Abstract
Aims
Opioid misuse and overuse have contributed to a widespread overdose crisis and many patients and physicians are considering medical cannabis to support opioid tapering and chronic pain control. Using a five‐step modified Delphi process, we aimed to develop consensus‐based recommendations on: 1) when and how to safely initiate and titrate cannabinoids in the presence of opioids, 2) when and how to safely taper opioids in the presence of cannabinoids and 3) how to monitor patients and evaluate outcomes when treating with opioids and cannabinoids.
Results
In patients with chronic pain taking opioids not reaching treatment goals, there was consensus that cannabinoids may be considered for patients experiencing or displaying opioid‐related complications, despite psychological or physical interventions. There was consensus observed to initiate with a cannabidiol (CBD)‐predominant oral extract in the daytime and consider adding tetrahydrocannabinol (THC). When adding THC, start with 0.5‐3 mg, and increase by 1‐2 mg once or twice weekly up to 30‐40 mg/day. Initiate opioid tapering when the patient reports a minor/major improvement in function, seeks less as‐needed medication to control pain and/or the cannabis dose has been optimised. The opioid tapering schedule may be 5%–10% of the morphine equivalent dose (MED) every 1 to 4 weeks. Clinical success could be defined by an improvement in function/quality of life, a ≥30% reduction in pain intensity, a ≥25% reduction in opioid dose, a reduction in opioid dose to <90 mg MED and/or reduction in opioid‐related adverse events.
Conclusions
This five‐stage modified Delphi process led to the development of consensus‐based recommendations surrounding the safe introduction and titration of cannabinoids in concert with tapering opioids.
What’s known
Patients taking opioids, and the physicians prescribing them, are leveraging medical cannabis to support opioid tapering. However, there is limited clinical guidance on how to safely titrate cannabinoids in the presence of opioids for the treatment of chronic pain.
What’s new
Using a modified Delphi process to find expert consensus‐based recommendations, this article provides a practical guidance algorithm on how to safely and effectively titrate cannabinoids and taper opioids in patients with chronic pain.
1. INTRODUCTION
Opioids are commonly prescribed for chronic pain management and although data support their role for managing acute and cancer‐related pain, the evidence to support use in chronic pain is not robust.1, 2, 3, 4 Prescription opioid misuse has contributed to a widespread opioid overdose crisis resulting in the deaths of hundreds of thousands of individuals worldwide.5, 6
Medical cannabis containing different concentrations of cannabinoids—such as Δ7‐tetrahydrocannabinol (THC) and cannabidiol (CBD)—is increasingly being used for the management of chronic pain.8 Cannabinoids have a lower risk for dependence compared with opioids and the predicted median lethal dose for THC is >1000 fold higher than the effective dose.7, 9, 10 Previous studies have found that cannabinoids can improve pain‐related outcomes, quality of life and, importantly, have an opioid‐sparing effect.8, 11, 12, 13, 14, 15, 16, 17, 18 In addition, it has been reported that patients commonly use medical cannabis as a substitute for opioid medication.19, 20, 21, 22, 23, 24 However, the effectiveness of cannabis substitution for opioids is not universally observed.25, 26
Although these findings are noteworthy, the majority of clinical studies investigating cannabis and cannabinoids as substitutes or adjuncts to opioids are cross‐sectional or small sample‐size randomised controlled trials. This lack of high‐quality evidence makes providing classical evidence‐based recommendations inaccessible. Despite the paucity of clinical trial evidence, physicians and patients are using cannabis to support opioid tapering. In many countries, patients with chronic pain have access to cannabis, and patients have reported self‐administering cannabis to reduce their opioid dose in the absence of clinical guidance.21, 27, 28
Although cannabis has a lower risk of dependence compared with opioids, it is not an inert therapy.29, 30, 31, 32 At high doses, CBD‐related side effects can include fatigue, diarrhoea and changes in appetite and weight.33 THC‐related side effects can include sedation, syncope, tachycardia, risk of cannabis‐use disorder, psychosis and anxiety.34, 35 With patients having access to prescribed cannabinoids and self‐treating with cannabis to reduce their opioid dose, clinical guidance on safe cannabinoid initiation and titration is urgently required. Randomised placebo‐controlled clinical trials examining how to co‐manage cannabinoids and opioids are unlikely to be provided in the near future. Hence, there is an immediate unmet need for guidance on this topic.36
To provide guidance to healthcare professionals on how to safely manage opioids and cannabinoids in patients with chronic pain, we employed a modified Delphi process to develop a consensus‐based guidance algorithm. The modified Delphi process has been used extensively in healthcare settings to provide consensus‐based recommendations surrounding important clinical questions.37 A previous Delphi study related to opioids and cannabis was undertaken between 2015 and 2016 and aimed to develop consensus guidelines for responding to patients on long‐term opioids using cannabis; however, the experts disagreed on many of the proposed topics.38
The purpose of the present initiative was to develop consensus‐based recommendations on 1) when and how to safely initiate and titrate cannabinoids in the presence of opioids, 2) when and how to safely taper opioids in the presence of cannabinoids and 3) how to monitor patients and evaluate clinical outcomes when treating with opioids and cannabinoids.
