Abstract
Background:
Epidermal growth factor receptor-mutated (EGFR+) non-small-cell lung cancer (NSCLC) patients failing tyrosine kinase inhibitors (TKI) can benefit from next-line targeted therapies, but implementation is challenging.
Methods:
EGFR+ NSCLC patients treated with first/second-generation (1G/2G) TKI at our institution with a last follow-up after osimertinib approval (February 2016), were analyzed retrospectively, and the results compared with published data under osimertinib.
Results:
A total of 207 patients received erlotinib (37%), gefitinib (16%) or afatinib (47%). The median age was 66 years, with a predominance of female (70%), never/light-smokers (69%). T790M testing was performed in 174/202 progressive cases (86%), positive in 93/174 (53%), and followed by osimertinib in 87/93 (94%). Among the 135 deceased patients, 94 (70%) received subsequent systemic treatment (43% chemotherapy, 39% osimertinib), while 30% died without, either before (4%) or after progression, due to rapid clinical deterioration (22%), patient refusal of further therapy (2%), or severe competing illness (2%). Lack of subsequent treatment was significantly (4.5x, p < 0.001) associated with lack of T790M testing, whose most frequent cause (in approximately 50% of cases) was also rapid clinical decline. Among the 127 consecutive patients with failure of 1G/2G TKI started after November 2015, 47 (37%) received osimertinib, with a median overall survival of 36 months versus 24 and 21 months for patients with alternative and no subsequent therapies (p = 0.003).
Conclusion:
Osimertinib after 1G/2G TKI failure prolongs survival, but approximately 15% and 30% of patients forego molecular retesting and subsequent treatment, respectively, mainly due to rapid clinical deterioration. This is an important remediable obstacle to sequential TKI treatment for EGFR+ NSCLC. It pertains also to other actionable resistance mechanisms emerging under 1G/2G inhibitors or osimertinib, whose rate for lack of next-line therapy is similar (approximately 35% in the FLAURA/AURA3 trials), and highlights the need for closer monitoring alongside broader profiling of TKI-treated EGFR+ NSCLC in the future.
Keywords: EGFR+ NSCLC, EGFR T790M mutation, overall survival, rebiopsy, second line, tyrosine kinase inhibitor
Introduction
Epidermal growth factor receptor (EGFR) mutations drive growth in 10–15% of non-small-cell lung cancers (NSCLC) and were instrumental in major therapeutic advances during the last decade.1 EGFR-directed tyrosine kinase inhibitors (TKI) have consistently shown superior efficacy and tolerability over conventional chemotherapy for these tumors, with responses in the majority of cases and a median overall survival (OS) currently exceeding 2.5 years.2–4 After failure of the first/second-generation (1G/2G) compounds erlotinib, gefitinib, afatinib, or dacomitinib, the third-generation drug osimertinib has also demonstrated superiority over alternative options and is the treatment of choice for the approximately 50% of patients with resistance mediated by the EGFR T790M mutation.5 However, experience in daily clinical practice shows that a considerable number of potentially eligible patients are never exposed to osimertinib, for example, because they do not undergo T790M testing at the time of disease progression. In the phase III randomized FLAURA trial, the percentage of patients in the control arm, who received osimertinib as first subsequent therapy after failure of 1G TKI was 31%.2 Given the superior efficacy of next-line osimertinib and other targeted drugs over conventional chemotherapy for eligible patients harboring sensitizing resistance mutations,3,6 accommodation of tandem TKI treatment in patient management is important for longer survival.7 More recently, upfront administration of osimertinib emerged as an alternative strategy for NSCLC patients with EGFR exon19 deletions (del19) or L858R,8 based on better systemic and intracranial efficacy as well as longer OS compared with 1G inhibitors in the FLAURA study.2,9,10 Nonetheless, sequential administration of targeted therapies remains of critical importance for EGFR+ NSCLC regardless of the initial TKI choice, because many patients are eligible for next-line precision drugs also after failure of osimertinib. Here, we systematically analyze the feasibility and clinical impact of molecular retesting, sequential targeted therapies, and any next-line treatment for NSCLC failing EGFR inhibitors, along with critical factors that determine successful implementation in the real-world setting.
Patients and methods
Study population and study endpoints
This retrospective study included all non-consecutive stage IV NSCLC patients with activating EGFR exon 18–21 mutations who received 1G/2G TKI in the Thoraxklinik Heidelberg between 2010 and 2019 and had their last follow up after osimertinib approval as second-line therapy in Europe (1 February 2016). Three main types of analyses were performed: (1) molecular workup at disease progression and implementation of sequential targeted therapies were examined in all evaluable patients; (2) administration of any subsequent treatment was analyzed in the subset of deceased patients, because the entire therapeutic trajectory was available for them; and (3) survival analyses according to subsequent treatment were performed in the consecutive (and therefore unbiased) subset of patients with failure of 1G/2G TKI started after 15 November 2015 (this translates to an earliest possible date of TKI switch after 1 February 2016, since the earliest restaging is performed 8 weeks after treatment start, and rebiopsy with subsequent molecular analysis needed at least 2 weeks). The robustness of results across patient subsets was confirmed by additional sensitivity analyses provided in the Supplemental material. Patients with EGFR exon 20 insertions were excluded, because they respond poorly to currently approved compounds and are managed mainly with other treatments.11 Cases with ongoing responses or switch to osimertinib without, or despite, negative EGFR T790M testing were also excluded from analysis.
