Abstract
Impaired lung epithelial cell regeneration following injury may contribute to the development of pulmonary fibrosis. Epithelial-mesenchymal transition (EMT) is a critical event in embryonic development, wound healing following injury, and even cancer progression. Previous studies have shown that the combination of transforming growth factor beta-1 (TGFβ1) and fibroblast growth factor 2 (FGF2) induces EMT during cancer metastasis. However, this synergy remains to be elucidated in inducing EMT associated with wound healing after injury. We set out this study to determine the effect of FGF2 on TGFβ1-induced EMT in human lung epithelium. BEAS-2B and A549 cells were treated with TGFβ1, FGF2 or both. EMT phenotype was investigated morphologically and by measuring mRNA expression levels using quantitative real-time PCR. E-cadherin expression was assayed by western blot and immunofluorescence staining. Cell migration was confirmed using a wound-healing assay. TGFβ1 induced a morphological change and a significant increase in cell migration of BEAS-2B cells. TGFβ1 significantly reduced E-cadherin (CDH1) mRNA expression and markedly induced expression of N-cadherin (CDH2), tenascin C (TNC), fibronectin (FN), actin alpha 2 (ACTA2) and COL1A1. While FGF2 alone did not significantly alter EMT gene expression, it enhanced TGFβ1-induced suppression of CDH1 and upregulation of ACTA2, but not TNC, FN and CDH2. FGF2 significantly inhibited TGFβ1-induced COL1A1 expression. Furthermore, FGF2 maintained TGFβ1-induced morphologic changes and increased the migration of TGFβ1-treated cells. This study suggests a synergistic effect between TGFβ1 and FGF2 in inducing EMT in lung epithelial cells, which may play an important role in wound healing and tissue repair after injury.
Keywords: Epithelial cells, Epithelial-mesenchymal transition, Fibroblast Growth Factor 2, Lung injury, Transforming growth factor beta 1
1. Introduction
Pulmonary fibrosis, including idiopathic pulmonary fibrosis (IPF), represents a chronic and dysregulated wound healing repair response to a past stimulus of lung injury, leading to irreversible scarring and remodeling of the lung (1). Injury to lung epithelial cells leads to release of pro-fibrotic factors including transforming growth factor beta-1 (TGFβ1) that contribute to activation of fibroblasts (2,3). Subsequently, activated fibroblasts (myofibroblasts) express contractile proteins such as actin alpha 2 (ACTA2) as well as matrix proteins such as fibronectin and collagen, leading to excessive deposition of extracellular matrix (ECM) that contribute to lung remodeling, architectural distortion, and abnormalities in gas exchange (4). When lung tissue is invaded/wounded by foreign antigens such as viruses and bacteria, a series of signaling pathways activate the immune system, resulting in inflammatory responses that lead to epithelial-mesenchymal transition (EMT) (5,6). Numerous studies have demonstrated that EMT is implicated in pulmonary fibrosis in mouse models (7–9) and in humans (10,11). However, the importance of EMT in pulmonary fibrosis has been challenged by other studies in animal models (12–14) and humans (15).
Epithelial-mesenchymal transition (EMT) is a process by which differentiated epithelial cells lose their epithelial characteristics and acquire a migratory mesenchymal phenotype. During EMT, epithelial cells decrease expression of epithelial-specific genes such as tight junctional protein including E-cadherin (CDH1), increase mesenchymal-specific genes such as α-smooth muscle actin (α-SMA), and acquire a mesenchymal spindle-shaped morphology (16). Mesenchymal markers used to define EMT are contractile and ECM genes such as ACTA2, collagen I (COL1A1), vimentin (VIM), fibronectin (FN), tenascin C (TNC), and connective tissue growth factor (CTGF), pro-migratory genes such as N-cadherin (CDH2), and the fibroblast proliferation transcription factors Snail (SNAI1) and Slug (SNAI2) (4,17,18). EMT is classified into three subtypes. Type I EMT occurs during organ development and is essential in gastrulation and neural crest cell migration. Type II EMT is associated with wound healing and organ fibrosis, where epithelial cells acquire a myofibroblast phenotype and migrate to heal injured tissues. If the injury is mild and acute, this process ends once the tissue is repaired. However, persistent EMT has been argued to promote myofibroblast differentiation leading to fibrosis (19,20). Type III EMT occurs in cancer metastasis, where neoplastic epithelial cells are transformed into invasive metastatic mesenchymal cells (21,22).
The profibrotic cytokine, TGFβ1 was first defined as a major inducer of EMT in normal mouse mammary epithelial (NMuMG) cells (23) and has been implicated in mediating EMT in vitro in epithelial cells from the kidney (24–26), eye (27,28), and lung (29–40). Other EMT inducers such as fibroblast growth factors 2 (FGF2) and FGF4 are key regulators of EMT during development and cancer progression in the lung (41,42). It has been reported that FGF2 reduces E-cadherin in human ovarian cancer cells (43), and induces the expression of mesenchymal markers (VIM, α-SMA and SNAI1) in corneal endothelial cells (44) and proximal tubular epithelial cells (42,45). A number of studies have shown the synergistic effect of combined treatment of TGFβ1 and FGF2 in inducing EMT in NMuMG cells (46), rat Hertwig’s epithelial root sheath (HERS) cells (47), mouse lung epithelial type II cell line MLE-12 (48), and human lung adenocarcinoma cell lines (49–51).
We have previously shown that FGF2 is crucial for epithelial repair and recovery after bleomycin-induced lung injury in mice (52). We have also found that FGF2 overexpression is protective against bleomycin-induced lung injury in vivo and inhibits TGFβ1-induced collagen I and α-SMA expression in primary mouse and human lung fibroblasts in vitro (53). These findings suggest that FGF2 may be protective against lung injury either through inhibition of TGFβ1 signaling, or by augmenting epithelial recovery through enhancement of type II EMT. While previous studies have used the combination of TGFβ1 and FGF2 to induce type III EMT, no studies have shown a synergistic effect of FGF2 and TGFβ1 in type II EMT in lung epithelial cells. To test whether FGF2 alters the response to TGFβ1 in lung epithelial cells, we investigated the effect of FGF2 on TGFβ1-induced EMT gene expression in both bronchial and alveolar lung epithelial cells in vitro. We hypothesized that FGF2 would induce EMT and may play an important role in wound healing and repair of lung epithelial cells after injury. We found that FGF2 enhanced the majority of TGFβ1-induced EMT markers and wound healing in human lung epithelial cells. Interestingly, collagen I gene expression was dramatically suppressed after the addition of FGF2 to TGFβ1-treated cells. These findings suggest that a possible mechanism of the reparative effect of FGF2 is through augmentation of type II EMT and suppression of collagen production. These data also provide insight into the potential therapeutic use of FGF2 in lung injury and pulmonary fibrosis.
2. Materials and Methods
2.1. Cell culture
BEAS-2B and A549 were purchased from the American Type Culture Collection (ATCC, VA, USA). All procedures were performed at the University of Chicago Medical Center. BEAS-2B cells were plated on pre-coated plates with a mixture of 0.01 mg/ml fibronectin, 0.03 mg/ml bovine collagen type I and 0.01 mg/ml bovine serum albumin (BSA) dissolved in bronchial epithelial basal medium (BEBM) and were grown in bronchial epithelial growth medium (BEGM; Lonza, MD, USA). A549 cells were plated in Ham’s F-12 medium (Life Technologies, CA, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (10,000 U/ml). Cells were incubated in a humidified incubator at 37°C with 5% CO2.
