Abstract
Purpose:
To test the effects of an encapsulated cell-based delivery of a neuroprotective agent, ciliary neurotrophic factor (CNTF), on progression of macular telangiectasia type 2, a neurodegenerative disease with no proven effective therapy.
Design:
Randomized sham-controlled clinical trial.
Participants:
Ninety-nine study eyes of 67 eligible participants were enrolled.
Methods:
Single-masked randomized clinical trial of 24 months’ duration conducted from May 2014 through April 2017 in 11 clinical centers of retinal specialists in the United States and Australia. Participants were randomized 1:1 to surgical implantation of intravitreal sustained delivery of human CNTF versus a sham procedure.
Main Outcome Measures:
The primary outcome was the difference in the area of neurodegeneration as measured in the area of the ellipsoid zone disruption (or photoreceptor loss) measured on spectral-domain (SD) OCT images at 24 months from baseline between the treated and untreated groups. Secondary outcomes included comparison of visual function changes between treatment groups.
Results:
Among the 67 participants who were randomized (mean age, 62±8.9 years; 41 women [61%]; 58 white persons [86%]), 65 (97%) completed the study. Two participants (3 study eyes) died and 3 participants (4 eyes) were found ineligible. The eyes receiving sham treatment had 31% greater progression of neurodegeneration than the CNTF-treated eyes. The difference in mean area of photoreceptor loss was 0.05±0.03 mm2 (P = 0.04) at 24 months. Retinal sensitivity changes, measured using microperimetry, were correlated highly with the changes in the area of photoreceptor loss (r = 0.86; P < 0.0001). The mean retinal sensitivity loss of the sham group was 45% greater than that of the treated group (decrease, 15.81±8.93 dB; P = 0.07). Reading speed deteriorated in the sham group (−13.9 words per minute) with no loss in the treated group (P = 0.02). Serious adverse ocular effects were found in 2 of 51 persons (4%) in the sham group and 2 of 48 persons (4%) in the treated group.
Conclusions:
In participants with macular telangiectasia type 2, a surgical implant that released CNTF into the vitreous cavity, compared with a sham procedure, slowed the progression of retinal degeneration. Further research is needed to assess longer-term clinical outcomes and safety.
Idiopathic macular telangiectasia type 2 is a bilateral, slowly progressive, degenerative condition of the retina that leads to marked decreases in visual function with blurred and distorted vision, affecting activities of daily life usually in patients in middle age.1 This condition progresses slowly and rarely results in total blindness. However, the affected individuals have profoundly reduced health-related quality-of-life measures compared with an age-matched normal reference group.2 Initially, macular telangiectasia type 2 was considered a vascular disease because of telangiectatic retinal vessels observed around the center of the macula,3 but it is now considered to be a primary neurodegenerative condition that also affects blood vessels and Müller cells leading to the loss of photoreceptors, as demonstrated in donor eyes.4 Subsequent studies of other donor eyes with a documented clinical diagnosis of macular telangiectasia type 2 also confirmed the loss of Müller cells and photoreceptors in the macular area.5 Central vision loss (legal blindness) may occur in late stages, caused by retinal atrophy or neovascular complications.1 There is no known effective therapy for this condition, which is estimated in 3 population-based studies in Australia (data collected 2003–2007), the United States (1988–1990), and Africa (2005–2007) to have a prevalence of 0.0045% to 0.022%, 0.01%, and 0.06%, respectively.6–8
Ciliary neurotrophic factor (CNTF)9,10 is a neurotrophic factor that can reduce photoreceptor cell loss in animal models of outer retinal degeneration.11–14 The Neurotech-501 encapsulated cell therapy implant (Neurotech Pharmaceuticals, Inc, Cumberland, RI) is a novel, cell-based drug delivery system. Encapsulated in a semipermeable hollow fiber membrane, genetically modified human retinal pigment epithelium cells release CNTF into the vitreous cavity of the eye. An open-label phase 1 study that evaluated the adverse event of this treatment for macular telangiectasia type 2 demonstrated that the treated eyes tolerated the procedure well.15 The drug seemed to be active and the adverse events profile was good at 5 years after insertion of the implant. This article reports the results of a multicenter randomized sham-controlled phase 2 trial designed to evaluate CNTF for the treatment of macular telangiectasia type 2.
Methods
This multicenter, randomized controlled clinical trial (ClinicalTrials.gov identifier, NCT01949324) conducted at 11 outpatient clinical sites of both academic and community retinal specialists in Australia and the United States evaluated the effects of CNTF on the course of macular telangiectasia type 2. The study visits included baseline screening, then visits on day 1, week 1, and months 3, 6, 12, 18, and 24 after surgery. Full details of the protocol and the statistical analyses plan arc available in the Protocol and Statistical Analyses Plan of the Supplemental Materials (available at www.aaojournal.org). This clinical trial was conducted according to the tenets of the Declaration of Helsinki and the protocol was approved at all sites by the local or central institutional review boards. All study participants provided written informed consent.
Participants
Eligible study participants were between 21 and 80 years of age with a diagnosis of macular telangiectasia type 2. The criteria for diagnosis included fluorescein angiographic leakage of the retinal vessels, retinal opacification, crystalline deposits, cavities in the inner and outer retina, and hyperpigmentation not involving the foveal center. The study eye had to have a disruption in the ellipsoid zone layer (evidence of photoreceptor loss) between 0.16 and 4.00 mm2 as measured on the en face spectral-domain (SD) OCT images by the central reading center. The best-corrected visual acuity (BCVA) had to be 20/50 or better (Early Treatment Diabetic Retinopathy Study visual acuity test score of 64 or better). An exclusion criterion included presence of subretinal neovascular proliferation. The other inclusion and exclusion criteria are listed in Table S1 (available at www.aaojournal.org).
Race also was noted in this study because Food and Drug Administration regulations require sponsors of Investigational New Drugs to report the total number of participants initially planned for inclusion in the study and the number entered into the study to date, tabulated by age group, gender, and race. In addition, the Food and Drug Administration requires the reporting at the end of the study. Determination of race was made by the participant and based on fixed categories where multiple races could be chosen, and if other, a “specify” field was included.
