Abstract
Objective
Previous research reported cognitive and psychomotor impairments in long‐term users of benzodiazepine receptor agonists (BZRAs). This article explores the role of acute intoxication and clinical complaints.
Methods
Neurocognitive and on‐road driving performance of 19 long‐term (≥6 months) regular (≥twice weekly) BZRA users with estimated plasma concentrations, based on self‐reported use, exceeding the therapeutic threshold (CBZRA+), and 31 long‐term regular BZRA users below (CBZRA−), was compared to that of 76 controls.
Results
BZRA users performed worse on tasks of response speed, processing speed, and sustained attention. Age, but not CBZRA or self‐reported clinical complaints, was a significant covariate. Road‐tracking performance was explained by CBZRA only. The CBZRA + group exhibited increased mean standard deviation of lateral position comparable to that at blood‐alcohol concentrations of 0.5 g/L.
Conclusions
Functional impairments in long‐term BZRA users are not attributable to self‐reported clinical complaints or estimated BZRA concentrations, except for road‐tracking, which was impaired in CBZRA + users. Limitations to address are the lack of assessment of objective clinical complaints, acute task related stress, and actual BZRA plasma concentrations. In conclusion, the results confirm previous findings that demonstrate inferior performance across several psychomotor and neurocognitive domains in long‐term BZRA users.
Keywords: benzodiazepines, benzodiazepine receptor agonists, long‐term use, neurocognition, on‐road driving, psychomotor functioning
1. INTRODUCTION
Benzodiazepine receptor agonists (BZRAs) are a class of drugs prescribed mainly for the symptomatic treatment of insomnia and anxiety. They act as positive allosteric modulators of the gamma‐aminobutyric acid type a (GABAa) receptors in the central nervous system (CNS) where they potentiate the actions of the inhibitory neurotransmitter GABA, thus acting as CNS depressants. Although CNS suppression is the intended therapeutic effect, psychomotor and cognitive side‐effects such as unsteady gait, slowed response speed, impaired sustained attention and anterograde amnesia also occur (Jongen, Vuurman, Ramaekers, & Vermeeren, 2018; Uzun, Kozumplik, Jakovljević, & Sedić, 2010). These side‐effects can negatively impact daily functioning. This is especially apparent in the elderly where the risks of falling and cognitive decline have been linked to the use of BZRAs (Paterniti, Dufouil, & Alpérovitch, 2002; Sorock & Shimkin, 1988; Uzun et al., 2010). In the context of traffic safety, the use of BZRAs has been linked to increased crash risk and has been observed to impair road tracking during standardized on‐the‐road driving testing (Jongen et al., 2018; Leufkens & Vermeeren, 2014; Roth, Eklov, Drake, & Verster, 2014; Vermeeren, 2004; Verster, Veldhuijzen, & Volkerts, 2005).
Prolonged and regular (i.e., daily or near daily) BZRA use has the potential to induce physical dependence (Owen & Tyrer, 1983). It is therefore advised that treatment duration is limited to 2–4 weeks, including gradually tapering off the dose (Ashton, 1994). However, in clinical practice prolonged use of BZRAs is frequently observed. Approximately 12%–30% of all first time users progress to long‐term use (Bushnell, Stürmer, Gaynes, Pate, & Miller, 2017; Gerlach, Maust, Leong, Mavandadi, & Oslin, 2018). Arguably, this phenomenon is in part due to the practice of “doctor shopping” by patients who do not wish to discontinue treatment (Cook, Biyanova, Masci, & Coyne, 2007; Peirce, Smith, Abate, & Halverson, 2012). Also, there seems to be some empirical basis for prolonged BZRA treatment of clinical anxiety as it has been reported that, unlike the sedating properties, tolerance does not develop to the anxiolytic effects (Vinkers & Olivier, 2012). Given this reality of frequently occurring long‐term BZRA use, it is important to determine its potential consequences.
Several studies have investigated the effects of long‐term BZRA use on performance. A recent study by van der Sluiszen et al. (2019) on neurocognitive and driving performance of long‐term (≥6 months) BZRA users found that this group exhibited significant impairments on tasks of vigilance, executive functioning, and reaction speed. In line with these findings, a meta‐analysis by Barker, Greenwood, Jackson, and Crowe (2004a) reported that long‐term BZRA users (≥1 year) are impaired on neuropsychological tests of attention, problem solving, visuospatial cognition, general intelligence, psychomotor speed, and nonverbal memory. A follow‐up investigation found that impaired performance was still apparent in these domains as well as in verbal memory, motor control, and speed of processing, 6months after last use (Barker, Greenwood, Jackson, & Crowe, 2004b). The persistence of the observed impairments led the authors to the suggestion that this might be indicative of a permanently acquired cognitive deficit caused by prolonged BZRA exposure.
By contrast, a prior study by Lucki, Rickels, and Geller (1986) did not find any significant differences in psychomotor or cognitive functioning between patients treated with BZRAs and BZRA‐free patients, with the exception of slower visual temporal processing in the former. These findings suggest that prolonged BZRA use has little consequences for cognitive or psychomotor performance. Instead, they suggest that the reported performance decrements in BZRA users relative to healthy controls might be attributable to a systematic difference between BZRA users and controls, other than BZRA use. The clinical condition for which the BZRA is prescribed is the most obvious candidate. The study by van der Sluiszen et al. (2019) showed that BZRA treated individuals still report increased levels of anxiety, depression, and insomnia. It is known that anxiety (Yu et al., 2018), depression (McDermott & Ebmeier, 2009), and insomnia (Fortier‐Brochu, Beaulieu‐Bonneau, Ivers, & Morin, 2012) can have a negative impact on cognitive and psychomotor test performance. However, Barker et al. (2004a, 2004b) did not consider clinical complaints as a potential confounder. The study by van der Sluiszen et al. (2019) did assess clinical complaints but did not consider them as covariates in the analysis.
Besides controlling for clinical complaints, it is also important to control for the acute effects of BZRAs on performance. It has been reported that tolerance to the performance impairing effects of benzodiazepines is only partial (Pomara, Tun, DaSilva, & Hernando, 1998). Therefore, it seems likely that the observed impairments in long‐term BZRA users are partly attributable to these “residual acute effects.” The studies by Lucki et al. (1986) and Barker et al. (2004a) aimed to control for this by excluding participants that used a BZRA within 4 h of laboratory testing. However, depending on the half‐life and dose of the respective BZRA, this approach is arguably inadequate to completely control for residual acute effects. van der Sluiszen et al. (2019) pointed out the problem of heterogeneity of medication use that is inherent to a clinical sample of BZRA users. The patients included in the study used different BZRAs (i.e., different potencies), at different doses, at different frequencies, and at different times relative to the laboratory tests and on‐road driving. All these factors determine the achieved drug plasma concentrations and should therefore be taken into consideration when controlling for potential residual acute BZRA effects.
A previous investigation by Verster and Roth (2013a) concluded that no significant relationship exists between individual BZRA blood plasma concentrations and road tracking performance during on‐road driving. It is conceivable that the psychomotor and cognitive effects of BZRAs are subject to a wide range of inter‐individual variability. However, it should be noted that all of the reported plasma concentrations fell well within the therapeutic window for the respective BZRA. It can therefore not be excluded that a relationship exists between BZRAs blood plasma concentrations and road‐tracking performance all together. The notion that no significant behavioral or cognitive effects are expected below the therapeutic threshold remains unchanged, while effects are expected when blood plasma concentrations exceed it, albeit to varying degrees. It is therefore argued that it is important to assess whether or not participants have BZRA plasma concentrations exceeding the therapeutic threshold at the time of cognitive testing in order to control for potential residual acute BZRA effects in long‐term users.
Uncovering what contributes to the observed functional impairments in long‐term BZRA users is important since different causal factors imply different clinical management strategies. If impairments reported in long‐term BZRA users are attributable to residual acute effects or acquired functional deficits due to prolonged exposure, cessation or limitation of BZRA use would be advised. However, if clinical complaints account for the observed impairments, continuation could be the best decision (Shinfuku et al., 2019). Also, for the individual assessment of the fitness to drive of long‐term BZRA users, it is important to elucidate which factors might impair driving performance to allow for efficient screening. The present study revisited the dataset from van der Sluiszen et al. (2019) that included on‐road driving and neurocognitive performance of long‐term benzodiazepine users and healthy controls. The current investigation compares on‐road driving performance and neurocognitive functioning of long‐term regular BZRA users to that of healthy controls in order to elucidate whether clinical complaints and residual acute effects, operationalized as estimated BZRA plasma concentrations, account for the previously reported performance decrements in BZRA users.
2. METHODS
2.1. Participants
Medical, driving and neurocognitive data of 55 long‐term (>6months), chronic (≥2 times per week) BZRA users, aged 21–75, were retrieved from a previous study into the effects of long‐term use of sedative medications on driving ability initiated by the Dutch government (van der Sluiszen et al., 2019; Verster, van de Loo, et al., 2016). In this study, all information regarding eligibility was gathered through completion of an extensive medical questionnaire which was subsequently reviewed by a clinician. Participants were required to be in the possession of a valid driver's license, of which a photocopy was obtained, and to drive at least 500 km/year. In addition, normal or corrected to normal vision, and a body mass index between 17 and 35 kg/m2 were necessary prerequisites. In addition to the medical questionnaire, the requirement regarding visual acuity was checked on site using an eye chart examination. Participants were excluded if they consumed >21 alcoholic beverages per week, smoked >20 cigarettes per day, or used any psychoactive substances recreationally and regularly, or recently prior to testing as determined by an alcohol breathalyzer test and a urine test. Only those BZRA users of whom a complete medication profile could be retrieved, that is, type of medication, dose, frequency, and time of last use, were included in the current analysis. In addition to the BZRA user group, data of 76 control participants was retrieved. Control participants were free of psychoactive medications and diagnosed psychiatric, neurological, and substance abuse disorders, as determined by the inspection of the medical questionnaire by a clinician.
2.2. Estimation and classification of BZRA plasma concentrations
In order to quantify expected residual acute drug effects, the average steady state drug plasma concentration was estimated for each BZRA user from use as reported by the participant (drug, dose, and time of dosing) and established pharmacokinetic parameters of the drug (see Equation (1), [Wakamatsu, Aoki, Sakiyama, Ohnishi, & Sugita, 2013]). These and other parameters were also used to estimate drug plasma concentration at the start of the testing day (see Equation (2) [Wakamatsu et al., 2013]). The pharmacokinetic parameters entered into Equations (1) and (2) and their literature references are listed in Table 1.
| (1) |
Equation 1. C̅ss: Average drug plasma concentration at steady state (ng/ml); F: Bioavailability (% absorbed); D: dose (mg); CL: Clearance rate (ml/min/kg); τ: the dosing interval (hours).
| (2) |
Equation 2. Css(t): estimated drug plasma concentration at steady state at time t (ng/ml); t: time since last use relative to start of testday (hours); F: bioavailability (% of drug absorbed); D: drug dose (mg); Ka: absorption rate constant (h−1); Vd: Apparent volume of distribution (L/kg); Kel: elimination rate constant (h−1).