2.2. Epithelial-mesenchymal transition induction
Cells were plated at ~30-40% confluence in 6-well plates. After overnight culture, cells were treated with 2 ng/ml of TGFβ1 (Fisher Scientific, NJ, USA), 2 nM of FGF2 (PeproTech, NJ, USA) and 1 nM heparin sulfate (Fisher Scientific), or TGFβ1, FGF2 and heparin for 4 days in complete medium. The control group was cultured with the complete medium only. The dose of FGF2 used was based on our prior studies (53). Medium with or without treatments was changed after 48 hours. The experiments were designed so that the cells reached confluence one day prior to harvesting and were conducted independently 3-6 times each in duplicate. In order to determine the dose and time course of TGFβ1 used in our experiments, BEAS-2B cells were treated with 2 ng/ml or 5 ng/ml of TGFβ1 for 3, 4 and 5 days and expression of CDH1, ACTA2, and COL1A1 were assessed by qRT-PCR. We found that 2 ng/ml was sufficient to repress CDH1 and induce ACTA2 and COL1A1, but ACTA2 started to be only detectable after 4 days of treatment (data not shown).
2.3. EMT assay in the presence of FGFR-specific tyrosine kinase inhibitor
BEAS-2B cells were incubated with TGFβ1 (2 ng/ml) alone, FGF2 (2 nM) alone, PD173074 (0.1 μM, Cayman Chemical, MI, USA) alone or FGF2 (2 nM) and TGFβ1 with or without PD173074 for 4 days prior to collection of RNA. The dose of PD173074 used was based on our prior studies (53).
2.4. RNA isolation and quantitative real-time PCR
Cells were lysed in RLT buffer and total RNA was extracted using the RNeasy plus mini kit (Qiagen, CA, USA) according to the manufacturer’s instructions. cDNA was made using the iScript Reverse Transcription Supermix (BioRad, CA, USA). Quantitative RT-PCR was performed on an Applied Biosystems StepOne thermocycler using Taqman® Fast Advanced Master Mix (Applied Biosystems, CA, USA) and Taqman® gene expression assays. All samples were normalized to GAPDH and then scaled relative to controls using the standard delta Ct (ΔCt) method. Data are reported as fold change over control.
2.5. Protein isolation and immunoblotting
Protein was extracted from cultured epithelial cells in radioimmunoprecipitation assay lysis buffer with freshly added 2% Protease Inhibitor Cocktail (Sigma-Aldrich, MO, USA) and Phosphatase Inhibitor Cocktail I and II (Sigma-Aldrich). Total protein (20-40 μg) was separated on 4-20% polyacrylamide gels (BioRad) and transferred to PVDF membranes. Membranes were blocked for one hour at room temperature in TBST (50 mM Tris, pH7.4, 150 mM NaCl, 0.1% Tween-20) containing 5% BSA, and then probed with primary antibodies against E-cadherin (BD Transduction Laboratories, KY, USA) overnight at 4°C. Immunoblotting for β-tubulin (Abcam, Cambridge, USA) was used as a loading control. Membranes were then incubated for one hour at room temperature in HRP-linked secondary antibodies with 5% nonfat milk and developed using SuperSignal West Femto (Thermo Scientific) or Pico (Thermo Scientific) Substrate. Protein bands were quantified using Image Lab (BioRad), normalized to tubulin, and reported as fold change relative to controls.
2.6. Migration assay
BEAS-2B cells were grown to ~90% confluent in complete media at the time of wounding. Five 1 mm diameter circular wounds were created using a custom-made rubber tool (54). The non-adherent cells washed off and fresh medium with the same treatments as described previously was added to the wells. The wound closure was measured immediately after scratch wounding (0 h), at 24 h, 48 h, and 72 h. This experiment was then repeated by pretreating the cells for 3 days then wounding the cells and wound closure was measured at 0 h, 24 h, 40 h and 48 h. Pictures were taken using a digital camera attached to a phase-contrast inverted-stage microscope (Nikon, IL). Wound areas were measured using Image J (NIH), and values were normalized to time = 0 values.
2.7. Immunofluorescence staining
BEAS-2B cells were grown on coated glass coverslips and stimulated with the same treatments for 4 days as described above. Cells were fixed with 4 % paraformaldehyde for 10 minutes at room temperature. Then cells were blocked in 0.1% BSA and 5% serum prepared in PBS + 0.2% Tween-20 for 1h at room temperature. Coverslips were stained with 1:50 of monoclonal mouse anti-E-cadherin antibody (BD Transduction Laboratories) and incubated overnight at 4°C, followed by 1:500 of secondary antibody (goat anti-mouse conjugated with Alexa488). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Scientific) and coverslips mounted with SlowFade™ Gold Antifade Mountant (Thermo Scientific). Images were captured with 3i Marianas spinning disk confocal microscope and the two channels merged using Image J software.
2.8. Statistical Analysis
The data showed the mean ± standard deviation and the significant differences in mean values were determined using one-way ANOVA followed by Tukey’s multiple comparisons test. A p-value of less than 0.05 was considered to be significant. Statistical analysis was performed using GraphPad Prism 7.04 software.
3. Results
3.1. FGF2 does not alter morphological changes in BEAS-2B induced by TGFβ1.
We first examined whether FGF2 alters the morphology of cultured bronchial epithelial cells in response to TGFβ1. The addition of FGF2 to BEAS-2B cells did not alter their cobblestone-like morphology (Figure 1A, B). Upon treatment with TGFβ1, cells developed a fibroblast-like shape (Figure 1C). Compared to cells treated with TGFβ1 alone, the addition of FGF2 did not revert BEAS-2B cells back to a cobblestone-like morphology (Figure 1 D).
Figure 1. Morphological changes induced by TGFβ1 and FGF2.
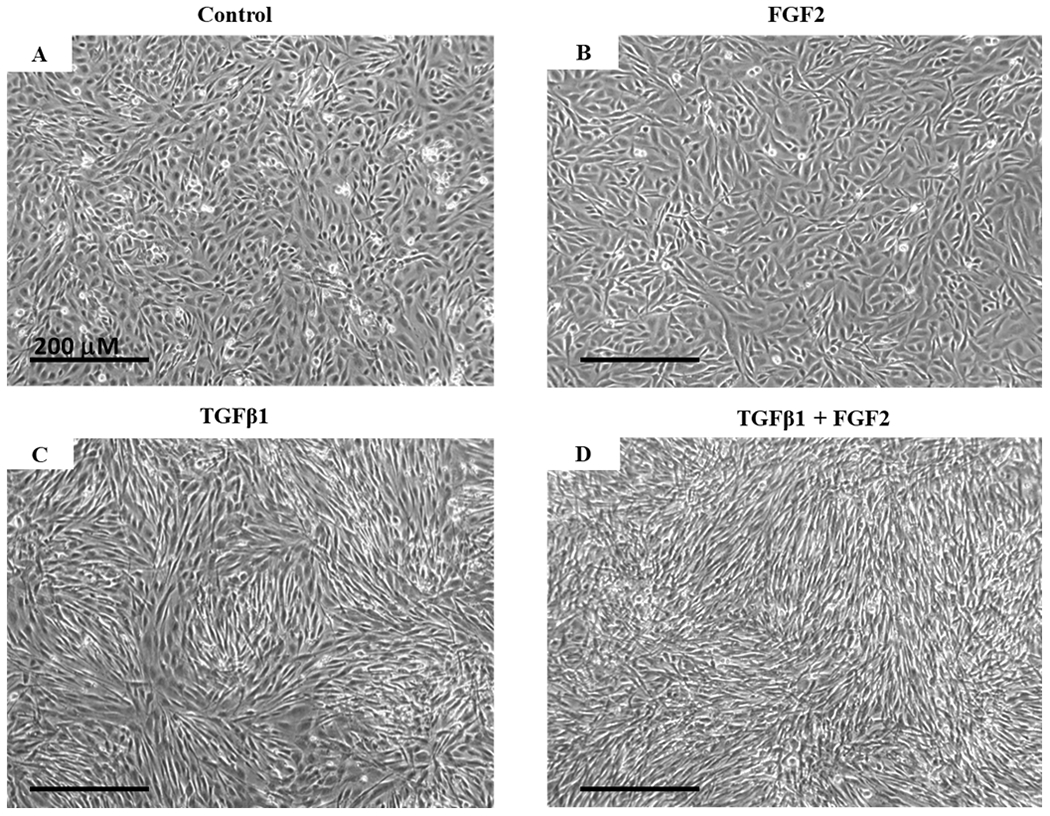
BEAS-2B cells were grown in complete growth media (Control) or stimulated with TGFβ1 (2 ng/ml), FGF2 (2 nM) + heparin sulphate (1 nM), or TGFβ1 + FGF2 + heparin sulphate for 4 days. Representative phase-contrast images (10× original magnification) show the morphological change of BEAS-2B cells from cobblestone-like shape as in the control (A) and FGF2 treatment alone (B) to the fibroblast-like shape in the presence of TGFβ1 (C) which is unaltered after adding FGF2 (D).