Randomization
This was a single-masked randomized trial because the sham treatment was designed to mimic the treatment procedure to mask (or blind) the participant to whether the study eye(s) received the implant or the sham therapy. A permuted block design was used (block size 4) to achieve balance between the treatment groups. Randomization was conducted using a proprietary internet-based web randomization system (IWRS) (Advantage EDCSM: The Emmes Corporation, Rockville, MD). In participants who had 1 eligible eye, each eligible eye was randomized 1:1 to a group in which a CNTF implant was placed, or to one in which a sham procedure was performed. For those participants with both eyes eligible, the right eye was randomized to the active treatment or a sham procedure, whereas the left eye received the alternative treatment.
Intervention
The effects of CNTF on the course of macular telangiectasia type 2 were evaluated. The Ncurotech-501 implants producing CNTF at 20 ng/day were provided by Neurotech Pharmaceuticals, Inc. All study participants were taken to the operating room for the placement of the intraocular implant of CNTF or the sham procedure. All surgeons were trained and certified for this implant procedure. The implant was inserted surgically in the study eye through a small (2-mm) inferotemporal circumferential incision at the pars plana into the vitreous cavity outside the visual axis. The implant was secured to the sclera by a suture passed through a titanium anchor loop at the end of the implant and then through the sclera. The incision site was closed with 3 sutures and covered with conjunctiva. More details about the implant arc found in Appendix B (available at www.aaojournal.org). Eyes assigned to the sham procedure underwent similar preoperative preparation; a conjunctival incision was made and then closed with a suture, but the globe was not penetrated.
Outcome Measures
Primary Outcome.
The primary outcome was the change in the area of the ellipsoid zone disruption as measured on SD OCT images at 24 months from the baseline. A masked and certified technician obtained SD OCT images at baseline and at each follow-up visit. Images were graded at the Duke Reading Center (Duke University, Durham, NC) by masked and trained readers using a standardized protocol. In each SD OCT volume, the cross-sectional annotation of the ellipsoid zone layer boundaries on individual images were interpolated to create an en face ellipsoid zone thickness map allowing the measurement of the area of photoreceptor loss.16
Secondary Outcomes.
Secondary outcomes evaluated changes relative to baseline measurements in the following: change in ellipsoid zone (photoreceptor loss) from baseline to month 12, proportion of study eyes with 35% or more increased from baseline in the ellipsoid zone disruption at months 12 and 24, changes in BCVA from baseline to months 12 and 24, proportion of study eyes with 15-letter or more (or with 10-letter or more) loss from baseline at months 12 and 24 months, change in retinal sensitivity (in decibels) as measured by microperimetry from baseline to months 12 and 24, and change in monocular reading speed at baseline to months 12 and 24. Humphrey visual fields (30-2; Carl Zeiss Meditec, Dublin, CA) obtained at baseline and each study visit also were evaluated for change at months 12 and 24.
At each study visit, certified study personnel conducted comprehensive dilated eye examinations that included BCVA using the Early Treatment Diabetic Retinopathy Study visual acuity test, measurement of monocular reading speed using the International Reading Speed Texts (IReST; Precision Vision, La Salle, IL),17 Humphrey visual fields (30-2), and evaluation of retinal sensitivity using microperimetry using the Macular Integrity Assessment (CenterVue, San Jose, CA) microperimeter. The retinal sensitivity thresholds obtained by microperimetry were mapped onto a fundus image. The mapping of the ellipsoid zone loss on the SD OCT images resulted in an en face image that was overlaid onto the microperimetry fundus image to evaluate for one-to-one correspondence. The aggregate sensitivity is considered a measure of the retinal function, especially over the area of the scotoma. Aggregate retinal sensitivity is derived by first summing and averaging the values on the test points on microperimetry that is outside of the scotoma (considered the background retinal sensitivity). The levels of the retinal sensitivity within the scotoma are subtracted from this mean. The sum of these differences results in the value known as the aggregate sensitivity. The greater the difference, the larger the loss of the retinal sensitivity in the area of the photoreceptor loss.
Exploratory Outcomes and Safety Outcomes.
Exploratory outcomes to investigate the efficacy of CNTF include change in the National Eye Institute Visual Function Questionnaire overall and subscale scores from baseline to months 12 and 24. The safety outcomes include change in electroretinography results that were found in the phase 1 study of CNTF therapy for macular telangiectasia type 2 and changes in visual fields that were detected in a clinical trial of CNTF therapy for retinitis pigmentosa. The electroretinography results were evaluated in a subset of participants, whereas Humphrey visual fields (30-2) were evaluated for all participants for changes from baseline to months 12 and 24. All study personnel in the outpatient clinics who performed the visual function testing (visual acuity, microperimetry, Humphrey visual field, reading speed, and electroretinography results) and who performed the ocular imaging were masked to the treatment allocation.
Sample Size Calculation
The sample size calculation was based on both the number of participants and the number of eligible eyes. For the primary outcome of the change in the area of loss of the ellipsoid zone area as determined on SD OCT imaging, it was estimated at month 24 that the area of photoreceptor loss in the sham group would enlarge by an average of 0.28 mm2 from baseline to month 24.18 A total sample size was estimated to require 68 participants (34 per treatment group), assuming both eyes were eligible for 30% of the participants and the correlation between eyes was 10%.19 This was estimated to provide approximately 80% power, with a 1-sided type 1 error rate of 0.05, to detect a difference in means between groups of 0.084 mm2 (0.280 mm2 vs. 0.196 mm2, or a 30% reduction),18,19 with a standard deviation of 0.15 mm2 in each treatment group. We assumed that 10% of patients would be lost to follow-up. No interim analyses were conducted. The 1-side type 1 error was chosen because of the previous experience with a phase 1 study in which participants followed up for more than 5 years showed minimal harmful side effects. Collectively, more than 250 participants with diseases of retinal neurodegeneration also were treated with the CNTF implant with minimal adverse effects in the past, although removal of the implant was required in a few cases and most were removed because the study protocols required removal at the exit of the study. The minimal clinically important difference between the 2 treatment groups was determined based on the natural history available,18,19 assuming that the study is powered for a modest 30% treatment effect between the treatment groups.
