Abstract
Problem
Immune responses of fetal membranes involve the production of chemoattractant mediators causing infiltration of maternal and fetal leukocytes, intrauterine inflammation and potentially the disruption of maternal‐fetal tolerance. Prolactin (PRL) has deep immunoregulatory effects in the fetal‐maternal interface. We aimed to test the in vitro PRL effect upon chemotactic capacities of human fetal membranes.
Method of Study
Fetal membranes and umbilical cord blood were collected from healthy non‐laboring caesarean deliveries at term. Fetal membranes were cultured in Transwell® frames to mimic the barrier function between choriodecidual and amniotic sides. Tissues were treated with PRL, Lipopolysaccharide (LPS), or both simultaneously. Then, RANTES, MCP‐1, MIP‐1α, IP‐10, and PECAM‐1 were quantified in a conditioned medium by choriodecidual or amniotic sides. The chemotaxis of subsets of migrating mononuclear cells from umbilical cord blood was evaluated in a Boyden Chamber in response to the conditioned medium by both sides.
Results
Lipopolysaccharide stimulates the production of RANTES, MCP‐1, MIP‐1α, and PECAM‐1 in choriodecidua, while MIP‐1α and PECAM‐1 only increase in amnion. PRL decrease RANTES, MCP‐1, and MIP‐1 only in choriodecidua, but PECAM‐1 was decreased mainly in amnion. The leukocyte migration was regulated significantly in response to the conditioned medium by the amnion, increase in the conditioned medium after LPS treatment, contrary with, the leukocyte migration decreased in a significant manner in response to conditioned medium after PRL and LPS‐PRL co‐treatment. Finally, T cells were the most responsive subset of cells.
Conclusions
Prolactin modified in a tissue‐specific manner the chemotactic factor and the leukocyte migration differentially in fetal membranes.
Keywords: amnion, leukocytes, LPS, migration, prolactin, T cells
1. INTRODUCTION
Fetal membranes constitute a selective barrier with physical and immunological functions essential for maintaining the fetus in an immunologically privileged milieu of placenta and are composed of two compartments of choriodecidua and amnion.1 These adjacent structures are non‐vascularized, however, infiltrating leukocytes are observed during labor.2The onset of labor is led by the secretion of cytokines such as TNF‐α, IL‐1β, and IL‐6 produced by the myometrium, cervix, and fetal membranes. This inflammatory environment causes the activation of the myometrium, cervical dilation, and rupture of fetal membranes in a coordinated manner.3 Secondary to this initial inflammatory stage, gestational tissues secrete chemoattractant such as IL‐8, RANTES, MCP‐1, and MIP‐1α, which recruit leukocytes from maternal and fetal circulation.2 Progressive accumulation of immune cells in the myometrium, decidua, fetal membranes, and amniotic cavity enhance the inflammatory environment and tissue remodeling to induce labor.3Several works have demonstrated that fetal membranes possess immunological capabilities that play an important part in the immune defense against pathogens.4, 5, 6 However, higher immune response caused by intrauterine infections can trigger a premature onset of labor before the 37 weeks of gestation.7 In this scenario, inflammatory responses increase the number of immunocompetent cells (neutrophils, monocytes, and lymphocytes) into the fetal membranes, promoting a premature rupture of chorioamniotic membranes.8, 9On the other hand, Prolactin (PRL) is a neuroendocrine hormone that was originally described as a product of secretion by the pituitary gland, and involved in the development of breast and milk production.10 During pregnancy, decidua is the main source of extra‐pituitary PRL production, that crosses the fetal membranes and stores in the amniotic cavity, reaching levels of 4000 ng/mL, which are 10 times greater than maternal and fetal serum concentrations.11, 12, 13 PRL performs important functions at the maternal‐fetal interface regulating the osmolarity in fetal membranes,14 promoting the proliferation and migration of extravillous trophoblast,15 maintaining higher levels of progesterone16 and performing immunomodulatory effects.10 PRL decreases the secretion of inflammatory cytokines such as IL‐6 in decidual cells,16 decreases the expression and secretion of TNF‐α and IL‐1β induced by Lipopolysaccharide (LPS) from Escherichia coli in the placenta,17 and decreases the secretion of prostaglandin E2 from fetal membranes.14 In our laboratory, we demonstrated that PRL inhibits the secretion of TNF‐α, IL‐1β and MMP‐9,18 and limits the secretion of pro‐inflammatory cytokines induced by LPS in fetal membranes with differential effects between choriodecidual and amniotic sides.19, 20
Considering the background, the goal in this work was to evaluate the effect of PRL on the chemoattractant potential of LPS‐activated fetal membranes in an in vitro model.
2. MATERIAL AND METHODS
2.1. Biological samples
This study was approved by the Human Ethics, Biosecurity and Research Committees of the Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes” (212250–3210–21205–01–15) and UMAE Hospital de Gineco‐Obstetricia No. 4 “Luis Castelazo Ayala” (IMSS R‐2017–785–013) in Mexico City. All patients accepted to participate in the study by signing a written informed consent form.
Fetal membranes, and cord blood samples from healthy women at term (range 37.5–39.5 week of pregnancy), uncomplicated, and singleton pregnancies were collected after elective cesarean section, and were performed before the onset of labor. No patient was under medical treatment or received medication. Patients with a history of cervicovaginal infection, penicillin allergy, chronic hypertension, polyhydramnios, oligohydramnios, diabetes mellitus, or preeclampsia were excluded from the study. Additionally, microbial analyses of all samples were conducted to preclude the presence of chorioamniotic infection. All sample tissues were examined by macroscopic and routine histopathology techniques to confirm the normal morphology of these tissues and the absence of inflammation signals. The clinical data of participants are summarized in Table 1.
TABLE 1.
Demographic and clinical characteristics of the study population
| Study population (n = 6) | |
|---|---|
| Clinical data | Mean ± SD (Range) |
| Maternal age (years) | 32.0 ± 5.2 (20–39) |
| Maternal pregestacional weigth (kg) | 66.1 ± 8.0 (57–81) |
| Maternal gestacional weigth (kg) | 72.6 ± 6.2 (57–81) |
| Gestational age (weeks) | 38.6 ± 1.0 (37–39) |
| Pregnancies (number) | 2.1 ± 1.0 (1–3) |
| Newborngender (Female‐Male %) | 50–50 |
SD, Standard deviation.
