Abstract
Background
Risk factors associated with coronavirus disease 2019 (COVID-19) severity in patients with multiple sclerosis (MS) have been described. Recent improvements in supportive care measures and increased testing capacity may modify the risk of severe COVID-19 outcome in MS patients. This retrospective study evaluates the severity and outcome of COVID-19 in MS and characterizes temporal trends over the course of the pandemic in the United States.
Methods
We conducted a comparative cohort study using de-identified electronic health record (EHR) claims-based data. MS patients diagnosed with COVID-19 between February 2, 2020 and October 13, 2020 were matched (1:2) to a control group using propensity score analysis. The primary outcome was a composite of intensive care unit (ICU) admission, mechanical ventilation, and/or death.
Results
A total of 2,529 patients (843 MS and 1,686 matched controls) were included. Non-ambulatory and pre-existing comorbidities were independent risk factors for COVID-19 severity. The risk for the severe composite outcome was lower in the late cohorts compared with the early cohorts.
Conclusions
The majority of MS patients actively treated with a disease-modifying therapy (DMT) had mild disease. The observed trend toward a reduction in severity risk in recent months suggests an improvement in COVID-19 outcome.
Keywords: Multiple sclerosis, COVID-19, SARS-CoV-2, Disease-modifying therapy, Outcome
Graphical abstract
1. Introduction
The rapid spread of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a public health emergency of international concern. (Willis and Robertson, 2020, Pérez, 2020) The clinical spectrum of COVID-19 infection ranges from asymptomatic to fatal, (Crescenzo et al., 2020) and different factors have been associated with poor prognosis including age, gender, and pre-existing comorbid conditions such as hypertension, diabetes, and cardiovascular disease. (Ghajarzadeh et al., 2020, Moss et al., 2020, Ciotti et al., 2020, Guevara et al., 2020)
Immunomodulatory and immunosuppressive therapies qualitatively and quantitatively alter various components of the immune system and are associated with an increased susceptibility to viral and bacterial infections. (Bsteh et al., 2020) Although the use of disease-modifying therapy (DMT) for the treatment of multiple sclerosis (MS) during the ongoing pandemic has remained a concern, (Moss et al., 2020, Brownlee, 2020) available evidence supports an acceptable level of safety regarding the use most DMTs. (Louapre et al., 2020) It has been postulated that the use of DMTs might induce regulation of the hyperinflammatory stage associated with the most severe manifestations of COVID-19, (Rostami Mansoor and Ghasemi-Kasman, 2020) but the impact of DMT use on COVID-19 severity remains unclear. (Dalla Costa et al., 2020) The use of anti-CD20 therapies has been associated with increased frequency of severe COVID-19 in some studies, (Sormani et al., 2021, Simpson-Yap et al., 2020) but existing evidence is conflicting. (Louapre et al., 2020, Hughes et al., 2020, Amor et al., 2020)
Epidemiological data in MS suggest a similar risk of severe COVID-19 infection relative to the general population. (Ciotti et al., 2020, Louapre et al., 2020) However, improvements in testing capacity, supportive care, and medical treatments leading to a reduction in the overall case-fatality rate in the general population have led to speculation that other COVID-19 outcomes might have also improved in recent months. (Serling-Boyd et al., 2020) Characterization of temporal trends in COVID-19 outcome in MS can help provide a rationale for an individualized approach to therapy and inform expert recommendations regarding treatment strategies and the potential implications of DMT use in patients with MS.
In this study, we assess COVID-19 characteristics and temporal trends in clinical outcome in a group of systematically identified patients with MS and matched controls during the first 8 months of the ongoing pandemic in the United States.
2. Methods
2.1. Data source and collection
This study was performed using longitudinal, electronic health record (EHR) claims data from the OPTUM® Health Data and Analytics dataset (October 2020 release), a large U.S. population-based insurance health network licensed by The University of Texas Health Science Center at Houston. The database contains de-identified patient information from a geographically diverse population of >160 million individuals spanning all 50 states and contains real-time updates of EHR data including demographics, diagnoses, procedures, medications, laboratory values, and inpatient and outpatient services from commercially insured and managed Medicare beneficiaries with Part D only or both medical and part D coverage. The study was deemed exempt from institutional review board oversight and informed consent was not obtained because it only uses de-identified patient data. All clinical, demographic, and outcome data were extracted using pertinent international classification of disease (ICD)-10 codes, current procedural terminology (CPT) codes, and national drug codes (NDC) using the inverted index technique described by Huang et al. (Huang et al., 2021) Because EDSS assessments are not observable in claims data as no ICD-10 code proxies exist, wheelchair dependence was used as an EDSS surrogate to classify MS-related disability level (EDSS score ≥7) as defined by ICD-10 code Z99.3 and/or CPT codes for wheelchair requirement (97542). (Ghiani et al., 2020, Toliver et al., 2021)
