Abstract
This review summarizes recent therapeutic advances in cervical (CeAD) and intracranial artery dissection (IAD) research. Despite unproven benefits, but in the absence of any signal of harm, in patients, with acute ischemic stroke attributable to CeAD, intravenous thrombolysis and, in case of large-vessel occlusion, endovascular revascularization should be considered. Future research will clarify which patients benefit most from either treatment modality. For stroke prevention, the recently published randomized controlled TREAT-CAD study showed that, against the initial hypothesis, aspirin was not shown non-inferior to anticoagulation with vitamin K antagonists (VKAs). With the results of two randomized controlled trials (CADISS and TREAT-CAD) available now, the evidence to consider aspirin as the standard therapy of CeAD is weak. Further analyses might clarify whether the assumption supports, in particular, that patients presenting with cerebral ischemia, clinical or subclinical with magnetic resonance imaging surrogates, might benefit most from VKA treatment. In turn, it remains to be shown, whether in CeAD patients presenting with pure local symptoms and without hemodynamic compromise, antiplatelets are sufficient, and whether a dual antiplatelet therapy during the first weeks of treatment is recommendable. The observation that ischemic strokes occurred (or recurred) very early after CeAD diagnosis, consistently across randomized and observational studies, supports the recommendation to start antithrombotic treatment immediately, whatever antithrombotic agent is chosen in each individual case. The lack of a license for the use in CeAD patients and the paucity of data are still arguments against the use of direct oral anticoagulants in CeAD. Nevertheless, due to their beneficial safety and efficacy profile proven in atrial fibrillation, these agents are a worthwhile treatment option to be tested in further CeAD treatment trials. In IAD, the experience with the use of antithrombotic agents is limited. As the risk of suffering intracranial hemorrhage is higher in IAD than in CeAD, the use of antithrombotic therapy in IAD remains controversial.
Keywords: stroke, cervical artery dissection, intracranial dissection, stroke-therapy
Introduction
Cervical artery dissection (CeAD) is a major cause of acute ischemic stroke (AIS) in young and middle-aged adults. It accounts for up to 25% of acute ischemic stroke in these patients, and for 2–2.5% of AIS in the general population.1,2 In non-Asian countries, dissection of the intracranial arteries (IAD) is considerably less frequent than CeAD. There are no reliable data on incidence of IAD in populations of European ethnic origin.3 Incidence of CeAD, however, is reported to be 2.6–3.0/100,000 per year.4,5 Most of the cervico-cerebral arterial dissection research, particularly on etiology and therapy has focused on CeAD; IAD on the other hand remains poorly understood in many clinically and scientifically important aspects.
With this review, we primarily aimed to summarize recent advances in CeAD research. We put most emphasis on novel aspects, particularly in CeAD therapy.
The hallmark of CeAD is the occurrence of an intramural hematoma of the cervical portion of the internal carotid artery dissection (ICAD; see Figure 1) or the vertebral artery dissection (VAD; see Figure 2).6 Studies based on large hospital-based CeAD patient cohorts have shown that ICAD can be expected roughly twice as often as VAD.7 The exact mechanisms that lead to hematoma formation are poorly understood. The most likely mechanism is a subintimal tear with consequent blood stream between the layers of intima and media which then leads to a luminal stenosis. Another hypothesis is the intramural rupture of vasa vasorum. This may lead to dissection of the media and adventitia with more eccentric hematoma growth or formation of a dissecting aneurysm.
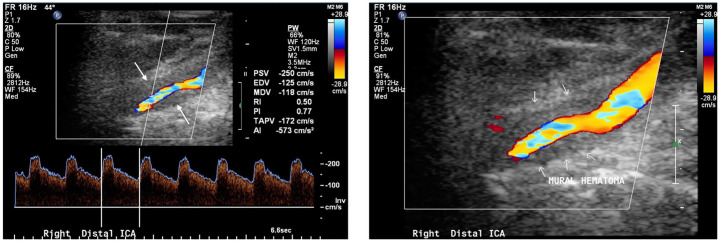
Figure 1.
Color-coded duplex sonography depicting the right internal carotid artery in a female patient who suffered bilateral internal carotid artery dissection.
The hypoechogenic mural hematoma is clearly visible (arrows). The reduced true lumen leads to stenotic, accelerated flow in the affected artery.
ICA, internal carotid artery.
