Abstract
Background
Understanding the risk factors responsible for the increased infection among HCWs can mitigate the transmission of COVID-19 among HCWs and patients alike. The aim of this study is to evaluate factors associated with SARS-CoV-2 infection among healthcare Workers.
Methods
Healthcare workers and hospital administrators were asked to participate in this cross-sectional survey study that was conducted in Jaber Al Ahmad Hospital (JAH) between August to October 2020. Participants were invited to undergo SARS-CoV-2-specific antibody testing and to complete a questionnaire targeted to factors that may be associated with acquisition of SARS-CoV-2. Descriptive analysis and multivariate logistic regression were done.
Results
847 healthcare workers participated in the study and 20.5% of them had previous SARS-CoV-2 infection. The average age of participants was 35.7 years (SD = 7.9); 52.4% were female, and 55.8% were doctors. Multivariate analysis showed that working as a nurse (adjusted OR 1.77, 95% CI = 1.15, 2.71), and wearing gloves (adjusted OR 2.93, 95% CI = 1.19, 7.22) were significantly associated with an increased likelihood of contracting SARS-CoV-2 infection while controlling for other factors. Most personal protective equipment (PPE) were reported to be available always or most of the time, with the least available PPE item being coveralls (74.4%).
Conclusions
After adjusting for confounding factors, being a nurse and prolonged glove use were associated with increased likelihood of SARS-CoV-2 infection. Prospective cohort studies are required to further elucidate the reasons for our findings in order to minimize the transmission of infection among healthcare workers.
Keywords: Healthcare workers, Infection control, COVID-19, SARS-CoV-2
Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues to have a major impact on healthcare services worldwide, despite ongoing vaccination efforts. New variants are continuously emerging with devastating effects [1]. Also, vaccine supply shortages, delivery logistical issues and anti-vaccination sentiments have made reaching global herd immunity a challenge [2,3]. Healthcare workers remain pivotal to the functionality of healthcare systems. In fact, global policy decisions regarding public health measures, such as lockdowns and airport closures, are guided to maintain functional capacity of healthcare systems [4].
Epidemiological seroprevalence studies have consistently demonstrated that healthcare workers are at a higher risk of SARS-CoV-2 transmission compared to the general public [5,6]. This emphasizes the importance of infection prevention and control in avoiding nosocomial SARS-CoV-2 outbreaks and disruptions to essential services [7,8]. However, there are several challenges facing infection control departments within hospitals that include protective personal equipment shortages [7], lack of clarity regarding SARS-CoV-2 transmission patterns [9] and the difficulty of implementing guidelines issued by international governing bodies that are continuously changing [10,11].
To minimize healthcare workers’ susceptibility to respiratory infections, such as COVID-19, a comprehensive risk factor assessment is merited [12]. Several authors have addressed this through multiple types of questionnaires [13,14]. However, most of the questionnaires used have focused on certain demographics or particular infection control practices. Also, most of the studies that have surveyed healthcare workers did not utilize laboratory testing to confirm the status of the controls [15], despite the fact that asymptomatic SARS-CoV-2 infection may play a role in super-spreader transmission events [16,17]. The aim of this study is to better understand and evaluate the association between several infection control factors and the risk of SARS-CoV-2 infection and potentially assess effectiveness of infection control measures.
Methods
Study design and population
This was a cross-sectional survey where participants were invited to undergo SARS-CoV-2-specific antibody testing and complete a questionnaire at a designated COVID-19 hospital in Kuwait (Jaber Al Ahmad Hospital (JAH)) between August to October 2020 (Note: that recruitment period was prior to the vaccination period in Kuwait). JAH is a large general hospital with a bed capacity of 1150 beds and provides comprehensive medical, surgical, dental, obstetrics, and pediatrics services. In February 2020, it became the national designated COVID-19 center. Invitations to participate in the study were extended to all healthcare workers and hospital administrators over the age of 18 across all health districts in Kuwait through social media. Participants who reported that they were not active in their role during the pandemic period were excluded from the study.
