Abstract
Natural products have been used in the treatment of illnesses throughout the history of humankind. Exploitation of bioactive compounds from natural sources can aid in the discovery of new drugs, provide the scaffold of new medicines. In the face of challenging diseases, such as the COVID-19 pandemic, for which there was no effective treatment, nature could offer insights as to novel therapeutic options for control measures. However, the environmental impact and supply chain of bioactive production must be carefully evaluated to ensure the detrimental effects will not outweigh the potential benefits gained. History has already proven that highly bioactive compounds can be rare and not suitable for medicinal exploitation; therefore, the sustainability must be accessed before expensive, time-demanding, and large trials can be initialized. A sustainable option to readily produce a phytotherapy with minimal environmental stress is the use of agro-industry wastes, a by-product produced in high quantities. In this review we evaluate the sustainability issues associated with the production of phytotherapy as a readily available tool for pandemic control.
Keywords: SARS-CoV-2, Coronavirus, Citrus, Apple pomace, Supportive treatment, Phytotherapy
SARS-CoV-2; Coronavirus; Citrus; Apple pomace; Supportive treatment; Phytotherapy.
1. Introduction
On 11th March 2020, a huge new challenge to humankind was established, the COVID-19 pandemic. First reported in late 2019, COVID-19 is a viral disease caused by a novel virus of Coronaviridae family [1], namely, Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) [2]. Since its discovery and to date (June 2021) a staggering 175 million people have been infected by SARS-CoV-2 and 3.7 million have lost their lives [1]. The rate and impact of infection cannot be underestimated and its abatement is an immediate global societal need today [3].
In record time, the scientific community was able to provide a vaccine for COVID-19 to the population. Nearly one year was required to develop 5 different vaccines that are being applied. To date, nearly 2.2 billion doses have already been administrated [1], yet, only 0.8% of low-income countries population have received any dose. Low and middle-income countries are struggling with sequential waves of COVID-19 contaminations due to absence of means to obtain and distribute vaccines. Such countries are not able to produce the vaccine and are highly dependent of commercial trade [4]. Even though those countries represents 84% of global population, they also represent only 35% of vaccine orders [5]. Yet, in order to control a pandemic, all global population need to receive treatment in the shorter time possible.
The challenge in providing global treatment in not merely financial, but also involves the technological capacities of each country to manufacture vaccines, presence of necessary infrastructure for its storage and distribution, and the supply chain of inputs and medicinal products [6]. The supply chain of COVID-19 vaccines is not capable to provide global immunization on short time. In addition, medical products, such as ventilators and sedative drugs, had their demand increased drastically during pandemic, and their supply chain were strained, causing shortage in many regions [7, 8, 9].
In pandemic scenarios guarantee a robust supply chain for industry and medical treatment is essential in the process of diseases control, economy maintenance, and society adaptation to the “new normal”. Sustainable technologies are one of the research lines that need to be improved in order to achieve such goals [10]. Green methods not only aid the environmental management, but also can build up local empowerment, reducing the dependency of low and middle income countries to international imports and global dependent supply chains, such as those used to provide the COVID-19 vaccine inputs [6, 10]. While global vaccine distribution is not possible, provide a sustainable supportive treatment is mandatory to preserve life. As long as supportive treatments are not implemented in the absence of vaccine, more are the chances for the development of resistant strains of the virus [11, 12].
A sustainable supportive treatment that can be developed against COVID-19 is by the use of natural bioproducts, mainly those obtained from industrial wastes, which can provide a renewable source of material. Natural products have been present in human history since ancient times. There are records of their usage dating back nearly 5,000 years, when Chinese Encyclopedias described the medicinal applications of several plants, fungi and even animal exudations [13]. The potential of natural bioproducts can even be observed today, for example, the Aspirin®, acetylsalicylic acid, is a semi-synthetic derivative based on the natural exudations from the White Willow tree (Salix alba), rich in the glycoside salicin that is easily metabolized into salicylic acid [14]. In addition, half of the habitable area on Earth is used by agriculture, within which approximately 23% is designated to crops with a total production larger than 1 billion tons per year [15]. Of the massive production of crops, nearly half of them become waste. Agro-industrial wastes are extremely difficult to be properly disposed and are usually not properly treated, causing severe damage to the environment and animal health [16, 17, 18].
The exploitation of natural products to develop supportive treatments had already proved its feasibility during human history [19]. Yet, it has been poorly explored in the combat to COVID-19 pandemic [20, 21], even though the World Health Organization recognizes the existence and importance of phytotherapy, particularly in low-income countries [22]. Does natural based treatment be a reliable and sustainable tool in middle of the chaotic scenario of COVID-19 pandemic? In this review we aim to provide an insight into the value of natural products in COVID-19 treatment and the sustainability issues of exploiting these natural products.
2. The COVID-19 case
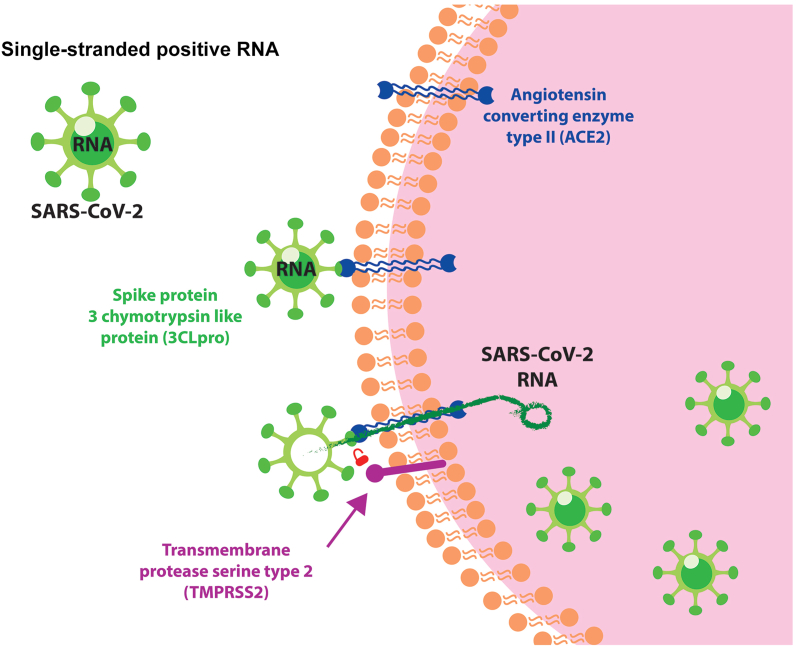
The COVID-19 pandemic has become a seriously health public issue, even though its estimated mortality rate is lower than others coronavirus which were also pandemic in the past (SARS and MERS) [23]. One of the issues is the vulnerability of people which has comorbidities such as diabetes, hypertension, and many other cardiovascular diseases [24], that currently have a high occurrence rate globally [25]. The susceptibility of non-communicable comorbidities patients to get severe forms of COVID-19 is related to the inoculation and replication system of the virus. The mechanism of SARS-CoV-2 infection and replication (Figure 1) is via the expression downregulation of the angiotensin converting enzyme type II (ACE2). Because of this interaction SARS-CoV-2 affects the homeostasis of the cardiovascular, diabetic and respiratory processes. Once the virus interacts with ACE2 through the 3 chymotrypsin-like proteases (3CLpro) present on its surface, the virus enters the host cell, which is promoted by protein cleavage of the transmembrane protease serine type 2 (TMPRSS2). At this point on, SARS CoV-2 RNA is released inside the cell and the virus starts to replicate [20].
Figure 1.
SARS-CoV-2 main inoculation mechanism.
COVID-19 generates several problems to society not only related to mortality. Psychological diseases had exponentially increased, manpower in several production lines had drastically decreased, global economy was severely affected, supply chains in diverse industrial lines collapsed with the increasing demands. The world is not the same as before November, 2019. There are several ways to treat viral diseases, such as drug repurposing, supportive drug development, and vaccine development. Drug repurposing was the first strategy used against COVID-19 since the development processes for a new drug or vaccine usually take several years, if not decades. It is a common alternate, particularly as a strategy for pandemic disease control [26]. However, in the case of COVID-19, the rapid drug repurposing of traditional anti-malarials (chloroquine and hydroxychloroquine) backfired as they show little or no efficacy against SARS-CoV-2 [27]. In the case of hydroxychloroquine, the use of these drugs even increased the incidences of heart attack. Currently, the repurposed drugs have questionable efficacy in the treatment of COVID-19 [28].
