Abstract
COVID-19 is caused by the SARS-CoV-2, and its presentation ranges from mild upper respiratory illness to critical disease including acute respiratory distress syndrome and multiorgan dysfunction. While it was initially believed to primarily target the respiratory system, numerous studies have demonstrated it to cause a hypercoagulable state that predisposes to arterial and venous thrombosis. We present a case where a patient with COVID-19 developed acute lower limb ischaemia due to arterial thrombosis in the setting of full-dose enoxaparin, followed by heparin infusion protocol. The patient developed recurrent ischaemia despite thrombolysis in addition to anticoagulation, and eventually required open thrombectomy before making a full recovery.
Keywords: vascular surgery, COVID-19, cardiovascular medicine
Background
COVID-19 is caused by the SARS-CoV-2. Its presentation ranges from mild upper respiratory illness to critical disease including respiratory failure and multiorgan dysfunction. It is also associated with coagulopathy, with venous thromboembolism (VTE) and pulmonary embolism seen in 13.9%–26.1% of critically ill patients with COVID-19.1 2 Although patients with COVID-19 admitted to the hospital are routinely prescribed thromboprophylaxis, thromboembolism can still occur. We describe a case of threatening limb ischaemia in a patient infected with COVID-19 who was on therapeutic dose anticoagulation at the time of diagnosis. He went on to develop recurrent limb ischaemia despite treatment with two consecutive and different types of anticoagulation as well as a successful thrombolysis. He ultimately required open thrombectomy for the recurrent disease and made a full recovery.
Case presentation
A 60-year-old man with a medical history of hypertension, hyperlipidaemia, intermittent asthma, gastritis, arthritis and no smoking history presented to the emergency department with worsening dyspnoea for the past 4 days. He reported testing positive for SARS-CoV-2, a few months prior. He was noted to be in significant respiratory distress, diaphoretic and tachycardic. His oxygen saturation was 83% and only improved to 95% on proning with high-flow nasal cannula and non-rebreather oxygen mask.
Initial laboratory values are summarised in table 1. X-ray of the chest showed patchy bilateral airspace consolidations. Electrocardiogram (EKG) showed normal sinus rhythm at 96 beats/min. Nasopharyngeal swab tested positive for SARS-CoV-2. The patient was started on remdesivir, dexamethasone, azithromycin and ceftriaxone. Given the patient’s clinical presentation, COVID-19 positive status and elevated D-dimer, there was a high clinical suspicion for pulmonary embolism. He was, however, deemed too clinically unstable to undergo CT pulmonary angiogram at the time of presentation, as he was only able to saturate 90% while lying prone on high-flow oxygen. He was transferred to the intensive care unit (ICU) and empirically started on treatment dose enoxaparin 70 mg (1 mg/kg) every 12 hours on admission.
Table 1.
Initial laboratory results on admission
| Reference range | ||
| Haemoglobin (g/L) | 135–175 | 151 |
| White blood cell count (×109/L) | 3.8–11 | 6.9 |
| Absolute neutrophil count (×109/L) | 2–6 | 6.2* |
| Absolute lymphocyte count (×109/L) | 1–2 | 0.4* |
| Platelets (×109/L) | 130–400 | 172 |
| Blood urea nitrogen (mg/dL) | 9–20 | 32* |
| Serum creatinine (mg/dL) | 0.7–1.3 | 1.8* |
| Troponin (ng/mL) | <0.03 | 0.086* |
| Prothrombin time (s) | 9.5–13.1 | 11.5 |
| International normalised ratio | 0.8–1.2 | 1.03 |
| Partial thromboplastin time (s) | 24-3–35.9 | 32.1 |
| D-dimer (ng/mL) | ≤500 | 1242* |
| Lactate dehydrogenase (U/L) | 313–618 | 1650* |
| C reactive protein (mg/dL) | <1.0 | 15.5* |
| Ferritin (ng/mL) | 17.9–464 | 916* |
| Creatine kinase-MB (ng/ml) | 0.0–3.3 | 0.96 |
| Lactic acid (mmol/L) | 0.70–2.00 | 1.39 |
*Outside reference range
On day 5 of admission, the patient reported a few hours’ history of altered sensation, pain and reduced ability to move his right foot. On physical examination, the right foot was pale in appearance and cool to touch compared with the left foot. There was weakness in the right ankle and toe extension and flexion. On palpation, popliteal pulse was reduced, and dorsalis pedis (DP) and posterior tibial (PT) pulses were absent on the right. DP pulses were absent, while PT pulses were detectable with handheld doppler. He was immediately switched from therapeutic dose enoxaparin to heparin infusion protocol. He underwent angiogram on day 6 of admission, and was diagnosed with right popliteal artery, tibial and peroneal artery thrombosis (figure 1). He underwent right popliteal artery catheter-directed thrombolysis, with palpable pulses and patent popliteal artery shown on completion angiogram the next day (figure 2). On day 8 of admission, the patient developed severe respiratory distress requiring endotracheal intubation. On day 9, he developed right foot ischaemia due to recurrent thrombosis of right popliteal, tibial and peroneal arteries (postoperative day 3). This was despite being on heparin infusion protocol since day 5 of admission. He underwent open thrombectomy of the right popliteal, tibial and peroneal arteries, and the right foot remained well perfused on examinations postoperatively. He was extubated successfully, and his anticoagulation was switched from heparin to apixaban.
