Abstract
Aim
To determine the influence of high-efficacy disease modifying therapy (DMT) on the development of IgG SARS-CoV-2 antibody response in COVID-19 convalescent people with multiple sclerosis (pwMS).
Methods
Seventy-four pwMS taking high-efficacy DMTs (specifically natalizumab, fingolimod, alemtuzumab, ocrelizumab, cladribine and ublituximab) and diagnosed with COVID-19 and 44 healthy persons (HC) were enrolled. SARS-CoV2 antibodies were tested with Elecsys® Anti-SARSCoV-2 S assay.
Results
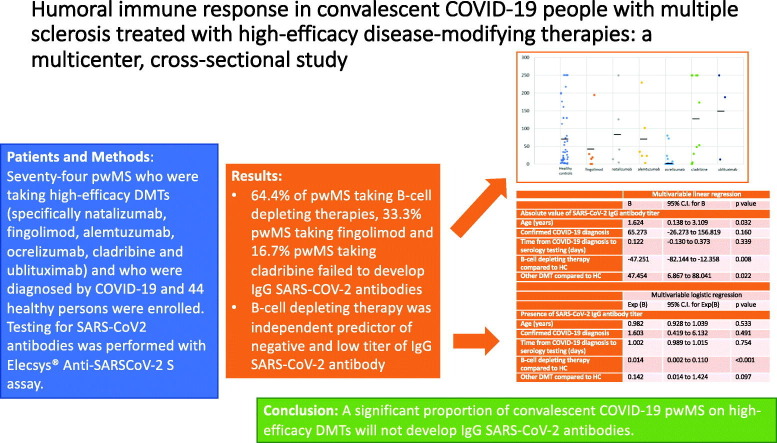
pwMS taking high-efficacy DMTs had a significantly higher chance of having negative titer of SARS-CoV2 antibodies compared to healthy controls (33 negative pwMS [44.6%] compared to one negative HC [2.3%], p < 0.001). pwMS taking B-cell depleting therapy (ocrelizumab and ublituximab) had a significantly higher chance of having negative titer of SARS-CoV2 antibodies compared to pwMS on all other DMTs (29 negative pwMS on B-cell therapy [64.4%] compared to four negative pwMS on all other DMTs [13.8%], p < 0.001). Out of other DMTs, two (33.3%) pwMS taking fingolimod and two (16.7%) pwMS taking cladribine failed to develop IgG SARS-COV-2 antibodies. B-cell depleting therapy independently predicted negative titer of IgG SARS-CoV-2 antibody (Exp[B] =0.014, 95%CI 0.002–0.110, p < 0.001).
Conclusions
A significant proportion of convalescent COVID-19 pwMS on high-efficacy DMTs will not develop IgG SARS-CoV-2 antibodies. B-cell depleting therapies independently predict negative and low titer of IgG SARS-CoV-2 antibody.
Keywords: Multiple sclerosis, COVID-19, Disease modifying therapy, SARS-CoV-2 IgG antibody
Graphical abstract
1. Introduction
The COVID-19 pandemic significantly impacted the management of people with multiple sclerosis (pwMS), reflected in reduced access to health care and rehabilitation facilities and modifications in available disease-modifying therapies (DMT) (García-Azorín et al., 2021; Baker et al., 2020; Colais et al., 2021). The main reason for this was the uncertainties with immunosuppressive effects of different DMTs at various levels of the immune system.
One of the unknowns causing the most concern was the development of humoral immunity in COVID-19 convalescent subjects. Studies in people without MS indicated that the majority of convalescent COVID-19 subjects develop an IgG SARS-CoV-2 antibody response and that a protective level prevails over a period of up to 9 months, regardless of age, gender, major blood types or clinical symptomatology (Achiron et al., 2021a). Only a few studies investigated the development of humoral immunity in COVID-19 convalescent pwMS, identifying potential impact of DMTs on development of humoral response (Zabalza et al., 2020; van Kempen et al., 2021; Conte, 2021). Zabalza and colleagues found that convalescent COVID-19 pwMS on anti-CD20 therapies had a lower proportion of positive serological tests (15.8%) than those with other DMTs (48.8%) or without DMTs (68.4%) (Zabalza et al., 2020). Although a serological response was noted in patients with all types of DMTs, including anti-CD20s, the proportion of positive serological tests varied depending on the DMT (Zabalza et al., 2020). As in the previous study, two other studies showed similar results (van Kempen et al., 2021; Conte, 2021).