Definitions
For the purposes of this study ‘Doctors’ were defined as physicians and dentists who were active clinically during the pandemic. ‘Nurses’ included staff nurses and student nurses who had direct and indirect patient contact throughout the study period. ‘Other medical staff’ were healthcare professionals whose role put them in contact with patients, such as physiotherapists, occupational therapists, and nutritionists. ‘Administrative staff’ were hospital workers whose role did not put them in contact with patients. Patients were classified as ‘COVID-19 positive’ if they reported a prior positive result for SARS-CoV-2 via polymerase chain reaction (PCR) or antibody testing or if they had a positive antibody testing during the study. Patients were considered ‘COVID-19 negative’ if they never had a positive result for SARS-CoV-2 via PCR or antibody testing.
Serological testing for SARS-CoV-2
After obtaining informed consent, specimens were collected and underwent SARS-CoV-2-specific antibody testing using the LIAISON® SARS-CoV-2 S1/S2 IgG and IgM (DiaSorin, Saluggia, Italy). This system uses chemiluminescent immunoassay (CLIA) for semi-automatic detection of SARS-CoV-2 antibodies in human samples. The manufacturer’s protocol was followed for sample analysis.
Questionnaire
The survey was distributed to the healthcare workers electronically via text message using the SurveyCTO online platform (Dobility, USA) prior to their serological testing. The survey was adapted from the World Health Organization’s survey [18]. The survey was adapted to better fit our local population’s environment. The questionnaire’s primary aim was to assess risk factors for SARS-CoV-2 infection in healthcare workers dealing with COVID-19 patients and to potentially estimate the efficacy of infection control measures. The survey was composed of the following domains: participants’ demographics, transmission dynamics, infection control training, personal protective equipment (PPE) availability, clinical practice and COVID-19 contact characteristics.
Ethics
Ethical approval for this study was obtained from the Standing Committee for Coordination of Health and Medical Research (Ethics Review Committee) at the Ministry of Health of Kuwait (reference no. 2020/1473).
Statistical analysis
Continuous data were described using mean (M) and standard deviation (SD). Categorical data were described using number (n) and percentage (%). To determine the potential risk factors of COVID-19 infection, we conducted univariate logistic regression. The associated risk was quantified using crude odds ratio (OR) and 95% confidence intervals (CI). Where zeros caused problems with computation of the OR or its Standard Error, 0.5 was added to all cells [19] and the significance test was calculated according to Sheskin [20]. In the univariate analysis, missing values (i.e., with no reported response) and not-needed-for-my-role values were excluded. However, when conducting multivariate analysis, we used multiple imputation to avoid listwise deletion. Prior to imputing the data, the frequency, percentage, and pattern of missing data were checked. Additionally, the outcome variable was compared between patients with and without missing values using Chi-square test. The pattern of missing data in this study (shown in Appendix A) was arbitrary because the missing values for the variables of any record were seen in a random fashion. Thus, multiple imputation by chained equations was appropriate. The basic idea is to impute incomplete variables, one at a time, using the completed variables. The imputation model used a logistic regression algorithm as all missing variables were categorical and included age, sex, job, nationality and all variables related to PPE availability and practice, contact with COVID-19 patients, as well as training. Missing values were imputed ten times with 1000 iterations. After imputation, adjusted OR and 95% CI were estimated using a multiple logistic regression model, which included the variables that were significantly associated with COVID-19 infection in the univariate analysis. A model was applied to each dataset. The estimated coefficients, SEs, and CIs from each model were pooled together using Rubin’s rule [21].
Results of statistical analysis were considered significant when the probability of error was .05 or less. Statistical analyses were performed using IBM SPSS Statistics (Version 27) [22] and R (Version 4.0.4) packages [23].
Results
During the study period, 847 healthcare workers completed the survey and underwent SARS-CoV-2-specific antibody testing. Of those, 174 (20.5%) participants were categorized as positive. At the time of the study, 79 (9.3%) tested positive for IgM, 133 (15.7%) for IgG, and 108 (12.8%) self-reported a previous positive PCR test. Participants were an average of 35.7 years of age (SD = 7.9), and mostly were female (52.4%), and doctors (55.8%) or nurses (18.4%). In addition, other medical staff (22.0%) took part in the study included lab technicians (6.8%), radiology technicians (3.4%), and pharmacists (2.5%). With regard to comorbidities, 37.9% reported at least one comorbidity; the most common were dyslipidemia (12.3%), obesity (10.9%), and bronchial asthma (9.2%). The majority (80%) reported that they were not on prescription medications.
