Abstract
Bone marrow mesenchymal stem cells (MSCs) give rise to osteoblasts and adipocytes, with an inverse relationship between the two. The MSCs from protease-activated receptor-2 knockout (PAR2 KO) mice have a reduced capacity to generate osteoblasts. Here we describe the observation that PAR2 KO osteoblastic cultures generate more adipocytes than wildtype (WT) cultures. Osteoblasts from PAR2 KO mice expressed lower levels of osteoblastic genes (Runx2, Col1a1 and Bglap), and higher levels of the adipocytic gene Pparg than WT osteoblasts. Bone marrow stromal cells from PAR2 KO mice generated fewer osteoblastic colonies (assessed by staining for alkaline phosphatase activity and mineral deposition) and more adipocytic (Oil Red-O positive) colonies than cultures from WT mice. Similarly, cultures of the bone marrow stromal cell line (Kusa 4b10) in which PAR2 was knocked down (F2rl1 KD), were less osteoblastic and more adipocytic than vector control cells. Putative regulators of PAR2-mediated osteogenesis and suppression of adipogenesis were identified in an RNA-sequencing (RNA-seq) investigation; these include C1qtnf3, Gpr35, Grem1, Snorc and Tcea3, which were more highly expressed, and Cnr1, Enpep, Hmgn5, Il6 and Ramp3 which were expressed at lower levels, in control than in F2rl1 KD cells. Interleukin-6 (IL-6) levels were higher in medium harvested from F2rl1 KD cells than from control cells, and a neutralising anti-IL-6 antibody reduced the number of adipocytes in F2rl1 KD cultures to that of control cultures. Thus, PAR2 appears to be a mediator of the reciprocal relationship between osteogenesis and adipogenesis, with IL-6 having a regulatory role in these PAR2-mediated effects.
Keywords: Protease-activated receptor-2, Lineage determination, Osteoblast, Adipogenesis, Interleukin-6, Mesenchymal stem cells
Highlights
-
•
PAR2 promotes osteogenic differentiation of MSCs at the expense of adipogenesis.
-
•
PAR2 deficiency increases secretion of IL-6 by MSCs during early differentiation.
-
•
Elevated IL-6 secretion stimulates adipogenesis with no effect on osteogenesis.
-
•
Ten genes have been identified as putative novel mediators of PAR2 effects on MSCs.
1. Introduction
In bone marrow, mesenchymal stem cells (MSCs) give rise to both osteoblasts and adipocytes, as well as other lineages (Owen, 1988; Dirckx and Maes, 2019). This differentiation is largely mutually exclusive, such that one lineage predominates at the expense of the other (Bethel et al., 2013). Current evidence suggests that this process may contribute to the pathology of various bone conditions (Berendsen and Olsen, 2014). For instance, the increase in the adiposity of bone marrow and gradual bone loss with aging have been attributed to a decrease in osteogenesis in favour of adipogenesis (Kawai et al., 2009). Despite this, the phenomenon is still poorly understood and the roles of many mediators remain to be fully elucidated.
Protease-activated receptor-2 (PAR2) is a seven transmembrane domain G protein-coupled receptor that is activated by proteolytic cleavage of its extracellular domain. The receptor is widely expressed; it has been detected in the foetal (Jenkins et al., 2000) as well as postnatal skeleton, where it is expressed by a range of cells including osteoblasts (Abraham et al., 2000). We have previously reported that during growth, but not in the more mature skeleton, PAR2 knockout (KO) mice have a higher bone mass phenotype than wildtype (WT) controls, which could be attributed to lower osteoclast numbers and reduced bone resorption, as osteoblast parameters were also lower in the absence of PAR2 (Georgy et al., 2012). Additionally, the cortical bone healing response is delayed in these mice. We have shown that activation of PAR2 in calvarial osteoblasts induces the expression of collagen type I (Georgy et al., 2010) and that in its absence, primary bone marrow cultures form significantly fewer alkaline phosphatase (ALP) positive (i.e. osteoblastic) colony forming units (Georgy et al., 2012). These observations suggest a complex role for PAR2 in both osteogenesis and bone resorption, rendering bone remodeling defective in its absence.
We have recently undertaken a study investigating a possible role for PAR2 in prostate cancer bone metastases, based on the fact that prostate cancer cells express multiple activators of PAR2 (Ramsay et al., 2008). We treated mouse calvarial osteoblasts with medium conditioned by MDA-PCa-2b human prostate cancer cells (Navone et al., 1997), and observed that this medium enhances osteogenesis in WT, but not PAR2 KO cells (Pagel et al., manuscript submitted for publication). During the course of this study, we observed that the presence of PAR2 appeared to suppress adipogenesis. No similar effect on adipocyte differentiation has been described previously, although it has been observed that PAR2 null mice are generally resistant to obesity induced by a high fat diet when compared to their wildtype counterparts, an effect that can be attributed to regulation of the behaviour of mature adipocytes (Badeanlou et al., 2011). The current work reports our initial findings and other experiments that were designed to investigate the role of PAR2 in the reciprocal relationship between osteoblastic and adipogenic differentiation of MSCs. For this purpose, studies were undertaken in primary calvarial osteoblasts and bone marrow stromal cells derived from WT and PAR2 KO mice. Additionally, PAR2 was stably knocked down in the bipotential mouse bone marrow stromal cell line, Kusa 4b10 (Allan et al., 2003), to provide a model to investigate potential regulators of PAR2-mediated effects.
2. Materials and methods
2.1. Materials
The MDA-PCa-2b (ATCC® CRL-2422™) human prostate cancer cell line (Navone et al., 1997) was purchased from American Type Culture Collection (Manassas, VA, USA). BRFF-HPC1™ medium for the MDA-PCa-2b cell line was purchased from Athena Enzyme Systems (Baltimore, MD, USA). Foetal calf serum (FCS) was from Hyclone™ Thermo Fisher Scientific (Waltham, MA, USA) for MDA-PCa-2b and primary osteoblastic and marrow stromal cultures and from Bovogen Biologicals (East Keilor, Victoria, Australia) for the Kusa 4b10 cells. Collagenase A and the 5-bromo-2′-deoxyuridine (BrdU) incorporation kit were from Roche Diagnostics (Indianapolis, IN, USA). All other cell culture media, L- glutamine, trypsin and antibiotics were from Gibco™ Life Technologies (Scoresby, Victoria, Australia). All PCR reagents, Wizard® Genomic DNA Purification kit, Total RNA Isolation system and GoScript™ reverse transcription mix were obtained from Promega (Madison, Wisconsin, USA). PRISM® BigDye® terminator v3.1 was from Applied Biosystems (Carlsbad, California, USA). Anti-interleukin-6 (IL-6), non-immune rat IgG and IL-6 ELISA kit were purchased from Thermo Fisher Scientific. All other reagents including MISSION™ shRNA lentiviral transduction particles, AEBSF, ascorbic acid, β-glycerophosphate, Fura-2 AM, hexadimethrine bromide, probenecid and puromycin were obtained from Sigma Aldrich (now Merck, Darmstadt, Germany).
2.2. Animals
Mice used for this research were housed and maintained in the animal house facility of the Faculty of Veterinary and Agricultural Sciences, University of Melbourne. The use of all animals in this study was approved by the Gene Technology and Biosafety Committee and the Animal Ethics Committee of the Faculty of Veterinary and Agricultural Sciences, the University of Melbourne (AEC 1112164). Mice were housed in a controlled environment with free access to food and water, and all work was conducted in compliance with the Australian Code for the Care and Use of Animals for Scientific Purposes (2013) and the National Health and Medical Research Council Guidelines for the Generation, Breeding, Care and Use of Genetically Modified and Cloned Animals for Scientific Purposes (2007). WT and PAR2 KO mice were maintained as heterozygous breeding stock (F2rl1+/−) on a C57BL/6J background (Lindner et al., 2000). Homozygous (WT or KO) mice were either kept for breeding or bone marrow stromal cell isolation. Wildtype and PAR2 KO pups from these homozygous breeders were used for primary osteoblast cultures.