Data collection and statistical analysis
Histological diagnosis of NSCLC was performed at the Institute of Pathology Heidelberg on tissue specimens according to the criteria of the current World Health Organization (WHO) Classification (2015) for lung cancer.12 Molecular profiling of tissue and liquid biopsies was performed using DNA-based next-generation sequencing (NGS) with a laboratory turnaround time <10 working days, as described previously.13,14 Clinical data were systematically collected from the patients’ records with a cutoff on 30 June 2020. Since all patients were treated in-house, there were no missing data regarding the study endpoints (molecular retesting, subsequent treatment, survival) or loss-to-follow-up cases. The progression date under 1G/2G TKI was verified by the investigators with review of radiologic images, that is, chest/abdomen computed tomography (CT) and brain magnetic resonance imaging (MRI)-based restaging every 6–12 weeks, without formal RECIST re-evaluation, as several studies have demonstrated very good agreement between real-world and RECIST-based assessments.15,16 OS was calculated from start of treatment for stage IV disease. Follow-up time was calculated by the reverse Kaplan–Meier method.17 Time-to-next-treatment (TNT) was calculated from the start of 1G/2G EGFR TKI until initiation of next-line therapy or death. Survival data were analyzed according to Kaplan–Meier and compared between patient groups with the logrank test. Numerical data were analyzed with the Student’s t-test, categorical data with the chi-square test, and effects of variables on survival were quantified by Cox regression. Confidence intervals (CI) for proportions were computed according to Clopper–Pearson.18 Statistical calculations were performed with SPSS v24 (IBM, Armonk, NY, USA), and plots generated with GraphPad Prism v7 (La Jolla, CA, USA).
Ethics
This study was approved by the ethics committee of Heidelberg University (S-145/2017 and S-469/2017). Since this was a non-interventional, retrospective study, informed consent was obtained whenever possible, but its need for every participant was waived by the ethics committee.
Results
Evaluable study patients
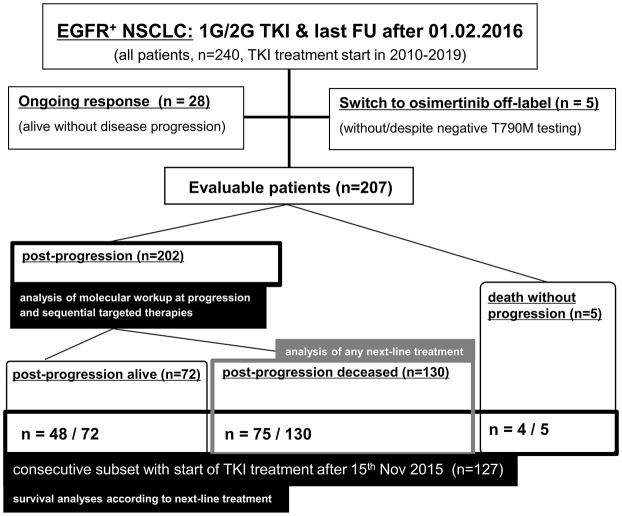
Overall, 240 stage IV NSCLC patients with 1G/2G TKI treatment for activating EGFR exon 18–21 mutations and last follow up after 1 February 2016 were identified (Figure 1). After exclusion of cases with ongoing responses (n = 28) or switch to osimertinib regardless of EGFR T790M testing (n = 5, details given in the Supplemental material), 207 evaluable patients remained for analysis of molecular work-up at disease progression and implementation of sequential targeted therapies (Figure 2). Among these, 135 had died at the time of data cutoff and were used for analyzing the administration of any subsequent therapy (Figure 3). The characteristics of evaluable patients are summarized in Table 1 and were very similar between the entire population and the subset of consecutive patients with failure of 1G/2G TKI started after 15 November 2015 (n = 127), which was used for survival analyses according to subsequent treatment (Figure 4). Most were females (70%), with a median age of 66 years, never/light-smoking history (69% and 66%), lung adenocarcinomas (>95%), and EGFR del19 (approximately 65%). Over 80% had received an EGFR inhibitor already from the first line, most frequently afatinib (approximately 50–60%).
Figure 1.
Flow diagram of the entire study population and subsets used to calculate each endpoint.
1G/2G, first/second-generation; EGFR+, epidermal growth factor receptor-mutated; FU, follow up; NSCLC, non-small-cell lung cancer; TKI, tyrosine kinase inhibitors.
Figure 2.
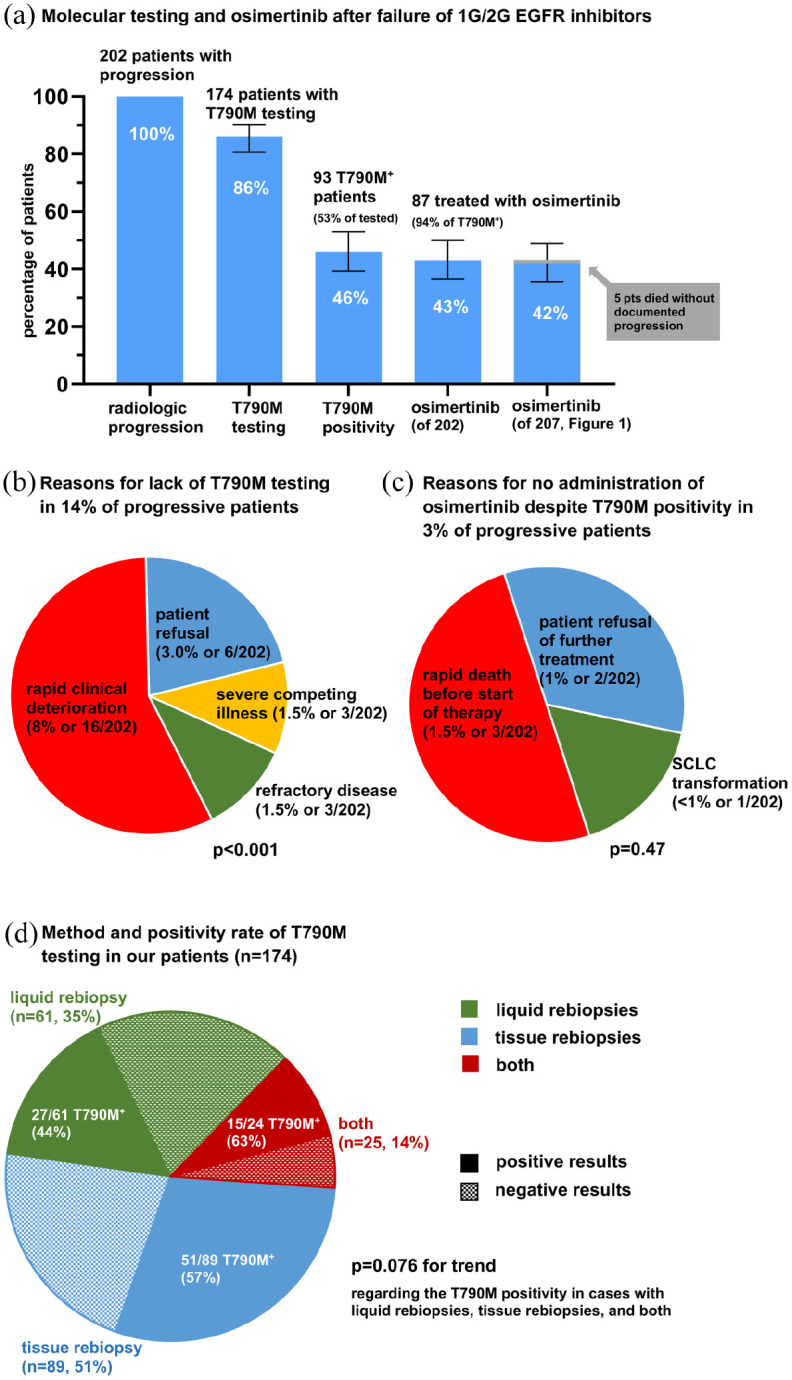
