Abstract
Background
Ethiopia reported the first case of COVID-19 on 13th March, 2020 with community transmission ensuing by mid-May. A national, population-based serosurvey against anti-SARS-CoV-2 IgG was conducted to measure the prevalence of prior COVID-19 infections and better approximate the burden across major towns in Ethiopia.
Methods
We conducted a cross-sectional, population-based serosurvey from June 24 to July 8, 2020 in 14 major urban areas. Two-stage cluster sampling was used to randomly select enumeration areas and households. All persons aged ≥15 years were enrolled. Serum samples were tested by Abbott™ ARCHITECT™ assay for SARS-CoV-2 IgG antibodies. National COVID-19 surveillance data on the median date of the serosurvey is analyzed for comparison.
Findings
Adjusted seroprevalence was 3.5% (95% CI: 3.2%-3.8%) after controlling for age, sex and test kit performance. Males (3.7%) and females (3.3%) were nearly equally infected, while middle-aged adults '40-65 years' had the highest (4.0%) prevalence. Gambella (7.5%), Dire Dawa (6.2%) and Jigjiga (6.1%) were the most affected towns. About 6.7% and 8.0% of seropositives had symptoms and chronic underlying illness, respectively. A surveillance system had identified 4,416 RT-PCR confirmed cases in Addis Ababa.
Interpretation
This serosurvey shows that a majority of urban Ethiopians remain uninfected with SARS-CoV-2. Most anti-SARS-CoV-2 IgG positive cases were asymptomatic with no underlying illness, keeping case detection to a minimum.
Keywords: Coronavirus Infections / epidemiology, Immunoglobulin G / blood, Seroepidemiological Studies, Ethiopia / epidemiology
Background
Ethiopia activated its Public Health Emergency Operation Centre (PHEOC) on January 27, 2020 following reports of SARS-CoV-2 infections from a couple of countries in the world and started wide scale surveillance activity throughout the country. Surveillance was conducted with tight controls of all the national borders including the main gate, Addis Ababa International Airport, and all land crossing borders across more than 20 port of entry checkpoints through screening of all individuals entering the country and isolation of suspect cases for two weeks. It has adopted the suspect case definition from WHO interim guidelines and has started to test all individuals fulfilling the suspect case definition with RT-PCR tests after cases were reported from health facilities and from the community through toll free calls. Through these intensified surveillance activities, Ethiopia reported its first case of COVID-19 on a 48 year old male who entered the country nine days before the case detection, on March 04, from Burkina Faso.
After reporting its first case on March 13, 2020, Ethiopia experienced a very slow increase of new cases until early July, when the 5-day rolling average increased from 1.2 in March to 106 positive tests in July. Starting in mid-May 2020, signs of sustained community transmission were observed in parts of Addis Ababa. From July onwards, the number of reported infections further increased to a 5-day rolling average >600 by the end of July. Ethiopia's exponential increase in cases followed the trend seen in most of Africa. (Twahirwa Rwema et al., 2020) The country implemented early risk mitigation measures to curb the spread of the virus. The most notable measures were school closure, promotion of physical distancing and frequent hand-washing practice supported by a nationwide state of emergency. Moreover, the country enforced mandatory quarantine for new arrivals and mandatory face covering as early as March 2020.
Due to individuals avoiding medical care when ill, variable test availability and practices, incomplete case reporting to public health authorities, and asymptomatic infections, it is generally believed that officially reported cases represent the “tip of the iceberg” when compared to the true severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection incidence. (Ramanathan et al., 2020) With very limited testing and surveillance capacity, it is difficult to use case counts as a measure of disease burden. (El-Sadr and Justman, 2020) Testing for SARS-CoV-2 responsive antibodies in representative populations is essential for detection of past covid-19 cases including mild and asymptomatic cases, which helps for making public policy decisions to open-up or to continue enforcing national, state and local government rules to “shelter-in place”.
From June 24 to July 17, 2020, we conducted a population-based survey in fourteen purposively selected major towns in Ethiopia to measure the seroprevalence of antibodies to SARS-CoV-2 and better approximate the number of people with a history of infections. The survey also intended to identify individuals’ demographic and clinical characteristics that could be associated to high prevalence. Prior seroprevalence studies conducted in Ethiopia were having limitations in scope, design complexity and laboratory test method validity.(Deyessa et al., 2020)(Chan, 2021) In this survey, we provide new and well-measured data at a national level that better describes the disease burden across major towns of Ethiopia during July 2020 (a period with wide community transmission) using a larger sample size with the necessary design complexity than used in the previous reports.
Methods
As of January 2021, the population of Ethiopia was estimated to be 116,401,322 people, of which 21.3 % of the population are urban residents. Ethiopia's population density is 115 per Km2 (298 people per mi2) as of 2021. The sex ratio of the total population was 0.991 (991 males per 1,000 females), which is lower than the global sex ratio. The population median age is 19.5 years with about 46.3% of the people below 15 years of age. Total life expectancy (both sexes) at birth for Ethiopia is 56.2 years. 48.93% of the adult population (aged 15 years and above) in Ethiopia is able to read and write.(Ethiopia population (2021) live — Countrymeters, n.d.)(Ethiopia Population (2021) - Worldometer, n.d.)