2.3. Cell culture
2.3.1. MDA-PCa-2b cells
The MDA-PCa-2b cell line was grown in BRFF-HPC1 medium containing 20% FCS, L-glutamine (2 mM) and gentamicin (50 μg/mL; ‘complete BRFF-HPC1’). Cultures were maintained at 37 °C in a humid environment under 5% CO2 in air. Every 3–4 days medium was changed, and fresh medium was added. For the production of medium conditioned by MDA-PCa-2b cells (MDA-CM), medium was added to 60–80% confluent cell cultures, incubated for 48 h and then collected into sterile centrifuge tubes. Non-adherent cells were pelleted by centrifugation at 300g for 2 min, following which supernatants were transferred to fresh sterile tubes that were stored at 4 °C for use (undiluted) in experiments. The medium used to prepare MDA-CM was complete BRFF-HPC1 for use in all subsequent assays except BrdU incorporation assays in bone marrow stromal cells, for which the BRFF-HPC1 contained bovine serum albumin (0.1% w/v) instead of FCS.
2.3.2. Primary mouse osteoblasts
Primary osteoblasts were isolated by sequential collagenase digestion from the calvariae of neonatal WT and PAR2 KO mice as described (Pagel et al., 2003), and cultured in complete Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) FCS, l-glutamine (2 mM) and gentamicin (50 μg/mL). Cell isolates with ALP activity greater than 10 nmol Pi/min/ng cellular DNA were trypsinised, pooled, plated in complete DMEM in 12-well plates (6 × 104 cells/well) for adipocyte counts or 6-well plates (105 cells/well) for RNA extraction. After incubation for 48 h, medium was replaced with complete MDA-CM or control (unconditioned complete BRFF-HPC1) medium and cells were incubated for an additional 1, 4 or 12 days prior to RNA extraction or 21 days prior to staining with Oil Red-O for adipocyte counts. One third of the medium in each well was replaced with fresh medium every 3–4 days. For cultures to be stained with Oil Red-O, the media contained ascorbic acid (0.44 mg/l) and β-glycerophosphate (5 mM).
2.3.3. Primary mouse bone marrow stromal cells
Bone marrow stromal cells were isolated from 6 to 8 week old WT and PAR2 KO male mice as described (Anjos-Afonso and Bonnet, 2008). Cells were cultured in complete α-minimum essential medium (α-MEM) containing 10% (v/v) FCS, l-glutamine (2 mM) and gentamicin (50 μg/mL). After 24 h, adherent cells were trypsinised and plated in complete α-MEM in 96 well plates (104 cells/well) for BrdU incorporation assays, in 60 mm dishes (106 cells/dish) for colony characterisation, or in 6-well plates (106 cells/well) for RNA extraction, then incubated for 48 h prior to treatment with MDA-CM.
2.3.4. Kusa 4b10 cells
Kusa 4b10 cells were kindly provided by Professor Natalie Sims (St. Vincent's Institute of Medical Research, Melbourne, Australia). PAR2 expression was stably knocked down in Kusa 4b10 cells using MISSION® lentiviral small hairpin RNA (shRNA) constructs as per the manufacturer's protocol. All five PAR2 targeting shRNA clones were used in combination each at a multiplicity of infection (MOI) of 2 (a total cumulative MOI of 10). To control for the cellular effects of the transduction process as well as the RNA induced silencing complex (RISC) and RNA interference (RNAi) pathway, MISSION® Non-Target shRNA Control Transduction Particles were used at an MOI of 10 (vector control). Lentiviral particles also contained a puromycin resistance gene which was used to select for successfully transduced clones. Puromycin selection was done using a concentration of 6 mg/mL based on a previously generated puromycin kill curve. Resistant colonies were picked, expanded and assayed to confirm PAR2 knockdown at both RNA and protein levels using quantitative reverse transcription PCR (RT-qPCR) and intracellular calcium mobilisation in response to the PAR2 activator, trypsin, respectively. Based on the results, a final clone from each knockdown (F2rl1 KD) and vector control (vector control) group was selected, expanded and stored. Long term knockdown of PAR2 in the selected clone was ensured by another round of evaluation following thawing and culture for 21 days. Cells were maintained in antibiotic-free complete α-MEM at 37 °C under 5% CO2 (Walker et al., 2008). For osteoblast differentiation, cells were cultured in α-MEM containing 15% FCS, ascorbic acid (50 μg/mL) and β-glycerophosphate (10 mM). If RNA isolation or Oil Red-O staining was planned, β-glycerophosphate was omitted. Media were changed once every three days throughout both maintenance and differentiation stages. For differentiation experiments, cells were subcultured and regularly seeded at a density of 3000 cells/cm2 (Allan et al., 2003) in 12-well plates if mineralisation assays or morphological measurements were to be performed or in 6-well plates for RNA isolation. On day 3 (differentiation day 0), the medium was changed to osteoblast differentiation medium.
2.4. Adipocyte assays
To assess the extent of adiposity in cell cultures, cells in 12 well plates were stained using Oil Red-O and haematoxylin using the method described by the manufacturer (Thermo Fisher Scientific, Waltham, MA, USA). Cultures were then photographed at 40× magnification using an Olympus 1 × 70 inverted microscope (Tokyo, Japan) fitted with a ProgRes® C3 camera (Jenoptik, Thuringia, Germany). The number of mature adipocytes was manually counted using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and expressed as either absolute adipocyte number/well (or field of view) or as a proportion of total cell counts. Where adipocyte area measurements were taken, this was done by manually tracing individual cell perimeters in ImageJ and reporting measurements similarly as an absolute or normalised value.
2.5. Characterisation of colonies in cultures of bone marrow stromal cells
Bone marrow stromal cell cultures were incubated in MDA-CM or control medium as described above for the primary osteoblast cultures used in adipocyte assays, but for 9 or 17 days. At 9 days, cultures were washed twice in phosphate-buffered saline (PBS), fixed with 4% (w/v) paraformaldehyde in PBS and stained for ALP activity using an ALP staining kit (Sigma), according to the manufacturer's instructions. The same plates were stained with Oil Red-O as described above. ALP-positive and Oil Red-O positive colonies were counted using an inverted microscope and counts expressed as a percentage of the total colony number. In the process of performing the colony counts, an undefined cell population with rounded morphology was also occasionally encountered (round cells). These colonies were counted and reported separately. Colonies containing more than one cell type, e.g. both ALP positive and Oil Red-O positive cells, were included and counted under both categories. RNA was extracted from some cultures at 9 days. Cultures maintained for 17 days were fixed in 4% (w/v) paraformaldehyde in PBS followed by von Kossa and Oil Red-O staining and counting of von Kossa-positive and Oil Red-O positive colonies.
2.6. BrdU incorporation assay
Primary bone marrow stromal cells were treated with serum-free MDA-CM or BRFF-HPC1 for 2 days then incubated with BrdU for the last 2 h of the experiment. BrdU incorporation was measured using a kit according to the manufacturer's instructions.
2.7. Protease activity
Medium was collected from Kusa 4b10 cells cultured for 5 days in osteogenic medium, followed by 24 h in α-MEM containing 2% FCS. Trypsin-like activity of the medium was measured using Nα-benzoyl-L-arginine-p-nitroanilide dihydrochloride (Sigma), and kallikrein-like activity was measured using the chromogenic substrate Chromozym-pk (Roche Diagnostics).
2.8. Mineralisation assay
Kusa 4b10 cells cultured in 12 well plates were fixed in ice cold 70% (v/v) ethanol and stained by Alizarin Red-S as described (Allan et al., 2003). After photography, samples were destained using 5% (w/v) cetylpyridinium chloride and the concentration of the extracted stain was quantitated. Where indicated, Alizarin Red-S values were normalised to total cell counts obtained from parallel cultures stained with Oil Red-O and haematoxylin.