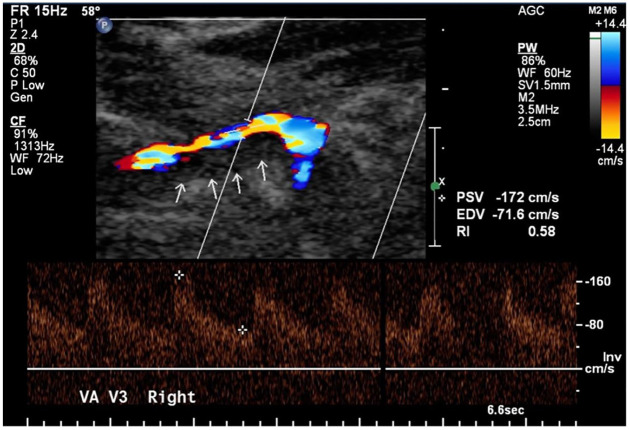
Figure 2.
Color-coded duplex sonography depicting the right vertebral artery (V3 segment) in a female patient.
The hypoechogenic mural hematoma is clearly visible (arrows).
VA, vertebral artery.
Mean age of CeAD occurrence is 45 years, with men being about 5 years older than women when experiencing CeAD.8,9 Although these figures derived from large hospital-based CeAD patient cohorts suggest that CeAD does not occur in elderly patients, a large observational study has shown about 7% (i.e. 1 out of 14) of CeAD patients are aged 60 years and beyond.10 This is important to remember in clinical practice, as findings from the aforementioned study also suggest that important clinical features (i.e. red flags hinting at CeAD) might be missing in CeAD patients >60 years: one of these is the commonly reported headache and or neck pain which was reported significantly less frequently in patients aged >60 years compared with younger CeAD patients. Likewise, the history of migraine (without aura) and the history of a potential mechanical trigger event prior to CeAD occurrence were reported less frequently in patients aged >60 years compared with younger patients.10 Thus, patient age per se should not mislead clinicians and accordingly, diagnostics should be prompted if CeAD is a probable cause of the presenting symptoms.
Large observational studies based on multicentric, international hospital-based CeAD patient cohorts suggest that two thirds of CeAD patients present with signs and symptoms of cerebral ischemia (i.e. acute ischemic stroke, transient ischemic attack, retinal ischemia).11 Mural hematoma growth or thrombus formation at the site of dissection can lead to stenosis or occlusion of the dissected artery, causing hemodynamic impairment with ischemic stroke. However, in most CeAD patients, cerebral ischemic events are caused by arterial embolism from the site of dissection.6 Aside from cerebral ischemia, CeAD patients typically present with so-called local symptoms.5 Most frequently, these are headache and/or neck pain, ipsilateral Horner syndrome, pulsatile tinnitus, or cranial-nerve impairment. While pain is a common feature of ICAD and VAD, the remaining symptoms are much more likely in ICAD. Horner syndrome occurs through ipsilateral, local compression of sympathetic nerve fibers surrounding the internal carotid artery.12 Likewise, lower cranial-nerve impairment is considered a local compression phenomenon in ICAD. Pulsatile tinnitus is explainable through changes and turbulences in local bloodflow (i.e. stenosis or aneurysm) at the site of dissection, which can then be perceived as ipsilateral pulsatile murmurs by the patients.13 Local symptoms mostly resolve throughout the healing process of the affected artery. However, the prevention or treatment of cerebral ischemia is our primary and most important therapeutic target.
Acute recanalization therapies: more work to be done
Both intravenous thrombolysis (IVT) as well as endovascular therapy (EVT, i.e. mechanical thrombectomy) have been proven efficacious and safe in the acute treatment of ischemic stroke of all causes.14,15 In particular, major improvements in devices, techniques and acute patient management pathways have revolutionized EVT in patients with intracranial large-vessel occlusion (LVO). Although CeAD patients have not explicitly been excluded from most randomized controlled trials (RCTs) testing IVT or EVT, there are no RCT data on either therapy in this group of patients. Thus, we must still rely on observational data to inform our decision making in treatment of AIS attributable to CeAD.
Concerns that either IVT or EVT might lead to CeAD-specific complications have been mentioned in the literature.2 First, IVT might, in theory, lead to an enlargement of the mural hematoma, leading to hemodynamic worsening with unfavorable clinical courses, as seen in patients with aortic dissection and IVT treatment for myocardial infarction.16,17 Second, EVT might risk entering the false lumen at the site of dissection and might even lead to perforation of the affected artery. For both concerns we have insufficient proof from the current literature. Nonetheless, as the stroke mechanism in CeAD is different from stroke in large-artery atherosclerosis or cardioembolic stroke, for example, proof of efficacy and safety of IVT and EVT in stroke in general might not necessarily translate into a benefit of these treatments in patients with stroke attributable to CeAD. In the face of lacking data from RCTs, exploratory and observational data can provide clinically useful results.