3.2. FGF2 enhances TGFβ1 induced EMT-related gene expression.
Even though FGF2 did not alter morphologic changes induced by TGFβ1, it is possible that it may alter TGFβ1-induced gene expression. To test this, we treated BEAS-2B cells for 4 days with FGF2 (2 nM), TGFβ1 (2 ng/ml), or TGFβ1 + FGF2. FGF2 treatment alone led to a non-significant decrease in CDH1 and a non-significant increase in both ACTA2 and CDH2 when compared to control. We observed a significant decrease in CDH1 (Figure 2A) and a significant increase in ACTA2 (Figure 2B) and CDH2 (Figure 2C) mRNA expression after treating BEAS-2B cells with TGFβ1. Addition of FGF2 to TGFβ1 resulted in a further significant decrease in CDH1 expression (Figure 2A) and a significant increase in ACTA2 compared to TGFβ1 treatment alone (Figure 2B). The addition of FGF2 to TGFβ1 did not alter the expression of CDH2 induced by TGFβ1 alone (Figure 2C).
Figure 2. FGF2 enhances TGFβ1-induced EMT gene expression in BEAS-2B cells.
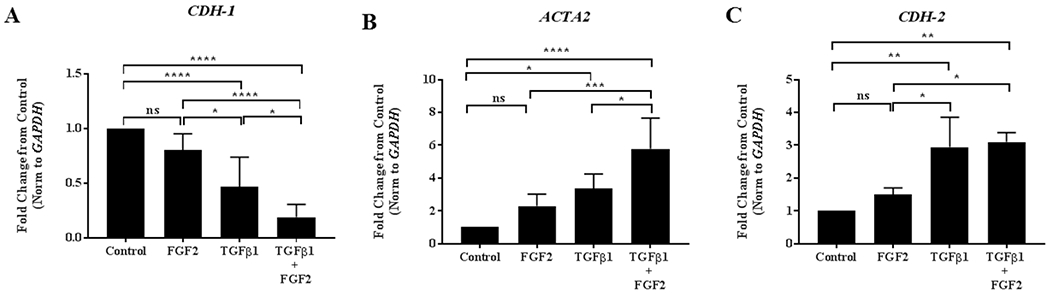
BEAS-2B cells were incubated for 4 days in the absence or presence of 2 ng/ml TGFβ1 alone, FGF2 (2 nM) + heparin sulphate (1 nM), or TGFβ1+ FGF2 + heparin. Quantitative real-time PCR analysis was performed for CDH1 (A), ACTA2 (B) and CDH2 (C). ΔCt values were normalized to GAPDH and expressed as fold change from untreated controls. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test; ns = not significant, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, and **** indicates p < 0.0001.
We then assessed the effects of TGFβ1 and FGF2 on E-cadherin protein levels in BEAS-2B cells. Immunoblotting of total cell lysates obtained after 4 days of incubation with TGFβ1, FGF2, or TGFβ1 + FGF2 demonstrated that E-cadherin protein levels were not significantly altered by FGF2 treatment alone and were significantly decreased in response to TGFβ1 (Figure 3A, B). The addition of FGF2 to TGFβ1 led to further suppression of E-cadherin (Figure 3A, B). Immunofluorescence for E-cadherin revealed a loss of grid-like localization of E-cadherin at the cell-cell contact surface following FGF2 treatment and further loss of cell-cell contact induced by TGFβ1 that was not altered by the addition of FGF2 (Figure 3C).
Figure 3. TGFβ1 induced decrease in E-Cadherin protein expression is augmented by FGF2.
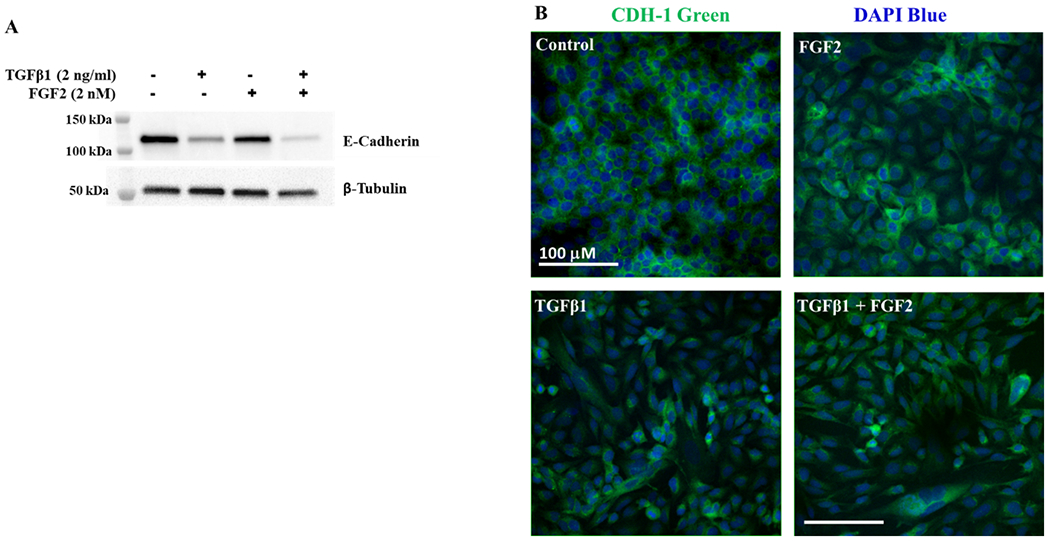
(A) Total cell lysates from BEAS-2B cells stimulated for 4 days with or without TGFβ1 (2 ng/ml) alone, FGF2 (2 nM) + heparin sulphate (1 nM), or TGFβ1 + FGF2 + heparin, were immunoblotted for E-cadherin. Blots were reprobed for β-tubulin as a loading control. (B) Densitometry for E-cadherin was normalized to β-tubulin and is expressed as fold change from untreated control. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test; ns = not significant, * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001. (C) Representative images showing immunofluorescent staining for E-cadherin in BEAS-2B cells stimulated with or without TGFβ1 alone, FGF2alone or TGFβ1 and FGF2 for 4 days. The confocal images were obtained at 20× original magnification for E-cadherin (green) and DAPI (blue).
3.3. FGF2 inhibits TGFβ1-induced collagen, but not fibronectin or tenascin C.
The effect FGF2 on the expression of extracellular matrix (ECM) proteins such as fibronectin (FN), tenascin C (TNC) and collagen I (COL1A1) was then examined. Treatment with FGF2 alone did not significantly alter the expression of FN, TNC, or COL1A1, but TGFβ1 treatment led to a highly significant induction of each of these genes (Figure 4A–C). FGF2 treatment had no effect on TGFβ1-induced expression of FN (Figure 4A) and TNC (Figure 4B), but interestingly there was a significant decrease in the expression of COL1A1 (Figure 4C) compared to TGFβ1 treatment alone unlike other EMT genes studied in this report.
Figure 4. FGF2 inhibits TGFβ1 induced collagen, but not fibronectin or tenascin-C.