Statistical Analyses
Data are presented as mean (standard deviation). The primary outcome was analyzed on the intention-to-treat (ITT) population, which included all participants, and the per-protocol (PP) population, which excluded all participants who were ineligible at baseline because of subretinal neovascular proliferation and inadequate area of photoreceptor loss, as well as those who had protocol deviations that precluded their data validity. The mean difference in change in continuous measurements between each treatment group and the corresponding 1-sided P values were computed using a mixed-effects model incorporating both random effects (accounting for correlation between eyes) and fixed effects (treatment group). Logistic regression incorporating the generalized estimation equation methodology was performed for categorical outcomes to determine if there is a significant difference in the odds between the treatment groups for each of these end points. Mixed-effects model analyses were conducted to account for correlation between eyes. Statistical significance was based on a 1-sided P < 0.05.
The primary efficacy analysis involved a single hypothesis test at the standard significance level of 5%. All values produced for secondary efficacy analyses, safety analyses, and treatment group comparisons of demographic and baseline characteristics are considered descriptive statistics that support the results of the primary efficacy analysis, and not as formal tests of hypotheses. For this reason, adjustments for multiple comparisons are not needed and were not performed. Generally, analysis models of secondary efficacy end points include the main effect of treatment groups. All secondary efficacy analyses were performed on the ITT population only.
All analyses were performed using commercially available statistical software (SAS version 9.3; SAS Institute, Cary, NC). Adverse events data were evaluated by tabulations of treatment-emergent events and separated out by eye (treated or not treated) for ocular adverse events.
Results
Study Participants and Baseline Characteristics
Between May 14, 2014, and April 22, 2015, 112 participants were screened and 99 study eyes of 67 eligible participants were randomized and underwent either sham or implant surgery (Fig 1). The study was conducted for a follow-up period of 24 months with the clinical trial ending on April 30, 2017. The mean age ± standard error of the participants was 62±8.9 years; 61% were women, and 86% were white (Table 2). In 35 participants, a single study eye (16 implant, 19 sham) was randomized, and 32 participants had 2 randomly assigned study eyes (Table 2). After enrollment, 4 study eyes (3 participants) were found to be ineligible because of neovascularization (3 eyes) or lesion size outside the acceptable range (1 eye). Except for the 2 participants (3 study eyes; both participants underwent CNTF implantation) who died, all study participants were evaluated through 24 months. The baseline mean ± standard deviation area of photoreceptor loss was 0.70±0.42 mm2 for treated eyes and 0.77±0.55 mm2 for sham eyes (P = 0.24; Table 3). The mean ± standard deviation BCVAs at baseline were 76.9±5.9 letters and 76.1±6.75 letters (P = 0.39; Snellen equivalent, 20/30) in the implant and sham groups, respectively. The mean monocular reading speed at baseline using the International Reading Speed Texts18 was reduced at 94.3±46.1 words per minute (wpm) for the treated group compared with 107.3±43.2 wpm for the sham group, which was not statistically significantly different (P = 0.13). The expected fluent reading speed is approximately 160 wpm at a reading level of sixth grade.20 The National Eye Institute Visual Function Questionnaire completed by all participants showed an overall mean value of 80.54±11.2 for the sham group and 72.78±15.2 for the treated group (P = 0.25), lower than the maximum score of 100. For the near activities subscale, the baseline mean was 70.39±21.65 for the sham group and 62.5±20.18 for the treated group (P = 0.46).
Figure 1.
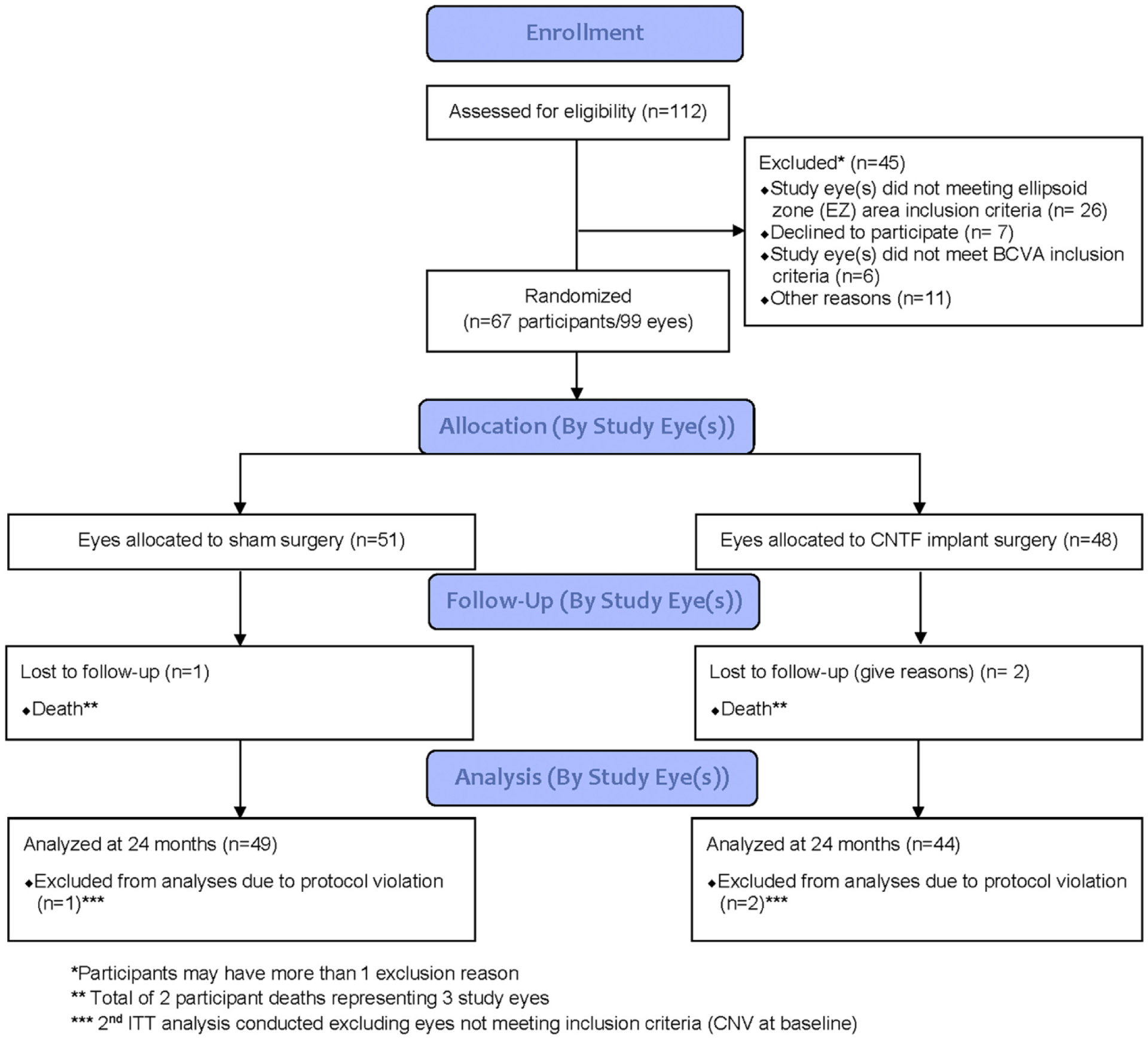
Consolidated Standards of Reporting Trials (CONSORT) diagram demonstrating the patient flow and the participants included in the analyses of this study of sham-controlled trial of implant of ciliary neurotrophic factor (CNTF) for the treatment of macular telangiectasia type 2. BCVA = best-corrected visual acuity; CNV = choroidal neovascularization; EZ = ellipsoid zone; ITT = intention to treat.