For IVT, among a population of consecutive stroke patients treated with IVT, patients with CeAD as the underlying cause were compared with all those patients with a cause other than CeAD; i.e. non-CeAD-stroke patients.18 Unexpectedly, IVT seemed less beneficial in CeAD patients than in non-CeAD patients, as indicated by the following findings: CeAD patients (n = 55) were less likely to achieve an excellent 3-month functional outcome (i.e. modified Rankins Scale score, mRS, 0 or 1) than non-CeAD patients (n = 1007). After adjustment for age, sex, and stroke severity, this lower recovery rate in CeAD patients remained statistically significant [odds ratio (OR) 0.50; (95% confidence interval (CI) 0.27–0.95); p = 0.03]. More importantly, the rates of symptomatic intracranial hemorrhages (ICHs) as an IVT complication were comparable in both groups. The same was true for recurrent acute ischemic strokes.18 Thus, although caution is required for drawing conclusions from observational data, these findings suggest less benefit of IVT in CeAD patients compared with non-CeAD stroke patients, but at least also, no signal of harm has been detected. Interestingly, the CeAD group had a high rate of large-artery occlusions.18 As occlusions are an important predictor of worse outcome,11 this baseline characteristic might explain the lower likelihood of a favorable outcome in CeAD patients.
Data from the multicenter CADISP (Cervical Artery Dissection and Ischemic Stroke Patients) registry, allowed the comparison of CeAD stroke patients treated with IVT with those not treated with IVT.19 Counterintuitively, the likelihood of a favorable outcome was virtually identical (OR 0.95; 95% CI, 0.45–2.00) when comparing IVT versus no IVT in CeAD patients, also after accounting for differences in outcome predictive baseline variables, including propensity-score matching. Of note, safety concerns were absent. Specifically, and most importantly, symptomatic ICH, as well as major extracranial hemorrhage in IVT-treated CeAD patients occurred at a lower rate than could have been expected from prior RCTs (i.e. the NINDS and ECASS III-studies).19 Low rates of symptomatic ICH in IVT-treated CeAD patients were also reported by Tsivgoulis et al.20 in a comprehensive meta-analysis of reported case series (2%; 95% CI 0–5%).
In 2016, a systematic literature review across 10 observational studies reporting on 174 IVT-treated CeAD patients and 672 CeAD patients treated without IVT (control group)21 showed that the likelihood of favorable outcome (i.e. mRS 0, 1, 2) and the odds for an excellent functional outcome (i.e. mRS 0–1) were similar in both groups.21 Moreover, there was a higher frequency of intracranial bleeds in IVT-treated CeAD patients, although none of those were symptomatic, while one symptomatic intracranial bleed occurred among CeAD patients who did not have IVT treatment.21
Thus, based on the evidence available so far, guidelines do not recommend against IVT in CeAD patients and thus, in the absence of contraindications, IVT should be considered in CeAD patients similarly to non-CeAD AIS patients.
Occlusions of the dissected artery, often accompanied by an intracranial large-artery occlusion, were present in about one third of CeAD patients in observational studies.11 Furthermore, occlusions of the dissected artery are an important and independent outcome predictor in CeAD patients.11 Thus, it makes sense to evaluate whether EVT in these patients is as safe and efficacious as in stroke patients in general.
Counterintuitively, observational studies so far have not shown a beneficial effect of EVT (with or without prior IVT) over IVT alone in CeAD patients. In a Swiss multicenter observational study, 24 IVT-treated CeAD patients were compared with 38 EVT-treated patients (including those with IVT-bridging therapy):22 there was an excellent 3-month functional outcome, i.e. mRS 0–1, as the primary outcome occurred numerically more frequent in EVT- than in IVT-treated patients (adjusted OR 2.23; 95% CI 0.52–9.59). However, this difference did not reach statistical significance. At least, partial or complete recanalization was achieved more frequently in EVT-treated patients [84.2% versus 66.7%; OR 3.20 (95% CI 0.90–11.38)].22 On the contrary, symptomatic ICH occurred solely in EVT-treated patients. These results were included in a subsequent meta-analysis with existing observational studies. Overall, outcomes of 102 EVT-treated (including IVT-bridging) CeAD patients were compared with those of 110 IVT-treated CeAD patients (n = 8 studies). Favorable 3-month outcome was comparable in both groups (OR 0.97; 95% CI 0.39–2.44).22 However, given the rather small sample sizes of the included studies, and the substantial heterogeneity in treatment effects across all studies (I2 = 35%), these findings must be interpreted carefully. Another important aspect is the optimal treatment of tandem occlusions (i.e. occlusion of the cervical portion of the dissected artery as well as intracranial LVO) which requires a two-step approach. Frequently, the intracranial lesion is treated first, to re-establish brain perfusion as quickly as possible.23 The available, very limited data indicate that following emergency, carotid artery (dissection) stenting (CAS) could potentially be beneficial: in a multicenter, retrospective analysis of observational data including 136 CeAD patients with tandem occlusion, acute CAS was compared with no stenting.23 Rates of recanalization (modified treatment in cerebral infarction (mTICI) 2b/3) were higher in CAS-treated patients; however, this did not translate into improved 90-day outcome when compared with non-CAS patients.