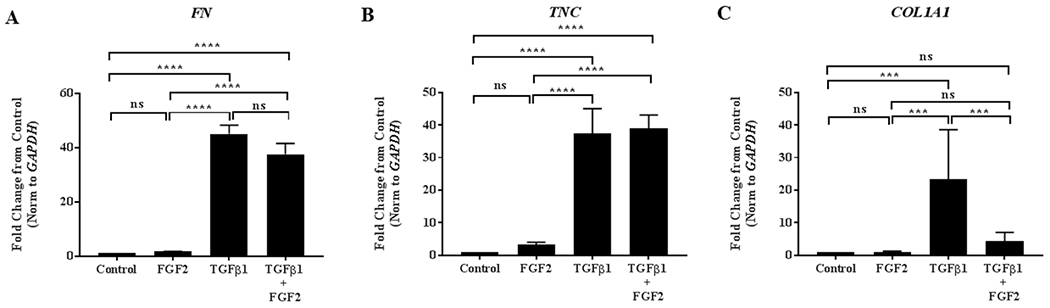
BEAS-2B cells were incubated for 4 days in the absence or presence of 2 ng/ml TGFβ1 alone, FGF2 (2 nM) + heparin sulphate (1 nM), or TGFβ1 + FGF2 + heparin. Quantitative real-time PCR analysis was performed for FN (A), TNC (B) and COL1A1 (C). ΔCt values were normalized to GAPDH and expressed as fold change from untreated controls. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test; ns = not significant, *** indicates p < 0.001, and **** indicates p <0.0001.
3.4. FGF2 has similar effects on EMT gene expression induced by TGFβ1 in A549 cells.
We then tested whether the effect of FGF2 on TGFβ1-induced gene expression in BEAS-2B cells was unique to bronchial epithelial cells or conserved in other epithelial cell types in the lung. The alveolar epithelial A549 cells were treated with FGF2, TGFβ1, or TGFβ1 + FGF2 for 4 days prior to the collection of total mRNAs. We observed that FGF2 enhanced TGFβ1-induced downregulation of CDH1 (Figure 5A) and upregulation of ACTA2 (Figure 5B) compared to control. In addition, FGF2 suppressed TGFβ1-induced COL1A1 mRNA expression in A549 cells compared to TGFβ1 treatment alone similar to what was observed in BEAS-2B cells (Figure 5C).
Figure 5. The effect of FGF2 on TGFβ1-induced EMT gene expression is conserved in A549 cells.
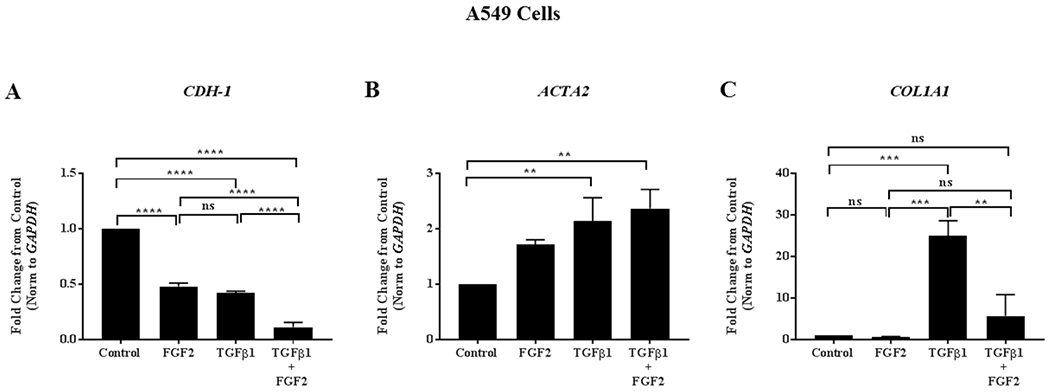
A549 cells were grown in complete Ham’s F12 media (Control) or stimulated with TGFβ1 (2 ng/ml), FGF2 (2 nM) + heparin sulphate (1 nM), or TGFβ1 + FGF2 + heparin sulphate for 4 days. Quantitative real-time PCR analysis was performed for CDH1 (A), ACTA2 (B) and COL1A1 (C) ΔCt values were normalized to GAPDH and expressed as fold change from untreated controls. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test; ns = not significant, ** indicates p < 0.01, *** indicates p < 0.001, and **** indicates p < 0.0001.
3.5. PD173074 inhibited the effect of FGF2 on TGFβ1-treated cells in both types of epithelial cells.
To confirm that the effect of FGF2 is mediated by FGF receptor (FGFR) signaling, BEAS-2B and A549 cells were treated with the FGFR-specific tyrosine kinase inhibitor PD173074 (0.1 μM) in combination with FGF2, TGFβ1, or TGFβ1 + FGF2. We found that PD173074 significantly blocked the effect of FGF2 on TGFβ1-induced repression of CDH1 (Figure 6A) and induction of ACTA2 (Figure 6B), and reversed FGF2-mediated inhibition of TGFβ1 induction of COL1A1 expression non-significantly in BEAS-2B cells (Figure 6C). Similarly, PD173074 inhibited FGF2 effect on TGFβ1-induced EMT in A549 cells significantly for CDH1 (Figure 6D) and non-significantly for ACTA2 (Figure 6E) and COL1A1 (Figure 6F). PD173074 alone did not alter the expression of the above genes in both types of epithelial cells.
Figure 6. The effect of FGF2 on TGFβ1 induced EMT gene expression in lung epithelial cells is blocked by the FGFR-specific tyrosine kinase inhibitor PD173074.
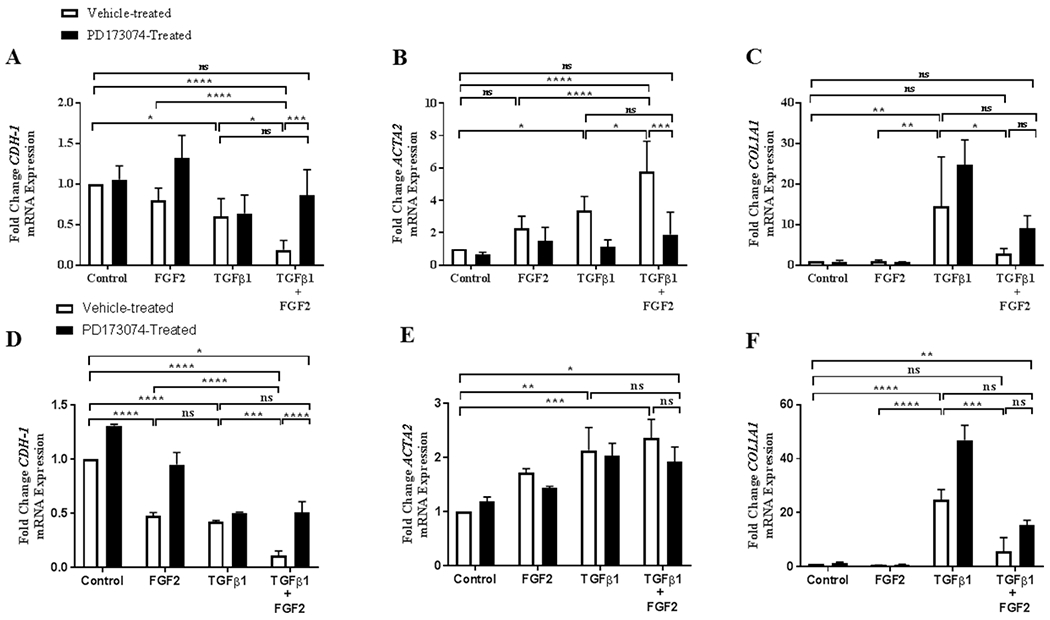
BEAS-2B cells were incubated with TGFβ1 (2 ng/ml), FGF2 (2 nM) + heparin sulphate (1 nM), PD173074 (0.1 μM) alone or FGF2 + heparin + TGFβ1 +/− PD173074 for 4 days. Quantitative real-time PCR analysis was performed for CDH1 (A), ACTA2 (B) and COL1A1 (C). A549 cells were incubated with TGFβ1 (2 ng/ml), FGF2 (2 nM) alone, PD173074 (0.1 μM) alone or FGF2 (2 nM) + TGFβ1 +/− PD173074 for 4 days. Quantitative real-time PCR analysis was performed for CDH1 (D), ACTA2 (E) and COL1A1 (F). ΔCt values were normalized to GAPDH and expressed as fold change from untreated controls. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test; ns = not significant, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, and **** indicates p < 0.0001.