Results of the univariate analysis of the variables relating to demographic and clinical characteristics are presented in Table 1 . Among healthcare workers, the odds of contracting SARS-CoV-2 infection was highest among nurses (OR 1.87, 95% CI 1.22−.86), and other medical staff (apart from doctors or nurses) (OR 1.52, 95% CI 1.01–2.30). Since 81.5% of Indians were nurses or other medical staff, nationality will not be included in the multivariate model. Other variables namely: age, sex, comorbidities, and medications were not associated with COVID-19 status in our study.
Table 1.
Demographics and clinical characteristics of healthcare workers and their associated risk to COVID-19 infection.
| Covid-19 status |
Crude OR (95% CI) | |||
|---|---|---|---|---|
| Negative | Positive | |||
| N = 673 | N = 174 | |||
| Age in years, M ± SD | 35.6 ± 8.1 | 36.1 ± 7.1 | 1.01 (0.99, 1.03) | |
| Male, n (%) | 319 (47.4) | 84 (48.3) | 1.04 (0.74, 1.45) | |
| Nationality, n (%) | ||||
| Kuwait | 339 (50.4) | 63 (36.2) | 1 | |
| India | 141 (21.0) | 59 (33.9) | 2.25 (1.50, 3.38)* | |
| Egypt | 121 (18.0) | 31 (17.8) | 1.38 (0.86, 2.22) | |
| Other | 72 (10.7) | 21 (12.1) | 1.57 (0.90, 2.74) | |
| Job, n (%) | ||||
| Doctor | 393 (58.4) | 80 (46.0) | 1 | |
| Nurse | 113 (16.8) | 43 (24.7) | 1.87 (1.22, 2.86)* | |
| Other medical staff | 142 (21.1) | 44 (25.3) | 1.52 (1.01, 2.30)* | |
| Administrative staff | 25 (3.7) | 7 (4.0) | 1.38 (0.58, 3.29) | |
| Comorbidities, n (%) | ||||
| Diabetes | 32 (4.8) | 12 (6.9) | 1.48 (0.75, 2.94) | |
| Hypertension | 54 (8.0) | 22 (12.6) | 1.66 (0.98, 2.81) | |
| High cholesterol | 83 (12.3) | 21 (12.1) | 0.98 (0.59, 1.63) | |
| Bronchial asthma | 56 (8.3) | 22 (12.6) | 1.59 (0.94, 2.69) | |
| Blood diseases | 19 (2.8) | 4 (2.3) | 0.81 (0.27, 2.41) | |
| Autoimmune diseases | 17 (2.5) | 9 (5.2) | 2.10 (0.92, 4.81) | |
| Obesity | 72 (10.7) | 20 (11.5) | 1.08 (0.64, 1.83) | |
| Others | 22 (3.3) | 7 (4.0) | 1.24 (0.52, 2.95) | |
| Medications, n (%) | ||||
| Statins | 36 (5.3) | 3 (1.7) | 0.31 (0.09, 1.02) | |
| Steroids | 15 (2.2) | 3 (1.7) | 0.77 (0.22, 2.69) | |
| Immunosuppressives | 8 (1.2) | 2 (1.1) | 0.97 (0.20, 4.59) | |
| Others | 80 (11.9) | 28 (16.1) | 1.42 (0.89, 2.27) | |
There were no missing data observations in any of the variable.
p value < .05.
The individual’s perception of PPE training adequacy and PPE availability were evaluated. Most participants reported that different items of PPE were available always or most of the time (74–99%), the least available PPE item was coverall (74.4%) (Table 2 ). PPE availability was not associated with SARS-CoV-2 infection in our study. Similarly, relevant training was not associated with SARS-CoV-2 infection. The most frequently reported training was that on the five recommended moments of hand hygiene (93.8%). Only two types of training were received by less than 60% of participants, namely care of COVID-19 patients (52.6%), and how to perform the N95 mask seal check (55.9%).
Table 2.
