Abstract
Background
There is a concern that influenza vaccination may increase the incidence of immune‐related adverse events in patients receiving immune checkpoint inhibitors (ICIs). The aim of this systematic review was to summarize the available data on the safety and efficacy of influenza vaccination in cancer patients receiving ICIs.
Methods
Studies reporting safety and efficacy outcomes of influenza vaccination in cancer patients receiving ICIs were included. Only descriptive statistics were conducted to obtain a pooled rate of immune‐related adverse events in vaccinated patients.
Results
Ten studies assessing the safety and eight assessing the efficacy of influenza vaccination in cancer patients receiving ICIs were identified, for a total of 1124 and 986 vaccinated patients, respectively. Most patients had melanoma or lung cancer and received a single agent anti‐PD‐1, but also other tumour types and immunotherapy combinations were represented. No severe vaccination‐related toxicities were reported. The pooled incidence of any grade immune checkpoint inhibitor–related adverse events was 28.9%. In the 6 studies specifying the incidence of grade 3‐4 toxicities, the pooled incidence was 7.5%. No grade 5 toxicities were reported. No pooled descriptive analysis was conducted in studies reporting efficacy outcomes due to the heterogeneity of endpoints and data reporting. Nevertheless, among the eight studies included, seven reported positive efficacy outcomes of influenza vaccination.
Conclusion
The results of this systematic review support the safety and efficacy of influenza vaccination in cancer patients receiving ICIs. These results are particularly relevant in the context of the SARS‐CoV‐2 pandemic.
Keywords: anti‐PD‐1, COVID‐19, immune checkpoint inhibitors, immunotherapy, influenza vaccination, lung cancer, melanoma, SARS‐CoV‐2 pandemic
1. BACKGROUND
Immune checkpoint inhibitors (ICIs) have become a mainstay of cancer immunotherapy in recent years for a number of solid and haematologic malignancies, such as melanoma, lung cancer, renal cell carcinoma and Hodgkin lymphoma.1 They increase antitumour immunity by blocking intrinsic downregulators of immunity, such as cytotoxic T‐lymphocyte antigen 4 (CTLA‐4) and programmed cell death 1 (PD‐1) or its ligand, programmed cell death ligand 1 (PD‐L1). The interaction between PD‐1 and PD‐L1 inhibits T cells in peripheral tissue, while CTLA‐4 is generally believed to inhibit T‐cell activation at a proximal step in the immune response.2 With the introduction of ICIs in everyday clinical practice, a new category of anticancer therapy–related adverse events has emerged. Unlike traditional chemotherapy, ICIs can induce a spectrum of adverse events of autoimmune pathogenesis (irAEs), due to nonspecific activation of the immune system targeting healthy tissues and organs.2 Although the exact pathophysiology underlying irAEs remains to be further characterized, it is believed to be closely related to the function that immune checkpoints play in maintaining immunological homeostasis and avoiding autoimmune reactions.2 The backbone of immune‐related toxicity management is corticosteroid therapy. Guidelines for the management of irAEs are provided by the most influent scientific societies such as the European Society for Medical Oncology (ESMO),3 the American Society of Clinical Oncology (ASCO) 4 and the National Comprehensive Cancer Network (NCCN).5
There is a concern that influenza vaccination may increase the incidence of irAEs in patients with cancer receiving ICIs.6 In an early report on the safety of influenza vaccination, among 23 patients receiving anti‐PD‐1 monoclonal antibodies, 6 (26.1%) had severe irAEs following the administration of the influenza vaccine, including rare events such as encephalitis (8.7%) and neuropathy (4.3%).6 The authors of that report speculated that PD‐1 blockade together with vaccination could boost the breakage of tolerance by enhancing the mechanisms associated with irAEs.6 However, cancer patients are at higher risk for developing complications related to influenza infection,7, 8, 9 and vaccination is the most important protective strategy against this infection.10, 11, 12, 13 A Cochrane review of influenza vaccines in patients with cancer receiving chemotherapy revealed lower mortality and infection‐related outcomes with influenza vaccination.14
The aim of this systematic review was to summarize and discuss the currently available data assessing the safety and efficacy of influenza vaccination in cancer patients receiving ICIs.