The survey methodology was based on two-stage cluster sampling. In Ethiopia, each town is divided into enumeration areas (EAs) with clear geographic demarcation during the construction of census maps. Each EA has on average 200 households. After determining the sample size with assumed seroprevalence of 5% for the capital, Addis Ababa, and 1% for the rest of towns, 219 EAs (76 EAs in Addis Ababa and 143 EAs in other towns) were selected randomly by the Central Statistical Authority (CSA). All households within the chosen EAs were listed and then 40 households were randomly selected.
All individuals ≥15 years of age in the randomly selected households were eligible to participate. Trained interviewers explained the purpose of the survey, the confidentiality of the data, and the fact that the test result would not be returned to respondents. All consenting participants were interviewed and asked to provide a blood sample. Trained nurse interviewers completed a questionnaire eliciting information on demographic characteristics, prior exposure to COVID-19, protective behaviors, presence of underlying chronic illness, and symptom history since the introduction of the virus into the country.
A trained laboratory professional collected 3-5mL of venous blood in a serum separator tube. Daily collected blood samples were triple packed and transported at 2-8°C from the field in less than six hours to a nearby designated laboratory for temporary storage until being shipped to the International Clinical Laboratories (ICL) Main Branch for analysis. To determine seroprevalence, we used the Abbott™ ARCHITECT™ SARS-CoV-2 IgG assay, a validated (Bryan et al., 2020) chemiluminescent microparticle immunoassay (CMIA) with catalog number 6R86-22 and intended for the qualitative detection of IgG antibodies to SARS-CoV-2 in human serum. The assay is designed to detect immunoglobulin class G (IgG) antibodies to the nucleocapsid protein of SARS-CoV-2 in serum and plasma from individuals who are suspected to have had coronavirus disease (COVID-19) with a reported Sensitivity and Specificity of 100% and 99.6% respectively.(Abbott Laboratories, 2020)
The primary variables of interest in the analysis were evidence of SARS-CoV-2 infection, age, gender, educational level, COVID-19 symptoms, reported prevention behaviors, and the following underlying health conditions: diabetes, hypertension, heart disease, tuberculosis, Human Immunodeficiency Virus/Acquired Immunodeficiency syndrome (HIV/AIDS), and chronic respiratory disease. All analysis incorporated sampling weights that adjusted for unequal probabilities of selection and response rates, which were calculated based on age-sex strata using the national CSA data prepared in 2018.
Finally, further adjustment was made to the data to consider test kit performance using a formula for adjusted prevalence adopted from another study after in-country performance evaluation of test kits was done on 241 samples. (Sempos and Tian, 2021)
Of the 241 samples used to evaluate the kit performance and not included in this survey, 128 were known reverse transcription polymerase chain reaction (RT-PCR) positives from COVID-19 treatment centers and 113 were RT-PCR negative samples. For the test kit evaluation, of the 128 positive samples, we used only the 55 RT-PCR positive samples that elapsed more than 14 days post RT-PCR test. For this purpose, Real-Time Fluorescent RT-PCR kit was used for detecting SARS-CoV-2. It is a molecular in vitro diagnostic test that aids in the detection and diagnosis of SARS-CoV-2 and is based on widely used nucleic acid amplification technology. (Real-Time Fluorescent RT-PCR Kit for Detecting SARS-CoV-2, n.d.) Negative samples were collected in June 2019, months before the COVID-19 pandemic began in Wuhan, China, and the samples included a variety of infectious disease positive samples, including hepatitis, HIV, chikungunya fever, and dengue fever.
Prevalence of symptoms and chronic illnesses for seropositive individuals and their adherence to prevention guidelines was estimated. We reviewed the cumulative number of infections reported by the National COVID-19 Surveillance System as of July 1st, the median day for the serosurvey study period, in order to provide a comparison to the serosurvey data. In addition, the minimum case fatality rate was estimated using the fraction of deaths reported from the national surveillance system on the median date of the serosurvey over the number of infections obtained from the survey.
Logistic regression was also done to determine the association of some sociodemographic factors with risk of SARS-CoV-2 infection. All statistical analyses were run using IBM® SPSS® Statistics for Windows, Version 25. The study was conducted as part of routine surveillance activities and therefore, did not require ethical clearance from the local IRB. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.1
Results
Of 17,200 sampled individuals from 14 study towns, 16,932 had complete data and valid laboratory results. Local validation of the Abbott™ ARCHITECT™ SARS-CoV-2 IgG assay found a sensitivity of 54.5% (95% confidence interval (CI): 40.5%-68.0%) and a specificity of 100%.