Figure 1.
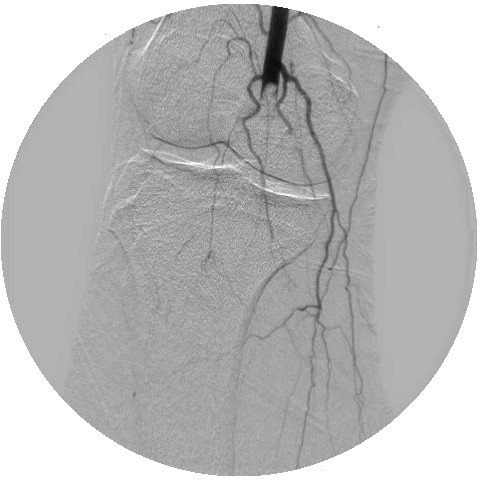
Right lower extremity angiogram on day 6 showing acute thrombosis of the right popliteal artery.
Figure 2.
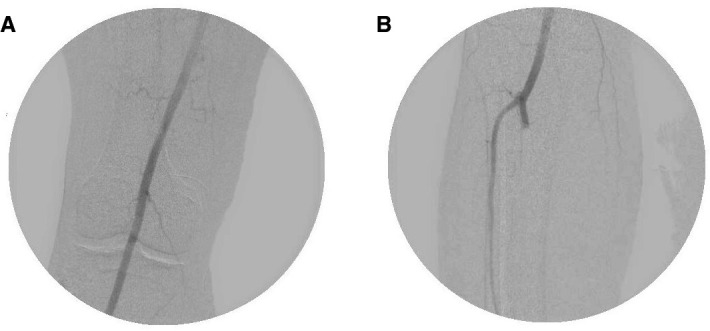
Right lower extremity angiogram on day 7. The (A) right popliteal artery was patent with no evidence of aneurysm, while the (B) tibioperoneal trunk was occluded at the bifurcation before the thrombolysis catheter was repositioned.
Discussion
COVID-19 infection causes inflammatory response and is strongly associated with arterial and venous thrombosis, especially in patients with moderate-to-severe disease. Thrombotic events occur in 11.5% of hospitalised, non-ICU patients and in 29.4% of patients with COVID-19 in the ICU. Severe arterial thrombosis including lower limb ischaemia have been reported in patients diagnosed with COVID-19, and it has been reported in 18.6% of critically ill patients.2 3 We reported a case of acute limb ischaemia in the setting of therapeutic dose anticoagulation use. To the best of our knowledge, this is the first case where a patient with COVID-19 developed recurrent limb ischaemia despite treatment with two consecutive types of anticoagulation as well as thrombolysis, and ultimately required open thrombectomy before making a full recovery. Our case described here suggests that alternative approaches to thromboprophylaxis may need to be considered in patients with COVID-19 with recurrent thrombosis that does not respond to anticoagulation and thrombolysis alone.
In a study on patients with COVID-19 pneumonia who developed acute limb ischaemia, revascularisation was successful in 12 of 17 (70.6%) patients who underwent operative treatment.4 The lower-than-expected revascularisation success rate could be due to a link between COVID-19 and hypercoagulable state, leading to increased risk of arterial occlusion. The mechanism by which COVID-19 infection predisposes patients to thromboembolic events is poorly understood. A myriad of factors including overwhelming inflammatory response or ‘cytokine storm’ syndromes, platelet activation, complement activation and enhanced thrombin generation has been proposed to contribute to the hypercoagulable state.5 Marked coagulation derangement including low antithrombin, high D-dimer and high fibrin degradation products have been noted in patients infected with COVID-19.6 Endothelial damage has been attributed to the hypercoagulable state seen in COVID-19, with viral inclusion bodies observed under light microscopy. Endotheliitis, hypercoagulability and prolonged immobility form Virchow’s triad, another potential explanation as to why these patients are prone to form arterial thrombosis.7 Both heparin and enoxaparin which were used in this patient potentiate antithrombin III as the mechanism of promoting anticoagulation. This may explain why patients experience breakthrough thromboembolism while on full-dose anticoagulation.8 Further research is needed to assess the efficacy of different anticoagulants in the setting of COVID-19 infection and hypercoagulable states.
