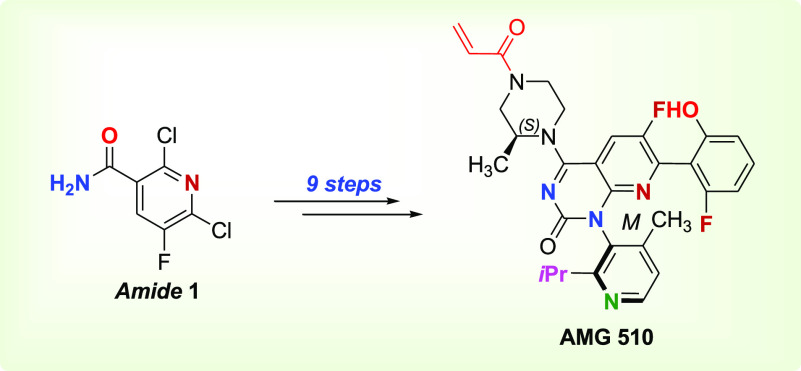
Important Compound Classes
Title
Improved Synthesis of KRAS G12C Inhibitor Compound
Patent Publication Number
WO 2021/097207 A1 and WO 2020/232130 A1
Publication Date
May 20, 2021 and November 19, 2020
Priority Application
62/935,515, 62/847,862, 62/867,747
Priority Date
November 14, 2019; May 14, 2019; June 27, 2019 US.
Inventors
Corbett, M. T.; Caille, S.; Henary, H.; Lipford, J. R.; Cee, V. J.
Assignee Company
Amgen Inc. [US/US]; One Amgen Center Drive, Thousand Oaks, California 91320-1799 (US).
Disease Area
Cancer
Biological Target
KRAS G12C Mutant
Summary
The present Patent Highlight showcases the improved, efficient, and scalable process to prepare the KRAS G12C inhibitor AMG 510 (compound 9). AMG 510 (Lumakras and Sotorasib the generic name) is the first ever FDA approved drug that inhibits KRAS, a cancer target that has been termed “undruggable” even after over 40 years of cancer research. AMG 510 is approved for KRAS G12C-mutated non-small cell lung cancer (NSCLC), requiring a daily 960 mg dose. Consequently, an efficient synthesis is desirable to provide a timely scale-up of the drug to fulfill increased anticipated market demands. Furthermore, this report may spur the investigation of structure-based design efforts that could facilitate the discovery of enhanced potency, permeability, solubility, and oral bioavailability for derivatized AMG 510.
Cancer is one of the deadliest diseases of the 21st century and the second leading cause of death worldwide. Genetic mutations can alter the proper expression and function of genes and proteins that are vital to cell growth, proliferation, and differentiation, which have been identified as the predominant cause of most cancers. Mutations in the RAS oncogene are the most common activating mutation, which is associated with the development of cancerous tumors in human. The RAS gene family consists of three isoforms: KRAS, HRAS, and NRAS. However, 85% of RAS-driven cancers are caused by mutations in the KRAS isoform. The inhibition of KRAS mutant protein has been targeted as a potential therapeutic for cancer, which until now has been challenging for finding a reliable KRAS mutant cancer therapy. Among the KRAS mutant tumors, 80% of all oncogenic mutations occur within codon 12, with G12C as the actionable biomarker in NSCLC.
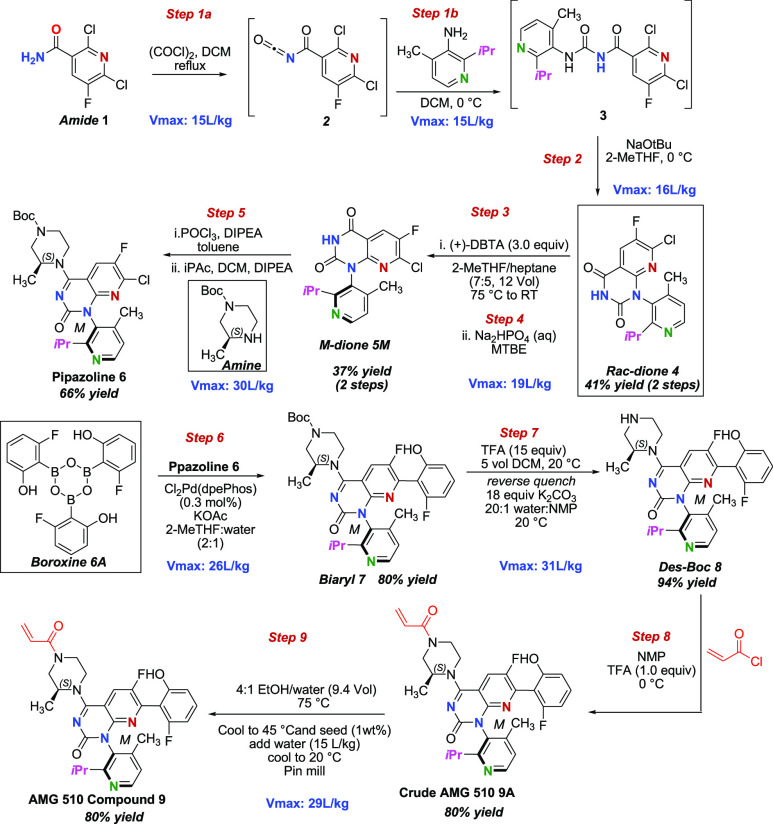
As shown in Scheme 1, AMG 510 was prepared from an improved, efficient, and scalable process. Amide 1 reacted with oxalyl chloride to give intermediate 2, which was carried through Step 1b to give a DCM solution of intermediate 3 that was not isolated but used directly in the next step. Urea compound 3 cyclized under basic condition to give rac-dione compound 4 (atropisomers) in 41% yield over two steps. Chiral separation was carried out by reacting (+)-2,3-dibenzoyl-d-tartaric acid with rac-dione 4 in 2-MeTHF warmed to 75 °C until the mixture was fully dissolved and eventually cooled to 20 °C, which led to solids of M-dione compound 5M in 37% yield over two steps. Compound 5 was treated with phosphoryl chloride and N,N-diisopropylethylamine (DIPEA) in toluene followed by (S)-1-boc-3-methylpiperazine, which afforded pipazoline 6 in 66% yield. Compound 6 was cross coupled under palladium catalyzed conditions with boroxine 6A, which gave the biaryl compound 7 in 80% yield. Charcoal filtration removed residual palladium and was followed by boc-deprotection reaction that afforded compound 8 in 94% yield. Treatment with acryloyl chloride gave crude 9A, which was purified to afford pure AMG 510.
