Abstract
Systemic lupus erythematosus (SLE) is an archetype autoimmune disease characterized by a myriad of immunoregulatory abnormalities that drives injury to multiple tissues and organs. Due to the involvement of various immune cells, inflammatory cytokines, and related signaling pathways, researchers have spent a great deal of effort to clarify the complex etiology and pathogenesis of SLE. Nevertheless, current understanding of the pathogenesis of SLE is still in the early stages, and available nonspecific treatment options for SLE patients remain unsatisfactory. First discovered in 1993, microRNAs (miRNAs) are small RNA molecules that control the expression of 1/3 of human genes at the post-transcriptional level and play various roles in gene regulation. The aberrant expression of miRNAs in SLE patients has been intensively studied, and further studies have suggested that these miRNAs may be potentially relevant to abnormal immune responses and disease progression in SLE. The aim of this review was to summarize the specific miRNAs that have been observed aberrantly expressed in several important pathogenetic processes in SLE, such as DCs abnormalities, overactivation and autoantibody production of B cells, aberrant activation of CD4+ T cells, breakdown of immune tolerance, and abnormally increased production of inflammatory cytokines. Our summary highlights a novel perspective on the intricate regulatory network of SLE, which helps to enrich our understanding of this disorder and ignite future interest in evaluating the molecular regulation of miRNAs in autoimmunity SLE.
Keywords: microRNAs, systemic lupus erythematosus, epigenetics, autoimmune disease, DNA methylation
Introduction
Systemic lupus erythematosus (SLE) is a severe autoimmune inflammatory disease with a broad range of clinical manifestations characterized by loss of tolerance to self-antigens, activation of dysregulated autoreactive T cells and B cells, production of autoantibodies (auto-Abs) and perturbed cytokine activities (1). Approximately 50% of SLE patients develop life-threatening complications, such as nephritis, vasculitis, pulmonary hypertension, interstitial lung disease, and cerebral stroke (2). Current studies suggest that SLE is associated with dysregulation of the innate and adaptive immune responses that are likely rooted in the intricate interactions among environmental stimulants, sex hormone imbalance, genetic predisposition, epigenetic regulation, immunological factors, and other undefined factors, resulting in breach of self-tolerance characterized by uncontrolled activation and expansion of dendritic cells (DCs) and lymphocytes, coupled with the production of copious amounts of anti-nuclear and antiphospholipid antibodies. Even so, current understanding of immunological events that trigger the onset of clinical manifestations of SLE is still in the early stages. At present, primary treatment for SLE is based on conventional nonspecific immunosuppressants, but this treatment option is unsatisfactory because of the associated side effects including infection, malignancy, metabolic disturbances, and infertility (1). Meanwhile, given the multitude of active pathways in a disease as heterogeneous as SLE (1), the role of single target approaches with inhibitors such as antiCD20, antiinterferonα and antiIL6 may be too limited. More extensive and in-depth study of SLE from different perspectives will contribute to a more comprehensive understanding of this disease and potentially open up exciting new therapeutic possibilities for treating this multifactorial disease.
MicroRNAs (miRNAs) are a large family of endogenous, single-stranded, small (~22 nucleotides), nonprotein-coding RNA molecules that modulate gene expression at the post-transcriptional level and protein synthesis in higher eukaryotes (3). In 1993, Lee et al. (4) firstly found that the Lin-4 gene encoded some small RNAs, rather than proteins, which controlled the temporal development of Caenorhabditis elegans. With the development of molecular cloning and bioinformatics technology, more than 3,800 miRNAs have been identified so far, which are widely distributed in plants, animals, and viruses. Moreover, a recent study estimated that there are 2,300 mature miRNAs in humans (5), whose genes constitute about 1%–3% of the human genome sequence and approximately 1/3 of human genes expression is regulated by mature miRNAs (3, 6). It has been reported that the epigenetic ability of miRNAs can regulate a variety of biological processes, including embryo development, cell differentiation, proliferation, and apoptosis, signal transduction, and metabolism (7). In terms of the regulation of the immune system, increasing evidences suggested that miRNAs are involved in the regulation of innate and adaptive immune cells (8). It is not surprising that dysregulation of miRNA expression has been implicated in the progression of a broad range of diseases, some of which have been identified as diagnostic and/or prognostic biomarkers of various diseases, including cancer (9), diabetes (10), viral infection, cardiovascular diseases (11), and kidney diseases (12). In addition, miRNAs can affect the occurrence and development of autoimmune diseases through different pathways including the release of inflammatory mediators, innate immune responses, lymphocyte function, the signaling of toll-like receptors (TLRs), and nuclear factor (NF)-κB (13).
In 2007, Dai et al. (14) found differences in the miRNA expression profiles of SLE patients and normal controls, as seven miRNAs were down-regulated (miR-196a, miR-L7-5p, miR-409-3p, miR-141, miR-383, miR-112, and miR-184) and nine were up-regulated (miR-189, miR-61, miR-78, miR-21, miR-142-3p, miR-342, miR-299-3p, miR-198, and miR-298), suggesting that miRNAs are potential diagnostic markers of SLE and may be important factors related to the pathogenesis of the disease. With the publication of work of Dai et al. (14), more and more studies have demonstrated that the aberrantly expressed miRNAs have the ability to promote different immunological events of SLE, but the exact mechanism of these miRNAs is still largely unknown. The miRNAs in immune cells during active SLE has opened up potential new avenues for a more comprehensive understanding of SLE and may provide new therapeutic clues to improve patient outcomes, which remains to be confirmed and requires further investigation.
Alterations to miRNAs have been exploited as potential tools and targets for novel therapeutic approaches for many diseases. The first miRNAbased therapeutic agent was approved in 2013 for the treatment of familial hypercholesterolemia. Many miRNA-targeted therapies have been clinically advanced, including phase I clinical trials of miR-34, a mimic of the tumor suppressor miRNA for the treatment of cancer (15), and phase II clinical trials of anti-miRNAs targeting miR-122 for the treatment of hepatitis (NCT01646489, NCT01727934, NCT01872936, NCT01200420). MiRNAs have an intriguing potential role in the development and deterioration of SLE, which may allow for the development of more effective therapies with fewer side effects to mitigate this disorder. Therefore, this article reviews the current understanding of miRNAs and summarizes the impact of miRNA dysregulation in several important immunological events in SLE, including the dysfunction of immune-related cells, aberrant immune cell signaling, and the production of inflammatory cytokines. The summary of this study helps to enrich the current understanding of the intricate immunological regulation network of SLE and to stimulate future interest in evaluating the molecular regulation of SLE.
Biogenesis and Function of miRNAs
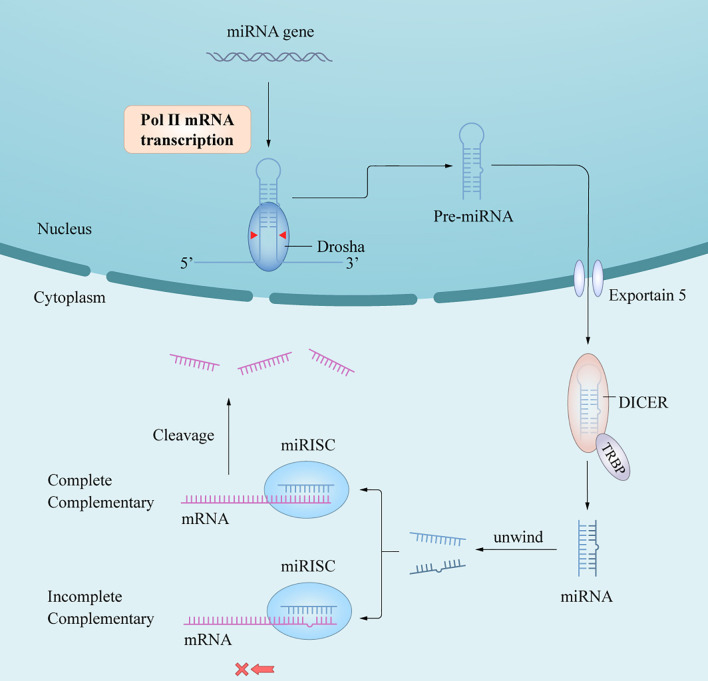
In humans and animals, synthesis of mature miRNAs is initiated by the transcription of nuclear genes into primary RNA transcripts (pri-miRNAs) (3), which are cleaved by the ribonuclease III (RNase III) enzyme Dorsha and the protein DiGeorge syndrome critical region 8 into precursor miRNAs (pre-miRNAs) (6). After being transported from the nucleus into the cytoplasm by exportin-5/Ran-GTP, pre-miRNAs are processed by Dicer and trans-activation response RNA-binding protein to yield miRNA duplexes. One of the functional chains is loaded into the RNA-induced silencing complex (RISC) to form an asymmetric RISC assembly (16), which interacts with the target messenger RNA (mRNA) to regulate the expression of target genes after transcription (17) (Figure 1) via two mechanisms of action depending on whether the single-stranded miRNA in the RISC assembly is completely complementary to the target mRNA 3’-untranslated region (3’-UTR); if so, the mRNA is targeted and degraded by the RISC assembly, and if not, the mRNA blocks translation of the target gene. The miRNAs mainly follow the first mechanism in plants and the second in animals. A complex regulatory network is formed between the miRNA and target gene mRNAs, thus affecting the course of disease through post-transcriptional regulation without changing the gene sequence (18). In short, the extent of complementarity between the miRNA seed region and the target mRNA 3’-UTR determines the mechanism of miRNA-mediated gene regulation/translation repression or miRNA cleavage and degradation.
Figure 1.
A schematic of miRNA biogenesis. miRNA biogenesis begins in the nucleus, where genes are mainly transcribed by RNA polymerase II into pri-miRNAs. Pri-miRNAs are processed into pre-miRNA by the RNase III enzyme, Dorsha and DGCR8. After being transported into cytoplasm by Exportin-5/Ran-GTP, pre-miRNAs are further processed by Dicer and TRBP to yield miRNAs duplex. An functional miRNA strand is unwound from the miRNAs duplex and then loaded into the RISC to form an miRISC assembly, which are the functional forms of miRNAs and the regulator of gene expression in posttranscriptional level by interacting with the target mRNA.