The American Society of Hematology currently recommends prophylactic intensity anticoagulation over intermediate or therapeutic intensity anticoagulation in patients with COVID-19 who do not have suspected or confirmed VTE.9 Emerging evidence demonstrates high incidence of thromboembolism in patients with COVID-19 despite prophylactic dose anticoagulation. In an Italian retrospective study on laboratory-proven patients with COVID-19, at least one thromboembolic event occurred in 20 out of 314 (6.4%, 95% CI 4.2% to 9.6%) patients in general ward and in 8 out of 48 (16.7%, 95% CI 8.7% to 29.6%) ICU patients. This is despite thromboprophylaxis used in 75% of general ward patients and in 100% of ICU patients.10 On the other hand, pre-emptive therapeutic anticoagulation is associated with 2.3 times higher risk of mortality compared with those on prophylactic dose.11 The current literature on the characteristics of patients that warrant intermediate or therapeutic dose anticoagulation is sparse. Ongoing international trials such as the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia, Antithrombotic Therapy to Ameliorate Complications of COVID-19, and Anti-thrombotics for Adults Hospitalized With COVID-19 aim to address the question whether therapeutic dose anticoagulation is warranted in moderately to critically ill patients.12 Results from studies that compare different anticoagulant types and doses will guide clinicians in selecting optimal thromboprophylaxis tailored to each patient with COVID-19.
Learning points.
Hospitalised patients with COVID-19 are at significant risk of arterial and venous thrombosis, and thromboembolism can occur even in those on successive therapeutic dose anticoagulation.
Successful revascularisation after thrombolysis does not preclude recurrent thrombosis, and clinicians should maintain a high index of suspicion for recurrence in patients with COVID-19 with confirmed thromboembolism.
All hospitalised patients with COVID-19, unless clinically contraindicated, should be on minimum of prophylactic dose anticoagulation.
More clinical data are needed to guide clinicians on the optimal choice and dosing of anticoagulation in patients with COVID-19.
Footnotes
Twitter: @EvaTengMD
Contributors: FH, MP and ET attended to the patient and wrote the manuscript. DW reviewed and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Hippensteel JA, Burnham EL, Jolley SE. Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br J Haematol 2020;190:e134–7. 10.1111/bjh.16908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a new York City health system. JAMA 2020;324:799–801. 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kashi M, Jacquin A, Dakhil B, et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res 2020;192:75–7. 10.1016/j.thromres.2020.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg 2020;72:1864–72. 10.1016/j.jvs.2020.04.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moschonas IC, Tselepis AD. SARS-CoV-2 infection and thrombotic complications: a narrative review. J Thromb Thrombolysis 2021;52:111–23. 10.1007/s11239-020-02374-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020;58:1116–20. 10.1515/cclm-2020-0188 [DOI] [PubMed] [Google Scholar]
- 7.Cheruiyot I, Kipkorir V, Ngure B, et al. Arterial thrombosis in coronavirus disease 2019 patients: a rapid systematic review. Ann Vasc Surg 2021;70:273–81. 10.1016/j.avsg.2020.08.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gubitosa JC, Xu P, Ahmed A, et al. COVID-19-Associated acute limb ischemia in a patient on therapeutic anticoagulation. Cureus 2020;12:e10655. 10.7759/cureus.10655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Society of hematology: should DOACs, LMWH, UFH, fondaparinux, argatroban, or bivalirudin at intermediate-intensity or therapeutic-intensity vs. prophylactic intensity be used for patients with COVID-19 related critical illness who do not have suspected or confirmed VTe? 2020. Available: https://guidelines.ash.gradepro.org/profile/3CQ7J0SWt58 [Accessed 29 Mar 2021].
- 10.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motta JK, Ogunnaike RO, Shah R, et al. Clinical outcomes with the use of prophylactic versus therapeutic anticoagulation in coronavirus disease 2019. Crit Care Explor 2020;2:e0309. 10.1097/CCE.0000000000000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of health: antithrombotic therapy in patients with COVID-19, 2021. Available: https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/ [Accessed 11 Aug 2021].