2. METHODS
Reporting of this study conforms to broad EQUATOR guidelines15; specifically, Preferred Reporting Items for Systematic Reviews and meta‐Analyses (PRISMA) guidelines were used for the conduct and reporting of this systematic review (Figure 1).16, 17, 18
FIGURE 1.
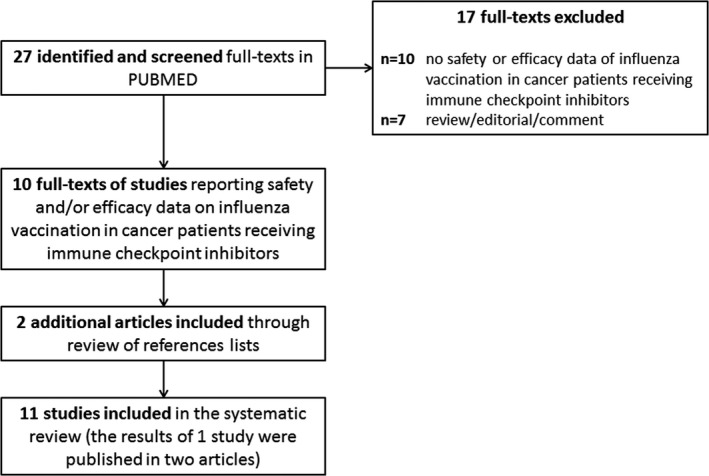
The PRISMA flow chart summarizing the process for the identification of the eligible studies
Studies reporting data on the safety and efficacy of influenza vaccination in cancer patients receiving ICIs were included in this systematic review. The following data were extracted from each report: study design, type of vaccine, number of vaccinated patients, incidence and severity of ICI‐related AEs (ie irAEs) in vaccinated patients, incidence of severe vaccination‐related AEs, number of nonvaccinated patients and differences in outcomes between nonvaccinated and vaccinated patients (in studies comparing the two populations).
Studies were identified by a computerized search on the PubMed search engine with the string ("pembrolizumab"[Supplementary Concept] OR "pembrolizumab"[All Fields] OR ("nivolumab"[MeSH Terms] OR "nivolumab"[All Fields] OR "nivolumab s"[All Fields]) OR "anti‐PD‐1"[All Fields] OR ("ipilimumab"[MeSH Terms] OR "ipilimumab"[All Fields]) OR (("cell cycle checkpoints"[MeSH Terms] OR ("cell"[All Fields] AND "cycle"[All Fields] AND "checkpoints"[All Fields]) OR "cell cycle checkpoints"[All Fields] OR "checkpoint"[All Fields] OR "checkpoints"[All Fields]) AND ("antagonists and inhibitors"[MeSH Subheading] OR ("antagonists"[All Fields] AND "inhibitors"[All Fields]) OR "antagonists and inhibitors"[All Fields] OR "inhibitors"[All Fields] OR "inhibitor"[All Fields] OR "inhibitor s"[All Fields]))) AND (("influenza s"[All Fields] OR "influenza, human"[MeSH Terms] OR ("influenza"[All Fields] AND "human"[All Fields]) OR "human influenza"[All Fields] OR "influenza"[All Fields] OR "influenzae"[All Fields] OR "influenzas"[All Fields]) AND ("vaccin"[Supplementary Concept] OR "vaccin"[All Fields] OR "vaccination"[MeSH Terms] OR "vaccination"[All Fields] OR "vaccinable"[All Fields] OR "vaccinal"[All Fields] OR "vaccinate"[All Fields] OR "vaccinated"[All Fields] OR "vaccinates"[All Fields] OR "vaccinating"[All Fields] OR "vaccinations"[All Fields] OR "vaccination s"[All Fields] OR "vaccinator"[All Fields] OR "vaccinators"[All Fields] OR "vaccine s"[All Fields] OR "vaccined"[All Fields] OR "vaccines"[MeSH Terms] OR "vaccines"[All Fields] OR "vaccine"[All Fields] OR "vaccins"[All Fields])). The search was performed on the 16 December 2020 with no date restriction and no filters. Conference abstracts were included in our analysis, and additional studies were identified following review of references lists. Only English‐language publications were considered for inclusion.