The Roles of miRNAs in DCs Abnormalities in SLE
In general, DCs have a unique sentinel function, continuously detecting danger signals from the environment through innate pattern-recognition receptors such as TLRs, which have the ability to capture antigens through binding to microbes or endogenous tissues (8). Inappropriate or dysfunctional antigen presentation by DCs might promote the breakdown of Tcell and Bcell tolerance in SLE (19). Patients with SLE show multiple DC abnormalities, including a decrease in the number of circulating normal myeloid DC (mDC) but an increase in the number of plasmacytoid DC (pDC). Similar to mDCs, pDCs upregulate the expressions of T-cell costimulatory molecules such as CD80, CD86 and CD40 upon antigen stimulation, and serve as antigen presenting cells to prime and activate T cells (20). Distinctively, the pDC subset specialize in producing type I interferon in response to single stranded RNA and hypomethylated CpG DNA, via TLR7 and TLR9 (21, 22). These unique features allow pDCs to play a crucial role in the pathogenesis of SLE and have been shown to correlate with disease manifestations including the SLE hallmark anti-dsDNA autoantibodies. Recent studies have described the involvement of let-7c, miR-155, miR-150 in regulating the functions of pDC in response to TLRs stimulation (Figure 2A).
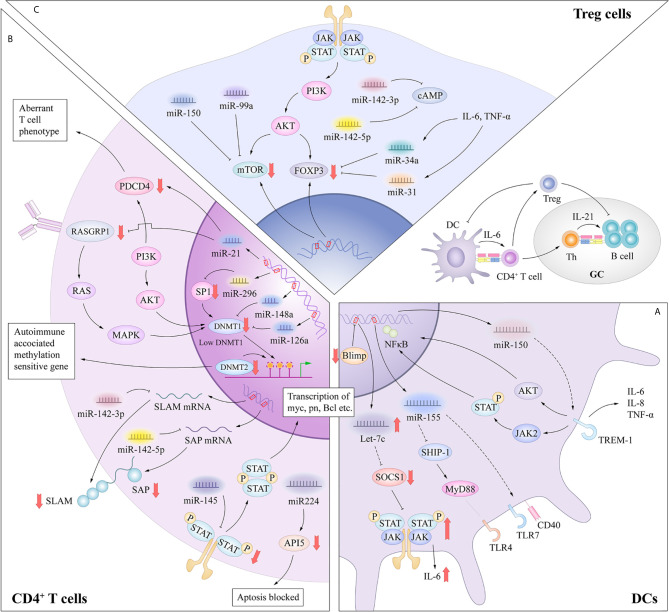
Figure 2.
(A) MiRNAs in dysfunctional antigen presentation by DCs. In the DC-specific absence of Blimp1, an increase in let-7 miRNA results in a broad spectrum of proinflammatory DC phenotype, mediated in part through suppression of SOCS1 expression. The CD40 expression was significantly upregulated with a negative correlation to the miR-155 in SLE primary target SHIP-1 expression. MiRNA-150 inhibited the expression of TREM-1 which amplify the function of TLR4. (B) miRNAs in aberrant activation of CD4+ T cells. MiR-21 contributed to the aberrant phenotype of T cells through interaction with PDCD4 or indirectly inhibiting DNMT1 expression through targeting RASGRP1. miRNAs such as miR-126, miR-29b and miR-148a can directly inhibit DNMT1 expression by targeting the protein coding region. These processes result in the overexpression of autoimmune-associated methylation-sensitive genes, which contribute to the autoreactivity and overstimulation of CD4+ T cells in SLE. miR-142-3p specifically targets the SLAM family, while miR-142-5p targets the 3’-UTR of SAP. Thus, decreased miR-142-3p/5p expression contributes to the up-regulation of CD84 and IL-10/SAP, resulting in the increased T cell function and IgG production in co-cultured B cells. Aberrant expression of miR-145 and miR-224 can promote T cell activation-induced cellular apoptosis and SLE-associated nephritis by overexpression of STAT1 and underexpression of API5. (C) miRNAs in functional inhibitory of Treg cells. The release of IL-6 or TNF-α can increase the expression levels of miR-34a, which can attenuate Foxp3 expression by targeting its 3ʹ-UTR. MiR-142-5p positively regulates intracellular levels of cAMP to maintain the suppressive function of Treg cells. MiR-142-3p can restrict cAMP levels in CD4+ T cells, which compromises the inhibitory function of Treg cells. MiR-99a and miR150 could regulate the function of Treg cells by targeting mTOR.
B lymphocyte–induced maturation protein-1 (Blimp1) is identified as an important transcriptional repressor of let-7c miRNA (23). Expression of let-7c miRNA influences the differentiation and functional homeostasis in B cells and T cells respectively (24). In the DC-specific absence of Blimp1, an increase in let-7 miRNA results in a broad spectrum of proinflammatory DC phenotype, mediated in part through suppression of suppressor of cytokine signaling 1 (SOCS1) expression (23). According to this research, let-7c miRNA enrich our understanding of the mechanisms underlying of polymorphisms in Blimp1 associated with risk for human autoimmune disorders such as SLE and inflammatory bowel disease. The pDCs derived from symptomatic mice showed functional hypersensitivity to TLR7, as represented by the elevated upregulation of CD40, CD86 and MHC class II molecules. In addition, Yan et al. (25) showed an enhanced induction of miR-155 in SLE mice in response to TLR7 stimulation, and CD40 expression was significantly upregulated with a negative correlation to the miR-155 primary target SH2 domain-containing inositol 5’-phosphatase 1 (SHIP-1) expression. According to the research of Gao et al. (26), miRNA-150 inhibited the expression of TREM-1 which potently amplified the function of TLR4 and then enhanced the inflammation responses in splenic cDCs in lupus prone mice. These researches enrich our understanding of pathogenesis of DCs dysfunction in SLE. Compared with the role of miRNAs in adaptive immune cells, the contribution of miRNAs to DC activation has been examined in only a few studies, and further research is needed in this field.
The Roles of miRNAs in Overactivation and Autoantibody Production of B Cells in SLE
Abnormalities of B cells are important characteristics of the pathogenesis of SLE. Although it is well-known that B cells have the ability to produce autoantibodies, they also mediate venomous functions through antibody-independent activities, including the presentation of antigen to T cells, co-stimulatory functions via the expression of accessory molecules engaging stimulatory receptors on T cells and the production of cytokines (27). Furthermore, B cell depletion therapy can have beneficial effects on patients with these disorders (28), highlighting the importance of B cells in the pathogenesis of autoimmune diseases. These autoreactive antibodies promote the pathogenesis of through cause acute and chronic inflammation and tissue necrosis with the participation of complement, or cause tissue cell destruction by directly interacting with their antigens, thus leading to multi-system damage of SLE (29, 30). Besides, B cells can contribute to SLE pathogenesis through additional pathways. For example, B cells can work as antigen presenting cells and correlate with activation of other crucial lymphocytes in SLE; Certain subtype of B cells may secrete anti-inflammatory cytokines in SLE. Therefore, the study of B lymphocytes can potentially unravel important pathogenic mechanisms of SLE. Previous studies have delineated several signaling pathways that contributed to the over-reactivity of B cells in SLE, including Janus kinase/signal transducer and activator of transcription (JAK-STAT), B cell receptor/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), and TLRs (31), although the detailed molecular mechanisms remain to be elucidated. Mice that are deficient in various inhibitory molecules that dampen B-cell receptor (BCR) signaling, such as SHIP-1 (32), Lck/Yes novel tyrosine kinase (LYN) (33), or Fcγ receptor IIb (FcγRIIb) (34), develop systemic autoimmunity. Studies of the aberrant expression of several miRNAs in the B cells of SLE patients have reported that miR-7, miR-155, miR-30a, and miR-15a were up-regulated, while miR-1246 were down-regulated (Figure 3), and researches have preliminarily demonstrated their role in SLE.
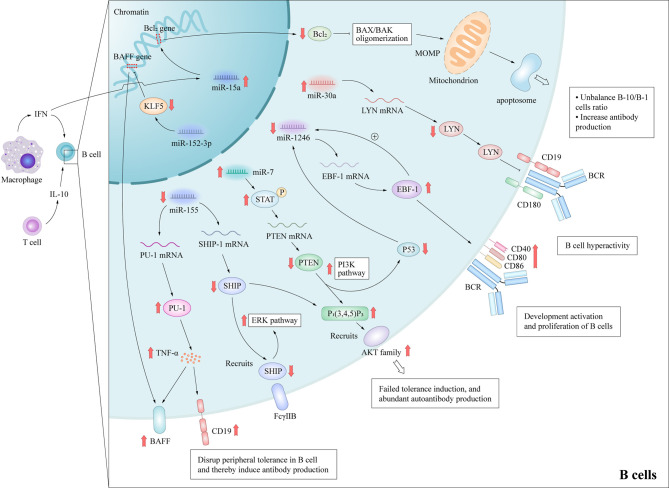
Figure 3.
Molecular mechanisms by which miRNAs promote B cell hyperactivity in SLE patients. These aberrant expressed miRNAs promote B cells abnormal activation and autoantibodies production in SLE by affecting important protein molecules in the B cell signaling pathway. MiR-15a interacts the Bcl-2 gene directly, which leads to the decrease of Bcl-2 and activation of intrinsic apoptotic pathway of regulatory B-10 cells. MiR-7 mediates the suppression of PTEN/AKT signaling, and then promotes B cell differentiation into plasma cells and spontaneous germinal center formation. Decreasing miR-155 in B cells contributes to the SHIP-1 reduction, which leads to the production of serum IgG anti-dsDNA antibodies. MiR-155 also suppresses PU.1 and TNF-α, and then inhibits BAFF and CD19 protein expression, and promotes the B cell proliferation and antibody production in SLE. MiR-30a increases in B cells of SLE patients and directly decreases the expression of Lyn, an important mediator of B cell activation, via targeting with the 3’-UTR of Lyn mRNA. MOMP, Mitochondrial outer membrane permeabilization; BAX/BAK, Bcl-2-associated X protein/Bcl-2 homologous antagonist killer.