The time record develop of vaccines was a flash of hope in pandemic ending, yet, none of them are fully optimized. Vaccines are considered well-consolidated and fully optimized, such as those for HPV and hepatitis B (Table 1), if they have high efficacy, can be easily transported to any place, require only standard freezing, and do not degrade easily under storage. On the contrary, the Ebola vaccine has not been fully optimized as its storage and transportation logistics make it inaccessible in a global perspective [29, 30, 31]. The Ebola vaccine requires a storage temperature at -60 °C and its distribution in times of emergency is extremely complicated in countries that do not have a stablished ultra-cold chain for products distribution. For instance, during the STRIVE test, performed in Sierra Leone, West Africa, the urgent implementation of an ultra-cold chain for distribution of Ebola vaccine was evaluated. The implementation cost was significantly high for the country. Critical issues highlighted in STRIVE test were that ultra-cold chain requires overly expensive infrastructure, electricity supply, and specialized human resource to track and monitor the vaccine throughout the chain [32]. The results of STRIVE test elucidates that less than 30 countries have the structure to quickly implement ultra-cold chain for the distribution of vaccines that requires such low temperatures [32, 33]. This is one of the many reasons that underdeveloped countries are struggling with their vaccination campaigns [32].
Table 1.
Vaccines features related to sustainability.
| Vaccine or Developer representant | Long term storage duration | Short term storage duration | Efficacy | Ref. |
|---|---|---|---|---|
| HPV | 3 years/2–25 °C | 1 year/≤ 37 °C | Close to 100% | [27] |
| Hepatitis B | 3 years/2–8 °C | 1 month/≤ 37 °C | 80–100% | [27] |
| Ebola rSVΔG-ZEBON-GP | -60 °C | 12 hours/2–8 °C | 97–100% | [26, 28] |
| Oxford/Astrazeneca | -4 °C | - | 62–90% | [29] |
| Moderna | 6 months/-20 °C | 30 days/2–8 °C | 94% | [30] |
| Pfizer/BioNTech | -70 °C | 5 days/2–8 °C | 95% | [31] |
| Sputnik V | -4 °C | - | 91.4% | [32] |
Even though the scientific community has managed to develop a vaccine in a record time, there is still much uncertainty. There is no precise information about how long the vaccine will be effective, part of the global population is afraid to be vaccinated, middle and low-income countries cannot afford massive vaccine programs and are depending on other countries for vaccine production. While a few countries are vaccinating their population rapidly and on schedule, too many others are unable to follow the same immunization rhythm, and still have increasing infection rates, which are creating more resistant variants of the SARS-CoV-2 virus [34]. In face of such challenges imposed by COVID-19, every available possibility to aid pandemic restrain must be explored. Sustainable options for supportive treatments can be the hope until the vaccines are fully optimized or equally available worldwide.
3. Natural metabolites and the SARS-CoV-2
Throughout human history viral diseases have been notoriously difficult to fight as they incorporate themselves into a host system and can quickly mutate to become resistant to drugs or vaccines. For instance, there is no effective prophylactic or cure for the SARS-CoV (recognized in 2002) and HIV (recognized in 1981) until today [27, 35], highlight the complexity involved in creating safe treatments and vaccines for viral diseases.
Several viral infections are treated after infection through symptom management or inhibit virus replication [36]. These strategies are also known as supportive treatments. The aim of these strategies is to prevent disease development by treating the infection in its early stages. The supportive treatment can be done by neither synthetic and semi-synthetic drugs, and phytomedicine [27]. In general, phytotherapies are associated with less side-effects to the patient and have higher acceptance by civilians as a alternative for primary health care [37]. Plants undisputedly play a major role as sources of medicinal substances, though many other organisms have been used, such as marine organisms [38], microorganisms [39], endophytic fungi [40], higher plants [19], and metabolically engineered organisms [41, 42].
Currently, several existing, catalogued, natural products are being tested via in silico assays as potential anti-COVID-19 drugs [20, 43]. In silico trials are a fundamental step in the prospection of bioactive compounds, as these computational simulations lessen the need for time-consuming laboratorial procedures. The goal of these tests is to predict which metabolites may interact with the enzyme and protein involved in the SARS-CoV-2 inoculation mechanism, with the viral RNA directly (Figure 1), or treat the infection symptoms (e.g. inflammation of upper respiratory tract and obstructive pulmonary disease) [44].
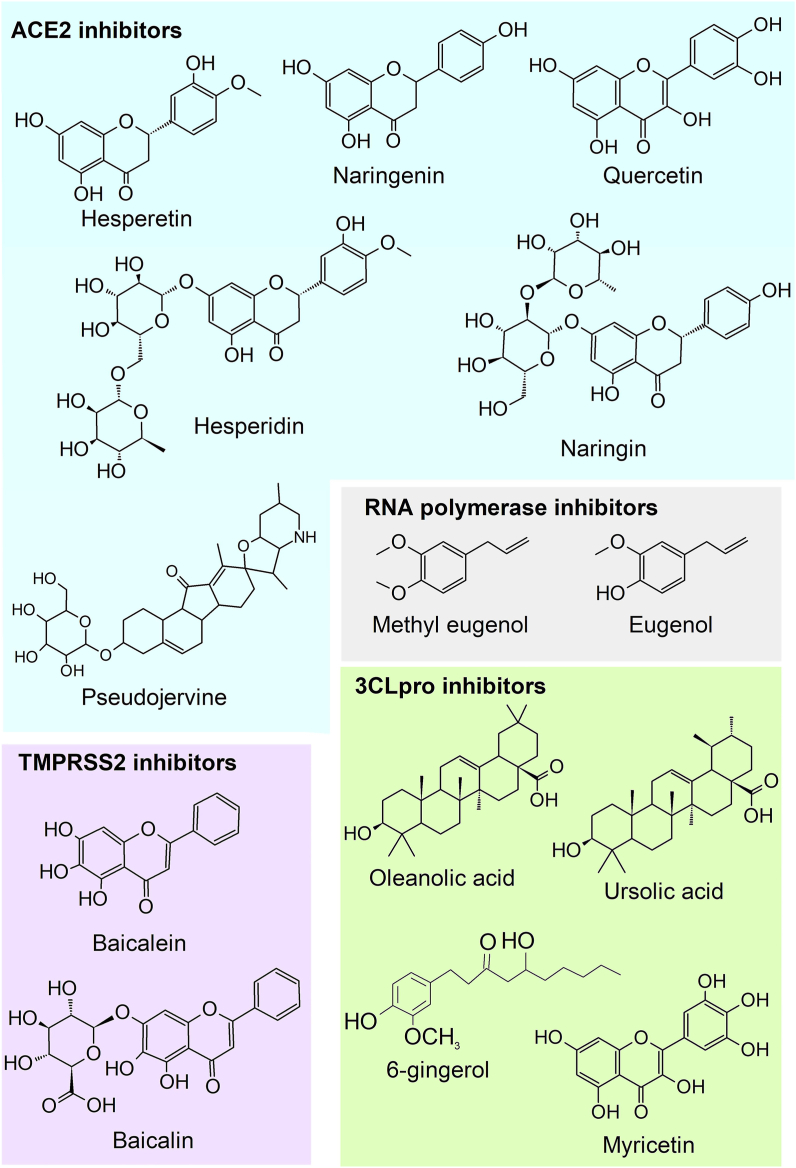
Some of the best in silico results for anti-SARS-CoV-2 involve glycosylated flavonols, flavanones, terpenoids, and alkaloids, such as naringin, naringenin [45], baicalin, hesperitin [46], eugenol, pseudojervine [20], 6-gingerol, quercetin [45], and myricetin (Figure 2) [47]. These compounds have better inhibition, in silico, than most repurposed drug candidates. Briefly, their main active site against SARS-CoV-2 is through inhibition of ACE2 and TMPRSS. Chemical classes, such as terpenoids, have lower binding energy with 3CLpro, reducing virus ability to bind in cellular membranes acting directly in the virus structure [48, 49]. In addition, terpenic acids, such as oleanolic acid, also show promising results as RNA polymerase inhibitors of SARS-CoV-2. The RNA polymerase inhibition is a particularly interesting mechanism in drug development due to its specificity to the virus as opposed to host proteins (ACE2 and TMPRSS2) [50].
Figure 2.
Natural metabolites within in silico results against SARS-CoV-2.
The search for natural products that are bioactive against SARS-CoV-2 is mainly in the in silico phase, and the most prominent results are focused on isolated compounds, without proper analysis of their sustainability for such application [20]. Closer evaluation of natural metabolites with suggested anti-COVID-19 activity identifies several other problems associated with the usage of isolated compounds. For instance, pseudojervine has a low lethal concentration for humans [51]; gingerol promotes a burning sensation [52]; baicalein and baicalin are products of a very specific specialization of the cinnamic acid pathway, being extremely difficult to obtain from plants [53].
