Abstract
Purpose:
To evaluate the effects of low-level laser therapy (LLLT) on the retina with diabetic retinopathy (DR).
Methods:
Eight Wistar rats were used as a control group, and 64 rats were injected intraperitoneally with 55 mg/kg of streptozotocin to induce diabetes and served as a diabetic group. After the establishment of the DR, the rats were separated into (a) 32 rats with DR; did not receive any treatment, (b) 32 rats with DR were exposed to 670 nm LLLT for 6 successive weeks (2 sessions/week). The retinal protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, total antioxidant capacity (TAC), hydrogen peroxide (H2O2), and histological examination.
Results:
LLLT improved retinal proteins such as neurofilament (NF) proteins (200 KDa, 160 KDa, and 86 KDa), neuron-specific enolase (NSE) (46 KDa). Moreover, the percentage changes in TAC were 46.8% (P < 0.001), 14.5% (P < 0.01), 4.8% and 1.6% (P > 0.05), and in H2O2, they were 30% (P < 0.001), 25% (P < 0.001), 20% (P < 0.01), and 5% (P > 0.05) after 1, 2, 4, and 6 weeks, compared with the control. DR displayed swelling and disorganization in the retinal ganglion cells (RGCs) and photoreceptors, congestion of the capillaries in the nerve fiber layer, thickening of the endothelial cells’ capillaries, and edema of the outer segment of the photoreceptors layer. The improvement of the retinal structure was achieved after LLLT.
Conclusion:
LLLT could modulate retinal proteins such as NSE and NFs, improve the RGCs, photoreceptors, and reduce the oxidative stress that originated in the retina from diabetes-induced DR.
Keywords: Diabetic retinopathy, Hydrogen peroxide, Low-level laser therapy, Retinal protein, Total antioxidant capacity
INTRODUCTION
Diabetic retinopathy (DR) is a prominent reason for eyesight loss, especially in developed countries, and there is an extensive clinical and research concern to manage the related microvascular complication.1 The microvascular complications of DR can lead to neuron degeneration, oxidative stress, inflammation, hemodynamic changes within the retina, and interruption of the retinal function and anatomy.2,3,4,5,6
The utilizing of the long wavelengths (600–1000 nm) in the region of far-red to near-infrared light is termed low-level laser therapy (LLLT), low-intensity laser therapy, low-power laser therapy, cold laser, soft laser, photobiostimulation, and photobiomodulation (PBM).7,8 This wavelength permits high tissue penetration required for wound healing as in diabetic ulcer, reduction in neurologic neck pain, optical nerve recovery, central nervous system (CNS) injury, and after ischemic stroke.9,10
Moreover, it was involved in treating a wide range of eye diseases such as retinopathy of prematurity, age-related macular degeneration, and possibly other eye diseases.11 Fortunately, the previous studies in animals with streptozotocin-induced diabetes and diabetic humans have detected beneficial effects of LLLT to alleviate the progression of DR. These included a marked decline in ganglion cell death, a 50% enhancement in the amplitude of photopic b wave ERG, reduction of the leukostasis on the retinal vessels, and inhibition of oxidative stress.12,13,14
Neurofilaments (NFs) are the cytoskeletal component of the mature neurons in the retina formed of three subunits with molecular weights of 68 KDa, 160 KDa, and 200 KDa.15,16 Their immunoreactivity has been described in the axons and the processes of retinal ganglion cells (RGCs).16 The anomalous expression, processing, and structure of NFs are regarded as an etiologic factor in DR. Indeed, any alteration in the synthesis of these proteins could lead to severe damages in axon structure and function.15,16 Neuron-specific enolase (NSE) is a soluble protein present in the interphotoreceptor matrix that lies between the retinal pigmented epithelium (RPE) and the neural retina and contains arrays of proteins and glycoproteins. NSE appears as a single protein fraction at 46 KDa when subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). It was considered a survival factor for photoreceptors neurons. Elevation in NSE was considered a robust biomarker of DR.17
The present study assesses the effects of LLLT on retinal protein structure, total antioxidant capacity (TAC), hydrogen peroxide (H2O2), and different retinal layers after diabetes-induced DR.
METHODS
This study was done per the guides of the National Institutes of Health, the Association for Research in Vision and Ophthalmology and with approval of the Institutional Animal Care and Use Committee at Cairo University for the use of laboratory animals in ophthalmic and vision research. Animals were housed and maintained in a standard laboratory lighting cycle (12 h light-12 h dark) with a balanced diet and free access to water at a temperature of 25°C ± 2°C.
Experimental animals
Seventy-two Wistar rats weighing 200 ± 20 g and 10 ± 12 weeks age were involved in this study. All rats’ eyes were examined by slit-lamp biomicroscope before induction of diabetes. The results exhibited no signs of any intraocular or periocular infection or intraocular inflammation such as infectious conjunctivitis, infectious blepharitis, keratitis, scleritis, and endophthalmitis, in all eyes. Eight rats were considered a control group (did not receive any treatment), and 64 rats were injected intraperitoneally with a single dose of 55 mg/kg of streptozotocin (C8H15N3O7, Sigma Aldrich, USA) in 0.1 M freshly prepared citrate buffer pH = 4.4. The blood glucose concentration was measured after 24 h by using a rapid blood glucose test (Accu-Check, Roche Diabetes Care, Inc., Indianapolis; USA). The diabetic group was followed up for 8 weeks until the establishment of DR. Rats with blood glucose concentration exceeded 280 ± 50 mg/dl were designated as diabetic.
The DR group was then divided into two subgroups: (a) DR group (n = 32 rats); did not receive any treatment and divided into four subgroups (n = 8 rats) and euthanized after 1, 2, 4, and 6 weeks (b) DR group (n = 32 rats) was exposed to LLLT for 6 successive weeks.
Low-level laser therapy
Thirty-two rats with DR were anesthetized by intramuscular injection with 45 mg/kg ketamine hydrochloride (Ketalar®, Sandoz Canada Inc.) mixed with was 21 mg/kg pentobarbital sodium (Rematal®Sodium Taj Pharmaceuticals Limited, India). The low-level laser (670 nm) was generated from a continuous-wave diode-pumped solid-state laser (Cobolt, DPSSL-DRIVER II, China) (2 sessions/week, 3 days apart, for successive 6 weeks) and calibrated to deliver a beam intensity of 5 mW/cm2, for 90 s using a perpendicular laser prop with a spot size of 3 mm and a convex lens for beam focusing on the central retina. The distance between the laser prop and rat eye was ~7–9 cm. The weekly radiant exposure energy (energy density) was thus 900 mJ/cm2 or (~1 J/cm2) for each eye (n = 64 eyes). The rats were divided into four subgroups with n = 8 animals per group and euthanized successively after 1, 2, 4, and 6 weeks of starting LLLT.
