Abstract
Purpose:
To compare the effects of two types of mesenchymal stem cells (MSCs), activated omental cells (AOCs), and adipose tissue-derived stem cells (ADSCs) in the healing process of animal model of ocular surface alkali injury.
Methods:
An alkaline burn was induced on the ocular surfaces of eighteen rats divided randomly into three groups. The first and second groups received subconjunctival AOCs and ADSCs, respectively. The control group received normal saline subconjunctival injection. On the 90th day after the injury, the eyes were examined using slit-lamp biomicroscopy. Corneal neovascularization and scarring were graded in a masked fashion. Histological evaluation of the corneal scar was performed, and the number of inflammatory cells was evaluated.
Results:
Corneal neovascularization scores revealed higher neovascularization in the control (0.49 ± 0.12) than the AOC (0.80 ± 0.20, P = 0.01) and ADSC groups (0.84 ± 0.24, P = 0.007). There were no statistically significant differences between the neovascularization score of the AOC and ADSC groups (P > 0.05). According to histologic evaluation, stromal infiltration was significantly more in the control group compared to AOC and ADSC groups (P < 0.05).
Conclusions:
Our results suggest that MSCs, even with different sources, can be used to promote wound healing after corneal chemical burns. However, the ease of harvesting ADSC from more superficial fat sources makes this method more clinically applicable.
Keywords: Activated omental cell, Adipose tissue-derived stem cell, Corneal alkaline burn, Corneal neovascularization, Limbal stem cell deficiency, Mesenchymal stem cell, Ocular cell therapy, Omentum
INTRODUCTION
Transparency of self-renewing corneal epithelium relies on healthy limbal stem cells. It can be destroyed by severe ocular surface injuries such as autoimmune, traumatic, or genetic diseases.1 One of the leading causes of limbal stem cell deficiency is alkaline burn. Alkali can penetrate the corneal epithelium and reaches the underlying stromal tissues. Consequently, increased PH damages the limbus, clinically seen as limbal “blanching.” In severe cases, corneal conjunctivalization, vascularization, and chronic inflammation may lead to persistent epithelial defects and poor vision.2,3 To overcome these complications, cell-based therapy is a promising therapeutic approach.4 Mesenchymal stem cells (MSCs), a type of pluripotent stem cell, as a source for repopulation of limbal stem cell and corneal stromal repair, have attracted many researchers in recent decades.
MSCs are originally derived from the bone marrow5 and subsequently isolated from various tissues like adipose tissue6 or omentum.7,8 They perform various beneficial effects which promote wound healing including cell differentiation and immunomodulatory properties that decrease the inflammation.1,9,10,11 Omentum, a fatty tissue in mammals, called the “policeman of the abdomen,” can migrate to the injured site, adhere to the wound, and accelerate the healing process.12,13,14 Some omental cells express markers of adult stem cells (SDF–1α, CXCR4, WT–1) as well as embryonic pluripotent cells (Oct–4, Nanog, SSEA–1),15 while others can be activated by foreign bodies and become a rich source of growth and angiogenic factors.16 Furthermore, immunomodulator cells present in the omentum and play an important role in reducing inflammation by suppressing Th17 cells.14 The effects of omental cells in corneal limbal alkaline burns were evaluated in previous studies.17,18
Adipose tissue-derived stem cells (ADSCs) are another kind of MSCs that have the capacity to differentiate into specific cells, depending on the microenvironment. Moreover, some of their paracrine effects such as anti-angiogenesis, anti-inflammation, and anti-fibrosis properties were described previously.11,19,20
To determine which kind of MSCs are more effective, we compared autologous activated omental cell (AOCs) with allogeneic ADSCs on the healing process of corneal alkali injury. Since all previous studies18,19 evaluated the effects of these cells in a brief period, we decided to observe the long-term outcome of these cells in corneal wound healing.