Molecular testing, T790M positivity and next-line osimertinib after failure of 1G/2G-generation EGFR inhibitors in the entire study population. (a) Rate of T790M testing, T790M positivity and next-line osimertinib administration among patients with documented radiologic disease progression under 1G/2G EGFR TKI (n = 202, Figure 1). Error bars indicate 95% CI. (b) Reasons for lack of T790M testing in 14% of progressive patients (chi-square p < 0.001). Patient refusal was due to severe side effects from first-line TKI (5/6), and reluctance to undergo bronchoscopy (1/6). Severe competing illness leading to decision against further anticancer therapy was dementia in two cases, and glioblastoma multiforme in the third. Refractory disease was primary progressive disease at the first restaging after start of 1G/2G EGFR TKI in 2/3 cases, and small-cell transformation at the time of TKI failure in 1/3. (c) Reasons for lack of treatment with osimertinib despite T790M positivity in 3% of progressive patients (chi-square p = 0.47). (d) Utilization and positivity rate of liquid rebiopsies, tissue rebiopsies, and their combination for T790M testing in our patients (chi-square p = 0.076 for trend regarding positivity).
1G/2G, first/second-generation; CI, confidence intervals; EGFR, epidermal growth factor receptor; SCLC, small-cell lung cancer; TKI, tyrosine kinase inhibitors.
Figure 3.
Systemic treatment after failure of 1G/2G EGFR inhibitors in the subset of deceased patients. (a) Rate for implementation of any subsequent treatment, subsequent CHT, and subsequent osimertinib after failure of 1G/2G EGFR inhibitors. Error bars indicate 95% CI. (b) Association between administration of any next-line therapy and performance of T790M testing (chi-square p < 0.001). (c) Association between administration of any next-line therapy and results of T790M testing (chi-square p = 0.041). (d) Reasons for lack of T790M testing in 30% of deceased patients (chi-square p < 0.001). Severe competing illness leading to withdrawal of further treatment was dementia in two cases, and glioblastoma multiforme in the third.
1G/2G, first/second-generation; CHT, chemotherapy; CI, confidence intervals; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitors.
Table 1.
Characteristics of study patients.
| Patients with 1G/2G EGFR TKI treatment and last follow-up after 01.02.2016 | All cases (n = 207) | TKI start after 15 November 2015 (n = 127) | |
|---|---|---|---|
| Age (Median; IQR) | 66; 19 | 66; 21 | |
| Sex (Female %) | 70% | 71% | |
| PS at diagnosisa | ECOG 0 n (%) | 96 (51%) | 55 (48%) |
| ECOG 1 n (%) | 92 (48%) | 61 (52%) | |
| ECOG 2 n (%) | 2 (1%) | 0 | |
| Smoking statusa,b | never/light-smokers, n (%) | 141 (69%) | 84 (66%) |
| Histologyc | adenocarcinoma, n (%) | 203 (98%) | 124 (98%) |
| Metastatic sitesd | any extrathoracic site | 124 (60%) | 86 (68%) |
| brain | 50 (24%) | 40 (31%) | |
| EGFR mutation | del19 | 134 (65%) | 81 (64%) |
| L858R | 54 (26%) | 34 (27%) | |
| other EGFR mutations | 19 (9%) | 12 (9%) | |
| Systemic treatment | gefitinib, n (%) | 33 (16%) | 12 (9%) |
| erlotinib, n (%) | 77 (37%) | 42 (33%) | |
| afatinib, n (%) | 97 (47%) | 72 (57%) | |
| EGFR TKI in 1st line, n (%) | 172 (83%) | 112 (88%) | |
| CHT, n (%) | 97 (47%) | 50 (39%) | |
| Local treatment | palliative radiotherapy, n (%) | 107 (52%) | 61 (48%) |
| palliative surgery, n (%)e | 20 (10%) | 8 (6%) | |
| Follow-up time in months (median;IQR) | 49.9 (31.5–78.1) | 36.1 (27.0–46.6) | |
ECOG PS available for 190/207 (92%) and 117/127 (92%) of cases; smoking status available for 204/207 (99%) and 125/127 (98%) of cases.
“Light” smoking status refers to <10 pack-years.
Other histologies were squamous (3/207, 2/127) and NSCLC-NOS (3/207, 3/127).
At diagnosis of metastatic disease, either primary metastatic in 176/207 (85%) and 102/127 (80%) of cases, or by relapse of previous nonmetastatic NSCLC.
Excluding thoracoscopic surgery for pleural effusion.
1G/2G, first/second-generation; CHT, chemotherapy; del19, EGFR exon 19 deletions; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; n, number; NSCLC-NOS, non-small cell lung cancer-not otherwise specified; TKI, tyrosine kinase inhibitor.
