Abstract
Gastric antral vascular ectasia (GAVE) is one of the uncommon causes of upper gastrointestinal bleeding. Major treatment of GAVE includes pharmacotherapy, endoscopy, and surgery. The efficacy and safety of pharmacotherapy have not been sufficiently confirmed; and surgery is just considered when conservative treatment is ineffective. By comparison, endoscopy is a common treatment option for GAVE. This paper reviews the currently used endoscopic approaches for GAVE, mainly including argon plasma coagulation (APC), radiofrequency ablation (RFA), and endoscopic band ligation (EBL). It also summarizes their efficacy and procedure-related adverse events. The endoscopic success rate of APC is 40–100%; however, APC needs several treatment sessions, with a high recurrence rate of 10–78.9%. The endoscopic success rates of RFA and EBL are 90–100% and 77.8–100%, respectively; and their recurrence rates are 21.4–33.3% and 8.3–48.1%, respectively. Hyperplastic gastric polyps and sepsis are major adverse events of APC and RFA; and Mallory–Weiss syndrome is occasionally observed after APC. Adverse events of EBL are rare and mild, such as nausea, vomiting, esophageal or abdominal pain, and hyperplastic polyps. APC is often considered as the first-line choice of endoscopic treatment for GAVE. RFA and EBL have been increasingly used as alternatives in patients with refractory GAVE. A high recurrence of GAVE after endoscopic treatment should be fully recognized and cautiously managed by follow-up endoscopy. In future, a head-to-head comparison of different endoscopic approaches for GAVE is warranted.
Keywords: argon plasma coagulation, endoscopic band ligation, gastric antral vascular ectasia, radiofrequency ablation
Introduction
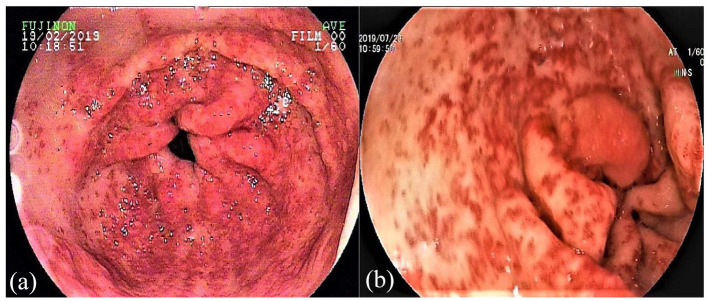
Gastric antral vascular ectasia (GAVE), which was first described by Rider et al.1 in 1953, refers to dilated blood vessels located in the antrum and radiated to the pylorus. It was also named ‘watermelon stomach’ by Jabbari et al.2 in 1984, as its typical appearance is similar to the stripes seen on watermelons. The most common lesion site of GAVE is the antrum, but it is also observed in other parts of the gastrointestinal tract, including the cardia, duodenum, and jejunum.3 According to the endoscopic characteristics, GAVE can be divided into two types: striped and diffuse (Figure 1). Histology shows hyaline thrombosis, fibrin hyalinization, and spindle hyperplasia within dilated submucosal capillaries of the gastric antrum.4 The pathophysiology of GAVE may be attributed to an increased prostaglandin E2 level,5 a cross-linking of protein antibody in gastric mucosa,6 an injury of gastric mucosa caused by dyskinesia,7 or the effect of vasoactive substances, such as gastrin or serotonin.8
Figure 1.
Endoscopic images of striped (a) and diffuse (b) GAVE.
GAVE, gastric antral vascular ectasia.
GAVE is often associated with chronic liver disease and autoimmune and connective tissue diseases, such as liver cirrhosis, hepatocellular carcinoma, hypothyroidism, sclerosis, and systemic lupus erythematosus. Most of the patients are elderly, while the ratio of female to male is reported to be 2:1.9
GAVE accounts for about 4% of non-variceal upper gastrointestinal bleeding.10 It can be clinically asymptomatic or manifested as occult and dominant upper gastrointestinal bleeding with hematemesis or melena. Many patients present with refractory anemia in spite of blood transfusion and intravenous or oral iron supplementation.
There are several treatment options for GAVE. The efficacy of pharmacologic therapy, such as octreotide,11 tranexamic acid,12 bevacizumab,13 estrogen-progesterone,14 and thalidomide,15 has not yet been sufficiently confirmed. Endoscopy has gradually become the first-line treatment option for GAVE in recent years, including cryotherapy, Nd-YAG laser, argon plasma coagulation (APC), radiofrequency ablation (RFA), and endoscopic band ligation (EBL). Surgery can be considered if conservative treatment fails. Antrectomy can effectively control chronic bleeding,16 but most patients, especially those with comorbid diseases, have a high risk of procedural complications.
To the best of our knowledge, there is lack of consensus statement or guideline recommendation regarding the management of GAVE. This review aims to summarize common endoscopic procedures for the treatment of GAVE and their efficacy and complications. Literature regarding APC, RFA, and EBL for the treatment of GAVE were searched via the PubMed and EMBASE databases from the earliest available publications until 5 June 2021. Search items were [(gastric antral vascular ectasia) OR (watermelon stomach)] AND [(argon plasma coagulation) OR (APC)] OR [(radiofrequency ablation) OR (RFA)] OR [(endoscopic band ligation) OR (EBL)]. Initially, a total of 520 papers were identified. Data on APC, RFA, or EBL alone were collected. The major outcomes of interests included endoscopic success rate, blood transfusion requirement, recurrence rate, and adverse events after endoscopic treatment for GAVE.
Argon plasma coagulation
APC is the most commonly used endoscopic approach for GAVE in recent years. It is a non-contact electrocoagulation device that uses argon plasma to transduce electrical energy to local tissues. The depth of the treated lesions is approximately 1–3 mm, which is sufficient to coagulate the superficial vessels.17 The electric power output of 40–60 W and the argon flow rate of 0.8–2 l/min are effective to eliminate the lesions18 (Supplemental Table 1).