TABLE 1.
| BZRA | F | t₁/₂ abs | Ka | Vd | CL | Kel | References |
|---|---|---|---|---|---|---|---|
| Alprazolam | 90 | 19.2 | 2.17 | 0.84 | 67.2 | 0.080 | Greenblatt and Wright (1993); Smith, Kroboth, Vanderlugt, Phillips, and Juhl (1984); Wright (1995) |
| Brotizolam | 70 | 10.2 | 4.08 | 0.66 | 111 | 0.168 | Jochemsen, Wesselman, Hermans, Van Boxtel, and Breimer (1983); Langley and Clissold (1988); Scavone, Greenblatt, Harmatz, and Shader (1986) |
| Clonazepam | 90 | 24.6 | 1.69 | 2.95 | 42 | 0.014 | Berlin and Dahlström (1975); Crevoisier, Delisle, Joseph, and Foletti (2003); Greenblatt et al. (2005); Wishart et al. (2018) |
| Clorazepate | 91 | 18.6 | 2.24 | 1.28 | 13.2 | 0.010 | Ochs, Steinhaus, Locniskar, Knüchel, and Greenblatt (1982); Shader et al. (1981); Wishart et al. (2018) |
| Diazepam | 94 | 31.8 | 1.31 | 1.83 | 21 | 0.011 | Divoll, Greenblatt, Ochs, and Shader (1983); Eatman et al. (1977); Greenblatt, Allen, Harmatz, and Shader (1980); Greenblatt, Harmatz, Friedman, Locniskar, and Shader (1989) |
| Lorazepam | 90 | 32.4 | 1.28 | 1.15 | 57 | 0.050 | Greenblatt (1981); Greenblatt, Divoll, Harmatz, and Shader (1982); Wishart et al. (2018) |
| Lormetazepam | 94 | 102 | 0.41 | 6.8 | 240 | 0.035 | Hildebrand, Hellstern, Hümpel, Hellenbrecht, and Saller (1990); Kampf, Huempel, Lerche, and Kessel (1981); Lombardo, Obach, Shalaeva, and Gao (2002) |
| Midazolam | 50 | 18 | 2.31 | 1.3 | 330 | 0.253 | Greenblatt et al. (1984); Malacrida, Fritz, Suter, and Crevoisier (1992); Wishart et al. (2018) |
| Nitrazepam | 100 | 16.2 | 2.57 | 2.55 | 54 | 0.021 | Greenblatt et al. (1985); Jochemsen et al. (1982) |
| Oxazepam | 93 | 37.8 | 1.1 | 1.5 | 87 | 0.058 | Greenblatt (1981); Sonne et al. (1988) |
| Temazepam | 95 | 117 | 0.36 | 1.4 | 71.4 | 0.051 | Divoll, Greenblatt, Harmatz, and Shader (1981); Schwarz (1979); Wishart et al. (2018) |
| Zolpidem | 70 | 37.8 | 2.24 | 0.54 | 348 | 0.644 | Greenblatt et al. (2013); Langtry and Benfield (1990); Olubodun et al. (2002); Salvà and Costa (1995) |
| Zopiclone | 80 | ‐ | 3.49 | ‐ | 228 | 0.172 | Caille, Du Souich, Spenard, Lacasse, and Vezina (1984); Fernandez, Martin, Gimenez, and Farinotti (1995); Gaillot, Heusse, Hougton, Aurele, and Dreyfus (1983); Noble, Langtry, and Lamb (1998) |
Abbreviations: BZRA, benzodiazepine receptor agonists; CL, Clearance rate (ml/h/kg); F, bioavailability (% of drug absorbed); Kel, elimination rate constant (h−1), calculated as Kel = CL/Vd; t₁/₂ abs, absorption half‐life (min); Ka, absorption rate constant (h−1), calculated as Ka = Ln (2)/t₁/₂ abs; Vd, Apparent volume of distribution (L/kg).
For three BZRA users, it was not possible to estimate the drug plasma concentrations because of irregular drug use. For another two users, the concentration estimates could not be determined because reliable pharmacokinetic parameters of bromazepam could not be retrieved from the literature. Hence, a total of 50 BZRA users were included for the analysis. Six BZRA users indicated using two BZRAs daily. The doses of both BZRAs were first converted to the diazepam equivalents (Ashton, 1994) before being entered into the equations. The estimated equivalent plasma concentrations were then added for the final estimate.
Estimated plasma concentrations were compared to the therapeutic threshold of the respective drug (Schulz, Iwersen‐Bergmann, Andresen, & Schmoldt, 2012) in order to determine whether any CNS depressant effects would be likely. The correspondence rate of the two drug plasma concentration estimates, that is, C̅ss and Css(t), in this respect was 100%. In other words, BZRA users with estimated C̅ss levels that exceeded the therapeutic threshold were also found to have estimated Css(t) levels exceeding this threshold (CBZRA+, N = 19), and vice versa (CBZRA−, N = 31). A summary of group descriptives, self‐reported clinical complaint severity, and medication use is provided in Table 2. A complete overview of type, dose and frequency of BZRA use, as well as the estimated drug plasma concentrations per BZRA user is provided in Appendix 1.
TABLE 2.
Sample descriptives
| Control Participants | Patients (CBZRA‐) | Patients (CBZRA+) | |
|---|---|---|---|
| N | 76 | 31 | 19 |
| Gender [F:M] | 35:41 | 19:12 | 9:10 |
| Mean age (SD) | 55.6 (12.7) | 52.8 (12.0) | 56.5 (11.4) |
| Mean annual distance (SD) [km] | 13,499(9276) | 12,798 (8886) | 15,553 (24,189) |
| BDI (SD) a | 2.51 (2.69) | 12.39 (10.49) | 8.89 (6.54) |
| STAI‐T (SD) b | 27.33 (5.65) | 43.23 (11.59) | 40.89 (12.55) |
| PSQI (SD) c | 2.86 (2.33) | 8.9 (4.87) | 8.47 (5.35) |
| GSQS (SD) d | 1.54 (2.05) | 5.19 (4.39) | 4.84 (4.44) |
| Median alcoholic beverages per week (IQR) | 4.5 (8.5) | 2 (7) | 4 (6) |
| Mean equivalent BZRA plasma concentration (SD) [ng/ml] e | ‐ | 46.9 (57.3) | 257.2 (574.1) |
| Mean equivalent BZRA steady state plasma concentration (SD) [ng/ml] f | ‐ | 46.7 (49.7) | 245 (529.5) |
| Duration of use [years] | ‐ | 8.6 (8.4) | 6.9 (7.0) |
| CNS co‐medications g : | ‐ | 23 (75%) | 14 (74%) |
| Tri‐ and tetracyclic antidepressants h | ‐ | 3 (10%) | 3 (16%) |
| Selective serotonin/Serotonin‐norepinephrine reuptake inhibitors i | ‐ | 13 (42%) | 9 (47%) |
| Antipsychotics j | ‐ | 5 (16%) | 1 (5%) |
| Opioids k | ‐ | 4 (13%) | 1 (5%) |
| Lamotrigine | ‐ | 1 (3%) | ‐ |
| Lithium | ‐ | 2 (6%) | 1 (5%) |
| Paracetamol | ‐ | 3 (10%) | 4 (21%) |
| Pramipexole | ‐ | 1 (3%) | ‐ |
| Pregabaline | ‐ | 1 (3%) | ‐ |
| Tranylcypromine | ‐ | 1 (3%) | ‐ |
| Trazodone | ‐ | 1 (3%) | ‐ |
Beck's depression inventory.
State‐trait anxiety inventory–trait.
Pittsburgh sleep quality index.
Groningen sleep quality scale.
Mean estimated equivalent diazepam plasma concentration at start of test day.
Mean estimated equivalent diazepam average steady state plasma concentration.
Number of participants that used at least one other central nervous system medication (classified as N‐class by the anatomical, therapeutic, and chemical (ATC) classification system) daily or multiple times per week.
amitriptyline (n = 2), clomipramine (n = 1), mirtazapine (n = 2) and nortriptyline (n = 1).
Citalopram (n = 8), duloxetine (n = 2), escitalopram (n = 3), fluoxetine (n = 3), paroxetine (n = 4), sertraline (n = 1), and venlafaxine (n = 1).
Olanzapine (n = 1), quetiapine (n = 7), risperidone (n = 1).
Codeine (n = 2), oxycodone (n = 2), and tramadol (n = 1).
Appendix A1.