Figure 4.
OS according to next-line treatment in the consecutive subset of EGFR+ NSCLC patients. Within the subset of consecutive patients with 1G/2G TKI start after 15 November 2015 (n = 127, Figure 1), median OS from the start of systemic treatment for stage IV disease was significantly longer for patients that received next-line osimertinib, compared with patients that received alternative or no subsequent therapies: 36.0 (95% CI 26.3–45.7) versus 23.5 (18.9–28.2) versus 20.6 (14.4–26.8) months, respectively, logrank test for trend p = 0.0031.
1G/2G, first/second-generation; CI, confidence interval; EGFR+, epidermal growth factor receptor-mutated; NSCLC, non-small-cell lung cancer; OS, overall survival; TKI, tyrosine kinase inhibitors.
Analysis of EGFR T790M testing, T790M positivity, and next-line osimertinib
Among patients with radiologic disease progression under 1G/2G EGFR TKI (n = 202, Figure 1), the rate of EGFR T790M testing was 86% (174/202), the percentage of EGFR T790M-positive cases 46% (93/174 or 53% of tested patients), and the rate of next-line osimertinib treatment 43% (87/93, or 94% of tested T790M positive patients, Figure 2a). Sensitivity analyses showed very similar results within the subset of deceased patients (Supplemental Figure S1a). The main reason for lack of T790M testing in 14% of progressive patients was rapid clinical deterioration (16/202 cases, p < 0.001), followed by patient refusal (6/202), severe competing illness (3/202), and refractory disease (3/202), namely primary progression under 1G/2G TKI (n = 2) or small-cell transformation (n = 1, Figure 2b). Reasons for T790M positive patients not receiving osimertinib were rapid death before treatment could be initiated (n = 3), refusal of further treatment (n = 2), and concomitant small-cell transformation with switch to chemotherapy (n = 1, Figure 2c). Tissue rebiopsies were used for T790M testing in about half of patients (51% or 89/174), liquid rebiopsies (ctDNA analyses) in 35% (n = 61/174), while 14% (24/174) underwent both (Figure 2d). The T790M positivity rate was 44% for liquid rebiopsies (27/61), 57% for tissue rebiopsies (51/89), and 63% for patients who underwent both (15/24, with 12/24 positive tissue rebiopsies, and 10/24 positive liquid rebiopsies, chi-square test for trend p = 0.076).
Analysis of subsequent treatment
Among deceased patients, subsequent treatment was offered to 70% (94/135) and consisted mainly of chemotherapy (58/94, 43% of 135 deceased patients) and osimertinib (53/94, 39% of 135, Figure 3a), while immunotherapy (5/94) and other TKI (4/94) were used less frequently. The 30% of patients (41/135) that died without any next-line therapy were significantly (4.5×) enriched among cases foregoing T790M testing (15% or 16/106 patients without next-line therapy among T790M-tested versus 69% or 20/29 patients without next-line therapy among non-T790M-tested cases, p < 0.001, Figure 3b). The association between lack of next-line treatment and results of T790M testing was weaker (9% or 53/58 patients without next-line therapy among T790M-positive versus 23% or 11/48 patients without next-line therapy among T790M-negative cases, p = 0.041, Figure 3c). The main reason for failure to enter next-line treatment was rapid clinical deterioration (30/135 cases or 22%, p < 0.001, Figure 3d), while 3/135 (2%) patients refused further treatment, 3/135 (2%) patients had serious competing illness precluding further anticancer therapy, and 5/135 (4%) patients died during the first TKI line without radiologic progression (Figure 1). Sensitivity analyses showed a very similar rate of next-line treatment in the entire study population as that observed in the subset of deceased patients (73% versus 70%, Supplemental Figure S1b).
Survival according to subsequent treatment
Among the consecutive patients failing TKI that had started after 15 November 2015 (n = 127, Figure 1), median OS from the start of systemic treatment for stage IV disease was 25.5 months (95% CI 21.0–30.1 months, 79/127 events). Patients that received next-line osimertinib had significantly longer median OS than patients with alternative (mostly chemotherapy) or no next-line treatment: 36.0 months (26.3–45.7, 26/47 events) versus 23.5 months (18.9–28.2, 23/37 events) versus 20.6 months (14.4–26.8, 30/43 events), respectively, logrank p = 0.0031 (Figure 4). The mere performance of T790M testing [hazard ratio (HR) 0.31, p < 0.001] showed a stronger association with OS than next-line osimertinib (HR = 0.52, p = 0.006) and classical predictors, such as a better initial Eastern Cooperative Oncology Group (ECOG) performance status, lower serum lactate dehydrogenase (LDH), absence of brain metastases at diagnosis of metastatic disease, and del19 instead of other EGFR mutations (HR = 0.51–0.67, Supplemental Table S1). Median time from start of 1G/2G EGFR TKI to start of any subsequent therapy was 15.1 months (95% CI 12.3–17.8 months, Supplemental Figure S2).
Discussion
Even though the superiority of osimertinib after failure of 1G/2G EGFR inhibitors was demonstrated by the AURA3 trial already in 2017,5 practical implementation remains challenging with widely variable success rates in the literature. In contrast to large randomized clinical trials spanning over several countries, the present study analyzes an all-comers patient population under the homogenous, but real-world conditions of a single large academic institution with the aim of defining bottlenecks and priorities close to the circumstances of daily clinical practice.