The biological samples were obtained and transported in sterile conditions at birth. Umbilical cord blood was collected in Heparin‐containing tubes (BD Vacutainer, Becton Dickinson) by venipuncture of the umbilical vein. Fetal membranes were cut five centimetres above the placenta and transferred to sterile sodium chloride solution 0.9% (PiSA®).
2.2. Culture and stimulation of chorioamniotic membranes
All samples were manipulated under sterile conditions. We used the two‐compartment tissue culture system, which has been previously standardized by our group.21 Briefly, we exhaustively rinse the chorioamniotic membranes with sodium chloride solution 0.9% to eliminate the remaining blood clothes; we cut explants of whole membranes that were manually mounted and held with silicone rubber rings to be placed into a Transwell® frame (Costar) with the polycarbonate membrane previously removed. Thus, created two independent compartments that allowed us to test the compartmentalized secretion of soluble factors. The choriodecidua were faced to the upper chamber of the Transwell® and the amnios to the lower chamber.
The mounted explants were placed in a 12‐well tissue culture plate (Costar). One mL of DMEM with phenol red (Biowest; Nuaillé, FRA) and supplemented with 0.2% lactalbumin hydrolysate, 1 mM sodium pyruvate, 1× antibiotic‐antimycotic solution (penicillin 100 U/mL, streptomycin 100 µg/mL and amphotericin B 0.25 µg/mL) (GIBCO, BRL) were added to each side of the chamber and were incubated in 5% CO2 at 37°C for a day. At the beginning of culture, 4000 ng/mL of PRL (Peprotech) were added to the upper and lower chambers. At the second day, old medium was replaced by 1 mL of fresh culture medium in accord with the experimental treatments that included: (1) culture media; (2) PRL [4000 ng/mL]; (3) LPS [500 ng/mL]; (4) LPS +PRL; additionally, we include an anti‐inflammatory control that comprises LPS +Dexamethasone [200 nM] cotreatment and the cotreatment of LPS +Methotrexate [50 µM], a non‐specific inhibitor of PRL‐receptor signaling. The LPS stimulation was added just on the choriodecidua in all treatments. Samples were incubated 24 h in 5% CO2 at 37°C and the medium from both compartments were collected, divided into aliquots, and stored at −70°C until assay.
2.3. Quantification of chemotactic factors
The concentrations of chemokines MCP‐1 (900‐K31; Peprotech), MIP‐1α (900‐K35; Peprotech), RANTES (900‐K33; Peprotech), IP‐10 (900‐K39; Peprotech), and the adhesion molecule PECAM‐1 (DY806‐05; R&D Systems) present in cell culture supernatants from maternal and fetal compartments were determined by Sandwich ELISA according to the manufacturer´s instructions.
2.4. Isolation of umbilical cord blood mononuclear cells (UCBMC)
Using umbilical cord blood samples from donors previously collected into heparinized tubes were diluted 1:1 with sterile sodium chloride solution 0.9% (PiSA®). Then, 25 mL of diluted blood was carefully underlaid in 12 mL of Lymphoprep® (Stemcell Technologies) to isolate the leukocytes by density gradient, then centrifuged at 400 g for 40 min at room temperature with the brake turned off. Following the procedure, the band of leukocytes (which represent UCBMC) was recovered and resuspended in Roswell Park Memorial Institute Medium (RPMI‐1640; GIBCO Invitrogen) without any supplementation. Leukocyte viability was assessed by trypan blue exclusion and the leukocyte count was determined using a Hemocytometer. Viability was greater than 95%. Leukocyte suspension was stored at 37°C and used within an hour after isolation.
2.5. Chemotaxis assay
The chemotaxis assay was performed using a Boyden Chamber, where 350 µL of each conditioned medium coming from amnios and choriodecidua were placed in the lower chamber as a chemotactic factor; immediately 1 × 106 UCBMC in 350 µL RPMI were added on top of the polycarbonate membrane Transwell® insert (8 µm pore size; Costar) that correspond to the upper chamber (Figure 1).
FIGURE 1.
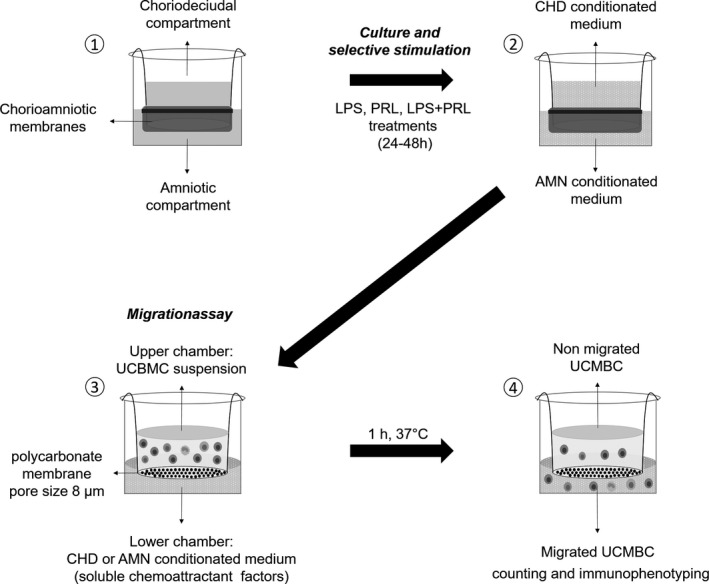
Diagram of experimental design. The conditioned medium by choriodecidua (CHD) and amnion (AMN), was generated by the culture of fetal membranes in a two independent chambers model (1), and the subsequent pre‐treatment with PRL (0–24 h) and the selective stimulation with LPS alone or only PRL and a set of transwells with the endotoxin and PRL co‐treated at the same time (24–48 h) (2). General conditions of migration assay of UCBMC in response to conditioned medium (3) to the posterior counting and Immunophenotyping of migrant UCBMC (4)
Boyden chambers were incubated from 60 minutes at 37°C in humidified air containing 5% CO2. Afterwards, Transwell inserts containing the non‐migrated leukocytes were removed.
Every experimental condition in chemotaxis assay was performed by triplicate; two replicates were used to cell counting and the other one left for immunophenotyping.