Table listing individual medication profiles
| Subj | BZRA | Body weight (kg) | Dose (mg) | Last dose (h)a | Dosing interval (h) | Therapeutic Treshold (ng/ml) | Css(t) (ng/ml)b | C̅ss (ng/ml)c | Css(t) DIA (ng/ml)d | C̅ss DIA (ng/ml)e |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Temazepam | 60 | 20 | 14 | 24 | 20 | 181,4 | 184,8 | 91,6 | 91,4 |
| 2 | Temazepam | 78 | 20 | 12,5 | 24 | 20 | 149,7 | 142,2 | 75,6 | 70,3 |
| 3 | Oxazepam | 78 | 10 | 24 | 200 | 27,8 | 57,1 | 14,3 | 28,9 | |
| 4 | Lorazepam | 100 | 1 | 15 | 24 | 80 | 5,6 | 6,6 | 59,3 | 68,7 |
| 5 | Lorazepam | 78 | 1 | 11,75 | 24 | 80 | 8,4 | 8,4 | 89,4 | 88,1 |
| 6 | Oxazepam | 125 | 30 | 12,5 | 24 | 200 | 101,2 | 106,9 | 52,3 | 54,0 |
| 7 | Lorazepam | 90 | 1 | 2,5 | 8 | 80 | 24,0 | 21,9 | 256,5 | 229,0 |
| 8 | Zolpidem | 70 | 10 | 11 | 24 | 80 | 0,2 | 12,0 | 0,1 | 8,0 |
| 9 | Oxazepam | 105 | 10 | 1,75 | 24 | 200 | 65,9 | 42,4 | 34,0 | 21,4 |
| 10 | Temazepam | 75 | 10 | 10,5 | 8 | 20 | 181,9 | 221,8 | 91,9 | 109,7 |
| 11 | Zopiclon | 65 | 7,5 | 12 | 24 | 10 | 10,9 | 16,9 | 8,7 | 13,2 |
| 12 | Oxazepam | 70 | 5 | 1 | 24 | 200 | 110,8f | 95,4f | 57,2g | 48,2g |
| Oxazepam | 10 | 10,5 | 24 | 200 | ‐ | ‐ | ‐ | ‐ | ||
| 13 | Temazepam | 125 | 20 | 10,5 | 24 | 20 | 102,1 | 88,7 | 51,6 | 43,9 |
| 14 | Diazepam | 84 | 5 | 11,5 | 24 | 100 | 112,3 | 111,0 | 114,7 | 111,0 |
| 15 | Lormetazepam | 95 | 3 | 11,5 | 24 | 2 | 4,9 | 4,6 | 47,1 | 42,9 |
| 16 | Nitrazepam | 85 | 2,5 | 11 | 24 | 30 | 23,1 | 22,7 | 22,2 | 21,3 |
| 17 | Oxazepam | 105 | 10 | 13,75 | 24 | 200 | 37,4 | 42,4 | 19,3 | 21,4 |
| 18 | Oxazepam | 74 | 7,5 | 2,5 | 24 | 200 | 72,1 | 45,1 | 37,7 | 23,1 |
| 19 | Zolpidem | 60 | 15 | 15 | 24 | 80 | 0,0 | 21,0 | 0,0 | 14,1 |
| 20 | Temazepam | 122 | 20 | 12,25 | 24 | 20 | 96,8 | 90,9 | 48,9 | 45,0 |
| 21 | Oxazepam | 94 | 10 | 2,75 | 12 | 200 | 115,0 | 94,8 | 59,4 | 47,9 |
| 22 | Oxazepam | 81 | 10 | 11,5 | 24 | 200 | 55,2 | 55,0 | 28,5 | 27,8 |
| 23 | Lorazepam | 68 | 0,5 | 11 | 24 | 80 | 5,0 | 4,8 | 53,2 | 50,5 |
| 24 | Zopiclon | 78 | 3,75 | 12,75 | 24 | 10 | 3,3 | 7,0 | 2,7 | 5,5 |
| 25 | Oxazepam | 70 | 10 | 0,25 | 4,8 | 200 | 307,8 | 318,1 | 158,9 | 160,8 |
| 26 | Zopiclon | 68 | 7,5 | 10 | 24 | 10 | 13,7 | 16,1 | 10,9 | 12,6 |
| 27 | Zolpidem | 81 | 10 | 12,5 | 24 | 80 | 0,1 | 10,3 | 0,0 | 6,9 |
| 28 | Zopiclon | 75,5 | 7,5 | 8,5 | 24 | 10 | 14,8 | 14,5 | 11,9 | 11,4 |
| 29 | Brotizolam | 76 | 0,25 | 13,25 | 24 | 1 | 0,4 | 0,9 | 21,9 | 46,4 |
| 30 | Zolpidem | 76 | 10 | 11,5 | 24 | 80 | 0,1 | 11,0 | 0,1 | 7,4 |
| 31 | Alprazolam | 58 | 0,5 | 5 | 12 | 5 | 10,4 | 9,6 | 222,2 | 201,0 |
| 32 | Midazolam | 68 | 3,75 | 11 | 24 | 40 | 1,5 | 3,5 | 3,7 | 8,7 |
| 33 | Oxazepam | 81 | 10 | 1,5 | 6 | 200 | 236,5 | 220,0 | 122,1 | 111,2 |
| 34 | Temazepam | 74 | 10 | 11,75 | 24 | 20 | 81,6 | 74,9 | 41,3 | 37,1 |
| 35 | Midazolam | 57 | 15 | 11 | 24 | 40 | 7,0 | 16,6 | 17,9 | 41,6 |
| 36 | Lorazepam | 60 | 1 | 13 | 24 | 80 | 10,2 | 11,0 | 109,2 | 114,5 |
| 37 | Zopiclon | 94 | 7,5 | 10,5 | 24 | 10 | 6,8 | 11,7 | 5,4 | 9,1 |
| 38 | Temazepam | 71 | 20 | 10 | 24 | 20 | 141,8 | 116,9 | 71,7 | 57,8 |
| 39 | Zolpidem | 55 | 10 | 12 | 24 | 80 | 0,1 | 15,2 | 0,1 | 10,2 |
| 40 | Lorazepam | 71 | 1,25 | 11,5 | 24 | 80 | 11,7 | 11,6 | 124,3 | 121,0 |
| 41 | Lorazepam | 140 | 1 | 1,5 | 8 | 80 | 15,6 | 14,1 | 166,9 | 147,2 |
| 42 | Alprazolam | 82 | 1 | 1,25 | 12 | 5 | 19,0 | 13,6 | 405,1 | 284,3 |
| 43 | Lormetazepam | 78 | 2 | 9,5 | 24 | 2 | 4,3 | 3,7 | 24,4 | 20,9 |
| 44 | Zolpidem | 107 | 10 | 11,5 | 24 | 80 | 0,1 | 7,8 | 21,6g | 26,3g |
| Oxazepam | 107 | 10 | 11,5 | 24 | 200 | 41,8 | 41,6 | ‐ | ‐ | |
| 45 | Zopiclon | 102 | 7,5 | 12 | 24 | 10 | 4,0 | 10,7 | 2413,6g | 2335,6g |
| Clorazepate | 102 | 30 | 2 | 6 | 200 | 3427,3 | 3379,4 | ‐ | ‐ | |
| 46 | Zolpidem | 55 | 10 | 12,5 | 24 | 80 | 0,1 | 15,2 | 690,3g | 688,4g |
| Clonazepam | 55 | 2 | 12,5 | 24 | 22 | 32,4 | 32,5 | ‐ | ‐ | |
| 47 | Zolpidem | 89 | 10 | 11,75 | 24 | 80 | 0,1 | 9,4 | 195,9g | 199,3g |
| Lorazepam | 89 | 2,5 | 11,75 | 24 | 80 | 18,4 | 18,5 | ‐ | ‐ | |
| 48 | Oxazepam | 110 | 10 | 1,75 | 24 | 200 | 62,9 | 40,5 | 32,4 | 20,5 |
| 49 | Alprazolam | 71 | 0,25 | 1,5 | 12 | 5 | 5,5 | 3,9 | 116,9 | 82,1 |
| 50 | Oxazepam | 110 | 10 | 11,75 | 24 | 200 | 40,1 | 40,5 | 36,8g | 35,3g |
| Lormetazepam | 110 | 2 | 11,75 | 24 | 2 | 2,8 | 2,7 | ‐ | ‐ |
Elapsed time since last use relative to start of test day.
Estimated drug plasma concentration at steady state at the start of the test day.
Estimated average drug plasma concentration at steady state.
Estimated equivalent diazepam plasma concentration at steady state at the start of the test day.
Estimated equivalent average diazepam plasma concentration at steady state.
Different doses of the same drug used at different times were calculated separately and the results were then added. Added estimate is listed.
Sum of equivalent diazepam plasma concentrations.
2.3. Materials
2.3.1. Self‐reported clinical questionnaires
The Beck Depression Inventory (BDI) consists of 21 items containing a statement that is related to depressive symptomatology. The participant indicates how relatable each item is on a scale from 0 to 3. Higher scores indicate more severe depressive symptoms (Beck, Steer, & Carbin, 1988).
The Groningen Sleep Quality Scale (GSQS) assesses subjective sleep quality in 14 items that are rated as true or false. Higher scores are indicative of poorer subjective sleep quality (Mulder‐Hajonides van der Meulen & Van den Hoofdakker, 1990).
Pittsburgh Sleep Quality Index (PSQI) is a 19‐item self‐report questionnaire for the assessment of sleep quality. Participant rate the relatability of the presented items on a 0 to 3 scale. Summary scores range from 0 to 21, with higher scores indicating poorer sleep quality (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989).
State‐Trait Anxiety Inventory—Trait (STAI‐T). The Trait component of the STAI is a 20‐item self‐report questionnaire for the assessment of trait anxiety, as opposed to state anxiety which is assessed by the complementary part of the STAI. Respondents rate the relatability of the presented items on a 1–4 scale. Summary scores range from 20 to 80, with higher scores indicating higher trait anxiety (Spielberger, 2010).
2.3.2. Psychomotor tasks and visual perception tasks
Trail Making Test (TMT) is a pen‐and‐paper psychomotor test consisting of two parts. In part A, participants have to connect the numbers 1 to 25 in ascending order without lifting the pen as fast as possible. In part B, participants have to do the same, except that they have to alternate between the numbers 1–13 and the letter A–L (i.e., 1‐A‐2‐B‐3‐C,…). Completion time serves as the main outcome measure. The maximum allowed completion time for part A and B were 5 and 6 min, respectively (Reitan, 1958).
Digit Symbol Substitution test (DSST). During the DSST, a list of nine abstract symbols is presented to the participants. Each symbol corresponds a number, that is, 1–9. Below, the participant is presented with a long list with the numbers 1–9 in a random order. The participants are asked to fill in as many of the corresponding symbols below the number as they can within 90 s. Participants are instructed to complete the list sequentially. The total number of correct answers serves as the main outcome. A pen‐and‐paper version of the DSST was administered (Wechsler, 1955).
Adaptive Tachistoscopic Traffic Perception Test (ATTPT). The ATTPT was included in order to assess perceptual performance. During the ATTPT, a traffic scene is flashed on a computer screen. After the short scene presentation, participants need to indicate which elements were present, that is, cyclists, cars, traffic lights and signs, and/or pedestrians. Response accuracy was used as the main outcome measure (Schuhfried, 2009).
Reaction test (RT) is a computerized reaction time test that consists of three parts. In part 1 (RT1), participants need to respond as fast as possible when a circle presented in the middle of the screen lights up yellow. In the second part (RT2), participants need to respond as fast as possible to the presentation of a high pitched tone. The last part (RT3), requires participants to respond whenever the yellow circle and high pitched tone are presented simultaneously, and to withhold a response when this is not the case (e.g., circle lights up red, yellow light and tone are not simultaneously). The main outcome measure of this set of tasks is the mean reaction time (Prieler, 2008).
Adaptive Determination Test (ADT) is a complex computerized reaction time test. Participants are presented with a grid of eight empty circles on a computer screen. During the test, the circles will light up in any of five colors. Participants need to press the corresponding colored button as fast as possible on a specially equipped key board. Simultaneously, high and low pitched tones are presented. Each of the two pitches has a corresponding button on the keyboard that needs to be pressed as fast as possible following tone presentation. Furthermore, in the right and left bottom corners of the screen, two rectangles are presented. When they light up, the participant needs to press the corresponding foot pedal. Stimulus presentation speeds up as performance gets better and slows down if reactions take longer or mistakes are made. Number of correct responses was used as the outcome measure. The task duration was set at 10 min (Neuwirth & Benesch, 2003).