A first finding is that main obstacles to sequential therapy with osimertinib in EGFR+ NSCLC are lack of T790M testing in approximately 15%, and T790M negativity in approximately 45% of progressive patients, while almost all (94%) T790M positive patients receive the drug (Figure 2a). Of these, the rate of T790M positivity for progressive patients is more-or-less similar across 1G/2G EGFR inhibitors and current analytical methods,19,20 with the 50–55% observed in our study corresponding well to the literature21,22; therefore, the main bottleneck appears to be initiation of T790M testing. Traditionally, the main issue here has been tissue availability, since many advanced lung cancer patients are not suitable or willing to undergo invasive procedures.23 Meanwhile, this problem is largely solved by liquid biopsies, which are not only feasible for every patient, but also provide results earlier than tissue rebiopsies due to the faster sample collection.24 Their marginally lower sensitivity of 75–80% compared with tissue testing is offset by their wider applicability, and their use together with conventional tumor rebiopsies maximizes yield (Figure 2d).25–27 However, the clinical impairment of many patients remains an important limitation: in our study, rapid clinical deterioration was the main reason for both lack of T790M testing (Figure 2b), and lack of any subsequent treatment (Figure 3d), which correlated (Figure 3b), and also prevented some T790M positive cases from receiving osimertinib (Figure 2c). Along the same lines, the mere performance of T790M testing was associated more strongly with OS than exposure to next-line osimertinib, type of EGFR mutation, brain status, and other classical predictors (Supplemental Table S1),28 which is indirect evidence that, in routine clinical practice, the threshold for T790M testing is influenced by the patient’s condition and perceived life expectancy. Of note, this clinical deterioration is typically disease-related, as <10% (Figure 2b) of our patients did not receive next-line therapy due to some other severe competing illness. For non-EGFR+ NSCLC patients treated with palliative chemotherapy, the attrition is even greater, with only 30–50% entering the second line,29–31 which has been a major argument for maintenance therapy and more frequent restaging every 6–8 weeks.32 However, the TKI treatment of EGFR+ patients is continuous anyway, and shortening of imaging intervals for them is limited by the increased radiation and logistic burden due to the longer survival. Alternative methods for improved surveillance of these patients could be serial liquid biopsies and/or performance of tissue rebiopsies earlier, that is, at the first sign of radiologic progression.33,34 In addition, monitoring of electronic patient-reported outcomes (ePROs) under chemotherapy for various solid tumors was associated with significantly longer survival in a pivotal study, and could therefore represent a cost-efficient method to improve care of EGFR+ NSCLC patients, whose quality of life is also known to fluctuate under treatment.35,36 Closer monitoring could facilitate an increase of the T790M testing rate up to a theoretical maximum of approximately 95%, and of the treatment rate for T790M positive cases to >95% (Figure 2). Of note, the 82%–86% rate of T790M testing in our study (Figure 2 and Supplemental Figure S1a) was very similar to that of a prospective study in Japanese cancer centers (81%),25 while our approximately 30% rate of patients without next-line treatment (Figure 3) was very similar to that observed in the standard arm of the FLAURA trial (32%, Supplemental Table S2)2 and other certified German lung cancer centers (30%).37 The markedly lower rates of T790M testing and any next-line treatment, for example 19%–30% and 31%–38%, respectively, reported in some registry studies,38,39 presumably suggest an additional need to improve awareness and access regarding novel diagnostic and therapeutic options in the community outside specialized centers (Figure 5).
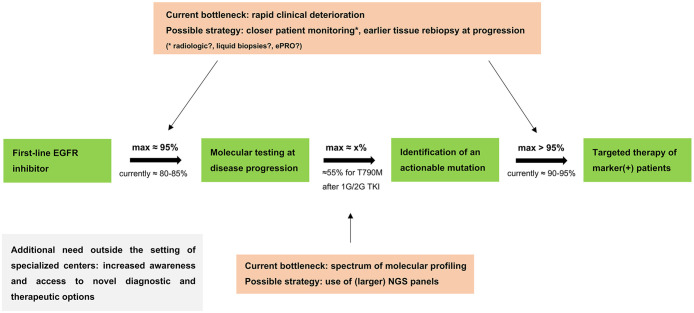
Figure 5.
Implementation of sequential targeted therapies for metastatic EGFR+ NSCLC. Critical parameters, possible improvement strategies and feasibility limit for implementation of sequential targeted therapies in metastatic EGFR+ NSCLC. The maximum testing rate of 95% is taken from Supplemental Table S2 and excludes only patients with EGFR TKI discontinuation due to reasons precluding further treatment (i.e. “other reasons” or patient decision); the actual testing rate of 80–85% is taken from Figure 2a, Supplemental Figure S1a and the literature cited in the Discussion; the actual treatment rate for T790M positive patients with osimertinib of 90–95% is taken from Figure 2a and Supplemental Figure S1A; the maximum treatment rate for T790M positive patients of >95% additionally considers that 3/6 T790M positive patients foregoing osimertinib treatment suffered early death before the drug could be started (Figure 2c), which could potentially have been prevented by an earlier change in therapeutic strategy facilitated by closer patient monitoring; the x% rate of marker-positivity is mutation-specific, for example, approximately 55% for EGFR T790M under 1G/2G EGFR TKI (Figure 2a).21,22 For any next-line targeted therapy, the theoretical upper limit of implementation is the product of these three parameters (0.95*0.95*x), for example, approximately 50% for osimertinib after 1G/2G EGFR TKI. The rate of molecular testing affects the feasibility of implementation for all next-line targeted therapies. This framework appears to be very similar for 1G/2G EGFR inhibitors and osimertinib, as shown in Supplemental Table S2 and explained in the Discussion.
1G/2G TKI, first/second-generation tyrosine kinase inhibitors; EGFR, epidermal growth factor receptor; EGFR+, epidermal growth factor receptor-mutated; ePRO, electronic patient-reported outcomes; NGS, next-generation sequencing; NSCLC, non-small-cell lung cancer.