A recent study reported that he up-regulation of miR-7 in the B cells of SLE patients can negatively regulate the expression of phosphatase and tensin homolog (PTEN), which results in up-regulated activation of PI3K/AKT signaling (35, 36). MiR-7-mediated down-regulation of PTEN/AKT signaling promoted the differentiation of B cells into plasmablasts/plasma cells and formation of spontaneous germinal center in a MRLlpr/lpr mouse model (37). The treatment of miR-7 antagomir showed its therapeutic value in vivo in MRLlpr/lpr mice, which consequently alleviated the clinical manifestations of organ damage in lupus mice model (37). Thai et al. (38) reported that ablation of miR-155 in lupus-prone mice with death receptor deficiency (Faslpr) reversed the reduced expression of SHIP-1 to normal levels, which acted as downstream of inhibitory cell-surface receptors, such as FcγRIIb. Subsequently, these processes contributed to decreasing serum levels of IgG anti-dsDNA antibodies and kidney inflammation, and then reduced autoantibody responses in lupus-like diseases. In addition, miR-155 acts as a suppressor of autoimmunity through transcriptional repression of PU.1 (a crucial regulator of B-cell development) and TNF-α, which in turn suppresses B cell-activating factor belonging to the TNF family and CD19 protein expression (39). Like miR-7 and miR-155, Liu et al. (40) observed that miR-30a expression was significantly increased in the B cells of SLE patients and miR-30a directly decreased expression of LYN by targeting the 3’-UTR of LYN mRNA. LYN is a member of the Src family of non-receptor tyrosine kinases (41) and a key mediator in several pathways of B cell activation, such as CD19 and CD180 (33). In addition, significantly decreased LYN levels have been observed in the B cells of SLE patients (42). Thus, high miR-30a expression can regulate B cell proliferation and antibody production in SLE patients, suggesting that miR-30a might be involved in pathogenesis of SLE. B cell lymphoma-2 (Bcl-2) is an important component of the apoptotic pathway. In the human genome, four members of the miR15/16 family share the same 9-bp Bcl-2-complementarity sequence. This functional redundancy indicates that Bcl-2 expression is regulated by a very fine mechanism. MiR-15a has been demonstrated to potentially regulate the balance of B-10 and B-1 cell subsets and was positively correlated to autoantibody levels in lupus due to differential expression in B cell subpopulations (43). Down-regulation of Bcl-2 expression by miR-15a overexpression activates the apoptotic pathway of the B-10 subset (44), which has been shown to suppress lupus in a B/W mouse model and other inflammatory diseases via preferential production of IL-10 (45). The induced loss of this regulatory B cell subset may lead to more prominent autoantibody production (46).
On the contrary, miR-1246 expression was negatively correlated with the activation of B cells in SLE patients (47). Further research verified that decreased miR-1246 expression reduced the inhibitory effect on the expression of early B cell factor 1 (EBF1), which contributed to the development, activation, and proliferation of B cells via activation of the AKT signaling pathway. The upregulated expression of EBF1 increases the production of the B cell surface co-stimulatory molecules CD40, CD80, and CD86, which then enhances B cell function.
As listed above, the underlying mechanism of miRNA-7 or miRNA-155 contribute to B cell hyperresponsiveness and autoantibody responses have been well studied. Further, single intervention with miRNA-7 or miRNA-155 alleviate the disease manifestation or inhibits lupus development in mice model suggesting that their critical role in lupus progression and their potential as treatment strategy in SLE. The miRNA-15a, miRNA-30a and miRNA-1246 are demonstrated to be involved in certain factors that contribute to B cells overactivation. Further studies are needed to show the role of miRNAs in SLE and whether it has the potential to be developed into new therapeutic targets.
The Roles of miRNAs in Aberrant Activation of CD4+ T Cells in SLE
The critical role of T cells in the pathogenesis of SLE has been confirmed by initiating and amplifying the inflammatory process through directly contacting with other immune cells in lymphoid organs, secreting pro-inflammatory cytokines or mediating direct effects on target tissues. Naive CD4+ T cells can differentiate into various Teff cell subsets, including Th1, Th17, Th2 and follicular helper T (Tfh) cells. Continuously stimulated T cells in lupus are likely to contribute to the disease by secreting inflammatory cytokines and supporting B cells to produce a wide variety of high affinity autoantibodies through contact-dependent mechanisms (mediated by CD40L:CD40, OX40L:OX40, and so on), which is an important characteristic of SLE. In addition, stimulation of autoreactive CD4+ T cells can foster the differentiation of CD8+ T cells into cytotoxic T lymphocytes along with the employment of inflammatory cytokines. However, the mechanism that causes the aberrant activation, differentiation and function of T cells in SLE remains largely unclear.
Genome-wide analysis has revealed that global DNA methylation levels are reduced by 15%–20% in the CD4+ T cells of patients with active SLE (48), especially genes involved in disease pathogenesis and progression, such as ITGAL, CD40LG, CD70, and PPP2CA. DNA methylation is an elementary determinant of the chromatin structure that is established during development by de novo DNA methyltransferases (DNMTs) with potent suppressive effects on transcription. DNMT1 serves to maintain the methylation status of proliferating cells (49). Moreover, the T cells of mice treated with procainamide and other inhibitors of DNA methylation can induce SLE in recipient mice (50). The pathological significance of the autoreactivity induced by inhibiting DNA methylation in T cells was further investigated. Pan et al. (51) demonstrated increased expression of miR-21 and miR-148a in SLE patients and SLE-prone MRL/lpr mice, and that both miRNAs reduced DNMT1 expression, which contributed to epigenetic changes via DNA hypomethylation. MiR-21 indirectly inhibits DNMT1 expression by targeting the important autoimmune gene RAS guanyl nucleotide-releasing protein 1, which is a critical regulator of the upstream RAS/mitogen-activated protein kinase pathway signaling cascade of DNMT1 in T cells (51). Another study confirmed that enhanced miR-21 expression also contributed to the aberrant phenotype of T cells in SLE which could be through interaction with its predicted target gene, programmed cell death protein 4 (52). Silencing of miRNA-21 in vivo can efficiently alter the course of autoimmune splenomegaly in lupus mice (53). On the other hand, miR-148a directly inhibits DNMT1 expression by targeting the protein coding region of the transcript. These data clearly showed that abnormally expressed miRNAs in SLE patients had a critical functional link with the aberrant DNA hypomethylation in lupus CD4+ T cells, resulting in the overexpression of autoimmune-associated methylation-sensitive genes, such as those that encode CD70 (tumor necrosis factor (ligand) superfamily, member 7 [TNFSF7]), CD40 ligand (TNFSF5), and lymphocyte function-associated antigen 1 (LAF-1, integrin αLβ2, CD11a/CD18) (54), which contributed to the autoreactivity and overstimulation of CD4+ T cells in SLE (51). Many studies have found that expression of miRNAs, such as miR-126 (55) and miR-29b (56), was markedly altered in the CD4+ T cells of SLE patients and was involved, either directly or indirectly, in decreasing DNA methylation levels, which led to aberrant activation and differentiation of CD4+ T cells.
In addition to aberrant DNA methylation, miRNAs in the CD4+ T cells of patients with SLE can regulate T cell activation in other ways. A recent study found that miR-142-3p/5p expression was decreased in the CD4+ T cells of patients with SLE (57). MiR-142-3p specifically targets members of the signaling lymphocytic activation molecule (SLAM) family, including interleukin (IL-10) and CD84, while miR-142-5p targets the 3’-UTR of SLAM-associated protein (SAP). Thus, decreased miR-142-3p/5p expression contributes to the up-regulation of CD84 and IL-10/SAP, resulting in increased T cell function and immunoglobulin (Ig) G production in co-cultured B cells. Reduced expression of miR-142-3p/5p in the CD4+ T cells of patients with SLE activates T cells and hyperstimulates B cells (58). Besides, overexpression of signal transducer and activator of transcription 1 (STAT1) and under-expression of apoptosis inhibitory protein 5 (API5) in the T cells of SLE patients are targeted by miR-145 and miR-224, respectively. Thus, aberrant expression of miR-145 and miR-224 can promote T cell activation-induced cellular apoptosis by suppressing API5 expression and SLE-associated nephritis by enhancing STAT1 expression (59) (Figure 2B).
According to current studies, a critical functional link between miRNAs and the lupus CD4+ T cells have been connected by the potential interplay between miRNAs and critical molecules such as SLAM family, STAT-1, DNMT1 which contribute to T cells abnormalities and hypomethylation in SLE. Moreover, transfection of miRNAs or miRNA inhibitors have beneficial effects on alleviating CD4+T cell disease phenotype, highlighting the important role of miRNAs in lupus-like CD4+T phenotype transformation. Further researches are needed to evaluate the place of miRNAs in the complicated regulatory networks of DNA hypomethylation in SLE.
The Roles of miRNAs in Breakdown of Immune Tolerance in SLE
SLE is characterized by a wide array of immune tolerance breakdown with systemic inflammation involving the dysregulation of immune responses. Tregs are a unique subpopulation of CD4+ T cells with an indispensable role in maintaining self-tolerance by suppressing autoreactive lymphocytes and suppressing excessive immune responses by controlling the responses of Teffs (60, 61). Tregs characteristically express CD25 (IL-2 receptor α chain) and the lineage-specific transcription factor forkhead box P3 (Foxp3). An imbalance between effector T cells (Teffs) and regulatory T cells (Tregs) is central to the pathogenesis of SLE. Pathogenic Teffs in SLE are mainly Tfh and Th17 cells. Tfh cells assist the activation of B cells to produce autoantibodies, which results in multiple organ damage. Th17 cells secrete pro-inflammatory cytokine that amplify immuno-inflammatory responses, resulting in tissue damage. Previous studies have reported that the proportions of Tfh and Th17 cells were increased in SLE patients and correlated with disease severity (62), although the underlying mechanisms remain unclear.
MiR-125a is commonly downregulated in the peripheral CD4+ T cells of patients with various autoimmune diseases, such as SLE and Crohn’s disease, which suppresses several factors of Teffs, including STAT3, IFN-γ, and IL-13 (63). A recent study reported consistent delivery of miR-125a into splenic T cells in a mouse model of SLE with the use of a nano-delivery system, which significantly alleviated disease progression by reversing the imbalance of Teffs and Tregs (64). These findings point to miR-125a as a critical factor to restrict the development of SLE by stabilizing Treg-mediated immune homeostasis. Xie et al. (65) reported that miR-34a in the peripheral blood mononuclear cells (PBMCs) of SLE patients played a potential role in disease activity and expression levels were positively correlated with several serum disease indexes, including rheumatoid factor, antistreptolysin antibody, erythrocyte sedimentation rate, and C-reactive protein. Further research demonstrated that miR-34a attenuated human and murine Foxp3 expression (66, 67) by targeting the 3ʹ-UTR, and then limited the differentiation of Tregs, which impaired the balance of Tregs and Th17 cells. The release of IL-6 or tumor necrosis factor (TNF)-α in the inflammatory environment can activate the NF-κB pathway and increase the expression levels of miR-34a by enhancing promoter activity, resulting in Foxp3 downregulation and Treg/Th17 imbalance (65). Meanwhile, miR-31 overexpression also inhibits differentiation of Tregs by targeting Foxp3 and other molecules that are indispensable for the development of Tregs, such as G protein-coupled receptor class C group 5 member A and protein phosphatase 6c (68, 69). MiR-142-5p positively regulates intracellular levels of cyclic adenosine monophosphate (cAMP) to maintain the suppressive function of Tregs (58). In contrast, miR-142-3p can restrict cAMP levels in CD4+ T cells, which compromises the inhibitory function of Tregs (70). Many studies have confirmed that the expression levels of the abovementioned miRNAs were markedly altered in the Tregs of SLE patients and aberrant regulation of Tregs was involved in the development of SLE. Other miRNAs, such as miR-99a, miR-17 (71, 72), and miR150 (73), also have the ability to regulate the function of Tregs either directly or indirectly according to researches, which potentially provides new clues for future research on the development of SLE (Figure 2C).