On the other hand, part of the specialized metabolites with promising in silico results have a more consolidate data regarding their medicinal application and are more easily obtained from natural and synthetic sources. Examples of it are methyl eugenol, oleanolic acid, and ursolic acid which are widely distributed within plant species. It is estimated that these metabolites have already been identified in at least 300 (methyl eugenol) [54] or 1500 (oleanolic and ursolic acid) [55] distinct species of several botanic families. Most of these botanic species are inserted in the human diet, as fruits or vegetables, or used as medicinal herbs. Examples of natural sources containing these metabolites include Beta vulgaris L., Oleandra neriifolia L., Ilex paraguariensis St. hil., Glechoma hederacea L., Crataegus pinnatifida Bge., Vaccinium spp. (blueberry), Olea europaea L. (olive), and apple peels [56]. The broad distribution of methyl eugenol, oleanolic and ursolic acid in several botanic species makes them great promising lead-compounds. It is worth mentioning that methyl eugenol preliminary in silico studies evidence methyl eugenol binding energy with SARS-CoV-2 spike protein at -8.29 kcal mol−1 [57], a result similar to the repurposed drugs [58]. The botanic families with major occurrence of those triterpenoids (ursolic and oleanolic acids) include Asteraceae, Apiaceae, and Lamiaceae. Depending on the species and cultivars, the concentration of these compounds can be as high as 100 mg per 100 g of fresh raw material (fruits or leaves) [59, 60]. In addition to their recent suggestion as possible anti-SARS-CoV-2 metabolites [48, 57], methyl eugenol, oleanolic acid and ursolic acid were already known for possessing several biological activities, such as anti-inflammatory, antioxidant, anti-leishmaniasis, antibacterial, neuroprotective, and antiviral. For instance, ursolic acid has bioactivity against hepatitis B and C and influenza viruses [61], while oleanolic acid has anti-HIV activity and was used as a scaffold for anti-HIV drugs [55].
Another interesting chemical classes already tested in silico is the flavonoids, which natural abundance can become useful in the supply chain for a phytomedicine against COVID-19. The flavonoids naringin, naringenin, hesperetin, and hesperidin binding energy with SARS-CoV-2 proteins ranged from 6.09 to 7.0 [20], also results close to suggested repurposed drugs, such as Hydroxychloroquine and Azithromycin [58]. These compounds are the major flavonoids within several Citrus genus (Rutaceae) species. In fact, their premature identification as possible phototherapeutics against COVID-19 was because they are common in Chinese medicinal herbs, and therefore are catalogued in their databases [62].
In addition to controlling SARS-CoV-2 replication in the organism, natural metabolites can also be used to control other clinical manifestations caused by the virus. During COVID-19 infection, of moderate and severe presentation, patients experience several clinical conditions that deteriorate health and recovery chances. SARS-CoV-2 clinical manifestations include neuroinvasion, neurotropism, cellular death, inflammatory process of tissue, pneumonia, reduction of lung capacity, lung obstruction, arrhythmia, kidney failure, and arthralgia [1]. For such clinical conditions, several natural products are already known. Phytotherapy bioproducts have been used in the treatment of viral infections, as they are able to control clinical manifestations induced by virus and their viral load in the organism [63]. For instance, phytotherapy derived from honey (Echinacea species) are recognized as anti-inflammatory, cardio protectors, and antivirals against influenza, HIV, herpes and rubella [64]. As Echinacea metabolites are serine protease inhibitors, they can be used in the treatment of the beforementioned clinical conditions of SARS-CoV-2. [44].
China is one of the major countries which applies phytotherapy in disease treatment. In this country, which is the epicenter of the SARS-CoV-2 pandemic, medicinal herbs were adopted as supportive treatment to COVID-19, depending on the clinical stage. In Chinese medicine, COVID-19 was treated as a viral influenza-like disorder. The clinical stages are divided into four: Prevention (healthy or asymptomatic patients); early (mild symptoms, such as fever, sore throat, shortness of breath, intestinal disorders); late (pneumonia, obstructing the lung); and recovery (sequelae of diseases, such as deficiency of the lung) [65]. Small clinical trials were developed in Wuhan, northeast Chongqing, and Taiwan, using a mixture of Chinese herbs, which included Scutellariae species [66, 67, 68]. In all cases, the phytotherapy were used in the late stage of the disease, to treat pneumonia and lung obstruction. Improvement of clinical conditions were monitored with chest tomography and COVID-19 RT-PCR test. Despite the small size of patients evaluated, all researchers reported that the application of Chinese traditional herbs alleviated lung obstruction and reduce the hospitalization period [66, 67, 68].
The chemical composition of the traditional Chinese Herbs preparation adopted in such treatments was investigated by Tsai et al. (2021) [66] and Lee et al. (2021) [69]. The major basis of Chinese phytomedicine against COVID-19 was based in the Glycyrrhiza species and Scutellaria baicalensis. The Glycyrrihyza extracts (licorice) and its major metabolite glycyrrhizin are recognized medicinal bioproduct with large economomic importance to pharmaceutical and cosmetic industries. Currently, both bioproducts are already in vitro test against COVID-19, with a suggested dose for clinical randomized trials varing from 10 to 50 mg of glycyrrhizin [70]. In the Scutellaria baicalensis, the major bioactive compound identified was baicalin, with a concentration of 2.38 mg mL−1. In addition, other compounds, such as caffeoylquinic acids, glycosylated flavonoids, flavanones, and flavones were also detected [66]. Baicalin-rich extracts tested in vitro against COVID-19 also had proeminet results with a EC50 of 10.27 μM, nearly 10 times higher than hydroxychloroquine [20].
Those traditional Chinese herbal preparations were also demonstrated to have multiple mechanisms in the treatment of COVID-19 symptoms and sequela, acting as an antiviral and anti-inflammatory. Although the precise mechanism is uncertain, the chemical profile of the herb indicates that it possible that it acts in different sites to inhibit SARS-CoV-2 proliferation [68, 69].
The results of this phytotherapy alternative tested in China for COVID-19 were preliminary, however, it was only possible because of the remarkable presence of the Traditional Chinese Medicine in the health system. Clinical conditions such as flu and other viral diseases are commonly treated with supportive phytotherapy [65]. In other words, medicinal herb preparations are already available in the health system. However, a phytotherapy option must have a sustainable characteristic that enable efficacy tests, clinical trials, and production.
4. Natural products perspectives against COVID-19 pandemic
Discovery of bioactive natural products and their application in disease treatment requires as much care as that of any other synthetic drug. In a pandemic scenario, in which time is crucial to preserve life, several of these questions are related to cost-benefit and risk-benefit. For instance, in the case of chloroquine and hydroxychloroquine, the cost-benefit was accessible, as it was a drug that is well-consolidated in the market. However, the risk-benefit was not accurately measured. The consequence was a great insecurity in whether those drugs are safe or not. After widespread use by society, research have come forth reporting the cardiovascular dangers of those drugs and their little to no efficacy against COVID-19 [71, 72].
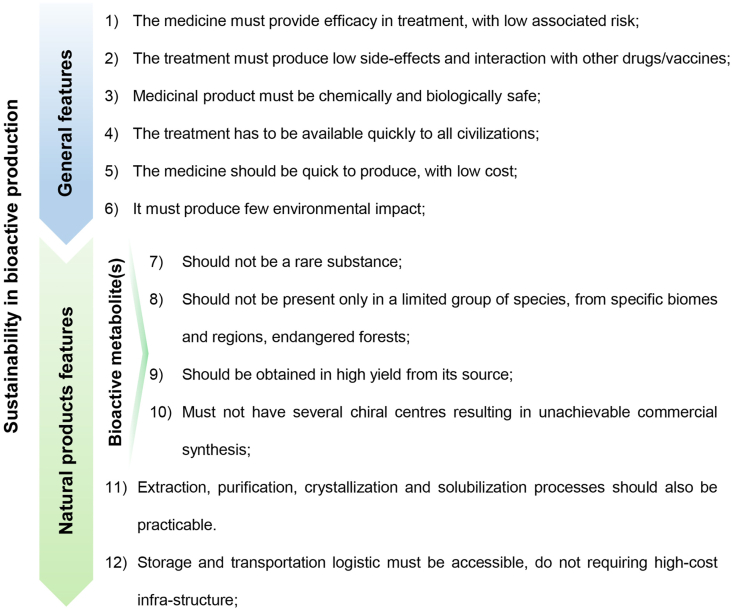
For a bioproduct to be sustainable in a pandemic scenario, several aspects must be carefully considered (Figure 3), that includes general and specific features of drug and phytotherapy development [73]. Attendance with such aspects can provide treatments capable of being delivered efficiently worldwide thus restraining the pandemic scenario. These aspects briefly involve supply chain, safety, and optimization for worldwide commercialization.