2. METHODS
2.1. The modified delphi process
A modified Delphi process was used to develop this consensus guidance document and associated algorithm.39, 40, 41 The methods used for this report have been previously published in abstract form.42 The participants were recruited based on extensive clinical experience with prescribing and managing patients on medical cannabis and/or extensive research expertise with cannabis. The consensus process incorporated a five‐step modified Delphi method similar to previous reports43, 44, 45 and took place between July 2019 and November 2019 (Figure 1 and Table 1). In step one, a core scientific committee of cannabinoid subject matter experts from the United States and Canada (n = 9) identified key areas of focus. From these areas of focus, an initial draft of consensus questions was developed, and these questions were incorporated into four domains:
FIGURE 1.
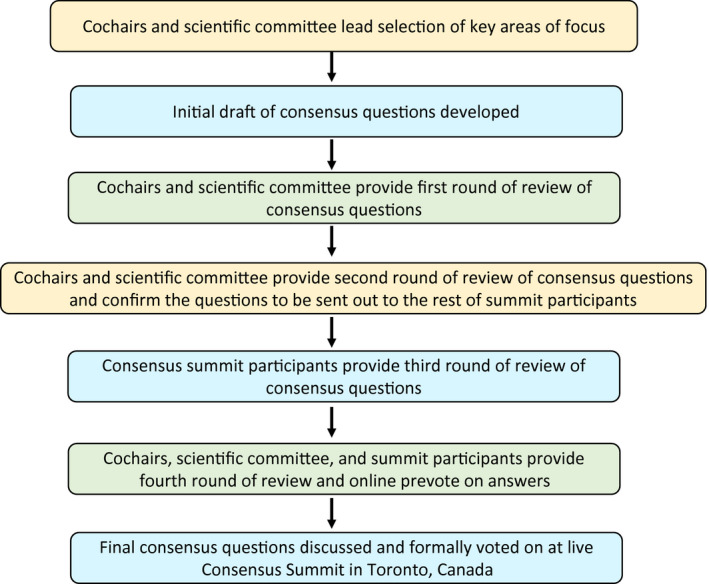
Flow of modified Delphi process
TABLE 1.
Participation during the modified Delphi process
| Modified Delphi process development | Consensus question development | Consensus voting | Manuscript development |
|---|---|---|---|
| AS, BKS, CO | AS, BKS, MJM, ZDC, HC, BLF, JP, KR, DS, RS, MO, SAA, CC, PD, KE, AD, DF, AB, VN, ZW, AB, DEM, CO | AS, MJM, BLF, KR, DS, RS, MO, SAA, CC, PD, KE, AD, DF, AB, VN, ZW, AB, DEM, CO | AS, BKS, MJM, ZDC, HC, BLF, JP, KR, DS, RS, MO, SAA, CC, PD, KE, AD, DF, AB, VN, ZW, AB, DEM, CO |
When to consider introducing cannabinoids in patients with chronic pain taking opioids
How to introduce cannabinoids in patients with chronic pain taking opioids
When and how to taper opioids in patients with chronic pain taking cannabinoids
Evaluating clinical outcomes and guiding patient monitoring and safety
In step two, the core scientific committee reviewed the initial draft of questions and provided comments. Following the inclusion of the suggested changes to the consensus questions, a teleconference was conducted to gain verbal approval from the scientific committee to send out the questions for review by the rest of the consensus summit participants (n = 13).
In step three, the consensus summit participants were provided a reference package and sent the consensus questions for their review and associated comments. Twelve of the 13 participants provided their comments and suggestions.
Following the inclusion of these updates into the consensus questions, step four was initiated and all summit invitees, which included the core scientific committee and the participants, reviewed the consensus questions and prevoted using an online software. Sixteen of the 22 summit invitees provided a prevote. These prevote results were then used at the live event to focus the discussion on topics where a lack of consensus was apparent. In step five, a formal voting session took place at an in‐person meeting in Toronto, Canada: The Opioids and Cannabinoids Consensus Summit. The voting was public but anonymous using live polling software (Slido, www.slido.com). Nineteen participants took part in the live voting session; however, the opportunity to abstain from answering questions was available.
For consensus to be declared, a predetermined threshold of ≥75% of the voters had to agree on a specific answer, or ≥75% of the voters had to strongly agree or agree (or strongly disagree or disagree) on an answer. This consensus threshold is similar to previous studies using a modified Delphi method.43, 46 At the in‐person event, revisions to the questions and associated answers, and revotes, were permitted. The voters were instructed that the patient they were considering was a patient with chronic pain taking opioids who was not currently using cannabis, recreationally or medically, to treat their chronic pain. The voters were instructed that the term cannabinoids refers to the most studied of the cannabinoids, that is, THC and CBD. The voters were instructed to assume that there were no patient access or financial limitations to consider when choosing a given answer.
