patients with rheumatoid arthritis: a drug use study from a prospective national biologics registry. Arthritis Research & Therapy. 2017;19 doi: 10.1186/s13075-017-1461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burmester GR, Buttgereit F, Bernasconi C. Continuing versus tapering glucocorticoids after achievement of low disease activity or remission in rheumatoid arthritis (SEMIRA): a double-blind, multicentre, randomised controlled trial. Lancet. 2020;396(10246):267–276. doi: 10.1016/S0140-6736(20)30636-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.