Abstract
Chronic limb-threatening ischemia (CLTI) is associated with mortality, amputation, and impaired quality of life. These Global Vascular Guidelines (GVG) are focused on definition, evaluation, and management of CLTI with the goals of improving evidence-based care and highlighting critical research needs. The term CLTI is preferred over critical limb ischemia, as the latter implies threshold values of impaired perfusion rather than a continuum. CLTI is a clinical syndrome defined by the presence of peripheral artery disease (PAD) in combination with rest pain, gangrene, or a lower limb ulceration >2 weeks duration. Venous, traumatic, embolic, and nonatherosclerotic etiologies are excluded. All patients with suspected CLTI should be referred urgently to a vascular specialist. Accurately staging the severity of limb threat is fundamental, and the Society for Vascular Surgery Threatened Limb Classification system, based on grading of Wounds, Ischemia, and foot Infection (WIfI) is endorsed. Objective hemodynamic testing, including toe pressures as the preferred measure, is required to assess CLTI. Evidence-based revascularization (EBR) hinges on three independent axes: Patient risk, Limb severity, and ANatomic complexity (PLAN). Average-risk and high-risk patients are defined by estimated procedural and 2-year all-cause mortality. The GVG proposes a new Global Anatomic Staging System (GLASS), which involves defining a preferred target artery path (TAP) and then estimating limb-based patency (LBP), resulting in three stages of complexity for intervention. The optimal revascularization strategy is also influenced by the availability of autogenous vein for open bypass surgery. Recommendations for EBR are based on best available data, pending level 1 evidence from ongoing trials. Vein bypass may be preferred for average-risk patients with advanced limb threat and high complexity disease, while those with less complex anatomy, intermediate severity limb threat, or high patient risk may be favored for endovascular intervention. All patients with CLTI should be afforded best medical therapy including the use of antithrombotic, lipid-lowering, antihypertensive, and glycemic control agents, as well as counseling on smoking cessation, diet, exercise, and preventive foot care. Following EBR, long-term limb surveillance is advised. The effectiveness of nonrevascularization therapies (eg, spinal stimulation, pneumatic compression, prostanoids, and hyperbaric oxygen) has not been established. Regenerative medicine approaches (eg, cell, gene therapies) for CLTI should be restricted to rigorously conducted randomizsed clinical trials. The GVG promotes standardization of study designs and end points for clinical trials in CLTI. The importance of multidisciplinary teams and centers of excellence for amputation prevention is stressed as a key health system initiative.
Keywords: Chronic limb-threatening ischemia, Critical limb ischemia, Peripheral artery disease, Diabetes, Foot ulcer, Endovascular intervention, Bypass surgery, Practice guideline, Evidence-based medicine
INTRODUCTION
Rationale and goals
Chronic limb-threatening ischemia (CLTI) represents the end stage of peripheral artery disease (PAD), a problem of growing prevalence and increased health care costs around the globe.1 CLTI is a highly morbid disease, incurring significant mortality, limb loss, pain, and diminished health-related quality of life (HRQL) among those afflicted. Multiple health care specialists are involved in the management of CLTI, yet lack of public awareness and the frequent failure to make an early diagnosis continue to be major obstacles to effective treatment. Variability in practice patterns is high, contributing to a broad disparity in the use of treatments and clinical outcomes. For example, a study from the United States suggested that many patients do not even receive angiography in the year before major limb amputation.2 These data also demonstrate a broad variation in the use of open or endovascular interventions by region of the country and hospital referral center.2 More expensive (and more invasive) care is not associated with better outcomes.3 Instead, what is lacking is a uniform definition of clinical stages of disease and key patient-focused outcomes, contributing to an incomplete picture of the epidemiology of CLTI and a limited evidence base to guide daily practice.
At the same time, rapidly evolving technologies in diagnostics, devices, drugs, and biologics offer new opportunities to improve treatment and to address unmet needs in this vulnerable population. A PubMed search of the term “critical limb ischemia” revealed >5000 citations, with a clear inflection point at the turn of the millennium, demonstrating an explosion of interest. A new framework is urgently needed to establish evidence-based medical practices in this changing field. The rationale for this global guideline on the management of CLTI was based on this nexus of factors and the recognition of its growing impact on public health across all nations and socioeconomic strata. Vascular specialists play a dominant role in the treatment of CLTI. Accordingly, in 2013, when several leading vascular societies determined to launch the Global Vascular Guidelines (GVG) initiative, CLTI was considered the first priority disease area of focus. The primary goal of this practice guideline on CLTI is to improve the quality of care for all patients with CLTI as well as for those at risk for CLTI. An important secondary goal is to identify key research priorities in need of further basic, translational, clinical, and health services investigation to advance those aims.
GVG structure
The three major global vascular surgical societies, the European Society for Vascular Surgery (ESVS), the Society for Vascular Surgery (SVS), and the World Federation of Vascular Societies (WFVS), joined efforts to launch the GVG initiative. In this process, the ESVS represents national vascular societies from Europe and the SVS represents national, regional, and local vascular societies in North America. The WFVS represents a large number of non-European, non-North American vascular surgical societies from across the world. These include the Australian and New Zealand Society for Vascular Surgery, the Japanese Society for Vascular Surgery, the Vascular Society of India, the Vascular Society of Southern Africa, the Asian Society for Vascular Surgery, and the Latin American Society of Vascular Surgery and Angiology (this list is not exhaustive). As the primary sponsors, the ESVS, SVS, and WFVS developed the organizational structure, policies on conflict of interest, and committed financial support for the GVG program. All financial support for the GVG was derived directly from the sponsoring societies and without the direct involvement of industry or other external stakeholders. Representatives from the three leading societies were asked to serve as Co-Editors as well as members of the Steering Committee to oversee all aspects of the project and its subsequent communications. Oversight from the societies was limited to budgetary and administrative aspects, including their respective document review policies before public dissemination of the final guideline. The Steering Committee recruited a large and diversified writing group; developed the scope and section briefs for the guideline; identified priority questions for commissioned evidence reviews; and participated in all stages of writing, consensus debate, and editing of the manuscript.
Conflict of interest policy
A primary consideration on inception of the GVG was to create a robust yet practical approach to conflict of interest to enable an unbiased effort at guideline development by experts in the field. A central element to this, in concert with the exclusion of direct commercial funding sources, was full disclosure and specific limits on relevant financial relationships for members of the writing group, Steering Committee, and Co-Editors. A full description of the GVG Conflict of Interest policy is provided at the beginning of this supplement. Financial disclosures for all contributing authors were collected and updated by the Steering Committee. They are detailed in the table of Contributing Authors listed at the beginning of the guideline.
Leadership and writing group
The Co-Editors and Steering Committee were selected by the three major sponsoring societies and were tasked with the recruitment of a multidisciplinary, international writing group of recognized experts. In total, the final writing group comprised 58 individuals from 24 countries across 6 continents. This group represents specialists in vascular surgery, vascular medicine, interventional cardiology and radiology, angiology, epidemiology, podiatry, and orthopedics as well as a methodologist with expertise in guideline development. Authors were assigned to individual sections of the guideline, and all authors reviewed the complete final document before societal review.
Methodology
The Steering Committee drafted a Table of Contents that was divided into distinct sections. Briefs were created to outline the scope and content of each section. Potential authors were then solicited and vetted, and two authors were chosen to co-lead the writing effort for each section. The co-lead authors communicated directly with the Steering Committee on their progress and on iterative cycles of revision as needed. All of the authors of each section reviewed and approved their final versions before compilation of the full document.
The Steering Committee examined the state of recent evidence reviews in the field, including those commissioned by the participating societies, and determined the need for additional evidence reviews and updating. These were commissioned to an external group (Mayo Clinic Evidence-Based Practice Research Program) who performed four systematic reviews that summarized evidence from randomized and nonrandomized studies.4–7 These systematic reviews underwent peer review and were published in the Journal of Vascular Surgery, one of which is published as an accompaniment to the guideline document in this supplement.7
Consensus development during the process occurred through confidential electronic communications, teleconferences, and multiple in-person meetings of the Steering Committee and members of the writing group. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to determine the quality of evidence and strength of recommendations.8 A strong (Grade 1) recommendation implies that the guideline developers are confident as to the balance of benefits and harm and that this recommendation should apply to the majority of patients. A conditional recommendation (Grade 2) implies less certainty and indicates that a different course of action is reasonable. The guideline developers used an imperative verb to denote strong recommendations and used the term “consider” to denote a conditional recommendation. The level of evidence for each recommendation is considered high quality (A), moderate quality (B), or low quality (C). The guideline also includes good practice recommendations. These ungraded good practice recommendations are supported by a wealth of indirect evidence but no direct evidence, and the benefit of pursuing the recommended actions is considered to outweigh any plausible harm. The intention of these good practice recommendations was to draw attention to and remind providers of known and noncontroversial surgical principles or principles about general medical care. For example, there are good practice statements about performing a comprehensive history and physical examination in patients with CLTI.9
The final grading of all guideline recommendations was determined by the guideline developers and the methodologist. After approval by the full writing group, the sections were compiled into one document and reviewed concurrently by the document oversight bodies of each of the three sponsoring societies. An open comment period was subsequently enabled on a secure website (http://vsweb.org/GlobalVascularGuidelines) to provide an opportunity for external stakeholders to review the document. The Co-Editors collated all reviews and made final revisions to the document, which was then approved by the sponsoring societies before publication and dissemination.
Target population
The target population of patients includes adults with CLTI, defined as a patient with objectively documented PAD and any of the following clinical symptoms or signs:
Ischemic rest pain with confirmatory hemodynamic studies
Diabetic foot ulcer (DFU) or any lower limb ulceration present for at least 2 weeks
Gangrene involving any portion of the lower limb or foot
Specifically excluded are patients with pure venous ulcers, pure traumatic wounds, acute limb ischemia (symptoms present for 2 weeks or less), embolic disease, and nonatherosclerotic chronic vascular conditions of the lower extremity (eg, vasculitis, Buerger disease, radiation arteritis).
Target audience
The primary target audience for this guideline includes all clinicians who are directly involved in the management of patients with CLTI, to include surgeons (vascular, general, plastic, and orthopedic), interventionalists (radiologists, cardiologists), podiatrists, wound care providers, rehabilitation medicine specialists, orthotists and physical therapists, and trainees in these disciplines.
Secondary audiences include referring providers, such as primary care physicians, medical specialists, nurses, and other allied health providers, who may care for the at-risk population and who are critical for awareness and timely specialist referral of patients with suspected CLTI. Other key targets for this guideline are third parties with influence over the current and future treatment of CLTI, including government agencies, payers (funders), industry stakeholders, investigators, and research organizations.
CLTI: A new paradigm for treatment and research
This clinical practice guideline (CPG) intentionally seeks to create a new conceptual framework for the treatment of CLTI. It encompasses nomenclature, disease staging, and a platform for evidence-based revascularization (EBR) that will allow future evolution and quality improvement in the field. A brief introduction to the key elements introduced in this document is provided here.
Nomenclature.
Consistent and meaningful nomenclature is of fundamental importance for assessing the state of evidence and guiding future research efforts. To this end, the GVG promotes the use of the term CLTI, defined by the target population, to denote the universe of patients with advanced lower limb ischemia, wounds, neuropathy, and infection who are commonly referred to vascular specialists for evaluation and management. Prior terms, such as “critical” and “severe” limb ischemia, connote specific hemodynamic thresholds and fail to recognize the full spectrum and inter-relatedness of components beyond ischemia that contribute to major limb amputation and long-term disability. This is addressed fully in Section 1 of the guideline.
Disease staging in CLTI.
Improved disease staging is mandatory for designing clinical trials, conducting comparative effectiveness research, identifying critical gaps in knowledge, and developing effective algorithms for treatment. CLTI represents a broad range of clinical severity (limb threat) and anatomic complexity of disease. The GVG incorporates the SVS Lower Extremity Threatened Limb Classification System10 as a preferred staging system for CLTI, which is discussed more fully in Section 1 and other related areas of the document.
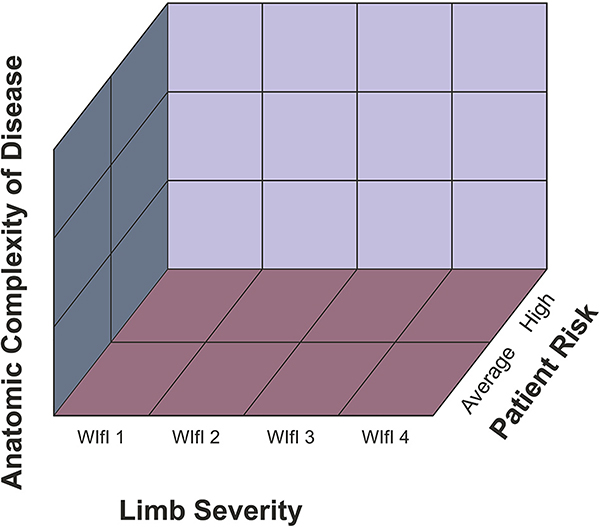
EBR and the PLAN concept.
The GVG espouses a goal of EBR for CLTI to improve the quality of vascular care and to reduce disparities in treatment and outcomes. However, the existing database to support EBR is found to be lacking in many domains. There have been few high-quality randomized controlled trials (RCT) or comparative effectiveness studies in the field. This remains a major unmet need requiring broad support from national health agencies, payers, industry, professional organizations, and research foundations. The writing group sought the best available evidence to generate consensus recommendations while also providing a foundation for future iterations based on a patient- and limb-centric approach to treatment rather than on the prevailing lesion-focused lexicon in the field.
The PLAN concept of EBR (Section 6) stresses a structured management approach based on Patient risk, Limb severity, and ANatomic pattern of disease, in that order of priority. The authors believe that adequate stratification along these three independent axes is clinically relevant and of fundamental importance to improve evidence quality and to achieve EBR for patients with CLTI. Further development of this approach requires prospective validation and refinement of tools to accurately stage patient risk, limb threat, and anatomic patterns of disease, as discussed in detail in the document.
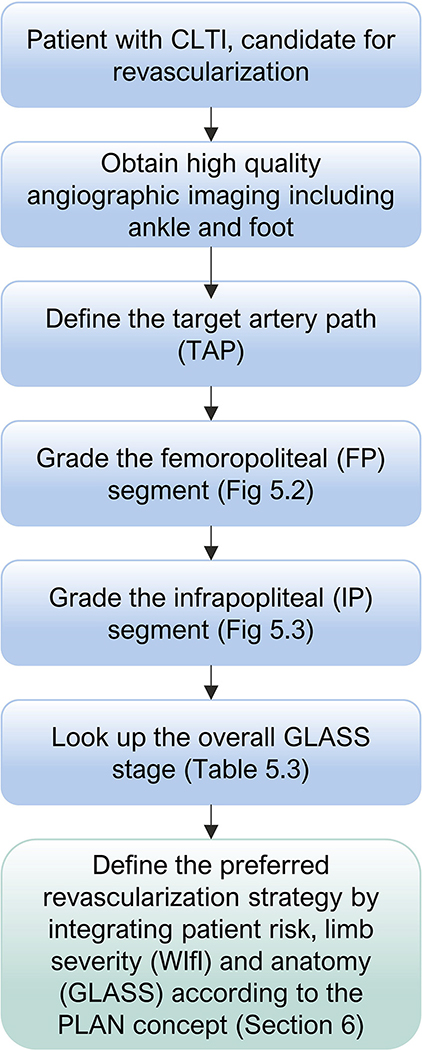
Global Limb Anatomic Staging System (GLASS).
A new anatomic scheme for the threatened limb is proposed. Commonly used anatomic classification schemes for PAD are lesion or segment focused11 or aim to quantify the overall burden of disease,12 rather than integrating the complex patterns of disease found in most patients with CLTI. Successful revascularization in CLTI, particularly in patients with tissue loss, nearly always requires restoration of in-line (pulsatile) flow to the foot. Moreover, there is a general lack of understanding of the relationships between patterns of disease, hemodynamic improvement after treatment, anatomic durability, clinical stage, and outcomes that continues to plague the field. With this in mind, a new approach was developed to facilitate clinical decision-making in CLTI–the GLASS (Section 5). To be most useful, GLASS incorporates a set of baseline assumptions to avoid overcomplexity and to permit its ready utility in everyday clinical practice and in future research.
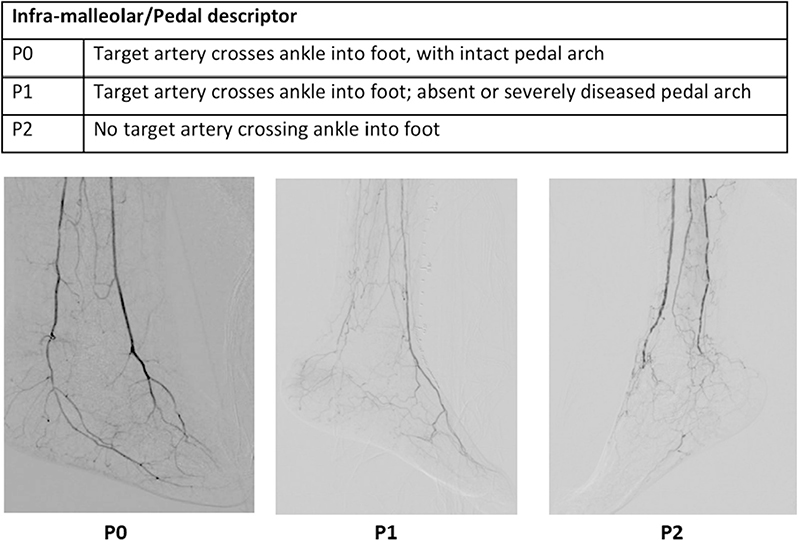
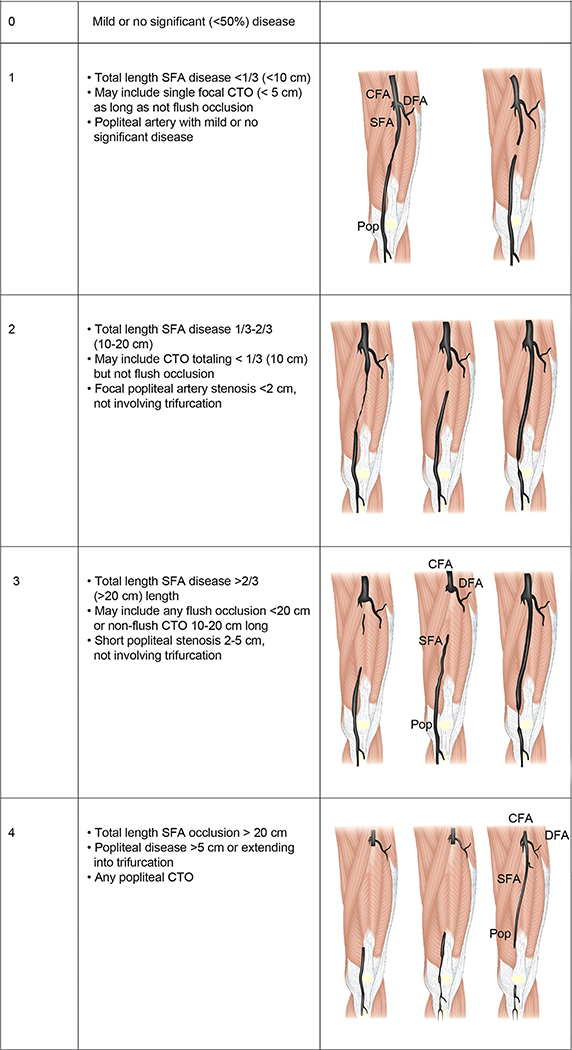
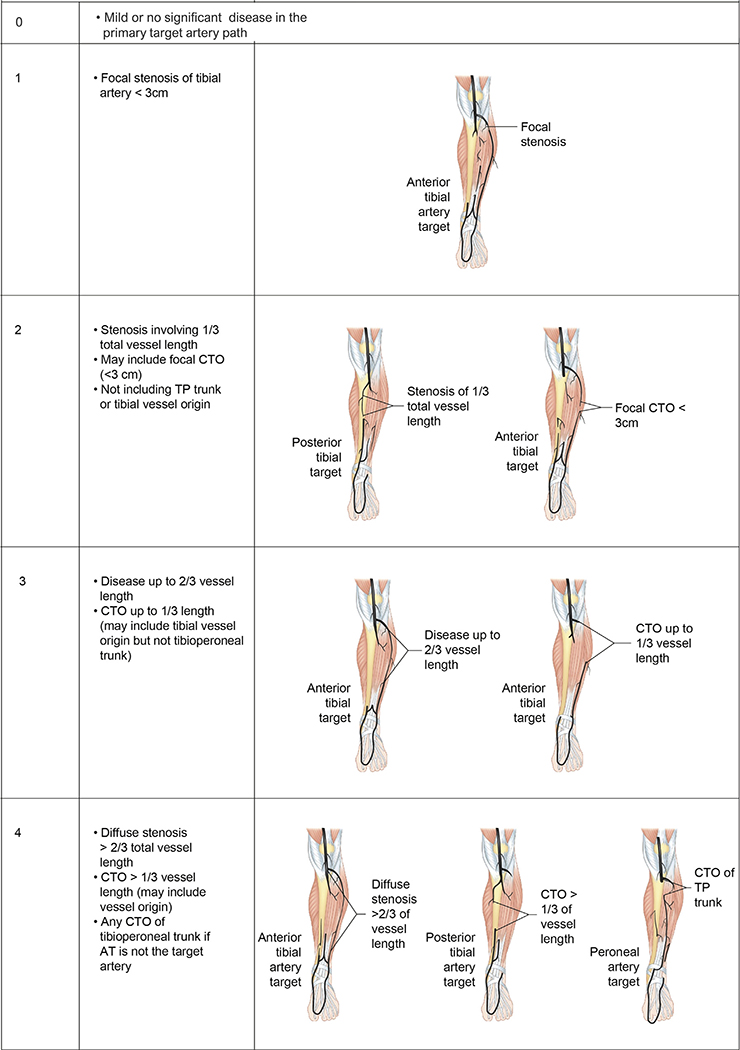
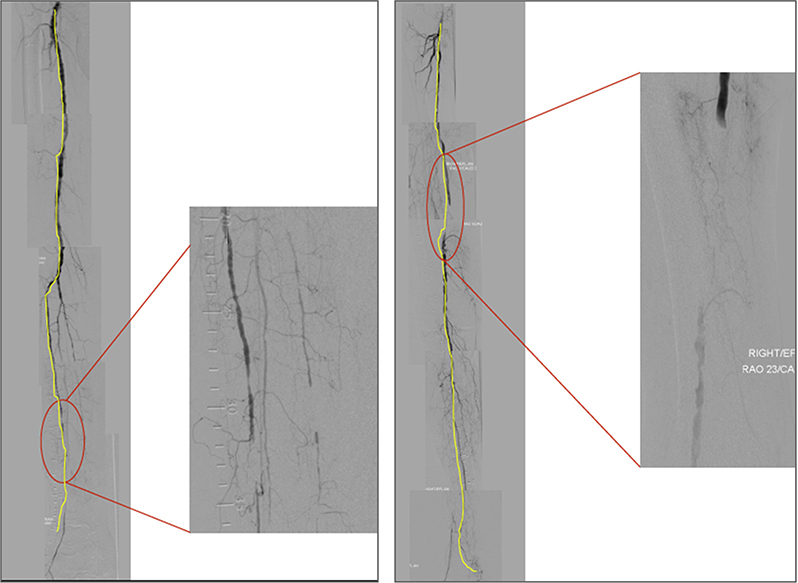
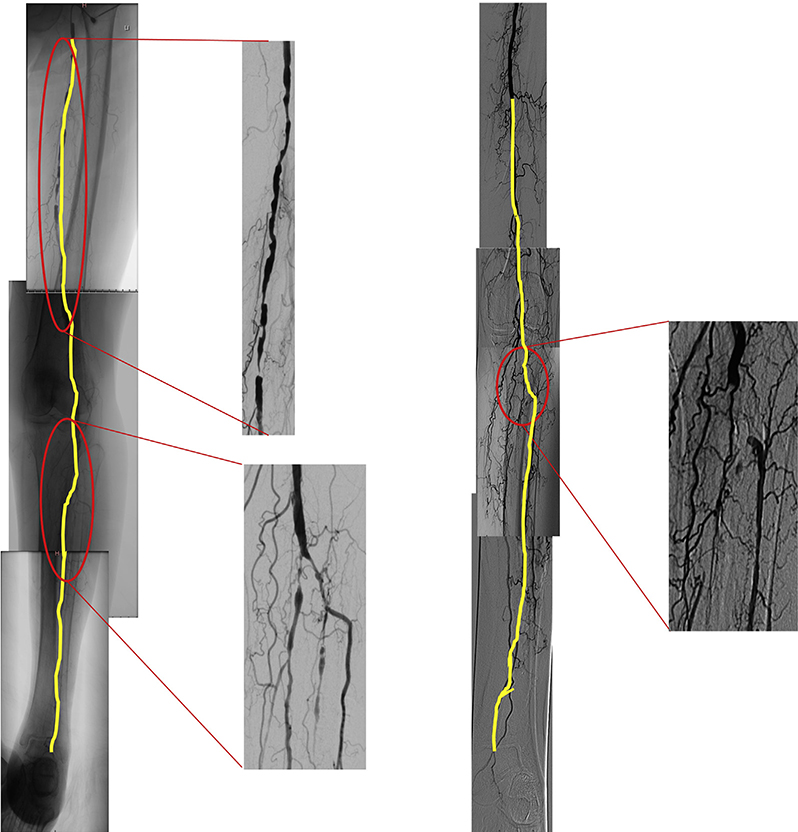
GLASS incorporates two novel and important concepts, the target arterial path (TAP) and estimated limb-based patency (LBP). Based on appropriate angiographic imaging, the TAP is defined by the treating surgeon or interventionalist as the optimal arterial pathway to restore in-line (pulsatile) flow to the ankle and foot. It may incorporate either the least diseased or an angiosome-preferred path, as chosen by the treating clinician. LBP is defined as maintenance of in-line flow throughout the TAP, from groin to ankle. LBP allows more direct comparison of anatomic outcomes across revascularization strategies in CLTI. The complexity of disease traversed by the TAP is integrated in the GLASS. Femoropopliteal (FP) and infrapopliteal (IP) arterial segments are individually graded on a scale of 0 to 4. Using a consensus-based matrix, these segmental grades are combined into three overall GLASS (I-III) stages for the limb.
GLASS includes a simplified approach to inflow (aortoiliac [AI]) disease, a dichotomous stratification for severe calcification within segment, and a simple modifier for pedal (inframalleolar [IM]) disease. GLASS stages (I-III) were defined on the basis of expected technical success and anatomic durability for infrainguinal endovascular intervention and reflect the overall complexity of disease within the TAP. The consensus process for developing and assigning GLASS stages was informed by an updated systematic review of revascularization outcomes in CLTI.7 Thus, GLASS stages I to III correlate with low-, intermediate-, or high-complexity infrainguinal disease patterns, with expected correlation to immediate technical success and 1-year LBP for endovascular intervention. The relevance of these GLASS anatomic stages in different clinical scenarios is integrated within the PLAN framework for decision-making. GLASS is designed for subsequent refinement, reclassification, and validation based on data from prospective studies that employ the scheme and report appropriate outcome measures. A mobile app to quickly derive GLASS stage from angiographic imaging in real time will be released in proximity to the guideline publication.
End points and trial designs.
Existing limitations of the evidence base in CLTI were obvious and broadly acknowledged during the GVG development process. The importance of developing consensus around key outcome measures, with a focus on patient-oriented end points, is critical to advancing the field. It is anticipated that currently enrolling RCTs, including Bypass vs Angioplasty in Severe Ischaemia of the Leg (BASIL-2) trial, Balloon vs Stenting in Severe Ischaemia of the Leg (BASIL-3) trial, and Best Endovascular vs Best Surgical Therapy for Patients with Critical Limb Ischemia (BEST-CLI), will allow important advances in the management of CLTI, with significant overlap among these efforts.13–15 In Section 11 of the guideline, a full consideration of this important topic is provided as a framework, with specific recommendations for study and RCT designs going forward.
Interdisciplinary team in CLTI.
There has been growing recognition of the value of multidisciplinary and interdisciplinary team-based care to optimize the outcomes for patients with CLTI. The components of such teams vary considerably across centers and regions of practice, but certain critical skill sets, expertise, facilities, and resources are required to create a Center of Excellence for CLTI management. Consideration of this important topic is addressed in Section 12 of the guideline.
Dissemination, translation to practice, and future revisions of the guideline
Translation of expert guidelines into clinical practice is known to be a major obstacle to evidence-based medicine. Reasons are multifactorial and include limited provider and patient engagement, lack of consensus, economic conflicts, and resource constraints. The international scope of the GVG mandated an attempt to survey differences in practice patterns, resources, and potential hurdles to implementation around the globe (Section 13). Dissemination of the guideline by the sponsoring societies is planned to include an array of print media, web and social media, mobile apps, and communications at multiple national and regional meetings to facilaitate discussion. The incorporation of suggested staging systems and end points into national and multinational registries will greatly facilitate use and future refinement of this effort. It is anticipated that the GVG will be translated into the other major world languages.
To remain current and evidence based, practice guidelines must be periodically reviewed and updated. Ongoing RCTs and prospective cohort studies will provide critical new evidence in the management of CLTI during the next several years. The sponsoring societies of the GVG recognize the importance of stewardship of this practice guideline, both as new key evidence arises and as a planned interval exercise.
Supporting materials
Evidence-based recommendations made in this guideline are supported by key references listed in the text. A summary of the relevant findings from the studies used to support each recommendation is provided as a Supplementary Table (online only) to the guideline.
A scientific manuscript summarizing a commissioned evidence review on the outcomes of revascularization in CLTI is also published within the guidelines supplement.7 This manuscript underwent independent peer review by the Journal of Vascular Surgery. The Supplementary Tables of that document summarizing the individual source studies and the various outcomes analyzed by time interval are also available online (https://www.jvascsurg.org/article/S0741–5214(18)30854–1/fulltext).
SUMMARY OF RECOMMENDATIONS
| Recommendation | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 1. Definitions and nomenclature | ||||
| 1.1 | Use objective hemodynamic tests to determine the presence and to quantify the severity of ischemia in all patients with suspected CLTI. | 1 (Strong) | C (Low) | de Graaff,16 2003 Brownrigg,17 2016 Wang,18 2016 |
| 1.2 | Use a lower extremity threatened limb classification staging system (eg, SVS’s WIfI classification system) that grades wound extent, degree of ischemia, and severity of infection to guide clinical management in all patients with suspected CLTI. | 1 (Strong) | C (Low) | See Table 1.2 in full guideline. |
| 2. Global epidemiology and risk factors for CLTI | ||||
| No recommendations | ||||
| 3. Diagnosis and limb staging in CLTI | ||||
| 3.1 | Perform a detailed history to determine symptoms, past medical history, and cardiovascular risk factors in all patients with suspected CLTI. | Good practice statement | ||
| 3.2 | Perform a complete cardiovascular physical examination of all patients with suspected CLTI. | Good practice statement | ||
| 3.3 | Perform a complete examination of the foot, including an assessment of neuropathy and a probe-to-bone test of any open ulcers, in all patients with pedal tissue loss and suspected CLTI. | Good practice statement | ||
| 3.4 | Measure AP and ABI as the first-line noninvasive test in all patients with suspected CLTI. | 1 (Strong) | B (Moderate) | Lijmer,19 1996 Dachun,20 2010 |
| 3.5 | Measure TP and TBI in all patients with suspected CLTI and tissue loss (Fig 3.1 in full guideline). | 1 (Strong) | B (Moderate) | Aboyans,21 2008 Salaun,22 2018 |
| 3.6 | Consider using alternative methods for noninvasive assessment of perfusion, such as PVR, transcutaneous oximetry, or skin perfusion pressure, when ankle and toe pressures, indices, and waveforms cannot be assessed. | 2 (Weak) | C (Low) | Aboyans,21 2008 Shirasu,23 2016 Saluan,22 2018 |
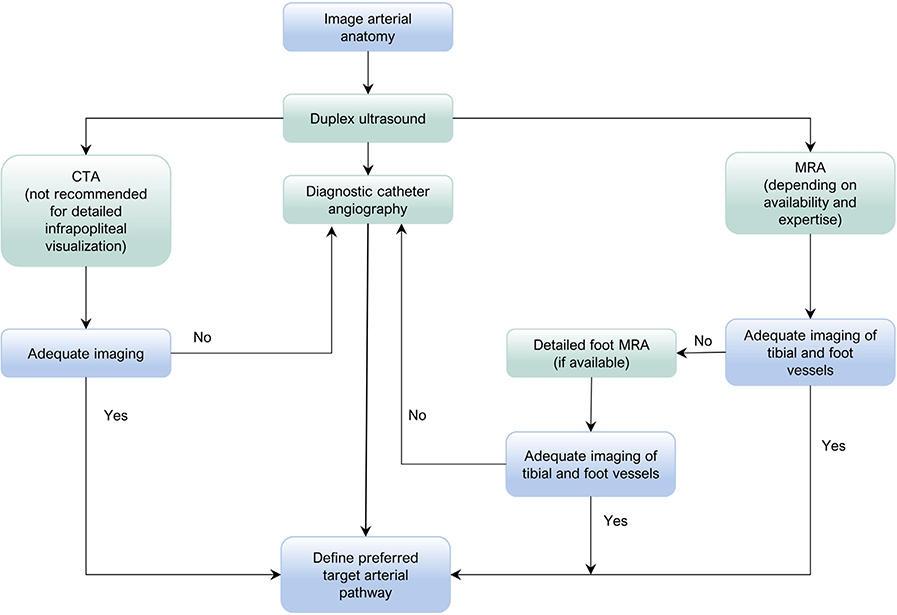
| 3.7 | Consider DUS imaging as the first arterial imaging modality in patients with suspected CLTI. | 2 (Weak) | B (Moderate) | Hingorani,24 2008 |
| 3.8 | Consider noninvasive vascular imaging modalities (DUS, CTA, MRA) when available before invasive catheter angiography in patients with suspected CLTI who are candidates for revascularization. | 2 (Weak) | B (Moderate) | Larch,25 1997 Adriaensen,26 2004 Hingorani,27 2004 Collins,28 2007 Hingorani,24 2008 Met,29 2009 |
| 3.9 | Obtain high-quality angiographic imaging of the lower limb (with modalities and techniques to be determined by local availabilty of facilities and expertise). This should include the ankle and foot in all patients with suspected CLTI who are considered potential candidates for revascularization. | Good practice statement | ||
| 4. Medical management | ||||
| 4.1 | Evaluate cardiovascular risk factors in all patients with suspected CLTI. | 1 (Strong) | B (Moderate) | I.C.A.I. Group,30 1997 |
| 4.2 | Manage all modifiable risk factors to recommended levels in all patients with suspected CLTI. | 1 (Strong) | B (Moderate) | Armstrong,31 2014 Faglia,32 2014 |
| 4.3 | Treat all patients with CLTI with an antiplatelet agent. | 1 (Strong) | A (High) | Antithrombotic Trialists’ Collaboration,33 2002 Antithrombotic Trialists’ Collaboration,34 2009 |
| 4.4 | Consider clopidogrel as the single antiplatelet agent of choice in patients with CLTI. | 2 (Weak) | B (Moderate) | CAPRIE,35 1996 Hiatt,36 2017 |
| 4.5 | Consider low-dose aspirin and rivaroxaban, 2.5 mg twice daily, to reduce adverse cardiovascular events and lower extremity ischemic events in patients with CLTI. | 2 (Weak) | B (Moderate) | Anand,37 2018 |
| 4.6 | Do not use systemic vitamin K antagonists for the treatment of lower extremity atherosclerosis in patients with CLTI. | 1 (Strong) | B (Moderate) | Anand,38 2007 |
| 4.7 | Use moderate- or high-intensity statin therapy to reduce all-cause and cardiovascular mortality in patients with CLTI. | 1 (Strong) | A (High) | Leng,39 2000 Heart Protection Study Collaborative Group,40 2002 Meade,41 2002 Aung,42 2007 Mills,43 2011 Rodriguez,44 2017 |
| 4.8 | Control hypertension to target levels of <140 mm Hg systolic and <90 mm Hg diastolic in patients with CLTI. | 1 (Strong) | B (Moderate) | ACCORD Study Group,45 2010 Bavry,46 2010 Wright,47 2015 (SPRINT) Moise,48 2016 |
| 4.9 | Consider control of type 2 DM in CLTI patients to achieve a hemoglobin A1c of <7% (53 mmol/mol [International Federation of Clinical Chemistry]). | 2 (Weak) | B (Moderate) | Selvin,49 2004 Nathan,50 2005 van Dieren,51 2014 Fox,52 2015 American Diabetes Association,53 2018 |
| 4.10 | Use metformin as the primary hypoglycemic agent in patients with type 2 DM and CLTI. | 1 (Strong) | A (High) | Palmer,54 2016 |
| 4.11 | Consider withholding metformin immediately before and for 24 to 48 hours after the administration of an iodinated contrast agent for diabetic patients, especially those with an estimated glomerular filtration rate <30 mL/min/1.73 m2. | 2 (Weak) | C (Low) | Nawaz,55 1998 Goergen,56 2010 Stacul,57 2011 |
| 4.12 | Offer smoking cessation interventions (pharmacotherapy, counseling, or behavior modification therapy) to all patients with CLTI who smoke or use tobacco products. | 1 (Strong) | A (High) | Dagenais,58 2005 Athyros,59 2013 Blomster,60 2016 |
| 4.13 | Ask all CLTI patients who are smokers or former smokers about status of tobacco use at every visit. | 1 (Strong) | A (High) | Kondo,61 2011 Newhall,62 2017 |
| 4.14 | Prescribe analgesics of appropriate strength for CLTI patients who have ischemic rest pain of the lower extremity and foot until pain resolves after revascularization. | Good practice statement | ||
| 4.15 | In CLTI patients with chronic severe pain, use paracetamol (acetaminophen) in combination with opioids for pain control. | Good practice statement | ||
| 5. The Global Limb Anatomic Staging System (GLASS) for CLTI | ||||
| 5.1 | Use an integrated, limb-based anatomic staging system (such as the GLASS) to define complexity of a preferred target artery path (TAP) and to facilitate evidence-based revascularization (EBR) in patients with CLTI. | Good practice statement | ||
| 6. Strategies for EBR | ||||
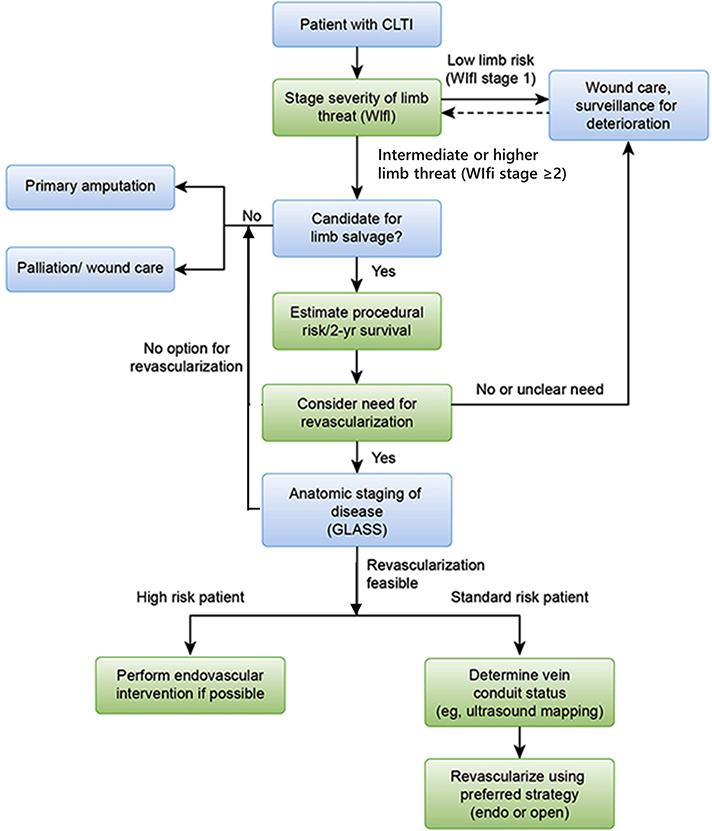
| 6.1 | Refer all patients with suspected CLTI to a vascular specialist for consideration of limb salvage, unless major amputation is considered medically urgent. | Good practice statement | ||
| 6.2 | Offer primary amputation or palliation to patients with limited life expectancy, poor functional status (eg, nonambulatory), or an unsalvageable limb after shared decision-making. | Good practice statement | ||
| 6.3 | Estimate periprocedural risk and life expectancy in patients with CLTI who are candidates for revascularization. | 1 (Strong) | C (Low) | Biancari,63 2007 Schanzer,64 2008 Bradbury,65 2010 Meltzer,66 2013 Simons,67 2016 |
| 6.4 | Define a CLTI patient as average surgical risk when anticipated periprocedural mortality is <5% and estimated 2-year survival is >50%. | 2 (Weak) | C (Low) | |
| 6.5 | Define a CLTI patient as high surgical risk when anticipated periprocedural mortality is ≥5% or estimated 2-year survival is ≤50%. | 2 (Weak) | C (Low) | |
| 6.6 | Use an integrated threatened limb classification system (such as WIfI) to stage all CLTI patients who are candidates for limb salvage. | 1 (Strong) | C (Low) | Cull,68 2014 Zhan,69 2015 Causey,70 2016 Darling,71 2016 Robinson,72 2017 |
| 6.7 | Perform urgent surgical drainage and debridement (including minor amputation if needed) and commence antibiotic treatment in all patients with suspected CLTI who present with deep space foot infection or wet gangrene. | Good practice statement | ||
| 6.8 | Repeat limb staging after surgical drainage, debridement, minor amputations, or correction of inflow disease (AI, common and deep femoral artery disease) and before the next major treatment decision. | Good practice statement | ||
| 6.9 | Do not perform revascularization in the absence of significant ischemia (WIfI ischemia grade 0) unless an isolated region of poor perfusion in conjunction with major tissue loss (eg, WIfI wound grade 2 or3) can be effectively targeted and the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading. | Good practice statement | ||
| 6.10 | Do not perform revascularization in very-low-risk limbs (eg, WIfI stage 1) unless the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading. | 2 (Weak) | C (Low) | Sheehan,73 2003 Cardinal,74 2008 Lavery,75 2008 Snyder,76 2010 |
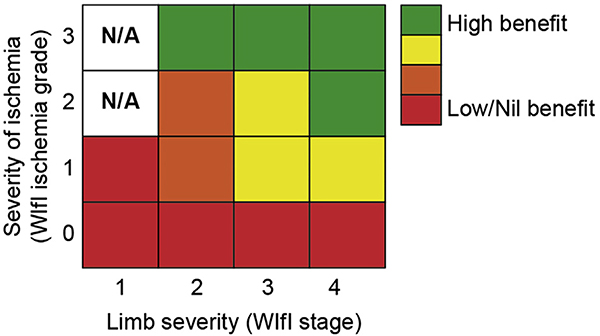
| 6.11 | Offer revascularization to all average-risk patients with advanced limb-threatening conditions (eg, WIfI stage 4) and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). | 1 (Strong) | C (Low) | Abu Dabrh,5 2015 |
| 6.12 | Consider revascularization for average-risk patients with intermediate limb threat (eg, WIfI stages 2 and 3) and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). | 2 (Weak) | C (Low) | Zhan,69 2015 Causey,70 2016 Darling,71 2016 Robinson,72 2017 |
| 6.13 | Consider revascularization in average-risk patients with advanced limb threat (eg, WIfI stage 4) and moderate ischemia (eg, WIfI ischemia grade 1). | 2 (Weak) | C (Low) | |
| 6.14 | Consider revascularization in average-risk patients with intermediate limb threat (eg, WIfI stages 2 and 3) and moderate ischemia (eg, WIfI ischemia grade 1) if the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading. | 2 (Weak) | C (Low) | |
| 6.15 | Obtain high-quality angiographic imaging with dedicated views of ankle and foot arteries to permit anatomic staging and procedural planning in all CLTI patients who are candidates for revascularization. | Good practice statement | ||
| 6.16 | Use an integrated limb-based staging system (eg, GLASS) to define the anatomic pattern of disease and preferred TAP in all CLTI patients who are candidates for revascularization. | Good practice statement | ||
| 6.17 | Perform ultrasound vein mapping when available in all CLTI patients who are candidates for surgical bypass. | 1 (Strong) | C (Low) | Seeger,77 1987 Wengerter,78 1990 Schanzer,79 2007 |
| 6.18 | Map the ipsilateral GSV and small saphenous vein for planning of surgical bypass. Map veins in the contralateral leg and both arms if ipsilateral vein is insufficient or inadequate. |
Good practice statement | ||
| 6.19 | Do not classify a CLTI patient as being unsuitable for revascularization without review of adequate-quality imaging studies and clinical evaluation by a qualified vascular specialist. | Good practice statement | ||
| 6.20 | Correct inflow disease first when both inflow and outflow disease are present in a patient with CLTI. | Good practice statement | ||
| 6.21 | Base the decision for staged vs combined inflow and outflow revascularization on patient risk and the severity of limb threat (eg, WIfI stage). | 1 (Strong) | C (Low) | Harward,80 1995 Zukauskas,81 1995 |
| 6.22 | Correct inflow disease alone in CLTI patients with multilevel disease and low-grade ischemia (eg, WIfI ischemia grade 1) or limited tissue loss (eg, WIfI wound grade 0/1) and in any circumstance in which the risk-benefit of additional outflow reconstruction is high or initially unclear. | 1 (Strong) | C (Low) | |
| 6.23 | Restage the limb and repeat the hemodynamic assessment after performing inflow correction in CLTI patients with inflow and outflow disease. | 1 (Strong) | C (Low) | |
| 6.24 | Consider simultaneous inflow and outflow revascularization in CLTI patients with a high limb risk (eg, WIfI stages 3 and 4), or in patients with severe ischemia (eg, WIfI ischemia grades 2 and 3). | 2 (Weak) | C (Low) | |
| 6.25 | Use an endovascular-first approach for treatment of CLTI patients with moderate to severe (eg, GLASS stage IA) aorto-iliac (AI) disease, depending on the history of prior intervention. | 1 (Strong) | B (Moderate) | Jongkind,82 2010 Ye,83 2011 Deloose,84 2017 |
| 6.26 | Consider surgical reconstruction for the treatment of average-risk CLTI patients with extensive (eg, GLASS stage II) AI disease or after failed endovascular intervention. | 2 (Weak) | C (Low) | Ricco,85 2008 Chiu,86 2010 Indes,87 2013 |
| 6.27 | Perform open CFA endarterectomy with patch angioplasty, with or without extension into the PFA, in CLTI patients with hemodynamically significant (>50% stenosis) disease of the common and deep femoral arteries. | 1 (Strong) | C (Low) | Kang,88 2008 Ballotta,89 2010 |
| 6.28 | Consider a hybrid procedure combining open CFA endarterectomy and endovascular treatment of AI disease with concomitant CFA involvement (GLASS stage IB). | 2 (Weak) | C (Low) | Chang,90 2008 |
| 6.29 | Consider endovascular treatment of significant CFA disease in selected patients who are deemed to be at high surgical risk or to have a hostile groin. | 2 (Weak) | C (Low) | Baumann,91 2011 Bonvini,92 2011 Gouëffic,93 2017 Siracuse,94 2017 |
| 6.30 | Avoid stents in the CFA and do not place stents across the origin of a patent deep femoral artery. | Good practice statement | ||
| 6.31 | Correct hemodynamically significant (≥50% stenosis) disease of the proximal deep femoral artery whenever technically feasible. | Good practice statement | ||
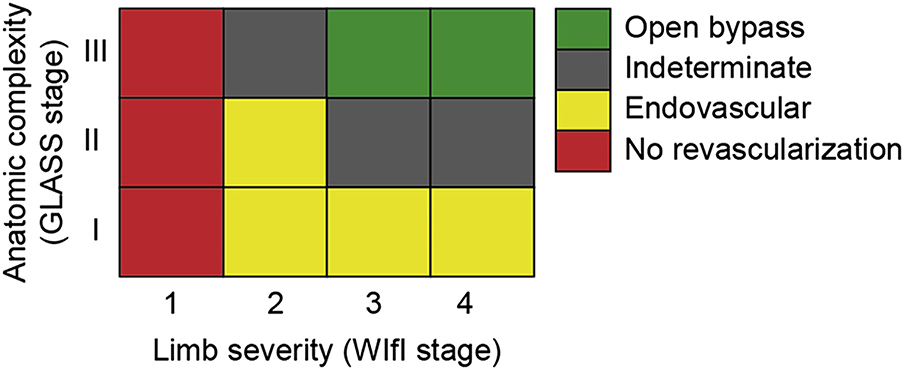
| 6.32 | In average-risk CLTI patients with infrainguinal disease, base decisions of endovascular intervention vs open surgical bypass on the severity of limb threat (eg, WIfI), the anatomic pattern of disease (eg, GLASS), and the availability of autologous vein. | 1 (Strong) | C (Low) | Almasri,7 2018 |
| 6.33 | Offer endovascular revascularization when technically feasible for high-risk patients with advanced limb threat (eg, WIfI stage 4) and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). | 2 (Weak) | C (Low) | Abu Dabrh,5 2015 Zhan,69 2015 Causey,70 2016 Darling,71 2016 Robinson,72 2017 |
| 6.34 | Consider endovascular revascularization for high-risk patients with intermediate limb threat (eg, WIfI stages 2 and 3) and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). | 2 (Weak) | C (Low) | |
| 6.35 | Consider endovascular revascularization for high-risk patients with advanced limb threat (eg, WIfI stage 4) and moderate ischemia (eg, WIfI ischemia grade 1) if the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading, when technically feasible. | 2 (Weak) | C (Low) | |
| 6.36 | Consider endovascular revascularization for high-risk patients with intermediate limb threat (eg, WIfI stages 2 and 3) and moderate ischemia (eg, WIfI ischemia grade 1) if the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading, when technically feasible. | 2 (Weak) | C (Low) | |
| 6.37 | Consider open surgery in selected high-risk patients with advanced limb threat (eg, WIfI stage 3 or 4), significant perfusion deficits (ischemia grade 2 or 3), and advanced complexity of disease (eg, GLASS stage III) or after prior failed endovascular attempts and unresolved symptoms of CLTI. | 2 (Weak) | C (Low) | |
| 6.38 | Consider angiosome-guided revascularization in patients with significant wounds (eg, WIfI wound grades 3 and 4), particularly those involving the midfoot or hindfoot, and when the appropriate TAP is available. | 2 (Weak) | C (Low) | Azuma,95 2012 Sumpio,96 2013 Biancari,97 2014 Chae,98 2016 Jongsma,99 2017 |
| 6.39 | In treating femoro-popliteal (FP) disease in CLTI patients by endovascular means, consider adjuncts to balloon angioplasty (eg, stents, covered stents, or drug-eluting technologies) when there is a technically inadequate result (residual stenosis or flow-limiting dissection) or in the setting of advanced lesion complexity (eg, GLASS FP grade 2–4). | 2 (Weak) | B (Moderate) | Schillinger,100 2006 Saxon,101 2008 Dake,102 2011 Rosenfield,103 2015 Almasri,7 2018 |
| 6.40 | Use autologous vein as the preferred conduit for infrainguinal bypass surgery in CLTI. | 1 (Strong) | B (Moderate) | Almasri,7 2018 |
| 6.41 | Avoid using a nonautologous conduit for infrainguinal bypass unless there is no endovascular option and no adequate autologous vein. | 2 (Weak) | C (Low) | Almasri,7 2018 |
| 6.42 | Perform intraoperative imaging (angiography, DUS, or both) on completion of open bypass surgery for CLTI and correct significant technical defects if feasible during the index operation. | 1 (Strong) | C (Low) | Mills,104 1992 Bandyk,105 1994 |
| 7. Nonrevascularization treatments of the limb | ||||
| 7.1 | Consider spinal cord stimulation to reduce the risk of amputation and to decrease pain in carefully selected patients (eg, rest pain, minor tissue loss) in whom revascularization is not possible. | 2 (Weak) | B (Moderate) | Ubbink,106 2013 |
| 7.2 | Do not use lumbar sympathectomy for limb salvage in CLTI patients in whom revascularization is not possible. | 2 (Weak) | C (Low) | Karanth,107 2016 |
| 7.3 | Consider intermittent pneumatic compression therapy in carefully selected patients (eg, rest pain, minor tissue loss) in whom revascularization is not possible. | 2 (Weak) | B (Moderate) | Abu Dabrh,4 2015 |
| 7.4 | Do not offer prostanoids for limb salvage in CLTI patients. Consider offering selectively for patients with rest pain or minor tissue loss and in whom revascularization is not possible. | 2 (Weak) | B (Moderate) | Vietto,108 2018 |
| 7.5 | Do not offer vasoactive drugs or defibrinating agents (ancrod) in patients in whom revascularization is not possible. | 1 (Strong) | C (Low) | Smith,109 2012 |
| 7.6 | Do not offer HBOT to improve limb salvage in CLTI patients with severe, uncorrected ischemia (eg, WIfI ischemia grade 2/3). | 1 (Strong) | B (Moderate) | Kranke,110 2015 Game,111 2016 Santema,112 2018 |
| 7.7 | Continue to provide optimal wound care until the lower extremity wound is completely healed or the patient undergoes amputation. | Good practice statement | ||
| 8. Biologic and regenerative medicine approaches in CLTI | ||||
| 8.1 | Restrict use of therapeutic angiogenesis to CLTI patients who are enrolled in a registered clinical trial. | 1 (Strong) | B (Moderate) | Abu Dabrh,4 2015 Peeters,113 2015 |
| 9. The role of minor and major amputations | ||||
| 9.1 | Consider transmetatarsal amputation of the forefoot in CLTI patients who would require more than two digital ray amputations to resolve distal necrosis, especially when the hallux is involved. | 2 (Weak) | C (Low) | Elsherif,114 2018 |
| 9.2 | Offer primary amputation to CLTI patients who have a pre-existing dysfunctional or unsalvageable limb, a poor functional status (eg, bedridden), or a short life expectancy after shared decision-making with the patient and health care team. | 1 (Strong) | C (Low) | Aziz,115 2015 Siracuse,116 2015 |
| 9.3 | Consider secondary amputation for patients with CLTI who have a failed or ineffective reconstruction and in whom no further revascularization is possible and who have incapacitating pain, nonhealing wounds, or uncontrolled sepsis in the affected limb after shared decision-making with the patient and health care team. | 2 (Weak) | C (Low) | Reed,117 2008 |
| 9.4 | Consider revascularization to improve the possibility of healing an amputation at a more distal functional amputation level (eg, AKA to BKA), particularly for patients with a high likelihood of rehabilitation and continued ambulation. | 2 (Weak) | C (Low) | Rollins,118 1985 Miksic,119 1986 |
| 9.5 | Consider a TKA or AKA in patients who are nonambulatory for reasons other than CLTI (ie, bedridden patients with flexion contracture, dense hemiplegia, cancer) and are unlikely to undergo successful rehabilitation to ambulation. | 2 (Weak) | C (Low) | Ayoub,120 1993 Taylor,121 2008 |
| 9.6 | Involve a multidisciplinary rehabilitation team from the time a decision to amputate has been made until successful completion of rehabilitation has been achieved. | 1 (Strong) | C (Low) | Webster,122 2012 |
| 9.7 | Continue to observe CLTI patients who have undergone amputation at least yearly to monitor progression of disease in the contralateral limb and to maintain optimal medical therapy and risk factor management. | 1 (Strong) | C (Low) | Bradley,123 2006 Glaser,124 2013 |
| 10. Postprocedural care and surveillance after infrainguinal revascularization for CLTI | ||||
| 10.1 | Continue best medical therapy for PAD, including the long-term use of antiplatelet and statin therapies, in all patients who have undergone lower extremity revascularization. | 1 (Strong) | A (High) | Abbruzzese,125 2004 Henke,126 2004 Brown,127 2008 Bedenis,128 2015 Suckow,129 2015 |
| 10.2 | Promote smoking cessation in all CLTI patients who have undergone lower extremity revascularization. | 1 (Strong) | A (High) | Hobbs,130 2003 Willigendael,131 2005 |
| 10.3 | Consider DAPT (aspirin plus clopidogrel) in patients who have undergone infrainguinal prosthetic bypass for CLTI for a period of 6 to 24 months to maintain graft patency. | 2 (Weak) | B (Moderate) | Brown,127 2008 Belch,132 2010 Gassman,133 2014 Bedenis,128 2015 |
| 10.4 | Consider DAPT (aspirin plus clopidogrel) in patients who have undergone infrainguinal endovascular interventions for CLTI for a period of at least 1 month. | 2 (Weak) | C (Low) | Cassar,134 2005 Bhatt,135 2006 Tepe,136 2012 Strobl,137 2013 |
| 10.5 | Consider DAPT for a period of 1 to 6 months in patients undergoing repeated catheter-based interventions if they are at low risk for bleeding. | 2 (Weak) | C (Low) | Cassar,134 2005 Tepe,136 2012 Strobl,137 2013 |
| 10.6 | Observe patients who have undergone lower extremity vein bypass for CLTI on a regular basis for at least 2 years with a clinical surveillance program consisting of interval history, pulse examination, and measurement of resting APs and TPs. Consider DUS scanning where available. | Good practice statement | ||
| 10.7 | Observe patients who have undergone lower extremity prosthetic bypass for CLTI on a regular basis for at least 2 years with interval history, pulse examination, and measurement of resting APs and TPs. | Good practice statement | ||
| 10.8 | Observe patients who have undergone infrainguinal endovascular interventions for CLTI in a surveillance program that includes clinical visits, pulse examination, and noninvasive testing (resting APs and TPs). | Good practice statement | ||
| 10.9 | Consider performing additional imaging in patients with lower extremity vein grafts who have a decrease in ABI ≥0.15 and recurrence of symptoms or change in pulse status to detect vein graft stenosis. | Good practice statement | ||
| 10.10 | Offer intervention for DUS-detected vein graft lesions with an associated PSV of >300 cm/s and a PSV ratio >3.5 or grafts with low velocity (midgraft PSV <45 cm/s) to maintain patency. | 1 (Strong) | B (Moderate) | Mills,138 2001 |
| 10.11 | Maintain long-term surveillance after surgical or catheter-based revision of a vein graft, including DUS graft scanning where available, to detect recurrent graft-threatening lesions. | 1 (Strong) | B (Moderate) | Landry,139 2002 Nguyen,140 2004 |
| 10.12 | Consider arterial imaging after endovascular intervention for failure to improve (wound healing, rest pain) or a recurrence of symptoms to detect restenosis or progression of pre-existing disease. | 2 (Weak) | C (Low) | Bui,141 2012 |
| 10.13 | Consider reintervention for patients with DUS-detected restenosis lesions >70% (PSV ratio >3.5, PSV >300 cm/s) if symptoms of CLTI are unresolved or on a selective basis in asymptomatic patients after catheter-based interventions. | 2 (Weak) | C (Low) | Humphries,142 2011 |
| 10.14 | Provide mechanical offloading as a primary component for care of all CLTI patients with pedal wounds. | 1 (Strong) | A (High) | Elraiyah,143 2016 |
| 10.15 | Provide counseling on continued protection of the healed wound and foot to include appropriate shoes, insoles, and monitoring of inflammation. | 1 (Strong) | A (High) | Elraiyah,143 2016 |
| 11. Study designs and trial end points in CLTI | ||||
| 11.1 | Use a research framework such as the IDEAL for gathering new data and evidence on the surgical and endovascular management of CLTI. | |||
| 11.2 | Encourage funders, journal reviewers, and editors to prioritize prospective, multicenter, controlled, and preferably randomized studies over retrospective case series, studies using historical controls, or other less rigorous research methodologies. | |||
| 11.3 | When RCTs are not feasible, use the OPG benchmarks from the SVS’s Critical Limb Ischemia Working Group to evaluate the efficacy of novel endovascular CLTI techniques and devices. | |||
| 11.4 | To facilitate sufficient enrollment, limit RCT exclusion criteria to those who are deemed essential to trial integrity. | |||
| 11.5 | Design RCTs, prospective cohort studies, and registries that are specific to CLTI. | |||
| 11.6 | Use an integrated, limb-based threatened limb classification system (eg, WIfI) and a whole limb anatomic classification scheme (eg, GLASS) to describe the characteristics and outcomes of CLTI patients who are enrolled. | |||
| 11.7 | Describe outcomes in CLTI trials using a combination of objective and clinically relevant events, subjective PROMs and HRQL assessments, and anatomic and hemodynamic end points. | |||
| 11.8 | Require regulatory trials aimed at obtaining premarket approval for devices for use in CLTI to study CLTI patients and to present data on objective and clinically relevant end points, PROMs and HRQL assessments, and anatomic and hemodynamic end points. | |||
| 11.9 | Follow up patients in trials for a time sufficient (this will usually be >2 years) to allow appropriate comparison of the impact of the different interventions on the natural history of CLTI. Measure and declare completeness of follow-up coverage to quantify risk of attrition bias. | |||
| 11.10 | Include a time-integrated measure of clinical disease severity (such as freedom from CLTI) in the CLTI trial design to describe the total impact of comparator CLTI interventions. | |||
| 11.11 | Publish all CLTI trial protocols together with the full statistical analysis plans in peer-reviewed journals to allow independent, public, and transparent scrutiny and to prevent nonreporting of negative trials. | |||
| 11.12 | Conduct postmarketing surveillance data collection using well-designed, large observational studies and registries. | |||
| 11.13 | Share clinical trial data to allow subsequent individual patient data analyses, meta-analyses, and subgroup analyses; updating of OPGs; and validation of decision-making tools, such as the WIfI system and GLASS. | |||
| 11.14 | Assess the quality of evidence in CLTI research using frameworks such as GRADE that consider multiple certainty domains and are not based solely on study design. | |||
| 12. Creating a Center of Excellence for amputation prevention | ||||
| No recommendations | ||||
ABI, Ankle-brachial index; AI, aortoiliac; AKA, above-knee amputation; AP, ankle pressure; BKA, below-knee amputation; CFA, common femoral artery; CLTI, chronic limb-threatening ischemia; CTA, computed tomography angiography; DAPT, dual antiplatelet therapy; DM, diabetes mellitus; DUS, duplex ultrasound; EBR, evidence-based revascularization; FP, femoropopliteal disease; GLASS, Global Limb Anatomic Staging System; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; GSV, great saphenous vein; HBOT, hyperbaric oxygen therapy; HRQL, health-related quality of life; IDEAL, Idea, Development, Exploration, Assessment, and Long-term study; IPC, intermittent pneumatic compression; LS, lumbar sympathectomy; MRA, magnetic resonance angiography; OPGs, objective performance goals; PAD, peripheral artery disease; PFA, profunda femoris artery; PROMs, patient-reported outcomes measures; PSV, peak systolic velocity; PVR, pulse volume recording; RCTs, randomized controlled trials; SCS, spinal cord stimulation; SVS, Society for Vascular Surgery; TAP, target arterial path; TBI, toe-brachial index; TKA, through-knee amputation; TP, toe pressure; WIfI, Wound, Ischemia, and foot Infection.
Table 1.2.