Endoscopic success of APC
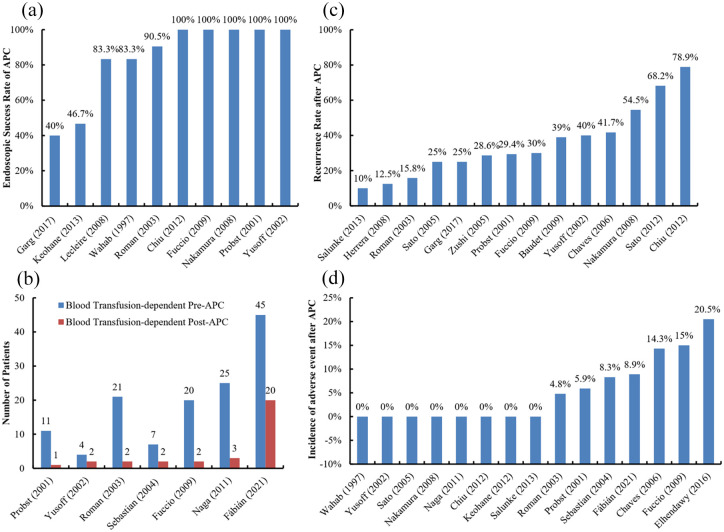
Endoscopic success, which is often defined as complete hemostasis or elimination of the majority of visible lesions under endoscopy, can reach 40–100% by APC19–28 [Figure 2(a)]. In a study, 21 patients were included, of whom 19 (90.5%) achieved endoscopic success after multiple treatment sessions, but 2 had persistent bleeding and required blood transfusion. It should be noted that 19 patients who achieved endoscopic success presented with iron deficiency anemia alone, but 2 patients who failed were more severe and manifested as acute gastrointestinal bleeding.21 In another study, the endoscopic success rate was 83.3% (25/30). Only 1 patient received anticoagulant drugs before GAVE was successfully treated by APC, but no patient received antiplatelet drugs. The reasons for endoscopic failure might be that all of the 5 patients had striped lesions, and 3 of them took antiplatelet drugs.20 By comparison, Garg et al.27 reported a low success rate of 40% for endoscopic resolution of GAVE. Interestingly, all endoscopic success was just achieved in cirrhotic patients. None of non-cirrhotic patients had endoscopic resolution of GAVE. This may be because diffuse GAVE is more common in liver cirrhosis.16,20 It should be acknowledged that APC is more effective for diffuse GAVE, which is mainly characterized as small lesions with local hemorrhage, but achieves incomplete coagulation for striped GAVE, which is often characterized as large lesions.29
Figure 2.
Outcomes of GAVE patients undergoing APC.
Panel a, endoscopic success rate; Panel b, change in blood transfusion requirement; Panel c, recurrence rate; Panel d, incidence of adverse events.
APC, argon plasma coagulation; GAVE, gastric antral vascular ectasia.
Transfusion after APC
The transfusion-dependent rate after APC is 9.1–50%21–23,25,30–32 [Figure 2(b)]. The causes of transfusion after APC are often varied. In a study by Yussof et al.,23 of the 4 patients who were dependent on blood transfusion before APC, 2 required blood transfusion 8 and 12 months after the last APC due to the recurrence of endoscopically confirmed GAVE. In another study, 2 patients still required continuous blood transfusion after APC, mainly due to bleeding from varices in the esophagus and rectum.21 In the study by Sebastian et al.,30 two patients required blood transfusion after APC, but did not develop GAVE recurrence. One was due to bleeding from non-steroidal anti- inflammatory drug (NSAID)-related ulcers; another did not have any definite cause of bleeding. As a result, except for GAVE recurrence, other causes for transfusion after APC should be considered. It should be noted that follow-up endoscopy is necessary to clarify the reasons for transfusion requirement.
Recurrence after APC
Dilated capillaries can recur a few months after APC for GAVE. The incidence of recurrence is 10–78.9%21–27,33–38 [Figure 2(c)]. The annual recurrence rate of GAVE is 34–50.3%.24,25 Such a wide difference may be related to the heterogeneity in the definition of recurrence, duration of follow-up, and characteristics of included patients. Herrera et al.36 reported a low recurrence rate of 12.5%, but the recurrence was evaluated according to the patient’s clinical manifestations without endoscopy follow-up. Similarly, Roman et al.21 reported a recurrence rate of 15.8% by using the same definition of recurrence without endoscopic follow-up. In addition, it should be noted that 57.9% (11/19) of the patients died without information regarding recurrence, which contributed to a low recurrence rate. By comparison, Nakamura et al.24 followed 22 patients by blood tests every month and endoscopy every 3 months, and found that the 1-, 2-, and 3-year cumulative recurrence-free rates after APC were 49.7%, 35.5%, and 35.5%, respectively. It should be noted that in the aforementioned study, recurrence was strictly defined as more than 50% of lesions recurred or active bleeding was observed under endoscopy. On the other hand, the studies, in which a majority of patients had chronic liver disease, found a very high recurrence rate of 66.6–78.9%.26,38 Whether chronic liver disease can affect GAVE recurrence after APC is still uncertain. In a study of GAVE patients with liver cirrhosis, some variables, including gender, age, the etiology of cirrhosis, CTP score, medication (blockers, anticoagulant, or antiplatelet drugs), INR, prothrombin rate, and platelet count, were analyzed. However, none of them can significantly predict GAVE recurrence after APC.25
Adverse events of APC
The incidence of adverse events after APC is 0–20.5%19,21–26,28,30,31,33,35,39–41 [Figure 2(d)]. Procedure-related adverse events are often dependent on the operator’s skills, power output and argon flow rate, distance between equipment and mucosa, and residence time at the same lesion site.