Retinal protein extraction
The rats from each subgroup (n = 8; 16 eyes) were decapitated under anesthesia after the estimated periods. The eye globes were enucleated and immersed in phosphate-buffered saline (PBS) on ice. Ten eyes were cut at the posterior edge of the limbus and the cornea, while the remaining eyes (n = 6) were kept for histological examination. The retinas were removed, sliced on ice, and immersed in liquid nitrogen. Each 5 mg of tissue was homogenized with 300 μl protein lysis buffer (RIPA) with protease inhibitor (Sigma-Aldrich, Inc., St. Louis, MO) and then placed on ice for 45 min. The sample was sonicated for 20 s and then centrifuged for 20 min at 12,000 rpm. The supernatant was separated for SDS-PAGE, TAC, and H2O2analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Retinal protein composition was analyzed using SDS-PAGE using 3% stacking gel and 12% separating gel.18 The gel was scanned using a scanner model SG-800 imaging densitometer (Bio-Rad Laboratories Inc., USA).
Measurement of total antioxidative capacity
The total antioxidative capacity was determined by the interaction of antioxidants in 20 μl of the supernatant of the retina's samples with 0.5 ml of H2O2(0.1%) as a substrate. The antioxidants presented in the retinal samples remove a defined concentration of H2O2, and the remaining H2O2 is enzymatically determined by the change of 3, 5, dichloro-2-hydroxy benzene sulfonate (0.5 ml) into a colored product measured at 505 nm using a spectrophotometer (Thermo Fisher Scientific, Madison WI 53711, USA, Evo 600).19
Measurement of hydrogen peroxide concentration
The H2O2 present in 50 μl of the supernatant will react with 0.5 ml of a chromogen (1.0 mM/L of 3,5 dichloro-2-hydroxy benzene sulfonate in 100 mM/L of phosphate buffer, pH 7.0) and 2 mM/L of 4-amino antipyrin (0.5 ml) in the presence of peroxidase (>2000 U/L) to form a colored complex that is measured at 510 nm and represented the total H2O2 molecules present in the sample.20
Histological examination
The rats’ eyes (n = 6 eyes/subgroup) were enucleated and injected at the corneoscleral junction with 4% glutaraldehyde in 0.1 M PBS (pH 7.4) containing 5.4% glucose at 4°C. After 30 min, the posterior chamber of the eye containing the retina was immersed in fresh prepared glutaraldehyde buffered solution. After half an hour, the posterior part of the eye was dissected into sections (about 1 mm3) and then further fixed for 8 h with fresh glutaraldehyde buffered solution (pH 7.4).
The sections were washed for 1 h with several changes of PBS at 4°C, fixed in 1.33% osmium tetroxide, dehydrated in cold ethanol grads (50%, 70%, 80%, 90%, and 96%), and then embedded in freshly prepared Araldite CY212 mixtures. Semithin sections were cut (about 1.0 μm) by ultratome (LKB Produkter, Sweden), fitted on glass slides, and stained with toluidine blue for light microscope examination.
Statistical evaluation
The result was specified as mean ± standard deviation, and the variations between different groups were analyzed employing a commercially available software package (SPSS 11 for Windows; SPSS Inc., Chicago, Illinois, USA). A one-way analysis of variance and Student's t-test was used for comparing the control with different groups. The result was considered significant at a P < 0.05.
RESULTS
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis for retinal proteins
The SDS-PAGE scanning patterns for retinal proteins of the control, DR, and DR after 1 week of starting the LLLT (2 sessions/week) are illustrated in Figure 1. The retinal protein for the control group was separated into nine fractions varies on their molecular weights and intensities. The DR group was characterized by shifting of the two fractions at 260 KDa and 200 KDa towards the high molecular weight region with broadening and increase in the intensity of the fraction at 160 KDa. In addition, pronounced changes in the molecular weight region between 87 KDa and 15 KDa, especially the two peaks at 68 KDa and 46 KDa, showed an increment in their intensities that decreased slightly after LLLT. By extending the DR to 2 weeks [Figure 2], the fraction at 260 KDa was separated into two fractions (285 KDa and 250 KDa), and the two fractions at 200 KDa and 68 KDa disappeared. Moreover, there were marked increases in intensities of the three fractions at 160 KDa, 99 KDa, and 46 KDa which noticeably declined after LLLT (4 sessions).
Figure 1.
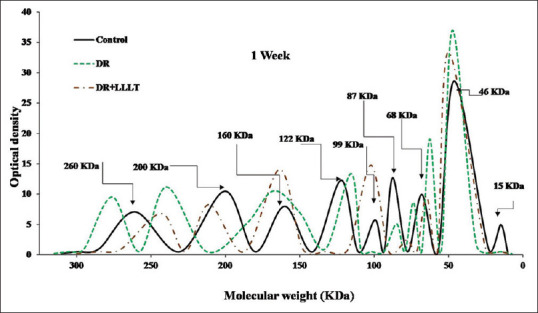
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis scanning pattern of the retinal protein after 1 week for the control, diabetic retinopathy (DR) group, and DR group exposed to low level laser therapy
Figure 2.
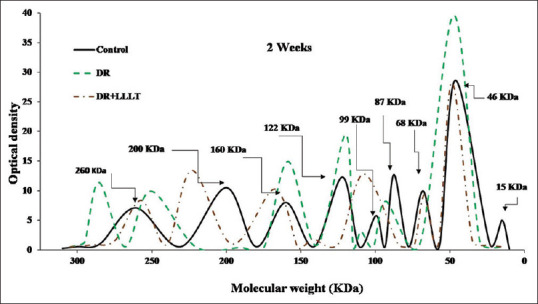
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis scanning pattern of the retinal protein after 2 weeks for the control, diabetic retinopathy (DR) group, and DR group exposed to low level laser therapy
Furthermore, after 4 weeks [Figure 3], the DR group was characterized by splitting of the peak at 260 KDa, an increase in the intensities at 160 KDa, 68 KDa, and 46 KDa. At the same time, the group of LLLT (8 sessions) showed noticeable improvement with a slight increase in the intensities and shift in the molecular weights of some fractions. Moreover, after 6 weeks [Figure 4], the DR group was characterized by severe alteration in all protein fractions. The fraction at 260 KDa was split into two peaks at 268 KDa and 229 KDa in addition to a marked increase in the intensity of the fraction at 160 KDa and 46 KDa. In contrast, all over the scanning pattern showed noticeable improvement in all fractions after LLLT (12 sessions).