Availability of PPE and training of healthcare workers and their associated risk to COVID-19 infection.
| COVID-19 status |
Missing dataa (%) | Crude OR (95% CI) | ||
|---|---|---|---|---|
| Negative | Positive | |||
| Total = 673 | Total = 174 | |||
| n (%) | n (%) | |||
| Availability of PPEb | ||||
| Alcohol-based hand rub | 615 (98.6) | 155 (98.7) | (7.7) | 1.13 (0.24, 5.30) |
| Soap and water | 589 (97.7) | 147 (95.5) | (10.6) | 0.50 (0.20, 1.26) |
| Surgical mask | 589 (97.4) | 151 (98.1) | (10.3) | 1.37 (0.39, 4.75) |
| Face shield | 516 (93.1) | 131 (92.3) | (16.3) | 0.88 (0.44, 1.76) |
| Gloves | 553 (95.0) | 142 (96.6) | (13.6) | 1.49 (0.57, 3.92) |
| Goggles | 405 (77.4) | 110 (80.3) | (20.1) | 1.19 (0.74, 1.90) |
| Gown | 501 (91.4) | 129 (93.5) | (17.7) | 1.34 (0.64, 2.82) |
| Coverall | 346 (71.5) | 90 (75.0) | (26.0) | 1.20 (0.76, 1.89) |
| Headcover | 441 (86.1) | 117 (90.7) | (22.0) | 1.57 (0.82, 2.99) |
| Shoe cover | 356 (74.3) | 94 (77.7) | (26.3) | 1.20 (0.75, 1.93) |
| N95 mask or other type of respirator | 373 (73.7) | 97 (77.0) | (23.4) | 1.19 (0.75, 1.89) |
| Training during the pandemic | ||||
| Infection prevention and control | 479 (72.2) | 127 (75.1) | (1.8) | 1.16 (0.79, 1.71) |
| Care of Covid-19 patients | 341 (51.3) | 97 (57.7) | (1.7) | 1.30 (0.92, 1.83) |
| Five recommended moments of hand hygiene | 623 (93.5) | 162 (94.7) | (1.2) | 1.24 (0.59, 2.60) |
| PPE donning and doffing | 539 (81.5) | 147 (85.5) | (1.7) | 1.33 (0.83, 2.12) |
| How to perform the N95 mask fit test | 369 (55.5) | 98 (57.3) | (1.3) | 1.08 (0.77, 1.51) |
Percentages were calculated out of available observations i.e. after exclusion of missing observations and participants who reported “not needed for my role”.
Missing data refers to missing observations i.e. where a participant did not respond.
Participants who answered always or most of the time.
More than two thirds of participants (69.4%) reported contact with patients known to have COVID-19 infection always or most of the time. However, there was no statistically significant relationship between SARS-CoV-2 infection among participants and the degree of reported contact with known COVID-19 patients, even with reported close contact (less 1 m), or contact with bodily fluids (p > .05 for all three variables), (Table 3). On the other hand, wearing three types of PPE always or most of the time was significantly associated with an increased likelihood of COVID-19 infection: gloves (OR 3.63, 95% CI 1.55–8.51), goggles (OR 1.79, 95% CI 1.10–2.89), and gowns (OR 1.77, 95% CI 1.01–3.11). Practice related to other types of PPE was not associated with risk of SARS-CoV-2 infection. In the univariate analysis, missing values (i.e. with no reported response) and not-needed-for-my-role values were excluded. Overall, 9.9% of values were missing across 33 variables among 437 (51.6%) participants. The distribution of missing values are provided in Table 2, Table 3 . None of the variables in Table 1 included any missing values. The number of participants who reported “not needed for my role” ranged between one and 37 (4.4%), the latter was reported for the variable “contact with bodily fluids of patients known to have COVID-19 infection”. The outcome variable (COVID-19 status) did not significantly differ between participants with missing values (n = 437, 51.6%) and those without (n = 410, 48.4%), (X2 p > .05).
Table 3.