2.2. Study Population
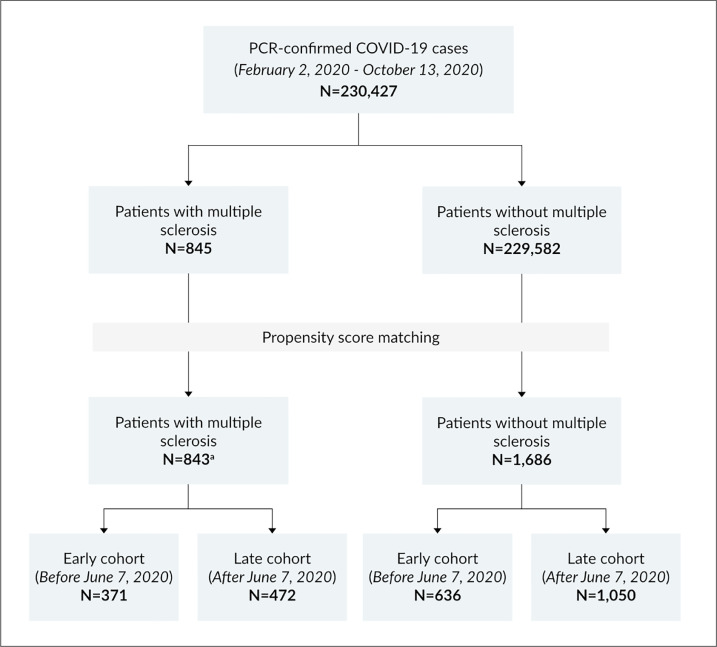
A total of 230,427 patients with a diagnosis of COVID-19 based on ICD-10 codes U07.1, U07.2, U07.3 and confirmed by polymerase chain reaction (PCR) from nasopharyngeal swabs and/or positive serology were identified within the OPTUM® database between February 2, 2020 and October 13, 2020 (Fig. 1 ). Among them, patients with MS were identified by the presence of ≥3 MS-related encounters from any combination of inpatient, outpatient, or DMT use within one year of COVID-19 diagnosis and at least one outpatient MS diagnosis code as defined by ICD-10 code dG35. (Culpepper et al., 2019) DMT use was confirmed by manual review of EHR data. Based on these criteria, 843 patients with MS were identified and matched on a 1:2 basis within the index date by age, gender, and race to a non-MS control group using propensity score analysis (Supplementary Fig. 1). (Zhang et al., 2019) Subjects in each group were subdivided into an early cohort of patients diagnosed with COVID-19 in the first 126 days between February 2, 2020 and June 7, 2020 (first 126 days) months, and a late cohort that included all patients diagnosed in the subsequent 126 days between June 8, 2020 and October 13, 2020). All patients had complete follow-up to death or recovery.
Fig. 1.
Flow diagram of patient selection from the OPTUM® Health Data and Analytics dataset. Abbreviations: COVID-19, coronavirus disease 2019; N, number; PCR, polymerase chain reaction. a Two patients with multiple sclerosis were excluded due to missing gender data.
2.3. Variables and Measurements
We collected patient demographics, pre-existing comorbid conditions, and current DMT information. Reported clinical symptoms relevant to a COVID-19 diagnosis based on their respective ICD-10 codes were systematically extracted for each patient. The primary endpoint was the participant's clinical status recorded within 30 days of a COVID-19 diagnosis. Severity outcome was defined as mild (the patient was not hospitalized), moderate (the patient was hospitalized and did not require supplemental oxygen or intensive care unit admission), or severe (intensive care unit admission, mechanical ventilation, and/or death).
We used the World Health Organization (WHO) definition of disabling chronic neurological disorders (Dua et al., 2006) and included conditions that affect both mental and physical function and fulfilled WHO criteria as causing persistent disability, limited the individual's functioning, and interfered with the person's ability to engage in activities, including dementia, movement disorders, prior stroke with long-term sequelae, neuromuscular disorders, and primary CNS malignancy (Supplementary Table 1). Although MS is considered a chronic neurologic disorder by WHO criteria, it was not included as an additional variable in the analysis. MS patients were divided into two categorical groups defined by the presence or the absence of pre-existing neurologic comorbidities and their outcomes were compared to non-MS controls.
2.4. Statistical Analyses
Descriptive statistics were used to summarize demographic and clinical characteristics. Continuous variables were described in terms of means and standard deviations (SD), and categorical variables were summarized as counts and percentages. Group comparisons were performed using the Mann-Whitney U test for numerical and ordinal variables, and the Fisher's exact test or χ when appropriate for categorical variables.
Summary measures for association between demographic, clinical and outcome variables, and COVID-19 outcome were assessed by univariable logistic regression models. To determine which variables are independently associated with the composite (severe) outcome of ICU admission, mechanical ventilation, and/or death, a multivariable logistic regression model was performed with the following variables as predictors: age, gender, smoking status, comorbid conditions and neurologic comorbidities, all of which have a known association with COVID-19 outcomes. (Bsteh et al., 2020, García-Azorín et al., 2020, Safavi et al., 2020) Results are expressed as odds ratios (OR) with 95% confidence intervals (CIs). Goodness-of-fit was evaluated using the Hosmer-Lemeshow test.
The impact of disease-modifying therapy (DMT) was evaluated separately for each drug, adjusting for age, gender, smoking status, and the presence of comorbid conditions in univariable and multivariable regression models. The “no therapy” group was used as the reference category. Alemtuzumab and cladribine were grouped in the “other” category due to the low number in of patients in each DMT class to draw meaningful conclusions. Among the 511 patients with missing DMT data, we were unable to differentiate between untreated patients and actively treated patients for whom DMT data was unavailable. Therefore, the primary analyses were performed using only complete baseline data with no imputation to avoid due to the large amount of missing DMT data. Patients on no DMT were used as reference group in the primary DMT analysis, but patients with missing DMT data were excluded introducing systematic bias into our results. However, a sensitivity analysis test was run using a model with the same variables in which 5% and 10% of patients without DMT data were randomly assigned to the each of the treatment groups (Supplementary Table 2).