Figure 3.
Sustainability features for bioactive production.
A major concern in pandemic control is that the treatment has to be available as fast as possible to all society. The high financial cost of vaccines is one of the drawbacks in the global control of COVID-19 [4]. The supply chain regards not only the final availability of the product, but also the availability of any material necessary to manufacture it. In developing bioproducts, the initial concern in the supply chain is the availability of the bioactive compound or bioactive extract and its raw source.
For medicinal purposes, several in vitro, in vivo, pre-clinical, and clinical assays must be performed with the drug of interest. Thus, even before pre-clinical testing, a significant amount of the medicine must be available for test. For instance, in pre-clinical trials of a phytotherapy it is recommended that nearly 50 kg of the raw material be available [74]. Raw material in phytotherapy can be any vegetative or reproductive organ, and even byproducts, such as oleoresins and essential oils. In natural conditions, obtaining that amount of raw material can be extremely challenging. For example, the Copaifera genus oleoresin is a worldwide popular phytotherapy, however, each Copaifera tree can present a very distinct quantity of oleoresin. It is estimated that, depending on the species and geographical region, the yield of oleoresin can vary from 0.1 to 60 L per tree, with sampling intervals of at least three months [75, 76]. In addition, approximately only 25% of Copaifera trees produce oleoresin and their spatial distribution in native forests is close to 1.7 trees per hectare [76, 77]. Obtaining Copaifera oleoresin becomes even harder as the extraction procedure requires digging a hole in the trunk, which can result in tree death [76].
The Copaifera example highlights how difficult it can be to obtain raw natural product material in natural conditions. The same problem can be observed in several herbs from the Chinese Traditional Medicine. The Scutellaria baicalensis is a species endemic in China, with lower occurrence in Japan, Korea, and Southeast Asia. Regardless of its inclusion in the Chinese Pharmacopoeia and extensive clinical usage in China, species domestication was not incentivized until approximately 2010, the moment in which wild populations started to significantly decrease [78, 79]. The increasing cultivation by small farmers with no proper domestication study also introduced poor quality S. baicalensis, with less than 90 mg of baicalin per gram of root, into the market [78].
Those issues with S. baicalensis cultivation indicates that the herb would not be sustainable in a scale larger than its current demand, which could present problems in attending pre-clinical and clinical tests of efficacy against COVID-19. Exponential exploitation of S. baicalensis could cause a severe environmental impact, with wild populations decreasing uncontrollably, or safety problems if the reproducibility of S. baicalensis extract quality cannot be ensured. Note that indications of S. baicalensis in the treatment of COVID-19 applied the plant extract [69]. Despite baicalin and baicalein can be present in high quantities in plant extracts (36 mg–90 mg per gram of root), it can only be obtained in few species of Scutellaria genus [80, 81]. Thus, application of baicalein and baicalin in anti-COVID-19 phytomedicine development would have to rely on semi-synthetic or synthetic production. As these compounds were already known there are already options for synthetic routes and cell cultures, with relatively low cost and high yield for commercialization [53, 80, 82].
Basically, the same issues are observed with the Glycyrrhiza species. As an endemic species in China, their density worldwide is low and the wild populations are threatened since 2010 due to their exploitation [83]. The bright side is that as a phytomedicine already exploited by humankind, there is already safety studies regarding their consumption by humans [84]. As glycyrrhizin and licorice extracts are mainly obtain from the root of few Glycyrrhiza species, its sustainable application during pandemic in larger communities would requires synthetic and semi-synthetic production routes as well.
Despite the outstanding potential and applicability of natural products in the development of medicine, an recurrent issue is their availability and environmental impact [85]. As important as developing a new treatment for COVID-19 with licorice or S. baicalensis, their efficacy against pandemic will be based in their supply chain. Even if they have formidable benefits to the treatment, if they cannot be supplied in sufficient content, it will be of little help, as many other natural products before. An example of this issue is the Paclitaxel®. Paclitaxel is an iconic case of a highly bioactive natural product with several drug development sustainability issues [86]. Taxol is a remarkable natural anti-neoplastic compound, firstly obtained in the bark of Taxus brevifolia. However, its concentration in the bark was only 0.05% and after few decades of exploitation, the species was nearly extinct [86]. In addition, Taxol is a very complex substance, with 11 asymmetric centers, not produced by any other plant species. Its total synthesis, microbial production, or in cell cultures showed no economic feasibility [86, 87]. It took several decades to find an alternative source from another plant species (Taxus baccata) where a similar substance would be extracted from the leaves and be able to be converted to Taxol in only some economically viable chemical synthetic steps [88]. Nevertheless, the pharmaceutical formulation of such a nonpolar compound was only achieved by several of the most aggressive collateral effects until recent advances in nano-formulations [86, 87].
5. Challenges and opportunities
Consolidate a natural product in pharmaceutical industry from its natural source have always being a challenging task. Even today, there are few medicinal drugs of such type commercialized by large companies. Most of them are local phytomedicine from small to medium size producers [88]. The great challenge in this task, as previously illustrated is to obtain a robust source of the raw material, without causing environmental impact, and with homogeneous characteristics (e.g. chemical composition, which in plants is extremely variable by biotic and abiotic stress) [85, 88].
Produce a pharmaceutical drug from natural source are difficult as taxonomy identification of plant in wild is tough. Several methods such as morphological, microscopical and DNA analysis need to be performed to ensure the identity of the tree. If a different species is used it is possible to not achieve the desire effect, or even intoxicate the patient. In many cases, the species will be of difficult access, or not domesticated. And even if there a dense population of the species, plants chemical composition changes during their life cycles and different seasons [85]. An strategy to ensure a robust supply chain with more homogeneous composition through time is to rely on already domesticated crops or even on the agro-industrial wastes.
Considering the robustness of a supply chain, licorice and S. baicalensis are not suitable to be used during pandemic as a natural source. Both bioproducts are mainly undomesticated, with a dense population centered on China. For instance, licorice roots global productions was 40,911 tons in 2004 [89], a yield easily surpassed by domesticated crops, such as ginger roots (production of 1,256,777 tons in 2004) [90].
Previously we seen that compounds such flavonoids and terpenoids had promising results in preliminary test against COVID-19 [20]. What if there is a robust and homogenous source of them? Compounds such as baicalin, baicalein, naringenin, naringin, eugenol and methyl eugenol can be found in several other higher plants [86, 91]. In addition, these compounds were also reported to be present in agro-industrial waste of Citrus [92, 93] and apple pomace [94, 95].
The major advance of agro-industrial waste as raw material in treatment development is that they are constantly produced. In addition, their management also follows a sustainable perspective as less residues will be discharged in nature [85, 96]. For instance, in 2019, the production of apples was 87,236,221 tons and oranges reached 78,699,604 tons. Considering the residue-to-product rate for apple pomace is 0.25 and for orange pomace is 0.37, the residues of just these two fruits reaches more than half a billion tons in a single year (apple = 348,944,844 tons; orange = 212,701,632 tons) [90]. Use of this huge amount of raw material for extracting natural bioactive products could bypass most of the sustainability issues already described. Thus, even in face of the supply chain crisis caused by COVID-19 pandemic, those raw materials can be of interesting for treatment development.
5.1. Citrus
As one of the major commercialized natural products, Citrus already have a well-established production chain. Several studies have evaluated the reuse of its industrial waste, although its waste usage to promote value-added products is still not as consolidated as the production of industrialized products, such as juices or frozen pulps [97, 98]. Citrus waste valorization has many branches, such as the extraction of phenolic compounds and flavonoids, carotenoids, pectin, limonoids, and even essential oils [99, 100]. Depending on the waste characteristics and the bioproduct wanted, there are plenty extraction options applicable to large-scale industries and different types of biorefineries [101]. Citrus agro-industrial waste management has been encouraged since the waste can cause several environmental problems, such as the severe reduction in soil fertility due the presence of alkalis, antimicrobial compounds, and organic matter with low nitrogen content (which difficult microbial decomposition) [102]. The administration of Citrus wastes is such an important issue that as juice commercialization increases, several researches have been performed to develop methods to afford the creation of value-added products [98, 103].