One-year major limb amputation rate by Society for Vascular Surgery (SVS) Wound, Ischemia, and foot Infection (WIfI) clinical stage
| Study (year): No. of limbs at risk | Stage 1 | Stage 2 | Stage 3 | Stage 4 |
|---|---|---|---|---|
|
| ||||
| Cull68 (2014): 151 | 37 (3) | 63 (10) | 43 (23) | 8 (40) |
| Zhan69 (2015): 201 | 39 (0) | 50 (0) | 53 (8) | 59 (64)a |
| Darling71 (2016): 551 | 5 (0) | 110 (10) | 222 (11) | 213 (24) |
| Causey70 (2016): 160 | 21 (0) | 48 (8) | 42 (5) | 49 (20) |
| Beropoulis163 (2016): 126 | 29 (13) | 42 (19) | 29 (19) | 26 (38) |
| Ward166 (2017): 98 | 5 (0) | 21 (14) | 14 (21) | 58 (34) |
| Darling164 (2017): 992 | 12 (0) | 293 (4) | 249 (4) | 438 (21) |
| Robinson72 (2017): 280 | 48 (2.1) | 67 (7.5) | 64 (7.8) | 83 (17) |
| Mathioudakis165 (2017): 217 | 95 (4) | 33 (3) | 87 (5) | 64 (6) |
| Tokuda167 (2018): 163 | 16 (0) | 30 (10) | 56 (10.7) | 61 (34.4) |
| N = 2982 (weighted mean) | 307 (3.2) | 757 (7.0) | 859 (8.7) | 1059 (23.3) |
| Median (1-year major limb amputation) | 0% | 9% | 9.4% | 29% |
The number of limbs at risk in each WIfI stage is given, with percentage of amputations at 1 year in parentheses. Means in totals (in parentheses) are weighted.
Falsely elevated because of inadvertent inclusion of stage 5 (unsalvageable) limbs.
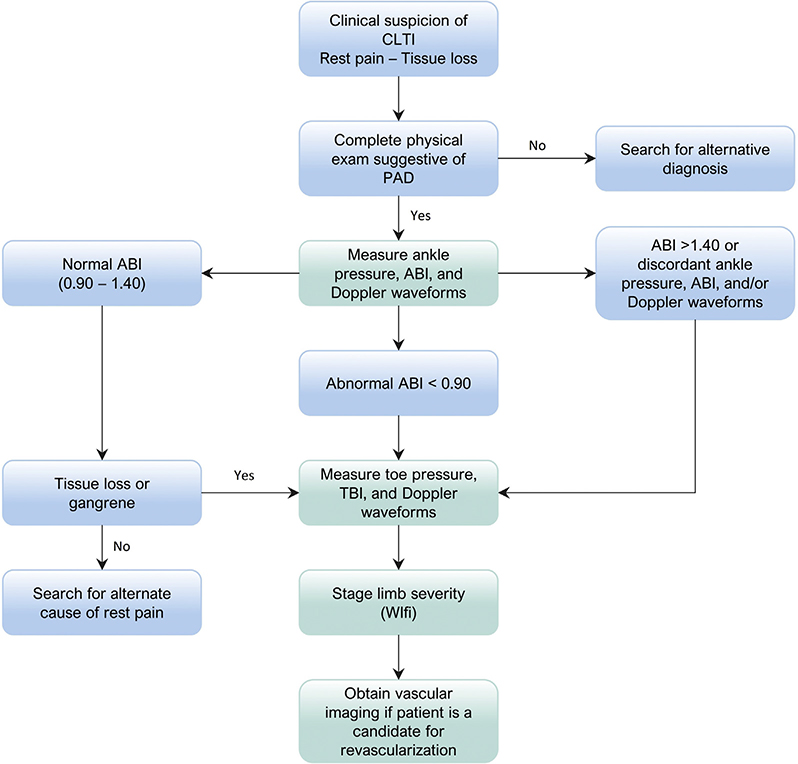
Fig 3.1.
Flow diagram for the investigation of patients presenting with suspected chronic limb-threatening ischemia (CLTI). ABI, Ankle-brachial index; PAD, peripheral artery disease; TBI, toe-brachial index; WIfI, Wound, Ischemia, and foot Infection.
1. DEFINITIONS AND NOMENCLATURE
Defining and describing the severity of PAD
The term “critical limb ischemia” (CLI) is outdated and fails to encompass the full spectrum of patients who are evaluated and treated for limb-threatening ischemia in modern practice. Instead, the new term CLTI is proposed to include a broader and more heterogeneous group of patients with varying degrees of ischemia that can often delay wound healing and increase amputation risk.
For development of a clearer concept of CLTI, the following are excluded from the population as defined in this guidelines document: patients with purely venous ulcers, acute limb ischemia, acute trash foot, ischemia due to emboli, acute trauma, or mangled extremity and those with wounds related to nonatherosclerotic conditions. These include vasculitides, collagen vascular disease, Buerger's disease, neoplastic disease, dermatoses, and radiation arteritis.
Previous leg ischemia definition and classification systems
CLI.
In 1982, a working group of vascular surgeons defined CLI as ischemic rest pain with an ankle pressure (AP) <40 mm Hg, or tissue necrosis with an AP <60 mm Hg, in patients without diabetes.144 Patients with diabetes were specifically excluded because of the confounding effects of neuropathy and susceptibility to infection. This definition has long been debated because it failed to capture a large group of patients who were at risk for amputation from a broader range of ischemia.145,146 To address this limitation, multiple and disparate lower limb ischemia and wound/DFU classification systems have been developed and promulgated during the past 5 decades, many of which remain in use today. These and other commonly used classifications and their associated components and grades of severity are summarized in Table 1.1.10,147–158 Among vascular surgeons, the Fontaine and Rutherford classifications have been the most widely adopted, whereas orthopedists, podiatric surgeons, and diabetic foot specialists traditionally applied the Wagner and University of Texas classifications. The strengths and limitations of each have been widely discussed in previous key publications.10,150,159–161 Although each of these systems has advantages, the use of multiple classification systems has hindered the development of optimal treatment algorithms. It has also contributed to the fragmentation and variability of care provided for patients with DFUs as well as for nondiabetic patients across the spectrum of CLTI.
Table 1.1.
Classification schemes used for chronic limb ischemia and ulceration
| Classification system | Ischemic rest pain | Ulcer | Gangrene | Ischemia | Infection | Key features and comments |
|---|---|---|---|---|---|---|
|
| ||||||
| Ischemia and PAD classifications | ||||||
| Fontaine (1954) | Yes (class III/IV) | Class IV/IV; ulcer and gangrene grouped together | Class IV/IV; ulcer and gangrene grouped together | Cutoff values for CLI based on European consensus document: Ischemic rest pain >2 weeks with AP <50 mm Hg or TP <30 mm Hg Ulcer and gangrene: AP <50 mm Hg, TP <30 mm Hg, absent pedal pulses in patient with diabetes |
No | Pure ischemia model No clear definitions of spectrum of hemodynamics; minimal description of wounds; infection omitted |
| Rutherford (1997) | Yes (category 4/6) | Category 5: minor tissue loss, nonhealing ulcer, focal gangrene with diffuse pedal ischemia | Category 6: major tissue loss extending above TM level, functional foot no longer salvageable (although, in practice, often refers to extensive gangrene, potentially salvageable foot with significant efforts) | Yes; cutoffs for CLI Category 4: resting AP <40 mm Hg; flat or barely pulsatile ankle or forefoot PVR; TP <30 mm Hg Category 5/6: AP <60 mm Hg; flat or barely pulsatile ankle or forefoot PVR; TP <40 mm Hg |
No | Pure ischemia model PAD classification system includes milder forms of PAD (categories 1–3). Categories 4–6 based on cutoff values for CLI; no spectrum of ischemia, does not acknowledge potential need for revascularization, with CLI cutoff depending on wound extent/infection; not intended for patients with diabetes; wound classes not sufficiently detailed; omits infection as a trigger |
| Second European Consensus (1991) | Yes; pain >2 weeks requiring analgesia; AP ≤50 mm Hg or TP ≤30 mm Hg | Yes, if AP ≤50 mm Hg or TP ≤30 mm Hg | Yes, if AP ≤50 mm Hg or TP ≤30 mm Hg | One hemodynamic cutoff for ulcer and gangrene, with or without diabetes | No | Ischemia threshold too low, especially for patients with diabetes; wounds not graded; infection not considered |
| TASC I (2000) | Yes, if ischemia criteria met | Yes, if ischemia criteria met | Yes, if ischemia criteria met | One hemodynamic cutoff, with no differentiation of diabetics from nondiabetics | No | Focused primarily on arteriographic anatomy without detailed stratification of the limb itself (wounds and infection not graded) |
| TASC II (2007) | Yes, if AP <50 mm Hg or TP <30 mm Hg | Yes, if ischemia criteria met of AP <70 mm Hg or TP <50 mm Hg | Yes, if ischemia criteria met of AP <70 mm Hg or TP <50 mm Hg | Yes, but noted “there is not complete consensus regarding the vascular haemodynamic parameters required to make the diagnosis of CLI” | No | Focused primarily on arteriographic anatomy without detailed stratification of the limb itself (wounds and infection not graded); issues with hemodynamic criteria noted |
| DFU classifications | ||||||
| Meggitt-Wagner (1976, 1981) | No | Grade 0: pre- or post-ulcerative lesion Grade 1: partial/full-thickness ulcer Grade 2: probing to tendon or capsule Grade 3: deep ulcer with osteitis Grade 4: partial foot gangrene Grade 5: whole foot gangrene |
Ulcer and gangrene grouped together; gangrene due to infection not differentiated from gangrene due to ischemia; also includes osteomyelitis | No | No for soft tissue component; included only as osteomyelitis | Orthopedic classification intended for diabetic feet No hemodynamics; gangrene from infection not differentiated from that due to ischemia; osteomyelitis included; soft tissue infection not separated from bone infection |
| University of Texas (1998) | No | Yes: grade 0-III ulcers Grade 0: pre- or post-ulcerative completely epithelialized lesion Grade I: superficial, not involving tendon, capsule, or bone Grade II: penetrating to tendon/capsule Grade III: penetrating to bone or joint |
No | Yes: binary ± based on ABI <0.8 | Yes ± wounds, with frank purulence or >2 of the following (warmth, erythema, lymphangitis, edema, lymphadenopathy, pain, loss of function) considered infected | Primarily intended for DFUs; includes validated ulcer categories; PAD and infection included, but only as ± variable with no grades/spectrum |
| S(AD) SAD system (1999) | No | Yes: 0–3 based on area and depth Grade 0: skin intact Grade 1: superficial, < 1 cm2 Grade 2: penetrates to tendon, periosteum, joint capsule, 1–3 cm2 Grade 3: lesions in bone or joint space, >3 cm2 |
No | Pulse palpation only, no objective hemodynamic testing | Yes; 1 = no infection, 2 = cellulitis, 3 = osteomyelitis | Intended for DFUs; also includes neuropathy; does not mention gangrene; no hemodynamic information, perfusion assessment based on pulse palpation only |
| PEDIS (2004) | No | Yes: grades 1–3 Grade 1: superficial full-thickness ulcer, not penetrating deeper than the dermis Grade 2: deep ulcer, penetrating below the dermis to subcutaneous structures involving fascia, muscle, or tendon Grade 3: all subsequent layers of the foot involved including bone and joint (exposed bone, probing to bone) |
No | Yes: 3 grades, CLI cutoff Grade 1: no PAD symptoms, ABI >0.9, TBI >0.6, TcPo2 >60 mm Hg Grade 2: PAD symptoms, ABI <0.9, AP >50 mm Hg, TP >30 mm Hg, TcPo2 30–60 mm Hg Grade 3: AP < 50 mm Hg, TP <30 mm Hg, TcPo2 <30 mm Hg |
Yes: grades 1–4 based on IDSA classification | Primarily intended for DFUs; ulcer grades validated; includes perfusion assessment, but with cutoff for CLI; gangrene not separately categorized; includes validated IDSA infection categories |
| Saint Elian (2010) | No | Yes: grades 1–3 based on depth Grade 1: superficial wound disrupting entire skin Grade 2: moderate or partial depth, down to fascia, tendon, or muscle but not bone or joints Grade 3: severe or total, wounds with bone or joint involvement Multiple categories including area, ulcer number, location, and topography |
No | Yes: grades 0–3 Grade 0: AP >80 mm Hg, ABI 0.9–1.2 Grade 1: AP 70–80 mm Hg, ABI 0.7–0.89, TP 55–80 mm Hg Grade 2: AP 55–69 mm Hg, ABI 0.5–0.69, TP 30–54 mm Hg Grade 3: AP <55 mm Hg, ABI <0.5, TP <30 mm Hg |
Yes: grades 0–3 Grade 0: none Grade 1: mild; erythema 0.5–2 cm, induration, tenderness, warmth, and purulence Grade 2: moderate; erythema >2 cm, abscess, muscle tendon, joint, or bone infection Grade 3: severe; systemic response (similar to IDSA) |
Detailed system intended only for DFUs; comprehensive ulcer classification system with hemodynamic categories for gradations of ischemia; gangrene not considered separately Infection system similar to IDSA |
| IDSA (2012) | No | No | No | No | Yes: uninfected, mild, moderate, and severe | Validated system for risk of amputation related to foot infection but not designed to address wound depth/complexity or degree of ischemia |
| Recommended CLTI classification | ||||||
| SVS WIfI threatened limb classification (2014) | Yes, if confirmed by hemodynamic criteria | Yes: grades 0–3 Grouped by depth, location, and size and magnitude of ablative/wound coverage procedure required to achieve healing |
Yes: grades 0–3 Grouped by extent, location, and size and magnitude of ablative or wound coverage procedure required to achieve healing |
Yes: ischemia grades 0–3 Hemodynamics with spectrum of perfusion abnormalities; no cutoff value for CLI Grade 0 unlikely to require revascularization |
Yes: IDSA system (grades 0–3); grades corelate with amputation risk | Includes PAD ± diabetes with a range of wounds, ischemia, and infection, scaled from 0–3 No single cutoff for CLI as CLTI is considered a spectrum of disease Need for revascularization depends on degree of ischemia, wound, and infection severity Ulcers/gangrene categorized by extent and complexity of anticipated ablative surgery/coverage |
ABI, Ankle-brachial index; AP, ankle pressure; CLI, critical limb ischemia; DFU, diabetic foot ulcer; CLTI, chronic limb-threatening ischemia; IDSA, Infectious Diseases Society of America; PAD, peripheral artery disease; PEDIS, perfusion, extent depth, infection, and sensation; PVR, pulse volume recording; SVS, Society for Vascular Surgery; TASC, TransAtlantic Inter-Society Consensus; TBI, toe-brachial index; TcPo2, transcutaneous oximetry; TM, transmetatarsal; TP, toe pressure; WIfI, Wound, Ischemia, foot Infection.
Lower extremity threatened limb classification system
The definitions summarized in Table 1.1 were developed primarily to describe patients suffering from pure ischemia due to atherosclerosis. This was when the predominant risk factor was tobacco smoking and before the global epidemic of diabetes mellitus (DM). As such, these definitions were ischemia-dominant models of limb threat. However, because patients with DM now make up the majority of patients with CLTI, absolute perfusion now needs to be considered in the context of neuropathy, wound characteristics, and infection. To address this unmet need, the SVS Lower Extremity Guidelines Committee created the SVS Lower Extremity Threatened Limb Classification System. This system stratifies amputation risk according to wound extent, degree of ischemia, and presence and severity of foot infection (Wound, Ischemia, and foot Infection [WIfI]).10 Although it may require some adjustments, WIfI appears to correlate strongly with important clinical outcomes. This includes those set forth in the SVS objective performance goals (OPGs) that focus on limb amputation, 1-year amputation-free survival (AFS), and wound healing time (Table 1.2).10,68–72,162–167
The WIfI classification system is currently being evaluated in multicenter trials including the U.S. National Institutes of Health-funded BEST-CLI trial13 and the UK National Institute for Health Research Health Technology Assessment-funded BASIL-2 and BASIL-3 trials.14,15 WIfI is also being incorporated into the U.S. SVS Vascular Quality Initiative registry of lower extremity interventions.
Hemodynamic criteria
Although previous guidelines have suggested a range of AP and toe pressure (TP) thresholds for defining limb-threatening ischemia, such thresholds must be used with great caution and considered in the clinical context because of multiple confounding factors and the lack of a clear and reliable relationship to outcomes. Patients with limb-threatening ischemia should be defined primarily in terms of their clinical presentation, supplemented by physiologic studies that demonstrate a degree of ischemia sufficient to cause pain, to impair wound healing, and to increase amputation risk.
In addition to patients who meet the proposed new definition of CLTI, there are a significant number of patients whose PAD is so severe that they are likely to be at increased risk for development of CLTI in the foreseeable future.168 Although data are lacking, it is logical to suggest that such individuals should be monitored closely for clinical disease progression.
CLTI
We propose that CLTI be defined to include a broader and more heterogeneous group of patients with varying degrees of ischemia that may delay wound healing and increase amputation risk. A diagnosis of CLTI requires objectively documented atherosclerotic PAD in association with ischemic rest pain or tissue loss (ulceration or gangrene).
Ischemic rest pain is typically described as affecting the forefoot and is often made worse with recumbency while being relieved by dependency. It should be present for >2 weeks and be associated with one or more abnormal hemodynamic parameters. These parameters include an ankle-brachial index (ABI) <0.4 (using higher of the dorsalis pedis [DP] and posterior tibial [PT] arteries), absolute highest AP <50 mm Hg, absolute TP <30 mm Hg, transcutaneous partial pressure of oxygen (TcPO2) <30 mm Hg, and flat or minimally pulsatile pulse volume recording (PVR) waveforms (equivalent to WIfI ischemia grade 3). Pressure measurements should be correlated with Doppler arterial waveforms, keeping in mind that AP and ABI are frequently falsely elevated because of medial calcinosis, especially in people with DM and end-stage renal disease (ESRD). For this reason, a combination of tests may be needed. In patients with DM or ESRD, toe waveforms and systolic pressures are preferred. One study demonstrated that AP alone failed to identify 42% of patients with CLTI. TP and TcPO2 measurements were more accurate than AP and also were more predictive of 1-year amputation risk (TP <30 mm Hg or TcPO2 <10 mm Hg).169
Tissue loss related to CLTI includes gangrene of any part of the foot or nonhealing ulceration present for at least 2 weeks. It should be accompanied by objective evidence of significant PAD (eg, WIfI ischemia grade ≥1). This definition excludes purely neuropathic, traumatic, or venous ulcers lacking any ischemic component. However, the WIfI scheme recognizes that a wide range of ischemic deficit may be limb threatening when it coexists with varying degrees of wound complexity and superimposed infection. CLTI is present if either ischemic rest pain or tissue loss with appropriate hemodynamics is present.
Some patients may have relatively normal hemodynamics when the limb or foot is considered as a whole but nevertheless suffer ulceration as a result of diminished local perfusion (ie, angiosomal or regional ischemia without adequate collateral flow). It is recognized that such ulcers may contribute to limb threat, and current tools to assess regional ischemia require further development to better define such circumstances and their treatment. The relationship between regional ischemia and patterns of IP and pedal disease also requires more in-depth study.12,170
The GVG recommends use of the SVS WIfI classification (Section 3) in a manner analogous to the TNM system of cancer staging to stage the limb in patients with CLTI. The WIfI classification is intuitive and has been made user-friendly by the availability of free online application software provided by the SVS (SVS Interactive Practice Guidelines; https://itunes.apple.com/app/id1014644425).
Data accrued in nearly 3000 patients to date and summarized in Table 1.2 suggest that the four WIfI clinical stages of limb threat correlate with the risk of major limb amputation and time to wound healing. It has also been suggested that novel WIfI composite and mean scores may predict other clinically significant events as well.164 The WIfI system appears to contain the key limb status elements needed to gauge the severity of limb threat at presentation.
In addition, recent data suggest that WIfI can assist in predicting which patients might fare better with open surgical bypass compared with endovascular therapy.171,172 One study reported that when endovascular therapy alone was applied to WIfI stage 4 patients, results were worse than in lower clinical stage patients.172 Specifically, the wound healing rate was only 44%, the major limb amputation rate was 20%, and 46% of patients required multiple, repetitive endovascular procedures. In a nonrandomized, single-center comparison of WIfI stage 4 patients, researchers found that freedom from major limb amputation was superior in patients who underwent bypass compared with those who underwent endovascular therapy.171 If these results can be confirmed, WIfI may prove to be a useful tool in deciding whether to offer endovascular therapy or bypass.
Another study used WIfI in a fashion analogous to TNM staging for cancer and reassigned patients to stages after 1 month of therapy. The investigators found that at 1 month and 6 months, wound, ischemia, and infection grades correlated with AFS, whereas baseline ischemia grade did not.173 These data suggest that restaging with WIfI at 1 month and 6 months after intervention may help identify a cohort of patients undergoing therapy for CLTI that remains at higher risk for major limb amputation and may merit targeted reintervention.
Ultimately, the optimal staging system for CLTI is expected to evolve with additional clinical application and larger scale, multicenter, and multinational data analysis.
| Recommendation | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 1.1 | Use objective hemodynamic tests to determine the presence and to quantify the severity of ischemia in all patients with suspected CLTI. | 1 (Strong) | C (Low) | de Graaff,16 2003 Brownrigg,17 2016 Wang,18 2016 |
| 1.2 | Use a lower extremity threatened limb classification staging system (eg, SVS’s WIfI classification system) that grades wound extent, degree of ischemia, and severity of infection to guide clinical management in all patients with suspected CLTI. | 1 (Strong) | C (Low) | See Table 1.2 |
2. GLOBAL EPIDEMIOLOGY AND RISK FACTORS FOR CLTI
In 2010, estimates suggested that >200 million people worldwide were living with PAD. This represented a 23.5% increase since 2000, an increase that is believed to be largely attributable to aging populations and the growing prevalence of risk factors, in particular DM.1 These figures are thought to almost certainly underestimate the true burden of disease as they are largely based on community-based studies that define PAD on the basis of reduced ABI. Although CLTI is widely believed to be a growing global health care problem, reliable epidemiologic data are extremely limited.
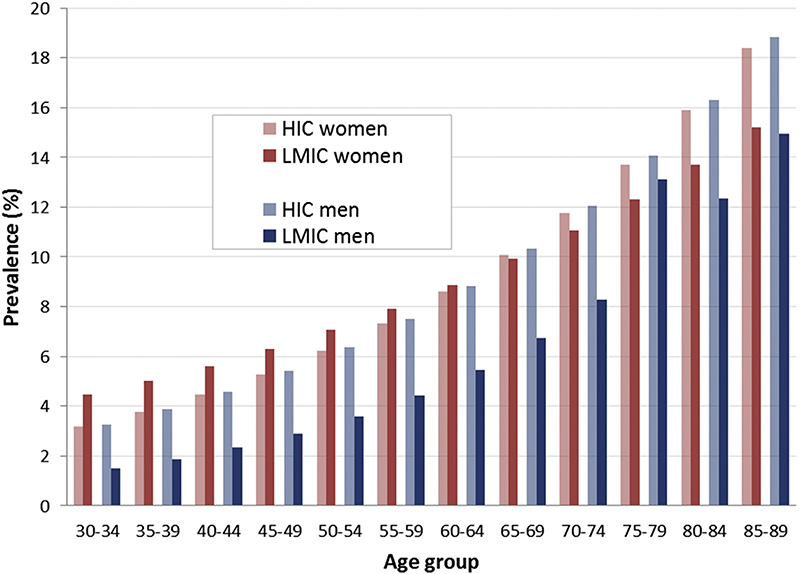
Men have been reported to have a higher prevalence of PAD in high-income countries (HICs; Fig 2.1), whereas women seem to have a higher prevalence of PAD in low- and middle-income countries (LMICs).1 As life expectancy increases, the burden of PAD seems likely to rise in LMIC. However, in certain geographic regions, notably in the western Pacific and Southeast Asia, most PAD cases are reported in people younger than 55 years.1
Fig 2.1.
Prevalence of peripheral artery disease (PAD; ankle-brachial index [ABI] <0.9) by age and sex in high-income countries (HICs) and in low- and middle-income countries (LMICs).1
In a meta-analysis from the United States, the prevalence of PAD in men ranged from 6.5% (aged 60–69 years) to 11.6% (aged 70–79 years) to 29.4% (>80 years).174 There were similar age-related increases in PAD prevalence in women (5.3%, 11.5%, and 24.7% in these age categories, respectively).174 Given that the life expectancy of women still exceeds that of men, the overall burden of PAD (total number of individuals affected) is likely to be greater in women than in men. The epidemiology of PAD is likely to be similar in other developed countries, such as the United Kingdom, and regions, such as the European Union.175,176 However, as these populations become more multicultural, differences in disease burden between different communities within these nations seem likely to become apparent, further complicating the epidemiology of the condition.177
Data on the epidemiology of PAD and in particular of CLTI in other parts of the world are even more limited. In one Japanese community study of people older than 40 years, the prevalence of ABI <0.9 was very low (1.4%).178 In a population-based cohort of 4055 Chinese men and women older than 60 years, the prevalence of PAD (ABI <0.9) was 2.9% and 2.8%, respectively.179 Another population-based cohort of 1871 individuals younger than 65 years in two countries from Central Africa showed that the overall prevalence of PAD was 14.8%.180
There is a considerable body of evidence showing that PAD is more common among black individuals than among whites.181–184 There is also evidence that Asians and Hispanics have a lower prevalence of PAD than whites do.184 It is not clear whether these differences have a genetic basis or simply reflect differential exposure to traditional risk factors. However, disease risk profiles appear to change as populations migrate, suggesting that environment is more important than genetic makeup. Another explanation may be that ABI is intrinsically lower in black individuals, resulting in a falsely high prevalence of PAD.185
There are far more international data on the epidemiology of intermittent claudication (IC) than of CLTI. The annual incidence of IC in 60-year-old men has been shown to range from 0.2% in Iceland to 1.0% in Israel.186 A study using data from a large, insured U.S. population estimated the annual incidence of PAD, defined by the presence of a diagnosis or procedure insurance claim, to be 2.4% in a cohort of adults older than 40 years.187 Studies reporting on the epidemiology of PAD based on ABI rather than on the presence of symptomatic disease suggest that the prevalence of asymptomatic PAD may be similar in men and women, although IC appears to be more prevalent in men.188,189 Differences in presentation between men and women with IC may influence the accuracy of prevalence estimates.190
Risk factors for PAD.
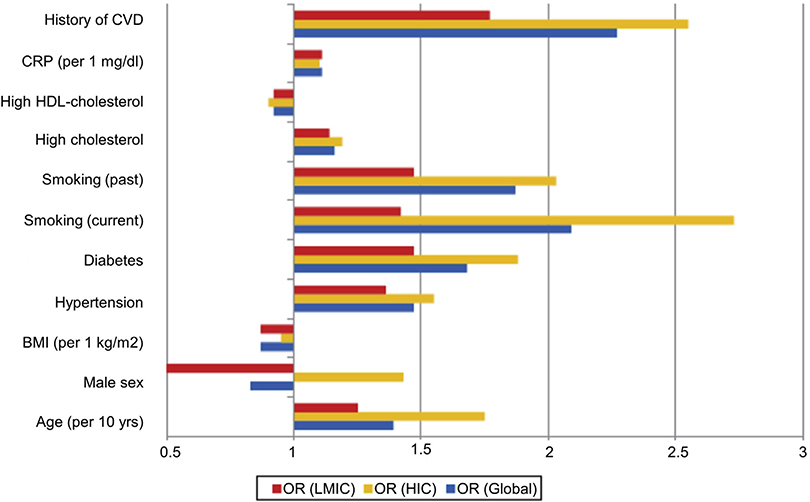
Modifiable risk factors for PAD have been comprehensively studied in HICs and include smoking, DM, hypertension, hypercholesterolemia, and air pollution. A global study suggested that although these risk factors may be equally applicable to LMICs, for most, the strength of the association was greater in HICs. This may be because HIC studies often include a larger number of older patients and because the exposure time tends to be shorter in LMICs.1
Smoking is unarguably a significant risk factor in the development and progression of PAD. Nevertheless, whereas smoking rates are falling in most HICs, this is not the case in LMICs (Fig 2.2). DM is also strongly associated with the development of PAD, and risk increases with the duration of DM in affected individuals. Patients with DM are widely recognized to be at markedly higher risk of amputation.191,192 The rapidly increasing worldwide prevalence of type 2 DM is concerning and likely to have a significant impact on the future incidence and prevalence of PAD and CLTI as well as their morbid end points.
Fig 2.2.
Odds ratios (ORs) for peripheral artery disease (PAD) in high-income countries (HICs) and low- and middle-income countries (LMICs). BMI, Body mass index; CRP, C-reactive protein; CVD, cardiovascular disease; HDL, high-density lipoprotein. (Reprinted from Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26.)
The link between obesity and PAD is inconsistent. Many studies have suggested the existence of an “obesity paradox,” with lower rates of PAD being observed in patients with a higher body mass index (BMI).186 By contrast, other studies that have adjusted for smoking, which is associated with a generally lower BMI,193 reported a positive correlation between BMI and PAD. Hypertension is associated with the development of PAD and is another common risk factor in the adult population.
The association between dyslipidemia and the development and progression of atherosclerosis has been extensively studied. Whereas elevated levels of total cholesterol and low-density lipoprotein cholesterol (LDL-C) are widely accepted as risk factors for PAD, reduced high-density lipoprotein cholesterol levels also appear to be associated with increased mortality in PAD patients.194 A ratio of the two may also be a useful predictor of PAD.195 Whereas hypertriglyceridemia appears to be atherogenic,196 its role in the development and progression of PAD remains incompletely defined.
Chronic kidney disease (CKD), particularly ESRD, is a strong risk factor for PAD and limb loss, especially in association with DM. Affected patients frequently have heavily calcified arteries and a distal pattern of arterial disease.186 The association between alcohol consumption and PAD is inconsistent, making it difficult to draw any firm conclusions.197 However, heavy alcohol consumption is often associated with other risk factors for PAD, such as smoking, and as with DM, the presence of alcoholic neuropathy increases the risk of tissue loss for any given perfusion deficit.
Recent data suggest that air pollution from sources such as motor vehicles, power plants, wood burning, and some industrial processes may be associated with increased cardiovascular morbidity and mortality.198 Likewise, chronic inflammation, characterized by elevated levels of C-reactive protein and other biomarkers, has been shown to be associated with PAD.186 Homocysteine levels are higher in several case-control PAD cohort studies, although the benefits of folate supplementation appear to be negligible.186,199
The significance of family history and genetic makeup is uncertain.200,201 Studies have yielded varying results, with some identifying a small number of candidate genes or even single-nucleotide polymorphisms and others failing to identify any association at all.
Finally, people of lower socioeconomic status and educational attainment tend to have a higher prevalence of IC and probably also of CLTI, although the association is not always strong and can often be explained in part by their increased exposure to other risk factors, such as smoking.180,183,202 However, there is increasing evidence that chronic mental and psychosocial stress may have direct effects on cardiovascular health.203
Incidence and prevalence of CLTI.
As noted before, high-quality data on the epidemiology of CLTI are lacking, especially from LMICs, with many estimates being extrapolated from the incidence and prevalence of IC, amputation, and DM. Unfortunately, such estimates can be highly misleading for a number of reasons. First, IC does not progress to CLTI in a predictable manner. Second, CLTI probably represents <10% of all PAD patients, and those undergoing amputation for CLTI are at very high risk of premature death (and so more likely to be absent from population-based studies). Third, the clinical and hemodynamic data required to reliably diagnose CLTI are difficult to obtain in large populations. This is particularly true in patients with DM, who often have incompressible vessels. Thus, although it is estimated that approximately half of all patients with a DFU in western Europe and North America also have significant PAD, the disease may often appear relatively mild (not fulfilling the criteria for CLTI) on hemodynamic assessment.204
For many years, the annual incidence of what has typically been termed CLI was estimated at 500 to 1000 new cases per million individuals in Western countries.205 Unfortunately, there are no reliable contemporary epidemiologic data that take into account recent changes in lifestyle (such as reduced smoking rates), identification and medical management of cardiovascular risk factors, prevalence of obesity and diabetes, and overall increasing life expectancy around the world.
In 2013, a meta-analysis involving 6 studies and close to 83,000 patients showed the overall prevalence of severe chronic limb ischemia (defined by Fontaine stage, AP <70 mm Hg, and ABI <0.60) to be 0.74% (95% confidence interval [CI], 0.26–1.46), with marked heterogeneity between studies (prevalence, 0.11%-1.59%).206
In an analysis of the U.S. MarketScan database (Truven Health Analytics, Ann Arbor, Mich), composed of approximately 12 million Americans aged 40 years and older receiving care from Medicare and Medicaid between 2003 and 2008, the prevalence and annual incidence of CLTI were estimated at 1.33% and 0.35%, respectively. This equates to around 3500 new cases per million individuals per year.187 The study defined primary CLTI as patients with no prior PAD or subsequent PAD diagnostic code >30 days after a CLTI diagnostic code. Secondary CLTI included patients with prior PAD (or subsequent PAD diagnostic codes within 30 days of a CLTI diagnostic code). The annual incidence rate of primary and secondary CLTI was 0.19% and 0.16%. CLTI patients represented 11.08% (95% CI, 11.03%-11.13%) of total PAD patients annually. As noted before, although one might expect similar rates of CLTI in other developed nations and regions, data from LMICs are lacking. Even within HICs, the epidemiology of CLTI is likely to be complex and evolving.
Amputation and CLTI.
A number of studies have used major lower limb amputation as a surrogate for CLTI on the basis that most (>80%) are due to CLTI. However, it can be difficult to distinguish reliably between minor (below the ankle) and major (above the ankle) amputations in some administrative data. Furthermore, the number of amputations that are performed for trauma, tumor, or infection, including patients with DM and neuropathy (but without PAD), is likely to vary considerably from country to country, particularly in comparing HICs and LMICs.
In the United States in 2015, an estimated 504,000 individuals (of a total estimated population of 295.5 million) were living with a major amputation due to PAD, a number that was projected to more than double by 2050.207 In Minnesota, a state with low overall rates of cardiovascular disease (CVD), one study showed that between 2005 and 2008, the age-adjusted annual incidence of ischemic lower limb amputation (amputations not due to trauma or cancer) remained unchanged at 20 per 100,000.208
A systematic review found that the rate of major amputation varied considerably (3.6 to 68.4 per 100,000 per year) across the world, probably because of differences in ethnicity, social deprivation, and, in particular, the prevalence of DM.209 In some countries, including England, the incidence of amputations unrelated to DM appears to be decreasing.210 However, in most parts of the world, the incidence of DM-related limb amputations is increasing.211
Natural history of untreated CLTI.
A meta-analysis (13 studies and 1527 patients) of the natural history of untreated CLTI found that during a median follow-up of 12 months, both the mortality rate and the per-patient amputation rate were 22%, although there was marked heterogeneity between studies.5 With regard to disease progression, one study estimated that only 5% to 10% of patients with either asymptomatic PAD or IC went on the develop CLTI during a 5-year period.212 However, another meta-analysis suggested that this progression rate may be significantly higher at 21% (range, 12%-29%) during 5 years.213 Approximately 50% of patients presenting with CLTI have no prior history of PAD.214,215
Patients with CLTI present with a wide spectrum of clinical, hemodynamic, and anatomic disease. Outcomes depend on the availability and quality of primary and secondary care and may be further influenced by factors such as social stigmatization and cultural and religious beliefs. Those living in regions with poor access to health care often present late with advanced disease and unsalvageable limbs. Indeed, it has been estimated that approximately half of all patients with CLTI do not undergo revascularization.216 Even in HICs with advanced health care systems, such as Germany and the United States, many patients with suspected CLTI do not receive angiography or any attempt at revascularization.217 This may be because patients are too sick or frail, are thought to have no revascularization option, or present too late. Unfortunately, whereas reasonable data are available on amputation rates, data on processes of care that can help explain the shortfall and differences in revascularization and amputation are lacking.
The recently published VASCUNET report showed large (almost sixfold) differences but an overall decline in major amputation rates in 12 European and Australasian countries between 2010 and 2014.218 DM prevalence, age distribution, and mortality rates were also found to vary between countries. Despite limitations inherent to the use of registry data, these findings are important and may indicate disparities in access to vascular surgical intervention across the countries studied. Further research is clearly required to improve limb salvage in different demographic and geographic settings.218
In patients with known PAD, the risk for development of CLTI appears to be greater in men, in patients who have had a stroke or are in heart failure, and in patients with DM.187 Patients who present de novo with CLTI (no prior diagnosis of PAD) seem more likely to be older and male and to have pre-existing CVD (including hypertension, myocardial infarction, heart failure, or stroke) and renal failure.187 Not surprisingly, because of the associated high prevalence of neuropathy, DM had the strongest association with a new presentation of CLTI (odds ratio [OR], 7.45; 95% CI, 7.19–7.72). The medical management of patients who have or are at risk of having CLTI is covered elsewhere in the guideline (Section 4). Still, there is growing evidence that aggressive medical management of risk factors can significantly improve the overall prognosis for patients with PAD. This may in part explain the decline in mortality observed in patients with IC and CLTI in The Netherlands between 1998 and 2010.219
The risk of amputation is high in CLTI patients, even in those undergoing a successful revascularization.220 Unsurprisingly, patients who present late and with the greatest degree of tissue loss are at highest risk. In one analysis, the rates of amputation at 4 years were 12.1%, 35.3%, and 67.3% for Rutherford class 4, class 5, and class 6, respectively.217
Anatomic patterns of disease.
CLTI is usually the result of multilevel arterial occlusive disease. Involvement of parallel vascular beds, such as the superficial femoral artery (SFA) and profunda femoris artery (PFA), is also common. Below-knee arteries typically become increasingly involved as the overall severity of disease worsens. However, FP and IP disease does not always progress in parallel. The general requirement is that there needs to be two levels of arterial occlusive disease to cause CLTI. However, an increasingly observed exception is diffuse disease involving the IP and pedal arteries in patients with DM or CKD. In patients with CLTI and IP disease, the PT artery tends to be the most diseased, often with relative sparing of the peroneal artery. In patients with DM, there may also be sparing of the DP artery. A number of specific factors appear to drive the distribution of lower limb PAD (Fig 2.3). Thus, women may be more prone to development of FP disease, whereas elderly male patients and those with diabetes are more likely to develop IP disease.221 There is also some evidence that black people and Asians are more likely to develop distal disease.222,223
Fig 2.3.
Association of risk factors with the level of atherosclerotic target lesions. The red overlay on the anatomic cartoon illustrates the association of risk factor with patterns of atherosclerotic disease.217 (Reprinted from Diehm N, Shang A, Silvestro A, Do DD, Dick F, Schmidli J, et al. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg 2006;31:59–63.)
CVD and mortality risk.
Despite some evidence of recent improvements in HICs, patients who develop PAD and CLTI remain at high risk of premature death. Thus, in a German study, 4-year mortality was 18.9% in Rutherford class 1 to class 3, 37.7% in class 4, 52.2% in class 5, and 63.5% in class 6.217 However, interestingly, up to 40% of the deaths were not cardiovascular, perhaps because better medical therapy and management of risk factors have improved overall survival from CVD.224,225
In 2014, the Global Burden of Disease (2010) database was used to estimate PAD deaths, disability-adjusted life-years, and years of life lost in 21 regions worldwide between 1990 and 2010. In 1990, the age-specific PAD death rate per 100,000 population ranged from 0.05 among those aged 40 to 44 years to 16.63 among those aged 80 years or older. In 2010, the corresponding estimates were 0.07 and 28.71. Death rates increased consistently with age in 1990 and 2010, and the rates in 2010 were higher than they were in 1990 in all age categories.
The overall relative change in median disability-adjusted life-years was greater for men and women in developing than in developed nations. The overall relative change in the median years of life lost rate in developed countries was larger in women than in men. Researchers concluded that disability and mortality associated with PAD increased during the 20 years of the study and that this increase in burden was greater among women than men. In addition, the burden of PAD is no longer confined to the elderly population and now includes young adults. Finally, the relative increase in PAD burden in developing regions of the world is striking and exceeds the increases in developed nations.226
Management strategies in CLTI.
A study based in South Carolina identified patients who underwent revascularization for CLTI in 1996 and 2005 and examined the requirement for subsequent amputations and further revascularizations. Although revascularization procedures increased by 33%, the 1-year and 3-year amputation rates did not change significantly between 1996 (34% and 43%) and 2005 (34% and 40%). However, the percentage of patients who required further revascularization in the same calendar year increased from 8% to 19%. Investigators concluded that the shift to endovascular interventions increased the number of secondary procedures required to maintain limb salvage rates. Although the absolute number of amputations appeared to decrease despite the increasing population at risk, they concluded that it could be misleading to suggest a direct relationship to the increase in revascularization rates. Thus, whereas the number of amputations fell by approximately 500, the number of revascularization procedures rose by only 187.227 As noted before, improved risk factor management and use of best medical therapy are likely to have been important factors. The increased number of revascularization procedures may also be due to the increasing availability of endovascular technology and techniques. Indeed, there is some suggestion that practitioners have become more liberal with the use of all revascularization techniques, including bypass and angioplasty.228 Data from the United Kingdom suggest that an increasing number of patients are undergoing attempts at revascularization.228
Undoubtedly, there is an increase in the number and proportion of revascularization procedures performed using an endovascular approach. In the South Carolina study, the endovascular approach was used in 26% of CLTI revascularization procedures performed in 1996 compared with 51% in 2005.227 It is difficult to establish whether this change in management strategy has resulted in the salvage of more limbs and prevention of premature deaths. Such questions can only be answered by RCTs. There are, however, consistent data to suggest that more modern vascular strategies (including a more widespread adoption of endovascular techniques as first- or second-line therapies) are associated with an increased number of patients requiring repeated revascularization (increasing from 8% to 19% in the South Carolina study).227 Alternative explanations may be that vascular surgeons are becoming more aggressive at retreating patients or that patients are living longer.
Summary.
PAD is an increasingly common condition worldwide. Most patients remain asymptomatic, but it is estimated that up to 10% will progress to or present de novo with CLTI (although that figure appears to vary widely). The number of women with PAD continues to increase, and women may be more likely to develop symptomatic disease. Modifiable risk factors include DM, smoking, hypertension, dyslipidemia, CKD, obesity, and sedentary lifestyle.
Despite advances in risk factor management and best medical therapy, PAD and especially CLTI are associated with markedly increased cardiovascular morbidity and mortality, especially in LMICs. Left untreated, the overall risk of limb loss in CLTI is estimated at approximately 25% at 1 year.5 However, it will probably be much higher than that for some groups, such as those with extensive tissue loss at presentation. The key to preventing limb loss is aggressive risk factor management and best medical therapy together with timely EBR. There are major differences in amputation rates between and within countries. An increasing number of patients appear to be undergoing revascularization (both endovascular and bypass surgery) in HICs, and at least in part, this may account for a reduction in amputation. However, improvements in cardiovascular risk management, processes of care, and vascular and endovascular technology may be equally important.
| Research priorities | |
|---|---|
|
| |
| 2.1 | Quantify and track the incidence, prevalence, demographics, and risk factors associated with CLTI in different global regions. |
| 2.2 | Describe the contemporary natural history of CLTI (including risk to the limb, cardiovascular events, and all-cause mortality in that population) in different global regions. |
| 2.3 | Describe the contemporary management strategies used in the treatment of CLTI around the world and the associated outcomes. |
| 2.4 | Describe and monitor the incidence and prevalence of nontraumatic lower limb amputation around the globe (eg, the Global Amputation Study, https://GAS.vascunet.org). |
| 2.5 | Establish a reliable system to monitor the number of major amputations in as many countries and regions as possible. Time trends and differences around the globe could then be studied. |
3. DIAGNOSIS AND LIMB STAGING IN CLTI
Diagnosis and evaluation
The diagnostic evaluation, staging, and imaging of patients with suspected CLTI, leading to EBR, is an integral part of successful treatment. Beyond history and examination, an important new tool is the SVS Threatened Limb Classification System (WIfI), which correlates with the probability of limb salvage and wound healing after revascularization. Fig 3.1 summarizes the recommended evaluation pathway for patients presenting with CLTI that should be followed whenever possible. In patients who are appropriate candidates for revascularization (Section 6), the GLASS (Section 5) anatomic scheme can be used to help define the optimal revascularization strategy.
Recent technologic advances have made the diagnosis and imaging of CLTI more accurate, which in turn allows better selection of patients and planning of revascularization. However, the authors are well aware that access to sophisticated diagnostic modalities and vascular imaging varies considerably around the globe, and as expected, this leads to a wide range of different approaches being employed in different health care settings.229 As such, it would not be possible or indeed desirable to make firm, proscriptive recommendations in this section. Rather, the aim is to set out broad principles and considerations that can reasonably be used to guide patient evaluation, diagnosis, limb staging, and imaging in most health care environments.
History
Ischemic rest pain usually affects the forefoot, is frequently worse at night, and often requires opiate analgesia for management. If present for >2 weeks and combined with hemodynamic evidence of severely impaired perfusion (eg, absolute AP <50 mm Hg, absolute TP <30 mm Hg), it is diagnostic of CLTI.230
Ischemic ulceration is frequently located on the toes and forefoot, but other areas may be affected in patients with diabetic neuropathy, altered biomechanics, or foot deformity. Gangrene usually occurs on the forefoot. A range of perfusion deficits may be limb threatening in different scenarios of tissue loss and concomitant infection (Section 1). Thus, all patients presenting with signs or symptoms of suspected CLTI should undergo a complete vascular assessment.
In addition to a carefully documented history of presenting limb complaints, it is important to record details of cardiovascular risk factors, drug history, and previous vascular and endovascular revascularization procedures and amputations.230,231 Assessment of frailty, functional status, and HRQL is also important.232,233
Physical examination
All patients with suspected CLTI should undergo a complete physical examination.234,235 Palpation of lower limb pulses can help determine the likely presence and distribution of arterial disease.236–240 Although they can be nonspecific, features such as coolness, dry skin, muscle atrophy, hair loss, and dystrophic toenails are frequently observed in patients with PAD. Buerger sign, pallor of the foot on elevation and rubor (so-called sunset foot) on dependency, is usually present in CLTI. The capillary refill time will usually exceed 5 seconds, especially when the patient is lying supine or the leg is elevated.239 It is important not to examine the patient with suspected CLTI sitting in a chair with the leg hanging down as that may lead to false reassurance regarding the perfusion of the foot.
Many patients with CLTI, especially those with DM, have “glove and stocking”239 sensory, motor, and autonomic neuropathy that may be asymptomatic or be associated with tingling, numbness, weakness, and burning pain in the feet and ankles. The presence of such neuropathy is a major risk factor for tissue loss and should be carefully sought and evaluated using monofilaments and, if available, a tuning fork (loss of vibration sense is an early feature).241–244 Neuropathy often leads to abnormal foot biomechanics and deformity, and neuropathic (neuroischemic) ulcers often occur at sites of abnormal pressure (load bearing). In patients with suspected CLTI who have a foot ulcer, a probe-to-bone test should be performed to assess depth and the probability of underlying osteomyelitis.245,246
| Recommendations | ||
|---|---|---|
|
| ||
| 3.1 | Perform a detailed history to determine symptoms, past medical history, and cardiovascular risk factors in all patients with suspected CLTI. | Good practice statement |
| 3.2 | Perform a complete cardiovascular physical examination of all patients with suspected CLTI. | Good practice statement |
| 3.3 | Perform a complete examination of the foot, including an assessment of neuropathy and a probe-to-bone test of any open ulcers, in all patients with pedal tissue loss and suspected CLTI. | Good practice statement |
Noninvasive hemodynamic tests
AP and ABI.
Measurement of AP and calculation of ABI (highest AP divided by highest brachial systolic pressure) is recommended as the first-line noninvasive hemodynamic test in all patients with suspected CLTI (Fig 3.1).19 Although many patients with CLTI will have an AP <50 mm Hg or a markedly reduced ABI (typically <0.4), an increasing proportion will not, especially those with DM and CKD, who may have incompressible crural arteries. ABI results should be reported as noncompressible if the value is >1.4. However, it is important to be aware that incompressibility can lead to artifactually elevated readings between 0.4 and 1.4.247–249 This should be suspected when the ABI falls in or near the normal range but is associated with dampened, monophasic waveforms (recognized acoustically or visually on a screen).23 These falsely normal APs and ABI values have been reported to be an independent predictor of major amputation.250 In such patients, TP and toe-brachial index (TBI) or other hemodynamic measurements, as described next, should always be obtained.251
TP and TBI.
TP is measured using an appropriately sized mini-cuff typically placed around the base of the great toe and attached to a standard manometer. A photoplethysmographic or continuous-wave Doppler flow detector is then used to determine when flow returns while the inflated cuff is slowly deflated. Various automated systems can be purchased. TPs are less often affected by incompressibility and, if possible, should be measured whenever falsely elevated APs or ABIs are detected or suspected, particularly when such values are nonconcordant with acoustic or visual waveform analysis. Studies have suggested that TP is more sensitive than AP in the diagnosis of CLTI and more predictive of amputation risk.21,22 Systolic TPs are generally 20 to 40 mm Hg lower than APs. TBIs <0.7 are considered abnormal and TPs <30 mm Hg are typically associated with advanced ischemia.22,230,252
Other methods for noninvasive diagnosis of CLTI
Alternative noninvasive testing methods can also be used to assist in the diagnosis of CLTI (Table 3.1). Whereas each method has its own advantages and limitations, depending on local availability and expertise, they can be used to augment APs and TPs and indices. Segmental pressures can provide information on anatomic localization of lower limb vascular disease in patients with CLTI but are used infrequently today, at least in HICs. Several other noninvasive tests, including laser Doppler flowmetry, TcPO2, skin perfusion pressure, and plethysmography, have been used to evaluate limb perfusion.16,253 However, these tests can be influenced by a variety of confounding factors and are not used routinely in most vascular laboratories around the world.
Table 3.1.
Comparison of methods of noninvasive testing in patients with chronic limb-threatening ischemia (CLTI)
| Techniques | Advantages | Limitations |
|---|---|---|
|
| ||
| AP or ABI | • Simple, inexpensive, quick, widely applicable • Provides data to predict wound healing and limb survival • Useful to monitor efficacy of therapeutic intervention |
• Because of incompressible tibial arteries, may be falsely elevated or normal in patients with diabetes, renal insufficiency, or advanced age • Does not provide localization of the disease |
| TP or TBI | • Simple, inexpensive, quick • Useful in the presence of small-vessel artery disease • Useful in noncompressible tibial arteries • Provides data to predict wound healing and limb survival • Useful to monitor efficacy of therapeutic intervention |
• Generally requires a hallux • Does not provide localization of the disease |
| Segmental pressures | • Useful in initial anatomic localization of CLTI disease • Useful in creating therapeutic plan based on disease localization • Provides data to predict wound healing and limb survival • Useful to monitor efficacy of therapeutic intervention |
• Not accurate in noncompressible tibial arteries |
| TcPo2 | • Useful to assess microcirculation • Can predict wound healing • May be useful for monitoring efficacy of revascularization |
• Limited accuracy in the presence of edema or infection • Requires skin heating to ≥40°C • Time-consuming • Limited data validation |
| Skin perfusion pressure | • Useful to assess microcirculation and wound healing potential • May be useful for monitoring efficacy of revascularization • Can be measured in a shorter time compared with TcPo2 |
• Probe size and shape may affect measurements • Limited data validation |
ABI, Ankle-brachial index; AP, ankle pressure; TBI, toe-brachial index; TcPo2, transcutaneous oximetry; TP, toe pressure.
Adapted from Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654.
| Recommendation | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 3.4 | Measure AP and ABI as the first-line noninvasive test in all patients with suspected CLTI. | 1 (Strong) | B (Moderate) | Lijmer,19 1996 Dachun,20 2010 |
| 3.5 | Measure TP and TBI in all patients with suspected CLTI and tissue loss (Fig 3.1). | 1 (Strong) | B (Moderate) | Aboyans,21 2008 Salaun,169 2018 |
| 3.6 | Consider using alternative methods for noninvasive assessment of perfusion, such as PVR, transcutaneous oximetry, or skin perfusion pressure, when ankle and toe pressures, indices, and waveforms cannot be assessed. | 2 (Weak) | C (Low) | Aboyans,21 2008 Shirasu,23 2016 Saluan,169 2018 |
Wound and tissue loss classification systems
A number of limb and wound classification systems have been developed to try to improve clinical decision-making and clinical outcomes.254–256 The WIfI system10 is based on three key factors: wound, ischemia, and foot infection (Tables 3.2–3.5). WIfI correlates with limb salvage, amputation risk, and wound healing and can identify patients who are likely to benefit from revascularization.68,69
Table 3.2.
Wound grading in Wound, Ischemia, and foot Infection (WIfI) classification
| Grade | Ulcer | Gangrene |
|---|---|---|
|
| ||
| 0 | No ulcer | No gangrene |
| Clinical description: ischemic rest pain (requires typical symptoms + ischemia grade 3); no wound. | ||
| 1 | Small, shallow ulcer on distal leg or foot; no exposed bone, unless limited to distal phalanx | No gangrene |
| Clinical description: minor tissue loss. Salvageable with simple digital amputation (1 or 2 digits) or skin coverage. | ||
| 2 | Deeper ulcer with exposed bone, joint, or tendon; generally not involving the heel; shallow heel ulcer, without calcaneal involvement | Gangrenous changes limited to digits |
| Clinical description: major tissue loss salvageable with multiple (≥3) digital amputations or standard TMA ± skin coverage. | ||
| 3 | Extensive, deep ulcer involving forefoot and/or midfoot; deep, full-thickness heel ulcer ± calcaneal involvement | Extensive gangrene involving forefoot and/or midfoot; full-thickness heel necrosis ± calcaneal involvement |
| Clinical description: extensive tissue loss salvageable only with a complex foot reconstruction (nontraditional transmetatarsal, Chopart, or Lisfranc amputation); flap coverage or complex wound management needed for large soft tissue defect. | ||
TMA, Transmetatarsal amputation.
Table 3.5.
Clinical stages of major limb amputation risk based on Wound, Ischemia, and foot Infection (WIfI) classification
| Risk of amputation | Proposed clinical stages | WIfI spectrum score |
|---|---|---|
|
| ||
| Very low | Stage 1 | W0 I0 fI0,1 |
| W0 I1 fI0 | ||
| W1 I0 fI0,1 | ||
| W1 I1 fI 0 | ||
| Low | Stage 2 | W0 I0 fI2 |
| W0 I1 fI1 | ||
| W0 I2 fI0,1 | ||
| Wo I3 fI0 | ||
| W1 I0 fI2 | ||
| W1 I1 fI1 | ||
| W1 I2 fi0 | ||
| W2 I0 fI0/1 | ||
| Moderate | Stage 3 | W0 I0 fI3 |
| W0 I2 fI1,2 | ||
| W0 I3 fI1,2 | ||
| W1 I0 fI3 | ||
| W1 I1 fI2 | ||
| W1 I2 fI1 | ||
| W1 I3 fI0,1 | ||
| W2 I0 fI2 | ||
| W2 I 1 fI0,1 | ||
| W2 I2 fi0 | ||
| W3 I0 fi0,1 | ||
| High | Stage 4 | W0 I1,2,3 fI3 |
| W1 I1 fI3 | ||
| W1 I2,3 fI2,3 | ||
| W2 I0 fi3 | ||
| W2 I1 fI2,3 | ||
| W2 I2 fi1,2,3 | ||
| W2 I3 fI0,1,2,3 | ||
| W3 I0 fI2,3 | ||
| W3 I1,2,3 fI0,1,2,3 | ||
Clinical descriptors:Stage 1: minimal ischemia; no/minor tissue loss.-Stages 2–4 reflect increasing stages of ischemia, wound, and infection. Stage 5 (not shown in table): unsalvageable foot (most often due to wound extent or severity of infection).
A limb-staging classification system, such as WIfI, should be used in all patients presenting with suspected CLTI (Tables 3.2–3.5). Limb staging should be repeated after vascular intervention, foot surgery, or treatment of infection and whenever there is suspected clinical deterioration.
Imaging of vascular anatomy
Vascular imaging should be performed in all patients with suspected CLTI (Table 3.6) to determine the presence, extent, and severity of arterial disease and to help inform decisions about revascularization. Although there have been huge advances in imaging techniques in recent years, access to these latest modalities, and so practice, varies considerably between and even within countries.
Table 3.6.
Comparison of different imaging modalities for patients with chronic limb-threatening ischemia (CLTI)
| Techniques | Advantages | Limitations |
|---|---|---|
|
| ||
| DUS | • Noninvasive • Inexpensive • Quick, widely available worldwide • Useful to monitor efficacy of therapeutic intervention |
• Highly operator dependent • Limitations to the visualization of iliac arteries due to body habitus, bowel gas • Calcification may produce incomplete examination • Most DUS studies were performed in mixed populations; thus, the validity of DUS imaging for CLTI patients alone is uncertain |
| CTA | • Noninvasive • Excellent patient acceptance Ability to evaluate previously stented arteries • Mostly applicable in patients with contraindications to MRA |
• Image interference from calcified arteries • Potentially nephrotoxic contrast agents Radiation exposure • Less reliable for imaging of IP vessels • Patients with CLTI who require a complete assessment of their lower extremity (including foot) arteries for planning of a revascularization are under-represented in the current studies. The clinical value of CTA in the CLTI target population remains uncertain. |
| MRA | • Noninvasive • Eliminates exposure to ionizing radiation • Unaffected by arterial calcification • Three-dimensional images of the entire arterial tree are presented in a maximum intensity projection format produced on a workstation • Easily produced arterial map aids in planning of revascularization strategies |
• Patients with pacemakers and defibrillators and some cerebral clips cannot be scanned safely • Tendency to overestimate stenosis • Metal clips can cause artifacts that mimic vessel occlusions • Venous contamination can obscure arteries below the knee |
| Catheter angiography (DSA) | • Provides a complete map of the lower limb arteries • Images are easily displayed and interpreted by most physicians in charge of patients with CLTI • Selective catheter placement during lower extremity angiography enhances imaging, reduces contrast material dose, and enhances sensitivity in patients with CLTI |
• Exposure to ionizing radiation and contrast media • Alternatively, carbon dioxide and magnetic resonance contrast agents (eg, gadolinium) can be used instead of conventional contrast media • Complications of catheterization despite improvements in catheter and guidewire technology |
CTA, Computed tomography angiography; DSA, digital subtraction angiography; DUS, duplex ultrasound; IP, infrapopliteal; MRA, magnetic resonance angiography.
In patients with CLTI who are candidates for revascularization (Section 6), imaging should allow complete anatomic staging using, for example, GLASS (Section 5). Adequate imaging of the tibial and pedal vessels is of critical importance, particularly in planning intervention in patients with tissue loss. History and physical examination often help guide the optimal imaging approach. For those with tibial disease, particularly in the setting of tissue loss, computed tomography angiography (CTA) and magnetic resonance angiography (MRA) may offer useful information but may fail to completely image the ankle and foot vessels with sufficient resolution for procedural planning. Many vascular specialists believe that digital subtraction angiography (DSA) remains the “gold standard.” CTA offers more precise quantification of arterial calcification compared with MRA and DSA. Selective intra-arterial dual-energy CTA combines the low contrast material dose of conventional angiography with computed tomography; if it is available, it may allow crural artery visualization in patients with renal insufficiency.257 This technology is in evolution and not routinely available.
Duplex ultrasound imaging (DUS).
DUS imaging is usually the first imaging modality of choice and in some health care settings may be the only modality available. DUS provides information on the anatomic location and extent of disease as well as information about flow volume and velocity.258,259 There may be difficulty in directly imaging the AI segments because of body habitus, bowel gas, and movement. However, the presence of “inflow” disease can often be inferred from common femoral artery (CFA) waveforms. In the IP arterial segments, assessment can be technically challenging, particularly when vessel calcification and overlying tissue loss are present. Some vascular specialists advocate the use of ultrasound contrast agents to improve visualization; however, clinical studies to date are limited.260 Although multiple studies have shown DUS to be inferior to other imaging techniques, such as DSA, it offers many advantages as a first-line imaging modality, including its noninvasive nature, low cost, no iodinated contrast media, no ionizing radiation, and no fixed installation (mobility).25,261,262 The main disadvantages of DUS are that it is time-consuming and highly operator dependent, and it does not produce a continuous lesion map. DUS is also poor at estimating collateral blood supply and reserve. Furthermore, the stored images can be difficult to interpret at a later point in time.
CTA.
In recent years, CTA has advanced considerably in terms of accuracy and acquisition times. Modern CTA quickly generates high-resolution, contrast-enhanced images that can be viewed in multiple planes or as three-dimensional reconstructions.26,263–265 In a meta-analysis comparing CTA with DSA that predominantly included patients with IC, CTA was found to have high sensitivity and specificity in the AI (95% and 96%, respectively) and FP (97% and 94%) segments but was somewhat inferior in the IP segment (95% and 91%).29 The researchers highlighted the difficulties encountered with blooming artifact in calcified arteries (where motion-related artifact causes calcium deposits to appear larger than they truly are), which would probably result in lowered accuracy of this modality in the CLTI population, particularly in the IP segment. As such, in many centers, CTA is primarily used to image and plan intervention in AI and FP segments.266
| Recommendation | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 3.7 | Consider DUS imaging as the first arterial imaging modality in patients with suspected CLTI. | 2 (Weak) | B (Moderate) | Hingorani,24 2008 |
| 3.8 | Consider noninvasive vascular imaging modalities (DUS, CTA, MRA) when available before invasive catheter angiography in patients with suspected CLTI who are candidates for revascularization. | 2 (Weak) | B (Moderate) | Larch,25 1997 Adriaensen,26 2004 Hingorani,27 2004 Collins,28 2007 Hingorani,24 2008 Met,29 2009 |
Contrast-induced nephropathy can be a significant problem,57,267,268 and patients with pre-existing renal insufficiency are at particular risk.269 Various guidelines have been written,270,271 and many hospitals have local operating policies to try to mitigate the risks. Unfortunately, practices vary considerably, making it impossible to identify firm recommendations, outside of recognizing the risk. Finally, CTA is associated with significant doses of ionizing radiation.26,272
MRA.
MRA has the potential to produce images that are comparable in quality to DSA images but without exposure to ionizing radiation or iodinated contrast material, making contrast-induced nephropathy extremely rare.27–29,57,263–269,272–276 Time-resolved techniques can accurately image flow patterns, which can be helpful in assessing IP runoff. In a meta-analysis, MRA also showed improved specificity and sensitivity over CTA and DUS.276 Whereas conventional time-of-flight MRA sequences may overestimate the degree of arterial stenosis, newer techniques suggest that noncontrast-enhanced MRA remains an excellent imaging modality for patients with CLTI, accurately assessing distal lower extremity vessels.277 However, failure of MRA to visualize vessel wall calcification may underestimate the difficulty of surgical and endovascular revascularization. Contrast-enhanced MRA (CE-MRA) using gadolinium-based contrast agents is generally preferred because of the high contrast to noise ratio, better spatial resolution, more rapid acquisition, and less artifact. Time-resolved MRA is particularly useful in imaging of IP disease.274 Finally, MRA produces a three-dimensional map of the overall arterial tree, with the possibility of additional accurate mapping of the IP and foot vessels in more specialized centers. Other challenges of MRA include the potential overestimation of stenoses, problems visualizing in-stent restenosis, compatibility with implanted devices such as pacemakers and defibrillators, longer image acquisition times, and image artifact. Patients often have a lower tolerance for MRA than for CTA because of claustrophobia. Accurate interpretation of the images by a dedicated subspecialist, such as a vascular radiologist, is essential in aiding revascularization strategies. MRA equipment is expensive, although it can be used for other nonvascular magnetic resonance-based investigations. Thus, in some developing and developed countries, access to MRA and to dedicated subspecialists who are available to interpret the images is scarce.229 Finally, gadolinium contrast enhancement has been associated with cases of nephrogenic systemic fibrosis, primarily in individuals with an estimated glomerular filtration rate of <30 mL/min/1.73 m2.278
Foot MRA.
CLTI patients have a high incidence of IP and pedal artery disease. The precise location, length, and severity of disease as well as the patency of runoff vessels should ideally be delineated before revascularization planning. In highly specialized centers, compared with DSA, foot CE-MRA yielded a sensitivity of 92% for the detection of significant disease in IP and pedal vessels.279 Magnetic resonance perfusion imaging may have a role in assessing overall foot perfusion before and after intervention.280,281 As for limitations of foot CE-MRA, in slow-flow states, there may be significant venous overlay obscuring arterial anatomy, and the availability of the modality is limited.