Psychomotor Vigilance Test (PVT) is a prolonged (10 min) simple reaction time test. Participants press the response button when a stimulus is presented. Stimuli are presented at random intervals with an average of 8 s between subsequent stimulus presentations. Median reaction time and number of attentional lapses (reaction time > 500 ms) serve as the outcome measures of this test (Dinges & Powell, 1985).
2.3.3. Standardized on‐the‐road driving test
The standardized on the road driving test consists of a 100 km drive on a highway in actual traffic in an instrumented test vehicle (O'Hanlon, 1984; Ramaekers, 2017; Verster & Roth, 2011). Participants are accompanied by a licensed driving instructor who has access to dual controls. During the drive, participants are instructed to keep a steady position in the right traffic lane and to maintain a steady speed of 95 km/h. The lateral position of the vehicle relative to the traffic lane demarcation on its left and the velocity are logged every 250 ms. From this, the standard deviation of speed (SDS) and the standard deviation of the lateral position (SDLP) are calculated. The SDLP serves as the main outcome measure and is essentially a measure of road tracking ability. Higher SDLP values correspond to more lane weaving, hence worse performance. The SDLP has been shown sensitive to the impairing effects of alcohol and acute BZRA administration on road tracking ability (Roth et al., 2014; Vermeeren, 2004; Verster et al., 2005, 2016a; Verster, Veldhuijzen, & Volkerts, 2004).
2.4. Procedure
All participants were informed about the purpose, procedure and associated risks of study participation prior to providing written informed consent. Hereafter, participants were medically screened based on a medical questionnaire that was reviewed by a clinician to ensure the minimal requirements for safely operating a vehicle in actual traffic were met. Next, participants were invited to a training day and a testing day. The training day served to familiarize the participants with the tasks in order to ascertain a smooth course of the testing day during which the data was collected. In addition, at the start of the training day participants completed the BDI, PSQI, and STAI‐T, as well as a test of visual acuity, before practicing the test battery. At the start of the test day, medication, drugs, alcohol, nicotine, and caffeine use in the last 24 h were documented. In addition, recent use of alcohol and common recreational and medicinal drugs was tested using an alcohol breathalyzer test and a urine drugs test respectively. Next, participants completed the GSQS and then completed the test battery in the same order as on the training day, that is, pen‐and‐paper psychomotor tests (TMT and DSST), computerized perception and reaction time tests (ATTPT, RT, and ADT), the on‐the‐road driving test, and lastly the PVT. At the end of the testing day participants also completed a set of driving simulator scenarios. However, these data are not considered here. The study was approved by the medical ethics committee of the Maastricht University Medical Center and was executed in accordance to the declaration of Helsinki (1964) and its most recent amendments (2013). For a more detailed description of the testing procedure, the reader is referred to the study by van der Sluiszen et al. (van der Sluiszen et al., 2019) or the technical report (Verster et al., 2016b).
3. STATISTICAL ANALYSIS
3.1. Dimension reduction
To simplify the interpretability of the various test outcomes (i.e., TMT, DSST, ATTPT, RT, ADT, PVT, and the on‐the‐road driving test) and to minimize the multiple testing problem, an ordinary least squares factor analysis (FA) was performed on the various outcome measures of both groups, that is BZRA users and controls, combined. The extracted solution was then rotated using Direct Oblimin rotation (δ = 0). The factor scores for each participant were estimated using least squares regression and the resulting estimates were saved as a new variable. Missing variable scores were replaced by the mean before being entered in the FA. To determine the number of factors to extract, the parallel analysis method was employed (Franklin, Gibson, Robertson, Pohlmann, & Fralish, 1995). One thousand random permutations of the raw dataset were performed to determine the percentiles of the eigenvalues of random data. Eigenvalues of the raw dataset exceeding the eigenvalues of the permutated datasets marking the border of the 95th percentile were counted and the total number was used as the number of factors to be extracted during the FA.
Depression, insomnia, and anxiety are highly comorbid disorders (Taylor, Lichstein, Durrence, Reidel, & Bush, 2005). Therefore, and in order to have parsimonious predictors in the general linear model (see below), the scores on the self‐assessment clinical complaint questionnaires, that is BDI, STAI, PSQI, and GSQS, were bundled into one composite score using a principal component analysis. A parallel analysis also confirmed that a single component solution was appropriate. Component scores based on regression coefficients were saved as a clinical complaint composite score.
3.2. General linear modelling
Next, one‐way analyses of covariance (ANCOVA) were performed for each extracted factor. Variables that loaded only moderately or weakly (r = [−0.5, 0.5]) on any of the factors yielded by the factor analysis were also considered separately as dependent variables. Group (i.e., BZRA user or control) was entered as random factor. The clinical complaint composite score was entered as a continuous covariate. Also, estimated drug plasma concentration was entered as a categorical covariate (CBZRA) with three levels, that is, (0) no BZRA/control, (1)BZRA levels below the effective concentration (i.e., CBZRA‐), and (2)BZRA levels above the effective concentration (i.e., CBZRA+). Finally, considering the wide age range of the participants, age was also included as a covariate. Non‐significant covariates were first removed from the model before interpreting the results. Dunett's t‐test was used for post hoc testing in case of multiple comparisons, with the control group as the appointed reference. Levene's test for equality of error variances, the White's test for heteroscedasticity, and normality checks were performed in order to assure that the model's assumptions were met. All statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS; IBM corporation, 2017).
4. RESULTS
4.1. Missing data
The test score of the TMT part B could not be retrieved for one BZRA user of the CBZRA + subgroup. For another BZRA user in this subgroup, the PVT data could not be recovered. Two control participants and one BZRA user of the CBZRA + subgroup did not complete the on‐the‐road driving test because of technical difficulties with the test vehicle. Therefore, no SDLP or SDS data was available for these participants. For another additional three control participants and one BZRA user in the CBZRA + subgroup no velocity data of the on‐the‐road driving test was available, hence the SDS could not be determined for these participants.
4.2. Dimension reduction
Based on the parallel analysis, it was decided that a three factor solution was appropriate for the factor analysis. The correlation coefficients of the variables and the factors after the Direct Oblimin rotation are shown in Table 3. The first factor was found to correlate highly with performance on single response reaction time tasks, that is RT and PVT, and was labeled Response latency. The second factor seems to capture the performance on the TMT, DSST, and ADT, all of which depend on the speed of stimulus processing for the selection of the appropriate response, and was therefore labeled Processing speed. The third factor is explained mostly by performance on the ADT, PVT and on‐the‐road driving test. It is proposed that these tasks share a requirement of sustained attention, hence the factor was labeled as such.
TABLE 3.
Structure matrix obtained after the factor analysis of the listed outcome measures
| Response latency | Processing speed | Sustained attention | |
|---|---|---|---|
| TMT A | |||
| Mean completion time (Sec) | 0.304** | −0.782** | 0.181** |
| TMT B | |||
| Mean completion time (Sec) | 0.345** | −0.666** | 0.023 |
| DSST | |||
| Correct responses (#) | −0.352** | 0.761** | 0.279** |
| ATTPT | |||
| accuracy (%) | −0.003 | 0.092 | 0.073 |
| RT1 | |||
| Mean reaction time (msec) | 0.941** | −0.291** | −0.076 |
| RT2 | |||
| Mean reaction time (msec) | 0.773** | −0.213** | −0.052 |
| RT3 | |||
| Mean reaction time (msec) | 0.679** | −0.427** | −0.033 |
| ADT | |||
| Correct responses (#) | −0.443 | 0.749** | 0.424** |
| PVT | |||
| Median reaction time (msec) | 0.632** | −0.310** | −0.412** |
| Lapses (#) | 0.604** | −0.299** | −0.352** |
| On‐the‐road driving test | |||
| SDLP (cm) | 0.184* | −0.098 | −0.467** |
| SDS (km/h) | 0.307** | −0.193* | −0.222** |
Note: The Pearson correlation coefficients quantifying the linear relation between each outcome measure with the three extracted factors are shown.
Abbreviations: ADT, Adaptive Determination Test; ATTPT, Adaptive Tachistoscopic Traffic Perception Test; DSST, Digit Symbol Substitution test; PVT, Psychomotor Vigilance Test; RT, Reaction test; SDLP, standard deviation of the lateral position; SDS, standard deviation of speed; TMT, Trail Making Test.
*p < 0.05; **p < 0.01.
The SDLP was found to correlate only moderately with its main factor, Sustained attention, and was therefore also considered separately as a dependent variable in the group comparisons, as was the SDS. The ATTPT response accuracy was also considered separately as dependent variable because it correlated very weakly with any of the extracted factors.
Finally, the parallel analysis confirmed that a single component solution would yield an optimal solution for the principal component analysis of the clinical complaint measures. The component loadings of the BDI (r = 0.840, p < 0.01), PSQI (r = 0.871, p < 0.01), STAI‐T (r = 0.882, p < 0.01), and GSQS (r = 0.693, p < 0.01) were all indicative of a strong and positive relationships with the principal component.
4.3. Group comparisons
The ANCOVA of Response latency, found that Age (F (1,125) = 6.3, p = 0.01, f = 0.22) and Group (F (1,125) = 9.22, p < 0.01, f = 0.27) significantly contributed to the model. Inspection of the estimated marginal means revealed that controls (M = −0.2, SE = 0.1) seemed to outperform BZRA users (M = 0.3, SE = 0.13, p < 0.003). The clinical complaint composite score (F (1,121) = 0.23, p = 0.88) and CBZRA (F (1,121) = 0.56, p = 0.81) did not contribute significantly to the model (Figure 1).
FIGURE 1.
