Abstract
Prader‐Willi Syndrome (PWS) is a rare and incurable congenital neurodevelopmental disorder, resulting from the absence of expression of a group of genes on the paternally acquired chromosome 15q11‐q13. Phenotypical characteristics of PWS include infantile hypotonia, short stature, incomplete pubertal development, hyperphagia and morbid obesity. Hypothalamic dysfunction in controlling body weight and food intake is a hallmark of PWS. Neuroimaging studies have demonstrated that PWS subjects have abnormal neurocircuitry engaged in the hedonic and physiological control of feeding behavior. This is translated into diminished production of hypothalamic effector peptides which are responsible for the coordination of energy homeostasis and satiety. So far, studies with animal models for PWS and with human post‐mortem hypothalamic specimens demonstrated changes particularly in the infundibular and the paraventricular nuclei of the hypothalamus, both in orexigenic and anorexigenic neural populations. Moreover, many PWS patients have a severe endocrine dysfunction, e.g. central hypogonadism and/or growth hormone deficiency, which may contribute to the development of increased fat mass, especially if left untreated. Additionally, the role of non‐neuronal cells, such as astrocytes and microglia in the hypothalamic dysregulation in PWS is yet to be determined. Notably, microglial activation is persistently present in non‐genetic obesity. To what extent microglia, and other glial cells, are affected in PWS is poorly understood. The elucidation of the hypothalamic dysfunction in PWS could prove to be a key feature of rational therapeutic management in this syndrome. This review aims to examine the evidence for hypothalamic dysfunction, both at the neuropeptidergic and circuitry levels, and its correlation with the pathophysiology of PWS.
Keywords: hypothalamus, microglia, neuropeptides, obesity, Prader‐Willi Syndrome
Prader‐Willy Syndrome is a genetic obesity syndrome characterized by severe hyperphagia. Here, we provided an overview of defective hypothalamic neurocircuitries and peptides that underlie the gross obesity found in this disorder.
1. INTRODUCTION
Prader‐Willi Syndrome (PWS) is a rare congenital neurodevelopmental disorder caused by the loss of genes and non‐coding RNAs on chromosome 15q11‐q13.1 The phenotypic features affiliated with PWS are due to a multifactorial disruption of homeostatic processes at cellular and tissue level. Many features are present from a young age; motoric and linguistic milestones are typically delayed, and all individuals have a certain degree of mental retardation.1, 2, 3 In addition, PWS patients suffer from multiple endocrine abnormalities. PWS infants present with hypotonia which, among others, causes an impaired feeding behaviour due to poor suck and swallowing. By contrast, children and adults are characterised by gross hyperphagia and poor satiety, which lead to a severe obese phenotype.1, 2 The estimated prevalence of PWS is 1/10 000‐1/30 000 cases worldwide. Well‐defined, widely accepted diagnostic criteria are available and can strengthen the suspicion as early as in the foetal period; however, genetic testing remains the pillar of PWS diagnosis currently.2 Clinical studies have provided substantial evidence for hypothalamic dysfunction in PWS like uncontrollable hunger4, 5 and impaired growth6, 7 and sexual development.8 However, the current shred of evidence cannot pinpoint if the disorder is primarily due to hypothalamic defects or the disruptive hypothalamic function is a consequence of imbalance elsewhere. Further, the molecular mechanisms behind hypothalamic dysfunction in PWS are yet to be determined. In this review, we aim to discuss the evidence for hypothalamic dysfunction and the correlation with the clinical and pathophysiological aspects of PWS.
2. THE GENETICS OF PWS
The PWS phenotype results from the loss of paternally expressed components on chromosome 15q11‐q13. The same genes and non‐coding RNAs derived from the mother are inactivated by imprinting; and thus, not expressed under normal conditions. So far, it is impossible to attribute the phenotypic traits to a single gene; but rather, the symptomology of PWS is a consequence of the entire deletion. In this section, we will briefly discuss our current understanding of the role of each gene and its connections with the disruption of hypothalamic function. In addition, a schematic representation of the expression map of chromosome 15 can be found in Figure 1. Importantly, PWS‐associated loss of expression can be extended to a non‐imprinted region, resulting in a more severe phenotype.9 Conventionally, the extension of the deletion led to subdivision of the genotypes. Namely, PWS T1 genotype refers to those that lack expression of both, the critical and non‐imprinted region; whereas PWS T2 is associated with deletion exclusively of the critical region.9
FIGURE 1.
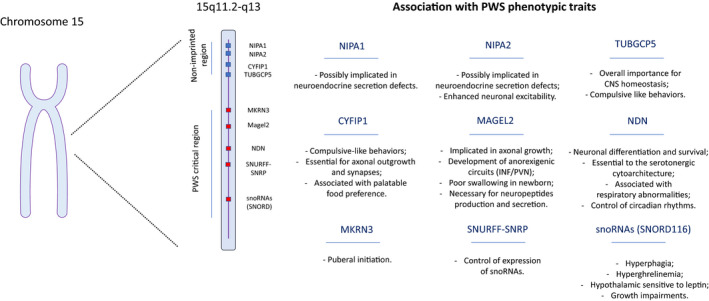
Schematic expression map of the PWS genomic region. PWS is caused by loss of expression of paternally inherited genes located in chrmosome 15. The extension of the deletion is critical for the severity of the phenotype, and patients that lack expression of genes in the non‐imprinted region are reported to present more serious symptons. In addition, the contribuition of the PWS‐causative genes to the phenotypic traits of the disease is given
2.1. NIPA1
NIPA1 encodes a transmembrane protein recruited upon fluctuations in intracellular magnesium concentrations.10, 11 There is no evidence for the participation of this gene in energy homeostasis in the hypothalamus or periphery. Its localization with endosomes suggests a role in the secretion pathways.10 A recent report has demonstrated that neurons from PWS patients have decreased secretory granules and neuropeptides production. However, the authors attribute this defect to the MAGEL2 gene.12 Since PWS T1 have more severe phenotypic traits it is possible that the additional loss of NIPA1 hampers the neuroendocrine maturation and secretion.12
2.2. NIPA2
NIPA2 shares structural and functional similarities with NIPA1. It is also a transmembrane protein sensitive to magnesium fluctuations.13 Likewise, there are no in‐depth studies that pinpoints a role for this protein in hypothalamic control of metabolism. However, some reports suggest that NIPA2 has a role in neuronal homeostasis, and thus its deletion might impact optimal neuronal functioning. Maternal protein restriction leads to increased expression of NIPA2 in the foetal hypothalamus in rats,14 but the implications of these findings are yet to be clarified. The authors also report an enrichment of mitochondrial metabolism proteins, which reinforces the hypothesis of the participation of NIPA2 in mitophagy.15 Further, NIPA2 absence has been linked to increased neuronal excitability in the cortex.16
2.3. CYFIP1
CYFIP1 is involved in cytoskeleton regulation and neuronal development, especially axonal outgrowth.17 It is particularly enriched in synaptosomes,18, 19 which reinforces the idea of a neuropeptidergic imbalance in PWS. CYFIP haploinsufficiency is also linked with compulsive‐like behaviour and it can be connected with palatable food preference.20 Therefore, CYFIP1 expression may relate to PWS symptomatology. In addition to neuronal physiology, CYFIP1 impacts myelination as well, through disruptions of oligodendrocyte biology.21
2.4. TUBGCP5
This protein encoding gene has a notorious role in centrosome formation,22 and therefore impacts the normal process of cellular division. Thus far, it has not been particularly implicated in hypothalamic circuits. Remarkably, disruption of this protein is implicated in microencephaly22; indicating an important role in brain development. Similar to CYFIP1, dysfunction or absence of TUBGCP5 is associated with the promotion of compulsive behaviours in neuropsychiatric disorders, such as the autism spectrum disorder.23
2.5. MKRN3
The Makoring RING protein 3 (MKRN3) is a protein encoding allele, and its deletion is common to all PWS sub genotypes. Here, it is important to highlight the fact that a paternal deletion of MKRN3, MAGEL2 and NDN (the last two will be discussed in sequence) does not result in PWS.24 This study reports two PWS subjects with PWS‐like features, but who do not display the core of PWS deletion. One of them, which lacks expression of the three mentioned genes had cognitive impairments and obesity,24 suggesting that those genes have a potential role in the phenotypic traits explored in this review.
MRKN3 in the hypothalamus is distinctly involved in the initiation of puberty.25 Hypothalamic MKRN3 is highly expressed in early life, both in rodents and nonhuman primates’ restraining signals of pubertal initiation. With the onset of puberty, its expression gradually decreases, which allows the expression of kisspeptin. When untimely released, this leads to gonadotropin‐releasing hormone (GnRH) secretion and sexual development.25, 26 Loss‐of function mutations in MRNK3 were consistently linked with central precocious puberty (CPP).27, 28 This is specially intriguing, since CPP is extremely rare in PWS. As far we were able to trace, there are five confirmed cases of CPP in genetically‐confirmed PWS patients29, 30, 31, 32 and the exact mechanism behind this phenomenon is still poorly understood. Unquestionably, central control of reproduction is complex and goes beyond the MRNK3‐Kisspeptin‐GnRH axis and even hormones classically associated with metabolism have major reproductive controlling roles.33, 34 Indeed, although evidence is still very fragmentary for any conclusions in PWS biology the marked hyperghrelinemia (discussed further down) can be a key point to understanding this puzzle. A variety of studies demonstrate a suppressive role of ghrelin in the hypothalamic‐pituitary‐gonadal axis both in vitro and in vivo35, 36, 37 and initial hints indicate that in humans a similar pattern is found.38, 39
2.6. MAGEL2
Melanoma antigen L2 (MAGEL2) is perhaps one of the most intensively explored genes among the PWS deletion. Its cellular function is to facilitate endosomal membrane protein recycling.40 It is expressed throughout the central nervous system (CNS), and abundantly present in the hypothalamus.41 Studies in MAGEL2 null mice have been insightful and many of the PWS features can be recapitulated in this model. For instance, impaired axonal growth and development of anorexigenic hypothalamic neuronal populations (pro‐opiomelanocortin and oxytocin related circuitry).42, 43 At the cellular level, its loss results in a decreased neuropeptide production and secretory capacity.12 Moreover, MAGEL2 null new born mice also display poor sucking as PWS infants.44
2.7. NDN
Necdin (NDN) is a nuclear protein exclusively expressed in differentiated neurons.45 This protein is mainly implicated with the molecular identity of neuronal cells45 and their survival.46, 47 The understanding of the spatial and temporal events that underlie neuronal dysfunction in PWS pathology is very limited. Interestingly, among all PWS‐related animal models only those with NDN deletion recapitulate the respiratory defects found in PWS‐patients48, 49 (i.e. sleep apnea).50 Recent findings demonstrate that NDN deletion disturbs the serotonergic cytoarchitecture leading to abnormal respiratory patterns.51 Lastly, in the murine hypothalamus the NDN transcript is abundantly present in the superchiasmatic nuclei, the master regulator of circadian rhythms.52 NDN presence controls the expression of key clock genes and therefore largely impacts the circadian regulation. From the metabolic perspective, these findings have several implications. Circadian integration of metabolism optimizes energy consumption and expenditure across the light/dark circles. Disruption of these patterns are tightly associated with obesity and its comorbidities, such as cardiovascular diseases53 and diabetes.54
2.8. SNUFF‐SNRPN
Little is known about the contribution of this protein to the pathophysiology of PWS. This is perhaps the least explored protein encoding gene in the PWS critical genomic region. However, a report from Cao and colleagues demonstrates that a deletion that causes loss of function of SNUFF‐SNRPN is sufficient to promote PWS‐like symptoms.55 The authors speculate this is because SNUFF‐SNRPN is responsible for the expression of key small nucleoar RNAs (sno RNAs – discussed next), that are central factors in the PWS symptomatology traits.55
2.9. sno RNAs
In addition to the protein‐coding alleles, non‐coding RNAs are found in the critical genomic region of PWS, especially snoRNAs. The exact biology of these RNAs is yet to be defined, but it has been proposed that they are involved in the modification of other RNAs.56 One special cluster of these biomolecules – the Snord116‐ is highly implicated in PWS and has gained crescent attention. Snord116del mice are of special interest in PWS research, because those mice in which the snoRNA116 deletion is of paternal heritage display hyperphagia.57 However, the Snor116del mice do not show increased body weight, even when fed an obesogenic diet. In fact, these mice present characteristically reduced body weight, delayed sexual maturation and high rates of mortality prior to weaning. The hypothalamic dysfunction associated with Snord116del can be explained by endocrine imbalance and sensitivity to adipostatic signals. These will be discussed into more detail in the sections below.
Taken together, it is possible to conclude that none of the individual features of the syndrome can be attributed to any single causative gene. Rather, the PWS symptomatology is a direct result of the cumulative deletion. Although animal models allowed us to have insights into the participation of each gene in the phenotype, we are far from understanding PWS at the molecular level. Certainly, the rarity of the syndrome and the limitations associated with human studies are the main sources of obstacles currently.
3. HYPOTHALAMIC NEUROCIRCUITS IN PWS
The insatiable hunger experienced by PWS subjects is strongly indicating a malfunction of the hypothalamic control of feeding behaviour. Functional magnetic resonance imaging (fMRI) studies of the hypothalamus and cortex have given a better understanding of satiety‐related events in PWS. PWS patients display abnormal brain networks, engaged with physiological control of eating and the motivational component of feeding.58, 59, 60, 61 Holsen et al 58 exposed healthy controls and PWS participants to visual food stimuli in a pre‐ and post‐meal condition and evaluated the activation of neuronal networks related to food motivation. Interestingly, PWS patients presented greater cortical activation to food stimuli in a post‐meal state. By contrast, healthy subjects presented an opposite pattern, with stronger function in the pre‐meal state.58 Consistently, upon glucose consumption PWS subjects have delayed satiety‐associated neural circuit activation in the hypothalamus and extra hypothalamic areas.59 These findings suggest that perception of nutritional status is delayed in PWS patients, likely leading to a defective hypothalamic response to nutrients. Interestingly, it has been shown that the hyperfunction of those neuronal networks is particularly associated with high caloric foods rather than low caloric stimuli.61 A schematic overview of the neurocircuitry engaged in feeding at the hypothalamic62, 63, 64 and cortical65, 66, 67, 68, 69, 70, 71, 72, 73, 74 levels can be found in Figure 2.