Minor adverse events mainly include epigastric pain,40 abdominal distention,40 and mild ulcer bleeding.30,37 They can be alleviated by oral proton pump inhibitors in combination with over-the-counter analgesics in a few days. Hyperplastic gastric polyps25,35,37 and fever40 after APC have also been reported. Major adverse events are uncommon, including Mallory–Weiss syndrome,32 scarring of ulcer,22,32 and sepsis.21,35 Probst et al.22 reported that one patient developed deep antrum ulcers followed by circumferential scarring of the antrum and asymptomatic stenosis 6 months after APC, and planned to undergo Billroth I surgery. Roman et al.21 found that one patient developed sepsis after APC and then died of infectious peritonitis 4 months after APC.
Hybrid-APC
Hybrid-APC is a modified procedure that creates a ‘safety cushion’ by injecting saline into the submucosa and achieves deeper lesions. In a prospective study, hybrid-APC was used in 9 patients, of whom 8 received 1 session and 1 received 2 sessions. After 6 months, the hemoglobin level reached a normal level in all of the 9 patients without any serious complication.42 As a result, hybrid-APC may be a safer choice for the treatment of GAVE as compared with traditional APC.
Combination of polidocanol and APC
Polidocanol injection can be used for the management of small-bowel angioectasia,43 chronic venous disorder,44 and gastrointestinal ulcer bleeding45 with excellent results. A combination of polidocanol and APC can treat mucosa and submucosa lesions to radically treat GAVE.46 In a recent retrospective study, 15 GAVE patients were treated with a combination of polidocanol and APC. Only polidocanol was used as an initial choice of treatment; and repeated APC was employed for the remaining lesions 3–4 days later. All patients achieved a successful hemostasis. However, two (13%) developed treatment-related adverse events, of whom one developed ulceration and another hematoma; and both received conservative treatment. In addition, two (13%) recurred at 36 months and 48 months after hemostasis.46
Radiofrequency ablation
Since the first report by Ganz et al.47 in 1999, RFA has been widely used for superficial gastrointestinal diseases, such as angiodysplasia, chronic radiation proctitis, and precancerous lesions.48 In recent years, RFA has become an alternative technique to APC for the treatment of GAVE (Supplemental Table 2).
The RFA device includes an energy generator and an ablation catheter.49 The catheters are used to achieve appropriate tissue contact; and they include through-the-scope catheter, HALO60, and HALO90, whose ablation area is 1.2 cm2, 1.5 cm2, and 2.6 cm2, respectively. The HALO90 ULTRA catheter can even ablate a larger area of 5.2 cm2 each time and achieve rapid hemostasis.50
Gross et al.51 first reported that 6 patients with GAVE were treated with RFA under the HALO90 system in 2008, of whom 4 had failed APC treatment. The mean hemoglobin level increased from 8.6 g/dl to 10.2 g/dl during a mean follow-up period of 2 months after RFA. Of these patients, 5 (83.3%) did not require blood transfusion. All patients tolerated the RFA procedure without complications. However, it should be acknowledged that the sample size is small and follow-up duration is short for the first report. Since then, more studies have explored the efficacy of RFA, especially in patients with refractory GAVE who present with iron deficiency anemia requiring transfusion or gastrointestinal bleeding in spite of several other treatments.
Endoscopic success of RFA
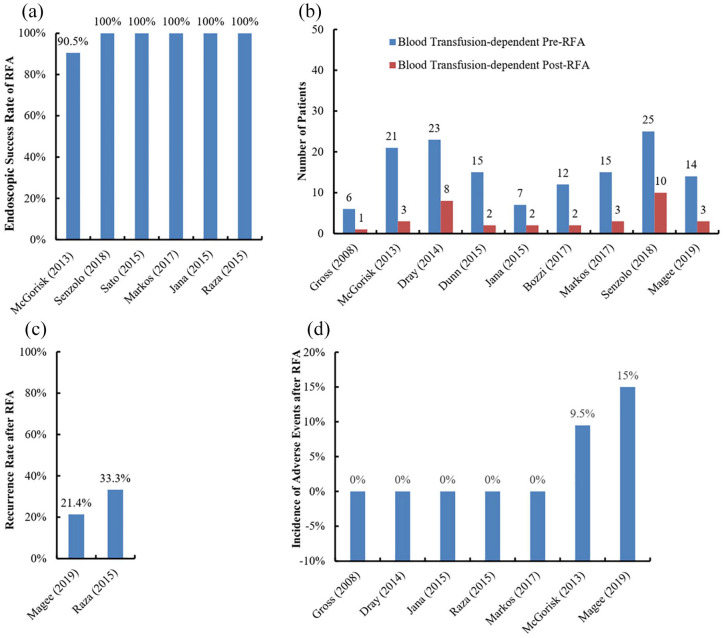
The endoscopic success rate of RFA is up to 90%29,49,52–54 [Figure 3(a)]. However, there is a probability of persistent gastrointestinal bleeding after complete ablation by the HALO90 ablation system.49,52
Figure 3.
Outcomes of GAVE patients undergoing RFA.
Panel a, endoscopic success rate; Panel b, change in blood transfusion requirement; Panel c, recurrence rate; Panel d, incidence of adverse events.
GAVE, gastric antral vascular ectasia; RFA, radiofrequency ablation.
Transfusion after RFA
Transfusion requirement after RFA remained in 13.3–40% of GAVE patients29,49,51,52,54–58 [Figure 3(b)]. In a large case series by Dray et al.,56 23 GAVE patients required transfusion before RFA; and 65.2% (15/23) of them were weaned off transfusion within 6 months after RFA.
It seems that neither initial endoscopic findings nor previous treatments significantly affect transfusion requirement after RFA.56 The major causes of transfusion requirement after RFA are persistent hemorrhage49,52 and recurrent bleeding during follow-up.55 Other sources of bleeding, such as jejunal arteriovenous malformation,49 necessitate transfusion requirement.