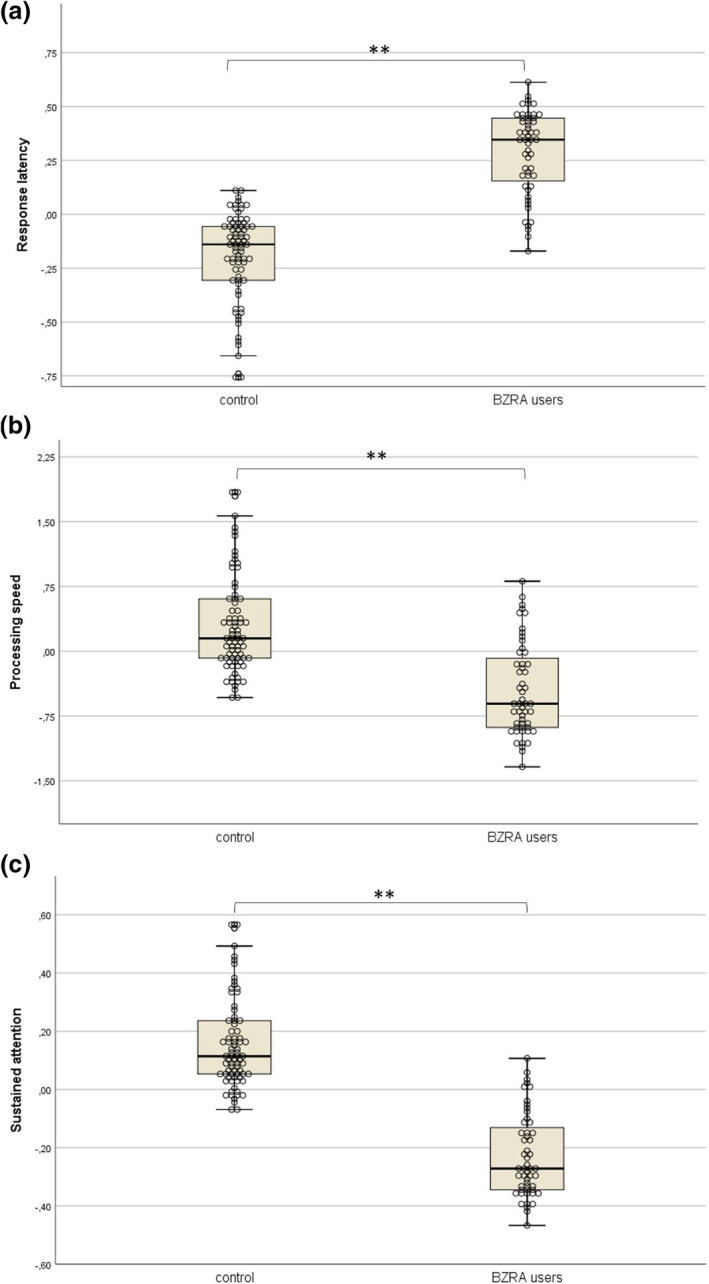
Unstandardized predicted values of factor scores corrected for the effect of age. (a) Response latency: higher scores indicate worse performance. (b) Processing speed: higher scores mean better performance. (c) Sustained attention: higher scores indicate better performance. *Estimated marginal mean difference was found to be statistically significant (p < 0.05). **Estimated marginal mean difference was found to be statistically significant (p < 0.01)
The analysis of Processing speed revealed both factor Group (F (1,125) = 96.97, p < 0.001, f = 0.88) and the covariate Age (F (1,125) = 48.08, p < 0.001, f = 0.62) were significant predictors, while CBZRA did not significantly contribute to the model (F (1,121) = 1.29, p = 0.26). The clinical complaint composite score was found to be marginally significant (F (1,121) = 3.21, p = 0.08) and was therefore initially kept in the model. However, after removal of the estimated drug concentration covariate, the contribution of the clinical complaint composite score was clearly not significant (F (1,121) = 2.3, p = 0.13) and the covariate was therefore removed from the model (Figure 2). Inspection of the estimated marginal means showed that BZRA users (M = −0.48, SE = 0.09) performed significantly worse compared to controls (M = 0.33, SE = 0.08, p < 0.001; Figure 1).
FIGURE 2.
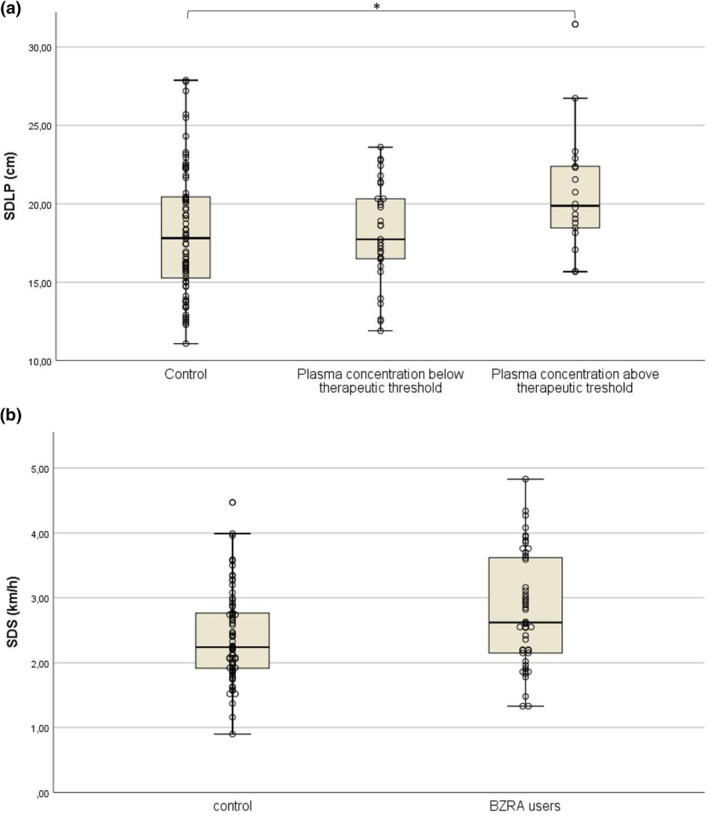
(a) The standard deviation of the lateral position (SDLP) values of the on‐the‐road driving test for controls and benzodiazepine receptor agonist (BZRA) users grouped by estimated BZRA plasma concentrations, that is estimated BZRA plasma concentrations below the therapeutic threshold (CBZRA‐) and BZRA plasma concentrations exceeding the therapeutic threshold (CBZRA+). (b) Unstandardized predicted values of standard deviation of speed (SDS) of controls and patients corrected for the effect of Age. *Estimated marginal mean difference relative to control was found to be statistically significant (p < 0.05). **Estimated marginal mean difference was found to be statistically significant (p < 0.01)
For the ANCOVA of Sustained attention, the covariates CBZRA (F (1,121) = 0.21, p = 0.644) and clinical complaint composite score (F (1,121) = 0.88, p = 0.35) did not contribute significantly to the model and were removed. After their removal, it was found that both the covariate age (F (1,125) = 5.28, p = 0.023, f = 0.21) and the random factor group (F (1,125) = 9.04, p = 0.003, f = 0.27) significantly predicted performance of this factor, with controls (M = 0.16, SE = 0.08) outperforming BZRA users (M = −0.24, SE = 0.13, p = 0.003; Figure 1).
The separate analysis of SDLP demonstrated that age (F (1,118) = 0.5, p = 0.48) and clinical complaint composite score (F (1,118) = 0.36, p = 0.55) were nonsignificant predictors and were removed from the model. After their removal, it was found that CBZRA (F (1,120) = 5.38, p = 0.022) significantly predicted the SDLP. However, after the correction for this covariate, it was found that the random factor Group was not significant (F (1,120) = 2.48, p = 0.118). A post hoc one‐way ANOVA with CBZRA as the sole factor again confirmed that it significantly predicted SDLP (F (2,120) = 3,52, p = 0.033). Post hoc, Dunnett's t‐test revealed that the CBZRA + subgroup had significantly higher SDLP values (M = 20.74, SE = 0.91) compared to control participants (M = 18.19, SE = 0.46, p = 0.012, d = 0.65), while the difference between controls and the CBZRA− subgroup was insignificant (M = 18.14, SE = 0.58, p = 0.737; Figure 2).
The SDS was also analyzed separately because of its weak correlation with any of the factors. It was found that neither clinical complaint composite score (F (1,114) = 0.15, p = 0.697) or CBZRA (F (1,114) = 0.05, p = 0.812) significantly contributed to the model and were removed. Hereafter, it was found that both age (F (1,118) = 6.4, p = 0.013, f = 0.23) and group (F (1,118) = 9.01 p = 0.003, f = 0.28) were significant predictors. Control participants (M = 2.39, SE = 0.09) outperformed BZRA users (M = 2.81, SE = 0.11, p = 0.003; Figure 2).
A separate ANCOVA with ATTPT accuracy as the dependent variable showed that neither age (F (1,121) = 2.81, p = 0.096), clinical complaint composite score (F (1,121) = 0.21, p = 0.649), nor CBZRA (F (1,121) = 0, p = 0.995) significantly contributed to the model and were consequentially removed. Hereafter, it was found that no performance differences were apparent between the BZRA users and control group (F (1,126) = 0.94, p = 0.333).
A post‐hoc comparison of self‐reported alcohol use between the BZRA user groups and control participants was performed. Control participants (Mdn = 4.5) indicated to consume significantly more alcoholic beverages per week than BZRA users (Mdn = 3 U = 1479.5, p = 0.015), as demonstrated by a Mann‐Whitney U‐test. The two BZRA user groups (CBZRA−: Mdn = 2, CBZRA+: Mdn = 4, U = 282.5, p = 0.804) did not differ with respect to self‐reported number of alcoholic beverages per week.
5. DISCUSSION
The goal of the current analysis was to investigate the neurocognitive and driving performance of long‐term, regular BZRA users, controlled for estimated BZRA plasma concentrations and severity of clinical complaints. In addition, considering the wide age range of participants and the known effects of age on cognitive and psychomotor functioning, age was also controlled for. Performance of long‐term and regular BZRA users on various psychomotor tasks and a standardized on‐the‐road driving test was compared to that of healthy controls.
Dimension reduction of the dependent variables through ordinary least squares factor analysis yielded a three factor solution. The first factor, Response latency, correlated strongly and positively with simple reaction time tests consisting of one type of target stimulus and one response option. The second factor, Processing speed, correlated strongly with tasks that share a level of complexity relative to simple reaction time tasks in that multiple response options are presented from which the correct one should be selected as fast as possible. This operation requires higher level functioning such as conscious stimulus identification, working memory and response matching. Hence, performance depends on the speed with which a stimulus is identified and the correct response is selected. The third factor, Sustained attention, was best explained by the ADT, PVT, and on‐the‐road driving test performance, albeit modest. Arguably, what these tasks share is the requirement of sustained attention. The ADT places high demands on the participants' attentional resources. The stimulus presentation rate adapts to the participants performance which assures a constant high level of difficulty. This arguably induces mental fatigue. The PVT and on‐the‐road driving tests are prolonged, monotonous tasks which require effort to remain vigilant.
Separate analyses of covariance demonstrated that healthy controls performed better than BZRA users on all three factors. However, clinical symptoms and estimated BZRA plasma concentration grouped relative to the therapeutic threshold did not significantly contribute to the model, which suggests that there is no direct influence of BZRA use or clinical symptomatology on neurocognitive performance. Similar results were found for the separate analysis of SDS. A separate analysis of the ATTPT failed to show any difference in accuracy between groups or as a function of any of the covariates.