Data were independently extracted by two investigators (FS and AB) to ensure homogeneity of collection and to rule out the effect of subjectivity in data gathering and entry. Disagreements were resolved by iteration, discussion and consensus.
Only descriptive statistics were conducted to obtain a pooled response rate of irAEs by severity in vaccinated patients.
3. RESULTS
The agreement rate between the two investigators who independently extracted data was 100% after iteration and consensus. Twelve records reporting data from 11 studies were identified: safety outcomes were assessed in 10 studies, while efficacy endpoints were reported in 8 studies (Figure 1).
Among the 10 studies assessing the safety of influenza vaccination in patients with cancer receiving ICIs, for a total of 1124 vaccinated subjects (Table 1),6, 19, 20, 21, 22, 23, 24, 25, 26, 27 the majority of patients had melanoma or lung cancer, but also several other types of cancers were represented (data not shown). Most patients received an anti‐PD‐1 as single agent, but also patients receiving combined anti‐PD‐1 and anti‐CTLA‐4 treatment were included.23, 24 Common Terminology Criteria for Adverse Events (CTCAE) versions 4.0 or 5.0 were used in all but one study,27 where safety was assessed using the FDA toxicity grading scale for clinical trials; notably, this information was missing in 3 studies.19, 24, 25 No severe vaccination‐related adverse events were reported. The pooled incidence of any grade ICI‐related irAEs was 28.9%. In the 6 studies reporting the incidence of grade 3‐4 toxicities, the pooled incidence was 7.5%.6, 20, 21, 22, 23, 27 No grade 5 toxicities were reported. Among 5 studies assessing the incidence of irAEs in vaccinated and nonvaccinated patients, two studies reported a statistically significant lower incidence in the vaccinated group20, 24 and one study a statistically significant higher frequency of irAEs in vaccinated patients, albeit with a very sample size of only 23 vaccinated patients.6 One study comparing the safety of ICIs in 385 vaccinated and 149 nonvaccinated patients showed a trend towards lower incidence of irAEs in vaccinated patients25; finally, one small study reported a trend towards lower incidence of irAEs in nonvaccinated patients.26
TABLE 1.
Summary of safety endpoints’ results in patients who received anti‐PD‐1/PD‐L1 immunotherapy and influenza vaccine
| First author and date of publication | Study design | Safety endpoints | Type of vaccine | Number of vaccinated patients | Immune‐related adverse events in the vaccinated population | Severe vaccination‐related AEs | Number of nonvaccinated patients | Differences with nonvaccinated group | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) | Grade 5 (%) | Any Grade (%) | ||||||||
| Bayle et al 202019 | Prospective case series | Incidence of irAEs in vaccinated patients | NR | 30 | 15 (50%) | 0 | 0 | 15 (50%) | NR | NA | NA | ||
| Failing et al 202020 | Retrospective case‐control study | Incidence of irAEs in the vaccinated group compared with the nonvaccinated group | High or standard dose, inactivated, nonadjuvanted trivalent or quadrivalent | 70 | NR | NR | 4 (5.7%) | 0 (0%) | 18 (25.7%) | NR | 92 | OR: 0.4 (95% CI, 0.2‐0.9) | |