Recurrence after RFA
The recurrence rate after RFA is 21.4–33.3%53,55 [Figure 3(c)]. In a retrospective study, 9 patients with refractory GAVE were included. After the eradication of the lesions, they were followed clinically with serial measurement of their hemoglobin concentration. Three patients (33.3%) recurred and needed repeated treatment. One had the first recurrence 3 months after the first treatment and the second recurrence 3 months after the repeated ablation, while the other 2 had recurrence 11 months after the last treatment. All of the 3 patients had chronic kidney diseases; and 1 of them was also complicated with liver cirrhosis.53 In another study by Magee et al.,55 14 patients were followed for 12 months after RFA, of whom 3 (21.4%) had recurrences that manifested as recurrent anemia and required blood transfusion or iron supplement. It should be noted that in the two studies mentioned herein, repeated endoscopy after complete ablation was performed only in the case of increased blood transfusion requirement. As a result, more accurate information regarding recurrence of striped or diffuse GAVE lesions after complete ablation needs to be further confirmed by regular follow-up endoscopy.
Adverse events of RFA
The incidence of adverse events after RFA is 0–15%29,49,51–53,56,59 [Figure 3(d)]. Like other endoscopic thermal therapies (ETTs), such as heat probe electrocoagulation and APC, adverse events associated with RFA are primarily attributed to mucosal injury, including hyperplastic polyps, superficial ulcers, hemorrhagic ulcers, and even sepsis. The formation of fragile sessile and pedunculated polyps may be related to both high gastrin status and repeated thermal injury.60 Superficial and hemorrhagic ulcers may be conservatively controlled without any intervention.52 Sepsis may be treated by antibiotics.61 Gastroesophageal junction tear related to RFA with HALO90 has been also reported.62
RFA versus APC
RFA has some technical advantages, including a contact therapy modality which can counteract severe antral contractions,51 a wider ablation area which can avoid incomplete treatment, and a reproducible depth of treatment with preset power output and energy density that can achieve a uniform ablation and eliminate the influence of operator.49,63 However, whether RFA is superior to APC for the treatment of GAVE is still controversial. Some studies have found that RFA has higher endoscopic and clinical success rates,64,65 requires less procedural times66 and treatment sessions,65 reduces costs, shortens the length of hospital stay,54,59 prolongs the length of retreatment,66 and improves hemoglobin levels more effectively.64,66 In contrast, another study considered that APC might be more dominant in increasing hemoglobin level and reducing transfusion requirement.64
In a large cohort study, 24 underwent APC alone and 28 underwent RFA alone. There was no significant difference in the improvement of hemoglobin level, number of treatment sessions, procedural times, and retreatment intervals between the two groups.63 It should be noted there was a potential bias of patient selection due to the retrospective nature of this study; it was more likely that patients who underwent RFA were more severe. Unfortunately, until now, there is no randomized controlled trial comparing RFA versus APC.
Endoscopic band ligation
EBL is the standard treatment for esophageal varices located in the submucosa.67 As a result, it is theoretically feasible and effective for GAVE located at the gastric mucosa and submucosa68 (Supplemental Table 3). EBL is usually initiated from the pylorus until most of the lesions located at the antrum are treated.69 The number of bands applied for the treatment of GAVE is dependent upon the areas of the lesions and the choices of the endoscopists. After EBL, submucosal capillary thrombosis develops, further leading to various degrees of ischemic necrosis followed by superficial ulcer and finally submucosal fibrosis.70
Endoscopic success of EBL
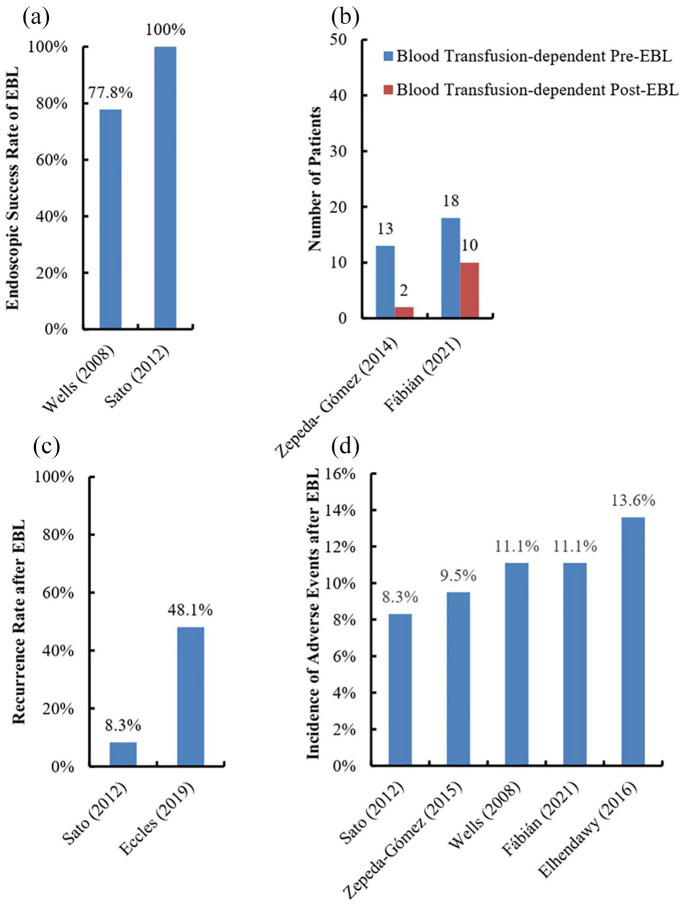
The endoscopic success rate of EBL is 77.8–100%38,68 [Figure 4(a)]. In a study, a total of 9 patients were included, of whom 7 and 2 manifested as overt and occult bleeding, respectively. Of these, bleeding events were successfully controlled in 7 patients after an average of 1.9 EBL sessions, while hemostasis failed in the remaining 2 patients.68
Figure 4.
Outcomes of GAVE patients undergoing EBL.
Panel a, endoscopic success rate; Panel b, change in blood transfusion requirement; Panel c, recurrence rate; Panel d, incidence of adverse events.
EBL, endoscopic band ligation; GAVE, gastric antral vascular ectasia.