FIGURE 2.
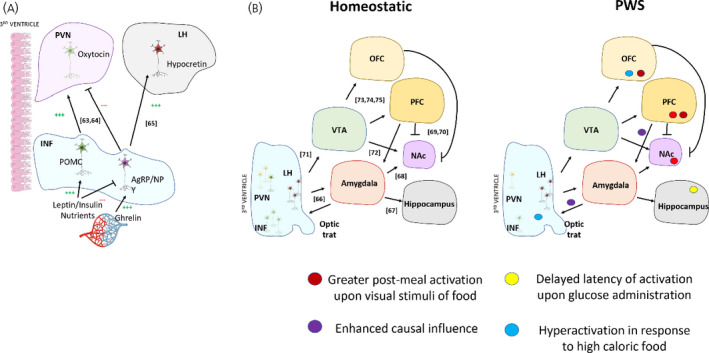
Schematic representation of the neurocircuitry mediating hunger and hedonic components of feeding. Diagrammatic representation of a coronal hypothalamic human section highlighting the infundibular nucleus (INF), paraventricular nucleus of hypothalamus (PVN) and lateral hypothalamus (LH) neuronal populations responsible for the homeostatic control of energy homeostasis. In brief, peripheral factors (hormones and nutrients) activate INF neurons, which will propagate the orexigenic/anorexigenic response throughout the hypothalamus generating autonomic and neuroendocrine outputs consistent with primary signal247 (A). Schematic overview of the hypothalamic and cortical (hedonic) components responsible with feeding. Electrical stimulation of LH neurons leads to inputs in cortical structures involved with behavior choices of feeding and reward centers. The combination of the homeostatic drive of feeding, learned behavior and hedonic components constitute the choice of eating.74 Next, representation of the known disruptions of this neurocircuitry in PWS (B). INF, Infundibular nucleus; LH, lateral hypothalamus; NAc, nucleus accumbens; OFC, orbitofrontal cortex; PFC, prefrontal cortex; PVN, paraventricular nucleus; PWS, Prader Willi Syndrome; VTA, ventral tegmental area
Undoubtedly, neurocircuitries in the brain of patients with PWS are already malfunctioning in the early life stage. This idea is supported by the study of Zhang et al who found alterations in the baseline brain activity of PWS children compared to control siblings. In this study, a combination of fMRI with causality analysis was employed to understand the behavioural and mechanistic components of hyperphagia in PWS. The study showed significantly increased impacts of amygdala onto the hypothalamus. Moreover, cortical influences on the amygdala were also increased.60 The cortical‐amygdala‐hypothalamic axis is implicated in the coupling of the behavioural component of feeding with the neuronal populations engaged with initiation or termination of eating in the hypothalamus.75 The authors suggest that the enhanced cortical inputs into the amygdala play a crucial role in an imbalanced cognitive processing, which can lead to an increased eating motivation in PWS. The combination of abnormal cortical inputs, and increased influence onto hypothalamic circuits can lead to the insatiable state of hunger found in PWS patients. Those findings are especially interesting when the hyperstimulation of the hypothalamic circuits takes place in the baseline condition, meaning that a hunger state is evoked regardless of any food stimuli (i.e. visual).60
The hypothalamus is a highly heterogeneously structured area engaged in behavioural, autonomic, and endocrine functions in response to the environment.76, 77, 78 Extensive studies in rodents and humans demonstrated that disruption of different hypothalamic neurocircuitries that control energy homeostasis is underlying the development of metabolic diseases.79, 80, 81, 82, 83, 84 The infundibular nucleus (INF, or arcuate nucleus in rodents) plays a key role in the central management of feeding behaviour and energy expenditure. Under physiological conditions, feeding activates the anorexigenic neuronal population located at the INF, characterized by the expression of pro‐opiomelanocortin (POMC).85 Antagonistically, an appetite‐stimulating neuronal population is characterized by the expression of neuropeptide Y (NPY) and agouti related protein (AgRP).86 Moreover, the activity of the regulatory neuronal populations associated with energy homeostasis are modulated by humoral feedback from the periphery. Receptors for peripheral metabolic hormones such as insulin, leptin and ghrelin are expressed on POMC and AgRP/NPY neurons.87, 88 The opposed nature of these neuronal populations creates a counter‐regulatory system of energy homeostasis. The INF neuronal populations project to the paraventricular nucleus of the hypothalamus (PVN),89 in which the satiety or hunger signals will be perpetuated through neuroendocrine mediators or (para)sympathetic outflow to peripheral metabolic organs. Arginine vasopressin (AVP)‐ and oxytocin (OXT)‐ expressing neurons are two major neuronal populations located in the PVN and are largely implicated in inhibition of feeding behavior.90 Additionally, other neuronal populations that reside in the PVN are implicated in the global metabolic control. Corticotropin‐releasing hormone‐ (CRH) expressing neurons inhibit food intake and promote energy expenditure.91 Moreover, CRH neurons interact with leptin‐sensitive inputs from the arcuate nucleus in mice.92 Furthermore, thyrotropin‐releasing hormone‐ (TRH) expressing neurons in the PVN co‐express leptin receptors and participate in energy metabolism due to their involvement in the hypothalamus‐pituitary‐thyroid axis impact on thyroid function.93 TRH neurons propagate the anorexigenic signal93, 94 and have a protagonist role in thermoregulation.95 Additionally, orexin neurons in the lateral hypothalamus (LH) are involved in feeding‐regulation,96, 97, 98 although they play a less potent role than the NPY and AgRP populations.99 In the fasting state, the levels of the precursor peptide of orexin in the hypothalamus are elevated,100 whereas central administration of orexin leads to food intake in a dose‐dependent manner.98 Moreover, orexin has also been involved in the motivational component of feeding as well.101
Likely, the gross obesity of PWS patients has multiple mechanisms that culminate in the disease phenotype. Among those factors, it is possible to highlight the disruption of satiety control,4, 5, 58 endocrine dysregulation (central and peripheral),6, 7 reduced energy expenditure due to hypotonia and behavioural features of the syndrome. Indeed, studies with post‐mortem hypothalamic tissue specimens have shown that in PWS patients, neurons in a variety of hypothalamic areas are affected, including the INF, PVN and LH.102, 103
4. HYPOTHALAMIC NEUROPEPTIDERGIC SYSTEM IN PWS
Extensive studies in rodents and humans demonstrated that disruption of the hypothalamic neuropeptides engaged in energy homeostasis promote the obese phenotype.104, 105, 106, 107 Mutations in the melanocortin 4 receptor (MC4R) are the most common cause of monogenic obesity.80, 108, 109, 110 In this case, obesity is a consequence of the lack of POMC‐derived peptide signalling in MC4R‐expressing neurons, resulting in a chronic orexigenic state.108, 109 A study with post‐mortem human brain tissue also showed that the expression of NPY and AgRP has a close correlation with the individual’s body mass index (BMI).111 It is thought‐provoking to address the importance and limitations of immunohistochemistry on post‐mortem tissue, which is the major source of literature on neuropeptides at the protein level in PWS. Due to the rarity of the PWS post‐mortem tissue and the lack of representative animal models that fully recapitulates its phenotype functional interpretation is a challenge. Of notice, hypothalamic post‐mortem material has been extensively used in other fields of research and has proved to be reliable, such as in non‐genetic obesity,112 diabetes,84 and mood disorders.113 However, the analysis of neuropeptides’ immunoreactivity without other parameters or support of the literature requires caution. This is especially due to alterations in the balance of synthesis, maturation, and secretion of these peptides. Increased levels of a neuropeptide can be explained as increased production or a defective or decreased transport and release. In the same way, reduced immunoreactivity can also indicate rapid turnover of the protein and not necessarily decreased production. This problem can be illustrated by the discrepancy between an increased vasopressin content in the SCN together with an diminished vasopressin production in female depressed patients.114 Therefore, human post‐mortem material is a reliable and stable source of study, especially in rare pathophysiology such as PWS; however the interpretation of data requires a global overview of the studied systems.
4.1. Hypothalamic orexigenic neuropeptides in PWS
NPY neuron numbers are consistently downregulated in obese and PWS‐obese subjects.103 At first glance, reduction in NPY cell counts seems counterintuitive since an insatiable hunger is a major feature of PWS.4 The molecular mechanisms behind this reduction are yet to be clarified. Diminished orexigenic signalling might be explained by increased plasma levels of adipostatic hormones such as leptin and insulin,115, 116 responsible for inhibition of neuronal activity of NPY and AgRP neurons. Evidence from murine studies demonstrates a reduction of NPY transcript in the hypothalami of high fat diet‐fed animals.117, 118, 119 It is believed that the chronic exposure of these adipostatic factors leads to malfunctioning and eventual death of the orexigenic neuronal populations.117 The role of peripheral endocrine factors in the pathophysiology of PWS will be addressed in a further section.
Interestingly, in the study with post‐mortem hypothalamic tissues, Goldstone et al 103 reported unchanged expression of AgRP in PWS. The authors found a tendency for reduction of AgRP immunoreactivity in the INF of PWS and non‐genetic obese individuals, which was lost when the values were adjusted according to premorbid illness duration. This finding is consistent with reports of murine models of obesity, in which the AgRP expression is unaltered.120 In contrast, a recent study examined the transcriptomic signature of PWS patients and found a 3‐fold upregulation in the AgRP transcript.121 Additionally, genes that are overrepresented in the PWS hypothalami overlap with the murine AgRP identity.121 The discrepancy between both studies might be explained by a defective post translational processing of AgRP in PWS. The enzyme prohormone convertase PC1 is responsible for the posttranslational cleavage of the AgRP transcript.122 Furthermore, PWS patients have reduced levels of prohormone convertase PC2,123, 124 largely implicated in the posttranslational processing of hypothalamic neuroendocrine mediators.125 Interestingly, Burnett et al 126 found a reduction in PC1 expression in neurons derived from induction of pluripotent stem cells obtained from a PWS patients, compared to those derived from unaffected controls. Thus, it is likely that even though there is an enrichment of an AgRP‐related transcripts in the hypothalami of PWS, due to defective peptide maturation unaltered levels of protein expression are reported. However, a more solid proof of concept on this hypothesis is needed. It is also worth to mention that PC1 is not only responsible for AgRP processing, but takes place in POMC, ghrelin and insulin post translational modifications.127 Thus, the infundibular orexigenic neuronal population is hypoactive in PWS, either due to defective secretory mechanisms or (possibly) chronic exposure to peripheral inputs that negatively regulate those populations. Further investigation is necessary to understand this hypoactivity and the underlying mechanisms that allow hyperphagia in this condition.
The LH is a recognized orexigenic centre which receives INF projections.128 Neuropeptides produced by neuronal populations in this area, such as hypocretin (orexin) exert appetite‐stimulating functions.129 Interestingly, studies on post‐mortem hypothalamic specimens did not find alterations in the distribution and abundance of hypocretin‐expressing neurons in PWS compared to matched controls.130 Consistently, hypocretin levels are unaltered in the cerebrospinal fluid of PWS patients. Surprisingly, hypocretin plasma levels are increased in PWS.131 It is uncertain to what extent the levels of plasma hypocretin accurately reflect the central presence of this neuropeptide. Collectively, those data indicate increased activity of this neuronal population. Hypocretin receptors are densely expressed throughout the CNS and peripheral tissues98, 132 Orexin receptors are also found in the human adipose tissue,133 and a study has shown that hypocretin signals promote adipose tissue expansion (through the promotion of adipogenesis) and fat deposition in mature adipocytes (through inhibition of lipolysis).134 Thus, elevated levels of plasma hypocretin in PWS might contribute to the hypertrophy of adipose tissue in PWS, and consequently, promote weight gain. Interestingly, hypocretin has a role in sleep regulation,135, 136 which is also markedly disturbed in PWS.137 Therefore, the involvement of the hypocretin system in PWS is beyond the regulation of energy metabolism and may play a more complex role in PWS pathophysiology.
An overview of the main findings regarding the neuropeptides explored so far is summarized in Table 1.
TABLE 1.
Summary of hypothalamic anorexigenic and orexigenic neuropeptides in Prader‐Willi Syndrome
| Neuropeptide | CNS | Hypoactivity/Hyperactivity | Plasma concentrations |
Reference (##) |
|---|---|---|---|---|
| NPY | Decreased expression and cell count | Hypoactivity | – | 103 |
| AgRP | Decreased expression and unchanged cell count | Hypoactivity | – | 103, 121 |
| Hypocretin | Unchanged cell count | Unaltered/ Hyperactivity (?) | Increased | 130, 131 |
| POMC | Decreased expression | Hypoactivity | – | 121 |
| OXT | Decreased expression and cell count | Hypoactivity | Increased | 102, 142 |
| BDNF | Decreased expression | Hypoactivity | Decreased | 121, 146 |
Abbreviations: AgRP, agouti related protein; BDNF, brain derived neurotrophic factor; NPY, Neuropeptide Y; OXT, oxytocin; POMC, proopiomelanocortin.
4.2. Hypothalamic anorexigenic neuropeptides in PWS
Surprisingly, although impaired satiety is a hallmark in PWS,5, 58 the well‐known anorexigenic POMC has been poorly explored in the disorder. Transcriptomic analysis revealed that, in line with the defective satiety, POMC‐associated genes are downregulated in the hypothalamus of PWS patients.121 Animal models have shown that impaired POMC function is central to the development of features of PWS.42 This is translated into defective anorexigenic neurocircuitry and impaired leptin sensitivity in this neuronal population.42 As PC1/2 expression is diminished in PWS neurons, it is plausible to hypothesize that lower levels of α‐MSH can be found in the hypothalami of PWS patients. Therefore, a marked reduction of expression of α‐MSH and cell number is expected in PWS.