The present study aims to determine the influence of high-efficacy DMT on development of the IgG SARS-CoV-2 antibody response in COVID-19 convalescent pwMS.
2. Objectives
The primary objective was to investigate the differences in presence and titers of IgG SARS-CoV-2 antibody between COVID-19 convalescent pwMS taking high-efficacy DMT and COVID-19 convalescent healthy persons.
The secondary objectives were to investigate the differences in the presence and titers of IgG SARS-CoV-2 antibodies depending on the different DMTs. Furthermore, predictors of seroconversion after recovery from COVID-19 were examined.
3. Methods
3.1. Study participants and design
This case-control study was performed in three University hospitals, two in Croatia (University Hospital Center Zagreb, Zagreb and University Hospital Center “Sestre Milosrdnice”, Zagreb) and one in Slovenia (University Medical Centre Ljubljana, Ljubljana). Consecutive pwMS taking high-efficacy DMTs (specifically natalizumab, fingolimod, alemtuzumab, ocrelizumab, cladribine and ublituximab) and diagnosed with COVID-19 by their general practitioners were invited to participate in this study. COVID-19 infection was confirmed if the patient tested positive for polymerase chain reaction (PCR) or point-of-care (POC) antigen test and suspected if the person had typical clinical presentation and was a known contact of a person with confirmed COVID-19. Age and sex-matched healthy persons with PCR- or POC-confirmed COVID-19 served as healthy controls. Healthy controls did not take any medications influencing the immune system nor had any neurological illnesses.
The study was approved by the Slovenian National Medical Ethics Committee and the Ethical Committee of the University Hospital Center, Zagreb.
3.2. Data collection
The following data were collected through a structured interview with each pwMS: demographic data (age, sex, height, weight, smoking status, and comorbidities), MS related data (date of 1st MS symptom, MS phenotype [RRMS, SPMS, PPMS], EDSS, high-efficacy DMT, and duration of treatment) and COVID-19 related data (date of diagnosis, diagnostic test [PCR or POC], symptoms, treatment, and hospitalization).
3.3. Detection of SARS-CoV-2 IgG antibodies
Testing for SARS-CoV2 antibodies was performed with Elecsys® Anti-SARSCoV-2 S assay (Roche Diagnostics Int, Rotkreuz, Switzerland) in a period of ≥2 weeks following the first occurrence of symptoms in all patients. Blood samples were drawn during the regular clinic visit of the patient. The assay was performed per the manufacturer's instructions, (Elecsys® Anti-SARS-CoV-2, 2021) using Cobas e 801 analytical unit for immunoassay tests (F. Hoffmann-La Roche Ltd.). Antibody titer ≥0.8 U/mL was considered positive, as recommended by the manufacturer.
3.4. Statistical analysis
Statistical analysis was performed with the IBM SPSS v25 software. The data distribution was tested with the Kolmogorov-Smirnov test. The differences between qualitative variables were tested with the chi-square test. The differences between the quantitative variables were tested with the parametric independent sample t-test and non-parametric Mann-Whitney test. The association between variables was tested with Spearman's correlation coefficient. Multivariable logistic regression was performed for binary outputs (seroconversion). p-values < 0.05 were considered significant.
4. Results
Seventy-four COVID-19 convalescent pwMS and 44 COVID-19 convalescent healthy controls were included in the analysis. Demographic characteristics and symptoms as well as diagnosis and treatment of the COVID-19 in pwMS are provided in Table 1 . In 57 (77.0%) pwMS and in all healthy controls, the diagnosis of COVID-19 was confirmed by PCR or POC. All pwMS with suspected COVID-19 had typical clinical presentation and had a known contact of a person with confirmed COVID-19. There was no difference in age (39.7 ± 9.2 vs. 43.0 ± 11.0 years, respectively; p = 0.089), sex (63.5% vs. 63.6% of women, respectively; p = 0.989) or time from COVID-19 diagnosis to antibody testing (63 [12–175] vs. 64.5 [12–347] days, respectively; p = 0.800) between pwMS and healthy controls.
Table 1.
