Summary
This systematic review examined the impact of exercise intervention programs on selected cardiometabolic health indicators in adults with overweight or obesity. Three electronic databases were explored for randomized controlled trials (RCTs) that included adults with overweight or obesity and provided exercise‐training interventions. Effects on blood pressure, insulin resistance (homeostasis model of insulin resistance, HOMA‐IR), and magnetic resonance measures of intrahepatic fat in exercise versus control groups were analyzed using random effects meta‐analyses. Fifty‐four articles matched inclusion criteria. Exercise training reduced systolic and diastolic blood pressure (mean difference, MD = −2.95 mmHg [95% CI −4.22, −1.68], p < 0.00001, I 2 = 63% and MD = −1.93 mmHg [95% CI −2.73, −1.13], p < 0.00001, I 2 = 54%, 60 and 58 study arms, respectively). Systolic and diastolic blood pressure decreased also when considering only subjects with hypertension. Exercise training significantly decreased HOMA‐IR (standardized mean difference, SMD = −0.34 [−0.49, −0.18], p < 0.0001, I 2 = 48%, 37 study arms), with higher effect size in subgroup of patients with type 2 diabetes (SMD = −0.50 [95% CI: −0.83, −0.17], p = 0.003, I 2 = 39%). Intrahepatic fat decreased significantly after exercise interventions (SMD = −0.59 [95% CI: −0.78, −0.41], p < 0.00001, I 2 = 0%), with a larger effect size after high‐intensity interval training. In conclusion, exercise training is effective in improving cardiometabolic health in adults with overweight or obesity also when living with comorbitidies.
Keywords: hypertension, insulin resistance, morbid obesity, NAFLD, physical activity, type 2 diabetes
1. INTRODUCTION
Obesity is a global and growing multifactorial disease as well as a public health issue.1 The pandemic expansion of obesity is accompanied by the concomitant growth of many related comorbidities, themselves determinants of increased cardiovascular risk.2 Indeed, increased blood glucose, high blood pressure, altered lipid profile, and increased intrahepatic fat are all cardiometabolic risk markers closely related to obesity, with insulin resistance as a common pathophysiological pathway.3 All international guidelines recommend to prescribe physical exercise for the management of type 2 diabetes,4 arterial hypertension,5 and obesity‐related comorbidities such as nonalcoholic fatty liver disease (NAFLD).6 However, the effect of exercise training on these aspects of cardiometabolic health have not been specifically addressed in subjects with overweight or obesity. In 2006, a Cochrane review based on a very limited number of randomized controlled trials (RCTs) available showed that groups of adults with overweight or obesity participating in physical exercise intervention programs experienced advantages for cardiometabolic health (i.e., blood pressure lowering and improved lipid profile), particularly if involved in higher‐intensity exercise training.7 In 2013, Pattyn et al.,8 focusing on subjects with metabolic syndrome, displayed the efficacy of aerobic training in improving cardiovascular risk factors. In 2012, Cornelissen et al.9 described the significant effect of resistance training in reducing systolic and diastolic blood pressure, but this meta‐analysis did not address individuals with overweight or obesity. Moreover, previous systematic reviews and meta‐analysis outlined significant effect of regular exercise in improving insulin sensitivity in patients with type 2 diabetes, but limited data are available in patients with overweight and obesity with or without type 2 diabetes.10, 11
High‐intensity interval training (HIIT) is currently receiving increasing attention regarding its metabolic and cardiovascular effects. Recently, Batacan et al.12 highlighted the beneficial effect of HIIT on cardiometabolic health in subjects with overweight or obesity. On the other hand, also high intensity continuous training showed powerful effect in reducing intrahepatic fat, a main marker of metabolic dysfunction in people with obesity.13 NAFLD is currently recognized as a major health concern, considering that it can give rise to serious forms of liver disease such as cirrhosis and hepatic carcinoma.14 Prior reviews showed that exercise training alone is effective in reducing different measures of intrahepatic fat in patients with NAFLD,13, 15, 16 particularly for higher energy expenditure programmes.17
In summary, a specific focus on overweight or obesity is lacking in most previous reviews and meta‐analyses. Therefore, given the growing number of individuals affected by obesity‐related comorbidities18, 19, 20, 21 and the central role of physical exercise in their management,22 it seemed suitable to perform an update and synthesis about the effect of exercise training programs on cardiometabolic health parameters in adults with overweight or obesity. The main objective of this work is to evaluate through systematic review and meta‐analysis the effect of exercise training on blood pressure values, insulin resistance, and magnetic resonance (MR) measures of intrahepatic fat in patients with overweight or obesity with or without obesity‐related comorbidities.
2. METHODS
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines and is registered in the PROSPERO database (registration number CRD42019157823).
2.1. Search strategy
Three electronic databases (PubMed, Web of Science, and Cochrane Library) were searched for original articles published up to April 2020 using the strategy “obesity AND physical activity AND age AND comorbidities.” Previous systematic reviews were screened to identify relevant subject headings and keywords to include within each subject category. The specific keywords used for the search are listed in Table S1. Limits were set to include RCTs, only with adults, and that were published in English. Reference lists from the resulting articles were also screened to identify additional articles.
2.2. Study selection, inclusion, and exclusion
RCTs were included if they involved adults (≥18 years including older adults) with overweight (body mass index [BMI] ≥ 25 kg/m2) or obesity (BMI ≥ 30 kg/m2) participating in physical activity interventions, that is, exercise training programs. Original articles focusing on the primary prevention of weight gain/obesity were not included. Presence of obesity comorbidities was not an exclusion criterion, provided that the article focused on subjects with obesity. Specifically, subjects with the following comorbidities were not excluded: type 2 diabetes, hypertension, dyslipidaemia, metabolic syndrome, NAFLD, and nonalcoholic steatohepatitis (NASH). No minimum intervention length criterion was applied. Exercise training programs included sessions with one or more types of exercise (aerobic and/or resistance and/or HIIT). Exercise sessions could have been fully supervised, partially supervised or nonsupervised. Physical activity programs in combination with other interventions (e.g., diet, dietary supplements, or drugs) with appropriate controls were included. Comparators included no intervention or usual care (i.e., intervention that any patient would have received in the framework of obesity management).
Abstracts and full texts were independently assessed for eligibility by two authors (FB and AE) with uncertainty regarding eligibility discussed among authors.
2.3. Data extraction and synthesis
Data were extracted by two authors (FB and AE) using standardized forms. Characteristics of each included original article were recorded: reference, study design, participants included in intervention and control groups, population characteristics (age, BMI, % female, and comorbidities for intervention and control groups), description of intervention (program duration, number of sessions/week, type of training/modality, and supervision/delivery), and comparison, outcomes, and duration of follow‐up.
The findings pertaining to systolic blood pressure, diastolic blood pressure, insulin resistance, and intrahepatic fat (measured only by MR) of each included original article are reported. In addition, the study authors' conclusions were extracted, and an overview of the quality of the original studies and current authors' assessment of conclusions is provided for each included article.
Effects on systolic and diastolic blood pressure, Homeostasis Model Assessment Insulin Resistance Index (HOMA‐IR), and intrahepatic fat were examined using random effects meta‐analyses (Review Manager Version 5.3). Analyses are presented as mean difference (MD) for systolic and diastolic blood pressure and as standardized mean difference (SMD) for HOMA‐IR and intrahepatic fat. Sensitivity analysis by using one‐study‐removed procedure was also conducted. Effect sizes were considered large, medium, small, and negligible when SMD was >0.8, between 0.5 and 0.8, between 0.2 and 0.5, and below 0.2, respectively. Heterogeneity was calculated using the I 2 test. Heterogeneity was considered low, moderate, and high when I 2 was <50%, between 50% and 75%, and ≥75%, respectively.23 Publication bias was assessed with visual inspection of the funnel plot. Limit for statistical significance was fixed at a p value of 0.05. Absolute change (pre‐ to postintervention values) in intervention and control groups was indicated as mean and standard deviation (SD). For missing data, authors were contacted. Alternatively, conversion of confidence intervals in SD or calculation of SD of absolute change (when studies provided the exact p value for intragroup or intergroup analyses) was performed by formulas and transformation methods of the Cochrane handbook.24 When these values were not reported, we calculated the MD as the difference in mean pre‐ and postintervention and its SD using the formula: SD = square root ([SD pretreatment]2 + [SD posttreatment]2) – [2r × SD pretreatment × SD posttreatment]). Because the pretest–posttest correlation coefficients (r) were not reported in the studies, a conservative r value of 0.5 was assumed throughout. Data from figures were extracted using an online tool (WebPlotDigitizer; https://automeris.io/WebPlotDigitizer/). If a study included more than two experimental groups, which were compared with one control group, the number of subjects in the control group was divided by the number of included intervention arms.