To evaluate the proper functioning of the migration assay and determine whether the Prolactin present in the conditioned media could act as a chemoattractant, internal controls were performed, consisting of the use of supplemented DMEM non conditioned by fetal membranes (negative control), supplemented DMEM +10% Fetal Calf Serum (FCS; positive control), and supplemented DMEM +4000 ng/mL of PRL.
2.6. Migrant Leukocyte counting
UCBMC that migrated through the filter into the bottom wells were collected, stored, and then stained with the CyQUANT® GR dye kit (Invitrogen) following manufacturer's instructions and quantified by interpolation from the standard curve using a BioTek® Synergy HTX microplate reader.
2.7. Immunophenotyping of migrant UCBMC
After we collected and centrifuged the migrated cells to get a pellet, these were stained and analyzed by flow cytometry to identify the phenotype of attracted heterologous leukocytes using conjugated monoclonal antibodies BD Multitest®: total leukocytes/anti‐CD45‐PerCP (2D1/HLe‐1), T cells/anti‐CD3‐FITC (SK7), B cells/anti‐CD19‐APC (SJ25C1), NK cells/anti‐CD16+‐PE (B73.1)/anti‐CD56‐PE (NCAM 16.2; BD Bioscience) and Monocytes/anti‐CD14‐PE/Cy7 (HCD14; Biolegend). The flow cytometer FACSAria III (BD Bioscience) was set to analyze at least 10 000 events, the results were analyzed using the BD FACSDiva 8.0.2 software (BD Bioscience).
2.8. Statistical analysis
The chemokine immunoassay results were analyzed by non‐parametric analysis of variance Kruskal–Wallis. PECAM immunoassay results, the differences between experimental treatments in the number of total leukocyte migration and immunophenotyping of immune cells were analyzed by one‐way analysis of variance (ANOVA) using Sigma Stat v 11 (Systat Software, SigmaStat Version 13, from Systat Software, Inc.; systatsoftware.com). A p‐value ≤ 0.05 was considered statistically significant.
3. RESULTS
Taking into consideration that in this study, we used biological samples from pregnant women, demographics sensible data of the patients that participate in this study are not publicly available due to privacy or ethical restrictions.
3.1. Differential secretion profile of chemokines in the conditioned mediums by fetal membranes
After experimental treatments, the concentration of secreted chemokines was measured in supernatants. We observed a differential secretion profile between each compartment in the chorioamniotic membranes culture system. RANTES and IP‐10 were mainly detected in the choriodecidual conditioned mediums, while MCP‐1 and MIP‐1α were detected in both choriodecidual and amniotic conditioned mediums (Figure 2).
FIGURE 2.
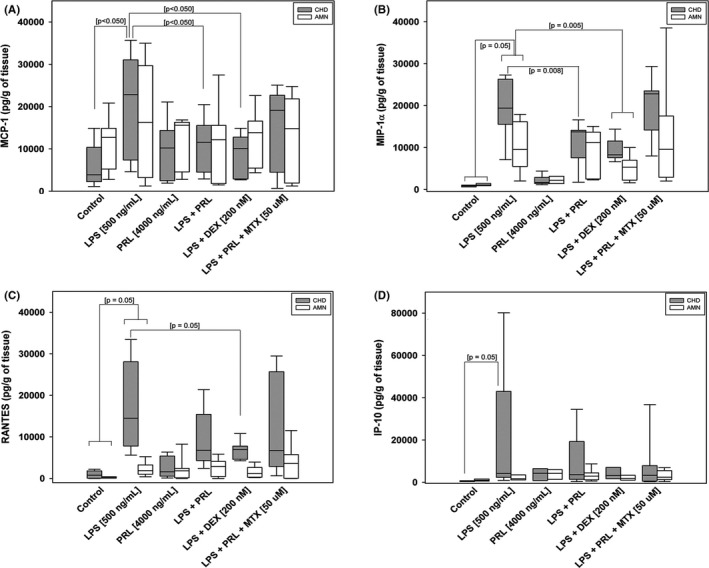
Effect of co‐treatment with PRL on the secretion of chemokines to the culture medium. A, Secretion profile of MCP‐1. B, MIP‐1‐α, C, RANTES, and D, IP‐10 after 24 h of the selective stimulation measure by ELISA in choriodecidual (CHD) and amniotic (AMN) compartments. Graphs show medians with an interquartile range of six independent experiments by triplicate
In comparison with control, LPS treatment increased significantly the secretion of RANTES (18‐fold), MCP‐1 (6‐fold), MIP‐1α (23‐fold), and IP‐10 (7.1 fold) in the choriodecidual region (p < 0.001). On the other hand, the same stimulus only significantly increased the secretion of MIP‐1α (8‐fold) in the amniotic region compared to control (p < 0.001). As a control, PRL does not induce the production of secreted chemokines in both compartments. Interestingly, the co‐treatment with PRL plus LPS, PRL significantly diminish the secretion of MCP‐1 and MIP‐1α induced by LPS, in 50% and 70% respectively (p < 0.001), compared to only LPS treatment (Figure 2A,B); but the secretion of RANTES and IP‐10 was not decreased in response to this co‐treatment (Figure 2C,D). For amnion, none of the chemokines induced by LPS was significantly downregulated by the co‐treatment with LPS plus PRL.
The co‐treatment with LPS and Dexamethasone was used as an experimental control of anti‐inflammation, which decreased the secretion of RANTES, MCP‐1, and MIP‐1α induced by LPS in choriodecidua (p < 0.001); and the MIP‐1α secretion induced by LPS in amnion (p < 0.001), as expected.
On the contrary, the co‐treatment with Methotrexate significantly reversed the inhibitory effect of PRL increasing the secretion of MIP‐1α in choriodecidua at similar level induced by the LPS stimulus. The MCP‐1 and RANTES secretion showed a tendency to increase in response to co‐treatment with Methotrexate in choriodecidua and amnion. These results indicate that chemokine down‐regulation is PRL dependent because Methotrexate acts as a pan‐JAK inhibitor, blocking the canonical signaling of PRL which depends on the JAK/STAT activation pathway.