2.9. Intracellular calcium mobilisation
This was assayed fluorometrically using Fura-2 AM (Molecular Probes, Invitrogen) as described (Pagel et al., 2003) with minor modifications. Due to strong adhesion of Kusa 4b10 cells to the flask surface, trypsinised cells were suspended in maintenance medium and stirred overnight (~15 h) in small Techne® vessels (Cole-Parmer, UK) at minimum speed while being incubated at 37 °C in 5% CO2 to allow recovery of cells from trypsin. Probenecid (2.5 mM) was included in the assay buffers. Following loading with Fura-2 AM, cells were plated into a 96 well plate (100 μL/well; ~2 × 105 cells/well). Cells were treated with trypsin (75 nM) followed by 5 μL 0.5% (v/v) Triton X-100 in PBS to confirm fluorochrome loading. Calcium responses were measured in a BMG FLUOstar Galaxy microplate reader. The ratio of fluorescence emission at 510 nm after excitation at 340 nm and 380 nm (340:380) was plotted against time. To determine the extent of response to the agonists, area under the curve (AUC) was calculated.
2.10. IL-6 ELISA
Measurement of IL-6 concentration in the supernatants of F2rl1 KD and vector control cells was carried out by ELISA according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA, USA). Supernatants were collected from days 1 to 7 from cultures under osteogenic conditions and kept refrigerated at 4 °C until use (3 replicates/day).
2.11. Neutralising IL-6 in cultures of Kusa 4b10 cells
To study the role of IL-6 in the adipogenic conversion of Kusa 4b10 cells, a rat anti-mouse IL-6 neutralising antibody (Thermo Fisher Scientific, Waltham, MA, USA) was added to the medium. An antibody to antigen molar ratio of 10:1 was used, which equalled 17.2 ng anti-IL-6 per mL of medium, selected to neutralise maximum measured IL-6 concentrations (~250 pg/mL on day 3). A matched non-immune IgG at an equal molar concentration was used as control. Media were changed every three days with fresh antibody added just before each use.
2.12. RNA quantitation
Total RNA was isolated using the Total RNA Isolation mini columns according to the manufacturer's instructions (Promega, Madison, Wisconsin, USA). RNA was eluted in 50–100 μL nuclease free water and stored at −80 °C if not used immediately. Detection of PAR2 activators was carried out using standard PCR following a method previously described (Georgy et al., 2010). Appropriate positive controls were included depending on the protease being assayed (Supplemental Table 1). Messenger RNA copy number and relative expression of a number of genes were measured by use of RT-qPCR. For this purpose, up to 1 μg RNA was reverse transcribed into complementary DNA (cDNA) using the GoScript™ reverse transcription system (Promega). PCRs were performed using 100–500 ng cDNA per reaction following a procedure described previously (Georgy et al., 2010). Results are presented as copy numbers or mean normalised expression (MNE) calculated as previously described (Pagel et al., 2009). Ppia, Hprt or Gapdh were used as the reference genes either alone or in combination as a geometric mean, i.e. Best Keeper Index (BKI) (Pfaffl et al., 2004).
PCR primers for detecting the following genes were as described: Col1a1, Bglap and Runx2 (Georgy et al., 2010); Acr, Klk4, Klk5, Klk6, Klk14, Prss1, Tmprss2, Tmprss11d and Tpsab1 (Georgy et al., 2012). All other PCR primers (Supplemental Table 2) were designed in NCBI's native Primer-Blast environment (https://www.ncbi.nlm.nih.gov/tools/primer-blast) and synthesised by Geneworks Australia (now Integrated DNA Technologies, IA, USA).
Total RNA was collected from WT and PAR2 KO primary osteoblasts cultured for 48 h in the presence or absence of MDA-CM (n = 3/genotype and treatment) using Total RNA Isolation mini columns according to the manufacturer's instructions (Promega). RNA samples were submitted to the Australian Genomic Research Facility (AGRF), Parkville, Australia for library preparation and RNA sequencing. For each sample 100 bp paired end RNA seq reads were generated using an Illumina HiSeq2000 platform (Illumina, San Diego, CA, USA). After trimming to remove adapters and bases with a quality score < 20, reads were mapped to the Mus musculus reference genome (build GRCm38/mm10; Dec, 2011) using TopHat version 2.0.9 (Trapnell et al., 2009). Mapped reads were assembled into transcripts, their abundances were estimated and reported as fragments per kilobase of transcript per million fragments sequenced (FPKM) and confidence intervals for FPKM values were generated using Cufflinks version 2.1.1 (Trapnell et al., 2010). A single reference annotation-based transcript assembly was prepared using Cuffmerge utility version 1.0.0 (Trapnell et al., 2012). Differential gene expression between experimental groups was compared using Cuffdiff version 2.1.1 (Trapnell et al., 2010; Trapnell et al., 2012). RNA-sequencing data generated from this study is deposited with GEO, accession number GSE135865 (Pagel and Mackie, 2019).
2.13. Statistical analysis
All analyses were carried out blindly where possible. For experiments with primary osteoblast and bone marrow stromal cell cultures, the number of replicates (n) was the number of cell isolates and batches of MDA-CM. Normally distributed continuous variables were analysed using a t-test or, if multiple comparisons were required, a one-way analysis of variance (ANOVA) with contrast analysis (pre-planned comparisons) to determine group interactions. Non-normal data were analysed by either a Mann-Whitney U test if only two groups were to be compared or a Kruskal-Wallis test followed by Dunn's pairwise comparisons if multiple groups were compared. SPSS statistics version 23, (IBM Corp.©, Armonk, NY, USA) was used for analyses. Graphs were prepared using GraphPad prism v5 (GraphPad software Inc., La Jolla, CA, USA). For RNAseq analysis a minimum fold change of 2 and a maximal false discovery rate (FDR) adjusted p value of <0.001 were considered significant.
3. Results
3.1. Loss of PAR2 promotes prostate cancer cell-induced adipocyte differentiation in primary osteoblast cultures
In experiments designed to identify the role of PAR2 in responses of primary calvarial osteoblasts to prostate cancer cell-conditioned medium (Pagel et al., manuscript submitted for publication), we found that treatment for 21 days with medium conditioned by MDA-PCa-2b cells (MDA-CM) in mineralising conditions stimulated an increase in the number of Oil Red-O positive cells (adipocytes) in PAR2 KO but not in WT cultures (Fig. 1.A). RT-qPCR evaluations at 1, 4 and 12 days also revealed that MDA-CM stimulated expression of the adipocyte-associated genes Pparg and Lpl on day 4 in PAR2 KO but not in WT cultures (Fig. 1.A); no differences between genotypes were observed at other time points examined. Investigation of the expression of various lineage-associated genes in untreated cultures 24 h following plating showed significantly lower expression of the osteoblast-associated genes Runx2, Col1a1 and Bglap as well as higher expression of Pparg in PAR2 KO than in WT cultures (Fig. 1.B and C). These observations indicate that PAR2 KO osteoblasts were more adipogenic and less osteoblastic than WT osteoblasts even without MDA-CM treatment.
Fig. 1.
Adipogenesis in WT and PAR2 KO calvarial cultures. (A) WT and PAR2 KO cells were cultured in MDA-CM or complete BRFF-HPC1 (control) medium. Adipocyte differentiation was quantified by counting the number of adipocytes per well (Ad.N) at 21 days, and using RT-qPCR to measure expression of adipocyte lineage associated genes at 4 days. (B, C) Expression of osteoblast lineage associated genes (B) and adipocyte lineage associated genes (C) in WT and PAR2 KO calvarial osteoblasts treated with control medium for 24 h. n = 3–5; *p ≤ 0.05, **p ≤ 0.01 when data analysed using Mann-Whitney U test; error bars represent SEM.
3.2. Loss of PAR2 predisposes to adipogenesis at the expense of osteogenesis in bone marrow stromal cell cultures
Following on our observations with primary osteoblasts, we chose to investigate cells at an earlier stage of differentiation, i.e. bone marrow stromal cells. Treatment for 2 days with MDA-CM had no effect on BrdU incorporation (cell proliferation) in either WT or PAR2 KO stromal cultures, and no difference was observed between genotypes (Fig. 2.A).