3. RESULTS
The results described below highlight what we believe are the key consensus findings. The full complement of questions and answers are available in the Supplementary Tables. A treatment guidance algorithm for healthcare professionals was developed based on the answers where consensus was reached (Figure 2).
FIGURE 2.
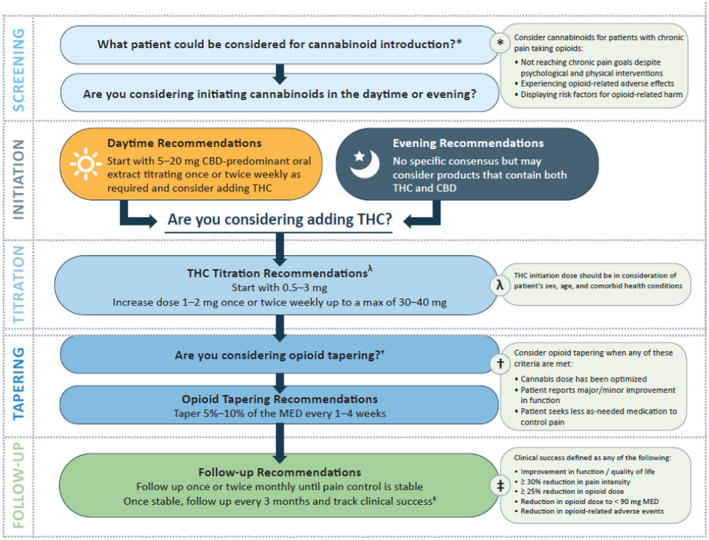
Consensus‐based algorithm for titrating cannabinoids and tapering opioids in patients with chronic pain
3.1. Domain 1: When to consider introducing cannabinoids in patients with chronic pain taking opioids
The first domain asked questions about patient factors that may influence the suitability of a patient for treatment with cannabinoids. The key consensus findings are:
If a patient has a history of psychosis, is pregnant or breastfeeding or has had an adverse reaction to cannabinoids, cannabinoids should be avoided. Although, when considering a patient with a history of psychosis, THC appears to be the more significant causal agent and CBD may in fact reduce psychosis.47, 48 Physicians may consider medical cannabis in a patient: taking opioids at any morphine equivalent dose (MED), not reaching chronic pain goals, experiencing opioid‐related adverse effects and/or displaying risk factors for opioid‐related harm. It is important to note that this consensus initiative does not aim to suggest that all patients taking opioids should reduce their opioids. In a recent commentary, the Centre for Disease Control guidelines for opioid tapering were clarified to highlight that clinicians should avoid increasing the opioid dosage to ≥90 MED, but not necessarily discontinue opioids in patients on a high dose.49 Dialogue and shared decision‐making with the patient and carefully evaluating the benefits and risks associated with tapering and discontinuation of opioids are strongly encouraged. In addition, before the introduction of any additional pharmaceutical intervention, evidence‐based psychological and physical therapy interventions to reduce opioid use should be attempted and maximised.50, 51
There was consensus that no age restrictions were recommended for CBD or THC use. With respect to CBD, the group quickly reached consensus as high doses of CBD have been shown to be safe in children, albeit in a patient population dissimilar to typical patients with chronic pain.52, 53 Conversely, there was debate surrounding the minimum age recommendation for THC. When using THC, careful consideration in the younger population should be made as the nervous system is not fully developed until 25 years of age.54 However, the consensus summit participants debated that if a young patient is already on opioids, it did not seem rational to withhold cannabinoid therapy until they turn a specific age. Similarly, although there was no maximum age agreed to, careful consideration must be given when considering cannabinoids to the elderly people, while also recognising that the elderly people are particularly susceptible to the adverse effects of opioids.
3.2. Domain 2: How to introduce cannabinoids in patients with chronic pain taking opioids
The second domain asked questions regarding how to administer, initiate, titrate and dose cannabinoids in the presence of opioids. The key consensus findings are:
There was consensus that the preferred format of administering cannabinoids was the oral route, using oil extracts or capsules. Sublingual tinctures may also be considered. There was consensus strongly recommending against smoking cannabis. Caution should be exercised in jurisdictions where regulated and standardised medical cannabis products are unavailable.
In the daytime, it was agreed that a patient should initiate with CBD oral dosing at a range of 5‐20 mg. For THC, there was consensus that the initial THC dose range should be 0.5‐3 mg and then titrated up by 1‐2 mg every 1 or 2 weeks to reach chronic pain goals.
