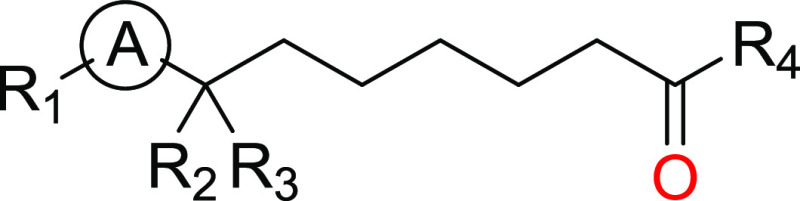
Important Compound Classes
Title
Inhibitors of Histone Deacetylase Useful for the Treatment or Prevention of HIV Infection
Patent Publication Number
WO 2020/096916 A2
Publication Date
May 14, 2020
Priority Application
US 62/757,422
Priority Date
November 8, 2018
Inventors
Yu, W.; Kozlowski, J. A.; Clausen, D. J.; Liu, J.; Yu, Y.; Wang, M.; Li, B.
Assignee Company
Merch Sharp & Dohme Corp., USA
Disease Area
HIV infection
Biological Target
Histone deacetylase (HDAC)
Summary
The regulation of gene expression through the inhibition of the nuclear enzyme histone deacetylase (HDAC) is one of several possible regulatory mechanisms whereby chromatin can be affected. The dynamic homeostasis of the nuclear acetylation of histone can be regulated by the opposing activity of the enzymes histone acetyl transferase (HAT) and HDAC. Acetylation reduces the positive charge of histones, thereby expanding the structure of the nucleosome and facilitating the interaction of transcription factors with the DNA. The removal of the acetyl group restores the positive charge, condensing the structure of the nucleosome. Whereas histone acetylation can activate DNA transcription, enhancing the gene expression, histone deacetylase can reverse the process and can serve to repress the gene expression. Inhibition of the histone deacetylase can also increase the activation of DNA transcription.
With the introduction of combination antiretroviral therapy (ART), HIV became a controllable chronic disease. The combination of ART (cART) targets specific stages of the viral life cycle and is effective at combatting active viral load down to detectable levels. Histone deacetylase inhibitors have shown promise in vitro in activating virus production from latent infected cells; therefore, this class of drugs is being studied as a part of a strategy aimed at a cure for HIV.
The present application describes a series of novel histone deacetylase inhibitors for the treatment of HIV infection. Furthermore, the application discloses compounds and their preparation, use, pharmaceutical composition, and treatment.
Definitions
Ⓐ = a five-membered heteroaryl ring, which is optionally substituted with halo, cyano, R5, R6, (C=O)N(R5)2, NR5(C=O)N(R5)2, (C=O)R6, or (C1–3alkyl)O(C=O)R4;
R1 = phenyl, bicyclic aryl, tricyclic aryl, or heteroaryl, which may be monocyclic, bicyclic, or tricyclic, wherein said phenyl and heteroaryl groups are optionally substituted with one to four groups independently selected from the group consisting of halo, oxo, cyano, C2–3alkenyl, R5, R6, OR5, N(R5)2, (C=O)NHR6, NR5(C=O)N(R5)2, NR5(C=NR5)N(R5)2, SO2R5, SO2N(R5)2, NR5SO2R5, and NR5SO2N(R5)2;
R2 = H, N(R5)2, NR5(C=O)NR5, NR5(C=O)N(R5)2, NR5(C=NR5)N(R5)2, NR5(C=O)R6, NR5(C1–3alkyl)R6, and NR5(C=O)(C1–3alkyl)R6;
R3 = H, C1–6alkyl, or CH2OR5;
R4 = C1–6alkyl or C3–6cycloalkyl, which is optionally substituted with one to three substituents independently selected from the group consisting of halo and OH or C3–6cycloalkyl;
R5 = H, cyano, or C1–6alkyl, which is optionally substituted with NH2, N(CH3)2, NH(CH3)2, N(CH2CH3)2, or 1–3 halo; and
R6 = heterocyclyl, which may be monocyclic, bicyclic, or tricyclic, C3–6cycloalkyl, phenyl, or heteroaryl, which may be monocyclic, bicyclic, or tricyclic, wherein said heterocyclyl, C3–6cycloalkyl, phenyl, and heteroaryl groups are optionally substituted with one to four groups independently selected from the group consisting of halo, cyano, oxo, R5, OR5, (C=O)R5, (C=O)OR5, N(R5)2, (C=O)N(R5)2, NR5(C=O)R5, NR5(C=O)N(R5)2, NR5(C=NR5)N(R5)2, SO2R5, SO2N(R5)2, NR5SO2R5 and NR5SO2N(R5)2, C2–3alkenyl, benzyl, benzyl-OR5, CH2(C3–6cycloalkyl), CH2(heteroaryl), heteroaryl, heterocyclyl, and C3–6cycloalkyl.