Fig. 2.
Osteoblast and adipocytic lineage precursors in wild type and PAR2 KO bone marrow stromal cell cultures. (A) BrdU incorporation on day 2 measured as absorbance at 450 nm [BrdU (A450)]. (B) WT and PAR2 KO (KO) bone marrow stromal cells were treated with MDA-CM for 9 days and stained for alkaline phosphatase activity and with Oil Red-O. Purple arrows indicate alkaline phosphatase positive cells; red arrows indicate Oil Red-O positive adipocytes; black arrows indicate round cells; two similarly treated examples presented for each genotype, bar = 300 μm. (C) Numbers of ALP positive, Oil Red-O positive and round cell colonies on day 9, expressed as percentages of the total number of colonies. (D) RT-qPCR analysis of Sox9 expression levels on day 9. (E) Von Kossa and Oil Red-O positive colonies on day 17. n = 3–4; *p ≤ 0.05 when data analysed using Kruskal-Wallis test with Dunn's multiple comparison post-hoc test; error bars represent SEM.
When WT and PAR2 KO primary marrow stromal cultures were treated with MDA-CM and stained either for ALP activity or adipogenesis (Oil Red-O), we observed that some of the colonies were neither ALP nor Oil Red-O positive and consisted of round cells, which were counted separately (Fig. 2.B). Treatment with MDA-CM for 9 days had no effect on the percentage of ALP positive, Oil Red-O positive or round cell colonies (Fig. 2.C).
However, ALP positive colonies were less frequent and round cell colonies more frequent in untreated PAR2 KO cultures than in untreated WT cultures. It was considered possible that the round cell colonies may be chondrocytes, but there was no difference in the level of Sox9 expression between the two genotypes (Fig. 2.D). At day 17, at which time there was still no effect of MDA-CM treatment on colony composition in either genotype, untreated PAR2 KO cultures contained fewer mineralised (von Kossa positive) colonies and more Oil Red-O positive colonies than did WT cultures (Fig. 2.E). No round cell colonies were observed at this time point, suggesting that the round cell colonies that were abundant in PAR2 KO cultures at day 9 may have been adipocyte precursors prior to the onset of lipid synthesis. Since we have not observed similar colonies when we have previously cultured WT and PAR2 KO bone marrow stromal cells in standard medium (Georgy et al., 2012), it is likely that they are caused by the BRFF-HPC1 culture medium used to culture the MDA-PCa-2b cells. One component of this medium that may be responsible is dihydrotestosterone.
These observations point to a role for PAR2 in the osteoblastic differentiation of marrow stromal cells which seems to be at least partly dependent on the suppression of their adipogenic capacity.
3.3. PAR2 deficiency increases adipocyte differentiation of mouse bone marrow stromal Kusa 4b10 cells
For investigation of the mechanism of PAR2-mediated regulation of mesenchymal stem cell fate, we selected the Kusa 4b10 mouse bone marrow stromal cell line (Allan et al., 2003). To confirm the relevance of this cell line for this purpose, expression of PAR2 by Kusa 4b10 cells was confirmed using reverse transcription polymerase chain reaction (RT-PCR; Fig. 3.A). Similarly, mRNA expression of various PAR2 activating proteases was demonstrated (Fig. 3.A and Supplemental Table 1); genes encoding coagulation factor VII, kallikrein-related peptidases KLK6 and KLK14 were expressed following culture for 2 or 7 days in osteoblast differentiation medium. The presence of PAR2-activating proteases was confirmed by the demonstration that both kallikrein-like and trypsin-like activity (~0.00172 U/mL and 0.0348 U/mL, respectively) were detectable in medium harvested from Kusa 4b10 cells on day 6.
Fig. 3.
Characterisation of Kusa 4b10 cells before and after PAR2 knockdown. (A) Expression of PAR2 mRNA and some of its activators by Kusa 4b10 cells cultured for 2 or 7 days in osteoblast differentiation medium (D2, D7) and by positive control tissues (PC). (B) RT-qPCR analysis of F2rl1 expression as well as intracellular Ca2+ mobilisation (fluorescence ratio 340:380) in response to trypsin after knockdown of PAR2 from Kusa 4b10 cells (F2rl1 KD) in comparison to vector control cells (Vector Ctl). Area under the curve (AUC) and mean 340:380 ratios are shown for Ca2+ mobilisation assay; n = 12 (F2rl1/Hprt copy number); n = 4–5 wells (calcium mobilisation assay). (C) The total number of cells (Tt.N), number and area of adipocytes (Ad.N & Ad.Ar), as well as Alizarin Red-S concentrations (AR-S) for F2rl1 KD and vector control cells at various times up to 21 days of culture in osteoblast differentiation medium; cell count and area measurements were carried out on one field of view per well at 40× magnification; n = 8–12 wells. (D) Oil Red-O and haematoxylin staining at day 14 (upper panel; bar = 100 μm) and Alizarin Red-S staining at day 17 (lower panel; bar = 5 mm). (E) Expression of adipocyte specific (Pparg) and osteoblast specific (Runx2) transcripts by F2rl1 KD and vector control cultures at 0, 1, 3 and 5 days; n = 6 wells. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 when data analysed using Mann-Whitney U test (A, D) and Student's t-test (C); error bars represent SEM.
PAR2 expression in Kusa 4b10 cells was stably knocked down using lentiviral small hairpin RNA (shRNA) constructs. Knockdown of PAR2 (F2rl1) mRNA was confirmed using RT-qPCR; knockdown of PAR2 protein was demonstrated by a decrease in the magnitude of intracellular calcium mobilisation in response to trypsin in F2rl1 KD cells as compared with vector control cells (Fig. 3.B).
Analysis of vector control and F2rl1 KD cells cultured in osteoblast differentiation medium for up to 21 days showed that PAR2 deficiency in Kusa 4b10 cells resulted in lower total cell counts at days 14 and 17 and increased adiposity at all time points preceding day 21, as demonstrated by higher absolute adipocyte number as well as adipocyte number and area normalised to total cell counts (Fig. 3.C and D). As expected, PAR2 deficiency caused a significant reduction in the amount of Alizarin Red-S recovered from F2rl1 KD cultures in comparison to vector control cultures at all time points indicating their reduced capacity for mineralisation. A similar effect was also observed after normalisation of values to total cell counts, however not at 17 days (Fig. 3.C).
Expression of Pparg expression was significantly higher in F2rl1 KD than in vector control cells at 1 and 3 days (Fig. 3.E). Levels of Runx2 expression, on the other hand, appeared higher in the vector control than in the F2rl1 KD cells at 5 days, the first day at which it was detectable. The relatively later adipogenic biomarker, Lpl, was minimally expressed until day 5, when its expression was significantly higher in the F2rl1 KD group. These results were in line with those observed when bone marrow stromal cells were used indicating a crucial role for PAR2 in the osteoblastic differentiation of MSCs and inhibition of adipogenesis. Based on these results, day 5 was selected for further gene expression analyses.
3.4. Gene expression analyses identify putative mediators of PAR2-induced effects on mesenchymal stem cell fate
Primary calvarial osteoblasts from WT and PAR2 KO mice were cultured in control medium or MDA-CM for 48 h, then analysed by RNA-sequencing (RNA-seq). RNA seq data were filtered and sorted in order to identify significantly regulated genes which were genes that showed a minimum of 2-fold up- or downregulation and a value of the FDR-adjusted p-value of the test statistic (q) of <0.001. Genes that were significantly regulated between control medium and MDA-CM treated cells within genotypes and between genotypes following treatment with MDA-CM were selected for further study. Genes not previously associated with the reciprocal relationship between osteogenesis and adipogenesis were shortlisted for further analysis. Twenty-seven genes of interest (GOIs) selected this way were then organised into two categories as follows. Category 1 GOIs were those that were upregulated by MDA-CM in WT but not KO cultures or downregulated in the KO but not WT cultures; only genes for which the endpoint expression levels were significantly higher in WT than in KO cells were included. Category 2 GOIs included genes that were upregulated uniquely in the KO cells, and more highly expressed at the endpoint in the KO cultures. None of the genes downregulated in the WT group met the criteria. Following the knockdown of PAR2 in Kusa 4b10 cells, RT-qPCR analysis of the selected genes was performed 5 days after the introduction of osteoblast differentiation medium.