This initiation and titration protocol deserves further discussion. CBD was recommended as the initiating cannabinoid for daytime dosing partly because there is limited sedation or intoxication associated with CBD. 55 In addition, CBD unaccompanied by THC has been observed to support opioid tapering and reduce cue‐induced cravings for opioids.56, 57 Published CBD doses range from tens of mg to thousands of mg yet from a practical standpoint, the cost of CBD may become a limitation at high doses. As such, there was no consensus on how high up the CBD dose should be titrated. It is important to note that the available evidence for CBD alone to improve chronic pain control is weak.58, 59, 60, 61 Preliminary studies examining topical application of CBD have observed that this route may be efficacious for treating pain, although further research is required.62, 63
In contrast to CBD, the available evidence for THC to improve chronic pain control is relatively strong.8, 64 During the consensus summit, it was noted that some patients can reach pain control goals with CBD alone, but it was quickly pointed out that many CBD preparations, both medical and recreational, contain a small percentage of THC and thus it could be the low concentration of THC providing the pain relief associated with CBD, especially at high CBD doses. Healthcare professionals may consider adding THC soon after CBD initiation if the patient is not reaching their chronic pain control goals.
The initiating dose range of 0.5‐3 mg THC was chosen based on clinical observations that patients typically begin to experience psychoactive effects at 2‐2.5 mg of THC, which is similar to the reported effective dose of dronabinol, a synthetic isomer of THC,65 and many patients would prefer to avoid the psychoactive effect. In addition, there is individual variability regarding the response to THC, and taking a ‘start low and go slow’ approach is recommended.
There are reported sex differences in the response to THC.66, 67, 68 For example, a recent placebo‐controlled study observed that women experience similar psychoactive effects at a lower THC dose in comparison to men,69 and previous observational studies observed that women accelerate to complicated cannabis use faster than men.70, 71 As such, sex, age and comorbid health conditions have been specifically highlighted in Figure 2 as factors to consider when initiating THC.
Our consensus findings on how to initiate and titrate cannabinoids in the presence of opioids are similar to a previously published editorial on cannabinoid dosing for chronic pain management.72 A caveat to the ‘start low and go slow’ approach may be considered with a patient at high risk for opioid‐related harm. The need to rapidly increase cannabinoids may be apparent as the opioid taper may need to be more aggressive. Therefore, under certain circumstances, it may be appropriate to start low but go fast with cannabinoid introduction and titration. Patient‐specific factors, such as response and access to close monitoring, may allow for a more aggressive cannabinoid titration and be a valuable strategy in a patient where the opioid risks are high.
For night‐time use, there was no consensus on the CBD or THC dose, or the THC:CBD distribution ratio, although there was discussion around the potential importance of THC for sleep quality, which may support chronic pain relief.73
For breakthrough pain, vaporisation of dried cannabis flower was recommended. It is important to note that vaporising dried flower differs from vaping through an electronic cigarette device. Vaporisation of dried flower is ideally accomplished with approved medical devices, although many different types of vaporisers of differing quality could be used.74 In contrast, vaping cannabis using electronic cigarette devices may expose the patient to unsafe additives and increase the risk of a novel lung disease, EVALI (e‐cigarette or vaping product use‐associated lung injury).75, 76 Until such a time that electronic cigarette devices are proven safe, inhalation of medical cannabis for breakthrough pain should be undertaken exclusively with medically approved vaporisers using dried cannabis flowers.
3.3. Domain 3: When and how to taper opioids in patients with chronic pain taking cannabinoids
The third domain discussed questions around when and how to taper opioids in patients being administered opioids and cannabinoids. The key consensus findings are:
Depending on the patient, begin the opioid taper when any of the following criteria are met: the patient has an improvement in pain/function, the cannabinoid dose has been optimised or the patient begins to seek less ‘as needed’ medication. Importantly, it was recommended to not begin tapering opioids at cannabinoid initiation or a specific cannabinoid dose.
When initiating the opioid taper, a gradual opioid dose reduction of 5%‐10% of the MED every 1 to 4 weeks was agreed upon. The timeline for the frequency of dose reduction is broad to allow for patient tailoring, and is similar to previously published opioid tapering recommendations.1, 3 More recently, the US Department of Health & Human Services have published opioid‐tapering guidelines.77, 78 These guidelines do not provide specific MED targets and encourage a highly individualised opioid tapering approach and collaborative shared decision‐making with the patient. Indeed, there may be patients who could benefit from a 20% to 50% rapid taper after titrating to an effective cannabinoid dose, depending on their objectives and needs.
3.4. Domain 4: Evaluating clinical outcomes and guiding patient monitoring and safety
The fourth domain examined questions around monitoring, safety and efficacy measures in patients with chronic pain taking opioids and cannabinoids. The key consensus findings for this domain are:
During the early phase of opioids and cannabinoids co‐administration, it was recommended to follow‐up with patients once or twice monthly until the patient is stable. Once the healthcare professional and the patient are comfortable, it was agreed that follow‐up could occur every 3 months. It was also agreed that the monitoring of these patients could be led by healthcare professionals other than the treating physician, and that these follow‐ups could be done via phone call or home visit depending on what works best for the healthcare professional and patient. At this point, even though a patient may be stable with respect to their chronic pain control, variations in dosing and administration of medical cannabis may be required for optimal pain control.