The SDLP, the main outcome of the on‐the‐road driving test, did not load strongly on any of the factors and was therefore considered separately. This analysis showed that when the difference between controls and BZRA users was corrected for the effect of estimated BZRA plasma concentrations, the group difference was no longer apparent. A post hoc analysis of variance and subsequent multiple comparisons demonstrated that BZRA users with estimated BZRA plasma concentration exceeding the therapeutic threshold exhibited significantly higher SDLP values. This suggests that residual acute effects of BZRA use can have a negative impact on road tracking ability, which contrasts the results regarding the Sustained attention factor, where residual acute drug effects were not found to significantly explain performance differences, despite the SDLP being one of the greater contributors to that factor.
Arguably, what differentiates the SDLP from the Sustained attention factor is the task duration and the stimuli and response modes.
The duration of the on‐road driving test is significantly longer than the duration of the other two largest contributors, the ADT and PVT. This characteristic is conceivably of central importance to detect any residual acute drug effects caused by BZRA use. During the driving test, SDLP increases as a function of time on task (Verster & Roth, 2011, 2013b), likely due to driver fatigue. It is plausible that BZRA users might be less able to counteract fatigue during prolonged monotonous tasks. Also, the stimuli and response modes might explain the discrepant findings. Unlike the PVT and ADT, the on‐road driving test does not involve discrete stimuli and response options. The “stimulus” during the on‐road driving test is the deviation from the aimed direction. This is not a discrete stimulus since the magnitude of the deviation is of central importance for determining the magnitude of the correction, which also makes the steering correction a graded, non‐discrete response. Arguably, this added level of complexity, together with the prolonged test duration, makes the on‐road driving test the most sensitive test in the battery to pick up impairments in sustained attention.
The mean SDLP was found to be 2.55 cm higher compared to the control group. Previous research demonstrated that a difference of 2.5 cm is comparable to the increase in SDLP observed at blood‐alcohol concentration (BAC) of 0.5 g/L compared to placebo (Jongen et al., 2017). This BAC has been found to be associated with a significantly increase in crash risk (Borkenstein, Crowther, & Shumate, 1974). It follows that an increase in SDLP of ≥2.5 cm implies a significantly increase in crash risk (Ramaekers, 2017). In conclusion, long‐term and regular BZRA users with BZRA plasma concentration exceeding the therapeutic threshold show impaired road‐tracking ability which potentially increases their respective crash risk.
Overall, the results confirm the previous findings by Barker et al. (2004a, 2004b) that long‐term benzodiazepine users are impaired on tests of psychomotor functioning compared to healthy controls. In addition, the current results suggest that the observed impairments cannot always be explained by residual acute effects of BZRA's, with the exception of road tracking (SDLP). This latter notion suggests that residual acute BZRA effects can still play a causal role in the observed performance differences between BZRA users and controls during prolonged tasks with indiscrete stimuli and graded response requirements.
Despite previous findings that BZRA blood plasma concentrations appeared to correlate poorly with road racking performance during the standardized on‐road driving test (Verster & Roth, 2013a), the current findings suggests that BZRA blood plasma concentrations can be useful for the estimation of drug effects when interpreted relative to the therapeutic threshold, that is, in a binary sense rather than as a continuous linear correlate. However, it should be stressed that the BZRA plasma concentrations are estimated based on average pharmacokinetic parameters of the respective drugs as described in the literature. Absorption, distribution, metabolism, and excretion are generally subject to considerable inter‐individual variation. Also, age is known to slow metabolic rate of drugs, but was not taken into account for the estimations. Another potential limitation regarding the BZRA estimates is the role of co‐medications. Despite the observation that the relative frequency of number and types of CNS co‐medications was similar in both BZRA user groups, a more detailed evaluation of potential hepatic drug interactions was not performed. The potential of certain co‐medications to inhibit or enhance the actions of the cytochrome P450 enzymes necessary for the breakdown of BZRAs might have resulted in over‐ or under estimations of the BZRA plasma concentrations. Future research should ideally include actual blood plasma samples for the determination of the BZRA plasma concentrations. However, provided the comparable age distribution and large difference in estimated equivalent BZRA plasma concentrations between the two BZRA user subgroups, the estimates are arguably sufficiently accurate to allow for a group comparison. Another conceivable shortcoming of this investigation is the regular consumption of alcoholic beverages. Although the regular consumption of alcohol is unlikely to have contributed to the observed impairments in BZRA users, since BZRA users reported to have less drinks per week than controls, it might have attenuated the differences in performance. This is because of the notion of cross‐tolerance for the effects of ethanol and BZRAs, which has been demonstrated in rodents (Chan, Schanley, Aleo, & Leong, 1985; Le, Khanna, Kalant, & Grossi, 1986). For now, it remains unclear at what dose and frequency alcohol consumption can induce cross‐tolerance in humans. It is therefore plausible that some BZRA users, especially those with the maximally allowed weekly intake, might experience less functional impairment introduced by the use of their respective BZRA.
Despite the observation that the Clinical Complaint Composite Score was not found to significantly predict performance, the potential role of clinical complaints cannot be excluded (van der Sluiszen et al., 2017; Verster & Roth, 2014; Wingen, Ramaekers, & Schmitt, 2006). It should be noted that the applied depression, anxiety, and sleep quality assessments that make up the complaint composite score predominantly inquire about the BZRA users' experiences in the recent past (i.e., yesterday, last few days, weeks, and months) and were administered before execution of the test battery. Hence, none of the applied questionnaires were used to quantify task related stress during or immediately after completion of the tasks. Increased anxiety in response to being subjected to a test is generally known as test anxiety and has been repeatedly demonstrated to be associated with poorer test performance (Cook et al., 2007; Eysenck, 1985; Hembree, 1988) and often co‐exists with anxiety and depression related complaints (Akinsola & Nwajei, 2013). Previous research (Eysenck, 1985) has demonstrated that performance on tasks requiring higher order cognitive functions is more affected by test anxiety than performance on tasks drawing on low level functions. In line with this, it was found that the impairment of Processing speed, the factor summarizing the most complex tasks, due to the effect of Group (BZRA users vs. controls) was of large magnitude (f = 0.88), while the magnitude of impairments on the lower level Response latency and Sustained attention factors were small to medium (f = 0.27 in both instances). It therefore remains plausible that the performance of BZRA users suffered from test anxiety. Future research should include measures of task related stress such as the Dundee stress state questionnaire (Matthews et al., 1999) in order to more specifically investigate the role of this potential confounder.
6. CONCLUSION
Long‐term regular BZRA users are impaired on various tasks of psychomotor functioning. Specifically, it was found that response latency, processing speed during more complex psychomotor tasks, and sustained attention are impaired as compared to healthy controls. These impairments were not explained by estimated BZRA plasma concentrations, which makes the role of acute drug effects seem less likely. However, road tracking during a standardized driving test in real traffic did appear to be significantly impaired in BZRA users with therapeutically relevant estimated BZRA levels, but not for the other BZRA user subgroup with low estimated BZRA levels. The magnitude of the effect was comparable to the impairment observed while driving at a BAC of 0.5 g/L. These findings suggest that residual acute BZRA effects are relevant to task performance, depending on the tasks characteristics. Severity of depressive, anxiety, and sleep complaints combined did not explain any of the observed impairments. However, it is argued that based on the applied assessment instruments, the role of acute task related stress cannot be ruled out. Future research should include assessment of experienced task related stress in order to control for this potential confounder. It is also advised to collect blood plasma samples in order to prevent any inaccuracies inherent to the estimations of the BZRA plasma concentrations based on medication profiles.
CONFLICTS OF INTEREST
J.C. Verster has received grants from Janssen, Nutricia, Red Bull, Sequential, and Takeda and acted as a consultant/advisor for 82Labs, Canadian Beverage Association, Centraal Bureau Drogisterijbedrijven, Clinilabs, Coleman Frost, Danone, Deenox, Eisai, Janssen, Jazz, Purdue, Red Bull, Sanofi‐Aventis, Sen‐Jam Pharmaceutical, Sepracor, Takeda, Transcept, Trimbos Institute, Vital Bevrages, and ZBiotics. A. Vermeeren and J.G. Ramaekers have received funding over the last 4 years from Eisai, Jazz, Merck, and Transcept.
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are not publicly available due to privacy sensitivity, but are available from the corresponding author on reasonable request.