In recent years, other cells with regulatory capabilities were revealed such as B regulatory cells and NK cells. As we mentioned above, miR-15a overexpression contributes to activating the apoptotic pathway of the B-10 subset (44) and then weaken its suppress effects on SLE and other inflammatory diseases (45). In another study, the recognition between iNKT cells and B cells through CD1d is associated with the tolerance of NKT cells (74). The increased miR-155 contribute to inhibiting CD1d expression in B cells by directly targeting the 3’-UTR of CD1d, and thus impair the tolerance of NKT cells (75).
The Roles of miRNAs in Abnormally Increased Production of Inflammatory Cytokines in SLE
Cytokines are a family of small proteins that play crucial roles as messengers of immune pathways and in the regulation of leukocyte activation. It is thought that the balance between proinflammatory and anti-inflammatory cytokines influences the clinical manifestations in many inflammatory diseases such as SLE and rheumatoid arthritis. These cytokines are mainly produced by helper T (Th) cells, which can be classified based on functional effects into T helper (Th) 1 (IFN-γ, IL-2, TNF-α), Th2 (IL-4, IL-5 and IL-6), Th17 (IL-17), and Tregs (IL-10). High levels of inflammatory cytokines may lead to the exacerbation of inflammatory responses, apoptosis, and production of autoantibodies that initiate and sustain SLE disease activity (76, 77). Dysregulation of chemokine production has been linked to the clinical manifestations and disease activity of SLE (78). Multifactorial components contribute to the immune modulation of cytokines, such as genetic polymorphisms, environmental factors, and hormonal alterations, among others, leading to irreversible impairment of self-immunological tolerance.
Type I IFNs are a family of cytokines produced by innate immune cells, especially plasmacytoid DCs and tissue cells, when viral components are perceived via retinoic acid-inducible-like receptors and TLRs. Increasing serum levels of IFN in lupus patients was described more than 40 years ago (79). Among the key immunological alterations in SLE, type I IFNs and related signaling pathways have been shown to play pivotal roles in disease pathogenesis (80, 81). Overexpression of type I IFNs can cease tolerance and induce autoimmune diseases via increased expression of major histocompatibility complex I (MHC I) molecules (82, 83), which enhances the cross-presentation of exogenous antigens. Likewise, the expression of other molecules related to the immune response promoted by IFN include MHC II, CD40, CD80, and CD86 in addition to chemokines and related cognate receptors, such as chemokine (C-X-C motif) ligand 10 and CXC chemokine receptor 3 (84). In response to IFN stimulation, DCs mature and transform into active antigen-presenting cells (APCs). Potent APCs induce the differentiation of naive CD4+ T cells, promote the development of CD8+ memory T cells and differentiation of Teffs, and suppress the functions of Tregs, which can collectively lead to the expansion of autoreactive T cells (85). The function of B cells is also impacted by type I IFNs, which is specifically manifested by extended survival and activation, leading to enhanced antibody production (86), ultimately resulting in the development of autoimmune diseases.
Under normal physiological conditions, immune cells spontaneously and negatively regulate TLR signaling through various mechanisms, so as to avoid abnormal activation and to maintain immunological balance (87). Extracellular miRNAs were shown to act as cell-to-cell regulators through a nonconventional mechanism of scilicet interactions with innate immune RNA receptors, such as TLR 7 and 8 (88, 89). MiR-146a is a negative regulator of TLR signaling (90), which can be induced by various stimuli, such as lipopolysaccharides, imiquimod R837, type A CpG oligonucleotides, and type I IFNs (51). Hou et al. (91) used bioinformatics tools to demonstrate that mature miR-146a reduced expression of multiple components in the type I IFN signaling cascades, including interleukin-1 receptor-associated kinase 1, tumor necrosis factor receptor-associated factor 6, IFN regulatory factor 5, and STAT1, thereby directly attenuating downstream activation of type I IFNs. Therefore, it appears that miR-146a deficiency is a causal factor that contributes to abnormal activation of the type I IFN pathway in SLE. Moreover, the coordinated activation of the type I IFN pathway was notably reduced after the introduction of miR-146a into the PBMCs of SLE patients. Collectively, these results suggested that exogeneous regulation of miR-146a is a promising therapeutic strategy for SLE. In 2010, Wang et al. (92) reported a positive association between miR-155 and IFN-α. MiR-155 feedback induced by viral infection promotes type I IFN signaling by targeting suppressor of cytokine signaling 1, a canonical negative regulator of type I IFN signaling, and mediates the enhancing effect of miR-155 on type I IFN-mediated antiviral responses. However, further studies are needed to clarify the role of miR-155 in type I IFN signaling and to investigate any possible correlations with SLE. Otherwise, the results of a luciferase reporter assay using Rat-1 fibroblasts stably expressing miR-181b revealed that miR-181b directly and negatively regulated IFN-α (93).
When regulated upon activation, normal T cell express and secrete CCL5 (RANTES) which is a key chemokine for T cell recruitment to inflammatory tissues, and active expression is known to enhance levels and detrimental effects of inflammatory factors in arthritis, nephritis, and a myriad of other inflammatory disorders (94). Downregulated expression of miR-125a in SLE patients contributes to blunting the negative regulation of RANTES expression by targeting Kruppel-like factor 13 in active T cells. Hence, miR-125a could potentially serve as a therapeutic target for the treatment of SLE via regulation of inflammatory chemokine production (95).
Conclusions and Perspectives
SLE is a heterogeneous chronic inflammatory autoimmune disorder, which is characterized by aberrant activation of lymphocytes, auto-antibodies, and inflammatory cytokine production (1). The highly heterogeneous nature of SLE has hampered a comprehensive understanding of the etiology of the disease in terms of both the underlying pathogenic processes and manifestations. Although researchers have spent a great deal of effort to figure it out, it is clear that there is still much to understand regarding the intricate network of SLE. MiRNAs are a family of small noncoding RNA molecules that provide quantitative regulation of genes at the post-transcriptional level by targeting mRNA translation or degradation (3). This summary of the current research results has shown that various, ubiquitous and functional miRNAs are involved in most immunological events leading to SLE. The upregulation of let-7c contributes to proinflammatory features of DCs in SLE (23); MiR-7 is associated with the overactivation of B cells and subsequent autoantibody production (37); MiR-21 up-regulation contributes to T cell hyperactivity (51); MiR-34a is associated with defection of Tregs and immune tolerance breakdown (65); Transfection of miR-98 alleviates the increased production of inflammatory cytokines (96). Different miRNAs can target the same mRNA, and one miRNA can target multiple mRNAs. Table 1 and Figure 4 list miRNAs that are preliminarily reported as being affiliated with immunoregulation in SLE. More extensive and in-depth studies on miRNAs are ongoing and the miRNAs involved in the pathogenesis of SLE are not limited to those described here.
Table 1.
The roles of miRNAs in the pathogenesis of SLE.