METHODS
Eighteen adult male Sprague Dawley rats (250–300 g) were enrolled in the study. They had free access to food and water and were kept under a 12-h light-dark cycle and temperature-controlled conditions (25°C). The study was approved by the Ethics Committee of Shiraz University of Medical Sciences and adhered to the guidelines of the International Council for Laboratory Animal Science, Brussels, BELGIUM (ICLAS). All procedures were performed on the animals under general anesthesia with ketamine (100 mg/kg body weight) and xylazine (5 mg/kg body weight). Topical 0.5% tetracaine was applied on the eyes prior to the induction of corneal alkali injury. They were randomly divided into three groups of six rats. The corneal alkaline burn was induced on the one eye of each animal. Donut-shaped filter paper rings (with 8 mm outside and 4 mm inside diameter) soaked in 0.5 N NaOH were placed on the corneas for 20 s. Then the eyes were rinsed with sterile normal saline until pH returned to normal.
Two days after alkaline burn induction, equal amounts (0.1 ml) of either autologous AOCs, allogeneic ADSC, or normal saline was injected around the limbus in Groups 1, 2, and 3, respectively.
Preparation of autologous activated omental cells
AOCs were isolated from rat omentum according to previous reports.14,16,18,21 Five days before alkaline burn induction, the rats in Group 1 were injected intraperitoneally with 5 ml of polydextran particle slurry (Bio-gel P-60, Biorad Laboratories, Richmond, California) 1:1 in phosphate-buffered saline (PBS). Seven days later (two days after alkali injury), the rats were anesthetized, and the expanded omentum of each rat was harvested aseptically and gently chopped into small pieces. These pieces were then digested by 1 mg/ml of collagenase type I (Sigma, St. Louis, MO) for 30 min at 37°C. A ficoll gradient was used to wash and separate AOCs. The cells lying on the interface were passed through a cell strainer. After washing and centrifugation, they were suspended in sterile saline and immediately moved to the operating room for injection in the AOC group. Each rat received its autologous AOCs.
Preparation of allogeneic adipose tissue-derived stem cell
To prepare allogeneic ADSCs, we anesthetized one female donor rat. Fragments of adipose tissue isolated from its pelvic area were washed with PBS, minced into small pieces, and digested by 0.2% collagenase type I (GIBCO, USA) at 37°C. The digested tissue was centrifuged (1500 RPM) and the pellet, including the adherent stromal cells, was carefully put on Ficoll-Paque (Biosera, UK) and centrifuged again. The second white layer, stromal vascular fraction (SVF), was transferred into a tube and washed with PBS. The SVF pellet was re-suspended in DMEM culture medium (GIBCO, USA) containing 10% fetal bovine serum (GIBCO, USA) and 1% penicillin/streptomycin (Biosera, UK). Nonadherent cells were discarded after 24 h of culturing. The adherent cells were cultured by changing the medium every 4 days and harvested in passage 3 after nearly 30 days of culturing. The quality of cultured cells was assessed morphologically by their spindle shape and positivity for the expression of mesenchymal specific markers: CD44 and CD90.
Surgical procedures
Similar to a previous study,18 two days after burn induction, Group 1 received subconjunctival injection of 0.5 × 106, freshly isolated autologous AOCs/eye in 0.1 ml sterile saline. The total volume was divided among 3 different locations at 2, 6, and 10 o’clock around the corneas about 2 mm from the limbus. In Group 2, 0.5 × 106 ADSCs/eye in 0.1 ml sterile saline were injected exactly in the same way as described for Group 1. In the control group, the rats received only 0.1 ml PBS subconjunctivally 2 days after the alkaline burn. All animals were treated with topical chloramphenicol 0.5% eye drops (Chlobiotic, Sina Daru, Tehran, Iran) one drop three times a day for 3 days.