Figure 3.
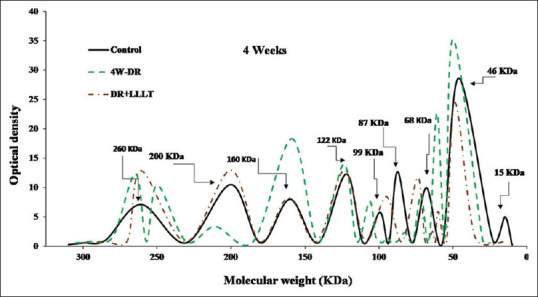
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis scanning pattern of the retinal protein after 4 weeks for the control, diabetic retinopathy (DR) group, and DR group exposed to low level laser therapy
Figure 4.
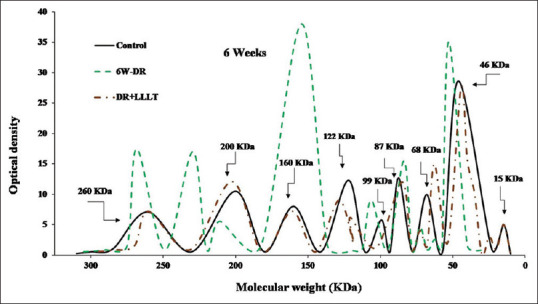
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis scanning pattern of the retinal protein after 6 weeks for the control, diabetic retinopathy (DR) group, and DR group exposed to low level laser therapy
Total antioxidant capacity
Figure 5 represents the TAC in the retina with DR and after exposure to LLLT compared with the control group. The DR group showed a highly significant increase (P < 0.001) in the TAC after 1 week and 2 weeks with a percentage change of 79.0% and 45.2%. These increments were followed by noticeable decreases after 4 weeks and 6 weeks with percentage changes of 24.2% (P < 0.01) and −40.3% (P < 0.001) compared with the control [Table 1]. Moreover, LLLT of the retina showed a significant increase in TAC after 1 week (P < 0.001) and 2 weeks (P < 0.01) with percentage changes of 46.8% and 14.5%, respectively. Furthermore, there were nonsignificant changes in TAC after 4 weeks and 6 weeks with a percentage change of 4.8% and 1.6% (P > 0.05) as compared with the control.
Figure 5.
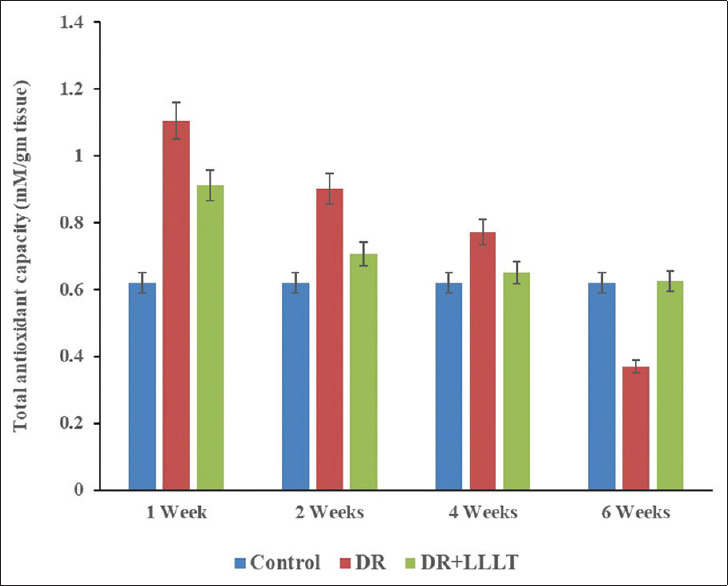
Total antioxidant capacity of the retinal protein after 1, 2, 4, and 6 weeks for the control, diabetic retinopathy (DR) group, and DR group exposed to low level laser therapy
Table 1.
Total antioxidant capacity and hydrogen peroxide concentration for the control, diabetic retinopathy (DR) and DR exposed to low level laser therapy
| Groups | Periods (week) | TAC (mM/g tissue) | Percentage change (%) | H2O2 (mM/g tissue) | Percentage change (%) |
|---|---|---|---|---|---|
| Control | 0.62±0.3 | 0.20±0.01 | |||
| DR | 1 | 1.11±0.05* | 79.0 | 0.27±0.03* | 35 |
| 2 | 0.90±0.03* | 45.2 | 0.40±0.02* | 100 | |
| 4 | 0.77±0.03* | 24.2 | 0.41±0.02* | 105 | |
| 6 | 0.37±0.02* | −40.3 | 0.51±0.03* | 155 | |
| DR+LLLT | 1 | 0.91±0.02*** | 46.8 | 0.26±0.02*** | 30 |
| 2 | 0.71±0.03*** | 14.5 | 0.25±0.01*** | 25 | |
| 4 | 0.65±0.02** | 4.8 | 0.24±0.02*** | 20 | |
| 6 | 0.63±0.03** | 1.60 | 0.21±0.01** | 5 |
*Significant difference when compared with the control group, **Significant difference when compared with DR group, ***Significant difference when compared with both control and DR group. The values are expressed in mean±SD. TAC: Total antioxidant capacity, DR: Diabetic retinopathy, LLLT: Low-level laser therapy, H2O2: Hydrogen peroxide, SD: Standard deviation
Hydrogen peroxide concentration
The concentrations of H2O2 in the retinal samples for the control, DR group, and LLLT group are illustrated in Figure 6. The data showed a highly significant elevation (P < 0.001) in the H2O2 concentration in the retina of the DR group with percentage changes of after 35%, 100%, 105%, and 155% after 1, 2, 4, and 6 weeks, respectively [Table 1] compared to the control. In addition, LLLT significantly improved H2O2 concentration with percentage changes of 30% (P < 0.001), 25% (P < 0.001), 20% (P < 0.01), and 5% (P >0 0.05) after 1, 2, 4, and 6 weeks, respectively, compared with the control [Table 1].