Out of 19 genes examined in category 1, C1qtnf3, Gpr35, Grem1, Snorc and Tcea3 were consistently expressed at higher levels in vector control than in F2rl1 KD cells (Fig. 4.A). The category 2 GOIs included 8 genes, of which Cnr1, Enpep, Hmgn5, Il6 and Ramp3 demonstrated higher levels of expression in F2rl1 KD than in vector control cells (Fig. 4.B). These observations support a role for these GOIs as novel putative mediators of PAR2 induced effects on the differentiation of MSCs. A summary of available information on these GOIs is presented in Supplemental Table 3.
Fig. 4.
RT-qPCR analysis of the selected GOIs in F2rl1 KD and vector control cells cultured in osteogenic medium for 5 days. (A) Category 1 GOIs. (B) Category 2 GOIs. n = 6 wells; *p ≤ 0.05, **p ≤ 0.01 when data analysed using Mann-Whitney U test; error bars represent SEM.
3.5. Suppression of IL-6 expression contributes to PAR2 induced effects on adipocyte differentiation
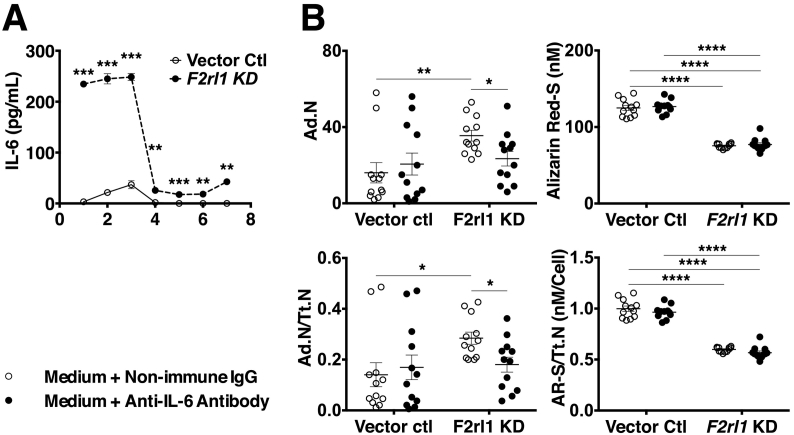
From the list of GOIs that displayed altered expressed in response to loss of PAR2, Il6 was selected for further experiments. Media collected on days 1–7 from vector control and F2rl1 KD cells cultured in osteoblast differentiation medium contained significantly higher concentrations of IL-6 protein in the F2rl1 KD cultures throughout the whole culture period (Fig. 5.A). The magnitude of this difference was greatest during the first three days of treatment with osteogenic medium. The change of medium at day 3 resulted in a substantial reduction in the IL-6 concentration in both cultures which did not reach pre-change concentrations again. Regardless, values remained higher in the F2rl1 KD conditioned medium at all times.
Fig. 5.
The role of IL-6 in PAR2 dependent regulation of MSC differentiation. (A) ELISA measurement of IL-6 concentrations in the media harvested from F2rl1 KD and vector control cells at days 1 to 7 of culture in osteogenic medium; n = 3 wells. (B) Effect of Anti IL-6 antibody (17.2 ng/mL) on adipocyte number and mineral content of F2rl1 KD and vector control cells at day 7 of culture in osteogenic medium; n = 12 wells. *p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001 when data analysed using Mann-Whitney U test (A) and one-way ANOVA with preplanned (contrast) tests (B); error bars represent SEM.
Inclusion of an anti-IL-6 neutralising antibody (Clone MP520F3; Thermo Fisher Scientific) in the osteogenic medium resulted in partial reversal of the effects of PAR2 deficiency in Kusa 4b10 cells indicating that PAR2 regulation of adipocyte differentiation is at least partially mediated by suppression of IL6 expression. This involved a significant reduction in adipocyte numbers to values that were no longer statistically different from those of non-immune IgG- or anti-IL-6 antibody-treated vector control cultures (Fig. 5.B). Mineralisation, however, did not appear to respond to the presence of anti-IL-6 in the medium in either F2rl1 KD or vector control cultures (Fig. 5.B) nor did total cell counts (data not shown).
4. Discussion
To explore the role of PAR2 in the reciprocal relationship between osteoblast and adipocyte differentiation, we performed a series of experiments using cells isolated from WT and PAR2 knockout mice as well as the vector control and F2rl1 KD Kusa 4b10 cells. This revealed a key role for PAR2 in the process, whereby PAR2 supports osteoblastogenesis of bone marrow MSCs at the expense of adipogenesis. Our investigations point to a number of putative mediators for these effects. Of note, on the basis of our results, suppression of IL-6 expression by MSCs appears to be one mechanism by which PAR2 inhibits adipogenesis.
Having made the unexpected observation that treatment with media conditioned by osteogenic prostate cancer MDA-PCa-2b cells stimulated adipocyte differentiation in PAR2 KO calvarial cultures, we compared levels of osteoblast- and adipocyte-associated genes between untreated WT and PAR2 KO calvarial cells, and observed that PAR2-deficient cells were already less osteoblastic and more adipocytic than WT cells. Primary calvarial cultures are considered to be comprised mostly of pre-osteoblasts, which express high levels of alkaline phosphatase and a number of other osteoblast associated markers (Yamamoto et al., 2002). The observation that PAR2 KO calvarial cells are more adipogenic than the WT cells is interesting because it suggests that transdifferentiation from pre-osteoblasts to pre-adipocytes had occurred. Indeed, pre-osteoblasts are considered to have the capacity to transdifferentiate into pre-adipocytes, and the reverse is also considered to occur (Berendsen and Olsen, 2014). We then turned our attention to the precursors of these cells, MSCs. Studies with bone marrow stromal cells from WT and PAR2 KO mice demonstrated that, indeed, PAR2-deficient MSCs generate fewer osteoblastic colonies and more adipocytic colonies than do WT MSCs.
We generated a PAR2-deficient mouse MSC line (F2rl1 KD) using the Kusa 4b10 cell line, in order to investigate molecules that may mediate the effect of PAR2 deficiency on adipogenesis. The F2rl1 KD cells were found to behave in a similar manner to primary mouse bone marrow stromal cultures from PAR2 KO mice, that is, they were less osteogenic and more adipogenic than their PAR2-expressing counterparts. When we used these cells to investigate expression of GOIs identified in an RNA-seq experiment conducted as part of the parallel cancer study with primary osteoblasts, we identified 10 genes that showed the expected expression patterns when comparing PAR2-expressing with PAR2-deficient cells.
Five category 1 GOIs, C1qtnf3, Gpr35, Grem1, Snorc, and Tcea3 were found to be expressed more highly in vector control than in F2rl1 KD Kusa 4b10 cells, and thus may be mediators of PAR2's support for osteogenesis and suppression of adipogenesis.
Two of the category 1 genes, C1qtnf3 and Gpr35, are known to be expressed in white adipose tissue (Li et al., 2018; Agudelo et al., 2018). It may seem contradictory that these genes showed lower expression levels in the more adipogenic F2rl1 KD cells than in vector control cells, however it is known that white adipose tissue is phenotypically distinct from bone marrow adipose tissue (Sebo et al., 2019). C1qtnf3 encodes complement C1q/tumour necrosis factor-related protein 3 (CTRP3), which is generally described as an adipokine because it exerts systemic effects, including anti-inflammatory effects and regulation of glucose metabolism (Li et al., 2018). Clinically, it has recently been shown that low serum CTRP3 levels are associated with osteoporosis (Demirtas et al., 2020), an observation in keeping with a putative role for this factor as a mediator of PAR2's pro-osteogenic and anti-adipogenic effects. G protein-coupled receptor 35, encoded by Gpr35, was until recently considered to be an orphan G protein-coupled receptor (Milligan, 2018). In white adipose tissue, this receptor mediates responses to the tryptophan metabolite kynurenic acid, including stimulation of lipid metabolism and anti-inflammatory gene expression (Agudelo et al., 2018). This gene has recently been shown to be expressed by bone marrow MSCs and its knockout causes decreased bone mass in mice (Zhang et al., 2021), similarly in keeping with an anti-adipogenic role for the protein it encodes.