In summary, MRA is still an evolving technology with new contrast-enhanced and noncontrast-enhanced sequences being reported in the literature. Time will tell whether these advances will overcome some of the current limitations. However, access to the most modern imaging techniques is highly variable around the world.
| Recommendation | ||
|---|---|---|
|
| ||
| 3.9 | Obtain high-quality angiographic imaging of the lower limb (with modalities and techniques to be determined by local available facilities and expertise). This should include the ankle and foot in all patients with suspected CLTI who are considered potential candidates for revascularization. | Good practice statement |
Catheter DSA.
With the advent of DUS, CTA, and MRA, diagnostic DSA is probably performed less commonly now, but many vascular specialists still consider it the gold standard imaging modality in patients with suspected CLTI, particularly when IP disease is likely to be present.282 Enthusiasts for DSA will also point out that it allows intervention at the same setting. Other vascular specialists, however, argue that diagnostic DSA is outdated. The DSA technique should minimize the amount of iodinated contrast material and the dose of ionizing radiation used while maximizing imaging of the distal vasculature.268,283–285 In general, diagnostic DSA is widely available, and the complication rate is low.283,286
CO2 angiography.
CO2 angiography can be used in patients with an allergy to contrast material or in individuals with severe CKD; unfortunately, it frequently causes significant discomfort of the patient. CO2 angiography is generally considered inferior to iodinated angiography but can still provide useful diagnostic images. There is a general trend of imaging performance progressively degrading down the leg.287 Power injectors may improve safety and quality.
Perfusion angiography.
This is a new technique performed with use of a dedicated imaging suite and workstation to provide time-resolved perfusion imaging of the foot to aid in the diagnosis and impact of revascularization techniques. Perfusion angiography provides quantifiable information of the functional status of foot perfusion and is a positive step toward functional imaging of the foot.288
Summary
All patients presenting with CLTI should have a full history and physical examination followed by noninvasive hemodynamic testing. These studies can be easily performed in most centers around the world. The authors recommend that all patients undergo limb staging by a classification system, such as WIfI, that integrates multiple key elements (eg, wound, ischemia, infection) and correlates with the risk of amputation and the likelihood of wound healing. The next step in appropriate candidates (Section 6) is to obtain high-quality diagnostic images to guide revascularization. This will depend heavily on the availability of equipment and local expertise (Fig 3.2). Where it is available, DUS is the preferred first noninvasive imaging modality. However, for more complete noninvasive anatomic imaging, either MRA or CTA can be considered.
Fig 3.2.
Suggested algorithm for anatomic imaging in patients with chronic limb-threatening ischemia (CLTI) who are candidates for revascularization. In some cases, it may be appropriate to proceed directly to angiographic imaging (computed tomography angiography [CTA], magnetic resonance angiography [MRA], or catheter) rather than to duplex ultrasound (DUS) imaging.
Catheter DSA represents the gold standard imaging technique, especially below the knee. In many centers, however, DSA is typically used only when MRA or CTA is not available, when MRA or CTA imaging is suboptimal and fails to adequately define the arterial anatomy, or for those patients expected to proceed to endovascular intervention. No patient with suspected CLTI who is a suitable candidate for limb salvage should be denied revascularization without first undergoing complete diagnostic angiography that includes the ankle and foot.
| Research priorities | |
|---|---|
|
| |
| 3.1 | Define optimal methods for measuring foot perfusion and its correlation with stages of disease and response to treatment. |
| 3.2 | Validate contrast-enhanced ultrasound in patients with CLTI. |
| 3.3 | Define optimal strategies to reduce the incidence of contrast-induced nephropathy in patients with CLTI. |
| 3.4 | Improve noninvasive imaging of the ankle and foot vascular tree using MRA. |
4. MEDICAL MANAGEMENT
CLTI is an end-stage manifestation of systemic atherosclerosis. It is frequently accompanied by clinically significant CVD, resulting in exceedingly high mortality from stroke and myocardial infarction. In the absence of aggressive identification and treatment of risk factors and associated comorbid conditions, the prognosis of CLTI is usually poor, with a mortality rate of 20% to 26% within 1 year of diagnosis.5,30,154,213,219,220,230,289
In a study of 574 patients with CLTI who did not undergo revascularization after 2 years, 31.6% had died, primarily of CVD, and 23% required major amputation.290
The goal of treatment of patients with CLTI is not only to salvage a functional limb but to reduce cardiovascular morbidity and mortality through aggressive risk factor modification and best medical therapy.31,32,224 Whereas certain risk factors, such as age and sex, cannot be modified, others can, including hyperlipidemia, hypertension, diabetes, smoking, and sedentary lifestyle.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 4.1 | Evaluate cardiovascular risk factors in all patients with suspected CLTI. | 1 (Strong) | B (Moderate) | I.C.A.I. group,30 1997 |
| 4.2 | Manage all modifiable risk factors to recommended levels in all patients with suspected CLTI. | 1 (Strong) | B (Moderate) | Armstrong,224 2014 Faglia,32 2014 |
Antithrombotic therapy.
Antiplatelet agents are strongly recommended for all patients with symptomatic PAD to reduce the risk of major adverse cardiovascular events (MACE).33,34,291 The Antithrombotic Trialists’ Collaboration performed a meta-analysis of antiplatelet agent trials before 1997.33 It included 135,000 patients with cerebrovascular disease, coronary disease, or PAD (IC) who were treated with antiplatelet agents and 77,000 control patients. The antiplatelet therapy group had a 22% reduction in MACEs, and 75 to 150 mg of aspirin per day was as effective as higher doses but with a lower risk of bleeding.33 A more recent meta-analysis studied the specific benefit of aspirin in 16 secondary prevention trials comprising 17,000 patients.34 This study confirmed the benefit of antiplatelet agents with an 18.2% reduction in MACEs in both men and women. The Critical Leg Ischaemia Prevention Study (CLIPS) group compared the benefit of 100 mg of aspirin per day in 185 patients with symptoms of PAD and an ABI <0.85 or a TBI <0.6 with placebo and reported a 64% risk reduction in vascular events compared with a 24% reduction in the placebo group.291
However, there is a growing body of literature indicating that alternatives to aspirin, such as ticlopidine, dipyridamole, and clopidogrel, may be more effective.35,292–294 The Clopidogrel versus Aspirin in Patients at Risk for Ischaemic Events (CAPRIE) trial, although not specifically designed to address CLTI, compared 75 mg of clopidogrel per day with 325 mg of aspirin per day in patients with PAD. Researchers noted an 8.7% decrease in MACEs with clopidogrel compared with aspirin. There was no significant difference in bleeding risks between the two agents.35
Other antiplatelet agents, such as ticagrelor and vorapaxar, have also been shown to reduce MACEs in patients with PAD.292–294 However, benefit over clopidogrel has not been demonstrated.36,294–298 The Examining Use of Ticagrelor in Peripheral Artery Disease (EUCLID) trial compared ticagrelor with clopidogrel in 13,885 patients with symptomatic PAD and an ABI ≤0.8.36 Although both drugs had a similar safety profile, ticagrelor was not superior to clopidogrel. The Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients with Atherosclerosis-Thrombolysis in Myocardial Infarction 50 (TRA2°P-TIMI 50) examined the effects of the protease-activated receptor 1 antagonist vorapaxar on secondary prevention of ischemia events in patients with stable atherosclerosis, including symptomatic PAD.295 Acute limb ischemia, a prespecified study end point, was reduced by 41% among the PAD cohort.298 However, vorapaxar has been associated with an increase in intracranial hemorrhage in patients who have had a prior stroke or transient ischemic attack.296 In a meta-analysis, vorapaxar added to aspirin yielded little improvement in the reduction of MACEs in patients with atherosclerosis and was associated with a slightly higher incidence of intracranial hemorrhage.294 Finally, a meta-analysis that reviewed the use of ticagrelor, ticlopidine, aspirin, cilostazol, picotamide, vorapaxar, and clopidogrel as single antiplatelet therapy or dual antiplatelet therapy (DAPT) in patients with PAD found that clopidogrel monotherapy resulted in the best overall safety and efficacy (reduction of MACEs).297
The long-term use of DAPT or systemic anticoagulation with vitamin K antagonists is not indicated for PAD.299,300 The role of direct oral anticoagulants is currently the subject of intense investigation. The Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial, a multicenter randomized trial of 7470 individuals with stable, mild to moderate PAD, found that low-dose rivaroxaban (an oral factor Xa inhibitor) in combination with aspirin reduced MACEs (death, myocardial infarction, or stroke) and major adverse limb events (MALEs) compared with aspirin alone.37 Patients who had previous lower extremity revascularization, amputation, or history of IC and ABI of <0.9 and documented peripheral stenosis of >50% or carotid stenosis of >50% were included in the study. Overall, 8.5% of study patients had an ABI of <0.7. In this population, there was a significant reduction in MALEs, major amputation, and acute limb ischemia compared with aspirin alone.301 This drug combination was associated with a small but statistically significant increase in clinically relevant bleeding. Whereas the study results are promising, the benefits and risks of the low-dose rivaroxaban and low-dose aspirin combination in patients with CLTI have not yet been adequately defined. In addition, this drug combination is not globally available at this time.
The ongoing VOYAGER trial (ClinicalTrials.gov identifier NCT02504216) is comparing the same two antithrombotic regimens in PAD patients undergoing peripheral revascularization.302
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 4.3 | Treat all patients with CLTI with an antiplatelet agent. | 1 (Strong) | A (High) | Antithrombotic Trialists’ Collaboration,33 2002 Antithrombotic Trialists’ Collaboration,34 2009 |
| 4.4 | Consider clopidogrel as the single antiplatelet agent of choice in patients with CLTI. | 2 (Weak) | B (Moderate) | CAPRIE,35 1996 Hiatt,36 2017 |
| 4.5 | Consider low-dose aspirin and rivaroxaban, 2.5 mg twice daily, to reduce adverse cardiovascular events and lower extremity ischemic events in patients with CLTI. | 2 (Weak) | B (Moderate) | Anand,37 2018 |
| 4.6 | Do not use systemic vitamin K antagonists for the treatment of lower extremity atherosclerosis in patients with CLTI. | 1 (Strong) | B (Moderate) | Anand,38 2007 |
Lipid-lowering therapy.
The Heart Protection Study (HPS) evaluated the effect of blood lipid lowering on cardiovascular events in PAD and included patients with CLTI.40 Other studies, although similar, limited inclusion to patients with IC.41 The HPS included 20,536 high-risk individuals with a total cholesterol concentration of at least 135 mg/dL (3.5 mmol/L). The participants were randomized to 40 mg/d of simvastatin or a placebo. In the simvastatin group, there was a 25% (95% CI, 16%-33%) relative risk (RR) reduction in the first major vascular event among patients who had no history of a coronary event at baseline.40 In addition, lipid lowering was shown to be most effective in patients with a blood cholesterol concentration >135 mg/dL (> 3.5 mmol/L). There was also a significant reduction in cardiovascular events (P < .0001) among a subgroup of individuals with PAD.
A Cochrane review evaluated 18 lipid-lowering trials comprising 10,049 PAD patients.39,42 Whereas the majority had IC and only some trials included CLTI, the results appear relevant to the CLTI population. Only one study showed a negative effect of lipid lowering. When this study was excluded, analysis showed that lipid-lowering therapy significantly reduced the risk of total cardiovascular events in PAD (OR, 0.74; CI, 0.55–0.98).42 This was primarily due to a positive effect on total coronary events (OR, 0.76; CI, 0.67–0.87).
The impact of statin agents may extend beyond their lipid-lowering effect by reducing inflammation in patients with PAD.303,304 An individual-patient data meta-analysis of 54 prospective cohort studies demonstrated that inflammatory biomarkers independently predict vascular risk with a magnitude of effect at least as large as that of blood pressure or cholesterol.305 Even after adjustment for age, sex, and traditional risk factors, patients with PAD are known to have increased levels of inflammatory cytokines, acute phase reactants, and soluble adhesion molecules.306 However, although the attributable vascular risk associated with inflammation is large and animal models using targeted anti-inflammatory therapies have shown promise, it remains unknown whether inhibiting inflammation alone will lower vascular event rates.
The landmark Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) examined the use of intensive statin therapy (rosuvastatin 20 mg daily vs placebo) in a primary prevention trial.307,308 In total, there were 17,802 individuals who had low levels of LDL-C but an elevated vascular risk based on a proinflammatory biomarker (high levels of high-sensitivity C-reactive protein). Investigators demonstrated a 44% reduction in major vascular events, including a 54% reduction in myocardial infarction, a 48% reduction in stroke, a 46% reduction in arterial revascularization, a 43% reduction in deep venous thrombosis or pulmonary embolism, and a 20% reduction in mortality. The greatest absolute risk and the greatest absolute risk reduction were observed among those with the highest levels of high-sensitivity C-reactive protein. There are now multiple studies showing a decrease in cardiovascular events in patients with established atherosclerosis treated with intensive statin therapy.43,224,309,310 A large retrospective cohort study from the U.S. Veterans Affairs population demonstrated reduced mortality and major amputation rates among patients with established PAD receiving intensive-dose statins.311 Statin therapy can be associated with muscle aching, the most common adverse effect limiting its use. In the setting of this complication, statin dose can be lowered to the maximum tolerated dose, and a second nonstatin cholesterol-lowering drug can be added to reduce cholesterol levels even further.
Recent (2013, 2018) American College of Cardiology/American Heart Association guidelines on treatment of blood cholesterol recommend the use of moderate- to high-intensity statins for all individuals with established atherosclerotic CVD including PAD.312,313 Both rosuvastatin (20–40 mg) and atorvastatin (40–80 mg) have been shown to be effective.310 The 2018 guideline describes “very high risk” individuals to include those with symptomatic PAD and at least one other high-risk condition (age ≥65 years, familial hypercholesterolemia, history of coronary revascularization, DM, hypertension, CKD, current smoking, congestive heart failure)–a categorization that applies to the overwhelming majority of patients with CLTI. For this population, high-intensity/maximally tolerated statin dosing is recommended, and if on-treatment LDL-C levels remain ≥70 mg/dL (1.8 mmol/L), the addition of ezetimibe is considered reasonable.313
New lipid-lowering agents have entered the armamentarium. Proprotein convertase subtilisin/kexin type 9 (PCSK9) directs the degradation of LDL receptors in the liver and has become a drug target. The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) RCT demonstrated an additional benefit of evolocumab (a PCSK9 inhibitor) in reducing MACEs in PAD patients already receiving statin therapy.314 The composite end point of cardiovascular death, myocardial infarction, stroke, hospital admission for unstable angina, or coronary revascularization was statistically reduced in PAD patients treated with the PCSK9 inhibitor evolocumab (hazard ratio [HR], 0.79; P = .0040). There was also a reduction in the risk of MALEs, including acute limb ischemia and major amputation. Further studies will be needed in PAD subpopulations including CLTI.
Further studies of these agents are desirable in high-risk PAD subpopulations including CLTI.
| Recommendation | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 4.7 | Use moderate- or high-intensity statin therapy to reduce all-cause and cardiovascular mortality in patients with CLTI. | 1 (Strong) | A (High) | Leng,39 2000 Heart Protection Study Group,40 2002 Meade,41 2002 Aung,42 2007 Mills,43 2011 Rodriguez,44 2017 |
Management of hypertension.
It is universally accepted that control of hypertension reduces MACEs in patients with PAD. The International Verapamil-SR/Trandolapril Study (INVEST) analyzed the impact of control of hypertension on all-cause death, nonfatal myocardial infarction, and nonfatal stroke in 22,576 hypertensive patients with stable coronary artery disease (CAD), of whom 2699 also had PAD.46 PAD patients had a significantly higher incidence of sustaining a primary end point MACE compared with those without PAD (16.3% vs 9.2%). In addition, among those with PAD, a MACE was less likely to occur in patients with systolic blood pressure <145 mm Hg and diastolic pressures <90 mm Hg. Further reduction of blood pressure to below 130 mm Hg systolic and 80 mm Hg diastolic provides even greater protection from cardiovascular events.48 The Systolic Blood Pressure Intervention Trial (SPRINT) compared blood pressure control with a systolic pressure of 120 mm Hg (intensive control) or 140 mm Hg (standard control) in 2510 patients with a mean age of 79.9 years observed for a mean of 3.14 years.315 The study documented a significantly lower incidence of composite cardiovascular events of death with intensive control. However, intensive blood pressure control may result in greater morbidity associated with periods of clinically significant hypotension.45,47 Optimal blood pressure control for patients with CLTI has not been established, and although maintaining systolic pressure <140 mm Hg and diastolic pressure <90 mm Hg is important, lower pressures may be beneficial to further reduce MACEs.
The first-line category of oral antihypertensive does not appear to be of significance. Angiotensin-converting enzyme inhibitors (ACEIs), calcium channel blockers, and diuretics, when successful in lowering blood pressure to target, reduce cardiovascular events to a similar extent.316,317 Although the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) and Heart Outcomes Prevention Evaluation (HOPE) study suggested that in the absence of heart failure, monotherapy with an ACEI (ramipril) reduces the rate of MACEs in high-risk patients, there is recent evidence to suggest that this class of drug may result in a higher amputation rate for patients with CLTI.318 In an analysis of the Medicare database for 2007 to 2008, there were 22,954 patients who underwent lower extremity revascularization. Of these, 64.6% were treated for CLTI. Compared with those not taking an ACEI, patients who presented with rest pain and were taking an ACEI after the index procedure had a higher risk of amputation. Other studies have not noted an increased risk of amputation associated with ACEIs but have suggested an increased rate of reintervention. A propensity score-matched cohort study of 17,495 Danish patients compared those receiving ACEIs with those who were not after vascular reconstruction. Observed for a mean of 1.6 years, the patients treated with ACEIs had a lower all-cause mortality (20.4% vs 24.9%) but underwent more reintervention (24% vs 23.1%).319 Using the same general methodology, these investigators found that the use of beta blockers after primary vascular reconstruction was associated with a decrease in the incidence of major amputation but a higher rate of myocardial infarction and stroke without an increase in all-cause mortality.320
Globally, adequate control of hypertension remains a significant challenge. In LMICs, the availability of oral antihypertensives is limited and costs are high, resulting in poor overall blood pressure control. Strategies are urgently required to improve availability and affordability of drugs so that vascular specialists can treat their patients to target.321
There have been concerns that drugs reducing heart rate and blood pressure will worsen ischemia in patients with PAD. Although beta blockade has not been directly evaluated in CLTI, it has been the subject of several clinical trials in IC and has been shown to be effective in lowering blood pressure without worsening symptoms.322,323
Management of diabetes.
Type 2 DM is a significant risk factor for PAD,324,325 and the extent of vascular disease appears related to the duration and severity of hyperglycemia. Glycemic control is therefore essential in all diabetic patients with PAD. Metformin monotherapy is generally recognized as the best initial oral hypoglycemic agent. When additional therapy is needed, any other class of oral hypoglycemic agent, including sulfonylurea, thiazolidinedione, dipeptidyl peptidase 4 inhibitor, or α-glucosidase, can be added with equal effectiveness.54
Sodium-glucose co-transporter 2 (SGLT-2) inhibitors are a newer class of agents that have been associated with beneficial effects on cardiovascular complications, renal disease, and mortality in type 2 diabetics. However, one large trial (10,142 subjects) demonstrated an approximately 2-fold increased risk of lower limb amputations associated with the use of canaglifozin, an SGLT-2 inhibitor, prompting a “black-box” warning.326–328 The mechanism is unclear and may be generically related to diuretic actions in this population.329 Caution is advised in the use of this agent in diabetic patients with advanced PAD and/or CLTI.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 4.9 | Consider control of type 2 DM in CLTI patients to achieve a hemoglobin A1c of <7% (53 mmol/mol [International Federation of Clinical Chemistry]). | 2 (Weak) | B (Moderate) | Selvin,49 2004 Nathan,50 2005 van Dieren,51 2014 Fox,52 2015 American Diabetes Association,53 2018 |
| 4.10 | Use metformin as the primary hypoglycemic agent in patients with type 2 DM and CLTI. | 1 (Strong) | A (High) | Palmer,54 2016 |
| 4.11 | Consider withholding metformin immediately before and for 24 to 48 hours after the administration of an iodinated contrast agent for diabetic patients, especially those with an estimated glomerular filtration rate <30 mL/min/1.73 m2. | 2 (Weak) | C (Low) | Nawaz,55 1998 Goergen,56 2010 Stacul,57 2011 |
Whereas there are some data to suggest that the dipeptidyl peptidase 4 inhibitors may reduce the risks of myocardial infarction and stroke, the impact on PAD in patients with CLTI has not yet been defined.330 The goal for most adults with DM is to maintain a glycosylated hemoglobin A1c level of <7% (equivalent to International Federation of Clinical Chemistry units of 53 mmol/mol).49–52 However, less stringent goals (eg, hemoglobin A1c level <8%) may be appropriate for individuals with advanced vascular complications or limited life expectancy.53
Type 2 DM patients with abnormal renal function treated with metformin may be at higher risk for contrast-induced nephropathy and lactic acidosis. Whereas the matter is the subject of continued debate, it is reasonable to withhold metformin for 24 to 48 hours after the administration of an iodinated contrast agent.55–57,270,271
Lifestyle modifications.
In addition to controlling risk factors as discussed, it is important to encourage CLTI patients to adopt a healthier lifestyle. Stopping smoking (tobacco and other recreational drugs) completely and permanently, adopting a healthy diet and weight control, and regular exercise must be stressed as extremely important for both life and limb.331,332
Tobacco.
The adverse impact of tobacco use on cardiovascular health has been well established. Despite the use of best medical therapy, male and female smokers (even those smoking 1–10 cigarettes per day) have a significantly higher rate of disease progression and MACEs.58–60 Thus, all patients presenting with CLTI should be asked about smoking and referred to a smoking cessation program if they are still smoking. To encourage compliance with advice to stop smoking, patients should be challenged about smoking at every medical encounter.61,62 The safety of electronic cigarettes has not been established, including for patients with PAD, and until more evidence becomes available should not be considered in patients with CLTI.333
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 4.12 | Offer smoking cessation interventions (pharmacotherapy, counseling, or behavior modification therapy) to all patients with CLTI who smoke or use tobacco products. | 1 (Strong) | A (High) | Dagenais,58 2005 Athyros59 2013 Blomster,60 2016 |
| 4.13 | Ask all CLTI patients who are smokers or former smokers about status of tobacco use at every visit. | 1 (Strong) | A (High) | Kondo,61 2011 Newhall,62 2017 |
Diet and exercise.
Although diet and exercise have not been specifically evaluated in CLTI, there is compelling evidence that they affect the progression of atherosclerosis. Diets that are high in carbohydrates and saturated fats are associated with a higher risk of MACEs.334 A diet that reduces the intake of saturated fats and increases the intake of monounsaturated fats, omega-3 fatty acids, antioxidants, and other natural plant sterols and stanols is associated with a reduction in plaque burden and MACEs.335–337 Patients should be encouraged to adopt a low-fat or Mediterranean diet.338 Unfortunately, fruits and vegetables are not always available or affordable, especially in LMICs.339
Although CLTI studies are not available, numerous trials have confirmed the benefits of supervised exercise in IC.340 Exercise-based cardiac rehabilitation reduces the risk of subsequent myocardial infarction and cardiovascular mortality.341 It therefore seems reasonable to suggest that a postrevascularization walking-based exercise program would also benefit CLTI patients who are cleared for full weight-bearing.
Management of pain.
Although pain is an important issue for most CLTI patients, it is often poorly managed. Poor pain control can reduce HRQL levels to those seen in patients with terminal cancer and has a major adverse impact on functional capacity.
As no RCTs have been conducted in CLTI, good practice recommendations have to be extrapolated from other conditions in which severe pain is a major factor. The management of ischemic pain in CLTI is often complicated by the coexisting neuropathic pain, particularly in patients with DM. However, the management of neuropathic pain is not covered here.
Guidelines usually recommend a tiered approach to pain management, with a “tradeoff” between benefits and harms (eg, constipation, drowsiness).342,343 Patients should be offered paracetamol (acetaminophen) in combination with opioids and in proportion to the severity of pain. All patients receiving opioids should also be offered laxatives and antinausea medication. If the maximum tolerated analgesic dose does not produce adequate pain relief, alternative approaches should be considered. These include tricyclic antidepressants, gabapentin, and pregabalin, all of which are used effectively for neuropathic pain. However, if the clinician is unfamiliar with the use of these compounds, early referral to a pain management service for patients with pain not controlled by opioids is required.
| Recommendations | ||
|---|---|---|
|
| ||
| 4.14 | Prescribe analgesics of appropriate strength for CLTI patients who have ischemic rest pain of the lower extremity and foot until pain resolves after revascularization. | Good practice statement |
| 4.15 | In CLTI patients with chronic severe pain, use paracetamol (acetaminophen) in combination with opioids for pain control. | Good practice statement |
| Research priorities | |
|---|---|
|
| |
| 4.1 | Define the optimal antithrombotic regimen (safety and efficacy) in patients with CLTI to reduce cardiovascular and limb-specific events. |
| 4.2 | Define treatment targets and optimal dosing for lipid-lowering agents in the CLTI population. |
| 4.3 | Identify biomarkers predictive of clinical events in the CLTI population that may serve as targets for therapy. |
| 4.4 | Identify effective smoking cessation strategies for patients with advanced PAD and CLTI. |
| 4.5 | Identify the type of analgesia that is most effective in patients with chronic pain secondary to CLTI. |
5. THE GLOBAL LIMB ANATOMIC STAGING SYSTEM (GLASS)
Rationale.
An accurate assessment of limb threat and stratification of the anatomic pattern of disease are the foundations of EBR. This is true not only in everyday practice but also in outcomes assessment and research. The authors propose a new, clinically oriented framework for classifying the pattern of arterial disease in CLTI. The GLASS is a fundamental departure from current approaches used in PAD and more analogous to the SYNTAX system for CAD.344,345
Current PAD anatomic classification schemes either describe the location and severity of individual arterial lesions11,156 or quantify the overall burden and morphology of disease.12,151,170 Lesion- or segment-based grading systems are useful for comparing endovascular device performance in well-defined clinical situations. They are not, however, useful for defining EBR strategies in CLTI, especially given the complex, multilevel, and increasingly distal disease patterns typically seen in current clinical practice.
Successful revascularization in CLTI, particularly in patients with tissue loss, nearly always requires restoration of pulsatile in-line flow to the foot. Because individual lesion-based schemes correlate poorly with effective revascularization in CLTI, vascular specialists must integrate approaches for arterial segments into a management strategy for the whole limb. Factors that determine a successful anatomic outcome are intrinsically different for bypass grafting and endovascular intervention. Bypass surgery requires adequate inflow and outflow and, perhaps most important, a suitable autologous conduit. By contrast, the success of endovascular intervention is largely defined by the complexity of atherosclerosis within the anticipated target arterial path (TAP) that provides in-line flow to the foot. When the TAP includes multiple lesions in series, technical success and sustained patency for the limb as a whole must be estimated as a product function of each lesion traversed.
GLASS is based on defining the TAP in each individual patient by high-quality imaging and requires selection of a preferred infrapopliteal (IP) artery. The TAP is generally selected on the basis of the least diseased crural artery providing runoff to the foot. It can also be selected on the basis of other relevant factors, such as angiosome preference or avoidance of a previously instrumented vessel. Whereas the relationship between the pattern of occlusive disease, patency of the chosen intervention, and clinical success in CLTI is a complex one, an integrated limb-based anatomic staging system like GLASS is critical to define it. The preferred TAP for endovascular intervention and the preferred target artery for open bypass surgery may not always be the same; clinical decision-making thus hinges on a comparative estimate of risk and success for each. Like SYNTAX, GLASS stage is designed to correlate primarily with endovascular outcomes. As such, it does not incorporate factors like venous conduit quality or distal runoff that are more directly relevant for bypass grafting.
GLASS provides a basis for clinical practice and supports future research in CLTI. When it is combined with tools for stratification of patient risk and severity of limb threat (Sections 1 and 3), GLASS facilitates the development of specific evidence-based revascularization (EBR) guidelines in CLTI (Section 6). In developing GLASS, the writing group was informed by a commissioned systematic review of revascularization outcomes in CLTI and expert opinion. Still, the authors acknowledge that the new grading system requires prospective validation in a variety of patient populations and health care environments. The system is expected to undergo revisions as outcomes are reported. Important factors for refinement include the current state of limited high-quality evidence in the field, ongoing changes in both epidemiology and technology, and differences in disease patterns and practice around the world.
Assumptions and approach.
As CLTI is usually the result of complex multilevel occlusive disease, certain simplifying assumptions are required to develop a usable anatomic staging system (Table 5.1). First, because existing schemes for AI disease appear adequate, the focus of GLASS is on infrainguinal disease (a simplified inflow disease scheme is presented in Table 5.2). In GLASS, the CFA and PFA are seen as inflow arteries, and the infrainguinal system begins at the origin of the SFA. This is justified by the distinct approaches used in the treatment of CFA and PFA disease (Section 6) and long-term results that are similar to those for AI interventions.
Table 5.1.
Key definitions and assumptions in the Global Limb Anatomic Staging System (GLASS)
| Restoration of in-line flow to the ankle and foot is a primary goal. |
| Target arterial path (TAP): the selected continuous route of in-line flow from groin to ankle. The TAP typically involves the least diseased IP artery but may be angiosome based.a |
| Limb-based patency (LBP): maintained patency of the TAP |
| Inflow disease (AI and CFA) is considered separately and assumed corrected when using the infrainguinal staging system for clinical decision-making. |
| Grade within segment is determined by presence of any one of the defined descriptors within that grade (ie, the worst disease attribute within the segment defines grade). |
| Calcification is considered only if severe; increases within segment grade by 1. |
| IM disease (pedal) modifier: describes status of IM vessels (including terminal divisions of the peroneal artery) providing outflow into the foot. |
AI, Aortoiliac; CFA, common femoral artery; IM, inframalleolar; IP, infrapopliteal.
The generic case of rest pain is used as a default for defining TAP as the least diseased IP artery, or a specific IP target artery based on clinical circumstances (eg, angiosome directed in setting of wounds) may be selected by the clinician.
Table 5.2.
Aorto-iliac (inflow) disease staging in GLASS
| I Stenosis of the common and/or external iliac artery, chronic total occlusion of either common or external iliac artery (not both), stenosis of the infrarenal aorta; any combination of these |
| II Chronic total occlusion of the aorta; chronic total occlusion of common and external iliac arteries; severe diffuse disease and/or small-caliber (<6 mm) common and external iliac arteries; concomitant aneurysm disease; severe diffuse in-stent restenosis in the AI system |
| A, no significant CFA disease; B, significant CFA disease (>50% stenosis) |
AI, Aortoiliac; CFA, common femoral artery.
A simplified staging system for inflow (AI and CFA) disease is suggested. Hemodynamically significant disease (>50% stenosis) of the CFA is considered a key modifier (A/B).
For GLASS to be useful in everyday clinical practice and to form the basis of practice-changing research, it is important that it does not rely on complex methods of lesion characterization. With regard to vessel calcification, GLASS adopts a dichotomous subjective scale in which severe calcification (eg, >50% of circumference; diffuse, bulky, or “coral reef” plaques) increases the within-segment grade by one numeric level. This is a subjective determination made by the treating physician that the severity of calcification significantly increases technical complexity (and expected technical failure rates) for endovascular intervention. Alternative approaches for quantifying arterial calcification in PAD have been suggested but are more complex, and none of these has been validated for discriminating clinical outcomes.346,347 With regard to IM disease, GLASS employs a three-level modifier (Fig 5.1) to describe the status of arteries crossing the ankle (including the terminal divisions of the peroneal artery) and the pedal arch. Currently, the IM disease modifier is not considered within the primary assignment of limb stages in GLASS, given the absence of strong evidence on how it affects treatment outcomes. It should, however, be captured in future studies to better define how to incorporate pedal outflow disease into anatomic staging in CLTI.
Fig 5.1.
Inframalleolar (IM)/pedal disease descriptor in Global Limb Anatomic Staging System (GLASS). Representative angiograms of P0 (left), P1 (middle), and P2 (right) patterns of disease.
GLASS also makes the following assumptions:
Restoring durable (pulsatile) in-line flow to the affected part, particularly in patients with tissue loss, is a primary goal of revascularization in CLTI.
Using high-quality imaging (Section 3), the vascular specialist chooses and defines a TAP that is most likely to achieve that in-line flow.
The TAP will usually involve the least diseased IP artery.
Other IP arteries (not selected for the TAP) are equally diseased or more so.
In addition, although it is an important research question, the current version of GLASS does not consider multivessel IP revascularization because evidence of its role is still lacking. Where the clinician is considering such revascularization, GLASS staging is based on the primary IP target, as defined by the clinician before the intervention.
In defining infrainguinal anatomic stages (I-III), GLASS combines grades (0–4) for the FP (origin of the SFA to the origin of the anterior tibial [AT] artery; Fig 5.2) and IP (origin of the tibioperoneal trunk and the AT artery to the malleoli; Fig 5.3) segments in series. Stages were developed to correlate with estimated LBP, defined as maintenance of in-line flow through the entire length of the TAP, from the SFA origin to the malleoli. LBP is considered to be lost when any one of the following occurs:
Fig 5.2.
Femoropopliteal (FP) disease grading in Global Limb Anatomic Staging System (GLASS). Trifurcation is defined as the termination of the popliteal artery at the confluence of the anterior tibial (AT) artery and tibioperoneal trunk. CFA, Common femoral artery; CTO, chronic total occlusion; DFA, deep femoral artery; Pop, popliteal; SFA, superficial femoral artery.
Fig 5.3.
Infrapopliteal (IP) disease grading in Global Limb Anatomic Staging System (GLASS). AT, Anterior tibial; CTO, chronic total occlusion; TP, tibioperoneal.
Anatomic failure: occlusion, critical stenosis, or reintervention affecting any portion of the defined TAP; or
Hemodynamic failure: a significant drop in ABI (≥0.15) or TBI (≥0.10), or identification of ≥50% stenosis in the TAP, in the presence of recurrent or unresolved clinical symptoms (eg, rest pain, worsening or persistent tissue loss).
LBP is an important new concept allowing more direct comparison between revascularization approaches in CLTI. Estimating LBP after surgical or endovascular intervention is central to the development of EBR (Section 6). The writing group defined three GLASS stages based on the likelihood of immediate technical failure (ITF)347 and 1-year LBP after endovascular intervention of the selected TAP. GLASS stages for the limb thus reflect a gradient of infrainguinal disease complexity:
Stage I: low-complexity disease: expected ITF < 10% and 1-year LBP > 70%
Stage II: intermediate-complexity disease: expected ITF < 20% and 1-year LBP 50% to 70%
Stage III: high-complexity disease: expected ITF > 20%; or 1-year LBP < 50%
Consensus process and assignment of limb stages.
To assign GLASS stages (I-III) in the two-dimensional matrix shown in Table 5.3, a multinational, multispecialty group of vascular specialists (GVG writing group and invited external experts) as well as evidence summaries7 and other published material79,160,348–404 were surveyed. Representative examples of GLASS stage I to stage III disease are illustrated in the angiograms depicted in Figs 5.4 to 5.6. Table 5.4 provides a descriptive summary of the three GLASS stages.
Table 5.3.
Assignment of Global Limb Anatomic Staging System (GLASS) Stage
| Infrainguinal GLASS stage (I-III) | ||||||
|
| ||||||
| FP Grade | 4 | III | III | III | III | III |
| 3 | II | II | II | III | III | |
| 2 | I | II | II | II | III | |
| 1 | I | I | II | II | III | |
| 0 | NA | I | I | II | III | |
| 0 | 1 | 2 | 3 | 4 | ||
| IP Grade | ||||||
NA, Not applicable.
After selection of the target arterial path (TAP), the segmental femoropopliteal (FP) and infrapopliteal (IP) grades are determined from high-quality angiographic images. Using the table, the combination of FP and IP grades is assigned to GLASS stages I to III, which correlate with technical complexity (low, intermediate, and high) of revascularization.
Fig 5.4.
Representative angiograms of Global Limb Anatomic Staging System (GLASS) stage I disease patterns. The target arterial path (TAP) is outlined in yellow. Left panel, TAP includes the anterior tibial (AT) artery. Femoropopliteal (FP) grade is 0. Infrapopliteal (IP) grade is 2 (3-cm chronic total occlusion; chronic total occlusion of AT artery and total length of disease <10 cm). Right panel, TAP includes the peroneal artery. FP grade is 2 (chronic total occlusion <10 cm; total length of disease <). IP grade is 0.
Fig 5.6.
Representative angiograms of Global Limb Anatomic Staging System (GLASS) stage III disease patterns. The target arterial path (TAP) is outlined in yellow. Left panel, TAP includes the peroneal artery. Femoropopliteal (FP) grade is 4 (superficial femoral artery [SFA] disease length, 10–20 cm; popliteal stenosis <5 cm; heavily calcified). Infrapopliteal (IP) grade is 2 (stenosis of tibioperoneal trunk and proximal peroneal <10 cm). Right panel, TAP includes the anterior tibial (AT) artery. FP grade is 4 (popliteal chronic total occlusion extending into trifurcation). IP grade is 3 (chronic total occlusion of target artery origin).
Table 5.4.
Descriptive summary of Global Limb Anatomic Staging System (GLASS) stages of infrainguinal arterial disease
| Estimated PVI outcomes |
|||
|---|---|---|---|
| Stage | Technical failure | 1-year LBP | Anatomic pattern |
|
| |||
| I | <10% | >70% | Short- to intermediate-length FP disease and/or short-length IP disease; no or minimal popliteal disease |
| II | <20% | 50%–70% | Intermediate- to long-length FP disease; may include popliteal stenosis and/or short- to intermediate-length IP disease |
| III | >20% | <50% | Extensive FP or IP occlusions, alone or in combination with any disease in the other segment; popliteal CTO |
CTO, Chronic total occlusion; FP, femoropopliteal; IP, infrapopliteal; LBP, limb-based patency; PVI, peripheral [endo-]vascular intervention.
Managing CLTI with GLASS.
Use of the GLASS system involves the following steps (Fig 5.7):
Fig 5.7.
Flow chart illustrating application of Global Limb Anatomic Staging System (GLASS) to stage infrainguinal disease pattern in chronic limb-threatening ischemia (CLTI). FP, Femoropopliteal; IP, infrapopliteal; PLAN, patient risk estimation, limb staging, anatomic pattern of disease; TAP, target arterial path; WIfI, Wound, Ischemia, and foot Infection.
Obtain high-quality angiographic imaging to include the ankle and foot (Section 3).
Identify the TAP.
Determine the FP GLASS grade (0–4) (Fig 5.2).
Determine the IP GLASS grade (0–4) (Fig 5.3).
Decide whether there is severe calcification (eg, >50% of circumference; diffuse, bulky, or coral reef plaques likely to compromise endovascular outcomes) within the FP and IP segments of the TAP. If present, increase the segment grade by one.
Combine FP and IP grades to determine the overall GLASS stage (Table 5.3).
Use the pedal modifier (P0, P1, or P2) to describe the status of IM arteries.
For the individual patient with CLTI, an EBR strategy (Section 6) is based on the full integration of
estimated patient risk and long-term survival;
severity of limb threat (eg, using WIfI) (Sections 1 and 3); and
anatomic pattern and severity of disease in the affected limb (eg, GLASS).
| Recommendation | ||
|---|---|---|
|
| ||
| 5.1 | Use an integrated, limb-based anatomic staging system (such as the GLASS) to define complexity of a preferred TAP and to facilitate EBR in patients with CLTI. | Good practice statement |
| Research priorities | |
|---|---|
|
| |
| 5.1 | What are the expected procedural, hemodynamic, and clinical outcomes of revascularization across the spectrum of infrainguinal disease severity? Better evidence is needed to validate the GLASS, particularly for endovascular strategies in intermediate (II) and severe (III) stages of infrainguinal disease. |
| 5.2 | What is the effect of severe IM and pedal arch disease on revascularization outcomes in CLTI? Is there a clinically useful way to grade this level of disease? |
| 5.3 | Is there evidence that other measures, such as outflow bed resistance or below-knee runoff scores, are predictive of procedural or clinical outcomes? How do these compare with target path lesion complexity assessed by angiography? |
| 5.4 | Is there a simple, reproducible method for quantification of calcification that has predictive value for infrainguinal interventions? |
| 5.5 | Are there specific patient factors (eg, demographic or comorbidity) associated with anatomic patterns of disease in CLTI? |
| 5.6 | Are there anatomic patterns of disease in which an endovascular approach is futile? |
| 5.7 | How does lesion morphology (eg, concentric vs eccentric) influence treatment success for different endovascular interventions? |
| 5.8 | Is there a correlation between GLASS stage and clinical presentation (WIfI)? |
| 5.9 | What is the comparative value of direct (angiosome based) vs indirect revascularization in the setting of tissue loss, and how should it drive selection of the preferred TAP? Is this specific to wound location or WIfI stage? |
Limitations and future direction.
The authors acknowledge the limitations of the available data in developing this initial version of GLASS. Severe calcification, particularly in the tibial arteries, is a negative predictor of technical success for intervention and signifies a higher risk for amputation.405,406 However, a simplified and validated scoring system for calcification that is associated with procedural outcomes is still lacking.346 At the same time, pedal artery disease appears to be increasing in both prevalence and importance, particularly in CLTI patients experiencing major tissue loss or infection (WIfI stage 4).407,408
Pedal interventions remain relatively uncommon, and data on outcomes are extremely limited. Patients with no IM revascularization target are placed in a high-risk subgroup, although they are assigned a simplified modifier (P2) in the current version of GLASS. In the future, it is anticipated that better data will allow a more sophisticated incorporation of calcification and pedal disease. Other important issues, including the benefits of revascularizing multiple IP arteries, the relative quality of runoff distal to the revascularization and extending to the wound-related artery or angiosome, and the complex relationship between hemodynamic and clinical success, also require further study.
In assigning GLASS stages, the authors assume that pre-procedural decision-making is frequently driven by the estimation of the anticipated technical and clinical success after endovascular intervention. As a result, the preferred TAP for endovascular intervention and bypass surgery may not always be the same. Thus, treatment outcomes for surgical bypass should also be reported and analyzed on the basis of the actual procedure performed, including inflow artery, outflow artery, and conduit used.
6. STRATEGIES FOR EBR
Effective revascularization is the cornerstone of limb salvage in CLTI. Although multiple techniques are available, there are limited high-quality data to support EBR. A new, systematic paradigm is required to improve decision-making, clinical outcomes, and cost-effectiveness.
To aid clinical decision-making in everyday practice and to facilitate future EBR research in CLTI, the authors propose a three-step integrated approach (PLAN; Figs 6.1 and 6.2) based on
Fig 6.1.
Paradigm for evidence-based revascularization (EBR) in the treatment of chronic limb-threatening ischemia (CLTI). Patient risk, Limb severity, and ANatomic stage are integrated in the PLAN approach. WIfI, Wound, Ischemia, and foot Infection.
Fig 6.2.
PLAN framework of clinical decision-making in chronic limb-threatening ischemia (CLTI); infrainguinal disease. Refer to Fig 6.4 for preferred revascularization strategy in standard-risk patients with available vein conduit, based on limb stage at presentation and anatomic complexity. Approaches for patients lacking suitable vein are reviewed in the text. GLASS, Global Limb Anatomic Staging System; WIfI, Wound, Ischemia, and foot Infection.
Patient risk estimation
Limb staging
ANatomic pattern of disease
PLAN: Patient risk estimation.
The first step involves assessing the patient for candidacy for limb salvage, periprocedural risk, and life expectancy.
CLTI is associated with advanced age, multiple comorbidities, and frailty. The goals of treatment include relief of pain, healing of wounds, and preservation of a functional limb. However, revascularization may incur significant morbidity and mortality, requiring multiple hospitalizations, prolonged outpatient care, and thus considerable health and social care costs. Whereas the majority of patients with CLTI should be considered candidates for limb salvage, some may be appropriately treated with primary amputation or palliation after shared decision-making. Patients, families, and caregivers should have access to appropriate expertise in making these challenging decisions. Although maintenance of independent ambulatory status is an important goal, predicting functional outcomes after revascularization may be challenging, particularly in patients who are severely deconditioned. Palliative care consultants, where available, may be a valuable resource to optimize symptom management in patients with limited goals of care.
| Recommendations | ||
|---|---|---|
|
| ||
| 6.1 | Refer all patients with suspected CLTI to a vascular specialist for consideration of limb salvage, unless major amputation is considered medically urgent. | Good practice statement |
| 6.2 | Offer primary amputation or palliation to patients with limited life expectancy, poor functional status (eg, nonambulatory), or an unsalvageable limb after shared decision-making. | Good practice statement |
Palliative therapy should rarely include revascularization except in special circumstances, such as
treatment of hemodynamically significant inflow disease, if needed to improve the likelihood of a successful amputation at the most distal possible level; and
relief of intractable pain or to improve wound healing after shared decision-making with the patient, family, and vascular treatment team.
Estimation of operative risk and life expectancy plays a critical role in EBR. Tradeoffs between risk, invasiveness, hemodynamic gain, and anatomic durability of the vascular intervention are commonly made in everyday practice. Risk stratification tools can assist by providing objective criteria for such decisions. Multiple tools have been developed and applied to the CLTI population (Table 6.1).63–67,225,409–412 End points modeled have included all-cause mortality, major amputation, AFS, and perioperative events. The list of predictors identified in these models includes advanced age (>75 or 80 years), CKD, CAD, congestive heart failure, DM, smoking, cerebrovascular disease, tissue loss, BMI, dementia, and functional status. Frailty, a recently identified functional measure, is also of clear importance in the CLTI population.413,414 Patients with ESRD are at the highest risk in many reports and yet have been specifically excluded in some CLTI studies.415,416 All of these tools have been developed retrospectively using data from patients who have undergone revascularization, thereby excluding those who were managed conservatively or selected for primary amputation. Whereas some were validated in external data sets of similar patients, none has been prospectively tested across the spectrum of CLTI presenting for initial evaluation and treatment. As such, no specific tool and model can be recommended in preference to others.
Table 6.1.
Comparison of risk stratification tools for the chronic limb-threatening ischemia (CLTI) population
| Tool | End points | Critical factors | Reference |
|---|---|---|---|
|
| |||
| Taylor et al | Mortality, ambulatory failure (median follow-up of 2 years) | Age, race, ESRD, CAD, COPD, DM, dementia, baseline ambulatory status | Taylor,409 2006 |
| Finnvasc | Perioperative (30-day) mortality, limb loss | DM, CAD, gangrene, urgent operation | Biancari,63 2007 |
| PREVENT III | AFS (1 year) | ESRD, tissue loss, age >75 years, CAD, anemia | Schanzer,64 2008 |
| BASIL | Survival (2 years) | Age, CAD, smoking, tissue loss, BMI, Bollinger score, serum creatinine concentration, AP (number measured and highest value), prior stroke/TIA | Bradbury,65 2010 |
| CRAB | Perioperative (30-day) mortality, morbidity | Age >75 years, prior amputation or revascularization, tissue loss, ESRD, recent MI/angina, emergency operation, functional dependence | Meltzer,66 2013 |
| Soga et al | Survival (2 years) | Age, BMI, nonambulatory status, ESRD, cerebrovascular disease, tissue loss, left ventricular ejection fraction | Soga,225 2014 |
| VQI | AFS (1 year) | Age, tissue loss, DM, CHF, serum creatinine concentration, ambulatory status, urgent operation, weight, bypass conduit used | Simons,67 2016 |
| VQI | Survival (30 days, 2 and 5 years) | Age, CKD, ambulatory status, CAD, CHF, COPD, tissue loss, diabetes, smoking, beta-blocker use | Simons,412 2018 |
AFS, Amputation-free survival; AP, ankle pressure; BASIL, Bypass vs Angioplasty in Severe Ischaemia of the Leg; BMI, body mass index; CAD, coronary artery disease; CNF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CRAB, Comprehensive Risk Assessment for Bypass; DM, diabetes mellitus; ESRD, end-stage renal disease; MI, myocardial infarction; PREVENT III, Project of Ex-vivo Vein graft Engineering via Transfection III; TIA, transient ischemic attack; VQI, Vascular Quality Initiative.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.3 | Estimate periprocedural risk and life expectancy in patients with CLTI who are candidates for revascularization. | 1 (Strong) | C (Low) | Biancari,63 2007 Schanzer,64 2008 Bradbury,65 2010 Meltzer,66 2013 Simons,67 2016 |
| 6.4 | Define a CLTI patient as average surgical risk when anticipated periprocedural mortality is <5% and estimated 2-year survival is >50%. | 2 (Weak) | C (Low) | |
| 6.5 | Define a CLTI patient as high surgical risk when anticipated periprocedural mortality is ≥5% or estimated 2-year survival is ≤50%. | 2 (Weak) | C (Low) | |
Specific recommendations about preoperative cardiac and anesthetic evaluation before limb revascularization are beyond the scope of this document. The reader is referred to Section 4 and to other published guidelines.417,418
PLAN: Limb staging.
CLTI patients present with a broad spectrum of disease severity. Staging of the limb is central to EBR (Section 3), and use of the SVS Threatened Limb Classification System (WIfI) is recommended (Section 1).10,68–72,171 This is the only system that fully integrates wound severity, ischemia, and infection to stage CLTI.
The severity of ischemia and the benefits of revascularization do not map in an exclusively concordant fashion with amputation risk across the spectrum of CLTI, as expressed in the original WIfI consensus document.10 Expert opinion, now supported by reports from institutional series,69,70,72 suggests that the presumed benefit of revascularization in CLTI is linked to both the severity of ischemia and the degree of limb threat (Fig 6.3). All symptomatic patients who have severe (eg, WIfI grade 3) ischemia should undergo attempted revascularization, presuming they are appropriate candidates for limb salvage.5 In settings of advanced tissue loss or infection (eg, WIfI stage 4 limbs), revascularization may also be of benefit in the presence of moderate ischemia (eg, WIfI ischemia grades 1 and 2). Conversely, patients with lesser degrees of tissue loss or infection (eg, WIfI stages 1 to 3) and mild to moderate ischemia are often successfully treated with infection control and wound and podiatric care. Revascularization may be considered selectively in these patients if their wounds fail to progress (or regress) despite appropriate limb care after 4 to 6 weeks or if they have signs or symptoms of clinical deterioration. In such cases, all elements of the initial staging and treatment plan, including treatment of underlying moderate ischemia, should be re-evaluated. Whenever possible, the limb should be restaged after surgical drainage or débridement and after the infective component is stabilized. During the course of treatment, periodic restaging of the limb is important in guiding subsequent decisions, particularly when there is lack of progress in healing or any deterioration of symptoms.
Fig 6.3.
The benefit of performing revascularization in chronic limb-threatening ischemia (CLTI) increases with degree of ischemia and with the severity of limb threat (Wound, Ischemia, and foot Infection [WIfI] stage). WIfI stage 1 limbs do not have advanced ischemia grades, denoted as not applicable (N/A).
WIfI also provides a useful and necessary tool through which one can compare and contrast the quality of different revascularization strategies in CLTI. This has become an issue of critical importance as an everincreasing array of technologies and treatment strategies are being used. The magnitude and durability of increased perfusion required to resolve the clinical situation, and to maintain satisfactory limb health (eg, preservation of a functional foot, freedom from recurrent CLTI), will vary considerably across the spectrum. The extent of benefit for revascularization (Fig 6.3) is also linked to anatomic durability of the selected intervention. These concepts are central to PLAN and to the development of EBR strategies in CLTI.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.6 | Use an integrated threatened limb classification system (such as WIfI) to stage all CLTI patients who are candidates for limb salvage. | 1 (Strong) | C (Low) | Cull,68 2014 Zhan,69 2015 Causey,70 2016 Darling,71 2016 Robinson,72 2017 |
| 6.7 | Perform urgent surgical drainage and debridement (including minor amputation if needed) and commence antibiotic treatment in all patients with suspected CLTI who present with deep space foot infection or wet gangrene. | Good practice statement | ||
| 6.8 | Repeat limb staging after surgical drainage, débridement, minor amputations, or correction of inflow disease (AI, common and deep femoral artery disease) and before the next major treatment decision. | Good practice statement | ||
| 6.9 | Do not perform revascularization in the absence of significant ischemia (WIfI ischemia grade 0), unless an isolated region of poor perfusion in conjunction with major tissue loss (eg, WIfI wound grade 2 or 3) can be effectively targeted and the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading. | Good practice statement | ||
| 6.10 | Do not perform revascularization in very-low-risk limbs (eg, WIfI stage 1) unless the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading. | 2 (Weak) | C (Low) | Sheehan,73 2003 Cardinal,74 2008 Lavery,75 2008 Snyder,76 2010 |
| 6.11 | Offer revascularization to all average-risk patients with advanced limb-threatening conditions (eg, WIfI stage 4) and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). | 1 (Strong) | C (Low) | Abu Dabrh,5 2015 |
| 6.12 | Consider revascularization for average-risk patients with intermediate limb threat (eg, WIfI stages 2 and 3) and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). | 2 (Weak) | C (Low) | Zhan,69 2015 Causey,70 2016 Darling,71 2016 Robinson,72 2017 |
| 6.13 | Consider revascularization in average-risk patients with advanced limb threat (eg, WIfI stage 4) and moderate ischemia (eg, WIfI ischemia grade 1). | 2 (Weak) | C (Low) | |
| 6.14 | Consider revascularization in average-risk patients with intermediate limb threat (eg, WIfI stages 2 and 3) and moderate ischemia (eg, WIfI ischemia grade 1) if the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading. | 2 (Weak) | C (Low) | |
PLAN: Anatomic pattern of disease (and conduit availability).
Although secondary to the broader context of patient risk and limb threat severity, the anatomic pattern of arterial occlusive disease is a dominant consideration in EBR. The overall pattern and severity of disease in the limb (eg, as described by GLASS; Section 4) help define the optimal strategy for vascular intervention. Furthermore, the availability and quality of autologous vein conduit (especially the great saphenous vein [GSV]) are key considerations for bypass surgery and should be defined before revascularization decisions are taken in average-risk patients.13,77,79
“No-option” anatomy.
The majority of CLTI patients are anatomically suitable for revascularization, and establishing direct in-line flow to the foot is the primary technical goal. One important exception is ischemic rest pain, for which correction of inflow disease alone or treatment of FP disease even without continuous tibial runoff to the foot may provide relief of symptoms. This may also be the case in patients presenting with minor degrees of tissue loss (eg, WIfI stage 2). Thus, the definition of a no-option anatomic pattern of disease is dependent on clinical context. Lack of a target artery crossing the ankle and absence of a suitable pedal or plantar artery target (eg, GLASS P2 modifier) may be considered no-option disease patterns in patients with advanced CLTI (eg, WIfI stages 3 and 4). Angiography may occasionally fail to detect a patent distal artery target, and there are reports of successful tibial and pedal bypass grafting based on exploration of an artery identified on Doppler ultrasound examination that was not identified on contrast arteriography.419,420 Careful selection and experienced surgical judgment are required before proceeding to surgery in such instances.
EBR strategies in CLTI.
The technical options for treating complex patterns of disease in a minimally invasive fashion have increased markedly in recent years and led some to advocate an “endovascular-first” approach for most or all patients with CLTI, reserving bypass surgery as a secondary option. However, existing evidence argues strongly for a selective revascularization algorithm based on specific clinical and anatomic scenarios, as described here. Currently enrolling RCTs are eagerly awaited to provide higher quality data in support of EBR in patients with CLTI.13–15
The Bypass vs Angioplasty in Severe Ischaemia of the Leg (BASIL) trial (now called BASIL-1) remains the only multicenter RCT to have directly compared an endovascular-first with a bypass surgery-first strategy in limb-threatening ischemia due to infrainguinal disease.159,421 BASIL was conducted across 27 hospitals in the United Kingdom and enrolled 452 participants between 1999 and 2004. All but six patients in the endovascular arm received plain balloon angioplasty (PBA) alone; approximately 25% of the bypasses were prosthetic; around one-third of the procedures were IP; and just more than 50% of patients were observed for >5 years. Considering the follow-up period as a whole, an intention-to-treat analysis showed no significant difference between the two arms in terms of AFS and overall survival. However, for the approximately 70% of patients who lived for >2 years, HRs for overall survival (0.65; P = .009) and AFS (0.85; P = .108) were better for those treated initially with bypass surgery. An analysis by treatment received showed that prosthetic bypasses performed very poorly (worse than PBA) and that patients having bypass after failed PBA had a highly significantly worse AFS and overall survival compared with those patients who received bypass as their first allocated treatment.160
A systematic review comparing open and endovascular treatments for CLTI found only nine studies meeting standard criteria, three of which were RCTs (among which only BASIL met all of the study quality benchmarks).6 Researchers concluded that low-quality evidence (due to heterogeneity and imprecision) suggested similar mortality and amputation outcomes but better expected patency for bypass surgery. Other comparative reviews have yielded broadly similar conclusions.227,422–425 OPGs for endovascular interventions in CLTI based on open surgical data from high-quality sources have been suggested and provide minimum standards of safety and efficacy until direct comparative data become available.162
To obtain updated data on outcomes after endovascular and open bypass surgery in CLTI, a review was conducted of comparative studies and noncomparative studies that met more inclusive criteria.7 These criteria included prospective study design, 50 or more patients with critical or severe limb ischemia (Rutherford class 4–6 definition), infrainguinal procedure, minimum follow-up of 1 year, at least 50 procedures of each subtype (endovascular or open), and adequate anatomic description of lesion location and types of subinterventions (eg, percutaneous transluminal angioplasty, stent, atherectomy) employed. In total, 44 studies enrolling 8602 patients were reviewed in detail and results tabulated to display outcomes across anatomic subsets and from 30 days to 5-year follow-up intervals. Most of the studies were assessed as having moderate to high risk of bias, and the study quality was variable.
Review of the attributes of these studies revealed several notable limitations: few studies of SFA intervention were included because of inadequate numbers of CLTI patients (vs those with IC); the majority of FP bypass studies included prosthetic grafts; and although a good number of studies (20) addressed endovascular intervention for IP disease, the severity of disease was generally mild to moderate (GLASS IP grades 1 and 2), with no studies including GLASS IP grade 4 disease. Thus, the current state of evidence in CLTI remains severely limited, particularly for assessing endovascular outcomes in commonly encountered, complex (especially distal) disease patterns. Caveats aside, the compendium of data suggests similar mortality, amputation, and AFS rates for endovascular and bypass surgery at 1 year, with improved patency for bypass using vein compared with endovascular interventions or prosthetic bypass grafts at 1 year and beyond.
Additional evidence, including a larger body of retrospective studies and registries, provides further insights into specific factors associated with inferior outcomes for individual techniques and informs current vascular practice.79,365,366,369,372,373,376,385,391,393,395,402,407,426–438 Surgical bypass with nonautologous conduits to IP targets in CLTI performs poorly. Similarly, patency rates for endovascular intervention are poor in settings of diffuse tibial disease and popliteal and trifurcation occlusions and are diminished in small, diffusely diseased or heavily calcified FP arteries. Several studies suggest that endovascular outcomes for advanced tissue loss (eg, gangrene, WIfI stage 4, WIfI ischemia grade 3, or foot infection grades 2 and 3) are inferior, with high early rates of major amputation.171,439 Patients with ESRD experience higher rates of limb loss across all interventions. These factors must be carefully considered in each individual case, evaluating the available treatment options against the patient risk, limb stage, functional status, and presumptive importance of a hemodynamically durable intervention for resolving the clinical scenario at hand.
Finally, a nonselective endovascular-first approach carries some risk of both clinically ineffective and cost-ineffective treatment and potential for harm. Whereas a significant percentage of CLTI patients are appropriate candidates for endovascular intervention, those with severe anatomic patterns and higher stages of limb threat may not be well served by a nonselective approach for several reasons. First, ineffective revascularization can lead to poor symptom relief, limited durability of benefit, delayed wound healing, inadequate clearance of infection, or progression of tissue loss in the foot. There are both patient and system costs to inadequately treated CLTI. Another important consideration is the potential effect of endovascular failures on the outcomes of secondary bypass surgery in CLTI. Although data in this regard are limited, several multicenter data sets including BASIL160 and large regional registries440,441 suggest that the outcomes of bypass surgery in patients who have undergone failed endovascular interventions are significantly inferior to those in patients who underwent primary bypass surgery. The inferior outcomes associated with “secondary bypass” are similar whether the initial failure was percutaneous or a prior bypass graft. This may be a particularly high penalty to pay if clinical success of the initial procedure was short-lived. These studies cannot establish causality vs association, but they strongly suggest that the success of the initial vascular intervention is of importance in CLTI and that endovascular failure, like open bypass failure, carries consequences. Thus, an important consideration is to avoid risking potential loss of bypass targets in performing endovascular interventions. Conversely, surgical bypass may incur significant morbidity and mortality despite the potential attractiveness of greater durability. Factors that may increase the risk of wound complications, graft failure, or other major postoperative complications must be carefully weighed. These considerations informed the consensus recommendations on specific EBR strategies.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.15 | Obtain high-quality angiographic imaging with dedicated views of ankle and foot arteries to permit anatomic staging and procedural planning in all CLTI patients who are candidates for revascularization. | Good practice statement | ||
| 6.16 | Use an integrated limb-based staging system (eg, GLASS) to define the anatomic pattern of disease and preferred TAP in all CLTI patients who are candidates for revascularization. | Good practice statement | ||
| 6.17 | Perform ultrasound vein mapping when available in all CLTI patients who are candidates for surgical bypass. | 1 (Strong) | C (Low) | Seeger,77 1987 Wengerter,78 1990 Schanzer,79 2007 |
| 6.18 | Map the ipsilateral GSV and small saphenous vein for planning of surgical bypass. Map veins in the contralateral leg and both arms if ipsilateral vein is insufficient or inadequate. |
Good practice statement | ||
| 6.19 | Do not classify a CLTI patient as being unsuitable for revascularization without review of adequate-quality imaging studies and clinical evaluation by a qualified vascular specialist. | Good practice statement | ||
EBR: Treatment of inflow disease.