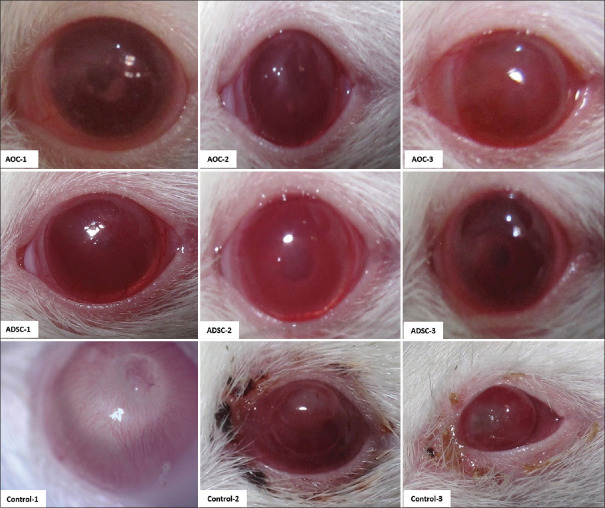
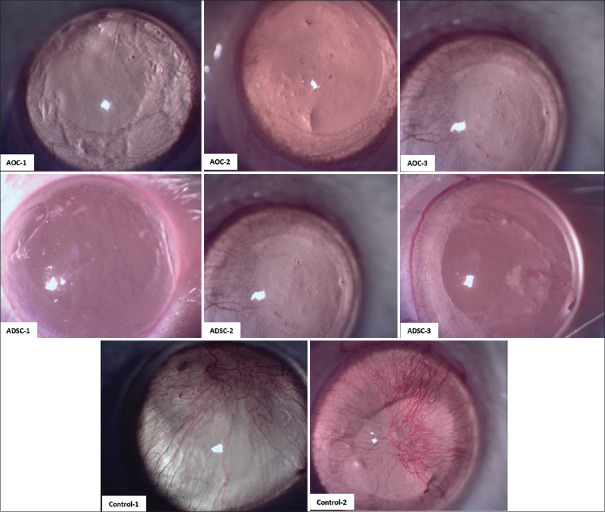
All three groups were followed up and examined on the 90th day after the procedure. Gross and slit-lamp biomicroscopy photos were captured by Canon G12 camera [Figures 1 and 2].
Figure 1.
Effects of activated omental cell (AOC), adipose tissue-derived stem cell (ADSC) on limbal alkaline corneal injury after three months. Gross images by Canon G12 camera. AOCs, ADSCs, and control group are illustrated in the first, second, and third rows, respectively. Two of six eyes in the control group developed auto evisceration. Another two eyes developed thin descemetocele. No eyes in cell injected groups developed these complications
Figure 2.
Slit-lamp images of rat corneas in activated omental cell, adipose tissue-derived stem cell, and control Group (C). Note the central corneal clarity and minimal amount of neovascularization in the cell injected groups. Compared to dense corneal neovascularization in the control group
Captured slit-lamp images of all rats were analyzed and processed with the MatLab application (R2017a) in the aspect of corneal neovascularization. Gabor filter and supervised classification were used in the texture analysis and detection corneal neovascularization.22 Data from central 4 mm diameter of the corneas were scored from 0 to 1 (0 indicated severe vascularization and 1 revealed clear cornea) in a masked fashion. Dilated limbal vessels which did not penetrate the corneal stroma were not considered representative of corneal neovascularization.
On the 90th day of the study, all the subjects were euthanized, and enucleation was performed. The eyes were fixed in 10% formaldehyde. Then 5-micrometer histologic sections from the center of the cornea were stained with H&E and evaluated with light microscope at ×100 magnification (Olympus, BX41 Japan). The pictures were taken with a digital camera.
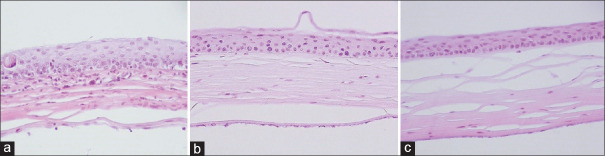
Histologic evaluation was done in the aspects of corneal infiltration, vascularization, and scar. Three fields in the paracenteal area of each sample were used for neutrophil cell counting. Accordingly, the amount of infiltration was graded as mild, moderate, or severe.23 The amount of vascularization was assessed and graded under ×400 magnification of light microscope: near normal: no vessel in the stromal tissue; mild: 1–2 vessels in the anterior stroma; moderate: 3–5 vessels in up to the half depth of the stroma; and severe: the presence of neovascularization in the entire thickness of the cornea. The severity of scaring was graded as mild, moderate, or severe, according to the increase in the number of observed keratocytes as well as regularity of collagen lamellae in comparison to the same areas in normal cornea [Figure 3].
Figure 3.
Effects of activated omental cell (AOC), and adipose tissue-derived stem cell (ADSC) on corneal histology after three months of limbal corneal alkali injury. Light microscopy findings with representative H&E stained corneal sections from the AOCs (b), ADSCs (c), and control Group (a), with a ×100 magnification. Note less amount of leukocyte infiltration in the corneal stroma and almost normal epithelium, more organized collagen lamellae with minimum scar in AOCs and ADSCs injected groups compared to the control group
Statistical analysis
Two eyes in the control group were excluded because of auto-evisceration. All data were analyzed using SPSS software version 22.0 (IBM Corp, Armonk, N.Y., USA), and the data were expressed as mean ± standard deviation. The normal distribution of variables was verified using the Shapiro–Wilk.