2.4. Quality assessment
Study quality was assessed with a standardized tool including 14 criteria, as previously described.25 Study quality was defined as good, fair, and poor when 0, 1, or ≥2 criteria considered as “fatal flaws” were not fulfilled.25 Three assessment items represented fatal flaws if answered “No/Not reported/Can't determine”: (i) randomization (#1), (ii) dropout rate <20% (#7), and (iii) intent‐to‐treat analysis (#14). Quality assessment was conducted independently by two reviewers (FB ad AE) using this standardized tool. Any disagreement between the reviewers was resolved through discussion.
3. RESULTS
The systematic review flow diagram is presented in Figure 1. The comprehensive (databases and reference lists) search yielded 6768 articles; 1418 were removed because they were duplicates. Of the remaining 5350, 5108 were eliminated based on titles and abstracts alone. The full text was retrieved from 242 articles, and 54 satisfied the inclusion criteria.
FIGURE 1.
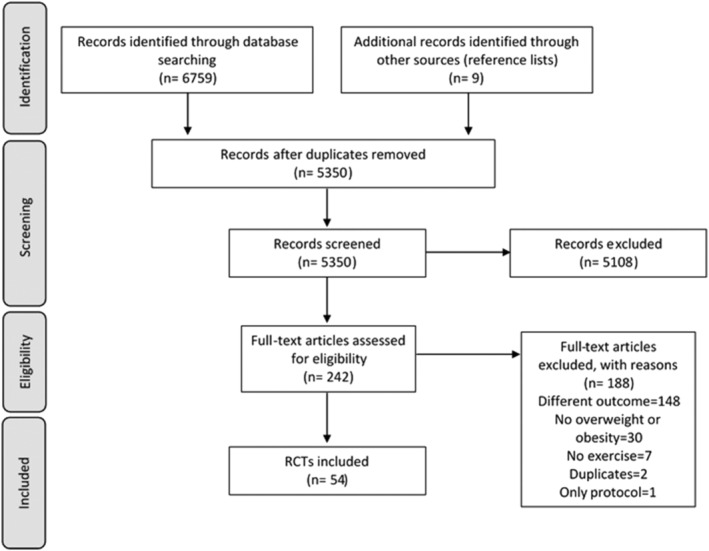
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) flow diagram
3.1. Study characteristics
Characteristics and findings of the included original studies are presented in Tables S3–S4. RCTs were published between 2006 and April 2020. Fifty‐four RCTs were included. The median (range) total sample size of the included studies was 38 (10–404) subjects. The median (range) age was 52 (22–70) years. Fifty‐three studies reported BMI at baseline, with the median (range) being 31.9 (26.3–43.7) kg/m2. In the single study26 not reporting BMI values, the presence of obesity (BMI ≥ 30 kg/m2) was an inclusion criteria. Males and females were present in 34 studies18, 21, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 in which the median (range) percentage of females was 59.0 (15.9–92.1)%. Eight studies included only male,58, 59, 60, 61, 62, 63, 64, 65 and 12 studies included only female adults.66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 Five studies included subjects with several comorbidities including arterial hypertension, type 2 diabetes, and metabolic syndrome.27, 43, 46, 47, 53 Six studies included patients with hypertension and metabolic syndrome.26, 36, 44, 54, 57, 68 Four studies were performed on subjects affected by hypertension and NAFLD,41, 51, 55, 78 three studies included subjects affected by NAFLD and type 2 diabetes,28, 29, 50 and two included also patients with dyslipidaemia.18, 52 Two studies enrolled patients with only type 2 diabetes,49, 58 12 studies with only hypertension,30, 31, 33, 39, 42, 48, 62, 63, 64, 66, 71, 75 one with NAFLD,45 and one with NASH.32 Interventions are described in detail in Table S3. Duration of exercise training ranged from 4 to 48 weeks, with a median duration of 12 weeks and a median of 3 (range 2–7) sessions/week. Aerobic training was performed in 36 studies, resistance training in 12 studies, a combination of aerobic and resistance training in eight studies, and HIIT in 11 studies. Exercise duration was prescribed in minutes or as energy expenditure (kcal), and the exercise intensity defined as percentage of maximal aerobic capacity (VO2max) or heart rate (HRmax or HRReserve). The mean (range) exercise prescription was 45 (17–90) min per session or 1698 (668–2430) kcal/week at 60 (45–85)% VO2max, 70 (50–85)% HRmax, or 60 (45–80)% HRReserve for aerobic training. Resistance training was prescribed with a median (range) of 3 (2–4) sets of 10 (8–15) repetitions for 8 (4–12) exercises at 70 (60–85)% of 1 RM. HIIT was prescribed as a median (range) of 5 (3–11) bouts of 128 (30–480) s at 90 (85–95)% VO2max or 90 (80–100)% HRmax with a median (range) recovery duration of 120 (45–180) s at 50 (20–70)% VO2max or 70 (70–70)% HRmax. Four studies evaluated exercise intensity as rate of perceived exertion (RPE) measured with Borg scale with a median value of 14 (13–18). One study prescribed exercise training at individualized VO2, corresponding to maximal fat oxidation (34.5%, range 24.4–46.8% VO2max). Exercise sessions were fully supervised in 28 studies, partially supervised in 14 studies, and not supervised in five studies. Information about supervision was not reported in seven studies. Supervision was conducted by physical therapists, physicians, fitness instructors, exercise physiologists or specialists, or kinesiologists. Control groups were characterized by no exercise prescription, sham exercise (i.e., stretching), usual care, or dietary prescription, if the intervention group received a diet prescription plus exercise. One study compared exercise and rosiglitazone versus rosiglitazone alone considered as control group. Reported outcomes included systolic blood pressure (36 studies), diastolic blood pressure (35 studies), HOMA‐IR (30 studies), and intrahepatic fat (13 studies). Except for two RCTs that assessed outcome after the conclusion of the exercise intervention program 6‐54 and 8‐month follow‐ups,30 all RCTs reported measures recorded immediately after the intervention period. When studies reported more than one follow‐up measure, those assessed immediately after the conclusion of the intervention period were chosen.
3.2. Study findings
Findings of the included studies are listed in Table S4.
3.2.1. Blood pressure
Included outcomes were systolic and diastolic blood pressure assessed as office measures. Only two RCTs48, 63 used 24‐h blood pressure monitoring (ABPM), and the mean of daytime measures was included in meta‐analyses. Nine studies included only subjects with prehypertension or hypertension, six studies included only subjects with normal blood pressure, and 16 studies included mixed populations, that is, subjects with or without hypertension.
3.2.2. Systolic blood pressure
To assess the overall effect of exercise on systolic blood pressure, all studies were included in the meta‐analysis, whatever the blood pressure status (60 study arms, 1334 subjects in the experimental groups, and 942 subjects in the control groups). As shown in Figure S1, exercise training programs were effective in reducing systolic blood pressure (MD: −2.95 mmHg [95% CI: −4.22, −1.68], p < 0.00001, I 2 = 63%). When including only studies with hypertensive subjects (17 study arms), blood pressure was reduced on average by −3.39 mmHg (95% CI −5.31, −1.46), p = 0.0006, I 2 = 58% (Figure 2). When including only studies with normotensive subjects (eight study arms), the effect was not significant (MD: −2.03 mmHg [95% CI: −5.32, 1.26], p = 0.23, I 2 = 31%). Funnel plots all looked symmetric (Figure S5a).
FIGURE 2.