Transfusion after EBL
Transfusion requirement after EBL remained in 15.4%–55.6% of GAVE patients32,69 [Figure 4(b)]. In a retrospective study, the indication of blood transfusion was defined as the amount of blood lost reached beyond 30% of the total blood volume in patients with acute hemorrhage, or if the hemoglobin concentration was less than 7 g/dl in patients with chronic hemorrhage. During a mean follow-up duration of 18 months, 55.6% (10/18) of patients still required blood transfusion after an average of 2.2 treatment sessions.32 In another study, 2 of 14 patients included did not wean off blood transfusion, probably because both of them had chronic renal failure with diffuse GAVE.69
Recurrence after EBL
The recurrence rate after EBL is 8.3–48.1%38,71 [Figure 4(c)]. In one study, where 12 patients were treated with EBL, endoscopy revealed that only 1 (8.3%) patient developed the recurrence of GAVE during a mean follow-up duration of 14.8 months.38 In contrast, in another retrospective study where 27 patients were treated with EBL, 13 (48.1%) recurred during a mean follow-up duration of 18.2 months.71 A higher transfusion requirement before EBL was the only predictor of recurrence.71
Adverse events of EBL
The incidence of adverse events after EBL is 8.3–13.6%32,38,40,68,69 [Figure 4(d)]. Common adverse events after EBL include nausea and vomiting68 and mild esophagus or epigastric pain.69 Most of them do not require any intervention and disappear within a few days. Other adverse event, such as bleeding from ulcers, can be solved by conservative treatments.38 Hyperplastic polyps have also been occasionally reported.32
EBL versus ETT
EBL should be more advantageous on occlusion of mucosal and submucosal vessels as compared with ETT. Some studies have demonstrated that EBL was significantly superior to ETT in hemostasis,68 eradication of visible lesions on endoscopy,28 treatment sessions,32,68,72 improvement of hemoglobin,72 transfusion requirement,68,72 and recurrence.38 In contrast, other studies found no difference between the two groups in terms of treatment sessions,28,38 blood transfusion requirement,32 improvement of hemoglobin,28,32,68 and adverse events.72 In a randomized controlled trial, 88 patients with GAVE were assigned to either the APC or EBL group. They found that the EBL group needed less treatment sessions and a lower amount of blood transfusion than the APC group; however, there was no significant difference in hemoglobin improvement or mild adverse events between the two groups.40
Conclusion
At the moment, APC is often the first-line choice of endoscopic treatment for GAVE, but it requires more treatment sessions due to its higher risk of recurrence. RFA and EBL have been considered as alternative approaches. Notably, some comparative studies suggest that RFA and EBL may be superior to APC in terms of hemostasis, treatment sessions, and recurrence. However, the optimal endoscopic treatment option for GAVE has not yet been sufficiently confirmed by high-quality randomized controlled trials. In addition, underlying diseases, other sources of bleeding, indications of blood transfusion, and definitions of treatment success vary widely among cohorts, which are also major limitations of the currently published studies.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223211039696 for Endoscopic treatment for gastric antral vascular ectasia by Mengyuan Peng, Xiaozhong Guo, Fangfang Yi, Xiaodong Shao, Le Wang, Yanyan Wu, Chunmei Wang, Menghua Zhu, Ou Bian, Mostafa Ibrahim, Saurabh Chawla and Xingshun Qi in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223211039696 for Endoscopic treatment for gastric antral vascular ectasia by Mengyuan Peng, Xiaozhong Guo, Fangfang Yi, Xiaodong Shao, Le Wang, Yanyan Wu, Chunmei Wang, Menghua Zhu, Ou Bian, Mostafa Ibrahim, Saurabh Chawla and Xingshun Qi in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-3-taj-10.1177_20406223211039696 for Endoscopic treatment for gastric antral vascular ectasia by Mengyuan Peng, Xiaozhong Guo, Fangfang Yi, Xiaodong Shao, Le Wang, Yanyan Wu, Chunmei Wang, Menghua Zhu, Ou Bian, Mostafa Ibrahim, Saurabh Chawla and Xingshun Qi in Therapeutic Advances in Chronic Disease
Footnotes
Authors’ contributions: Conceptualization: Xingshun Qi;
Investigation: Mengyuan Peng, Xiaozhong Guo, Fangfang Yi, Xiaodong Shao, Le Wang, Chunmei Wang, Yanyan Wu, Menghua Zhu, Ou Bian, Mostafa Ibrahim, Saurabh Chawla, and Xingshun Qi;
Writing–original draft: Mengyuan Peng and Xingshun Qi;
Writing–review and editing: Mengyuan Peng, Xiaozhong Guo, Fangfang Yi, Xiaodong Shao, Le Wang, Chunmei Wang, Yanyan Wu, Menghua Zhu, Ou Bian, Mostafa Ibrahim, Saurabh Chawla, and Xingshun Qi;
Supervision: Xiaozhong Guo and Xingshun Qi.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Xingshun Qi  https://orcid.org/0000-0002-9448-6739
https://orcid.org/0000-0002-9448-6739
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mengyuan Peng, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China; Postgraduate College, Jinzhou Medical University, Jinzhou, P.R. China.
Xiaozhong Guo, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China.
Fangfang Yi, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China.
Xiaodong Shao, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China.
Le Wang, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China.
Yanyan Wu, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China.
Chunmei Wang, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China.
Menghua Zhu, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China.
Ou Bian, Department of No.1 Cadre Ward, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), Shenyang, P.R. China.
Mostafa Ibrahim, Department of Gastroenterology and Hepatology, Theodor Bilharz Research Institute, Cairo, Egypt.
Saurabh Chawla, Department of Medicine, Division of Digestive Diseases, Emory University School of Medicine, Atlanta, Georgia, USA.
Xingshun Qi, Department of Gastroenterology, General Hospital of Northern Theater Command (formerly General Hospital of Shenyang Military Area), No. 83 Wenhua Road, Shenyang, Liaoning Province 110840, China.