Inflow disease is defined here as proximal to the origin of the SFA and meeting one or more of the following criteria:
absent femoral pulse
blunted CFA waveform on Doppler ultrasound
>50% stenosis by angiography in the aorto-iliac arteries or CFA
aorta to CFA systolic pressure gradient >10 mm Hg at rest
The decision to perform staged vs multilevel revascularization for patients with combined inflow and outflow disease is individualized on the basis of severity of limb threat (especially presence of tissue loss), anatomic complexity, and patient risk. In settings of rest pain and minor tissue loss, inflow correction alone may suffice to achieve the desired clinical outcome. As procedural complexity increases, perioperative morbidity and mortality rise as well. Most patterns of AI disease may be successfully treated using an endovascular approach, frequently employing bare-metal or covered stents.82–84 Surgery is often reserved for extensive occlusions or after failure of endovascular procedures. The choice of an open surgical inflow procedure should be based on patient risk, anatomic pattern of disease, and other clinical factors. Direct anatomic bypass (eg, aortofemoral) grafting may be preferred to extra-anatomic reconstruction in average-risk patients with severe ischemia (WIfI ischemia grades 2 and 3) because of greater anatomic and hemodynamic durability.85–87
CFA endarterectomy can be performed with low morbidity and excellent long-term durability.88,89 It remains the optimal approach to treatment of hemodynamically significant CFA disease, which often includes bulky calcific plaque. In some cases, femoral interposition grafting may be preferred. In all cases, durable in-line PFA flow should be maximized. CFA endarterectomy may be combined with proximal intervention to treat combined disease in a “hybrid” fashion.90 Although long-term outcome data are sparse, reports suggest that endovascular treatment of CFA disease may be a safe alternative in selected patients (eg, high surgical risk, hostile groin anatomy).91–94
Surgical treatment (eg, profundaplasty or bypass grafting) of PFA disease is an important component of CLTI revascularization with a major impact on the long-term prognosis for the limb. The indications for and optimal approaches to treatment of nonorificial (ie, not in continuity with the CFA) or long-segment PFA disease are not established. There is limited evidence regarding the use of endovascular interventions for PFA disease. However, it may be considered a secondary approach in settings of hostile groin anatomy or in other high-risk circumstances.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.20 | Correct inflow disease first when both inflow and outflow disease are present in a patient with CLTI. | Good practice statement | ||
| 6.21 | Base the decision for staged vs combined inflow and outflow revascularization on patient risk and the severity of limb threat (eg, WIfI stage). | 1 (Strong) | C (Low) | Harward,80 1995 Zukauskas,81 1995 |
| 6.22 | Correct inflow disease alone in CLTI patients with multilevel disease and low-grade ischemia (eg, WIfI ischemia grade 1) or limited tissue loss (eg, WIfI wound grade 0/1) and in any circumstance in which the risk-benefit of additional outflow reconstruction is high or initially unclear. | 1 (Strong) | C (Low) | |
| 6.23 | Restage the limb and repeat the hemodynamic assessment after performing inflow correction in CLTI patients with inflow and outflow disease. | 1 (Strong) | C (Low) | |
| 6.24 | Consider simultaneous inflow and outflow revascularization in CLTI patients with a high limb risk (eg, WIfI stages 3 and 4) or in patients with severe ischemia (eg, WIfI ischemia grades 2 and 3). | 2 (Weak) | C (Low) | |
| 6.25 | Use an endovascular-first approach for treatment of CLTI patients with moderate to severe (eg, GLASS stage IA) AI disease, depending on the history of prior intervention. | 1 (Strong) | B (Moderate) | Jongkind,82 2010 Ye,83 2011 Deloose,84 2017 |
| 6.26 | Consider surgical reconstruction for the treatment of average-risk CLTI patients with extensive (eg, GLASS stage II) AI disease or after failed endovascular intervention. | 2 (Weak) | C (Low) | Ricco,85 2008 Chiu,86 2010 Indes,87 2013 |
| 6.27 | Perform open CFA endarterectomy with patch angioplasty, with or without extension into the PFA, in CLTI patients with hemodynamically significant (>50% stenosis) disease of the common and deep femoral arteries. | 1 (Strong) | C (Low) | Kang,88 2008 Ballotta,89 2010 |
| 6.28 | Consider a hybrid procedure combining open CFA endarterectomy and endovascular treatment of AI disease with concomitant CFA involvement (eg, GLASS stage IB inflow disease). | 2 (Weak) | C (Low) | Chang,90 2008 |
| 6.29 | Consider endovascular treatment of significant CFA disease in selected patients who are deemed to be at high surgical risk or to have a hostile groin. | 2 (Weak) | C (Low) | Baumann,91 2011 Bonvini,92 2011 Gouëffic,93 2017 Siracuse,94 2017 |
| 6.30 | Avoid stents in the CFA and do not place stents across the origin of a patent deep femoral artery. | Good practice statement | ||
| 6.31 | Correct hemodynamically significant (≥50% stenosis) disease of the proximal deep femoral artery whenever technically feasible. | Good practice statement | ||
EBR: Treatment of infrainguinal disease in average-risk patients.
Outflow (infrainguinal) disease starts at the SFA origin (Section 5). An average-risk patient is defined as one in whom the anticipated periprocedural mortality is <5% and the anticipated 2-year survival is >50% (Recommendation 6.4). These patients are potential surgical or endovascular candidates, depending on individual clinical and anatomic factors.
Fig 6.4 provides a summary of preferred infrainguinal revascularization strategies for an average-risk patient with available vein conduit based on the presenting combination of limb stage (WIfI) and anatomic pattern of disease (GLASS). Open bypass surgery and endovascular therapy have complementary roles, with notable lack of consensus across the intermediate ranges of clinical and anatomic complexity. Comparative effectiveness studies employing these staging schemes are urgently needed to improve the quality of evidence for interventions in specific clinical scenarios.
Fig 6.4.
Preferred initial revascularization strategy for infrainguinal disease in average-risk patients with suitable autologous vein conduit available for bypass. Revascularization is considered rarely indicated in limbs at low risk (Wound, Ischemia, and foot Infection [WIfI] stage 1). Anatomic stage (y-axis) is determined by the Global Limb Anatomic Staging System (GLASS); limb risk (x-axis) is determined by WIfI staging. The dark gray shading indicates scenarios with least consensus (assumptions–inflow disease either is not significant or is corrected; absence of severe pedal disease, ie, no GLASS P2 modifier).
| Recommendation | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.32 | In average-risk CLTI patients with infrainguinal disease, base decisions of endovascular intervention vs open surgical bypass on the severity of limb threat (eg, WIfI), the anatomic pattern of disease (eg, GLASS), and the availability of autologous vein. | 1 (Strong) | C (Low) | Almasri,7 2018 |
Patients lacking adequate autologous (GSV) conduit must be considered separately as this is a critical factor in determining the likely success and durability of bypass surgery. For those with no suitable venous conduit, prosthetic or venous allografts are the only options. Given the inferior performance of these conduits in CLTI, endovascular intervention is preferred when possible.160 Use of prosthetic or biologic conduits (eg, cryopreserved vein allografts) for infrainguinal bypass in CLTI may be reasonable in highly selected cases, such as in patients with untreatable anatomy for endovascular intervention or prior endovascular failure, with acceptable runoff, and in patients who are able to tolerate aggressive antithrombotic therapy.
In many patients lacking GSV, arm/spliced vein bypass conduits may be an option. However, the results of arm/spliced vein bypass are highly dependent on the operator’s training and experience. The determination of when and how to employ these alternative vein conduits is surgeon specific. In general, large single-center and multicenter reports demonstrate that arm and spliced vein bypasses perform better than nonautologous grafts to distal targets and are inferior to autologous GSV conduits.7,79,442,443 However, these higher risk vein grafts require closer surveillance and more reinterventions to maintain primary assisted patency.444
EBR: Treatment of infrainguinal disease in high-risk patients.
A high-risk patient is defined as one in whom the anticipated perioperative mortality is >5% or the anticipated 2-year survival is <50%. Because endovascular intervention can be performed with reduced morbidity, it may often be preferred in high-risk patients who are otherwise candidates for functional limb salvage. Shared decision-making is of great importance in high-risk patients to allow the patient, family, and other stakeholders to express value judgments on the tradeoffs between risk and effectiveness in relation to the desired goals.
EBR: Infra-malleolar disease.
Severe IM disease creates a major challenge to effective revascularization.407 The P2 modifier in GLASS describes the circumstance in which no named artery crosses the ankle into the foot and there is no suitable target for bypass surgery. Although technically successful endovascular interventions in the pedal arch have been reported, their durability and hemodynamic and clinical effectiveness remain unknown.438 Diabetic patients often have a segment of preserved pedal artery that may be a target for bypass. Open bypass surgery has also been successfully employed to tarsal and plantar arteries, but again, techniques and outcomes are not established. Given the technical difficulty and the likely reduced hemodynamic impact and durability, the appropriate role for interventions at this level is not determined. The impact of IM disease on the success of proximal revascularization, whether open or endovascular, is likewise unknown. Although the presence of an intact pedal arch appears important for both, clinical success may still be attained in the presence of significant IM disease. The severity of limb threat (tissue loss or infection) is likely to be a critical modifier of the relationship between IM disease severity and postprocedural clinical outcomes.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.33 | Offer endovascular revascularization when technically feasible for high-risk patients with advanced limb threat (eg, WIfI stage 4) and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). | 2 (Weak) | C (Low) | Abu Dabrh,5 2015 Zhan,69 2015 Causey,70 2016 Darling,71 2016 Robinson,72 2017 |
| 6.34 | Consider endovascular revascularization for high-risk patients with intermediate limb threat (eg, WIfI stages 2 and 3) and significant perfusion deficits (eg, WIfI ischemia grades 2 and 3). | 2 (Weak) | C (Low) | |
| 6.35 | Consider endovascular revascularization for high-risk patients with advanced limb threat (eg, WIfI stage 4) and moderate ischemia (eg, WIfI ischemia grade 1) if the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading, when technically feasible. | 2 (Weak) | C (Low) | |
| 6.36 | Consider endovascular revascularization for high-risk patients with intermediate limb threat (eg, WIfI stages 2 and 3) and moderate ischemia (eg, WIfI ischemia grade 1) if the wound progresses or fails to reduce in size by ≥50% within 4 weeks despite appropriate infection control, wound care, and offloading, when technically feasible. | 2 (Weak) | C (Low) | |
| 6.37 | Consider open surgery in selected high-risk patients with advanced limb threat (eg, WIfI stage 3 or 4), significant perfusion deficits (ischemia grade 2 or 3), and advanced complexity of disease (eg, GLASS stage III) or after prior failed endovascular attempts and unresolved symptoms of CLTI. | 2 (Weak) | C (Low) | |
EBR: Role of angiosome-guided revascularization.
Whereas few would argue about the desirability of maximizing perfusion at the site of tissue loss, there is considerable debate about the utility of angiosome-guided revascularization.445,446 First, unambiguous assignment of foot wounds to an individual angiosome is possible in only a minority of cases.447 Toe lesions, which typically represent more than half of the lesions encountered, have a dual blood supply (AT and PT), although for more proximal foot lesions, unique angiosome assignment may be achieved in up to 75% to 80% of patients. Then there is the practical question of whether the desired target artery for the angiosome is available and the comparative hemodynamic and clinical effectiveness of “direct” vs “indirect” revascularization. Tibial and peroneal bypasses perform equally well for limb salvage, and DP bypass can be effective for some hindfoot lesions.448 Systematic reviews have yielded conflicting results,96–99 and data are inextricably confounded by the quality of the pedal arch and the nature of the revascularization performed.95,449 Whereas wound healing may be improved when direct revascularization is achievable, major amputation rates and patency are not consistently different. To date, none of the analyses take into account the confounding effect of limb staging, for example, using WIfI. In summary, angiosome-guided revascularization may be of importance in the setting of endovascular intervention for midfoot and hindfoot lesions but is likely to be irrelevant for ischemic rest pain and of marginal value for most forefoot lesions and minor ulcers. The role of multivessel (tibial) revascularization is also currently unknown. However, it may be reasonable in selected patients with advanced limb threat (eg, WIfI stages 3 and 4) undergoing endovascular therapy if it can be safely accomplished without risking loss of a bypass target or compromising runoff to the foot.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.38 | Consider angiosome-guided revascularization in patients with significant wounds (eg, WIfI wound grades 3 and 4), particularly those involving the midfoot or hindfoot, and when the appropriate TAP is available. | 2 (Weak) | C (Low) | Azuma,95 2012 Sumpio,96 2013 Biancari,97 2014 Chae,98 2016 Jongsma,99 2017 |
EBR: Preferred endovascular techniques for infrainguinal disease.
PBA, drug-coated balloon (DCB) angioplasty, stent placement (bare-metal stent, drug-eluting stent [DES], or covered stent), and atherectomy may all be reasonable options in specific circumstances and lesion anatomies. However, unfortunately, there are few high-quality comparative data to guide the choice of a specific endovascular approach in CLTI.7,380,387–389,396,450–455
PBA may be inferior to DCB angioplasty and stents for the treatment of intermediate-length SFA disease (FP grades 2–4) in patients with IC and possibly rest pain.100–103 However, there are inadequate data to support a preferred endovascular approach for FP disease in CLTI.
PBA remains a reasonable primary endovascular approach for anatomically suitable IP disease as current evidence is inadequate to support other, more expensive techniques. Atherectomy is not superior to PBA and is associated with greatly increased costs.453 Combination approaches, such as atherectomy followed by DCB angioplasty, add significant cost and lack high-quality comparative data. Several modestsized trials suggest potential short-term benefit for DESs in short (ie, <3 cm) tibial lesions, but one cannot generalize these data to the population of CLTI patients as a whole, who typically present with much more extensive disease.7,456 DES may be a preferred endovascular “bailout” after technical complications (eg, dissection) or failed PBA for short, proximal IP lesions. Although early studies suggested a potential advantage for DCBs in tibial arteries, an RCT showed no benefit of DCB angioplasty over PBA, with a nonsignificant higher rate of amputations in the DCB angioplasty group.396 The results of further, ongoing studies are awaited. In summary, PBA currently remains the standard of care for the endovascular treatment of IP disease in CLTI.
Technical advances in endovascular intervention include improved wires, low-profile catheters, and retrograde access to allow treatment of complex disease patterns down to the distal calf and foot. Specialized catheters may facilitate crossing of difficult chronic total occlusions and ensure re-entry into the true lumen. Retrograde access techniques using either fluoroscopic or ultrasound guidance may increase the ability to cross chronic total occlusions at the IP and popliteal levels. The “pedal loop technique” has been described to achieve complete arch reconstitution in the presence of IM disease, and some reports suggest that it may be of value in highly selected patients.438,457 The clinical efficacy of these techniques remains to be defined in CLTI as hemodynamic durability remains the primary limitation of endovascular interventions in high-complexity target path anatomy.
EBR: Preferred approaches for infrainguinal bypass.
An acceptable target for bypass surgery in CLTI should provide adequate runoff to the lower limb and foot to resolve the clinical situation. In the setting of WIfI stages 3 and 4, it is recommended that the selected target artery provide continuous in-line flow to the ankle and foot.
Good-quality GSV is the optimal autologous conduit for infrainguinal bypass surgery. Alternative (small saphenous vein or arm vein) or spliced veins are acceptable bypass conduits, although there is a higher frequency of reinterventions, and durability is inferior to single-segment GSV grafts. There is no evidence to support a preferred configuration (reversed, nonreversed translocated, in situ) for vein bypass grafting.
Prosthetic conduits may be useful in selected patients lacking other revascularization options. Heparin-bonded expanded polytetrafluoroethylene grafts may be superior to standard expanded polytetrafluoroethylene grafts for below-knee bypass.458,459 Other adjuncts, such as a distal vein cuff, may also improve patency of prosthetic bypass to tibial targets, although the data are limited in scope and quality.460 In general, clinical outcomes of prosthetic grafting in CLTI are highly sensitive to runoff and severity of limb presentation. Bypass using nonautologous conduit to poor-quality tibial or pedal targets in CLTI is discouraged as patency rates are extremely poor. Defining the optimal approach for below-knee bypass in patients lacking venous conduit remains a major challenge in the field; if these patients are not suitable for endovascular intervention, the individual surgeon’s experience may dictate practice. Further advances in bioengineered arterial conduits are needed to meet this clinical dilemma.
| Recommendation | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.39 | In treating FP disease in CLTI patients by endovascular means, consider adjuncts to balloon angioplasty (eg, stents, covered stents, or drug-eluting technologies) when there is a technically inadequate result (residual stenosis or flow-limiting dissection) or in the setting of advanced lesion complexity (eg, GLASS FP grade 2–4). | 2 (Weak) | B (Moderate) | Schillinger,100 2006 Saxon,101 2008 Dake,102 2011 Rosenfield,103 2015 Almasri,7 2018 |
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 6.40 | Use autologous vein as the preferred conduit for infrainguinal bypass surgery in CLTI. | 1 (Strong) | B (Moderate) | Almasri,7 2018 |
| 6.41 | Avoid using a nonautologous conduit for bypass unless there is no endovascular option and no adequate autologous vein. | 2 (Weak) | C (Low) | Almasri,7 2018 |
| 6.42 | Perform intraoperative imaging (angiography, DUS, or both) on completion of open bypass surgery for CLTI and correct significant technical defects if feasible during the index operation. | 1 (Strong) | C (Low) | Mills,104 1992 Bandyk,105 1994 |
| Research priorities | |
|---|---|
|
| |
| 6.1 | In patients presenting with the full spectrum of CLTI, prospectively validate and refine patient risk stratification models. |
| 6.2 | Conduct comparative effectiveness studies directly comparing strategies of revascularization—and specific techniques and technologies—in well-defined subgroups of patients (eg, WIfI and GLASS stages) with CLTI. |
| 6.3 | Define the circumstances in which angiosome-targeted or multivessel revascularization provides clinical benefit in CLTI. |
| 6.4 | Develop and test strategies for the management of no-option CLTI patients. |
| 6.5 | Conduct appropriately controlled prospective trials to determine the safety and efficacy of drug-eluting technologies specifically in the CLTI population, with adequate (at least 2 year) long term follow up. |
7. NONREVASCULARIZATION TREATMENTS OF THE LIMB
Although the optimal treatment of CLTI is undoubtedly revascularization, unfortunately, a significant proportion of patients are not suitable for revascularization for anatomic or physiologic reasons. Whereas major amputation may be suitable for some of these patients, there is clearly a significant number who might benefit from nonrevascularization-based treatments.
There is, however, a paucity of strong evidence regarding these treatment options. The majority of studies are low quality and uncontrolled, combined with considerable study heterogeneity, making systematic review and meta-analysis difficult or even impossible. This heterogeneity is reflected by large variations in patient factors, lesions of interest, intervention protocols, study designs, and end points (limb salvage, AFS, target lesion patency, pain relief, quality of life determinants, ulcer healing, and evolution of tissue lesions).4
This section reviews nonrevascularization interventions, pharmacotherapy, and conservative management.
Interventional nonrevascularization treatments
Spinal cord stimulation (SCS).
Mechanism of action.
SCS, originally used to treat chronic pain, was first described by Cook et al461 in the treatment of PAD. In SCS, electrodes are implanted in the lumbar epidural space and connected to a generator to stimulate sensory fibers.462 SCS promotes activation of cell signaling pathways that cause the release of vasodilatory molecules, leading to a decrease in vascular resistance and relaxation of smooth muscle cells.462 This improved peripheral microcirculatory status has been shown to result in increased capillary flow and density of perfusing capillaries, higher skin temperature and local TcPO2, normalization of pulse wave morphology, and improved skin nutrition.106 In addition, SCS suppresses sympathetic vasoconstriction and pain transmission.462
Evidence.
A 2013 Cochrane review analyzed data from 444 patients in six controlled studies investigating the use of SCS in CLTI.106,463–468 The general quality of studies was good, and all studies used limb salvage as the primary end point (major AFS at 12 months). When the results were pooled, limb salvage rates were found to be significantly higher in the SCS group (RR for major amputation, 0.71; 95% CI, 0.56–0.90).106 Results were better when patients were selected on the basis of their initial TcPO2. Significant pain relief was also found in both treatment groups, although the SCS group required less analgesia. In addition, there was no significant effect on ulcer healing. Overall mortality was not evaluated, but the overall complication rate was 17% (95% CI, 12–22%). Implantation problems occurred in 9% (95% CI, 4%-15%), reintervention for changes in stimulation occurred in 15% (95% CI, 10%-20%), and infection of a lead or pulse generator pocket accounted for 3% (95% CI, 0%-6%).106
Researchers concluded that SCS offered a modest positive effect on pain relief and an 11% reduction in the amputation rate compared with conservative management at 1 year.106 They stress, however, that the positive benefits should be weighed against the high cost and possible complications. In fact, the Cochrane review found the cost to be significantly higher in the SCS group by $8824. Klomp et al469 calculated the number needed to treat to save one limb as 13, at $111,705 per limb saved and $312,754 per quality-adjusted life-year gained. They concluded that SCS is not a cost-effective treatment of CLTI.
Lumbar sympathectomy (LS)
Mechanism of action.
Sympathetic denervation of the lumbar sympathetic ganglia is performed either through open or laparoscopic retroperitoneal access or through percutaneous chemical blockade. LS increases blood flow to the lower limb by inducing vasodilation of the collateral circulation and shunting of blood through cutaneous arteriovenous anastomoses by its reduction of sympathetic tone. This, in turn, improves tissue oxygenation and decreases tissue damage and pain. Pain is also decreased by interruption of sympathetic nociceptive coupling and by a direct neurolytic action on nociceptive fibers.470
Evidence.
In their systematic review, Sanni et al470 reported that RCTs failed to identify any objective benefits for LS in patients with CLTI. They concluded, however, that LS may be considered an alternative to amputation in patients with otherwise viable limbs because it is minimally invasive and cost-effective, with a low complication rate.470 Chemical sympathectomy and surgical sympathectomy also appear to perform equally well, with some suggestion that LS can benefit diabetic patients.
Of the three RCTs that focus on LS in PAD, only two reported on its use in CLTI,471,472 with the third reporting on its use in IC.473 Cross et al472 found that chemical sympathectomy provided relief of rest pain in 67% of patients undergoing LS compared with 24% of controls at 6 months. However, in a contrasting study, Barnes et al471 found that LS combined with AI revascularization did not provide any additional benefits compared with revascularization alone. In fact, the majority of cohort studies reporting LS in CLTI474–483 consistently demonstrate subjective improvements in approximately 60% of patients with regard to pain relief and ulcer healing.470 Moreover, a Cochrane systematic review was unable to find any RCTs that evaluated the effect of LS (open, laparoscopic, or chemical) compared with no intervention in CLTI due to nonreconstructible PAD.107 Overall, data are limited, but there is no evidence to suggest that LS reduces the risk of major amputation in patients with CLTI. It remains unclear whether any subgroup of CLTI patients may have improved pain control or ulcer healing with LS.
Intermittent pneumatic compression (IPC)
Mechanism of action.
In patients treated with IPC, arterial blood flow is increased in the distal limbs by an increase in the arteriovenous pressure gradient, which stimulates the endothelial vasodilators, thus suspending the venoarteriolar reflex and stimulating collateral artery growth.484 As a result, the arterial flow, peak systolic velocity (PSV), end-diastolic velocity, and pulse volume are all increased.485
Several methods of lower limb IPC use various protocols. These include the ArtAssist (ACI Medical, San Marcos, Calif) device, which provides sequential compression to the foot and calf; the Aircast ArterialFlow (DJO Global, Vista, Calif) device, which compresses the calf; and devices that deliver leg compression synchronized with ventricular contraction of the heart (Syncarbon [Contilabo, Saint Gobain, France] and Vascular Pump [Rheomedix, Philadelphia, Pa]).484
Evidence.
Two controlled studies486,487 and several case series488–495 have been published regarding IPC, but there is no robust evidence from high-quality trials. In one, investigators entered 171 patients with CLTI into a 3-month IPC program.494 They reported improved pain relief, increased TPs by a mean of 15 mm Hg, and increased popliteal artery flow by a mean of 20 cm/s. The median AFS was 18 months, with 94% limb salvage at 3.5 years. They determined that IPC is a cost-effective intervention at a cost of $4454 per patient.494 In a retrospective observational study involving 107 patients, researchers from the Mayo Clinic found 40% wound healing at 6 months.493
In another study, a non-RCT involving 48 patients, investigators found that 58% of patients who underwent IPC benefited from complete healing and limb salvage compared with 17% in the control group (OR, 7.00; 95% CI, 1.82–26.89).486 In a prospective trial, changes in quality of life were reviewed before and after IPC treatment.495 Researchers reported a significant improvement in pain, physical functioning, and general health perception. Another systematic review found that IPC might be associated with improved limb salvage, wound healing, and pain management as well as with a low risk of complications.484 However, this review also noted a high risk of bias in these studies, with large variations in the type of compression and optimum parameters used.484
Wound healing varied considerably (4%-96% at 3 months) in studies that used the same IPC device. In contrast, mortality rates were more consistent.484 It has been suggested that outcomes with IPC may be worse for patients with renal failure, with the prognosis for this group being worse for both limb salvage and mortality.484
Guidelines on nonrevascularization interventions
The TransAtlantic Inter-Society Consensus II (TASC II) document on the management of PAD concluded that there is low-level evidence available for the recommendation of SCS.156 Likewise, guidelines from the ESVS state that the benefit of SCS is unproven, with insufficient evidence to recommend its use in the treatment of CLTI.496
Although the TASC II document did not include LS in the treatment of CLTI, it did mention its potential role in the management of complex regional pain syndrome.156 The ESVS guidelines conclude that LS should not be considered an option to prevent amputation but can be considered in patients who are not amenable to revascularization to relieve symptoms.496 The American Heart Association’s guidelines on the management of PAD do not mention LS.496 Finally, the international guidelines make no reference to IPC at all.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 7.1 | Consider SCS to reduce the risk of amputation and to decrease pain in carefully selected patients (eg, rest pain, minor tissue loss) in whom revascularization is not possible. | 2 (Weak) | B (Moderate) | Ubbink,106 2013 |
| 7.2 | Do not use LS for limb salvage in CLTI patients in whom revascularization is not possible. | 2 (Weak) | C (Low) | Karanth,107 2016 |
| 7.3 | Consider IPC therapy in carefully selected patients (eg, rest pain, minor tissue loss) in whom revascularization is not possible. | 2 (Weak) | B (Moderate) | Abu Dabrh,4 et al 2015 |
Pharmacotherapy
Prostanoids.
Mechanism of action.
Prostanoids include a family of inflammatory mediators, mainly prostaglandin E1 (PGE1), prostacyclin (PGI2), and iloprost. Prostanoids act by inhibiting the activation of platelets and leukocytes, by inhibiting the adhesion and aggregation of platelets, and by promoting vasodilation and vascular endothelial cytoprotection through antithrombotic and profibrinolytic activities.108,497,498
Evidence.
A meta-analysis evaluating the use of PGE1 vs placebo in the treatment of 254 patients with CLTI demonstrated favorable results at 6 months, with ulcer healing or pain reduction (47.8% vs 25.2% placebo) and reduction in major amputation or death (22.6% vs 36.2% placebo) associated with PGE1 use.499 Subsequently, a 2018 Cochrane paper reviewed 33 prostanoid studies with various formulations, doses, and administration routes.108 These included intravenous (IV) administration of PGE1 (synthetic form, alprostadil) for 21 days and an intra-arterial administration; IV administration of PGI2 for 4 to 7 days; IV administration of iloprost (synthetic analogue of PGI2) for 14 to 28 days, oral administration for 28 days to 1 year, and low-dose infusion; IV administration of lipoecaprost for 50 days; and IV administration of ciprostene (a PGI2 analogue) for 7 days.108,497 Compared with placebo, prostanoids appeared to have some efficacy for treating rest pain (RR, 1.30; 95% CI 1.06 to 1.59) and ulcer healing (RR, 1.24; 95% CI 1.04 to 1.48). As a group, however, prostanoids did not have a significant impact on amputations or mortality, although not all studies defined major vs minor amputations.498 Prostanoids were associated with a statistically significant increase in side effects (RR, 2.35; 95% CI, 1.99–2.78).498 The side effects were mostly minor, including headache, facial flushing, nausea, vomiting, and diarrhea.
The authors of the Cochrane systematic review concluded that there is no strong evidence on the efficacy and safety of prostanoids in patients with CLTI on the basis of a high-quality meta-analysis of homogeneous, long-term RCTs.497 They also called on the need for further high-quality trials.498 A subgroup analysis of the Cochrane meta-analysis, however, suggested that iloprost appeared to reduce major amputation (RR, 0.69; 95% CI, 0.52–0.93) and fared better with rest pain (RR, 1.54; 95% CI, 1.19–1.99) and ulcer healing (RR, 1.80; 95% CI, 1.29–2.50). The authors stated that whereas previous meta-analyses of iloprost had been more positive,500 only a few of the studies used in those previous meta-analyses could be included in the Cochrane review because of study methodology issues. In fact, in clinical practice, iloprost appears to benefit approximately 40% of patients in whom revascularization is not possible.156,500
Since the Cochrane review was published, a newer RCT comparing a placebo with the use of PGI2 analogue taprostene intravenously for 2 weeks failed to demonstrate any difference in pain relief, ulcer size improvement, or prevention of amputation.501 There are no data to support the use of prostanoids to reduce the risk of major amputation in CLTI patients in whom revascularization is not possible.
Vasoactive drugs.
Naftidrofuryl.
A Cochrane review of eight RCTs examined the IV administration of naftidrofuryl in 269 patients.109 The treatment tended to reduce rest pain and to improve skin necrosis, but this was not statistically significant. The studies were found to be of low methodologic quality, with varying levels of severity of CLTI, varying lengths of duration of treatment (from 3 to 42 days), and different measures of effect. This resulted in varying end points that precluded a meaningful pooling of results.109 Thus, there is currently insufficient evidence to support the use of naftidrofuryl in the treatment of CLTI.498
Pentoxifylline.
This drug improves blood flow by increasing red blood cell deformity and decreasing viscosity. A European RCT involving 314 patients found a significant reduction in rest pain, sleep disturbance, and analgesia requirements.502 In a separate Norwegian study using the same dosing regimen, there was no statistically significant difference either in pain-free levels or in absolute walking distance between the two groups.503 Researchers concluded that further investigation is necessary to evaluate the role of pentoxifylline in the treatment of patients with CLTI. Thus, there is currently a lack of consistent evidence to recommend the use of pentoxifylline in the treatment of CLTI.498
Cilostazol.
This drug has been well studied in claudicants but not as much in CLTI. One small study demonstrated that cilostazol improves microvascular circulation and skin perfusion pressure in ischemic limbs.504 Another uncontrolled study that used cilostazol in conjunction with endovascular revascularization reported higher rates of AFS and limb salvage but not higher rates of survival or freedom from further revascularization.505 In the absence of RCTs in patients with CLTI, there is insufficient evidence that cilostazol improves clinical outcomes in patients with CLTI.504,505
Vasodilators
Because vasodilators can cause shunting of blood away from ischemic areas to nonischemic areas, they are of no value to patients with CLTI.156
Defibrinating agents
Two small RCTs compared ancrod, a defibrinating agent, with placebo in CLTI.506,507 Although one study showed positive changes in APs and TPs, both studies failed to demonstrate any improvements in clinical outcome.
Hyperbaric oxygen therapy (HBOT)
There are numerous plausible mechanisms for HBOT to have a therapeutic role in CLTI. These include increased oxygen transport capacity of plasma (independent of red blood corpuscle number and function), improved function of the leukocyte oxygen-dependent peroxidase system, reduced tissue edema due to the osmotic effect of oxygen, stimulation of progenitor stem cell mobilization and angiogenesis, and improved fibroblast function.508 If there is superimposed infection, HBOT also inhibits bacterial growth (particularly anaerobes), generates free radicals that destroy bacterial cellular structures, and improves the oxygen-dependent transport of antibiotics.509
In 2015, a Cochrane review of the role of HBOT in healing of chronic wounds was published,110 involving 12 trials and 577 patients. Ten of the 12 trials studied the effect of HBOT on ulcer healing in patients with diabetes. The 2015 review concluded that HBOT increased the rate of ulcer healing in DFUs at 6 weeks but not at longer term follow-up, with no significant difference in the risk of major amputation.110
Three other studies involved patients with ischemic ulcers, but each study used varying definitions of ischemia.510–512 Abidia et al511 randomized 18 patients with an ABI of <0.8 or TBI of <0.7 and found improvement in wound healing in the treatment group. Löndahl et al512 randomized 94 patients with adequate distal perfusion or nonreconstructible arterial disease. They found that 57% of patients had a TP of <60 mm Hg (median, 52 mm Hg). Complete ulcer healing occurred in 52% of the patients treated with HBOT compared with 29% of controls at 12 months (P < .02). Stratification based on TPs did not appear to affect healing rates. A subsequent publication by this group demonstrated that preintervention TcPO2 correlated with ulcer healing and that individuals with a TcPO2 of <25 mm Hg did not heal.513 There was no significant difference in major amputations between the two groups, with three amputations in the HBOT cohort and one in the control cohort.
One study randomized 70 patients with DFUs to either HBOT or standard care.510 The mean ABI and TcPO2 were 0.65 and 23 mm Hg in the HBOT cohort and 0.64 and 21 mm Hg in the non-HBOT group. All patients with an ABI <0.9 or TcPO2 <50 mm Hg were considered ischemic, underwent an iloprost infusion, and were examined for possible revascularization. Thirteen patients in each group underwent a revascularization procedure. At the completion of the therapy, resting TcPO2 increased by a mean of 12.1 in the HBOT group and 5.0 in the control group (P < .0002). There was a significant reduction in major amputations in the HBOT group (P < .016).510
A large longitudinal cohort study using data from a wound healing group in the United States61 included patients with DFUs and adequate foot perfusion as determined by clinicians. A total of 793 patients underwent HBOT. Propensity scoring was used to compensate for the lack of randomization. The study found that individuals treated with HBOT were less likely to have healing of ulcers (HR, 0.68; 95% CI, 0.63–0.73) and more likely to undergo lower limb amputation (HR, 2.37; 95% CI, 1.84–3.04).514
A subsequent multicenter RCT (Does Applying More Oxygen Cure Lower Extremity Sores? [DAMO2CLES]) undertaken in 25 hospitals in the Netherlands and Belgium randomized 120 patients with an ischemic foot wound and diabetes to standard care with or without a course of HBOT. Ischemia was defined as AP <70 mm Hg, TP <50 mm Hg, or TcPO2 <40 mm Hg. All patients were assessed for revascularization, and when applicable, this was generally performed before HBOT. Primary outcomes were limb salvage, wound healing at 12 months, and time to wound healing. Mortality and AFS were also analyzed. Limb salvage (47/60 in the standard care cohort and 53/60 in the standard care with HBOT cohort), index wound healing at 12 months (28/60 in the standard care cohort vs 30 in the standard care with HBOT cohort), and AFS (41/60 in the standard care cohort vs 49 in the standard care with HBT cohort) were not significantly different between the two groups. A high proportion (35%) of those allocated to HBOT were unable to undergo HBOT or did not complete at least 30 treatments, mostly for medical comorbidities or logistical reasons, reinforcing the significant medical comorbidities present in these patients.112
Overall, whereas controversy remains, there may be a role for the use of HBOT to accelerate ulcer healing in diabetic patients with nonhealing neuropathic ulcers and low-grade ischemia who have failed to respond to conventional wound care. However, HBOT does not prevent major limb amputation and should not be used as an alternative to revascularization in patients with CLTI.
Guidelines on nonrevascularization pharmacotherapy
The TASC II document notes that although previous studies with prostanoids in CLTI suggested improved healing of ischemic ulcers and reduction in amputation, trials do not demonstrate a benefit for prostanoids in promoting AFS.156 The current PAD guidelines and recommendations of the American College of Cardiology Foundation and the American Heart Association state that parenteral administration of PGE1 or PGE2 may be considered to reduce pain and to improve ulcer healing in CLI but that the beneficial effect is likely to occur only in a small subset of patients.515
Finally, international guidelines do not address vasoactive drugs, vasodilators, or defibrinating agents. However, the TASC II guideline advocated for considering HBOT in selected patients who have not responded to revascularization.156
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 7.4 | Do not offer prostanoids for limb salvage in CLTI patients. Consider offering selectively for patients with rest pain or minor tissue loss and in whom revascularization is not possible. | 2 (Weak) | B (Moderate) | Vietto,108 2018 |
| 7.5 | Do not offer vasoactive drugs or defibrinating agents (ancrod) in patients in whom revascularization is not possible. | 1 (Strong) | C (Low) | Smith,109 2012 |
| 7.6 | Do not offer HBOT to improve limb salvage in CLTI patients with severe, uncorrected ischemia (eg, WIfI ischemia grade 2/3). | 1 (Strong) | B (Moderate) | Kranke,110 2015 Game,111 2016 Santema,112 2018 |
| Recommendation | ||
|---|---|---|
|
| ||
| 7.7 | Continue to provide optimal wound care until the lower extremity wound is completely healed or the patient undergoes amputation. | Good practice statement |
Conservative management
Wound care.
CLTI is associated with a markedly shortened life expectancy, and not surprisingly, patients with unreconstructed CLTI experience poorer outcomes in terms of survival and limb salvage. In a retrospective study involving 105 patients with unreconstructed CLTI, 46% of patients lost the limb and 54% died within 1 year.516 Of the patients with a nonamputated leg, 72% were dead within 1 year. Thus, despite advances in revascularization techniques and anesthetics, endovascular or surgical revascularization may not be appropriate in some patients, even if it is technically possible, because of significant comorbidities and reduced life expectancy.
A group of 169 patients with stable tissue loss who were unsuitable for revascularization based on medical and anatomic reasons were entered into a dedicated wound management program.290 At 1 year, 77% of patients remained amputation free, 52% had ulcer healing, and only 28% required minor amputation. Investigators concluded that conservative management might serve a subset of CLTI patients. In fact, circumstances other than revascularization have been identified as important for conservative management, including adequate nutrition, absence of infection, removal of mechanical features interfering with wound healing (by surgical débridement, hydrotherapy, or larvae therapy), negative dressing therapy, and noncontact low-frequency ultrasound.517
More recently, a group of 602 diabetic patients with foot ulcers and low TPs or APs were observed.518 During the variable follow-up period of 1 to 276 weeks, 38% of patients had healed primarily, 12% had minor amputation, 17% healed after major amputation, and 33% died unhealed.
CONCLUSIONS
Despite the lack of evidence to support nonrevascularization methods in CLTI, they are still widely used in real-world practice. In a mail-in questionnaire of vascular surgeons in the United Kingdom published in 2009, 75% believed that LS had a role in clinical practice for inoperable PAD,519 although in current practice LS is rarely used for CLTI. Similarly, in a report on outcomes in patients with nonreconstructible CLTI, 88% received prostanoid infusions, 14% low-molecular-weight heparin or oral anticoagulants, 3% SCS, 17% HBOT, and 69% wound treatment. In addition, 13% of patients underwent toe or other foot-sparing amputations; at 24 months, the major amputation rate was 9.3%, with a mortality rate of 23.2%.520 It is possible that these examples of real-world nonevidence-based practice represent the desire to help this challenging population of patients when traditional methods either are unsuitable or have failed. Still, these treatments are mostly unsupported by evidence and should be considered alternatives only on an individual basis and after careful consideration of benefit and risks.
| Research priorities | |
|---|---|
|
| |
| 7.1 | Assess whether pneumatic compression is effective in improving AFS and resolution of rest pain in patients with CLTI. |
| 7.2 | Better define individuals with CLTI who are likely to benefit from nonrevascularization therapies. |
| 7.3 | Define the role of exercise therapy for the nonrevascularization treatment of patients with CLTI. |
| 7.4 | Define the population of CLTI patients who experience benefit from HBOT in terms of wound healing, pain relief, or other meaningful outcomes. |
8. BIOLOGIC AND REGENERATIVE MEDICINE APPROACHES IN CLTI
Biologic or regenerative medicine therapies include gene therapy and cellular therapy. These treatments offer the potential to promote wound healing and to prevent amputation in patients who otherwise have no options for revascularization.
Therapeutic angiogenesis is defined as the growth of new blood vessels from pre-existing blood vessels in response to growth factor stimulation. This has been shown to occur in animal models of hind limb ischemia and can be induced either by angiogenic proteins such as vascular endothelial growth factor or by cellular therapy using stem cells or bone marrow aspirate. The concept of angiogenesis was introduced into the clinical realm by Jeffrey Isner in the early 1990s.521 Various growth factors, including vascular endothelial growth factor, hepatocyte growth factor (HGF), and fibroblast growth factor (FGF), have been shown to promote angiogenesis in animal models. The short half-life of these proteins has led to the use of gene therapy to maintain sustained expression in the ischemic limb. Most clinical trials to date have used intramuscular injection of either a gene or cellular therapy. In the case of gene therapy, expression of the protein is maintained for 2 to 6 weeks. Ongoing research in this arena includes alternative vectors to safely enhance long-term gene expression.
The putative mechanism of cellular therapy involves either the differentiation of stem cells into vascular cells, after injection into the hypoxic extremity, or induction of angiogenic growth factor expression, again due to relative tissue hypoxia in the ischemic extremity. General concerns about the safety of angiogenic therapy have been related to the potential for “off-target” angiogenesis, which can result in promotion of occult tumor growth or accelerated progression of diabetic proliferative retinopathy. To date, these concerns have not occurred in angiogenic clinical therapy trials that have been completed.
Trials of gene and stem cell therapy in CLTI
Gene therapy.
Fibroblast growth factor (FGF).
This has been extensively studied in the context of severe limb ischemia. The TALISMAN phase 2 trial (NCT00798005) enrolled 125 patients and reported a significant improvement in AFS at 12 months of 73% in patients treated with FGF plasmid compared with 48% in placebo-treated patients with no options for revascularization (P = .009).522 Complete ulcer healing at 6 months occurred in 14% of the placebo group and 20% of the treatment group (not significant).522 In a separate study, the investigators demonstrated proof of concept of gene therapy when they identified the FGF plasmid, messenger RNA, and protein in the amputation specimens of patients with CLTI who received FGF plasmid injections before amputation.523
These findings led to a phase 3 trial, the TAMARIS trial (NCT00566657).524 This trial enrolled 525 patients from 30 countries who had either an ischemic ulcer or minor gangrene. However, the TAMARIS trial failed to show a difference in AFS compared with placebo in patients with CLTI (63% in the treatment group vs 67% in the placebo group).524 The AFS for both groups was similar to that for the FGF-treated patients in the phase 2 TALISMAN trial (Table 8.1). The likely explanation for the different results observed in the phase 2 TALISMAN and phase 3 TAMARIS trials is a type II error in the earlier study.
Table 8.1.
Major trials of gene therapy and cell therapy
| End points |
||||||
|---|---|---|---|---|---|---|
| Trial | Treatment | No. of participants | AFS at 12 months (treated vs placebo) | Other end points | Treatment vs placebo | Reference |
|
| ||||||
| Gene therapy | ||||||
| TALISMAN | FGF | 125 | 73% vs 48% (P = .009) | Nikol,522 2008 | ||
| TAMARIS | FGF | 525 | 63% vs 67% (P = .48) | Belch,524 2011 | ||
| HGF-STAT | HGF | 104 | No difference | Change in TcPo2at 6 months | 25.2 mm Hg in high-dose group vs 9.4 mm Hg in placebo group (P = .0015) | Powell,526 2008 |
| HGF-0205 | HGF | 27 | No difference | Change in TBI at 6 months | +0.05 vs −0.17 (P = 0.047) | Powell,525 2010 |
| Shigematsu et al | HGF | 40 | No difference | Improvement in rest pain or reduction in ulcer size | 70.4% vs 30.8% (P = .014) | Shigematsu,527 2010 |
| Cell therapy | ||||||
| Iafrati et al | Autologous bone marrow | 97 | No difference | Improvement in pain at 6 months | 58% vs 26% (P = .025) | Iafrati,528,530 2011, 2016 |
| Improvement in TBI at 6 months | 0.48 vs 0.012 (P = .02) | |||||
| RESTORE-CLI | Expanded autologous stem cells | 72 | No difference | Combined outcomes (1-year freedom from major amputation, mortality, increased wound size, new gangrene) | 40% vs 67% (P = .045) | Powell,532 2012 |
| MOBILE | Autologous bone marrow cells | 152 | 80% vs 69% (P = not significant) | Murphy,529,5312011, 2016 | ||
| JUVENTAS | BMMNCs | 160 | 77% vs 84% (at 6 months) No difference |
Major amputation at 6 months | 19% vs 13% (P = not significant) | Teraa,535 2015 |
AFS, Amputation-free survival; BMMNCs, bone marrow mononuclear cells; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; JUVENTAS, Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation; MOBILE, MarrowStim treatment of limb ischemia in subjects with severe peripheral arterial disease; TBI, toe-brachial index; TcPo2, transcutaneous oximetry.
Hepatocyte growth factor (HGF).
Several clinical trials have evaluated HGF plasmid in the treatment of patients with CLTI and no option for revascularization. Early phase 2 trials (NCT00189540, NCT00060892) have shown that HGF plasmid gene therapy can improve TcPO2 and pain scores in patients with CLTI compared with placebo, but this did not result in improved AFS.525,526 A Japanese trial of 40 patients demonstrated a significant improvement in a composite end point of improvement of rest pain in patients without ulcers or reduction in ulcer size in those with ulcers at 12 weeks (70.4% vs 30.8%; P = .014).527 The AFS at 12 months was not reported. There are currently no U.S. Food and Drug Administration (FDA)-approved gene therapies for treatment of patients with CLTI.
Stem cell therapy.
Preclinical studies using animal hind limb ischemia models have shown that stem cells injected intramuscularly into the hind limb can promote improved blood flow through an angiogenic mechanism. Early studies in humans have similarly shown improved vascularity in the treated extremity, as measured by ABI, although the mechanism by which this occurs in humans is unknown. Cellular therapies can be divided into autologous and allogeneic. Several phase 1 and phase 2 trials have recently been completed, including ones from Harvest Technologies (NCT00498069) and Biomet (NCT01049919).528,529 Both of these report promising early results of phase 1 trials using autologous bone marrow mononuclear cells (BMMNCs) in the treatment of CLTI.528,529 In addition, both companies have developed point-of-care cell preparation systems. After bone marrow harvest, the BMMNCs are extracted for direct intramuscular injection into the ischemic limb.
Iafrati et al528 reported the results of 97 patients. In patients treated with intramuscular bone marrow concentrate, there was a 64% AFS at 6 months compared with 65% in the control group. The treated patients had a significant improvement in pain relief and TBI.528,530 Another trial of 152 patients found little difference in AFS between the treatment group and control group at 6 months (80% vs 69%; P = .224).529,531 Both of these phase 3 trials are being conducted through investigator device exemptions from the Center for Devices and Radiological Health of the FDA.
Another trial, the RESTORE-CLI (phase 2) trial, used expanded autologous stem cell therapy, ixmyelocel-T, in the treatment of CLTI patients for whom revascularization was not an option.532 Bone marrow aspirate (50 mL) was taken from study patients and sent to the sponsor, where the cells were cultured in a bioreactor and expanded during a 2-week period; when expanded, the cell population is enriched with mesenchymal precursors and alternatively activated macrophages. It was then returned to the trial site for intramuscular injection into the ischemic limb of the patient. The trial enrolled 72 patients with either ischemic rest pain or tissue loss. At 12 months, 40% of patients who were treated with ixmyelocel-T experienced one or more treatment failure events (defined as death, major amputation, doubling of wound size from baseline, or new-onset gangrene) compared with 67% of placebo-treated patients (P = .045, Fisher exact test). There was no difference in AFS.532 Treatment failure events were particularly pronounced in patients who presented with tissue loss at baseline. In the subgroup of patients presenting with wounds, 45% of patients treated with ixmyelocel-T experienced a treatment failure event compared with 88% of control patients (P = .01).532
In a small study of 28 patients with CLTI, Losordo et al533 completed a placebo-controlled trial to compare CD34-positive cells selected by leukopheresis after mobilization with granulocyte colony-stimulating factor. The investigators showed a trend toward reduction in all amputations (both major and minor). At 12 months, 31% of treated patients underwent amputation compared with 75% of placebo-treated patients (P = .058). There was no difference between the two groups when only major amputation was evaluated, although the number of patients in the trial was small.533
In another small trial, the Bone Marrow Autograft in Limb Ischemia (BALI) study randomized 38 patients with CLI to treatment with bone marrow-derived mononuclear cells vs placebo at seven centers in France.534 A single treatment employing 30 separate intramuscular injections in the ischemic limb was performed. There was no statistical difference in major amputation at 6 or 12 months or in ulcers or pain relief at 6 months. Interestingly, TcPO2 values increased in both treated and placebo patients. Using a “jackknife” method of logistic regression, the authors suggest some benefit in major amputation for the treated group. However, the total number of patients and events in this trial was small, and the results can be considered only exploratory at best.
The Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial randomized 160 patients with severe limb ischemia to three intra-arterial infusions of either BMMNCs or placebo, 3 weeks apart.535 No major differences were found in major amputations at 6 months (19% in patients receiving BMMNCs vs 13% in the placebo cohort) or in AFS at 6 months (77% in patients receiving BMMNCs vs 84% in the placebo group). No differences were found in the safety outcomes or secondary outcomes of the two groups.535
The recently completed phase 1 allogeneic cell therapy trial sponsored by Pluristem (NCT00951210) has shown promising safety and potential efficacy (personal communication). This open label trial of allogeneic placental stem cells (PLX-PAD cells) will be entering phase 2 placebo-controlled trials. The PLX-PAD cells are mesenchymal-like stromal cells derived from the full-term placenta and are expanded using the sponsor’s proprietary bioreactor. The cells are believed to be immune privileged and would potentially offer an “off-theshelf” treatment option.
Finally, a meta-analysis of randomized placebo-controlled trials of stem cell therapy involved 499 patients in 10 trials.113 Follow-up in all of the included trials was <12 months, and only three studies observed patients for at least 6 months. This meta-analysis demonstrated no improvement in major amputation rates or AFS associated with stem cell therapy. Secondary outcomes (ABI, TcPO2, and pain scores) were significantly better in the treatment group.113
Safety of therapeutic angiogenesis
Early concerns about off-target angiogenesis and the potential for progression of diabetic proliferative retinopathy or occult tumor growth previously resulted in significant restrictions in the inclusion and exclusion criteria for entry into these studies. As early studies demonstrated an acceptable safety record for this therapy and potential concerns about off-target angiogenic complications lessened, these restrictions have since decreased.
Unanswered questions in the field
Trial design and completion hurdles.
Trials involving CLTI patients face multiple hurdles that have resulted in delays in completion. The overall comorbid burden of the population of CLTI patients results in a high incidence of adverse events throughout the length of the study. Likewise, the heterogeneous nature of CLTI results in a highly variable natural history. Patients with ischemic tissue loss have a major amputation rate at 1 year of up to 35% compared with <10% in patients with rest pain. In addition, the FDA recommends that AFS should be the primary efficacy end point in a phase 3 CLTI trial. This has resulted in studies with an expected enrollment requirement of at least 500 patients. The reason for these large numbers in a phase 3 trial is that biologic treatment of CLTI is a limb-sparing procedure. As such, it is not expected to significantly influence mortality, although mortality is a component of the primary end point. Consequently, because of the heterogeneous and frail nature of the population of CLTI patients, larger numbers of patients are needed to complete a clinical trial that can detect any potential efficacy on amputation at 1 year.
Selection of patients.
Many trials have recruited individuals who are considered to have no option for revascularization. Unfortunately, there is no consistent definition of no-option CLI. Published studies referred to in this section have broadly included individuals who were considered poor candidates for surgical or endovascular revascularization. This was due to either technical factors (inadequate venous conduit; unfavorable anatomy, such as absence of a patent artery in the calf that is in continuity with the foot) or patient-related factors (poor operative risk, but pain or tissue loss was unlikely to require amputation within 4 weeks). In several studies, imaging was assessed by an independent vascular specialist.
The development of advanced endovascular techniques gives many patients who were previously considered to have no option for revascularization a new opportunity to be considered potentially suitable for endovascular intervention. Nonetheless, there are few data supporting many of these techniques. Novel methods to measure circulating stem or progenitor cells before therapy may prove helpful in serving as companion diagnostics to identify those individuals who may or may not respond to angiogenic therapy.536
Conclusions
There have been promising early safety and efficacy trial data for both gene and cellular therapies in patients with CLTI. Despite these early promising results, no phase 3 trials have shown this therapy to be effective. Still, current trial design has improved, and there are multiple phase 3 clinical trials that either are actively enrolling or are in early stages of development. These involve potentially disruptive technologies that, if proven effective, could dramatically alter how patients with CLTI are cared for in the future. Until further evidence is available, these therapies should be considered investigational.
| Research priorities | |
|---|---|
|
| |
| 8.1 | Identify surrogate markers (biomarkers, imaging) that would assist in understanding the possible mechanisms of action of gene- and cell-based therapies in CLTI. |
| 8.2 | Determine whether gene- or cell-based therapies can serve as an adjunct to revascularization to improve clinical outcomes in subsets of CLTI patients. |
9. THE ROLE OF MINOR AND MAJOR AMPUTATIONS
CLTI is associated with a reduced life expectancy, a significant curtailment in ambulation, and a high likelihood of limb loss. Preservation of a patient’s ability to walk is an important aspect of care in CLTI, and vascular reconstruction is the most direct method for achieving functional limb salvage in these often critically ill patients. When properly applied, open surgical and endovascular techniques have proved useful and successful for the preservation of limb function. A successful limb salvage intervention is associated with low postprocedural morbidity and mortality, preservation or restoration of independent ambulation, improved quality of life for the patient, and lower cost to the health care system. Although most patients require a single procedure to accomplish this, many will need minor amputations to remove distal necrotic or infected tissue to achieve a completely healed and functional extremity. This is especially true of diabetics, who have a lifetime risk of foot ulceration of 25%, with 50% of ulcers becoming infected.154 Treatment of these patients requires both in-line pulsatile flow to the foot and wound débridement or minor amputation.537
Minor amputations.
Minor amputations of the foot include digital and ray amputation of the toe, transmetatarsal amputation of the forefoot, and Lisfranc and Chopart amputations of the midfoot. Each of these can be useful to preserve foot function in appropriately selected patients. Although there is a significant risk of need for reamputation at a higher level in diabetics, the use of minor amputations, including single-digit and ray amputations, can preserve foot function in the majority of patients.538–540 There are some instances in which transmetatarsal amputation may be a better first procedure, including necrosis of the great toe requiring long ray amputation or ray amputation of the first and fifth toes, but ensuring adequate distal perfusion and appropriate offloading of the forefoot are the major principles for preservation of foot function.114,541
There are, however, situations in which an aggressive attempt at limb salvage would be unlikely to succeed, would pose too great a physiologic stress on the patient, or would be of limited value because of other causes of limb dysfunction. For these patients, major amputation may be considered a reasonable option. Because a well-planned primary amputation can often result in a high likelihood of independent ambulation for many patients, this procedure should not be considered a failure of vascular surgery. Rather, it should be viewed as another path to the goal of preserving the walking ability in carefully selected patients or for resolution of ischemic pain, ulceration, and infection.
Primary amputation.
Primary amputation in patients with CLTI is defined as lower extremity amputation without an antecedent open or endovascular attempt at limb salvage. There are four major goals of primary amputation for patients with CLTI: (1) relief of ischemic pain; (2) removal of all lower extremity diseased, necrotic, or grossly infected tissues; (3) achievement of primary healing; and (4) preservation of independent ambulatory ability for patients who are capable. In addition, there are five major indications for primary amputation.
Nonreconstructible arterial disease, as confirmed by clear distal imaging studies that fail to identify patent distal vessels needed for a successful intervention. In the setting of severe distal ischemia, in particular in association with ischemic ulceration, gangrene, or infection, the inability to improve straight-line distal perfusion often results in major amputation even with a patent bypass graft. Bypasses to arteries that do not have at least large, angiographically apparent collateral vessel outflow provide little additional flow to the foot for distal limb salvage.542 Patients without any appropriate targets for successful distal revascularization are frequently better served with a primary major amputation.
Destruction of the major weight-bearing portions ofthe foot, rendering it incompatible with ambulation. The weight-bearing portions of the foot consist of the calcaneus, the first and fifth metatarsal heads, and a functional arch. Patients with gross destruction of the calcaneus and overlying skin should be considered for primary amputation because a functional foot can infrequently be salvaged. After aggressive heel ulcer excision and extensive calcanectomy, complete wound healing is infrequent and chronic pain is common.543,544
Nonfunctional lower extremity due to paralysis or unremediable flexion contractures. These patients are unlikely to benefit from attempts at revascularization, and there will be little change in quality of life despite a successful intervention.
Severe comorbid conditions or limited life expectancy due to a terminal illness. The goal of treatment for these patients is relief from ischemic pain, if present, and an improvement in the remaining quality of life. Extensive distal revascularization, prolonged hospitalization, and protracted recovery should be avoided. Assessment of the patient’s frailty may be of value to determine whether primary major amputation is more appropriate than distal revascularization.545,546
Multiple surgical procedures needed to restore a viable lower extremity. As the technology and techniques of vascular surgery have improved, surgeons have advanced beyond revascularization to complex vascular and soft tissue reconstruction. This approach usually involves multiple surgical procedures to increase distal flow, removal of all necrotic tissue, and reconstruction of these areas with free flaps. The course of treatment is prolonged, involving multiple returns to the operating room, long periods of inactivity, and a difficult recovery. For these patients, if multiple procedures with high morbidity are required, primary amputation should be strongly considered to permit early ambulation. A detailed discussion with the patient to develop a comprehensive treatment plan with shared decision-making is important for such advanced vascular disease.
For all patients considered for primary amputation, also consider revascularization to improve inflow in an attempt to reduce the level of the amputation.118,119 For example, those patients with extensive infrainguinal arterial occlusion, including the common and proximal PFA, might benefit from restoration of flow into the deep femoral system to reduce the amputation level from the upper thigh to the level of the knee. In such cases, despite some additional risk, proximal revascularization has the potential to offer a tangible and significant benefit to the patient.
Secondary amputation.
For those in whom one or more attempts at revascularization have failed and the likelihood of a successful and durable redo procedure is limited, major amputation with a goal of rehabilitation to independent ambulation should be considered.
Level of amputation.
Selecting the level of amputation that will heal primarily is critical to successful prosthetic rehabilitation and maximal functional mobility. Thus, a great deal of consideration must go into selecting the initial level of amputation. Preoperative tissue perfusion assessment can make it possible to lower the level of amputation, although there is no accurate method to predict the optimal level of amputation.547 In addition, whereas assessment of preoperative tissue perfusion can aid in decision-making, it still remains largely a clinical decision. Many techniques to evaluate tissue perfusion have been tried, including laser Doppler flowmetry, thermography, skin perfusion pressure, fluorometric quantification of a fluorescein dye, TcPO2, and indocyanine green fluorescence angiography. In particular, TcPO2 has been extensively evaluated, and it has been shown that wound complications increase as TcPO2 levels fall below 40 mm Hg.547 Currently, there is still no single definitive method of evaluating tissue perfusion that can accurately predict the wound healing potential or failure at the site of amputation.
Healing rates of amputations and reamputations.
Achieving primary healing is challenging in ischemic lower limbs, and it is difficult to predict early failure (Table 9.1). Multiple débridements and reamputations are required in 4% to 40% of patients, depending on the level of amputation.548–550 Likewise, readmission rates of 20% have been reported even after minor amputations (toe and distal forefoot), with the majority of reamputations occurring within 1 month.548–550 Reported long-term healing rates after transmetatarsal amputations are approximately 53%.551 These amputations should not be offered to patients who have poor rehabilitation potential.
Table 9.1.
Major amputation of the lower extremity
| Level of amputation | Below knee | Through knee | Above knee |
|---|---|---|---|
|
| |||
| Primary healing | 30%–92% | 60%–81% | 60%–95% |
| Perioperative mortality | 4%–10% | 1%–17% | 10%–20% |
| Revision to higher level | 12%–20% | 1.5%–20% | 8%–12% |
| Ambulation | 40%–80% | 57%–70% | 20%–40% |
The role of partial foot or midfoot (eg, Lisfranc, Chopart) amputations remains controversial. Prosthetic specialists discourage the use of these procedures as they have higher rates of delayed healing, require more revisions, and develop deformities and ulcers, and patients often struggle to achieve their full rehabilitation potential. Conversely, these amputations preserve a weight-bearing heel and allow amputees the ability to mobilize for short distances without prostheses.552
Transtibial amputation (below-knee amputation [BKA]) and transfemoral amputation (above-knee amputation [AKA]) are performed with an almost equal frequency in patients with CLTI. Reports have shown primary healing rates for BKA of approximately 60%, with 15% leading to a transfemoral amputation.121,548 The transfemoral amputation has the highest probability of successful primary healing and therefore has been the amputation of choice in individuals who are less likely to ambulate with a prosthesis.
Recent data from the American College of Surgeons National Surgical Quality Improvement Program show improved results with a 12.6% early failure rate for BKA compared with 8.1% for AKA.553 A similar trend is found in data from the National Vascular Registry of the United Kingdom, which show that one in eight AKAs and one in six BKAs remain unhealed at 30 days.554
Knee disarticulation.
The biomechanical advantages of a knee disarticulation or through-knee amputation (TKA) compared with an AKA are well recognized, although it remains an infrequently performed amputation. A well-performed TKA offers healing rates that are comparable to those of AKA and provides bedridden and wheelchair-bound patients with a higher level of mobilization and transfer, counterbalance, and reduced potential for contractures. Even in patients who have rehabilitation potential, the current prosthetic technology permits excellent functional mobility, making TKA a good amputation choice when a BKA is unlikely to heal. The aesthetic disadvantage of a TKA is that the prosthetic knee will be marginally distal to the normal contralateral knee in a sitting position.
Mortality.
Survival after major lower limb amputation is poor, as seen in a systematic review that reported 30-day postoperative mortality rates of 4% to 22%.555 Even after minor amputations, the 1-year and 5-year mortality rates are reported to be 16% and 25%, respectively, for those with limb ischemia.556 Mortality rates for minor amputations are higher in diabetics, with type 2 diabetics having a 5-year mortality of >50%.557 The 5-year mortality after major amputations varies from 30% to 70% and is significantly worse for AKA than for BKA.558,559 The mortality is even higher in bilateral lower limb amputees, with a 5-year survival rate of <40%.560 These mortality rates demonstrate the high rate of comorbidities and the frailty of this group of patients.
In patients with diabetes who have had major amputations, survival is often worse than in some malignant diseases. Survival rates have been reported as 78% at 1 year, 61% at 3 years, 44% at 5 years, and 19% at 10 years.561
In 2010, recognizing the need to do more to reduce perioperative mortality, the Vascular Society of Great Britain and Ireland introduced a quality improvement framework to reduce mortality from amputation surgery to <5% by 2015, which was later revised to <10% in 2016.562 Recent data from the United Kingdom’s National Vascular Registry showed mortality rates of 11.6% for AKA and 6.1% for BKA by establishment of dedicated multidisciplinary amputation services that provided expeditious and comprehensive preoperative and postoperative care.550 These rates are similar to results from the American College of Surgeons National Surgical Quality Improvement Program of 12.7% for AKA and 6.5% for BKA, with an overall 9.1% mortality of 6389 patients studied.563
Fate of contralateral limb after lower extremity amputation.
Published reports of the risk of contralateral amputation vary from 2.2% to 44%, with a lower risk if the index amputation is a minor amputation.124 In most patients, the reason for contralateral amputation is disease progression, although the medical management of unilateral amputees can also be suboptimal, with one-third of patients not prescribed a statin and an antiplatelet agent.123 Continued follow-up of these patients at least yearly after amputation with attention to the contralateral limb is important.124
Prosthetic rehabilitation, mobility, and quality of life.
When an amputation is inevitable, and whenever possible, a prosthetic specialist should be involved in decision-making with the surgical team regarding the optimal level of amputation that will ensure the best opportunity for healing, survival, and maximum functional mobility. Advances in prosthetics have resulted in a prosthesis for every stump. However, to use the prosthesis effectively, the stump must be created to truly function as a dynamic sensorimotor end organ and not simply as an inert filler in the socket.
Muscle-stabilizing procedures can help create a stump with its proprioception intact and any of the procedures can be used, including myoplasty, myodesis, and osteomyoplasty. The stump evolves with time, and the prosthetic requirements continue to change. The patient requires regular adjustments in the prosthesis and often complete revisions. A poorly fitting prosthesis can be as disabling as the actual amputation.
The quality of life after amputation is significantly influenced by pain, social isolation, depression, and the patient’s lifestyle before amputation. Mobility has a direct effect on quality of life. It is a key determinant to the social reintegration of the amputee and has a beneficial effect on late mortality.
Energy expenditures of ambulation increase with ascending levels of amputation. Energy consumption during ambulation is increased by 10% to 40% after BKA and by 50% to 70% after AKA.564 The potential for rehabilitation is better with BKA than with AKA. Therefore, it is worthwhile to try to salvage a BKA in a patient who has the potential to ambulate fully. In studies involving >100 patients, ambulatory status at 6 to 12 months after amputation varies from 16% to 74%.551 At 2 years, only 40% of BKA patients achieve full mobility.121
Maintaining ambulation is one of the most important factors in preserving independence. A significant amount of evidence is available to suggest that early postsurgical prosthetic fitting leads to early mobility.565 However, to achieve and to maintain daily functional ambulation, multidisciplinary inputs are needed from physiotherapists, occupational therapists, prosthetists, social workers, recreational therapist nurses, psychologists, and the surgeon. Despite initial successful prosthetic rehabilitation, prosthetic use deteriorates over time, and most patients eventually become household walkers only.566
Delivery of amputation service.