3.2. The differential soluble fraction of PECAM‐1 in the conditioned mediums by fetal membranes
In the choriodecidual compartment, the experimental treatments did not show a significant difference among them. Conversely, in the amniotic compartment, the treatment with LPS increased 1.4‐fold of the PECAM‐1 levels in a significant manner in respect to control (p < 0.001), the co‐treatment with LPS and PRL decreased at 52% the PECAM‐1 levels in comparison with the levels induced by LPS treatment (p < 0.001).
The PECAM‐1 levels in the simple PRL treatment were not differed to control. In the co‐treatment with LPS plus Dexamethasone the PECAM‐1 levels were significantly minor to LPS levels, even to control levels in both choriodecidual and amniotic compartments (p < 0.001). The co‐treatment with Methotrexate was not able to revert the PRL effect in comparison to the co‐treatment with LPS (Figure 3).
FIGURE 3.
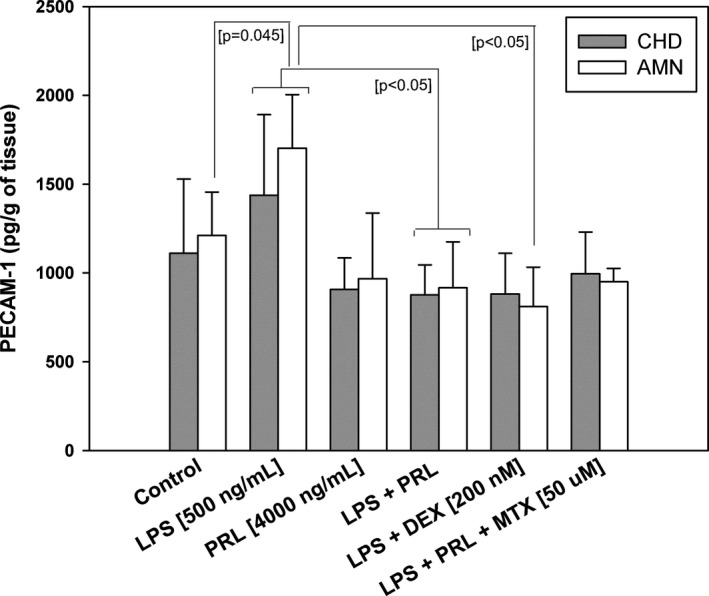
The soluble fraction of PECAM‐1 in conditioned mediums. In vitro secretion profile of PECAM‐1 measure in the amniotic (AMN) and choriodecidual (CHD) compartments after different treatments stimulation. Each bar represents Mean ±SD of three independent experiments by triplicate
3.3. Differential chemotactic capacities of choriodecidua and amnion conditioned mediums
After the migration assays, where the conditioned mediums by amnion and choriodecidua were used, we counted the migrant cells in the lower chamber.
The internal controls of the chemotaxis assay demonstrate that the supplemented DMEM non‐conditioned medium (negative control) and PRL [4000 ng/mL] added to non‐conditioned medium does not have chemotactic activity per se in contrast with the positive control (FCS 10%) (Figure 4A).
FIGURE 4.
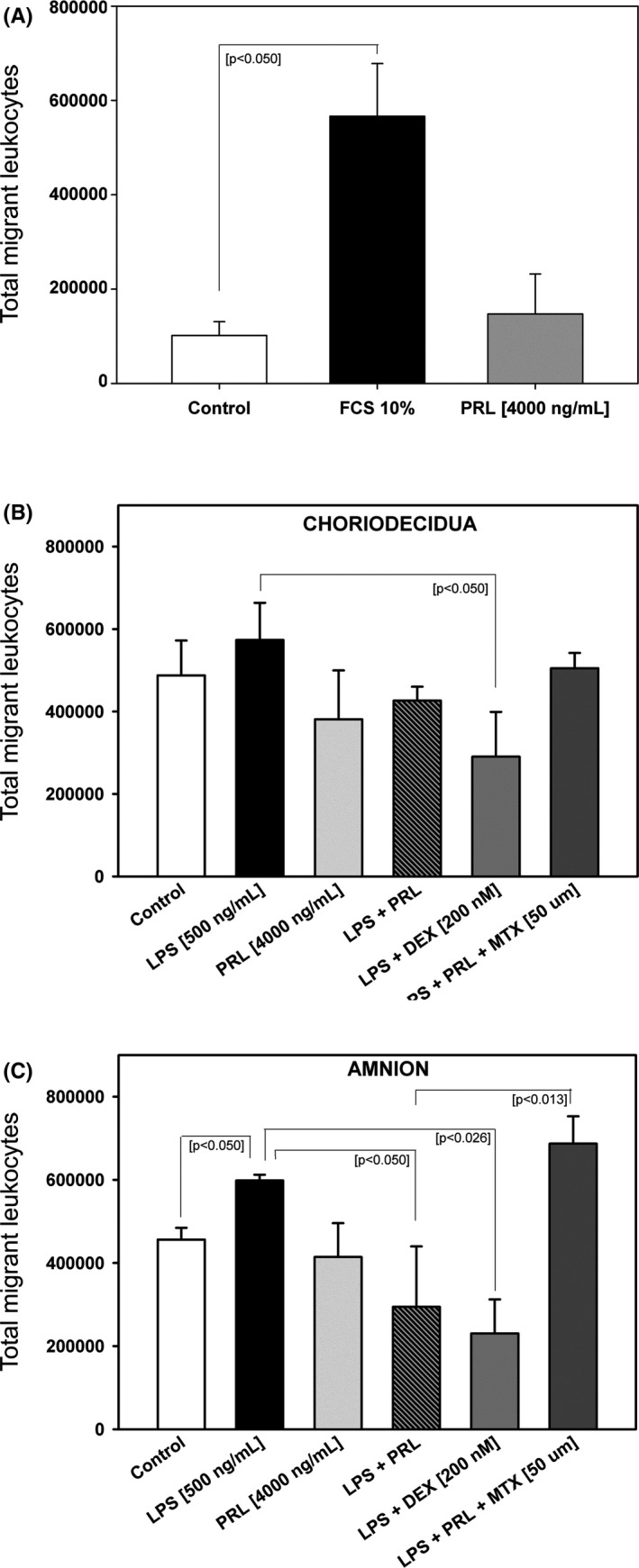
PRL treatment of fetal membranes decreases the total leukocyte migration in response to the conditioned medium by amnion. A, Internal assay controls, each bar represents the Mean ±SD of three independent experiments. Statistical differences between positive control (FCS 10%) and negative control are indicated by *p < 0.001. B, In vitro migration profile of total leukocytes in response to conditioned mediums from choriodecidua (CHD). C, In vitro migration profile of total leukocytes in response to conditioned mediums from amnion (AMN). Each bar represents Mean ±SD of three independent experiments by duplicate
In experimental control conditions, there are chemotactic factors that increase the migration of leukocytes. A similar pattern in both amnion and choriodecidual conditioned medium was observed for the total migrant cells in response to different experimental treatments, but the statistical differences were only found in amnion (Figure 4B,C).