References
- 1.Rider JA, Klotz AP, Kirsner JB.Gastritis with veno-capillary ectasia as a source of massive gastric hemorrhage. Gastroenterology 1953; 24: 118–123. [PubMed] [Google Scholar]
- 2.Jabbari M, Cherry R, Lough JO, et al. Gastric antral vascular ectasia: the watermelon stomach. Gastroenterology 1984; 87: 1165–1170. [PubMed] [Google Scholar]
- 3.Calès P, Voigt JJ, Payen JL, et al. Diffuse vascular ectasia of the antrum, duodenum, and jejunum in a patient with nodular regenerative hyperplasia. Lack of response to portosystemic shunt or gastrectomy. Gut 1993; 34: 558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suit PF, Petras RE, Bauer TW, et al. Gastric antral vascular ectasia. A histologic and morphometric study of “the watermelon stomach”. Am J Surg Pathol 1987; 11: 750–757. [PubMed] [Google Scholar]
- 5.Saperas E, Perez Ayuso RM, Poca E, et al. Increased gastric PGE2 biosynthesis in cirrhotic patients with gastric vascular ectasia. Am J Gastroenterol 1990; 85: 138–144. [PubMed] [Google Scholar]
- 6.Ceribelli A, Cavazzana I, Airò P, et al. Anti-RNA polymerase III antibodies as a risk marker for early Gastric Antral Vascular Ectasia (GAVE) in systemic sclerosis. J Rheumatol 2010; 37: 1544. [DOI] [PubMed] [Google Scholar]
- 7.Sallam H, McNearney TA, Chen JD.Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma). Aliment Pharmacol Ther 2006; 23: 691–712. [DOI] [PubMed] [Google Scholar]
- 8.Lowes JR, Rode J.Neuroendocrine cell proliferations in gastric antral vascular ectasia. Gastroenterology 1989; 97: 207–212. [DOI] [PubMed] [Google Scholar]
- 9.Hsu WH, Wang YK, Hsieh MS, et al. Insights into the management of gastric antral vascular ectasia (watermelon stomach). Therap Adv Gastroenterol 2018; 11: 1756283x17747471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulai GS, Jensen DM, Kovacs TO, et al. Endoscopic treatment outcomes in watermelon stomach patients with and without portal hypertension. Endoscopy 2004; 36: 68–72. [DOI] [PubMed] [Google Scholar]
- 11.Michopoulos S, Zampeli E, Giannopoulos C, et al. Effective pharmacological management of gastrointestinal vascular lesions with long acting octreotide: a case series. Gastroenterology 2011; 140: S388. [Google Scholar]
- 12.McCormick PA, Ooi H, Crosbie O.Tranexamic acid for severe bleeding gastric antral vascular ectasia in cirrhosis. Gut 1998; 42: 750–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Bawardy B, Kamath PS, Simonetto D, et al. Intravenous bevacizumab for the treatment of refractory bleeding from small bowel angioectasia and Gastric Antral Vascular Ectasia (GAVE): initial experience of a tertiary center. Gastroenterology 2018; 154: S-662–S-663. [Google Scholar]
- 14.Tran A, Villeneuve JP, Bilodeau M, et al. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol 1999; 94: 2909–2911. [DOI] [PubMed] [Google Scholar]
- 15.Bayudan AM, Chen CH.Thalidomide is an effective treatment for refractory gastrointestinal bleeding from vascular malformations. Gastroenterology 2019; 156: S-748. [Google Scholar]
- 16.Ito M, Uchida Y, Kamano S, et al. Clinical comparisons between two subsets of gastric antral vascular ectasia. Gastrointest Endosc 2001; 53: 764–770. [DOI] [PubMed] [Google Scholar]
- 17.Zuckerman GR, Cornette GL, Clouse RE, et al. Upper gastrointestinal bleeding in patients with chronic renal failure. Ann Intern Med 1985; 102: 588–592. [DOI] [PubMed] [Google Scholar]
- 18.Morris ML, Tucker RD, Baron TH, et al. Electrosurgery in gastrointestinal endoscopy: principles to practice. Am J Gastroenterol 2009; 104: 1563–1574. [DOI] [PubMed] [Google Scholar]
- 19.Wahab PJ, Mulder CJ, den Hartog G, et al. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy 1997; 29: 176–181. [DOI] [PubMed] [Google Scholar]
- 20.Lecleire S, Ben-Soussan E, Antonietti M, et al. Bleeding gastric vascular ectasia treated by argon plasma coagulation: a comparison between patients with and without cirrhosis. Gastrointest Endosc 2008; 67: 219–225. [DOI] [PubMed] [Google Scholar]
- 21.Roman S, Saurin JC, Dumortier J, et al. Tolerance and efficacy of argon plasma coagulation for controlling bleeding in patients with typical and atypical manifestations of watermelon stomach. Endoscopy 2003; 35: 1024–1028. [DOI] [PubMed] [Google Scholar]
- 22.Probst A, Scheubel R, Wienbeck M.Treatment of watermelon stomach (GAVE syndrome) by means of endoscopic Argon Plasma Coagulation (APC): long-term outcome. Z Gastroenterol 2001; 39: 447–452. [DOI] [PubMed] [Google Scholar]
- 23.Yusoff I, Brennan F, Ormonde D, et al. Argon plasma coagulation for treatment of watermelon stomach. Endoscopy 2002; 34: 407–410. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Mitsunaga A, Konishi H, et al. Long-term follow-up of gastric antral vascular ectasia treated by argon plasma coagulation. Gastrointest Endosc 2008; 50: 1495–1502. [Google Scholar]
- 25.Fuccio L, Zagari RM, Serrani M, et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia-related bleeding in patients with liver cirrhosis. Digestion 2009; 79: 143–150. [DOI] [PubMed] [Google Scholar]