Based on current international practice,562,566 the following best practice recommendations will help decrease mortality and improve functional outcomes:
The indication for any nonurgent amputation should be discussed at a multidisciplinary team meeting after a full functional and vascular assessment.
Patients should be informed as to the rationale of any amputation as well as the postamputation care pathway.
Patients should have access to a second opinion (by avascular specialist from another institution).
A preoperative assessment by a rehabilitation and occupational physiotherapist as well as by a prosthetic specialist should be organized.
Procedures should be performed on an elective list (within 48 hours of the decision).
Amputations should be performed by or in the presence of a board-certified consultant surgeon.
A named discharge coordinator should ensure that there is a defined postamputation care pathway.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 9.1 | Consider transmetatarsal amputation of the forefoot in CLTI patients who would require more than two digital ray amputations to resolve distal necrosis, especially when the hallux is involved. | 2 (Weak) | C (Low) | Elsherif,114 2017 |
| 9.2 | Offer primary amputation to CLTI patients who have a pre-existing dysfunctional or unsalvageable limb, a poor functional status (eg, bedridden), or a short life expectancy after shared decision-making with the patient and health care team. | 1 (Strong) | C (Low) | Aziz,115 2015 Siracuse,116 2015 |
| 9.3 | Consider secondary amputation for patients with CLTI who have a failed or ineffective reconstruction and in whom no further revascularization is possible and who have incapacitating pain, nonhealing wounds, or uncontrolled sepsis in the affected limb after shared decision-making with the patient and health care team. | 2 (Weak) | C (Low) | Reed,117 2008 |
| 9.4 | Consider revascularization to improve the possibility of healing an amputation at a more distal functional amputation level (eg, AKA to BKA), particularly for patients with a high likelihood of rehabilitation and continued ambulation. | 2 (Weak) | C (Low) | Rollins,118 1985 Miksic,119 1986 |
| 9.5 | Consider a TKA or AKA in patients who are nonambulatory for reasons other than CLTI (ie, bedridden patients with flexion contracture, dense hemiplegia, cancer) and are unlikely to undergo successful rehabilitation to ambulation. | 2 (Weak) | C (Low) | Ayoub,120 1993 Taylor,121 2008 |
| 9.6 | Involve a multidisciplinary rehabilitation team from the time a decision to amputate has been made until successful completion of rehabilitation has been achieved. | 1 (Strong) | C (Low) | Webster,122 2012 |
| 9.7 | Continue to observe CLTI patients who have undergone amputation at least yearly to monitor progression of disease in the contralateral limb and to maintain optimal medical therapy and risk factor management. | 1 (Strong) | C (Low) | Bradley,123 2006 Glaser,124 2013 |
| Research priorities | |
|---|---|
|
| |
| 9.1 | Identify the best noninvasive test to predict the optimal level of amputation with respect to primary healing. |
| 9.2 | Determine whether the primary healing rates, postprocedure mobility with prosthesis, and quality of life data justify a TKA over an AKA. |
| 9.3 | Investigate whether there is a difference in stump healing between the skew flap, long posterior flap, and equal anterior and posterior flap techniques of BKA. |
| 9.4 | Investigate whether the quality of life after partial foot amputations is inferior to or even better than after BKA or AKA. |
| 9.5 | Determine the optimal early prosthesis fitting and rehabilitation strategies for independent ambulation. |
10. POSTPROCEDURAL CARE AND SURVEILLANCE AFTER INFRAINGUINAL REVASCULARIZATION FOR CLTI
This section reviews evidence for adjunctive medical therapies, surveillance, reintervention, and postprocedural care after infrainguinal revascularization for CLTI.
Medical therapies
All patients who have undergone revascularization for CLTI should continue with best medical therapies to slow the progression of atherosclerosis and mitigate the adverse impact of risk factors as recommended in Section 4. In addition, the role of specific pharmacotherapy for maintaining the benefits of revascularization has been the subject of a number of studies.
Endovascular interventions.
Long-term antiplatelet therapy remains a cornerstone to reduce atherothrombotic events and to improve patency and limb salvage rates after peripheral interventions.35,135 Contemporary management involves the choice between single antiplatelet therapy and DAPT. Aspirin has been a mainstay of treatment because it is efficacious and cost-effective. Clopidogrel is also effective as a single agent.35,567 Use of DAPT after intervention has become standard in the treatment of CAD134,568 and has migrated to other arenas of vascular intervention. Clopidogrel is a prodrug requiring conversion by cytochrome P450 enzymes, the activity of which may be affected by genetic polymorphisms or drug-drug interactions. It has been estimated that between 4% and 30% of individuals treated with conventional doses of clopidogrel do not attain the full antiplatelet response.569 Of note, it has been reported that patients with PAD may have a higher prevalence of resistance to clopidogrel than coronary intervention patients.136
Despite an absence of level 1 evidence, DAPT is frequently employed for 1 to 6 months after peripheral interventions.134,136 The Clopidogrel and Aspirin in the Management of Peripheral Endovascular Revascularization (CAMPER) study was designed to compare aspirin with DAPT but was stopped because of poor enrollment.137,570 The MIRROR trial was a double-blind RCT comparing clinical outcomes of aspirin and placebo vs aspirin and clopidogrel for 6 months after FP intervention. Of the 80 patients who were randomized, 42% had CLTI.136 Decreased target lesion revascularization was observed in patients randomized to the DAPT arm, although there was no significant difference in patency rate. A meta-analysis suggested that DAPT might be associated with a reduced risk of major amputations after revascularization, with increased bleeding risk vs monotherapy.297 A propensity-adjusted analysis from the Vascular Quality Initiative associated DAPT use with improved survival after revascularization for CLTI.571 The efficacy of DAPT may depend on multiple factors, including procedure-related, anatomic, and patient factors. Subgroups of patients who may derive more benefit from DAPT include those with complex disease patterns, those with prior failed interventions, and those at lower risk of bleeding complications (eg, younger patients). Adequately powered RCTs are needed to better define the risks and benefits of DAPT after peripheral intervention as well as optimal dosing and duration of treatment.
The phosphodiesterase inhibitor cilostazol has antiplatelet and antiproliferative properties, and several studies have suggested that it may reduce the incidence of restenosis after catheter interventions. Iida et al572 reported that cilostazol treatment reduced angiographic restenosis after FP intervention (angioplasty with provisional stenting) in an open label randomized trial of 200 patients, of whom 90% had intermittent claudication. A meta-analysis suggested an association between cilostazol use and reduced rates of in-stent restenosis after FP stenting in “high-risk” patients, pooling studies that included 75% claudicants.573 Conversely, an open label RCT found no effect of cilostazol treatment in reducing restenosis after IP interventions for severe limb ischemia.574 No clear recommendation can be made at present regarding the potential benefit of cilostazol after endovascular interventions for CLTI.
Vein and prosthetic bypass grafts.
After vein graft implantation, patency of the graft is likely to be enhanced by lifestyle modifications and medical therapy. Most studies of vein graft patency include patients with both CLTI and claudication. Meta-analyses from prospective studies130,131 along with multiple case series demonstrate a consistent association between the avoidance of smoking and enhanced vein graft patency. Statin medications have not been evaluated in randomized trials for enhancement of vein graft patency, although some retrospective studies suggest that they may be of benefit.125,126 In a cohort study, statin use was not associated with better limb outcomes, although overall survival was improved.129
Although antiplatelet agents are commonly used, there is inconclusive evidence that they specifically enhance lower extremity vein graft patency. A Dutch trial of 2690 patients randomized to oral anticoagulants (target international normalized ratio of 3–4.5) or 80 mg of aspirin per day after lower extremity bypass found better vein graft patency at 12 and 24 months for the oral anticoagulants on subgroup analysis.575 However, there were twice as many bleeding complications in the anticoagulant-treated patients. In contrast, a multicenter U.S. trial comparing warfarin plus aspirin with aspirin alone found no improvement in vein graft patency and a higher rate of bleeding in the combined treatment arm.576 A study of 56 patients with poor-quality venous conduits compared aspirin alone with a combination of aspirin and warfarin and found improved patency in the aspirin plus warfarin group.577 Finally, a systematic review found no effect of aspirin or dipyridamole compared with placebo on vein graft patency at 1 year.127,128 Vein graft patients receiving aspirin or aspirin plus clopidogrel have similar patency, and there is a higher rate of mild to moderate bleeding with DAPT.132 A more recent systematic review concluded that antiplatelet therapy has a beneficial effect on primary patency of peripheral bypass grafts compared with placebo or no treatment.128 It appears, then, that there is limited evidence to support a specific antithrombotic regimen in patients after vein bypass grafting for CLTI. Single antiplatelet therapy, recommended as standard for long-term PAD management, should be continued in these patients. Treatment with warfarin may be considered in patients with high-risk vein grafts (eg, spliced vein conduit, poor runoff) who are not at increased risk for bleeding.
In contrast, there is consistent evidence supporting the use of antiplatelet therapy in patients who have undergone prosthetic bypass grafting. Two Cochrane reviews have supported the use of aspirin and other antiplatelets in maintaining lower extremity bypass graft patency, and greater benefits have been seen with prosthetic grafts.127,128 Other studies have demonstrated similar findings.133 In particular, one randomized trial (Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Arterial Disease [CASPAR]) showed that DAPT with clopidogrel and aspirin led to significantly improved patency in prosthetic grafts but not in venous grafts.132 However, this was accompanied by an increased risk of mild to moderate bleeding. Another study demonstrated that the use of anticoagulants such as vitamin K antagonists did not improve the prosthetic graft patency, although they were beneficial in venous conduits.575,578 In a single-center study, investigators suggested the use of therapeutic vitamin K antagonists to prolong the patency of prosthetic grafts with low velocities.579
Surveillance and reintervention
After endovascular treatment.
Despite the high initial technical success rates of endovascular interventions, early failure of these minimally invasive procedures is common.100,365,580–583 This has led to high rates of secondary interventions and questions of clinical efficacy to support them.
Currently, guidelines support DUS surveillance and prophylactic reintervention for asymptomatic vein graft stenosis to promote long-term patency.138,584–589 Conversely, strategies for surveillance and guidelines for reintervention after angioplasty have primarily been left up to the individual practitioner. There are many determinants of failure after angioplasty, including indication (claudication vs CLTI), lesion length, lesion severity (occlusion vs stenosis), calcification, location, concomitant inflow and outflow vessel disease, use of stents, and residual stenosis or recoil at the time of the initial procedure. As a result, predicting which interventions are more prone to failure has proved challenging, and there is scarce evidence to support indications for repeated interventions in CLTI.
Modalities for surveillance include clinical follow-up visits (assessment of symptoms, inspection of the extremity, pulse examination), ABI measurements, and DUS scan (PSV measurement and velocity ratio). Other imaging modalities, such as DSA, CTA, and MRA, are not reasonable for surveillance because of invasiveness, cost, and limited access as well as exposure to ionizing radiation and contrast dye and potential risks from the procedure itself.
Surveillance by clinical follow-up alone may be insufficient to detect restenosis as patients may remain asymptomatic until the target artery has occluded, akin to bypass grafts. Likewise, ABI measurement alone has limited value, given the difficulty in determining the level of restenosis, the limitation in diabetics with calcified vessels, and the variability of correlation when there is a drop in ABI (>0.15) with lesion severity.590,591 The addition of DUS provides anatomic information using direct visualization of the vessel as well as physiologic information based on spectral waveforms, pressure, and velocity measurements. The combination of PSV and velocity ratio measurements offers high positive predictive value for identifying moderate and severe restenosis when it is correlated with angiography.592,593
The value of DUS in a postprocedural surveillance program needs to be balanced by the potential harm associated with performing unnecessary procedures on asymptomatic restenotic lesions that may have an otherwise benign natural history. The cost associated with maintaining such a program should also be considered. One strategy is to pursue DUS surveillance at regular intervals (3–6 months) and to consider reintervention for severe recurrent asymptomatic lesions (>70%) before they progress to complete occlusion. This approach is supported by data suggesting that restenotic lesions are markers of subsequent failure.142,594,595
Several studies have shown that reintervention on occluded lesions brings higher rates of distal embolization and subsequent reocclusion in comparison to intervening on restenotic but patent vessels.596,597 Although these seem to be reasonable incentives for surveillance, DUS may not identify all of these lesions before failure; for example, not all angioplasty site reocclusions are preceded by severe restenotic lesions.141,598,599 To date, there are inadequate data demonstrating clinical benefit of a DUS surveillance program after endovascular intervention for CLTI. Still, there are likely to be subgroups of patients who may benefit more than others from close surveillance and early reintervention. These may include patients who have experienced multiple failed angioplasties; patients who have previously undergone failed bypasses or for whom conduits are unavailable; patients who had presented with severe ischemia (eg, WIfI grade 3), unresolved tissue loss, or appearance of new inflow lesions; and patients with known poor runoff or long target vessel occlusions that are prone to failure.
Vein and prosthetic bypass grafts.
Vein grafts primarily fail when stenotic lesions develop within the venous conduit or at anastomotic sites of the conduit to the inflow and outflow arteries. Stenotic lesions can also develop in the outflow artery remote from the distal anastomotic site. Approximately one-third of lower extremity vein grafts develop lesions that threaten graft patency, and most occur within 2 years of graft placement. Vein grafts are never entirely free of the risk for development of intragraft or anastomotic stenosis. The risk of vein graft stenosis is greater with smaller caliber conduits, with nonsaphenous or spliced venous conduits, and in grafts with anastomosis to more distal (tibial or pedal) arteries. Surveillance of lower extremity autologous vein grafts is based on this natural history and assumes that a patent, hemodynamically uncompromised reconstruction is optimal for wound healing and limb viability. Secondary reconstructions for thrombosed lower extremity vein grafts are technically more complex and less durable than revision of a failing but patent bypass.
Vein graft surveillance programs may be solely clinical or clinical and vascular laboratory based. The TASC II working group recommended that patients treated with lower extremity vein grafts be observed for at least 2 years with a surveillance program consisting of an interval history to detect new symptoms, pulse examination, and measurement of resting and postexercise ABI, when possible.156 Most vascular laboratory-based surveillance programs focus on DUS detection of stenotic lesions within the graft or at the anastomotic sites. Although there is considerable information on DUS surveillance of lower extremity vein grafts for CLTI, there are few prospective data.
The Vein Graft Surveillance Randomised Trial (VGST), a prospective trial from the United Kingdom, randomized 594 patients with patent vein grafts 30 days after surgery to either clinical surveillance or combined DUS surveillance and clinical surveillance. The majority of operations (two-thirds) were femoral-popliteal bypasses for CLTI. Conduits were ipsilateral reversed saphenous vein in >90%. Thus, technical complexity of surgery in the VGST may not reflect that of open reconstructions performed for CLTI in the modern endovascular era. At 18 months, the investigators found no differences in primary, primary assisted, or secondary patency between the two surveillance strategies.589 A smaller study from Sweden randomized 156 patients with lower extremity arterial reconstructions to intensive surveillance, including DUS scanning (n = 79), or routine clinical surveillance (n = 77). There were 40 polytetrafluoroethylene grafts, equally distributed between the two groups. Only two grafts in each group were performed for claudication, and two-thirds were to the popliteal artery. Among the vein grafts in the study, there was improved assisted primary and secondary patency in the intensive surveillance group that had DUS scanning.585
The benefit of a vein graft surveillance program with DUS scanning is suggested in large single-institution case series as well as in one large multi-institution prospective study.79,138,140,600,601 These studies and others have demonstrated large differences between primary patency and assisted primary patency of vein grafts monitored with a DUS-based surveillance program.139 They also demonstrate that electively revised vein grafts have excellent long-term patency, even comparable to that of grafts that have never undergone revision. In contrast, salvage of vein grafts that have already thrombosed is associated with markedly reduced secondary patency. Improved quality of life has been associated with maintained patency of vein grafts performed for CLTI.233 Despite these observations, it must be acknowledged that the clinical benefit of DUS-based surveillance after vein bypass for CLTI is still unclear. A systematic review found low-quality evidence for DUS surveillance of infrainguinal vein grafts.602
The underlying principle of clinical surveillance of vein grafts is that recurrence of symptoms, change in pulse status, or decrease in ABI >0.15 indicates an at-risk graft that should be considered for revision. It is also suggested that vein grafts with >70% stenosis identified by DUS scanning be considered for revision as such lesions are unlikely to improve and associated grafts have an adverse natural history.138,600 These lesions are defined by an associated PSV of >300 cm/s, a PSV ratio (defined as PSV at the lesion divided by PSV in a proximal segment) of >3.5, or a midgraft PSV <45 cm/s. Vein graft stenoses treated with open surgical techniques (patch angioplasty or interposition grafting) have excellent long-term patency and associated limb salvage.139 The technical success and short-term patency of surveillance-detected lesions treated with catheter-based techniques are high, although long-term data are lacking. In general, longer lesions and lesions detected within 3 months of graft implantation are best treated surgically. Short lesions and those treated after 3 months of graft implantation may be treated either surgically or with catheter-based techniques, primarily balloon angioplasty, and possibly with drug-coated balloons.603,604 With either mode of treatment, recurrence of stenosis within the vein graft or its anastomoses is possible. Thus, continued surveillance after reintervention is indicated to detect recurrent and new stenotic lesions. After treatment of a vein graft stenosis, the treated graft should undergo surveillance at intervals similar to those for primarily placed grafts.139 Treatment of recurrent lesions in previously revised vein grafts can also provide continued long-term patency and limb salvage.139
Long-term patency of infrainguinal prosthetic bypass grafts is inferior to that of venous bypass grafts. Evidence as to the efficacy of prosthetic graft surveillance programs is more inconclusive. In one study, 69 patients with infrainguinal prosthetic bypasses were assessed by ultrasound after 4 weeks and every 3 months thereafter (total follow-up was 3 years).605 The ultrasound examination appeared to be of limited value, with 12 of 14 failing grafts not correctly predicted. In a retrospective analysis of 118 above-knee prosthetic grafts, most bypass occlusions again occurred without previously detected lesions.606 A quarter of patients developed a graft-related stenosis detected by ultrasound. Successful intervention of the stenotic lesions was associated with a lower bypass occlusion rate of 21% at 2 years (vs 41% for the entire series). Hence, in the authors’ opinion, ultrasound surveillance was justified. In another study of 89 grafts in 66 patients (FP and femorotibial), specific criteria for DUS proved predictive for patency of prosthetic tibial bypasses but not of popliteal bypasses.607 These criteria included PSV >300 cm/s at graft anastomoses, adjacent PSV ratio >3.0, uniform PSVs <45 cm/s, and monophasic flow throughout the graft.
One study sought to describe modes of failure and associated limb loss after infrainguinal polytetrafluoroethylene bypass grafting as well as benefits of warfarin on graft patency.579 The study involved 121 patients (86% with CLTI) with 131 infrainguinal (above-knee and below-knee) bypasses. Of these, 77% of the below-knee bypasses had anastomotic adjuncts (vein cuff or patch). Postoperative DUS was performed at 1 month, 4 months, and 7 months and then twice yearly. Multivariate analysis showed that low graft flow (midgraft velocity <45 cm/s) was more commonly associated with graft failure than stenosis detected by DUS. Therapeutic anticoagulation with warfarin increased patency in patients with low-flow grafts but not in patients with high-flow grafts.579
A consensus document from Mohler et al608 supports surveillance of prosthetic reconstructions at baseline and at 6-month intervals, similar to vein reconstructions. DUS imaging criteria were recommended for patients after femoral-femoral bypass grafting, particularly for those with a PSV >300 cm/s in the inflow iliac artery and a midgraft velocity <60 cm/s predictive of graft failure.609 When DUS-directed intervention was performed, patency at 5 years (assisted patency) was 88%. Patency appeared to be improved in comparison to most reports in the literature of patency without surveillance. DUS surveillance of prosthetic grafts does not reliably detect correctable lesions that precede failure as it does in vein bypass grafts. Instead, surveillance may serve as a predictor of graft thrombosis by the detection of midgraft velocities below 45 cm/s. Prosthetic grafts with low velocity may benefit from warfarin to improve patency, which may justify surveillance. The use of warfarin was recommended if the mean graft velocity was below 60 cm/s to reduce the incidence of expanded polytetrafluoroethylene bypass graft thrombosis.579 No specific recommendations can be made, however, regarding surveillance and reintervention for prosthetic grafts, and this information can only serve as a guideline.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 10.1 | Continue best medical therapy for PAD, including the long-term use of antiplatelet and statin therapies, in all patients who have undergone lower extremity revascularization. | 1 (Strong) | A (High) | Abbruzzese,125 2004 Henke,126 2004 Brown,127 2008 Bedenis,128 2015 Suckow,129 2015 |
| 10.2 | Promote smoking cessation in all CLTI patients who have undergone lower extremity revascularization. | 1 (Strong) | A (High) | Hobbs,130 2003 Willigendael,131 2005 |
| 10.3 | Consider DAPT (aspirin plus clopidogrel) in patients who have undergone infrainguinal prosthetic bypass for CLTI for a period of 6 to 24 months to maintain graft patency. | 2 (Weak) | B (Moderate) | Brown,127 2008 Belch,132 2010 Gassman,133 2014 Bedenis,128 2015 |
| 10.4 | Consider DAPT (aspirin plus clopidogrel) in patients who have undergone infrainguinal endovascular interventions for CLTI for a period of at least 1 month. | 2 (Weak) | C (Low) | Cassar,134 2005 Bhatt,135 2006 Tepe,136 2012 Strobl,137 2013 |
| 10.5 | Consider DAPT for a period of 1 to 6 months in patients undergoing repeated catheter-based interventions if they are at low risk for bleeding. | 2 (Weak) | C (Low) | Cassar,134 2005 Tepe,136 2012 Strobl,137 2013 |
| 10.6 | Observe patients who have undergone lower extremity vein bypass for CLTI on a regular basis for at least 2 years with a clinical surveillance program consisting of interval history, pulse examination, and measurement of resting APs and TPs. Consider DUS scanning where available. | Good practice statement | ||
| 10.7 | Observe patients who have undergone lower extremity prosthetic bypass for CLTI on a regular basis for at least 2 years with interval history, pulse examination, and measurement of resting APs and TPs. | Good practice statement | ||
| 10.8 | Observe patients who have undergone infrainguinal endovascular interventions for CLTI in a surveillance program that includes clinical visits, pulse examination, and noninvasive testing (resting APs and TPs). | Good practice statement | ||
| 10.9 | Consider performing additional imaging in patients with lower extremity vein grafts who have a decrease in ABI ≥0.15 and recurrence of symptoms or change in pulse status to detect vein graft stenosis. | Good practice statement | ||
| 10.10 | Offer intervention for DUS-detected vein graft lesions with an associated PSV of >300 cm/s and a PSV ratio >3.5 or grafts with low velocity (midgraft PSV <45 cm/s) to maintain patency. | 1 (Strong) | B (Moderate) | Mills,138 2001 |
| 10.11 | Maintain long-term surveillance after surgical or catheter-based revision of a vein graft, including DUS graft scanning where available, to detect recurrent graft-threatening lesions. | 1 (Strong) | B (Moderate) | Landry,139 2002 Nguyen,140 2004 |
| 10.12 | Consider arterial imaging after endovascular intervention for failure to improve (wound healing, rest pain) or a recurrence of symptoms to detect restenosis or progression of pre-existing disease. | 2 (Weak) | C (Low) | Bui,141 2012 |
| 10.13 | Consider reintervention for patients with DUS-detected restenosis lesions >70% (PSV ratio >3.5, PSV >300 cm/s) if symptoms of CLTI are unresolved or on a selective basis in asymptomatic patients after catheter-based interventions. | 2 (Weak) | C (Low) | Humphries,142 2011 |
Management of the limb after revascularization
Treatment of lower extremity tissue loss both acutely and in the longer term is complex and mandates a team approach. Physicians, surgeons, and nurses must work collaboratively rather than in individual silos of care.610–612 In these cases, wound healing is protracted, with the median time to healing ranging from 147 days for forefoot wounds to 188 days for midfoot wounds and 237 days for hindfoot wounds.613 The likelihood and duration of healing are also determined by the presence of concomitant infection and ischemia.192
The Threatened Limb Classification System from the SVS has been validated in several studies.68–70,164,166 It is a promising, pragmatic means to assess the likelihood of morbidity for at-risk legs and to communicate severity. The structure of the WIfI system is designed using a scale of none (0), mild (1), moderate (2), or severe (3), similar to the TNM system in cancer assessment.10,68,69,164 The system can be visualized as three intersecting rings of risk, enabling the team to collectively identify which risk is more dominant at any given time.
Tissue loss-dominant conditions.
The primary issue after revascularization in CLTI is often management of tissue loss (wound healing). Therapy is based primarily on appropriate débridement, offloading, and a simple moisture-retentive dressing strategy.233 Pressure offloading is one of the single most important and yet neglected aspects of therapy. Whereas the total contact cast remains the gold standard for offloading noninfected, nonischemic wounds, other techniques may also be considered, depending on available resources.614,615
More significant degrees of tissue loss may require a strategy of filling the defect followed by skin grafting.616,617 Once the wound heals and the patient is no longer “tissue loss dominant,” care then shifts to maximizing ulcer-free and activity-rich days in diabetic foot remission.618 This may include protecting the tissue by external (shoes, insoles, and inflammation monitoring) and internal (reconstructive surgery, physical therapy, and rehabilitation) means.619–622 The role and timing of foot amputations (eg, digital, forefoot, or midfoot) are discussed in Section 9.
Ischemia-dominant conditions.
The management and monitoring of ischemia play a central role in healing as well as in recurrence and involve regular vascular assessment and monitoring for potential intervention.
Infection-dominant conditions.
Infection is often the primary factor leading to amputation, accentuated by tissue loss and ischemia. Addressing this triad involves surgical and medical therapy based on established criteria. Each member of the wound care team must work to categorize, stage, and grade the severity of each component of the “wound triad” initially and at all follow-up encounters. Appropriate and regular documentation of the wound status is crucial, including diagrams and photographs to document progress. Often, one or more of these conditions can be found to be more “dominant” and can then be targeted for care. These conditions are dynamic and will change over time. During follow-up, recurrence may be related to tissue loss (deformity, inappropriate shoes, or change in activity). As a result, nonhealing may be due to ongoing or recurrent ischemia, and intervening in the development of an infection may require additional surgical or medical intervention.
| Recommendations | Grade | Level of evidence | Key references | |
|---|---|---|---|---|
|
| ||||
| 10.14 | Provide mechanical offloading as a primary component for care of all CLTI patients with pedal wounds. | 1 (Strong) | A (High) | Elraiyah,143 2016 |
| 10.15 | Provide counseling on continued protection of the healed wound and foot to include appropriate shoes, insoles, and monitoring of inflammation. | 1 (Strong) | A (High) | Elraiyah,143 2016 |
11. STUDY DESIGNS AND TRIAL END POINTS IN CLTI
IDEAL: A framework for research
The evidence base underpinning the surgical and endovascular management of CLTI is weak compared with that available for coronary interventions and pharmacologic cardiovascular risk reduction. In addition, methodologies (phase 1 to 4 trials) that have been successfully used by the pharmaceutical industry to generate level 1 evidence cannot be easily transferred to the evaluation of revascularization strategies for CLTI, and so different approaches are required. The Idea, Development, Exploration, Assessment, and Long-term study (IDEAL) framework provides a system for evaluating new surgical and interventional therapies that can be adapted for use in CLTI (Table 11.1).623–627
Table 11.1.
IDEAL: Stages of surgical and endovascular innovation for chronic limb-threatening ischemia (CLTI)
| Stage | 1. Idea | 2a. Development | 2b. Exploration | 3. Assessment | 4. Long-term study |
|---|---|---|---|---|---|
|
| |||||
| Patients | Single digit, highly selected and homogeneous | Few; selected and homogeneous | Many; more heterogeneous | Many; expanded but well-defined indications | All eligible |
| Vascular specialists | Very few; innovators | Few; innovators and early adopters | Many; innovators, early adopters, early majority | Many; early majority | All eligible |
| Output | Description | Description | Measurement and some comparison | Comparison | Regional and international variance; quality assurance; risk stratification and adjustment |
| Procedure | Inception | Development | Refinement | Fully evolved | Fully evolved |
| Method | Structured case report | Prospective development study | Prospective cohort study; feasibility or explanatory RCT | RCT | Registries and databases |
| Outcomes | Proof of concept; technical achievement; disasters; notable successes | Technical success; emphasis on safety and reproducibility | Safety; objective clinical and patient-reported outcomes | Objective clinical and patient-reported outcomes; cost-effectiveness | Rare events; long-term outcomes; quality assurance |
| Ethical approval | Yes, usually | Yes, always | Yes, always | Yes, always | Yes, always |
RCT, Randomized controlled trial.
| Recommendation | |
|---|---|
|
| |
| 11.1 | Use a research framework such as the IDEAL for gathering new data and evidence on the surgical and endovascular management of CLTI. |
Depending on the stage of surgical innovation, the IDEAL framework describes a wide range of different methodologies that can be used to provide varying levels of evidence that serve different purposes. However, once the assessment stage has been reached, RCTs remain by far the most reliable means of comparing the clinical effectiveness and cost-effectiveness of alternative treatment strategies and should be the method of choice whenever it is practically and financially feasible. Funding of such trials by governmental or professional organizations to assess existing or new technologies further enhances the value of the resulting data by avoiding actual or perceived commercial sponsor bias. Still, RCTs have limitations, including cost, long completion times, potentially incomplete applicability to populations of patients outside the defined inclusion criteria, and restricted ability to address epidemiologic study questions.
As a result, a number of alternative methodologic approaches are available and can be employed in certain circumstances.628 For example, large administrative databases and prospective registries (particularly population-based ones) have the benefit of relative low cost, simplicity, and improved external validity, although they can carry a substantial risk of treatment bias and confounding. Given that the observed treatments are typically not randomly assigned but rather chosen on the basis of a mix of the patient’s characteristics and the provider’s inclination, reliable comparisons between dissimilar groups can be a problem. Additional risks include important sampling errors and improper or imprecise assignment of causality to a particular observed end point, although some of these limitations can be mitigated by employing multivariate analysis. Still, the increasing use of registries designed to capture the outcomes of patients with vascular disease reflects their value in identifying trends in practice patterns. Added value can be found in capturing the experience of particular subsets of patients undergoing defined treatments or techniques. However, because registries are highly dependent on robust follow-up and capture of detailed information of the patient on a consistent basis, they are also susceptible to reporting and attrition bias that can paint an unreliable picture with regard to the clinical effectiveness and cost-effectiveness of a particular treatment strategy.
| Recommendation | |
|---|---|
|
| |
| 11.2 | Encourage funders, journal reviewers, and editors to prioritize prospective, multicenter, controlled, and preferably randomized studies over retrospective case series, studies using historical controls, or other less rigorous research methodologies. |
Objective performance goals OPGs
The SVS Critical Limb Ischemia Working Group developed a standardized set of outcome measurements, OPGs, derived from CLTI patients undergoing open bypass in several RCTs.162 The OPGs include major adverse limb events (MALE) and postoperative death as a measure of early safety and AFS to define longer term clinical effectiveness. Additional safety and efficacy OPGs were created for specific outcome variables of interest, and risk-stratified guidelines based on clinical, anatomic, and conduit criteria were identified for defined subgroups. The main aim of the OPG initiative was to establish benchmark values against which novel endovascular therapies could be initially evaluated without undertaking full RCTs. However, without good-quality RCTs, OPGs cannot be refreshed and, over time, will increasingly come to rely on historical controls. As such, RCTs are still required to determine both the clinical effectiveness and cost-effectiveness once safety and efficacy OPGs have been met.
| Recommendation | |
|---|---|
|
| |
| 11.3 | When RCTs are not feasible, use the OPG benchmarks from the SVS’s Critical Limb Ischemia Working Group to evaluate the efficacy of novel endovascular CLTI techniques and devices. |
RCTs
An appropriately designed RCT remains the optimal means of providing critical confirmatory evidence before the widespread adoption of novel interventions.629–631 The paucity of such studies in CLTI,13–15,632 however, underscores the many challenges that aspiring investigators face, particularly in trying to complete trials on time and on budget.
Trial design.
The adaptive features of a pragmatic trial design allow investigators greater flexibility with regard to specific treatment decisions. They will also generally lead to results that are more universally applicable, particularly in time-intensive and laborious studies that unfold during a period of potentially changing treatment paradigms. Conversely, a nonpragmatic design can more definitively generate supportive evidence for a particular technology or treatment scheme. It can also facilitate direct comparisons within a given revascularization strategy. One should determine to what degree a particular study is targeting real-world applicability and balance the theoretical, statistical, and practical impact of choosing one design over another.
Inclusion and exclusion criteria.
Therapeutic goals can differ according to whether the CLTI patient presents with ischemic rest pain only or with minor or major tissue loss. More important, the goals in all CLTI patients differ significantly from those in patients presenting with IC. Therefore, it should be clear that it is rarely if ever appropriate to combine IC patients and CLTI patients in the same study. Similarly, it is clearly inappropriate to extrapolate data gathered in patients with IC to those with CLTI and vice versa.
Because CLTI represents a wide spectrum of disease, it is important that trials describe patients who are enrolled in terms of limb threat (Sections 1 and 3) and anatomic burden of disease (Section 5). Amputation rates are significantly higher in patients with tissue loss than in those with rest pain. This makes the group of patients with tissue loss a potentially more attractive one for a study in terms of being able to demonstrate the clinical effectiveness and cost-effectiveness of a new intervention with an achievable sample size and within a realistic time. However, as the severity of tissue loss progresses, opportunities to detect therapeutic benefit may begin to decrease as some patients with advanced disease will inevitably progress to amputation or death regardless of the intervention provided. As such, the CLTI patient group, in which there is a real prospect of showing true benefit for a new intervention, may be more limited than is often initially appreciated.
| Recommendations | |
|---|---|
|
| |
| 11.4 | To facilitate sufficient enrollment, limit RCT exclusion criteria to those that are deemed essential to trial integrity. |
| 11.5 | Design RCTs, prospective cohort studies, and registries that are specific to CLTI. |
| 11.6 | Use an integrated, limb-based threatened limb classification system (eg, WIfI) and a whole limb anatomic classification scheme (eg, GLASS) to describe the characteristics and outcomes of CLTI patients who are enrolled. |
Outcomes
Efficacy vs effectiveness.
It is important to distinguish between clinical efficacy and clinical effectiveness. Clinical efficacy is the patient benefit observed under ideal circumstances. Does the procedure work in a selected group of homogeneous patients when it is performed by a selected group of clinicians? This is best demonstrated by an explanatory trial. Clinical effectiveness is the patient benefit observed from a procedure in the real world. It is best demonstrated by a pragmatic trial. With regard to CLTI, although the majority of published (usually industry-funded) trials fall into the clinical efficacy category, the results are often presented and overinterpreted as if they represent clinical effectiveness. This has incorrectly led to new treatments being adopted as the standard of care solely on the basis of limited evidence gathered in highly selected patients and centers.
Types of end points.
Most CLTI trial end points can be broadly divided into the following categories:
Objective clinical: AFS, MALEs
Subjective clinical: patient-reported outcomes measures (PROMs), including generic and disease-specific HRQL instruments633
Hemodynamic: ankle and toe pressures and indices
Anatomic: patency; target lesion, vessel, and limb revascularization
To describe the overall quality of revascularization for CLTI, RCTs should use a menu of outcomes derived from all four of the categories (Table 11.2).
Table 11.2.
Bypass vs Angioplasty in Severe Ischaemia of the Leg (BASIL-2), Balloon vs Stenting in Severe Ischaemia of the Leg (BASIL-3), and Best Endovascular vs Best Surgical Therapy for Patients with Critical Limb Ischemia (BEST-CLI) trial end points
| End points | BASIL-2 and BASIL-3 | BEST-CLI |
|---|---|---|
|
| ||
| Primary | AFS | MALE-free survival |
| Secondary | Freedom from all-cause mortality In-hospital and 30-day morbidity and mortality MALE MACE Relief of ischemic pain Psychological morbidity HRQL: generic and disease-specific instruments Reintervention and crossover intervention rates Healing of tissue loss (ulcers, gangrene) Extent and healing of minor amputations Hemodynamic changes; absolute APs and TPs, ABI, TBI HRQL (VascuQoL and EQ-5D) Health economic analysis |
Freedom from all-cause mortality RAFS Freedom from MALE and POD AFS Freedom from myocardial infarction Freedom from stroke Freedom from reinterventions (major and minor) in index leg No. of reinterventions (major and minor) per limb salvaged Freedom from hemodynamic failure Freedom from clinical failure Freedom from CLTI HRQL (VascuQoL and EQ-5D) Health economic analysis |
ABI, Ankle-brachial index; AFS, amputation-free survival; APs, ankle pressures; CLTI, chronic limb-threatening ischemia; EQ-5D, EuroQol-5 Dimension questionnaire; HRQL, health-related quality of life; MACE, major adverse cardiac event; MALE, major adverse limb event; POD, perioperative death; RAFS, reintervention- and amputation-free survival; TBI, toe-brachial index; TPs, toe pressures; VascuQoL, Vascular Quality of Life questionnaire.
It is also important for RCTs to include a full health economic analysis for the cost-effectiveness of the comparator interventions to be determined. This is preferably based on quality-adjusted life-years. It is then up to each health care system to determine whether and how such data should be used in relation to individual “willingness to pay” thresholds, which are typically based on economic, societal, and political considerations. For example, in the United Kingdom, bearing in mind the proportion of gross domestic product that the country has decided to spend on health care and the Department of Health’s agreed social value judgments, the National Health Service will not usually fund interventions that are associated with an incremental cost-effectiveness ratio in excess of £20,000 per quality-adjusted life-year. This figure represents the United Kingdom’s willingness to pay threshold.
Objective clinical outcomes.
AFS has been recommended as a suitable primary CLTI efficacy end point by TASC II, the U.S. FDA, the UK National Institute for Health and Care Excellence, and the SVS Critical Limb Ischemia Working Group. It has been used in a number of CLTI RCTs, including Project of Ex-vivo Vein graft Engineering via Transfection III (PREVENT III),634 all three BASIL trials, and BEST-CLI. As with most end points, however, AFS has its limitations. For example, AFS does not distinguish between transfemoral and transtibial amputation, and because the performance and timing of amputation can be discretionary and not easily blinded, AFS does not necessarily capture the full clinical impact of particular revascularization strategies. Thus, the severity of pain and use of analgesia, the success of healing of minor amputations and tissue loss, and the requirement for reintervention are all important clinical parameters not characterized by AFS. In addition, its appropriateness in patients with rest pain only has been questioned, and as a composite, AFS life tables do not distinguish between effect of the intervention on limb salvage and overall mortality. Therefore, whereas it is reasonable to use AFS and other related composite end points, such as MALEs, as the determinants of sample size calculations, they should be accompanied by a range of single, composite, objective, and subjective clinical end points.
Subjective outcomes.
Given the growing appreciation of the importance of the patients’ perception of their treatment experience, incorporating HRQL and PROMs into trial designs is strongly recommended. A number of well-validated generic HRQL instruments are now available in a range of languages. These include the 12-Item Short-Form Health Survey and the EuroQol-5 Dimension questionnaire as well as more disease-specific instruments, such as the Vascular Quality of Life tool. Some researchers have advocated that future RCTs be based on anticipated PROMs and HRQL benefits.
Hemodynamic outcomes.
Measuring hemodynamic parameters in CLTI patients can be challenging because CLTI is defined in part by the hemodynamic consequences of the disease (Section 1). Thus, it is important to attempt to describe the outcome of various interventions for CLTI in terms of their impact on hemodynamic measures, including ankle and toe pressures and indices.
Anatomic outcomes.
Anatomic outcomes such as patency have been widely used in regulatory trials designed to obtain premarketing authorizations despite the well-recognized problematic relationship between these outcomes and clinical success. The related outcome measures of clinically driven target lesion and target vessel revascularization are inappropriate in the context of CLTI, given the frequency of complex multilevel disease and the high degree of subjectivity surrounding decisions to reintervene. Patency as an outcome metric is further limited by the lack of consensus with regard to definitions after endovascular interventions. The role of patency and other anatomic end points within CLTI trial methodology needs to be better defined.
| Recommendations | |
|---|---|
|
| |
| 11.7 | Describe outcomes in CLTI trials using a combination of objective and clinically relevant events, subjective PROMs and HRQL assessments, and anatomic and hemodynamic end points. |
| 11.8 | Require regulatory trials aimed at obtaining premarket approval for devices for use in CLTI to study CLTI patients and to present data on objective and clinically relevant end points, PROMs and HRQL assessments, and anatomic and hemodynamic end points. |
Follow-up.
Determining the end points as well as the frequency and time during which they will be collected will depend on the study aims, design, and budget. Given the importance of evaluating the impact of comparator interventions on the natural history of CLTI, a follow-up period of at least 2 to 3 years is strongly recommended as it is unlikely that 6-month or 12-month follow-up periods will provide adequate assessment of clinical durability.
Clinical outcomes can be measured either in absolute proportions or by cumulative outcome estimates using the Kaplan-Meier analysis. Absolute proportions provide the most transparent and reliable outcome measure. Unfortunately, because they evaluate identical follow-up periods in all participants, they also limit follow-up to the observation period of the last included patient. In contrast, cumulative estimates can integrate variable follow-up periods, thereby avoiding loss of available information. These estimates, however, are based on specific assumptions and are therefore vulnerable to attrition bias.635,636 Consequently, incomplete follow-up might lead to relevant but easily missed false outcome estimates that can affect study groups differently.637 To evaluate the risk of attrition bias, completion of follow-up should be measured independently of the study design and systematically declared against a predefined study end date using the follow-up index or the C index.
| Recommendation | |
|---|---|
|
| |
| 11.9 | Follow up patients in trials for a time sufficient (this will usually be >2 years) to allow appropriate comparison of the impact of the different interventions on the natural history of CLTI. Measure and declare completeness of follow-up coverage to quantify risk of attrition bias. |
Time-to-event analysis.
Given the chronic and recurrent nature of CLTI, there is a compelling need to develop end points that move beyond the historical paradigm of a simple time-to-first-event analysis. End points such as AFS can reliably capture the centrally important end-stage events of limb amputation and death. Likewise, MALE and other end points focused on reintervention or other patient-related outcomes can capture the early clinical impact of treatment failure. Unfortunately, these and other time-to-first-event end points collectively may present an incomplete assessment of the total impact various CLTI treatment strategies over time.
The primary goal of a time-integrated measure for CLTI disease severity should be to more accurately assess long-term relief from commonly occurring multiple events in a manner that is analogous to disease-free survival after cancer treatment. Without such a time-integrated approach, even an otherwise well designed CLTI trial may prove to be an incomplete and potentially misleading assessment of overall clinical effectiveness and cost-effectiveness. As an example, consider two CLTI patients with ulceration.
Patient 1 has an endovascular intervention that heals his wound but after 2 months has recurrent symptoms and restenosis with a second intervention at 4 months. He develops another recurrence with pain and two gangrenous digits at 6 months. The patient subsequently requires a bypass graft at 7 months and a transmetatarsal amputation of the foot, resulting in clinical stabilization for 2 years. Outcomes: no death; no major amputation; time to first reintervention, 4 months; time to initial healing, 2 months; time to MALE, 7 months.
Patient 2 receives a bypass graft that heals his wound by 3 months. At 7 months, he presents with an asymptomatic graft stenosis and undergoes a surgical revision (3-cm interposition graft). He remains clinically stable for 2 years. Outcomes: no death; no major amputation; time to first reintervention/MALE, 7 months; time to initial healing, 3 months.
Patient 1 had clinical recurrences and two reinterventions and spent most of the year with symptoms. Patient 2 had a prophylactic reintervention and spent most of the year symptom free. A CLTI trial using only AFS and MALE as end points would have failed to differentiate these two notably different clinical experiences.
| Recommendation | |
|---|---|
|
| |
| 11.10 | Include a time-integrated measure of clinical disease severity (such as freedom from CLTI) in the CLTI trial design to describe the total impact of comparator CLTI interventions. |
Sample and effect size
CLTI patients who are entered into the “nonactive treatment” (placebo) group in RCTs often have outcomes that are better than expected compared with similar patients who are treated outside of research conditions. This makes it more difficult to demonstrate differences in clinical effectiveness and cost-effectiveness among the comparator interventions for CLTI. As a result, researchers must avoid the potential pitfall of basing the power calculation for their trial on an unrealistically large effect size. It is widely agreed that it is poor science and unethical to embark on a trial when there is no realistic prospect of answering the question being posed. An overpowered trial is equally undesirable as it is a misuse of resources, and patients may be disadvantaged by continuing to receive a treatment that is likely of little or no value or even potentially harmful to them. Despite this understanding, the CLTI literature is characterized by studies that present highly questionable, post hoc, subgroup analyses. To guard against this, all CLTI protocols along with full statistical analysis plans should be published in peer-reviewed journals to allow independent, public, and transparent scrutiny.
| Recommendation | |
|---|---|
|
| |
| 11.11 | Publish all CLTI trial protocols together with the full statistical analysis plans in peer-reviewed journals to allow independent, public, and transparent scrutiny and to prevent nonreporting of negative trials. |
Beyond the pivotal RCT
Given the challenges inherent in evaluating the wide array of novel endovascular modalities for CLTI, comparative trials of varying size and scope can be effective in establishing the utility of a particular technique, device, or overall revascularization strategy. As described within the OPGs, focused superiority or noninferiority RCTs can also be used to test a novel intervention against more established alternatives, and the safety and efficacy of new technologies can be effectively studied in a timely fashion. However, once the pivotal RCTs have been successfully completed, it is important that ongoing surveillance be rigorously undertaken with the use of well-designed, large, prospective, observational studies, including disease- or procedure-based national registries. Of note, some countries require manufacturers and importers to submit reports of device-related deaths, serious injuries, or malfunctions to the appropriate regulatory bodies.
Also important is cooperation among publicly and industry-funded investigators in designing and performing RCTs. Currently, this is happening with the BASIL and BEST-CLI trials, which will serve to facilitate subsequent individual patient data analyses, meta-analyses, and subgroup analyses. Ultimately, this type of data sharing will provide a powerful framework for refining OPGs and validating the use of tools to better define patient, limb, lesion, and anatomic risk in CLTI patients, such as WIfI and GLASS.
| Recommendations | |
|---|---|
|
| |
| 11.12 | Conduct postmarketing surveillance data collection using well-designed, large observational studies and registries. |
| 11.13 | Share clinical trial data to allow subsequent individual patient data analyses, meta-analyses, and subgroup analyses; updating of OPGs; and validation of decision-making tools, such as the WIfI system and GLASS. |
Strength of recommendation and level of evidence
Multiple methods to systematically assess the quality of research have been proposed and used by various bodies. Whereas each method has its advantages and disadvantages, the continued use of multiple methodologies that each produces slightly different strengths of recommendation on any given topic leads to inconsistency and confusion. As a result, there is a strong movement globally to use the GRADE system as a means of rating the level of evidence and thereby defining the appropriate strength of resulting recommendations.638 The GVG on CLTI also endorse the use of GRADE. Thus, it is in the best interests of public and commercial researchers who want their research to have maximum impact on practice to ensure that their studies are designed in such a way as to score well using the GRADE criteria.
| Recommendation | |
|---|---|
|
| |
| 11.14 | Assess the quality of evidence in CLTI research using frameworks such as GRADE that consider multiple certainty domains and are not based solely on study design. |
| Research priorities | |
|---|---|
|
| |
| 11.1 | Design well-constructed RCTs that address clinically relevant issues regarding the management of CLTI. |
| 11.2 | Clarify angiosome-based vs indirect tibial revascularization. |
| 11.3 | Identify the relative value of endovascular vs surgical therapy. |
| 11.4 | Validate specific anatomic scenarios outlined within GLASS. |
| 11.5 | Validate the WIfI system across specific grade levels. |
| 11.6 | Develop a reliable, real-time assessment tool for postintervention foot and wound perfusion. |
| 11.7 | Develop consensus definitions of postintervention patency and standardized patency-based end points relevant to CLTI interventions and trials. |
12. CREATING A CENTER OF EXCELLENCE FOR AMPUTATION PREVENTION
The major causes of amputation are related to diabetes and CLTI. Of the 200 million people worldwide with PAD, CLTI affects at least 2% to 3%.1 Whereas revascularization is the treatment of choice in preventing limb loss, procedure bias, lack of specialty training, market forces, and lack of consensus definitions remain major obstacles in achieving the best possible outcomes for CLTI care.639 The CLTI patient is particularly complex. Patients with PAD have an increased risk of CAD and cerebrovascular disease and an elevated risk of 5-year mortality.640 Historically, CLTI was primarily sequalee of smoking and a diet high in saturated fats. However, in the last few decades, the rise in CLTI has followed the global epidemic of diabetes. Because of this changing epidemiology, this section focuses mainly on establishing and monitoring teams for the patient with diabetes-related CLTI, but the concepts presented herein can be applied to all CLTI teams.
Diabetes-related CLTI is only one part of diabetic foot syndrome, which is a common but complex group of complications from diabetes. These include neuropathy, ulceration, Charcot foot, soft tissue and bone infection, and PAD including CLTI and gangrene. It is well known that diabetes increases the risk of myocardial infarction by 50% and stroke by 25%; however, the greatest increased risk is for a foot or leg amputation.618 Diabetic foot syndrome is also a costly comorbidity representing approximately one-third of the total cost of diabetes.641 One study found the mean 1-year cost from a public payer perspective in the United States to be $44,200.642 Roughly 75% of the cost was due to inpatient hospitalizations, for which the average length of stay for DFU and lower extremity amputation exceeded that of myocardial infarction, stroke, and diabetic ketoacidosis.643–645
The patient with diabetic foot syndrome has a poor prognosis. It is frequently associated with loss of quality of life, work, independence, and income for both the patient and the primary caregiver. The relative 5-year mortality rate after a lower extremity amputation is a staggering 70%.646 For patients with DFU, it is 55%; and for patients with PAD alone, the 5-year relative mortality rate is 32%.647 Thus, although diabetes is an endocrine disease, common complications of diabetes are related to microvascular or macrovascular disease. For this reason, diabetic foot syndrome should be more appropriately thought of as part of the cardiovascular complications of diabetes.
Many institutions and government agencies have responded to the growing complexity, options, and sub-specialization of treating medical conditions by creating disease-specific Centers of Excellence. A Center of Excellence is a virtual or physical location with a team of highly skilled experts who are often involved in research and innovation to advance their field.648 Whereas there have been experts in the field of PAD who have opined on what a Center of Excellence for CLTI, diabetic foot care, or amputation prevention might encompass, there are currently no governmental agencies or professional societies that have established such guidelines.
Center of Excellence.
In 2010, building on the work of the International Working Group on the Diabetic Foot, three tiers of care were proposed for an amputation prevention team–basic, intermediate, and Center of Excellence (Table 12.1).649 The basic model of care is performed in an office setting with a general practitioner, internist, or endocrinologist and a specialist nurse. An intermediate model of care is set in a hospital or multidisciplinary clinic and consists of various specialists to heal wounds and to prevent limb loss. This model is similar to a wound care center in the United States or a diabetic foot clinic in Europe. A Center of Excellence model is typically found in a tertiary care hospital with a predetermined team of specialists operating under clinical practice pathways, policies, and procedures. The Center of Excellence has advanced diagnostics and can intervene rapidly to prevent limb loss.
Table 12.1.
The three tiers of care for amputation prevention and diabetic foot care centers
| Clinical level of care | Setting | Potential clinicians | Role |
|---|---|---|---|
|
| |||
| Basic model of care | General practitioner’s office, health center, small community hospital | General practitioner Internist Endocrinologist Podiatrist Diabetic nurse Physical therapist |
Close collaboration with a referral center |
| Intermediate model of care | Regional hospital or multidisciplinary clinic | Endocrinologist Vascular surgeon Interventionalist Orthopedic surgeon Podiatric surgeon Diabetic nurse Wound nurse Physical therapist Diabetes educator Nutritionist |
Active collaboration with other departments in the hospital and extramural facilities |
| Center of Excellence | Large teaching hospital, tertiary referral center | Endocrinologist Vascular surgeon Interventionalist Podiatric surgeon Orthopedic surgeon Infectious disease specialist Orthotist Diabetes educator Nutritionist Wound nurse Physical therapist |
Collects and reports outcomes; facilitates regional education |
Adapted from Rogers LC, Andros G, Caporusso J, Harkless LB, Mills JL Sr, Armstrong DG. Toe and flow. J Am Podiatr Med Assoc 2010;100:342–8.
Currently, in many countries, there are no criteria required to designate oneself a Center of Excellence for health care. Anyone or any institution can use the terminology, and doing so does not guarantee that excellent care is being delivered. Based on experience in creating Centers of Excellence, a set of criteria are proposed to determine Center of Excellence designation in CLTI and amputation prevention, as outlined in Table 12.2.
Table 12.2.
Criteria for Center of Excellence designation in chronic limb-threatening ischemia (CLTI) or amputation prevention
| Center of Excellence criteria | Description |
|---|---|
|
| |
| Multidisciplinary team of specialists | Specialists who can surgically and medically manage PAD and infections and provide the general or intensive medical care needed for the complex CLTI patient |
| Protocol-driven care | A team that follows written, evidence-based clinical practice pathways, policies, and procedures |
| Outcomes monitoring and reporting | Establishes a process for data collection and reports that data to the community or in the literature |
| Methods of improvement | Establishes a process for continual improvement based on outcomes and new techniques or therapies |
| Educational resource | Serves as an educational resource for the medical community through mentoring, publishing, and symposia |
PAD, Peripheral artery disease.
Team setting, components, and function.
No single specialist possesses all the necessary skills to manage diabetic foot syndrome. Therefore, it is important to create a team of specialists with the required skills. Whereas some of the services required to treat CLTI and to prevent amputation can be performed in the outpatient setting, many needed services are intensive and require access to an acute care hospital.
An understanding of the natural history of amputation in diabetes can assist in determining how to build an effective team (Fig 12.1).650 Diabetes leads to peripheral neuropathy, although the timing of its onset is related to long-term control of blood glucose level. Peripheral neuropathy leads to unfelt repetitive trauma and in combination with foot deformity causes DFU.651 Approximately half of these patients have significant PAD with their DFU. Still, more often than not, infection serves as the final event leading up to the amputation.652
Fig 12.1.
The elevating risk of the “stairway to an amputation” or the natural history of diabetes-related amputations. (Adapted from Rogers LC, Armstrong DG. Podiatry care. In: Cronenwett JL, Johnston KW, editors. Rutherford’s vascular surgery. 7th ed. Philadelphia: Saunders Elsevier; 2010. p. 1747–60.)
Fitzgerald et al610 described the seven essential skills for limb salvage teams. These were modified to identify nine skills needed for the comprehensive management of diabetic foot. Table 12.3 lists the essentials skills as well as the type of specialist who should be added to the team to complete a given task. The simplest method to construct a team for a Center of Excellence is to ensure that each of these skills is covered by an expert on the team. In addition, several authors have described an irreducible minimum to the team that includes vascular surgery and surgical podiatry. These two specialties have been nicknamed the “toe and flow” team.610,649
Table 12.3.
The nine essential skills to prevent amputations in diabetes and the possible specialty responsible
| Essential skills | Possible team members |
|---|---|
|
| |
| The ability to perform hemodynamic and anatomic vascular assessment | Vascular surgeon Interventionalist (cardiologist or radiologist) Vascular medicine |
| The ability to perform a peripheral neurologic workup | Neurologist Endocrinologist Podiatrist |
| The ability to perform site-appropriate culture technique | Infectious disease specialist Surgeon Wound nurse Physical therapist |
| The ability to perform wound assessment and staging or grading of infection and ischemia | Vascular surgeon Podiatrist Surgeon Infectious disease specialist Wound nurse Physical therapist |
| The ability to perform site-specific bedside and intraoperative incision and drainage or débridement | Podiatric surgeon Orthopedic surgeon Plastic surgeon Surgeon Vascular surgeon |
| The ability to initiate and to modify culture-specific and patient-appropriate antibiotic therapy | Infectious disease specialist Endocrinologist Primary care physician Vascular surgeon Podiatrist Surgeon |
| The ability to perform revascularization | Vascular surgeon Interventionalist (cardiologist or radiologist) |
| The ability to perform soft tissue or osseous reconstruction of deformities and defects | Podiatric surgeon Plastic surgeon Orthopedic surgeon Surgeon |
| The ability to perform appropriate postoperative monitoring to reduce risks of reulceration and infection | Podiatrist Wound nurse |
Adapted from Fitzgerald RH, Mills JL, Joseph W, Armstrong DG. The diabetic rapid response acute foot team: 7 essential skills for targeted limb salvage. Eplasty 2009;9:e15.
Team-driven protocols.
It is simply not enough to have a designated team. The team must be used in an effective manner, and outcomes should be monitored in a structured fashion. Fig 12.2 illustrates a useful pathway in setting up the structure of the team, establishing goals, and ensuring that the goals are met. Published CPGs from medical and surgical societies establish best practices, but they are not always feasible for practice in all settings. Current CPGs exist for PAD in diabetes, diabetic foot infections, DFUs, offloading of DFUs, inpatient management of the diabetic foot and the Charcot foot, and prevention of diabetic foot problems.158,653–658 Whereas these CPGs can serve as a template, localities are encouraged to create their own clinical practice pathways specific to the facility or system in which they practice.
Fig 12.2.
A schematic on how to organize the diabetic foot care within a multidisciplinary team.
The clinical practice pathways are used to identify the team structure and patient flow, when to engage various members, and what to do if the patient is not improving as expected. Policies and procedures are then created to assist providers and staff in complying with the pathway. Quality assurance goals are also created for measurable policies and procedures. Certain outcomes are self-explanatory, such as limb salvage rate, whereas others should be followed to ensure the quality of care delivered by the Center of Excellence. These can include the high-low amputation ratio,659 median days to heal for foot wounds, healing percentage, and quality of life measures. Table 12.4 lists the most important measurable outcomes for limb salvage and their calculation. These data may not always be easy to track. Existing electronic health record systems are lacking in their ability to track and to report most of these or other custom measures. Centers of Excellence often resort to developing their own software or keeping track of data manually in spreadsheets.
Table 12.4.
Major outcome measures for chronic limb-threatening ischemia (CLTI) and amputation prevention
| Quality assurance measure | Calculation |
|---|---|
|
| |
| Limb salvage rate | No. of total patients – No. of major amputations (BKA or AKA) No. of total patients |
| Major to minor amputation ratio | No. of major amputations performed (BKA or AKA) No. of limb-sparing amputations performed |
| Healing percentage, all wounds | No. of wounds healed Total No. of wounds – palliative care patients |
| Healing percentage, DFUs | No. of DFUs healed Total No. of DFUs – palliative care patients |
| Median days to heal, all wounds | Calculate days to heal for all wounds. Exclude amputated and palliative care patients. |
| Median days to heal, DFUs | Calculate days to heal for all DFUs. Exclude amputated and palliative care patients. |
| Noninvasive vascular study, DFUs | No. of NIVSs performed No. of new DFU patients |
| Revascularization success, open bypass | No. of open bypass patients – No. of open bypass failures No. of open bypass patients |
| Revascularization success, endovascular | No. of endovascular patients – No. of endovascular failures No. of endovascular patients |
AKA, Above-knee amputation; BKA, below-knee amputation; DFUs, diabetic foot ulcers; NIVSs, noninvasive vascular studies.
Palliative care patients are defined as those in whom healing is not the treatment goal, that is, terminal or hospice patients.
Finally, performance improvement plans must be drafted and initiated when the quality assurance goals are not met. Fig 12.3 shows an example of how this system would be applied to vascular disease screening in DFUs.
Fig 12.3.
An example of using the organized care model for peripheral artery disease (PAD) screening in diabetic foot ulcers (DFUs). CPG, Clinical practice guideline; CPP, clinical practice pathway; P&P, policies and procedures; PI, performance improvement; QA, quality assurance.
Team impact.
In 2005, the World Health Organization and the International Diabetes Federation declared that up to 80% of diabetes-related amputations are preventable.660,661 Currently, the only intervention to address this has been the formation of multidisciplinary teams to prevent unnecessary amputations. In fact, the multidisciplinary team to prevent diabetes-related amputations dates back to at least 1934, when Elliott P. Joslin, an endocrinologist in Boston, established his team to treat diabetic gangrene.662
In the United States, an organized team in a public hospital reduced lower extremity amputations 72% during 2 years. In the Veterans Affairs medical centers, several factors were significant in the reduction of lower extremity amputations, including use of a specialized team and establishment of a high-risk foot clinic.663,664 In a military medical center, amputations were reduced by 82% as a result of a specialized limb preservation service.665 Another report showed a reduction improvement in diabetes-related foot outcomes with an integrated interdisciplinary team in a large academic medical center.666 In several other studies, adding podiatry to the team was found to be helpful in reducing amputations and significantly reducing the cost associated with diabetic foot.641,663,667,668
The impact of a limb salvage team is not limited to any geographic area. In The Netherlands, investigators reported a 34% nationwide reduction in amputations after setting up multidisciplinary teams.669 In Brazil, the establishment of >20 interdisciplinary foot clinics nationwide is leading to improved care.670 In Italy, investigators reported a reduction in hospitalizations and amputations in the diabetic foot after implementing a multidisciplinary referral team.670,671 In Spain, a multidisciplinary foot team reduced amputations during 3 years compared with the previous 6 years.672 The United Kingdom has also seen reduced amputations secondary to better-organized diabetic foot care with specialized clinics that follow multidisciplinary care pathways and protocols.673,674 Lastly, in Finland, a decrease in major amputations was correlated with rising interest in limb salvage and an increase in distal vascular procedures.675 In a subsequent study, researchers reported a reduction in amputations and length of stay when inpatient care was reorganized.676
Summary.
Centers of Excellence can be implemented with a well-organized team approach to diabetic foot syndrome and, in particular, the foot with CLI. Creating an integrated team whose primary focus is limb salvage and that receives all referrals for suspected CLTI is key. Teams can improve processes, time to intervention, and outcomes. The setting and structure of the team will ultimately depend on the availability and local need. However, to be most successful, Centers of Excellence should have team members who are capable of performing the nine essential skills as outlined in Table 12.3.
Centers of Excellence have published pathways and policies and procedures to determine the function and involvement of various members. Equally important to setting up the team is measuring the Center’s performance. This is best accomplished with concrete quality assurance goals and the implementation of a performance improvement plan to be used when these goals are not met.
13. GLOBAL PERSPECTIVES IN CLTI
The preceding sections of this guideline make recommendations regarding the diagnosis and treatment of CLTI based on data published in peer-reviewed journals and, where such data are lacking, consensus expert opinion. Vascular specialists managing CLTI across the globe serve the needs of diverse communities and cultures, working within a wide range of health care environments. Most vascular specialists will strive to keep up to date with the published evidence base and are greatly facilitated in doing so through the use of modern information technology systems. However, the reality is that most publications on CLTI are written in English, and the data contained therein overwhelmingly derive from relatively few countries, mainly HICs (western Europe, North America, Japan), that have mature, well-resourced health and social care systems as well as clinical research infrastructure. Most vascular specialists treating patients with CLTI do not, of course, work in such favorable environments. As such, they often have to adapt foreign “evidence-based recommendations” to their own particular situation to provide the best possible care to their patients with the resources available. The GVG authors recognize this and, specifically, that some of the recommendations contained within this guideline are likely to remain aspirational for many vascular specialists working in diverse health care settings across the globe. The authors therefore thought it important to examine the state of CLTI care from a broader perspective. To that end, a questionnaire enquiring about the presentation, diagnosis, and management of CLTI was sent to vascular specialists (n = 50) working in a range of lower, middle, and higher income countries. This section primarily comprises a description of the responses received (n = 22), supported by published locoregional data where available. The authors and the Steering Committee of the GVG appreciate and recognize these contributors for providing survey responses for this Section (Table 13.1).