REFERENCES
- Akinsola, E. F., & Nwajei, A. D. (2013). Test anxiety, depression and academic performance: Assessment and management using relaxation and cognitive restructuring techniques. Psychology, 4(6), 18. [Google Scholar]
- Ashton, H. (1994). Guidelines for the rational use of benzodiazepines. Drugs, 48(1), 25–40. [DOI] [PubMed] [Google Scholar]
- Barker, M. J., Greenwood, K. M., Jackson, M., & Crowe, S. F. (2004a). Cognitive effects of long‐term benzodiazepine use. CNS Drugs, 18(1), 37–48. [DOI] [PubMed] [Google Scholar]
- Barker, M. J., Greenwood, K. M., Jackson, M., & Crowe, S. F. (2004b). Persistence of cognitive effects after withdrawal from long‐term benzodiazepine use: A meta‐analysis. Archives of Clinical Neuropsychology, 19(3), 437–454. [DOI] [PubMed] [Google Scholar]
- Beck, A. T., Steer, R. A., & Carbin, M. G. (1988). Psychometric properties of the Beck depression inventory: Twenty‐five years of evaluation. Clinical Psychology Review, 8(1), 77–100. [Google Scholar]
- Berlin, A., & Dahlström, H. (1975). Pharmacokinetics of the anticonvulsant drug clonazepam evaluated from single oral and intravenous doses and by repeated oral administration. European Journal of Clinical Pharmacology, 9(2–3), 155–159. [DOI] [PubMed] [Google Scholar]
- Borkenstein, R. F., Crowther, R. F., & Shumate, R. (1974). The role of the drinking driver in traffic accidents (The Grand Rapids Study). Blutalkohol, 11(Suppl), 1–131. [Google Scholar]
- Bushnell, G. A., Stürmer, T., Gaynes, B. N., Pate, V., & Miller, M. (2017). Simultaneous antidepressant and benzodiazepine new use and subsequent long‐term benzodiazepine use in adults with depression, United States, 2001‐2014. JAMA psychiatry, 74(7), 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse, D. J., Reynolds, C. F., III, Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Caille, G., Du Souich, P., Spenard, J., Lacasse, Y., & Vezina, M. (1984). Pharmacokinetic and clinical parameters of zopiclone and trimipramine when administered simultaneously to volunteers. Biopharmaceutics & Drug Disposition, 5(2), 117–125. [DOI] [PubMed] [Google Scholar]
- Chan, A. W., Schanley, D. L., Aleo, M. D., & Leong, F. W. (1985). Cross‐tolerance between ethanol and chlordiazepoxide. Alcohol, 2(2), 209–213. [DOI] [PubMed] [Google Scholar]
- Cook, J. M., Biyanova, T., Masci, C., & Coyne, J. C. (2007). Older patient perspectives on long‐term anxiolytic benzodiazepine use and discontinuation: A qualitative study. Journal of General Internal Medicine, 22(8), 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevoisier, C., Delisle, M., Joseph, I., & Foletti, G. (2003). Comparative single‐dose pharmacokinetics of clonazepam following intravenous, intramuscular and oral administration to healthy volunteers. European Neurology, 49(3), 173–177. [DOI] [PubMed] [Google Scholar]
- Dinges, D. F., & Powell, J. W. (1985). Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behavior Research Methods, Instruments, & Computers, 17(6), 652–655. [Google Scholar]
- Divoll, M., Greenblatt, D. J., Harmatz, J. S., & Shader, R. I. (1981). Effect of age and gender on disposition of temazepam. Journal of Pharmaceutical Sciences, 70(10), 1104–1107. [DOI] [PubMed] [Google Scholar]
- Divoll, M., Greenblatt, D. J., Ochs, H. R., & Shader, R. I. (1983). Absolute bioavailability of oral and intramuscular diazepam: Effects of age and sex. Anesthesia & Analgesia, 62(1), 1–8. [PubMed] [Google Scholar]
- Eatman, F., Colburn, W., Boxenbaum, H., Posmanter, H., Weinfeld, R., Ronfeld, R., … Kaplan, S. (1977). Pharmacokinetics of diazepam following multiple‐dose oral administration to healthy human subjects. Journal of Pharmacokinetics and Biopharmaceutics, 5(5), 481–494. [DOI] [PubMed] [Google Scholar]
- Eysenck, M. W. (1985). Anxiety and cognitive‐task performance. Personality and Individual Differences, 6(5), 579–586 10.1016/0191-8869(85)90007-8. [DOI] [Google Scholar]
- Fernandez, C., Martin, C., Gimenez, F., & Farinotti, R. (1995). Clinical pharmacokinetics of zopiclone. Clinical Pharmacokinetics, 29(6), 431–441. [DOI] [PubMed] [Google Scholar]
- Fortier‐Brochu, É., Beaulieu‐Bonneau, S., Ivers, H., & Morin, C. M. (2012). Insomnia and daytime cognitive performance: A meta‐analysis. Sleep Medicine Reviews, 16(1), 83–94. [DOI] [PubMed] [Google Scholar]
- Franklin, S. B., Gibson, D. J., Robertson, P. A., Pohlmann, J. T., & Fralish, J. S. (1995). Parallel analysis: A method for determining significant principal components. Journal of Vegetation Science, 6(1), 99–106. [Google Scholar]
- Gaillot, J., Heusse, D., Hougton, G., Aurele, J. M., & Dreyfus, J. (1983). Pharmacokinetics and metabolism of zopiclone. Pharmacology, 27(Suppl. 2), 76–91. [DOI] [PubMed] [Google Scholar]
- Gerlach, L. B., Maust, D. T., Leong, S. H., Mavandadi, S., & Oslin, D. W. (2018). Factors associated with long‐term benzodiazepine use among older adults. JAMA Internal Medicine, 178(11), 1560–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt, D. J. (1981). Clinical pharmacokinetics of oxazepam and lorazepam. Clinical Pharmacokinetics, 6(2), 89–105. [DOI] [PubMed] [Google Scholar]
- Greenblatt, D. J., Abernethy, D. R., Locniskar, A., Harmatz, J. S., Limjuco, R. A., & Shader, R. I. (1984). Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology: The Journal of the American Society of Anesthesiologists, 61(1), 27–35. [PubMed] [Google Scholar]
- Greenblatt, D. J., Abernethy, D. R., Locniskar, A., Ochs, H. R., Harmatz, J. S., & Shader, R. I. (1985). Age, sex, and nitrazepam kinetics: Relation to antipyrine disposition. Clinical Pharmacology & Therapeutics, 38(6), 697–703. [DOI] [PubMed] [Google Scholar]
- Greenblatt, D. J., Allen, M. D., Harmatz, J. S., & Shader, R. I. (1980). Diazepam disposition determinants. Clinical Pharmacology & Therapeutics, 27(3), 301–312. [DOI] [PubMed] [Google Scholar]
- Greenblatt, D. J., Blaskovich, P. D., Nuwayser, E., Harmatz, J. S., Chen, G., & Zinny, M. A. (2005). Clonazepam pharmacokinetics: Comparison of subcutaneous microsphere injection with multiple‐dose oral administration. The Journal of Clinical Pharmacology, 45(11), 1288–1293. [DOI] [PubMed] [Google Scholar]
- Greenblatt, D. J., Divoll, M., Harmatz, J. S., & Shader, R. I. (1982). Pharmacokinetic comparison of sublingual lorazepam with intravenous, intramuscular, and oral lorazepam. Journal of Pharmaceutical Sciences, 71(2), 248–252. [DOI] [PubMed] [Google Scholar]
- Greenblatt, D. J., Harmatz, J. S., Friedman, H., Locniskar, A., & Shader, R. I. (1989). A large‐sample study of diazepam pharmacokinetics. Therapeutic Drug Monitoring, 11(6), 652–657. [DOI] [PubMed] [Google Scholar]
- Greenblatt, D. J., Harmatz, J. S., Roth, T., Singh, N. N., Moline, M. L., Harris, S. C., & Kapil, R. P. (2013). Comparison of pharmacokinetic profiles of zolpidem buffered sublingual tablet and zolpidem oral immediate‐release tablet: Results from a single‐center, single‐dose, randomized, open‐label crossover study in healthy adults. Clinical Therapeutics, 35(5), 604–611. [DOI] [PubMed] [Google Scholar]
- Greenblatt, D. J., & Wright, C. E. (1993). Clinical pharmacokinetics of alprazolam. Clinical Pharmacokinetics, 24(6), 453–471. [DOI] [PubMed] [Google Scholar]
- Hembree, R. (1988). Correlates, causes, effects, and treatment of test anxiety. Review of Educational Research, 58(1), 47–77. [Google Scholar]
- Hildebrand, M., Hellstern, A., Hümpel, M., Hellenbrecht, D., & Saller, R. (1990). Plasma levels and urinary excretion of lormetazepam in patients with liver cirrhosis and in healthy volunteers. European Journal of Drug Metabolism and Pharmacokinetics, 15(1), 19–26. [DOI] [PubMed] [Google Scholar]
- IBM corporation . (2017). IBM SPSS statistics for windows (version 25.0 Armonk). New York, NY: IBM Corp. [Google Scholar]
- Jochemsen, R., Hogendoorn, J., Dingemanse, J., Hermans, J., Boeijinga, J., & Breimer, D. (1982). Pharmacokinetics and bioavailability of intravenous, oral, and rectal nitrazepam in humans. Journal of Pharmacokinetics and Biopharmaceutics, 10(3), 231–245. [DOI] [PubMed] [Google Scholar]
- Jochemsen, R., Wesselman, J., Hermans, J., Van Boxtel, C., & Breimer, D. (1983). Pharmacokinetics of brotizolam in healthy subjects following intravenous and oral administration. British Journal of Clinical Pharmacology, 16(S2), 285S–290S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen, S., Vermeeren, A., van der Sluiszen, N. N. J. J. M., Schumacher, M. B., Theunissen, E. L., Kuypers, K. P. C., … Ramaekers, J. G. (2017). A pooled analysis of on‐the‐road highway driving studies in actual traffic measuring standard deviation of lateral position (i.e., “weaving”) while driving at a blood alcohol concentration of 0.5 g/L. Psychopharmacology, 234(5), 837–844. 10.1007/s00213-016-4519-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen, S., Vuurman, E. F. P. M., Ramaekers, J. G., & Vermeeren, A. (2018). Comparing the effects of oxazepam and diazepam in actual highway driving and neurocognitive test performance: A validation study. Psychopharmacology, 235(4), 1283–1294. 10.1007/s00213-018-4844-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf, D., Huempel, M., Lerche, U., & Kessel, M. (1981). Effects of uremia and hemodialysis on lormetazepam disposition. Clinical Pharmacology & Therapeutics, 30(1), 77–85. [DOI] [PubMed] [Google Scholar]
- Langley, M. S., & Clissold, S. P. (1988). Brotizolam. Drugs, 35(2), 104–122. [DOI] [PubMed] [Google Scholar]
- Langtry, H. D., & Benfield, P. (1990). Zolpidem. Drugs, 40(2), 291–313. [DOI] [PubMed] [Google Scholar]
- Le, A., Khanna, J., Kalant, H., & Grossi, F. (1986). Tolerance to and cross‐tolerance among ethanol, pentobarbital and chlordiazepoxide. Pharmacology Biochemistry and Behavior, 24(1), 93–98. [DOI] [PubMed] [Google Scholar]
- Leufkens, T. R., & Vermeeren, A. (2014). Zopiclone's residual effects on actual driving performance in a standardized test: A pooled analysis of age and sex effects in 4 placebo‐controlled studies. Clinical Therapeutics, 36(1), 141–150. [DOI] [PubMed] [Google Scholar]
- Lombardo, F., Obach, R. S., Shalaeva, M. Y., & Gao, F. (2002). Prediction of volume of distribution values in humans for neutral and basic drugs using physicochemical measurements and plasma protein binding data. Journal of Medicinal Chemistry, 45(13), 2867–2876. [DOI] [PubMed] [Google Scholar]
- Lucki, I., Rickels, K., & Geller, A. M. (1986). Chronic use of benzodiazepines and psychomotor and cognitive test performance. Psychopharmacology, 88(4), 426–433. [DOI] [PubMed] [Google Scholar]
