Abstract
Raynaud's phenomenon is a rare side effect of CGRP monoclonal antibodies. These molecular treatments are a relatively new class of drugs for the prevention of migraine. It is likely that we will see this side effect more often in the future. Patients with a background of Raynaud's phenomenon may experience worsening of their symptoms if started on these treatments.
Keywords: CGRP monoclonal antibodies, chronic migraine, erenumab, Raynaud's phenomenon
Raynaud's phenomenon is a rare side effect of CGRP monoclonal antibodies. These molecular treatments are a relatively new class of drugs for the prevention of migraine. It is likely that we will see this side effect more often in the future. Patients with a background of Raynaud's phenomenon may experience worsening of their symptoms if started on these treatments.

1. INTRODUCTION
A 45‐year‐old woman with chronic migraine undergoing treatment with the calcitonin gene‐related peptide (CGRP) monoclonal antibody, erenumab 70 mg monthly subcutaneous injections developed Raynaud's phenomenon (RP). The patient subsequently discontinued the treatment due to severe symptoms of RP, a rare side effect associated with CGRP monoclonal antibodies.
Chronic migraine (CM) is a debilitating neurological disorder with a prevalence of 0.5%–5% in the general population.1 It is associated with a significant negative impact on quality of life (QOL) and mental health.2 Monoclonal antibodies targeting the CGRP pathway have been shown to be effective in episodic and chronic migraine. These molecular treatments work by binding either to the CGRP receptor or the CGRP ligand.3 Raynaud's phenomenon (RP) is characterized by brief reduction of blood flow to the extremities due to vasoconstriction.4, 5 The relationship of RP and migraine is previously documented. Zahavi et al. reported RP in association with migraine in 26% (29/111) of patients.4 RP secondary to administration of migraine‐specific therapies, such as CGRP monoclonal antibodies, has been recently reported in a few cases.
2. CASE
We present the case of a 45‐year‐old right‐handed woman who developed chronic daily headache (CDH) with migraine features in 2018. She had migraine in her teens, often associated with her menstrual cycle. There is no history of migraine aura. The headaches progressively increased in frequency and severity in her 30's. In January 2018, after a viral infection, she developed unremitting headache with associated migraine symptoms and chronic daily headache (CDH). The pain is usually holocranial. She also has bilateral facial pain. With worsenings, there is associated phonophobia, aggravation by physical activity and severe fatigue. The patient denied photophobia, nausea, vomiting, and cranial autonomic symptoms. Poor sleep and physical activity worsen the headaches. The clinical examination was normal, including fundoscopy. Her routine blood tests including full blood count, biochemical profile, renal, liver, thyroid function, vitamin B12, and folate were within the normal limits. MRI brain and MR venogram (MRV) of the intracranial vessels were unremarkable. The patient has a previous history of varicose vein surgery and panic attacks. There is no history of rheumatological disease. She had sinus surgery in 2009, with no improvement in her headache and associated migraine symptoms. Her other medication consists of duloxetine 30mg daily, paracetamol PRN, and naproxen PRN.
A diagnosis of chronic daily headache (CDH) with migraine features was made in 2018, and she was started on prophylactic medication. She had failed five migraine prophylactic drugs due to side effects and/or lack of efficacy: propranolol (minimal benefit), amitriptyline (weight gain), topiramate (some benefit, but significant cognitive impairment at doses above 50 mg twice daily), flunarizine (intolerable side effects), and venlafaxine (worsening of headaches). Therefore, as per national and international guidelines, she was started on erenumab 70 mg, a monthly subcutaneous injection. The patient reported 40% improvement in headache severity and overall migraine symptoms but with no crystal clear days.
Two weeks after the second injection of erenumab, she developed intermittent blue discoloration of both hands, which worsened over a period of 7–8 months on erenumab treatment (see Figure 1). There was no associated pain or sensory disturbance. The symptoms were worse in cold weather and improved in the summertime. Hand movements also improved the symptoms. The patient had never experienced such symptoms prior to erenumab administration. A diagnosis of RP secondary to erenumab was made. The patient initially declined discontinuation of erenumab, as she feared worsening of headaches and associated symptoms. However, she discontinued treatment after 8 months due to the side effect of RP, both voluntarily and on medical advice. The RP symptoms have improved by approximately 70% and she is now off erenumab for more than 1 year. The patient is currently having Botox treatment (PREEMPT Protocol) and is due to her fourth round of injections. There is an improvement of approximately 40%–50% in terms of headache and migraine severity. She has not tried an alternative CGRP monoclonal antibody.
FIGURE 1.