Gremlin 1, encoded by Grem1, is a secreted bone morphogenetic protein (BMP) antagonist; it has been identified as a marker of a bone marrow MSC population that can differentiate into osteoblasts, chondrocytes and reticular marrow stromal cells, but not adipocytes (Worthley et al., 2015). Our observation that Grem1 is expressed at lower levels in F2rl1 KD than in vector control cells reinforces the conclusions that PAR2 is required for normal early osteoblastic differentiation of bone marrow MSCs, and that PAR2 suppresses adipogenesis in these cells.
Snorc encodes a cartilage-specific transmembrane proteoglycan (Heinonen et al., 2011). The fact that it is expressed at higher levels in the vector control cells than in the F2rl1 KD cells suggests that Kusa4b10 cells retain some chondrogenic capacity, which is lost in the absence of PAR2.
Tcea3 encodes transcription elongation factor 3, which is expressed in embryonic stem cells, as well as in cardiomyocytes and skeletal muscle myoblasts; it is required for normal skeletal muscle cell differentiation (Xu et al., 2009; Kazim et al., 2019). To our knowledge its expression by osteoblast or adipocyte lineage cells has not been described.
Five category 2 GOIs, Cnr1, Enpep, Hmgn5, Il6 and Ramp3 were found to be expressed at higher levels in F2rl1 KD than in vector control Kusa 4b10 cells, and thus may be inhibitors of osteogenesis and/or stimulators of adipogenesis that are suppressed by signaling through PAR2.
Cannabinoid receptor 1 (encoded by Cnr1) is expressed by adipocytes from visceral fat; it stimulates glucose uptake and IL-7 expression, and inhibits adiponectin expression in these cells (Pagano et al., 2007; Ge et al., 2013). Expression of Cnr1 has also been described in bone marrow stromal cells and at lower levels in osteoblasts (Idris et al., 2009). In marrow stromal cell cultures, expression of Cnr1 is required for normal osteoblast differentiation and suppression of adipocyte differentiation (Idris et al., 2009), so it is unlikely that its up-regulation in F2rl1 KD cells in the current study contributes to their increased adipogenesis.
Enpep encodes aminopeptidase A, which converts angiotensin II, the main effector of the renin-angiotensin system of blood pressure regulation, to angiotensin III, the precursor for angiotensin IV (Li et al., 2017). This enzyme has a widespread tissue distribution, but to our knowledge its expression has not previously been identified in cells of the osteoblast lineage or adipocytes (Mentzel et al., 1996). As far as we are aware, effects of angiotensin III and IV on skeletal MSCs have not been described. It is therefore not possible to predict the functional implications of the enhanced conversion of angiotensin II to angiotensin III that presumably results from up-regulation of Enpep in Kusa 4b10 cells in the absence of PAR2.
Hmgn5 encodes high-mobility group nucleosome-binding domain 5, which modulates the structure of chromatin and regulates transcription. This gene has been most studied in the context of cancer, where it is generally up-regulated with respect to the relevant normal tissue, and enhances cell proliferation and apoptosis (Shi et al., 2016). It has not been described in skeletal MSCs, primary osteoblasts or adipocytes, however it has been detected in osteosarcoma tissue.
Ramp3 encodes receptor activity modifying protein 3 (RAMP3), which dimerises with the calcitonin receptor or the calcitonin-like receptor to form receptors for amylin or adrenomedullin, respectively (Naot and Cornish, 2008). To our knowledge, expression of Ramp3 has not been described in MSCs. However, it is expressed in adipose tissue (Nagae et al., 2000), and its ligand amylin stimulates proliferation and lipid accumulation in 3T3-L1 preadipocytes (Miegueu et al., 2013). Ramp3 expression has been described in rodent osteoblasts, and adrenomedullin stimulates their proliferation (Naot and Cornish, 2008). Since Ramp3 has positive effects on both lineages, it is difficult to determine whether its up-regulation could contribute to the pro-adipogenic effect of PAR2 deficiency in Kusa 4b10 cells. Perhaps the outcome (adipogenesis or osteogenesis) depends on the stage of differentiation at which Ramp3 is expressed.
Whether or not up-regulation (category 1 GOIs) or suppression (category 2 GOIs) of any or all of these genes by PAR2 mediates its effects on Kusa 4b10 cell differentiation will require further experiments for confirmation. In the case of Il6, we have undertaken further investigation of a potential role in mediating the effects of PAR2 deficiency.
We were intrigued by the observation that Il6 expression was elevated in the absence of PAR2, since in many cells (e.g. neutrophils and oral keratinocytes) activation of PAR2 leads to increased secretion of Il6 (Lourbakos et al., 2001; Shpacovitch et al., 2004), although the current result is consistent with our previous demonstration that activation of PAR2 causes suppression of Il6 expression in bone marrow cultures treated with osteoclastogenic factors (Smith et al., 2004). Here we demonstrate that the elevated expression of Il6 mRNA in F2rl1 KD cells is matched by higher levels of secretion of IL-6 into the medium, and that this can account for the higher level of adipogenesis, but not the lower level of mineralisation caused by PAR2 deficiency. Interleukin-6 is an inflammatory cytokine that has been extensively studied in bone, in particular in the context of its role in stimulating osteoclast differentiation (Ponzetti and Rucci, 2019). Previous studies on the effect of IL-6 on osteoblast differentiation have yielded contradictory results, with both stimulatory and inhibitory effects having been observed (Bellido et al., 1997; Kaneshiro et al., 2014). Interleukin-6 treatment of 3T3-L1 preadipocytes or primary preadipocytes from human subcutaneous adipose tissue inhibits their differentiation (Xie et al., 2010; Almuraikhy et al., 2016). Our contrasting result (stimulation of adipocyte differentiation by IL-6), may result from differences between adipogenic precursors from bone marrow and those from other locations, or perhaps the absence of PAR2 alters the capacity of MSCs to respond to IL-6.
The results of the current study have identified a number of novel PAR2-regulated genes. While IL-6 is well known to be regulated by activation of PAR2, albeit usually in the opposite direction to that identified in Kusa 4b10 cells, the other genes investigated in the current study have not previously been identified as being regulated by PAR2. Moreover, of the 10 genes that were consistently found to be regulated by the presence of PAR2 in primary osteoblasts and Kusa 4b10 cells, several had not previously been identified in one or more of the cell types under investigation, i.e. skeletal MSCs, osteoblasts and adipocytes; indeed, expression of Tcea3, Enpep and Hmgn5 has not been described in any of these cells. A theme that emerged for some of the regulated genes is that in skeletal MSCs the presence of PAR2 appears to be associated with dampening of inflammation, since two of the genes that were down-regulated with PAR2 knockdown, C1qtnf3 and Gpr35, mediate anti-inflammatory responses and two of the genes that were up-regulated with PAR2 knockdown, Cnr1 and Il6, mediate pro-inflammatory responses.
In conclusion, the presence of PAR2 in bone marrow-derived MSCs cultured under osteogenic conditions is required for normal osteoblast differentiation and suppression of adipocyte differentiation. This effect is in part mediated by suppression of IL-6 synthesis and secretion. When PAR2 is deficient, the elevated IL-6 secretion stimulates adipocyte differentiation, but does not mediate the inhibition of mineralisation.