Diminished anorexigenic oxytocin expressing neurons were also observed in the PVN of PWS.102 A clearly abnormal neuroanatomy and decreased abundance of oxytocin‐expressing neurons was reported in the PVN of PWS patients which was consistently recapitulated in animal models of PWS.43, 44 Diminished expression of oxytocin in PWS was confirmed on the RNA level,121 corroborating the findings based on immnunostained neuronal counts. Postnatal central administration of this neuropeptide in PWS animal models has been shown to successfully restore metabolic outcomes of the syndrome and promoted positive effects on behavior.44 To this date, five clinical trials employed oxytocin in the treatment of PWS.138, 139, 140, 141, 142 In short, the administration of oxytocin has effects on a behavioural level but is not yet proven to be an effective treatment of all PWS symptoms, and much more evidence is required before it can be used as a therapeutic tool. In contrast with the hypothalamic pattern, plasma oxytocin is reported to be increased in PWS individuals.143 Moreover, in concordance with this, increased plasma levels of oxytocin have been reported in PWS children when compared to unaffected siblings.143 The discrepancy between the increase in plasma oxytocin levels and the reduction in cell counts and expression at central level exemplify our limited knowledge on the hypothalamic pathophysiology of PWS. Oxytocin has an inactive and an active form, which is not dissociated in the screening of plasma samples.144 PWS patients have been reported to have diminished oxytocin receptor expression,145 which adds to the level of complexity. Bochukova et al 121 demonstrated a clear reduction of hypothalamic brain‐derived neurotrophic factor (BDNF) in PWS. The authors propose that the element of neurodegeneration in the pathogenesis of PWS might be associated with this phenomenon. BDNF and its receptor TrKB (encoded by ntrk2) expression were found to be decreased in the ventromedial nucleus (VMH) of the hypothalamus.121 Furthermore, plasma levels of BDNF in fasting conditions are decreased in PWS compared to healthy controls.146 Notably, beyond its trophic role, BDNF and its receptor have been implicated in suppression of feeding behavior.147 Diminished levels of this peptide seem to be consistent with the phenotype observed in PWS, reported to have an unhealthy microenvironment for neuronal populations and satiety deficiencies.148, 149
An overview of the alterations in neuropeptides can be found in Table 1.
5. HYPOTHALAMUS‐REGULATORY METABOLIC HORMONES IN PWS
As discussed so far, the hypothalamic neuronal populations engaged with energy homeostasis are severely affected in PWS. Hypothalamic neuronal malfunction might be directly induced by the genetic defects of PWS but can also be indirectly caused by the neuroendocrine dysregulation which occurs in conjunction with the primary PWS phenotype. Circulating metabolic hormones (i.e. ghrelin, leptin, insulin and adiponectin) inform the hypothalamic neurocircuits about the nutritional status of the organism.150 Here we will discuss the current understanding of the role of endocrine factors in the aetiology of PWS.
5.1. Ghrelin
Ghrelin is an orexigenic hormone produced by enteroendocrine cells.151, 152 Central or peripheral administration of ghrelin induces eating and promotes adiposity.153, 154 In fasted conditions, ghrelin levels are elevated and by contrast, re‐feeding or oral glucose administration reduces the total plasma concentrations of this hormone. Ghrelin acts in the hypothalamus via NPY/AgRP neuron154 signalling while POMC‐expressing neurons do not express functional receptors for this hormone.155 Genetic ablation of ghrelin or its receptor leads to resistance to diet‐induced obesity in murine models.156 Furthermore, hypothalamic resistance to ghrelin signalling is observed upon obesogenic cues.157 Intriguingly, ghrelin levels are reduced in human non‐genetic obesity.158 Whether humans also have hypothalamic resistance to ghrelin is yet to be determined.
Differently from non‐genetic obesity, ghrelin is found to be upregulated in PWS, and has been implicated as an underlying cause of hyperphagia in PWS.159, 160, 161, 162 DelParigi et al 160 reported a 2.5‐fold increase in PWS plasma levels of ghrelin compared to lean controls; and a 4.5‐fold increase compared to obese subjects. The difference remained significant even after adjustment for percent of body fat.160 Ghrelin levels remained elevated in PWS patients in comparison to matched controls even after the consumption of satiating dose liquid meals.160 This finding matches with the persistent urge to eat found in PWS. Moreover, plasma ghrelin levels and subjective rating of hunger have a positive correlation in PWS.160 It would be interesting to study the ghrelin levels in PWS infants before the onset of hyperphagia. However, no differences were found between non‐obese PWS infants (under the age of 5) and matched controls regarding their ghrelin levels.163 In a different study, PWS obese children (average age 9.5 years) had elevated plasma ghrelin.164 It is still possible that hyperghrelinemia occurs prior to the onset of obesity.163 Nevertheless, Feigerlova et al 161 also concluded that ghrelin levels are consistently increased in all analysed ages (0‐17 years old).
Currently, no animal model is available that can fully mimic the phenotype of PWS in humans, possibly due to the complex genetic components of the syndrome which complicate the development of reliable animal models.165 Yet, there are numerous studies that investigate the role of specific genes present in the PWS critical deletion region. Such PWS mice models are e.g. employed to investigate hyperphagic related features of the syndrome, as in the Snord116del mice.57, 166 In ad libitum conditions, these mice show increased levels of ghrelin (approximately 2‐fold increase when compared to wild type).57 This is the same magnitude of increase expected in wild type mice upon 24 hours fasting. Another study showed Snord116del mice in a ghrelin‐deficient background did not impact the mortality or maturation of sexual traits evoked by the Snord116 loss.167 However, the ghrelin‐deficient background promoted leanness and reduction of body fat observed in the single mutant.167 Although with divergent phenotypical traits of the PWS pathophysiology (i.e. leanness), this model seems to be particularly interesting to comprehend, at least partially, the hyperphagic state of the syndrome.168 It is interesting to note that Snordel116 mice have reduced PC1 expression.126 This protein acts in ghrelin posttranslational maturation,127 as in POMC.169 Although ghrelin levels are elevated in Snord116del mice, there is an increased ratio between the pro‐ghrelin and the mature hormone, due to diminished expression of PC1.126 Therefore, concomitantly to the hyperghrelinemia, Snord116del mice are less efficient in ghrelin’s post translational processing. The exact implications of those findings in PWS patients are yet to be determined.
It is worth to notice that extra hypothalamic actions of ghrelin can promote hunger/eating behaviour. The ghrelin receptor is expressed in dopaminergic neurons in the ventral tegmental area (VTA),170 and local injection of the peptide in the VTA evokes feeding in a dose dependent manner.171 Ghrelin administration increases dopamine turnover in the mesolimbic system upon food consumption, suggesting a role of it in the hedonic component of eating,172, 173 at least in rodents. Yet, it is clear that the impact of ghrelin in extra‐hypothalamic areas remains to be clarified both in rodents and humans.
In summary, there is no solid evidence for a relation between hyperghrelinemia and hypothalamic dysfunction in PWS. The lack of experimental models and limited human‐derived samples limits our knowledge of ghrelin`s role in the induction of hyperphagia in PWS. In addition, if hyperghrelinemia is the major cause of the drastic hyperphagic state in PWS it is undetermined until now what the main neuronal population behind this orchestration is. This phenotype might be due to ghrelin`s actions on hypothalamic orexigenic neurons, mesolimbic neurons that have direct inputs into the hypothalamus or a synergic effect among them.
5.2. Leptin
Leptin is an adipokine that regulates energy homeostasis by signalling hypothalamic centers.115 Leptin is a potent anorexigenic hormone, and it is well recognized for activation of POMC‐expression neurons while suppressing the NPY and AgRP neuronal activity.85 Leptin also exerts its functions through glial cells.89, 174 Recently the importance of leptin receptors in both astrocytes174 and microglia89 has been demonstrated. It is well‐known that lack of leptin leads to a severe obese phenotype and deletion of its receptor also leads to obesity and diabetic traits.175 Moreover, central resistance to leptin signalling is a hallmark of obesity in animal models and human subjects.176, 177
The participation of leptin in the clinical features of PWS is less clear than what is known for ghrelin. Lindgren et al 178 demonstrated that leptin expression is increased in the adipose tissue of PWS children. This is paralleled by increased plasma levels of leptin in PWS infants.178 In agreement with these findings, Butler et al 179 reported that obese individuals have higher leptin levels compared to their lean counterparts. However, in the lean group, women presented with higher plasma levels of leptin, which was not observed within the PWS population. No sex differences were observed among non‐obese PWS individuals. Furthermore, the only difference in leptin plasma levels found between control and PWS subjects is between lean males and non‐obese PWS males. The authors proposed that this difference might be related to hypogonadism, characteristic of PWS males.179 Of note, leptin is a major regulator of reproduction.180 Goldstone et al 181 also did not find differences in plasma leptin between lean and PWS adult women. These differences on plasma levels of leptin cannot be explained by defective secretion or defects in functionality of the receptor.181
Snord116del mice have unaltered leptin levels.168 Hypothalamic genes associated with leptin signalling genes also remain unchanged in these mutants compared to their wild type littermates.168 Interestingly, adenovirus‐mediated deletion of Snord116 in the hypothalamus leads to increased expression of the suppressor of cytokine signalling 3 (SOCS3) in obese animals.168 This gene is responsible for the suppression of leptin signaling.182 However, the cited work cannot dissociate the contribution of the obese phenotype versus the deletion itself in the elevated SOCS3 expression.168 Another animal model that is often employed in PWS research is the MAGEL2 null mouse.183 This knockout is insensitive to the anorexigenic effects of leptin,184 hence POMC neurons fail to be activated upon leptin exposure. However, whether this is a congenital defect is still up for debate. Moreover, POMC neuron fibers that project to the PVN are reduced in the MAGEL2 mutant mice.42
5.3. Insulin
Insulin is a hormone secreted by β‐cells of the pancreatic islets. Molecular resistance to insulin or plasma insufficiency leads to the development of type 2 diabetes mellitus (T2DM).185 Hypothalamic insulin action takes place in synergism with leptin signalling, and therefore has also a potent anorexigenic effect.186 The morbid obesity in PWS is believed to be the main cause of T2DM in PWS population. Indeed, the prevalence of T2DM is much higher in PWS as compared to the general population. A report from Vanderpump shows a prevalence of approximately 3 to 6 percent of T2DM in an English cohort.187 By contrast, concomitant studies reported that approximately 25% of PWS individuals are diabetic in other European cohorts,188, 189 and even a higher percentage is suggested in an Asian population, with approximately 30% of T2DM incidence in PWS.190 Despite the clear incidence of T2DM within PWS population, there are discrepant reports in the literature on the role of defective glucose‐regulation in the pathophysiology of PWS. Furthermore, increased plasma concentrations of additional metabolism‐controlling hormones (i.e. ghrelin and adiponectin)191 are thought to have great influence in glycaemic control in PWS pathophysiology.
Butler et al 192 determined that fasting glucose and insulin levels were comparable between PWS and non‐syndromic obese individuals. PWS patients were reported to have significantly higher concentrations of glucose and insulin compared to lean individuals.192 Moreover, PWS patients were reported to have hyperinsulinemia response to an oral glucose tolerance test (OGTT) when compared to normal weight controls,193 while obese non‐PWS controls displayed comparable OGTT readout to PWS individuals in this study.193 In another study, an intravenous glucose tolerance test (IGVTT) showed comparable glucose assimilation coefficient between PWS and non‐syndromic obese population.194 These data on glucose assimilation and insulin concentration upon oral glucose challenge indicate that the insulin sensitivity among non‐syndromic obese and PWS should also be comparable. Interestingly, after a protein meal ingestion, PWS patients showed a similar insulin peak to healthy weighted controls, whereas obese non‐PWS controls had a clear insulin peak upon the meal consumption.195
Schuster et al 196 demonstrated that PWS infants have lower insulin response to oral glucose test compared to BMI and age matched individuals. In the same study, no differences were found in glucose or insulin levels on PWS adults when compared to lean or obese controls. The authors discuss that the PWS limited group size might influence the interpretation of this data. Interestingly, on OGTT, PWS presented delayed peak of glucose and insulin when compared to obese controls. It was proposed that this difference might be explained by reduced pancreatic β‐cell responsiveness to glucose fluctuations in PWS population. Consistently with previous literature, the authors found no differences in glucose assimilation assessed by IVGTT.194 However, insulin and peptide C plasma levels were reduced in PWS.196 This led to reduced insulin to glucose ratios during IGVTT in PWS compared to obese control group. Furthermore, the authors were the first to demonstrate increased hepatic insulin extraction and insulin clearance in PWS compared to obese controls.