The longer OS of T790M positive patients treated with next-line osimertinib in our real-world cohort (Figure 4) underlines the importance of sequential TKI administration for clinical outcome, as already demonstrated by the randomized AURA3 trial,5 the international observational GioTag study,40 and other smaller series.41,42 However, besides EGFR T790M, up to 15–20% of EGFR+ patients treated with 1G/2G TKI will develop other actionable genetic alterations, for which novel, more active than chemotherapy drugs are available through clinical trials, early access programs, or off-label (e.g., MET or HER2 amplifications, PIK3CA, or BRAF mutations).43,44 Our ability to capture and tackle these resistance mechanisms will depend on the very same principles demonstrated here for EGFR T790M, but require broader molecular profiling instead of T790M-only assays (Figure 5).13 Furthermore, the same principles remain relevant with osimertinib as well,8 because feasibility of detection for the diverse resistance alterations acquired in 20–30% of progressive patients (e.g., MET amplifications, EGFR C797S, etc.), and utilization of suitable targeted therapies (e.g., next-line treatment with MET inhibitors),45–47 follow the same rules. The rates for TKI discontinuation precluding further treatment (i.e., for reasons other than tumor progression and side effects, approximately 5–10%), and lack of subsequent anticancer therapy (approximately 35%) appear to be similar under osimertinib in the FLAURA and AURA3 studies, as those observed under 1G/2G EGFR inhibitors (Supplemental Table S2).2,5,9,48 For example, among FLAURA patients treated with upfront osimertinib, 2.8% had to discontinue treatment for reasons other than disease progression, adverse events, and patient decision, which is similar to the 3.8% rate observed in the current study (Supplemental Table S2).2 Furthermore, the rate for patient refusal of further therapy without disease progression or treatment-limiting toxicity can be reasonably assumed to be uniform, approximately 2–3% according to Supplemental Table S2, despite an outlier in the experimental FLAURA arm, as no clinically relevant difference was observed in PROs between osimertinib and standard TKI in this study.49 Therefore, the described findings constitute a general framework for the potential benefit of EGFR+ NSCLC patients from novel drugs after failure of any EGFR inhibitor, with a maximum theoretical patient testing rate of approximately 95% (Figure 5). According to our results and the literature, the three main bottlenecks for tandem targeted therapies in EGFR+ NSCLC currently are rapid clinical deterioration, the spectrum of molecular profiling, and awareness or access regarding novel options outside specialized centers (Figure 5). While the indications, techniques, and extent of molecular profiling at baseline or progression are elaborated in current recommendations from several societies,50,51 the need for improved monitoring of patients under treatment does not receive a similar attention yet. Our results suggest that this will be crucial for pre-empting consequences of rapid clinical deterioration, in order to maximize testing rates and utilization of all novel drugs (Figure 5). Particularly promising in this respect are recent data from the FLAURA trial showing that longitudinal ctDNA assays can detect treatment failure several weeks earlier than radiologic imaging.52
The main limitations of our study are the retrospective, single-institution design, and small patient number. Specific strengths are the homogenous, standardized testing and treatment of our patients within the same academic institution, the dissection of the entire study population into those subsets that are most suitable for estimation of each endpoint (Figure 1), and the sensitivity analyses that demonstrated robustness of findings (Supplemental Figure S1). Whenever possible, we have compared our estimated parameters with the findings of other investigators or published data from clinical trials, and found good agreement, which suggests generalizability. The results presented provide a comprehensive picture of clinical implementation for sequential targeted therapies in EGFR+ NSCLC, define obstacles, prioritize potential measures to overcome them, and highlight the importance for patient survival in the real-world setting.
Supplemental Material
Supplemental material, sj-pdf-1-tam-10.1177_1758835921996509 for Real-world implementation of sequential targeted therapies for EGFR-mutated lung cancer by Nikolaus Magios, Farastuk Bozorgmehr, Anna-Lena Volckmar, Daniel Kazdal, Martina Kirchner, Felix J. Herth, Claus-Peter Heussel, Florian Eichhorn, Michael Meister, Thomas Muley, Rami A. Elshafie, Jürgen R. Fischer, Martin Faehling, Mark Kriegsmann, Peter Schirmacher, Helge Bischoff, Albrecht Stenzinger, Michael Thomas and Petros Christopoulos in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank Petra Kettenring of the Clinical Trial Unit in the Thoraxklinik Heidelberg for assistance with the collection of patient data and samples.
Footnotes
Conflict of interest statement: FB reports personal fees from Novartis, MSD, Chugai Pharma, Roche, and AstraZeneca and research grants from AstraZeneca, BMS, and Roche.
ALV reports personal fees from AstraZeneca.
DK reports personal fees from AstraZeneca, personal fees from Bristol-Myers Squibb GmbH, personal fees from Pfizer Pharma GmbH, outside the submitted work.
FJH reports advisory board fees and honoraria from Lilly, Roche, AstraZeneca, Novartis, Boehringer, Chiesi, Teva, Pulmonx BTG, and Olympus as well as research funding from Lilly, Roche, AstraZeneca, Novartis, Boehringer, Chiesi, and Teva.
CPH reports consultation, lecture and other fees from Novartis, Basilea, Bayer, Grifols, Boehringer, Pierre Fabre, Covidien, Siemens, Chiesi, Intermune, MEDA Pharma, Bracco, Pfizer, MSD, Roche, Lilly, AstraZeneca, Schering-Plough, Essex, Gilead, MeVis, Fresenius, and Astellas as well as ownership of GSK stocks
TM reports research funding from Roche and patents with Roche.
JRF reports advisory board honoraria from Boehringer, Roche, Celgene, and AstraZeneca.
PS reports advisory board honoraria from Pfizer, Roche, Novartis, and AstraZeneca as well as speaker’s honoraria and research funding from Roche, AstraZeneca, and Novartis.
AS reports advisory board honoraria and/or speaker fees: Astra Zeneca, Bayer, Eli Lilly, Roche, BMS, Illumina, MSD, Novartis, Pfizer, Seattle Genetics, Takeda, and Thermo Fisher, and research grants from BMS, Bayer, and Chugai.
MT reports advisory board honoraria from Novartis, Lilly, BMS, MSD, Roche, Celgene, Takeda, AbbVie, Boehringer, speaker’s honoraria from Lilly, MSD, Takeda, research funding from AstraZeneca, BMS, Celgene, Novartis, Roche and travel grants from BMS, MSD, Novartis, Boehringer.
PC reports lecture/advisory board fees from AstraZeneca, Boehringer, Chugai, Novartis, Pfizer, Roche and Takeda, as well as research funding from AstraZeneca, Novartis, Roche, and Takeda.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by AstraZeneca and the German Center for Lung Research (DZL).