| Gwynn et al 202021 | Prospective case series | Incidence of irAEs in vaccinated patients | Standard dose, inactivated, quadrivalent | 24 | 2 (8.3%) | 4 (16.6%) | 1 (4.2%) | 1 (4.2%) | 0 (0%) | 7a (29.2%) | NR | NA | NA |
| Keam et al 202022 | Prospective case series | Incidence of irAEs in vaccinated patients treated with ICI compared with CT | Standard dose, quadrivalent | 47 | 4 (8.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (8.5%) | 0 (0%) | NA | NA |
| Chong et al 201923 | Retrospective case series | Incidence and severity of new onset irAEs in vaccinated patients | High or standard dose, inactivated, nonadjuvanted trivalent or quadrivalent | 370b | 5 (1.4%) | 40 (10.8%) | 27 (7.3%) | 3 (0.8%) | 0 (0%) | 75 (20.3%) | 0 (0%) | NA | NA |
| Awadalla et al 201924 | Retrospective case‐control study |
Vaccination rate in patients who had ICI‐related myocarditis compared with those who had not Incidence of irAEs in vaccinated and nonvaccinated cases |
NR | 105c | NR | NR | NR | NR | NR | 38 (36%)d | NR | 197 |
Vaccination rate: 25% in myocarditis group vs 40% in control (P = .01 for rate comparison) Rate of any grade irAEs other than myocarditis: 36% in vaccinated vs 55% unvaccinated cases (P = .10) |
| Gopalakrishnan et al25 | Retrospective case series | Incidence of irAEs in vaccinated and nonvaccinated patients | NR | 385 | NR | NR | NR | NR | NR | 144 (37.4%) | NR | 149 | Rate of irAEs: 37.4% in vaccinated vs 42.6% in nonvaccinated patients (P = .067) |
| Läubli et al 20186 | Prospective case series with retrospective control cohort | Incidence of irAEs in vaccinated patients | Standard dose, inactivated, nonadjuvanted trivalent | 23 | 6 (26.1%) | 6 (26.1%) | 0 (0%) | 12 (52.2%) | 0 (0%) | 40 | Frequency of irAEs was significantly higher in vaccinated patients | ||
| Wijn et al 201826 | Retrospective case‐control study | Incidence of irAEs in the vaccinated group compared with the nonvaccinated group | Standard dose, inactivated, nonadjuvanted trivalent | 42 | NR | NR | NR | NR | NR | 11 (26.2%) | NR | 85 | RR: 1.20 (95% CI, 0.51‐2.65) |
| Kanaloupitis et al 201727 | Prospective case series | Incidence of irAEs in vaccinated patients | NR | 28 | 0 | 1 (3.6%) | 0 | 0 | 0 | 1 (3.6%) | 0 (0%) | NA | NA |
Abbreviations: and RR, rate ratio; CT, chemotherapy; ICI, immune checkpoint inhibitors; irAE, immune‐related adverse event; NA, not applicable; NR, not reported.
Not equal to the sum of grade 1‐4 AEs because a single patients may have more than one AE.
Including 82 patients treated with anti‐CTLA‐4 + anti‐PD‐1, 42 patients with anti‐PD‐1 + experimental drugs and 15 with anti‐CTLA‐4 followed by anti‐PD‐1.
Including also patients treated with anti‐CTLA‐4 + anti‐PD‐1.
Excluding myocarditis.
The results of the eight studies assessing the efficacy of influenza vaccination in 986 patients with cancer receiving ICIs are summarized in Table 2.6, 19, 21, 22, 23, 25, 27, 28, 29 No pooled descriptive analysis was conducted due to the heterogeneity of efficacy endpoints and reporting of data. Nevertheless, among the eight studies included in this systematic review, seven reported positive efficacy outcomes of influenza vaccination in cancer patients receiving ICIs.6, 19, 21, 22, 23, 25, 27, 28
TABLE 2.