- 26.Chiu YC, Lu LS, Wu KL, et al. Comparison of argon plasma coagulation in management of upper gastrointestinal angiodysplasia and gastric antral vascular ectasia hemorrhage. BMC Gastroenterol 2012; 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg S, Aslam B, Nickl N.Endoscopic resolution and recurrence of gastric antral vascular ectasia after serial treatment with argon plasma coagulation. World J Gastrointest Endosc 2017; 9: 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keohane J, Berro W, Harewood GC, et al. Band ligation of gastric antral vascular ectasia is a safe and effective endoscopic treatment. Dig Endosc 2013; 25: 392–396. [DOI] [PubMed] [Google Scholar]
- 29.Markos P, Bilic B, Ivekovic H, et al. Radiofrequency ablation for gastric antral vascular ectasia and radiation proctitis. Indian J Gastroenterol 2017; 36: 145–148. [DOI] [PubMed] [Google Scholar]
- 30.Sebastian S, McLoughlin R, Qasim A, et al. Endoscopic argon plasma coagulation for the treatment of gastric antral vascular ectasia (watermelon stomach): long-term results. Dig Liver Dis 2004; 36: 212–217. [DOI] [PubMed] [Google Scholar]
- 31.Naga M, Esmat S, Naguib M, et al. Long-term effect of Argon Plasma Coagulation (APC) in the treatment of Gastric Antral Vascular Ectasia (GAVE). Arab J Gastroenterol 2011; 12: 40–43. [DOI] [PubMed] [Google Scholar]
- 32.Fábián A, Bor R, Szabó E, et al. Endoscopic treatment of gastric antral vascular ectasia in real-life settings: argon plasma coagulation or endoscopic band ligation? J Dig Dis 2021; 22: 23–30. [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Yamazaki K, Toyota J, et al. Efficacy of argon plasma coagulation for gastric antral vascular ectasia associated with chronic liver disease. Hepatol Res 2005; 32: 121–126. [DOI] [PubMed] [Google Scholar]
- 34.Zushi S, Imai Y, Fukuda K, et al. Endoscopic coagulation therapy is useful for improving encephalopathy in cirrhotic patients with gastric antral vascular ectasia. Dig Endosc 2005; 17: 32–35. [Google Scholar]
- 35.Chaves DM, Sakai P, Oliveira CV, et al. Watermelon stomach: clinical aspects and treatment with argon plasma coagulation. Arq Gastroenterol 2006; 43: 191–195. [DOI] [PubMed] [Google Scholar]
- 36.Herrera S, Bordas JM, Llach J, et al. The beneficial effects of argon plasma coagulation in the management of different types of gastric vascular ectasia lesions in patients admitted for GI hemorrhage. Gastrointest Endosc 2008; 68: 440–446. [DOI] [PubMed] [Google Scholar]
- 37.Baudet JS, Diaz-Bethencourt D, Soler M, et al. [Long-term follow-up of patients with gastric antral vascular ectasia treated with argon plasma coagulation]. Med Clin (Barc) 2009; 133: 217–220. [DOI] [PubMed] [Google Scholar]
- 38.Sato T, Yamazaki K, Akaike J.Endoscopic band ligation versus argon plasma coagulation for gastric antral vascular ectasia associated with liver diseases. Dig Endosc 2012; 24: 237–242. [DOI] [PubMed] [Google Scholar]
- 39.Salunke S, Phull P.Efficacy of argon plasma coagulation therapy for gastric antral vascular ectasia. Gut 2012; 61: A370. [Google Scholar]
- 40.Elhendawy M, Mosaad S, Alkhalawany W, et al. Randomized controlled study of endoscopic band ligation and argon plasma coagulation in the treatment of gastric antral and fundal vascular ectasia. United European Gastroenterol J 2016; 4: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabian A, Bor R, Szabo E, et al. Endoscopic treatment of gastric antral vascular ectasia: a retrospective multicentre clinical study. United European Gastroenterol J 2016; 4: A134. [Google Scholar]
- 42.Hernandez Mondragon OVV, Blanco Velasco G, Blancas Valencia JM.Safety and efficacy of hybrid-APC in Gastric Antral Vascular Ectasia (GAVE): a pilot study. United European Gastroenterol J 2016; 4: A10. [Google Scholar]
- 43.Igawa A, Oka S, Tanaka S, et al. Major predictors and management of small-bowel angioectasia. BMC Gastroenterol 2015; 15: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabe E, Pannier F.Indications, contraindications and performance: European guidelines for sclerotherapy in chronic venous disorders. Phlebology 2014; 29(Suppl. 1): 26–33. [DOI] [PubMed] [Google Scholar]
- 45.Asaki S.Efficacy of endoscopic pure ethanol injection method for gastrointestinal ulcer bleeding. World J Surg 2000; 24: 294–298. [DOI] [PubMed] [Google Scholar]
- 46.Tamari H, Oka S.Clinical usefulness of combination therapy with polidocanol injection and argon plasma coagulation for gastric antral vascular ectasia. JGH Open 2021; 5: 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganz RA, Utley DS, Stern RA, et al. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc 2004; 60: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 48.Becq A, Camus M, Rahmi G, et al. Emerging indications of endoscopic radiofrequency ablation. United European Gastroenterol J 2015; 3: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jana T, Thosani N, Fallon MB, et al. Radiofrequency ablation for treatment of refractory gastric antral vascular ectasia (with video). Endosc Int Open 2015; 3: E125–E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarty TR, Rustagi T.New indications for endoscopic radiofrequency ablation. Clin Gastroenterol Hepatol 2018; 16: 1007–1017. [DOI] [PubMed] [Google Scholar]
- 51.Gross SA, Al-Haddad M, Gill KR, et al. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc 2008; 67: 324–327. [DOI] [PubMed] [Google Scholar]
- 52.McGorisk T, Krishnan K, Keefer L, et al. Radiofrequency ablation for refractory gastric antral vascular ectasia (with video). Gastrointest Endosc 2013; 78: 584–588. [DOI] [PubMed] [Google Scholar]