| Events | miRNA | Levels | Mechanism | Biological function | Experimental subject | Reference |
|---|---|---|---|---|---|---|
| DCs | let-7c | ↑ | suppresses SOCS1 expression | contributes to a broad spectrum of proinflammatory DC phenotype | DCBlimp1ko mice | (23) |
| miR-155 | ↑ | enhances TLR7-induced CD40 expression | contributes to the hyperactivated TLR7 response in lupus pDCs | Lupus-Prone NZB/W F1 mice | (25) | |
| miR-150 | ↓ | inhibits the expression of TREM-1 | enhances the inflammation responses in splenic cDCs in lupus prone mice | MRLlpr/lpr mice and C57BL/6 wild type mice | (26) | |
| B cell | miR-1246 | ↓ | reduces the inhibitory effect on the expression of EBF1 and activates AKT signaling pathway | contributes to the development, activation, and proliferation of B cells | PBMCs from SLE patients and healthy controls | (47) |
| miR-15a | ↑ | targets important genes involving cell cycle (e.g. cyclin D1) or cell apoptosis (e.g. Bcl-2) | disrupts the balance between regulatory B cells (B-10) and the pathogenic B cells (B-2) and increases the anti-dsDNA autoantibody levels | SLE mouse model | (46) | |
| miR-30a | ↑ | decreases the expression of Lyn such as CD19 and CD180 | regulates the B cell proliferation and antibody production | PBMCs from SLE patients and healthy controls | (40) | |
| hsa-miR-345 | ↑ | inhibits IRF8 | regulates the differentiation of B cells | PBMCs from SLE patients and healthy controls | (97) | |
| hsa-miR-224 | ↑ | contributes to IRF4 overexpression and increass production of IL-21 | B cell hyperresponsiveness | |||
| hsa-miR-10a | ↑ | inhibits IL-8 during the inactive phase | blocks the generation of autoreactive antibodies by B lymphocytes | |||
| miR-155 | ↑ | contributes to the reduction of SHIP-1 expression partly | increases serum IgG anti-dsDNA antibodies and kidney inflammation | Faslpr lupus-prone mice and their PBMCs | (38) | |
| increases the expression of PU.1 and TNF-α, which in turn promotes BAFF and CD19 protein expression | disrupts peripheral tolerance in B cells and thereby induces autoantibody production increase the SLEDAI-2K | PBMCs from SLE patients and healthy controls | (39) | |||
| miR-152-3p | ↑ | increases the BAFF expression by targeting and decreases the expression of KLF5 | causes B-cell self-reactivity and autoantibody production | PBMCs from SLE patients and healthy controls | (98) | |
| miR-7 | ↑ | negatively regulates the PTEN expression and down-regulates activation of PI3K/AKT signaling | promotes B cell differentiation into plasmablasts/plasma cells and spontaneous germinal center (GC) formation | MRL lpr/lpr lupus mice and their PBMCs | (35–37) | |
| T cell | miR-21 | ↑ | targets RASGRP1 to diminish Ras-MAPK pathway signaling and inhibit DNMT1 expression | contributes to DNA hypomethylation and T cell hyperactivity | SLE patients, lupus-prone MRL/lpr mice and healthy human PBMCs | (51–53) |
| decreases expression level of PDCD4 to regulate IL-10 and CD40L | contributes to aberrant T cell phenotype | PBMCs from SLE patients and healthy controls | ||||
| miR-148a | ↑ | targets DNMT1 to regulate CD70 and LFA-1 | contributes to DNA hypomethylation and T cell hyperactivity | SLE patients, lupus-prone MRL/lpr mice and healthy human PBMCs | (51) | |
| miR-126 | ↑ | targets DNMT1 to induce demethylation and up-regulate CD70 and CD11a | contributes to DNA hypomethylation and T cell hyperactivity | PBMCs from SLE patients and healthy controls | (55, 99) | |
| miR-29b | ↑ | targets Sp1 to negatively regulate DNMT1 and increases the expression of CD70 and CD11a gene | contributes to DNA hypomethylation and T cell hyperactivity | PBMCs from SLE patients and healthy controls | (56) | |
| miR-145 | ↓ | targets STAT-1 mRNA and ehances its expression level | associates with lupus nephritis significantly | PBMCs from SLE patients and healthy controls | (59) | |
| miR-224 | ↑ | targets API5 mRNA and decreases its expression level | accelerates T cell activation-induced cell death in Jurkat cells | |||
| miR-142-5p | ↓ | targets SLAM associated protein (SAP) | T cell activation and B cell hyperresponsiveness | PBMCs from SLE patients and healthy controls | (57) | |
| miR-142-3p | ↓ | targets SLAM family (IL-10 and CD84) | T cell activation and B cell hyperresponsiveness | |||
| immune tolerance | miR-21 | ↑ | regulates FOXP3 expression positively | negatively regulates Treg cell development | umbilical cord blood mononuclear cells (UCBMC) | (68) |
| miR-142-3p | ↑ | reduces the level of intracellular cAMP by inhibiting AC9 production | limits the suppressor function of Treg cells | splenocytes from naive BALB/c mice | (70) | |
| miR-31 | ↑ | targets Gprc5a, Ppp6C and Foxp3 | negatively regulates Treg cell generation and differentiation | PBMCs from murine and SLE patient | (68, 69) | |
| miR-17 | ↓ | targets TGFβRII and CREB1 | inhibits iTreg differentiation and facilitates effector T-cell responses | mice and PBMCs from SLE patients | (71, 72) | |
| miR-142-5p | ↓ | enhances Pde3b transcription and reduces the level of intracellular cAMP | key determinant of Treg function and peripheral immune tolerance | spleen and peripheral lymph nodes cells from mice | (58) | |
| miR-34a | ↑ | targets and attenuates the expression of Foxp3 gene | inhibits Treg cell differentiation and disrupts Treg/Th17 balance | PBMCs from murine and SLE patient | (65–67) | |
| miR-125a | ↓ | insufficiently targets and increases the expression of STAT3, Ifng, Il13 | labilizes the immunoregulatory capacity of Treg cells | mice, SLE patient and their PBMCs | (63, 64) | |
| miR-15a | ↑ | activating the apoptotic pathway of the B-10 subset | weaken suppress effects of B-10 cells on SLE and other inflammatory diseases | Female (NZB × NZW)F1 or B/W mice | (46) | |
| miR-155 | ↑ | inhibiting CD1d expression in B cells | impair the tolerance of NKT cells | Female C57BL/6J and MRL/lpr lupus-prone mice | (75) | |
| inflammatory chemokine | miR-146a | ↓ | impairs negative regulation of multiple components expression in the type I IFN pathway, including IRAK1, TRAF6, IRF-5 and STAT-1 | directly activates downstream of type I IFN | C57BL/6 mice, HEK293 cells | (90, 91) |
| miR-125a | ↓ | increases KLF13 gene expression and hence RANTES expression | regulates inflammatory chemokines production and contribute to organ inflammation | PBMCs from SLE patients and healthy controls | (95) | |
| miR-181b | ↓ | directly regulates AID and IFN-α mRNAs | impairs negative regulation to IFN-α | PBMCs from SLE patients and healthy controls | (93) | |
| miR-410 | ↓ | impairs negative regulation to transcription activity of STAT3 | increases the expression levels of IL-10 | PBLs from SLE patients and healthy controls | (100) | |
| miR-302d | ↓ | increases IRF9 gene expression and enhances ISG expression | negatively correlates with IFN score | PBMCs from SLE patients and healthy controls | (101) | |
| miR-31 | ↑ | targets serine/threonine kinase 40 | promotes NF-κB signaling to enhance inflammatory cytokine production. | PBMCs and splenocytes in MRL/lpr mice | (102) | |
| miR-98 | ↓ | impairs targeting gene IL-6 | promotes STAT3-mediated cell proliferation and inflammatory cytokine production | PBMCs from SLE patients and healthy controls | (96) | |
| miR-654 | ↓ | inhibits MIF expression by binding to MIF mRNA | selectively suppresses the phosphorylation of ERK and AKT, reduces downstream inflammatory cytokine production | PBMCs from SLE patients and healthy controls | (103) |
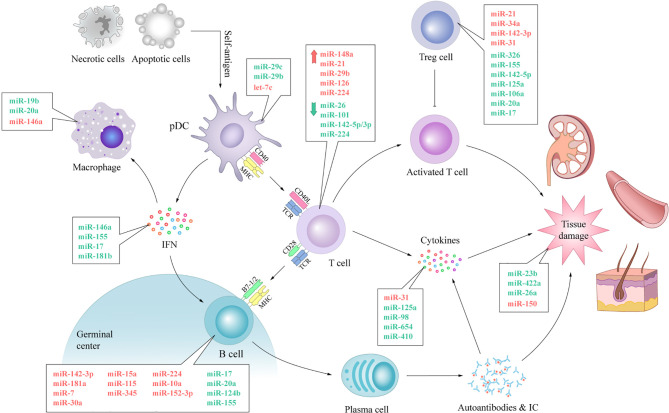
Figure 4.
The expression of miRNAs and their involvement in immunological dysfunction of SLE. The aberrant expression of miRNAs can be noticed in almost every vital process of SLE. The expression levels of anti-inflammatory miRNAs, such as miR-19b, miR-146a, miR-142-5p, miR-124b and miR-422a, decrease, while the expression levels of pro-inflammatory miRNAs, such as miR-146a, miR-224, miR-29b, miR-31 and miR-150, increase. Dysregulated miRNAs disturb the normal biological procedure of immune response by swaying the expression of pivotal protein molecule (e.g. CD40, CD40L, IFN, BAFF) directly or indirectly. These processes lead to autoimmune and tissue damage with aberrantly activated T lymphocytes, over-activated B cells, autoantibody accumulation and abnormally increased inflammatory cytokines in SLE. IC, immune complex; TCR, T cell receptor.
Based on the results of current studies, the exact relationship between miRNAs and the pathogenesis of SLE cannot be concluded arbitrarily. Although dysregulation of miRNAs has been reported to be involved in most important events in the progression of SLE, there is no conclusive evidence that abnormal miRNA expression is a cause or merely a consequence of SLE. Many of current studies are limited to the analysis of how miRNA affects SLE at the cytological level. These studies demonstrated that abnormally expressed miRNAs in PBMCs could lead to lupus-like cellular phenotypes characterized by overexpression of TLRs (25, 57, 104) or co-stimulatory molecules (51, 73), enhanced cell signal transduction pathways (23, 59, 65, 69, 70), increased inflammatory cytokines (66, 67, 91), which have been found in abundance in SLE. However, whether small miRNAs can be a cause for the development of this intricate disease in healthy individuals has not been demonstrated in the present study. Even so, the role of miRNAs in molecular regulatory network of SLE is fascinating and have suggested an exciting avenue to enrich our understanding of SLE. Investigations into miRNAs and related molecular mechanisms involved in SLE will help to clarify the pathogenesis of this complex disease and potentially facilitate the identification of new treatment modalities. MiRNA-based therapeutic agents have been developing for the treatment of a variety of diseases (15, 105). Gene knockout and transfection of several miRNAs have been demonstrated to alleviate disease activity of SLE in mice (23, 38). In addition, small-molecule drugs that target the biogenesis of miRNA-155 for the treatment of SLE have been recently discovered (106). However, these approaches have not yet reached the level of clinical application. Current studies have made significant progress to analysis how miRNA affects SLE at the cytological level, but there are still many efforts to be invested in how far miRNAs may have played a role and whether it has the potential to be developed into new therapeutic targets. Thus, it is still too early to make conclusions about the therapeutic effect of miRNAs on lupus. Despite the considerable therapeutic potential, the results of basic studies are still a long way from being translating to clinical care, thus further research in this challenging field is urgently needed.