Table 2.
Baseline Characteristics of Participants by Treatment Assignment (1-Eye or 2-Eye Contribution)
| Treatment Group | ||||
|---|---|---|---|---|
| Single Eye in Study | Two Eyes in Study | |||
| Baseline Characteristics (by Treatment Groups) | Sham | Human Ciliary Neurotrophic Factor Neurotech-501 Implant | Sham plus Human Ciliary Neurotrophic Factor Neurotech-501 Implant | Total |
| Total no. | 19 | 16 | 32 | 67 |
| Gender, no. (%) | ||||
| Male | 8 (42.1) | 7 (43.8) | 11 (34.4) | 26 (38.8) |
| Female | 11 (57.9) | 9 (56.3) | 21 (65.6) | 41 (61.2) |
| Race, no. (%) | ||||
| Asian | 1 (5.3) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| Black | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (1.5) |
| White | 16 (84.2) | 12 (75.0) | 30 (93.8) | 58 (86.6) |
| Other | 2 (10.5) | 4 (25.0) | 1 (3.1) | 7 (10.4) |
| Race, no. (%) | ||||
| Not Hispanic or Latino | 18 (94.7) | 15 (93.8) | 31 (96.9) | 64 (95.5) |
| Hispanic or Latino | 0 (0.0) | 1 (6.3) | 1 (3.1) | 2 (3.0) |
| Unknown | 1 (5.3) | 0 (0.0) | 0 (0.0) | 1 (1.5) |
| Age at surgery (yrs) | ||||
| Median (range) | 61 (47–73) | 60 (45–79) | 64 (44–76) | 62.0 (44–79) |
No statistically significant differences between groups (all P > 0.05).
Table 3.
Baseline Ophthalmic Characteristics by Treatment Group
| Treatment Group | |||
|---|---|---|---|
| Sham (n = 51) | Human Ciliary Neurotrophic Factor Neurotech-501 Implant (n = 48) | Total (n = 99) | |
| Best-corrected visual acuity (letters) | |||
| No. | 51 | 48 | 99 |
| Mean (SD) | 76.12 (6.75) | 76.94 (5.92) | 76.52 (6.34) |
| Median (minimum—maximum) | 76.00 (65.00–90.00) | 76.50 (63.00–92.00) | 76.00 (63.00–92.00) |
| Ellipsoid zone loss (mm2) | |||
| No. | 51 | 48 | 99 |
| Mean (SD) | 0.77 (0.55) | 0.70 (0.42) | 0.74 (0.49) |
| Median (minimum—maximum) | 0.62 (0.15–2.79) | 0.62 (0.17–1.98) | 0.62 (0.15–2.79) |
| Reading speed (words per minute) | |||
| No. | 49 | 47 | 96 |
| Mean (SD) | 107.26 (43.17) | 94.29 (46.13) | 100.91 (44.89) |
| Median (minimum—maximum) | 102.58 (29.00–191.62) | 95.97 (0.00–202.63) | 98.76 (0.00–202.63) |
| Pupil diameter (mm) | |||
| No. | 50 | 46 | 96 |
| Mean (SD) | 3.72 (0.84) | 3.61 (0.93) | 3.67 (0.88) |
| Median (minimum—maximum) | 4.00 (2.00–6.00) | 3.50 (2.00–6.00) | 4.00 (2.00–6.00) |
| IOP (mmHg) | |||
| No. | 51 | 48 | 99 |
| Mean (SD) | 14.59 (3.07) | 14.73 (3.21) | 14.66 (3.13) |
| Median (minimum—maximum) | 14.00 (8.00–21.00) | 14.00 (8.00–23.00) | 14.00 (8.00–23.00) |
IOP = intraocular pressure; SD = standard deviation.
All participants and eyes were included in the ITT analyses. Per-protocol analyses excluded the 4 study eyes of participants not meeting the eligibility criteria and an additional 3 eyes that had undergone an incorrect imaging method (2 eyes) or were not gradable (1 eye) for the photoreceptor loss on the SD OCT.