- Malacrida, R., Fritz, M. E., Suter, P. M., & Crevoisier, C. (1992). Pharmacokinetics of midazolam administered by continuous intravenous infusion to intensive care patients. Critical Care Medicine, 20(8), 1123–1126. [DOI] [PubMed] [Google Scholar]
- Matthews, G., Joyner, L., Gilliland, K., Campbell, S., Falconer, S., & Huggins, J. (1999). Validation of a comprehensive stress state questionnaire: Towards a state big three. Personality psychology in Europe, 7, 335–350. [Google Scholar]
- McDermott, L. M., & Ebmeier, K. P. (2009). A meta‐analysis of depression severity and cognitive function. Journal of Affective Disorders, 119(1–3), 1–8. [DOI] [PubMed] [Google Scholar]
- Mulder‐Hajonides van der Meulen, R., & Van den Hoofdakker, R. (1990). The Groningen sleep quality scale. Scandinavian Journal of Psychology. [Google Scholar]
- Neuwirth, W., & Benesch, M. (2003). Determination test. Mödling, Austria: Dr G. Schuhfried GmbH. [Google Scholar]
- Noble, S., Langtry, H. D., & Lamb, H. M. (1998). Zopiclone. Drugs, 55(2), 277–302. [DOI] [PubMed] [Google Scholar]
- O'Hanlon, J. (1984). Driving performance under the influence of drugs: Rationale for, and application of, a new test. British Journal of Clinical Pharmacology, 18(S1), 121S–129S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs, H. R., Steinhaus, E., Locniskar, A., Knüchel, M., & Greenblatt, D. J. (1982). Desmethyldiazepam kinetics after intravenous, intramuscular, and oral administration of clorazepate dipotassium. Klinische Wochenschrift, 60(8), 411–415. [DOI] [PubMed] [Google Scholar]
- Olubodun, J. O., Ochs, H. R., Trüten, V., Klein, A., von Moltke, L. L., Harmatz, J. S., … Greenblatt, D. J. (2002). Zolpidem pharmacokinetic properties in young females: Influence of smoking and oral contraceptive use. The Journal of Clinical Pharmacology, 42(10), 1142–1146. [DOI] [PubMed] [Google Scholar]
- Owen, R., & Tyrer, P. (1983). Benzodiazepine dependence. Drugs, 25(4), 385–398. [DOI] [PubMed] [Google Scholar]
- Paterniti, S., Dufouil, C., & Alpérovitch, A. (2002). Long‐term benzodiazepine use and cognitive decline in the elderly: The epidemiology of vascular aging study. Journal of Clinical Psychopharmacology, 22(3), 285–293. [DOI] [PubMed] [Google Scholar]
- Peirce, G. L., Smith, M. J., Abate, M. A., & Halverson, J. (2012). Doctor and pharmacy shopping for controlled substances. Medical care, 494–500. [DOI] [PubMed] [Google Scholar]
- Pomara, N., Tun, H., DaSilva, D., & Hernando, R. (1998). The acute and chronic performance effects of alprazolam and lorazepam in the elderly: Relationship to duration of treatment and self‐rated sedation. Psychopharmacology Bulletin, 34(2), 139. [PubMed] [Google Scholar]
- Prieler, J. (2008). RT Test Manual, version 31, Mödling: Schuhfried GmbH. [Google Scholar]
- Ramaekers, J. G. (2017). Drugs and driving research in medicinal drug development. Trends in Pharmacological Sciences, 38(4), 319–321. [DOI] [PubMed] [Google Scholar]
- Reitan, R. M. (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual & Motor Skills, 8(3), 271–276. [Google Scholar]
- Roth, T., Eklov, S. D., Drake, C. L., & Verster, J. C. (2014). Meta‐analysis of on‐the‐road experimental studies of hypnotics: Effects of time after intake, dose, and half‐life. Traffic Injury Prevention, 15(5), 439–445. [DOI] [PubMed] [Google Scholar]
- Salvà, P., & Costa, J. (1995). Clinical pharmacokinetics and pharmacodynamics of zolpidem. Clinical Pharmacokinetics, 29(3), 142–153. [DOI] [PubMed] [Google Scholar]
- Scavone, J., Greenblatt, D., Harmatz, J., & Shader, R. (1986). Kinetic and dynamic interaction of brotizolam and ethanol. British Journal of Clinical Pharmacology, 21(2), 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhfried, G. (2009). Adaptive tachistoscopic traffic perception test, version 22, Mödling: Schuhfried GmbH. [Google Scholar]
- Schulz, M., Iwersen‐Bergmann, S., Andresen, H., & Schmoldt, A. (2012). Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Critical Care, 16(4), R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, H. (1979). Pharmacokinetics and metabolism of temazepam in man and several animal species. British Journal of Clinical Pharmacology, 8(Suppl 1), 23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shader, R. I., Greenblatt, D. J., Ciraulo, D. A., Divoll, M., Harmatz, J. S., & Georgotas, A. (1981). Effect of age and sex on disposition of desmethyldiazepam formed from its precursor clorazepate. Psychopharmacology, 75(2), 193–197. [DOI] [PubMed] [Google Scholar]
- Shinfuku, M., Kishimoto, T., Uchida, H., Suzuki, T., Mimura, M., & Kikuchi, T. (2019). Effectiveness and safety of long‐term benzodiazepine use in anxiety disorders: A systematic review and meta‐analysis. International Clinical Psychopharmacology, 34(5), 211–221. [DOI] [PubMed] [Google Scholar]
- Smith, R., Kroboth, P., Vanderlugt, J., Phillips, J., & Juhl, R. (1984). Pharmacokinetics and pharmacodynamics of alprazolam after oral and IV administration. Psychopharmacology, 84(4), 452–456. [DOI] [PubMed] [Google Scholar]
- Sonne, J., Loft, S., Døssing, M., Vollmer‐Larsen, A., Olesen, K., Victor, M., … Andreasen, P. (1988). Bioavailability and pharmacokinetics of oxazepam. European Journal of Clinical Pharmacology, 35(4), 385–389. [DOI] [PubMed] [Google Scholar]
- Sorock, G. S., & Shimkin, E. E. (1988). Benzodiazepine sedatives and the risk of falling in a community‐dwelling elderly cohort. Archives of Internal Medicine, 148(11), 2441–2444. [PubMed] [Google Scholar]
- Spielberger, C. D. (2010). State‐Trait anxiety inventory. In The Corsini encyclopedia of psychology, 1‐1. [Google Scholar]
- Taylor, D. J., Lichstein, K. L., Durrence, H. H., Reidel, B. W., & Bush, A. J. (2005). Epidemiology of insomnia, depression, and anxiety. Sleep, 28(11), 1457–1464. 10.1093/sleep/28.11.1457 [DOI] [PubMed] [Google Scholar]
- Uzun, S., Kozumplik, O., Jakovljević, M., & Sedić, B. (2010). Side effects of treatment with benzodiazepines. Psychiatria Danubina, 22(1), 90–93. [PubMed] [Google Scholar]
- van der Sluiszen, N. N. J. J. M., Vermeeren, A., Verster, J. C., van de Loo, A. J. A. E., van Dijken, J. H., Veldstra, J. L., … Ramaekers, J. G. (2019). Driving performance and neurocognitive skills of long‐term users of benzodiazepine anxiolytics and hypnotics. Human Psychopharmacology: Clinical and Experimental, 34(6), e2715. 10.1002/hup.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluiszen, N. N. J. J. M., Wingen, M., Vermeeren, A., Vinckenbosch, F., Jongen, S., & Ramaekers, J. G. (2017). Driving performance of depressed patients who are untreated or receive long‐term antidepressant (SSRI/SNRI) treatment. Pharmacopsychiatry, 50(05), 182–188. [DOI] [PubMed] [Google Scholar]
- Vermeeren, A. (2004). Residual effects of hypnotics. CNS Drugs, 18(5), 297–328. 10.2165/00023210-200418050-00003 [DOI] [PubMed] [Google Scholar]
- Verster, J. C., Peters, L. V., van de Loo, A. J. A. E., Bouwmeester, H., Tiplady, B., Alford, C., & Roth, T. (2016a). Next‐morning effects of hypnotic drugs on attention, psychomotor performance, and memory functioning: Implications for traffic safety. Journal of Sleep Research, 25, 126–127 [Google Scholar]
- Verster, J. C., & Roth, T. (2011). Standard operation procedures for conducting the on‐the‐road driving test, and measurement of the standard deviation of lateral position (SDLP). International Journal of General Medicine, 4, 359–371. 10.2147/IJGM.S19639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster, J. C., & Roth, T. (2013a). Blood drug concentrations of benzodiazepines correlate poorly with actual driving impairment. Sleep Medicine Reviews, 17(2), 153–159 [DOI] [PubMed] [Google Scholar]
- Verster, J. C., & Roth, T. (2013b). Vigilance decrement during the on‐the‐road driving tests: The importance of time‐on‐task in psychopharmacological research. Accident Analysis & Prevention, 58, 244–248. [DOI] [PubMed] [Google Scholar]
- Verster, J. C., & Roth, T. (2014). Insomnia and driving ability. Sleep, 37(9), 1411–1412. 10.5665/sleep.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster, J. C., van de Loo, A. J. A. E., Vermeeren, A., van der Sluiszen, N. N. J. J. M., Brookhuis, K. A., Veldstra, J. L., … Ramaekers, J. G. (2016b). Beïnvloeding van de rijvaardigheid bij langdurig gebruik van ICADTS‐categorie III geneesmiddelen. Retrieved from https://www.rijksoverheid.nl/documenten/rapporten/2016/12/01/beinvloeding-van-de-rijvaardigheid-bij-langdurig-gebruik-van-icadts-categorie-iii-geneesmiddelen [Google Scholar]
- Verster, J. C., Veldhuijzen, D. S., & Volkerts, R. (2004). Residual effects of sleep medication on driving ability. Sleep Medicine Reviews, 8(4), 309–325. [DOI] [PubMed] [Google Scholar]
- Verster, J. C., Veldhuijzen, D. S., & Volkerts, R. (2005). Is it safe to drive a car when treated with anxiolytics? Evidence from onthe‐road driving studies during normal traffic. Current Psychiatry Reviews, 1(2), 215–225. [Google Scholar]
- Vinkers, C. H., & Olivier, B. (2012). Mechanisms underlying tolerance after long‐term benzodiazepine use: A future for subtype‐selective GABAA receptor modulators? Advances in pharmacological sciences. 2012, 416864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu, A., Aoki, K., Sakiyama, Y., Ohnishi, T., & Sugita, M. (2013). Predicting pharmacokinetic stability by multiple oral administration of atypical antipsychotics. Innovations in clinical neuroscience, 10(3), 23. [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1955). Manual for the Wechsler adult intelligence scale. New York, NY: Psychological corporation. [Google Scholar]
- Wingen, M., Ramaekers, J. G., & Schmitt, J. A. (2006). Driving impairment in depressed patients receiving long‐term antidepressant treatment. Psychopharmacology, 188(1), 84–91. [DOI] [PubMed] [Google Scholar]
- Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., … Sayeeda, Z. (2018). DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Research, 46(D1), D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, C. E. (1995). Clinical pharmacokinetics of alprazolam extended release: A summary. Current Therapeutic Research, 56(9), 947–956. [Google Scholar]
- Yu, Y., Jiang, C., Xu, H., Yang, Q., Li, J., Xu, Y., … Lv, F. (2018). Impaired cognitive control of emotional conflict in trait anxiety: A preliminary study based on clinical and non‐clinical individuals. Frontiers in Psychiatry, 9, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to privacy sensitivity, but are available from the corresponding author on reasonable request.