When considering the safety of a patient taking both opioids and cannabinoids, healthcare professionals should screen for opioid withdrawal, illicit opioid use, cannabinoid‐related adverse effects, use of other illicit substances and symptoms of psychosis. Cannabinoid titration should be halted: when the patient's goals are met; when cannabinoid treatment reaches an efficacy plateau (ie, no change in pain relief after a dose increase); or if the patient experiences a cannabinoid‐related adverse event. If the patient experiences a cannabinoid‐related adverse event, it is recommended to reduce the dose of the associated cannabinoid. If a patient experiences opioid withdrawal symptoms, it was agreed that the healthcare professional should consider slowing or pausing the patient's opioid taper.
When considering treatment success, no consensus was found on the need to use a validated questionnaire to establish efficacy. However, there was consensus on initiating and documenting discussions with the patient around the degree of pain relief, sleep quality and everyday functionality throughout treatment. In concert with this, it was agreed that clinical success when titrating cannabinoids and tapering opioids is most clearly defined by an improvement in patient function.
4. DISCUSSION
Globally, cannabis is being used to support a reduction or cessation of opioid use for pain. This utilisation of cannabis is occurring despite a lack of placebo‐controlled, randomised clinical trials and highlights a practical unmet need for expert guidance on the safe co‐management of cannabinoids and opioids. The aim of the present project was to develop consensus‐based recommendations on how to safely and effectively manage cannabinoid initiation and titration with opioid tapering. The primary consensus findings are provided as an algorithm (Figure 2) to be applied in clinical practice by the healthcare team.
An important stipulation when considering these consensus‐based recommendations is that a patient's personal considerations should always be taken into account, and that the treating physician's clinical rationale and individual assessment of the patient are paramount. Many of the consensus recommendations are presented as ranges to allow the healthcare professional to tailor the cannabinoid and opioid management strategy on an individual basis. Additionally, it is important to maximise psychological and physical therapy treatment interventions before initiation of cannabinoids or any additional pharmaceutical therapy.
A limitation of the consensus‐based recommendations provided herein is that they are based primarily on expert opinion developed through a modified Delphi process, and not placebo‐controlled, randomised clinical trials. However, as there is a lack of high‐quality literature investigating opioids and medical cannabis, the authors of this document leveraged their real‐world experience across tens of thousands of patient interactions to support the development of this guidance algorithm with a focus on safety. As new evidence becomes available related to the use of cannabinoids and opioids in patients with chronic pain, the recommendations made within this document will be updated and refined.
We also note that the education of healthcare professionals surrounding the safe and effective use of medical cannabis is lacking.79, 80 We hope that the introduction of this algorithm will initiate more extensive conversations about key educational needs surrounding the safe and effective use of medical cannabis, which may include focused dialogue on the divergent, but perhaps complementary, physiological effects of CBD and THC that lead to the support of opioid tapering and improved pain control. We would recommend learning modules on chronic pain treatment with medical cannabis be completed before prescribing.
The unmet need for expert guidance on co‐managing cannabinoids and opioids prompted the development of this consensus‐based document. Additionally, in the midst of an opioid crisis, the validation of strategies to reduce exposure to opioids is of interest for public health policy. We employed a modified Delphi process to add robustness to our scientific process as these expert consensus‐based recommendations are derived primarily from real‐world clinical experience in lieu of placebo‐controlled, randomised clinical trials. This modified Delphi process led to the development of a series of consensus‐based recommendations surrounding the introduction and titration of cannabinoids in concert with the tapering of opioids. These recommendations should be evaluated prospectively to examine if target reductions in opioids may be met. Future rigorous experimental studies in this area will be integral in helping shape formal guidelines.
Funding information
This research was supported by Spectrum Therapeutics, a Canopy Growth Company. Spectrum Therapeutics provided an unrestricted grant to the medical division of CTC Communications Corp to develop and run this study. Spectrum provided the faculty names, but did not influence the design, development or conduct of the study. Spectrum did not influence the collection, management, analysis or interpretation of the data, nor influence the preparation, review, approval of the manuscript or decision to submit the manuscript for publication.
Supporting information
ACKNOWLEDGEMENTS
We acknowledge Michael Boivin from CommPharm Consulting for support with this project.
Sihota A, Smith BK, Ahmed S‐A, et al. Consensus‐based recommendations for titrating cannabinoids and tapering opioids for chronic pain control. Int J Clin Pract.2021;75:e13871. 10.1111/ijcp.13871
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
REFERENCES
- 1.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain ‐ United States, 2016. MMWR Recomm Reports. 2016;65(1):1‐49. 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long‐term opioid therapy for chronic pain: a systematic review for a national institutes of health pathways to prevention workshop. Ann Intern Med. 2015;162(4):276‐286. 10.7326/M14-2559 [DOI] [PubMed] [Google Scholar]
- 3.Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18):E659‐E666. 10.1503/cmaj.170363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inturrisi CEClinical Pharmacology of Opioids for Pain. 2002. [DOI] [PubMed]
- 5.WHO . Information sheet on opioid overdose. 2018.