Figure 6.
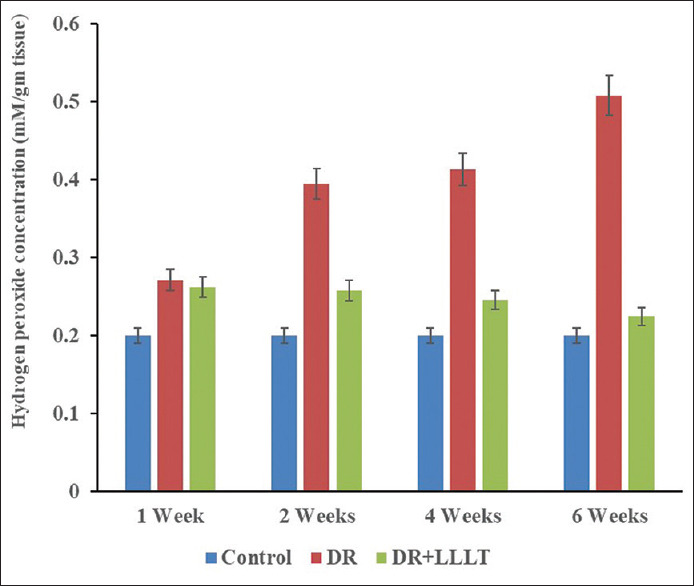
Hydrogen peroxide concentration of the retinal protein after 1, 2, 4, and 6 weeks for the control, diabetic retinopathy (DR) group, and DR group exposed to low level laser therapy
Histological analysis
Light micrograph of the control retina [Figure 7a] showed normal retinal layers without any changes. Histological examination after 1 week of DR [Figure 7b] revealed the presence of severe edema in the cytoplasm of the RPE layer and swelling in the RGCs. After LLLT [Figure 7c], the retinal layer showed some edema in the RPE, fragmentation of nuclear chromatin of the cell bodies in the inner nuclear layer (INL), and swelling in the RGCs with intact cytoplasm. After 2 weeks, the retina with DR [Figure 7d] showed congestion of the retinal capillaries in the nerve fiber layer (NFL), thickening of the lining endothelial cells capillaries, and swelling of the RGCs. Two weeks after LLLT [Figure 7e], there was revealed improvement in the RPE layer and INL, but the swelling of RGCs still persisted. Moreover, there was a thickened basement membrane of the capillaries in the NFL. The histological follow-up after 4 weeks of DR [Figure 7f] displayed edema in-between the outer segments of the photoreceptors layer (PRL), disorganization of the photoreceptors, and swelling of RGCs with a migration of the chromatin. Furthermore, histological examination revealed unchanged retinal layers after treatment with LLLT [Figure 7g]. After 6 weeks [Figure 7h], the retina with DR showed edema of the outer segment of PRL, edema of the outer plexiform layer and thick-wall capillary in the outer plexiform layer. In addition, many ganglion cell nuclei showed migration of chromatin and scanty cytoplasm while after LLLT [Figure 7i], the retinas showed improvement in all layers and no deviation from the control.
Figure 7.
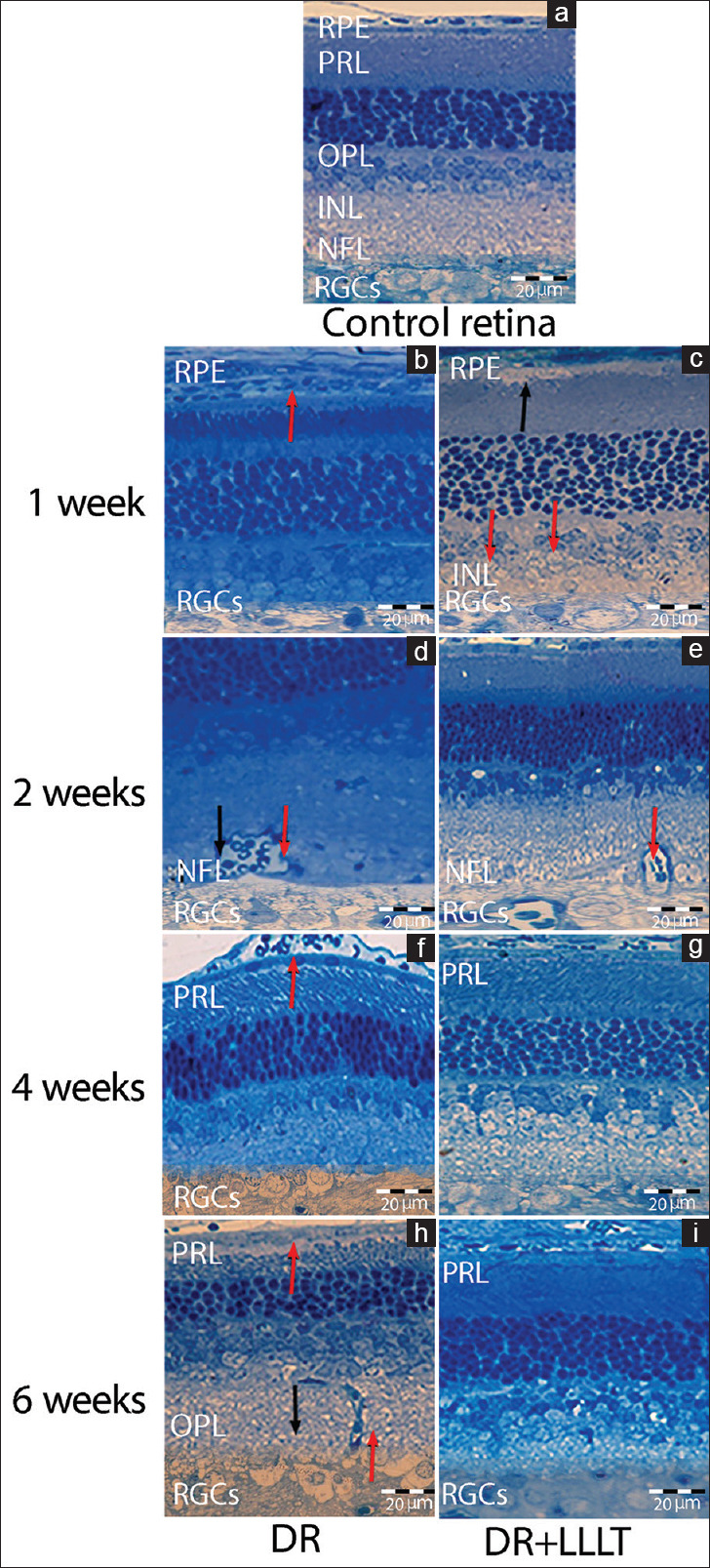
(a) Light micrograph of the control retina. After 1 week: (b) Diabetic retinopathy (DR) showed severe edema in the cytoplasm of retinal pigmented epithelium (RPE) (red arrow) and swelling in the retinal ganglion cells (RGCs), (c) DR group exposed to low level laser therapy (DR + LLLT) showed edema in the RPE (black arrow), fragmentation in the inner nuclear layer (red arrows), swelling in the RGCs. Two weeks: (d) DR showed congestion of the retinal capillaries in the nerve fiber layer (NFL), thickening of the lining endothelial cells capillaries (black arrow) and swelling of the RGCs (red arrow), (e) DR + LLLT showed swelling in RGCs and the capillaries of NFL (red arrow). Four weeks: (f) DR showed edema and disorganization in the photoreceptors layer (PRL), swelling of RGCs, (g): DR + LLLT showed normal retina. Six weeks: (h) DR showed edema of the outer segment of PRL (red arrow), outer plexiform layer (black arrow), with thick-wall capillary (red arrow) and scanty cytoplasm of RGCs (i) DR + LLLT showed normal retina. (Toluidine blue, scale bar: 20 μm)
DISCUSSION
DR has been previously defined as a neurovascular disorder that is characterized by the presence of defects in the retinal vessels, retinal neurons, amacrine cells, PRL, and RGCs.21,22 The increase in oxidative stress due to blood sugar elevation leads to neuronal dysfunction, vascular leakage, and mitochondrial damage.21,23,24,25 Previous experiments on rats with short-term diabetes reported retinal protein changes, and early treatment is necessary.26 In diabetic albino rats, whole-body exposure to 670 nm alleviated retinal neuron degeneration that was contributed to DR.12 Besides, the beneficial effect of LLLT was demonstrated in patients with noncenter-involving diabetic macular edema.13 Moreover, PBM induces retinal protection against abnormalities induced by diabetes in pigmented animals.1
In the current study, rats with diabetes-induced DR were exposed to 670 nm, and retinal protein composition, oxidative stress (TAC and H2O2), along with retinal histology were evaluated.