A recent study described a heightened fasting insulin levels and sensitivity in PWS infants compared to BMI and age‐matched controls.197 However, this study lacks the comparison between PWS and lean subjects. Therefore, it is impossible to determine if in this cohort, whether PWS are normoinsulinemic during fasting. In concordance with the insulin sensitivity data, a more recent report described that PWS individuals have lower fasting insulin levels when compared to obese controls. Not only insulin concentrations were lessened, but also PWS individuals were also proven to have greater insulin sensitivity, which were compared to lean controls.191 Thus, although the prevalence of T2DM is greater in PWS, an unexpected increase in insulin sensitivity is found within this population as well. The link between insulin resistance and T2DM is long recognized.198 In addition, molecular resistance to insulin signalling is one of the most accurate predictors of development of T2DM and the main therapeutic target for this disorder.199, 200 There are potential mechanisms that are used to explain this paradoxical clinical feature of PWS. Firstly, although PWS patients display abnormal fat deposition, that occurs preferentially in subcutaneous depots rather in visceral ones.191, 201, 202, 203 The visceral fat deposition, which is observed in obese controls, has a positive correlation with lowered sensitivity to insulin signalling.204 Moreover, it is known that PWS patients are deficient in growth hormone.6, 205 This hormone has glucoregulatory roles and, it is particularly interesting to pinpoint that there is a transient induction of insulin resistance in puberal development due to elevated secretion of growth hormone.206 Lastly, increased insulin sensitivity in PWS population can eventually be explained by increased levels of adiponectin.202 This is an adipose tissue‐derived hormone and targets insulin‐producing cells in the pancreas.207 Adiponectin is associated with increased insulin sensitivity and has anti‐inflammatory properties.208 Compared to non‐syndromic obese population, PWS individuals have higher plasma concentrations of this hormone. This is further correlated with insulin sensitivity in a PWS cohort.202 It is proposed that increased overall fatty acid oxidation promoted by adiponectin signalling in skeletal muscle leads to increased insulin sensitivity, as previously stated.209 However, whether this is true for the pathophysiology of PWS remains unknown.
Our current understanding of the endocrine imbalance on the hypothalamic malfunction is still extremely poor. One of the biggest gaps to be filled in that sense is the “chicken‐or‐egg question” between the endocrine imbalance and the defective neuronal functioning. Is disrupted hypothalamic function the primordial cause of the obese phenotype, which is accompanied by endocrine alterations? Or is the defective endocrine production and/or secretion the main cause of the hypothalamic function?
6. HYPOTHALAMIC GLIAL CELLS AND INFLAMMATORY PATHWAYS IN PWS
The consumption of an obesogenic diet leads to structural and functional damage of the neuronal populations engaged with energy homeostasis regulation.82, 87, 210 The malfunction of those neurons is closely related to the activation of microglia cells.83, 211 Microglia are the resident immune cells of the CNS. The homeostatic functions of microglia are responsible for microenvironmental cleansing, mostly though phagocytosis of cellular debris and unwanted particles.212 This promotes a healthy microenvironment for optimal neuronal function throughout the CNS.213 Beyond the homeostatic functions of immunosurveillance, microglia play a central role in CNS pathologies, such as Alzheimer’s disease,214 Parkinson’s disease,215 multiple sclerosis216 and disorders in the CNS circuitry in control of energy homeostasis.82, 83, 89, 217, 218
Hypothalamic microglial activation towards a proinflammatory state is triggered by dietary composition and peripheral hormones.218 In the obese hypothalamus, activated microglia accumulate and produce neurotoxic and proinflammatory mediators, such as cytokines (mainly tumor necrosis factor; interleukin‐6; interleukin‐1 beta) and nitric oxide.219 Thus, the malfunction and numerical loss of INF neurons might be directly driven by microglial activation. Moreover, by looking into the post‐mortem hypothalamic tissues of type 2 diabetic patients, we have recently demonstrated that anti‐diabetic treatment is associated with a reduction of microglia cell number within the INF.84 This suggests that changes in microglial biology might also be implicated in the reversion of metabolic syndrome features. The obese phenotype is associated with a global inflammatory process that affects not only the hypothalamus.220 Resident and infiltrating immune cells trigger an inflammatory response in metabolic relevant organs, such as the liver, pancreas, and adipose tissue.220 Furthermore, both animal studies and preliminary evidence on human brain material demonstrate that inflammatory changes are first observed in the hypothalamus.107 Those alterations are also known to precede the peripheral metabolic disorders.
Currently, the role of inflammatory mediators in the pathophysiology of PWS is poorly explored and understood. Questions, such as whether the PWS patients have similar inflammatory outcomes as the non‐syndromic obese subjects, and whether there is any biological system that is differentially affected in either condition (i.e. is inflammation in the hypothalamus differently regulated in PWS and non‐genetic obesity?) still remain. Although plasma levels of cytokines are comparable among PWS and obese controls.191 The transcriptional signature of the hypothalami of PWS patients revealed a marked inflammatory process, concomitant with dampening of neuronal‐associated transcripts.121 The same study demonstrated reduced cell counts of astrocytes in PWS hypothalami. Astrocytes accumulate within the hypothalamus in conditions like obesity and T2DM221 and can contribute to the maintenance of an inflammatory process.222 This might suggest that the hypothalamic inflammatory‐associated changes found in PWS are more closely related to microglia than to other cell types, quite different from non‐genetic conditions. Beyond that, further studies are needed to find out whether the inflammatory changes in microglia are triggered by endocrine or dietary components, and whether the genes present in the PWS critical deletion region participate in the homeostatic or inflammatory changes of microglia.
Of importance, neuropeptides are known regulators of microglia function.223, 224, 225 The participation of neurotransmitters and neuropeptides as instructors of microglial function has gained crescent interest of the scientific community.226 However, the contribution of hypothalamic neuropeptides as immunomodulators is still poorly understood. It is also interesting that microglia cells comprehend a very heterogenous population in situ, and therefore it is difficult to translate the relevance of the in vitro findings into physiological terms.227 Therefore, the magnitude of the neuropeptidergic damping found in PWS hypothalami on the microglia biology is far to be fully understood. The two major antagonist neuropeptides expressed on the infundibular nucleus explored here (NPY and POMC) are known immunomodulators, especially in murine models. The NPY effects on microglia is broader explored in the retina, rather than in the hypothalamus.228 In brief, NPY is capable to inhibit LPS‐induced proinflammatory mediators (such as nitric oxide production and cytokines/interleukins) and has a suppressor effect on phagocytic capacity of microglia.229, 230 Likewise, the anorexigenic neuropeptides here mentioned have been broadly implicated in promoting an anti‐inflammatory phenotype. POMC‐derived peptides suppress LPS‐induced cytokine expression/secretion.231, 232 A similar pattern was found in other myeloid populations, as peripheral macrophages and neutrophils.225, 233, 234 Further research is necessary for the comprehension of the melanocortinergic system on non‐neuronal cells. Interestingly, among the neuropeptides addressed in this review, oxytocin is the one that has been further explored in microglial immunity. In primary microglia, oxytocin treatment leads to diminished production of LPS‐induced neurotoxic factors.235, 236 In concordance, intranasal oxytocin administration in adult mice limits microglial inflammatory response upon intraperitoneal LPS injection.236 It has been proposed that this phenotype is achieved through inhibition of signalling pathways classically associated with a proinflammatory response, such as the Nuclear Factor Kappa Beta and the mechanistic Target of Rapamycin.237, 238 Finally, the endocrine mediators explored here (leptin, ghrelin, and insulin) are also known regulators of microglia cells. In brief, it is broadly recognized that these hormones can modulate microglia cytokine production239, 240, 241 and phagocytic capacity.89, 152, 242 Therefore, the microglial biology might be a fundamental link between the molecular disruption of hypothalamic dysfunction and the clinical aspects of PWS.
7. CONCLUSION AND FUTURE PROSPECTIVE
Hypothalamic dysfunction is a hallmark of PWS. Defective hypothalamic neurocircuitry leads to the hyperphagic state, and consequently morbid obesity. This is translated into diminished expression of hypothalamic neuropeptides engaged in energy homeostasis regulation. Ultimately, there is a marked numerical loss of the neurons that compose both orexigenic (NPY‐expressing neurons) and anorexigenic populations (oxytocin‐expressing and presumably POMC‐expressing neurons). Furthermore, the neurocircuitry engaged in food intake and motivation are abnormally activated in PWS. Whether the disruption of the homeostatic control of eating behaviour is due to defective neuronal biology, an unhealthy microenvironment leading to neuronal dysfunction or primarily due to endocrine imbalance, is yet to be determined.
So far, symptomatic management of the disease is the only therapeutic option. The most successful approach in that sense is growth hormone therapy, which has been largely implicated in the attenuation of PWS related features (i.e. stature, body composition and motor and cognitive development). Despite the improvement of those symptoms growth hormone therapy fails to completely resolve the hyperphagia and obesity. It is also relevant to address that it is unknown to what extend improvement of hypothalamic dysfunction by growth hormone therapy is a consequence of its local signalling or overall amelioration of symptoms. Therefore, therapeutic approaches designed to specifically attenuate the hyperphagic state and obesity itself in PWS are necessary. Among the most promising agents in this respect are the glucagon‐like 1 receptor (GLP‐1R) agonists that have been extensively studied in obesity and T2DM and induce both weight loss and improved glycaemic control.243 Additional research is necessary to adopt GLP‐1R based therapies for PWS, but preliminary data show positive results. Salehi and collaborators reported that short‐term use of exenatide (a GLP‐1R agonist) promoted anorectic effects in a small PWS cohort (13‐25 years old).244 Importantly, the authors did not observe BMI changes during the short term administration and suggested further studies with long‐term administration as follow up, as well a combination of exenatide with behavioural modifications.244 Further, a more recent case report from Kim and colleagues demonstrated rapid and marked weight loss in a PWS adolescent (female, 18 years old) upon liraglutide (another GLP‐1R agonist) administration.245 Remarkably, the effects of GLP‐1R activation modulate hypothalamic neuronal populations engaged in energy homeostasis.246
Even though the therapeutic approach in PWS is still evolving, it is clear by now that hypothalamic dysfunction needs to be a key component in this equation regardless of the question whether it has a causal role. Overall, the effects of the temporal and spatial changes of hypothalamic neuronal and glial populations are not fully understood. Challenges such as the implications of the deletion at its full length rather than the role of each individual gene is one of the main obstacles in PWS field. Accumulating evidence indicates that neuroinflammation may be a major causative event on hypothalamic neuronal malfunctioning; and thus, it might be decisive in the design of new symptomatology management strategies. Further research is still necessary to comprehend the participation of glial cells, especially microglia, in the pathophysiology of PWS. Whether glial or neuronal cells are the first to be affected remains an open question at this moment.
AUTHOR CONTRIBUTIONS
Felipe Correa da SIlva: Writing‐original draft; Writing‐review & editing. Eric Fliers: Supervision; Writing‐original draft; Writing‐review & editing. Dick Swaab: Supervision; Writing‐original draft; Writing‐review & editing. Chun‐Xia Yi: Conceptualization; Supervision; Writing‐original draft; Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jne.12994.
ACKNOWLEDGEMENT
The authors would like to thank Irina Milanova for careful revision of the manuscript.