Author Contributions
MC, KM, and LS were involved in the conception of the study. MC and KM were involved in writing the article. MQ, ZH, ZD, YL, XL, QZ and CL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by Foundation of Popularization project Department of Sichuan Health commission (19PJYY0731), Foundation of “apricot grove scholar” of Chengdu University of Traditional Chinese Medicine (2019yky10), Xinlin scholars Science Foundation of Chengdu University of Traditional Chinese Medicine (ARQN2019007), Science and technology project fund for Returned Students of Sichuan Province (00809504), The project of 2020 High-level Overseas Chinese Talent Returning Funding.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| SLE | Systemic lupus erythematosus |
| miRNAs | microRNAs |
| auto-Abs | autoantibodies |
| DCs | dendritic cells |
| TLR | Toll-like receptor |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| pri-miRNAs | primary RNA transcripts |
| RNase III enzyme | ribonuclease III enzyme |
| pre-miRNA | precursor miRNAs |
| RISC | RNA-induced silencing complex |
| mRNA | messenger RNA |
| 3’-UTR | 3’-untranslated region |
| mDC | myeloid DC |
| pDC | plasmacytoid DC |
| Blimp1 | B lymphocyte–induced maturation protein-1 |
| SOCS | suppressor of cytokine signaling |
| SHIP-1 | SH2 domain-containing inositol 5’-phosphatase 1 |
| JAK-STAT | Janus kinase/signal transducer and activator of transcription |
| PI3K | phosphatidylinositol 3-kinase |
| AKT | protein kinase B |
| BCR | B cell receptor |
| LYN | Lck/Yes novel tyrosine kinase |
| FcγRIIb | Fcγ receptor IIb |
| PTEN | phosphatase and tensin homolog |
| Bcl-2 | B cell lymphoma-2 |
| EBF1 | expression of Early B cell factor 1 |
| Tfh cells | follicular helper T cells |
| DNMTs | DNA methyltransferases |
| RASGRP1 | rasguanyl-nucleotide-releasingprotein1 |
| TNFSF | tumour necrosis factor superfamily |
| LAF-1 | lymphocyte function-associated antigen 1 |
| SLAM | signaling lymphocytic activation molecule |
| IL-10 | interleukin 10 |
| SAP | SLAM associated protein |
| IgG | immunoglobulin G |
| STAT-1 | activator of transcription-1 |
| API5 | apoptosis inhibitory protein 5 |
| Foxp3 | forkhead box P3 |
| Teff cell | effector T cell |
| Treg cells | regulatory T cells |
| PBMCs | peripheral blood mononuclear cells |
| TNF α | tumour necrosis factor α |
| cAMP | cyclic adenosine monophosphate |
| Th cell | T helper cell |
| IFN-1 | type 1 interferon |
| MHC I | major histocompatibility complex I |
| CXCL | chemokine (C-X-C motif) ligand |
| CXCR 3 | CXC chemokine receptors 3 |
| APCs | antigen-presenting cells |
| RANTES | regulated on activation, normal T cell expressed and secreted |
References
- 1.Dorner T, Furie R. Novel Paradigms in Systemic Lupus Erythematosus. Lancet (2019) 393(10188):2344–58. 10.1016/S0140-6736(19)30546-X [DOI] [PubMed] [Google Scholar]
- 2.Kiriakidou M, Ching CL. Systemic Lupus Erythematosus. Ann Intern Med (2020) 172(11):ITC81–96. 10.7326/AITC202006020 [DOI] [PubMed] [Google Scholar]
- 3.Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An Overview of Micrornas: Biology, Functions, Therapeutics, and Analysis Methods. J Cell Physiol (2019) 234(5):5451–65. 10.1002/jcp.27486 [DOI] [PubMed] [Google Scholar]
- 4.Lee RC, Feinbaum RL, Ambros V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs With Antisense Complementarity to Lin-14. Cell (1993) 75(5):843–54. 10.1016/0092-8674(93)90529-y [DOI] [PubMed] [Google Scholar]
- 5.Alles J, Fehlmann T, Fischer U, Backes C, Galata V, Minet M, et al. An Estimate of the Total Number of True Human MiRNAs. Nucleic Acids Res (2019) 47(7):3353–64. 10.1093/nar/gkz097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol (2018) 141(4):1202–7. 10.1016/j.jaci.2017.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozomara A, Birgaoanu M, Griffiths-Jones S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res (2019) 47(D1):D155–D62. 10.1093/nar/gky1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan VS, Nie YJ, Shen N, Yan S, Mok MY, Lau CS. Distinct Roles of Myeloid and Plasmacytoid Dendritic Cells in Systemic Lupus Erythematosus. Autoimmun Rev (2012) 11(12):890–7. 10.1016/j.autrev.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 9.Van Roosbroeck K, Calin GA. Cancer Hallmarks and MicroRNAs: The Therapeutic Connection. Adv Cancer Res (2017) 135:119–49. 10.1016/bs.acr.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 10.Sebastiani G, Nigi L, Grieco GE, Mancarella F, Ventriglia G, Dotta F. Circulating MicroRNAs and Diabetes Mellitus: A Novel Tool for Disease Prediction, Diagnosis, and Staging? J Endocrinol Invest (2017) 40(6):591–610. 10.1007/s40618-017-0611-4 [DOI] [PubMed] [Google Scholar]
- 11.Thum T. MicroRNA Therapeutics in Cardiovascular Medicine. EMBO Mol Med (2012) 4(1):3–14. 10.1002/emmm.201100191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters LJF, Floege J, Biessen EAL, Jankowski J, van der Vorst EPC. MicroRNAs in Chronic Kidney Disease: Four Candidates for Clinical Application. Int J Mol Sci (2020) 21(18):3–4. 10.3390/ijms21186547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long H, Wang X, Chen Y, Wang L, Zhao M, Lu Q. Dysregulation of MicroRNAs in Autoimmune Diseases: Pathogenesis, Biomarkers and Potential Therapeutic Targets. Cancer Lett (2018) 428:90–103. 10.1016/j.canlet.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 14.Dai Y, Huang YS, Tang M, Lv TY, Hu CX, Tan YH, et al. Microarray Analysis of Microrna Expression in Peripheral Blood Cells of Systemic Lupus Erythematosus Patients. Lupus (2007) 16(12):939–46. 10.1177/0961203307084158 [DOI] [PubMed] [Google Scholar]
- 15.Hong DS, Kang YK, Borad M, Sachdev J, Ejadi S, Lim HY, et al. Phase 1 Study of MRX34, A Liposomal MiR-34a Mimic, in Patients With Advanced Solid Tumours. Br J Cancer (2020) 122(11):1630–7. 10.1038/s41416-020-0802-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuyama H, Suzuki HI. Systems and Synthetic MicroRNA Biology: From Biogenesis to Disease Pathogenesis. Int J Mol Sci (2019) 21(1):2–3. 10.3390/ijms21010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tafrihi M, Hasheminasab E. MiRNAs: Biology, Biogenesis, Their Web-Based Tools, and Databases. MicroRNA (2019) 8(1):4–27. 10.2174/2211536607666180827111633 [DOI] [PubMed] [Google Scholar]
- 18.Krol J, Loedige I, Filipowicz W. The Widespread Regulation of MicroRNA Biogenesis, Function and Decay. Nat Rev Genet (2010) 11(9):597–610. 10.1038/nrg2843 [DOI] [PubMed] [Google Scholar]
- 19.Liao X, Reihl AM, Luo XM. Breakdown of Immune Tolerance in Systemic Lupus Erythematosus by Dendritic Cells. J Immunol Res (2016) 2016:6269157. 10.1155/2016/6269157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colonna L, Dinnall JA, Shivers DK, Frisoni L, Caricchio R, Gallucci S. Abnormal Costimulatory Phenotype and Function of Dendritic Cells Before and After the Onset of Severe Murine Lupus. Arthritis Res Ther (2006) 8(2):R49. 10.1186/ar1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JM, Park SH, Kim HY, Kwok SK. A Plasmacytoid Dendritic Cells-Type I Interferon Axis Is Critically Implicated in the Pathogenesis of Systemic Lupus Erythematosus. Int J Mol Sci (2015) 16(6):14158–70. 10.3390/ijms160614158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu YJ. IPC: Professional Type 1 Interferon-Producing Cells and Plasmacytoid Dendritic Cell Precursors. Annu Rev Immunol (2005) 23:275–306. 10.1146/annurev.immunol.23.021704.115633 [DOI] [PubMed] [Google Scholar]
- 23.Kim SJ, Gregersen PK, Diamond B. Regulation of Dendritic Cell Activation by MicroRNA Let-7c and BLIMP1. J Clin Invest (2013) 123(2):823–33. 10.1172/JCI64712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic Function of Blimp-1 in Dendritic Cells. J Exp Med (2011) 208(11):2193–9. 10.1084/jem.20110658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan S, Yim LY, Tam RC, Chan A, Lu L, Lau CS, et al. MicroRNA-155 Mediates Augmented CD40 Expression in Bone Marrow Derived Plasmacytoid Dendritic Cells in Symptomatic Lupus-Prone NZB/W F1 Mice. Int J Mol Sci (2016) 17(8):7–9. 10.3390/ijms17081282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao S, Yuan L, Wang Y, Hua C. Enhanced Expression of TREM-1 in Splenic Cdcs in Lupus Prone Mice and it was Modulated by Mirna-150. Mol Immunol (2017) 81:127–34. 10.1016/j.molimm.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 27.Shen P, Fillatreau S. Antibody-Independent Functions of B Cells: A Focus on Cytokines. Nat Rev Immunol (2015) 15(7):441–51. 10.1038/nri3857 [DOI] [PubMed] [Google Scholar]
- 28.Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, et al. Sustained B Cell Depletion by CD19-Targeted CAR T Cells Is a Highly Effective Treatment for Murine Lupus. Sci Transl Med (2019) 11(482):8–9. 10.1126/scitranslmed.aav1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores-Mendoza G, Sanson SP, Rodriguez-Castro S, Crispin JC, Rosetti F. Mechanisms of Tissue Injury in Lupus Nephritis. Trends Mol Med (2018) 24(4):364–78. 10.1016/j.molmed.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 30.Munoz LE, Janko C, Schulze C, Schorn C, Sarter K, Schett G, et al. Autoimmunity and Chronic Inflammation - Two Clearance-Related Steps in the Etiopathogenesis of SLE. Autoimmun Rev (2010) 10(1):38–42. 10.1016/j.autrev.2010.08.015 [DOI] [PubMed] [Google Scholar]
- 31.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New Insights Into the Immunopathogenesis of Systemic Lupus Erythematosus. Nat Rev Rheumatol (2016) 12(12):716–30. 10.1038/nrrheum.2016.186 [DOI] [PubMed] [Google Scholar]