Analysis of retina protein with SDS-electrophoresis was documented previously for the investigation of different pathological conditions.27,28,29 However, there is a lack of literature concerned with the analysis of retinal protein with SDS-PAGE in the case of DR and LLLT. In the present work, the control retina's electrophoretic pattern reveals the presence of three different characteristic fractions that appeared at 200 KDa, 160 kDa and 68 kDa, termed as NFs corresponded to the main intermediate filaments in mature neurons.16 These NFs are described as NF-High, NF-Middle, and NF-Low and polymerize in the neuronal soma to form the so-called NF-triplet of 10 nm heteropolymer filaments. NFs run parallel along the axon's length, serving primarily as a structural function and are correlated with axonal caliber.30,31 Furthermore, NFs perform a critical role in the dynamics, functions, and vitality of the neural cytoskeleton.32 NFs have been detected mainly in the axons and processes of RGCs but undetected in photoreceptors.33,34 Moreover, the abnormal configuration of NFs in the soma and dendrites of some RGCs can produce neuronal degeneration and cell death.35 In the retina, NFs change in DR by accumulation in axonal swellings, which may be related to the changes in retrograde axonal transport. NF phosphorylation is increased by mild glutamate toxicity in cerebellar granule cells, suggesting that this is a potential mechanism of neurotoxicity in diabetes.36
In agreement with these data,32,33,34,35,36 our study detected a pronounced change in the molecular weights and intensities at 200 KDa, 160 KDa, and 68 KDa during the progression of DR indicating the abnormal composition of NFs triplet fractions. In addition, swelling in the RGCs after 1, 2, and 4 weeks and migration of the nucleic chromatin were detected by histological examination as shown in Figure 7b, d, f and i reflecting severe RGCs damage. Furthermore, these changes in the DR group have an adverse effect on RGCs’ survival and may induce apoptosis.37
Furthermore, the electrophoretic pattern showed another protein band at molecular weight 46 KDa that can be employed to indicate neuronal damage and cone dysfunction. This protein fraction was identified as NSE.17 Numerous studies have correlated with the elevated NSE levels in serum and cerebrospinal fluid in CNS-related conditions as in cases of stroke, head trauma, and sclerosis.38,39 However, two limited studies have demonstrated an increase in the synthesis and release of NSE in the serum of patients with DR when compared to non-DR patients and may be used as a sign for the incidence of DR.40,41 In addition, the elevated level of NSE in vitreous humor is thought to indicate photoreceptors damage and neuron degeneration.42 Despite these findings, studies on the relationship between NSE level and DR are rather limited within the literature.38,41 Generally, all these previous findings are in concordance with our results, which indicated that NSE intensity (at 46 KDa) of the DR group were significantly elevated than those of the control group all over the follow-up periods.17,40,41 The elevation of NSE levels in retinal samples revealed retinal neuronal injury, cone dysfunction, and photoreceptors damage. This extra release of NSE in the retinal sample may be interpreted as a marker for retinal neuron distresses and as a response of neuroprotection.43 Moreover, the histological examination of the retina with DR mainly after 4 and 6 weeks, showed edema and disorganization in the photoreceptor layer, as shown in Figure 7f and h.
Oxidative stress is the most important mechanism that has been responsible for diabetic end-organ damage. TAC and H2O2 levels have been used to indicate the relation between oxidative stress and tissue damage. Oxidative stress occurs when the equilibrium between antioxidants and oxidants is shifted in the preference of the latter. Oxidation has a critical role in cellular metabolism to maintain cell survival and activity.44 During oxidation, reactive oxygen species (ROS) are produced and normally scavenged by endogenous antioxidative systems.45 However, if antioxidant defenses are deficient, dysfunction and apoptosis of protein, lipids, and DNA will occur.45
Some studies have demonstrated increased antioxidants levels associated with DR.46 On the other hand, reduced levels of antioxidants have been observed in the retina of diabetic rats.25 In this regard, our data showed an elevated level of total retinal antioxidants after the 1st and 2nd weeks of DR (P < 0.001), followed by a severe reduction after the 4th (P < 0.01) and 6th weeks (P < 0.001). Alternatively, these elevations and reductions in TAC may be responsible for the alteration that occurred in retinal protein and histology.