Funding
Funding was provided by the National Health and Medical Research Council of Australia (Project grant no. 614206). The funding source had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
RS and NT were each supported by an International Postgraduate Research Scholarship and an Australian Postgraduate Award, and PK was supported by scholarships from the University of Melbourne.
CRediT authorship contribution statement
R. Sanaei: Methodology, Investigation, Formal analysis, Visualization, Writing – original draft. P.K. Kularathna: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. N. Taghavi: Methodology, Resources, Writing – review & editing. J.D. Hooper: Conceptualization, Funding acquisition, Writing – review & editing. C.N. Pagel: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – review & editing. E.J. Mackie: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgment
Global PAR2 KO (F2rl1−/−) mice were kindly provided by Professor Shaun Coughlin, Cardiovascular Research Institute, University of California, San Francisco. RNA-sequencing data generated from this study is deposited with GEO, accession number GSE135865.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bonr.2021.101113.
Appendix A. Supplementary data
Expression of PAR2 and its activating proteases by Kusa 4b10 cells.
References
- Owen M. Marrow stromal stem cells. J. Cell Sci. 1988;1988(Supplement 10):63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- Dirckx N., Maes C. Local and circulating osteoprogenitor cells and lineages. In: Bilezikian J.P., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Wiley Blackwell; Hoboken, NJ, USA: 2019. pp. 134–153. [Google Scholar]
- Bethel M., Chitteti B.R., Srour E.F., Kacena M.A. The changing balance between osteoblastogenesis and adipogenesis in aging and its impact on hematopoiesis. Curr. Osteopor. Rep. 2013;11(2):99–106. doi: 10.1007/s11914-013-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen A.D., Olsen B.R. Osteoblast–adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell. Mol. Life Sci. 2014;71(3):493–497. doi: 10.1007/s00018-013-1440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Devlin M.J., Rosen C.J. Fat targets for skeletal health. Nat. Rev. Rheumatol. 2009;5(7):365–372. doi: 10.1038/nrrheum.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins A.L., Chinni C., De Niese M.R., Blackhart B., Mackie E.J. Expression of protease-activated receptor-2 during embryonic development. Dev. Dyn. 2000;218(3):465–471. doi: 10.1002/1097-0177(200007)218:3<465::AID-DVDY1013>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Abraham L.A., Chinni C., Jenkins A.L., Lourbakos A., Ally N., Pike R.N., Mackie E.J. Expression of protease-activated receptor-2 by osteoblasts. Bone. 2000;26(1):7–14. doi: 10.1016/s8756-3282(99)00237-9. [DOI] [PubMed] [Google Scholar]
- Georgy S.R., Pagel C.N., Ghasem-Zadeh A., Zebaze R.M.D., Pike R.N., Sims N.A., Mackie E.J. Proteinase-activated receptor-2 is required for normal osteoblast and osteoclast differentiation during skeletal growth and repair. Bone. 2012;50(3):704–712. doi: 10.1016/j.bone.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Georgy S.R., Pagel C.N., Wong D.M., Sivagurunathan S., Loh L.H., Myers D.E., Hollenberg M.D., Pike R.N., Mackie E.J. Proteinase-activated receptor-2 (PAR2) and mouse osteoblasts: regulation of cell function and lack of specificity of PAR2-activating peptides. Clin. Exp. Pharmacol. Physiol. 2010;37(3):328–336. doi: 10.1111/j.1440-1681.2009.05294.x. [DOI] [PubMed] [Google Scholar]
- Ramsay A.J., Reid J.C., Adams M.N., Samaratunga H., Dong Y., Clements J.A., Hooper J.D. Prostatic trypsin-like kallikrein-related peptidases (KLKs) and other prostate-expressed tryptic proteinases as regulators of signalling via proteinase-activated receptors (PARs) Biol. Chem. 2008;389(6):653–668. doi: 10.1515/BC.2008.078. [DOI] [PubMed] [Google Scholar]
- Navone N.M., Olive M., Ozen M., Davis R., Troncoso P., Tu S.M., Johnston D., Pollack A., Pathak S., von Eschenbach A.C., Logothetis C.J. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin. Cancer Res. 1997;3(12 Pt 1):2493–2500. [PubMed] [Google Scholar]
- Anjos-Afonso F., Bonnet D. Isolation, culture, and differentiation potential of mouse marrow stromal cells. Curr. Protoc. Stem Cell Biol. 2008;7(1):2B.3.1–2B.3.11. doi: 10.1002/9780470151808.sc02b03s7. Chapter 2. [DOI] [PubMed] [Google Scholar]
- Badeanlou L., Furlan-Freguia C., Yang G., Ruf W., Samad F. Tissue factor–protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation. Nat. Med. 2011;17(11):1490–1497. doi: 10.1038/nm.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan E.H., Ho P.W.M., Umezawa A., Hata J.I., Makishima F., Gillespie M.T., Martin T.J. Differentiation potential of a mouse bone marrow stromal cell line. J. Cell. Biochem. 2003;90(1):158–169. doi: 10.1002/jcb.10614. [DOI] [PubMed] [Google Scholar]
- Lindner J.R., Kahn M.L., Coughlin S.R., Sambrano G.R., Schauble E., Bernstein D., Foy D., Hafezi-Moghadam A., Ley K. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J. Immunol. 2000;165(11):6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- Pagel C.N., De Niese M.R., Abraham L.A., Chinni C., Song S.-J., Pike R.N., Mackie E.J. Inhibition of osteoblast apoptosis by thrombin. Bone. 2003;33(4):733–743. doi: 10.1016/s8756-3282(03)00209-6. [DOI] [PubMed] [Google Scholar]
- Walker E.C., McGregor N.E., Poulton I.J., Pompolo S., Allan E.H., Quinn J.M., Gillespie M.T., Martin T.J., Sims N.A. Cardiotrophin-1 is an osteoclast-derived stimulus of bone formation required for Normal bone remodeling. J. Bone Miner. Res. 2008;23(12):2025–2032. doi: 10.1359/jbmr.080706. [DOI] [PubMed] [Google Scholar]
- Pagel C.N., Song S.J., Loh L.H., Tudor E.M., Murray-Rust T.A., Pike R.N., Mackie E.J. Thrombin-stimulated growth factor and cytokine expression in osteoblasts is mediated by protease-activated receptor-1 and prostanoids. Bone. 2009;44(5):813–821. doi: 10.1016/j.bone.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W., Tichopad A., Prgomet C., Neuvians T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26(6):509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S.L. TopHat: discovering splice junctions with RNA-seq. Bioinformatics. 2009;25(9):1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., Van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28(5):511. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and cufflinks. Nat. Protoc. 2012;7(3):562. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel C.N., Mackie E.J. Accession Number: GSE135865. 2019. Protease-activated receptor-2 promotes osteogenesis in skeletal mesenchymal stem cells at the expense of adipogenesis. [dataset] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Furuya K., Hanada K. Progressive development of the osteoblast phenotype during differentiation of osteoprogenitor cells derived from fetal rat calvaria: model for in vitro bone formation. Biol. Pharm. Bull. 2002;25(4):509–515. doi: 10.1248/bpb.25.509. [DOI] [PubMed] [Google Scholar]
- Li Y., Wright G.L., Peterson J.M. C1q/TNF-related protein 3 (CTRP3) function and regulation. Compr. Physiol. 2018;7(3):863–878. doi: 10.1002/cphy.c160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo L.Z., Ferreira D.M.S., Cervenka I., Bryzgalova G., Dadvar S., Jannig P.R., Pettersson-Klein A.T., Lakshmikanth T., Sustarsic E.G., Porsmyr-Palmertz M., Correia J.C., Izadi M., Martinez-Redondo V., Ueland P.M., Midttun O., Gerhart-Hines Z., Brodin P., Pereira T., Berggren P.O., Ruas J.L. Kynurenic acid and Gpr35 regulate adipose tissue energy homeostasis and inflammation. Cell Metab. 2018;27(2):378–392. doi: 10.1016/j.cmet.2018.01.004. e5. [DOI] [PubMed] [Google Scholar]