Correa‐da‐Silva F, Fliers E, Swaab D, Yi C‐X. Hypothalamic neuropeptides and neurocircuitries in Prader Willi syndrome. J Neuroendocrinol. 2021;33:e12994. 10.1111/jne.12994
Funding information
AMC PhD Scholarship (FCS, 2019, Amsterdam University Medical Center), the Dutch Diabetes Research Foundation (CXY, Diabetes Fonds, 2015.82.1826)
REFERENCES
- 1.Holm VA, Cassidy SB, Butler MG, et al. Prader‐Willi syndrome: consensus diagnostic criteria. Pediatrics. 1993;91(2):398‐402. http://www.ncbi.nlm.nih.gov/pubmed/8424017 [PMC free article] [PubMed] [Google Scholar]
- 2.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader‐Willi syndrome. Genet Med. 2012;14(1):10‐26. 10.1038/gim.0b013e31822bead0 [DOI] [PubMed] [Google Scholar]
- 3.Whittington J, Holland A, Webb T, Butler J, Clarke D, Boer H. Academic underachievement by people with Prader‐Willi syndrome. J Intellect Disabil Res. 2004;48(2):188‐200. 10.1111/j.1365-2788.2004.00473.x [DOI] [PubMed] [Google Scholar]
- 4.Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader‐Willi syndrome. Am J Med Genet Part A. 2011;155(5):1040‐1049. 10.1002/ajmg.a.33951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler MG, Theodoro MF, Bittel DC, Donnelly JE. Energy expenditure and physical activity in Prader‐Willi syndrome: comparison with obese subjects. Am J Med Genet Part A. 2007;143A(5):449‐459. 10.1002/ajmg.a.31507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eiholzer U, Schlumpf M, Nordmann Y, L’Allemand D. Early manifestations of Prader‐Willi syndrome: influence of growth hormone. J Pediatr Endocrinol Metab. 2001;14(Suppl 6):1441‐1444. [PubMed] [Google Scholar]
- 7.Myers SE, Carrel AL, Whitman BY, Allen DB. Sustained benefit after 2 years of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader‐Willi syndrome. J Pediatr. 2000;137(1):42‐49. 10.1067/mpd.2000.105369 [DOI] [PubMed] [Google Scholar]
- 8.Eldar‐Geva T, Hirsch HJ, Benarroch F, Rubinstein O, Gross‐Tsur V. Hypogonadism in females with Prader‐Willi syndrome from infancy to adulthood: variable combinations of a primary gonadal defect and hypothalamic dysfunction. Eur J Endocrinol. 2010;162(2):377‐384. 10.1530/EJE-09-0901 [DOI] [PubMed] [Google Scholar]
- 9.Roberts SE, Dennis NR, Browne CE, et al. Characterisation of interstitial duplications and triplications of chromosome 15q11–q13. Hum Genet. 2002;110(3):227‐234. 10.1007/s00439-002-0678-6 [DOI] [PubMed] [Google Scholar]
- 10.Goytain A, Hines RM, El‐Husseini A, Quamme GA. NIPA1(SPG6), the basis for autosomal dominant form of hereditary spastic paraplegia, encodes a functional Mg2+ transporter. J Biol Chem. 2007;282(11):8060‐8068. 10.1074/jbc.M610314200 [DOI] [PubMed] [Google Scholar]
- 11.Butler MG. Magnesium supplement and the 15q11.2 BP1–BP2 microdeletion (Burnside–Butler) syndrome: a potential treatment? Int J Mol Sci. 2019;20(12):2914. 10.3390/ijms20122914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Victor AK, Klein J, et al. Loss of MAGEL2 in Prader‐Willi syndrome leads to decreased secretory granule and neuropeptide production. JCI Insight. 2020;5(17 ). 10.1172/jci.insight.138576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goytain A, Hines RM, Quamme GA. Functional characterization of NIPA2, a selective Mg 2+ transporter. Am J Physiol Physiol. 2008;295(4):C944‐C953. 10.1152/ajpcell.00091.2008 [DOI] [PubMed] [Google Scholar]
- 14.Frapin M, Guignard S, Meistermann D, et al. Maternal protein restriction in rats alters the expression of genes involved in mitochondrial metabolism and epitranscriptomics in fetal hypothalamus. Nutrients. 2020;12(5):1464. 10.3390/nu12051464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W, Zhang W, Ma H, Yang M. NIPA2 regulates osteoblast function by modulating mitophagy in type 2 diabetes osteoporosis. Sci Rep. 2020;10(1):3078. 10.1038/s41598-020-59743-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu N‐N, Xie H, Xiang‐wei W‐S, Gao K, Wang T‐S, Jiang Y‐W. The absence of NIPA2 enhances neural excitability through BK (big potassium) channels. CNS Neurosci Ther. 2019;25(8):865‐875. 10.1111/cns.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cioni J‐M, Wong HH‐W, Bressan D, Kodama L, Harris WA, Holt CE. Axon‐axon interactions regulate topographic optic tract sorting via CYFIP2‐dependent WAVE complex function. Neuron. 2018;97(5):1078‐1093.e6. 10.1016/j.neuron.2018.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenport EC, Szulc BR, Drew J, et al. Autism and schizophrenia‐associated CYFIP1 regulates the balance of synaptic excitation and inhibition. Cell Rep. 2019;26(8):2037‐2051.e6. 10.1016/j.celrep.2019.01.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachmann SO, Sledziowska M, Cross E, et al. Behavioral training rescues motor deficits in Cyfip1 haploinsufficiency mouse model of autism spectrum disorders. Transl Psychiatry. 2019;9(1):29. 10.1038/s41398-018-0338-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babbs RK, Beierle JA, Ruan QT, et al. Cyfip1 haploinsufficiency increases compulsive‐like behavior and modulates palatable food intake in mice: dependence on Cyfip2 genetic background, parent‐of origin, and sex. G3: Genes|Genomes|Genetics. 2019;9(9):3009‐3022. 10.1534/g3.119.400470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva AI, Haddon JE, Ahmed Syed Y, et al. Cyfip1 haploinsufficient rats show white matter changes, myelin thinning, abnormal oligodendrocytes and behavioural inflexibility. Nat Commun. 2019;10(1):3455. 10.1038/s41467-019-11119-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maver A, Čuturilo G, Kovanda A, Miletić A, Peterlin B. Rare missense TUBGCP5 gene variant in a patient with primary microcephaly. Eur J Med Genet. 2019;62(12):103598. 10.1016/j.ejmg.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 23.De Wolf V, Brison N, Devriendt K, Peeters H. Genetic counseling for susceptibility loci and neurodevelopmental disorders: the del15q11.2 as an example. Am J Med Genet Part A. 2013;161(11):2846‐2854. 10.1002/ajmg.a.36209 [DOI] [PubMed] [Google Scholar]
- 24.Kanber D, Giltay J, Wieczorek D, et al. A paternal deletion of MKRN3, MAGEL2 and NDN does not result in Prader‐Willi syndrome. Eur J Hum Genet. 2009;17(5):582‐590. 10.1038/ejhg.2008.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abreu AP, Macedo DB, Brito VN, Kaiser UB, Latronico AC. A new pathway in the control of the initiation of puberty: the MKRN3 gene. J Mol Endocrinol. 2015;54(3):R131‐R139. 10.1530/JME-14-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin‐expressing neurons. J Clin Invest. 2020. . 10.1172/JCI136564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Settas N, Dacou‐Voutetakis C, Karantza M, Kanaka‐Gantenbein C, Chrousos GP, Voutetakis A. Central precocious puberty in a girl and early puberty in her brother caused by a novel mutation in the MKRN3 gene. J Clin Endocrinol Metab. 2014;99(4):E647‐E651. 10.1210/jc.2013-4084 [DOI] [PubMed] [Google Scholar]
- 28.Macedo DB, Abreu AP, Reis ACS, et al. Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene makorin ring finger 3. J Clin Endocrinol Metab. 2014;99(6):E1097‐E1103. 10.1210/jc.2013-3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig NG, Radaeli RF, Silva MMX, et al. A boy with Prader‐Willi syndrome: unmasking precocious puberty during growth hormone replacement therapy. Arch Endocrinol Metab. 2016;60(6):596‐600. 10.1590/2359-3997000000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crinò A, Di Giorgio G, Schiaffini R, et al. Central precocious puberty and growth hormone deficiency in a boy with Prader‐Willi syndrome. Eur J Pediatr. 2008;167(12):1455‐1458. 10.1007/s00431-008-0679-0 [DOI] [PubMed] [Google Scholar]
- 31.Linnemann K, Schröder C, Mix M, Krüger G, Fusch C. Prader‐Labhart‐Willi syndrome with central precocious puberty and empty sella syndrome. Acta Paediatr. 1999;88(11):1295‐1297. 10.1080/080352599750030482 [DOI] [PubMed] [Google Scholar]
- 32.Tauber M, Barbeau C, Jouret B, et al. Auxological and endocrine evolution of 28 children with Prader‐Willi syndrome: effect of GH therapy in 14 children. Horm Res Paediatr. 2000;53(6):279‐287. 10.1159/000053184 [DOI] [PubMed] [Google Scholar]
- 33.Barreiro ML, Tena‐Sempere M. Ghrelin and reproduction: a novel signal linking energy status and fertility? Mol Cell Endocrinol. 2004;226(1–2):1‐9. 10.1016/j.mce.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 34.Hausman GJ, Barb CR, Lents CA. Leptin and reproductive function. Biochimie. 2012;94(10):2075‐2081. 10.1016/j.biochi.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 35.Repaci A, Gambineri A, Pagotto U, Pasquali R. Ghrelin and reproductive disorders. Mol Cell Endocrinol. 2011;340(1):70‐79. 10.1016/j.mce.2011.02.022 [DOI] [PubMed] [Google Scholar]
- 36.Fernández‐Fernández R, Tena‐Sempere M, Navarro VM, et al. Effects of ghrelin upon gonadotropin‐releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology. 2005;82(5–6):245‐255. 10.1159/000092753 [DOI] [PubMed] [Google Scholar]
- 37.Bianco ME, Josefson JL. Hyperglycemia during pregnancy and long‐term offspring outcomes. Curr Diab Rep. 2019;19(12):143. 10.1007/s11892-019-1267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kluge M, Schüssler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab. 2007;92(8):3202‐3205. 10.1210/jc.2007-0593 [DOI] [PubMed] [Google Scholar]
- 39.Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated ghrelin inhibits spontaneous luteinizing hormone pulsatility and responsiveness to naloxone but not that to gonadotropin‐releasing hormone in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrinol Metab. 2008;93(9):3633‐3639. 10.1210/jc.2008-0049 [DOI] [PubMed] [Google Scholar]
- 40.Tacer KF, Potts PR. Cellular and disease functions of the Prader‐Willi syndrome gene MAGEL2. Biochem J. 2017;474(13):2177‐2190. 10.1042/BCJ20160616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao Y‐H, Fountain MD, Fon Tacer K, et al. USP7 acts as a molecular rheostat to promote WASH‐dependent endosomal protein recycling and is mutated in a human neurodevelopmental disorder. Mol Cell. 2015;59(6):956‐969. 10.1016/j.molcel.2015.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maillard J, Park S, Croizier S, et al. Loss of Magel2 impairs the development of hypothalamic Anorexigenic circuits. Hum Mol Genet. 2016;25(15):3208‐3215. 10.1093/hmg/ddw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ates T, Oncul M, Dilsiz P, et al. Inactivation of Magel2 suppresses oxytocin neurons through synaptic excitation‐inhibition imbalance. Neurobiol Dis. 2019;121:58‐64. 10.1016/j.nbd.2018.09.017 [DOI] [PubMed] [Google Scholar]
- 44.Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet. 2010;19(24):4895‐4905. 10.1093/hmg/ddq424 [DOI] [PubMed] [Google Scholar]
- 45.MacDonald HR, Wevrick R. The necdin gene is deleted in Prader‐Willi syndrome and is imprinted in human and mouse. Hum Mol Genet. 1997;6(11):1873‐1878. 10.1093/hmg/6.11.1873 [DOI] [PubMed] [Google Scholar]
- 46.Kuwako K‐I. Disruption of the paternal necdin gene diminishes TrkA signaling for sensory neuron survival. J Neurosci. 2005;25(30):7090‐7099. 10.1523/JNEUROSCI.2083-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tennese AA, Gee CB, Wevrick R. Loss of the Prader‐Willi syndrome protein necdin causes defective migration, axonal outgrowth, and survival of embryonic sympathetic neurons. Dev Dyn. 2008;237(7):1935‐1943. 10.1002/dvdy.21615 [DOI] [PubMed] [Google Scholar]
- 48.Muscatelli F. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader‐Willi syndrome. Hum Mol Genet. 2000;9(20):3101‐3110. 10.1093/hmg/9.20.3101 [DOI] [PubMed] [Google Scholar]
- 49.Gérard M, Hernandez L, Wevrick R, Stewart CL. Disruption of the mouse necdin gene results in early post‐natal lethality. Nat Genet. 1999;23(2):199‐202. 10.1038/13828 [DOI] [PubMed] [Google Scholar]
- 50.Hertz G, Cataletto M, Feinsilver SH, Angulo M. Sleep and breathing patterns in patients with Prader Willi syndrome (PWS): effects of age and gender. Sleep. 1993;16(4):366‐371. 10.1093/sleep/16.4.366 [DOI] [PubMed] [Google Scholar]
- 51.Matarazzo V, Caccialupi L, Schaller F, et al. Necdin shapes serotonergic development and SERT activity modulating breathing in a mouse model for Prader‐Willi syndrome. Elife. 2017;6 . 10.7554/eLife.32640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu R, Dong Y, Li J‐D. Necdin regulates BMAL1 stability and circadian clock through SGT1‐HSP90 chaperone machinery. Nucleic Acids Res. 2020;48(14):7944‐7957. 10.1093/nar/gkaa601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott EM. Circadian clocks, obesity and cardiometabolic function. Diabetes, Obes Metab. 2015;17:84‐89. 10.1111/dom.12518 [DOI] [PubMed] [Google Scholar]
- 54.Hogenboom R, Kalsbeek MJ, Korpel NL, et al. Loss of arginine vasopressin‐ and vasoactive intestinal polypeptide‐containing neurons and glial cells in the suprachiasmatic nucleus of individuals with type 2 diabetes. Diabetologia. 2019;62(11):2088‐2093. 10.1007/s00125-019-4953-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao Y, AlHumaidi SS, Faqeih EA, Pitel BA, Lundquist P, Aypar U. A novel deletion of SNURF/SNRPN exon 1 in a patient with Prader‐Willi‐like phenotype. Eur J Med Genet. 2017;60(8):416‐420. 10.1016/j.ejmg.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 56.Kufel J, Grzechnik P. Small nucleolar RNAs tell a different tale. Trends Genet. 2019;35(2):104‐117. 10.1016/j.tig.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 57.Ding F, Li HH, Zhang S, et al. SnoRNA Snord116 (Pwcr1/MBII‐85) deletion causes growth deficiency and hyperphagia in mice. PLoS One. 2008;3(3):e1709. 10.1371/journal.pone.0001709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holsen LM, Zarcone JR, Brooks WM, et al. Neural mechanisms underlying hyperphagia in Prader‐Willi syndrome*. Obesity. 2006;14(6):1028‐1037. 10.1038/oby.2006.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shapira NA. Satiety dysfunction in Prader‐Willi syndrome demonstrated by fMRI. J Neurol Neurosurg Psychiatry. 2005;76(2):260‐262. 10.1136/jnnp.2004.039024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Wang J, Zhang G, et al. The neurobiological drive for overeating implicated in Prader‐Willi syndrome. Brain Res. 2015;1620:72‐80. 10.1016/j.brainres.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 61.Dimitropoulos A, Schultz RT. Food‐related neural circuitry in Prader‐Willi syndrome: response to high‐ versus low‐calorie foods. J Autism Dev Disord. 2008;38(9):1642‐1653. 10.1007/s10803-008-0546-x [DOI] [PubMed] [Google Scholar]