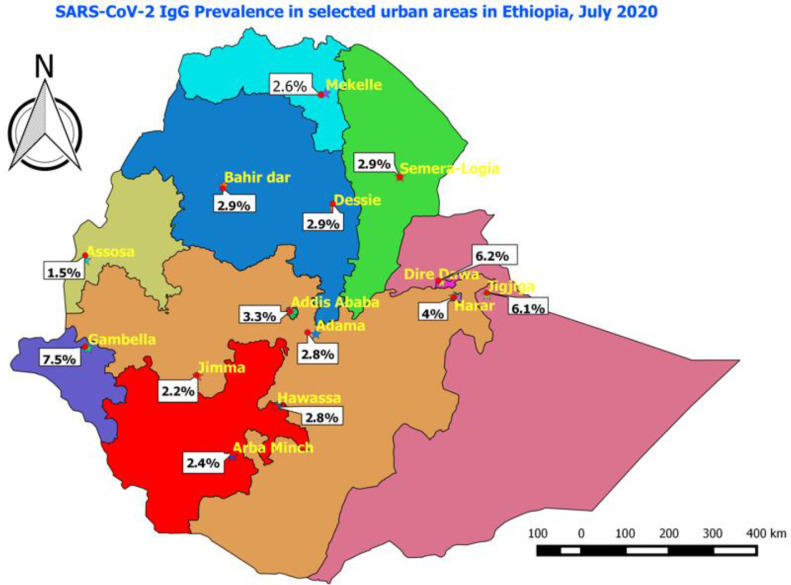
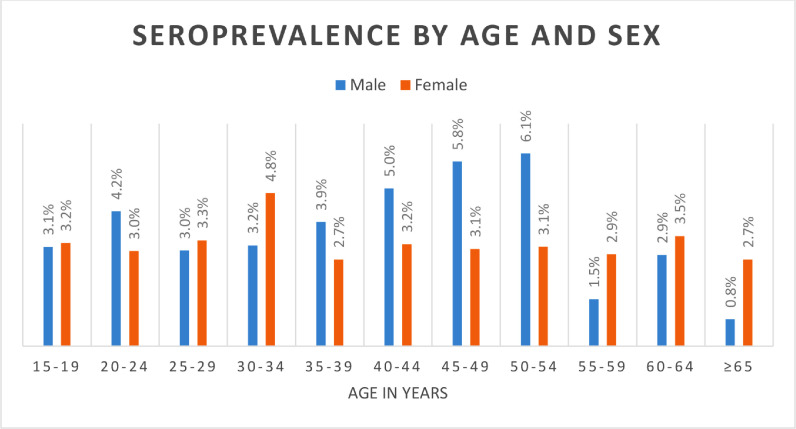
Two-thirds (66%) of the surveyed population were female and 78% of them were below 40 years with a mean age of 33 years [SD=14.6] (Table 1 ). From the total surveyed individuals, 314 people tested positive, yielding a crude prevalence of 1.9% (95% CI: 1.7-2.1), which remained the same with population level age-sex distribution adjustment. Further adjusting for local test kit performance, the seroprevalence was 3.5% [95% CI: 3.2-3.8] (Table 3). Males (3.7%) and females (3.3%) had comparable percent positivity. Middle age adults aged 41-65 years had the highest percent positivity (4.0%) while older adults >65 years had the lowest percent positivity (1.8%). Towns near the international borders, including Gambella (7.5%), Dire Dawa (6.2%) and Jigjiga (6.1%), had higher seroprevalence while the central towns, including Addis Ababa (the capital), had lower seroprevalence. (Table 3)
Table 1.
Age-sex distributions of the surveyed population and the general population in the study areas
| Characteristics | Study population (n) | Percent (%) | General population (n) | Percent (%) |
|---|---|---|---|---|
| Age (years) | ||||
| 15-20 | 3278 | 19.4 | 1,112,751 | 16.5 |
| 21-40 | 9852 | 58.2 | 3,784,028 | 56.3 |
| 41-65 | 3070 | 18.1 | 1,469,929 | 21.9 |
| >65 | 674 | 4.0 | 357,114 | 5.3 |
| Missing | 58 | 0.3 | ||
| Sex | ||||
| Male | 5829 | 34.4 | 3,357,792 | 49.9 |
| Female | 11102 | 65.6 | 3,366,031 | 50.1 |
| Missing | 1 | 0.0 | 0.0 | |
| Total | 16932 | 100 | 6,723,823 | 100 |
Table 3.