Data analyses were performed in SPSS v.26 (IBM Corp, Armonk, New York, NY). All 2-sided p values <0.05 were considered statistically significant.
3. Results
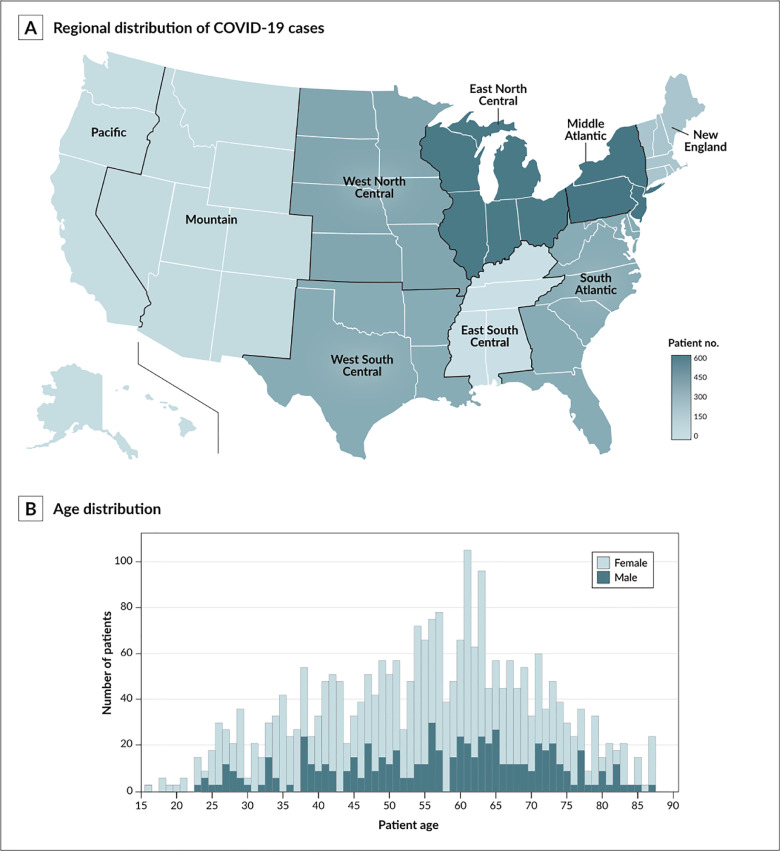
A total of 2,529 patients with a diagnosis of COVID-19 were included in the study (843 MS and 1,686 non-MS matched controls). Table 1 shows a description of both cohorts. The geographic distribution of all subjects is illustrated in Fig. 2 A. The average age in both groups was 55.2 ± 15.4 years (range 16-89 years), with 73.1% female participants (Fig. 2B).
Table 1.
Clinical and demographic characteristics of patients with COVID-19.
| Valuea | |||
|---|---|---|---|
| Characteristic | MS cohort (n=843) | Non-MS cohort (n=1686) | p value |
| Demographics | |||
| Age, mean (SD), y | 55.2 (15.4) | 55.2 (15.4) | 1.0 |
| Female:male ratio (% female) | 616:227 (73.1) | 1,232:454 (73.1) | 1.0 |
| Race/ethnicity, n (%) | |||
| Caucasian | 583 (69.2) | 1166 (69.2) | 1.0 |
| African American | 164 (19.5) | 328 (19.5) | 1.0 |
| Hispanic/Latino | 37 (4.4) | 74 (4.4) | 1.0 |
| Asian | 1 (0.1) | 2 (0.1) | 1.0 |
| Other | 58 (6.9) | 116 (6.9) | 1.0 |
| Smoking status, current, n (%) | 34 (4.0) | 50 (3.0) | 0.16 |
| Comorbid conditions, n (%) | |||
| Hypertension | 431 (51.1) | 813 (48.2) | 0.17 |
| Diabetes mellitus | 176 (20.9) | 354 (21.0) | 0.95 |
| Cardiovascular disease | 336 (39.9) | 631 (37.4) | 0.24 |
| Pulmonary disease | 209 (24.8) | 416 (24.9) | 0.95 |
| Obesity | 99 (11.7) | 178 (10.6) | 0.37 |
| Wheelchair dependence | 67 (7.9) | 23 (1.4) | <0.001 |
| Neurologic comorbidities, n (%) | |||
| Dementia | 76 (9.0) | 53 (3.1) | <0.001 |
| Stroke | 33 (3.9) | 31 (1.8) | 0.002 |
| Movement disorders | 29 (3.4) | 19 (1.1) | <0.001 |
| Neuromuscular disorders | 35 (4.2) | 32 (1.9) | <0.001 |
| Primary CNS malignancy | 1 (0.1) | 0 (0) | 0.16 |
| Presenting symptoms, n (%) | |||
| Fever | 178 (61.1) | 340 (60.2) | 0.58 |
| Cough | 238 (58.2) | 471 (57.9) | 0.88 |
| Sore throat | 54 (6.4) | 120 (7.1) | 0.51 |
| Abdominal pain/diarrhea | 66 (7.8) | 112 (6.6) | 0.27 |
| Anosmia | 19 (2.3) | 34 (2.0) | 0.70 |
| Ageusia | 8 (0.9) | 26 (1.5) | 0.22 |
| Fatigue | 135 (16.0) | 176 (10.4) | <0.001 |
| Dyspnea | 123 (14.6) | 220 (13.0) | 0.28 |
| Headache | 79 (9.4) | 133 (7.9) | 0.21 |
| Corticosteroid treatment, n (%) | 174 (20.6) | 215 (12.8) | <0.001 |
Abbreviations: CNS, central nervous system; COVID-19, coronavirus disease 2019; n, number; SD, standard deviation; y, years.