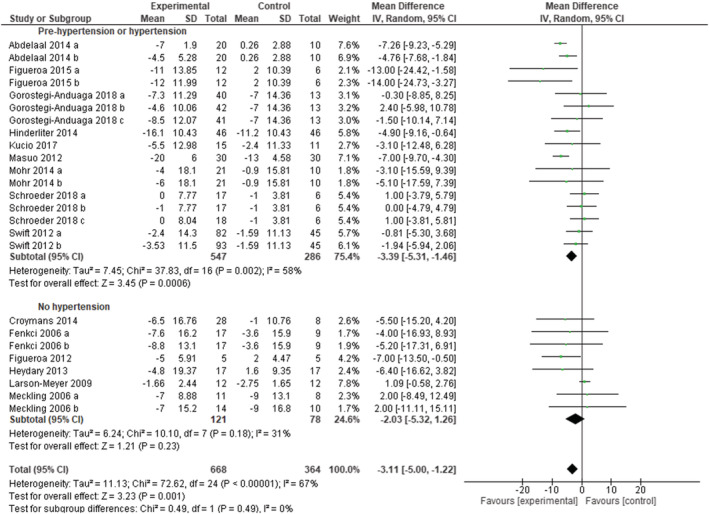
Forest plot of the effect of exercise training versus control on systolic blood pressure in adults with overweight or obesity, grouped by presence/absence of arterial hypertension. Presents mean difference between subjects participating in exercise training versus control in change of systolic blood pressure. Subgroup “Normotensive patients” includes randomized controlled trials (RCTs) that excluded patients with overweight or obesity and hypertension. Subgroup “Pre‐hypertension or hypertension” includes RCTs that included only patients with overweight or obesity and prehypertension or hypertension. Articles are presented in alphabetical order. Abdelaal 2014 (a): aerobic exercise; Abdelaal 2014 (b): resistance exercise. Fenkci (a): aerobic training; Fenkci (b): resistance training. Figueroa 2015 (a): high ankle blood pressure; Figueroa 2015 (b): low ankle blood pressure. Gorostegi‐Anduaga 2018 (a): high volume‐moderate intensity continuous training; Gorostegi‐Anduaga 2018 (b): high volume‐high intensity interval training; Gorostegi‐Anduaga 2018 (c): low volume‐high intensity interval training. Meckling (a): control diet + exercise versus control diet; Meckling (b): high protein diet + exercise versus high‐protein diet. Mohr 2014 (a): moderate intensity continuous training versus control. Mohr 2014 (b): high intensity interval training versus control. Schroeder 2018 (a): aerobic exercise; Schroeder 2018 (b): resistance exercise; Schroeder 2018 (c): combined exercise. Swift 2012 (a): lower energy expenditure (8 kcal/kg/week); Swift 2012 (b): higher energy expenditure (12 kcal/kg/week)
Sensitivity analyses were conducted according to study quality. Out of the 36 studies included in the meta‐analyses, 24 studies were rated as fair or good quality and 12 as poor quality (Table S2). When eliminating the poor‐quality studies, the overall effect on systolic blood pressure remained unchanged (MD: −3.04 mmHg [95% CI: −4.37, −1.71], p < 0.00001, I 2 = 54%). Sensitivity analyses performed by eliminating the two studies that used ABPM did not reveal substantial differences in the overall MD. One‐study removing sensitivity analyses did not change the overall result.
3.2.3. Diastolic blood pressure
A total of 58 study arms (1310 subjects in the experimental groups and 930 subjects in the control groups) were included to assess the overall effect of exercise training programs on diastolic blood pressure (whatever the initial blood pressure status). As shown in Figure S2, exercise training programs were effective in reducing diastolic blood pressure (MD: −1.93 mmHg [95% CI: −2.73, −1.13], p < 0.00001, I 2 = 54%). Blood pressure decreased significantly with exercise, both when including only studies with hypertensive subjects (16 study arms), (MD: −2.06 mmHg [95% CI: −3.42, −0.70], p = 0.003, I 2 = 76%), and only studies with normotensive subjects (eight study arms) MD: −2.09 mmHg (95% CI: −3.45, −0.74), p = 0.002, I 2 = 0%), as reported in Figure 3. Funnel plots demonstrated a symmetric distribution (Figure S5b).
FIGURE 3.
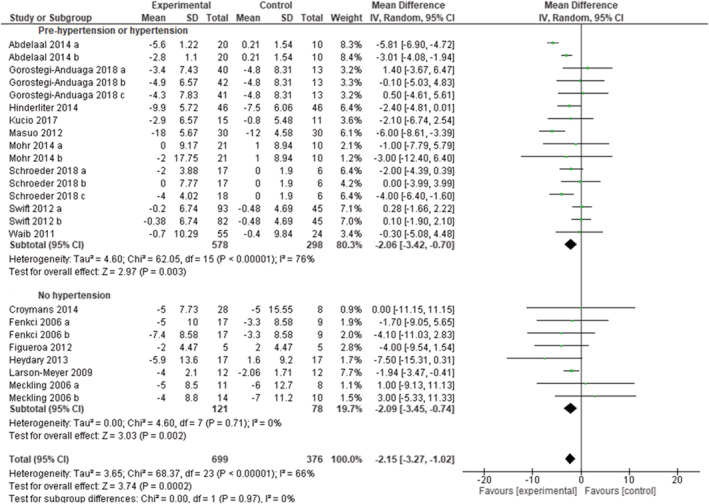
Forest plot of the effect of exercise training versus control on diastolic blood pressure in adults with overweight or obesity, grouped by presence/absence of arterial hypertension. Presents mean difference between subjects participating in exercise training versus control in change of diastolic blood pressure. Articles are presented in alphabetical order. Subgroup “Normotensive patients” includes randomized controlled trials (RCTs) that excluded patients with overweight or obesity and hypertension. Subgroup “Pre‐hypertension or hypertension” includes RCTs that included only patients with overweight or obesity and prehypertension or hypertension. Abdelaal 2014 (a): Aerobic exercise; Abdelaal 2014 (b): resistance exercise. Fenkci (a): aerobic training; Fenkci (b): resistance training; Gorostegi‐Anduaga 2018 (a): high volume‐moderate intensity continuous training; Gorostegi‐Anduaga 2018 (b): high volume‐high intensity interval training; Gorostegi‐Anduaga 2018 (c): low volume‐high intensity interval training. Meckling (a): control diet + exercise versus control diet; Meckling (b): high protein diet + exercise versus high protein diet. Mohr 2014 (a): moderate intensity continuous training versus control; Mohr 2014 (b): high intensity interval training versus control. Schroeder 2018 (a): aerobic exercise; Schroeder 2018 (b): resistance exercise; Schroeder 2018 (c): combined exercise. Swift 2012 (a): higher energy expenditure (12 kcal/kg/week); Swift 2012 (b): lower energy expenditure (8 kcal/kg/week)
Twenty‐three studies were rated as fair or good quality and 12 as poor quality (Table S2). When eliminating the poor‐quality studies, the overall effect of exercise was comparable (MD: −1.98 mmHg [95% CI: −2.87, −1.09], p < 0.0001, I 2 = 54%). Sensitivity analyses performed by eliminating the two studies that used ABPM did not reveal substantial differences in the overall MD. One study removing sensitivity analyses did not change the overall result.
3.2.4. Insulin resistance
Out of the 30 RCTs that reported HOMA‐IR, 18 studies included subjects without type 2 diabetes, eight studies included subjects with type 2 diabetes, one study assessed three arms of intervention based on the glycaemic status, and three studies provided no information or included mixed populations. A total of 37 study arms (740 subjects in the experimental groups and 697 subjects in the control groups) were included to assess the overall effect of exercise training programs on insulin resistance (whatever the initial glycemic status). As shown in Figure S3, exercise reduced HOMA‐IR (SMD: −0.34 [95% CI: −0.49, −0.18], p < 0.0001, I 2 = 48%). HOMA‐IR decreased significantly when we included only interventions performed in subjects with type 2 diabetes (SMD: −0.50 [95% CI: −0.83, −0.17], p = 0.003, I 2 = 39%) or only interventions performed in subjects without type 2 diabetes (SMD: −0.31 [95% CI: −0.49, −0.13], p = 0.0007, I 2 = 45%) (Figure 4). Funnel plots demonstrated a symmetric distribution (Figure S5c).
FIGURE 4.
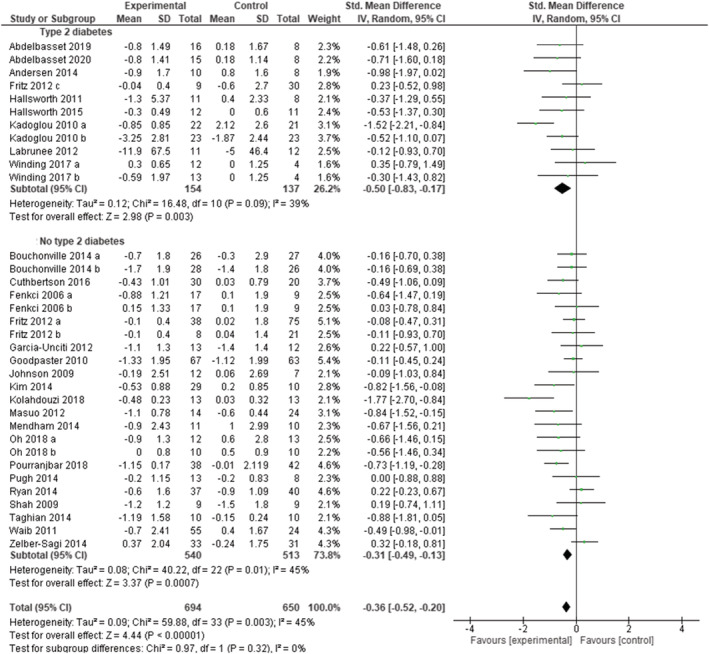
Forest plot of the effect of exercise training versus control on Homeostasis Model Assessment Insulin Resistance Index (HOMA‐IR) in adults with overweight or obesity, grouped by presence/absence type 2 diabetes. Presents mean difference between subjects participating in exercise training versus control in change of HOMA‐IR. Articles are presented in alphabetical order. Subgroup “No type 2 diabetes” includes randomized controlled trials (RCTs) that excluded patients with overweight or obesity and type 2 diabetes. Subgroup “Type 2 diabetes” includes RCTs that included only patients with overweight or obesity and type 2 diabetes. Articles are presented in alphabetical order. Bouchonville 2014 (a): exercise versus control. Bouchonville 2014 (b):diet + exercise versus diet; Fenkci (a): aerobic training; Fenkci (b): resistance training. Fritz 2012 (a): normal glucose tolerance; Fritz 2012 (b): impaired glucose tolerance; Fritz 2012 (c): type 2 diabetes. Kadoglou 2010 (a): exercise versus control; Kadoglou 2010 (b): rosiglitazone + exercise versus rosiglitazone. Oh 2018 (a): diet + exercise versus diet; Oh 2018 (b): exercise versus control; Winding 2014 (a): endurance training; Winding 2014 (b): high intensity interval training
Fifteen studies were rated as fair or good quality and 15 as poor quality. Removing the poor‐quality studies did not change the overall effect of exercise (SMD: −0.38 [95% CI: −0.56, −0.20], p < 0.0001, I 2 = 34%). One study removing sensitivity analyses did not change the overall result.