Quantitative statistical analysis between the animal model of corneal burns injected with stem cells activated omentum or control group was performed using independent Student's t-test for parametric data and Kruskal–Wallis for nonparametric, qualitative data. P ≤ 0.05 was considered to be statistically significant.
RESULTS
Corneal neovascularization score in the control group (0.49 ± 0.12) was statistically greater than the AOC group (0.80 ± 0.20, P = 0.01) and also greater than the ADSC group (0.84 ± 0.24, P = 0.007) [Figure 4]. There was no statistically significant difference between the AOC and ADSC injected groups regarding corneal neovascularization (P = 0.66).
Figure 4.
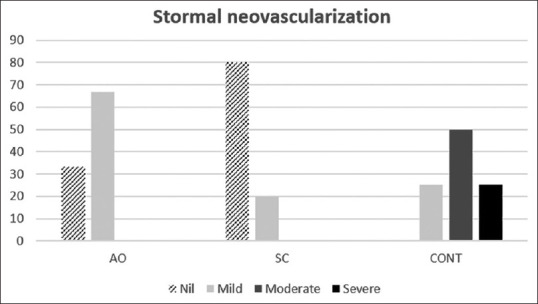
Severity of corneal neovascularization in three groups: activated omental cell, adipose tissue-derived stem cell, and CONT (control). The area of neovascularization was significantly smaller in the cell injected groups than in the control group suggesting an inhibitory effect of these kinds of cells on corneal neovascularization
Corneal scar and neovascularization were more severe in the control group compared to the ADSC group [P = 0.022 and P = 0.011, respectively, Table 1]. However, the statistical analysis failed to show a significant difference in these areas between the control and AOC groups P = 0.45].
Table 1.
Histologic findings of the corneal specimen in the aspects of corneal neovascularization, stromal scar and inflammatory cell infiltration 90 days after the injury
| Histologic findings | Number of eyes | Study groups | Near normal (%) | Mild (%) | Moderate (%) | Severe (%) |
|---|---|---|---|---|---|---|
| Neovascularization | 6 | AOC | 50 | 24 | 16 | |
| 4 | ADSC | 60 | 40 | |||
| 4 | Control | 25 | 75 | |||
| Stromal scar | 6 | AOC | 35 | 65 | ||
| 4 | ADSC | 80 | 20 | |||
| 4 | Control | 20 | 50 | 30 | ||
| Inflammatory cell infiltration | 6 | AOC | 33 | 33 | 33 | |
| 4 | ADSC | 80 | 20 | |||
| 4 | Control | 25 | 50 | 25 |
AOC: Activated omental cell, ADSC: Adipose tissue-derived stem cell
On histologic evaluation, in the control group, the severity of cellular infiltration into the stroma was significantly more compared with the AOC group (P = 0.038) and ADSC group (P = 0.018) [Figure 5], and the collagen bundles were more swollen and irregular than the cell treated groups.
Figure 5.
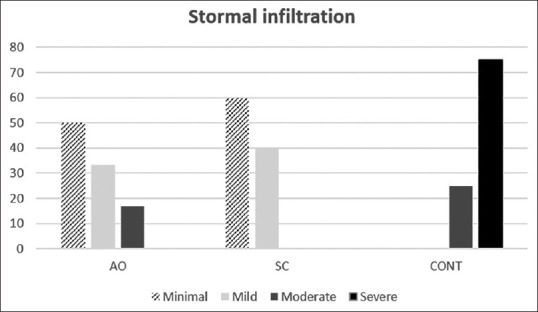
Severity of stromal infiltration in three groups: activated omental cell, adipose tissue-derived stem cell, and CONT (control)
DISCUSSION
After corneal chemical burn, inflammatory cells are recruited into the injured cornea by inflammatory mediators and chemotactic factors immediately released by the epithelial and other local cells. At 12–24 h after the original injury, the innate immune cells (such as neutrophils and macrophages) arrive in the stroma and release more inflammatory mediators.24 Therefore, inhibiting this inflammatory reaction in the early stage is critical for perfect healing.25 MSCs provide an effective pathway to suppress inflammation.