Primary Outcome
The primary outcome was photoreceptor loss measured on SD OCT images. The mean (SE) area of photoreceptor loss increased 0.27±0.05 mm2 from baseline to 24 months in the sham group compared with 0.22±0.05 mm2 from baseline to 24 months in the implant group in the ITT analyses. The eyes receiving sham treatment showed 31% greater progression of neurodegeneration than the CNTF-treated eyes. The difference in mean (SE) area of photoreceptor loss was 0.05±0.03 mm2 (P = 0.04; 95% 1-sided U = 0.10) at 24 months. For the PP analyses, the mean (SE) increase in area of photoreceptor loss at 24 months was 0.21±0.03 mm2 in the sham group compared with 0.15±0.03 mm2 in the implant group (difference, 0.06 mm2; P = 0.03; 95% 1-sided U = 0.12). There were 2 deaths and no participants were lost to follow-up. Multiple imputation for missing data (deaths before 24 months) was used as a sensitivity analysis with regard to the results. Using multiple imputation, the mean (SE) difference for the ITT population was 0.05±0.03 mm2 (P = 0.05).
Secondary Outcomes
Change of More than 35% Increase in the Ellipsoid Zone at Month 24.
At month 24, 44% of participants in the sham group versus 31% of participants in the treated group (P = 0.06) in the ITT population experienced a more than 35% increase in the ellipsoid zone loss from baseline. Post hoc analyses of the PP group showed the proportions to be 43% in the sham group versus 28% in the treated group (P = 0.05).
Best-Corrected Visual Acuity.
At 24 months, the mean ± SE changes from baseline in BCVA were −0.53±0.64 letters and −1.52±0.67 letters for the sham and implanted groups, respectively (difference, 0.99 letters; P = 0.12; 95% 1-sided U = 2.4) for the ITT population. For the PP analyses, the mean ± SE changes from baseline in BCVA were −0.40±0.57 letters and −0.85±0.59 letters for the sham and implanted groups, respectively (P = 0.28).
Microperimetry.
The changes in the photoreceptor loss compared with the changes in the retinal sensitivity measured on microperimctry were correlated highly (r = 0.85; P < 0.001) at 24 months. For the ITT analyses through 24 months, the difference in the retinal sensitivity loss was not statistically significantly different between the 2 treatment groups (difference, 15.81±10.66 dB; P = 0.07; 95% 1-sided U = 33.62). The retinal sensitivity loss was statistically significantly greater in the sham group, and the difference in change in aggregate retinal sensitivity loss between the sham and implant groups was 24.39±8.17 dB (P = 0.002; 95% 1-sided U = 38.04) in the PP analyses through 24 months, as demonstrated in post hoc analyses.
Reading Speed.
Mean monocular reading speed in words read per minute decreased in the sham-treated eyes by 13.90±4.69 (SE) wpm at 24 months. In the treated eyes, the reading speed did not change appreciably from baseline at 24 months (−1.30±4.81 wpm; difference, −12.60 words; P = 0.02; 95% 1-sided lower value, −22.71). For the post hoc PP analyses, the mean change from baseline in reading speed was reduced by 13.93±4.79 wpm and was reduced minimally for the treated eyes (0.92±4.91 wpm; difference, −13.00 words; P = 0.02; 95% 1-sided lower value, −23.31).
National Eye Institute Visual Function Questionnaire.
The analyses included only 35 participants who contributed 1 eye to the study. They included 19 in the treated group and 16 in the sham group. There was no statistically significant difference between the treatment groups for either the overall National Eye Institute Visual Function Questionnaire score (mean difference, 0.25±3.47) or the near vision subscale score (mean difference, 3.47±5.96) in this small sample size.
Safety Outcomes
Electroretinography.
To evaluate electroretinography further and to evaluate adverse events of the CNTF implant, 4 clinical sites performed electroretinography in both eyes in 18 study participants (5 participants randomized to sham only, 5 participants randomized to CNTF implant only, and 8 participants randomized to both) at baseline and visits 6, 12, and 24 months. Electroretinography testing was performed in both eyes for all participants. In 8 CNTF-treated study eyes (of 8 participants), the elcctroretinography B-wave amplitude was reduced in response to a dark-adapted dim flash at 6 months of follow-up. The responses to the high-luminance flashes, photopic single-flash, and 30-Hz flicker electroretinography scans were well preserved. By 24 months, this reduction returned to the baseline level in available participants. Follow-up electroretinography examinations were not available for 2 participants who died during the course of the study. The remaining 16 participants who underwent electroretinography examinations were found to have no abnormalities that were relevant to the safety issues.
Humphrey Visual Fields.
Humphrey visual fields were conducted in 85 study eyes (44 sham-treated eyes). The difference in retinal sensitivity between the sham- and the CNTF-treated eyes at months 12 and 24 was evaluated using the mean deviation and the pattern standard deviation. For the mean deviation results, the baseline mean ± SE values were −0.93±0.26 for the sham group and −0.66±0.26 for the CNTF-treated group (P = 0.32). At 24 months, the difference in the mean ± SE mean deviation between the 2 treatment groups was 0.33±0.34 (P = 0.17). The pattern standard deviation results showed no differences in the mean ± SE at baseline, 2.04±0.22 (sham) versus 2.35±0.22 (CNTF treated; P = 0.21), and at 24 months, the mean difference was 0.30 (P = 0.17). Because miosis is known to reduce visual field measurements, analyses were conducted with adjustment to miosis. The results again showed no statistically significant differences between the sham and treated groups.
Adverse Events
Adverse Ocular Events.
The implant was well tolerated, and no removal was required. In general, removal of the implant is required in cases of infection, inflammation, chronic pain, and dysfunction such as delayed dark adaptation resulting from severe miosis. Most ocular adverse effects were related to the surgical or sham procedure, and most of these resolved by the 3-month visit. These included conjunctival hemorrhage, ocular pain, dry eye, and others that are listed in Table 4. Two adverse events persisted throughout the duration of the study and were likely related to CNTF. These included self-reported delayed dark adaptation (18.8%) and miosis of the pupil (18.8%) in the treated eye. Five participants reporting difficulty with dark adaptation also reported miosis.
Table 4-.