3.2.5. Intrahepatic fat
Out of the 13 studies assessing the effect of exercise on MR measures of intrahepatic fat, nine studies included subjects with NAFLD, one study included subjects with NASH, and three studies included mixed populations (with and without NAFLD). A total of 17 study arms (329 subjects in the experimental groups and 189 subjects in the control groups) were included to assess the overall effect of exercise training programs on intrahepatic fat (whatever the initial status of participants and the type of exercise). Exercise reduced intrahepatic fat (SMD: −0.59 [95% CI: −0.78, −0.41], p < 0.00001, I 2 = 0%) (Figure S4). The funnel plot was symmetric (Figure S5d). Meta‐analyses stratified according to the type of exercise training (Figure 5) showed that HIIT, aerobic, and resistance alone or combined with aerobic training all decreased intrahepatic fat (SMD: −0.89 [95% CI: −1.36, −0.41], p = 0.0002, I 2 = 0%; SMD: −0.56 [95% CI: −0.77, −0.35], p < 0.00001, I 2 = 0%; and SMD: −0.40 [95% CI −1.06, 0.26], p = 0.24, I 2 = 0%, respectively).
FIGURE 5.
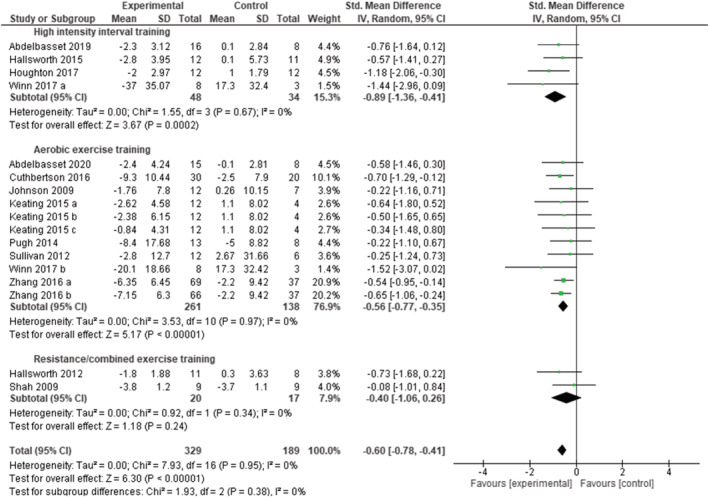
Pooled analysis for the effect of exercise training versus control on intrahepatic fat in adults with overweight or obesity, grouped by exercise modality. Presents mean difference between subjects participating in exercise training versus control in change of intrahepatic fat. Articles are presented in alphabetical order. Keating 2015 (a): high intensity, low volume aerobic exercise; Keating 2015 (b): low intensity, high volume aerobic exercise. Keating 2015 (c): low intensity, low volume aerobic exercise. Winn 2017 (a): high intensity interval training; Winn 2017 (b): moderate intensity continuous training; Zhang 2016 (a): moderate intensity training; Zhang (b): vigorous intensity training
Nine studies were rated as fair or good quality and four as poor quality. Removing the poor‐quality studies, the overall effect of exercise did not change (SMD: −0.59 [95% CI: −0.80, −0.38], p < 0.00001, I 2 = 0%). One study removing sensitivity analyses did not change the overall result.
3.3. Study quality
Study quality was rated as good, fair, and poor in 11 (20%), 20 (37%), and 23 (43%) studies, respectively (Table S2). Most RCTs did not perform intention to treat analysis (Criterion 14), blinding of participants was generally not applicable (Criterion 4), and blinding of allocation and of the people assessing outcomes (Criteria 3 and 5) was not accomplished in more than half of the RCTs.
4. DISCUSSION
This systematic review and meta‐analysis provides a comprehensive analysis on the effect of exercise training interventions on key aspects of cardiometabolic health in adults with overweight or obesity. Our results demonstrate that exercise training programs are effective in improving cardiometabolic health in adults with overweight or obesity, including patients living with obesity‐related comorbidities.
4.1. Blood pressure
An overall reduction in both systolic and diastolic blood pressure was observed. The reduction of systolic blood pressure, whatever the blood pressure status of participants, was about 2 mmHg. Similar reductions in blood pressure have been previously reported after exercise training, although the effect of exercise was not specifically assessed in subjects with overweight or obesity.79 Interestingly, we found that the decrease in blood pressure appeared to be larger in subjects with hypertension compared with normotensive patients, reaching on average 3 mmHg versus a nonsignificant effect. This is in line with results of Cornelissen and Smart that reported a larger effect of resistance exercise training on systolic blood pressure in subjects with hypertension compared with those with prehypertension or normal blood pressure.80 It should be noted, however, that even a small decrease in systolic blood pressure of 2 mmHg is associated with a 10% decrease in stroke mortality and 7% decrease in ischemic heart disease and vascular mortality in middle‐aged adults.81 Therefore, the observed reduction in blood pressure resulting from exercise training in subjects with overweight or obesity may bring substantial health benefits in the long term. Despite a relatively large number of studies included in our meta‐analysis (N = 54), the heterogeneity of participants, especially regarding their initial blood pressure status, was important and prevented us from comparing different types of exercise training. In studies enrolling only patients with hypertension, a majority of study arms was based on aerobic training; this enabled us to conclude that aerobic exercise is effective in reducing systolic and diastolic blood pressure in subjects with overweight or obesity and hypertension. A recent systematic review focusing on individuals with hypertension confirmed the efficacy of aerobic exercise and highlighted the potential role of HIIT in reducing blood pressure values with a greater magnitude.82 Also, resistance training seems to have similar effect in reducing blood pressure, with higher effect in patients with hypertension.83, 84, 85 These findings cannot yet be extended to hypertensive subjects with overweight or obesity because of the currently limited available data.