Table 13.1.
Contributors
| Country | Name | Affiliation |
|---|---|---|
|
| ||
| Argentina | Dr Juan Esteban Paolini | President, Argentine Association of Angiology and Cardiovascular Surgery, Caba |
| Brazil | Dr Tulio Pinho Navarro | Vascular and Endovascular Surgery, Belo Horizonte-MG |
| China | Dr Jinsong Wang | Vascular and Endovascular Surgery, Guangzhou, Guangdong |
| Colombia | Dr Alberto Munoz | Vascular and Endovascular Surgery, Bogota; Secretary General, WFVS-ALCVA (Latin American Society for Vascular Surgery and Angiology) |
| Costa Rica | Dr Roger Jimeìnez Juaìrez | Vascular and Endovascular Surgery, San Jose |
| Cuba | Dr Alejandro Hernandez Seara | National Institute of Angiology and Vascular Surgery, Havana |
| Ecuador | Dr Victor Hugo Jaramillo Vergara | Chief of Vascular Surgery Department, Hospital Carlos Andrade Marín, Quito |
| El Salvador | Dr Andres Reynaldo Hernandez Morales | Vascular Department Chairman, Institute Salvadorien del Seguro Social |
| India | Dr Varinder Bedi | Head of Department of Vascular and Endovascular Surgery, Sir Gangaram Hospital, New Delhi |
| India | Dr P. C. Gupta | Head of Department of Vascular and Endovascular Surgery, CARE Hospital, Hyderabad |
| India | Dr Kalkunte R. Suresh | Jain Institute of Vascular Sciences, Bangalore |
| Japan | Dr Tetsuro Miyata | Professor, Vascular and Endovascular Surgery, Tokyo |
| Malaysia | Dr Yew Pung Leong | Vascular and endovascular surgeon, The Vascular Centre, Sunway Medical Centre, Kuala Lumpur |
| Mexico | Dr José Antonio Munoa Prado | Vascular and Endovascular Surgery, Chiapa |
| New Zealand | Dr Thodur Vasudevan | Vascular surgeon; Chair, Board of Vascular Surgery, Waikato Hospital, Hamilton |
| Paraguay | Dr Agustin Saldivar Orrego | President of Paraguayan Society of Angiology and Vascular Surgery |
| Peru | Dr Fernando Batista Sanchez | Vascular and Endovascular Surgery, Lima |
| South Africa | Dr Martin Veller | Dean, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg |
| Spain | Dr Melina Vega de Ceniga | Senior consultant, Angiology and Vascular Surgery, Hospital de Galdakao-Usansolo, Bizkaia |
| Sri Lanka | Dr Mandika Wijeyaratne | Consultant vascular surgeon, Colombo |
| Tanzania | Dr Zulfiqarali G. Abbas | Consultant physician, Dar es Salaam; Chairman, Pan-African Diabetic Foot Study Group; Vice President, D-Foot International |
| Uruguay | Dr Marcelo Diamant | President of ALCVA (Asociación Latinoamericana de Cirugía Vascular y Angiología); vascular and endovascular surgeon |
All the respondents are vascular surgeons.
Whereas the information provided may not be considered the highest quality from an epidemiologic perspective, a number of important global issues emerged from the responses. This brief overview highlights the urgent need for better data on the impact of CLTI and how it is managed around the world. The majority of responses derive from a few key opinion leaders from Latin America, Asia, and Africa; thus, the following discussion may not reflect concerns of other populations, providers, and nations.
Definition and classification.
Clinical criteria, history, and examination are the mainstays of CLTI diagnosis across the world, with the use of adjunctive hemodynamic and perfusion measurements appearing to be highly variable. ABI testing was used by all except one respondent. However, although all respondents regularly dealt with diabetic vascular disease and the acknowledged limitations of APs in that setting, only two used TPs; none used TcPO2 routinely. All (except one who exclusively used WIfI) used either the Fontaine or Rutherford classification for staging, approximately in equal numbers. About one-third of respondents described employing WIfI in addition to another clinical classification system. In summary, therefore, across most of the world, there appears to be limited adherence to any one published definition or staging system for CLTI.
Epidemiology and risk factors.
Although accurate country-specific epidemiologic data are sparse, there seems little doubt that the increasing prevalence of DM (Fig 13.1) together with the growing use of tobacco and population aging is resulting in a significant increase in CLTI and amputations across much of the world, especially in LMICs.677
Fig 13.1.
International Diabetes Federation global diabetes projections. (From the International Diabetes Federation. IDF diabetes atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015.)
In 2013, Fowkes et al1 undertook a meta-analysis of 34 studies to compare the prevalence and risk factors between HICs and LMICs. This is well outlined in Section 2 of this document, but it is worth recalling some of the key presented data. They concluded, “Globally, 202 million people were living with peripheral artery disease in 2010, 69.7% of them in LMIC, including 54.8 million in Southeast Asia and 45.9 million in the western Pacific Region. During the preceding decade, the number of individuals with peripheral artery disease increased by 28.7% in LMIC and 13.1% in HIC. Also of note is the percentage of increase of PAD is higher in women than men in LMIC which is opposite of HIC.” The increase in PAD burden observed in women and in the younger, economically productive age groups is especially worrisome (Table 13.2).
Table 13.2.
Estimated number of people living with peripheral artery disease (PAD)
| Rate of change (%), 2000–2010 |
|||
|---|---|---|---|
| Age, years | HICs | LMICs | Worldwide |
|
| |||
| 25–29 | 3.02 | 11.91 | 10.34 |
| 30–34 | −1.52 | 7.62 | 5.82 |
| 35–39 | −4.12 | 22.49 | 16.19 |
| 40–44 | −3.28 | 32.05 | 22.59 |
| 45–49 | 7.14 | 25.83 | 20.51 |
| 50–54 | 12.15 | 42.40 | 32.37 |
| 55–59 | 31.31 | 55.53 | 47.49 |
| 60–64 | 16.85 | 29.90 | 25.06 |
| 65–69 | 4.90 | 20.29 | 14.35 |
| 70–74 | 8.02 | 29.73 | 20.05 |
| 75–79 | 11.68 | 41.36 | 26.75 |
| 80–84 | 51.98 | 45.77 | 48.92 |
| 85–89 | 34.80 | 47.86 | 39.84 |
| ≥90 | 37.22 | 58.82 | 44.09 |
| Total | 13.08 | 28.67 | 23.51 |
HICs, High-income countries; LMICs, Low- and middle-income countries.
Adapted from Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40.
The data on country-specific incidence of PAD and CLTI are sparse in these LMICs, unlike in HICs. There are no relevant epidemiologic data from large regions, but the updated data from Abbas are tabulated for perspective, reflecting PAD in diabetics in sub-Saharan Africa (Table 13.3).
Table 13.3.
Prevalence (%) of peripheral artery disease (PAD) in diabetics
| Country | 1990–2000 | 2000–2010 | 2010 |
|---|---|---|---|
|
| |||
| Benin | NA | NA | 42 |
| Ethiopia | 11.6 | NA | NA |
| Ivory Coast | NA | NA | 22 |
| Malawi | 15 | NA | NA |
| Nigeria | NA | 54 | 52 |
| South Africa | 10.2 | 8.2 | 30 |
| Sudan | 10 | NA | NA |
| Tanzania | 12.5 | 21 | 26 |
| Uganda | NA | NA | 39 |
| Zambia | NA | NA | 41 |
NA, Not available.
Adapted from Abbas ZG, Archibald LK. Recent international development: Africa. In: Boulton AJ, Cavanagh P, Rayman G, editors. The foot in diabetes. 4th ed. Hoboken, NJ: John Wiley & Sons; 2006. p. 379–385). Updated by Dr Abbas (Tanzania) with review of regional data and literature.
Lacking firm epidemiologic data, recent estimates of CLTI prevalence have used extrapolations from demographic and other available disease prevalence data, yielding global estimates of between 20 and 40 million individuals afflicted. About two-thirds of these are projected to be in LMICs. Unfortunately, documented data to support this are difficult to find in any indexed, peer-reviewed journals.
According to the survey respondents, the risk factors for CLTI in their regions are largely as expected, but DM is a predominant cause, more than in HICs. The prevalence reported by respondents varied from 40% to 90%. Interestingly, a cultural preference for walking barefoot or a lack of appropriate footwear is a significant problem in some countries. Approximately 60% to 80% of all the PAD patients seen by the respondents present with CLTI. The average age was around 65 years, and about 70% were men. Most respondents reported that 70% to 100% of CLTI patients presented with tissue loss; in three countries, it was <50%. Primary amputation was performed in 10% to 40% of CLTI patients, this being mainly (25%-90%) because of delayed presentation or referral. Only two countries reported a primary amputation rate of <10%. Postprocedural amputation rates were reported at around 5% to 10%, although two countries reported much higher rates (60%-70%) because of late presentation or aggressive disease patterns encountered.
Diagnostic evaluation.
DUS appears to be used almost universally, although three respondents preferred to proceed directly to other imaging modalities. Only five respondents performed DSA as their primary imaging modality. The remainder opted for MRA and CTA in about equal numbers. In patients with renal impairment, DSA was preferred by most, with half opting for iodinated contrast agents with appropriate renal protection measures and the other half favoring CO2 angiography. Two respondents performed only noninvasive testing in such patients before intervention.
Medical and noninterventional management (with or without revascularization).
Respondents reported widespread routine use of antiplatelet and lipid-lowering agents. ACEIs, vasoactive drugs (such as cilostazol and pentoxifylline), and anticoagulants were used selectively. IV prostanoids and vasodilators were used by some as adjuncts to revascularization and in those with nonreconstructible disease. Use of arterial assist devices (compression pump), HBOT, and SCS was uncommon. Lumbar sympathectomy was performed by a third of respondents, possible in patients with Buerger's disease (not specified).
Anatomic classification, risk stratification, and predictors of limb salvage.
The almost uniform answer to the question How satisfied are you with present systems? was “somewhat satisfied.” Interestingly, only six respondents used TASC to inform decisions about revascularization strategies and procedures in patients with CLTI. There was strong support for a new approach to patient and limb risk stratification and for a new anatomic classification system.
REVASCULARIZATION
Although, overall, there has been a shift toward endovascular intervention, there is considerable variation in practice across the respondents–varying from 5% to 80% for both endovascular and “open” procedures! All stated that the preferred conduit for both above-knee and below-knee bypass continues to be autogenous vein. Prosthetic grafts are used selectively above the knee, but none advocate their use for distal bypass. None of the respondents endorsed “routine stenting” in the femoral-popliteal region, and all endovascular options (balloon angioplasty, DCB, stenting) are used selectively. Balloon angioplasty is preferred for endovascular intervention in infrapopliteal vessels; four respondents selectively use DCB, but none were in favor of stents below the knee.
Postprocedural surveillance and follow-up.
All the respondents said they had defined follow-up protocols for patients undergoing infrainguinal revascularization. All patients (surgical and endovascular) are observed at least every 3 months for a year and then at variable intervals thereafter. Clinical evaluation and ABI are the mainstays of surveillance. Use of other noninvasive methods (PVR, DUS) is variable. Specific protocols for vein and prosthetic bypass grafts seem to be standardized per available data in a minority of centers. Approach to surveillance-detected lesions is similarly variable but mostly dictated by the patient’s symptoms rather than by the result of physiologic testing. Arteriography is reserved for clinically significant lesions. Postprocedural drug therapy, for example, with antiplatelet and ipid-lowering agents, appears concordant with current published recommendations. Because most CLTI patients had tissue loss, almost all the centers provided intensive wound services within their department as part of a multidisciplinary team approach. Nearly all agreed that wound infection is a significant determinant of outcome after revascularization and possible cause of amputation even after successful revascularization.
Health economics.
CLTI has a serious adverse economic impact on patients, their families, and wider communities right across the world but especially so in LMICs. Although these countries are often grouped together, the division between middle income and lower income is variable and imprecise. Furthermore, there is often considerable inequality within each LMIC, and respondents reported that most patients with CLTI (30%-90%) appear to come from the poor socioeconomic backgrounds. The following data from the Indian National Sample Survey Office could represent the situation in many LMICs678:
Only 18% of the urban population and 14% the of rural population are covered by some form of health insurance.
Governmental health expenditure is <2% of gross domestic product overall.
People in villages mainly depend on “household income or savings” (68%) and “borrowings” (25%) to fund hospitalization expenses.
Around 1% of the poor in rural areas have to sell their physical assets to meet health expenditure, and >5% seek help of friends and relatives. This is also in line with earlier studies showing that millions are pushed into poverty each year by medical expenditure and that such expenses are among the leading causes of indebtedness among the poor.
In cities, people rely much more on their income or savings (75%) than on borrowings (18%) to fund their treatment. Previous studies have repeatedly shown that India has one of the most privatized health care systems in the world, with out-of-pocket expenses accounting for the bulk of medical spending.
In India, the cost of IP bypass is U.S. $1500 to $3000, and costs of balloon angioplasty are similar. The use of a stent or DCB would add another U.S. $500 to $1000, and wound care adds at least U.S. $500. Such out-of-pocket expenses are probably unaffordable for most CLTI patients. Importantly, these costs depend on recycling of single-use devices like sheaths, angioplasty balloons, and guidewires. Without such practice, the cost would increase by at least 50%, and far fewer patients, especially poorer ones, would have access to treatment, resulting in much greater loss of life and limb. Recycling of single-use devices (not just vascular devices) is common in Asia, Africa, Latin America, and eastern Europe, and proper regulation of the practice, including appropriate consent procedures, is important to mitigate patient harm.679
Summary of global perspectives.
Based on the responses to the questionnaire and the limited published and unpublished data at times, we can draw the following conclusions.
CLTI is a significant and increasing global problem, especially in LMICs, where the incidence in women appears to be rising more quickly than in men.
Diabetes and unabated smoking are the major causes of CLTI globally.
Although vascular specialists try to follow the published evidence base, economic and social constraints mean that the approach to CLTI must to tailored to the working environment.
CLTI and diabetic foot problems are associated with high amputation rates in LMICs because of delayed presentation and referral and limited access to affordable care.
Economic constraints are an important limitation in the adoption of advanced vascular technologies, and practical issues such as recycling of single-use devices require oversight from a public health perspective.
Few countries maintain national registries or other CLTI data sets.
Most countries do not have a standardized approach to CLTI, with considerable locoregional variation in practice.
Most countries do not have well-organized and supported vascular societies where best practice and research can be shared and disseminated.
Dissemination and implementation.
A large number of vascular specialists from around the world have contributed to the GVG, and that global involvement sets the present guideline document apart from all previous consensus statements. The paradigms and tools, such as WIfI, PLAN, and GLASS, set out in the GVG will, it is hoped, meet the needs of the global vascular community as expressed by our questionnaire respondents. However, some guideline recommendations will not be achievable by vascular specialists working in LMICs. The GVG recommendations should not, therefore, be viewed as an inflexible global “standard of care.” Following publication, it will be important to disseminate the GVG as quickly and widely as possible, simultaneously through a range of different channels, and to obtain validation and feedback from the global community. Dissemination will be assisted by publication of the full GVG as a supplement to the Journal of Vascular Surgery and European Journal of Vascular and Endovascular Surgery, publication of an executive summary with the recommendations in a range of other journals in a number of different languages, presentations at conferences, and free online access to the documents linked from societies’ web pages.
ADDENDUM
As this guideline goes to press (April, 2019), the safety of paclitaxel-eluting devices for the treatment of peripheral arterial disease has come under intense scrutiny. The GVG Steering Committee, recognizing the importance of this issue to the vascular community, has unanimously approved the statement below. Given time constraints, this statement was not reviewed by the entire GVG Writing Group. This statement was approved by the three major sponsoring societies (ESVS, SVS, WFVS).
STATEMENT ON THE SAFETY OF PACLITAXEL-ELUTING DEVICES FOR THE TREATMENT OF CLTI
Recently the safety of paclitaxel (PTX)-eluting devices for the treatment of patients with peripheral arterial disease (PAD) has come into question. A meta-analysis of randomized controlled trials investigating these devices in the femoral and/or popliteal arteries identified an increased mortality at two years and beyond in patients treated with the PTX devices versus controls.680 These trials largely enrolled patients with intermittent claudication, with a small minority (11%) being within the spectrum of CLTI. Ongoing efforts from regulatory bodies and other independent groups seek to further clarify the validity of these observations. In the interim the US Food and Drug Administration has urged caution in the use of PTX devices for treatment of PAD.
The GVG Steering Committee believes that the risks and benefits of treatments for CLTI, including drug-eluting devices, need to be examined with appropriately controlled, prospective studies that are specific to the CLTI population. In this regard, the execution of randomized controlled-trials involving PTX-eluting devices in CLTI, with appropriate safety monitoring and regulatory oversight, are important to the vascular community. Such trials should incorporate appropriate informed consent discussions with subjects, including the potential increased risk of mortality, and should mandate long-term follow-up for at least 2 years. Outside of such trials, given the indeterminate risk and efficacy of these devices in patients with CLTI, and the availability of alternative modalities, we believe appropriate caution should be exercised.
Supplementary Material
Fig 5.5.
Representative angiograms of Global Limb Anatomic Staging System (GLASS) stage II disease patterns. The target arterial path (TAP) is outlined in yellow. Left panel, TAP includes the anterior tibial (AT) artery. Femoropopliteal (FP) grade is 1 (superficial femoral artery [SFA] occlusion <5 cm). Infrapopliteal (IP) grade is 2 (two focal stenoses of AT artery, total length <10 cm). Right panel, TAP includes the peroneal artery. FP grade is 0 (no significant stenosis). IP grade is 3 (chronic total occlusion of peroneal artery, 3–10 cm).
Table 3.3.
Ischemia grading in Wound, Ischemia, and foot Infection (WIfI) classification
| Grade | ABI | Ankle systolic pressure | TP, TcPo2 |
|---|---|---|---|
|
| |||
| 0 | ≥0.80 | >100 mm Hg | ≥60 mm Hg |
| 1 | 0.6–0.79 | 70–100 mm Hg | 40–59 mm Hg |
| 2 | 0.4–0.59 | 50–70 mm Hg | 30–39 mm Hg |
| 3 | ≤0.39 | <50 mm Hg | <30 mm Hg |
ABI, Ankle-brachial index; TP, toe pressure; TcPo2, transcutaneous oximetry.
Flat or minimally pulsatile forefoot pulse volume recording is grade 3. Measure TP or TcPo2 if ABI incompressible (>1.3). Patients with diabetes should have TP measurements. If arterial calcification precludes reliable ABI or TP measurements, ischemia should be documented by TcPo2, skin perfusion pressure, or pulse volume recording. If TP and ABI measurements result in different grades, TP will be the primary determinant of ischemia grade.
Table 3.4.
Foot infection grading in Wound, Ischemia, and foot Infection (WIfI) classification
| Clinical manifestation of infection | SVS | IDSA/PEDIS infection severity |
|---|---|---|
|
| ||
| No symptoms or signs of infection | 0 | Uninfected |
| Infection present, as defined by the presence of at least two of the following items: • Local swelling or induration • Erythema >0.5 to ≤2 cm around the ulcer • Local tenderness or pain • Local warmth • Purulent discharge (thick, opaque to white, or sanguineous secretion) |
1 | Mild |
| Local infection involving only the skin and the subcutaneous tissue (without involvement of deeper tissues and without systemic signs as described below). Exclude other causes of an inflammatory response of the skin (eg, trauma, gout, acute Charcot neuro-osteoarthropathy, fracture, thrombosis, venous stasis). |
||
| Local infection (as described above) with erythema >2 cm or involving structures deeper than skin and subcutaneous tissues (eg, abscess, osteomyelitis, septic arthritis, fasciitis) and no systemic inflammatory response signs (as described below). | 2 | Moderate |
| Local infection (as described above) with the signs of SIRS, as manifested by two or more of the following: • Temperature >38°C or <36°C • Heart rate >90 beats/min • Respiratory rate >20 breaths/min or Paco2 <32 mm Hg • White blood cell count >12,000 or <4000 cells/mm3 or 10% immature (band) forms |
3 | Severea |
IDSA, Infectious Diseases Society of America; Paco2, partial pressure of arterial carbon dioxide; PEDIS, perfusion, extent, depth, infection, and sensation; SIRS, systemic inflammatory response syndrome; SVS, Society for Vascular Surgery.
Ischemia may complicate and increase the severity of any infection. Systemic infection may sometimes be manifested with other clinical findings, such as hypotension, confusion, and vomiting, or evidence of metabolic disturbances, such as acidosis, severe hyperglycemia, and new-onset azotemia.
ACKNOWLEDGMENT
The Steering Committee wishes to acknowledge the outstanding administrative support of Ms Patricia Burton and Ms Kristin Hitchcock from the Society for Vascular Surgery throughout the course of the global guidelines project.
Independent peer-review and oversight has been provided by members of the ESVS Guideline Committee (Drs Gert Jan de Borst, Guidelines Committee chair, Jos van den Berg, Frederico Bastos Goncalves, Stavros Kakkos, Igor Koncar, Jes Lindholt, Henrik Sillesen), SVS Document Oversight Committee (Drs Thomas L. Forbes, chair, Ali AbuRahma, Kwame Anankwah, Neal Barshes, Ruth Bush, Ronald L. Dalman, Mark Davies, Alik Farber, Anil Hingorani, Mahmoud Malas, J. Sheppard Mondy, Eva Rzucidlo, Marc Schermerhorn), and the Council of the World Federation of Vascular Societies (Drs Alberto Muñoz, Vidyasagaran Thiruvengadam, Martin Björck, Peter Subramaniam, P Rajaruthnam, Varinder Bedi, Thanyani Mulaudzi, Kimihiro Komori, T. Vidyasagaran, Nobuyoshi Azuma, John Henry Nicholas Wolfe, John Wolfe, Arkadiusz Jawien, Pramook Mutirangura, Bernie Bourke, Arkadiusz Jawien, Alvaro Balcazar, Juan Esteban Paolini, Douglas Cavaye, Nelson de Luccia, Marcelo Diamant). 0741–5214
TABLE OF ABBREVIATIONS AND ACRONYMS
- ABI
Ankle-brachial index
- AFS
Amputation-free survival
- AI
Aortoiliac
- AKA
Above-knee amputation
- AP
Ankle pressure
- AT
Anterior tibial
- BKA
Below-knee amputation
- BMI
Body mass index
- BMMNCs
Bone marrow mononuclear cells
- CAD
Coronary artery disease
- CE-MRA
Contrast-enhanced MRA
- CFA
Common femoral artery
- CKD
Chronic kidney disease
- CLI
Critical limb ischemia
- CLTI
Chronic limb-threatening ischemia
- CPGs
Clinical practice guidelines
- CT
Computed tomography
- CTA
Computed tomography angiography
- CTO
Chronic total occlusion
- CVD
Cardiovascular disease
- DAPT
Dual antiplatelet therapy
- DCB
Drug-coated balloon
- DES
Drug-eluting stent
- DFU
Diabetic foot ulcer
- DM
Diabetes mellitus
- DP
Dorsalis pedis
- DSA
Digital subtraction angiography
- DUS
Duplex ultrasound
- EBR
Evidence-based revascularization
- EQ-5D
EuroQuol-5 Dimension questionnaire
- ESRD
End-stage renal disease
- ESVS
European Society for Vascular Surgery
- FGF
Fibroblast growth factor
- FP
Femoropoplitea
- GLASS
Global Limb Anatomic Staging System
- GRADE
Grading of Recommendations Assessment Development, and Evaluation
- GSV
Great saphenous vein
- GVG
Global Vascular Guidelines
- HBOT
Hyperbaric oxygen therapy
- HGF
Hepatocyte growth factor
- HICs
High-income countries
- HRQL
Health-related quality of life
- IC
Intermittent claudication
- IM
Inframalleolar
- IP
Infrapopliteal
- IPC
Intermittent pneumatic compression
- LBP
Limb-based patency
- LDL-C
Low-density lipoprotein cholesterol
- LMICs
Low- and middle-income countries
- LS
Lumbar sympathectomy
- MACE
Major adverse cardiovascular event
- MALE
Major adverse limb event
- MRA
Magnetic resonance angiography
- OPG
Objective performance goal
- PAD
Peripheral artery disease
- PBA
Plain balloon angioplasty
- PFA
Profunda femoris artery
- PLAN
Patient risk estimation, limb staging, anatomic pattern of disease
- PROM
Patient-reported outcomes measure
- PSV
Peak systolic velocity
- PT
Posterior tibial
- PVR
Pulse volume recording
- RCT
Randomized controlled trial
- SCS
Spinal cord stimulation
- SF-12
12-Item Short-Form Health Survey
- SFA
Superficial femoral artery
- SLI
severe limb ischemia
- SCLI
subcritical limb ischemia
- SVS
Society for Vascular Surgery
- SYNTAX
[System for coronary disease]
- TAP
Target arterial path
- TBI
Toe-brachial index
- TcPO2
Transcutaneous oximetry
- TKA
Through-knee amputation
- TP
Toe pressure
- VascuQoL
Vascular Quality of Life tool
- WFVS
World Federation of Vascular Societies
- WIfI
Wound, Ischemia, foot Infection
| Author | Affiliation | Section | Conflict of interest disclosures |
|---|---|---|---|
|
| |||
| Andrew Bradbury (Co-Editor) | University of Birmingham, UK | 1, 6, 11 (co-lead) | Daiichi Sankyo (honoraria), STD Pharmaceuticals (honoraria) |
| Michael Conte (Co-Editor) | University of California, San Francisco, San Francisco, Calif | 5, 6 (co-lead), 11 | SymicBio (advisor) Abbott Vascular (advisor) |
| Philippe Kolh (Co-Editor) | University of Liège, Liège, Belgium | 4, 6 (co-lead) | AstraZeneca (lecturer at symposium) |
| Florian Dick (Steering Committee) | Vascular Surgery, Kantonsspital St. Gallen, Switzerland | 5, 6 | None |
| Robert Fitridge (Steering Committee) | The University of Adelaide, Adelaide, South Australia, Australia | 1, 7 (co-lead), 6, 8 (co-lead) | None |
| Joseph L. Mills, Sr. (Steering Committee) | Baylor College of Medicine, Houston, Tex | 1 (co-lead), 6 | Nangio TX (stock) Innomed (consultant) |
| Jean-Baptiste Ricco (Steering Committee) | University of Poitiers Medical School, Poitiers, France | 1, 2, 3 (co-lead), 6 | Bayer (Scientific Advisory Committee) |
| Kalkunte R. Suresh (Steering Committee) | Jain Institute of Vascular Sciences, Bangalore, India | 6,10 (co-lead), 13 (lead) | None |
| John White (Steering Committee) | Advocate Lutheran General Hospital, Niles, Ill | 6, 9 (co-lead) | None |
| Victor Aboyans | Department of Cardiology, Dupuytren University Hospital, France | 1, 2 (co-lead), 7 | Bayer (honoraria, Scientific Advisory Committee) Amgen Novartis (honoraria) Pfizer/BMS Alliance Sanofi |
| Murat Aksoy | Department of Vascular Surgery American Hospital, Turkey | 1, 3, 5 | None |
| Vlad-Adrian Alexandrescu | University of Liège CHU Sart-Tilman Hospital, Belgium | 5 | None |
| David Armstrong | University of Southern California, USA | 1, 8, 10, 12 (co-lead) | None |
| Nobuyoshi Azuma | Asahikawa Medical University, Japan | 6 | Astellas Sanofi Daiichi Sankyo Otsuka Terumo (honoraria) |
| Jill Belch | Ninewells Hospital University of Dundee, UK | 3, 4 (co-lead) | Bayer (Advisory Board member and chair of scientific meeting, nonpromotional) Amgen (Advisory Board member and speaker at events) AstraZeneca (Advisory Board member) Sanofi (consultant) Rexgenero (end points adjudicator and advisor) |
| Michel Bergoeing | Escuela de Medicina Pontificia Universidad Catolica de Chile, Chile | 4, 5 | Altura Medical Inc Novate Medical Inc PQ Bypass Inc (honoraria) |
| Martin Bjorck | Department of Surgical Sciences, Vascular Surgery, Uppsala University, Sweden | 4, 11 | None |
| Nabil Chakfé | University Hospital of Strasbourg, France | 6 | I own stocks in a startup developed on a patent for a venous stent; still in development |
| Stephen Cheng | The University of Hong Kong | 2, 5 | None |
| Joseph Dawson | Royal Adelaide Hospital & University of Adelaide, Australia | 7 (co-lead) | None |
| Eike Sebastian Debus | University Heart Center Hamburg, University Hospital Hamburg-Eppendorf, Germany | 10 | Executive Committee member Voyager PAD study (Bayer) |
| Andrew Dueck | Schulich Heart Centre, Sunnybrook Health Sciences Centre, University of Toronto | 5 | Employed by Medtronic (chaired an aortic surgery update meeting) |
| Susan Duval | Cardiovascular Division, University of Minnesota Medical School, US | 2 | Merck (Scientific Advisory Board; statistical consultant) AstraZeneca (statistical consultant) Merck research grant |
| Hans Henning Eckstein | Technical University of Munich, Germany | 9, 10 | None |
| Roberto Ferraresi | Interventional Cardiovascular Unit, Cardiology Department, Istituto Clinico Città Studi, Milan, Italy | 5 | Boston Scientific, Abbott, Medtronic, Biotronik, Cook (speakers bureau) Boston Scientific (advisor) Medtronic (consultant) |
| Raghvinder Gambhir | King’s College Hospital, London, UK | 8, 9 (co-lead) | None |
| Mauro Garguilo | Diagnostica e Sperimentale, University of Bologna, Italy | 6, 10 | Medtronic Vascular Inc (consultant, lecturer) William Cook Europe ApS (consultant, lecturer) |
| Patrick Geraghty | Washington University School of Medicine, US | 5, 11 | Bard/Lutonix trial PI (payment goes to Washington University) Bard/Lutonix (consultant) Boston Scientific (Advisory Board member) Cook Medical trial PI (ayment goes to Washington University) Intact Vascular (previously Advisory Board member, now trial PI; payment goes to Washington University) Pulse Therapeutics (stockholder; startup, no current products in market) |
| Steve Goode | Sheffield Vascular Institute, UK | 3 (co-lead), 5, 11 | Boston Scientific (Scientific Advisory Committee) Vascular Solutions royalties) |
| Bruce Gray | Greenville Health System, US | 5, 10 | National Cardiovascular Data Registry (Peripheral Vascular Interventions registry committee) |
| Wei Guo | 1, 6, 10 | ||
| Prem Chand Gupta | Care Hospital, Banjara Hills, Hyderabad, India | 2, 10 | None |
| Robert Hinchliffe | University of Bristol, UK | 2 (co-lead), 8, 11 | Nonfunded member of the trial steering committee for a randomized clinical trial of stem cells for the treatment of critical limb ischemia (PACE study, Pluristem Therapeutics, Inc) |
| Prasad Jetty | Division of Vascular and Endovascular Surgery, The Ottawa Hospital and the University of Ottawa, Ottawa, Canada | 10 | None |
| Kimihiro Komori | Nagoya University Graduates School of Medicine, Japan | 2, 6 | None |
| Lawrence Lavery | UT Southwestern Medical Center, US | 3, 8 | Research grant, Cardinal Consultant/advisor: Aplion Medical Users, Harbor MedTech, Boehringer Ingelheim, Medline Industries Speakers bureau: Osiris, Integr, Smith-Nephew |
| Wei Liang | Renji Hospital, School of Medicine, Shanghai Jiaotong University, China | 1, 9 | None |
| Robert Lookstein | Division of Vascular and Interventional Radiology, Icahn School of Medicine at Mount Sinai | 6 | Boston Scientific (consultant and Advisory Board) Medtronic (consultant and Advisory Board) BTG (consultant) Gore (consultant) |
| Matthew Menard | Brigham and Women’s Hospital, US | 11 (co-lead) | Janssen (Scientific Advisory Committee) Aralez Pharmaceuticals, Inc (Scientific Advisory Board) |
| Sanjay Misra | Mayo Clinic, USA | 3 | FLEXSTENT DSMB board member COVR (Medical Advisory Committee) Patent from Bergheim Ingersoll |
| Tetsuro Miyata | Sanno Hospital and Sanno Medical Center, Japan | 1, 8 | Kaken Pharmaceutical Co, Ltd, Astellas Pharma Inc, Taisho Toyama Pharmaceutical Co, Ltd, Mitsubishi Tanabe Pharma Co, Amgen Astellas BioPharma KK, Daiichi Sankyo Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Pfizer Inc, Nippon Shinyaku Co, Ltd, LeMaitre Vascular GK, Cardinal Health Japan, Bristol-Myers Squibb, W. L. Gore & Associates, Toray Industries, Inc, Bayer Yakuhin, Ltd, Mochida Pharmaceutical Co, Ltd (speakers bureau) |
| Greg Moneta | Oregon Health & Science University | 1, 2, 10 (co-lead) | None |
| Jose Antonio Munoa Prado | Clinic Venart, Mexico | 6 | None |
| Alberto Munoz | Colombia National University, Colombia | 3, 6 | Pint Pharma (speakers bureau) |
| Juan Esteban Paolini | Sanatorio Dr Julio Mendez, University of Buenos Aires, Argentina | 6, 7 | None |
| Manesh Patel | Division of Cardiology, Duke University Health System | 5, 11 | Research Grants: AstraZeneca, Bayer, Janssen, Medtronic, Procyrion, Heartflow, NIH; Advisory Board/Consultant: Bayer, Janssen, and Amgen |
| Frank Pomposelli | St. Elizabeth’s Medical Center, US | 5, 6 | Cruzar Medical (consultant) |
| Richard Powell | Dartmouth-Hitchcock, US | 8 (co-lead), 11 | Anges (consultant) |
| Peter Robless | Mt. Elizabeth Hospital, Singapore | 4, 7 | None |
| Lee Rogers | Amputation Prevention Centers of America, US | 12 | RestorixHealth, Inc (consultant) Advanced Tissue, LLC (consultant) |
| Andres Schanzer | University of Massachusetts, US | 3,4 | Cook Medical (proctor) |
| Peter Schneider | Kaiser Foundation Hospital Honolulu and Hawaii Permanente Medical Group, US | 5 (co-lead), 6, 10 | Chief Medical Officer, Intact Vascular and Cagent Cook Medical (modest royalty) Participant in research sponsored by Gore, Silk Road Medical, Medtronic, Boston Scientific (no personal financial relationship) |
| Spence Taylor | Greenville Health Center/USC School of Medicine Greenville, US | 9 | None |
| Melina Vega De Ceniga | Hospital de Galdakao-Usansolo, Bizkaia, Spain | 7 (co-lead), 11 | None |
| Martin Veller | University of the Witwatersrand, Johannesburg, South Africa | 2,9 | Bayer (honoraria) |
| Frank Vermassen | Ghent University Hospital, Belgium | 6, 10 | Lecturer for Medtronic, Abbott Vascular, Bard, Terumo, Boston Scientific, Philips Consultant for Medtronic, Terumo, Boston Scientific, Philips |
| Jinsong Wang | The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China | 2, 3, 8 | None |
| Shenming Wang | The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China | 2, 3, 8 | Gore, Bayer (consultant) |
GVG GUIDELINE WRITING GROUP CONFLICT OF INTEREST POLICY: INDUSTRY RELATIONSHIPS
I. Introduction. The organizations participating in the Global Vascular Guidelines are committed to the precept of developing trustworthy clinical practice guidelines through transparency and full disclosure by those participating in the process of guideline development.
The tenets of the policy as set forth are reflective of the desire to maintain a balanced approach in the guidelines development process. Ensuring that industry will have no direct influence on the clinical content and recommendations of the clinical guideline is fundamental to a trustworthy and independent document. Conversely, it is acknowledged that a healthy relationship between content experts and industry, when properly managed and transparent, may bring value to the process and the final document.
II. Scope. All Co-Editors, Steering Committee members, and authors are required to disclose relationships with industry and other relevant entities as defined in Section IV.
- Industry income–Monies received from biomedical companies, device manufacturers, pharmaceutical companies, or other companies producing products related to the field.
- Industry relationships
- - Serve as an officer, board member, trustee, owner, or employee of a company;
- - Direct owner of stock, stock options, or bonds of a company (excludes diversified mutual funds);
- - Consultancy, scientific advisory committee membership, or lecturer for a company (required to disclose regardless of income; if income, must disclose amount; please note that disclosure is not required for an honorarium paid by a university, hospital, or medical society for a lecture that has received an unrestricted funding);
- - Investigator for a company, including holding research grants from the company (disclosure of research funding paid directly to your institution is not required as it does not constitute industry income);
- - Personal income from patents (intellectual property).
IV. Reporting Time Frame and Disclosure Timing. Disclosure is required from all members of the writing group for the past 12 months. Authors are discouraged from adding new relationships during the guideline development process; if relevant relationships are added, they must be disclosed immediately to the cochairs and verbally disclosed during any conference calls or meeting and added to the author disclosure grid. In the event that the required balance is not met, additional members may be added or removed to achieve balance.
Disclosures are made in writing or online before the writing initiative to determine eligibility of members to serve and throughout the guideline development process to ensure transparency.
V. Conflict of Interest Requirements by Role. The Co-Editors of the Global Vascular Guidelines should have less than $10,000 USD in industry income in aggregate during their work on the guidelines or subsequent revisions.
The majority (>50%) of the steering committee members and guideline authors should have less than $10,000 USD in industry income in aggregate during their work on the guidelines or subsequent revisions.
The minority of steering committee members and authors allowed additional industry income may have no more than $50,000 per annum (USD) in aggregate during their work on the guidelines or subsequent revisions.
Guideline reviewers are required to adhere to the same criteria for conflict of interest as the steering committee members and guideline authors.
VI. Review of Disclosures. The Conflict of Interest Committee for each sponsoring organization will review disclosures for relevant conflicts of interest. A member of the steering committee will be appointed to ensure ongoing compliance by committee members and authors.
- Direct industry funding will not be accepted by participating societies to support the Global Vascular Guidelines initiative.
- Part-time, full-time, and paid industry consultants (ie, advocacy, government affairs, and lobbyists) are prohibited from serving as members of the guidelines writing group and as document reviewers.
Additional material for this article may be found online at www.jvascsurg.org.
Joint guidelines of the Society for Vascular Surgery, European Society for Vascular Surgery, and World Federation of Vascular Societies
Endorsed by the American Podiatric Medical Association, British Cardiovascular Society, British Society for Endovascular Therapy, British Society of Interventional Radiology, Circulation Foundation, College of Podiatry, Society of Interventional Radiology, Society for Vascular Nursing, the Society for Vascular Technology of Great Britain and Ireland, and the Vascular Society of Great Britain and Ireland
REFERENCES
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 2.Goodney PP, Holman K, Henke PK, Travis LL, Dimick JB, Stukel TA, et al. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg 2013;57: 1471–9. 1480.e1–3; discussion: 1479–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodney PP, Travis LL, Brooke BS, DeMartino RR, Goodman DC, Fisher ES, et al. Relationship between regional spending on vascular care and amputation rate. JAMA Surg 2014;149:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Dabrh AM, Steffen MW, Asi N, Undavalli C, Wang Z, Elamin MB, et al. Nonrevascularization-based treatments in patients with severe or critical limb ischemia. J Vasc Surg 2015;62:1330–9.e13. [DOI] [PubMed] [Google Scholar]
- 5.Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg 2015;62:1642–51.e3. [DOI] [PubMed] [Google Scholar]
- 6.Abu Dabrh AM, Steffen MW, Asi N, Undavalli C, Wang Z, Elamin MB, et al. Bypass surgery versus endovascular interventions in severe or critical limb ischemia. J Vasc Surg 2016;63:244–53.e11. [DOI] [PubMed] [Google Scholar]
- 7.Almasri J, Adusumalli J, Asi N, Lakis S, Alsawas M, Prokop LJ, et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg 2018;68:624–33. [DOI] [PubMed] [Google Scholar]
- 8.Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc 2017;92:423–33. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Alonso-Coello P, Schunemann HJ, Djulbegovic B, Nothacker M, Lange S, et al. Guideline panels should seldom make good practice statements: guidance from the GRADE Working Group. J Clin Epidemiol 2016;80: 3–7. [DOI] [PubMed] [Google Scholar]
- 10.Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg 2014;59:220–34. e1–2. [DOI] [PubMed] [Google Scholar]
- 11.Jaff MR, White CJ, Hiatt WR, Fowkes GR, Dormandy J, Razavi M, et al. An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II): the TASC Steering Committee. Catheter Cardiovasc Interv 2015;86:611–25. [DOI] [PubMed] [Google Scholar]
- 12.Bollinger A, Breddin K, Hess H, Heystraten FM, Kollath J, Konttila A, et al. Semiquantitative assessment of lower limb atherosclerosis from routine angiographic images. Atherosclerosis 1981;38:339–46. [DOI] [PubMed] [Google Scholar]
- 13.Menard MT, Farber A, Assmann SF, Choudhry NK, Conte MS, Creager MA, et al. Design and rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) trial. J Am Heart Assoc 2016;5:e003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popplewell MA, Davies H, Jarrett H, Bate G, Grant M, Patel S, et al. Bypass versus angioplasty in severe ischaemia of the leg-2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials 2016;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt BD, Popplewell MA, Davies H, Meecham L, Jarrett H, Bate G, et al. BAlloon versus Stenting in severe Ischaemia of the Leg-3 (BASIL-3): study protocol for a randomised controlled trial. Trials 2017;18:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Graaff JC, Ubbink DT, Legemate DA, Tijssen JG, Jacobs MJ. Evaluation of toe pressure and transcutaneous oxygen measurements in management of chronic critical leg ischemia: a diagnostic randomized clinical trial. J Vasc Surg 2003;38:528–34. [DOI] [PubMed] [Google Scholar]
- 17.Brownrigg JR, Hinchliffe RJ, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: a systematic review. Diabetes Metab Res Rev 2016;32(Suppl 1):128–35. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Hasan R, Firwana B, Elraiyah T, Tsapas A, Prokop L, et al. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J Vasc Surg 2016;63: 29S–36S.e2. [DOI] [PubMed] [Google Scholar]
- 19.Lijmer JG, Hunink MG, van den Dungen JJ, Loonstra J, Smit AJ. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol 1996;22:391–8. [DOI] [PubMed] [Google Scholar]
- 20.Dachun X, Jue L, Liling Z, Yawei X, Dayi H, Pagoto SL, et al. Sensitivity and specificity of the ankle-brachial index to diagnose peripheral artery disease: a structured review. Vasc Med 2010;15:361–9. [DOI] [PubMed] [Google Scholar]
- 21.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg 2008;48: 1197–203. [DOI] [PubMed] [Google Scholar]
- 22.Salaun P, Desormais I, Lapebie FX, Riviere AB, Aboyans V, Lacroix P, et al. Comparison of ankle pressure, systolic toe pressure, and transcutaneous oxygen pressure to predict major amputation after 1 year in the COPART cohort. Angiology 2018. 3319718793566. [DOI] [PubMed] [Google Scholar]
- 23.Shirasu T, Hoshina K, Akagi D, Miyahara T, Yamamoto K, Watanabe T. Pulse volume recordings to identify falsely elevated ankle brachial index. Asian Cardiovasc Thorac Ann 2016;24:517–22. [DOI] [PubMed] [Google Scholar]
- 24.Hingorani AP, Ascher E, Marks N, Puggioni A, Shiferson A, Tran V, et al. Limitations of and lessons learned from clinical experience of 1,020 duplex arteriography. Vascular 2008;16: 147–53. [DOI] [PubMed] [Google Scholar]
- 25.Larch E, Minar E, Ahmadi R, Schnurer G, Schneider B, Stumpflen A, et al. Value of color duplex sonography for evaluation of tibioperoneal arteries in patients with femoropopliteal obstruction: a prospective comparison with anterograde intraarterial digital subtraction angiography. J Vasc Surg 1997;25:629–36. [DOI] [PubMed] [Google Scholar]
- 26.Adriaensen ME, Kock MC, Stijnen T, van Sambeek MR, van Urk H, Pattynama PM, et al. Peripheral arterial disease: therapeutic confidence of CT versus digital subtraction angiography and effects on additional imaging recommendations. Radiology 2004;233:385–91. [DOI] [PubMed] [Google Scholar]
- 27.Hingorani A, Ascher E, Markevich N, Kallakuri S, Schutzer R, Yorkovich W, et al. A comparison of magnetic resonance angiography, contrast arteriography, and duplex arteriography for patients undergoing lower extremity revascularization. Ann Vasc Surg 2004;18:294–301. [DOI] [PubMed] [Google Scholar]
- 28.Collins R, Cranny G, Burch J, Aguiar-Ibanez R, Craig D, Wright K, et al. A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technol Assess 2007;11. iii–iv, xi,–xiii, 1–184. [DOI] [PubMed] [Google Scholar]
- 29.Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJ. Diagnostic performance of computed tomography angiography in peripheral arterial disease: a systematic review and meta-analysis. JAMA 2009;301:415–24. [DOI] [PubMed] [Google Scholar]
- 30.Long-term mortality and its predictors in patients with critical leg ischaemia. The I.C.A.I. Group (Gruppo di Studio dell’Ischemia Cronica Critica degli Arti Inferiori). The Study Group of Criticial Chronic Ischemia of the Lower Exremities. Eur J Vasc Endovasc Surg 1997;14:91–5. [PubMed] [Google Scholar]
- 31.Armstrong EJ, Wu J, Singh GD, Dawson DL, Pevec WC, Amsterdam EA, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg 2014;60:1565–71. [DOI] [PubMed] [Google Scholar]
- 32.Faglia E, Clerici G, Scatena A, Caminiti M, Curci V, Morabito A, et al. Effectiveness of combined therapy with angiotensin-converting enzyme inhibitors and statins in reducing mortality in diabetic patients with critical limb ischemia: an observational study. Diabetes Res Clin Pract 2014;103:292–7. [DOI] [PubMed] [Google Scholar]
- 33.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antithrombotic Trialists’ Collaboration, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–39. [DOI] [PubMed] [Google Scholar]
- 36.Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med 2017;376: 32–40. [DOI] [PubMed] [Google Scholar]
- 37.Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219–29. [DOI] [PubMed] [Google Scholar]
- 38.Anand S, Yusuf S, Xie C, Pogue J, Eikelboom J, Budaj A, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med 2007;357:217–27. [DOI] [PubMed] [Google Scholar]
- 39.Leng GC, Price JF, Jepson RG. Lipid-lowering for lower limb atherosclerosis. Cochrane Database Syst Rev 2000;2: CD000123. [DOI] [PubMed] [Google Scholar]
- 40.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.12114036 [Google Scholar]
- 41.Meade T, Zuhrie R, Cook C, Cooper J. Bezafibrate in men with lower extremity arterial disease: randomised controlled trial. BMJ 2002;325:1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aung PP, Maxwell HG, Jepson RG, Price JF, Leng GC. Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database Syst Rev 2007;4:CD000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills EJ, O’Regan C, Eyawo O, Wu P, Mills F, Berwanger O, et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: a meta-analysis of >40 000 patients. Eur Heart J 2011;32:1409–15. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol 2017;2:47–54. [DOI] [PubMed] [Google Scholar]
- 45.ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bavry AA, Anderson RD, Gong Y, Denardo SJ, Cooper-Dehoff RM, Handberg EM, et al. Outcomes among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the INternational Verapamil-SR/Trandolapril STudy. Hypertension 2010;55:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moise N, Huang C, Rodgers A, Kohli-Lynch CN, Tzong KY, Coxson PG, et al. Comparative cost-effectiveness of conservative or intensive blood pressure treatment guidelines in adults aged 35–74 years: the Cardiovascular Disease Policy Model. Hypertension 2016;68:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–31. [DOI] [PubMed] [Google Scholar]
- 50.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Dieren S, Kengne AP, Chalmers J, Beulens JW, Davis TM, Fulcher G, et al. Intensification of medication and glycaemic control among patients with type 2 diabetes–the ADVANCE trial. Diabetes Obes Metab 2014;16:426–32. [DOI] [PubMed] [Google Scholar]
- 52.Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, de Boer IH, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2015;38:1777–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes–2018. Diabetes Care 2018;41(Suppl 1):S55–64. [DOI] [PubMed] [Google Scholar]
- 54.Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA 2016;316:313–24. [DOI] [PubMed] [Google Scholar]
- 55.Nawaz S, Cleveland T, Gaines PA, Chan P. Clinical risk associated with contrast angiography in metformin treated patients: a clinical review. Clin Radiol 1998;53:342–4. [DOI] [PubMed] [Google Scholar]
- 56.Goergen SK, Rumbold G, Compton G, Harris C. Systematic review of current guidelines, and their evidence base, on risk of lactic acidosis after administration of contrast medium for patients receiving metformin. Radiology 2010;254: 261–9. [DOI] [PubMed] [Google Scholar]
- 57.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol 2011;21:2527–41. [DOI] [PubMed] [Google Scholar]
- 58.Dagenais GR, Yi Q, Lonn E, Sleight P, Ostergren J, Yusuf S. Impact of cigarette smoking in high-risk patients participating in a clinical trial. A substudy from the Heart Outcomes Prevention Evaluation (HOPE) trial. Eur J Cardiovasc Prev Rehabil 2005;12:75–81. [PubMed] [Google Scholar]
- 59.Athyros VG, Tziomalos K, Katsiki N, Gossios TD, Giouleme O, Anagnostis P, et al. The impact of smoking on cardiovascular outcomes and comorbidities in statin-treated patients with coronary artery disease: a post hoc analysis of the GREACE study. Curr Vasc Pharmacol 2013;11:779–84. [DOI] [PubMed] [Google Scholar]
- 60.Blomster JI, Woodward M, Zoungas S, Hillis GS, Harrap S, Neal B, et al. The harms of smoking and benefits of smoking cessation in women compared with men with type 2 diabetes: an observational analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron modified release Controlled Evaluation) trial. BMJ Open 2016;6:e009668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kondo T, Osugi S, Shimokata K, Honjo H, Morita Y, Maeda K, et al. Smoking and smoking cessation in relation to all-cause mortality and cardiovascular events in 25,464 healthy male Japanese workers. Circ J 2011;75:2885–92. [DOI] [PubMed] [Google Scholar]
- 62.Newhall K, Suckow B, Spangler E, Brooke BS, Schanzer A, Tan TW, et al. Impact and duration of brief surgeon-delivered smoking cessation advice on attitudes regarding nicotine dependence and tobacco harms for patients with peripheral arterial disease. Ann Vasc Surg 2017;38:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Biancari F, Salenius JP, Heikkinen M, Luther M, Ylonen K, Lepantalo M. Risk-scoring method for prediction of 30-day postoperative outcome after infrainguinal surgical revascularization for critical lower-limb ischemia: a Finnvasc registry study. World J Surg 2007;31:217–25; discussion: 226–7. [DOI] [PubMed] [Google Scholar]
- 64.Schanzer A, Mega J, Meadows J, Samson RH, Bandyk DF, Conte MS. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg 2008;48:1464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a survival prediction model to facilitate clinical decision making. J Vasc Surg 2010;51(Suppl):52S–68S. [DOI] [PubMed] [Google Scholar]
- 66.Meltzer AJ, Graham A, Connolly PH, Meltzer EC, Karwowski JK, Bush HL, et al. The Comprehensive Risk Assessment for Bypass (CRAB) facilitates efficient perioperative risk assessment for patients with critical limb ischemia. J Vasc Surg 2013;57:1186–95. [DOI] [PubMed] [Google Scholar]
- 67.Simons JP, Goodney PP, Flahive J, Hoel AW, Hallett JW, Kraiss LW, et al. A comparative evaluation of risk-adjustment models for benchmarking amputation-free survival after lower extremity bypass. J Vasc Surg 2016;63: 990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cull DL, Manos G, Hartley MC, Taylor SM, Langan EM, Eidt JF, et al. An early validation of the Society for Vascular Surgery lower extremity threatened limb classification system. J Vasc Surg 2014;60:1535–41. [DOI] [PubMed] [Google Scholar]
- 69.Zhan LX, Branco BC, Armstrong DG, Mills JL Sr. The Society for Vascular Surgery lower extremity threatened limb classification system based on Wound, Ischemia, and foot Infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg 2015;61:939–44. [DOI] [PubMed] [Google Scholar]
- 70.Causey MW, Ahmed A, Wu B, Gasper WJ, Reyzelman A, Vartanian SM, et al. Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program. J Vasc Surg 2016;63: 1563–73.e2. [DOI] [PubMed] [Google Scholar]
- 71.Darling JD, McCallum JC, Soden PA, Meng Y, Wyers MC, Hamdan AD, et al. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system following infrapopliteal endovascular interventions for critical limb ischemia. J Vasc Surg 2016;64: 616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson WP, Loretz L, Hanesian C, Flahive J, Bostrom J, Lunig N, et al. Society for Vascular Surgery Wound, Ischemia, foot Infection (WIfI) score correlates with the intensity of multimodal limb treatment and patient-centered outcomes in patients with threatened limbs managed in a limb preservation center. J Vasc Surg 2017;66: 488–98.e2. [DOI] [PubMed] [Google Scholar]
- 73.Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003;26:1879–82. [DOI] [PubMed] [Google Scholar]
- 74.Cardinal M, Eisenbud DE, Phillips T, Harding K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen 2008;16:19–22. [DOI] [PubMed] [Google Scholar]
- 75.Lavery LA, Barnes SA, Keith MS, Seaman JW Jr, Armstrong DG. Prediction of healing for postoperative diabetic foot wounds based on early wound area progression. Diabetes Care 2008;31:26–9. [DOI] [PubMed] [Google Scholar]
- 76.Snyder RJ, Cardinal M, Dauphinee DM, Stavosky J. A posthoc analysis of reduction in diabetic foot ulcer size at 4 weeks as a predictor of healing by 12 weeks. Ostomy Wound Manage 2010;56:44–50. [PubMed] [Google Scholar]
- 77.Seeger JM, Schmidt JH, Flynn TC. Preoperative saphenous and cephalic vein mapping as an adjunct to reconstructive arterial surgery. Ann Surg 1987;205:733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wengerter KR, Veith FJ, Gupta SK, Ascer E, Rivers SP. Influence of vein size (diameter) on infrapopliteal reversed vein graft patency. J Vasc Surg 1990;11:525–31. [DOI] [PubMed] [Google Scholar]
- 79.Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg 2007;46:1180–90; discussion: 1190. [DOI] [PubMed] [Google Scholar]
- 80.Harward TR, Ingegno MD, Carlton L, Flynn TC, Seeger JM. Limb-threatening ischemia due to multilevel arterial occlusive disease. Simultaneous or staged inflow/outflow revascularization. Ann Surg 1995;221:498–503; discussion: 503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zukauskas G, Ulevicius H, Triponis V. Sequential aortofe-moropopliteal/distal bypass for treatment of critical lower-limb ischaemia. Cardiovasc Surg 1995;3:671–8. [DOI] [PubMed] [Google Scholar]
- 82.Jongkind V, Akkersdijk GJ, Yeung KK, Wisselink W. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. J Vasc Surg 2010;52:1376–83. [DOI] [PubMed] [Google Scholar]
- 83.Ye W, Liu CW, Ricco JB, Mani K, Zeng R, Jiang J. Early and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto-iliac lesions. J Vasc Surg 2011;53:1728–37. [DOI] [PubMed] [Google Scholar]
- 84.Deloose K, Bosiers M, Callaert J, Verbist J, Vermassen F, Scheinert D, et al. Primary stenting is nowadays the gold standard treatment for TASC II A & B iliac lesions: the definitive MISAGO 1-year results. J Cardiovasc Surg (Torino) 2017;58:416–21. [DOI] [PubMed] [Google Scholar]
- 85.Ricco JB, Probst H. Long-term results of a multicenter randomized study on direct versus crossover bypass for unilateral iliac artery occlusive disease. J Vasc Surg 2008;47: 45–53; discussion: 53–4. [DOI] [PubMed] [Google Scholar]
- 86.Chiu KW, Davies RS, Nightingale PG, Bradbury AW, Adam DJ. Review of direct anatomical open surgical management of atherosclerotic aorto-iliac occlusive disease. Eur J Vasc Endovasc Surg 2010;39:460–71. [DOI] [PubMed] [Google Scholar]
- 87.Indes JE, Pfaff MJ, Farrokhyar F, Brown H, Hashim P, Cheung K, et al. Clinical outcomes of 5358 patients undergoing direct open bypass or endovascular treatment for aortoiliac occlusive disease: a systematic review and meta-analysis. J Endovasc Ther 2013;20:443–55. [DOI] [PubMed] [Google Scholar]
- 88.Kang JL, Patel VI, Conrad MF, Lamuraglia GM, Chung TK, Cambria RP. Common femoral artery occlusive disease: contemporary results following surgical endarterectomy. J Vasc Surg 2008;48:872–7. [DOI] [PubMed] [Google Scholar]
- 89.Ballotta E, Gruppo M, Mazzalai F, Da Giau G. Common femoral artery endarterectomy for occlusive disease: an 8-year single-center prospective study. Surgery 2010;147:268–74. [DOI] [PubMed] [Google Scholar]
- 90.Chang RW, Goodney PP, Baek JH, Nolan BW, Rzucidlo EM, Powell RJ. Long-term results of combined common femoral endarterectomy and iliac stenting/stent grafting for occlusive disease. J Vasc Surg 2008;48:362–7. [DOI] [PubMed] [Google Scholar]
- 91.Baumann F, Ruch M, Willenberg T, Dick F, Do DD, Keo HH, et al. Endovascular treatment of common femoral artery obstructions. J Vasc Surg 2011;53:1000–6. [DOI] [PubMed] [Google Scholar]
- 92.Bonvini RF, Rastan A, Sixt S, Noory E, Schwarz T, Frank U, et al. Endovascular treatment of common femoral artery disease: medium-term outcomes of 360 consecutive procedures. J Am Coll Cardiol 2011;58:792–8. [DOI] [PubMed] [Google Scholar]
- 93.Gouëffic Y, Della Schiava N, Thaveau F, Rosset E, Favre JP, Salomon du Mont L, et al. Stenting or surgery for de novo common femoral artery stenosis. JACC Cardiovasc Interv 2017;10:1344–54. [DOI] [PubMed] [Google Scholar]
- 94.Siracuse JJ, Van Orden K, Kalish JA, Eslami MH, Schermerhorn ML, Patel VI, et al. Endovascular treatment of the common femoral artery in the Vascular Quality Initiative. J Vasc Surg 2017;65:1039–46. [DOI] [PubMed] [Google Scholar]
- 95.Azuma N, Uchida H, Kokubo T, Koya A, Akasaka N, Sasajima T. Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery? Eur J Vasc Endovasc Surg 2012;43:322–8. [DOI] [PubMed] [Google Scholar]
- 96.Sumpio BE, Forsythe RO, Ziegler KR, van Baal JG, Lepantalo MJ, Hinchliffe RJ. Clinical implications of the angiosome model in peripheral vascular disease. J Vasc Surg 2013;58:814–26. [DOI] [PubMed] [Google Scholar]
- 97.Biancari F, Juvonen T. Angiosome-targeted lower limb revascularization for ischemic foot wounds: systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2014;47: 517–22. [DOI] [PubMed] [Google Scholar]
- 98.Chae KJ, Shin JY. Is angiosome-targeted angioplasty effective for limb salvage and wound healing in diabetic foot? A meta-analysis. PLoS One 2016;11:e0159523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jongsma H, Bekken JA, Akkersdijk GP, Hoeks SE, Verhagen HJ, Fioole B. Angiosome-directed revascularization in patients with critical limb ischemia. J Vasc Surg 2017;65:1208–19.e1. [DOI] [PubMed] [Google Scholar]
- 100.Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006;354:1879–88. [DOI] [PubMed] [Google Scholar]
- 101.Saxon RR, Dake MD, Volgelzang RL, Katzen BT, Becker GJ. Randomized, multicenter study comparing expanded polytetrafluoroethylene-covered endoprosthesis placement with percutaneous transluminal angioplasty in the treatment of superficial femoral artery occlusive disease. J Vasc Interv Radiol 2008;19:823–32. [DOI] [PubMed] [Google Scholar]
- 102.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv 2011;4:495–504. [DOI] [PubMed] [Google Scholar]
- 103.Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med 2015;373:145–53. [DOI] [PubMed] [Google Scholar]
- 104.Mills JL, Fujitani RM, Taylor SM. Contribution of routine intraoperative completion arteriography to early infrainguinal bypass patency. Am J Surg 1992;164:506–10; discussion: 510–1. [DOI] [PubMed] [Google Scholar]