Contact with COVID-19 patients, and PPE practice among healthcare workers and their associated risk to COVID-19 infection.
| Covid-19 status |
Missing data (%) | Crude OR (95% CI) | ||
|---|---|---|---|---|
| Negative | Positive | |||
| n (%) | n (%) | |||
| Contact with patients known to have Covid-19a | ||||
| Any contact | 381 (68.8) | 108 (71.5) | (15.3) | 1.14 (0.77, 1.70) |
| Close contact <1 m | 336 (63.3) | 91 (64.1) | (18.7) | 1.04 (0.70, 1.52) |
| Contact with bodily fluids | 264 (53.9) | 74 (54.4) | (21.7) | 1.02 (0.70, 1.50) |
| PPE practicea | ||||
| Alcohol-based hand rub | 607 (98.1) | 156 (100) | (8.3) | 6.44 (0.38, 109.37) |
| Soap and water | 574 (95.3) | 149 (96.8) | (10.5) | 1.45 (0.55, 3.83) |
| Five moments of hand hygiene | 560 (94.0) | 144 (94.7) | (11.5) | 1.16 (0.53, 2.54) |
| Surgical mask | 589 (98.3) | 154 (99.4) | (10.6) | 2.61 (0.33, 20.58) |
| Face shield | 435 (77.0) | 120 (83.9) | (14.8) | 1.56 (0.96, 2.54) |
| Gloves | 498 (86.6) | 141 (95.9) | (13.9) | 3.63 (1.55, 8.51)* |
| Goggles | 377 (71.4) | 107 (81.7) | (20.3) | 1.79 (1.10, 2.89)* |
| Gown | 452 (81.7) | 127 (88.8) | (16.4) | 1.77 (1.01, 3.11)* |
| Coverall | 317 (64.0) | 81 (67.5) | (24.2) | 1.17 (0.76, 1.78) |
| Headcover | 368 (70.9) | 100 (78.1) | (21.5) | 1.47 (0.93, 2.32) |
| Shoe cover | 281 (57.9) | 77 (62.1) | (25.5) | 1.19 (0.79, 1.78) |
| N95 mask or another type of respirator | 383 (75.0) | 95 (74.2) | (22.8) | 0.96 (0.62, 1.50) |
| N95 mask during an aerosol generating procedure | 423 (84.3) | 102 (85.7) | (23.3) | 1.12 (0.64, 1.98) |
| N95 mask seal test | 352 (71.1) | 82 (71.3) | (25.5) | 1.01 (0.64, 1.58) |
Percentages were calculated out of available observations i.e. after exclusion of missing observations and participants who reported “not needed for my role”.
Participants who answered always or most of the time.
p value < .05.
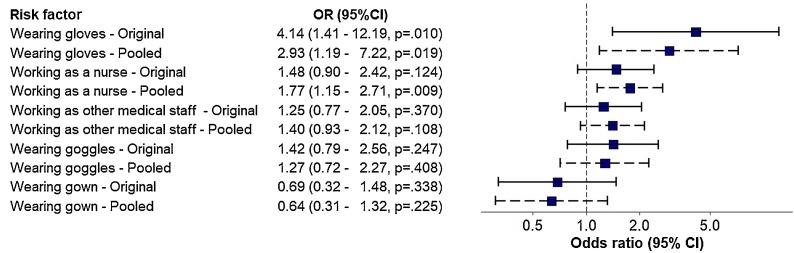
The significant variables in the univariate analysis were further analyzed using a Multivariate regression model. Cases with missing data were included in the multivariate analysis using data after multiple imputation which generated 10 data sets. The efficiency of estimating OR for the three variables in the model with missing data was 98.0% for gloves, 97.5% for goggles, and 97.3% for gowns. Adjusted odds ratios (OR) are shown in Fig. 1 . The model shows that working as a nurse (adjusted OR 1.77, 95% CI 1.15–2.71), and wearing gloves (adjusted OR 2.93, 95% CI 1.19–7.22) were significantly associated with an increased likelihood of contracting COVID-19 infection while controlling for other factors. The other variables in the model were not significant.
Fig. 1.
Error bar showing the adjusted odds ratio of risk factors for contracting COVID-19 infection among healthcare workers.
(Wearing refers to reported wearing of PPE always or most of the time, Original: multivariate regression model from the original data before imputation of missing values, Pooled: multivariate regression model from the imputed data.)
Discussion
Due to the nature of their work, healthcare workers are considered to be put at higher risk for COVID-19 infection due to their increase exposure to patients. Various risk factors have been shown to pose an increased transmission for COVID-19 in various hospital settings. In a convenient sample of hospital workers, 20.5% of surveyed healthcare workers had evidence of previous SARS-CoV-2 infection. Highest proportion of prior COVID-19 was observed among nurses (27.6%). Gloves were also associated with an increased risk of acquiring COVID-19 among the PPE surveyed.