3.6. FGF2 promotes epithelial cell migration alone or in combination with TGFβ1.
We then determined whether FGF2 enhances the migration of BEAS-2B cells after injury. BEAS-2B cells were treated with FGF2, TGFβ1, or TGFβ1 + FGF2 immediately after wounding. Treatment with TGFβ1 significantly reduced migration rates of BEAS-2B cells when given immediately after wounding at 48 h and 72 h, however, both FGF2 alone and the addition of FGF2 to TGFβ1-treated cells caused a non-significant increase in migration rate (Figure 7A, B). Therefore, we treated BEAS-2B cells with FGF2, TGFβ1, or TGFβ1 + FGF2 for 3 days prior to wounding. Both FGF2 and TGFβ1 alone significantly increased migration at 24 h, 40 h and 48 h after wounding compared to control, and this effect was substantially potentiated by addition of FGF2 to TGFβ1 (Figure 8A, B).
Figure 7. FGF2, but not TGFβ1, increases BEAS-2B migration of epithelial cells when added immediately after wounding.
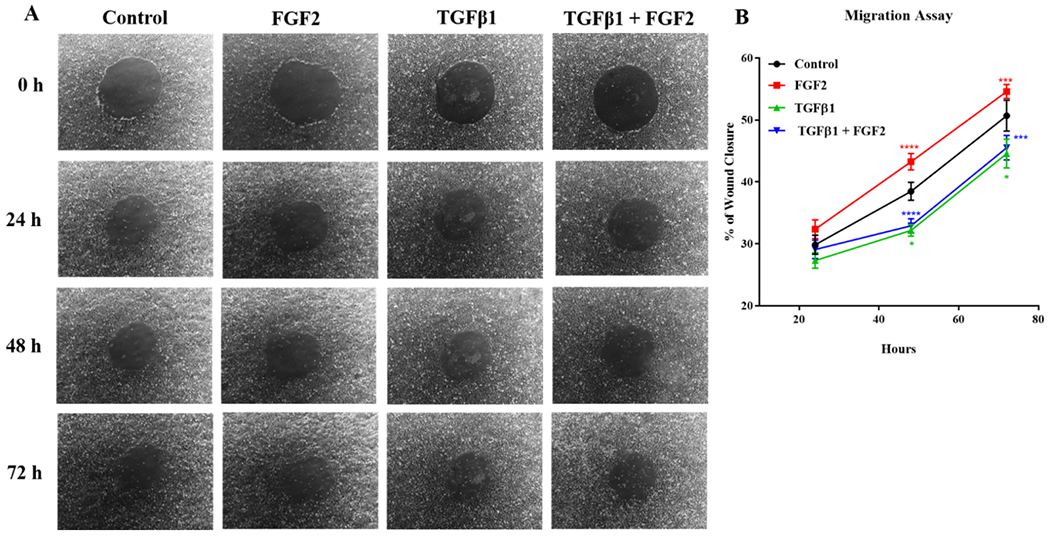
BEAS-2B were grown in complete media to confluence, and 1mm diameter circular wounds were generated and the cells were treated immediately with TGFβ1 (2 ng/ml), FGF2 (2 nM) + heparin sulphate (1 nM), or TGFβ1 + FGF2 + heparin. (A) Representative phase-contrast images (10× original magnification) of the same area were taken immediately after wounding (0 h) as well as 24 h, 48 h and 72 h later. (B) Wound area was imaged at 0 h, 24 h, 48 h and 72 h then the % of wound closure was measured using ImageJ software. Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test; Red asterisks mark significant differences for FGF2 vs. TGFβ1, blue asterisks mark significant differences for TGFβ1 + FGF2 vs. FGF2, and green asterisks mark significant differences for TGFβ1 vs. control. * indicates p < 0.05, *** indicates p < 0.001 and **** indicates p < 0.0001.
Figure 8. Pre-treatment with FGF2 and TGFβ1 significantly increases migration rates of BEAS-2B cells after wounding.
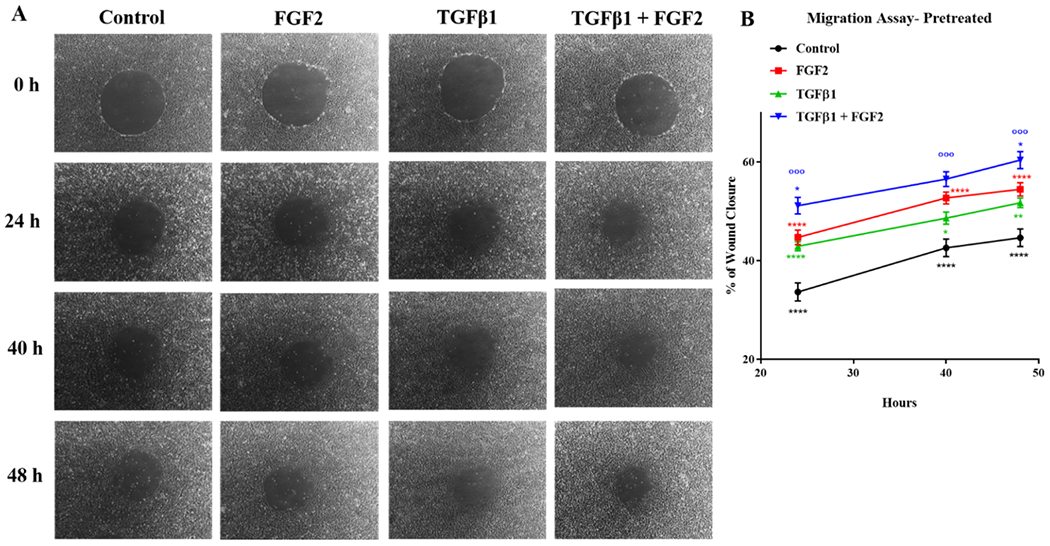
BEAS-2B cells were treated with TGFβ1 (2 ng/ml), FGF2 (2 nM) + heparin sulphate (1 nM), or TGFβ1 + FGF2 + heparin for 3 days. The cells then wounded and the % of wound closure was measured at 0 h, 24 h, 40 h and 48 h later. (A) Representative phase contrast images (10× original magnification) of the same area were taken immediately after wounding (0 h) as well as 24 h, 40 h and 48 h later. (B) Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test; Red asterisks mark significant differences for FGF2 vs. control, blue asterisks mark significant differences for TGFβ1 + FGF2 vs. FGF2, blue circles mark significant differences for TGFβ1 + FGF2 vs. TGFβ1, green asterisks mark significant differences for TGFβ1 vs. control and black asterisks mark significant differences for control vs TGFβ1 + FGF2. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, and **** indicates p < 0.0001.
4. Discussion
Several studies have demonstrated that human alveolar epithelial cells (29,30,33–39) and human bronchial epithelial cells (31,32,35,40) undergo EMT in response to TGFβ1 in vitro. A number of studies have reported that FGF2 induces EMT in malignant pleural mesothelioma cells (55), tubular epithelial cells (42,45) and lens epithelial cells (56). Although the synergistic effect between TGFβ1 and FGF2 in inducing type III EMT (46,47,49) and proliferation (57–59) has been described in other cell types, to our knowledge the synergistic effect between FGF2 and TGFβ1 in type II EMT in lung epithelial cells in vitro has not been previously described. In this study we found that FGF2 enhances TGFβ1 induced EMT in human bronchial epithelial cells (BEAS-2B) and alveolar type II epithelial cells (A549) in vitro, as shown by morphological and EMT-related gene expression. Interestingly, we also found that, unlike other EMT-related genes, collagen I expression is significantly inhibited by FGF2 (Figure 9).
Figure 9.