We observed that the LPS‐conditioned amnion medium significantly increased the chemotaxis of total UCBMC in comparison with the experimental control medium (p < 0.001), also co‐treatment with PRL decreased the leukocyte migration in the conditioned medium amnion (p < 0.001). According to the incapacity of PRL to induce secreted chemokines in fetal membranes by itself, the medium coming from PRL‐exposed tissues did not increase the chemotactic activity, neither choriodecidual nor amniotic conditioned mediums.
Besides, the co‐treatment with LPS and Dexamethasone decreased the cell migration in the conditioned medium for both, amnion and choriodecidua, but the media conditioned by the co‐treatment with Methotrexate, LPS, and PRL, increased the chemotaxis of UCBMC only in amnion conditioned medium (p < 0.01).
3.4. Subset of migrant UCBMC
To determine the differential sensitivity of the leukocyte's subsets in response to the conditioned medium, we analyzed the immunophenotype of chemoattracted UCBMC by flow cytometry. T lymphocytes were the population with the highest migration (Figure 5A), followed by Nk cells (Figure 5B); B lymphocytes (Figure 5C) and monocytes (Figure 5D) were found in a smaller proportion. The significant chemotactic regulation was not observed in B‐lymphocytes, NK cells, or monocytes, but in T cells, the pattern of migration was different between treatments.
FIGURE 5.
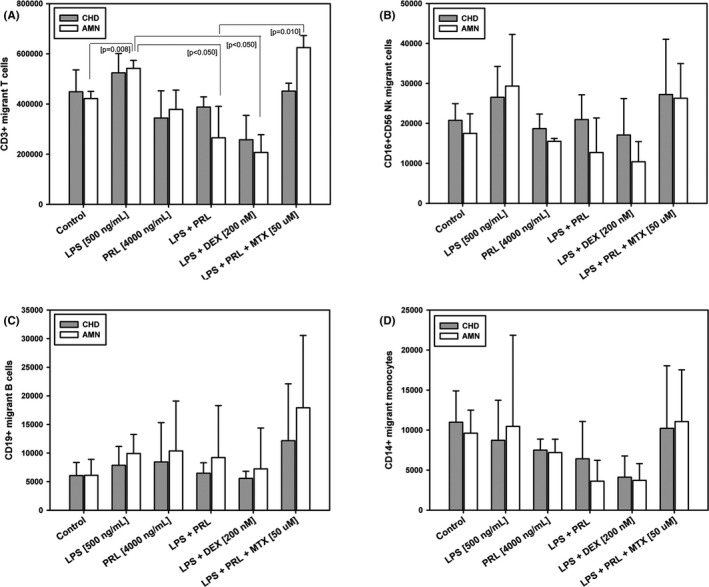
The phenotype of migrant leukocytes in response to conditioned mediums from CHD and AMN. Characterization of subpopulations of migrants leukocytes in response to conditioned mediums from choriodecidua (CHD) and amnion (AMN). (A) T lymphocytes, (B) Nk cells, (C) B lymphocytes, and (C) monocytes. Each bar represents the Mean ± SD of three independent experiments
We found significant changes in the number of chemoattracted T cells, increased 1.3‐fold in response to LPS treatment in the amnion conditioned medium (p < 0.001) versus experimental control medium. As shown in Figure 5A, in media conditioned by co‐treatment with LPS plus PRL or Dexamethasone, T cell chemotaxis decreased by 52% and 62% respectively compared to induced LPS‐chemotaxis (p < 0.001).
There were no significant changes in the mediums conditioned by choriodecidual treatments (Figure 5A), except in the anti‐inflammatory control (co‐treatment with LPS and Dexamethasone), in this case, the T cells decreased compared to the chemotaxis observed in the medium conditioned by the LPS treatment and control (p < 0.001).
4. DISCUSSION
Clinical and experimental evidence supports that leukocyte infiltration into the maternal‐fetal interface is a critical event during inflammation in term labor and preterm labor22, 23 with or without infection24, 25, 26 this cellular infiltration is regulated by chemokines and cellular adhesion molecules.27 Previous research demonstrated that fetal membranes have immunological competencies that include the secretion of the chemokine.28, 29
Our results demonstrate that under basal conditions, amnion and choriodecidua showed a differential secretion profile of RANTES, MCP‐1, MIP‐1α and IP‐10. However, once stimulation with LPS was done, both regions responded by increasing in a significant manner the differential secretion of all chemokines to their culture medium which supports the function and responsiveness of our ex vivo experimental model. This differential secretion profile agrees with previous works, where fetal membranes are challenged in vitro by bacterial products19 and virus.30 These results suggest different capacities of choriodecidua and amnion to recruit a specific subset of immune cells depending on the immunological challenge that is presented. Moreover, in our results, the increased secretion of RANTES, MCP‐1, MIP‐1α by LPS, were inhibited by PRL as we propose in our hypothesis, in agreement with our previous work, where PRL regulates in a dose‐dependent manner the differential secretion of chemokines LPS‐induced in chorioamniotic membranes.17
RANTES, MCP‐1, and MIP‐1α are members of CCL chemokines family; and they are the chemokines mostly detected in amniotic fluid during preterm labor with/without infection.8, 24, 31, 32 RANTES (CCL5) is a potent chemotactic factor to exert the activation of monocytes, T Lymphocytes, involved in the adhesion and migration of T cells into the endothelium.33 MCP‐1 (CCL2) attracts monocytes and macrophages into the inflammatory tissue, where it promotes the respiratory burst, lead the macrophage activation and polarization of M1 macrophage,31, 32 and MIP‐1α (CCL‐3) chemoattract neutrophils, basophils, lymphocytes, and monocytes which activate the release of histamine and cytokine production.34 The differential in vitro chemokine secretion profile for each region of the fetal membranes observed suggests selective and differential recruitment of immune cells.