Baseline characteristics of the MS cohort.
| Baseline characteristics of the MS cohort | |
|---|---|
| Sex (females) | 47 (63.5%) |
| Age (years) | 39.7 ± 9.2 |
| Height (cm) | 172.6 ± 17.3 |
| Weight (kg) | 76.5 ± 20.4 |
| Smoking | 8 (10.8%) |
| Hypertension | 9 (12.2%) |
| Diabetes mellitus | 2 (2.7%) |
| MS phenotype | |
| RRMS | 66 (89.2%) |
| SPMS | 1 (1.4%) |
| PPMS | 7 (9.5%) |
| Disease duration (years) | 8.4 ± 5.1 |
| EDSS | 2.5 (0–6.5) |
| COVID-19 characteristics | |
| Diagnosis conformed by | |
| PCR | 53 (71.6%) |
| POC test | 4 (5.4%) |
| Contact of confirmed COVID-19 person | 44 (59.5%) |
| Symptoms | |
| Fever <38 | 32 (43.2%) |
| Fever >38 | 25 (33.8%) |
| Headache | 35 (47.3%) |
| Muscle pain | 34 (45.9%) |
| Anosmia | 49 (66.2%) |
| Ageusia | 35 (47.3%) |
| GI symptoms | 8 (10.8%) |
| Dyspnea | 10 (13.5%) |
| Cough | 17 (23.0%) |
| Fatigue | 42 (56.8%) |
| Treatment | |
| Steroids | 5 (6.8%) |
| Azithromycin | 12 (16.2%) |
| Analgesics | 26 (35.1%) |
| Antipyretics | 46 (62.2%) |
| Hospitalization | 2 (2.7%) |
| O2 | 1 (1.4%) |
| Respirator | 0 |
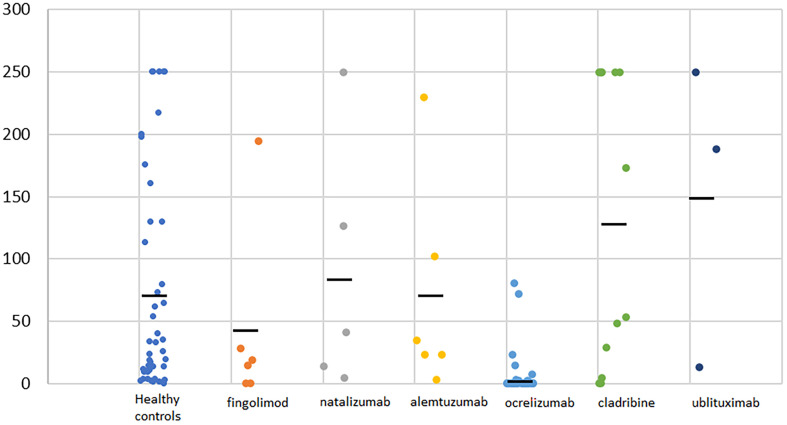
pwMS taking high-efficacy DMTs had a significantly higher probability of having a negative titer of SARS-CoV2 antibodies compared to healthy controls (33 negative pwMS [44.6%] compared to one negative HC [2.3%], p < 0.001). In participants from both groups with positive titers, there was no difference in the SARS-Cov2 antibodies titer between pwMS and HC (28.3 [1.8–250] vs 33.3 [1.6–250], p = 0.929). SARS-CoV-2 IgG antibody titer in COVID-19 convalescent healthy persons and COVID-19 convalescent pwMS by DMTs is presented in Fig. 1 . pwMS taking B-cell depleting therapy (ocrelizumab and ublituximab) had a significantly higher probability of having negative titer of SARS-CoV2 antibodies compared to pwMS taking all other high-efficacy DMTs (29 negative pwMS on B-cell therapy [64.4%] compared to four negative pwMS on all other DMTs [13.8%], p < 0.001) (Table 2 ). In pwMS with positive titers, those on B-cell depleting therapies revealed significantly lower titers of SARS-Cov2 antibodies (5.51 [1.8–250.0] vs 48.7 [3.4–250.0] p = 0.002). A positive correlation was established between IgG SARS-CoV-2 antibody titer and time elapsed from the last B-cell depleting therapy (ocrelizumab and ublituximab) until development of COVID-19 (rs = 0.412, p = 0.007). In all three ublituximab patients, timing of the last infusion in relation to COVID-19 was higher than 365 days (range from 368 to 378).