A further source of heterogeneity came from the interventions provided in addition to exercise training. One study, by Larson‐Meyer et al.,38 aimed to assess the effect of energy intake restriction versus energy intake restriction plus aerobic exercise, whereas the research of Kadoglu et al.34 was the only one combining exercise and drug treatment and one of the two with a long duration of the intervention (48 weeks). Nevertheless, the overall effect did not significantly change by eliminating these studies from the meta‐analysis. Although previous data have shown that the effect of exercise is higher when it is associated with weight loss,86 there are several exercise‐induced mechanisms that can explain blood pressure lowering, independently from weight loss.87 Indeed, changes in body composition, particularly when characterized by increased lean mass and decreased adipose tissue, especially ectopic fat, are able to improve the endocrine, metabolic, and inflammatory profile, with beneficial effects on blood pressure control.35, 88 In this connection, mice experiments demonstrated that increasing muscle mass improves obesity‐associated hypertension and that a plausible mechanism involves the relationship between glucose metabolism and the vascular and renal function.89 Accordingly, a previous meta‐analysis focused on the effect of dynamic resistance training in prehypertensive patients showed a significant reduction in systolic and diastolic blood pressure values, with a notable dose–response relationship.90 A link between myokines (i.e., myostatin) and arterial stiffness has also been recently described in healthy adolescents.91 Moreover, the improvement of endothelial function, arterial structure, and stiffness due to vasodilation and the reduction of vascular oxidative stress play also an important role in reducing blood pressure in response to exercise training.92, 93
4.2. Insulin resistance
Exercise training programs were effective in reducing insulin resistance in patients with overweight or obesity, with or without type 2 diabetes. Current results confirm findings of Thaane et al.10 who found a significant effect on insulin sensitivity, examining the effect of short term exercise training (<12 weeks) in subjects with overweight or obesity and type 2 diabetes. In addition, authors stated that the effect was poorly influenced by weight loss and was more pronounced for vigorous exercise. The efficacy of exercise was demonstrated also for dynamic measures of insulin sensitivity (i.e., clamp, oral glucose tolerance test, and insulin tolerance test), in patients with type 2 diabetes.11 It is known that physical exercise improves metabolic flexibility also by increasing insulin‐dependent glucose uptake, particularly in individuals with type 2 diabetes.94 Furthermore, physical exercise can enhance glucose metabolism not only in muscle but also in adipose and hepatic tissue,95 determining an overall improvement of insulin sensitivity.96 Overall, it is still unknown what the best exercise prescription would be (i.e., modality, volume, and intensity of exercise) to improve insulin sensitivity in patients with obesity and type 2 diabetes. Our systematic review found nine RCTs including only patients with type 2 diabetes, and only one performed resistance training, three performed HIIT, and five performed aerobic exercise. However, our subanalysis suggests that aerobic exercise, also including high intensity, reduces insulin resistance in patients with obesity and type 2 diabetes. Previous studies indicated that high‐intensity exercise had a greater beneficial effect than moderate intensity exercise on insulin resistance.97 Actually, high‐intensity exercise seems to have a more pronounced effect in recruiting glycolytic fibers,98 and in decreasing visceral fat,99 counteracting insulin resistance. Also for this outcome, heterogeneity was partly reduced by removing the study of Kadoglu et al.34 without changes in the overall effect. In this systematic review, it was not possible to identify the role played by exercise in improving insulin sensitivity, regardless of weight loss. Nevertheless, possible explanations for a higher insulin sensitivity can rely on the reduction in visceral adipose tissue and the virtuous hormonal cascade leading to a multiorgan improvement of glucose metabolism.87 On the other hand, also a quantitative and qualitative improvement in muscle mass induced by exercise training may explain the enhancement in insulin sensitivity observed in subjects with obesity with or without type 2 diabetes that followed exercise programs.11, 100, 101 From a clinical perspective, it is well‐known that enhancement of insulin sensitivity, rather than plasma glucose level, has a major role in improving diabetes outcomes, while insulin resistance is a better predictor of future cardiovascular events in nondiabetic individuals.99 Furthermore, there is compelling evidence that insulin resistance by itself is a cardiovascular risk factor in a variety of population groups, including the general population and patients with diabetes.99
4.3. Intrahepatic fat
Our results show the effective role of exercise training in reducing MR measures of intrahepatic fat in groups of adults with overweight or obesity. These findings are in line with previous reviews that pointed in the same direction. Keating et al. in 2012 showed that exercise training alone is effective in reducing intrahepatic fat in patients with NAFLD.35 Similarly, in 2017, Katsagoni et al. showed that exercise, alone or combined with dietary intervention, improves serum hepatic enzymes and intrahepatic fat (moderate to large effect size) in patients with NAFLD even in absence of weight loss.13 This well‐conducted systematic review included all types of intrahepatic fat measures (MR, ultrasound, and liver biopsy). Our work is focused on MR measures of intrahepatic fat, with 10 included studies using MR spectroscopy and the others with MR imaging. MR is the noninvasive method that better correlates with liver biopsy, recognized as the gold standard for the identification of liver steatosis. These MR techniques showed great accuracy and high intraindividual reproducibility.102 Hence, restricting meta‐analysis to studies that used these methods for intrahepatic fat quantification strengthen the reliability of our findings. In 2018, Smart et al. described the effect of exercise alone on surrogate markers of liver function, in subjects with overweight/obesity or fatty liver disease. The authors described a significant effect of exercise in reducing intrahepatic fat, particularly when selecting RCTs based on energy expenditure (at least 10,000 kcal for the total exercise program) and exercise without a diet intervention (only three studies included). Our pooled analyses showed that HIIT has the larger effect size in reducing intrahepatic fat, even though other exercise modalities also exhibited a statistically significant effect. Similar results were previously suggested by Katsagoni et al.,13 who showed that the effect on intrahepatic fat was larger for higher intensity and volume of training. These results can be achieved independently from weight loss.13 Several mechanisms can be responsible for the effect of exercise on NAFLD.103 In fact, it is well‐known that physical exercise is effective in decreasing hepatic lipogenesis104 and gluconeogenesis and in improving skeletal muscle glycogen synthesis, resulting in lower plasma and liver triglycerides105 and higher insulin sensitivity, also in patients with obesity.106 NAFLD shares common pathophysiological pathways with sarcopenia, such as insulin resistance and inflammation, and people that perform regular exercise have a lower probability to develop NAFLD. Indeed, a preserved muscle mass seems to be a key component for the exercise‐induced amelioration of fatty liver.107 Further experimental studies in rats report the increase of mitochondrial oxidation of lipids and the prevention or attenuation of the endoplasmic reticulum stress, a condition tightly linked to reactive oxygen species generation, chronic low‐grade inflammation, and apoptosis in the setting of metabolic diseases.13 The efficacy of exercise in reducing intrahepatic fat levels has important clinical implications. Indeed, liver fat content is considered a sensitive “barometer” for metabolic health.108 Intrahepatic fat accumulation leads to NAFLD, a condition recognized as the hepatic manifestation of the metabolic syndrome and metabolically related with adipose tissue insulin resistance and diabetes pathophysiology.109
5. LIMITATIONS AND PERSPECTIVES
Physical exercise and pharmacological treatments are both key components for the management of obesity and its related comorbidities. Their interactive effects might be explained by shared metabolic and vascular mechanisms, with a potential synergistic or compensatory effect on cardiometabolic health.110, 111, 112 However, future studies should explore the actual relationship between antidiabetic and/or anti‐obesity agents and exercise, in order to identify the best combination of pharmacological and nonpharmacological individualized treatment for each patient. Moreover, previous literature described that the response to lifestyle intervention for cardiometabolic health may be influenced by several factors such as sex and age, highlighting the importance of tailored actions.113, 114 Our current systematic review and meta‐analysis did not address these interesting aspects; thus, upcoming literature may focus on the analysis of the differential response to exercise therapy linked to age, sex, and the age–sex interaction.
Additional potential limitation is that data were often retrieved from secondary outcomes of the selected studies, introducing a potential inaccuracy tied to the power calculation of each study. Finally, most RCTs evaluated the effect of aerobic exercise; as a result, pooled analyses based on exercise modality were not performed for blood pressure and insulin resistance.
6. CONCLUSIONS
In conclusion, this systematic review and meta‐analysis showed a beneficial effect of physical exercise training in improving cardiometabolic health of adults with overweight or obesity. In these subjects, blood pressure, insulin resistance, and intrahepatic fat all decreased significantly after an exercise training program. Given the prevalence of these cardiometabolic risk factors in people with overweight or obesity, such improvements might bring substantial health benefits in this population. Our results indicate that blood pressure and insulin resistance may decrease to a larger extent in subjects with hypertension and with type 2 diabetes, respectively, reinforcing the importance of physical activity in people with overweight or obesity and comorbidities. Aerobic training has many well‐known benefits on cardiometabolic health and was indeed the most frequent type of exercise assessed in studies included in our review. We were able to assess the effect of different types of exercise training (aerobic, combined aerobic and resistance, and HIIT) on intrahepatic fat, and we found that all types of exercise were effective in reducing intrahepatic fat. Therefore, given the multiple benefits of exercise on major aspects of cardiometabolic health, individualized exercise prescription should be more broadly included in the management of people with overweight or obesity.
AUTHOR CONTRIBUTIONS
FB and AE performed the literature search, study selection, data extraction, and quality assessment. FB performed the meta‐analysis. All authors participated in the interpretation of data. FB and AE drafted the manuscript, and authors critically revised the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Table S1. Keywords included in database search strategy
Figure S1. Effect of exercise training vs. control on systolic blood pressure in adults with overweight or obesity.
Figure S2. Forest plot Effect of exercise training vs. control on diastolic blood pressure in adults with overweight or obesity.
Figure S3. Forest plot of the effect of exercise training programmes vs. control on HOMA‐IR in adults with overweight or obesity.
Figure S4. Forest plot of the effect of exercise training programmes vs. control on intrahepatic fat in adults with overweight or obesity.
Figure S5. Funnel plot.
Table S2. Summary of quality assessment of original studies.
Table S3. Characteristics of original studies
Table S4. Findings of original studies.
ACKNOWLEDGMENTS
The authors would like to thank the European Association for the Study of Obesity (EASO) for support in conducting this work.