Scheme 1. Synthetic Steps in the Manufacture of AMG 510.
Biological Assay and Patients’ Enrollment
Cellular phosphorylated extracellular signal-regulated kinase (pERK) assay for evaluation of serum concentration after dose administration. The first patient was enrolled on August 27, 2018, and by July 17, 2019 76 patients were enrolled and 34 had NSCLC. A total of 45 patients enrolled in the escalation cohort with daily doses of 180, 360, 720, and 960 mg; 31 patients enrolled in the 960 mg expansion cohort.
Biological Data
The pharmacokinetic (PK) profile of
compound 9 (AMG 510), 960 mg oral total daily dose, including
patients with NSCLC and colorectal cancer (CRC) is as follows: maximum
serum concentration (Cmax) 7.5 μg/mL
(98.3%), area under the curve (AUC) 65.3 h·μg/mL (81.7%
and elimination half-life (t1/2,z) 5.5
h (1.8). The table below shows the efficacy of AMG 510 in patients
with NSCLC, where PR = partial response; SD = stable disease; PD =
progressive disease; Evaluated patients = patients with measurable
treatment response; n = number of adverse effects; N = number of patients
receiving drug; RECIST = Response Evaluation Criteria In Solid Tumors.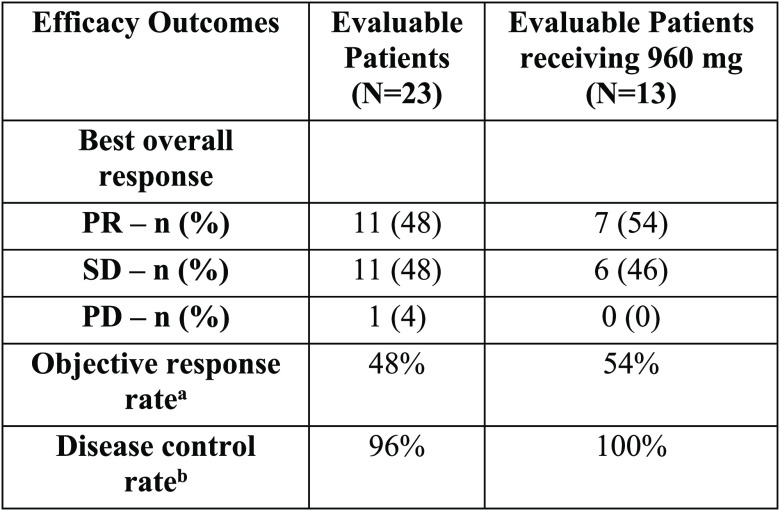 aEvaluation of response is based on modified
RECIST 1.1 criteria. bPR or SD at week 6.
aEvaluation of response is based on modified
RECIST 1.1 criteria. bPR or SD at week 6.
Recent Review Articles
-
1.
Erlanson D. A.; Webster K. R.. Curr. Opin. Chem. 2021, 62, 101.
-
2.
Zhao Y.; Xue J. Y.; Lito P.. Cancer Discov 2021, 11, 17.
-
3.
Mallapelle U.; Passiglia F.; Cremolini C.; Reale M. L.; Pepe F.; Pisapia P.. et al. Eur. J. Cancer 2021, 146, 74.
-
4.
Veluswamy R.; Mack P. C.; Houldsworth J.; Elhouly E.; Hirsch F. R.. J. Mol. Diagn. 2021, 23, 507.
-
5.
Xie M.; Xu X.; Fan Y.. Front. Oncol. 2021, 11, 672612.
-
6.
Erlanson D. A.; Webster K. R.. Curr. Opin. Chem. Biol. 2021, 62, 101.
The author declares no competing financial interest.
This paper was originally published on July 7, 2021, with an error in the AMG 510 structure. The corrected version was reposted on July 9, 2021.