Fig. 1.
SARS-CoV-2 IgG antibody titer in COVID-19 convalescent healthy persons and COVID-19 convalescent pwMS by disease modifying therapy. Black lines present average value of SARS-CoV-2 IgG antibody titer for every subgroup.
Table 2.
The number and percentages of pwMS with positive and negative titer of SARS-CoV2 antibodies depending on the DMT used.
| SARS-CoV2 antibodies titer |
||
|---|---|---|
| Negative | Positivea | |
| Fingolimod | 2 (33.3%) | 4 (66.7%) |
| Natalizumab | 0 | 5 (100%) |
| Alemtuzumab | 0 | 6 (100%) |
| Ocrelizumab | 29 (69%) | 13 (31%) |
| Cladribine | 2 (16.7%) | 10 (83.3%) |
| Ublituximab | 0 | 3 (100%) |
pwMS people with multiple sclerosis, DMT disease modifying therapy.
Antibody titer >0.8 U/mL was considered positive, as recommended by the manufacturer.
Results of the multivariable logistic regression analysis, investigating predictors of seroconversion after recovery from COVID-19 are presented in Table 3 . B-cell depleting therapy independently predicted negative results of the IgG SARS-CoV-2 antibody testing (Exp[B] =0.014, 95%CI 0.002–0.110, p < 0.001).
Table 3.
Results of the multivariable logistic regression analysis investigating predictors of seroconversion after recovery from COVID-19.
| N | Multivariable logistic regression |
|||
|---|---|---|---|---|
| Exp (B) | 95% C.I. for Exp(B) | p value | ||
| Presence of SARS-CoV-2 IgG antibody titer | ||||
| Age (years) | 118 | 0.982 | 0.928–1.039 | 0.533 |
| Confirmed COVID-19 diagnosis | 118 | 1.603 | 0.419–6.132 | 0.491 |
| Time from COVID-19 diagnosis to serology testing (days) | 118 | 1.002 | 0.989–1.015 | 0.754 |
| B-cell depleting therapy compared to HC | 89 | 0.014 | 0.002–0.110 | <0.001 |
| Other DMT compared to HC | 73 | 0.142 | 0.014–1.424 | 0.097 |
Statistically significant predictors are highlighted.
5. Discussion
This study has exposed the significant influence of B-cell depleting therapy on seroconversion in COVID-19 convalescent pwMS. Compared to healthy controls and pwMS taking other high-efficacy DMTs, pwMS taking B-cell depleting therapy had significantly higher probability of not developing seroconversion after COVID-19.
It is still unclear whether the use of lymphocyte-depleting agents benefits or harms the immune response against COVID-19. All real-world studies published so far identified similar risk factors for severe COVID-19, namely age, level of neurological disability, progressive MS phenotype and cardiovascular comorbidities (Louapre et al., 2020; Sormani et al., 2021; Salter et al., 2021). On the other hand, results regarding DMTs and risk of severe COVID-19 are not unanimous. While French and US studies did not identify DMTs to be associated with severe COVID-19, the Italian study identified corticosteroids and B-cell depleting therapy as independent predictors of severe COVID-19.
Another unanswered question is whether these DMTs may impact the antibody production against SARS-CoV-2 after recovery from COVID-19 or the immune reaction to vaccination. Data on both questions are limited. As mentioned earlier, several studies have demonstrated that convalescent COVID-19 pwMS on anti-CD20 therapies had a lower proportion of positive serological tests compared to those with other DMTs or without DMTs (Zabalza et al., 2020; van Kempen et al., 2021; Conte, 2021). Additionally, the Amsterdam MS cohort study revealed positive IgG SARS-COV-2 antibody in 64 patients (11.7%) (van Kempen et al., 2021). Although our data agree with the previous study, it has to be emphasized that two (33.3%) pwMS taking fingolimod and 2 (16.7%) pwMS taking cladribine failed to develop IgG SARS-COV-2 antibodies.