- 62.Mihály E, Fekete C, Tatro JB, Liposits Z, Stopa EG, Lechan RM. Hypophysiotropic thyrotropin‐releasing hormone‐synthesizing neurons in the human hypothalamus are innervated by neuropeptide Y, agouti‐related protein, and α‐melanocyte‐stimulating hormone 1. J Clin Endocrinol Metab. 2000;85(7):2596‐2603. 10.1210/jcem.85.7.6662 [DOI] [PubMed] [Google Scholar]
- 63.Siljee JE, Unmehopa UA, Kalsbeek A, Swaab DF, Fliers E, Alkemade A. Melanocortin 4 receptor distribution in the human hypothalamus. Eur J Endocrinol. 2013;168(3):361‐369. 10.1530/EJE-12-0750 [DOI] [PubMed] [Google Scholar]
- 64.Diano S, Horvath B, Urbanski HF, Sotonyi P, Horvath TL. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144(9):3774‐3778. 10.1210/en.2003-0274 [DOI] [PubMed] [Google Scholar]
- 65.Blouin AM, Fried I, Wilson CL, et al. Human hypocretin and melanin‐concentrating hormone levels are linked to emotion and social interaction. Nat Commun. 2013;4(1):1547. 10.1038/ncomms2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115(2):493‐500. 10.1037/0735-7044.115.2.493 [DOI] [PubMed] [Google Scholar]
- 67.Kim H, Shimojo S, O’Doherty JP. Is avoiding an aversive outcome rewarding? neural substrates of avoidance learning in the human brain. PLoS Biol. 2006;4(8):e233. 10.1371/journal.pbio.0040233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. 10.1126/science.1144599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol. 2007;98(3):1374‐1379. 10.1152/jn.00230.2007 [DOI] [PubMed] [Google Scholar]
- 70.Harris GC, Wimmer M, Aston‐Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556‐559. 10.1038/nature04071 [DOI] [PubMed] [Google Scholar]
- 71.Tiedemann LJ, Schmid SM, Hettel J, et al. Central insulin modulates food valuation via mesolimbic pathways. Nat Commun. 2017;8(1):16052. 10.1038/ncomms16052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gottfried JA. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104‐1107. 10.1126/science.1087919 [DOI] [PubMed] [Google Scholar]
- 73.Hornak J, O'Doherty J, Bramham J, et al. Reward‐related reversal learning after surgical excisions in orbito‐frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16(3):463‐478. 10.1162/089892904322926791 [DOI] [PubMed] [Google Scholar]
- 74.Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57(5):760‐773. 10.1016/j.neuron.2008.01.022 [DOI] [PubMed] [Google Scholar]
- 75.Fukuda M, Ono T. Amygdala‐hypothalamic control of feeding behavior in monkey: single cell responses before and after reversible blockade of temporal cortex or amygdala projections. Behav Brain Res. 1993;55(2):233‐241. 10.1016/0166-4328(93)90119-B [DOI] [PubMed] [Google Scholar]
- 76.Saper CB, Lowell BB. The hypothalamus. Curr Biol. 2014;24(23):R1111‐R1116. 10.1016/j.cub.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 77.Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone. 1999;2(3):17‐31. 10.1016/S1098-3597(99)90002-9 [DOI] [PubMed] [Google Scholar]
- 78.Coppari R, Ichinose M, Lee CE, et al. The hypothalamic arcuate nucleus: A key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1(1):63‐72. 10.1016/j.cmet.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 79.Vaisse C, Clement K, Guy‐Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20(2):113‐114. 10.1038/2407 [DOI] [PubMed] [Google Scholar]
- 80.Yeo GSH, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20(2):111‐112. 10.1038/2404 [DOI] [PubMed] [Google Scholar]
- 81.Iepsen EW, Zhang J, Thomsen HS, et al. Patients with obesity caused by melanocortin‐4 receptor mutations can be treated with a glucagon‐like peptide‐1 receptor agonist. Cell Metab. 2018;28(1):23‐32.e3. 10.1016/j.cmet.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 82.Yi C‐X, Walter M, Gao Y, et al. TNFα drives mitochondrial stress in POMC neurons in obesity. Nat Commun. 2017;8:15143. 10.1038/ncomms15143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao Y, Vidal‐Itriago A, Kalsbeek MJ, et al. Lipoprotein lipase maintains microglial innate immunity in obesity. Cell Rep. 2017;20(13):3034‐3042. 10.1016/j.celrep.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 84.Kalsbeek MJT, Wolff SEC, Korpel NL, et al. The impact of antidiabetic treatment on human hypothalamic infundibular neurons and microglia. JCI Insight. 2020;5(16 ). 10.1172/jci.insight.133868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cowley MA, Smart JL, Rubinstein M, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480‐484. 10.1038/35078085 [DOI] [PubMed] [Google Scholar]
- 86.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting‐activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271‐272. 10.1038/1082 [DOI] [PubMed] [Google Scholar]
- 87.Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. DMM Dis Model Mech. 2017;10(6):679‐689. 10.1242/dmm.026609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma X, Zubcevic L, Ashcroft FM. Glucose regulates the effects of leptin on hypothalamic POMC neurons. Proc Natl Acad Sci. 2008;105(28):9811‐9816. 10.1073/pnas.0800952105 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Gao Y, Vidal‐Itriago A, Milanova I, et al. Deficiency of leptin receptor in myeloid cells disrupts hypothalamic metabolic circuits and causes body weight increase. Mol Metab. 2018;7:155‐160. 10.1016/j.molmet.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; size changes in relation to age and sex. J Clin Endocrinol Metab. 1999;84(12):4637‐4644. 10.1210/jcem.84.12.6187 [DOI] [PubMed] [Google Scholar]
- 91.Arase K, York DA, Shimizu H, Shargill N, Bray GA. Effects of corticotropin‐releasing factor on food intake and brown adipose tissue thermogenesis in rats. Am J Physiol Metab. 1988;255(3):E255‐E259. 10.1152/ajpendo.1988.255.3.E255 [DOI] [PubMed] [Google Scholar]
- 92.Campbell RE, Grove KL, Smith MS. Distribution of corticotropin releasing hormone receptor immunoreactivity in the rat hypothalamus: coexpression in neuropeptide Y and dopamine neurons in the arcuate nucleus. Brain Res. 2003;973(2):223‐232. 10.1016/S0006-8993(03)02487-9 [DOI] [PubMed] [Google Scholar]
- 93.Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J Comp Neurol. 2009;516(5):423‐441. 10.1002/cne.22126 [DOI] [PubMed] [Google Scholar]
- 94.Vella KR, Ramadoss P, Lam FS, et al. NPY and MC4R signaling regulate thyroid hormone levels during fasting through both central and peripheral pathways. Cell Metab. 2011;14(6):780‐790. 10.1016/j.cmet.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Z, Boelen A, Kalsbeek A, Fliers E. TRH neurons and thyroid hormone coordinate the hypothalamic response to cold. Eur Thyroid J. 2018;7(6):279‐288. 10.1159/000493976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haynes AC, Jackson B, Chapman H, et al. A selective orexin‐1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96(1–2):45‐51. 10.1016/S0167-0115(00)00199-3 [DOI] [PubMed] [Google Scholar]
- 97.López M, Seoane LM, García MDC, Diéguez C, Señarís R. Neuropeptide Y, but not agouti‐related peptide or melanin‐concentrating hormone, is a target peptide for orexin‐A feeding actions in the rat hypothalamus. Neuroendocrinology. 2002;75(1):34‐44. 10.1159/000048219 [DOI] [PubMed] [Google Scholar]
- 98.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein‐coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573‐585. 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- 99.Edwards C, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin‐concentrating hormone and galanin. J Endocrinol. 1999;160(3):R7‐R12. 10.1677/joe.0.160r007 [DOI] [PubMed] [Google Scholar]
- 100.López M, Seoane L, del Carmen García M, et al. Leptin Regulation of prepro‐orexin and orexin receptor mRNA levels in the hypothalamus. Biochem Biophys Res Commun. 2000;269(1):41‐45. 10.1006/bbrc.2000.2245 [DOI] [PubMed] [Google Scholar]
- 101.Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin‐A in food motivation, reward‐based feeding behavior and food‐induced neuronal activation in rats. Neuroscience. 2010;167(1):11‐20. 10.1016/j.neuroscience.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 102.Swaab DF, Purba JS, Hofman MA. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader‐Willi syndrome: a study of five cases. J Clin Endocrinol Metab. 1995;80(2):573‐579. 10.1210/jcem.80.2.7852523 [DOI] [PubMed] [Google Scholar]
- 103.Goldstone AP, Unmehopa UA, Bloom SR, Swaab DF. Hypothalamic NPY and agouti‐related protein are increased in human illness but not in Prader‐Willi syndrome and other obese subjects. J Clin Endocrinol Metab. 2002;87(2):927‐937. 10.1210/jcem.87.2.8230 [DOI] [PubMed] [Google Scholar]
- 104.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087‐2102. 10.1053/j.gastro.2007.03.052 [DOI] [PubMed] [Google Scholar]
- 105.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro‐opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5(9):1066‐1070. 10.1038/12506 [DOI] [PubMed] [Google Scholar]
- 106.Melo HM, Seixas da Silva GDS, Sant’Ana MR, et al. Palmitate is increased in the cerebrospinal fluid of humans with obesity and induces memory impairment in mice via pro‐inflammatory TNF‐α. Cell Rep. 2020;30(7):2180‐2194.e8. 10.1016/j.celrep.2020.01.072 [DOI] [PubMed] [Google Scholar]
- 107.Thaler JP, Yi C‐X, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153‐162. 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farooqi IS, Drop S, Clements A, et al. Heterozygosity for a POMC‐null mutation and increased obesity risk in humans. Diabetes. 2006;55(9):2549‐2553. 10.2337/db06-0214 [DOI] [PubMed] [Google Scholar]
- 109.Farooqi IS, Keogh JM, Yeo GSH, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085‐1095. 10.1056/NEJMoa022050 [DOI] [PubMed] [Google Scholar]
- 110.Lotta LA, Mokrosiński J, Mendes de Oliveira E, et al. Human gain‐of‐function MC4R variants show signaling bias and protect against obesity. Cell. 2019;177(3):597‐607.e9. 10.1016/j.cell.2019.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alkemade A, Yi C‐X, Pei L, et al. AgRP and NPY expression in the human hypothalamic infundibular nucleus correlate with body mass index, whereas changes in αMSH are related to type 2 diabetes. J Clin Endocrinol Metab. 2012;97(6):E925‐E933. 10.1210/jc.2011-3259 [DOI] [PubMed] [Google Scholar]
- 112.Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL. High‐fat diet‐induced brain region‐specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132(3):361‐375. 10.1007/s00401-016-1595-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu X, Balesar R, Lu J, et al. Increased glutamic acid decarboxylase expression in the hypothalamic suprachiasmatic nucleus in depression. Brain Struct Funct. 2017;222(9):4079‐4088. 10.1007/s00429-017-1442-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou J‐N, Riemersma RF, Unmehopa UA, et al. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry. 2001;58(7):655. 10.1001/archpsyc.58.7.655 [DOI] [PubMed] [Google Scholar]
- 115.Elmquist JK, Maratos‐Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1(6):445‐450. 10.1038/2164 [DOI] [PubMed] [Google Scholar]
- 116.Wang J, Leibowitz KL. Central insulin inhibits hypothalamic galanin and neuropeptide Y gene expression and peptide release in intact rats. Brain Res. 1997;777(1–2):231‐236. 10.1016/S0006-8993(97)00963-3 [DOI] [PubMed] [Google Scholar]
- 117.Bergen HT, Mizuno T, Taylor J, Mobbs CV. Resistance to diet‐induced obesity is associated with increased proopiomelanocortin mRNA and decreased neuropeptide Y mRNA in the hypothalamus. Brain Res. 1999;851(1–2):198‐203. 10.1016/S0006-8993(99)02186-1 [DOI] [PubMed] [Google Scholar]
- 118.Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS. Differential expression of hypothalamic neuropeptides in the early phase of diet‐induced obesity in mice. Am J Physiol Metab. 2000;279(4):E838‐E845. 10.1152/ajpendo.2000.279.4.E838 [DOI] [PubMed] [Google Scholar]
- 119.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite‐regulating pathways in the hypothalamic regulation of body weight*. Endocr Rev. 1999;20(1):68‐100. 10.1210/edrv.20.1.0357 [DOI] [PubMed] [Google Scholar]
- 120.Guan X‐M, Yu H, Trumbauer M, Frazier E, Van der Ploeg LHT, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet‐induced obese mice. NeuroReport. 1998;9(15):3415‐3419. 10.1097/00001756-199810260-00015 [DOI] [PubMed] [Google Scholar]
- 121.Bochukova EG, Lawler K, Croizier S, et al. A Transcriptomic signature of the hypothalamic response to fasting and BDNF deficiency in Prader‐Willi syndrome. Cell Rep. 2018;22(13):3401‐3408. 10.1016/j.celrep.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Creemers JWM, Pritchard LE, Gyte A, et al. Agouti‐related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti‐related protein (AGRP)83–132: interaction between AGRP83–132 and melanocortin receptors cannot be influenced by syndecan‐3. Endocrinology. 2006;147(4):1621‐1631. 10.1210/en.2005-1373 [DOI] [PubMed] [Google Scholar]