Data are presented as number (percentage) of subjects, unless otherwise indicated.
Fig. 2.
Demographic characteristics of study patients. The general distribution of by United States division (as defined by the U.S. Census Bureau [1A]) and age at coronavirus disease 2019 (COVID-19) diagnosis (1B) are illustrated.
The most common presenting symptoms were fever and cough, followed by fatigue and shortness of breath (Table 1). Systemic and/or intravenous corticosteroid use was reported in 174 (20.6%) MS patients and 215 (12.8%) controls. At the time of COVID-19 testing, 259 MS patients (29.7%) reported no symptoms, compared to 612 patients (36.3%) in the control group. The overall distribution of mild, moderate, and severe COVID-19 outcome was similar between the two cohorts (Table 2 ). A total of 108 MS patients (12.8%) and 258 (15.3%) non-MS patients had severe COVID-19 presentation as defined by ICU admission, mechanical ventilation, and/or death. Among subjects who required mechanical ventilation, 13/23 (56.5%) MS and 15/28 (53.4%) non-MS controls had a fatal outcome. Reported rates of COVID-19–related complications, including stroke, sepsis, coagulopathy, etc. were comparable between the two cohorts.
Table 2.
Clinical characteristics and complications by COVID-19 severity.
| Mildapresentation | Moderatebpresentation | Severecpresentation | ||||
|---|---|---|---|---|---|---|
| MS (n=582) | Non-MS (n=1078) | MS group (n=142) | Non-MS (n=374) | MS (n=119) | Non-MS (n=234) | |
| Age, median (SD), y | 53.1 (14.8) | 53.4 (14.8) | 55.9 (16.3) | 59.20 (16.1) | 65.6 (12.3) | 66.9 (13.4) |
| F:M ratio | 3.0 | 3.0 | 2.8 | 2.2 | 1.3 | 1.5 |
| Race/ethnicity, N (% by race)d | ||||||
| Caucasian | 412 (70.7) | 696 (59.7) | 97 (16.6) | 306 (26.2) | 74 (12.7) | 164 (14.1) |
| African American | 105 (64.0) | 211 (64.3) | 25 (15.2) | 53 (16.2) | 34 (20.7) | 64 (19.5) |
| Hispanic | 27 (73.0) | 54 (73.0) | 5 (13.5) | 14 (18.9) | 5 (13.5) | 6 (8.1) |
| Asian | 1 (100.0) | 1 (50.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) |
| Complications, N(% of total) | ||||||
| ARDS | 9 (1.5) | 10 (0.9) | 18 (12.2) | 19 (5.1) | 40 (37.0) | 104 (44.4) |
| Renal failure | 12 (2.0) | 25 (2.3) | 34 (23.1) | 35 (9.4) | 49 (45.3) | 139 (59.4) |
| Liver failure | 0 (0.0) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 2 (1.9) | 9 (3.8) |
| Stroke | 1 (0.2) | 6 (0.6) | 2 (1.4) | 3 (0.8) | 4 (3.7) | 6 (2.6) |
| AMI | 1 (0.2) | 1 (0.1) | 1 (0.7) | 4 (1.1) | 1 (0.9) | 8 (3.4) |
| Sepsis | 9 (1.5) | 13 (1.2) | 39 (26.5) | 23 (6.1) | 56 (51.8) | 133 (56.8) |
| Pneumonia | 41 (6.9) | 55 (5.1) | 40 (27.2) | 63 (16.8) | 62 (57.4) | 147 (62.8) |
| Coagulopathy | 2 (0.3) | 2 (0.2) | 4 (2.7) | 5 (1.3) | 36 (33.3) | 79 (33.8) |
| SIRS | 4 (0.7) | 2 (0.2) | 11 (7.5) | 4 (1.1) | 45 (41.7) | 98 (41.9) |
Abbreviations: AMI, acute myocardial infarction; ARDS, acute respiratory distress syndrome; COVID-19, corona virus disease 2019; F, female; M, male; MS, multiple sclerosis; N, number; SD, standard deviation; SIRS, systemic inflammatory response syndrome.
Patients were not hospitalized.
Hospitalization required, but patients were not admitted to the intensive care unit and did not require mechanical ventilation.
Admission to the intensive care unit, mechanical ventilation required, and/or death.
Group totals by race are summarized in Table 1.
Of the 261 (31.0%) MS patients who were hospitalized, the average time from illness onset to discharge was 14.2 ± 4.5 days, and 157 (75.8%) of them were discharged home to self-care/home health, 17 (8.2%) to a skilled nursing facility or inpatient rehab, and 2 (1.0%) to hospice/palliative care (Supplementary Table 3). Of the 608 (33.2%) patients in the non-MS group who were hospitalized, the average time from illness onset to discharge was 16.5 ± 5.9 days, and 207 (73.4%) were discharged home to self-care/home health, 18 (6.4%) to a skilled nurse facility or inpatient rehab, and 1 (0.4%) to hospice/palliative care. All patients placed on hospice care during their admission died in the hospital.
