Important Compound Classes
Title
Small Molecule Inhibitors of KRAS G12C Mutant
Patent Publication Number
WO 2021/086833 A1
Publication Date
May 06, 2021
Priority Application
62/926,879 and 63/030,014 US
Priority Date
October 28, 2019 and 63/030,014
Inventors
Bharathan, I.; Gathiaka, S.; Graham, T. H.; Han, Y.; Henderson, T.; Hennessy, E.; Ma, X.; Otte, R.; Palani, A.; Sloman, D. L.
Assignee Company
Merck Sharp & Dohme Corp. [US/US]; 126 East Lincoln Avenue, Rahway, NJ 07065 (US).
Disease Area
Cancer
Biological Target
KRAS G12C Mutant
Summary
The present Patent Highlight relates to certain cyclic heteroaryl compounds and pharmaceutically acceptable salts that inhibit the G12C mutant of Kirsten rat sarcoma (KRAS) protein. These compounds have utility as therapeutic agents for the treatment of cancer and is related to the recent disclosure by the applicant in ACS Med. Chem. Lett. 2021, https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00321.
Rat sarcoma viral oncogene homologue (RAS), which is encoded by HRAS, NRAS, and KRAS, are small GTPase enzymes that function as cellular signal transducers cycling between an active GTP-bound and inactive GDP-bound state. RAS proteins regulate many cellular processes including cell proliferation and differentiation and play a vital role in human cancers. Activation of RAS is a common feature accounting for about 30% of human cancers. Oncogenic mutation of KRAS is closely linked to tumorigenesis, which is found in 22% of cancer patients, including lung (17%), colon (33%), and pancreatic cancers (61%).
Notably, patients with KRAS mutations are typically resistant to standard-of-care chemotherapy and have a poor prognosis. The lack of an ideal small-molecule binding pocket and high affinity toward the cellular guanosine triphosphate (GTP) renders the development of specific small-molecule drugs that directly target KRAS challenging. However, the recent approval of the KRAS G12C inhibitor (AMG 510, Lumakras) for the treatment of non-small cell lung cancer (NSCLC) is a beginning to address the extraordinary unmet clinical need that requires new therapies for directly targeting KRAS.
In general, there are theoretically three approaches to directly inhibiting KRAS with small molecules: obstructive GTP binding through the use of a competitive ligand; locking KRAS G12C in an inactive state through allosteric modulation; and disrupting the interaction of KRAS with its effector proteins and guanine nucleotide exchange factors (GEFs). The relatively small size and smooth surface of KRAS as well as the high flexibility of the KRAS switch regions have also made it difficult to develop allosteric or protein–protein interaction (PPI) inhibitors. Despite these difficulties, promising opportunity that selectively target mutant KRAS in patients are in clinical trials. Three novel KRAS G12C inhibitors with similar allosteric mechanisms, including MRTX849, JNJ-74699157, and GDC-6036. These inhibitors covalently bind to KRAS G12C at the key mutant residue cysteine 12, locking the GTPase in an inactive state and inhibiting KRAS signaling. The exemplary compounds modulate mutant KRAS, HRAS, and/or NRAS protein and may selectively inhibit the KRAS (G12C) protein.
Definitions
Y = N or C(H); Z = N or C(R5); M = C or N;
L = C1–C7 alkylene or C1–C7 heteroalkylene, where substituted or unsubstituted by 1 to 5 substituents selected from oxo, fluoro, C1–C3 alkyl, C1–C3 fluoroalkyl, amino, and hydroxy;
R1 = halo, H, C1–C4 alkyl, C1–C4 fluoroalkyl, or C1–C4 hydroxyalkyl;
R2 = H, CH3, or halo;
R3 = C1–C4 alkyl, C1–C4 cyanoalkyl, C1–C4 hydroxyalkyl, oxo, or carboxy;
W1 = −C(O)- or -S(O)2-;
W2 = 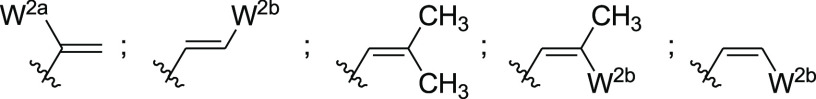 ;
;
R4 = H, C1–C4 alkyl, or C3–C5 cycloalkyl;
R5 = H or halo;
Ring Cy = phenyl, 5- to 6-membered monocyclic heteroaryl;
Ry = halo, hydroxy, C1–C3 alkyl, C1–C3 fluoroalkyl, cyano, hydroxy;
Ring Cz = phenyl or a 5- or 6-membered heteroaryl containing one or three heteroatoms;
Rz = halo, hydroxy, C1–C3 alkyl, C1–C3 fluoroalkyl, C1–C3 alkoxy, cyano.
Key Structures
Biological Assay
SOS-catalyzed nucleotide exchange assay and cellular phospho-extracellular signal-related kinase (phospho-ERK) assay.
Biological Data
The table below shows in vitro apparent
potency (IC50) in the SOS-catalyzed nucleotide exchange
assay with preincubation time of 60 min prior to addition of SOS and
in vitro potency in the cellular phosphor-ERK assay after 2 h incubation.
Recent Review Articles
-
1
Piffoux M.; Cassier P. A.. Br. J. Cancer 2021, 124, 333.
-
2
Fancelli S.; Caliman E.; Mazzoni F.; Brugia M.; Pillozzi S.. Cancer 2021, 13, 1091.
-
3
Malapelle U.; Pepe F.; Pisapia P.; Passiglia F.. Eur. J. Cancer 2021, 146, 74.
-
4
Duffy M. J.; Crown J.. Int. J. Cancer 2021, 148, 8.
-
5
Hamarsheh S.; Gross O.; Brummer T.; Zeiser R.. Nat. Commun. 2020, 11, 5439.
-
6
Kettle J. G.; Cassar D. J.. Opin. Ther. Pat. 2020, 30, 103.
The author declares no competing financial interest.