Battista F, Ermolao A, van Baak MA, et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: Focus on blood pressure, insulin resistance, and intrahepatic fat—A systematic review and meta‐analysis. Obesity Reviews. 2021;22(S4):e13269. 10.1111/obr.13269
REFERENCES
- 1.Timmis A, Townsend N, Gale CP, et al. European society of cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41(1):12‐85. 10.1093/eurheartj/ehz859 [DOI] [PubMed] [Google Scholar]
- 2.Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776‐786. 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord. 2018;23(2):149‐157. 10.1007/s40519-018-0481-6 [DOI] [PubMed] [Google Scholar]
- 4.Care D, Suppl SS. 5. Lifestyle management: Standards of medical care in diabetesd2019. Diabetes Care. 2019;42(January):S46‐S60. 10.2337/dc19-S005 [DOI] [PubMed] [Google Scholar]
- 5.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi MBM, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021‐3104. 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver (EASL) . EASL–EASD–EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. Diabetologia. 2016;59(6):1121‐1140. 10.1007/s00125-016-3902-y [DOI] [PubMed] [Google Scholar]
- 7.Shaw K, Gennat H, O'Rourke P, del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;(4):CD003817.. 10.1002/14651858.CD003817.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pattyn N, Cornelissen VA, Eshghi SRT, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta‐analysis of controlled trials. Sport Med. 2013;43(2):121‐133. 10.1007/s40279-012-0003-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta‐analysis of randomized, controlled trials. Hypertension. 2011;58(5):950‐958. 10.1161/HYPERTENSIONAHA.111.177071 [DOI] [PubMed] [Google Scholar]
- 10.Thaane T, Motala AA, McKune AJ. Effects of short‐term exercise in overweight/obese adults with insulin resistance or type 2 diabetes: a systematic review of randomized controlled trials. J Diabetes Metab. 2018;9(12):816.. 10.4172/2155-6156.1000816 [DOI] [Google Scholar]
- 11.Way KL, Hackett DA, Baker MK, Johnson NA. The effect of regular exercise on insulin sensitivity in type 2 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Metab J. 2016;40(4):253‐271. 10.4093/dmj.2016.40.4.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batacan RB, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high‐intensity interval training on cardiometabolic health: a systematic review and meta‐analysis of intervention studies. Br J Sports Med. 2017;51(6):494‐503. 10.1136/bjsports-2015-095841 [DOI] [PubMed] [Google Scholar]
- 13.Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non‐alcoholic fatty liver disease: a meta‐analysis. Metabolism. 2017;68:119‐132. 10.1016/j.metabol.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 14.Bertot LC, Adams LA. The natural course of non‐alcoholic fatty liver disease. Int J Mol Sci. 2016;17(5):774.. 10.3390/ijms17050774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating SE, Hackett DA, George J, Johnson NA. Exercise and non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. J Hepatol. 2012;57(1):157‐166. 10.1016/j.jhep.2012.02.023 [DOI] [PubMed] [Google Scholar]
- 16.Golabi P, Locklear CT, Austin P, et al. Effectiveness of exercise in hepatic fat mobilization in nonalcoholic fatty liver disease: systematic review. World J Gastroenterol. 2016;22(27):6318‐6327. 10.3748/wjg.v22.i27.6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smart NA, King N, McFarlane JR, Graham PL, Dieberg G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta‐analysis. Br J Sports Med. 2018;52(13):834‐843. 10.1136/bjsports-2016-096197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelbasset WK, Elsayed SH, Nambi G, et al. Effect of moderate‐intensity aerobic exercise on hepatic fat content and visceral lipids in hepatic patients with diabesity: a single‐blinded randomised controlled trial. Evidence‐Based Complement Altern Med. 2020;2020:1‐7. 10.1155/2020/1923575 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Leitner DR, Frühbeck G, Yumuk V, et al. Obesity and type 2 diabetes: Two diseases with a need for combined treatment strategies—EASO can lead the way. Obes Facts. 2017;10(5):483‐492. 10.1159/000480525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1‐7. 10.1016/j.phrs.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 21.Zelber‐Sagi S, Buch A, Yeshua H, et al. Effect of resistance training on non‐alcoholic fatty‐liver disease a randomized‐clinical trial. World J Gastroenterol. 2014;20(15):4382‐4392. 10.3748/wjg.v20.i15.4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petridou A, Siopi A, Mougios V. Exercise in the management of obesity. Metabolism. 2019;92:163‐169. 10.1016/j.metabol.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 23.Patsopoulos NA, Evangelou E, Ioannidis JPA. Sensitivity of between‐study heterogeneity in meta‐analysis: Proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148‐1157. 10.1093/ije/dyn065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Series CB. Cochrane Handbook for Systematic Reviews of Interventions.; 2019. 10.1002/9781119536604 [DOI]
- 25.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 SUPPL. 1):102‐138. 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity. 2009;17(12):2162‐2168. 10.1038/oby.2009.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorostegi‐Anduaga I, Corres P, MartinezAguirre‐Betolaza A, et al. Effects of different aerobic exercise programmes with nutritional intervention in sedentary adults with overweight/obesity and hypertension: EXERDIET‐HTA study. Eur J Prev Cardiol. 2018;25(4):343‐353. 10.1177/2047487317749956 [DOI] [PubMed] [Google Scholar]
- 28.Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non‐alcoholic fatty liver disease independent of weight loss. Gut. 2011;60(9):1278‐1283. 10.1136/gut.2011.242073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high‐intensity interval training reduces liver fat and improves cardiac function in non‐alcoholic fatty liver disease: a randomized controlled trial. Clin Sci. 2015;129(12):1097‐1105. 10.1042/CS20150308 [DOI] [PubMed] [Google Scholar]
- 30.Hinderliter AL, Sherwood A, Craighead LW, et al. The long‐term effects of lifestyle change on blood pressure: one‐year follow‐up of the ENCORE study. Am J Hypertens. 2014;27(5):734‐741. 10.1093/ajh/hpt183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho SS, Radavelli‐Bagatini S, Dhaliwal SS, Hills AP, Pal S. Resistance, aerobic, and combination training on vascular function in overweight and obese adults. J Clin Hypertens. 2012;14(12):848‐854. 10.1111/j.1751-7176.2012.00700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houghton D, Thoma C, Hallsworth K, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15(1):96‐102.e3. 10.1016/j.cgh.2016.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105‐1112. 10.1002/hep.23129 [DOI] [PubMed] [Google Scholar]
- 34.Kadoglou NPE, Iliadis F, Sailer N, et al. Exercise training ameliorates the effects of rosiglitazone on traditional and novel cardiovascular risk factors in patients with type 2 diabetes mellitus. Metabolism. 2010;59(4):599‐607. 10.1016/j.metabol.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 35.Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174‐182. 10.1016/j.jhep.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 36.Keadle SK, Lyden K, Staudenmayer J, et al. The independent and combined effects of exercise training and reducing sedentary behavior on cardiometabolic risk factors. Appl Physiol Nutr Metab. 2014;39(7):770‐780. 10.1139/apnm-2013-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrunée M, Antoine D, Vergès B, Robin I, Casillas JM, Gremeaux V. Effects of a home‐based rehabilitation program in obese type 2 diabetics. Ann Phys Rehabil Med. 2012;55(6):415‐429. 10.1016/j.rehab.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 38.Larson‐Meyer DE, Redman L, Heilbronn LK, Martin CK, Ravussin E. Caloric restriction with or without exercise: the fitness versus fatness debate. Med Sci Sports Exerc. 2010;42(1):152‐159. 10.1249/MSS.0b013e3181ad7f17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh M, Kim S, An KY, et al. Effects of alternate day calorie restriction and exercise on cardio‐metabolic risk factors in overweight and obese adults: an exploratory randomized controlled study. BMC Public Health. 2018;18(1):1‐10. 10.1186/s12889-018-6009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plotnikoff RC, Eves N, Jung M, Sigal RJ, Padwal R, Karunamuni N. Multicomponent, home‐based resistance training for obese adults with type 2 diabetes: a randomized controlled trial. Int J Obes (Lond). 2010;34(12):1733‐1741. 10.1038/ijo.2010.109 [DOI] [PubMed] [Google Scholar]