To test the hypothesis that each region of fetal membranes influenced by PRL can attract a specific phenotype of immune cells, we used the conditioned media coming from amnion compartment and choriodecidual compartment in Boyden Chamber assays and evaluated the chemotaxis of leukocytes isolation of umbilical cordon blood. Surprisingly, contrary with what we expected, only conditioned amnion media regulated differentially the total increased leukocyte migration in response to LPS treatment, and PRL decreased the total number of migrant's leukocytes.
This effect may be attributed to the downregulation of soluble PECAM‐1, which we observed only in amnion conditioned medium and the lower chemokine secretion in this side in comparison to the choriodecidual response. PECAM‐1 or CD31 is a transmembrane hemophilic receptor not only highly expressed in endothelial cells but also in granulocytes and leukocytes. The CD31 homophilic interaction between lymphocytes endothelium and neutrophils play a part in the recruitment, extravasation, and transmigration of immune cells into the inflamed tissues.35, 36 In fetal membranes from spontaneous vaginal term labor, the PECAM‐1 and ICAM‐1 mRNA expression are upregulated in the decidua, chorion, and amnion regions, indicating the role of the adhesion molecules in the inflammatory processes in these tissues.37 Furthermore, the extracellular domain of PECAM‐1 is released during tissue damage and endothelial cell apoptosis.38 In fact, the higher levels of PECAM‐1 in amniotic fluid suggest that the determination of PECAM‐1 levels could be used as a marker of intra‐amniotic inflammation and the possible development of preterm rupture of membranes.39
These results give an important biological significance to the differential role of each compartment from fetal membranes in their immune response. Unlike amnion, the choriodecidua is in direct contact with a greater number of maternal immune cells throughout the pregnancy. In vitro evidence, has supported that amnion possesses intrinsic immunoregulatory properties, the co‐culture with amniotic cells or conditioned media from the amniotic cells inhibits the activation/proliferation of T cells,40, 41 and the infiltration and survival of neutrophils.42, 43
It is known that several hormones can regulate the immune elements and the immunological competences of diverse tissues in the maternal‐fetal interface through pregnancy.44 Our results suggest that PRL could improve the intrinsic anti‐inflammatory properties that have been observed in the amnion, who has a critical location in the maternal fetal‐interface. We also report that the PRL present in the culture media did not induce the leukocyte migration per se, similar to what Dill and Walker reported45 in the mouse mammary gland in vitro and in vivo. However, unlike our results, they observed that PRL at 100 ng/mL improves the migration of T CD4+, T CD8+, B cells, macrophages, monocytes, and neutrophils during the mammary gland development. This effect of PRL upon the immune cells supports the hypothesis that the hyperprolactinemic environment prevalent along pregnancy has deep effects in the maternal immune system to maintain an anti‐inflammatory environment compatible with tolerance to fetus.
Gomez‐López et al29 previously reported selective chemotaxis of B cells in amnion extract tissues from term labor, while the recruitment of T cells, monocytes, and NK cells was driven by choriodecidual extract tissues. On the other hand, during vaginal term labor has been indentified a T cells subset in the rupture zone of chorioamniotic membranes.46, 47 In our study, lymphocytes T CD4+ were the responsive subpopulation to amnion‐conditioned media in response to LPS. Interestingly, T cells are the second most abundant population, after neutrophils, in amniotic fluid from women with spontaneous preterm labor and intra‐amniotic infection.48 Besides, Frascoli et al49 found the important role of fetal T cells from preterm labor, which exerts a strong pro‐inflammatory response to maternal antigens, furthermore, the authors demonstrate that T cells can be activated in vitro to produce INFγ and TNF‐α, and eventually induce the myometrial contractility.
In summary, illustrated in Figure 6, we propose that in vitro LPS‐challenge of fetal membranes turn on the inflammatory response, lead in part by the differential chemokine secretion. Although amnion secretes MCP‐1, MIP‐1α and PECAM, the choriodecidua is the main responsive region after LPS stimulation, consequently, leukocyte migration, mainly T cells, increases (1). Consequently, leukocyte migration (mainly T cells) of fetal membranes increases (2). We propose that PRL exerts a selective anti‐inflammatory effect, modifying the chemotaxis capacities of each compartment of fetal membranes, enhancing the intrinsic immunoregulatory properties of the amnion, regulating the traffic of T cells to the amniotic cavity, whose potential inflammatory response compromises local immunological tolerance and puts the normal continuation of pregnancy at risk.
FIGURE 6.
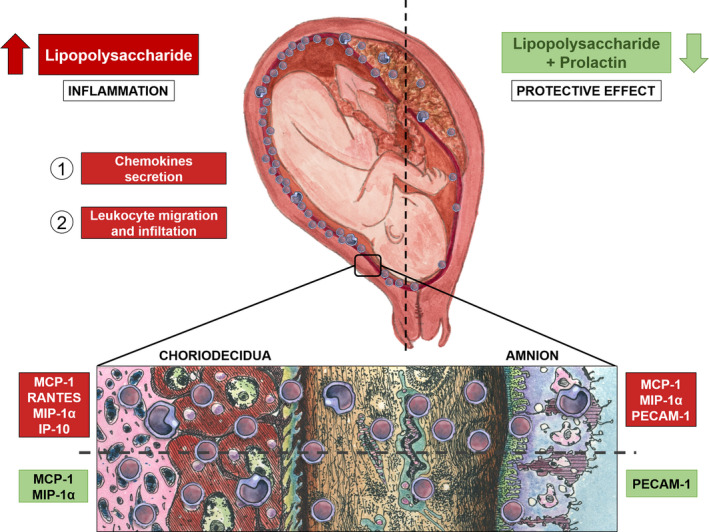
A comprehensive scheme of fetal membranes, chemotactic response. Differential chemokines secretion is turn on by choriodecidual and amniotic regions of term fetal membranes after LPS challenge (1), which leads to the selective increase in leukocyte migration (2). In the choriodecidua, RANTES, MCP‐1, MIP‐1α and IP‐10 increases, while in the amnion MCP‐1, MIP‐1α and PECAM‐1 increases after the infectious stimulus. PRL shows a diminishment in the MCP‐1 and MIP‐1α in choriodecidua, and PECAM‐1 in amnion. The chemotactic capacity of amnion upon leukocyte migration increases after the LPS challenge, on the contrary, PRL decreased the chemotactic capacity of the fetal membranes in this region as shown in the figure, indicating an anti‐inflammatory and protective effect of PRL during pregnancy, holding back positive feedback of inflammatory environment induced by professional immune cells attracted to the above‐mentioned tissues, which are key in the rupture of the fetal membranes, the remodeling of the cervix, and the dilation of the myometrium
In conclusion, PRL modifies the in vitro chemotactic capacities in fetal membranes, allowing us to speculate that this hormone exerts a protective effect that improves the immune response during adverse conditions such as infection, and supports the key role of PRL to maintain the local immune‐privilege during pregnancy at the maternal‐fetal interface.