Data availability: Data supporting the results presented in this study are available upon reasonable request.
ORCID iD: Petros Christopoulos  https://orcid.org/0000-0002-7966-8980
https://orcid.org/0000-0002-7966-8980
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nikolaus Magios, Department of Thoracic Oncology, Thoraxklinik at Heidelberg University Hospital, Heidelberg; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Farastuk Bozorgmehr, Department of Thoracic Oncology, Thoraxklinik at Heidelberg University Hospital, Heidelberg; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Anna-Lena Volckmar, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Daniel Kazdal, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Martina Kirchner, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Felix J. Herth, Department of Pneumology, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Claus-Peter Heussel, Department of Diagnostic and Interventional Radiology with Nuclear Medicine, Thoraxklinik at Heidelberg University Hospital, Heidelberg; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Florian Eichhorn, Department of Thoracic Surgery, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Michael Meister, Translational Research Unit, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Thomas Muley, Translational Research Unit, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Rami A. Elshafie, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany
Jürgen R. Fischer, Department of Thoracic Oncology, Lungenklinik Löwenstein, Löwenstein, Germany
Martin Faehling, Department of Cardiology, Angiology and Pneumology, Klinikum Esslingen, Esslingen, Germany.
Mark Kriegsmann, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Peter Schirmacher, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Helge Bischoff, Department of Thoracic Oncology, Thoraxklinik at Heidelberg University Hospital, Heidelberg; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Albrecht Stenzinger, Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Michael Thomas, Department of Thoracic Oncology, Thoraxklinik at Heidelberg University Hospital, Heidelberg; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
Petros Christopoulos, Department of Thoracic Oncology, Thoraxklinik at Heidelberg University Hospital, Röntgenstraße 1, Heidelberg, Baden-Württemberg 69126, Germany; Translational Lung Research Center Heidelberg (TLRC-H), Heidelberg, Germany Member of the German Center for Lung Research (DZL).
References
- 1.Okamoto I, Mitsudomi T, Nakagawa K, et al. The emerging role of epidermal growth factor receptor (EGFR) inhibitors in first-line treatment for patients with advanced non-small cell lung cancer positive for EGFR mutations. Ther Adv Med Oncol 2010; 2: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382: 41–50. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 2018; 36: 2244–2250. [DOI] [PubMed] [Google Scholar]
- 4.Park K, Wan-Teck Lim D, Okamoto I.et al. First-line afatinib for the treatment of EGFR mutation-positive non-small-cell lung cancer in the ‘real-world’ clinical setting. Ther Adv Med Oncol 2019; 11: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017; 376: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan M, Huang L-L, Chen J-H.et al. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther 2019; 4: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsh V.Turning EGFR mutation-positive non-small-cell lung cancer into a chronic disease: optimal sequential therapy with EGFR tyrosine kinase inhibitors. Ther Adv Med Oncol 2018; 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Recondo G, Facchinetti F, Olaussen KA, et al. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol 2018; 15: 694–708. [DOI] [PubMed] [Google Scholar]
- 9.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378: 113–125. [DOI] [PubMed] [Google Scholar]
- 10.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non–small-cell lung cancer. J Clin Oncol 2018; 36: 3290–3297. [DOI] [PubMed] [Google Scholar]
- 11.Vyse S, Huang PH.Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther 2019; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Nicholson AG.et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–1260. [DOI] [PubMed] [Google Scholar]
- 13.Volckmar AL, Leichsenring J, Kirchner M, et al. Combined targeted DNA and RNA sequencing of advanced NSCLC in routine molecular diagnostics: analysis of the first 3,000 Heidelberg cases. Int J Cancer 2019; 145: 649–661. [DOI] [PubMed] [Google Scholar]
- 14.Fassunke J, Ihle MA, Lenze D.et al. EGFR T790M mutation testing of non-small cell lung cancer tissue and blood samples artificially spiked with circulating cell-free tumor DNA: results of a round robin trial. Virchows Archiv 2017; 471: 509–520. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Nussbaum NC, Magee K, et al. Comparison of real-world response rate (rwRR) to RECIST-based response rate in patients with advanced non-small cell lung cancer (aNSCLC). Ann Oncol 2019; 30(Suppl. 5): V651. [Google Scholar]
- 16.Bartlett CH, Mardekian J, Cotter M.et al. Concordance of real world progression free survival (PFS) on endocrine therapy as first line treatment for metastatic breast cancer using electronic health record with proper quality control versus conventional PFS from a phase 3 trial. Cancer Res 2018; 78(Suppl. 4): Abstract P3-17-03. [Google Scholar]
- 17.Schemper M, Smith TL.A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996; 17: 343–346. [DOI] [PubMed] [Google Scholar]
- 18.Clopper CJ, Pearson ES.The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26: 404–413. [Google Scholar]
- 19.Yoon BW, Kim JH, Lee SH.et al. Comparison of T790M acquisition between patients treated with afatinib and gefitinib as first-line therapy: retrospective propensity score matching analysis. Transl Oncol 2019; 12: 852–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Zhou C.Comparison of cross-platform technologies for EGFR T790M testing in patients with non-small cell lung cancer. Oncotarget 2017; 8: 100801–100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma C, Wei S, Song Y.T790M and acquired resistance of EGFR TKI: a literature review of clinical reports. J Thorac Dis 2011; 3: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart EL, Tan SZ, Liu G.et al. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations—a review. Transl Lung Cancer Res 2014; 4: 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim L, Tsao MS.Tumour tissue sampling for lung cancer management in the era of personalised therapy: what is good enough for molecular testing? Eur Respir J 2014; 44: 1011–1022. [DOI] [PubMed] [Google Scholar]