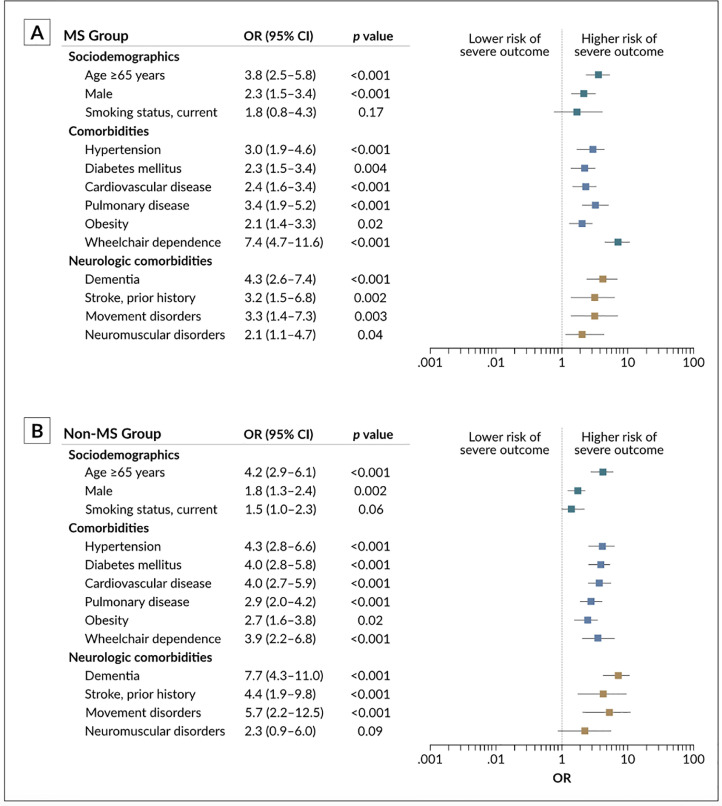
Older age (≥65 years), male gender, non-ambulatory status, and the presence of pre-existing comorbidities were associated with a higher risk for severe COVID-19 outcome (Fig. 3 ). Concerning the presence of neurologic comorbidities, univariable logistic regression models showed an odds ratio (OR) of 3.9 (95% CI, 2.5–6.1) for severe outcome in the MS cohort compared to 4.6 (95% CI, 3.8–7.5) in the control group. In a subgroup analysis, MS patients were divided into two categorical subgroups defined by the presence or the absence of pre-existing neurologic comorbidities (n=146 and n=700, respectively). The odds for a composite of ICU admission, mechanical ventilation, and/or death were higher among MS patients with pre-existing neurologic comorbidities compared to their counterparts with no prior history of these conditions (OR, 4.92 [95% CI, 3.29–7.35]).
Fig. 3.
Risk for severe COVID-19 outcome (defined as intensive care unit admission, mechanical ventilation, and/or death) expressed as the odds ratio (OR) with 95% confidence intervals (CI). Abbreviations: COVID-19, coronavirus disease 2019; MS, multiple sclerosis.
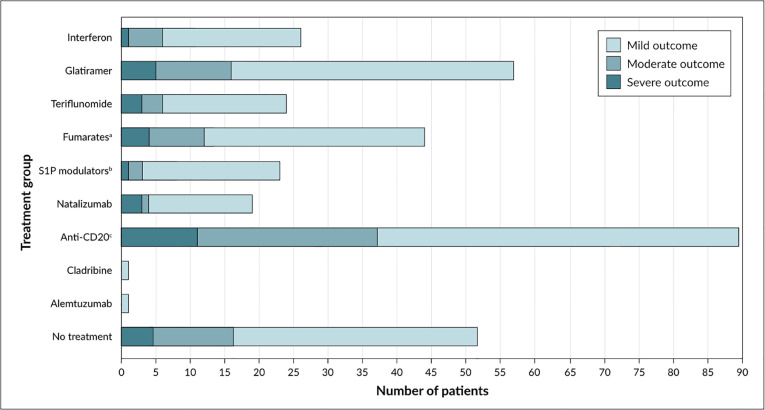
Of the 332 patients for whom disease-modifying therapy (DMT) data was available, 26.8% were treated with anti-CD20 therapies (rituximab, n=17; ocrelizumab, n=72), 15.0% with fumarates (dimethyl fumarate, n=45; diroximel fumarate, n=5), 5.1% with sphingosine-1-phosphate (S1P) modulators (fingolimod, n=14; siponimod, n=2; ozanimod, n=1), 5.4% (n=18) with natalizumab, 16.6% (n=55) with glatiramer acetate, 7.8% (n=26) with beta-interferons, 7.2% (n=24) with teriflunomide, 0.3% (n=1) with alemtuzumab, 0.3% (n=1) with cladribine, and 15.7% were on no treatment (Fig. 4 ). (Table 3 ). In a sensitivity analysis, the results were consistent when 5% and 10% of patients without DMT data were randomly assigned to each of the treatment groups. No patients in the non-MS group were treated with any of these drugs.
Fig. 4.
Distribution of COVID-19 severity cases (mild [no hospitalization], moderate [hospitalized], and severe [intensive care unit admission, mechanical ventilation, and/or death]) by disease-modifying treatment in patients with multiple sclerosis. Abbreviations: COVID-19, coronavirus disease 2019; S1P, sphingosine-1-phosphate. a Dimethyl fumarate, diroximel fumarate, monomethyl fumarate. b Fingolimod, siponimod, ozanimod.
Table 3.