The majority of data on EVT in CeAD patients has been collected and published prior to 2015, i.e. before EVT treatment and techniques had significantly improved, and thus led to the positive results of several landmark RCTs. More data on EVT treatment in CeAD patients beyond 2015 are needed to further test whether the superiority in EVT over IVT in the stroke populations with LVO due to miscellaneous, mostly non-CeAD, causes is also applicable to CeAD patients. Testing of EVT in CeAD patients is unlikely to happen in an RCT setting in the near future. Thus, we will have to rely on up-to-date, observational, comparative research, and such research is underway. Despite the low evidence to date, for clinical practice, currently, there is no scientifically supported reason to withhold EVT treatment from CeAD patients if otherwise there is no contraindication for treatment.
Antithrombotic treatment: treat early, but how?
The prevention of cerebral ischemic events without increasing risk of major bleeding (intracranial or extracranial) while under antithrombotic treatment is particularly important in CeAD patients. Results from a US study based on national registry data [n = 2791 CeAD patients who had presented without AIS or transischemic attack (TIA) at baseline] showed that 1.7% of the included patients suffered an AIS within 2 weeks from first CeAD diagnosis.24 These data suggest that the overall stroke rate might be low in CeAD; however, the study population comprised solely those patients who had not presented with stroke or TIA at baseline; thus, the majority of CeAD patients with a particular high stroke risk had been excluded. Most importantly, however, this study showed that risk for a first or recurrent ischemic stroke is the highest within 14 days to 4 weeks after CeAD diagnosis and decreases thereafter.24 These data are comparable with prior, yet smaller observational studies, and they underscore the recommendation that antithrombotic treatment should be established as early as possible. Historically, two treatment options have been used: (a) antiplatelets [i.e. aspirin (ASA), in most cases] or oral anticoagulation with vitamin K antagonists (VKAs) with or without prior bridging therapy using low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). For many years, meta-analyses across observational data were the only basis for an ongoing debate on the optimal treatment regimen.25–29 However, these meta-analyses yielded conflicting results regarding effectiveness and safety of either treatment (ASA versus VKA). Therefore, more important is the analyses of the results of two subsequent RCTs which have tested antiplatelets against anticoagulants in CeAD patients.
In 2015, investigators of the UK-based CADISS study (Cervical Artery Dissection in Stroke Study) reported the main results of the multicenter, randomized controlled, open-label study which was designed to show feasibility of a RCT in CeAD patients. CADISS included 250 CeAD patients and randomly allocated participants to either antiplatelets or VKAs.30 The specific choice of drug within either treatment arm was left to the discretion of the treating physicians. The intention-to-treat population comprised 126 patients in the antiplatelet group and 124 patients in the anticoagulation group. Antiplatelet treatment was heterogeneous, with 22% of patients receiving ASA alone, 33% receiving clopidogrel alone, 28% receiving both ASA and clopidogrel, and 16% receiving ASA and dipyridamole. One patient received dipyridamole only. In the anticoagulation group, 90% of patients received heparin and warfarin, whereas 10% received warfarin alone. Regarding the primary study endpoint (ipsilateral stroke or death) there was no statistically significant group difference.30 Within the 3-month study period, ischemic stroke occurred in 3 (of 126) patients in the antiplatelet group and in 1 (of 124) patient in the anticoagulation group [OR 0.335 (0.006–4.233), p = 0.63]. There was one major hemorrhage (subarachnoid hemorrhage) in the anticoagulation group. No major hemorrhage was observed in the antiplatelet group.30 In the subsequent long-term follow-up analysis (12 months) there were two additional ischemic strokes (one in each treatment arm) yielding again no statistically significant difference in the primary endpoint between groups.31
In clinical practice, these findings resulted in a tendency to turn away from the historically preferred VKAs toward ASA, taking into account the ease of use and the lower costs of ASA compared with VKAs.32
Furthermore, these results questioned the feasibility of an RCT trial based on clinical endpoints, as, calculated based on the CADISS data, roughly 10,000 participants (5000 per group) would have been needed for such a trial, to gather meaningful results.30