Numerous evidences show that AOCs and ADSCs have MSC properties14 and can enhance the tissue regeneration through a similar mechanism of action.26,27 MSCs are spindle-shaped cells that can rapidly expand in the culture media.28,29 Studies have shown that these cells enhance wound healing and promote tissue formation30,31 through different mechanisms. They can differentiate into other tissues such as the brain, muscle, fat, and liver,32 or even corneal epithelium.5,33,34 Gu et al. demonstrated that MSCs could differentiate into corneal epithelial-like cells, which express corneal-specific markers.33 Direct differentiation of these cells to corneal epithelial cells and even stromal cells was confirmed in other studies.34,35 In contrast, some researchers have denied the trans-differentiation hypothesis for MSCs and emphasized other properties of these cells.36,37,38
Furthermore, immunomodulatory properties of these cells are carried out by inhibiting the expression of pro-inflammatory factors such as cluster of differentiation 45+ (CD45+), interleukin -2, (IL-2), matrix metalloproteinase-2, and tumor necrosis factor-alpha.36,37,38 Another described immunomodulatory mechanism is suppressing the infiltration of adaptive CD4 + T cells and their related cytokines (IL-2, interferon-c).37 Wound healing can be promoted by these cells through supporting angiogenesis and expression of various growth factors and cytokine genes.7,36,36,37,38,39
Like other studies in this field,17,18 we observed that the corneal healing was effectively enhanced by autologous AOCs and allogeneic ADSC in comparison with the control group. Corneal neovascularization was significantly lower in the cell-injected groups, suggesting that AOCs or ADSCs were not pro-angiogenic in the corneal tissue. In other words, the microenvironment may play a critical role to determine the final designation of stem cells and cause them to secrete different kinds of hormones and growth factors, based on the nature of the tissue. Yao et al. showed that vascular endothelial growth factor levels were significantly fewer in the MSC-treated corneas than in the sham group, leading to a decrease in corneal neovascularization.
It has been shown that, after treatment of alkaline burned eyes with MSCs, the regenerated corneal epithelial cells produce a variety of anti-angiogenic factors such as angiostatin and restin that help to maintain avascularity and regression of the corneal blood vessels.40,41,42
Our study revealed that AOCs and ADSCs were equally effective in alkaline injury of the ocular surface in terms of prevention of corneal neovascularization and stromal scarring. No significant adverse effects were observed and, therefore, this modality can be applied as an effective treatment to promote healing and prevent complications in severe chemical eye injuries.
Although the autologous AOC can be prepared in a shorter period compared to allogeneic ADSC (7 days in comparison to 1 month, respectively), and the extracted AOCs are fresher than the ADSCs obtained from the culture plate, the main advantage of ADSC over AOC is that it can be harvested easily with minimal invasive surgery.
There were no significant differences in the final healing result of autologous AOCs with allogeneic ADSCs. According to previous studies, this can be due to the low expression of major histocompatibility Class II on these cells.43
Despite the ADSC group, on the histological examination, there were no statistically significant differences in the corneal scar and neovascularization between the control and AOC groups. This may be the result of two auto-eviscerated eyes that occurred in the control group that may play the role of a confounding factor which masked and underestimated the severity of the corneal scar and neovascularization in the control group. However, the possibility of clearer cornea in ADSC group due to more beneficial effects of these cells compare to AOCs could not be ruled out. This difference was not approved statistically.
This study was limited by the low number of animals, limited clinical assessment of the rats, semi-quantitative data, and nonextensive histological evaluation. Moreover, the assessment of the exact markers of AOCs and ADSCs is lacking.
In conclusion, we showed the effective use of two types of cells in the management of ocular surface burn. Further studies are required to compare the effects and possible mechanisms of action of these cells in enhancing the corneal regeneration.
Financial support and sponsorship
The present research was financially supported by a grant from Shiraz University of Medical Sciences (No. 94-01-19-9201).