- 41.Pugh CJA, Sprung VS, Kemp GJ, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol ‐ Hear Circ Physiol. 2014;307(9):H1298‐H1306. 10.1152/ajpheart.00306.2014 [DOI] [PubMed] [Google Scholar]
- 42.Schroeder EC, Franke WD, Sharp RL, Lee D. chul. Comparative effectiveness of aerobic, resistance, and combined training on cardiovascular disease risk factors: a randomized controlled trial. PLoS One. 2019;14(1):1‐14. 10.1371/journal.pone.0210292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stensvold D, Tjønna AE, Skaug EA, et al. Strength training versus aerobic interval training to modify risk factors of metabolic syndrome. J Appl Physiol. 2010;108(4):804‐810. 10.1152/japplphysiol.00996.2009 [DOI] [PubMed] [Google Scholar]
- 44.Straznicky EA, Lambert MT, Grima T, et al. 1. The effects of dietary weight loss with or without exercise training on liver enzymes in obese metabolic syndrome subjects. Diabetes, Obes Metab. 2012;14:139‐148. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55(6):1738‐1745. 10.1002/hep.25548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tjønna AE, Lee SJ, Rognmo Ø, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346‐354. 10.1161/CIRCULATIONAHA.108.772822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdelaal AAM, Mohamad MA. Obesity indices and haemodynamic response to exercise in obese diabetic hypertensive patients: randomized controlled trial. Obes Res Clin Pract. 2015;9(5):475‐486. 10.1016/j.orcp.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 48.Waib PH, Gonçalves MI, Barrile SR. Improvements in insulin sensitivity and muscle blood flow in aerobic‐trained overweight‐obese hypertensive patients are not associated with ambulatory blood pressure. J Clin Hypertens. 2011;13(2):89‐96. 10.1111/j.1751-7176.2010.00393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winding KM, Munch GW, Iepsen UW. The effect of low‐volume high‐intensity interval training. Diabetes Obes Metab. 2018;20(6):1‐27. [DOI] [PubMed] [Google Scholar]
- 50.Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy‐matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity—a randomized trial. Metabolism. 2018;78:128‐140. 10.1016/j.metabol.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 51.Zhang HJ, He J, Pan LL, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern Med. 2016;176(8):1074‐1082. 10.1001/jamainternmed.2016.3202 [DOI] [PubMed] [Google Scholar]
- 52.Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8‐week high‐intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health‐related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine (Baltimore). 2019;98(12):e14918.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balducci S, Zanuso S, Cardelli P, et al. Supervised exercise training counterbalances the adverse effects of insulin therapy in overweight/obese subjects with type 2 diabetes. Diabetes Care. 2012;35(1):39‐41. 10.2337/dc11-1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouchonville M, Armamento‐Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes (Lond). 2014;38(3):423‐431. 10.1038/ijo.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuthbertson DJ, Shojaee‐Moradie F, Sprung VS, et al. Dissociation between exercise‐induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non‐alcoholic fatty liver disease. Clin Sci. 2016;130(2):93‐104. 10.1042/CS20150447 [DOI] [PubMed] [Google Scholar]
- 56.Fritz T, Caidahl K, Lundstrom P, et al. Effects of Nordic walking on cardiovascular risk factors in overweight individuals with type 2 diabetes, impaired or normal glucose tolerance. Diabetes Metab Res Rev. 2013;29(1):25‐32. 10.1002/dmrr [DOI] [PubMed] [Google Scholar]
- 57.Goodpaster BH, DeLany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA ‐ J Am Med Assoc. 2010;304(16):1795‐1802. 10.1001/jama.2010.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersen TR, Schmidt JF, Thomassen M, et al. A preliminary study: effects of football training on glucose control, body composition, and performance in men with type 2 diabetes. Scand J Med Sci Sport. 2014;24(SUPPL.1):43‐56. 10.1111/sms.12259 [DOI] [PubMed] [Google Scholar]
- 59.Croymans DM, Krell SL, Oh CS, et al. Effects of resistance training on central blood pressure in obese young men. J Hum Hypertens. 2014;28(3):157‐164. 10.1038/jhh.2013.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heydari M, Boutcher YN, Boutcher SH. The effects of high‐intensity intermittent exercise training on cardiovascular response to mental and physical challenge. Int J Psychophysiol. 2013;87(2):141‐146. 10.1016/j.ijpsycho.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 61.Kim YS, Nam JS, Yeo DW, Kim KR, Suh SH, Ahn CW. The effects of aerobic exercise training on serum osteocalcin, adipocytokines and insulin resistance on obese young males. Clin Endocrinol (Oxf). 2015;82(5):686‐694. 10.1111/cen.12601 [DOI] [PubMed] [Google Scholar]
- 62.Kolahdouzi S, Baghadam M, Kani‐Golzar FA, et al. Progressive circuit resistance training improves inflammatory biomarkers and insulin resistance in obese men. Physiol Behav. 2019;205:15‐21. 10.1016/j.physbeh.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 63.Kucio C, Narloch D, Kucio E, Kurek J. The application of nordic walking in the treatment hypertension and obesity. Fam Med Prim Care Rev. 2017;19(2):144‐148. 10.5114/fmpcr.2017.67870 [DOI] [Google Scholar]
- 64.Masuo K, Rakugi H, Ogihara T, Lambert GW. Different mechanisms in weight loss‐induced blood pressure reduction between a calorie‐restricted diet and exercise. Hypertens Res. 2012;35(1):41‐47. 10.1038/hr.2011.134 [DOI] [PubMed] [Google Scholar]
- 65.Mendham AE, Duffield R, Marino F, Coutts AJ. A 12‐week sports‐based exercise programme for inactive Indigenous Australian men improved clinical risk factors associated with type 2 diabetes mellitus. J Sci Med Sport. 2015;18(4):438‐443. 10.1016/j.jsams.2014.06.013 [DOI] [PubMed] [Google Scholar]
- 66.Cao L, Jiang Y, Li Q, Wang J, Tan S. Exercise training at maximal fat oxidation intensity for overweight or obese older women: A randomized study. J Sport Sci Med. 2019;18(3):413‐418. [PMC free article] [PubMed] [Google Scholar]
- 67.Fenkci S, Sarsan A, Rota S, Ardic F. Effects of resistance or aerobic exercises on metabolic parameters in obese women who are not on a diet. Adv Ther. 2006;23(3):404‐413. 10.1007/BF02850161 [DOI] [PubMed] [Google Scholar]
- 68.Swift DL, Earnest CP, Katzmarzyk PT, Rankinen T, Blair SN, Church TS. The effect of different doses of aerobic exercise training on exercise blood pressure in overweight and obese postmenopausal women. Menopause. 2012;19(5):503‐509. 10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taghian F, Zolfaghari M, Hedayati M. Effects of aerobic exercise on serum retinol binding protein4, insulin resistance and blood lipids in obese women. Iran J Public Health. 2014;43(5):658‐665. [PMC free article] [PubMed] [Google Scholar]
- 70.Figueroa A, Gil R, Wong A, et al. Whole‐body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens Res. 2012;35(6):667‐672. 10.1038/hr.2012.15 [DOI] [PubMed] [Google Scholar]
- 71.Figueroa A, Kalfon R, Wong A. Whole‐body vibration training decreases ankle systolic blood pressure and leg arterial stiffness in obese postmenopausal women with high blood pressure. Menopause. 2015;22(4):423‐427. 10.1097/GME.0000000000000332 [DOI] [PubMed] [Google Scholar]
- 72.García‐Unciti M, Izquierdo M, Idoate F, et al. Weight‐loss diet alone or combined with progressive resistance training induces changes in association between the cardiometabolic risk profile and abdominal fat depots. Ann Nutr Metab. 2012;61(4):296‐304. 10.1159/000342467 [DOI] [PubMed] [Google Scholar]
- 73.Kim JW, Kim DY. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab Syndr Relat Disord. 2012;10(6):452‐457. 10.1089/met.2012.0036 [DOI] [PubMed] [Google Scholar]
- 74.Meckling KA, Sherfey R. A randomized trial of a hypocaloric high‐protein diet, with and without exercise, on weight loss, fitness, and markers of the metabolic syndrome in overweight and obese women. Appl Physiol Nutr Metab. 2007;32(4):743‐752. 10.1139/H07-059 [DOI] [PubMed] [Google Scholar]
- 75.Mohr M, Nordsborg NB, Lindenskov A, et al. High‐Intensity intermittent swimming improves cardiovascular health status for women with mild hypertension. Biomed Res Int. 2014;2014:1‐9. 10.1155/2014/728289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pourranjbar M, Arabnejad N, Naderipour K, Rafie F. Effects of aerobic exercises on serum levels of myonectin and insulin resistance in obese and overweight women. J Med Life. 2018;11(4):381‐386. 10.25122/jml-2018-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryan AS, Ge S, Blumenthal JB, Serra MC, Prior SJ, Goldberg AP. Aerobic exercise and weight loss reduce vascular markers of inflammation and improve insulin sensitivity in obese women. J Am Geriatr Soc. 2014;62(4):607‐614. 10.1111/jgs.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zelber‐Sagi S, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven‐year prospective follow‐up. J Hepatol. 2012;56(5):1145‐1151. 10.1016/j.jhep.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 79.Piercy KL, Troiano RP. Physical activity guidelines for Americans from the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes. 2018;11(11):e005263. 10.1161/CIRCOUTCOMES.118.005263 [DOI] [PubMed] [Google Scholar]