These results prompted the debate regarding the efficacy of the COVID-19 vaccines in inducing humoral immunity in MS patients treated with these DMTs. The study by Achiron and colleagues showed different rates of development of IgG SARS-CoV-2 antibody after vaccination with BNT162b2-COVID-19 vaccine (Achiron et al., 2021b). SARS-CoV-2 IgG antibody titer was high in healthy subjects, untreated pwMS, and pwMS taking cladribine, while most pwMS taking ocrelizumab and fingolimod failed to develop IgG SARS-COV-2 antibodies (Achiron et al., 2021b).
However, humoral immunity is just a fraction of the immune response to either SARS-CoV-2 infection or vaccination against COVID-19. Increasing interest is focused on the role of T-cell immunity in fighting SARS-CoV-2 infection and in resisting re-infection (Sheridan, 2021). The T-cell immunity might prove to be very important in pwMS taking B-cell depleting therapies. Supporting this is an Israeli study showing low rates of infection in pwMS receiving one or both doses of BNT162b2-COVID-19 vaccine, irrespective of DMT use (Achiron et al., 2021c). Future studies, especially the ones monitoring the effectiveness of COVID-19 vaccines in pwMS on B-cell depleting therapies will add more insight regarding this issue.
The limitations of this study were the unevenly distributed DMTs in pwMS and the relatively minuscule number of participants. Because the majority of patients were on B-cell depleting therapy, we were unable to address differences in humoral immunity for every given high-efficacy DMT. Nevertheless, the strengths of the study are comparison with healthy controls, multicenter design and the whole spectrum of different high-efficacy DMTs tested.
In conclusion, a significant proportion of convalescent COVID-19 pwMS on high-efficacy DMTs will not develop IgG SARS-CoV-2 antibodies. B-cell depleting therapies independently predict of negative and low titer of IgG SARS-CoV-2 antibody.
Authors' contributions
Mario Habek: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. Gregor Jakob Brecl: Data curation, Investigation, Methodology, Writing – review & editing. Vanja Bašić Kes: Data curation, Investigation, Methodology, Writing – review & editing. Dunja Rogić: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. Barbara Barun: Data curation, Investigation, Methodology, Writing – review & editing. Tereza Gabelić: Data curation, Investigation, Methodology, Writing – review & editing. Andreja Emeršič: Data curation, Investigation, Methodology, Writing – review & editing. Alenka Horvat Ledinek: Data curation, Investigation, Methodology, Writing – review & editing. Nevena Grbić: Data curation, Investigation, Methodology, Writing – review & editing. Ivana Lapić: Data curation, Investigation, Methodology, Writing – review & editing. Dragan Šegulja: Data curation, Investigation, Methodology, Writing – review & editing. Koraljka Đurić: Data curation, Investigation, Methodology, Writing – review & editing. Ivan Adamec: Data curation, Investigation, Methodology, Writing – review & editing. Magdalena Krbot Skorić: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing.
Financial & competing interest disclosure
MH: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
GJB: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Novartis, Pliva/Teva, Roche.
VBK: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Pliva/Teva, Roche.
DR: Reports no conflict of interest.
BB: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals.
TG: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
AE: Reports no conflict of interest.
AHL: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Bayer, Biogen, Sanofi Genzyme, Merck, Novartis, Pliva/Teva, Roche.
NG: Reports no conflict of interest.
IL: Reports no conflict of interest.
DŠ: Reports no conflict of interest.
KĐ: Reports no conflict of interest.
IA: Participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals.
MKS: received consultation and/or speaker fees from: Sanofi Genzyme, Roche.
Funding
No funding was received for this study.
References
- Achiron A., Gurevich M., Falb R., Dreyer-Alster S., Sonis P., Mandel M. SARS-COV-2 antibody dynamics and B-cell memory response over-time in COVID-19 convalescent subjects. Clin. Microbiol. Infect. 2021 May 8 doi: 10.1016/j.cmi.2021.05.008. S1198-743X(21)00229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021 Apr 22;14 doi: 10.1177/17562864211012835. 17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Dolev M., Menascu S., Zohar D.N., Dreyer-Alster S., Miron S., Shirbint E., Magalashvili D., Flechter S., Givon U., Guber D., Stern Y., Polliack M., Falb R., Gurevich M. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021 May;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Amor S., Kang A.S., Schmierer K., Giovannoni G. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID-19 pandemic. Mult. Scler. Relat. Disord. 2020 Aug;43:102174. doi: 10.1016/j.msard.2020.102174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colais P., Cascini S., Balducci M., Agabiti N., Davoli M., Fusco D., Calandrini E., Bargagli A.M. Impact of the COVID-19 pandemic on access to healthcare services amongst patients with multiple sclerosis in the Lazio region, Italy. Eur. J. Neurol. 2021 Apr 25 doi: 10.1111/ene.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte W.L. Attenuation of antibody response to SARS-CoV-2 infection in patients with multiple sclerosis on ocrelizumab: a case-control study. Mult Scler Relat Disord. 2021 May 7;52:103014. doi: 10.1016/j.msard.2021.103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elecsys® Anti-SARS-CoV-2. 2021. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html accessed May 27, 2020.