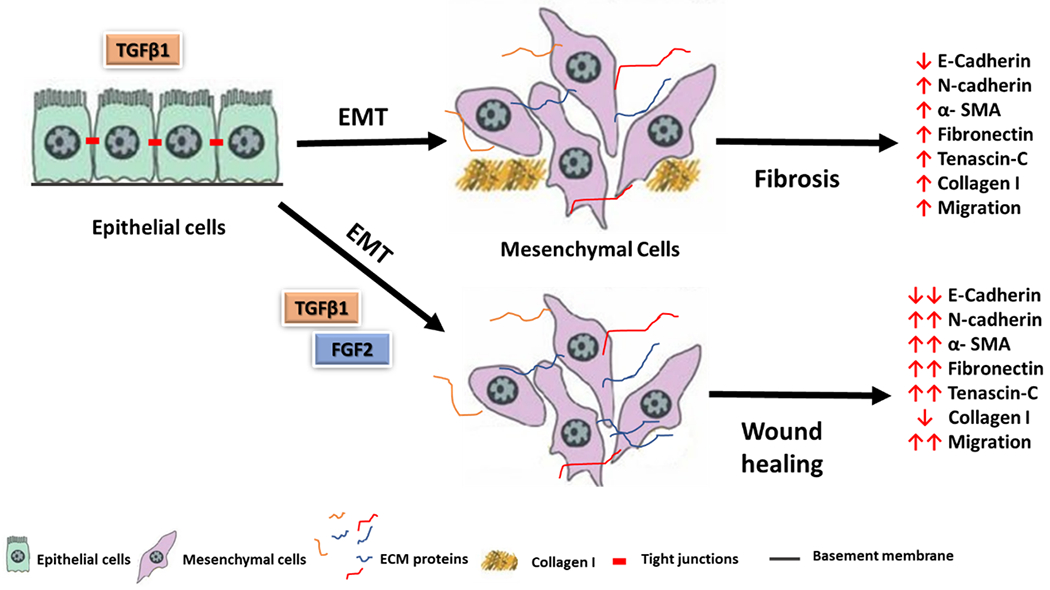
A schematic diagram of FGF2 and TGFβ1 for the induction of epithelial-mesenchymal transition in lung epithelial cells.
TGFβ1: transforming growth factor beta 1, FGF2: fibroblast growth factor 2, EMT; epithelial-mesenchymal transition, α-SMA; alpha smooth muscle actin, ECM; extracellular matrix proteins.
In this study, we stimulated both BEAS-2B and A549 cells with TGFβ1, FGF2 or both for up to 4 days. In response to TGFβ1, BEAS-2B cells lost their cobblestone morphology and adopted an elongated spindle-like shape, and this shape was unaltered by the addition of FGF2. TGFβ1 treatment also led to the downregulation of CDH1 and the upregulation of ACTA2 and CDH2. FGF2 alone did not significantly alter CDH1, ACTA2, or CDH2 expression, but had an additive effect on the changes in expression of these genes when added to TGFβ1-treated cells. Additionally, FGF2 augments the increase in the ECM mRNA expression of FN and TNC induced by TGFβ1. Similarly, we observed an identical pattern of EMT gene expression following stimulation with TGFβ1 +/− FGF2 in A549 cells. In accordance with the present results, Shirakihara et al. (46) showed that there was a synergistic effect between FGF2 and TGFβ1 on the induction of EMT in the mouse normal mammary epithelial (NMuMG) cells, without evidence of induction of EMT by FGF2 alone. They found that the morphology of NMuMG cells clearly changed from a cobblestone-like shape to a fibroblastic spindle shape with TGFβ1 treatment. Although FGF2 alone did not alter this shape, the addition of FGF2 to TGFβ1-treated cells maintained the spindled-shape morphology. Li et al. (60) and Kurimoto et al. (50) indicated that treatment with FGF2 + TGFβ1 down-regulated E-cadherin, upregulated vimentin and N-cadherin and increased migration ability in A549 cells. These findings suggest that the combination of FGF2 and TGFβ1 treatment and not FGF2 alone is an effective way of promoting the induction of an EMT phenotype.
The present study showed that FGF2 alone or in combination with TGFβ1, increased the migratory capacity of BEAS-2B cells after 24 h, 40 h and 48 h that were pre-treated for 3 days prior to wounding, however, the cell motility of immediately treated cells after wounding was not accelerated significantly when measured after 24 h, 48 h and 72 h. These results show that treatment with TGFβ1, FGF2, or both requires at least 3 days to promote increased motility and almost complete wound closure after injury. These findings are consistent with Shirakihara et al. (46) study which showed that TGFβ1 treatment alone increased cell motility, and the addition of FGF2 to TGFβ1-treated cells treated for 4 days strongly enhanced the motility of NMuMG cells. These results match those observed in Chen et al. (47) study who also reported that treatment with TGFβ1, FGF2, or both generate EMT phenotype in rat Hertwig’s epithelial root sheath (HERS) cells. They found that the migratory capacity highly increased after 48h and 72 h of the pretreated cells with TGFβ1, FGF2, or both for 3 days. These findings suggest that generating a well-established EMT phenotype in epithelial cells using the combined treatments of TGFβ1 and FGF2 requires prolonged induction.
Several studies have shown increased COL1A1 expression in BEAS-2B (31,32,61) as well as in A549 (29,35,38) epithelial cells in response to TGFβ1. This also accords with our observations, which showed that TGFβ1 treatment significantly increases COL1A1 expression in both BEAS-2B and A549 cells. However, interestingly, COL1A1 expression was dramatically suppressed with the addition of FGF2 to TGFβ1-treated BEAS-2B and A549 cells. These findings mirror those of our previous study demonstrating inhibition of TGFβ1-induced collagen expression by FGF2 in primary mouse and human lung fibroblasts in vitro (53). These results may provide an important insight into the anti-fibrotic effect of FGF2 through suppression of TGFβ1-induced collagen expression in both lung fibroblast and epithelial cells.
The FGFR-specific tyrosine kinase inhibitor PD173074 was used to block the inductive effect of FGF2 on TGFβ1-treated cells. PD173074 inhibitor was reported to show both high affinity and selectivity for the FGF receptor (FGFR) family (62). We found that the addition of PD173074 attenuates the inductive effect of FGF2 by reversing the reduction of CDH1 expression and COL1A1, and the induction of ACTA2. This finding suggests that the effect of FGF2 is dependent upon canonical signaling through FGFRs.
In conclusion, the data presented in this report suggests that the reparative effect of FGF2 may be mediated through synergism with TGFβ1 to augment type II EMT and promote wound healing and tissue repair. It also demonstrates that the anti-fibrotic effect of FGF2 may be through inhibition of collagen expression not only in lung fibroblasts, but also in lung epithelial cells. While further studies are needed to determine the cellular mechanism underlying the interaction between FGF2 and TGFβ1, this report suggests that targeting and augmenting FGF2 signaling may provide therapeutic benefit in lung injury and pulmonary fibrosis.
Acknowledgments
This work was funded by NIH grant K08HL125910, and AHA grants 14FTF19840029. LMFB was supported by The Culture Affairs and Mission Sector, Ministry of Higher Education and Scientific Research, Egypt.