Ocular Adverse Events Ordered by Most Frequent Preferred Term by Treatment Group
| Treatment Group | ||||||
|---|---|---|---|---|---|---|
| Sham | Human Ciliary Neurotrophic Factor Neurotech-501 Implant | All Eyes | ||||
| Ocular Adverse Event | Eyes with Event, No. (%) | No. of Total Events | Eyes with Event, No. (%) | No. of Total Events | Eyes with Event, No. (%) | No. of Total Events |
| Eye irritation | 43 (84.3) | 45 | 42 (87.5) | 46 | 85 (85.9) | 91 |
| Vision blurred | 34 (66.7) | 38 | 39 (81.3) | 45 | 73 (73.7) | 83 |
| Conjunctival hemorrhage | 17 (33.3) | 17 | 13 (27.1) | 13 | 30 (30.3) | 30 |
| Eye pain | 13 (25.5) | 14 | 15 (31.3) | 20 | 28 (28.3) | 34 |
| Eye swelling | 7 (13.7) | 7 | 14 (29.2) | 14 | 21 (21.2) | 21 |
| Eye pruritus | 10 (19.6) | 10 | 10 (20.8) | 10 | 20 (20.2) | 20 |
| Dry eye | 6 (11.8) | 6 | 8 (16.7) | 8 | 14 (14.1) | 14 |
| Foreign body sensation in eyes | 4 (7.8) | 5 | 7 (14.6) | 7 | 11 (11.1) | 12 |
| Eyelid edema | 6 (11.8) | 7 | 4 (8.3) | 4 | 10 (10.1) | 11 |
| Delayed dark adaptation | 0 | 0 | 9 (18.8) | 9 | 9 (9.1) | 9 |
| Miosis | 0 | 0 | 9 (18.8) | 9 | 9 (9.1) | 9 |
Pupil Size.
Previous studies revealed that approximately 20% of all eyes implanted with CNTF demonstrated miosis. The pupils were measured at baseline and annually in this study. At baseline, the mean ± standard deviation pupil diameter was 3.61±0.93 mm for the sham group and 3.72±0.84 mm for the treated group (P = 0.63). The mean ± SE difference of the pupil size between baseline and 24 months was −0.21±0.13 mm for the sham group and −1.13±0.14 mm for the treated group (difference, 0.92; P ≤ 0.001).
Serious Adverse Events.
Serious adverse events were reported for 12 participants, including 2 deaths resulting from aortic aneurysm and myocardial infarct (Table 5). Both deaths occurred in participants assigned to receive CNTF. Eight of these 12 participants contributed both a control eye and a treated eye, and the adverse events occurred in multiple organ systems, making causality attribution difficult. In general, the serious adverse events were not related to the eye and none was reported as related to CNTF, following guidelines for relatedness provided in the protocol. One participant (2 study eyes) reported an ophthalmic serious adverse event of extended hospital stay (1 day) because of blurred vision immediately after the procedure. This event resolved without sequelae.
Table 5.
Nonocular Serious Adverse Events by System Organ Class. Preferred Term, and Treatment Arm
| Treatment Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sham | Human Ciliary Neurotrophic Factor Neurotech-501 Implant | Human Ciliary Neurotrophic Factor Neurotech-501 Implant plus Sham | All Participants | |||||
| System Organ Class Preferred Term | Participants with Event, No. (%) | No. of Total Events | Participants with Event, No. (%) | No. of Total Events | Participants with Event, No. (%) | No. of Total Events | Participants with Event, No. (%) | No. of Total Events |
| Cardiac disorders | 1 (5.3) | 1 | 0 | 0 | 2 (6.3) | 2 | 3 (4.5) | 3 |
| Atrial fibrillation | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Cardiac arrest | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Stress cardiomyopathy | 1 (5.3) | 1 | 0 | 0 | 0 | 0 | 1 (1.5) | 1 |
| Endocrine disorders | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Thyroid mass | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Infections and infestations | 1 (5.3) | 1 | 0 | 0 | 1 (3.1) | 1 | 2 (3) | 2 |
| Pneumonia | 1 (5.3) | 1 | 0 | 0 | 1 (3.1) | 1 | 2 (3) | 2 |
| Injury, poisoning, and procedural complications | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Postlaminectomy syndrome | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Nervous system disorders | 0 | 0 | 1 (6.3) | 1 | 0 | 0 | 1 (1.5) | 1 |
| Normal pressure hydrocephalus | 0 | 0 | 1 (6.3) | 1 | 0 | 0 | 1 (1.5) | 1 |
| Psychiatric disorders | 1 (5.3) | 1 | 0 | 0 | 1 (3.1) | 1 | 2 (3) | 2 |
| Anxiety | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Suicide attempt | 1 (5.3) | 1 | 0 | 0 | 0 | 0 | 1 (1.5) | 1 |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 1 (6.3) | 1 | 0 | 0 | 1 (1.5) | 1 |
| Laryngeal cyst | 0 | 0 | 1 (6.3) | 1 | 0 | 0 | 1 (1.5) | 1 |
| Surgical and medical procedures | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Vulval operation | 0 | 0 | 0 | 0 | 1 (3.1) | 1 | 1 (1.5) | 1 |
| Vascular disorders | 1 (5.3) | 1 | 1 (6.3) | 1 | 0 | 0 | 2 (3) | 2 |
| Aortic aneurysm | 0 | 0 | 1 (6.3) | 1 | 0 | 0 | 1 (1.5) | 1 |
| Shock | 1 (5.3) | 1 | 0 | 0 | 0 | 0 | 1 (1.5) | 1 |
Discussion
In participants with macular telangiectasia type 2, a surgical implant that released CNTF into the vitreous cavity, compared with a sham procedure, slowed the progression of retinal degeneration. The corresponding visual function as measured by microperimetry demonstrated a greater decrease in retinal sensitivity in eyes receiving the sham treatment compared with the CNTF implant-treated eyes. The reading speed was stabilized in eyes receiving the implant, whereas those who underwent the sham treatment continued to show decrease in reading speed. Central visual acuity loss was minimal and did not differ between groups. Visual field and electroretinography testing suggested no safety concerns associated with the use of CNTF for persons with macular telangiectasia type 2.