The positivity rate of SARS-CoV-2 found among the healthcare workers in our study was 20.5%. This is much higher than the community rate of 12–15% for the same period in Kuwait [24]. However, this is in line with other studies conducted in high-risk setting of SARS-CoV-2 transmission ranging between 24.4% and 27% [6,25]. On the contrary, other studies have reported much lower seroprevalence rates, such as 2.36% and 4.04% [26,27]. It is important to note that in the latter studies, a larger cohort was surveyed and the hospitals being surveyed are larger. In these studies, the seroprevalence was similar to that found in the community at that same time. A potential reason behind our high SARS- CoV-2 positivity rate to be due to the fact that, early in the outbreak, some healthcare workers shared housing in order to isolate from family which increased the risk of transmission between healthcare workers. Also, this study was conducted at a hospital which cared for COVID-19 patients exclusively.
Of all the healthcare workers surveyed, we found the largest proportion of COVID-19 cases among the nurses in comparison to the other health professionals. Since 81.5% of Indians were nurses or other medical staff, the observed statistical significance of this nationality is likely due to the significant association of their profession with infection. These findings are consistent with those reported by Çelebi et al. and Rubbi et al. [28,29]. In those studies, this has been attributed to nurses having greater exposure to COVID-19 patients. This was corroborated by findings by Rubbi et al., where exposure less than 1 m and a contact time of at least 2 h was associated with COVID-19 infection in nurses [29]. In our study, the frequency and the degree of contact with COVID-19 patients was not found to be a significant factor for getting infected. However, it is difficult to quantify the exact exposure time as all hospitalized patients who are receiving care from the participating healthcare workers were SARS-CoV-2 infected. Also, we hypothesize that in our cohort community-acquired COVID-19 infection may have contributed to an increased infection rate in nurses. This finding has been observed by Wee et al., and Piccoli et al., who determined that household exposure to COVID-19 represented a higher risk or seropositivity for healthcare workers [5,30].
Most participants in our study reported that different types of PPE were available ‘always’ or ‘most of the time’, and we found no association between PPE availability and COVID-19 infection. Nguyen et al. conducted a large prospective cohort study in the UK and US, 99,795 healthcare workers self-reported COVID-19 data through a mobile application regarding COVID-19 risk factors and PPE usage. They found that even among healthcare workers who had access to adequate PPE, there was an increased susceptibility to COVID-19 infection [31]. They also found that adequate availability of PPE did not completely reduce the risk of infection in healthcare workers caring for COVID-19 patients and that reusing PPE was positively associated with an increased risk of infection for healthcare workers caring for COVID-19 patients [31].
Unexpectedly, we found that glove usage had a significant association with COVID-19 positivity among the healthcare workers in our study. Although this association has not been previously reported in other COVID-19 studies, it has been described in other infectious diseases [[32], [33], [34]]. The reasons for this may be multifaceted. One hypothesis is that extended glove usage results in greater contamination due to poor hand hygiene practices. Lindberg et al. explored this hypothesis further and reported that this may be due to glove usage giving a false sense of security to its users [35]. In addition, gloves are often improperly used by healthcare worker [32]. Picheansathian and Chotibang et al. have reported that gloves were often not replaced by healthcare workers between patients and procedures resulting in greater cross-contamination [36]. This is concerning, as a study by Ye et al. found that gloves were the second most contaminated surface in a hospital [37]. In fact, even the World Health Organization has issued a statement during the pandemic warning that improper glove use is likely to be linked with an increased risk for COVID-19 infection [38]. We believe that this is the first study to find an association between the frequency of glove use and COVID-19 transmission. We postulate that this may be due healthcare workers changing their gloves less frequently between patients and procedure, due to perceived PPE shortages during the pandemic [39].
In this study, we have found that being a nurse and glove use were positively associated with COVID-19 infection. Further studies are required, ideally prospective cohort studies, to further elucidate the reasons for this. This is important to minimize the transmission of infection among healthcare workers. The limitations of this study include the fact that some of the questionnaires contained missing data, which we imputated for. Due to the retrospective nature of our questionnaire, recall bias may have affected the accuracy of some of the reported responses. Also, the effect of a previous infection on the subsequent HCW behaviour and use of PPE cannot be determined.