It is well known that H2O2 considerably increases in vitreous humor samples of cases with DR.42,47 Consequently, our results agree with these findings as H2O2 dramatically increased in the retinal samples of the DR groups, suggesting the involvement of oxidative stress in the pathogenesis of DR that leads to neuron damage produced by rod cells.48 Another explanation for our findings could be that as H2O2 increases after the first 2 weeks, TAC is upregulated. However, after the 4th and 6th weeks, there is an exhaustion of these protective mechanisms.
It was believed that LLLT had healing effects on nerves by promoting photoreceptors.49 These photoreceptors have a capability for absorbing red to near-infrared light and triggering a metabolic reaction inside the mitochondria that is regulated by one of the respiratory chain enzymes termed as cytochrome C oxidase enzyme.50 The cytochrome C oxidase could absorb light photons, generate ROS, and adenosine triphosphate (ATP), causing an increase in cell respiration.14,51,52
Most of the retinal mitochondria are located at the inner segment layer of retinal photoreceptors. Thus, mitochondrial cytochrome C oxidase is believed to be a target of LLLT.53,54 Therefore, the biostimulation effect of LLLT is due to low energy deposited into tissues results in anti-inflammatory effects by upregulating mitochondrial ATP and improvement of the cell vitality.55,56,57 Some new data have shown low-level laser radiation can initiate many cellular bioactivities, such as proliferation, differentiation, and apoptosis.58,59,60 Of note, one previous report did not support the involvement of retinal photoreceptors in rats with DR.12 Their findings suggested that LLLT did not prevent diabetes-induced defects in ions movement in retinal photoreceptors and suggested the occurrence of beneficial effects that are independent of the mitochondrial photoreceptor. Our results contradict this study since NSE was elevated during the progression of the DR as shown in Figures 1–4. Throughout the development of DR, some biochemical alterations related to ischemia or hypoxia-induced oxidative stress may ensue in the retina. The oxidative stress deactivates many glycolytic enzymes, as well as NSE catalyzing the conversion of 2–phosphoglycerate into phosphoenolpyruvate, in neurons.61 The glycolytic enzymes are upregulated to compensate the high energy requirements for the survival of the neurons under the conditions of ischemia or hypoxia.62 Moreover, neurodegeneration is almost concomitant with neuroregeneration, specifically in the early stage, and this may explain why the edema and disorganization of photoreceptors did not appear after week 1 and week 2. In addition, as a result of chronic exposure to stress, NSE is readily leaked from the neurons into the blood.63 This also may explain the constant level of NSE in all DR groups, as illustrated in Figures 1–4. Furthermore, another previous work revealed a decline in a-wave amplitude values that begins from photoreceptors (rods and cones) and progressive decreases in the b-wave in experimental rats with DR suggested functional modifications and retinal ischemia. Consequently, the change in the b/a ratios is believed to be useful in evaluating the loss of retinal function.64
Furthermore, the improvement in NSE and retinal photoreceptors after LLLT indicating the contribution of mitochondrial photoreceptor in the process of healing by increasing the production of ATP in the mitochondria and proliferation of various cell type.56
On the other hand, the histological examination for the DR group revealed alteration in the retinal capillaries of the nerve NFL, thickening of the lining endothelial cells capillaries [Figure 7d], and thick-wall capillary in the outer plexiform layer [Figure 7h]. Generally, hyperglycemia-induce thickening of the basement membrane and increasing the vascular permeability and disruption of the blood-retinal barrier (BRB). This disruption of BRB causes an accumulation of extracellular fluid in the retina, edema, and neuronal cell loss.36,65 In DR, retinal capillary nonperfusion leads to tissue hypoxia and autoregulatory congestion of arterioles, and pressure reduction in the arterioles. Arteriolar congestion decreases the resistance to flow. Consequently, the hydrostatic pressure in the capillaries and venules is increased, leading to increased leakage of fluid from the intravascular compartment into the interstitial tissue compartment as well as widening and elongation of the vessels.56 Furthermore, LLLT stimulates microcirculation, which results in the improvement of capillary hydrostatic pressure, which in turn results in edema absorption and elimination of intermediary metabolites.56
Limitation of the study
The enzymatic activity, protein-binding interactions, detection of protein cofactors, and the quantitative measurements of different protein fractions cannot be determined on proteins isolated by SDS-PAGE due to the denaturation of proteins before electrophoresis. Instead, another method such as 2-DE techniques must be used to separate native proteins for investigations of structure-function relationships. Further study to compare the changes in NSE and NFs proteins quantitively may be helpful to confirm this work in particular. In addition, the effect of hyperglycemia on the DR groups and the elevation in death rate prevent the extension in the experiment time to >6 weeks.