In this situation, the use of surrogates (e.g. imaging surrogate markers) of clinical events can enable the feasibility of a trial. Surrogate markers that occur more frequently than the actual clinical events might help in significantly reducing the needed sample size within a trial, to a feasible degree.33 In stroke-therapy trials, new, primarily clinically silent, lesions on diffusion-weighted magnetic resonance imaging (MRI; DWI) may serve as surrogate for AIS. For example, the International Carotid Stenting Study–MRI sub-study (stenting versus endarterectomy in symptomatic carotid artery stenosis) including surrogate imaging markers (procedural DWI lesions) yielded virtually identical results compared with the main study.34 The inclusion of imaging surrogates enabled roughly a 90% reduction of the sample size in the MRI sub-study.34 More recently, further stroke-therapy trials have embraced the concept of including imaging surrogates in a composite study endpoint.35,36
The potential usefulness of DWI lesions as surrogates for ischemic stroke in a CeAD trial was shown in an observational study: Gensicke et al.37 reported new DWI lesions during follow up in 25% of the included patients with the vast majority of them being detected within 14 days after CeAD diagnosis was established and antithrombotic treatment was initiated. Thus, the frequency of DWI lesions was as much as 10-fold the frequency of clinical events observed in the CADISS trial. The fact that most DWI lesions occurred in the very early phase after CeAD diagnosis was established underscores the importance of an immediate initiation of antithrombotic treatment.
With these considerations in mind, the ‘Biomarkers and antithrombotic TREATment in Cervical Artery Dissection’ (TREAT-CAD) study was designed.38
TREAT-CAD adopted the innovative approach of including imaging markers in a composite study endpoint. TREAT-CAD was a multicenter therapy RCT comparing ASA with VKA in the treatment of CeAD. TREAT-CAD participants were randomly assigned to receive either ASA (300 mg/d) or VKA (with or without bridging with UFH or LMWH) for 3 months. TREAT-CAD hypothesized that ASA would be non-inferior to VKA in CeAD patients with regards the composite study endpoint.38 The latter was a combination of clinical (AIS, major intra- or extracranial hemorrhage, death) and imaging events [new DWI or SWI (susceptibility weighted imaging)/T2* lesions during follow up until 21 days as compared with baseline imaging].38
The main results of TREAT-CAD have been published in March 2021.39 The primary analyses in TREAT-CAD were performed in the per protocol population which comprised 173 patients (of 194 in the intention-to-treat population) of which 91 were allocated to ASA and 82 were allocated to VKA.39 The primary composite endpoint occurred in 21 (23%) patients in the ASA group, and in 12 (15%) in the VKA group [absolute difference 8% (95% CI −4 to 21), non-inferiority p = 0.55].39 Accordingly, non-inferiority of ASA was not shown. For methodological reasons, however, this does not mean that VKA could be declared superior to ASA. Interestingly, all ischemic strokes (n = 7) occurred in the ASA group, whereas the only major, though extracranial (gastrointestinal bleeding), hemorrhage occurred in the VKA group. There were no deaths in either group. The distribution of clinical events across treatment groups is impressive, though statistically not significant when analyzed separately from the imaging events.39 Furthermore, it is important to note, that five of the seven ischemic strokes in the ASA group, did occur (or recur) on the day after treatment onset, stressing the importance for the earliest initiation of antithrombotic treatment, whichever the clinician might chose.
Considering all currently available evidence, in particular, the results from CADISS and TREAT-CAD as the only two RCTs, the case for ASA as the standard therapy in CeAD patients is weak.
Pooled analyses of CADISS and TREAT-CAD might help to identify patients who would benefit most from VKA treatment, e.g. those with cerebral ischemia (clinical or subclinical) as presenting symptom. This assumption is based on the fact that in TREAT-CAD, with one exception, all new ischemic outcomes (clinically or novel MRI lesions) have occurred in patients who had either ischemic events or MRI lesions at baseline.39 The prognostic importance of these clinical and imaging characteristics has also been suggested in prior research.37,40
In turn, it might be possible, in CeAD patients presenting with purely local symptoms and without hemodynamic compromise caused by dissection, particularly no occlusion, another indicator of an unfavorable prognosis,11 that ASA might be sufficient.