- 105.Bandyk DF, Mills JL, Gahtan V, Esses GE. Intraoperative duplex scanning of arterial reconstructions: fate of repaired and unrepaired defects. J Vasc Surg 1994;20:426–32; discussion: 432–3. [DOI] [PubMed] [Google Scholar]
- 106.Ubbink DT, Vermeulen H. Spinal cord stimulation for non-reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev 2013;2:CD004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karanth VK, Karanth TK, Karanth L. Lumbar sympathectomy techniques for critical lower limb ischaemia due to non-reconstructable peripheral arterial disease. Cochrane Database Syst Rev 2016;12:CD011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vietto V, Franco JV, Saenz V, Cytryn D, Chas J, Ciapponi A. Prostanoids for critical limb ischaemia. Cochrane Database Syst Rev 2018;1:CD006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith FB, Bradbury A, Fowkes G. Intravenous naftidrofuryl for critical limb ischaemia. Cochrane Database Syst Rev 2012;7:CD002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 2015;6:CD004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Löndahl M, et al. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev 2016;32(Suppl 1):154–68. [DOI] [PubMed] [Google Scholar]
- 112.Santema KT, Stoekenbroek RM, Koelemay MJ, Reekers JA, van Dortmont LM, Oomen A, et al. Hyperbaric oxygen therapy in the treatment of ischemic lower-extremity ulcers in patients with diabetes: results of the DAMO2CLES multicenter randomized clinical trial. Diabetes Care 2018;41: 112–9. [DOI] [PubMed] [Google Scholar]
- 113.Peeters Weem SM, Teraa M, de Borst GJ, Verhaar MC, Moll FL. Bone marrow derived cell therapy in critical limb ischemia: a meta-analysis of randomized placebo controlled trials. Eur J Vasc Endovasc Surg 2015;50:775–83. [DOI] [PubMed] [Google Scholar]
- 114.Elsherif M, Tawfick W, Canning P, Hynes N, Sultan S. Quality of time spent without symptoms of disease or toxicity of treatment for transmetatarsal amputation versus digital amputation in diabetic patients with digital gangrene. Vascular 2018;26:142–50. [DOI] [PubMed] [Google Scholar]
- 115.Aziz H, Branco BC, Braun J, Hughes JD, Goshima KR, Trinidad-Hernandez M, et al. The influence of do-not-resuscitate status on the outcomes of patients undergoing emergency vascular operations. J Vasc Surg 2015;61:1538–42. [DOI] [PubMed] [Google Scholar]
- 116.Siracuse JJ, Jones DW, Meltzer EC, Graham AR, Salzler GG, Connolly PH, et al. Impact of "do not resuscitate" status on the outcome of major vascular surgical procedures. Ann Vasc Surg 2015;29:1339–45. [DOI] [PubMed] [Google Scholar]
- 117.Reed AB, Delvecchio C, Giglia JS. Major lower extremity amputation after multiple revascularizations: was it worth it? Ann Vasc Surg 2008;22:335–40. [DOI] [PubMed] [Google Scholar]
- 118.Rollins DL, Towne JB, Bernhard VM, Baum PL. Isolated profundaplasty for limb salvage. J Vasc Surg 1985;2:585–90. [DOI] [PubMed] [Google Scholar]
- 119.Miksic K, Novak B. Profunda femoris revascularization in limb salvage. J Cardiovasc Surg (Torino) 1986;27:544–52. [PubMed] [Google Scholar]
- 120.Ayoub MM, Solis MM, Rogers JJ, Dalton ML. Thru-knee amputation: the operation of choice for non-ambulatory patients. Am Surg 1993;59:619–23. [PubMed] [Google Scholar]
- 121.Taylor SM, Kalbaugh CA, Cass AL, Buzzell NM, Daly CA, Cull DL, et al. "Successful outcome" after below-knee amputation: an objective definition and influence of clinical variables. Am Surg 2008;74:607–12; discussion: 612–3. [DOI] [PubMed] [Google Scholar]
- 122.Webster JB, Hakimi KN, Czerniecki JM. Prosthetic fitting, use, and satisfaction following lower-limb amputation: a prospective study. J Rehabil Res Dev 2012;49:1493. [DOI] [PubMed] [Google Scholar]
- 123.Bradley L, Kirker S. Secondary prevention of arteriosclerosis in lower limb vascular amputees: a missed opportunity. Eur J Vasc Endovasc Surg 2006;32:491–3. [DOI] [PubMed] [Google Scholar]
- 124.Glaser JD, Bensley RP, Hurks R, Dahlberg S, Hamdan AD, Wyers MC, et al. Fate of the contralateral limb after lower extremity amputation. J Vasc Surg 2013;58:1571–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abbruzzese TA, Havens J, Belkin M, Donaldson MC, Whittemore AD, Liao JK, et al. Statin therapy is associated with improved patency of autogenous infrainguinal bypass grafts. J Vasc Surg 2004;39:1178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Henke PK, Blackburn S, Proctor MC, Stevens J, Mukherjee D, Rajagopalin S, et al. Patients undergoing infrainguinal bypass to treat atherosclerotic vascular disease are underprescribed cardioprotective medications: effect on graft patency, limb salvage, and mortality. J Vasc Surg 2004;39: 357–65. [DOI] [PubMed] [Google Scholar]
- 127.Brown J, Lethaby A, Maxwell H, Wawrzyniak AJ, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database Syst Rev 2008;4:CD000535. [DOI] [PubMed] [Google Scholar]
- 128.Bedenis R, Lethaby A, Maxwell H, Acosta S, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database Syst Rev 2015;2: CD000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suckow BD, Kraiss LW, Schanzer A, Stone DH, Kalish J, DeMartino RR, et al. Statin therapy after infrainguinal bypass surgery for critical limb ischemia is associated with improved 5-year survival. J Vasc Surg 2015;61:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hobbs SD, Bradbury AW. Smoking cessation strategies in patients with peripheral arterial disease: an evidence-based approach. Eur J Vasc Endovasc Surg 2003;26: 341–7. [DOI] [PubMed] [Google Scholar]
- 131.Willigendael EM, Teijink JA, Bartelink ML, Peters RJ, Buller HR, Prins MH. Smoking and the patency of lower extremity bypass grafts: a meta-analysis. J Vasc Surg 2005;42:67–74. [DOI] [PubMed] [Google Scholar]
- 132.Belch JJ, Dormandy J; CASPAR Writing Committee. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg 2010;52:825–33. e1–e2. [DOI] [PubMed] [Google Scholar]
- 133.Gassman AA, Degner BC, Al-Nouri O, Philippi L, Hershberger R, Halandras P, et al. Aspirin usage is associated with improved prosthetic infrainguinal bypass graft patency. Vascular 2014;22:105–11. [DOI] [PubMed] [Google Scholar]
- 134.Cassar K, Ford I, Greaves M, Bachoo P, Brittenden J. Randomized clinical trial of the antiplatelet effects of aspirinclopidogrel combination versus aspirin alone after lower limb angioplasty. Br J Surg 2005;92:159–65. [DOI] [PubMed] [Google Scholar]
- 135.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–17. [DOI] [PubMed] [Google Scholar]
- 136.Tepe G, Bantleon R, Brechtel K, Schmehl J, Zeller T, Claussen CD, et al. Management of peripheral arterial interventions with mono or dual antiplatelet therapy–the MIRROR study: a randomised and double-blinded clinical trial. Eur Radiol 2012;22:1998–2006. [DOI] [PubMed] [Google Scholar]
- 137.Strobl FF, Brechtel K, Schmehl J, Zeller T, Reiser MF, Claussen CD, et al. Twelve-month results of a randomized trial comparing mono with dual antiplatelet therapy in endovascularly treated patients with peripheral artery disease. J Endovasc Ther 2013;20:699–706. [DOI] [PubMed] [Google Scholar]
- 138.Mills JL Sr, Wixon CL, James DC, Devine J, Westerband A, Hughes JD. The natural history of intermediate and critical vein graft stenosis: recommendations for continued surveillance or repair. J Vasc Surg 2001;33:273–8; discussion: 278–80. [DOI] [PubMed] [Google Scholar]
- 139.Landry GJ, Moneta GL, Taylor LM, Edwards JM, Yeager RA, Porter JM. Long-term outcome of revised lower-extremity bypass grafts. J Vasc Surg 2002;35:56–63. [PubMed] [Google Scholar]
- 140.Nguyen LL, Conte MS, Menard MT, Gravereaux EC, Chew DK, Donaldson MC, et al. Infrainguinal vein bypass graft revision: factors affecting long-term outcome. J Vasc Surg 2004;40: 916–23. [DOI] [PubMed] [Google Scholar]
- 141.Bui TD, Mills JL Sr, Ihnat DM, Gruessner AC, Goshima KR, Hughes JD. The natural history of duplex-detected stenosis after femoropopliteal endovascular therapy suggests questionable clinical utility of routine duplex surveillance. J Vasc Surg 2012;55:346–52. [DOI] [PubMed] [Google Scholar]
- 142.Humphries MD, Pevec WC, Laird JR, Yeo KK, Hedayati N, Dawson DL. Early duplex scanning after infrainguinal endovascular therapy. J Vasc Surg 2011;53:353–8. [DOI] [PubMed] [Google Scholar]
- 143.Elraiyah T, Prutsky G, Domecq JP, Tsapas A, Nabhan M, Frykberg RG, et al. A systematic review and meta-analysis of off-loading methods for diabetic foot ulcers. J Vasc Surg 2016;63(Suppl):59S–68S. e1–2. [DOI] [PubMed] [Google Scholar]
- 144.Bell P The definition of critical ischaemia of a limb. Br J Surg 1982;69:S2. [PubMed] [Google Scholar]
- 145.Thompson MM, Sayers RD, Varty K, Reid A, London NJ, Bell PR. Chronic critical leg ischaemia must be redefined. Eur J Vasc Surg 1993;7:420–6. [DOI] [PubMed] [Google Scholar]
- 146.Hafner J, Schaad I, Schneider E, Seifert B, Burg G, Cassina PC. Leg ulcers in peripheral arterial disease (arterial leg ulcers): impaired wound healing above the threshold of chronic critical limb ischemia. J Am Acad Dermatol 2000;43:1001–8. [DOI] [PubMed] [Google Scholar]
- 147.Fontaine R, Kim M, Kieny R. [Surgical treatment of peripheral circulation disorders]. Helv Chir Acta 1954;21:499–533. [PubMed] [Google Scholar]
- 148.Meggitt B Surgical management of the diabetic foot. Br J Hosp Med 1976;16:227–32. [Google Scholar]
- 149.Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 1981;2:64–122. [DOI] [PubMed] [Google Scholar]
- 150.Second European Consensus Document on chronic critical leg ischemia. Circulation 1991;84(Suppl):IV1–26. [PubMed] [Google Scholar]
- 151.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517–38. [DOI] [PubMed] [Google Scholar]
- 152.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21:855–9. [DOI] [PubMed] [Google Scholar]
- 153.Macfarlane RM, Jeffcoate WJ. Classification of diabetic foot ulcers: the S(AD) SAD system. Diabetic Foot 1999;2:123–6. [Google Scholar]
- 154.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg 2000;31(Pt 2): S1–296. [PubMed] [Google Scholar]
- 155.Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004;20(Suppl 1):S90–5. [DOI] [PubMed] [Google Scholar]
- 156.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45(Suppl S):S5–67. [DOI] [PubMed] [Google Scholar]
- 157.Martinez-De Jesus FR. A checklist system to score healing progress of diabetic foot ulcers. Int J Low Extrem Wounds 2010;9:74–83. [DOI] [PubMed] [Google Scholar]
- 158.Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54: e132–73. [DOI] [PubMed] [Google Scholar]
- 159.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925–34. [DOI] [PubMed] [Google Scholar]
- 160.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg 2010;51(Suppl):18S–31S. [DOI] [PubMed] [Google Scholar]
- 161.Shishehbor MH, Hammad TA, Zeller T, Baumgartner I, Scheinert D, Rocha-Singh KJ. An analysis of IN.PACT DEEP randomized trial on the limitations of the societal guidelines-recommended hemodynamic parameters to diagnose critical limb ischemia. J Vasc Surg 2016;63:1311–7. [DOI] [PubMed] [Google Scholar]
- 162.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg 2009;50:1462–73. e1–3. [DOI] [PubMed] [Google Scholar]
- 163.Beropoulis E, Stavroulakis K, Schwindt A, Stachmann A, Torsello G, Bisdas T. Validation of the Wound, Ischemia, foot Infection (WIfI) classification system in nondiabetic patients treated by endovascular means for critical limb ischemia. J Vasc Surg 2016;64:95–103. [DOI] [PubMed] [Google Scholar]
- 164.Darling JD, McCallum JC, Soden PA, Guzman RJ, Wyers MC, Hamdan AD, et al. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system after first-time lower extremity revascularizations. J Vasc Surg 2017;65:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mathioudakis N, Hicks CW, Canner JK, Sherman RL, Hines KF, Lum YW, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing but not major amputation in patients with diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg 2017;65:1698–705.e1. [DOI] [PubMed] [Google Scholar]
- 166.Ward R, Dunn J, Clavijo L, Shavelle D, Rowe V, Woo K. Outcomes of critical limb ischemia in an urban, safety net hospital population with high WIfI amputation scores. Ann Vasc Surg 2017;38:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tokuda T, Hirano K, Sakamoto Y, Mori S, Kobayashi N, Araki M, et al. Use of the Wound, Ischemia, foot Infection classification system in hemodialysis patients after endovascular treatment for critical limb ischemia. J Vasc Surg 2018;67:1762–8. [DOI] [PubMed] [Google Scholar]
- 168.White JV, Rutherford RB, Ryjewski C. Chronic subcritical limb ischemia: a poorly recognized stage of critical limb ischemia. Semin Vasc Surg 2007;20:62–7. [DOI] [PubMed] [Google Scholar]
- 169.Salaun P, Desormais I, Lapébie FX, Rivière AB, Aboyans V, Lacroix P, et al. Comparison of ankle pressure, systolic toe pressure, and transcutaneous oxygen pressure to predict major amputation after 1 year in the COPART cohort. Angiology 2018. 3319718793566. [DOI] [PubMed] [Google Scholar]
- 170.Graziani L, Silvestro A, Bertone V, Manara E, Andreini R, Sigala A, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg 2007;33:453–60. [DOI] [PubMed] [Google Scholar]
- 171.Ramanan B, Ahmed A, Wu B, Causey MW, Gasper WJ, Vartanian SM, et al. Determinants of midterm functional outcomes, wound healing, and resources used in a hospital-based limb preservation program. J Vasc Surg 2017;66:1765–74. [DOI] [PubMed] [Google Scholar]
- 172.Kobayashi N, Hirano K, Yamawaki M, Araki M, Sakai T, Sakamoto Y, et al. Characteristics and clinical outcomes of repeat endovascular therapy after infrapopliteal balloon angioplasty in patients with critical limb ischemia. Catheter Cardiovasc Interv 2018;91:505–14. [DOI] [PubMed] [Google Scholar]
- 173.Leithead C, Novak Z, Spangler E, Passman MA, Witcher A, Patterson MA, et al. Importance of postprocedural Wound, Ischemia, and foot Infection (WIfI) restaging in predicting limb salvage. J Vasc Surg 2018;67:498–505. [DOI] [PubMed] [Google Scholar]
- 174.Hirsch AT, Allison MA, Gomes AS, Corriere MA, Duval S, Ershow AG, et al. A call to action: women and peripheral artery disease: a scientific statement from the American Heart Association. Circulation 2012;125:1449–72. [DOI] [PubMed] [Google Scholar]
- 175.Marrett E, DiBonaventura M, Zhang Q. Burden of peripheral arterial disease in Europe and the United States: a patient survey. Health Qual Life Outcomes 2013;11:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Howard DP, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell PM. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation 2015;132:1805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Olinic DM, Spinu M, Olinic M, Homorodean C, Tataru DA, Liew A, et al. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int Angiol 2018;37:327–34. [DOI] [PubMed] [Google Scholar]
- 178.Kojima I, Ninomiya T, Hata J, Fukuhara M, Hirakawa Y, Mukai N, et al. A low ankle brachial index is associated with an increased risk of cardiovascular disease: the Hisayama study. J Atheroscler Thromb 2014;21:966–73. [DOI] [PubMed] [Google Scholar]
- 179.Sheng CS, Li Y, Huang QF, Kang YY, Li FK, Wang JG. Pulse waves in the lower extremities as a diagnostic tool of peripheral arterial disease and predictor of mortality in elderly Chinese. Hypertension 2016;67:527–34. [DOI] [PubMed] [Google Scholar]
- 180.Desormais I, Aboyans V, Guerchet M, Ndamba-Bandzouzi B, Mbelesso P, Dantoine T, et al. Prevalence of peripheral artery disease in the elderly population in urban and rural areas of Central Africa: the EPIDEMCA study. Eur J Prev Cardiol 2014;22:1462–72. [DOI] [PubMed] [Google Scholar]
- 181.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation 1993;88:837–45. [DOI] [PubMed] [Google Scholar]
- 182.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010;56: 1506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guerchet M, Aboyans V, Mbelesso P, Mouanga AM, Salazar J, Bandzouzi B, et al. Epidemiology of peripheral artery disease in elder general population of two cities of Central Africa: Bangui and Brazzaville. Eur J Vasc Endovasc Surg 2012;44:164–9. [DOI] [PubMed] [Google Scholar]
- 184.Forbang NI, Hughes-Austin JM, Allison MA, Criqui MH. Peripheral artery disease and non-coronary atherosclerosis in Hispanics: another paradox? Prog Cardiovasc Dis 2014;57: 237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC Jr, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA). J Vasc Surg 2007;45:319–27. [DOI] [PubMed] [Google Scholar]
- 186.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–26. [DOI] [PubMed] [Google Scholar]
- 187.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, et al. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg 2014;60:686–95.e2. [DOI] [PubMed] [Google Scholar]
- 188.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71:510–5. [DOI] [PubMed] [Google Scholar]
- 189.Gallotta G, Iazzetta N, Milan G, Ruocco A, Napoli C, Postiglione A. Prevalence of peripheral arterial disease in an elderly rural population of southern Italy. Gerontology 1997;43:289–95. [DOI] [PubMed] [Google Scholar]
- 190.Sigvant B, Lundin F, Nilsson B, Bergqvist D, Wahlberg E. Differences in presentation of symptoms between women and men with intermittent claudication. BMC Cardiovasc Disord 2011;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moss SE, Klein R, Klein BE. The 14-year incidence of lower-extremity amputations in a diabetic population. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care 1999;22:951–9. [DOI] [PubMed] [Google Scholar]
- 192.Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE study. Diabetologia 2008;51:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ix JH, Biggs ML, Kizer JR, Mukamal KJ, Djousse L, Zieman SJ, et al. Association of body mass index with peripheral arterial disease in older adults: the Cardiovascular Health Study. Am J Epidemiol 2011;174:1036–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Martinez-Aguilar E, Orbe J, Fernandez-Montero A, Fernandez-Alonso S, Rodriguez JA, Fernandez-Alonso L, et al. Reduced high-density lipoprotein cholesterol: a valuable, independent prognostic marker in peripheral arterial disease. J Vasc Surg 2017;66:1527–33.e1. [DOI] [PubMed] [Google Scholar]
- 195.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001;285:2481–5. [DOI] [PubMed] [Google Scholar]
- 196.Peng J, Luo F, Ruan G, Peng R, Li X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis 2017;16:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vliegenthart R, Geleijnse JM, Hofman A, Meijer WT, van Rooij FJ, Grobbee DE, et al. Alcohol consumption and risk of peripheral arterial disease: the Rotterdam study. Am J Epidemiol 2002;155:332–8. [DOI] [PubMed] [Google Scholar]
- 198.Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet 2016;388:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khandanpour N, Loke YK, Meyer FJ, Jennings B, Armon MP. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2009;38:316–22. [DOI] [PubMed] [Google Scholar]
- 200.Prushik SG, Farber A, Gona P, Shrader P, Pencina MJ, D’Agostino RB Sr, et al. Parental intermittent claudication as risk factor for claudication in adults. Am J Cardiol 2012;109:736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wassel CL, Lamina C, Nambi V, Coassin S, Mukamal KJ, Ganesh SK, et al. Genetic determinants of the ankle-brachial index: a meta-analysis of a cardiovascular candidate gene 50K SNP panel in the candidate gene association resource (CARe) consortium. Atherosclerosis 2012;222: 138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991;20:384–92. [DOI] [PubMed] [Google Scholar]
- 203.Batty GD, Russ TC, Stamatakis E, Kivimaki M. Psychological distress and risk of peripheral vascular disease, abdominal aortic aneurysm, and heart failure: pooling of sixteen cohort studies. Atherosclerosis 2014;236:385–8. [DOI] [PubMed] [Google Scholar]
- 204.Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50:18–25. [DOI] [PubMed] [Google Scholar]
- 205.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease. Int Angiol 2007;26: 81–157. [PubMed] [Google Scholar]
- 206.Biancari F Meta-analysis of the prevalence, incidence and natural history of critical limb ischemia. J Cardiovasc Surg (Torino) 2013;54:663–9. [PubMed] [Google Scholar]
- 207.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 2008;89:422–9. [DOI] [PubMed] [Google Scholar]
- 208.Peacock JM, Keo HH, Duval S, Baumgartner I, Oldenburg NC, Jaff MR, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005–2008. Prev Chronic Dis 2011;8:A141. [PMC free article] [PubMed] [Google Scholar]
- 209.Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM, et al. Lower extremity amputations–a review of global variability in incidence. Diabet Med 2011;28:1144–53. [DOI] [PubMed] [Google Scholar]
- 210.Vamos EP, Bottle A, Edmonds ME, Valabhji J, Majeed A, Millett C. Changes in the incidence of lower extremity amputations in individuals with and without diabetes in England between 2004 and 2008. Diabetes Care 2010;33: 2592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lombardo FL, Maggini M, De Bellis A, Seghieri G, Anichini R. Lower extremity amputations in persons with and without diabetes in Italy: 2001–2010. PLoS One 2014;9: e86405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006;47:1239–312. [DOI] [PubMed] [Google Scholar]
- 213.Sigvant B, Lundin F, Wahlberg E. The risk of disease progression in peripheral arterial disease is higher than expected: a meta-analysis of mortality and disease progression in peripheral arterial disease. Eur J Vasc Endovasc Surg 2016;51:395–403. [DOI] [PubMed] [Google Scholar]
- 214.Nehler MR, Hiatt WR, Taylor LM Jr. Is revascularization and limb salvage always the best treatment for critical limb ischemia? J Vasc Surg 2003;37:704–8. [DOI] [PubMed] [Google Scholar]
- 215.Nehler MR, McDermott MM, Treat-Jacobson D, Chetter I, Regensteiner JG. Functional outcomes and quality of life in peripheral arterial disease: current status. Vasc Med 2003;8: 115–26. [DOI] [PubMed] [Google Scholar]
- 216.National Institute for Health and Care Excellence. Peripheral arterial disease: diagnosis and management. Clinical guideline CG147. Available at: https://www.nice.org.uk/guidance/cg147/chapter/Recommendations.Accessed October 30, 2018. [PubMed]
- 217.Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, et al. Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J 2015;36:932–8. [DOI] [PubMed] [Google Scholar]
- 218.Behrendt CA, Sigvant B, Szeberin Z, Beiles B, Eldrup N, Thomson IA, et al. International variations in amputation practice: a VASCUNET report. Eur J Vasc Endovasc Surg 2018;56:391–9. [DOI] [PubMed] [Google Scholar]
- 219.van Haelst ST, Koopman C, den Ruijter HM, Moll FL, Visseren FL, Vaartjes I, et al. Cardiovascular and all-cause mortality in patients with intermittent claudication and critical limb ischaemia. Br J Surg 2018;105:252–61. [DOI] [PubMed] [Google Scholar]
- 220.Baubeta Fridh E, Andersson M, Thuresson M, Sigvant B, Kragsterman B, Johansson S, et al. Amputation rates, mortality, and pre-operative comorbidities in patients revascularised for intermittent claudication or critical limb ischaemia: a population based study. Eur J Vasc Endovasc Surg 2017;54:480–6. [DOI] [PubMed] [Google Scholar]
- 221.Ortmann J, Nüesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg 2012;55:98–104. [DOI] [PubMed] [Google Scholar]
- 222.Hobbs SD, Wilmink AB, Bradbury AW. Ethnicity and peripheral arterial disease. Eur J Vasc Endovasc Surg 2003;25: 505–12. [DOI] [PubMed] [Google Scholar]
- 223.Diehm N, Shang A, Silvestro A, Do DD, Dick F, Schmidli J, et al. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg 2006;31:59–63. [DOI] [PubMed] [Google Scholar]
- 224.Armstrong EJ, Chen DC, Westin GG, Singh S, McCoach CE, Bang H, et al. Adherence to guideline-recommended therapy is associated with decreased major adverse cardiovascular events and major adverse limb events among patients with peripheral arterial disease. J Am Heart Assoc 2014;3:e000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Soga Y, Iida O, Takahaera M, Hirano K, Suzuki K, Kawasaki D, et al. Two-year life expectancy in patients with critical limb ischemia. JACC Cardiovasc Interv 2014;7:1444–9. [DOI] [PubMed] [Google Scholar]
- 226.Sampson UK, Fowkes FG, McDermott MM, Criqui MH, Aboyans V, Norman PE, et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart 2014;9:145–58.e21. [DOI] [PubMed] [Google Scholar]
- 227.Cull DL, Langan EM, Gray BH, Johnson B, Taylor SM. Open versus endovascular intervention for critical limb ischemia: a population-based study. J Am Coll Surg 2010;210:555–61. [DOI] [PubMed] [Google Scholar]
- 228.Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJ. Epidemiological study of lower limb amputation in England between 2003 and 2008. Br J Surg 2010;97:1348–53. [DOI] [PubMed] [Google Scholar]
- 229.European Society of Radiology. Summary of the proceedings of the International Summit 2015: general and subspecialty radiology. Insights Imaging 2015;7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wolfe JH, Wyatt MG. Critical and subcritical ischaemia. Eur J Vasc Endovasc Surg 1997;13:578–82. [DOI] [PubMed] [Google Scholar]
- 231.Conde ID, Erwin PA. Evaluation of the patient who presents with critical limb ischemia: diagnosis, prognosis, and medical management. Tech Vasc Interv Radiol 2014;17: 140–6. [DOI] [PubMed] [Google Scholar]
- 232.Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease-specific quality of life measure for use in lower limb ischemia. J Vasc Surg 2001;33:679–87. [DOI] [PubMed] [Google Scholar]
- 233.Nguyen LL, Moneta GL, Conte MS, Bandyk DF, Clowes AW, Seely BL. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg 2006;44: 977–83; discussion: 983–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e726–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Aboyans V, Ricco JB, Bartelink ME, Björck M, Brodmann M, Cohnert T, et al. Editor’s choice–2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55: 305–68. [DOI] [PubMed] [Google Scholar]
- 236.Criqui MH, Fronek A, Klauber MR, Barrett-Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985;71:516–22. [DOI] [PubMed] [Google Scholar]
- 237.Feigelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA. Screening for peripheral arterial disease: the sensitivity, specificity, and predictive value of noninvasive tests in a defined population. Am J Epidemiol 1994;140:526–34. [DOI] [PubMed] [Google Scholar]
- 238.McGee SR, Boyko EJ. Physical examination and chronic lower-extremity ischemia: a critical review. Arch Intern Med 1998;158:1357–64. [DOI] [PubMed] [Google Scholar]
- 239.Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA 2006;295:536–46. [DOI] [PubMed] [Google Scholar]
- 240.Cournot M, Boccalon H, Cambou JP, Guilloux J, Taraszkiewicz D, Hanaire-Broutin H, et al. Accuracy of the screening physical examination to identify subclinical atherosclerosis and peripheral arterial disease in asymptomatic subjects. J Vasc Surg 2007;46:1215–21. [DOI] [PubMed] [Google Scholar]
- 241.Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG. Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med 1998;158:289–92. [DOI] [PubMed] [Google Scholar]
- 242.Kamei N, Yamane K, Nakanishi S, Yamashita Y, Tamura T, Ohshita K, et al. Effectiveness of Semmes-Weinstein monofilament examination for diabetic peripheral neuropathy screening. J Diabetes Complications 2005;19:47–53. [DOI] [PubMed] [Google Scholar]
- 243.Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg 2009;50:675–82.e1. [DOI] [PubMed] [Google Scholar]
- 244.O’Brien T, Karem J. An initial evaluation of a proof-of-concept 128-Hz electronic tuning fork in the detection of peripheral neuropathy. J Am Podiatr Med Assoc 2014;104:134–40. [DOI] [PubMed] [Google Scholar]
- 245.Lam K, van Asten SA, Nguyen T, La Fontaine J, Lavery LA. Diagnostic accuracy of probe to bone to detect osteomyelitis in the diabetic foot: a systematic review. Clin Infect Dis 2016;63:944–8. [DOI] [PubMed] [Google Scholar]
- 246.Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K. Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diabetes Res Clin Pract 2017;124:84–92. [DOI] [PubMed] [Google Scholar]
- 247.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–654. [DOI] [PubMed] [Google Scholar]
- 248.Al-Qaisi M, Nott DM, King DH, Kaddoura S. Ankle brachial pressure index (ABPI): an update for practitioners. Vasc Health Risk Manag 2009;5:833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Gornik HL. Rethinking the morbidity of peripheral arterial disease and the "normal" ankle-brachial index. J Am Coll Cardiol 2009;53:1063–4. [DOI] [PubMed] [Google Scholar]
- 250.Silvestro A, Diehm N, Savolainen H, Do DD, Vogelea J, Mahler F, et al. Falsely high ankle-brachial index predicts major amputation in critical limb ischemia. Vasc Med 2006;11:69–74. [DOI] [PubMed] [Google Scholar]
- 251.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- 252.Tyrrell MR, Wolfe JH. Critical leg ischaemia: an appraisal of clinical definitions. Joint Vascular Research Group. Br J Surg 1993;80:177–80. [DOI] [PubMed] [Google Scholar]
- 253.Castronuovo JJ Jr, Adera HM, Smiell JM, Price RM. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg 1997;26:629–37. [DOI] [PubMed] [Google Scholar]
- 254.Abbas ZG, Lutale JK, Game FL, Jeffcoate WJ. Comparison of four systems of classification of diabetic foot ulcers in Tanzania. Diabet Med 2008;25:134–7. [DOI] [PubMed] [Google Scholar]
- 255.Parisi MC, Zantut-Wittmann DE, Pavin EJ, Machado H, Nery M, Jeffcoate WJ. Comparison of three systems of classification in predicting the outcome of diabetic foot ulcers in a Brazilian population. Eur J Endocrinol 2008;159: 417–22. [DOI] [PubMed] [Google Scholar]
- 256.Santema TB, Lenselink EA, Balm R, Ubbink DT. Comparing the Meggitt-Wagner and the University of Texas wound classification systems for diabetic foot ulcers: inter-observer analyses. Int Wound J 2016;13:1137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Swanberg J, Nyman R, Magnusson A, Wanhainen A. Selective intra-arterial dual-energy CT angiography (s-CTA) in lower extremity arterial occlusive disease. Eur J Vasc Endovasc Surg 2014;48:325–9. [DOI] [PubMed] [Google Scholar]
- 258.Moneta GL, Yeager RA, Lee RW, Porter JM. Noninvasive localization of arterial occlusive disease: a comparison of segmental Doppler pressures and arterial duplex mapping. J Vasc Surg 1993;17:578–82. [DOI] [PubMed] [Google Scholar]
- 259.Pinto F, Lencioni R, Napoli V, Petrucci R, Vignali C, Armillotta N, et al. Peripheral ischemic occlusive arterial disease: comparison of color Doppler sonography and angiography. J Ultrasound Med 1996;15:697–704. quiz: 705–6. [DOI] [PubMed] [Google Scholar]
- 260.Hou XX, Chu GH, Yu Y. Prospects of contrast-enhanced ultrasonography for the diagnosis of peripheral arterial disease: a meta-analysis. J Ultrasound Med 2018;37:1081–90. [DOI] [PubMed] [Google Scholar]
- 261.Ligush J Jr, Reavis SW, Preisser JS, Hansen KJ. Duplex ultrasound scanning defines operative strategies for patients with limb-threatening ischemia. J Vasc Surg 1998;28: 482–90; discussion: 490–1. [DOI] [PubMed] [Google Scholar]
- 262.Proia RR, Walsh DB, Nelson PR, Connors JP, Powell RJ, Zwolak RM, et al. Early results of infragenicular revascularization based solely on duplex arteriography. J Vasc Surg 2001;33:1165–70. [DOI] [PubMed] [Google Scholar]
- 263.Ota H, Takase K, Igarashi K, Chiba Y, Haga K, Saito H, et al. MDCT compared with digital subtraction angiography for assessment of lower extremity arterial occlusive disease: importance of reviewing cross-sectional images. AJR Am J Roentgenol 2004;182:201–9. [DOI] [PubMed] [Google Scholar]
- 264.Romano M, Mainenti PP, Imbriaco M, Amato B, Markabaoui K, Tamburrini O, et al. Multidetector row CT angiography of the abdominal aorta and lower extremities in patients with peripheral arterial occlusive disease: diagnostic accuracy and interobserver agreement. Eur J Radiol 2004;50:303–8. [DOI] [PubMed] [Google Scholar]
- 265.Edwards AJ, Wells IP, Roobottom CA. Multidetector row CT angiography of the lower limb arteries: a prospective comparison of volume-rendered techniques and intraarterial digital subtraction angiography. Clin Radiol 2005;60:85–95. [DOI] [PubMed] [Google Scholar]
- 266.Heijenbrok-Kal MH, Kock MC, Hunink MG. Lower extremity arterial disease: multidetector CT angiography meta-analysis. Radiology 2007;245:433–9. [DOI] [PubMed] [Google Scholar]
- 267.Thomsen HS, Morcos SK. ESUR guidelines on contrast media. Abdom Imaging 2006;31:131–40. [DOI] [PubMed] [Google Scholar]
- 268.Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169:2078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Solomon R The role of osmolality in the incidence of contrast-induced nephropathy: a systematic review of angiographic contrast media in high risk patients. Kidney Int 2005;68:2256–63. [DOI] [PubMed] [Google Scholar]
- 270.ACR Committee on Drugs and Contrast Media. ACR manualon contrast media, version 10.3. Available at: https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf.Accessed October 30, 2018.
- 271.van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, et al. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients: recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 2018;28:2856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Spinazzi A, Pozzi Mucelli R. [Administration of iodinated contrast in patients with pre-existing renal failure: a review]. Radiol Med 2004;107:88–97. [PubMed] [Google Scholar]
- 273.Carpenter JP, Baum RA, Holland GA, Barker CF. Peripheral vascular surgery with magnetic resonance angiography as the sole preoperative imaging modality. J Vasc Surg 1994;20:861–71. [DOI] [PubMed] [Google Scholar]
- 274.Mell M, Tefera G, Thornton F, Siepman D, Turnipseed W. Clinical utility of time-resolved imaging of contrast kinetics (TRICKS) magnetic resonance angiography for infrageniculate arterial occlusive disease. J Vasc Surg 2007;45:543–8; discussion: 548. [DOI] [PubMed] [Google Scholar]
- 275.Kreitner KF, Kunz RP, Herber S, Martenstein S, Dorweiler B, Dueber C. MR angiography of the pedal arteries with gadobenate dimeglumine, a contrast agent with increased relaxivity, and comparison with selective intraarterial DSA. J Magn Reson Imaging 2008;27:78–85. [DOI] [PubMed] [Google Scholar]
- 276.Menke J, Larsen J. Meta-analysis: accuracy of contrast-enhanced magnetic resonance angiography for assessing steno-occlusions in peripheral arterial disease. Ann Intern Med 2010;153:325–34. [DOI] [PubMed] [Google Scholar]
- 277.Altaha MA, Jaskolka JD, Tan K, Rick M, Schmitt P, Menezes RJ, et al. Non-contrast-enhanced MR angiography in critical limb ischemia: performance of quiescent-interval single-shot (QISS) and TSE-based subtraction techniques. Eur Radiol 2017;27:1218–26. [DOI] [PubMed] [Google Scholar]
- 278.Kribben A, Witzke O, Hillen U, Barkhausen J, Daul AE, Erbel R. Nephrogenic systemic fibrosis: pathogenesis, diagnosis, and therapy. J Am Coll Cardiol 2009;53:1621–8. [DOI] [PubMed] [Google Scholar]
- 279.Turowski B, Schramm P. An appeal to standardize CT- and MR-perfusion. Clin Neuroradiol 2015;25:205–10. [DOI] [PubMed] [Google Scholar]
- 280.Grözinger G, Pohmann R, Schick F, Grosse U, Syha R, Brechtel K, et al. Perfusion measurements of the calf in patients with peripheral arterial occlusive disease before and after percutaneous transluminal angioplasty using MR arterial spin labeling. J Magn Reson Imaging 2014;40: 980–7. [DOI] [PubMed] [Google Scholar]
- 281.Hingorani A, Ascher E, Markevich N, Kallakuri S, Hou A, Schutzer R, et al. Magnetic resonance angiography versus duplex arteriography in patients undergoing lower extremity revascularization: which is the best replacement for contrast arteriography? J Vasc Surg 2004;39:717–22. [DOI] [PubMed] [Google Scholar]
- 282.Lapeyre M, Kobeiter H, Desgranges P, Rahmouni A, Becquemin JP, Luciani A. Assessment of critical limb ischemia in patients with diabetes: comparison of MR angiography and digital subtraction angiography. AJR Am J Roentgenol 2005;185:1641–50. [DOI] [PubMed] [Google Scholar]
- 283.Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology 1981;138:273–81. [DOI] [PubMed] [Google Scholar]
- 284.Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med 2003;348:491–9. [DOI] [PubMed] [Google Scholar]
- 285.Baker CS, Wragg A, Kumar S, De Palma R, Baker LR, Knight CJ. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol 2003;41:2114–8. [DOI] [PubMed] [Google Scholar]
- 286.Waugh JR, Sacharias N. Arteriographic complications in the DSA era. Radiology 1992;182:243–6. [DOI] [PubMed] [Google Scholar]
- 287.Palena LM, Diaz-Sandoval LJ, Candeo A, Brigato C, Sultato E, Manzi M. Automated carbon dioxide angiography for the evaluation and endovascular treatment of diabetic patients with critical limb ischemia. J Endovasc Ther 2016;23:40–8. [DOI] [PubMed] [Google Scholar]
- 288.Jens S, Marquering HA, Koelemay MJ, Reekers JA. Perfusion angiography of the foot in patients with critical limb ischemia: description of the technique. Cardiovasc Intervent Radiol 2015;38:201–5. [DOI] [PubMed] [Google Scholar]
- 289.Vierthaler L, Callas PW, Goodney PP, Schanzer A, Patel VI, Cronenwett J, et al. Determinants of survival and major amputation after peripheral endovascular intervention for critical limb ischemia. J Vasc Surg 2015;62:655–64.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Marston WA, Davies SW, Armstrong B, Farber MA, Mendes RC, Fulton JJ, et al. Natural history of limbs with arterial insufficiency and chronic ulceration treated without revascularization. J Vasc Surg 2006;44:108–14. [DOI] [PubMed] [Google Scholar]
- 291.Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med 2007;261:276–84. [DOI] [PubMed] [Google Scholar]
- 292.Bonaca MP, Bhatt DL, Storey RF, Steg PG, Cohen M, Kuder J, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol 2016;67:2719–28. [DOI] [PubMed] [Google Scholar]
- 293.Bonaca MP, Creager MA, Olin J, Scirica BM, Gilchrist IC, Murphy SA, et al. Peripheral revascularization in patients with peripheral artery disease with vorapaxar: insights from the TRA 2°P-TIMI 50 Trial. JACC Cardiovasc Interv 2016;9: 2157–64. [DOI] [PubMed] [Google Scholar]
- 294.Sharma A, Helft G, Garg A, Agrawal S, Chatterjee S, Lavie CJ, et al. Safety and efficacy of vorapaxar in secondary prevention of atherosclerotic disease: a meta-analysis of randomized control trials. Int J Cardiol 2017;227:617–24. [DOI] [PubMed] [Google Scholar]
- 295.Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366: 1404–13. [DOI] [PubMed] [Google Scholar]
- 296.Bonaca MP, Scirica BM, Braunwald E, Wiviott SD, Goto S, Nilsen DW, et al. New ischemic stroke and outcomes with vorapaxar versus placebo: results from the TRA 2°P-TIMI 50 Trial. J Am Coll Cardiol 2014;64:2318–26. [DOI] [PubMed] [Google Scholar]
- 297.Katsanos K, Spiliopoulos S, Saha P, Diamantopoulos A, Karunanithy N, Krokidis M, et al. Comparative efficacy and safety of different antiplatelet agents for prevention of major cardiovascular events and leg amputations in patients with peripheral arterial disease: a systematic review and network meta-analysis. PLoS One 2015;10:e0135692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bonaca MP, Gutierrez JA, Creager MA, Scirica BM, Olin J, Murphy SA, et al. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the Trial to Assess The effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients with Atherosclerosis-Thrombolysis in Myocardial Infarction 50 (TRA2°P-TIMI 50). Circulation 2016;133:997–1005. [DOI] [PubMed] [Google Scholar]
- 299.Whayne TF. A review of the role of anticoagulation in the treatment of peripheral arterial disease. Int J Angiol 2012;21: 187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Fanari Z, Malodiya A, Weiss SA, Hammami S, Kolm P, Weintraub WS. Long-term use of dual antiplatelet therapy for the secondary prevention of atherothrombotic events: meta-analysis of randomized controlled trials. Cardiovasc Revasc Med 2017;18:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Anand SS, Caron F, Eikelboom JW, Bosch J, Dyal L, Aboyans V, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol 2018;71:2306–15. [DOI] [PubMed] [Google Scholar]
- 302.Capell WH, Bonaca MP, Nehler MR, Chen E, Kittelson JM, Anand SS, et al. Rationale and design for the Vascular Outcomes study of ASA along with rivaroxaban in endovascular or surgical limb revascularization for peripheral artery disease (VOYAGER PAD). Am Heart J 2018;199:83–91. [DOI] [PubMed] [Google Scholar]
- 303.Ross R Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 304.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.McDermott MM, Guralnik JM, Corsi A, Albay M, Macchi C, Bandinelli S, et al. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am Heart J 2005;150:276–81. [DOI] [PubMed] [Google Scholar]
- 307.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 308.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Am J Cardiol 2010;106: 204–9. [DOI] [PubMed] [Google Scholar]
- 309.Rogers SL, Magliano DJ, Levison DB, Webb K, Clarke PJ, Grobler MP, et al. A dose-specific meta-analysis of lipid changes in randomized controlled trials of atorvastatin and simvastatin. Clin Ther 2007;29:242–52. [DOI] [PubMed] [Google Scholar]
- 310.Foley TR, Singh GD, Kokkinidis DG, Choy HK, Pham T, Amsterdam EA, et al. High-intensity statin therapy is associated with improved survival in patients with peripheral artery disease. J Am Heart Assoc 2017;6:e005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Arya S, Khakharia A, Binney ZO, DeMartino RR, Brewster LP, Goodney PP, et al. Association of statin dose with amputation and survival in patients with peripheral artery disease. Circulation 2018;137:1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(Pt B):2889–934. [DOI] [PubMed] [Google Scholar]
- 313.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018November8. [Epub ahead of print]. [Google Scholar]
- 314.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation 2018;137:338–50. [DOI] [PubMed] [Google Scholar]
- 315.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016;315:2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288: 2981–97. [DOI] [PubMed] [Google Scholar]
- 317.Piller LB, Simpson LM, Baraniuk S, Habib GB, Rahman M, Basile JN, et al. Characteristics and long-term follow-up of participants with peripheral arterial disease during ALLHAT. J Gen Intern Med 2014;29:1475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Kray JE, Dombrovskiy VY, Vogel TR. Use of angiotensin-converting enzyme inhibitors and freedom from amputation after lower extremity revascularization. Vasc Health Risk Manag 2017;13:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Høgh A, Lindholt JS, Nielsen H, Jensen LP, Johnsen SP. Use of angiotensin-converting enzyme inhibitors and cardio-vascular outcomes following primary vascular surgery: a nationwide propensity score matched follow-up study. Vasc Endovascular Surg 2012;46:515–23. [DOI] [PubMed] [Google Scholar]
- 320.Hogh A, Lindholt JS, Nielsen H, Jensen LP, Johnsen SP. Beta-blocker use and clinical outcomes after primary vascular surgery: a nationwide propensity score-matched study. Eur J Vasc Endovasc Surg 2013;46:93–102. [DOI] [PubMed] [Google Scholar]
- 321.Attaei MW, Khatib R, McKee M, Lear S, Dagenais G, Igumbor EU, et al. Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet Public Health 2017;2:e411–9. [DOI] [PubMed] [Google Scholar]
- 322.Paravastu SC, Mendonca DA, Da Silva A. Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev 2013;9:CD005508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Thomas Manapurathe D, Krishna SM, Dewdney B, Moxon JV, Biros E, Golledge J. Effect of blood pressure lowering medications on leg ischemia in peripheral artery disease patients: a meta-analysis of randomised controlled trials. PLoS One 2017;12:e0178713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Britton KA, Mukamal KJ, Ix JH, Siscovick DS, Newman AB, de Boer IH, et al. Insulin resistance and incident peripheral artery disease in the Cardiovascular Health Study. Vasc Med 2012;17:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA 2012;308:1660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G,Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017August17;377(7):644–57. doi: 10.1056/NEJMoa1611925.Epub 2017 Jun 12. [DOI] [PubMed] [Google Scholar]
- 327.Li D, Yang JY, Wang T, Shen S, Tang H. Risks of diabetic footsyndrome and amputation associated with sodium glucose co-transporter 2 inhibitors: A Meta-analysis of Randomized Controlled Trials. Diabetes Metab 2018November;44(5):410–4. doi: 10.1016/j.diabet.2018.02.001.Epub 2018 Feb 13. [DOI] [PubMed] [Google Scholar]
- 328. https://www.fda.gov/Drugs/DrugSafety/ucm557507.htm.
- 329.Potier L, Roussel R, Velho G, Saulnier PJ, Bumbu A, Matar O,Schneider F, Ragot S, Marre M, Mohammedi K, Hadjadj S. Lower limb events in individuals with type 2 diabetes: evidence for an increased risk associated with diuretic use. Diabetologia 2019February26. doi: 10.1007/s00125-019-4835-z. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 330.Home P Cardiovascular outcome trials of glucose-lowering medications: an update. Diabetologia 2019;62:357–69. [DOI] [PubMed] [Google Scholar]
- 331.Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, et al. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation 2017;135:1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sandfort V, Bluemke DA, Vargas J, Brinker JA, Gerstenblith G, Kickler T, et al. Coronary plaque progression and regression in asymptomatic African American chronic cocaine users with obstructive coronary stenoses: a preliminary study. J Addict Med 2017;11:126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Orr KK, Asal NJ. Efficacy of electronic cigarettes for smoking cessation. Ann Pharmacother 2014;48:1502–6. [DOI] [PubMed] [Google Scholar]
- 334.Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050–62. [DOI] [PubMed] [Google Scholar]
- 335.Kok FJ, Kromhout D. Atherosclerosis–epidemiological studies on the health effects of a Mediterranean diet. Eur J Nutr 2004;43(Suppl 1):I/2–5. [DOI] [PubMed] [Google Scholar]
- 336.Bhupathiraju SN, Tucker KL. Coronary heart disease prevention: nutrients, foods, and dietary patterns. Clin Chim Acta 2011;412:1493–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Nosova EV, Conte MS, Grenon SM. Advancing beyond the "heart-healthy diet" for peripheral arterial disease. J Vasc Surg 2015;61:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Vincent-Baudry S, Defoort C, Gerber M, Bernard MC, Verger P, Helal O, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr 2005;82:964–71. [DOI] [PubMed] [Google Scholar]
- 339.Miller V, Yusuf S, Chow CK, Dehghan M, Corsi DJ, Lock K, et al. Availability, affordability, and consumption of fruits and vegetables in 18 countries across income levels: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet Glob Health 2016;4:e695–703. [DOI] [PubMed] [Google Scholar]
- 340.Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev 2014;7: CD000990. [DOI] [PubMed] [Google Scholar]
- 341.Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016;1: CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Shaffer EE, Pham A, Woldman RL, Spiegelman A, Strassels SA, Wan GJ, et al. Estimating the effect of intravenous acetaminophen for postoperative pain management on length of stay and inpatient hospital costs. Adv Ther 2016;33:2211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Helander EM, Menard BL, Harmon CM, Homra BK, Allain AV, Bordelon GJ, et al. Multimodal analgesia, current concepts, and acute pain considerations. Curr Pain Headache Rep 2017;21:3. [DOI] [PubMed] [Google Scholar]
- 344.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–27. [PubMed] [Google Scholar]
- 345.Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517–92. [DOI] [PubMed] [Google Scholar]
- 346.Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv 2014;83:E212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Patel MR, Conte MS, Cutlip DE, Dib N, Geraghty P, Gray W, et al. Evaluation and treatment of patients with lower extremity peripheral artery disease: consensus definitions from Peripheral Academic Research Consortium (PARC). J Am Coll Cardiol 2015;65:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Davies MG, Feeley TM, O’Malley MK, Colgan MP, Moore DJ, Shanik GD. Infrainguinal polytetrafluoroethylene grafts: saved limbs or wasted effort? A report on ten years’ experience. Ann Vasc Surg 1991;5:519–24. [DOI] [PubMed] [Google Scholar]
- 349.Donaldson MC, Mannick JA, Whittemore AD. Femoral-distal bypass with in situ greater saphenous vein. Long-term results using the Mills valvulotome. Ann Surg 1991;213:457–64; discussion: 464–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.O’Riordain DS, Buckley DJ, O’Donnell JA. Polytetrafluoroethylene in above-knee arterial bypass surgery for critical ischemia. Am J Surg 1992;164:129–31. [DOI] [PubMed] [Google Scholar]
- 351.Smith FC, Thomson IA, Hickey NC, Paterson IS, Tsang GM, Simms MH, et al. Adjuvant prostanoid treatment during femorodistal reconstruction. Ann Vasc Surg 1993;7:88–94. [DOI] [PubMed] [Google Scholar]
- 352.Sayers RD, Thompson MM, Dunlop P, London NJ, Bell PR. The fate of infrainguinal PTFE grafts and an analysis of factors affecting outcome. Eur J Vasc Surg 1994;8:607–10. [DOI] [PubMed] [Google Scholar]
- 353.Hearn AT, Smith JM, Welling RE, Lohr JM, Thibodeaux LC. Analysis of autogenous vein femoral-infrapopliteal bypass for limb salvage in the elderly. Cardiovasc Surg 1996;4: 105–9. [DOI] [PubMed] [Google Scholar]
- 354.Panayiotopoulos YP, Reidy JF, Taylor PR. The concept of knee salvage: why does a failed femorocrural/pedal arterial bypass not affect the amputation level? Eur J Vasc Endovasc Surg 1997;13:477–85. [DOI] [PubMed] [Google Scholar]
- 355.Wijesinghe LD, Beardsmore DM, Scott DJ. Polytetrafluoroethylene (PTFE) femorodistal grafts with a distal vein cuff for critical ischaemia. Eur J Vasc Endovasc Surg 1998;15: 449–53. [DOI] [PubMed] [Google Scholar]
- 356.Hamsho A, Nott D, Harris PL. Prospective randomised trial of distal arteriovenous fistula as an adjunct to femoroinfrapopliteal PTFE bypass. Eur J Vasc Endovasc Surg 1999;17:197–201. [DOI] [PubMed] [Google Scholar]
- 357.Johnson WC, Lee KK. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. J Vasc Surg 2000;32:268–77. [DOI] [PubMed] [Google Scholar]
- 358.Ruckert RI, Settmacher U, Kruger U, Scholz H. Femorodistal PTFE bypass grafting for severe limb ischaemia: results of a prospective clinical study using a new distal anastomotic technique. Eur J Vasc Endovasc Surg 2000;20:51–6. [DOI] [PubMed] [Google Scholar]
- 359.Soder HK, Manninen HI, Jaakkola P, Matsi PJ, Rasanen HT, Kaukanen E, et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol 2000;11:1021–31. [DOI] [PubMed] [Google Scholar]
- 360.Dorros G, Jaff MR, Dorros AM, Mathiak LM, He T. Tibioperoneal (outflow lesion) angioplasty can be used as primary treatment in 235 patients with critical limb ischemia: five-year follow-up. Circulation 2001;104:2057–62. [DOI] [PubMed] [Google Scholar]
- 361.Schneider PA, Caps MT, Ogawa DY, Hayman ES. Intraoperative superficial femoral artery balloon angioplasty and popliteal to distal bypass graft: an option for combined open and endovascular treatment of diabetic gangrene. J Vasc Surg 2001;33:955–62. [DOI] [PubMed] [Google Scholar]
- 362.Klinkert P, Van Dijk PJE, Breslau PJ. Polytetrafluoroethylene femorotibial bypass grafting: 5-year patency and limb salvage. Ann Vasc Surg 2003;17:486–91. [DOI] [PubMed] [Google Scholar]
- 363.Hynes N, Akhtar Y, Manning B, Aremu M, Oiakhinan K, Courtney D, et al. Subintimal angioplasty as a primary modality in the management of critical limb ischemia: comparison to bypass grafting for aortoiliac and femoropopliteal occlusive disease. J Endovasc Ther 2004;11:460–71. [DOI] [PubMed] [Google Scholar]
- 364.Pedersen G, Laxdal E, Hagala M, Amundsen SR, Dregelid E, Aune S. The impact of patient characteristics on long-term results of above-knee prosthetic femoropopliteal bypass for critical ischemia. Int Angiol 2005;24:349–54. [PubMed] [Google Scholar]
- 365.Surowiec SM, Davies MG, Eberly SW, Rhodes JM, Illig KA, Shortell CK, et al. Percutaneous angioplasty and stenting of the superficial femoral artery. J Vasc Surg 2005;41:269–78. [DOI] [PubMed] [Google Scholar]
- 366.Ascher E, Marks NA, Hingorani AP, Schutzer RW, Mutyala M. Duplex-guided endovascular treatment for occlusive and stenotic lesions of the femoral-popliteal arterial segment: a comparative study in the first 253 cases. J Vasc Surg 2006;44:1230–7. [DOI] [PubMed] [Google Scholar]
- 367.Bosiers M, Hart JP, Deloose K, Verbist J, Peeters P. Endovascular therapy as the primary approach for limb salvage in patients with critical limb ischemia: experience with 443 infrapopliteal procedures. Vascular 2006;14:63–9. [DOI] [PubMed] [Google Scholar]
- 368.Peeters P, Verbist J, Deloose K, Bosiers M. Results with heparin bonded polytetrafluoroethylene grafts for femorodistal bypasses. J Cardiovasc Surg (Torino) 2006;47:407–13. [PubMed] [Google Scholar]
- 369.DeRubertis BG, Pierce M, Chaer RA, Rhee SJ, Benjeloun R, Ryer EJ, et al. Lesion severity and treatment complexity are associated with outcome after percutaneous infra-inguinal intervention. J Vasc Surg 2007;46:709–16. [DOI] [PubMed] [Google Scholar]
- 370.Jensen LP, Lepantalo M, Fossdal JE, Roder OC, Jensen BS, Madsen MS, et al. Dacron or PTFE for above-knee femoropopliteal bypass. a multicenter randomised study. Eur J Vasc Endovasc Surg 2007;34:44–9. [DOI] [PubMed] [Google Scholar]
- 371.Bosiers M, Kallakuri S, Deloose K, Verbist J, Peeters P. Infragenicular angioplasty and stenting in the management of critical limb ischaemia: one year outcome following the use of the MULTI-LINK VISION stent. EuroIntervention 2008;3:470–4. [DOI] [PubMed] [Google Scholar]
- 372.Dosluoglu HH, Cherr GS, Lall P, Harris LM, Dryjski ML. Stenting vs above knee polytetrafluoroethylene bypass for TransAtlantic Inter-Society Consensus-II C and D superficial femoral artery disease. J Vasc Surg 2008;48:1166–74. [DOI] [PubMed] [Google Scholar]
- 373.Giles KA, Pomposelli FB, Hamdan AD, Blattman SB, Panossian H, Schermerhorn ML. Infrapopliteal angioplasty for critical limb ischemia: relation of TransAtlantic Inter-Society Consensus class to outcome in 176 limbs. J Vasc Surg 2008;48:128–36. [DOI] [PubMed] [Google Scholar]
- 374.Peeters P, Verbist J, Deloose K, Bosiers M. Will heparin-bonded PTFE replace autologous venous conduits in infrapopliteal bypass? Ital J Vasc Endovasc Surg 2008;15:143–8. [Google Scholar]
- 375.Peregrin JH, Smirova S, Koznar B, Novotny J, Kovac J, Lastovickova J, et al. Self-expandable stent placement in infrapopliteal arteries after unsuccessful angioplasty failure: one-year follow-up. Cardiovasc Intervent Radiol 2008;31: 860–4. [DOI] [PubMed] [Google Scholar]
- 376.Ah Chong AK, Tan CB, Wong MW, Cheng FS. Bypass surgery or percutaneous transluminal angioplasty to treat critical lower limb ischaemia due to infrainguinal arterial occlusive disease? Hong Kong Med J 2009;15:249–54. [PubMed] [Google Scholar]
- 377.Deloose K, Bosiers M, Peeters P. One year outcome after primary stenting of infrapopliteal lesions with the Chromis Deep stent in the management of critical limb ischaemia. EuroIntervention 2009;5:318–24. [DOI] [PubMed] [Google Scholar]
- 378.Randon C, Vermassen F, Jacobs B, Deryck F. Long-term results of femorodistal reconstructions with cryopreserved saphenous vein allografts. Interact Cardiovasc Thorac Surg 2009;8(Suppl 1):S27. [Google Scholar]
- 379.Setacci C, Chisci E, de Donato G, Setacci F, Iacoponi F, Galzerano G. Subintimal angioplasty with the aid of a re-entry device for TASC C and D lesions of the SFA. Eur J Vasc Endovasc Surg 2009;38:76–87. [DOI] [PubMed] [Google Scholar]
- 380.Siablis D, Karnabatidis D, Katsanos K, Diamantopoulos A, Spiliopoulos S, Kagadis GC, et al. Infrapopliteal application of sirolimus-eluting versus bare metal stents for critical limb ischemia: analysis of long-term angiographic and clinical outcome. J Vasc Interv Radiol 2009;20:1141–50. [DOI] [PubMed] [Google Scholar]
- 381.Rastan A, Schwarzwalder U, Noory E, Taieb FH, Beschorner U, Sixt S, et al. Primary use of sirolimus-eluting stents in the infrapopliteal arteries. J Endovasc Ther 2010;17:480–7. [DOI] [PubMed] [Google Scholar]
- 382.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010;51(Suppl):5S–17S. [DOI] [PubMed] [Google Scholar]
- 383.Feiring AJ, Krahn M, Nelson L, Wesolowski A, Eastwood D, Szabo A. Preventing leg amputations in critical limb ischemia with below-the-knee drug-eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am Coll Cardiol 2010;55:1580–9. [DOI] [PubMed] [Google Scholar]
- 384.Fransson T, Thorne J. In situ saphenous vein bypass grafting—still first line treatment? A prospective study comparing surgical results between diabetic and nondiabetic populations. Vasa 2010;39:59–65. [DOI] [PubMed] [Google Scholar]
- 385.Schmidt A, Ulrich M, Winkler B, Klaeffling C, Bausback Y, Bräunlich S, et al. Angiographic patency and clinical outcome after balloon-angioplasty for extensive infrapopliteal arterial disease. Catheter Cardiovasc Interv 2010;76: 1047–54. [DOI] [PubMed] [Google Scholar]
- 386.Lindholt JS, Gottschalksen B, Johannesen N, Dueholm D, Ravn H, Christensen ED, et al. The Scandinavian Propaten Trial—1-year patency of PTFE vascular prostheses with heparin-bonded luminal surfaces compared to ordinary pure PTFE vascular prostheses—a randomised clinical controlled multi-centre trial. Eur J Vasc Endovasc Surg 2011;41:668–73. [DOI] [PubMed] [Google Scholar]
- 387.Rastan A, Tepe G, Krankenberg H, Zahorsky R, Beschorner U, Noory E, et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J 2011;32:2274–81. [DOI] [PubMed] [Google Scholar]
- 388.Bosiers M, Scheinert D, Peeters P, Torsello G, Zeller T, Deloose K, et al. Randomized comparison of everolimuseluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg 2012;55:390–8. [DOI] [PubMed] [Google Scholar]
- 389.Shammas NW, Lam R, Mustapha J, Ellichman J, Aggarwala G, Rivera E, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther 2012;19:480–8. [DOI] [PubMed] [Google Scholar]
- 390.Baumann F, Bloesch S, Engelberger RP, Makaloski V, Fink H, Do DD, et al. Clinically-driven need for secondary interventions after endovascular revascularization of tibial arteries in patients with critical limb ischemia. J Endovasc Ther 2013;20:707–13. [DOI] [PubMed] [Google Scholar]
- 391.Baumann F, Bloesch S, Engelberger RP, Makaloski V, Fink HP, Do DD, et al. Clinically driven need for secondary interventions after endovascular revascularization of tibial arteries in patients with critical limb ischemia. Vasa 2013;42(Suppl 84):57. [DOI] [PubMed] [Google Scholar]
- 392.Liistro F, Porto I, Angioli P, Grotti S, Ricci L, Ducci K, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation 2013;128:615–21. [DOI] [PubMed] [Google Scholar]
- 393.Lo RC, Darling J, Bensley RP, Giles KA, Dahlberg SE, Hamdan AD, et al. Outcomes following infrapopliteal angioplasty for critical limb ischemia. J Vasc Surg 2013;57: 1455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.McKinsey JF, Zeller T, Rocha-Singh KJ, Jaff MR, Garcia LA; DEFINITIVE LE Investigators. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study. JACC Cardiovasc Interv 2014;7:923–33. [DOI] [PubMed] [Google Scholar]
- 395.Singh GD, Armstrong EJ, Yeo KK, Singh S, Westin GG, Pevec WC, et al. Endovascular recanalization of infrapopliteal occlusions in patients with critical limb ischemia. J Vasc Surg 2014;59:1300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zeller T, Baumgartner I, Scheinert D, Brodmann M, Bosiers M, Micari A, et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol 2014;64:1568–76. [DOI] [PubMed] [Google Scholar]
- 397.Bosiers M, Keirse K, Hendriks J, Peeters P, Maene L, Bouchez D, et al. The PES-BTK-70 study: final results of the assessment of the first self-expanding nitinol paclitaxel eluting stent in below-the-knee lesions. EuroIntervention 2015. 20150519(20150522). [Google Scholar]
- 398.Lee JH, Oh J, Ko YG, Rha SW, Kim KC, Park SH, et al. Impact of nitinol self-expanding stent on the patency of subintimal angioplasty for below-the-knee occlusions with critical limb ischemia (NEXSIA-BTK): multicenter, prospective registry. J Am Coll Cardiol 2015;66(Suppl 1): B323–4. [Google Scholar]
- 399.Rastan A, McKinsey JF, Garcia LA, Rocha-Singh KJ, Jaff MR, Noory E, et al. One-year outcomes following directional atherectomy of infrapopliteal artery lesions: subgroup results of the prospective, multicenter DEFINITIVE LE trial. J Endovasc Ther 2015;22:839–46. [DOI] [PubMed] [Google Scholar]
- 400.Yiu WK, Conte MS. Primary stenting in femoropopliteal occlusive disease–what is the appropriate role? Circ J 2015;79:704–11. [DOI] [PubMed] [Google Scholar]
- 401.Yiu WK, Conte MS. The roles of drug-eluting technology and atherectomy in infrapopliteal occlusive disease. Ital J Vasc Endovasc Surg 2015;22:1–2. [Google Scholar]