- Sebo Z.L., Rendina-Ruedy E., Ables G.P., Lindskog D.M., Rodeheffer M.S., Fazeli P.K., Horowitz M.C. Bone marrow adiposity: basic and clinical implications. Endocr. Rev. 2019;40(5):1187–1206. doi: 10.1210/er.2018-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas D., Acibucu F., Baylan F.A., Gulumsek E., Saler T. CTRP3 is significantly decreased in patients with primary hyperparathyroidism and closely related with osteoporosis. Exp. Clin. Endocrinol. Diabetes. 2020;128(3):152–157. doi: 10.1055/a-0899-5210. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptors not currently in the spotlight: free fatty acid receptor 2 and GPR35. Br. J. Pharmacol. 2018;175(13):2543–2553. doi: 10.1111/bph.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Shi T., He Y. GPR35 regulates osteogenesis via the Wnt/GSK3ß/ß-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2021;556:171–178. doi: 10.1016/j.bbrc.2021.03.084. [DOI] [PubMed] [Google Scholar]
- Worthley D.L., Churchill M., Compton J.T., Tailor Y., Rao M., Si Y., Levin D., Schwartz M.G., Uygur A., Hayakawa Y., Gross S., Renz B.W., Setlik W., Martinez A.N., Chen X., Nizami S., Lee H.G., Kang H.P., Caldwell J.M., Asfaha S., Westphalen C.B., Graham T., Jin G., Nagar K., Wang H., Kheirbek M.A., Kolhe A., Carpenter J., Glaire M., Nair A., Renders S., Manieri N., Muthupalani S., Fox J.G., Reichert M., Giraud A.S., Schwabe R.F., Pradere J.P., Walton K., Prakash A., Gumucio D., Rustgi A.K., Stappenbeck T.S., Friedman R.A., Gershon M.D., Sims P., Grikscheit T., Lee F.Y., Karsenty G., Mukherjee S., Wang T.C. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1–2):269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen J., Taipaleenmäki H., Roering P., Takatalo M., Harkness L., Sandholm J., Uusitalo-Järvinen H., Kassem M., Kiviranta I., Laitala-Leinonen T., Säämänen A.M. Snorc is a novel cartilage specific small membrane proteoglycan expressed in differentiating and articular chondrocytes. Osteoarthr. Cartil. 2011;19(8):1026–1035. doi: 10.1016/j.joca.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Xu X.Q., Soo S.Y., Sun W., Zweigerdt R. Global expression profile of highly enriched cardiomyocytes derived from human embryonic stem cells. Stem Cells. 2009;27(9):2163–2174. doi: 10.1002/stem.166. [DOI] [PubMed] [Google Scholar]
- Kazim N., Adhikari A., Davie J. The transcription elongation factor TCEA3 promotes the activity of the myogenic regulatory factors. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano C., Pilon C., Calcagno A., Urbanet R., Rossato M., Milan G., Bianchi K., Rizzuto R., Bernante P., Federspil G., Vettor R. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J. Clin. Endocrinol. Metab. 2007;92(12):4810–4819. doi: 10.1210/jc.2007-0768. [DOI] [PubMed] [Google Scholar]
- Ge Q., Maury E., Rycken L., Gerard J., Noel L., Detry R., Navez B., Brichard S.M. Endocannabinoids regulate adipokine production and the immune balance of omental adipose tissue in human obesity. Int. J. Obes. 2013;37(6):874–880. doi: 10.1038/ijo.2012.123. [DOI] [PubMed] [Google Scholar]
- Idris A.I., Sophocleous A., Landao-Bassonga E., Canals M., Milligan G., Baker D., Ralston S.H., Van't Hof R.J. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab. 2009;10(2):139–147. doi: 10.1016/j.cmet.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol. Res. 2017;125(Pt A):21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel S., Dijkman H.B., Van Son J.P., Koene R.A., Assmann K.J. Organ distribution of aminopeptidase a and dipeptidyl peptidase IV in normal mice. J. Histochem. Cytochem. 1996;44(5):445–461. doi: 10.1177/44.5.8627002. [DOI] [PubMed] [Google Scholar]
- Shi Z., Tang R., Wu D., Sun X. Research advances in HMGN5 and cancer. Tumour Biol. 2016;37(2):1531–1539. doi: 10.1007/s13277-015-4693-3. [DOI] [PubMed] [Google Scholar]
- Naot D., Cornish J. The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone. 2008;43(5):813–818. doi: 10.1016/j.bone.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Nagae T., Mukoyama M., Sugawara A., Mori K., Yahata K., Kasahara M., Suganami T., Makino H., Fujinaga Y., Yoshioka T., Tanaka I., Nakao K. Rat receptor-activity-modifying proteins (RAMPs) for adrenomedullin/CGRP receptor: cloning and upregulation in obstructive nephropathy. Biochem. Biophys. Res. Commun. 2000;270(1):89–93. doi: 10.1006/bbrc.2000.2390. [DOI] [PubMed] [Google Scholar]
- Miegueu P., St-Pierre D.H., Munkonda M.N., Lapointe M., Cianflone K. Amylin stimulates fatty acid esterification in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2013;366(1):99–107. doi: 10.1016/j.mce.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Lourbakos A., Potempa J., Travis J., D'Andrea M.R., Andrade-Gordon P., Santulli R., Mackie E.J., Pike R.N. Arginine-specific protease from Porphyromonas gingivalis activates protease-activated receptors on human oral epithelial cells and induces interleukin-6 secretion. Infect. Immun. 2001;69(8):5121–5130. doi: 10.1128/IAI.69.8.5121-5130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpacovitch V.M., Varga G., Strey A., Gunzer M., Mooren F., Buddenkotte J., Vergnolle N., Sommerhoff C.P., Grabbe S., Gerke V., Homey B., Hollenberg M., Luger T.A., Steinhoff M. Agonists of proteinase-activated receptor-2 modulate human neutrophil cytokine secretion, expression of cell adhesion molecules, and migration within 3-D collagen lattices. J. Leukoc. Biol. 2004;76(2):388–398. doi: 10.1189/jlb.0503221. [DOI] [PubMed] [Google Scholar]
- Smith R., Ransjö M., Tatarczuch L., Song S.-J., Pagel C.N., Morrison J.R., Pike R.N., Mackie E.J. Activation of protease-activated receptor-2 leads to inhibition of osteoclast differentiation. J. Bone Miner. Res. 2004;19(3):507–516. doi: 10.1359/JBMR.0301248. [DOI] [PubMed] [Google Scholar]
- Ponzetti M., Rucci N. Updates on osteoimmunology: what's new on the cross-talk between bone and immune system. Front. Endocrinol. (Lausanne) 2019;10:236. doi: 10.3389/fendo.2019.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T., Borba V.Z.C., Roberson P., Manolagas S.C. Activation of the janus Kinase/STAT (Signal transducer and activator of Transcription) signal transduction pathway by Interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. 1997;138(9):3666–3676. doi: 10.1210/endo.138.9.5364. [DOI] [PubMed] [Google Scholar]
- Kaneshiro S., Ebina K., Shi K., Higuchi C., Hirao M., Okamoto M., Koizumi K., Morimoto T., Yoshikawa H., Hashimoto J. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014;32(4):378–392. doi: 10.1007/s00774-013-0514-1. [DOI] [PubMed] [Google Scholar]
- Xie L., Ortega M.T., Mora S., Chapes S.K. Interactive changes between macrophages and adipocytes. Clin. Vaccine Immunol. 2010;17(4):651–659. doi: 10.1128/CVI.00494-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuraikhy S., Kafienah W., Bashah M., Diboun I., Jaganjac M., Al-Khelaifi F., Abdesselem H., Mazloum N.A., Alsayrafi M., Mohamed-Ali V., Elrayess M.A. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia. 2016;59(11):2406–2416. doi: 10.1007/s00125-016-4031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of PAR2 and its activating proteases by Kusa 4b10 cells.