Furthermore, such pooled analyses might also clarify whether the differences between CADISS and TREAT-CAD regarding the antiplatelet arm do matter: in CADISS, one third of patients had ASA plus clopidogrel (i.e. dual antiplatelets) while in TREAT-CAD, ASA monotherapy was applied, albeit in a higher daily dosage of 300 mg. Thus, it could be clarified whether the use of dual antiplatelets (e.g. in a regimen similar to the CHANCE41 or POINT42 trials) in CeAD should be evaluated further, as advocated recently.43
For the time being, the observation that ischemic strokes occur (or recur) very early after CeAD diagnosis, an observation which was consistent across randomized and observational studies, seems to support the recommendation to start the antithrombotic treatment immediately without any delay, whatever type of antithrombotic treatment is chosen in the individual case.
Considering the sharply increased use of direct oral anticoagulants (DOACs) instead of VKAs in the prevention of cardioembolic ischemic stroke, it is reasonable to discuss these treatment options in CeAD patients, as well. Currently, there are limited data from the small case series available reporting on DOAC treatment in CeAD patients.44–46 DOACs were reportedly relatively safe with a favorable outcome in the reported cases, though superiority to other antithrombotic agents is not shown yet. In addition, DOACs have not been licensed for the use in CeAD to the best of our knowledge. Thus, we recommend against the use of DOACs in CeAD in current clinical practice. However, considering their beneficial safety and efficacy profile proven in atrial fibrillation, DOACs are a worthwhile treatment to be tested in further CeAD therapy trials.
Apart from the therapeutic aspects, TREAT-CAD has yielded important information on the feasibility of a therapy RCT in CeAD; by including imaging surrogate markers, the sample size in TREAT-CAD could be reduced to a reasonable and achievable number. This should serve as a model for future CeAD trials. Further, TREAT-CAD has underscored the importance of the use of MRI in CeAD: in TREAT-CAD, patients were included only after MRI confirmed definite CeAD diagnosis.38 Thus, in only 4 (of 194, 2%) patients CeAD diagnosis was not confirmed in central reading.39 This compared with 52 (of 250, 21%) false CeAD diagnoses in the CADISS trials, in which inclusion of both computed tomography–angiography, as well as MRI, was allowed.30 If CeAD is clinically suspected, MRI, including contrast-enhanced angiography, and particularly T1-weighted fat-saturated sequences, should be performed to establish the diagnosis (see Figure 3).
Figure 3.
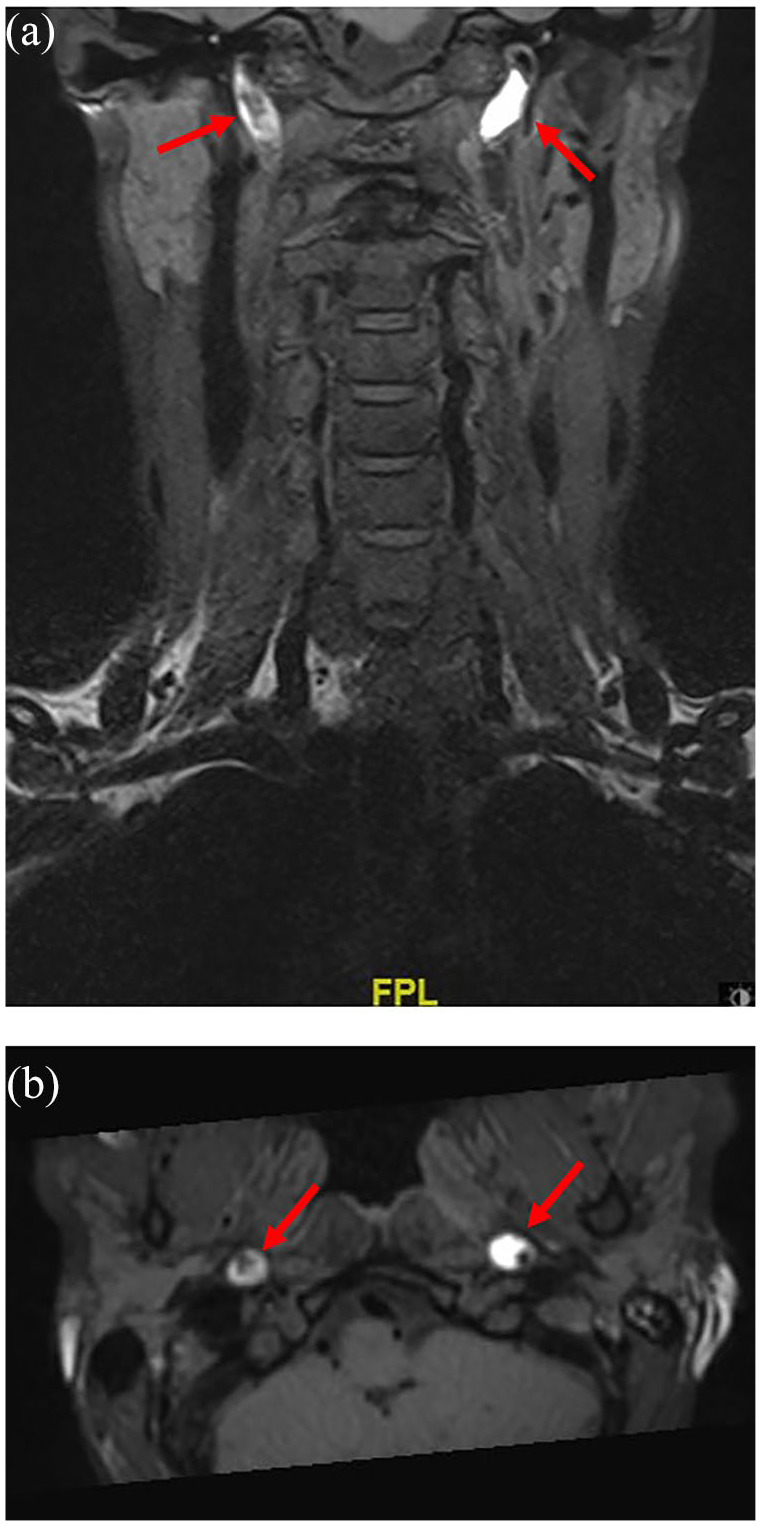
T1-weighted, fat-saturated magnetic resonance imaging in a female patient who suffered bilateral internal carotid artery dissection.
T1-weighted, fat-saturated magnetic resonance imaging: (a) coronal; (b) axial, in a female patient who suffered bilateral internal carotid artery dissection (also see Figure 1 for duplex sonography in the same patient). The T1-hyperintense mural hematoma in both internal carotid arteries is clearly visible (arrows).
In IAD, a feared complication of antithrombotic treatment is subarachnoid hemorrhage, of which the risk is higher in IAD as compared with that in CeAD patients.3 However, in a retrospective single-center study (81 patients included), VKA treatment in IAD patients appeared relatively safe.47 This study, however, was criticized for the neuroimaging criteria based on which patients were included and thus potentially increased the likelihood of false-positive IAD diagnosis.48 On the contrary, there are reports on IAD patients who had presented with cerebral ischemia who ultimately or concurrently (at baseline) developed subarachnoid hemorrhage, which generally puts the usefulness of antithrombotic therapy in such patients into question.49