- 123.Gabreëls BATF, Swaab DF, de Kleijn DPV, et al. Attenuation of the polypeptide 7B2, prohormone convertase PC2, and vasopressin in the hypothalamus of some Prader‐Willi patients: indications for a processing defect. J Clin Endocrinol Metab. 1998;83(2):591‐599. 10.1210/jcem.83.2.4542 [DOI] [PubMed] [Google Scholar]
- 124.Gabreëls BATF, Swaab DF, Seidah NG, van Duijnhoven HLP , Martens GJM, van Leeuwen FW . Differential expression of the neuroendocrine polypeptide 7B2 in hypothalami of Prader‐(Labhart)‐Willi syndrome patients. Brain Res. 1994;657(1–2):281‐293. 10.1016/0006-8993(94)90978-4 [DOI] [PubMed] [Google Scholar]
- 125.Zhou A, Webb G, Zhu X, Steiner DF. Proteolytic processing in the secretory pathway. J Biol Chem. 1999;274(30):20745‐20748. 10.1074/jbc.274.30.20745 [DOI] [PubMed] [Google Scholar]
- 126.Burnett LC, LeDuc CA, Sulsona CR, et al. Deficiency in prohormone convertase PC1 impairs prohormone processing in Prader‐Willi syndrome. J Clin Invest. 2016;127(1):293‐305. 10.1172/JCI88648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ugleholdt R, Poulsen M‐L, Holst PJ, et al. Prohormone convertase 1/3 is essential for processing of the glucose‐dependent insulinotropic polypeptide precursor. J Biol Chem. 2006;281(16):11050‐11057. 10.1074/jbc.M601203200 [DOI] [PubMed] [Google Scholar]
- 128.Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959;145(2):336‐352. 10.1113/jphysiol.1959.sp006145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Top M, Nolan MF, Lee K, et al. Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol. 2003;549(3):809‐821. 10.1113/jphysiol.2002.033290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fronczek R, Lammers GJ, Balesar R, Unmehopa UA, Swaab DF. The number of hypothalamic hypocretin (orexin) neurons is not affected in Prader‐Willi syndrome. J Clin Endocrinol Metab. 2005;90(9):5466‐5470. 10.1210/jc.2005-0296 [DOI] [PubMed] [Google Scholar]
- 131.Manzardo AM, Johnson L, Miller JL, Driscoll DJ, Butler MG. Higher plasma orexin a levels in children with Prader‐Willi syndrome compared with healthy unrelated sibling controls. Am J Med Genet Part A. 2016;170(8):2328‐2333. 10.1002/ajmg.a.37749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Lecea L, Sutcliffe JG. The hypocretins and sleep. FEBS J. 2005;272(22):5675‐5688. 10.1111/j.1742-4658.2005.04981.x [DOI] [PubMed] [Google Scholar]
- 133.Digby JE, Chen J, Tang JY, Lehnert H, Matthews RN, Randeva HS. Orexin receptor expression in human adipose tissue: effects of orexin‐A and orexin‐B. J Endocrinol. 2006;191(1):129‐136. 10.1677/joe.1.06886 [DOI] [PubMed] [Google Scholar]
- 134.Skrzypski M, T. Le T, Kaczmarek P, et al. Orexin A stimulates glucose uptake, lipid accumulation and adiponectin secretion from 3T3‐L1 adipocytes and isolated primary rat adipocytes. Diabetologia. 2011;54(7):1841‐1852. 10.1007/s00125-011-2152-2 [DOI] [PubMed] [Google Scholar]
- 135.Chow M, Cao M. The hypocretin/orexin system in sleep disorders: preclinical insights and clinical progress. Nat Sci Sleep. 2016;8:81‐86. 10.2147/NSS.S76711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nishino S, Okuro M, Kotorii N, et al. Hypocretin/orexin and narcolepsy: new basic and clinical insights. Acta Physiol. 2010;198(3):209‐222. 10.1111/j.1748-1716.2009.02012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cohen M, Hamilton J, Narang I. Clinically important age‐related differences in sleep related disordered breathing in infants and children with Prader‐Willi syndrome. PLoS One. 2014;9(6):e101012. 10.1371/journal.pone.0101012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tauber M, Mantoulan C, Copet P, et al. Oxytocin may be useful to increase trust in others and decrease disruptive behaviours in patients with Prader‐Willi syndrome: a randomised placebo‐controlled trial in 24 patients. Orphanet J Rare Dis. 2011;6(1):47. 10.1186/1750-1172-6-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tauber M, Boulanouar K, Diene G, et al. The use of oxytocin to improve feeding and social skills in infants with Prader‐Willi syndrome. Pediatrics. 2017;139(2):e20162976. 10.1542/peds.2016-2976 [DOI] [PubMed] [Google Scholar]
- 140.Einfeld SL, Smith E, McGregor IS, et al. A double‐blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet Part A. 2014;164(9):2232‐2239. 10.1002/ajmg.a.36653 [DOI] [PubMed] [Google Scholar]
- 141.Kuppens RJ, Donze SH, Hokken‐Koelega ACS. Promising effects of oxytocin on social and food‐related behaviour in young children with Prader‐Willi syndrome: a randomized, double‐blind, controlled crossover trial. Clin Endocrinol (Oxf). 2016;85(6):979‐987. 10.1111/cen.13169 [DOI] [PubMed] [Google Scholar]
- 142.Miller JL, Tamura R, Butler MG, et al. Oxytocin treatment in children with Prader‐Willi syndrome: a double‐blind, placebo‐controlled, crossover study. Am J Med Genet Part A. 2017;173(5):1243‐1250. 10.1002/ajmg.a.38160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Johnson L, Manzardo AM, Miller JL, Driscoll DJ, Butler MG. Elevated plasma oxytocin levels in children with Prader‐Willi syndrome compared with healthy unrelated siblings. Am J Med Genet Part A. 2016;170(3):594‐601. 10.1002/ajmg.a.37488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gainer H, Lively MO, Morris M. [10] Immunological and related techniques for studying neurohypophyseal peptide‐processing pathways. In: Methods in Neurosciences, Vol. 23. Academic Press; 1995:195‐207. 10.1016/S1043-9471(06)80121-7 [DOI] [Google Scholar]
- 145.Bittel DC, Kibiryeva N, Sell SM, Strong TV, Butler MG. Whole genome microarray analysis of gene expression in Prader‐Willi syndrome. Am J Med Genet Part A. 2007;143A(5):430‐442. 10.1002/ajmg.a.31606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bueno M, Esteba‐Castillo S, Novell R, et al. Lack of postprandial peak in brain‐derived neurotrophic factor in adults with Prader‐Willi syndrome. PLoS One. 2016;11(9):e0163468. 10.1371/journal.pone.0163468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liao G‐Y, Kinney CE, An JJ, Xu B. TrkB‐expressing neurons in the dorsomedial hypothalamus are necessary and sufficient to suppress homeostatic feeding. Proc Natl Acad Sci. 2019;116(8):3256‐3261. 10.1073/pnas.1815744116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marini AM, Jiang X, Wu X, et al. Role of brain‐derived neurotrophic factor and NF‐kappaB in neuronal plasticity and survival: from genes to phenotype. Restor Neurol Neurosci. 2004;22(2):121‐130. [PubMed] [Google Scholar]
- 149.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain‐derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15(3):331‐337. 10.1038/nm.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129(2):251‐262. 10.1016/j.cell.2007.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth‐hormone‐releasing acylated peptide from stomach. Nature. 1999;402(6762):656‐660. 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- 152.Corrêa da Silva F, Aguiar C, Pereira JAS, et al. Ghrelin effects on mitochondrial fitness modulates macrophage function. Free Radic Biol Med. 2019;145:61‐66. 10.1016/j.freeradbiomed.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 153.Druce MR, Wren AM, Park AJ, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes. 2005;29(9):1130‐1136. 10.1038/sj.ijo.0803001 [DOI] [PubMed] [Google Scholar]
- 154.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908‐913. 10.1038/35038090 [DOI] [PubMed] [Google Scholar]
- 155.Chen S‐R, Chen H, Zhou J‐J, et al. Ghrelin receptors mediate ghrelin‐induced excitation of agouti‐related protein/neuropeptide Y but not pro‐opiomelanocortin neurons. J Neurochem. 2017;142(4):512‐520. 10.1111/jnc.14080 [DOI] [PubMed] [Google Scholar]
- 156.Zigman JM. Mice lacking ghrelin receptors resist the development of diet‐induced obesity. J Clin Invest. 2005;115(12):3564‐3572. 10.1172/JCI26002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Naznin F, Toshinai K, Waise TMZ, et al. Diet‐induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J Endocrinol. 2015;226(1):81‐92. 10.1530/JOE-15-0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707‐709. 10.2337/diabetes.50.4.707 [DOI] [PubMed] [Google Scholar]
- 159.Cummings DE, Clement K, Purnell JQ, et al. Elevated plasma ghrelin levels in Prader‐Willi syndrome. Nat Med. 2002;8(7):643‐644. 10.1038/nm0702-643 [DOI] [PubMed] [Google Scholar]
- 160.DelParigi A, Tschöp M, Heiman ML, et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader‐Willi syndrome. J Clin Endocrinol Metab. 2002;87(12):5461‐5464. 10.1210/jc.2002-020871 [DOI] [PubMed] [Google Scholar]
- 161.Feigerlová E, Diene G, Conte‐Auriol F, et al. Hyperghrelinemia precedes obesity in Prader‐Willi syndrome. J Clin Endocrinol Metab. 2008;93(7):2800‐2805. 10.1210/jc.2007-2138 [DOI] [PubMed] [Google Scholar]
- 162.Goldstone AP, Thomas EL, Brynes AE, et al. Elevated fasting plasma ghrelin in Prader‐Willi syndrome adults is not solely explained by their reduced visceral adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89(4):1718‐1726. 10.1210/jc.2003-031118 [DOI] [PubMed] [Google Scholar]
- 163.Erdie‐Lalena CR, Holm VA, Kelly PC, Frayo RS, Cummings DE. Ghrelin levels in young children with Prader‐Willi syndrome. J Pediatr. 2006;149(2):199‐204. 10.1016/j.jpeds.2006.04.011 [DOI] [PubMed] [Google Scholar]
- 164.Haqq AM, Farooqi IS, O’Rahilly S, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader‐Willi syndrome. J Clin Endocrinol Metab. 2003;88(1):174‐178. 10.1210/jc.2002-021052 [DOI] [PubMed] [Google Scholar]
- 165.Bervini S, Herzog H. Mouse models of Prader‐Willi syndrome: a systematic review. Front Neuroendocrinol. 2013;34(2):107‐119. 10.1016/j.yfrne.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 166.Cavaille J, Buiting K, Kiefmann M, et al. Identification of brain‐specific and imprinted small nucleolar RNA genes exhibiting an unusual genomic organization. Proc Natl Acad Sci. 2000;97(26):14311‐14316. 10.1073/pnas.250426397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rodriguez JA, Bruggeman EC, Mani BK, et al. Ghrelin Receptor agonist rescues excess neonatal mortality in a Prader‐Willi syndrome mouse model. Endocrinology. 2018;159(12):4006‐4022. 10.1210/en.2018-00801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Polex‐Wolf J, Lam BYH, Larder R, et al. Hypothalamic loss of Snord116 recapitulates the hyperphagia of Prader‐Willi syndrome. J Clin Invest. 2018;128(3):960‐969. 10.1172/JCI97007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu X, Zhou A, Dey A, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci. 2002;99(16):10293‐10298. 10.1073/pnas.162352599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20(8):1772‐1785. 10.1210/me.2005-0084 [DOI] [PubMed] [Google Scholar]
- 171.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26(11):2274‐2279. 10.1016/j.peptides.2005.04.025 [DOI] [PubMed] [Google Scholar]
- 172.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12(1):6‐16. 10.1111/j.1369-1600.2006.00041.x [DOI] [PubMed] [Google Scholar]
- 173.Kawahara Y, Kawahara H, Kaneko F, et al. Peripherally administered ghrelin induces bimodal effects on the mesolimbic dopamine system depending on food‐consumptive states. Neuroscience. 2009;161(3):855‐864. 10.1016/j.neuroscience.2009.03.086 [DOI] [PubMed] [Google Scholar]
- 174.Kim JG, Suyama S, Koch M, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17(7):908‐910. 10.1038/nn.3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Burke SJ, Batdorf HM, Burk DH, et al. db/db mice exhibit features of human type 2 diabetes that are not present in weight‐matched C57BL/6J mice fed a western diet. J Diabetes Res. 2017;2017:1‐17. 10.1155/2017/8503754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight‐reduced subjects. Nat Med. 1995;1(11):1155‐1161. 10.1038/nm1195-1155 [DOI] [PubMed] [Google Scholar]
- 177.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet‐induced resistance to leptin action. Nat Med. 1995;1(12):1311‐1314. 10.1038/nm1295-1311 [DOI] [PubMed] [Google Scholar]
- 178.Lindgren AC, Marcus C, Skwirut C, et al. Increased leptin messenger RNA and serum leptin levels in children with Prader‐Willi syndrome and nonsyndromal obesity. Pediatr Res. 1997;42(5):593‐596. 10.1203/00006450-199711000-00007 [DOI] [PubMed] [Google Scholar]
- 179.Butler MG, Moore J, Morawiecki A, Nicolson M. Comparison of leptin protein levels in Prader‐Willi syndrome and control individuals. Am J Med Genet. 1998;75(1):7‐12. [PMC free article] [PubMed] [Google Scholar]
- 180.Chan JL, Mantzoros CS. Role of leptin in energy‐deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet. 2005;366(9479):74‐85. 10.1016/S0140-6736(05)66830-4 [DOI] [PubMed] [Google Scholar]
- 181.Goldstone AP, Brynes AE, Thomas EL, et al. Resting metabolic rate, plasma leptin concentrations, leptin receptor expression, and adipose tissue measured by whole‐body magnetic resonance imaging in women with Prader‐Willi syndrome. Am J Clin Nutr. 2002;75(3):468‐475. 10.1093/ajcn/75.3.468 [DOI] [PubMed] [Google Scholar]
- 182.Lubis AR, Widia F, Soegondo S, Setiawati A. The role of SOCS‐3 protein in leptin resistance and obesity. Acta Med Indones. 2008;40(2):89‐95. [PubMed] [Google Scholar]
- 183.Bischof JM, Stewart CL, Wevrick R. Inactivation of the mouse Magel2 gene results in growth abnormalities similar to Prader‐Willi syndrome. Hum Mol Genet. 2007;16(22):2713‐2719. 10.1093/hmg/ddm225 [DOI] [PubMed] [Google Scholar]
- 184.Mercer RE, Michaelson SD, Chee MJS, Atallah TA, Wevrick R, Colmers WF. Magel2 is required for leptin‐mediated depolarization of POMC neurons in the hypothalamic arcuate nucleus in mice. PLoS Genet. 2013;9(1):e1003207. 10.1371/journal.pgen.1003207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228‐236. 10.1038/nrendo.2011.183 [DOI] [PubMed] [Google Scholar]