Within the four flavanones with high in silico docking with SARS-CoV-2, naringin and hesperidin, are the most concentrated in Citrus, especially in its peels and pericarp. For instance, Citrus reticulata (Chinese mandarin orange) has one of the highest concentrations of both flavanones (Table 2). Citrus peel corresponds to nearly 40–65 % of its weight with respect to the whole fruit and is a major unavoidable food supply chain waste. An estimated 40 million tons of residue per year are produced [101]. As Citrus flavanones appear to have anti-SARS-CoV-2 activity [46, 104], the Citrus peel waste can become a reliable source of such metabolites for the development of anti-COVID-19 bioproducts. For instance, a country such as Brazil, which is struggling with consecutives waves of infection [105], and is the largest producer of oranges and its byproducts, Citrus waste potential as anti-COVID-19 treatment can be a light of hope.
Table 2.
Concentration (mg.g−1) of major anti-SARS-CoV-2 flavonoids in dried citrus peels.
| Species | Naringin | Naringenin | Hesperidin | Ref. |
|---|---|---|---|---|
| Citrus reticulata "Chaci" | 3.409 | n.i | 62.919 | [84] |
| Citrus reticulata "Erythosa" | 0.556 | n.i | 74.236 | [84] |
| Citrus reticulata "Subcompressa" | 0.582 | n.i | 100.525 | [84] |
| Citrus reticulata Blanco | 0.79 | 2.07 | 39.39 | [83] |
| Citrus reticulata Blanco var. porcan | 1.37 | 0.86 | 3.49 | [83] |
| Citrus reticulata | 0 | 0 | 0.026 | [85] |
| Citrus aurantifolia | 0.8 | 0.85 | 28.3 | [83] |
| Citrus aurantium x reticulata var. murcote | 0.96 | 1.8 | 2.27 | [83] |
| Citrus limettioides | 0.88 | 0.47 | 2.1 | [83] |
| Citrus sinensis L. Osbeck | 0 | 0 | 8.61 | [83] |
| Citrus sinensis L. Osbeck var. baía | 2.06 | 1.78 | 41.17 | [83] |
| Citrus clementine of Citrus reticulata (clementine) | 0 | 0.007 | 33.13 | [85] |
Flavonoid extraction from Citrus peel wastes can be accomplished by several methods, which varies from simple percolation with water to more refined methods, such as ultrasound assisted extraction (UAE) (Figure 4). Some issues to be considered in the extraction of anti-SARS-CoV-2 flavanones of Citrus peels include time, cost, toxicity, and purity obtained through the process [46]. Most applied methods include acid-base and Soxhlet based extraction. Despite their robust usage, extraction with those methods would not be recommended for such a hurry urgent pharmaceutical application, as they include the usage of large amounts of highly toxic solvents [101, 106]. These characteristics increase the presence of solvent residues in the final product.
Figure 4.
Major extraction methods of Citrus peels waste flavanones.
Other options of extractive procedures include percolation with hydroalcoholic solvent (50%–70% water content) [107]. In this case, the usage of methanol and ethanol produce similar extraction yields of Citrus flavanones. Taking into account the toxicity, ethanol is a good option for pharmaceutical approaches [108]. Usage of pure water as a solvent had also recently been presented as a viable option in a pilot scale study, though by hydrodistillation or even UAE [101, 107]. Despite the lower implementation cost of hydrodistillation and percolation, both methods usually require heating, which must be carefully controlled as flavonoids can undergo thermal degradation [46, 108]. It is worth mentioning that sustainable biorefineries with low energy consumption could be implemented in the case of hydrodistillation to enhance the total concentration of Citrus flavanones in extractions [108].
Ultrasound assisted extraction (UAE), hydrodynamic cavitation (HC), and microwave assisted extraction (MAE) enable efficient flavonoid extraction without the use of high temperatures, such as those commonly applied in hydrodistillation methods, thereby avoiding thermal degradation issues [107]. Within those methods, MAE presents the highest implementation difficulties for operational industries, as its equipment has lower capacity and higher associated cost when compared to the others [107, 109].
As a highly chemo diverse matrix, pre-treatment methods for Citrus waste management had been developed and are recommended for the extraction of specific phytochemicals. Among them, biocatalysis with microorganisms demonstrated reliability in obtaining higher concentrations of hesperitin and naringenin rather than glycosylated derivates [110, 111]. Thus, even after in vitro confirmation of the bioactivity of each flavanone form against COVID-19, it would be possible to specifically obtain any of them from Citrus peel waste with no greater addition of technology to the biorefineries.
5.2. Apple pomace
Apple pomace is the name given to the industrial residue of apple. This material is composed of peels, seeds, and pulp, usually from the species Malus × domestica Borkh. As a well domesticated and cultivated species around the world, each region will have an apple pomace of a specific variety and with distinct proportions of its fruit parts [112]. Apple pomace has been exploited in the last decades as a rich source of phytochemicals and vitamins. In fact, part of its commercial exploitation is in regards to the extraction of ursolic acid, to create high-value added bioproducts [94, 113].
This industrial residue has a highly chemo diverse composition, mainly with carbohydrates, fatty acids, and phenolic compounds. The most valorized metabolites obtained from apple pomace include pectin, chlorogenic acid, ursolic acid, and glycosylated flavanones, flavanone, flavones, and flavanols. Interestingly, the major occurrence of flavanones in apple pomace are as naringenin and hesperetin [103], as previously mentioned, compounds with great potential as anti-COVID-19 metabolites. With the large concentrations of naringenin (up to 75.6 mg kg−1 of dry weight), hesperetin (up to 676 mg kg−1 of dry weight) [103], and ursolic acid (average of 400 μg g−1 of fresh weight) [94] in apple pomace, this residue can be a reliable source, not only to isolate those compounds, but also to create bioactive extracts.
Issues concerning the application of apple pomace in COVID-19 treatment development the extraction, isolation, and purification process. The standard method used to isolate ursolic acid from apple pomace includes a pre-treatment with decoction, Soxhlet extraction with increasing polarity solvents, recrystallization and purification on a chromatographic column (Figure 5) [94]. The Soxhlet extraction has a yield of ursolic acid of 3.5% with a purity of nearly 60% [94]. Similar results can be achieved with the use of UAE (yield of 2.9% and purity up to 97%), which can reduce organic solvent residues in the final product, as the developed method uses only methanol [113]. Another advantage of the non-convection extraction method is related to energy consumption and safety, as Soxhlet requires boiling organic solvents. The major issue in the ursolic acid extraction from this matrix is the presence of carbohydrates, which interferes in the purification process. In any case, pre-treatment of the matrix to remove carbohydrates is a fundamental step in order to obtain a high value-added product and increase process efficiency [94, 112].
Figure 5.
Ursolic acid extraction methods.
6. Concluding remarks
The development of therapeutic strategies is a greater task than just finding a bioactive compound. It becomes even more critical if the new medicines must be readily available on a global scale. Even thought several vaccines against SARS-CoV-2 are already available, supportive treatments are still needed and urgent to control pandemic, as many countries are struggling to acquire any vaccine. As phytomedicine is more accepted by society than synthetic drug, now more than ever after hydroxychloroquine mistakes, natural products are being investigated as tools to develop new treatments more often. Preclinical trials have suggested that Traditional Chinese Medicinal Herbs, Scutellaria baicalensis and licorice roots can be promising in the treatment of COVID-19 patients. However, such herbs have a major supply issue, both of them are mainly cultivated in small scale and are not fully domesticated. Providing high quantities of natural product for quick response in a pandemic scenario requires a consistent supply source.
The in silico and in vitro tests also suggests that common occurrence natural metabolites, from flavonoids and terpenoids chemical classes, can be promising active compounds in a supportive treatment of COVID-19. The greater advance of such compounds is that they can be found in several plants, including domesticated species.
In this scenario, two natural products have drawn attention: the Citrus peel wastes and apple pomace. These bioproducts are rich source of suggested anti-COVID-19 natural metabolites. As they are agro-industrial waste of massive production worldwide, the supply chain is more robust than the ones of Scutellaria and licorice extracts. The domestication and high production make agro-industrial waste more sustainable than in natura bioactive, particularly for the immediate response required for diseases such as COVID-19.
Treatment development for such challenging diseases must be fast. Yet, the only concern cannot be in how fast we can obtain an anti-COVID-19 treatment but also the efficacy and sustainability of the new medicine. All development phases must be carefully accomplished and data must be analyzed thoroughly. Science must, and can, solve the problem, while avoiding creating new ones, such as in the case of Taxol. After ensuring initial risk of supply and efficacy, other questions must be addressed, such as production standardization, bioavailability, side-effects, and phytotherapy-synthetic drugs interaction. Using the best of our knowledge on chemical composition of biomass waste materials to take full advantage of natural resources seems to be the proper and sustainable way to increase development with conservation.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
VFVJ would like to acknowledge the University of York, Green Chemistry Centre of Excellence for scientific discussions.
Contributor Information
A.S. Matharu, Email: avtar.matharu@york.ac.uk.
V.F. Veiga-Junior, Email: valdir.veiga@gmail.com.