- 24.Sabari JK, Offin M, Stephens D.et al. A prospective study of circulating tumor DNA to guide matched targeted therapy in lung cancers. J Natl Cancer Inst 2019; 111: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seto T, Nogami N, Yamamoto N, et al. Real-world EGFR T790M testing in advanced non-small-cell lung cancer: a prospective observational study in Japan. Oncol Ther 2018; 6: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi K, Naoki K, Manabe T, et al. Comparison of detection methods of EGFR T790M mutations using plasma, serum, and tumor tissue in EGFR-TKI-resistant non-small cell lung cancer. Onco Targets Ther 2018; 11: 3335–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passiglia F, Rizzo S, Di Maio M, et al. The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: a systematic review and meta-analysis. Sci Rep 2018; 8: 13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christopoulos P, Kirchner M, Roeper J.et al. Risk stratification of EGFR+ lung cancer diagnosed with panel-based next-generation sequencing. Lung Cancer 2020; 148: 105–112. [DOI] [PubMed] [Google Scholar]
- 29.Sacher AG, Le LW, Lau A, et al. Real-world chemotherapy treatment patterns in metastatic non-small cell lung cancer: are patients undertreated? Cancer 2015; 121: 2562–2569. [DOI] [PubMed] [Google Scholar]
- 30.Zietemann V, Duell T.Every-day clinical practice in patients with advanced non-small-cell lung cancer. Lung Cancer 2010; 68: 273–277. [DOI] [PubMed] [Google Scholar]
- 31.Zietemann V, Duell T.Prevalence and effectiveness of first-, second-, and third-line systemic therapy in a cohort of unselected patients with advanced non-small cell lung cancer. Lung Cancer 2011; 73: 70–77. [DOI] [PubMed] [Google Scholar]
- 32.Socinski MA.Re-evaluating duration of therapy in advanced non-small-cell lung cancer: is it really duration or is it more about timing and exposure? J Clin Oncol 2009; 27: 3268–3270. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins S, Yang JC, Ramalingam SS, et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol 2017; 12: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 34.Franovic A, Raymond VM, Erlander MG.et al. Urine test for EGFR analysis in patients with non-small cell lung cancer. J Thorac Dis 2017; 9: S1323–S1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017; 318: 197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S-C, Lin C-C, Lai W-W.et al. Dynamic changes in quality of life after three first-line therapies for EGFR mutation-positive advanced non-small-cell lung cancer. Ther Adv Med Oncol 2018; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roeper J, Falk M, Tiemann M.et al. Risk of not receiving 2nd line therapy is high in EGFR mt+ pts: real world data of certified lung cancer centers on treatment sequence in EGFR mt+ pts. J Clin Oncol 2018; 36: e21220. [Google Scholar]
- 38.Nadler E, Pavilack M, Espirito JL, et al. Observational study of treatment patterns in patients with epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer after first-line EGFR-tyrosine kinase inhibitors. Adv Ther 2020; 37: 946–954. [DOI] [PubMed] [Google Scholar]
- 39.Chiang A, Fernandes A, Pavilack M.et al. MA15.11 real world biomarker testing and treatment patterns in patients with advanced NSCLC receiving EGFR-TKIs. J Thorac Oncol 2018; 13: S410–S411. [Google Scholar]
- 40.Hochmair MJ, Morabito A, Hao D, et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol 2018; 14: 2861–2874. [DOI] [PubMed] [Google Scholar]
- 41.Hochmair MJ, Buder A, Schwab S.et al. Liquid-biopsy-based identification of EGFR T790M mutation-mediated resistance to afatinib treatment in patients with advanced EGFR mutation-positive NSCLC, and subsequent response to osimertinib. Target Oncol 2019; 14: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roeper J, Lueders A, Netchaeva M, et al. Impact on OS and PFS of 2nd and 3rd generation TKI in EGFR mt+ and ALK+ pts: results of the NOWEL network. Ann Oncol 2017; 28(Suppl. 5): V486. [Google Scholar]
- 43.Tomasello C, Baldessari C, Napolitano M.et al. Resistance to EGFR inhibitors in non-small cell lung cancer: clinical management and future perspectives. Crit Rev Oncol Hematol 2018; 123: 149–161. [DOI] [PubMed] [Google Scholar]
- 44.Liao B-C, Griesing S and Yang JC-H. Second-line treatment of EGFR T790M-negative non-small cell lung cancer patients. Ther Adv Med Oncol 2019; 11: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol 2018; 29: viii740. [Google Scholar]
- 46.Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 2019; 121: 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmid S, Li JJN, Leighl NB. Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer 2020; 147: 123–129. [DOI] [PubMed] [Google Scholar]
- 48.Papadimitrakopoulou VA, Mok TS, Han J-Y.et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol 2020; 31: 1536–1544. [DOI] [PubMed] [Google Scholar]
- 49.Leighl NB, Karaseva N, Nakagawa K, et al. Patient-reported outcomes from FLAURA: osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer 2020; 125: 49–57. [DOI] [PubMed] [Google Scholar]
- 50.Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol 2020; 31: 1491–1505. [DOI] [PubMed] [Google Scholar]
- 51.Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement of the college of American pathologists/international association for the study of lung cancer/association for molecular pathology clinical practice guideline update. J Clin Oncol 2018; 36: 911–919. [DOI] [PubMed] [Google Scholar]
- 52.Reungwetwattana T, Gray J, Markovets A, et al. Longitudinal circulating tumour DNA (ctDNA) monitoring for early detection of disease progression and resistance in advanced NSCLC in FLAURA. Ann Oncol 2019; 30: ix199. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tam-10.1177_1758835921996509 for Real-world implementation of sequential targeted therapies for EGFR-mutated lung cancer by Nikolaus Magios, Farastuk Bozorgmehr, Anna-Lena Volckmar, Daniel Kazdal, Martina Kirchner, Felix J. Herth, Claus-Peter Heussel, Florian Eichhorn, Michael Meister, Thomas Muley, Rami A. Elshafie, Jürgen R. Fischer, Martin Faehling, Mark Kriegsmann, Peter Schirmacher, Helge Bischoff, Albrecht Stenzinger, Michael Thomas and Petros Christopoulos in Therapeutic Advances in Medical Oncology