The current hypothesis tested whether neurotrophin CNTF would decrease photoreceptor loss, which in turn would be reflected by visual functional benefit. The loss of ellipsoid zone integrity on OCT was chosen as an objective measure of photoreceptor loss. Breaks in the ellipsoid zone are considered to represent regions of photoreceptor loss.21 Photoreceptor loss was reduced significantly in the CNTF-treated eyes. In a previous study of 18 donor eyes that compared ex vivo OCT assessments with histopathologic findings, there was a good correlation of the ellipsoid zone segmentation on OCT with the histologic appearance of photoreceptor inner and outer segments.22 Furthermore, in 7 autopsy eyes from individuals affected with macular telangiectasia type 2, the SD OCT images also compared well with the histologic findings.4,5 In another study, the structural changes observed on OCT that reflected photoreceptor loss correlated well with the functional changes as measured by the area of retinal sensitivity change on Macular Integrity Assessment microperimetry. Together, these observations support the structure–function relationships that we observed in the present study.
Mean visual acuity loss (BCVA) at month 24 for treated and control eyes was approximately 1 letter in both groups. A previously reported study of the natural course of macular telangiectasia type 2 in 507 participants followed up for a mean ± SE duration of 4.2±1.6 years demonstrated the mean ± SE rate of visual acuity loss of 1.07±0.5 letters per year.23 Thus, the functional measure of BCVA, where the clinically meaningful difference is 15 letters, would not be an appropriate primary outcome of an intervention in this disease and underestimates the impact that this disease has on near vision tasks. However, when individual measures of visual acuity were evaluated for safety, no treated eye had visual acuity loss of 15 letters at the end of the study.
Because distance visual acuity is an insensitive measure of progression of functional loss in this condition, retinal sensitivity as measured by microperimetry and reading speed were evaluated. These data confirmed that these measures of function were reduced markedly in the study population. Consistent with the premise that CNTF slows the loss of photoreceptors, there was a reduction in point-wise functional loss (aggregate sensitivity) identified by microperimetry data overlays. A functional benefit also was seen in the stabilization of reading speed, as evidenced by no further loss in the number of words read per minute in the treated group. This finding is likely clinically meaningful, because one of the major early symptoms reported by patients with macular telangiectasia type 2 is difficulty with reading, despite preserved measure of distance visual acuity. Metamorphopsia (distortion) and the presence of scotomas in both eyes contribute to difficulty with near vision tasks that require scanning.
These results apply to a specific group of patients with macular telangiectasia type 2 with a specific lesion size. Would lesions smaller or larger or even those patients in whom there are not lesions evident benefit from this treatment? The generalizability of these study results is somewhat limited. Further evaluation is required to understand the therapeutic effectiveness in other affected persons.
An important question that may be difficult to answer is why CNTF seems to be beneficial specifically in eyes with macular telangiectasia type 2 and in no other clinical trials of retinal degenerations, such as retinitis pigmentosa.24 The mechanisms of action of the therapy are not fully understood, and the pathogenesis of macular telangiectasia type 2 and other retinal degenerations most likely are quite different. Is it possible that we are intervening at an earlier stage of this disease in macular telangiectasia type 2, whereas the participants in the study with retinitis pigmentosa or geographic atrophy associated with age-related macular degeneration25 were already in the more severe stage of disease? The study outcome measures also were different. The explanation is that the success or failure of CNTF for such complex diseases requires further research in each area.
The strengths of this study include the evaluation of the outcome at a centralized reading center by certified personnel who were masked to the treatment assignments. The tests of visual function were standardized and administered at each clinical site by certified and masked personnel. The microperimetry data were assessed at the reading center using a standardized protocol to assess the correlation of the functional and structural data. Previous studies have demonstrated similar high rates of correlation.17,21,26 Study follow-up was excellent, with all surviving participants completing all study visits.
The first potential limitation of the study design is the use of the 1-sided P value for estimation of sample size. This was undertaken because the previous phase 1 study15 showed no major adverse effect after 5 years of follow-up, and several studies of retinal neurodegeneration collectively enrolled 256 participants who also demonstrated no harmful effects. Results from a phase 2 study are not translated into clinical recommendations, and it is anticipated that 2-sided comparisons will be required for phase 3 studies. Currently, 2 phase 3 studies are enrolling participants in 2-sided comparison designs to test this hypothesis.
The second limitation of the study is the relatively short follow-up for a neurodegenerative disease with slow progression. These participants will be followed up for at least an additional 5 years in an extension study to gather long-term information on the effects of the CNTF secreting Neurotech-501 implant on this condition. The activity and durability of this implant were tested in both pharmacokinetic studies and in devices that have been explanted for as long as 5 years (Kauper et al, Invest Ophthalmol Vis Sci. 54:ARVO e-abstract 3295, 2013). The implant continued to show activity.
The third limitation is the inability for the investigators to know the minimal clinical important difference between the treatment groups for this primary end point. The sample size calculation was based on data calculated from a natural history study in macular telangiectasia type 2.18,19 A mean difference of 0.084 mm2 between the 2 treatment groups was chosen based on the assumption of powering for a modest 30% difference between the treatment groups. Further studies are required to evaluate the validity of this minimal clinically important difference.
Finally, what is the generalizability of these results of this group of patients with macular telangiectasia type 2 with specific lesion size of ellipsoid zone loss? Would lesions smaller or larger benefit from this treatment? The generalizability of these study results are somewhat limited. Further evaluation is required to understand the therapeutic effectiveness in other affected persons.
In conclusion, in patients with macular telangiectasia type 2, a surgical implant that released CNTF into the vitreous cavity, compared with a sham procedure, slowed the progression of retinal degeneration as measured by the loss of the ellipsoid zone band determined on SD OCT and maintained the reading speed. Further research is needed to assess longer-term clinical outcomes and safety.
Supplementary Material
Financial Disclosure(s):
The author(s) have made the following disclosure(s): G.J.: Consultant – Heidelberg Engineering.
C.J.: Employee – Neurotech Pharmaceuticals.
S.F.: Patent – software for segmenting the OCT images at Duke University.