- 6.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. Adults: 2015 national survey on drug use and health. Ann Intern Med. 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 7.Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol. 1973;25(3):363‐372. 10.1016/0041-008X(73)90310-4 [DOI] [PubMed] [Google Scholar]
- 8.The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research: Health and Medicine Division. http://www.nationalacademies.org/hmd/Reports/2017/health‐effects‐of‐cannabis‐and‐cannabinoids.aspx. Accessed December 1, 2019. [PubMed]
- 9.Ware MA, St A‐T. The abuse potential of the synthetic cannabinoid nabilone. Addiction. 2010;105(3):494‐503. 10.1111/j.1360-0443.2009.02776.x [DOI] [PubMed] [Google Scholar]
- 10.Katsidoni V, Anagnostou I, Panagis G. Cannabidiol inhibits the reward‐facilitating effect of morphine: involvement of 5‐HT1A receptors in the dorsal raphe nucleus. Addict Biol. 2013;18(2):286‐296. 10.1111/j.1369-1600.2012.00483.x [DOI] [PubMed] [Google Scholar]
- 11.Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M. Impact of co‐administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology. 2018;43(10):2046–2055. 10.1038/s41386-018-0011-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen S, Sabioni P, Trigo JM, et al. Opioid‐sparing effect of cannabinoids: a systematic review and meta‐analysis. Neuropsychopharmacology. 2017;42(9):1752–1765. 10.1038/npp.2017.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lake S, Walsh Z, Kerr T, et al. Frequency of cannabis and illicit opioid use among people who use drugs and report chronic pain: a longitudinal analysis. PLOS Med. 2019;16(11):e1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid‐opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90(6):844‐851. 10.1038/clpt.2011.188 [DOI] [PubMed] [Google Scholar]
- 15.Ware MA, Wang T, Shapiro S, et al. Cannabis for the management of pain: assessment of safety study (COMPASS). J Pain. 2015;16(12):1233‐1242. 10.1016/j.jpain.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 16.Haroutounian S, Ratz Y, Ginosar Y, et al. The effect of medicinal cannabis on pain and quality‐of‐life outcomes in chronic pain: a prospective open‐label study. Clin J Pain. 2016;32(12):1036‐1043. 10.1097/AJP.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 17.Sagy I, Bar‐Lev Schleider L, Abu‐Shakra M, Novack V. Safety and efficacy of medical cannabis in fibromyalgia. J Clin Med. 2019;8(6):807. 10.3390/jcm8060807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rod K. A pilot study of a medical cannabis ‐ opioid reduction program. Am J Psychiatry Neurosci. 2019;7(3):74. 10.11648/j.ajpn.20190703.14 [DOI] [Google Scholar]
- 19.Abuhasira R, Schleider LBL, Mechoulam R, Novack V. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Intern Med. 2018;49:44‐50. 10.1016/j.ejim.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 20.Lucas P, Walsh Z. Medical cannabis access, use, and substitution for prescription opioids and other substances: a survey of authorized medical cannabis patients. Int J Drug Policy. 2017;42:30‐35. 10.1016/j.drugpo.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 21.Lucas P, Baron EP, Jikomes N. Medical cannabis patterns of use and substitution for opioids & other pharmaceutical drugs, alcohol, tobacco, and illicit substances; results from a cross‐sectional survey of authorized patients. Harm Reduct J. 2019;16(1):9. 10.1186/s12954-019-0278-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas P, Walsh Z, Crosby K, et al. Substituting cannabis for prescription drugs, alcohol and other substances among medical cannabis patients: The impact of contextual factors. Drug Alcohol Rev. 2016;35(3):326‐333. 10.1111/dar.12323 [DOI] [PubMed] [Google Scholar]
- 23.Reddon H, DeBeck K, Socias ME, et al. Frequent cannabis use and cessation of injection of opioids, Vancouver, Canada, 2005–2018. Am J Public Health. 2020;110(10):e1‐e8. 10.2105/ajph.2020.305825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okusanya BO, Asaolu IO, Ehiri JE, Kimaru LJ, Okechukwu A, Rosales C. Medical cannabis for the reduction of opioid dosage in the treatment of non‐cancer chronic pain: a systematic review. Syst Rev. 2020;9(1):3. 10.1186/s13643-020-01425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wildes M, Bigand TL, Layton ME, Wilson M. Cannabis use and cognition in adults prescribed opioids for persistent pain. Pain Manag. Nurs. 2020;21(1):94‐99. [DOI] [PubMed] [Google Scholar]
- 26.Campbell G, Hall WD, Peacock A, et al. Effect of cannabis use in people with chronic non‐cancer pain prescribed opioids: findings from a 4‐year prospective cohort study. Lancet Public Health. 2018;3(7):e341‐e350. 10.1016/S2468-2667(18)30110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid‐based pain medication: patient self‐report. Cannabis Cannabinoid Res. 2017;2(1):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross‐sectional survey of patients with chronic pain. J Pain. 2016;17(6):739‐744. 10.1016/j.jpain.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 29.Hamilton I, Monaghan M. Cannabis and psychosis: are we any closer to understanding the relationship? Curr Psychiatry Rep. 2019;21(7):48. 10.1007/s11920-019-1044-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lev‐Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta‐analysis of longitudinal studies. Psychol Med. 2014;44(4):797‐810. 10.1017/S0033291713001438 [DOI] [PubMed] [Google Scholar]
- 31.Fischer B, Russell C, Sabioni P, et al. Lower‐risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am J Public Health. 2017;107(8):e1‐e12. 10.2105/AJPH.2017.303818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219‐2227. 10.1056/NEJMra1402309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2(1):139‐154. 10.1089/can.2016.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Data on cannabis for medical purposes. https://www.canada.ca/en/health‐canada/services/drugs‐medication/cannabis/research‐data/medical‐purpose.html. Accessed December 1, 2019.