Funding
Financial support for this study was provided by the Kuwait Foundation for the Advancement of Science (KFAS). Bader Sultan Medical Company provided the SARS-CoV-2 antibody kits.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgements
We thank the phlebotomists and lab technicians who have devoted a lot of their time to make sure all the healthcare workers have been tested.
Appendix A. Description of imputation method
References
- 1.The Lancet India’s COVID-19 emergency. Lancet. 2021;397:1683. doi: 10.1016/S0140-6736(21)01052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson R.M., Vegvari C., Truscott J., Collyer B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet Lond Engl. 2020;396:1614–1616. doi: 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torjesen I. Covid-19 vaccine shortages: what is the cause and what are the implications? BMJ. 2021;372:n781. doi: 10.1136/bmj.n781. [DOI] [PubMed] [Google Scholar]
- 4.Mehta S., Machado F., Kwizera A., Papazian L., Moss M., Azoulay É, et al. COVID-19: a heavy toll on health-care workers. Lancet Respir Med. 2021;9:226–228. doi: 10.1016/S2213-2600(21)00068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccoli L., Ferrari P., Piumatti G., Jovic S., Rodriguez B.F., Mele F., et al. Risk assessment and seroprevalence of SARS-CoV-2 infection in healthcare workers of COVID-19 and non-COVID-19 hospitals in Southern Switzerland. Lancet Reg Health Eur. 2021;1 doi: 10.1016/j.lanepe.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D., et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbas M., Robalo Nunes T., Martischang R., Zingg W., Iten A., Pittet D., et al. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10:7. doi: 10.1186/s13756-020-00875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung N.H.L. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021:1–18. doi: 10.1038/s41579-021-00535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fair J.M., LeClaire R.J., Dauelsberg L.R., Ewers M., Pasqualini D., Cleland T., et al. Systems dynamics and the uncertainties of diagnostics, testing and contact tracing for COVID-19. Methods. 2021 doi: 10.1016/j.ymeth.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppelaar E., Knibbe Jj, Miedema Hs, Burdorf A. Determinants of implementation of primary preventive interventions on patient handling in healthcare: a systematic review. Occup Environ Med. 2009;66:353–360. doi: 10.1136/oem.2008.042481. [DOI] [PubMed] [Google Scholar]
- 11.Maqbool A., Khan N.Z. Analyzing barriers for implementation of public health and social measures to prevent the transmission of COVID-19 disease using DEMATEL method. Diabetes Metab Syndr. 2020;14:887–892. doi: 10.1016/j.dsx.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calò F., Russo A., Camaioni C., De Pascalis S., Coppola N. Burden, risk assessment, surveillance and management of SARS-CoV-2 infection in health workers: a scoping review. Infect Dis Poverty. 2020;9:139. doi: 10.1186/s40249-020-00756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Abri Z.G.H., Al Zeedi M.A.S.A., Al Lawati A.A. Risk factors associated with COVID-19 infected healthcare workers in Muscat Governorate, Oman. J Prim Care Community Health. 2021;12 doi: 10.1177/2150132721995454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alajmi J., Jeremijenko A.M., Abraham J.C., Alishaq M., Concepcion E.G., Butt A.A., et al. COVID-19 infection among healthcare workers in a national healthcare system: the Qatar experience. Int J Infect Dis. 2020;100:386–389. doi: 10.1016/j.ijid.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemat A., Asady A., Raufi N., Zaki N., Ehsan E., Noor N.A.S., et al. A survey of the healthcare workers in Afghanistan during the COVID-19 pandemic. Am J Trop Med Hyg. 2021;104:537–539. doi: 10.4269/ajtmh.20-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolai L.A., Meyer C.G., Kremsner P.G., Velavan T.P. Asymptomatic SARS coronavirus 2 infection: invisible yet invincible. Int J Infect Dis. 2020;100:112–116. doi: 10.1016/j.ijid.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu X., Ran D., Wang J., Qin Y., Liu R., Shi X., et al. Unclear but present danger: an asymptomatic SARS-CoV-2 carrier. Genes Dis. 2020;7:558–566. doi: 10.1016/j.gendis.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO: Assessment of risk factors for coronavirus disease 2019 (COVID-19) in health workers: protocol for a case-control study. https://www.who.int/publications-detail-redirect/assessment-of-risk-factors-for-coronavirus-disease-2019-(covid-19)-in-health-workers-protocol-for-a-case-control-study, 2020. 2021). (Accessed 11 June 2021).