References
- 1.W.H. Organization . 2020. WHO (COVID-19) Homepage.https://covid19.who.int/ [Google Scholar]
- 2.Tahir ul Qamar M., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020:1–7. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Lancet COVID-19 vaccines: no time for complacency. Lancet. 2020;396:1607. doi: 10.1016/S0140-6736(20)32472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OECD . 2021. Using Trade to Fight COVID-19: Manufacturing and Distributing Vaccines; pp. 1–16. [Google Scholar]
- 5.Choi E.M. COVID-19 vaccines for low- and middle-income countries. Trans. R. Soc. Trop. Med. Hyg. 2021;115:447–456. doi: 10.1093/trstmh/trab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman R., Shah S., Jeurissen P., Jit M., Mossialos E. COVID-19 vaccine challenges: what have we learned so far and what remains to be done? Health Pol. 2021;125:553–567. doi: 10.1016/j.healthpol.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuman A.G., Fox E.R., Unguru Y. COVID-19 and drug shortages: a call to action. J. Manag. Care Spec. Pharm. 2020;26:945–947. doi: 10.18553/jmcp.2020.26.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deana C., Vetrugno L., Tonizzo A., Orso D., Piani T., Bove T., De Monte A. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp. Pharm. 2020:1–3. doi: 10.1177/0018578720931749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dar M., Swamy L., Gavin D., Theodore A. Mechanical-ventilation supply and options for the COVID-19 pandemic leveraging all available resources for a limited resource in a crisis. Ann. Am. Thorac. Soc. 2021;18:408–416. doi: 10.1513/AnnalsATS.202004-317CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooq M.U., Hussain A., Masood T., Habib M.S. Supply chain operations management in pandemics: a state-of-the-art review inspired by covid-19. Sustain. Times. 2021;13:1–33. [Google Scholar]
- 11.Figueroa J.P., Bottazzi M.E., Hotez P., Batista C., Ergonul O., Gilbert S., Gursel M., Hassanain M., Kim J.H., Lall B., Larson H., Naniche D., Sheahan T., Shoham S., Wilder-Smith A., Strub-Wourgaft N., Yadav P., Kang G. Urgent needs of low-income and middle-income countries for COVID-19 vaccines and therapeutics. Lancet. 2021;397:562–564. doi: 10.1016/S0140-6736(21)00242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontanet A., Autran B., Lina B., Kieny M.P., Karim S.S.A., Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.hai Zhang D., lun Wu K., Zhang X., qiong Deng S., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020;18:152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moors E.H.M., Cohen A.F., Schellekens H. Towards a sustainable system of drug development. Drug Discov. Today. 2014;19:1711–1720. doi: 10.1016/j.drudis.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 15.FAO, Food and Agriculture Organization of the United Nations. 2020. http://www.fao.org/faostat/en/#data/QC [PubMed] [Google Scholar]
- 16.Satari B., Karimi K. Citrus processing wastes: environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018;129:153–167. [Google Scholar]
- 17.Ravindran R., Jaiswal A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016;34:58–69. doi: 10.1016/j.tibtech.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Zakaria Z.A., Boopathy R., Dib J.R. Springer; Cham: 2020. Valorisation of Agro-Industrial Residues - Volume I: Biological Approaches. [Google Scholar]
- 19.Oliveira A.F.C.D.S., Teixeira R.R., Oliveira A.S., Souza A.P.M., Silva M.L., Paula S.O. Potential antivirals: natural products targeting replication enzymes of dengue and Chikungunya viruses. Molecules. 2017;22 doi: 10.3390/molecules22030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da S Antonio A., Wiedemann L.S.M., Da Veiga-Junior V.F. Natural products ’ role against COVID-19. RSC Adv. 2020;10:23379–23393. doi: 10.1039/d0ra03774e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan S.A., Al-Balushi K. Combating COVID-19: the role of drug repurposing and medicinal plants. J. Infect. Public Health. 2021;14:495–503. doi: 10.1016/j.jiph.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization (WHO) World Health Organization; Geneva: 2013. WHO Traditional Medicine Strategy 2002-2005.http://www.wpro.who.int/health_technology/book_who_traditional_medicine_strategy_2002_2005.pdf 2002. [Google Scholar]
- 23.Hu T., Liu Y., Zhao M., Zhuang Q., Xu L., He Q. A comparison of COVID-19, SARS and MERS. PeerJ. 2020;8:1–30. doi: 10.7717/peerj.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fathi M., Vakili K., Sayehmiri F., Mohamadkhani A., Hajiesmaeili M., Rezaei-Tavirani M., Eilami O. The prognostic value of comorbidity for the severity of COVID-19: a systematic review and meta-analysis study. PloS One. 2021;16:1–25. doi: 10.1371/journal.pone.0246190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Animaw W., Seyoum Y. Increasing prevalence of diabetes mellitus in a developing country and its related factors. PloS One. 2017;12:1–11. doi: 10.1371/journal.pone.0187670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar D., Chandel V., Raj S., Rathi B. In silico identification of potent FDA approved drugs against Coronavirus COVID-19 main protease: a drug repurposing approach | Kumar | Chemical Biology Letters. Chem. Biol. Lett. 2020;7:166–175. http://pubs.iscience.in/journal/index.php/cbl/article/view/1033 [Google Scholar]
- 27.Zhong H., Wang Y., Zhang Z.L., Liu Y.X., Le K.J., Cui M., Yu Y.T., Gu Z.C., Gao Y., Lin H.W. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol. Res. 2020;157:104872. doi: 10.1016/j.phrs.2020.104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasmi A., Peana M., Noor S., Lysiuk R., Menzel A., Gasmi Benahmed A., Bjørklund G. Chloroquine and hydroxychloroquine in the treatment of COVID-19: the never-ending story. Appl. Microbiol. Biotechnol. 2021;105:1333–1343. doi: 10.1007/s00253-021-11094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meo S.A., Bukhari I.A., Akram J., Meo A.S., Klonoff D.C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of pfizer/BioNTech and moderna vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1663–1679. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 30.Plotkin S., Robinson J.M., Cunningham G., Iqbal R., Larsen S. The complexity and cost of vaccine manufacturing – an overview. Vaccine. 2017;35:4064–4071. doi: 10.1016/j.vaccine.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO/KFDA) 2008. Workshop on Stability Evaluation of Vaccines, Seoul, Republic of Korea; p. 42.https://www.who.int/docs/default-source/biologicals/vaccine-quality/77-who-kfda-stability-who-web-version-30-mar-09.pdf?sfvrsn=12953057_1 Report. [Google Scholar]
- 32.Jusu M.O., Glauser G., Seward J.F., Bawoh M., Tempel J., Friend M., Littlefield D., Lahai M., Jalloh H.M., Sesay A.B., Caulker A.F., Samai M., Thomas V., Farrell N., Widdowson M.A. Rapid establishment of a cold chain capacity of -60°C or colder for the STRIVE Ebola vaccine trial during the Ebola outbreak in Sierra Leone. J. Infect. Dis. 2018;217:S48–S55. doi: 10.1093/infdis/jix336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith M.J., Ujewe S., Katz R., Upshur R.E.G. Emergency use authorisation for COVID-19 vaccines: lessons from Ebola. Lancet. 2020;6736:19–20. doi: 10.1016/S0140-6736(20)32337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koyama T., Weeraratne D., Snowdon J.L., Parida L. 2020. Emergence of Drift Variants that May A Ff Ect COVID-19 Vaccine Development and, Pathogens; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G., Sun J., Chang C. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith J., L Dp.M., J W A. Vaccine production, distribution, access, and uptake. Lancet. 2011;378:428–438. doi: 10.1016/S0140-6736(11)60478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaur N., Kumar V., Rishi P., Sehrawat N., Dilawari R., Kumar P., Aggarwal N.K. In: Yadav M., Kumar V., Sehrawat N., editors. Ind. Biotechnol. Plant Syst. Resour. Prod., Walter de Gruyter GmbH; Berlin: 2019. Phytomedicine: history, scope and future prospects; pp. 105–120. [Google Scholar]
- 38.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2020;37:175–223. doi: 10.1039/c9np00069k. [DOI] [PubMed] [Google Scholar]
- 39.Pham J.V., Yilma M.A., Feliz A., Majid M.T., Maffetone N., Walker J.R., Kim E., Cho H.J., Reynolds J.M., Song M.C., Park S.R., Yoon Y.J. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019;10:1–27. doi: 10.3389/fmicb.2019.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S., Chaturvedi P., Kulkarni M.G., Van Staden J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020;39:107462. doi: 10.1016/j.biotechadv.2019.107462. [DOI] [PubMed] [Google Scholar]