Crude and adjusted Seroprevalence disaggregated by socio-demographic factors, Ethiopia, July 2020
| Characteristics | Positive cases (n) | Sample size (n) | Crude Seroprevalence (95% CI) | Seroprevalence adjusted for age-sex and kit performance (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 162 | 8235 | 2.0% (1.6%-2.3%) | 3.7% (3.3%-4.1%) |
| Female | 158 | 8696 | 1.8% (1.6%-2.1%) | 3.3% (2.9%-3.7%) |
| Age (years) | ||||
| 15-20 | 60 | 3286 | 1.8% (1.4%-2.4%) | 3.3% (2.7%-4.0%) |
| 21-40 | 176 | 9501 | 1.9% (1.6%-2.1%) | 3.5% (3.1%-3.9%) |
| 41-65 | 75 | 3341 | 2.0% (1.5%-2.5%) | 4.0% (3.4%-4.7%) |
| >65 | 7 | 745 | 1.2% (0.6%-2.3%) | 1.8% (0.9%-3.0%) |
| Town | ||||
| Addis Ababa | 114 | 6227 | 1.8% (1.5%-2.1%) | 3.3% (2.9%-3.8%) |
| Bahir dar | 15 | 941 | 1.4% (0.8%-2.4%) | 2.9% (1.9%-4.2%) |
| Dessie | 11 | 702 | 1.3% (0.7%-2.4%) | 2.9% (1.8%-4.4%) |
| Adama | 10 | 687 | 1.5% (0.8%-2.6%) | 2.8% (1.7%-4.4%) |
| Jimma | 10 | 834 | 1.1% (0.6%-2.0%) | 2.2% (1.3%-3.4%) |
| Mekelle | 11 | 806 | 1.2% (0.7%-2.3%) | 2.6% (1.6%-4.0%) |
| Arba Minch | 12 | 891 | 1.3% (0.7%-2.2%) | 2.4% (1.5%-3.6%) |
| Semera-Logia | 14 | 912 | 2.0% (1.3%-3.1%) | 2.9% (1.9%-4.2%) |
| Gambella | 29 | 713 | 3.7% (2.5%-5.3%) | 7.5% (5.6%-9.7%) |
| Assosa | 6 | 785 | 1.0% (0.5%-2.0%) | 1.5% (0.8%-2.7%) |
| Harar | 19 | 863 | 2.1% (1.3%-3.3%) | 4.0% (2.8%-5.6%) |
| Dire Dawa | 23 | 684 | 3.2% (2.1%-4.8%) | 6.2% (4.5%-8.3%) |
| Jigjiga | 31 | 926 | 3.9% (2.8%-5.3%) | 6.1% (4.7%-7.9%) |
| Hawassa | 15 | 961 | 1.5% (0.9%-2.5%) | 2.8% (1.8%-4.0%) |
| ⁎⁎National | 320 | 16,932 | 1.9% (1.7%-2.1%) | 3.5% (3.2%-3.8%) |
| Education | ||||
| No education | 48 | 2531 | 1.9% (1.4%-2.5%) | 3.5% (2.8%-4.3%) |
| Primary | 87 | 3712 | 2.3% (1.9%-2.9%) | 4.2% (3.6%-4.9%) |
| Secondary | 79 | 4446 | 1.8% (1.4%-2.2%) | 3.3% (2.8%-3.9%) |
| TV+*ʃ | 50 | 3534 | 1.4% (1.1%-1.9%) | 2.6% (2.1%-3.2%) |
| Employment status | ||||
| Employed | 166 | 7937 | 2.1% (1.8%-2.4%) | 3.9% (3.4%-4.2%) |
| Not employed ʃ | 142 | 8476 | 1.7% (1.4%-2.0%) | 3.1% (2.7%-3.5%) |
TV+: Technical vocational or higher level
Reference
National prevalence: shows the crude and adjusted seroprevalence for all samples
In Addis Ababa, the capital city, applying a seroprevalence of 3.3%, we estimated 89,842 COVID-19 infections among people aged >14 years. To compare this estimate with the surveillance report, as of July 1st, the median period of the serosurvey sample collection, the national COVID-19 surveillance system had identified 4,416 RT-PCR confirmed cumulative infections in Addis Ababa. From these cases, 4,183 were aged>14 years, the group on which our seroprevalence study has also focused. (Table 5)
Table 5.
National COVID-19 Surveillance Data by Patient Demographics, Symptom History, Underlying Illness and Geography from March-June 2020.
| Characteristics | Number of cases | Percent (%) | Number of deaths | Percent (%) |
|---|---|---|---|---|
| Age (years) | ||||
| <15 | 355 | 5.2 | 2 | 2.0 |
| 15-20 | 914 | 13.5 | 3 | 3.0 |
| 21-40 | 4278 | 63.0 | 29 | 29.0 |
| 41-65 | 1003 | 14.8 | 34 | 34.0 |
| >65 | 189 | 2.8 | 29 | 29.0 |
| Missing | 45 | 0.7 | 3 | 3.0 |
| Sex | ||||
| Male | 4095 | 60.4 | 61 | 61 |
| Female | 2683 | 39.6 | 39 | 39 |
| Region | ||||
| Addis Ababa | 4416 | 65.1 | 77 | 77 |
| Amhara | 336 | 5.0 | 2 | 2 |
| Oromia | 404 | 6.0 | 13 | 13 |
| Tigray | 217 | 3.2 | 2 | 2 |
| SNNP | 90 | 1.3 | 2 | 2 |
| Afar | 98 | 1.4 | ||
| Gambella | 630 | 9.3 | ||
| Benishangul- Gumuz | 17 | 0.2 | ||
| Harar | 47 | 0.7 | 2 | 2 |
| Dire Dawa | 74 | 1.1 | 1 | 1 |
| Somali | 455 | 6.7 | 1 | 1 |
| Symptomatic | ||||
| Yes | 483 | 7.1 | 20 | 20 |
| No | 6301 | 92.9 | 80 | 80 |
| Chronic illness | ||||
| Yes | 237 | 3.5 | 12 | 12 |
| No | 6007 | 88.5 | 82 | 82 |
| Unknown | 540 | 8 | 6 | 6 |
On July 1st, the national surveillance system had reported 100 total cumulative deaths nationally, of which 77 were from Addis Ababa city with all deaths aged above 14 years (Table 5). Based on this, dividing the total number of deaths in Addis Ababa to the number of estimated (89,842) infections from this prevalence study, the estimated minimum infection fatality ratio (IFR) in Addis Ababa city is 0.086%, which is equal to nine deaths from every 10,000 cases.