Summary of efficacy endpoints’ results in patients who received anti‐PD‐1/PD‐L1 immunotherapy and influenza vaccine
| First author and date of publication | Study design | Efficacy endpoints | Type of vaccine | Number of vaccinated patients | Results |
|---|---|---|---|---|---|
| Bayle et al 202019 | Prospective case series | Antibody titres (seroprotective rate) | NR | 30 | Seroprotective rates: 67% against H1N1, and 63% against H3N2 |
| Gwynn et al 202021 | Prospective case series | Incidence of influenza or flu‐like symptoms | Standard dose, inactivated, quadrivalent | 24 | No patients reported influenza infection or flu‐like symptoms during the study period |
|
Keam et al 202022 Kang et al 202028 |
Prospective case series |
Antibody titres (seroprotective and seroconversion rates) Incidence of influenza |
Standard dose, quadrivalent | 47 |
Seroprotection rates ranged from 76% to 89% in ICI‐treated patients Seroconversion rates ranged from 52% to 65% in ICI‐treated patients Seroprotection and seroconversion rates were significantly higher in the ICI group than in the cytotoxic chemotherapy group for all strains, except for the H1N1 strain No patient had laboratory‐confirmed symptomatic influenza during the study period Cell‐mediated immune responses following influenza vaccination were stronger in patients receiving immunotherapy than in those receiving cytotoxic chemotherapy |
| Chong et al 201923 | Retrospective case series | Laboratory‐confirmed cases of influenza | High or standard dose, inactivated, nonadjuvanted trivalent or quadrivalent | 370a | The overall combined incidence of laboratory‐confirmed influenza among individuals tested across 3 seasons was 3.5% compared with an institution‐wide incidence during the same time of 10.7% |
| Gopalakrishnan et al25 | Retrospective case series |
Rate of flu prodromal Rate of admissions for flu‐related complications |
NR | 385 | Unvaccinated patients were less likely to experience flu prodrome (32.2% vs 43.7%, P = .067), but were more likely to be admitted for influenza‐related complications (62.4% 23.2%, P = .032) |
| Läubli et al 20186 | Prospective case series with retrospective control cohort |
Antibody titres (seroprotective rate) Incidence of influenza |
Standard dose, inactivated, nonadjuvanted trivalent | 23 |
No differences in terms of antibody titres compared with healthy age‐matched controls No influenza infection was diagnosed in any of the vaccinated patients |
| Bersanelli et al 201729 | Retrospective case series | Incidence of influenza syndrome | Inactivated, trivalent or quadrivalent | 79 | The incidence of influenza syndrome was 24.1% among patients receiving the vaccine compared with 11.8% in the unvaccinated control group (26/221), for an odds ratio = 2.4 (95% CI = 1.23‐4.59) |
| Kanaloupitis et al 201727 | Prospective case series |
Antibody titres Influenza infection rate, confirmed by rapid antigen testing, and influenza‐related hospitalizations |
NR | 28 |
IgM responses at 45 d to both influenza A and B common antigens were statistically significant (P < .05) IgG response to common influenza B antigens was increased at day 45 (P = .001) One of 28 patients contracted influenza B infection, confirmed by rapid antigen testing There were no influenza‐related hospitalizations |
Abbreviations: ICI, immune checkpoint inhibitors; NR, not reported.
Including 82 patients treated with anti‐CTLA‐4 + anti‐PD‐1, 42 patients with anti‐PD‐1 + experimental drugs and 15 with anti‐CTLA‐4 followed by anti‐PD‐1.
4. DISCUSSION
Influenza vaccination is the best strategy to protect cancer patients against this infection and showed to reduce mortality and flu‐related complications in those receiving chemotherapy.14 However, in one of the first reports on the safety of influenza vaccination in cancer patients receiving ICIs, major concerns about an increased risk of severe immunological complications were raised. Despite these data being based on only 23 subjects, many clinicians started advising their patients under ICIs against vaccination.6, 23 As highlighted in our systematic review, most subsequent and larger studies showed that the overall safety and efficacy of influenza vaccination in cancer patients receiving ICIs is not substantially different from that observed in the general population.
The SARS‐CoV‐2 pandemic had a major impact on health system reorganization and on the management of patients with cancer, who are at increased risk of infection‐related complications.30, 31, 32 Protection of cancer patients from influenza infection is extremely important: influenza vaccination has clearly shown to lower mortality and infection‐related outcomes in this setting.14 In the context of the current SARS‐CoV‐2 pandemic, protecting patients from influenza infection has additional relevance also considering that influenza symptoms overlap with those of COVID‐19 and may interfere with the proper prosecution of cancer treatments, ultimately decreasing their chances of survival.