Conflict of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank animal laboratory staff specially Mr. Omid Koohi for his assistance during surgeries and also taking good postoperative care of the animals. We would also like to express our appreciation for the help of Ms. Chenari in the preparation and purification of stem cells. In addtion, we would like to thank Ms. Hakimzadeh for her aid in the preparation of the pathology slides and Dr. Rousta for her contribution in statistical analysis. The authors wish to thank Dr. Nasrin Shokrpour at the Research Consultation Center of Shiraz University of Medical Sciences for her invaluable assistance in editing this manuscript.
REFERENCES
- 1.Tseng SC. Concept and application of limbal stem cells. Eye (Lond) 1989;3(Pt 2):141–57. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 2.Sharma N, Kaur M, Agarwal T, Sangwan VS, Vajpayee RB. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63:214–35. doi: 10.1016/j.survophthal.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Wagoner M, Kenyon K. Chemical injuries of the eye. Princ Pract Ophthalmol. 1994;5:234–5. [Google Scholar]
- 4.Mort RL, Douvaras P, Morley SD, Dorà N, Hill RE, Collinson JM, et al. Stem Cells and Corneal Epithelial Maintenance: Insights from the Mouse and Other Animal Models. Results Probl Cell Differ. 2012;53:357–94. doi: 10.1007/978-3-642-30406-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang TS, Cai L, Ji WY, Hui YN, Wang YS, Hu D, et al. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010;16:1304–16. [PMC free article] [PubMed] [Google Scholar]
- 6.Zannettino A, Paton S, Arthur A, Khor F, Itescu S, Gimble J, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–21. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 7.García-Gómez I, Goldsmith HS, Angulo J, Prados A, López-Hervás P, Cuevas B, et al. Angiogenic capacity of human omental stem cells. Neurol Res. 2005;27:807–11. doi: 10.1179/016164105X63674. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Kleineidam B, Kleinheinz J. Regenerative potential of human adipose-derived stromal cells of various origins. J Craniomaxillofac Surg. 2015;43:2144–51. doi: 10.1016/j.jcms.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Yao L, Bai H. Review: Mesenchymal stem cells and corneal reconstruction. Mol Vis. 2013;19:2237–43. [PMC free article] [PubMed] [Google Scholar]
- 10.Varga J, Brenner D, Phan SH. Fibrosis Research: Methods and Protocols. Springer E-Book 2005. Chicago, USA: Humana Press Inc; [Google Scholar]
- 11.Arnalich-Montiel F, Pastor S, Blazquez-Martinez A, Fernandez-Delgado J, Nistal M, Alio JL, et al. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26:570–9. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- 12.Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: Engraftment and involvement in wound healing. Eye (Lond) 2006;20:482–90. doi: 10.1038/sj.eye.6701913. [DOI] [PubMed] [Google Scholar]
- 13.Liebermann-Meffert D. The greater omentum. Anatomy, embryology, and surgical applications. Surg Clin North Am. 2000;80:275–93, xii. doi: 10.1016/s0039-6109(05)70406-0. [DOI] [PubMed] [Google Scholar]
- 14.Shah S, Lowery E, Braun RK, Martin A, Huang N, Medina M, et al. Cellular basis of tissue regeneration by omentum. PLoS One. 2012;7:e38368. doi: 10.1371/journal.pone.0038368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh AK, Patel J, Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, et al. Stromal cells cultured from omentum express pluripotent markers, produce high amounts of VEGF, and engraft to injured sites. Cell Tissue Res. 2008;332:81–8. doi: 10.1007/s00441-007-0560-x. [DOI] [PubMed] [Google Scholar]
- 16.Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G, Singh AK. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res. 2007;328:487–97. doi: 10.1007/s00441-006-0356-4. [DOI] [PubMed] [Google Scholar]
- 17.Shadmani A, Kazemi K, Khalili MR, Eghtedari M. Omental transposition in treatment of severe ocular surface alkaline burn: An experimental study. Med Hypothesis Discov Innov Ophthalmol. 2014;3:57–61. [PMC free article] [PubMed] [Google Scholar]
- 18.Bu P, Vin AP, Sethupathi P, Ambrecht LA, Zhai Y, Nikolic N, et al. Effects of activated omental cells on rat limbal corneal alkali injury. Exp Eye Res. 2014;121:143–6. doi: 10.1016/j.exer.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Pirounides D, Komnenou A, Papaioannou N, Gounari E, Stylianaki I, Alexandridis A, et al. The antiangiogenic properties of adipose-derived mesenchymal stem/stromal cells in corneal neovascularization in a rabbit model. Med Hypothesis Discov Innov Ophthalmol. 2020;9:74–84. [PMC free article] [PubMed] [Google Scholar]