Persons aged 41-65 years were more likely to be infected than people above the age of 65 years, with an odds ratio of 2.5 (95% CI: 1.1-5.5). Odds of infection for towns near the national border including Gambella, Dire Dawa and Jigjiga were 2.1 (95% CI: 1.4-3.3), 1.9 (95% CI: 1.2-3.0), and 2.3 (95% CI: 1.5-3.3) times higher compared to the capital city. Employed population groups have increased risk for infection by 30% (OR=1.3: 95% CI: 1.0-1.6)) compared to unemployed groups. Among employed subjects, public transport drivers (2.5%), private business organization employees (2.2%) and health care workers (1.9%) were the most affected groups, respectively. When we compare patients’ risk of infection by their highest level of education; completing only primary education has an increased risk for infection with an odds of 1.7 (95% CI: 1.2-2.3) compared to those with technical vocational or higher education, but on the other hand, not having any formal education or completing secondary education only does not have any association with increased or decreased risk of infection. (Table 2 ).
Table 2.
Demographic Characteristics versus risk of SARS-CoV-2 infection, Ethiopia, July 2020
| Characteristic | Number of positives | Percent | Number of negatives | Percent | OR (95% CI) |
|---|---|---|---|---|---|
| Age (years) | |||||
| 15-20 | 60 | 1.8 | 3226 | 98.2 | 2.0 (0.9-4.3) |
| 21-40 | 176 | 1.9 | 9325 | 98.1 | 2.0 (0.9-4.2) |
| 41-65 | 75 | 2.2 | 3266 | 97.8 | 2.4 (1.1-5.3)** |
| >65 ʃ | 7 | 0.9 | 738 | 99.1 | 1.0 |
| Towns | |||||
| Mekelle | 11 | 1.4 | 795 | 98.6 | 0.7 (0.4-1.4) |
| Semera Logia | 14 | 1.6 | 898 | 98.4 | 1.1 (0.7-1.9) |
| Bahir Dar | 15 | 1.6 | 926 | 98.4 | 0.8 (0.4-1.4) |
| Dessie | 11 | 1.6 | 691 | 98.4 | 0.7 (0.4-1.4) |
| Adama | 10 | 1.5 | 677 | 98.6 | 0.8 (0.4-1.6) |
| Jimma | 10 | 1.2 | 824 | 98.8 | 0.6 (0.3-1.2) |
| Assosa | 6 | 0.8 | 779 | 99.3 | 0.6 (0.3-1.2) |
| Gambella | 29 | 4.1 | 684 | 95.9 | 2.1 (1.4-3.3)** |
| Arbaminch | 12 | 1.3 | 879 | 98.7 | 0.7 (0.4-1.3) |
| Harari | 19 | 2.2 | 844 | 97.8 | 1.2 (0.7-2.0) |
| Dire Dawa | 23 | 3.4 | 661 | 96.6 | 1.9 (1.2-3.0)** |
| Jigjiga | 31 | 3.3 | 895 | 96.7 | 2.3 (1.5-3.3)** |
| Hawassa | 15 | 1.5 | 946 | 98.5 | 0.9 (0.5-1.5) |
| Addis Ababa ʃ | 114 | 1.8 | 6113 | 98.2 | 1.0 |
| Education | |||||
| No education | 48 | 1.9 | 2483 | 98.1 | 1.4 (0.9-2.0) |
| Primary | 87 | 2.3 | 3625 | 97.7 | 1.7 (1.2-2.3)** |
| Secondary | 79 | 1.8 | 4367 | 98.2 | 1.2 (0.9-1.8) |
| TV+*ʃ | 50 | 1.4 | 3484 | 98.6 | 1.0 |
| Employment status | |||||
| Employed | 166 | 2.1 | 7771 | 97.9 | 1.3 (1.0-1.6)** |
| HCW | 5 | 1.9 | 263 | 98.1 | |
| Drivers | 12 | 2.5 | 487 | 97.5 | |
| Gov't. employee | 24 | 1.3 | 1819 | 98.7 | |
| Private employee | 107 | 2.2 | 4961 | 97.8 | |
| Unemployed ʃ | 142 | 1.7 | 8334 | 98.3 | 1.0 |
| Jobless | 67 | 1.9 | 3517 | 98.1 | |
| Other | 86 | 1.6 | 5342 | 98.4 |
From 313 seropositive cases, 21 (6.7%) patients were symptomatic, with cough (1.9%), headache (1.3%), fever (1.3%) and nausea/vomiting (1.3%) being the most commonly reported symptoms. The national surveillance system reported 7.1% of nationally identified cases had symptoms. Chronic illness was found among 8% of seropositive patients, whereas the national surveillance reported 3.5% of cases had chronic medical illnesses (Table 4 ). From all seropositive cases; one person said he has contact history with a confirmed or suspected case and another one person confirmed he was tested by RT-PCR prior to this study, but the result was unknown. Comparing seropositive and seronegative subjects based on their manifestation of COVID-19 compatible clinical symptoms, presence of chronic underlying illness and their adherence to infection prevention measures, no significant difference was observed between the two groups.