- 80.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2(1):1‐9. 10.1161/JAHA.112.004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903‐1913. 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 82.Leal JM, Galliano LM, del Vecchio FB. Effectiveness of high‐intensity interval training versus moderate‐intensity continuous training in hypertensive patients: a systematic review and meta‐analysis. Curr Hypertens Rep. 2020;22(3):26.. 10.1007/s11906-020-1030-z [DOI] [PubMed] [Google Scholar]
- 83.Skrypnik D, Ratajczak M, Karolkiewicz J, et al. Effects of endurance and endurance‐strength exercise on biochemical parameters of liver function in women with abdominal obesity. Biomed Pharmacother. 2016;80:1‐7. 10.1016/j.biopha.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 84.Inder JD, Carlson DJ, Dieberg G, Mcfarlane JR, Hess NCL, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta‐analysis to optimize benefit. Hypertens Res. 2016;39(2):89‐94. 10.1038/hr.2015.111 [DOI] [PubMed] [Google Scholar]
- 85.Jin YZ, Yan S, Yuan WX. Effect of isometric handgrip training on resting blood pressure in adults: a meta‐analysis of randomized controlled trials. J Sports Med Phys Fitness. 2017;57(1–2):154‐160. 10.23736/S0022-4707.16.05887-4 [DOI] [PubMed] [Google Scholar]
- 86.Bacon SL, Sherwood A, Hinderliter A, Blumenthal JA. Effects of exercise, diet and weight loss on high blood pressure. Sport Med. 2004;34(5):307‐316. 10.2165/00007256-200434050-00003 [DOI] [PubMed] [Google Scholar]
- 87.Keating SE, Coombes JS, Stowasser M, Bailey TG. The role of exercise in patients with obesity and hypertension. Curr Hypertens Rep. 2020;22(10):77.. 10.1007/s11906-020-01087-5 [DOI] [PubMed] [Google Scholar]
- 88.Slentz CA, Bateman LA, Willis LH, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol ‐ Endocrinol Metab. 2011;301(5):1033‐1039. 10.1152/ajpendo.00291.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Butcher JT, Mintz JD, Larion S, et al. Increased muscle mass protects against hypertension and renal injury in obesity. J Am Heart Assoc. 2018;7(16):1‐12. 10.1161/JAHA.118.009358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.MacDonald HV, Johnson BT, Huedo‐Medina TB, et al. Dynamic resistance training as stand‐alone antihypertensive lifestyle therapy: a meta‐analysis. J Am Heart Assoc. 2016;5(10):e003231.. 10.1161/JAHA.116.003231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pucci G, Ministrini S, Nulli Migliola E, et al. Relationship between serum myostatin levels and carotid‐femoral pulse wave velocity in healthy young male adolescents. The Maciste Study. J Appl Physiol. 2021;130(4):987‐992. 10.1152/japplphysiol.00782.2020 [DOI] [PubMed] [Google Scholar]
- 92.Gronek P, Wielinski D, Cyganski P, et al. A review of exercise as medicine in cardiovascular disease: Pathology and mechanism. Aging Dis. 2020;11(2):327‐340. 10.14336/AD.2019.0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pucci G, Battista F, Schillaci G. Aerobic physical exercise and arterial de‐stiffening: a recipe for vascular rejuvenation. Hypertens Res. 2012;35(10):964–966. 10.1038/hr.2012.107 [DOI] [PubMed] [Google Scholar]
- 94.Schrauwen‐Hinderling VB, Kooi ME, Schrauwen P. Mitochondrial function and diabetes: consequences for skeletal and cardiac muscle metabolism. Antioxidants Redox Signal. 2016;24(1):1‐39. 10.1089/ars.2015.6291 [DOI] [PubMed] [Google Scholar]
- 95.Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21(1):1‐12. 10.1055/s-2000-8847 [DOI] [PubMed] [Google Scholar]
- 96.Smith RL, Soeters MR, Wüst RCI, Houtkooper RH. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev. 2018;39(4):489‐517. 10.1210/er.2017-00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ross R, Janssen I, Dawson J, et al. Exercise‐induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12(5):789‐798. 10.1038/oby.2004.95 [DOI] [PubMed] [Google Scholar]
- 98.Wallman K, Plant LA, Rakimov B, Maiorana AJ. The effects of two modes of exercise on aerobic fitness and fat mass in an overweight Population. Res Sport Med. 2009;17(3):156‐170. 10.1080/15438620903120215 [DOI] [PubMed] [Google Scholar]
- 99.Verheggen RJHM, Maessen MFH, Green DJ, Hermus ARMM, Hopman MTE, Thijssen DHT. A systematic review and meta‐analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17(8):664‐690. 10.1111/obr.12406 [DOI] [PubMed] [Google Scholar]
- 100.Bouchonville MF, Villareal DT. Sarcopenic obesity: how do we treat it? Curr Opin Endocrinol Diabetes Obes. 2013;20(5):412‐419. 10.1097/01.med.0000433071.11466.7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rubio‐Ruiz ME, Guarner‐Lans V, Pérez‐Torres I, Soto ME. Mechanisms underlying metabolic syndrome‐related sarcopenia and possible therapeutic measures. Int J Mol Sci. 2019;20(3):647. 10.3390/ijms20030647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16(13):1560‐1566. 10.3748/wjg.v16.i13.1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takahashi H, Kotani K, Tanaka K, Egucih Y, Anzai K. Therapeutic approaches to nonalcoholic fatty liver disease: exercise intervention and related mechanisms. Front Endocrinol (Lausanne). 2018;9(OCT):1‐17. 10.3389/fendo.2018.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trefts E, Williams AS, Wasserman DH. Exercise and the regulation of hepatic metabolism. Vol 135. 1st ed. Elsevier Inc.; 2015. 10.1016/bs.pmbts.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim K‐B, Kim K, Kim C, et al. Effects of exercise on the body composition and lipid profile of individuals with obesity: a systematic review and meta‐analysis. J Obes Metab Syndr. 2019;28(4):278‐294. 10.7570/jomes.2019.28.4.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rabøl R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci U S a. 2011;108(33):13705‐13709. 10.1073/pnas.1110105108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee YH, Jung KS, Kim SU, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008‐2011). J Hepatol. 2015;63(2):486‐493. 10.1016/j.jhep.2015.02.051 [DOI] [PubMed] [Google Scholar]
- 108.Bril F, Barb D, Portillo‐Sanchez P, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132‐1144. 10.1002/hep.28985 [DOI] [PubMed] [Google Scholar]
- 109.Godoy‐Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes. Diabetol Metab Syndr. 2020;12(1):1‐20. 10.1186/s13098-020-00570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Franz MJ, VanWormer JJ, Crain AL, et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755‐1767. 10.1016/j.jada.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 111.Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ, Reusch JEB. Saxagliptin restores vascular mitochondrial exercise response in the goto‐kakizaki rat. J Cardiovasc Pharmacol. 2015;65(2):137‐147. 10.1097/FJC.0000000000000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eckstein ML, Williams DM, O'Neil LK, Hayes J, Stephens JW, Bracken RM. Physical exercise and non‐insulin glucose‐lowering therapies in the management of Type 2 diabetes mellitus: a clinical review. Diabet Med. 2019;36(3):349‐358. 10.1111/dme.13865 [DOI] [PubMed] [Google Scholar]
- 113.Huang C, Sun S, Tian X, et al. Age modify the associations of obesity, physical activity, vision and grip strength with functional mobility in Irish aged 50 and older. Arch Gerontol Geriatr. 2019;84(38):103895. 10.1016/j.archger.2019.05.020 [DOI] [PubMed] [Google Scholar]
- 114.Pucci G, Alcidi R, Tap L, Battista F, Mattace‐Raso F, Schillaci G. Sex‐ and gender‐related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. 2017;120:34‐42. 10.1016/j.phrs.2017.03.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Keywords included in database search strategy
Figure S1. Effect of exercise training vs. control on systolic blood pressure in adults with overweight or obesity.
Figure S2. Forest plot Effect of exercise training vs. control on diastolic blood pressure in adults with overweight or obesity.
Figure S3. Forest plot of the effect of exercise training programmes vs. control on HOMA‐IR in adults with overweight or obesity.
Figure S4. Forest plot of the effect of exercise training programmes vs. control on intrahepatic fat in adults with overweight or obesity.
Figure S5. Funnel plot.
Table S2. Summary of quality assessment of original studies.
Table S3. Characteristics of original studies
Table S4. Findings of original studies.