- 20.Kotani T, Masutani R, Suzuka T, Oda K, Makino S, Ii M. Anti-inflammatory and anti-fibrotic effects of intravenous adipose-derived stem cell transplantation in a mouse model of bleomycin-induced interstitial pneumonia. Sci Rep. 2017;7:1–10. doi: 10.1038/s41598-017-15022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh AK, Pancholi N, Patel J, Litbarg NO, Gudehithlu KP, Sethupathi P, et al. Omentum facilitates liver regeneration. World J Gastroenterol. 2009;15:1057–64. doi: 10.3748/wjg.15.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soares JV, Leandro JJ, Cesar Júnior RM, Jelinek HF, Cree MJ. Retinal vessel segmentation using the 2-D Gabor wavelet and supervised classification. IEEE Trans Med Imaging. 2006;25:1214–22. doi: 10.1109/tmi.2006.879967. [DOI] [PubMed] [Google Scholar]
- 23.Nejabat M, Astaneh A, Eghtedari M, Mosallaei M, Ashraf MJ, Mehrabani D. Effect of honey in Pseudomonas aeruginosa induced stromal keratitis in rabbits. J Appl Anim Res. 2009;35:101–4. [Google Scholar]
- 24.Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Jr, Hong J, Lee J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–37. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 25.Nasser S, Morcos M, Gazia MA, Yousry M, Osman A. Comparative histological study on the effect of early and late administration of adipose derived stem cells on corneal alkali burn in adult male albino rats. Egypt J Histol. 2019;42:635–50. [Google Scholar]
- 26.Suga H, Glotzbach JP, Sorkin M, Longaker MT, Gurtner GC. Paracrine mechanism of angiogenesis in adipose-derived stem cell transplantation. Ann Plast Surg. 2014;72:234–41. doi: 10.1097/SAP.0b013e318264fd6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demirayak B, Yüksel N, Çelik OS, Subaşı C, Duruksu G, Unal ZS, et al. Effect of bone marrow and adipose tissue-derived mesenchymal stem cells on the natural course of corneal scarring after penetrating injury. Exp Eye Res. 2016;151:227–35. doi: 10.1016/j.exer.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Prockop DJ. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol. 2016;51:7–13. doi: 10.1016/j.matbio.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K, Ringdén O. Mesenchymal stem cells: Properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18:586–91. doi: 10.1016/j.coi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, André M, Cousin B, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29:503–10. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 32.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 33.Gu S, Xing C, Han J, Tso MO, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99–107. [PMC free article] [PubMed] [Google Scholar]
- 34.Reinshagen H, Auw-Haedrich C, Sorg RV, Boehringer D, Eberwein P, Schwartzkopff J, et al. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011;89:741–8. doi: 10.1111/j.1755-3768.2009.01812.x. [DOI] [PubMed] [Google Scholar]
- 35.Guo T, Wang W, Zhang J, Chen X, Li BZ, Li LS. Experimental study on repairing damage of corneal surface by mesenchymal stem cells transplantation. Zhonghua Yan Ke Za Zhi. 2006;42:246–50. [PubMed] [Google Scholar]
- 36.Ma Y, Xu Y, Xiao Z, Yang W, Zhang C, Song E, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24:315–21. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 37.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–55. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 38.Yao L, Li ZR, Su WR, Li YP, Lin ML, Zhang WX, et al. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7:e30842. doi: 10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda K, Falkenberg KJ, Woods AA, Choi YS, Morrison WA, Dilley RJ. Adipose-derived stem cells promote angiogenesis and tissue formation for in vivo tissue engineering. Tissue Eng Part A. 2013;19:1327–35. doi: 10.1089/ten.tea.2012.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson SL, Marcal H, Sarris M, Di Girolamo N, Coroneo MT, Wakefield D. The effect of mesenchymal stem cell conditioned media on corneal stromal fibroblast wound healing activities. Br J Ophthalmol. 2010;94:1067–73. doi: 10.1136/bjo.2009.165837. [DOI] [PubMed] [Google Scholar]
- 41.Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–48. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139–48. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 43.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic propertiesof differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]