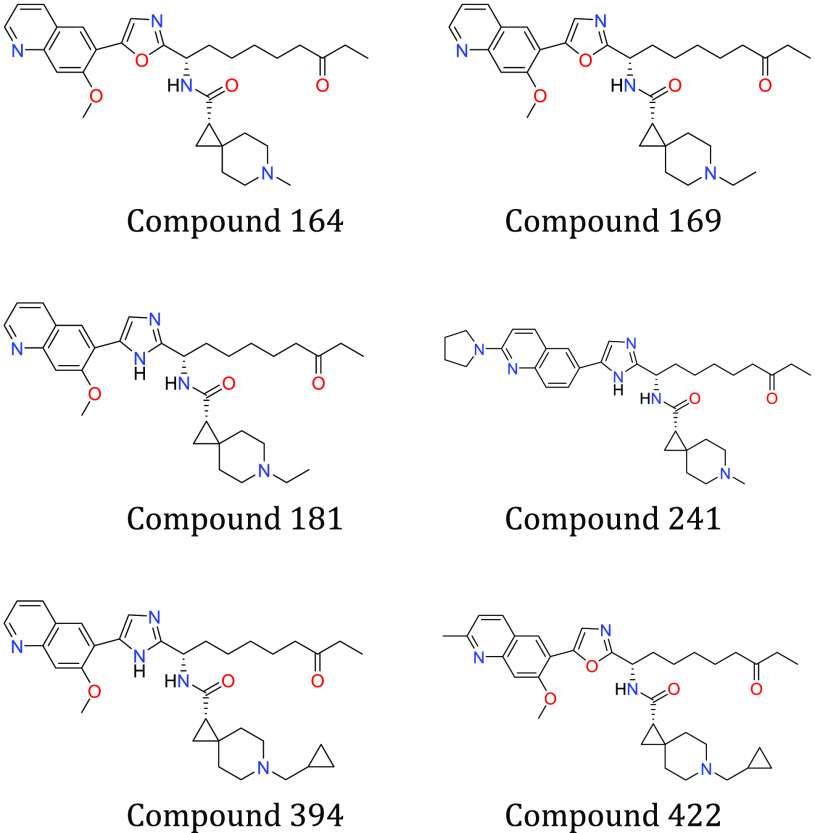
Key Structures
Biological Assay
The human HDAC inhibition assay was performed. The compounds described in this application were tested for their ability to inhibit HDAC (HDAC1, HDAC2, HDAC3, and HDAC6). The HDAC1, HDAC2, HDAC3, and HDAC6 IC50 (nM) are shown in the following table.
Biological Data
The table here shows
representative
compounds that were tested for HDAC1, HDAC2, HDAC3, and HDAC6 inhibition.
The biological data obtained from testing representative examples
are listed in the following table.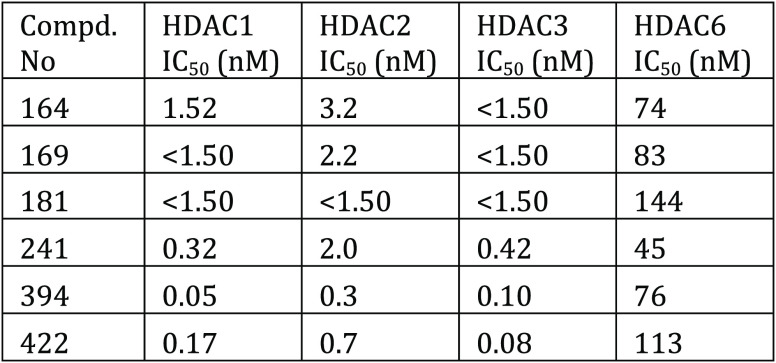
Claims
Total claims: 13
Compound claims: 8
Pharmaceutical composition claims: 2
Method of treatment claims: 2
Method of inhibition claims: 1
Recent Review Articles
-
1.
Zhang X.; Ma Q.; Wu H.; Khamis M. Y.; Li Y.; Ma L.; Liu H.. J. Med. Chem. 2021, 64, 1362.
-
2.
Pulya S.; Amin S. A.; Adhikari N.; Biswas S.; Jha T.; Ghosh B.. Pharmacol. Res. 2021, 163, 105274.
-
3.
Diteepeng T.; del Monte F.; Luciani M.. Eur. J. Clin. Invest. 2021, 51, e13504.
-
4.
Ramaiah M. J.; Tangutur A. D.; Manyam R. R.. Life Sci. 2021, 277, 119504.
-
5.
Vaidya G. N.; Rana P.; Venkatesh A.; Chatterjee D. R.; Contractor D.; Satpute D. P.; Nagpure M.; Jain A.; Kumar D.. Eur. J. Med. Chem. 2021, 209, 112844.
-
6.
Rroji O.; Kumar A.; Karuppagounder S. S.; Ratan R. R.. Neurobiol. Dis. 2021, 147, 105145.
The author declares no competing financial interest.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters special issue “Epigenetics 2022”.