Table 4.
Incidence of Symptoms, underlying chronic illnesses and adherence to COVID-19 prevention guidelines among Sero-positive individuals, Ethiopia, July 2020.
| Characteristics | Yes | Total valid samples | Percent yes (%) |
|---|---|---|---|
| Symptoms | 21 | 313 | 6.7 |
| Fever | 4 | 313 | 1.3 |
| Sore throat | 2 | 313 | 0.6 |
| Cough | 6 | 313 | 1.9 |
| Runny nose | 3 | 313 | 0.9 |
| Shortness of breath | 1 | 312 | 0.3 |
| Headache | 4 | 311 | 1.3 |
| Nausea/vomiting | 4 | 311 | 1.3 |
| Loss of taste/smell | 1 | 312 | 0.3 |
| Underlying illness | 25 | 313 | 8.0 |
| Hypertension | 12 | 312 | 3.8 |
| Heart disease | 6 | 313 | 1.9 |
| Diabetes mellitus | 8 | 313 | 2.6 |
| Tuberculosis | 2 | 158 | 1.3 |
| HIV AIDS | 1 | 313 | 0.3 |
| Chronic respiratory disease | 4 | 313 | 1.3 |
| Other chronic diseases | 4 | 308 | 1.3 |
| Pregnancy | 4 | 189 | 2.1 |
| Adherence to all COVID-19 Prevention measures | 271 | 313 | 86.6 |
| Increased hand washing frequency | 300 | 313 | 95.8 |
| Avoided social gatherings | 300 | 313 | 95.8 |
| Use face covering going outside home | 295 | 313 | 94.2 |
| Exercise physical distancing | 283 | 313 | 90.4 |
Discussion
Here, we report the prevalence of SARS-CoV-2 across the major towns of Ethiopia during July 2020 by a serological test for Anti-SARS-Cov-2 IgG antibodies. We estimated an adjusted prevalence of 3.5% (95% CI: 3.9%-4.5%) from 16,932 tested samples. We find that prevalence was similar among males and females, whereas middle-aged adults had the highest percent positivity. Regional towns around the national borders (Gambella, Dire Dawa, and Jigjiga) were more affected than towns from the central part of the country. Employment and education level affect the risk of infection. Here we observed that employment was associated with increased infection risk (OR=1.3: 95% CI: 1.0-1.6) while higher education was associated with lowered risk. The national surveillance system had identified 6,778 COVID-19 cases: 7.1% and 3.5% of those had symptoms and chronic underlying illnesses, respectively.
In this survey two-thirds of those tested were females and more than three-fourth of the surveyed population were between 15 and 40 years, which is comparable with the population level strata based on the latest CSA data. (Zekaria and Ababa, 2013) Overall seroprevalence across the 14 major towns in Ethiopia was comparable with some of the most affected countries and towns in the world, including Spain, Los Angeles, Milan, China and Brazil, based on results from community-based serosurveys or serosurveys from blood donors. (Pollán et al., 2020)(Filho et al., 2020)(Valenti et al., 2020)(Stadlbauer et al., 2020)(Xu et al., 2020) Seroprevalence in Malawi shows higher prevalence than our study, but it was done among healthcare workers, (Chibwana et al., 2020) who normally are expected to have more exposure than the general population.
According to our results, in Addis Ababa, the number of estimated infections as of July 1st was 21 times greater than the number of cases detected by the National COVID-19 Surveillance System. This huge under-detection is expected in a country like Ethiopia, where asymptomatic and mildly symptomatic infected persons who opt to stay at home go unnoticed because of the limited share of tests for these individuals. On the other hand, these community based serology tests will have the chance to detect a majority of past infections among tested subjects, as we go home to home to identify all exposed individuals. The estimate on this study is higher than that found in surveys conducted in Geneva and United States (US). (Havers et al., 2020)(Stringhini et al., 2020). A study done in Addis Ababa in April shows higher seroprevalence, but the difference is likely attributable to methodological differences, including test kit type and sample size. (Deyessa et al., 2020) The other study had used an IgG/IgM rapid test cassette where sensitivity will be expected to be much higher than CMIA based IgG tests. In addition, the several fold sample size differences used in these two studies might have its own effect on the seroprevalence estimation.