Disease-modifying treatment (DMT) as a risk factor for severe COVID-19 outcome in multiple sclerosis patients.a
| Univariate analysis, (n=332) | Multivariate analysis, (n=332) | |||
|---|---|---|---|---|
| DMT | OR (95% CI) | p value | OR (95% CI) | p value |
| Interferon | 0.39 (0.17–0.81) | 0.02 | 0.72 (0.34–1.40) | 0.26 |
| Glatiramer acetate | 0.36 (0.14–0.79) | 0.01 | 0.81 (0.42–1.83) | 0.42 |
| Teriflunomide | 0.54 (0.29–1.17) | 0.82 | 0.83 (0.39–1.97) | 0.67 |
| Fumaratesb | 0.47 (0.17–0.79) | 0.01 | 1.08 (0.49–2.09) | 0.74 |
| S1P modulatorsc | 0.57 (0.21–0.96) | 0.04 | 1.12 (0.52–2.34) | 0.85 |
| Natalizumab | 0.42 (0.18–0.62) | 0.01 | 1.18 (0.53–2.10) | 0.76 |
| Anti-CD20d | 0.81 (0.63–1.23) | 0.14 | 2.18 (1.53–4.58) | 0.01 |
| Other | 0.41 (0.22–1.07) | 0.07 | 1.24 (0.36–2.22) | 0.80 |
| No therapye | 1 (ref) | 1 (ref) | ||
Abbreviations: CI, confidence interval; COVID-19, corona virus disease 2019; OR, odds ratio.
Intensive care unit admission, mechanical ventilation, and/or death.
Dimethyl fumarate, diroximel fumarate, or monomethyl fumarate.
Fingolimod, siponimod, or ozanimod.
Ocrelizumab or rituximab.
The no therapy group was used as the reference category.
There was no significant difference in hospitalization rates between early and late MS (OR, 1.14 [95% CI, .85–1.91]) and non-MS cohorts (1.01 [95% CI, .83–1.27]). However, the odds of mechanical ventilation, ICU admission, death, and overall composite outcome were lower in the late groups in both cohorts compared to the early groups (Table 4 ).
Table 4.
Thirty-day outcome after COVID-19 diagnosis in early and late in cohorts.
| MS cohorta | Non-MS cohorta | |||||
|---|---|---|---|---|---|---|
| Early cohort (n=371) | Late cohort (n=472) | OR (95% CI) | Early cohort (n=636) | Late cohort (n=1050) | OR (95% CI) | |
| Hospitalization | 143 (38.5) | 197 (41.7) | 1.14 (0.85–1.91) | 209 (32.8) | 351 (33.4) | 1.01 (0.83–1.27) |
| ICU admission | 52 (14.0) | 35 (7.4) | 0.49 (0.31–0.77) | 85 (13.7) | 74 (7.0) | 0.49 (0.35–0.68) |
| Mechanical ventilation | 34 (9.2) | 25 (4.8) | 0.51 (0.29–0.88) | 65 (10.2) | 65 (6.2) | 0.58 (0.41–0.83) |
| Death | 40 (10.8) | 16 (3.4) | 0.30 (0.16–0.53) | 51 (8.0) | 25 (2.4) | 0.28 (0.17–0.45) |
| Composite outcomeb | 62 (16.7) | 46 (9.7) | 0.45 (0.30–0.67) | 181 (15.8) | 93 (8.8) | 0.52 (0.38–0.70) |
Abbreviations: CI, confidence interval; COVID-19, corona virus disease 2019; ICU, intensive care unit; MS, multiple sclerosis; n, number; OR, odds ratio.
Data are presented as number (percentage) of subjects, unless otherwise indicated.
ICU admission, mechanical ventilation, and/or death.
4. Discussion
In this study, we observed a similar constellation of presenting COVID-19 symptoms and comorbid conditions between patients with multiple sclerosis (MS) and matched controls. Hospitalization and mortality rates in both cohorts are consistent with data from smaller MS studies (Louapre et al., 2020, Parrotta et al., 2020) and the general population. (Zhou et al., 2020) Similar risk factors for severe COVID-19 outcome were identified including older age, male gender, and the presence of pre-existing comorbid conditions. (Parrotta et al., 2020, Petrilli et al., 2020)
Neurologic disability as measured by the Expanded Disability Status Scale (EDSS) score has been described as an important risk factor for severe COVID-19 outcome in patients with MS. (Moss et al., 2020, Louapre et al., 2020, Ciampi et al., 2020) EDSS scores ≥6 (a disability milestone representing ambulatory disability) have been associated with the highest variability of COVID-19 severe outcome, followed by age and obesity. (Louapre et al., 2020) It is worth nothing that EDSS assessments are not observable in claims data as no ICD-10 code proxies exist. Nevertheless, wheelchair dependence was an independent predictor of disease severity in this study, with higher odds for severe COVID-19 outcome in disabled MS patients relative to controls. This observation is in line with previous reports (Louapre et al., 2020) and suggests that diagnosis of wheelchair dependence may serve as an EDSS surrogate in real-world acute settings where EDSS evaluations are not routinely performed.
The presence of chronic neurologic conditions was an independent predictor of mortality in hospitalized COVID-19 patients in a recent study. (García-Azorín et al., 2020) To our knowledge, the implications of pre-existing neurologic comorbidities on COVID-19 severity have not been explored in MS populations. Our data suggest an increased risk for a composite of ICU admission, mechanical ventilation, and/or death in patients with dementia, movement disorders, and prior stroke in MS patients relative to those with no known history of these conditions. This observation may suggest that a higher burden of baseline neurologic disease may have a compounding effect on outcome and may carry a higher risk of COVID-19–associated morbidity and mortality in patients with MS.