Our systematic review has some limitations, mostly related to the heterogeneity of study designs and endpoints among the studies included in our analysis. One of the main limitations of our safety analysis is the variability of recording toxicities outside clinical trials, especially for low‐grade AEs. In fact, we found an incidence of low‐grade events which suggests a probable underreporting of such events. However, clinically significant AEs, such as those requiring intervention or hospitalizations, are usually reported properly also in observational and/or retrospective studies, and the rate of severe irAEs that we observed in our analysis was in line with that reported in clinical trials, with no deaths due to treatment‐related toxicity. Other potential biases include the lack of data about reasons for receiving or not influenza vaccination (possibility of self‐selection bias) and, for retrospective studies, the selection bias intrinsic to such study design. For the efficacy analysis, we could not conduct a pooled descriptive analysis due to the vast heterogeneity of efficacy endpoints and reporting of data.
Despite these limitations, and in line with previous reports,33, 34 the results of our systematic review support influenza vaccination in patients with cancer receiving immune checkpoint inhibitor therapy. These results are particularly relevant in the context of the SARS‐CoV‐2 pandemic.
CONFLICT OF INTEREST
The authors have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Matteo Lambertini acted as consultant for Roche, AstraZeneca, Lilly and Novartis, and received speaker honoraria from Sandoz, Roche, Takeda, Pfizer, Lilly and Novartis outside the submitted work. Francesco Spagnolo acted as consultant for Novartis, MSD, Sun Pharma and Pierre Fabre, and received speaker honoraria from Roche, Novartis, BMS, MSD, Merck, Sun Pharma, Sanofi and Pierre Fabre outside the submitted work. All the other authors declare no conflicts of interest.
Spagnolo F, Boutros A, Croce E, et al. Influenza vaccination in cancer patients receiving immune checkpoint inhibitors: A systematic review. Eur J Clin Invest. 2021;51:e13604. 10.1111/eci.13604
REFERENCES
- 1.Robert C. A decade of immune‐checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158‐168. [DOI] [PubMed] [Google Scholar]
- 3.Haanen JB, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2017;28:iv119‐iv142. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714‐1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy‐Related Toxicities, Version 1.2019. J Natl Compr Cancer Netw. 2019;17(3):255‐289. [DOI] [PubMed] [Google Scholar]
- 6.Läubli H, Balmelli C, Kaufmann L, et al. Influenza vaccination of cancer patients during PD‐1 blockade induces serological protection but may raise the risk for immune‐related adverse events. J Immunother Cancer. 2018;6(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooksley CD, Avritscher EBC, Bekele BN, et al. Epidemiology and outcomes of serious influenza‐related infections in the cancer population. Cancer. 2005;104(3):618‐628. [DOI] [PubMed] [Google Scholar]
- 8.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taha A, Vinograd I, Sakhnini A, et al. The association between infections and chemotherapy interruptions among cancer patients: prospective cohort study. J Infect. 2015;70(3):223‐229. [DOI] [PubMed] [Google Scholar]
- 10.Earle CC. Influenza vaccination in elderly patients with advanced colorectal cancer. J Clin Oncol. 2003;21(6):1161‐1166. [DOI] [PubMed] [Google Scholar]
- 11.Pollyea DA, Brown JMY, Horning SJ. Utility of influenza vaccination for oncology patients. J Clin Oncol. 2010;28(14):2481‐2490. [DOI] [PubMed] [Google Scholar]
- 12.Vinograd I, Eliakim‐Raz N, Farbman L, et al. Clinical effectiveness of seasonal influenza vaccine among adult cancer patients. Cancer. 2013;119(22):4028‐4035. [DOI] [PubMed] [Google Scholar]