- 41.Hug J.J., Krug D., Müller R. Bacteria as genetically programmable producers of bioactive natural products. Nat. Rev. Chem. 2020;4:172–193. doi: 10.1038/s41570-020-0176-1. [DOI] [PubMed] [Google Scholar]
- 42.Yang D., Park S.Y., Park Y.S., Eun H., Lee S.Y. Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 2020;38:745–765. doi: 10.1016/j.tibtech.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Sayed A.M., Khattab A.R., AboulMagd A.M., Hassan H.M., Rateb M.E., Zaid H., Abdelmohsen U.R. Nature as a treasure trove of potential anti-SARS-CoV drug leads: a structural/mechanistic rationale. RSC Adv. 2020;10:19790–19802. doi: 10.1039/d0ra04199h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalhori M.R., Saadatpour F., Arefian E., Soleimani M., Farzaei M.H., Aneva I.Y., Echeverría J. The potential therapeutic effect of RNA interference and natural products on COVID-19: a review of the coronaviruses infection. Front. Pharmacol. 2021;12:1–19. doi: 10.3389/fphar.2021.616993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng L., Zheng W., Li M., Huang J., Bao S., Xu Q., Ma Z. 2020. Citrus Fruits Are Rich in Flavonoids for Immunoregulation and Potential Targeting ACE2.https://www.preprints.org/manuscript/202002.0313/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meneguzzo F., Ciriminna R., Zabini F., Pagliaro M. Review of evidence available on hesperidin-rich products as potential tools against COVID-19 and hydrodynamic cavitation-based extraction as a method of increasing their production. Processes. 2020;8:1–18. [Google Scholar]
- 47.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A., Choudhir G., Shukla S.K., Sharma M., Tyagi P., Bhushan A., Rathore M. Identification of phytochemical inhibitors against main protease of COVID-19 using molecular modeling approaches. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1772112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev. Anti Infect. Ther. 2006;4:291–302. doi: 10.1586/14787210.4.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J., Song W., Huang H., Sun Q. Pharmacological therapeutics targeting RNA-dependent RNA polymerase , proteinase and spike Protein : from mechanistic studies to clinical trials for COVID-19. J. Clin. Med. 2020;2:1–23. doi: 10.3390/jcm9041131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minatani T., Ohta H., Sakai E., Tanaka T., Goto K., Watanabe D., Miyaguchi H. Analysis of toxic Veratrum alkaloids in plant samples from an accidental poisoning case. Forensic Toxicol. 2018;36:200–210. [Google Scholar]
- 52.Catchpole O.J., Grey J.B., Perry N.B., Burgess E.J., Redmond W.A., Porter N.G. Extraction of chili, black pepper, and ginger with near-critical CO2, propane, and dimethyl ether: analysis of the extracts by quantitative nuclear magnetic resonance. J. Agric. Food Chem. 2003;51:4853–4860. doi: 10.1021/jf0301246. [DOI] [PubMed] [Google Scholar]
- 53.Li Y.F., Yu B., Sun J.S., Wang R.X. Efficient synthesis of baicalin and its analogs. Tetrahedron Lett. 2015;56:3816–3819. [Google Scholar]
- 54.Tan K.H., Nishida R. Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012;12:1–60. doi: 10.1673/031.012.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pięt M., Paduch R. Ursolic and oleanolic acids as potential anticancer agents acting in the gastrointestinal tract. Mini-Reviews Org. Chem. 2018;16:78–91. [Google Scholar]
- 56.Ayeleso T.B., Matumba M.G., Mukwevho E. Oleanolic acid and its derivatives: biological activities and therapeutic potential in chronic diseases. Molecules. 2017;22 doi: 10.3390/molecules22111915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar A.H. Molecular docking of natural compounds from tulsi (ocimum sanctum) and neem (Azadirachta indica) against SARS-CoV-2 protein targets. Biol. Eng. Med. Sci. Reports. 2020;6:11–13. [Google Scholar]
- 58.Ibrahim M.A.A., Abdelrahman A.H.M., Allemailem K.S., Almatroudi A., Moustafa M.F., Hegazy M.E.F. In silico evaluation of prospective anti-COVID-19 drug candidates as potential SARS-CoV-2 main protease inhibitors. Protein J. 2021:1–4. doi: 10.1007/s10930-020-09945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.López-Hortas L., Pérez-Larrán P., González-Muñoz M.J., Falqué E., Domínguez H. Recent developments on the extraction and application of ursolic acid. A review. Food Res. Int. 2018;103:130–149. doi: 10.1016/j.foodres.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 60.Chan E.W.C., Soon C.Y., Tan J.B.L., Wong S.K., Hui Y.W. Ursolic acid: an overview on its cytotoxic activities against breast and colorectal cancer cells. J. Integr. Med. 2019;17:155–160. doi: 10.1016/j.joim.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Mlala S., Oyedeji A.O., Gondwe M., Oyedeji O.O. Ursolic acid and its derivatives as bioactive agents. Molecules. 2019;24:1–25. doi: 10.3390/molecules24152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Y., Bai C., He F., Xie Y., Zhou H. 2020. Review on the Potential Action Mechanisms of Chinese Medicines in Treating Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin L.T., Hsu W.C., Lin C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hossain K.S., Hossain M.G., Moni A., Rahman M.M., Rahman U.H., Alam M., Kundu S., Rahman M.M., Hannan M.A., Uddin M.J. Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capodice J.L., Chubak B.M. Traditional Chinese herbal medicine-potential therapeutic application for the treatment of COVID-19. Chin. Med. 2021;16:4–9. doi: 10.1186/s13020-020-00419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng Z.Z., Ma N.N., Li L., Jiang D. Efficacy of traditional Chinese medicine on COVID-19: two case reports. Med. Acupunct. 2021;33:92–102. doi: 10.1089/acu.2020.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y., Lang C., Huang D., Sun Q., Xiong Y., Huang X., Lv J., Luo Y., Shen L., Yang H., Huang G., Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020;92:797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsai K.C., Huang Y.C., Liaw C.C., Tsai C.I., Chiou C.T., Lin C.J., Wei W.C., Lin S.J.S., Tseng Y.H., Yeh K.M., Lin Y.L., Jan J.T., Liang J.J., Liao C.C., Chiou W.F., Kuo Y.H., Lee S.M., Lee M.Y., Su Y.C. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: a bedside-to-bench study. Biomed. Pharmacother. 2021;133:111037. doi: 10.1016/j.biopha.2020.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee D.Y.W., Li Q.Y., Liu J., Efferth T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: clinical experience and scientific basis. Phytomedicine. 2021;80:153337. doi: 10.1016/j.phymed.2020.153337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomaa A.A., Abdel-Wadood Y.A. The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomedicine. 2021;1:100043. doi: 10.1016/j.phyplu.2021.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldman A., Bomze D., Dankner R., Hod H., Meirson T., Boursi B., Maor E. Cardiovascular adverse events associated with hydroxychloroquine and chloroquine: a comprehensive pharmacovigilance analysis of pre-COVID-19 reports. Br. J. Clin. Pharmacol. 2021;87:1432–1442. doi: 10.1111/bcp.14546. [DOI] [PubMed] [Google Scholar]
- 72.Kamp T.J., Hamdan M.H., January C.T. Chloroquine or hydroxychloroquine for COVID-19: is cardiotoxicity a concern? J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hensel A., Bauer R., Heinrich M., Spiegler V., Kayser O., Hempel G., Kraft K. Challenges at the time of COVID-19: opportunities and innovations in antivirals from nature. Planta Med. 2020 doi: 10.1055/a-1177-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newman D.J. Developing natural product drugs: supply problems and how they have been overcome. Pharmacol. Ther. 2016;162:1–9. doi: 10.1016/j.pharmthera.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Plowden C. The ethnobotany of copaíba (Copaifera) oleoresin in the amazon. Econ. Bot. 2004;58:729–739. [Google Scholar]
- 76.de S Barbosa K., Scudeller V.V., Rosa A.L. In: Divers. Biológica e Sociocult. Do Baixo Rio Negro, Amaz., Manaus. Santos-Silva E.N., Scudeller V.V., editors. 2009. Potential de produção de óleo resina de Copaifera multijuga Hayne nos dois períodos climáticos amazônicos na Reserva de Desenvolvimento Sustentável do Tupé, Manaus-AM; pp. 143–153. [Google Scholar]