In conclusion, according to the results obtained from the analysis of retinal protein structure, oxidative stress, and histological examination, the NFs and NSE proteins may be potent markers that are intimately related to DR and define much about RGCs and PRCs damage. In addition, our findings afford evidence of the beneficial impact of 670 nm light therapy by improving the retinal protein structure, anatomical abnormalities, and the oxidative status originated in the retina from diabetes-induced DR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Saliba A, Du Y, Liu H, Patel S, Roberts R, Berkowitz BA, et al. Photobiomodulation mitigates diabetes-induced retinopathy by direct and indirect mechanisms: Evidence from intervention studies in pigmented mice. PLoS One. 2015;10:e0139003. doi: 10.1371/journal.pone.0139003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunce C, Wormald R. Causes of blind certifications in England and Wales: April 1999-March 2000. Eye (Lond) 2008;22:905–11. doi: 10.1038/sj.eye.6702767. [DOI] [PubMed] [Google Scholar]
- 3.Jackson GR, Barber AJ. Visual dysfunction associated with diabetic retinopathy. Curr Diab Rep. 2010;10:380–4. doi: 10.1007/s11892-010-0132-4. [DOI] [PubMed] [Google Scholar]
- 4.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 5.Guzman DC, Olguan HJ, Garcan EH, Peraza AV, de la Cruz DZ, Soto MP. Mechanisms involved in the development of diabetic retinopathy induced by oxidative stress. Redox Rep. 2017;22:10–6. doi: 10.1080/13510002.2016.1205303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015;33:183–4. doi: 10.1089/pho.2015.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heiskanen V, Hamblin MR. Correction: Photobiomodulation: Lasers vs. light emitting diodes? Photochem Photobiol Sci. 2018;18:259–259. doi: 10.1039/c8pp90049c. [DOI] [PubMed] [Google Scholar]
- 9.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, et al. Infrared laser therapy for ischemic stroke: A new treatment strategy: Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–9. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald M, Hodgetts S, Van Den Heuvel C, Natoli R, Hart NS, Valter K, et al. Red/near-infrared irradiation therapy for treatment of central nervous system injuries and disorders. Rev Neurosci. 2013;24:205–26. doi: 10.1515/revneuro-2012-0086. [DOI] [PubMed] [Google Scholar]
- 11.Geneva II. Photobiomodulation for the treatment of retinal diseases: A review. Int J Ophthalmol. 2016;9:145–52. doi: 10.18240/ijo.2016.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang J, Du Y, Lee CA, Talahalli R, Eells JT, Kern TS. Low-intensity far-red light inhibits early lesions that contribute to diabetic retinopathy: In vivo and in vitro. Invest Ophthalmol Vis Sci. 2013;54:3681–90. doi: 10.1167/iovs.12-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang J, Herda AA, Kern TS. Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br J Ophthalmol. 2014;98:1013–5. doi: 10.1136/bjophthalmol-2013-304477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YY, Nagata K, Tedford CE, McCarthy T, Hamblin MR. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J Biophotonics. 2013;6:829–38. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Q, Xie F, Siedlak SL, Nunomura A, Honda K, Moreira PI, et al. Biomedicine and diseases: Review neurofilament proteins in neurodegenerative diseases. Cell Mol Life Sci. 2004;61:3057–75. doi: 10.1007/s00018-004-4268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Ederra J, Garcra M, Hicks D, Vecino E. Comparative study of the three neurofilament subunits within pig and human retinal ganglion cells. Mol Vis. 2004;10:83–92. [PubMed] [Google Scholar]
- 17.Li A, Lane WS, Johnson LV, Chader GJ, Tombran-Tink J. Neuron-specific enolase: A neuronal survival factor in the retinal extracellular matrix? J Neurosci. 1995;15:385–93. doi: 10.1523/JNEUROSCI.15-01-00385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–61. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 21.Antonetti DA, Barber AJ, Bronson SK, Freeman WM, Gardner TW, Jefferson LS, et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes. 2006;55:2401–11. doi: 10.2337/db05-1635. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007;13:2699–712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- 23.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 24.Garhofer MG, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004;88:887–91. doi: 10.1136/bjo.2003.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quin G, Len AC, Billson FA, Gillies MC. Proteome map of normal rat retina and comparison with the proteome of diabetic rat retina: New insight in the pathogenesis of diabetic retinopathy. Proteomics. 2007;7:2636–50. doi: 10.1002/pmic.200600486. [DOI] [PubMed] [Google Scholar]
- 27.Darwish ST, Mohalal ME, Helal MM, El-Sayyad HI. Structural and functional analysis of ocular regions of five marine teleost fishes (Hippocampus hippocampus, Sardina pilchardus, Gobius niger, Mullus barbatus & Solea solea) Egy J Basic App Sci. 2015;2:159–66. [Google Scholar]
- 28.Funke S, Perumal N, Beck S, Gabel-Scheurich S, Schmelter C, Teister J, et al. Glaucoma related Proteomic Alterations in Human Retina Samples. Sci Rep. 2016;6:29759. doi: 10.1038/srep29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Marco S, Carnicelli V, Franceschini N, Di Paolo M, Piccardi M, Bisti S, et al. Saffron: A multitask neuroprotective agent for retinal degenerative diseases. Antioxidants (Basel) 2019;8:224. doi: 10.3390/antiox8070224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- 31.Nixon RA, Paskevich PA, Sihag RK, Thayer CY. Phosphorylation on carboxyl terminus domains of neurofilament proteins in retinal ganglion cell neurons in vivo: Influences on regional neurofilament accumulation, interneurofilament spacing, and axon caliber. J Cell Biol. 1994;126:1031–46. doi: 10.1083/jcb.126.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capano PC, Pernas-Alonso R, di Porzio U. Neurofilament homeostasis and motoneuron degeneration. Bioassays. 2001;23:24–33. doi: 10.1002/1521-1878(200101)23:1<24::AID-BIES1004>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 33.Straznicky C, Vickers JC, G ckers R, Costa M. A neurofilament protein antibody selectively labels a large ganglion cell type in the human retina. Brain Res. 1992;582:123–8. doi: 10.1016/0006-8993(92)90325-4. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Dong J, Cull G, Fortune B, Cioffi GA. Varicosities of intraretinal ganglion cell axons in human and nonhuman primates. Invest Ophthalmol Vis Sci. 2003;44:2–9. doi: 10.1167/iovs.02-0333. [DOI] [PubMed] [Google Scholar]
- 35.Dieterich DC, Trivedi N, Engelmann R, Gundelfinger ED, Gordon-Weeks PR, Kreutz MR. Partial regeneration and long-term survival of rat retinal ganglion cells after optic nerve crush is accompanied by altered expression, phosphorylation and distribution of cytoskeletal proteins. Eur J Neurosci. 2002;15:1433–43. doi: 10.1046/j.1460-9568.2002.01977.x. [DOI] [PubMed] [Google Scholar]