- 35.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta‐analysis. JAMA. 2015;313(24):2456‐2473. 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 36.Russo EB. Cannabis and pain. Pain Med. 2019;20(11):2083–2085. 10.1093/pm/pnz227 [DOI] [PubMed] [Google Scholar]
- 37.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008‐1015. 10.1046/j.1365-2648.2000.t01-1-01567.x [DOI] [PubMed] [Google Scholar]
- 38.Starrels JL, Young SR, Azari SS, et al. Disagreement and uncertainty among experts about how to respond to marijuana use in patients on long‐term opioids for chronic pain: results of a Delphi study. Pain Med. 2020;21(2):247‐254. 10.1093/pm/pnz153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalkey N. An experimental study of group opinion: the Delphi method. Futures. 1969;1(5):408–426. 10.1016/S0016-3287(69)80025-X [DOI] [Google Scholar]
- 40.Dalkey N, Helmer O. An Experimental application of the DELPHI method to the use of experts. Manage Sci. 1963;9(3):458–467. 10.1287/mnsc.9.3.458 [DOI] [Google Scholar]
- 41.Saad F, Canil C, Finelli A, et al. A Canadian consensus forum on the management of patients with advanced prostate cancer. Can Urol Assoc J. 2020;14(4):6082. 10.5489/cuaj.6082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.PAINWeek 2019 Accepted Abstracts by PAINWeek ‐ issuu. https://issuu.com/painweek/docs/accepted_full_abstracts_by_poster_number_8.26.19. Accessed December 16, 2019.
- 43.Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017 [Figure presented]. Eur Urol. 2018;73(2):178–211. 10.1016/j.eururo.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 44.Eubank BH, Mohtadi NG, Lafave MR, et al. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med Res Methodol. 2016;16(1):56. 10.1186/s12874-016-0165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart D, Gibson‐Smith K, MacLure K, et al. A modified Delphi study to determine the level of consensus across the European Union on the structures, processes and desired outcomes of the management of polypharmacy in older people. PLoS One. 2017;12(11):e018834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401‐409. 10.1016/j.jclinepi.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 47.McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225‐231. 10.1176/appi.ajp.2017.17030325 [DOI] [PubMed] [Google Scholar]
- 48.Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology. 2018;235(7):1923‐1932. 10.1007/s00213-018-4885-9 [DOI] [PubMed] [Google Scholar]
- 49.Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285‐2287. 10.1056/NEJMp1904190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tick H, Nielsen A, Pelletier KR, et al. Evidence‐based nonpharmacologic strategies for comprehensive pain care: the consortium pain task force white paper. Explore. 2018;14(3):177‐211. 10.1016/j.explore.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 51.Pain management best practices inter‐agency task force report. 2019.