Consequently, with the idea of reducing unnecessary risk, close monitoring instead of antithrombotic treatment in IAD patients without cerebral ischemia and without subarachnoid hemorrhage at baseline had been proposed.50 For both medical and interventional (endovascular, surgery) management of IAD, no evidence-based guidelines have been published thus far.51
Perspectives on the future of CeAD patient treatment, counseling and research
Neither CADISS and TREAT-CAD, nor preceding observational studies on CeAD patient treatment were designed to specify subgroups of patients who might benefit from either treatment approach, or who might benefit from more aggressive treatment and intensive follow up. In clinical routine, however, we experience that CeAD patients differ regarding baseline characteristics, presenting symptoms (purely local versus clinically silent cerebral ischemia versus ischemic stroke), vascular characteristics (patent versus stenotic versus occluded dissected artery), and probably also their family history, and thus potential genetic or inheritable modifiers. Many of these aspects go unrecognized in clinical decision making, albeit important characteristics have already proven to predict CeAD patient outcome: Horner syndrome in patients with ICAD has been proven to predict favorable outcome.12 On the contrary, occlusion of the dissected artery was proven to independently predict worse outcome in CeAD, and thus, probably calling for a more aggressive treatment approach including EVT.11 The individual weighting and thus impact of each of such baseline clinical or vascular characteristics is currently unknown. Another important aspect is the risk of recurrent dissection which was observed in a wide range from 0% to up to 25%1 of CeAD patients, although more recent data suggest early recurrences in 8%, which is more in line with our clinical experience.52
Identifiable risk factors for recurrent dissection are currently unknown. A family history of dissection or multiple CeAD recurrences might suggest that in these patients, genetics may play an important role. Indeed, in a recent study which performed whole-exome sequencing in such patients, pathogenic or likely pathogenic variants mostly coding for genes of the collagen family, have been reported.53 Another study reported on the variations of the biomarker signature of the extracellular matrix proteins in skin biopsies of patients suffering recurrent dissection.54 These and further genetic studies in CeAD patients do hint at identifiable, possibly genetically determined factors that could enable personalized prediction of risk of recurrent dissection, but also call for more intensive therapy or patient follow up. The available data to support this approach, however, are still insufficient and must be validated in further and particularly larger studies.55
Future CeAD research will have to focus on the individualized approach in both CeAD patient therapy as well as outcome prediction. Testing novel treatment approaches like the use of DOACs or dual antiplatelets seems worthwhile. However, patients included in CeAD therapy trials should ideally be selected or allocated to treatment regimens according to their individual risk profile based on clinical, vascular imaging and potentially genetic characteristics. Another open question that could be tested in such a trial is the optimal treatment duration; this would also benefit from more careful consideration of individual patient characteristics. From a patient perspective, the exploration of possibilities to individually assess risks of (recurrent) cerebral ischemia or recurrent dissection would be most important.