- 13.Blanchette PS, Chung H, Pritchard KI, et al. Influenza vaccine effectiveness among patients with cancer: a population‐based study using health administrative and laboratory testing data From Ontario, Canada. J Clin Oncol. 2019;37(30):2795‐2804. [DOI] [PubMed] [Google Scholar]
- 14.Bitterman R, Eliakim‐Raz N, Vinograd I, et al. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst Rev. 2018;2(2):CD008983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simera I, Moher D, Hoey J, et al. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA‐S: an extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst Rev. 2021;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayle A, Khettab M, Lucibello F, et al. Immunogenicity and safety of influenza vaccination in cancer patients receiving checkpoint inhibitors targeting PD‐1 or PD‐L1. Ann Oncol. 2020;31(7):959‐961. [DOI] [PubMed] [Google Scholar]
- 20.Failing JJ, Ho TP, Yadav S, et al. Safety of influenza vaccine in patients with cancer receiving pembrolizumab. JCO Oncol Pract. 2020;16(7):e573‐e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwynn ME, DeRemer DL, Saunders KM, et al. Immune‐mediated adverse events following influenza vaccine in cancer patients receiving immune checkpoint inhibitors. J Oncol Pharm Pract. 2019;26(3):647‐654. [DOI] [PubMed] [Google Scholar]
- 22.Keam B, Kang CK, Jun KI, et al. Immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;71(2):422‐425. [DOI] [PubMed] [Google Scholar]
- 23.Chong CR, Park VJ, Cohen B, et al. Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;70(2):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awadalla M, Golden DLA, Mahmood SS, et al. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopalakrishnan R, Johnson DB, York S, et al. Impact of the influenza vaccination on cancer patients undergoing therapy with immune checkpoint inhibitors (ICI). J Clin Oncol. 2018;36(15_suppl):3053. [Google Scholar]
- 26.Wijn DH, Groeneveld GH, Vollaard AM, et al. Influenza vaccination in patients with lung cancer receiving anti–programmed death receptor 1 immunotherapy does not induce immune‐related adverse events. Eur J Cancer. 2018;104:182‐187. [DOI] [PubMed] [Google Scholar]
- 27.Kanaloupitis DK, Chandran A, Ralph A, et al. Safety and efficacy of concurrent administration of influenza vaccine in patients undergoing anti‐PD‐1 immunotherapy. J Clin Oncol. 2017;35(15_suppl):e14607. [Google Scholar]
- 28.Kang CK, Kim H‐R, Song K‐H, et al. Cell‐mediated immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. J Infect Dis. 2020;222(11):1902‐1909. [DOI] [PubMed] [Google Scholar]
- 29.Bersanelli M, Giannarelli D, Castrignanò P, et al. INfluenza vaccine indication during therapy with immune checkpoint inhibitors: a transversal challenge, The INVIDIa study. Immunotherapy. 2018;10(14):1229‐1239. [DOI] [PubMed] [Google Scholar]
- 30.Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. 1990;2020(139):43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saponara M, Pala L, Conforti F, et al. Patients with locally advanced and metastatic cutaneous squamous cell carcinoma treated with immunotherapy in the era of COVID‐19: stop or go? Data from five Italian referral cancer centers. Ther Adv Med Oncol. 2020;12:1758835920977002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tagliamento M, Spagnolo F, Poggio F, et al. Italian survey on managing immune checkpoint inhibitors in oncology during COVID‐19 outbreak. Eur J Clin Invest. 2020;50(9):e13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bersanelli M, Buti S, De Giorgi U, et al. State of the art about influenza vaccination for advanced cancer patients receiving immune checkpoint inhibitors: when common sense is not enough. Crit Rev Oncol Hematol. 2019;139:87‐90. [DOI] [PubMed] [Google Scholar]
- 34.Desage A‐L, Bouleftour W, Rivoirard R, et al. Vaccination and Immune Checkpoint Inhibitors. Am J Clin. 2021;44(3):109. [DOI] [PubMed] [Google Scholar]