The estimated infection fatality ratio on this survey (0.09%) provides the lower limit of mortality, since surveillance data cannot track every community death and health facility deaths with unidentified causes. The estimated IFR was much lower compared to some other estimates from England (0.30%-0.49%), Stockholm (0.58%) and China (0.25%–3.0%), but those estimates were determined based on PCR positive denominators, unlike this study. (Wilson et al., 2020)(Estimating the infection fatality ratio in England - CEBM, n.d.)(Public Health Agency of Sweden, 2020) IFR estimates based on seroprevalence data denominators in Iceland and Geneva showed a higher IFR estimate than our study but the estimate in Denmark was lower than our study. (Perez-Saez et al., 2020)(Gudbjartsson et al., 2020) (Erikstrup et al., 2020)
Seroprevalence was nearly equal among males and females, but individuals aged 41-65 years had the highest percent positivity. This was in contrast to the number of cases identified by the National COVID-19 surveillance system by the end of June, where two-thirds of cases were males and aged 21-40 years. But the pattern was similar to that from a population-level seroprevalence in Los Angeles and Geneva where a majority of COVID-19 infections were among the middle-aged population. (Majiya H et al., 2020)(Havers et al., 2020) Although many studies suggested that there is no significant risk difference between males and females (Bryan et al., 2020)(Pollán et al., 2020), some other studies showed males are more prone to acquire infection than females due to humoral immunologic differences. (Chibwana et al., 2020)(Havers et al., 2020)
Addis Ababa had the largest number of cases from surveillance since the outbreak started in Ethiopia, but towns near the national border were found to have the highest seroprevalence. In contrast to our study, in the Kenyan and Spanish seroprevalence studies, the major central towns were the most affected areas. (Pollán et al., 2020)(Uyoga et al., 2020)
The very low proportion of symptomatic cases on this survey was consistent with surveillance data. But it was lower than other surveys, where only a small fraction of seropositive cases were asymptomatic. (Pollán et al., 2020)(Majiya H et al., 2020)(Wilson et al., 2020) This is related to the milder nature of the virus in Ethiopia and Africa in general which needs further investigation to better understand the possible reasons.(Social, environmental factors seen behind Africa's low COVID-19 cases |WHO |Regional Office for Africa, n.d.)
The prevalence of underlying chronic illnesses among seropositive cases was two times the prevalence reported by the national surveillance, but it was much lower compared to a systematic review and meta-analysis done elsewhere, which reported 40% of COVID-19 patients had underlying chronic illnesses and another study that showed 42% prevalence among hospital admitted COVID-19 patients. (Estimating the infection fatality ratio in England - CEBM, n.d.)(Public Health Agency of Sweden, 2020) This difference might be attributed to a higher proportion of unidentified chronic illnesses in Ethiopia, where health screening is not widely practiced. A Study done in the past in Ethiopia shows the prevalence of hypertension was found to be 3.5 times higher than that reported by the subjects and the prevalence of diabetes six times higher, indicating a large hidden burden of disease. (Prevett, n.d.)
Figure 1.
SARS-CoV-2 IgG Prevalence in selected urban areas in Ethiopia, July 2020
Figure 2.
Anti SARS-CoV-2 IgG prevalence by age and sex
Our findings showed that individuals above the secondary level of education have lower risk for infection than others. This was also observed in a study done in Rio de Janeiro, which showed the most educated are the ones most protected from having SARS-CoV-2 infection. (Filho et al., 2020) This could be due to better awareness about transmission mechanisms, adherence to infection prevention measures, lower housing density and lesser housing instability compared to the less educated and economically disadvantaged groups. Employment is also observed to have increased risk of infection compared to unemployment. This study further illustrates that public transport drivers, health care workers and private organization employees have more exposure as they spend more hours outdoors, possibly gathering with people, compared to individuals who spend much of their time at home. Studies in the US and Korea also showed similar findings and identified workplaces as major risk areas for exposure. (Baker et al., 2020)(Lee and Kim, 2020).
This survey had several limitations. By selecting 14 major towns and those aged above 14 years, we were unable to discuss rural burden and its impact on children, which limits its generalizability. Our local validation of the test kit found low sensitivity and good specificity. To correct for the low kit sensitivity and minimize the underestimation, we made adjustment to the prevalence estimate. However, this increases the degree of uncertainty and widens the confidence interval. Therefore, interpretation of the findings should consider the limitations of the test kit used in this study and its validation. Chronic illness and adherence to COVID-19 prevention guideline data might be affected by information bias as they are not obtained by clinical record review or behavioral observation. In addition, The IFR estimate provided in this survey will be affected by the seroprevalence estimation.
Generally, the low COVID-19 seroprevalence in Ethiopia warrants ongoing risk mitigation measures. Testing capacity and strategy must be expanded to limit under-detection of cases. Screening, quarantine and enforcement of all prevention guidelines should be improved across the country's border to minimize the high burden of disease in those areas. Additional workplace protections must be in place for essential workers. Messaging should be improved to ensure that messages are easily comprehensible for those with no or limited education.
Conflict of Interest: Authors declare no conflict of interest on this study
Funding Acknowledgement: Authors would like to thank Ethiopian public health institute for funding the whole budget required to conduct this study.
Ethical Approval: This study is done as part of the routine surveillance activity and didn't require ethical clearance from where the corresponding author is affiliated and, it is cleared by US CDC and was conducted consistent with applicable federal law and CDC policy.
Footnotes
U.S. CDC Author Disclaimer: The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention with which the authors are affiliated.
§ See e.g., 45 C.F.R. part 46.102(l) (2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
References
- Abbott Laboratories. ABBOTT Architect IgG SARS-CoV-2 manual. 2020 2020:12. https://doi.org/6R86-206R86-30.