- 32.Getahun A, Beavers NA, Larson SR, Shlomchik MJ, Cambier JC. Continuous Inhibitory Signaling by Both SHP-1 and SHIP-1 Pathways Is Required to Maintain Unresponsiveness of Anergic B Cells. J Exp Med (2016) 213(5):751–69. 10.1084/jem.20150537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodie EJ, Infantino S, Low MSY, Tarlinton DM. Lyn, Lupus, and (B) Lymphocytes, a Lesson on the Critical Balance of Kinase Signaling in Immunity. Front Immunol (2018) 9:401. 10.3389/fimmu.2018.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jhou JP, Chen SJ, Huang HY, Lin WW, Huang DY, Tzeng SJ. Upregulation of Fcgammariib by Resveratrol via NF-KappaB Activation Reduces B-Cell Numbers and Ameliorates Lupus. Exp Mol Med (2017) 49(9):e381. 10.1038/emm.2017.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo W, Hawse W, Conter L, Trivedi N, Weisel F, Wikenheiser D, et al. The AKT Kinase Signaling Network Is Rewired by PTEN to Control Proximal BCR Signaling in Germinal Center B Cells. Nat Immunol (2019) 20(6):736–46. 10.1038/s41590-019-0376-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu XN, Ye YX, Niu JW, Li Y, Li X, You X, et al. Defective PTEN Regulation Contributes to B Cell Hyperresponsiveness in Systemic Lupus Erythematosus. Sci Transl Med (2014) 6(246):246ra99. 10.1126/scitranslmed.3009131 [DOI] [PubMed] [Google Scholar]
- 37.Wang M, Chen H, Qiu J, Yang HX, Zhang CY, Fei YY, et al. Antagonizing MiR-7 Suppresses B Cell Hyperresponsiveness and Inhibits Lupus Development. J Autoimmun (2020) 109:102440. 10.1016/j.jaut.2020.102440 [DOI] [PubMed] [Google Scholar]
- 38.Thai TH, Patterson HC, Pham DH, Kis-Toth K, Kaminski DA, Tsokos GC. Deletion of Microrna-155 Reduces Autoantibody Responses and Alleviates Lupus-Like Disease in the Fas(Lpr) Mouse. Proc Natl Acad Sci USA (2013) 110(50):20194–9. 10.1073/pnas.1317632110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aboelenein HR, Hamza MT, Marzouk H, Youness RA, Rahmoon M, Salah S, et al. Reduction of CD19 Autoimmunity Marker on B Cells of Paediatric SLE Patients Through Repressing PU.1/TNF-Alpha/BAFF Axis Pathway by MiR-155. Growth Factors (2017) 35(2-3):49–60. 10.1080/08977194.2017.1345900 [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Dong J, Mu R, Gao Y, Tan X, Li Y, et al. MicroRNA-30a Promotes B Cell Hyperactivity in Patients With Systemic Lupus Erythematosus by Direct Interaction With Lyn. Arthritis Rheum (2013) 65(6):1603–11. 10.1002/art.37912 [DOI] [PubMed] [Google Scholar]
- 41.Ban T, Sato GR, Nishiyama A, Akiyama A, Takasuna M, Umehara M, et al. Lyn Kinase Suppresses the Transcriptional Activity of IRF5 in the TLR-Myd88 Pathway to Restrain the Development of Autoimmunity. Immunity (2016) 45(2):319–32. 10.1016/j.immuni.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 42.Vasquez A, Baena A, Gonzalez LA, Restrepo M, Munoz CH, Vanegas-Garcia A, et al. Altered Recruitment of Lyn, Syk and ZAP-70 Into Lipid Rafts of Activated B Cells in Systemic Lupus Erythematosus. Cell Signal (2019) 58:9–19. 10.1016/j.cellsig.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 43.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. MiR-15 and MiR-16 Induce Apoptosis by Targeting BCL2. Proc Natl Acad Sci U.S.A. (2005) 102(39):13944–9. 10.1073/pnas.0506654102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pekarsky Y, Balatti V, Croce CM. BCL2 and MiR-15/16: From Gene Discovery to Treatment. Cell Death Differ (2018) 25(1):21–6. 10.1038/cdd.2017.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang T, Mei Y, Li Z. Research Progress on Regulatory B Cells in Systemic Lupus Erythematosus. BioMed Res Int (2019) 2019:7948687. 10.1155/2019/7948687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan Y, Kasar S, Underbayev C, Vollenweider D, Salerno E, Kotenko SV, et al. Role of MicroRNA-15a in Autoantibody Production in Interferon-Augmented Murine Model of Lupus. Mol Immunol (2012) 52(2):61–70. 10.1016/j.molimm.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, et al. Requirement of Bic/MicroRNA-155 for Normal Immune Function. Science (2007) 316(5824):608–11. 10.1126/science.1139253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen SH, Lv QL, Hu L, Peng MJ, Wang GH, Sun B. DNA Methylation Alterations in the Pathogenesis of Lupus. Clin Exp Immunol (2017) 187(2):185–92. 10.1111/cei.12877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohan KN, Chaillet JR. Cell and Molecular Biology of DNA Methyltransferase 1. Int Rev Cell Mol Biol (2013) 306:1–42. 10.1016/B978-0-12-407694-5.00001-8 [DOI] [PubMed] [Google Scholar]
- 50.Richardson B, Sawalha AH, Ray D, Yung R. Murine Models of Lupus Induced by Hypomethylated T Cells (DNA Hypomethylation and Lupus…). Methods Mol Biol (2012) 900:169–80. 10.1007/978-1-60761-720-4_8 [DOI] [PubMed] [Google Scholar]
- 51.Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, et al. MicroRNA-21 and MicroRNA-148a Contribute to DNA Hypomethylation in Lupus CD4+ T Cells by Directly and Indirectly Targeting DNA Methyltransferase 1. J Immunol (2010) 184(12):6773–81. 10.4049/jimmunol.0904060 [DOI] [PubMed] [Google Scholar]
- 52.Stagakis E, Bertsias G, Verginis P, Nakou M, Hatziapostolou M, Kritikos H, et al. Identification of Novel MicroRNA Signatures Linked to Human Lupus Disease Activity and Pathogenesis: Mir-21 Regulates Aberrant T Cell Responses Through Regulation of PDCD4 Expression. Ann Rheum Dis (2011) 70(8):1496–506. 10.1136/ard.2010.139857 [DOI] [PubMed] [Google Scholar]
- 53.Garchow BG, Bartulos Encinas O, Leung YT, Tsao PY, Eisenberg RA, Caricchio R, et al. Silencing of Microrna-21 in Vivo Ameliorates Autoimmune Splenomegaly in Lupus Mice. EMBO Mol Med (2011) 3(10):605–15. 10.1002/emmm.201100171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weeding E, Sawalha AH. Deoxyribonucleic Acid Methylation in Systemic Lupus Erythematosus: Implications for Future Clinical Practice. Front Immunol (2018) 9:875. 10.3389/fimmu.2018.00875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao S, Wang Y, Liang Y, Zhao M, Long H, Ding S, et al. Microrna-126 Regulates DNA Methylation in CD4+ T Cells and Contributes to Systemic Lupus Erythematosus by Targeting DNA Methyltransferase 1. Arthritis Rheumatism (2011) 63(5):1376–86. 10.1002/art.30196 [DOI] [PubMed] [Google Scholar]
- 56.Qin H, Zhu X, Liang J, Wu J, Yang Y, Wang S, et al. MicroRNA-29b Contributes to DNA Hypomethylation of CD4+ T Cells in Systemic Lupus Erythematosus by Indirectly Targeting DNA Methyltransferase 1. J Dermatol Sci (2013) 69(1):61–7. 10.1016/j.jdermsci.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 57.Ding S, Liang Y, Zhao M, Liang G, Long H, Zhao S, et al. Decreased MicroRNA-142-3p/5p Expression Causes CD4+ T Cell Activation and B Cell Hyperstimulation in Systemic Lupus Erythematosus. Arthritis Rheum (2012) 64(9):2953–63. 10.1002/art.34505 [DOI] [PubMed] [Google Scholar]
- 58.Anandagoda N, Willis JC, Hertweck A, Roberts LB, Jackson I, Gokmen MR, et al. MicroRNA-142-Mediated Repression of Phosphodiesterase 3B Critically Regulates Peripheral Immune Tolerance. J Clin Invest (2019) 129(3):1257–71. 10.1172/JCI124725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu MC, Lai NS, Chen HC, Yu HC, Huang KY, Tung CH, et al. Decreased Microrna(MiR)-145 and Increased MiR-224 Expression in T Cells From Patients With Systemic Lupus Erythematosus Involved in Lupus Immunopathogenesis. Clin Exp Immunol (2013) 171(1):91–9. 10.1111/j.1365-2249.2012.04676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC. Regulatory T Cells in the Treatment of Disease. Nat Rev Drug Discov (2018) 17(11):823–44. 10.1038/nrd.2018.148 [DOI] [PubMed] [Google Scholar]
- 61.Shevyrev D, Tereshchenko V. Treg Heterogeneity, Function, and Homeostasis. Front Immunol (2019) 10:3100. 10.3389/fimmu.2019.03100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shan J, Jin H, Xu Y. T Cell Metabolism: A New Perspective on Th17/Treg Cell Imbalance in Systemic Lupus Erythematosus. Front Immunol (2020) 11:1027. 10.3389/fimmu.2020.01027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan W, Zhu S, Dai D, Liu Z, Li D, Li B, et al. MiR-125a Targets Effector Programs to Stabilize Treg-Mediated Immune Homeostasis. Nat Commun (2015) 6:7096. 10.1038/ncomms8096 [DOI] [PubMed] [Google Scholar]
- 64.Zhang J, Chen C, Fu H, Yu J, Sun Y, Huang H, et al. MicroRNA-125a-Loaded Polymeric Nanoparticles Alleviate Systemic Lupus Erythematosus by Restoring Effector/Regulatory T Cells Balance. ACS Nano (2020) 14(4):4414–29. 10.1021/acsnano.9b09998 [DOI] [PubMed] [Google Scholar]
- 65.Xie M, Wang J, Gong W, Xu H, Pan X, Chen Y, et al. NF-KappaB-Driven MiR-34a Impairs Treg/Th17 Balance via Targeting Foxp3. J Autoimmun (2019) 102:96–113. 10.1016/j.jaut.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 66.Goschl L, Scheinecker C, Bonelli M. Treg Cells in Autoimmunity: From Identification to Treg-Based Therapies. Semin Immunopathol (2019) 41(3):301–14. 10.1007/s00281-019-00741-8 [DOI] [PubMed] [Google Scholar]