Footnotes
Conflict of interest
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Desai O, Winkler J, Minasyan M, Herzog EL. The Role of Immune and Inflammatory Cells in Idiopathic Pulmonary Fibrosis. Front Med [Internet]. 2018March20 [cited 2019 Aug 27];5:43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29616220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet [Internet]. 2011December3 [cited 2019 Aug 4];378(9807):1949–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21719092 [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Wheeler SE, Velikoff M, Kleaveland KR, Lafemina MJ, Frank JA, et al. Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins. Am J Pathol. 2013November;183(5):1559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Luan F, Zhao Y, Hao H, Zhou Y, Han W, et al. Epithelial-mesenchymal transition: An emerging target in tissue fibrosis. Exp Biol Med (Maywood) [Internet]. 2016January [cited 2018 Mar 9];241(1):1–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26361988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rout-Pitt N, Farrow N, Parsons D, Donnelley M. Epithelial mesenchymal transition (EMT): A universal process in lung diseases with implications for cystic fibrosis pathophysiology [Internet]. Vol. 19, Respiratory Research. 2018. [cited 2019 Sep 24]. p. 136. Available from: 10.1186/s12931-018-0834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salton F, Volpe MC, Confalonieri M. Epithelial-mesenchymal transition in the pathogenesis of idiopathic pulmonary fibrosis. Med. 2019April1;55(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci [Internet]. 2006August29 [cited 2018 Oct 10];103(35):13180–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16924102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanjore H, Xu XC, Polosukhin V V., Degryse AL, Li B, Han W, et al. Contribution of Epithelial-derived Fibroblasts to Bleomycin-induced Lung Fibrosis. Am J Respir Crit Care Med [Internet]. 2009October1 [cited 2018 Oct 23];180(7):657–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19556518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degryse AL, Tanjore H, Xu XC, Polosukhin V V., Jones BR, McMahon FB, et al. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Cell Mol Physiol [Internet]. 2010October [cited 2018 Nov 7];299(4):L442–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20562227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward C, Forrest IA, Murphy DM, Johnson GE, Robertson H, Cawston TE, et al. Phenotype of airway epithelial cells suggests epithelial to mesenchymal cell transition in clinically stable lung transplant recipients. Thorax [Internet]. 2005October1 [cited 2018 Nov 7];60(10):865–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15972366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada T, Nabeshima K, Hamasaki M, Uesugi N, Watanabe K, Iwasaki H. Epithelial-mesenchymal transition in human lungs with usual interstitial pneumonia: Quantitative immunohistochemistry. Pathol Int [Internet]. 2010January [cited 2018 Nov 7];60(1):14–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20055947 [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Yang L, Cai L, Zhang M, Cheng X, Yang X, et al. Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an α-smooth muscle actin-Cre transgenic mouse. Respir Res [Internet]. 2007December7 [cited 2018 Nov 7];8(1):1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17207287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci [Internet]. 2011December27 [cited 2018 Nov 2];108(52):E1475–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22123957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyles RK, Derrett-Smith EC, Khan K, Shiwen X, Howat SL, Wells AU, et al. An Essential Role for Resident Fibroblasts in Experimental Lung Fibrosis Is Defined by Lineage-Specific Deletion of High-Affinity Type II Transforming Growth Factor β Receptor. Am J Respir Crit Care Med [Internet]. 2011January15 [cited 2018 Nov 7];183(2):249–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20709822 [DOI] [PubMed] [Google Scholar]
- 15.Yamada M, Kuwano K, Maeyama T, Hamada N, Yoshimi M, Nakanishi Y, et al. Dual-immunohistochemistry provides little evidence for epithelial–mesenchymal transition in pulmonary fibrosis. Histochem Cell Biol [Internet]. 2008April [cited 2018 Nov 7];129(4):453–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18236067 [DOI] [PubMed] [Google Scholar]
- 16.Kolahian S, Fernandez IE, Eickelberg O, Hartl D. Immune Mechanisms in Pulmonary Fibrosis. Am J Respir Cell Mol Biol [Internet]. 2016September1 [cited 2019 Feb 15];55(3):309–22. Available from: 10.1165/rcmb.2016-0121TR [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest [Internet]. 2003December [cited 2018 Apr 8];112(12):1776–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14679171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol [Internet]. 2006March27 [cited 2018 Apr 8];172(7):973–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16567498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluri R EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest [Internet]. 2009June1 [cited 2018 Apr 23];119(6):1417–9. Available from: http://www.jci.org/articles/view/39675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest [Internet]. 2009June1 [cited 2019 Mar 6];119(6):1429–37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19487819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest [Internet]. 2009June1 [cited 2018 Oct 31];119(6):1420–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19487818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tennakoon A, Izawa T, Kuwamura M, Yamate J. Pathogenesis of Type 2 Epithelial to Mesenchymal Transition (EMT) in Renal and Hepatic Fibrosis. J Clin Med [Internet]. 2015December30 [cited 2018 Apr 8];5(1):4. Available from: http://www.mdpi.com/2077-0383/5/1/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol [Internet]. 1994December [cited 2018 Oct 15];127(6 Pt 2):2021–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7806579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan J-M, Ng Y-Y, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, et al. Transforming growth factor-β regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int [Internet]. 1999October [cited 2018 Oct 16];56(4):1455–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10504497 [DOI] [PubMed] [Google Scholar]
- 25.Kaimori A, Potter J, Kaimori J-Y, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem [Internet]. 2007July27 [cited 2018 Oct 16];282(30):22089–101. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17513865 [DOI] [PubMed] [Google Scholar]
- 26.Zheng G, Lyons JG, Tan TK, Wang Y, Hsu T-T, Min D, et al. Disruption of E-Cadherin by Matrix Metalloproteinase Directly Mediates Epithelial-Mesenchymal Transition Downstream of Transforming Growth Factor-β1 in Renal Tubular Epithelial Cells. Am J Pathol [Internet]. 2009August1 [cited 2018 Oct 16];175(2):580–91. Available from: https://www.sciencedirect.com/science/article/pii/S000294401060572X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hales AM, Schulz MW, Chamberlain CG, McAvoy JW. TGF-beta 1 induces lens cells to accumulate alpha-smooth muscle actin, a marker for subcapsular cataracts. Curr Eye Res [Internet]. 1994December [cited 2018 Oct 11];13(12):885–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7720396 [DOI] [PubMed] [Google Scholar]
- 28.Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, et al. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol [Internet]. 2004February [cited 2018 Apr 8];164(2):651–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14742269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res [Internet]. 2005December9 [cited 2018 Oct 16];6(1):56. Available from: 10.1186/1465-9921-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol [Internet]. 2005May [cited 2018 Oct 15];166(5):1321–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15855634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doerner AM, Zuraw BL. TGF-beta1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Respir Res [Internet]. 2009October27 [cited 2018 Feb 26];10(1):100. Available from: 10.1186/1465-9921-10-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamitani S, Yamauchi Y, Kawasaki S, Takami K, Takizawa H, Nagase T, et al. Simultaneous Stimulation with TGF-β1 and TNF-α Induces Epithelial Mesenchymal Transition in Bronchial Epithelial Cells. Int Arch Allergy Immunol [Internet]. 2011. [cited 2018 Oct 28];155(2):119–28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21196756 [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Jang YS, Eom K-S, Hwang Y Il, Kang HR, Jang SH, et al. Transtorming Growth Factor β1 Induces Epithelial-to-Mesenchymal Transition of A549 Cells. J Korean Med Sci [Internet]. 2007October [cited 2018 Oct 28];22(5):898. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17982242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I Promotes Epithelial-to-Mesenchymal Transition in Lung Cancer Cells via Transforming Growth Factor–β Signaling. Am J Respir Cell Mol Biol [Internet]. 2008January [cited 2018 Oct 28];38(1):95–104. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17673689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Câmara J, Jarai G. Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-α. Fibrogenesis Tissue Repair [Internet]. 2010January5 [cited 2018 Apr 7];3(1):2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20051102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X-F, Zhang H-J, Wang H-B, Zhu J, Zhou W-Y, Zhang H, et al. Transforming growth factor-β1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Mol Biol Rep [Internet]. 2012April29 [cited 2018 Oct 28];39(4):3549–56. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21713404 [DOI] [PubMed] [Google Scholar]