- Baker MG, Peckham TK, Seixas NS. Estimating the burden of United States workers exposed to infection or disease: A key factor in containing risk of COVID-19 infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A. Performance characteristics of the abbott architect sars-cov-2 igg assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HTH. the Departments of Epidemiology and Biostatistics (M.A.H.), and the Cen-ter for Communicable Disease Dynam-ics, Departments of Epidemiology and of Immunology and Infectious Diseases 2021. https://doi.org/10.1056/NEJMoa2101765.
- Chibwana MG, Jere KC, Kamng'ona R, Mandolo J, Katunga-Phiri V, Tembo D. High SARS-CoV-2 seroprevalence in Health Care Workers but relatively low numbers of deaths in urban Malawi. MedRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.07.30.20164970. [DOI] [Google Scholar]
- Deyessa N, Wossen M, Ababa A, Ababa A, Services M, Ababa A. Sero-prevalence of anti-SARS-CoV-2 Antibodies in Addis Ababa. Ethiopia. 2020:1–16. [Google Scholar]
- El-Sadr WM, Justman J. Africa in the path of Covid-19. N Engl J Med. 2020;383:1–2. doi: 10.1056/NEJMp2008193. [DOI] [PubMed] [Google Scholar]
- Erikstrup C, Hother CE, Pedersen OBV, Mølbak K, Skov RL, Holm DK. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estimating the infection fatality ratio in England - CEBM. n.d. https://www.cebm.net/covid-19/estimating-the-infection-fatality-ratio-in-england/ (accessed September 29, 2020).
- Ethiopia Population (2021) - Worldometer. n.d. https://www.worldometers.info/world-population/ethiopia-population/ (accessed August 3, 2021).
- Ethiopia population (2021) live — Countrymeters. n.d. https://countrymeters.info/en/Ethiopia (accessed August 3, 2021).
- Filho LA, Szwarcwald CL, Mateos S de OG, de Leon ACMP, de Andrade Medronho R, Veloso VG. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro. Brazil. Rev Saude Publica. 2020;54:1–10. doi: 10.11606/s1518-8787.2020054002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med. 2020 doi: 10.1056/nejmoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;30329:1–11. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim M. Estimation of the number of working population at high-risk of COVID-19 infection in Korea. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majiya H, Aliyu-Paiko M, Balogu VT, Musa DA, Salihu IM, Kawu AA. Seroprevalence of COVID-19 in Niger State. MedRxiv. 2020 doi: 10.1101/2020.08.04.20168112. 2020.08.04.20168112. [DOI] [Google Scholar]
- Perez-Saez J, Lauer SA, Kaiser L, Regard S, Delaporte E, Guessous I. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis. 2020;3099:2–3. doi: 10.1016/S1473-3099(20)30584-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevett M. Chronic Non-communicable Disease… CHRONIC NON-COMMUNICABLE DISEASES IN ETHIOPIA-A HIDDEN BURDEN. n.d. [PMC free article] [PubMed]
- Public Health Agency of Sweden. The infection fatality rate of COVID-19 in Stockholm – Technical report 2020.
- Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- research that is available on the COVID-19 resource centre - including this for unrestricted research re-use a 2020:19–21.
- Real-Time Fluorescent RT-PCR Kit for Detecting SARS-CoV-2. n.d.
- Sempos CT, Tian L. Adjusting Coronavirus Prevalence Estimates for Laboratory Test Kit Error. Am J Epidemiol. 2021;190:109–115. doi: 10.1093/aje/kwaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Social, environmental factors seen behind Africa's low COVID-19 cases | WHO | Regional Office for Africa. n.d. https://www.afro.who.int/news/social-environmental-factors-seen-behind-africas-low-covid-19-cases (accessed April 23, 2021).
- Stadlbauer D, Amanat F, Chromikova V, Jiang K, Strohmeier S, Arunkumar GA. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr Protoc Microbiol. 2020;57:2425–2427. doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twahirwa Rwema JO, Diouf D, Phaswana-Mafuya N, Rusatira JC, Manouan A, Uwizeye E. COVID-19 Across Africa: Epidemiologic Heterogeneity and Necessity of Contextually Relevant Transmission Models and Intervention Strategies. Ann Intern Med. 2020:1–3. doi: 10.7326/m20-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyoga S, Adetifa IMO, Karanja HK, Nyagwange J, Tuju J, Wanjiku P. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. MedRxiv. 2020 doi: 10.1101/2020.07.27.20162693. 2020.07.27.20162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti L, Bergna A, Pelusi S, Facciotti F, Lai A, Tarkowski M. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 Milan outbreak. MedRxiv. 2020 doi: 10.1101/2020.05.11.20098442. 2020.05.11.20098442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, Kvalsvig A, Barnard LT, Baker MG. Using a Lag Time for. Fatality. 2020;26:2019–2021. doi: 10.3201/eid2606.200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Sun J, Nie S, Li H, Kong Y, Liang M. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- Zekaria S, Ababa A. Central Statistical Agency Population Projections for Ethiopia Population Census Commission ii 2013.