- 77.Rigamonte-Azevedo O.C., Wadt P.G.S., Wadt L.H.D.O. Oil resin production potential of Copaifera spp natural populations in the Southwestern Brazilian Amazon. Rev. Árvore. 2006;30:583–591. [Google Scholar]
- 78.Xu N., Meng F., Zhou G., Li Y., Wang B., Lu H. Assessing the suitable cultivation areas for Scutellaria baicalensis in China using the Maxent model and multiple linear regression. Biochem. Systemat. Ecol. 2020;90:104052. [Google Scholar]
- 79.Zhang L., Cao B., Bai C., Li G., Mao M. Predicting suitable cultivation regions of medicinal plants with Maxent modeling and fuzzy logics: a case study of Scutellaria baicalensis in China. Environ. Earth Sci. 2016;75:1–12. [Google Scholar]
- 80.Ohtsuki T., Himeji M., Fukazawa H., Tanaka M., Yamamoto H., Mimura A. High-yield production of scutellaria radix flavonoids (baicalein, baicalin and wogonin) by liquid-culture of Scutellaria baicalensis root-derived cells. Braz. Arch. Biol. Technol. 2009;52:291–298. [Google Scholar]
- 81.Bin Li H., Jiang Y., Chen F. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004;812:277–290. doi: 10.1016/j.jchromb.2004.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li J., Tian C., Xia Y., Mutanda I., Wang K., Wang Y. Production of plant-specific flavones baicalein and scutellarein in an engineered E. coli from available phenylalanine and tyrosine. Metab. Eng. 2019;52:124–133. doi: 10.1016/j.ymben.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Li J., Xiong C., He X., Lu Z., Zhang X., Chen X., Sun W. Using SSR-HRM to identify closely related species in herbal medicine products: a case study on licorice. Front. Pharmacol. 2018;9:1–11. doi: 10.3389/fphar.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang M., Wang W., Wei S. Investigation on medicinal plant resources of Glycyrrhiza uralensis in China and chemical assessment of its underground part. Zhongguo Zhongyao Zazhi. 2010;35:945–952. doi: 10.4268/cjcmm20100802. [DOI] [PubMed] [Google Scholar]
- 85.Shi V.G., Koh S.C.L., Baldwin J., Cucchiella F. Natural resource based green supply chain management. Supply Chain Manag. 2012;17:54–67. [Google Scholar]
- 86.Bergman M.E., Davis B., Phillips M.A. Medically useful plant terpenoids: biosynthesis, occurrence, and mechanism of action. Molecules. 2019;24:1–23. doi: 10.3390/molecules24213961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cragg G.M. Natural product drug discovery and development: the United States National Cancer Institute role. Puert. Rico Health Sci. J. 2002;21:97–111. [PubMed] [Google Scholar]
- 88.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., Rollinger J.M., Schuster D., Breuss J.M., Bochkov V., Mihovilovic M.D., Kopp B., Bauer R., Dirsch V.M., Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.International Food Policy Research Institute . Uzbekistan; Beijing: 2014. Licorice Industry in China: Implications for Licorice Producers. [Google Scholar]
- 90.Food and Agriculture Organization of the United Nations, FAOSTATS. 2020. http://www.fao.org/faostat/en/#data/QC [Google Scholar]
- 91.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5 doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Victor M.M., David J.M., Cortez M.V.M., Leite J.L., da Silva G.S.B. A high-yield process for extraction of hesperidin from orange (citrus sinensis L. osbeck) peels waste, and its transformation to diosmetin, A valuable and bioactive flavonoid. WasteBiomass Valoriz. 2020:331. 320. [Google Scholar]
- 93.Pereira R.M.S., López B.G.-C., Diniz S.N., Antunes A.A., Moreno Garcia D., Rocha Oliveira C., Marcucci M.C. Quantification of flavonoids in Brazilian orange peels and industrial orange juice processing wastes. Agric. Sci. 2017;8:631–644. [Google Scholar]
- 94.Cargnin S.T., Gnoatto S.B. Ursolic acid from apple pomace and traditional plants: a valuable triterpenoid with functional properties. Food Chem. 2017;220:477–489. doi: 10.1016/j.foodchem.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 95.Yates M., Gomez M.R., Martin-Luengo M.A., Ibañez V.Z., Martinez Serrano A.M. Multivalorization of apple pomace towards materials and chemicals. Waste to wealth. J. Clean. Prod. 2017;143:847–853. [Google Scholar]
- 96.Jin Q., Yang L., Poe N., Huang H. Integrated processing of plant-derived waste to produce value-added products based on the biorefinery concept. Trends Food Sci. Technol. 2018;74:119–131. [Google Scholar]
- 97.Morone P., Koutinas A., Gathergood N., Arshadi M., Matharu A. Food waste: challenges and opportunities for enhancing the emerging bio-economy. J. Clean. Prod. 2019;221:10–16. [Google Scholar]
- 98.Mackenzie L.S., Tyrrell H., Thomas R., Matharu A.S., Clark J.H., Hurst G.A. Valorization of waste orange peel to produce shear-thinning gels. J. Chem. Educ. 2019;96:3025–3029. [Google Scholar]
- 99.Remón J., Li T., Chuck C.J., Matharu A.S., Clark J.H. Toward renewable-based, food-applicable prebiotics from biomass: a one-step, additive-free, microwave-assisted hydrothermal process for the production of high purity xylo-oligosaccharides from beech wood hemicellulose. ACS Sustain. Chem. Eng. 2019;7:16160–16172. [Google Scholar]
- 100.Andritsou V., De Melo E.M., Tsouko E., Ladakis D., Maragkoudaki S., Koutinas A.A., Matharu A.S. Synthesis and characterization of bacterial cellulose from citrus-based sustainable resources. ACS Omega. 2018;3:10365–10373. doi: 10.1021/acsomega.8b01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharma K., Mahato N., Cho M.H., Lee Y.R. Converting citrus wastes into value-added products: economic and environmently friendly approaches. Nutrition. 2017;34:29–46. doi: 10.1016/j.nut.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 102.Mahato N., Sinha M., Sharma K., Koteswararao R., Cho M.H. 2019. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-products from Citrus Wastes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tocmo R., Pena-Fronteras J., Calumba K.F., Mendoza M., Johnson J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: a review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr. Rev. Food Sci. Food Saf. 2020:1–44. doi: 10.1111/1541-4337.12561. [DOI] [PubMed] [Google Scholar]
- 104.Cheng L., Zheng W., Li M., Huang J., Bao S., Xu Q., Ma Z. 2020. Citrus Fruits Are Rich in Flavonoids for Immunoregulation and Potential Targeting ACE2.www.preprints.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burki T. No end in sight for the Brazilian COVID-19 crisis. The Lancet Microbe. 2021;2 doi: 10.1016/S2666-5247(21)00095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zema D.A., Calabrò P.S., Folino A., Tamburino V., Zappia G., Zimbone S.M. Valorisation of citrus processing waste: a review. Waste Manag. 2018;80:252–273. doi: 10.1016/j.wasman.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 107.Anticona M., Blesa J., Frigola A., Esteve M.J. High biological value compounds extraction from citruswaste with non-conventional methods. Foods. 2020;9 doi: 10.3390/foods9060811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hilali S., Fabiano-Tixier A.S., Ruiz K., Hejjaj A., Ait Nouh F., Idlimam A., Bily A., Mandi L., Chemat F. Green extraction of essential oils, polyphenols, and pectins from orange peel employing solar energy: toward a zero-waste biorefinery. ACS Sustain. Chem. Eng. 2019;7:11815–11822. [Google Scholar]
- 109.Garcia-Garcia G., Rahimifard S., Matharu A.S., Dugmore T.I.J. Life-cycle assessment of microwave-assisted pectin extraction at pilot scale. ACS Sustain. Chem. Eng. 2019;7:5167–5175. [Google Scholar]
- 110.Nakajima V.M., Madeira J.V., Macedo G.A., Macedo J.A. Biotransformation effects on anti lipogenic activity of citrus extracts. Food Chem. 2016;197:1046–1053. doi: 10.1016/j.foodchem.2015.11.109. [DOI] [PubMed] [Google Scholar]
- 111.Shakour Z.T.A., Fayek N.M., Farag M.A. How do biocatalysis and biotransformation affect Citrus dietary flavonoids chemistry and bioactivity? A review. Crit. Rev. Biotechnol. 2020;8551 doi: 10.1080/07388551.2020.1753648. [DOI] [PubMed] [Google Scholar]
- 112.Nile S.H., Nile A., Liu J., Kim D.H., Kai G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019;131:110563. doi: 10.1016/j.fct.2019.110563. [DOI] [PubMed] [Google Scholar]
- 113.Fan J.P., Liao D.D., Zhang X.H. Ultrasonic assisted extraction of ursolic acid from apple pomace: a novel and facile technique. Separ. Sci. Technol. 2016;51:1344–1350. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.