- 186.Sato I. Insulin inhibits neuropeptide Y gene expression in the arcuate nucleus through GABAergic systems. J Neurosci. 2005;25(38):8657‐8664. 10.1523/JNEUROSCI.2739-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vanderpump MPJ, Tunbridge WMG, French JM, et al. The incidence of diabetes mellitus in an English community: a 20‐year follow‐up of the Whickham survey. Diabet Med. 1996;13(8):741‐747. [DOI] [PubMed] [Google Scholar]
- 188.Butler JV, Whittington JE, Holland AJ, Boer H, Clarke D, Webb T. Prevalence of, and risk factors for, physical ill‐health in people with Prader‐Willi syndrome: a population‐based study. Dev Med Child Neurol. 2002;44(04):248. 10.1017/S001216220100202X [DOI] [PubMed] [Google Scholar]
- 189.Laurier V, Lapeyrade A, Copet P, et al. Medical, psychological and social features in a large cohort of adults with Prader‐Willi syndrome: experience from a dedicated centre in France. J Intellect Disabil Res. 2015;59(5):411‐421. 10.1111/jir.12140 [DOI] [PubMed] [Google Scholar]
- 190.Lee J‐E. An update on Prader‐Willi syndrome with diabetes mellitus. J mucopolysaccharidosis rare Dis. 2016;2(2):35‐37. 10.19125/jmrd.2016.2.2.35 [DOI] [Google Scholar]
- 191.Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader‐Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab. 2011;96(1):E225‐E232. 10.1210/jc.2010-1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Butler MG, Swift LL, Hill JO. Fasting plasma lipid, glucose, and insulin levels in Prader‐Willi syndrome and obese individuals. Dysmorphol Clin Genet Off Publ Cent Birth Defects Inf Serv Inc 1990;4(1):23‐26. [PMC free article] [PubMed] [Google Scholar]
- 193.Parra A, Cervantes C, Schultz RB. Immunoreactive insulin and growth hormone responses in patients with Prader‐Willi syndrome. J Pediatr. 1973;83(4):587‐593. 10.1016/S0022-3476(73)80219-7 [DOI] [PubMed] [Google Scholar]
- 194.Bier DM, Kaplan SL, Havel RJ. The Prader‐Willi syndrome regulation of fat transport. Diabetes. 1977;26(9):874‐881. 10.2337/diab.26.9.874 [DOI] [PubMed] [Google Scholar]
- 195.Zipf WB, O’Dorisio TM, Cataland S, Dixon K. Pancreatic polypeptide responses to protein meal challenges in obese but otherwise normal children and obese children with Prader‐Willi syndrome*. J Clin Endocrinol Metab. 1983;57(5):1074‐1080. 10.1210/jcem-57-5-1074 [DOI] [PubMed] [Google Scholar]
- 196.Schuster DP, Osei K, Zipf WB. Characterization of alterations in glucose and insulin metabolism in Prader‐Willi subjects. Metabolism. 1996;45(12):1514‐1520. 10.1016/S0026-0495(96)90181-X [DOI] [PubMed] [Google Scholar]
- 197.Talebizadeh Z, Butler M. Insulin resistance and obesity‐related factors in Prader‐Willi syndrome: comparison with obese subjects. Clin Genet. 2004;67(3):230‐239. 10.1111/j.1399-0004.2004.00392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non‐insulin‐dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med. 1993;329(27):1988‐1992. 10.1056/NEJM199312303292703 [DOI] [PubMed] [Google Scholar]
- 199.Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61(4):778‐779. 10.2337/db12-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ferrannini E, Nannipieri M, Williams K, Gonzales C, Haffner SM, Stern MP. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes. 2004;53(1):160‐165. 10.2337/diabetes.53.1.160 [DOI] [PubMed] [Google Scholar]
- 201.Goldstone AP, Thomas EL, Brynes AE, et al. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader‐Willi syndrome female adults: evidence for novel influences on body fat distribution. J Clin Endocrinol Metab. 2001;86(9):4330‐4338. 10.1210/jcem.86.9.7814 [DOI] [PubMed] [Google Scholar]
- 202.Kennedy L, Bittel DC, Kibiryeva N, Kalra SP, Torto R, Butler MG. Circulating adiponectin levels, body composition and obesity‐related variables in Prader‐Willi syndrome: comparison with obese subjects. Int J Obes. 2006;30(2):382‐387. 10.1038/sj.ijo.0803115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Eiholzer U, Blum WF, Molinari L. Body fat determined by skinfold measurements is elevated despite underweight in infants with Prader‐Labhart‐Willi syndrome. J Pediatr. 1999;134(2):222‐225. 10.1016/S0022-3476(99)70419-1 [DOI] [PubMed] [Google Scholar]
- 204.Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5(6):2019‐2027. 10.3390/nu5062019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Eiholzer U, L’Allemand D, Schlumpf M, Rousson V, Gasser T, Fusch C. Growth hormone and body composition in children younger than 2 years with Prader‐Willi syndrome. J Pediatr. 2004;144(6):753‐758. 10.1016/j.jpeds.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 206.Moran A, Jacobs DR, Steinberger J, et al. Association between the insulin resistance of puberty and the insulin‐like growth factor‐I/growth hormone axis. J Clin Endocrinol Metab. 2002;87(10):4817‐4820. 10.1210/jc.2002-020517 [DOI] [PubMed] [Google Scholar]
- 207.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic β cells. Biochem Biophys Res Commun. 2003;312(4):1118‐1122. 10.1016/j.bbrc.2003.11.042 [DOI] [PubMed] [Google Scholar]
- 208.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459‐469. 10.1007/s00125-003-1074-z [DOI] [PubMed] [Google Scholar]
- 209.Lee B, Shao J. Adiponectin and lipid metabolism in skeletal muscle. Acta Pharm Sin B. 2012;2(4):335‐340. 10.1016/j.apsb.2012.06.008 [DOI] [Google Scholar]
- 210.Reis WL, Yi C‐X, Gao Y, Tschöp MH, Stern JE. Brain innate immunity regulates hypothalamic arcuate neuronal activity and feeding behavior. Endocrinology. 2015;156(4):1303‐1315. 10.1210/en.2014-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Valdearcos M, Douglass JD, Robblee MM, et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26(1):185‐197.e3. 10.1016/j.cmet.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Greenmyer JR, Gaultney RA, Brissette CA, Watt JA. Primary human microglia are phagocytically active and respond to borrelia burgdorferi with upregulation of chemokines and cytokines. Front Microbiol. 2018;9 . 10.3389/fmicb.2018.00811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wake H, Fields RD. Physiological function of microglia. Neuron Glia Biol. 2011;7(1):1‐3. 10.1017/S1740925X12000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Srinivasan K, Friedman BA, Etxeberria A, et al. Alzheimer’s patient microglia exhibit enhanced aging and unique transcriptional activation. Cell Rep. 2020;31(13):107843. 10.1016/j.celrep.2020.107843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Barcia C, Ros CM, Ros‐Bernal F, et al. Persistent phagocytic characteristics of microglia in the substantia nigra of long‐term Parkinsonian macaques. J Neuroimmunol. 2013;261(1–2):60‐66. 10.1016/j.jneuroim.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 216.van der Poel M, Ulas T, Mizee MR, et al. Transcriptional profiling of human microglia reveals grey–white matter heterogeneity and multiple sclerosis‐associated changes. Nat Commun. 2019;10(1):1139. 10.1038/s41467-019-08976-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Milanova IV, Kalsbeek MJT, Wang X‐L, et al. Diet‐induced obesity disturbs microglial immunometabolism in a time‐of‐day manner. Front Endocrinol (Lausanne). 2019;10 . 10.3389/fendo.2019.00424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat‐rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192‐4199. 10.1210/en.2004-1520 [DOI] [PubMed] [Google Scholar]
- 219.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9(6):2124‐2138. 10.1016/j.celrep.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111‐2117. 10.1172/JCI57132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.García‐Cáceres C, Yi C‐X, Tschöp MH. Hypothalamic astrocytes in obesity. Endocrinol Metab Clin North Am. 2013;42(1):57‐66. 10.1016/j.ecl.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 222.Bahniwal M, Little JP, Klegeris A. High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr Alzheimer Res. 2017;14(7 ). 10.2174/1567205014666170117104053 [DOI] [PubMed] [Google Scholar]
- 223.Dominguez‐Meijide A, Rodriguez‐Perez AI, Diaz‐Ruiz C, Guerra MJ, Labandeira‐Garcia JL. Dopamine modulates astroglial and microglial activity via glial renin‐angiotensin system in cultures. Brain Behav Immun. 2017;62:277–290. 10.1016/j.bbi.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 224.Duffy CM, Yuan CE, Wisdorf LE, et al. Role of orexin A signaling in dietary palmitic acid‐activated microglial cells. Neurosci Lett. 2015;606:140‐144. 10.1016/j.neulet.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Capsoni F, Ongari AM, Reali E, Catania A. Melanocortin peptides inhibit urate crystal‐induced activation of phagocytic cells. Arthritis Res Ther. 2009;11(5):R151. 10.1186/ar2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30(10):527‐535. 10.1016/j.tins.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 227.Pannell M, Szulzewsky F, Matyash V, Wolf SA, Kettenmann H. The subpopulation of microglia sensitive to neurotransmitters/neurohormones is modulated by stimulation with LPS, interferon‐γ, and IL‐4. Glia. 2014;62(5):667‐679. 10.1002/glia.22633 [DOI] [PubMed] [Google Scholar]
- 228.Zhao W, Li Q, Dong C, Li W, Bu W, Wu J. Neuropeptide Y protects cerebral cortical neurons by regulating microglial immune function. Neural Regen Res. 2014;9(9):959. 10.4103/1673-5374.133140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Álvaro AR, Rosmaninho‐Salgado J, Santiago AR, et al. NPY in rat retina is present in neurons, in endothelial cells and also in microglial and Müller cells. Neurochem Int. 2007;50(5):757‐763. 10.1016/j.neuint.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 230.Bedoui S, von Hörsten S , Gebhardt T. A role for neuropeptide Y (NPY) in phagocytosis: implications for innate and adaptive immunity. Peptides. 2007;28(2):373‐376. 10.1016/j.peptides.2006.07.029 [DOI] [PubMed] [Google Scholar]
- 231.Carniglia L, Durand D, Caruso C, Lasaga M. Effect of NDP‐α‐MSH on PPAR‐γ and ‐β expression and anti‐inflammatory cytokine release in rat astrocytes and microglia. PLoS One. 2013;8(2):e57313. 10.1371/journal.pone.0057313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Carniglia L, Ramírez D, Durand D, Saba J, Caruso C, Lasaga M. [Nle4, D‐Phe7]‐α‐MSH inhibits toll‐like receptor (TLR)2‐ and TLR4‐induced microglial activation and promotes a M2‐like phenotype. PLoS One. 2016;11(6):e0158564. 10.1371/journal.pone.0158564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. The neuropeptide α‐MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 1996;17(4):675‐679. 10.1016/0196-9781(96)00037-X [DOI] [PubMed] [Google Scholar]
- 234.Cutuli M, Cristiani S, Lipton JM, Catania A. Antimicrobial effects of α‐MSH peptides. J Leukoc Biol. 2000;67(2):233‐239. 10.1002/jlb.67.2.233 [DOI] [PubMed] [Google Scholar]
- 235.Szeto A, Sun‐Suslow N, Mendez AJ, Hernandez RI, Wagner KV, McCabe PM. Regulation of the macrophage oxytocin receptor in response to inflammation. Am J Physiol Metab. 2017;312(3):E183‐E189. 10.1152/ajpendo.00346.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yuan L, Liu S, Bai X, et al. Oxytocin inhibits lipopolysaccharide‐induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide‐treated mice. J Neuroinflammation. 2016;13(1):77. 10.1186/s12974-016-0541-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Inoue T, Yamakage H, Tanaka M, Kusakabe T, Shimatsu A, Satoh‐Asahara N. Oxytocin suppresses inflammatory responses induced by lipopolysaccharide through inhibition of the eIF‐2α‐ATF4 pathway in mouse microglia. Cells. 2019;8(6):527. 10.3390/cells8060527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu H, Leak RK, Hu X. Neurotransmitter receptors on microglia. BMJ. 2016;1(2):52‐58. 10.1136/svn-2016-000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Spielman L, Bahniwal M, Little J, Walker D, Klegeris A. Insulin modulates in vitro secretion of cytokines and cytotoxins by human glial cells. Curr Alzheimer Res. 2015;12(7):684‐693. 10.2174/1567205012666150710104428 [DOI] [PubMed] [Google Scholar]
- 240.Bulgarelli I, Tamiazzo L, Bresciani E, et al. Desacyl‐ghrelin and synthetic GH‐secretagogues modulate the production of inflammatory cytokines in mouse microglia cells stimulated by β‐amyloid fibrils. J Neurosci Res. 2009;87(12):2718‐2727. 10.1002/jnr.22088 [DOI] [PubMed] [Google Scholar]
- 241.Lafrance V, Inoue W, Kan B, Luheshi GN. Leptin modulates cell morphology and cytokine release in microglia. Brain Behav Immun. 2010;24(3):358‐365. 10.1016/j.bbi.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 242.Brabazon F, Bermudez S, Shaughness M, Khayrullina G, Byrnes KR. The effects of insulin on the inflammatory activity of BV2 microglia. PLoS One. 2018;13(8):e0201878. 10.1371/journal.pone.0201878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Garber AJ. Long‐acting glucagon‐like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Supplement_2):S279‐S284. 10.2337/dc11-s231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Salehi P, Hsu I, Azen CG, Mittelman SD, Geffner ME, Jeandron D. Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader‐Willi syndrome. Pediatr Obes. 2017;12(3):221‐228. 10.1111/ijpo.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kim Y‐M, Lee YJ, Kim SY, Cheon CK, Lim HH. Successful rapid weight reduction and the use of liraglutide for morbid obesity in adolescent Prader‐Willi syndrome. Ann Pediatr Endocrinol Metab. 2020;25(1):52‐56. 10.6065/apem.2020.25.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Burmeister MA, Ayala JE, Smouse H, et al. The hypothalamic glucagon‐like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes. 2017;66(2):372‐384. 10.2337/db16-1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zeltser LM. Feeding circuit development and early‐life influences on future feeding behaviour. Nat Rev Neurosci. 2018;19(5):302‐316. 10.1038/nrn.2018.23 [DOI] [PMC free article] [PubMed] [Google Scholar]