- 52.Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug‐resistant seizures in the dravet syndrome. N Engl J Med. 2017;376(21):2011‐2020. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- 53.Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the lennox‐gastaut syndrome. N Engl J Med. 2018;378(20):1888‐1897. 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 54.Arain M, Haque M, Johal L, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449‐461. 10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalton WS, Martz R, Lemberger L, Rodda BE, Forney RB. Influence of cannabidiol on delta‐9‐tetrahydrocannabinol effects. Clin Pharmacol Ther. 1976;19(3):300‐309. 10.1002/cpt1976193300 [DOI] [PubMed] [Google Scholar]
- 56.Capano A, Weaver R, Burkman E. Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. Postgrad Med. 2020;132(1):56‐61. [DOI] [PubMed] [Google Scholar]
- 57.Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue‐induced craving and anxiety in drug‐abstinent individuals with heroin use disorder: a double‐blind randomized placebo‐controlled trial. Am J Psychiatry. 2019;176(11):911‐922. 10.1176/appi.ajp.2019.18101191 [DOI] [PubMed] [Google Scholar]
- 58.Li X, Vigil JM, Stith SS, Brockelman F, Keeling K, Hall B. The effectiveness of self‐directed medical cannabis treatment for pain. Complement Ther Med. 2019;46:123‐130. 10.1016/j.ctim.2019.07.022 [DOI] [PubMed] [Google Scholar]
- 59.Stith SS, Vigil JM, Brockelman F, Keeling K, Hall B. The association between cannabis product characteristics and symptom relief. Sci Rep. 2019;9(1):2712. 10.1038/s41598-019-39462-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis‐based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double‐blind, randomized, placebo‐controlled study on 160 patients. Mult Scler. 2004;10(4):434‐441. 10.1191/1352458504ms1082oa [DOI] [PubMed] [Google Scholar]
- 61.Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole‐plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17(1):21‐29. 10.1191/0269215503cr581oa [DOI] [PubMed] [Google Scholar]
- 62.Hunter D, Oldfield G, Tich N, Messenheimer J, Sebree T. Synthetic transdermal cannabidiol for the treatment of knee pain due to osteoarthritis. Osteoarthr Cartil. 2018;26:S26. 10.1016/j.joca.2018.02.067 [DOI] [Google Scholar]
- 63.Xu DH, Cullen BD, Tang M, Fang Y. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol. 2020;21(5):390–402. 10.2174/1389201020666191202111534 [DOI] [PubMed] [Google Scholar]
- 64.Andreae MH, Carter GM, Shaparin N, et al. Inhaled cannabis for chronic neuropathic pain: a meta‐analysis of individual patient data. J Pain. 2015;16(12):1221‐1232. 10.1016/j.jpain.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beal JE, Olson R, Laubenstein L, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89‐97. 10.1016/0885-3924(94)00117-4 [DOI] [PubMed] [Google Scholar]
- 66.Cooper ZD, Craft RM. Sex‐dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology. 2018;43(1):34‐51. 10.1038/npp.2017.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cooper ZD, Haney M. Investigation of sex‐dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136(1):85‐91. 10.1016/j.drugalcdep.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooper ZD, Haney M. Sex‐dependent effects of cannabis‐induced analgesia. Drug Alcohol Depend. 2016;167:112‐120. 10.1016/j.drugalcdep.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matheson J, Sproule B, Di Ciano P, et al. Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology. 2020;237(2):305–316. 10.1007/s00213-019-05369-y [DOI] [PubMed] [Google Scholar]
- 70.Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: Age of onset of use, DSM‐IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35(2):102‐110. 10.1016/j.addbeh.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernandez‐Avila CA, Rounsaville BJ, Kranzler HR. Opioid‐, cannabis‐ and alcohol‐dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265‐272. 10.1016/j.drugalcdep.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 72.Boehnke KF, Clauw DJ. Brief commentary: cannabinoid dosing for chronic pain management. Ann Intern Med. 2019;170(2):118. 10.7326/M18-2972 [DOI] [PubMed] [Google Scholar]
- 73.Kuhathasan N, Dufort A, MacKillop J, Gottschalk R, Minuzzi L, Frey BN. The use of cannabinoids for sleep: a critical review on clinical trials. Exp Clin Psychopharmacol. 2019;27(4):383‐401. 10.1037/pha0000285 [DOI] [PubMed] [Google Scholar]
- 74.Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE. A vapourized δ9‐tetrahydrocannabinol (δ9‐THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Methods. 2014;70(1):120‐127. 10.1016/j.vascn.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 75.Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e‐cigarette use in Illinois and Wisconsin — preliminary report. N Engl J Med. 2020;382(10):903‐916. 10.1056/nejmoa1911614 [DOI] [PubMed] [Google Scholar]
- 76.Kalininskiy A, Bach CT, Nacca NE, et al. E‐cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017‐1026. 10.1016/S2213-2600(19)30415-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rubin R. HHS guide for tapering or stopping long‐term opioid use. JAMA. 2019;322(20):1947. 10.1001/jama.2019.18979 [DOI] [PubMed] [Google Scholar]
- 78.CDC . HHS Guide for Clinicians on the Appropriate Dosage Reduction or Discontinuation of Long‐Term Opioid Analgesics. https://www.oregonpainguidance.org/guideline/tapering/. Accessed December 5, 2019. [DOI] [PMC free article] [PubMed]
- 79.Morris NP. Educating physicians about marijuana. JAMA Intern Med. 2019;179(8):1017‐1018. 10.1001/jamainternmed.2019.1529 [DOI] [PubMed] [Google Scholar]
- 80.Evanoff AB, Quan T, Dufault C, Awad M, Bierut LJ. Physicians‐in‐training are not prepared to prescribe medical marijuana. Drug Alcohol Depend. 2017;180:151‐155. 10.1016/j.drugalcdep.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.