CONFLICT OF INTEREST
We declare no conflict of interest that could be perceived as impacting our impartiality to the informed investigation.
ACKNOWLEDGEMENTS
We are grateful to the placenta donors for this research. We thank Irma Sosa González and Graciela Villeda Gabriel from Infectology and Immunology Branch for performing the microbiological analysis of all samples. We thank Yolotzin Valdespino Vázquez and Del Castillo‐Hernández B from Pathology Branch for their support in the histological analysis of all samples. This study was supported by the Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes” funds to ZCV (INPer IER‐ 212250‐3210‐21205‐01‐15) and by the National Council of Science and Technology of Mexico (CONACyT) Grant No. CB2014‐242162 to VZC. NSE was supported by CONACYT (Social service scholarship 28033). We also thank Dr. Adalberto Parra for formulating the original hypothesis of this project. Finally, Arturo Linares Herrera and Evelyn García Linares for their support in the artistic design of Figure 6 have our thanks.
Núñez‐Sánchez E and María Del Pilar Flores‐Espinosa equally contributed to this study.
Funding information
This study was supported by the INPER IER (Grant No. 3210‐21205‐01‐14, awarded to VZC) and the National Council of Science and Technology of Mexico (CONACyT) (Grant No. CB2014‐24162 to VZC).
DATA AVAILABILITY STATEMENT
Taking into consideration that in this study, we used biological samples from pregnant women, demographics sensible data of the patients that participate in this study are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Menon R, Richardson LS, Lappas M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta. 2019;79:40‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One. 2013;8(2):e56946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: Redundancy and synergy in human parturition. Hum Reprod Update. 2016;22(5):535‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaga‐Clavellina V, Ruiz M, Flores‐Espinosa P. In vitro secretion profile of pro‐inflammatory cytokines IL‐1β, TNF‐α, IL‐6, and of Human Beta‐Defensins (HBD)‐1, HBD‐2, and HBD‐3 from human chorioamniotic membranes after selective stimulation with gardnerella vaginalis. Am J Reprod Immunol. 2012;67(1):34‐43. [DOI] [PubMed] [Google Scholar]
- 5.Thiex NW, Chames MC, Loch‐Caruso RK. Tissue‐specific cytokine release from human extra‐placental membranes stimulated by lipopolysaccharide in a two‐compartment tissue culture system. Reprod Biol Endocrinol. 2009;7:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia‐Lopez G, Flores‐Espinosa P, Zaga‐Clavellina V. Tissue‐specific human beta‐defensins (HBD)1, HBD2, and HBD3 secretion from human extra‐placental membranes stimulated with Escherichia coli. Reprod Biol Endocrinol. 2010;8:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science. 2014;345(6198):760‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynec. 2015;213(4 Suppl):S29‐S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez‐Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal‐maternal interface during pregnancy. J Leukoc Biol. 2010;88(4):625‐633. [DOI] [PubMed] [Google Scholar]
- 10.Costanza M, Binart N, Steinman L, Pedotti R. Prolactin: a versatile regulator of inflammation and autoimmune pathology. Autoimmun Rev. 2015;14(3):223‐230. [DOI] [PubMed] [Google Scholar]
- 11.Soares MJ, Konno T, Alam SMK. The prolactin family: effectors of pregnancy‐dependent adaptations. Trends Endocrinol Metab. 2007;18(3):114‐121. [DOI] [PubMed] [Google Scholar]
- 12.Ben‐Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29(1):1‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kletzky OA, Rossman F, Bertolli SI, Platt LD, Mishell DR. Dynamics of human chorionic gonadotropin, prolactin, and growth hormone in serum and amniotic fluid throughout normal human pregnancy. Am J Obstet Gynec. 1985;151(7):878‐884. [DOI] [PubMed] [Google Scholar]
- 14.Tyson JE, McCoshen JA, Dubin NH. Inhibition of fetal membrane prostaglandin production by prolactin: Relative importance in the initiation of labor. Am J Obstet Gynecol. 1985;151(8):1032‐1038. [DOI] [PubMed] [Google Scholar]
- 15.Stefanoska I, Jovanović Krivokuća M, Vasilijić S, Ćujić D, Vićovac L. Prolactin stimulates cell migration and invasion by human trophoblast in vitro. Placenta. 2013;34(9):775‐783. [DOI] [PubMed] [Google Scholar]
- 16.Bao L, Tessier C, Prigent‐Tessier A, et al. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology. 2007;148(5):2326‐2334. [DOI] [PubMed] [Google Scholar]
- 17.Olmos‐Ortiz A, Déciga‐García M, Preciado‐Martínez E, et al. Prolactin decreases LPS‐induced inflammatory cytokines by inhibiting TLR‐4/NFκB signaling in the human placenta. Mol Hum Reprod. 2019;25(10):660‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaga‐Clavellina V, Parra‐Covarrubias A, Ramirez‐Peredo J, Vega‐Sanchez R, Vadillo‐Ortega F. The potential role of prolactin as a modulator of the secretion of proinflammatory mediators in chorioamniotic membranes in term human gestation. Am J Obstet Gynec. 2014;211(1):48.e1‐48.e6. [DOI] [PubMed] [Google Scholar]
- 19.Flores‐Espinosa P, Preciado‐Martínez E, Mejía‐Salvador A, et al. Selective immuno‐modulatory effect of prolactin upon pro‐inflammatory response in human fetal membranes. J Reprod Immunol. 2017;123:58‐64. [DOI] [PubMed] [Google Scholar]
- 20.Flores‐Espinosa P, Vega‐Sánchez R, Mancilla‐Herrera I, et al. Prolactin selectively inhibits the LPS‐induced chemokine secretion of human foetal membranes. J Matern Fetal Neonatal Med. 2020;33(24):4083‐4084. [DOI] [PubMed] [Google Scholar]