- 37.Kawata M, Koinuma D, Ogami T, Umezawa K, Iwata C, Watabe T, et al. TGF-β-induced epithelial-mesenchymal transition of A549 lung adenocarcinoma cells is enhanced by pro-inflammatory cytokines derived from RAW 264.7 macrophage cells. J Biochem [Internet]. 2012February [cited 2018 Oct 16];151(2):205–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22161143 [DOI] [PubMed] [Google Scholar]
- 38.O’Beirne SL, Walsh SM, Fabre A, Reviriego C, Worrell JC, Counihan IP, et al. CXCL9 Regulates TGF-β1-Induced Epithelial to Mesenchymal Transition in Human Alveolar Epithelial Cells. J Immunol [Internet]. 2015September15 [cited 2018 Oct 28];195(6):2788–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26268659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi K, Koyama K, Suzukawa M, Igarashi S, Hebisawa A, Nagase T, et al. Epithelial-mesenchymal transition promotes reactivity of human lung adenocarcinoma A549 cells to CpG ODN. Allergol Int [Internet]. 2016September [cited 2018 Oct 16];65:S45–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27475623 [DOI] [PubMed] [Google Scholar]
- 40.Hackett T-L, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky D V., Murray LA, et al. Induction of Epithelial–Mesenchymal Transition in Primary Airway Epithelial Cells from Patients with Asthma by Transforming Growth Factor-β1. Am J Respir Crit Care Med [Internet]. 2009July15 [cited 2018 Oct 28];180(2):122–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19406982 [DOI] [PubMed] [Google Scholar]
- 41.Qi L, Song W, Li L, Cao L, Yu Y, Song C, et al. FGF4 induces epithelial-mesenchymal transition by inducing store-operated calcium entry in lung adenocarcinoma. Oncotarget [Internet]. 2016November8 [cited 2018 Oct 21];7(45):74015–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27677589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strutz F, Zeisberg M, Ziyadeh FN, Yang C-Q, Kalluri R, Müller GA, et al. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney Int [Internet]. 2002May [cited 2018 Mar 28];61(5):1714–28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11967021 [DOI] [PubMed] [Google Scholar]
- 43.Lau M-T, So W-K, Leung PCK. Fibroblast Growth Factor 2 Induces E-Cadherin Down-Regulation via PI3K/Akt/mTOR and MAPK/ERK Signaling in Ovarian Cancer Cells. Migliaccio A, editor. PLoS One [Internet]. 2013March15 [cited 2018 Oct 18];8(3):e59083. Available from: 10.1371/journal.pone.0059083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JG, Jung E, Heur M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J Biol Chem [Internet]. 2018March9 [cited 2018 Oct 17];293(10):3758–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29363574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masola V, Gambaro G, Tibaldi E, Brunati AM, Gastaldello A, D’Angelo A, et al. Heparanase and syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelial-mesenchymal transition in renal tubular cells. J Biol Chem [Internet]. 2012January6 [cited 2018 Oct 19];287(2):1478–88. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22102278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I, et al. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J [Internet]. 2011February16 [cited 2018 Oct 18];30(4):783–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21224849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J, Chen G, Yan Z, Guo Y, Yu M, Feng L, et al. TGF-β1 and FGF2 Stimulate the Epithelial-Mesenchymal Transition of HERS Cells Through a MEK-Dependent Mechanism. J Cell Physiol [Internet]. 2014November [cited 2018 Oct 20];229(11):1647–59. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24610459 [DOI] [PubMed] [Google Scholar]
- 48.Chen P-Y, Qin L, Li G, Tellides G, Simons M. Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFβ)-dependent smooth muscle cell phenotype modulation. Sci Rep [Internet]. 2016December16 [cited 2018 Oct 21];6(1):33407. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27634335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurimoto R, Iwasawa S, Ebata T, Ishiwata T, Sekine I, Tada Y, et al. Drug resistance originating from a TGF-β/FGF-2-driven epithelial-to-mesenchymal transition and its reversion in human lung adenocarcinoma cell lines harboring an EGFR mutation. Int J Oncol [Internet]. 2016May [cited 2018 Oct 22];48(5):1825–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26984042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurimoto R, Ebata T, Iwasawa S, Ishiwata T, Tada Y, Tatsumi K, et al. Pirfenidone may revert the epithelial-to-mesenchymal transition in human lung adenocarcinoma. Oncol Lett [Internet]. 2017July [cited 2018 Nov 29];14(1):944–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28693256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gholami MD, Falak R, Heidari S, Khoshmirsafa M, Kazemi MH, Zarnani A-H, et al. A Truncated Snail1 Transcription Factor Alters the Expression of Essential EMT Markers and Suppresses Tumor Cell Migration in a Human Lung Cancer Cell Line. Recent Pat Anticancer Drug Discov. 2019May27;14(2):158–69. [DOI] [PubMed] [Google Scholar]
- 52.Guzy RD, Stoilov I, Elton TJ, Mecham RP, Ornitz DM. Fibroblast Growth Factor 2 Is Required for Epithelial Recovery, but Not for Pulmonary Fibrosis, in Response to Bleomycin. Am J Respir Cell Mol Biol [Internet]. 2015January [cited 2018 Oct 23];52(1):116–28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24988442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koo HY, El-Baz LMF, House SL, Cilvik SN, Dorry SJ, Shoukry NM, et al. Fibroblast growth factor 2 decreases bleomycin-induced pulmonary fibrosis and inhibits fibroblast collagen production and myofibroblast differentiation. J Pathol. 2018;246(1):54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White SR, Fischer BM, Marroquin BA, Stern R. Interleukin-1beta mediates human airway epithelial cell migration via NF-kappaB. Am J Physiol Lung Cell Mol Physiol [Internet]. 2008December [cited 2018 Feb 26];295(6):L1018–27. Available from: 10.1152/ajplung.00065.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schelch K, Wagner C, Hager S, Pirker C, Siess K, Lang E, et al. FGF2 and EGF induce epithelial–mesenchymal transition in malignant pleural mesothelioma cells via a MAPKinase/MMP1 signal. Carcinogenesis [Internet]. 2018April5 [cited 2018 Oct 17];39(4):534–45. Available from: https://academic.oup.com/carcin/article/39/4/534/4904272 [DOI] [PubMed] [Google Scholar]
- 56.Tanaka T, Saika S, Ohnishi Y, Ooshima A, McAvoy JW, Liu C-Y, et al. Fibroblast growth factor 2: roles of regulation of lens cell proliferation and epithelial-mesenchymal transition in response to injury. Mol Vis [Internet]. 2004July15 [cited 2018 Oct 18];10:462–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15273655 [PubMed] [Google Scholar]
- 57.Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, Van Kooten C, et al. TGF-β1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int [Internet]. 2001February [cited 2018 Oct 30];59(2):579–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11168939 [DOI] [PubMed] [Google Scholar]
- 58.Bossé Y, Thompson C, Stankova J, Rola-Pleszczynski M. Fibroblast Growth Factor 2 and Transforming Growth Factor β1 Synergism in Human Bronchial Smooth Muscle Cell Proliferation. Am J Respir Cell Mol Biol [Internet]. 2006June [cited 2018 Oct 20];34(6):746–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16439802 [DOI] [PubMed] [Google Scholar]
- 59.Xiao L, Du Y, Shen Y, He Y, Zhao H, Li Z. TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front Biosci (Landmark Ed [Internet]. 2012June1 [cited 2018 Oct 30];17:2667–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22652804 [DOI] [PubMed] [Google Scholar]
- 60.Li F, Zhu T, Yue Y, Zhu X, Wang J, Liang L. Preliminary mechanisms of regulating PDL1 expression in non small cell lung cancer during the EMT process. Oncol Rep [Internet]. 2018June5 [cited 2018 Nov 29];40(2):775–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29901173 [DOI] [PubMed] [Google Scholar]
- 61.Hosper NA, van den Berg PP, de Rond S, Popa ER, Wilmer MJ, Masereeuw R, et al. Epithelial-to-mesenchymal transition in fibrosis: Collagen type I expression is highly upregulated after EMT, but does not contribute to collagen deposition. Exp Cell Res [Internet]. 2013November15 [cited 2018 Mar 28];319(19):3000–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23906925 [DOI] [PubMed] [Google Scholar]
- 62.Pardo OE, Latigo J, Jeffery RE, Nye E, Poulsom R, Spencer-Dene B, et al. The Fibroblast Growth Factor Receptor Inhibitor PD173074 Blocks Small Cell Lung Cancer Growth In vitro and In vivo. Cancer Res [Internet]. 2009November15 [cited 2018 Nov 5];69(22):8645–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19903855 [DOI] [PubMed] [Google Scholar]