- 19.Deeks J.J., Higgins J.P. 2010. Statistical algorithms in review manager 5. 11. [Google Scholar]
- 20.Sheskin D.J. 5th. Chapman and Hall/CRC; Boca Raton: 2000. Handbook of Parametric and Nonparametric Statistical Procedures. [Google Scholar]
- 21.Rubin D.B. John Wiley & Sons, Ltd.; 1987. Multiple imputation for nonresponse in surveys; pp. i–xxix. [DOI] [Google Scholar]
- 22.IBM SPSS . IBM Corp.; 2020. Satistics software for windows. [Google Scholar]
- 23.R: A. language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; n.d.
- 24.Bastaki H. 2021. Seroprevalance of COVID-19. [Google Scholar]
- 25.Venugopal U., Jilani N., Rabah S., Shariff M.A., Jawed M., Mendez Batres A., et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021;102:63–69. doi: 10.1016/j.ijid.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alserehi H.A., Alqunaibet A.M., Al-Tawfiq J.A., Alharbi N.K., Alshukairi A.N., Alanazi K.H., et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis. 2021;99 doi: 10.1016/j.diagmicrobio.2020.115273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J.H., Nielsen P.B., Pries-Heje M., et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Çelebi G., Pişkin N., Çelik Bekleviç A., Altunay Y., Salcı Keleş A., Tüz M.A., et al. Specific risk factors for SARS-CoV-2 transmission among health care workers in a university hospital. Am J Infect Control. 2020;48:1225–1230. doi: 10.1016/j.ajic.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubbi I., Pasquinelli G., Brighenti A., Fanelli M., Gualandi P., Nanni E., et al. Healthcare personnel exposure to COVID-19: an observational study on quarantined positive workers. Acta Bio Medica Atenei Parm. 2020;91 doi: 10.23750/abm.v91i12-S.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wee L.E., Sim X.Y.J., Conceicao E.P., Aung M.K., Goh J.Q., Yeo D.W.T., et al. Containment of COVID-19 cases among healthcare workers: the role of surveillance, early detection, and outbreak management. Infect Control Hosp Epidemiol. 2020;41:765–771. doi: 10.1017/ice.2020.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.-G., Ma W., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–83. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girou E., Chai S.H.T., Oppein F., Legrand P., Ducellier D., Cizeau F., et al. Misuse of gloves: the foundation for poor compliance with hand hygiene and potential for microbial transmission? J Hosp Infect. 2004;57:162–169. doi: 10.1016/j.jhin.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Pittet D., Dharan S., Touveneau S., Sauvan V., Perneger Tv. Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med. 1999;159:821–826. doi: 10.1001/archinte.159.8.821. [DOI] [PubMed] [Google Scholar]
- 34.Tenorio Ar, Badri Sm, Sahgal Nb, Hota B., Matushek M., Hayden Mk, et al. Effectiveness of gloves in the prevention of hand carriage of vancomycin-resistant enterococcus species by health care workers after patient care. Clin Infect Dis. 2001;32:826–829. doi: 10.1086/319214. [DOI] [PubMed] [Google Scholar]
- 35.Lindberg M., Skytt B., Lindberg M. Continued wearing of gloves: a risk behaviour in patient care. Infect Prev Pract. 2020;2 doi: 10.1016/j.infpip.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picheansathian W., Chotibang J. Glove utilization in the prevention of cross transmission: a systematic review. JBI Evid Synth. 2015;13:188–230. doi: 10.11124/jbisrir-2015-1817. [DOI] [PubMed] [Google Scholar]
- 37.Ye G., Lin H., Chen S., Wang S., Zeng Z., Wang W., et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J Infect. 2020;81:e1–5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO . World Health Organization; 2020. Glove use information leaflet. [Google Scholar]
- 39.Neuwirth M.M., Mattner F., Otchwemah R. Adherence to personal protective equipment use among healthcare workers caring for confirmed COVID-19 and alleged non-COVID-19 patients. Antimicrob Resist Infect Control. 2020;9:199. doi: 10.1186/s13756-020-00864-w. [DOI] [PMC free article] [PubMed] [Google Scholar]