The majority of MS patients actively treated with a disease-modifying therapy (DMT) had mild disease. Because cladribine and alemtuzumab were less commonly used, we were unable to determine whether each of these treatments is independently associated with COVID-19 outcome. Smaller case series have reported an increased susceptibility to COVID-19 in MS patients who are treated with anti-CD20 therapies without major effects on the severity of COVID-19 outcome. (Safavi et al., 2020, Sahraian et al., 2020, Montero-Escribano et al., 2020) We did not observe an association between DMT group and COVID-19 outcome in univariable comparisons, but anti-CD20 therapy use was associated with a higher risk of severe disease in multivariable regression models. Although that the possibility of sampling or selection bias cannot be excluded given the limited DMT data availability in the MS group and our observations should be interpreted with caution, this observation is in line with recent reports from larger MS cohorts. (Sormani et al., 2021, Simpson-Yap et al., 2020) Though it is possible that a more modest risk might exist between DMT exposure and COVID-19 severity, the majority of MS patients had mild disease regardless of the specific type of DMT used.
Over the span of the first 8 months of the ongoing pandemic, there was a non-significant increase in the hospitalization rate in both study cohorts. However, the odds for ICU admission, mechanical ventilation, death, and overall severe composite outcome were lower in more recent months. This improvement in COVID-19 outcomes is likely multifactorial and may reflect an increase in testing capacity allowing for detection of milder cases, lower volume of seriously ill patients after the initial surge of infections, improved supportive care and management, and/or a change in standard of care measures over time. Nonetheless, about half of all hospitalized MS patients had an acute kidney injury and/or sepsis, and more than a third required intensive care unit admission, mechanical ventilation, or had a fatal outcome, with 3.4% of MS patients dying within 30 days from a diagnosis of COVID-19, indicating the ongoing severe nature and guarded prognosis of this illness.
Our study had several limitations. Our results were estimated in an open cohort population using an administrative claims-based method, which is limited by the possibility of misclassification, underreporting and/or inconsistent ICD coding practices. Because our MS cohort was restricted to the first 843 patients entered into the registry, patient data outside the EHR reporting network were not available for analysis. Available evidence suggests the presence of racial and ethnic differences in COVID-19 outcome in MS populations, but race/ethnicity was not an independent risk factor of severe COVID-19 outcome in this study, which could be related to sampling bias due to the observational nature of our study. In addition, we were unable to stratify COVID-19 outcome across MS phenotypes or to ascertain the accuracy of various documented comorbid neurologic conditions such as stroke or movement disorders, because ICD-10 codes do not allow for this distinction, and the inconsistencies in DMT data availability within the MS cohort further limited our sample size.
Despite these limitations, our study has several strengths. The use of a multicenter EHR database allowed for real-time analysis of population-level trends in a broad geographical area across the United States and across different ages and socioeconomic backgrounds. An additional strength includes the use of a matched cohort design for the execution of the data analysis given the importance of age and gender in the development of autoimmune disorders. The consistent findings across all endpoints in our primary analyses support the validity of our findings.
5. Conclusions
Previously known risk factors such as age, male gender, and the presence of comorbid conditions also apply to patients with multiple sclerosis (MS). In this study, the presence of a pre-existing neurologic comorbidities and non-ambulatory status posed a significantly greater risk for poor outcome in the context of COVID-19 infection. In MS patients, the risk of severe outcome was also higher in individuals treated with anti-CD20 therapy. Nevertheless, the overall safety profile of DMTs is in agreement with current knowledge, and this observation should reinforce current recommendations of continuing current treatment and avoiding delays in DMT initiation in patients with MS with active disease or who are otherwise at risk for relapses or subsequent disability accumulation. Adequate and effective treatment of MS remain the primary consideration in DMT decision-making. The overall risk of ICU admission, mechanical ventilation, and death is lower in more recent months of the ongoing pandemic in the United States, which may reflect improvements in supportive care measures and increased testing capacity over time. These observations should help support expert guidelines regarding treatment strategies and potential implications of DMT use on individuals with MS. Despite the apparent improvement in COVID-19 outcomes, the risk for severe disease in MS patients remains substantial, and the results of future observational studies should be interpreted in the context of improving temporal trends.
Author disclosures
This manuscript has been approved by all authors and represents valid work; neither this manuscript nor one with substantially similar content under my authorship has been published or is being considered for publication elsewhere. We certify that we are the sole authors of this paper and hereby take public responsibility for the entire content of the manuscript.
Funding
No funding was received for the preparation of this manuscript.
Ethical approval
Because this case report does not contain identifying or protected health information, ethical approval was not obtained.
Author contributions
C.A.P, G.Q.Z, X.L., Y.H. contributed to acquisition of data, analysis and interpretation of data, and drafting and revising the manuscript. C.A.P, J.A.L., R.D.S., R.K.G., and J.W.L. contributed to interpretation of data and critical revision of the manuscript for important intellectual content.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.103217.