- 21.Zaga V, Estrada‐Gutierrez G, Beltran‐Montoya J, Maida‐Claros R, Lopez‐Vancell R, Vadillo‐Ortega F. Secretions of interleukin‐1β and tumor necrosis factor α by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod. 2004;71(4):1296‐1302. [DOI] [PubMed] [Google Scholar]
- 22.Thomson AJ, Telfer JF, Young A, et al. Leukocytes infiltrate the myometrium during human parturition: Further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229‐236. [PubMed] [Google Scholar]
- 23.Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro‐inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9(1):41‐45. [DOI] [PubMed] [Google Scholar]
- 24.Gomez‐Lopez N, Laresgoiti‐Servitje E, Olson DM, Estrada‐Gutiérrez G, Vadillo‐Ortega F. The role of chemokines in term and premature rupture of the fetal membranes: a review. Biol Reprod. 2010;82(5):809‐814. [DOI] [PubMed] [Google Scholar]
- 25.Takeda J, Fang X, Olson DM. Pregnant human peripheral leukocyte migration during several late pregnancy clinical conditions: a cross‐sectional observational study. BMC Pregnancy Childbirth. 2017;17(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez‐Lopez N, Romero R, Xu Y, et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol. 2018;79(4).e12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008;9(9):949‐952. [DOI] [PubMed] [Google Scholar]
- 28.Young A, Thomson AJ, Ledingham MA, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66(2):445‐449. [DOI] [PubMed] [Google Scholar]
- 29.Gomez‐Lopez N, Vadillo‐Perez L, Nessim S, Olson DM, Vadillo‐Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204(4):364.e9‐364.e16. [DOI] [PubMed] [Google Scholar]
- 30.Gervasi MT, Romero R, Bracalente G, et al. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med. 2012;25(10):2002‐2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobsson B, Mattsby‐Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Obstet Gynecol. 2003;82(5):423‐431. [DOI] [PubMed] [Google Scholar]
- 32.Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein‐1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra‐amniotic infection. J Matern Fetal Neonatal Med. 2005;17(6):365‐373. [DOI] [PubMed] [Google Scholar]
- 33.Athayde N, Romero R, Maymon E, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181(4):989‐994. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Gomez R, Galasso M, et al. Macrophage inflammatory protein‐1α in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32(2):108‐113. [DOI] [PubMed] [Google Scholar]
- 35.Marelli‐Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist's guide to CD31 function in T‐cells. J cell Sci. 2013;126(Pt11):2343‐2352. [DOI] [PubMed] [Google Scholar]
- 36.Vainer B, Nielsen OH. Chemotactic properties of ICAM‐1 and PECAM‐1 on neutrophil granulocytes in ulcerative colitis: effects of prednisolone and mesalazine. Aliment Pharmacol Ther. 2000;14(8):1023‐1031. [DOI] [PubMed] [Google Scholar]
- 37.Osman I, Crawford M, Jordan F, Young A, Norman J, Thomson A. Expression and localization of cell adhesion molecules in human fetal membranes during parturition. J Reprod Immunol. 2004;63(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 38.Ilan N, Mohsenin A, Cheung L, Madri JA. PECAM‐1 shedding during apoptosis generates a membrane‐anchored truncated molecule with unique signaling characteristics. FASEB J. 2001;15(2):362‐372. [DOI] [PubMed] [Google Scholar]
- 39.Shaarawy M, El‐Mallah SY, El‐Dawakhly AS, Mosaad M. The clinical value of assaying maternal serum and amniotic fluid intercellular adhesion molecule 1 (ICAM‐1) in cases of premature rupture of membranes. Cytokine. 1998;10(12):989‐992. [DOI] [PubMed] [Google Scholar]
- 40.Magatti M, Munari SD, Vertua E, Gibelli L, Wengler GS, Parolini O. Human amnion mesenchyme harbors cells with allogeneic T‐cell suppression and stimulation capabilities. Stem Cells. 2008;26(1):182‐192. [DOI] [PubMed] [Google Scholar]
- 41.Grzywocz Z, Hoser G, Sabalinska S, et al. Response of human normal and leukemia cells to factors released by amnion fragments in vitro. PLoS One. 2018;13(3).e0195035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan JL, Chan ST, Wallace EM, Lim R. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplant. 2014;23(3):319‐328. [DOI] [PubMed] [Google Scholar]
- 43.Zhou S, Chen J, Feng J. The effects of amniotic membrane on polymorphonuclear cells. Chin Med J (Engl). 2003;116(5):788‐790. [PubMed] [Google Scholar]
- 44.Robinson DP, Klein SL. Pregnancy and pregnancy‐associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dill R, Walker AM. Role of prolactin in promotion of immune cell migration into the mammary gland. J Mammary Gland Biol Neoplasia. 2017;22(1):13‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Khwad M, Pandey V, Stetzer B, et al. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13(3):191‐195. [DOI] [PubMed] [Google Scholar]
- 47.Gomez‐Lopez N, Vadillo‐Perez L, Hernandez‐Carbajal A, Godines‐Enriques M, Olson DM, Vadillo‐Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205(3):235.e15‐24. [DOI] [PubMed] [Google Scholar]
- 48.Gomez‐Lopez N, Romero R, Galaz J, et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra‐amniotic infection or intra‐amniotic inflammation. Am J Reprod Immunol. 2019;82(5).e13171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frascoli M, Coniglio L, Witt R, et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN‐γ and TNF‐α. Sci Transl Med. 2018;10(438):eaan2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Taking into consideration that in this study, we used biological samples from pregnant women, demographics sensible data of the patients that participate in this study are not publicly available due to privacy or ethical restrictions.