- García-Azorín D., Seeher K.M., Newton C.R., Okubadejo N.U., Pilotto A., Saylor D., Winkler A.S., Charfi Triki C., Leonardi M. Disruptions of neurological services, its causes and mitigation strategies during COVID-19: a global review. J. Neurol. 2021 May;22:1–14. doi: 10.1007/s00415-021-10588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., Deschamps R., Créange A., Wahab A., Pelletier J., Heinzlef O., Labauge P., Guilloton L., Ahle G., Goudot M., Bigaut K., Laplaud D.A., Vukusic S., Lubetzki C., De Sèze J., Covisep investigators, Derouiche F, Tourbah A, Mathey G, Théaudin M, Sellal F, Dugay MH, Zéphir H, Vermersch P, Durand-Dubief F, Françoise R, Androdias-Condemine G, Pique J, Codjia P, Tilikete C, Marcaud V, Lebrun-Frenay C, Cohen M, Ungureanu A, Maillart E, Beigneux Y, Roux T, Corvol JC, Bordet A, Mathieu Y, Le Breton F, Boulos DD, Gout O, Guéguen A, Moulignier A, Boudot M, Chardain A, Coulette S, Manchon E, Ayache SS, Moreau T, Garcia PY, Kumaran D, Castelnovo G, Thouvenot E, Taithe F, Poupart J, Kwiatkowski A, Defer G, Derache N, Branger P, Biotti D, Ciron J, Clerc C, Vaillant M, Magy L, Montcuquet A, Kerschen P, Coustans M, Guennoc AM, Brochet B, Ouallet JC, Ruet A, Dulau C, Wiertlewski S, Berger E, Buch D, Bourre B, Pallix-Guiot M, Maurousset A, Audoin B, Rico A, Maarouf A, Edan G, Papassin J, Videt D Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020 Sep 1;77(9):1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter A., Fox R.J., Newsome S.D., Halper J., Li D.K.B., Kanellis P., Costello K., Bebo B., Rammohan K., Cutter G.R., Cross A.H. Outcomes and risk factors associated with SARS-CoV-2 infection in a north American registry of patients with multiple sclerosis. JAMA Neurol. 2021 Mar;19 doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C. COVID-19 testing turns to T cells. Nat. Biotechnol. 2021;39:533–534. doi: 10.1038/s41587-021-00920-9. [DOI] [PubMed] [Google Scholar]
- Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., Radaelli M., Immovilli P., Capobianco M., Trojano M., Zaratin P., Tedeschi G., Comi G., Battaglia M.A., Patti F., Salvetti M., Musc-19 Study Group Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann. Neurol. 2021 Apr;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kempen Z.L.E., Strijbis E.M.M., Al M.M.C.T., Steenhuis M., Uitdehaag B.M.J., Rispens T., Killestein J. SARS-CoV-2 antibodies in adult patients with multiple sclerosis in the Amsterdam MS cohort. JAMA Neurol. 2021 Jul 1;78(7):880–882. doi: 10.1001/jamaneurol.2021.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalza A., Cárdenas-Robledo S., Tagliani P., Arrambide G., Otero-Romero S., Carbonell-Mirabent P., Rodriguez-Barranco M., Rodríguez-Acevedo B., Restrepo Vera J.L., Resina-Salles M., Midaglia L., Vidal-Jordana A., Río J., Galan I., Castillo J., Cobo-Calvo Á., Comabella M., Nos C., Sastre-Garriga J., Tintore M., Montalban X. COVID-19 in multiple sclerosis patients: susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2020 Dec 19 doi: 10.1111/ene.14690. [DOI] [PubMed] [Google Scholar]