- 402.Mustapha JA, Finton SM, Diaz-Sandoval LJ, Saab FA, Miller LE. Percutaneous transluminal angioplasty in patients with infrapopliteal arterial disease: systematic review and meta-analysis. Circ Cardiovasc Interv 2016;9: e003468. [DOI] [PubMed] [Google Scholar]
- 403.Spreen MI, Martens JM, Hansen BE, Knippenberg B, Verhey E, van Dijk LC, et al. Percutaneous Transluminal Angioplasty and Drug-Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia (PADI) trial. Circ Cardiovasc Interv 2016;9:e002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Spreen MI, Martens JM, Knippenberg B, van Dijk LC, de Vries JP, Vos JA, et al. Long-term follow-up of the PADI trial: percutaneous transluminal angioplasty versus drug-eluting stents for infrapopliteal lesions in critical limb ischemia. J Am Heart Assoc 2017;6:e004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Guzman RJ, Brinkley DM, Schumacher PM, Donahue RM, Beavers H, Qin X. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol 2008;51:1967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Kang IS, Lee W, Choi BW, Choi D, Hong MK, Jang Y, et al. Semiquantitative assessment of tibial artery calcification by computed tomography angiography and its ability to predict infrapopliteal angioplasty outcomes. J Vasc Surg 2016;64:1335–43. [DOI] [PubMed] [Google Scholar]
- 407.Kawarada O, Fujihara M, Higashimori A, Yokoi Y, Honda Y, Fitzgerald PJ. Predictors of adverse clinical outcomes after successful infrapopliteal intervention. Catheter Cardiovasc Interv 2012;80:861–71. [DOI] [PubMed] [Google Scholar]
- 408.Haine A, Haynes AG, Limacher A, Sebastian T, Saengprakai W, Fuss T, et al. Patency of the arterial pedalplantar arch in patients with chronic kidney disease or diabetes mellitus. Ther Adv Cardiovasc Dis 2018;12:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Taylor SM, Kalbaugh CA, Blackhurst DW, Cass AL, Trent EA, Langan EM 3rd, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg 2006;44:747–55; discussion: 755–6. [DOI] [PubMed] [Google Scholar]
- 410.Arvela E, Söderström M, Korhonen M, Halmesmäki K, Albäck A, Lepäntalo M, et al. Finnvasc score and modified Prevent III score predict long-term outcome after infrainguinal surgical and endovascular revascularization for critical limb ischemia. J Vasc Surg 2010;52:1218–25. [DOI] [PubMed] [Google Scholar]
- 411.Moxey PW, Brownrigg J, Kumar SS, Crate G, Holt PJ, Thompson MM, et al. The BASIL survival prediction model in patients with peripheral arterial disease undergoing revascularization in a university hospital setting and comparison with the FINNVASC and modified PREVENT scores. J Vasc Surg 2013;57:1–7. [DOI] [PubMed] [Google Scholar]
- 412.Simons JP, Schanzer A, Flahive JM, Osborne NH, Mills JL Sr, Bradbury AW, et al. Survival prediction in patients with chronic limb-threatening ischemia who undergo infrainguinal revascularization. J Vasc Surg 2018November26. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 413.Kraiss LW, Beckstrom JL, Brooke BS. Frailty assessment in vascular surgery and its utility in preoperative decision making. Semin Vasc Surg 2015;28:141–7. [DOI] [PubMed] [Google Scholar]
- 414.Kraiss LW, Al-Dulaimi R, Presson AP, Arya S, Lee GK, Goodney PP, et al. A Vascular Quality Initiative-based frailty instrument predicts 9-month postoperative mortality. J Vasc Surg 2016;64:551–2. [Google Scholar]
- 415.Kodama A, Sugimoto M, Kuma S, Okazaki J, Mii S, Komori K. Clinical outcomes after infrainguinal bypass grafting for critical limb ischaemia in patients with dialysis-dependent end-stage renal failure. Eur J Vasc Endovasc Surg 2014;48: 695–702. [DOI] [PubMed] [Google Scholar]
- 416.Fallon JM, Goodney PP, Stone DH, Patel VI, Nolan BW, Kalish JA, et al. Outcomes of lower extremity revascularization among the hemodialysis-dependent. J Vasc Surg 2015;62:1183–91.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;64:e77–137. [DOI] [PubMed] [Google Scholar]
- 418.Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management. The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014;35:2383–431. [DOI] [PubMed] [Google Scholar]
- 419.Pomposelli FB Jr, Jepsen SJ, Gibbons GW, Campbell DR, Freeman DV, Miller A, et al. Efficacy of the dorsal pedal bypass for limb salvage in diabetic patients: short-term observations. J Vasc Surg 1990;11:745–51; discussion: 751–2. [DOI] [PubMed] [Google Scholar]
- 420.Eiberg JP, Hansen MA, Jorgensen LG, Rasmussen JB, Jensen F, Schroeder TV. In-situ bypass surgery on arteriographically invisible vessels detected by Doppler-ultrasound for limb salvage. J Cardiovasc Surg (Torino) 2004;45:375–9. [PubMed] [Google Scholar]
- 421.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010;51:5S–17S. [DOI] [PubMed] [Google Scholar]
- 422.Korhonen M, Biancari F, Söderström M, Arvela E, Halmesmäki K, Albäck A, et al. Femoropopliteal balloon angioplasty vs. bypass surgery for CLI: a propensity score analysis. Eur J Vasc Endovasc Surg 2011;41:378–84. [DOI] [PubMed] [Google Scholar]
- 423.Jones WS, Dolor RJ, Hasselblad V, Vemulapalli S, Subherwal S, Schmit K, et al. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: systematic review of revascularization in critical limb ischemia. Am Heart J 2014;167:489–98.e7. [DOI] [PubMed] [Google Scholar]
- 424.Meltzer AJ, Sedrakyan A, Isaacs A, Connolly PH, Schneider DB. Comparative effectiveness of peripheral vascular intervention versus surgical bypass for critical limb ischemia in the Vascular Study Group of Greater New York. J Vasc Surg 2016;64:1320–6.e2. [DOI] [PubMed] [Google Scholar]
- 425.Siracuse JJ, Menard MT, Eslami MH, Kalish JA, Robinson WP, Eberhardt RT, et al. Comparison of open and endovascular treatment of patients with critical limb ischemia in the Vascular Quality Initiative. J Vasc Surg 2016;63:958–65.e1. [DOI] [PubMed] [Google Scholar]
- 426.Pomposelli FB, Kansal N, Hamdan AD, Belfield A, Sheahan M, Campbell DR, et al. A decade of experience with dorsalis pedis artery bypass: analysis of outcome in more than 1000 cases. J Vasc Surg 2003;37:307–15. [DOI] [PubMed] [Google Scholar]
- 427.Dick F, Diehm N, Galimanis A, Husmann M, Schmidli J, Baumgartner I. Surgical or endovascular revascularization in patients with critical limb ischemia: influence of diabetes mellitus on clinical outcome. J Vasc Surg 2007;45: 751–61. [DOI] [PubMed] [Google Scholar]
- 428.Fernandez N, McEnaney R, Marone LK, Rhee RY, Leers S, Makaroun M, et al. Multilevel versus isolated endovascular tibial interventions for critical limb ischemia. J Vasc Surg 2011;54:722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Antoniou GA, Chalmers N, Georgiadis GS, Lazarides MK, Antoniou SA, Serracino-Inglott F, et al. A meta-analysis of endovascular versus surgical reconstruction of femoropopliteal arterial disease. J Vasc Surg 2013;57:242–53. [DOI] [PubMed] [Google Scholar]
- 430.Baumann F, Engelberger RP, Willenberg T, Do DD, Kalka C, Baumgartner I, et al. Infrapopliteal lesion morphology in patients with critical limb ischemia: implications for the development of anti-restenosis technologies. J Endovasc Ther 2013;20:149–56. [DOI] [PubMed] [Google Scholar]
- 431.Iida O, Nakamura M, Yamauchi Y, Kawasaki D, Yokoi Y, Yokoi H, et al. Endovascular treatment for infrainguinal vessels in patients with critical limb ischemia: OLIVE registry, a prospective, multicenter study in Japan with 12-month follow-up. Circ Cardiovasc Interv 2013;6:68–76. [DOI] [PubMed] [Google Scholar]
- 432.Saqib NU, Domenick N, Cho JS, Marone L, Leers S, Makaroun MS, et al. Predictors and outcomes of restenosis following tibial artery endovascular interventions for critical limb ischemia. J Vasc Surg 2013;57:692–9. [DOI] [PubMed] [Google Scholar]
- 433.Ballotta E, Toniato A, Piatto G, Mazzalai F, Da Giau G. Lower extremity arterial reconstruction for critical limb ischemia in diabetes. J Vasc Surg 2014;59:708–19. [DOI] [PubMed] [Google Scholar]
- 434.Iida O, Nakamura M, Yamauchi Y, Fukunaga M, Yokoi Y, Yokoi H, et al. 3-Year outcomes of the OLIVE registry, a prospective multicenter study of patients with critical limb ischemia: a prospective, multi-center, three-year follow-up study on endovascular treatment for infra-inguinal vessel in patients with critical limb ischemia. JACC Cardiovasc Interv 2015;8:1493–502. [DOI] [PubMed] [Google Scholar]
- 435.Brothers TE, Zhang J, Mauldin PD, Tonnessen BH, Robison JG, Vallabhaneni R, et al. Predicting outcomes for infrapopliteal limb-threatening ischemia using the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg 2016;63:114–24.e5. [DOI] [PubMed] [Google Scholar]
- 436.Okamoto S, Iida O, Takahara M, Yamauchi Y, Hirano K, Soga Y, et al. Impact of perioperative complications after endovascular therapy in diabetic patients with critical limb ischemia due to isolated infrapopliteal lesions. J Endovasc Ther 2016;23:371–7. [DOI] [PubMed] [Google Scholar]
- 437.Iida O, Takahara M, Soga Y, Kodama A, Terashi H, Azuma N. Three-year outcomes of surgical versus endovascular revascularization for critical limb ischemia: the SPINACH study (Surgical Reconstruction Versus Peripheral Intervention in Patients With Critical Limb Ischemia). Circ Cardiovasc Interv 2017;10:e005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Nakama T, Watanabe N, Haraguchi T, Sakamoto H, Kamoi D, Tsubakimoto Y, et al. Clinical outcomes of pedal artery angioplasty for patients with ischemic wounds: results from the multicenter RENDEZVOUS registry. JACC Cardiovasc Interv 2017;10:79–90. [DOI] [PubMed] [Google Scholar]
- 439.Vogel TR, Dombrovskiy VY, Carson JL, Graham AM. In-hospital and 30-day outcomes after tibioperoneal interventions in the US Medicare population with critical limb ischemia. J Vasc Surg 2011;54:109–15. [DOI] [PubMed] [Google Scholar]
- 440.Nolan BW, De Martino RR, Stone DH, Schanzer A, Goodney PP, Walsh DW, et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg 2011;54:730–5; discussion: 735–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Jones DW, Schanzer A, Zhao Y, MacKenzie TA, Nolan BW, Conte MS, et al. Growing impact of restenosis on the surgical treatment of peripheral arterial disease. J Am Heart Assoc 2013;2:e000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Faries PL, LoGerfo FW, Arora S, Hook S, Pulling MC, Akbari CM, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg 2000;32:1080–90. [DOI] [PubMed] [Google Scholar]
- 443.Brochado Neto F, Sandri GA, Kalaf MJ, Matielo MF, Casella IB, Godoy MR, et al. Arm vein as an alternative autogenous conduit for infragenicular bypass in the treatment of critical limb ischaemia: a 15 year experience. Eur J Vasc Endovasc Surg 2014;47:609–14. [DOI] [PubMed] [Google Scholar]
- 444.Armstrong PA, Bandyk DF, Wilson JS, Shames ML, Johnson BL, Back MR. Optimizing infrainguinal arm vein bypass patency with duplex ultrasound surveillance and endovascular therapy. J Vasc Surg 2004;40:724–30; discussion: 730–1. [DOI] [PubMed] [Google Scholar]
- 445.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987;40:113–41. [DOI] [PubMed] [Google Scholar]
- 446.Attinger CE, Evans KK, Bulan E, Blume P, Cooper P. Angiosomes of the foot and ankle and clinical implications for limb salvage: reconstruction, incisions, and revascularization. Plast Reconstr Surg 2006;117(Suppl):261S–93S. [DOI] [PubMed] [Google Scholar]
- 447.Aerden D, Denecker N, Gallala S, Debing E, Van den Brande P. Wound morphology and topography in the diabetic foot: hurdles in implementing angiosome-guided revascularization. Int J Vasc Med 2014;2014:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Berceli SA, Chan AK, Pomposelli FB Jr, Gibbons GW, Campbell DR, Akbari CM, et al. Efficacy of dorsal pedal artery bypass in limb salvage for ischemic heel ulcers. J Vasc Surg 1999;30:499–508. [DOI] [PubMed] [Google Scholar]
- 449.Rashid H, Slim H, Zayed H, Huang DY, Wilkins CJ, Evans DR, et al. The impact of arterial pedal arch quality and angiosome revascularization on foot tissue loss healing and infrapopliteal bypass outcome. J Vasc Surg 2013;57:1219–26. [DOI] [PubMed] [Google Scholar]
- 450.Fusaro M, Cassese S, Ndrepepa G, Tepe G, King L, Ott I, et al. Drug-eluting stents for revascularization of infrapopliteal arteries: updated meta-analysis of randomized trials. JACC Cardiovasc Interv 2013;6:1284–93. [DOI] [PubMed] [Google Scholar]
- 451.Geraghty PJ, Mewissen MW, Jaff MR, Ansel GM; VIBRANT Investigators. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg 2013;58:386–95.e4. [DOI] [PubMed] [Google Scholar]
- 452.Todd KE, Ahanchi SS, Maurer CA, Kim JH, Chipman CR, Panneton JM. Atherectomy offers no benefits over balloon angioplasty in tibial interventions for critical limb ischemia. J Vasc Surg 2013;58:941–8. [DOI] [PubMed] [Google Scholar]
- 453.Ambler GK, Radwan R, Hayes PD, Twine CP. Atherectomy for peripheral arterial disease. Cochrane Database Syst Rev 2014;3:CD006680. [DOI] [PubMed] [Google Scholar]
- 454.Antoniou GA, Georgakarakos EI, Antoniou SA, Georgiadis GS. Does endovascular treatment of infrainguinal arterial disease with drug-eluting stents offer better results than angioplasty with or without bare metal stents? Interact Cardiovasc Thorac Surg 2014;19:282–5. [DOI] [PubMed] [Google Scholar]
- 455.Fanelli F, Cannavale A. Endovascular treatment of infrapopliteal arteries: angioplasty vs stent in the drug-eluting era. Eur Radiol 2014;24:793–8. [DOI] [PubMed] [Google Scholar]
- 456.Liu X, Zheng G, Wen S. Drug-eluting stents versus control therapy in the infrapopliteal disease: a meta-analysis of eight randomized controlled trials and two cohort studies. Int J Surg 2017;44:166–75. [DOI] [PubMed] [Google Scholar]
- 457.Gandini R, Del Giudice C, Simonetti G. Pedal and plantar loop angioplasty: technique and results. J Cardiovasc Surg (Torino) 2014;55:665–70. [PubMed] [Google Scholar]
- 458.Dorigo W, Pulli R, Castelli P, Dorrucci V, Ferilli F, De Blasis G, et al. A multicenter comparison between autologous saphenous vein and heparin-bonded expanded polytetrafluoroethylene (ePTFE) graft in the treatment of critical limb ischemia in diabetics. J Vasc Surg 2011;54:1332–8. [DOI] [PubMed] [Google Scholar]
- 459.Uhl C, Grosch C, Hock C, Topel I, Steinbauer M. Comparison of long-term outcomes of heparin bonded polytetrafluoroethylene and autologous vein below knee femoropopliteal bypasses in patients with critical limb ischaemia. Eur J Vasc Endovasc Surg 2017;54:203–11. [DOI] [PubMed] [Google Scholar]
- 460.Neville RF, Capone A, Amdur R, Lidsky M, Babrowicz J, Sidawy AN. A comparison of tibial artery bypass performed with heparin-bonded expanded polytetrafluoroethylene and great saphenous vein to treat critical limb ischemia. J Vasc Surg 2012;56:1008–14. [DOI] [PubMed] [Google Scholar]
- 461.Cook AW, Oygar A, Baggenstos P, Pacheco S, Kleriga E. Vascular disease of extremities. Electric stimulation of spinal cord and posterior roots. N Y State J Med 1976;76: 366–8. [PubMed] [Google Scholar]
- 462.Naoum JJ, Arbid EJ. Spinal cord stimulation for chronic limb ischemia. Methodist Debakey Cardiovasc J 2013;9: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Suy R, Gybels J, Van Damme H, Martin D, van Maele R, Delaporte C, editors. Spinal cord stimulation for ischemic rest pain. The Belgian randomized study. Darmstadt: Steinkopff; 1994. [Google Scholar]
- 464.Jivegard LE, Augustinsson LE, Holm J, Risberg B, Ortenwall P. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischaemia: a prospective randomised controlled study. Eur J Vasc Endovasc Surg 1995;9:421–5. [DOI] [PubMed] [Google Scholar]
- 465.Claeys LG, Horsch S. Transcutaneous oxygen pressure as predictive parameter for ulcer healing in endstage vascular patients treated with spinal cord stimulation. Int Angiol 1996;15:344–9. [PubMed] [Google Scholar]
- 466.Ubbink DT, Spincemaille GH, Prins MH, Reneman RS, Jacobs MJ; Dutch Spinal Cord Stimulation Study Group. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the Dutch multicentre randomized controlled trial. J Vasc Surg 1999;30:236–44. [DOI] [PubMed] [Google Scholar]
- 467.Spincemaille GH, Klomp HM, Steyerberg EW, Habbema JD. Spinal cord stimulation in patients with critical limb ischernia: a preliminary evaluation of a multicentre trial. Acta Chir Austriaca 2000;32:49–51. [Google Scholar]
- 468.Amann W, Berg P, Gersbach P, Gamain J, Raphael JH, Ubbink DT. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur J Vasc Endovasc Surg 2003;26:280–6. [DOI] [PubMed] [Google Scholar]
- 469.Klomp HM, Steyerberg EW, van Urk H, Habbema JD; ESES Study Group. Spinal cord stimulation is not cost-effective for non-surgical management of critical limb ischaemia. Eur J Vasc Endovasc Surg 2006;31:500–8. [DOI] [PubMed] [Google Scholar]
- 470.Sanni A, Hamid A, Dunning J. Is sympathectomy of benefit in critical leg ischaemia not amenable to revascularisation? Interact Cardiovasc Thorac Surg 2005;4:478–83. [DOI] [PubMed] [Google Scholar]
- 471.Barnes RW, Baker WH, Shanik G, Maixner W, Hayes AC, Lin R, et al. Value of concomitant sympathectomy in aortoiliac reconstruction. Results of a prospective, randomized study. Arch Surg 1977;112:1325–30. [DOI] [PubMed] [Google Scholar]
- 472.Cross FW, Cotton LT. Chemical lumbar sympathectomy for ischemic rest pain. A randomized, prospective controlled clinical trial. Am J Surg 1985;150:341–5. [DOI] [PubMed] [Google Scholar]
- 473.Fyfe T, Quin RO. Phenol sympathectomy in the treatment of intermittent claudication: a controlled clinical trail. Br J Surg 1975;62:68–71. [DOI] [PubMed] [Google Scholar]
- 474.Kim GE, Ibrahim IM, Imparato AM. Lumbar sympathectomy in end stage arterial occlusive disease. Ann Surg 1976;183: 157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Collins GJ Jr, Rich NM, Clagett GP, Salander JM, Spebar MJ. Clinical results of lumbar sympathectomy. Am Surg 1981;47: 31–5. [PubMed] [Google Scholar]
- 476.Norman PE, House AK. The early use of operative lumbar sympathectomy in peripheral vascular disease. J Cardiovasc Surg (Torino) 1988;29:717–22. [PubMed] [Google Scholar]
- 477.Baker DM, Lamerton AJ. Operative lumbar sympathectomy for severe lower limb ischaemia: still a valuable treatment option. Ann R Coll Surg Engl 1994;76:50–3. [PMC free article] [PubMed] [Google Scholar]
- 478.Alexander JP. Chemical lumbar sympathectomy in patients with severe lower limb ischaemia. Ulster Med J 1994;63:137–43. [PMC free article] [PubMed] [Google Scholar]
- 479.Mashiah A, Soroker D, Pasik S, Mashiah T. Phenol lumbar sympathetic block in diabetic lower limb ischemia. J Cardiovasc Risk 1995;2:467–9. [DOI] [PubMed] [Google Scholar]
- 480.Perez-Burkhardt JL, Gonzalez-Fajardo JA, Martin JF, Carpintero Mediavilla LA, Mateo Gutierrez AM. Lumbar sympathectomy as isolated technique for the treatment of lower limbs chronic ischemia. J Cardiovasc Surg (Torino) 1999;40:7–13. [PubMed] [Google Scholar]
- 481.Holiday FA, Barendregt WB, Slappendel R, Crul BJ, Buskens FG, van der Vliet JA. Lumbar sympathectomy in critical limb ischaemia: surgical, chemical or not at all? Cardiovasc Surg 1999;7:200–2. [DOI] [PubMed] [Google Scholar]
- 482.Matarazzo A, Rosati Tarulli V, Sassi O, Florio A, Tatafiore M, Molino C. Possibilities at present for the application of lumbar sympathectomy in chronic occlusive arterial disease of the lower limbs. Minerva Cardioangiol 2002;50:363–9. [PubMed] [Google Scholar]
- 483.Pieri S, Agresti P, Ialongo P, Fedeli S, Di Cesare F, Ricci G. Lumbar sympathectomy under CT guidance: therapeutic option in critical limb ischaemia. Radiol Med 2005;109: 430–7. [PubMed] [Google Scholar]
- 484.Moran PS, Teljeur C, Harrington P, Ryan M. A systematic review of intermittent pneumatic compression for critical limb ischaemia. Vasc Med 2015;20:41–50. [DOI] [PubMed] [Google Scholar]
- 485.Labropoulos N, Wierks C, Suffoletto B. Intermittent pneumatic compression for the treatment of lower extremity arterial disease: a systematic review. Vasc Med 2002;7:141–8. [DOI] [PubMed] [Google Scholar]
- 486.Kavros SJ, Delis KT, Turner NS, Voll AE, Liedl DA, Gloviczki P, et al. Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18-month follow-up. J Vasc Surg 2008;47:543–9. [DOI] [PubMed] [Google Scholar]
- 487.Tawfick WA, Hamada N, Soylu E, Fahy A, Hynes N, Sultan S. Sequential compression biomechanical device versus primary amputation in patients with critical limb ischemia. Vasc Endovascular Surg 2013;47:532–9. [DOI] [PubMed] [Google Scholar]
- 488.Dillon RS. Treatment of resistant venous stasis ulcers and dermatitis with the end-diastolic pneumatic compression boot. Angiology 1986;37:47–56. [DOI] [PubMed] [Google Scholar]
- 489.Dillon RS. Successful treatment of osteomyelitis and soft tissue infections in ischemic diabetic legs by local antibiotic injections and the end-diastolic pneumatic compression boot. Ann Surg 1986;204:643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Steinberg J Cardiosynchronous limb compression: effects on noninvasive vascular tests and clinical course of the ischemic limb. Angiology 1992;43:453–61. [DOI] [PubMed] [Google Scholar]
- 491.van Bemmelen PS, Gitlitz DB, Faruqi RM, Weiss-Olmanni J, Brunetti VA, Giron F, et al. Limb salvage using high-pressure intermittent compression arterial assist device in cases unsuitable for surgical revascularization. Arch Surg 2001;136: 1280–5. [DOI] [PubMed] [Google Scholar]
- 492.Louridas G, Saadia R, Spelay J, Abdoh A, Weighell W, Arneja AS, et al. The ArtAssist Device in chronic lower limb ischemia. A pilot study. Int Angiol 2002;21:28–35. [PubMed] [Google Scholar]
- 493.Montori VM, Kavros SJ, Walsh EE, Rooke TW. Intermittent compression pump for nonhealing wounds in patients with limb ischemia. The Mayo Clinic experience (1998–2000). Int Angiol 2002;21:360–6. [PubMed] [Google Scholar]
- 494.Sultan S, Hamada N, Soylu E, Fahy A, Hynes N, Tawfick W. Sequential compression biomechanical device in patients with critical limb ischemia and nonreconstructible peripheral vascular disease. J Vasc Surg 2011;54:440–6; discussion: 446–7. [DOI] [PubMed] [Google Scholar]
- 495.Chang ST, Hsu JT, Chu CM, Pan KL, Jang SJ, Lin PC, et al. Using intermittent pneumatic compression therapy to improve quality of life for symptomatic patients with infrapopliteal diffuse peripheral obstructive disease. Circ J 2012;76:971–6. [DOI] [PubMed] [Google Scholar]
- 496.Setacci C, de Donato G, Teraa M, Moll FL, Ricco JB, Becker F, et al. Chapter IV: treatment of critical limb ischaemia. Eur J Vasc Endovasc Surg 2011;42(Suppl 2):S43–59. [DOI] [PubMed] [Google Scholar]
- 497.Ruffolo AJ, Romano M, Ciapponi A. Prostanoids for critical limb ischaemia. Cochrane Database Syst Rev 2010;1: CD006544. [DOI] [PubMed] [Google Scholar]
- 498.Lambert MA, Belch JJ. Medical management of critical limb ischaemia: where do we stand today? J Intern Med 2013;274:295–307. [DOI] [PubMed] [Google Scholar]
- 499.Creutzig A, Lehmacher W, Elze M. Meta-analysis of randomised controlled prostaglandin E1 studies in peripheral arterial occlusive disease stages III and IV. Vasa 2004;33: 137–44. [DOI] [PubMed] [Google Scholar]
- 500.Loosemore TM, Chalmers TC, Dormandy JA. A meta-analysis of randomized placebo control trials in Fontaine stages III and IV peripheral occlusive arterial disease. Int Angiol 1994;13:133–42. [PubMed] [Google Scholar]
- 501.Belch JJ, Ray S, Rajput-Ray M, Engeset J, Fagrell B, Lepantalo M, et al. The Scottish-Finnish-Swedish PARTNER study of taprostene versus placebo treatment in patients with critical limb ischemia. Int Angiol 2011;30:150–5. [PubMed] [Google Scholar]
- 502.Intravenous pentoxifylline for the treatment of chronic critical limb ischaemia. The European Study Group. Eur J Vasc Endovasc Surg 1995;9:426–36. [DOI] [PubMed] [Google Scholar]
- 503.Efficacy and clinical tolerance of parenteral pentoxifylline in the treatment of critical lower limb ischemia. A placebo controlled multicenter study. Norwegian Pentoxifylline Multicenter Trial Group. Int Angiol 1996;15:75–80. [PubMed] [Google Scholar]
- 504.Miyashita Y, Saito S, Miyamoto A, Iida O, Nanto S. Cilostazol increases skin perfusion pressure in severely ischemic limbs. Angiology 2011;62:15–7. [DOI] [PubMed] [Google Scholar]
- 505.Soga Y, Iida O, Hirano K, Suzuki K, Kawasaki D, Miyashita Y, et al. Impact of cilostazol after endovascular treatment for infrainguinal disease in patients with critical limb ischemia. J Vasc Surg 2011;54:1659–67. [DOI] [PubMed] [Google Scholar]
- 506.Tonnesen KH, Sager P, Gormsen J. Treatment of severe foot ischaemia by defibrination with ancrod: a randomized blind study. Scand J Clin Lab Invest 1978;38:431–5. [DOI] [PubMed] [Google Scholar]
- 507.Lowe GD, Dunlop DJ, Lawson DH, Pollock JG, Watt JK, Forbes CD, et al. Double-blind controlled clinical trial of ancrod for ischemic rest pain of the leg. Angiology 1982;33: 46–50. [DOI] [PubMed] [Google Scholar]
- 508.Stoekenbroek RM, Santema TB, Legemate DA, Ubbink DT, van den Brink A, Koelemay MJ. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J Vasc Endovasc Surg 2014;47:647–55. [DOI] [PubMed] [Google Scholar]
- 509.Murad MH, Altayar O, Bennett M, Wei JC, Claus PL, Asi N, et al. Using GRADE for evaluating the quality of evidence in hyperbaric oxygen therapy clarifies evidence limitations. J Clin Epidemiol 2014;67:65–72. [DOI] [PubMed] [Google Scholar]
- 510.Faglia E, Favales F, Aldeghi A, Calia P, Quarantiello A, Oriani G, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer: a randomized study. Diabetes Care 1996;19: 1338–43. [DOI] [PubMed] [Google Scholar]
- 511.Abidia A, Laden G, Kuhan G, Johnson BF, Wilkinson AR, Renwick PM, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg 2003;25:513–8. [DOI] [PubMed] [Google Scholar]
- 512.Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care 2010;33: 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Löndahl M, Katzman P, Hammarlund C, Nilsson A, Landin-Olsson M. Relationship between ulcer healing after hyperbaric oxygen therapy and transcutaneous oximetry, toe blood pressure and ankle-brachial index in patients with diabetes and chronic foot ulcers. Diabetologia 2011;54:65–8. [DOI] [PubMed] [Google Scholar]
- 514.Margolis DJ, Gupta J, Hoffstad O, Papdopoulos M, Glick HA, Thom SR, et al. Lack of effectiveness of hyperbaric oxygen therapy for the treatment of diabetic foot ulcer and the prevention of amputation: a cohort study. Diabetes Care 2013;36:1961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:1425–43. [DOI] [PubMed] [Google Scholar]
- 516.Lepantalo M, Matzke S. Outcome of unreconstructed chronic critical leg ischaemia. Eur J Vasc Endovasc Surg 1996;11:153–7. [DOI] [PubMed] [Google Scholar]
- 517.Slovut DP, Sullivan TM. Critical limb ischemia: medical and surgical management. Vasc Med 2008;13:281–91. [DOI] [PubMed] [Google Scholar]
- 518.Elgzyri T, Larsson J, Thorne J, Eriksson KF, Apelqvist J. Outcome of ischemic foot ulcer in diabetic patients who had no invasive vascular intervention. Eur J Vasc Endovasc Surg 2013;46:110–7. [DOI] [PubMed] [Google Scholar]
- 519.Nesargikar PN, Ajit MK, Eyers PS, Nichols BJ, Chester JF. Lumbar chemical sympathectomy in peripheral vascular disease: does it still have a role? Int J Surg 2009;7:145–9. [DOI] [PubMed] [Google Scholar]
- 520.Martini R, Andreozzi GM, Deri A, Cordova R, Zulian P, Scarpazza O, et al. Amputation rate and mortality in elderly patients with critical limb ischemia not suitable for revascularization. Aging Clin Exp Res 2012;24(Suppl):24–7. [PubMed] [Google Scholar]
- 521.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 1996;348:370–4. [DOI] [PubMed] [Google Scholar]
- 522.Nikol S, Baumgartner I, Van Belle E, Diehm C, Visona A, Capogrossi MC, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther 2008;16:972–8. [DOI] [PubMed] [Google Scholar]
- 523.Baumgartner I, Chronos N, Comerota A, Henry T, Pasquet JP, Finiels F, et al. Local gene transfer and expression following intramuscular administration of FGF-1 plasmid DNA in patients with critical limb ischemia. Mol Ther 2009;17:914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 2011;377:1929–37. [DOI] [PubMed] [Google Scholar]
- 525.Powell RJ, Goodney P, Mendelsohn FO, Moen EK, Annex BH. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J Vasc Surg 2010;52:1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation 2008;118: 58–65. [DOI] [PubMed] [Google Scholar]
- 527.Shigematsu H, Yasuda K, Iwai T, Sasajima T, Ishimaru S, Ohashi Y, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Ther 2010;17:1152–61. [DOI] [PubMed] [Google Scholar]
- 528.Iafrati MD, Hallett JW, Geils G, Pearl G, Lumsden A, Peden E, et al. Early results and lessons learned from a multicenter, randomized, double-blind trial of bone marrow aspirate concentrate in critical limb ischemia. J Vasc Surg 2011;54: 1650–8. [DOI] [PubMed] [Google Scholar]
- 529.Murphy MP, Lawson JH, Rapp BM, Dalsing MC, Klein J, Wilson MG, et al. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg 2011;53: 1565–74.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Iafrati MD, O’Donnell TF Jr, Perler B, Illig KA, Hallett J, Woo K, et al. SS03. Bone marrow aspirate concentrate in critical limb ischemia: results of an abridged prospective randomized pivotal trial in no option CLI. J Vasc Surg 2016;63:47S. [Google Scholar]
- 531.Murphy M, Ross C, Kibbe M, Kelso R, Sharafuddin M, Tzeng E, et al. Administration of autologous bone marrow cells for limb salvage in patients with critical limb ischemia: results of the multicenter phase III MOBILE trial. New Orleans, La: American Heart Association Scientific Sessions; November 12–16, 2016. [Google Scholar]
- 532.Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, et al. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther 2012;20:1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Losordo DW, Kibbe MR, Mendelsohn F, Marston W, Driver VR, Sharafuddin M, et al. A randomized, controlled pilot study of autologous CD34þ cell therapy for critical limb ischemia. Circ Cardiovasc Interv 2012;5:821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Pignon B, Sevestre MA, Kanagaratnam L, Pernod G, Stephan D, Emmerich J, et al. Autologous bone marrow mononuclear cell implantation and its impact on the outcome of patients with critical limb ischemia—results of a randomized, double-blind, placebo-controlled trial. Circ J 2017;81:1713–20. [DOI] [PubMed] [Google Scholar]
- 535.Teraa M, Sprengers RW, Schutgens RE, Slaper-Cortenbach IC, van der Graaf Y, Algra A, et al. Effect of repetitive intra-arterial infusion of bone marrow mononuclear cells in patients with no-option limb ischemia: the randomized, double-blind, placebo-controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-arterial Supplementation (JUVENTAS) trial. Circulation 2015;131:851–60. [DOI] [PubMed] [Google Scholar]
- 536.Thom SR, Hampton M, Troiano MA, Mirza Z, Malay DS, Shannon S, et al. Measurements of CD34+/CD45-dim stem cells predict healing of diabetic neuropathic wounds. Diabetes 2016;65:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Sheahan MG, Hamdan AD, Veraldi JR, McArthur CS, Skillman JJ, Campbell DR, et al. Lower extremity minor amputations: the roles of diabetes mellitus and timing of revascularization. J Vasc Surg 2005;42:476–80. [DOI] [PubMed] [Google Scholar]
- 538.Kadukammakal J, Yau S, Urbas W. Assessment of partial first-ray resections and their tendency to progress to transmetatarsal amputations. J Am Podiatr Med Assoc 2012;102:412–6. [DOI] [PubMed] [Google Scholar]
- 539.Borkosky SL, Roukis TS. Incidence of repeat amputation after partial first ray amputation associated with diabetes mellitus and peripheral neuropathy: an 11-year review. J Foot Ankle Surg 2013;52:335–8. [DOI] [PubMed] [Google Scholar]
- 540.Thorud JC, Jupiter DC, Lorenzana J, Nguyen TT, Shibuya N. Reoperation and reamputation after transmetatarsal amputation: a systematic review and meta-analysis. J Foot Ankle Surg 2016;55:1007–12. [DOI] [PubMed] [Google Scholar]
- 541.Lavery LA, Lavery DC, Quebedeax-Farnham TL. Increased foot pressures after great toe amputation in diabetes. Diabetes Care 1995;18:1460–2. [DOI] [PubMed] [Google Scholar]
- 542.Ballotta E, Da Giau G, Gruppo M, Mazzalai F, Martella B, Militello C, et al. Revascularization to an isolated (blind) popliteal artery segment: a viable procedure for critical limb ischemia. Surgery 2009;145:426–34. [DOI] [PubMed] [Google Scholar]
- 543.Mohapatra A, Henry JC, Avgerinos ED, Chaer RA, Leers SA, Boitet A, et al. Heel wounds predict mortality but not amputation after infrapopliteal revascularization. Ann Vasc Surg 2018;51:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Oliver NG, Steinberg JS, Powers K, Evans KK, Kim PJ, Attinger CE. Lower extremity function following partial calcanectomy in high-risk limb salvage patients. J Diabetes Res 2015;2015:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Kodama A, Koyama A, Sugimoto M, Niimi K, Banno H, Komori K. Association between preoperative frailty and mortality in patients with critical limb ischemia following infrainguinal bypass surgery—usefulness of the Barthel index. Circ J 2017;82:267–74. [DOI] [PubMed] [Google Scholar]
- 546.Chopra A, Azarbal AF, Jung E, Abraham CZ, Liem TK, Landry GJ, et al. Ambulation and functional outcome after major lower extremity amputation. J Vasc Surg 2018;67: 1521–9. [DOI] [PubMed] [Google Scholar]
- 547.Arsenault KA, Al-Otaibi A, Devereaux PJ, Thorlund K, Tittley JG, Whitlock RP. The use of transcutaneous oximetry to predict healing complications of lower limb amputations: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2012;43:329–36. [DOI] [PubMed] [Google Scholar]
- 548.Dillingham TR, Pezzin LE, Shore AD. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil 2005;86:480–6. [DOI] [PubMed] [Google Scholar]
- 549.Kono Y, Muder RR. Identifying the incidence of and risk factors for reamputation among patients who underwent foot amputation. Ann Vasc Surg 2012;26:1120–6. [DOI] [PubMed] [Google Scholar]
- 550.Beaulieu RJ, Grimm JC, Lyu H, Abularrage CJ, Perler BA. Rates and predictors of readmission after minor lower extremity amputations. J Vasc Surg 2015;62:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Landry GJ, Silverman DA, Liem TK, Mitchell EL, Moneta GL. Predictors of healing and functional outcome following transmetatarsal amputations. Arch Surg 2011;146:1005–9. [DOI] [PubMed] [Google Scholar]
- 552.Quigley M, Dillon MP. Quality of life in persons with partial foot or transtibial amputation: a systematic review. Prosthet Orthot Int 2016;40:18–30. [DOI] [PubMed] [Google Scholar]
- 553.O’Brien PJ, Cox MW, Shortell CK, Scarborough JE. Risk factors for early failure of surgical amputations: an analysis of 8, 878 isolated lower extremity amputation procedures. J Am Coll Surg 2013;216:836–42. [DOI] [PubMed] [Google Scholar]
- 554.Waton S, Johal A, Heikkila K, Cromwell D, Loftus I. National Vascular Registry: 2015 annual report. London: The Royal College of Surgeons of England; November2015. [Google Scholar]
- 555.van Netten JJ, Fortington LV, Hinchliffe RJ, Hijmans JM. Early post-operative mortality after major lower limb amputation: a systematic review of population and regional based studies. Eur J Vasc Endovasc Surg 2016;51: 248–57. [DOI] [PubMed] [Google Scholar]
- 556.Uzzaman MM, Jukaku S, Kambal A, Hussain ST. Assessing the long-term outcomes of minor lower limb amputations: a 5-year study. Angiology 2011;62:365–71. [DOI] [PubMed] [Google Scholar]
- 557.Wilbek TE, Jansen RB, Jørgensen B, Svendsen OL. The diabetic foot in a multidisciplinary team setting. Number of amputations below ankle level and mortality. Exp Clin Endocrinol Diabetes 2016;124:535–40. [DOI] [PubMed] [Google Scholar]
- 558.Subramaniam B, Pomposelli F, Talmor D, Park KW. Perioperative and long-term morbidity and mortality after above-knee and below-knee amputations in diabetics and nondiabetics. Anesth Analg 2005;100:1241–7. [DOI] [PubMed] [Google Scholar]
- 559.Fortington LV, Geertzen JH, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg 2013;46:124–31. [DOI] [PubMed] [Google Scholar]
- 560.Inderbitzi R, Buettiker M, Enzler M. The long-term mobility and mortality of patients with peripheral arterial disease following bilateral amputation. Eur J Vasc Endovasc Surg 2003;26:59–64. [DOI] [PubMed] [Google Scholar]
- 561.Hoffmann M, Kujath P, Flemming A, Proß M, Begum N, Zimmermann M, et al. Survival of diabetes patients with major amputation is comparable to malignant disease. Diab Vasc Dis Res 2015;12:265–71. [DOI] [PubMed] [Google Scholar]
- 562.The Vascular Society of Great Britain and Ireland qualityimprovement framework for major amputation surgery. A best practice clinical care pathway for major amputation surgery. Available at: https://www.vascularsociety.org.uk/_userfiles/pages/files/Resources/Vasc_Soc_Amputation_Paper_V2.pdf.Accessed March 2017.
- 563.Karam J, Shepard A, Rubinfeld I. Predictors of operative mortality following major lower extremity amputations using the National Surgical Quality Improvement Program public use data. J Vasc Surg 2013;58:1276–82. [DOI] [PubMed] [Google Scholar]
- 564.Fisher SV, Gullickson G Jr. Energy cost of ambulation in health and disability: a literature review. Arch Phys Med Rehabil 1978;59:124–33. [PubMed] [Google Scholar]
- 565.Ali MM, Loretz L, Shea A, Poorvu E, Robinson WP, Schanzer A, et al. A contemporary comparative analysis of immediate postoperative prosthesis placement following below-knee amputation. Ann Vasc Surg 2013;27:1146–53. [DOI] [PubMed] [Google Scholar]
- 566.National Confidential Enquiry into Patient Outcome and Death. Lower limb amputation: working together. Available at: http://www.ncepod.org.uk/2014report2/downloads/WorkingTogetherFullReport.pdf.Accessed March 2017.
- 567.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358:527–33. [DOI] [PubMed] [Google Scholar]
- 568.Steinhubl SR, Berger PB, Mann IJ, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;288:2411–20. [DOI] [PubMed] [Google Scholar]
- 569.Nguyen TA, Diodati JG, Pharand C. Resistance to clopidogrel: a review of the evidence. J Am Coll Cardiol 2005;45: 1157–64. [DOI] [PubMed] [Google Scholar]
- 570.Dörffler-Melly J, Koopman MM, Prins MH, Büller HR. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev 2005;1:CD002071. [DOI] [PubMed] [Google Scholar]
- 571.Soden PA, Zettervall SL, Ultee KH, Landon BE, O’Malley AJ, Goodney PP, et al. Dual antiplatelet therapy is associated with prolonged survival after lower extremity revascularization. J Vasc Surg 2016;64:1633–44.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Iida O, Yokoi H, Soga Y, Inoue N, Suzuki K, Yokoi Y, et al. Cilostazol reduces angiographic restenosis after endovascular therapy for femoropopliteal lesions in the Sufficient Treatment of Peripheral Intervention by Cilostazol study. Circulation 2013;127:2307–15. [DOI] [PubMed] [Google Scholar]
- 573.Iftikhar O, Oliveros K, Tafur AJ, Casanegra AI. Prevention of femoropopliteal in-stent restenosis with cilostazol: a meta-analysis. Angiology 2016;67:549–55. [DOI] [PubMed] [Google Scholar]
- 574.Soga Y, Takahara M, Iida O, Yamauchi Y, Hirano K, Fukunaga M, et al. Efficacy of CilostAzol for Below-the-Knee Artery Disease after Balloon AnGioplasty in PatiEnts with Severe Limb Ischemia (CABBAGE Trial). Ann Vasc Surg 2017;45:22–8. [DOI] [PubMed] [Google Scholar]
- 575.Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin Study): a randomised trial. Lancet 2000;355:346–51. [PubMed] [Google Scholar]
- 576.Johnson WC, Williford WO. Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: a prospective randomized study. J Vasc Surg 2002;35:413–21. [DOI] [PubMed] [Google Scholar]
- 577.Sarac TP, Huber TS, Back MR, Ozaki CK, Carlton LM, Flynn TC, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg 1998;28:446–57. [DOI] [PubMed] [Google Scholar]
- 578.Geraghty AJ, Welch K. Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database Syst Rev 2011;6:CD000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Brumberg RS, Back MR, Armstrong PA, Cuthbertson D, Shames ML, Johnson BL, et al. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure. J Vasc Surg 2007;46:1160–6. [DOI] [PubMed] [Google Scholar]
- 580.Mewissen MW. Self-expanding nitinol stents in the femoropopliteal segment: technique and mid-term results. Tech Vasc Interv Radiol 2004;7:2–5. [DOI] [PubMed] [Google Scholar]
- 581.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Oliva V, et al. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. J Endovasc Ther 2006;13:701–10. [DOI] [PubMed] [Google Scholar]
- 582.Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation 2007;115: 2745–9. [DOI] [PubMed] [Google Scholar]
- 583.Laird JR, Jain A, Zeller T, Feldman R, Scheinert D, Popma JJ, et al. Nitinol stent implantation in the superficial femoral artery and proximal popliteal artery: twelve-month results from the Complete SE Multicenter Trial. J Endovasc Ther 2014;21:202–12. [DOI] [PubMed] [Google Scholar]
- 584.Idu MM, Blankstein JD, de Gier P, Truyen E, Buth J. Impact of a color-flow duplex surveillance program on infrainguinal vein graft patency: a five-year experience. J Vasc Surg 1993;17:42–53. [PubMed] [Google Scholar]
- 585.Lundell A, Lindblad B, Bergqvist D, Hansen F. Femoropopliteal-crural graft patency is improved by an intensive surveillance program: a prospective randomized study. J Vasc Surg 1995;21:26–34. [DOI] [PubMed] [Google Scholar]
- 586.Ihlberg L, Luther M, Tierala E, Lepantalo M. The utility of duplex scanning in infrainguinal vein graft surveillance: results from a randomised controlled study. Eur J Vasc Endovasc Surg 1998;16:19–27. [DOI] [PubMed] [Google Scholar]
- 587.Ihlberg L, Luther M, Alback A, Kantonen I, Lepantalo M. Does a completely accomplished duplex-based surveillance prevent vein-graft failure? Eur J Vasc Endovasc Surg 1999;18:395–400. [DOI] [PubMed] [Google Scholar]
- 588.Bandyk DF. Infrainguinal vein bypass graft surveillance: how to do it, when to intervene, and is it cost-effective? J Am Coll Surg 2002;194:S40–52. [DOI] [PubMed] [Google Scholar]
- 589.Davies AH, Hawdon AJ, Sydes MR, Thompson SG. Is duplex surveillance of value after leg vein bypass grafting? Principal results of the Vein Graft Surveillance Randomised Trial (VGST). Circulation 2005;112:1985–91. [DOI] [PubMed] [Google Scholar]
- 590.Baril DT, Rhee RY, Kim J, Makaroun MS, Chaer RA, Marone LK. Duplex criteria for determination of in-stent stenosis after angioplasty and stenting of the superficial femoral artery. J Vasc Surg 2009;49:133–9. [DOI] [PubMed] [Google Scholar]
- 591.Saarinen E, Laukontaus SJ, Albäck A, Venermo M. Duplex surveillance after endovascular revascularisation for critical limb ischaemia. Eur J Vasc Endovasc Surg 2014;47:418–21. [DOI] [PubMed] [Google Scholar]
- 592.Armstrong PA, Bandyk DF. Surveillance after peripheral artery angioplasty and stenting. In: Zierler E, editor. Strandness’s duplex scanning in vascular disorders. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. p. 169–76. [Google Scholar]
- 593.Baril DT, Marone LK. Rationale and benefits of surveillance after percutaneous transluminal angioplasty and stenting of iliac and femoral arteries. In: AbuRahma AF, Bandyk DF, editors. Noninvasive vascular diagnosis: a practical guide to therapy. London: Springer; 2013. p. 339–46. [Google Scholar]
- 594.Mewissen MW, Kinney EV, Bandyk DF, Reifsnyder T, Seabrook GR, Lipchik EO, et al. The role of duplex scanning versus angiography in predicting outcome after balloon angioplasty in the femoropopliteal artery. J Vasc Surg 1992;15:860–5. [PubMed] [Google Scholar]
- 595.Spijkerboer AM, Nass PC, de Valois JC, van der Graaf Y, Eikelboom BC, Mali WP. Evaluation of femoropopliteal arteries with duplex ultrasound after angioplasty. Can we predict results at one year? Eur J Vasc Endovasc Surg 1996;12:418–23. [DOI] [PubMed] [Google Scholar]
- 596.Kasirajan K, Gray B, Beavers FP, Clair DG, Greenberg R, Mascha E, et al. Rheolytic thrombectomy in the management of acute and subacute limb-threatening ischemia. J Vasc Interv Radiol 2001;12:413–21. [DOI] [PubMed] [Google Scholar]
- 597.Sarac TP, Hilleman D, Arko FR, Zarins CK, Ouriel K. Clinical and economic evaluation of the Trellis thrombectomy device for arterial occlusions: preliminary analysis. J Vasc Surg 2004;39:556–9. [DOI] [PubMed] [Google Scholar]
- 598.Sacks D, Robinson ML, Summers TA, Marinelli DL. The value of duplex sonography after peripheral artery angioplasty in predicting subacute restenosis. AJR Am J Roentgenol 1994;162:179–83. [DOI] [PubMed] [Google Scholar]
- 599.Tielbeek AV, Rietjens E, Buth J, Vroegindeweij D, Schol FP. The value of duplex surveillance after endovascular intervention for femoropopliteal obstructive disease. Eur J Vasc Endovasc Surg 1996;12:145–50. [DOI] [PubMed] [Google Scholar]
- 600.Mills JL, Bandyk DF, Gahtan V, Esses GE. The origin of infrainguinal vein graft stenosis: a prospective study based on duplex surveillance. J Vasc Surg 1995;21:16–25. [DOI] [PubMed] [Google Scholar]
- 601.Avino AJ, Bandyk DF, Gonsalves AJ, Johnson BL, Black TJ, Zwiebel BR, et al. Surgical and endovascular intervention for infrainguinal vein graft stenosis. J Vasc Surg 1999;29: 60–71. [DOI] [PubMed] [Google Scholar]
- 602.Abu Dabrh AM, Mohammed K, Farah W, Haydour Q, Zierler RE, Wang Z, et al. Systematic review and meta-analysis of duplex ultrasound surveillance for infrainguinal vein bypass grafts. J Vasc Surg 2017;66:1885–91.e8. [DOI] [PubMed] [Google Scholar]
- 603.Björkman P, Peltola E, Albäck A, Venermo M. Peripheral vascular restenosis: a retrospective study on the use of drug-eluting balloons in native arteries, vein grafts and dialysis accesses. Scand J Surg 2017;106:158–64. [DOI] [PubMed] [Google Scholar]
- 604.Jongsma H, Akkersdijk GP, de Smet A, Vroegindeweij D, de Vries JPM, Fioole B. Drug-eluting balloons and uncoated balloons perform equally to rescue infrainguinal autologous bypasses at risk. J Vasc Surg 2017;66: 454–60. [DOI] [PubMed] [Google Scholar]
- 605.Dunlop P, Sayers RD, Naylor AR, Bell PR, London NJ. The effect of a surveillance programme on the patency of synthetic infrainguinal bypass grafts. Eur J Vasc Endovasc Surg 1996;11:441–5. [DOI] [PubMed] [Google Scholar]
- 606.Aune S, Pedersen OM, Trippestad A. Surveillance of above-knee prosthetic femoropopliteal bypass. Eur J Vasc Endovasc Surg 1998;16:509–12. [DOI] [PubMed] [Google Scholar]
- 607.Calligaro KD, Doerr K, McAffee-Bennett S, Krug R, Raviola CA, Dougherty MJ. Should duplex ultrasonography be performed for surveillance of femoropopliteal and femorotibial arterial prosthetic bypasses? Ann Vasc Surg 2001;15:520–4. [DOI] [PubMed] [Google Scholar]
- 608.Mohler ER, Gornik HL, Gerhard-Herman M, Misra S, Olin JW, Zierler RE. ACCF/ACR/AIUM/ASE/ASN/ICAVL/SCAI/SCCT/SIR/SVM/SVS 2012 Appropriate Use Criteria for Peripheral Vascular Ultrasound and Physiological Testing Part I: Arterial Ultrasound and Physiological Testing: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American College of Radiology, American Institute of Ultrasound in Medicine, American Society of Echocardiography, American Society of Nephrology, Intersocietal Commission for the Accreditation of Vascular Laboratories, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Interventional Radiology, Society for Vascular Medicine, and Society for Vascular Surgery. J Am Coll Cardiol 2012;60:242–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Stone PA, Armstrong PA, Bandyk DF, Keeling WB, Flaherty SK, Shames ML, et al. Duplex ultrasound criteria for femorofemoral bypass revision. J Vasc Surg 2006;44: 496–502. [DOI] [PubMed] [Google Scholar]
- 610.Fitzgerald RH, Mills JL, Joseph W, Armstrong DG. The diabetic rapid response acute foot team: 7 essential skills for targeted limb salvage. Eplasty 2009;9:e15. [PMC free article] [PubMed] [Google Scholar]
- 611.Rogers LC, Andros G, Caporusso J, Harkless LB, Mills JL Sr, Armstrong DG. Toe and flow: essential components and structure of the amputation prevention team. J Vasc Surg 2010;52:23S–7S. [DOI] [PubMed] [Google Scholar]
- 612.Armstrong DG, Mills JL. Juggling risk to reduce amputations: the three-ring circus of infection, ischemia and tissue loss-dominant conditions. Wound Med 2013;1:13–4. [Google Scholar]
- 613.Pickwell KM, Siersma VD, Kars M, Holstein PE, Schaper NC. Diabetic foot disease: impact of ulcer location on ulcer healing. Diabetes Metab Res Rev 2013;29:377–83. [DOI] [PubMed] [Google Scholar]
- 614.Armstrong DG, Lavery LA, Nixon BP, Boulton AJ. It’s not what you put on, but what you take off: techniques for debriding and off-loading the diabetic foot wound. Clin Infect Dis 2004;39(Suppl 2):S92–9. [DOI] [PubMed] [Google Scholar]
- 615.Bus SA, Armstrong DG, van Deursen RW, Lewis J, Caravaggi CF, Cavanagh PR. IWGDF Guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. Available at: www.iwgdf.org/files/2015/website_footwearoffloading.pdf.Accessed October 30, 2018. [DOI] [PubMed]
- 616.Pappalardo J, Plemmons B, Armstrong D. Wound healing simplification: a vertical and horizontal philosophy illustrated. J Wound Technol 2013;19:38–9. [Google Scholar]
- 617.Rose JF, Giovinco N, Mills JL, Najafi B, Pappalardo J, Armstrong DG. Split-thickness skin grafting the high-risk diabetic foot. J Vasc Surg 2014;59:1657–63. [DOI] [PubMed] [Google Scholar]
- 618.Armstrong DG, Boulton AJ, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–75. [DOI] [PubMed] [Google Scholar]
- 619.Armstrong DG, Lavery LA, Frykberg RG, Wu SC, Boulton AJ. Validation of a diabetic foot surgery classification. Int Wound J 2006;3:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Armstrong DG, Holtz-Neiderer K, Wendel C, Mohler MJ, Kimbriel HR, Lavery LA. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med 2007;120:1042–6. [DOI] [PubMed] [Google Scholar]
- 621.Arad Y, Fonseca V, Peters A, Vinik A. Beyond the monofilament for the insensate diabetic foot. A systematic review of randomized trials to prevent the occurrence of plantar foot ulcers in patients with diabetes. Diabetes Care 2011;34: 1041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Ulbrecht JS, Hurley T, Mauger DT, Cavanagh PR. Prevention of recurrent foot ulcers with plantar pressure-based in-shoe orthoses: the CareFUL prevention multicenter randomized controlled trial. Diabetes Care 2014;37:1982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Cook JA, McCulloch P, Blazeby JM, Beard DJ, MarinacDabic D, Sedrakyan A. IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ 2013;346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Ergina PL, Barkun JS, McCulloch P, Cook JA, Altman DG. IDEAL framework for surgical innovation 2: observational studies in the exploration and assessment stages. BMJ : British Medical Journal 2013;346:f2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg J, et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg 2019;269:211–20. [DOI] [PubMed] [Google Scholar]
- 626.McCulloch P, Cook JA, Altman DG, Heneghan C, Diener MK. IDEAL framework for surgical innovation 1: the idea and development stages. BMJ 2013;346:f3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Sedrakyan A, Campbell B, Merino JG, Kuntz R, Hirst A, McCulloch P. IDEAL-D: a rational framework for evaluating and regulating the use of medical devices. BMJ 2016;353: i2372. [DOI] [PubMed] [Google Scholar]
- 628.Behrendt CA, Bertges D, Eldrup N, Beck AW, Mani K, Venermo M, et al. International Consortium of Vascular Registries consensus recommendations for peripheral revascularisation registry data collection. Eur J Vasc Endovasc Surg 2018;56:217–37. [DOI] [PubMed] [Google Scholar]
- 629.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med 2016;375:65–74. [DOI] [PubMed] [Google Scholar]
- 630.Bothwell LE, Podolsky SH. The emergence of the randomized, controlled trial. N Engl J Med 2016;375:501–4. [DOI] [PubMed] [Google Scholar]
- 631.Avery KN, Williamson PR, Gamble C, O’Connell Francischetto E, Metcalfe C, Davidson P, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open 2017;7:e013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess 2010;14: 1–210, iii–iv. [DOI] [PubMed] [Google Scholar]
- 633.Nordanstig J, Wann-Hansson C, Karlsson J, Lundström M, Pettersson M, Morgan MB. Vascular Quality of Life Questionnaire-6 facilitates health-related quality of life assessment in peripheral arterial disease. J Vasc Surg 2014;59:700–7.e1. [DOI] [PubMed] [Google Scholar]
- 634.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006;43:742–51.e1. [DOI] [PubMed] [Google Scholar]
- 635.Clark TG, Altman DG, Stavola BL. Quantification of the completeness of follow-up. Lancet 2002;359:1309–10. [DOI] [PubMed] [Google Scholar]
- 636.Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer 2003;89:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.von Allmen RS, Weiss S, Tevaearai HT, Kuemmerli C, Tinner C, Carrel TP, et al. Completeness of follow-up determines validity of study findings: results of a prospective repeated measures cohort study. PLoS One 2015;10:e0140817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Grading of Recommendations Assessment, Development,and Evaluation (GRADE) working group. Available at: www.gradeworkinggroup.org.Accessed October 30, 2018.
- 639.Conte MS, Farber A. Revascularization for chronic limb-threatening ischaemia. Br J Surg 2015;102:1007–9. [DOI] [PubMed] [Google Scholar]
- 640.Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord 2005;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Carls GS, Gibson TB, Driver VR, Wrobel JS, Garoufalis MG, DeFrancis RR, et al. The economic value of specialized lower-extremity medical care by podiatric physicians in the treatment of diabetic foot ulcers. J Am Podiatr Med Assoc 2011;101:93–115. [DOI] [PubMed] [Google Scholar]
- 642.Chan B, Cadarette S, Wodchis W, Wong J, Mittmann N, Krahn M. Cost-of-illness studies in chronic ulcers: a systematic review. J Wound Care 2017;26(Suppl 4):S4–14. [DOI] [PubMed] [Google Scholar]
- 643.Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care 2004;27:2129–34. [DOI] [PubMed] [Google Scholar]
- 644.Centers for Disease Control and Prevention. United Stateshospital discharge data, 2012. Available at: https://www.cdc.gov/nchs/nhds/.Accessed October 30, 2018.
- 645.Skrepnek GH, Mills JL, Lavery LA, Armstrong DG. Health care service and outcomes among an estimated 6.7 million ambulatory care diabetic foot cases in the U.S. Diabetes Care 2017;40:936–42. [DOI] [PubMed] [Google Scholar]
- 646.Gök Ü, Selek Ö, Selek A, Güdük A, Güner MÇ. Survival evaluation of the patients with diabetic major lower-extremity amputations. Musculoskelet Surg 2016;100:145–8. [DOI] [PubMed] [Google Scholar]
- 647.Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers. J Am Podiatr Med Assoc 2008;98:489–93. [DOI] [PubMed] [Google Scholar]
- 648.Sugerman D Centers of excellence. JAMA 2013;310:994. [DOI] [PubMed] [Google Scholar]
- 649.Rogers LC, Andros G, Caporusso J, Harkless LB, Mills JL Sr, Armstrong DG. Toe and flow. J Am Podiatr Med Assoc 2010;100:342–8. [DOI] [PubMed] [Google Scholar]
- 650.Rogers LC, Armstrong DG. Podiatry care. In: Cronenwett JL, Johnston KW, editors. Rutherford’s vascular surgery. 7th ed. Philadelphia: Saunders Elsevier; 2010. p. 1747–60. [Google Scholar]
- 651.Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999;22:157–62. [DOI] [PubMed] [Google Scholar]
- 652.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513–21. [DOI] [PubMed] [Google Scholar]
- 653.Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, et al. The Charcot foot in diabetes. J Am Podiatr Med Assoc 2011;101:437–46. [DOI] [PubMed] [Google Scholar]
- 654.Wukich DK, Armstrong DG, Attinger CE, Boulton AJ, Burns PR, Frykberg RG, et al. Inpatient management of diabetic foot disorders: a clinical guide. Diabetes Care 2013;36:2862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Snyder RJ, Frykberg RG, Rogers LC, Applewhite AJ, Bell D, Bohn G, et al. The management of diabetic foot ulcers through optimal off-loading. J Am Podiatr Med Assoc 2014;104:555–67. [DOI] [PubMed] [Google Scholar]
- 656.Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016;63(Suppl):3S–21S. [DOI] [PubMed] [Google Scholar]
- 657.National Institute for Health and Care Excellence. Diabeticfoot problems: prevention and management. Available at: https://www.nice.org.uk/guidance/ng19.Accessed October 30, 2018.
- 658.World Union of Wound Healing Societies, Position Document. Local management of diabetic foot ulcers. Available at: http://www.wuwhs2016.com/files/WUWHS_DFUs_web.pdf.Accessed October 30, 2018.
- 659.Wrobel JS, Robbins J, Armstrong DG. The high-low amputation ratio: a deeper insight into diabetic foot care? J Foot Ankle Surg 2006;45:375–9. [DOI] [PubMed] [Google Scholar]
- 660.International Diabetes Federation. The diabetic foot: amputations are preventable. Available at: http://www.idf.org/Position_statementsdiabetic_foot.Accessed July 30, 2008.
- 661.World Health Organization. World Diabetes Day: too manypeople are losing lower limbs unnecessarily to diabetes [press release]. Available at: https://www.who.int/mediacentre/news/releases/2005/pr61/en/.Accessed July 30, 2008.
- 662.Sanders LJ, Robbins JM, Edmonds ME. History of the team approach to amputation prevention: pioneers and milestones. J Vasc Surg 2010;52:3S–16S. [DOI] [PubMed] [Google Scholar]
- 663.Van Gils CC, Wheeler LA, Mellstrom M, Brinton EA, Mason S, Wheeler CG. Amputation prevention by vascular surgery and podiatry collaboration in high-risk diabetic and nondiabetic patients. The Operation Desert Foot experience. Diabetes Care 1999;22:678–83. [DOI] [PubMed] [Google Scholar]
- 664.Wrobel JS, Robbins JM, Charns MP, Bonacker KM, Reiber GE, Pogach L. Diabetes-related foot care at 10 Veterans Affairs medical centers: must do’s associated with successful microsystems. Jt Comm J Qual Patient Saf 2006;32:206–13. [DOI] [PubMed] [Google Scholar]
- 665.Driver VR, Madsen J, Goodman RA. Reducing amputation rates in patients with diabetes at a military medical center. The Limb Preservation Service model. Diabetes Care 2005;28:248–53. [DOI] [PubMed] [Google Scholar]
- 666.Armstrong DG, Bharara M, White M, Lepow B, Bhatnagar S, Fisher T, et al. The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev 2012;28:514–8. [DOI] [PubMed] [Google Scholar]
- 667.Sloan FA, Feinglos MN, Grossman DS. Receipt of care and reduction of lower extremity amputations in a nationally representative sample of U.S. elderly. Health Serv Res 2010;45(Pt 1):1740–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Schmidt BM, Wrobel JS, Munson M, Rothenberg G, Holmes CM. Podiatry impact on high-low amputation ratio characteristics: a 16-year retrospective study. Diabetes Res Clin Pract 2017;126:272–7. [DOI] [PubMed] [Google Scholar]
- 669.van Houtum WH, Rauwerda JA, Ruwaard D, Schaper NC, Bakker K. Reduction in diabetes-related lower-extremity amputations in The Netherlands: 1991–2000. Diabetes Care 2004;27:1042–6. [DOI] [PubMed] [Google Scholar]
- 670.Pedrosa H, Boulton AJ, Oliveira Dias MS. The diabetic foot in Brazil. In: Boulton AJ, Cavanagh PR, Rayman G, editors. The foot in diabetes. Hoboken, NJ: John Wiley & Sons; 2006. p. 364–74. [Google Scholar]
- 671.Anichini R, Zecchini F, Cerretini I, Meucci G, Fusilli D, Alviggi L, et al. Improvement of diabetic foot care after the implementation of the International Consensus on the Diabetic Foot (ICDF): results of a 5-year prospective study. Diabetes Res Clin Pract 2007;75:153–8. [DOI] [PubMed] [Google Scholar]
- 672.Rubio JA, Aragón-Sánchez J, Jiménez S, Guadalix G, Albarracín A, Salido C, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team for the diabetic foot. Int Low Extrem Wounds 2014;13:22–6. [DOI] [PubMed] [Google Scholar]
- 673.Canavan RJ, Unwin NC, Kelly WF, Connolly VM. Diabetes- and nondiabetes-related lower extremity amputation incidence before and after the introduction of better organized diabetes foot care. Continuous longitudinal monitoring using a standard method. Diabetes Care 2008;31:459–63. [DOI] [PubMed] [Google Scholar]
- 674.Williams DT, Powell-Chandler A, Qureshi Q, Zaidi A, Whitaker CJ. Improved limb salvage for patients with vascular disease and tissue loss associated with new model of provision targeted at the diabetic foot. Diabetes Res Clin Pract 2018;135:50–7. [DOI] [PubMed] [Google Scholar]
- 675.Eskelinen E, Eskelinen A, Albäck A, Lepäntalo M. Major amputation incidence decreases both in non-diabetic and in diabetic patients in Helsinki. Scand J Surg 2006;95:185–9. [DOI] [PubMed] [Google Scholar]
- 676.Laakso M, Honkasalo M, Kiiski J, Ala-Houhala M, Haapasalo H, Laine HJ, et al. Re-organizing inpatient care saves legs in patients with diabetic foot infections. Diabetes Res Clin Pract 2017;125:39–46. [DOI] [PubMed] [Google Scholar]
- 677.Hirsch AT, Duval S. The global pandemic of peripheral artery disease. Lancet 2013;382:1312–4. [DOI] [PubMed] [Google Scholar]
- 678.Health in India. Government of India. Available at: http://mospi.nic.in/sites/default/files/publication_reports/nss_rep574.pdf.Accessed December 7, 2018.
- 679.Kapoor A, Vora A, Nataraj G, Mishra S, Kerkar P, Manjunath CN. Guidance on reuse of cardio-vascular catheters and devices in India: a consensus document. Indian Heart J 2017;69:357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018:7e011245. doi: 10.1161/JAHA.118.011245.Accessed April 21, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.