- 67.Kumar P, Saini S, Khan S, Surendra Lele S, Prabhakar BS. Restoring Self-Tolerance in Autoimmune Diseases by Enhancing Regulatory T-Cells. Cell Immunol (2019) 339:41–9. 10.1016/j.cellimm.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rouas R, Fayyad-Kazan H, El Zein N, Lewalle P, Rothe F, Simion A, et al. Human Natural Treg MicroRNA Signature: Role of MicroRNA-31 and MicroRNA-21 in FOXP3 Expression. Eur J Immunol (2009) 39(6):1608–18. 10.1002/eji.200838509 [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Ke F, Liu Z, Bai J, Liu J, Yan S, et al. MicroRNA-31 Negatively Regulates Peripherally Derived Regulatory T-Cell Generation by Repressing Retinoic Acid-Inducible Protein 3. Nat Commun (2015) 6:7639. 10.1038/ncomms8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX, et al. MiR-142-3p Restricts Camp Production in CD4+CD25- T Cells and CD4+CD25+ TREG Cells by Targeting AC9 mRNA. EMBO Rep (2009) 10(2):180–5. 10.1038/embor.2008.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, et al. Molecular Dissection of the MiR-17-92 Cluster’s Critical Dual Roles in Promoting Th1 Responses and Preventing Inducible Treg Differentiation. Blood (2011) 118(20):5487–97. 10.1182/blood-2011-05-355644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin F, Hu H, Xu M, Zhan S, Wang Y, Zhang H, et al. Serum Microrna Profiles Serve as Novel Biomarkers for Autoimmune Diseases. Front Immunol (2018) 9:2381. 10.3389/fimmu.2018.02381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warth SC, Hoefig KP, Hiekel A, Schallenberg S, Jovanovic K, Klein L, et al. Induced MiR-99a Expression Represses Mtor Cooperatively With MiR-150 to Promote Regulatory T-Cell Differentiation. EMBO J (2015) 34(9):1195–213. 10.15252/embj.201489589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diana J, Beaudoin L, Gautron AS, Lehuen A. NKT and Tolerance. Methods Mol Biol (2011) 677:193–206. 10.1007/978-1-60761-869-0_14 [DOI] [PubMed] [Google Scholar]
- 75.Liu F, Fan H, Ren D, Dong G, Hu E, Ji J, et al. TLR9-Induced MiR-155 and Ets-1 Decrease Expression of CD1d on B Cells in SLE. Eur J Immunol (2015) 45(7):1934–45. 10.1002/eji.201445286 [DOI] [PubMed] [Google Scholar]
- 76.Guimaraes PM, Scavuzzi BM, Stadtlober NP, Franchi Santos L, Lozovoy MAB, Iriyoda TMV, et al. Cytokines in Systemic Lupus Erythematosus: Far Beyond Th1/Th2 Dualism Lupus: Cytokine Profiles. Immunol Cell Biol (2017) 95(9):824–31. 10.1038/icb.2017.53 [DOI] [PubMed] [Google Scholar]
- 77.Miyabe Y, Lian J, Miyabe C, Luster AD. Chemokines in Rheumatic Diseases: Pathogenic Role and Therapeutic Implications. Nat Rev Rheumatol (2019) 15(12):731–46. 10.1038/s41584-019-0323-6 [DOI] [PubMed] [Google Scholar]
- 78.Lu R, Munroe ME, Guthridge JM, Bean KM, Fife DA, Chen H, et al. Dysregulation of Innate and Adaptive Serum Mediators Precedes Systemic Lupus Erythematosus Classification and Improves Prognostic Accuracy of Autoantibodies. J Autoimmun (2016) 74:182–93. 10.1016/j.jaut.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune Interferon in the Circulation of Patients With Autoimmune Disease. N Engl J Med (1979) 301(1):5–8. 10.1056/NEJM197907053010102 [DOI] [PubMed] [Google Scholar]
- 80.Muskardin TLW, Niewold TB. Type I Interferon in Rheumatic Diseases. Nat Rev Rheumatol (2018) 14(4):214–28. 10.1038/nrrheum.2018.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Postal M, Vivaldo JF, Fernandez-Ruiz R, Paredes JL, Appenzeller S, Niewold TB. Type I Interferon in the Pathogenesis of Systemic Lupus Erythematosus. Curr Opin Immunol (2020) 67:87–94. 10.1016/j.coi.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Attallah AM, Strong DM. Differential Effects of Interferon on the MHC Expression of Human Lymphocytes. Enhanced Expression of HLA Without Effect on Ia. Int Arch Allergy Appl Immunol (1979) 60(1):101–5. 10.1159/000232328 [DOI] [PubMed] [Google Scholar]
- 83.Wu X, Fan Z, Chen M, Chen Y, Rong D, Cui Z, et al. Forkhead Transcription Factor FOXO3a Mediates Interferon-Gamma-Induced MHC II Transcription in Macrophages. Immunology (2019) 158(4):304–13. 10.1111/imm.13116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ronnblom L, Leonard D. Interferon Pathway in SLE: One Key to Unlocking the Mystery of the Disease. Lupus Sci Med (2019) 6(1):e000270. 10.1136/lupus-2018-000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuliasih Y, Rahmawati LD, Putri RM. Th17/Treg Ratio and Disease Activity in Systemic Lupus Erythematosus. Caspian J Intern Med (2019) 10(1):65–72. 10.22088/cjim.10.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sjostrand M, Johansson A, Aqrawi L, Olsson T, Wahren-Herlenius M, Espinosa A. The Expression of BAFF Is Controlled by IRF Transcription Factors. J Immunol (2016) 196(1):91–6. 10.4049/jimmunol.1501061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leifer CA, Medvedev AE. Molecular Mechanisms of Regulation of Toll-Like Receptor Signaling. J Leukoc Biol (2016) 100(5):927–41. 10.1189/jlb.2MR0316-117RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs Bind to Toll-Like Receptors to Induce Prometastatic Inflammatory Response. Proc Natl Acad Sci USA (2012) 109(31):E2110–6. 10.1073/pnas.1209414109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salvi V, Gianello V, Busatto S, Bergese P, Andreoli L, D’Oro U, et al. Exosome-Delivered MicroRNAs Promote IFN-Alpha Secretion by Human Plasmacytoid Dcs via TLR7. JCI Insight (2018) 3(10):5–6. 10.1172/jci.insight.98204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-KappaB-Dependent Induction of MicroRNA MiR-146, an Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc Natl Acad Sci USA (2006) 103(33):12481–6. 10.1073/pnas.0605298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hou J, Wang P, Lin L, Liu X, Ma F, An H, et al. MicroRNA-146a Feedback Inhibits RIG-I-Dependent Type I IFN Production in Macrophages by Targeting TRAF6, IRAK1, and IRAK2. J Immunol (2009) 183(3):2150–8. 10.4049/jimmunol.0900707 [DOI] [PubMed] [Google Scholar]
- 92.Wang P, Hou J, Lin L, Wang C, Liu X, Li D, et al. Inducible MicroRNA-155 Feedback Promotes Type I IFN Signaling in Antiviral Innate Immunity by Targeting Suppressor of Cytokine Signaling 1. J Immunol (2010) 185(10):6226–33. 10.4049/jimmunol.1000491 [DOI] [PubMed] [Google Scholar]
- 93.Kaga H, Komatsuda A, Omokawa A, Ito M, Teshima K, Tagawa H, et al. Downregulated Expression of Mir-155, MiR-17, and MiR-181b, and Upregulated Expression of Activation-Induced Cytidine Deaminase and Interferon-Alpha in PBMCs From Patients With SLE. Mod Rheumatol (2015) 25(6):865–70. 10.3109/14397595.2015.1030102 [DOI] [PubMed] [Google Scholar]
- 94.Appay V, Rowland-Jones SL. RANTES: A Versatile and Controversial Chemokine. Trends Immunol (2001) 22(2):83–7. 10.1016/s1471-4906(00)01812-3 [DOI] [PubMed] [Google Scholar]
- 95.Zhao X, Tang Y, Qu B, Cui H, Wang S, Wang L, et al. MicroRNA-125a Contributes to Elevated Inflammatory Chemokine RANTES Levels via Targeting KLF13 in Systemic Lupus Erythematosus. Arthritis Rheum (2010) 62(11):3425–35. 10.1002/art.27632 [DOI] [PubMed] [Google Scholar]
- 96.Yuan S, Tang C, Chen D, Li F, Huang M, Ye J, et al. MiR-98 Modulates Cytokine Production From Human Pbmcs in Systemic Lupus Erythematosus by Targeting IL-6 mRNA. J Immunol Res (2019) 2019:9827574. 10.1155/2019/9827574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinez-Ramos R, Garcia-Lozano JR, Lucena JM, Castillo-Palma MJ, Garcia-Hernandez F, Rodriguez MC, et al. Differential Expression Pattern of MicroRNAs in CD4+ and CD19+ Cells From Asymptomatic Patients With Systemic Lupus Erythematosus. Lupus (2014) 23(4):353–9. 10.1177/0961203314522335 [DOI] [PubMed] [Google Scholar]
- 98.Luo S, Ding S, Liao J, Zhang P, Liu Y, Zhao M, et al. Excessive MiR-152-3p Results in Increased BAFF Expression in SLE B-Cells by Inhibiting the KLF5 Expression. Front Immunol (2019) 10:1127. 10.3389/fimmu.2019.01127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao S, Sun G, Tony PW, Ma D, Zhao C. Expression and Methylation Status of the Syk Gene in Cervical Carcinoma. Arch Gynecol Obstet (2011) 283(5):1113–9. 10.1007/s00404-010-1546-6 [DOI] [PubMed] [Google Scholar]
- 100.Liu D, Zhang N, Zhang X, Qin M, Dong Y, Jin L. MiR-410 Down-Regulates the Expression of Interleukin-10 by Targeting STAT3 in the Pathogenesis of Systemic Lupus Erythematosus. Cell Physiol Biochem (2016) 39(1):303–15. 10.1159/000445625 [DOI] [PubMed] [Google Scholar]
- 101.Smith S, Fernando T, Wu PW, Seo J, Ni Gabhann J, Piskareva O, et al. MicroRNA-302d Targets IRF9 to Regulate the IFN-Induced Gene Expression in SLE. J Autoimmun (2017) 79:105–11. 10.1016/j.jaut.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 102.Wang Z, Heid B, Dai R, Ahmed SA. Similar Dysregulation of Lupus-Associated MiRNAs in Peripheral Blood Mononuclear Cells and Splenic Lymphocytes in MRL/Lpr Mice. Lupus Sci Med (2018) 5(1):e000290. 10.1136/lupus-2018-000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tu Y, Guo R, Li J, Wang S, Leng L, Deng J, et al. MiRNA Regulation of MIF in SLE and Attenuation of Murine Lupus Nephritis With MiR-654. Front Immunol (2019) 10:2229. 10.3389/fimmu.2019.02229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, et al. Negative Regulation of TLR4 via Targeting of the Proinflammatory Tumor Suppressor PDCD4 by the MicroRNA MiR-21. Nat Immunol (2010) 11(2):141–7. 10.1038/ni.1828 [DOI] [PubMed] [Google Scholar]
- 105.Esquela-Kerscher A, Slack FJ. Oncomirs - MicroRNAs With a Role in Cancer. Nat Rev Cancer (2006) 6(4):259–69. 10.1038/nrc1840 [DOI] [PubMed] [Google Scholar]
- 106.Azzaoui K, Blommers M, Gotte M, Zimmermann K, Liu H, Fretz H. Discovery of Small Molecule Drugs Targeting the Biogenesis of MicroRNA-155 for the Treatment of Systemic Lupus Erythematosus. Chimia (Aarau) (2020) 74(10):798–802. 10.2533/chimia.2020.798 [DOI] [PubMed] [Google Scholar]