- 53.Raza N, Diehl DL.Radiofrequency ablation of treatment-refractory Gastric Antral Vascular Ectasia (GAVE). Surg Laparosc Endosc Percutan Tech 2015; 25: 79–82. [DOI] [PubMed] [Google Scholar]
- 54.Senzolo M, Realdon S, Simoncin B, et al. Endoscopic radiofrequency ablation for the treatment of gastric antral vascular ectasia in cirrhotic patients: a bi-centric clinical and economical cost-effective analysis. Dig Liver Dis 2018; 50: 2. [Google Scholar]
- 55.Magee C, Lipman G, Alzoubaidi D, et al. Radiofrequency ablation for patients with refractory symptomatic anaemia secondary to gastric antral vascular ectasia. United European Gastroenterol J 2019; 7: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dray X, Repici A, Gonzalez P, et al. Radiofrequency ablation for the treatment of gastric antral vascular ectasia. Endoscopy 2014; 46: 963–969. [DOI] [PubMed] [Google Scholar]
- 57.Dunn JM, Boger P, Cheong E, et al. Radiofrequency ablation (RFA) is safe and effective for the treatment of gastric antral vascular ectasia refractory to APC-results of a national audit. Gut 2015; 64: A214. [Google Scholar]
- 58.Bozzi R, Bozzi F, Sannino M, et al. RFA and gastric antral vascular ectasia: results of our initial experience. Dig Endosc 2017; 29: 68–69.28425648 [Google Scholar]
- 59.Magee C, Graham D, McMaster J, et al. The cost-effectiveness of radiofrequency ablation for gastric antral vascular ectasia refractory to first-line endoscopic therapy. Gut 2019; 68: A31–A32. [DOI] [PubMed] [Google Scholar]
- 60.Quevedo R, Moehlen M, Joshi V.Gastric inflammatory polyps as a sequela of radiofrequency ablation for the treatment of gastric antral vascular ectasia. Am J Gastroenterol 2011; 106: S375. [Google Scholar]
- 61.Gaslightwala I, Diehl DL.Bacteremia and sepsis after radiofrequency ablation of gastric antral vascular ectasia. Gastrointest Endosc 2014; 79: 873–874. [DOI] [PubMed] [Google Scholar]
- 62.Gutkin E, Schnall A.Gastroesophageal junction tear from HALO 90® system: a case report. World J Gastrointest Endosc 2011; 3: 105–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.St Romain P, Boyd A, Zheng J, et al. Radiofrequency Ablation (RFA) vs. Argon Plasma Coagulation (APC) for the management of Gastric Antral Vascular Ectasia (GAVE) in patients with and without cirrhosis: results from a retrospective analysis of a large cohort of patients treated at a single center. Endosc Int Open 2018; 6: E266–E270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCarty TR, Rustagi T.Comparative effectiveness and safety of radiofrequency ablation versus argon plasma coagulation for treatment of gastric antral vascular ectasia: a systematic review and meta-analysis. J Clin Gastroenterol 2019; 53: 599–606. [DOI] [PubMed] [Google Scholar]
- 65.Rustagi T, McCarty T.Comparative effectiveness of radiofrequency ablation and argon plasma coagulation for treatment of gastric antral vascular ectasia: a systematic review and meta-analysis. Gastrointest Endosc 2015; 81: AB125. [DOI] [PubMed] [Google Scholar]
- 66.Puri N, Mathur AK, Lopez J, et al. Comparative study of argon plasma coagulation and radiofrequency ablation using HALO90 device for treatment of gastric antral vascular ectasia lesions. Gastrointest Endosc 2013; 77: AB266. [Google Scholar]
- 67.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017; 65: 310–335. [DOI] [PubMed] [Google Scholar]
- 68.Wells CD, Harrison ME, Gurudu SR, et al. Treatment of gastric antral vascular ectasia (watermelon stomach) with endoscopic band ligation. Gastrointest Endosc 2008; 68: 231–236. [DOI] [PubMed] [Google Scholar]
- 69.Zepeda-Gómez S, Sultanian R, Teshima C, et al. Gastric antral vascular ectasia: a prospective study of treatment with endoscopic band ligation. Endoscopy 2015; 47: 538–540. [DOI] [PubMed] [Google Scholar]
- 70.Polski JM, Brunt EM, Saeed ZA.Chronology of histological changes after band ligation of esophageal varices in humans. Endoscopy 2001; 33: 443–447. [DOI] [PubMed] [Google Scholar]
- 71.Eccles J, Falk V, Montano-Loza AJ, et al. Long-term follow-up in patients with gastric antral vascular ectasia (GAVE) after treatment with Endoscopic Band Ligation (EBL). Endosc Int Open 2019; 7: E1624–E1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chalhoub J, Umar J, Groudan K, et al. Endoscopic band ligation compared to thermal therapy for gastric antral vascular ectasia: a systematic review and meta-analysis. United European Gastroenterol J 2021; 9: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223211039696 for Endoscopic treatment for gastric antral vascular ectasia by Mengyuan Peng, Xiaozhong Guo, Fangfang Yi, Xiaodong Shao, Le Wang, Yanyan Wu, Chunmei Wang, Menghua Zhu, Ou Bian, Mostafa Ibrahim, Saurabh Chawla and Xingshun Qi in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223211039696 for Endoscopic treatment for gastric antral vascular ectasia by Mengyuan Peng, Xiaozhong Guo, Fangfang Yi, Xiaodong Shao, Le Wang, Yanyan Wu, Chunmei Wang, Menghua Zhu, Ou Bian, Mostafa Ibrahim, Saurabh Chawla and Xingshun Qi in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-3-taj-10.1177_20406223211039696 for Endoscopic treatment for gastric antral vascular ectasia by Mengyuan Peng, Xiaozhong Guo, Fangfang Yi, Xiaodong Shao, Le Wang, Yanyan Wu, Chunmei Wang, Menghua Zhu, Ou Bian, Mostafa Ibrahim, Saurabh Chawla and Xingshun Qi in Therapeutic Advances in Chronic Disease