- 36.Barber AJ. A new view of diabetic retinopathy: A neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–90. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet D, Garcia M, Vecino E, Lorentz JG, Sahel J, Hicks D. Brain-derived neurotrophic factor signalling in adult pig retinal ganglion cell neurite regeneration in vitro. Brain Res. 2004;1007:142–51. doi: 10.1016/j.brainres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Yan M, Zhang Y, Xie M, Yan L, Chen J. Serum neuron-specific enolase is elevated as a novel indicator of diabetic retinopathy including macular oedema. Diabet Med. 2015;32:102–7. doi: 10.1111/dme.12597. [DOI] [PubMed] [Google Scholar]
- 39.Qian J, Herrera JJ, Narayana PA. Neuronal and axonal degeneration in experimental spinal cord injury: In vivo proton magnetic resonance spectroscopy and histology. J Neurotrauma. 2010;27:599–610. doi: 10.1089/neu.2009.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haque A, Polcyn R, Matzelle D, Banik NL. New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci. 2018;8:33. doi: 10.3390/brainsci8020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim GH, Kim JE, Rhie SJ, Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. 2015;24:325–40. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asadova V, Gul Z, Buyukuysal RL, Yalcinbayir O. Assessment of neuron-specific enolase, S100B and malondialdehyde levels in serum and vitreous of patients with proliferative diabetic retinopathy. Int Ophthalmol. 2020;40:227–34. doi: 10.1007/s10792-019-01175-9. [DOI] [PubMed] [Google Scholar]
- 43.Dunker S, Sadun AA, Sebag J. Neuron specific enolase in retinal detachment. Curr Eye Res. 2001;23:382–5. doi: 10.1076/ceyr.23.5.382.5446. [DOI] [PubMed] [Google Scholar]
- 44.Williams M, Hogg RE, Chakravarthy U. Antioxidants and diabetic retinopathy. Curr Diab Rep. 2013;13:481–7. doi: 10.1007/s11892-013-0384-x. [DOI] [PubMed] [Google Scholar]
- 45.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izuta H, Matsunaga N, Shimazawa M, Sugiyama T, Ikeda T, Hara H. Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol Vis. 2010;16:130–6. [PMC free article] [PubMed] [Google Scholar]
- 47.Mandal LK, Choudhuri S, Dutta D, Mitra B, Kundu S, Chowdhury IH, et al. Oxidative stress-associated neuroretinal dysfunction and nitrosative stress in diabetic retinopathy. Can J Diabetes. 2013;37:401–7. doi: 10.1016/j.jcjd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci U S A. 2013;110:16586–91. doi: 10.1073/pnas.1314575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dias FJ, Fazan VP, Cury DP, de Almeida SR, Borie E, Fuentes R, et al. Growth factors expression and ultrastructural morphology after application of low-level laser and natural latex protein on a sciatic nerve crush type injury. PLoS One. 2019;14:E0210211. doi: 10.1371/journal.pone.0210211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lima PL, Pereira CV, Nissanka N, Arguello T, Gavini G, Maranduba CM, et al. Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome c oxidase. J Photochem Photobiol B. 2019;194:71–5. doi: 10.1016/j.jphotobiol.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Tsuka Y, Kunimatsu R, Gunji H, Nakajima K, Kimura A, Hiraki T, et al. Effects of Nd: YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: In vitro study. Lasers Med Sci. 2019;34:55–60. doi: 10.1007/s10103-018-2586-6. [DOI] [PubMed] [Google Scholar]
- 52.Ross G, Ross A. Photobiomodulation: An invaluable tool for all dental specialties. J Laser Dent. 2009;17:117–24. [Google Scholar]
- 53.Poyton RO, Ball KA. Therapeutic photobiomodulation: Nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov Med. 2011;11:154–9. [PubMed] [Google Scholar]
- 54.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355–61. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 55.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M. Low-level laser therapy for wound healing: Mechanism and efficacy. Dermatol Surg. 2005;31:334–40. doi: 10.1111/j.1524-4725.2005.31086. [DOI] [PubMed] [Google Scholar]
- 56.Tezel A, Kara C, Balkaya V, Orbak R. An evaluation of different treatments for recurrent aphthous stomatitis and patient perceptions: Nd: YAG laser versus medication. Photomed Laser Surg. 2009;27:101–6. doi: 10.1089/pho.2008.2274. [DOI] [PubMed] [Google Scholar]
- 57.Ferraresi C, Kaippert B, Avci P, Huang YY, de Sousa MV, Bagnato VS, et al. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. Photochem Photobiol. 2015;91:411–6. doi: 10.1111/php.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maldaner DR, Azzolin VF, Barbisan F, Mastela MH, Teixeira CF, Dihel A, et al. In vitro effect of low-level laser therapy on the proliferative, apoptosis modulation, and oxi-inflammatory markers of premature-senescent hydrogen peroxide-induced dermal fibroblasts. Lasers Med Sci. 2019;34:1333–43. doi: 10.1007/s10103-019-02728-1. [DOI] [PubMed] [Google Scholar]
- 59.Bagheri HS, Mousavi M, Rezabakhsh A, Rezaie J, Rasta SH, Nourazarian A, et al. Low-level laser irradiation at a high power intensity increased human endothelial cell exosome secretion via Wnt signaling. Lasers Med Sci. 2018;33:1131–45. doi: 10.1007/s10103-018-2495-8. [DOI] [PubMed] [Google Scholar]
- 60.Zamani AR, Mashayekhi MR, Jadid MF, Faridvand Y, Tajalli H, Rahbarghazi R. Photo-modulation of zinc phthalocyanine-treated breast cancer cell line ZR-75-1 inhibited the normal tumor activity in vitro. Lasers Med Sci. 2018;33:1969–78. doi: 10.1007/s10103-018-2563-0. [DOI] [PubMed] [Google Scholar]
- 61.Kaiser E, Kuzmits R, Pregant P, Burghuber O, Worofka W. Clinical biochemistry of neuron specific enolase. Clin Chim Acta. 1989;183:13–31. doi: 10.1016/0009-8981(89)90268-4. [DOI] [PubMed] [Google Scholar]
- 62.Mielke R, Schrkect R, Fink GR, Kessler J, Herholz K, Heiss WD. Regional cerebral glucose metabolism and postmortem pathology in Alzheimer's disease. Acta Neuropathol. 1996;91:174–9. doi: 10.1007/s004010050410. [DOI] [PubMed] [Google Scholar]
- 63.Marangos PJ, Schmechel DE. Neuron specific enolase, a clinically useful marker for neurons and neuroendocrine cells. Annu Rev Neurosci. 1987;10:269–95. doi: 10.1146/annurev.ne.10.030187.001413. [DOI] [PubMed] [Google Scholar]
- 64.Abdelkawi S, Ibrahim AE, Ghoneim DF, Hassan AA, A. Eldaiem MS. The combined effect of photobiomodulation therapy and chia seeds supplementation for the treatment of diabetic retinopathy in experimental rats. J Arab Soc Med Res. 2019;14:130–9. [Google Scholar]
- 65.Price TO, Eranki V, Banks WA, Ercal N, Shah GN. Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology. 2012;153:362–72. doi: 10.1210/en.2011-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]