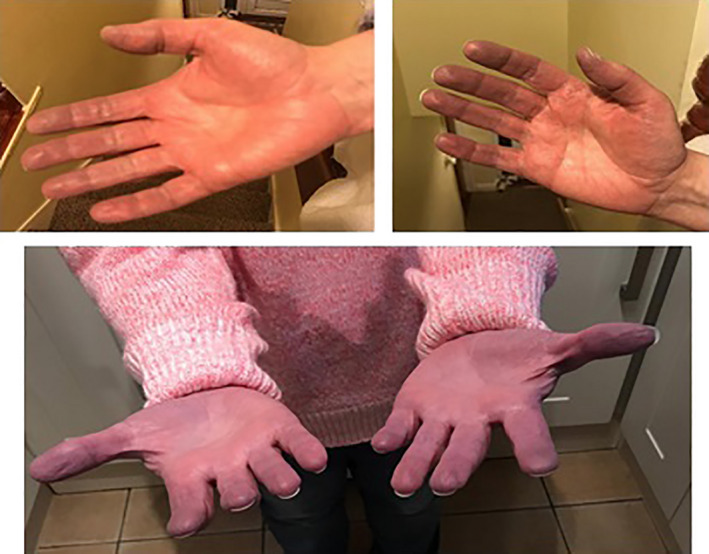
Raynaud's phenomenon secondary to erenumab in a patient with chronic migraine
3. DISCUSSION
The overall global prevalence of RP is approximately 10% in women (partly due to hormonal variations) and 4%–14% in men.4 Migraine and RP were found to co‐exist in 26% (29 of 111) of migraine patients in one study.4 Although the pathophysiology of RP is not well‐understood, a combination of neuronal and vascular factors (including intravascular anomalies) are known to play a role.6 Vasoconstriction is believed to be a major feature of RP and can be triggered by external stimuli, including cold water or weather. RP can also be triggered mainly by neural‐mediated changes.4 There are two main types: primary RP, which is a benign condition, and secondary RP, which is associated with several connective tissue diseases, in particular systemic sclerosis.5 The underlying mechanism in primary RP is considered to be a “local fault” in the thermoregulatory vessels without evidence of structural alteration or injury to vessels while the secondary RP are acquired conditions including those that can cause vascular injury.6
Calcitonin gene‐related peptide is a ubiquitous 37 amino acid neuropeptide found in two isoforms: α‐CGRP (mainly localized to the peripheral nervous system) and β‐CGRP (predominantly localized to enteric nervous system). Both isoforms are efficient vasodilators.2, 3, 7 Evidence suggests that the presence of increased CGRP in sites undergoing inflammatory response.7 Elevated CGRP levels are observed in saliva, CSF, and serum during migraine attacks and reduce after these bouts subside.2 Targeting the CGRP receptor with monoclonal antibodies is effective in the management of migraine.3, 7 It is believed that activation of the trigeminovascular system by migraine‐specific triggers causes vasodilation of cranial blood vessels, thereby activating sensory nerve fibers of the trigeminal system.2, 3 Pain is conveyed to the brainstem where several different neurotransmitters including CGRP and substance P are released and bind to the functional receptors causing neuronal inflammation, degranulation of mast cells, and leakage of blood vessels.2, 7
Although the CGRP monoclonal antibodies (erenumab, fremanezumab, galcanezumab, eptinezumab) were found to be safe and effective in clinical trials, more data are emerging regarding their safety profile and potential side effects in clinical practice.3 RP is becoming a relatively rare recognized side effect of CGRP monoclonal antibodies in patients with migraine.4 It has been recently reported in three patients (two women‐ 33 years & 45 years and one man‐ 65 years) undergoing CGRP monoclonal antibody treatment. Two patients were treated with monoclonal antibodies against the ligand (fremanezumab and galcanezumab) and in one case, the monoclonal antibody targeted the receptor (erenumab).4 There is a consensus now that the most likely mechanism by which CGRP monoclonal antibodies cause RP is primarily due to vasoconstriction, but this can only occur in conjunction with several other factors, including genetic and hormonal influences. CGRP monoclonal antibodies, therefore, antagonize CGRP's role as a potent vasodilator in this context.2, 3 When administered, erenumab binds to the functional receptor, subsequently blocking its function.3, 4 This prevents the cascade of reactions within the cell responsible for vasodilation, presumably leading to the development of RP in a small number of cases.3, 4
In summary, we report a case of chronic daily headache with migraine features who developed RP after receiving treatment with a CGRP receptor antagonist, further supporting the theory that RP is now a recognized rare side effect of CGRP monoclonal antibodies. This side effect information did not emerge from clinical trials. The presence of significant or debilitating RP in a small proportion of patients with migraine who are treated with CGRP monoclonal antibodies has clinical implications, including the necessity for cessation of treatment in some patients. Furthermore, this class of drugs could exacerbate the symptoms of RP in patients with a previous history of this condition. A previous background history of RP secondary to connective tissue disease might prevent patients from initiating these new class of migraine preventative medications.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
AHM: drafted the manuscript; AB: neurologist involved in patient's care; ET: headache specialist nurse involved in patient's care; MR: primary neurologist involved in patient's care and decisions regarding diagnosis and management. All authors contributed to chart review and data collection. All authors revised the draft manuscript and approved the final version.
CONSENT STATEMENT
Published with written consent of the patient.
ACKNOWLEDGEMENTS
We thank the patient for her consent and help in preparation of this case report.
Manickam AH, Buture A, Tomkins E, Ruttledge M. Raynaud's phenomenon secondary to erenumab in a patient with chronic migraine. Clin Case Rep. 2021;9:e04625. 10.1002/ccr3.4625
Funding information
This manuscript has not received financial support
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the authors upon reasonable request.
REFERENCES
- 1.Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American migraine prevalence and prevention study. Headache. 2012;52:1456‐1470. [DOI] [PubMed] [Google Scholar]
- 2.Durham PL. Calcitonin gene‐related peptide (CGRP) and migraine. Headache. 2006;46(Suppl 1);S3‐S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338‐350. [DOI] [PubMed] [Google Scholar]
- 4.Evans RW. Raynaud's phenomenon associated with calcitonin gene‐related peptide monoclonal antibody antagonists. Headache. 2019;59:1360‐1364. [DOI] [PubMed] [Google Scholar]
- 5.Ruaro B, Smith V, Sulli A, et al. Innovations in the assessment of primary and secondary Raynaud’s phenomenon. Front Pharmacol. 2019;10:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrick AL, Wigley FM. Raynaud's phenomenon. Best Pract Res Clin Rheumatol. 2020;34(1):101474. [DOI] [PubMed] [Google Scholar]
- 7.Durham PL. CGRP‐receptor antagonists—a fresh approach to migraine therapy? N Engl J Med. 2004;350:1073‐1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.