E.L.: Consultant – Janssen, Apellis, Roche, Stealth; Financial support – Roche, Apellis.
L.S.: Financial support – Aerpio, Alcon Laboratories, Allergan, Appellis, Digisight, Genentech, Ophthotech, Opthea, Roche, Tyrogenix; Equity owner – Ohr Pharmaceuticals, Regeneron.
P.R.: Consultant – Acuela, Apellis Boehringer-Ingelheim, Carl Zeiss Meditec, Cell Cure Neurosciences, Chengdu Kanghong Biotech, Isarna Therapeutics, Genentech, Healios K.K., Hemera Biosciences, F. Hoffman-LaRoche Ltd, Ocudyne, Ocunexus, Tyrogenex, Unity Biotechnology; Financial support – Astellas Institute for Regenerative Medicine (AIRM), Carl Zeiss Meditec, Genentech, Tyrogenex; Equity owner – Apellis, Digisight, Ocudyne.
D.E.: Consultant – Alimera, Allergan, Dutch Ophthalmic, Genentech, Santen.
Supported by the Lowy Medical Research Institute, La Jolla, California; and Neurotech Pharmaceuticals, Inc, Cumberland, Rhode Island, which provided the Neurotech-501 implants producing ciliary neurotropic factor (CNTF). The funding organization (Lowy Medical Research Institute) and the sponsor (Neurotech Pharmaceuticals, Inc) were consulted for design and conduct of the study. The collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication were the responsibilities of the research team, the MacTel Project investigators.
Abbreviations and Acronyms:
- BCVA
best-corrected visual acuity
- CNTF
ciliary neurotrophic factor
- ITT
intention-to-treat
- PP
per-protocol
- SD
spectral-domain
- SE
standard error
- wpm
words per minute
Footnotes
A complete listing of the members of the Macular Telangiectasia Type 2-Phase 2 CNTF Research Group is available at www.aaojoumal.org.
Supplemental material available at www.aaojournal.org.
References
- 1.Charbel Issa P, Gillies MC, Chew EY, et al. Macular telangiectasia type 2. Prog Retin Eye Res. 2013;34:49–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemons TE, Gillies MC, Chew EY, et al. The National Eye Institute Visual Function Questionnaire in the Macular Telangiectasia (MacTel) Project. Invest Ophthalmol Vis Sci. 2008;49:4340–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gass JD, Oyakawa RT. Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982;100:769–780. [DOI] [PubMed] [Google Scholar]
- 4.Powner MB, Gillies MC, Tretiach M, et al. Perifoveal Muller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117:2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powner MB, Gillies MC, Zhu M, et al. Loss of Müller cells and photoreceptors in macular telangiectasia type 2. Ophthalmology. 2013; 120:2344–2352. [DOI] [PubMed] [Google Scholar]
- 6.Aung KZ, Wickremasinghe SS, Makeyeva G, et al. The prevalence estimates of macular telangiectasia type 2: the Melbourne Collaborative Cohort Study. Retina. 2010;30:473–478. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Biodi BA, Meuer SM, et al. The prevalence of macular telangiectasia type 2 in the Beaver Dam eye study. Am J Ophthalmol. 2010;150:55–62.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallo FB, Leung I, Mathenge W, et al. The prevalence of type 2 idiopathic macular telangiectasia in two African populations. Ophthalmic Epidemiol. 2012; 19:185–189. [DOI] [PubMed] [Google Scholar]
- 9.Dorrell MI, Aguilar E, Jacobson R, et al. Antioxidant or neurotrophic factor treatment preserves function in a mouse model of neovascularization-associated oxidative stress. J Clin Invest. 2009;119:611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012;31:136–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaVail MM, Yasumura D, Matthes MT, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 12.Cayouette M, Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Human Gene Ther. 1997;8:423–430. [DOI] [PubMed] [Google Scholar]
- 13.LaVail MM, Unoki K, Yasumura D, et al. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci US A. 1992;89:11249–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen W, Fruttiger M, Zhu L, et al. Conditional Muller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci. 2012;32: 15715–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew EY, Clemons TE, Peto T, et al. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial. Am J Ophthalmol. 2015;159:659–666.e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee D, Lad EM, Vann RR, et al. Correlation between macular integrity assessment and optical coherence tomography imaging of ellipsoid zone in macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2017;58: BI0291–B10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn GA, Penka D, Gehrlich C, et al. New standardised texts for assessing reading performance in four European languages. Br J Ophthalmol. 2006;90:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallo FB, Peto T, Egan C, et al. The IS/OS junction layer in the natural history of type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2012;53:7889–7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauderman WJ, Barlow WE. Sample size calculations for ophthalmologic studies. Arch Ophthalmol. 1992;110:690–692. [DOI] [PubMed] [Google Scholar]
- 20.Rubin GS. Measuring reading performance. Vis Res. 2013;90: 43–51. [DOI] [PubMed] [Google Scholar]
- 21.Sallo FB, Peto T, Egan C, et al. “En face” OCT imaging of the IS/OS junction line in type 2 idiopathic macular telangiectasia. Invest Ophthalmol Vis Sci. 2012;53:6145–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curcio CA, Messinger JD, Sloan KR, et al. Human chorioretinal layer thicknesses measured in macula-wide, high-resolution histologic sections. Invest Ophthalmol Vis Sci. 2011;52: 3943–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peto T, Heeren TFC, Clemons TE, et al. Correlation of clinical and structural progression with visual acuity loss in macular telangiectasia type 2: MacTel Project report no. 6. The MacTel Research Group. Retina. 2018;38(Suppl 1):S8–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birch DG, Bennett LD, Duncan JL, et al. Long-term follow-up of patients with retinitis pigmentosa receiving intraocular ciliary neurotrophic factor implants. Am J Ophthalmol. 2016; 170:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K, Hopkins JJ, Heier JS, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011; 108: 6241–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charbel Issa P, Helb HM, Holz FG, Scholl HP. Correlation of macular function with retinal thickness in nonproliferative type 2 idiopathic macular telangiectasia. Am J Ophthalmol. 2008;145:169–175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