Appendix. Supplementary materials
References
- Amor S, Baker D, Khoury SJ, et al. SARS-CoV-2 and multiple sclerosis: Not all immune depleting DMTs are equal or bad. Ann. Neurol. 2020;87:794–797. doi: 10.1002/ana.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee WJ. COVID-19 and high-efficacy multiple sclerosis therapies: Time for business as usual? Mult. Scler. J. 2020;26:1267. doi: 10.1177/1352458520948211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsteh G, Bitschnau C, Hegen H, et al. Multiple sclerosis and COVID-19: how many are at risk? Eur. J. Neurol. 2020 doi: 10.1111/ene.14555. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi E, Uribe-San-Martin R, Cárcamo C. COVID-19 pandemic: The experience of a multiple sclerosis centre in Chile. Mult. Scler. Relat. Disord. 2020:42. doi: 10.1016/j.msard.2020.102204. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciotti JR, Grebenciucova E, Moss BP, et al. Multiple sclerosis disease-modifying therapies in the COVID-19 era. Ann. Neurol. 2020;88:1062–1064. doi: 10.1002/ana.25907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crescenzo F, Marastoni D, Bovo C, et al. Frequency and severity of COVID-19 in multiple sclerosis: A short single-site report from northern Italy. Mult. Scler. Relat. Disord. 2020:44. doi: 10.1016/j.msard.2020.102372. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpepper WJ, Marrie RA, Langer-Gould A, et al. Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology. 2019;92:E1016–E1028. doi: 10.1212/WNL.0000000000007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Costa G, Leocani L, Montalban X, et al. Real-time assessment of COVID-19 prevalence among multiple sclerosis patients: a multicenter European study. Neurol. Sci. 2020;41:1647–1650. doi: 10.1007/s10072-020-04519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dua T, Janca A, Kale R, et al. World Health Organization; Geneva: 2006. Public health principles and neurological disorders. [Google Scholar]
- García-Azorín D, Martínez-Pías E, Trigo J, et al. Neurological comorbidity Is a predictor of death in Covid-19 disease: A cohort study on 576 patients. Front. Neurol. 2020;11:1–8. doi: 10.3389/fneur.2020.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajarzadeh M, Mirmosayeb O, Barzegar M, et al. Favorable outcome after COVID-19 infection in a multiple sclerosis patient initiated on ocrelizumab during the pandemic. Mult. Scler. Relat. Disord. 2020;43:10222. doi: 10.1016/j.msard.2020.102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani M, Rouzic EM-L, Brüllinger L, et al. A claims-based proxy for the EDSS score in multiple sclerosis using German claims data (1473) Neurology. 2020;94:1473. [Google Scholar]
- Guevara C, Villa E, Rosas CS, et al. Treating patients with multiple sclerosis during the COVID-19 pandemic: Assessing the expert recommendations. Mult. Scler. Relat. Disord. 2020;43 doi: 10.1016/j.msard.2020.102224. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li X, Zhang G. ELII: A Novel Inverted Index for Fast Temporal Query with Application to a Large Covid-19 EHR Dataset. J. Biomed. Inform. 2021 doi: 10.1016/j.jbi.2021.103744. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R, Whitley L, Fitovski K, et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020;49 doi: 10.1016/j.msard.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77:1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Escribano P, Gómez-Iglesias P, Porta-Etessam J, et al. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series of 60 patients from Madrid, Spain. Mult. Scler. Relat. Disord.. 2020. [DOI] [PMC free article] [PubMed]
- Moss BP, Mahajan K, Bermel R, et al. Multiple sclerosis management during the COVID-19 pandemic. Mult. Scler. J. 2020;26:1163–1171. doi: 10.1177/1352458520948231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez CA. Looking ahead: The risk of neurologic complications due to COVID-19. Neurol Clin. Pract. 2020;10:371–374. doi: 10.1212/CPJ.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrotta E, Kister I, Charvet L, et al. COVID-19 outcomes in MS: Observational study of early experience from NYU multiple sclerosis comprehensive care center. Neurol. - Neuroimmunol. Neuroinflammation. 2020;7:1–9. doi: 10.1212/NXI.0000000000000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. m1966-m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami Mansoor S, Ghasemi-Kasman M. Impact of disease-modifying drugs on the severity of COVID-19 infection in multiple sclerosis patients. J. Med. Virol. 2020:1–6. doi: 10.1002/jmv.26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi F, Nourbakhsh B, Azimi AR. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult. Scler. Relat. Disord. 2020;43 doi: 10.1016/j.msard.2020.102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahraian MA, Azimi A, Navardi S, et al. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102472. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-219279. annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S, De Brouwer E, Kalincik T, et al. First results of the COVID-19 in MS Global Data Sharing Initiative suggest anti-CD20 DMTs are associated with worse COVID-19 outcomes. Present given 8th Jt ACTRIMS-ECTRIMS Meet 2020.
- Sormani MP, De Rossi N, Schiavetti I, et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann. Neurol. 2021:1–10. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toliver JC, Barner JC, Lawson KA, et al. Use of a claims-based algorithm to estimate disease severity in the multiple sclerosis Medicare population. Mult. Scler. Relat. Disord. 2021;49 doi: 10.1016/j.msard.2021.102741. [DOI] [PubMed] [Google Scholar]
- Willis MD, Robertson NP. Multiple sclerosis and the risk of infection: considerations in the threat of the novel coronavirus, COVID-19/SARS-CoV-2. J. Neurol. 2020;267:1567–1569. doi: 10.1007/s00415-020-09822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kim HJ, Lonjon G, et al. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019;7:16. doi: 10.21037/atm.2018.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.