For IAD, large, prospective, multicenter-registry-based studies like I-IDIS [ClinicalTrials.gov identifier: NCT02756091] might shed more light on optimal treatment.
Footnotes
Conflict of interest statement: STE reports grants from the Swiss National Science Foundation, Swiss Heart Foundation, the Freiwillige Akademische Gesellschaft Basel, the University of Basel, the University Hospital Basel, outside of the submitted work.
PL reports grants from Bayer GmbH Germany, Swiss National Science Foundation, ProPatient Foundation of the University Hospital Basel, travel grants from Bayer AG Switzerland, Pfizer AG Switzerland, advisory board compensation from Bayer AG Switzerland, Pfizer AG Switzerland, Daiichi Sankyo Switzerland, Bristol Myers Squibb Switzerland, Recordati SA Switzerland, Amgen Switzerland, and compensation for research activities from Sanofi Switzerland and Acticor SA France, all outside of the submitted work.
CT reports grants from the Swiss Heart Foundation, the University of Basel, the Bangerter-Rhyner Foundation and the Freiwillige Akademische Gesellschaft Basel and travel honoraria from Bayer AG, all outside of the submitted work.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Christopher Traenka  https://orcid.org/0000-0002-7600-1005
https://orcid.org/0000-0002-7600-1005
Contributor Information
Stefan T. Engelter, Neurology and Neurorehabilitation, University Department of Geriatric Medicine FELIX PLATTER, University of Basel, Basel, Switzerland Department of Neurology and Stroke Center, University Hospital Basel and University of Basel, Basel, Switzerland.
Philippe Lyrer, Department of Neurology and Stroke Center, University Hospital Basel and University of Basel, Basel, Switzerland.
Christopher Traenka, Department of Neurology and Stroke Center, University Hospital Basel and University of Basel, Petersgraben 4, Basel 4031, Switzerland; Neurology and Neurorehabilitation, University Department of Geriatric Medicine FELIX PLATTER, University of Basel, Basel, Switzerland.
References
- 1.Debette S, Leys D.Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol 2009; 8: 668–678. [DOI] [PubMed] [Google Scholar]
- 2.Engelter ST, Traenka C, Lyrer P.Dissection of cervical and cerebral arteries. Curr Neurol Neurosci Rep 2017; 17: 59. [DOI] [PubMed] [Google Scholar]
- 3.Debette S, Compter A, Labeyrie MA, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 2015; 14: 640–654. [DOI] [PubMed] [Google Scholar]
- 4.Bejot Y, Daubail B, Debette S, et al. Incidence and outcome of cerebrovascular events related to cervical artery dissection: the Dijon stroke registry. Int J Stroke 2014; 9: 879–882. [DOI] [PubMed] [Google Scholar]
- 5.Debette S.Pathophysiology and risk factors of cervical artery dissection: what have we learnt from large hospital-based cohorts? Curr Opin Neurol 2014; 27: 20–28. [DOI] [PubMed] [Google Scholar]
- 6.Engelter ST, Traenka C, Von Hessling A, et al. Diagnosis and treatment of cervical artery dissection. Neurol Clin 2015; 33: 421–441. [DOI] [PubMed] [Google Scholar]
- 7.Debette S, Grond-Ginsbach C, Bodenant M, et al. Differential features of carotid and vertebral artery dissections: the CADISP study. Neurology 2011; 77: 1174–1181. [DOI] [PubMed] [Google Scholar]
- 8.Metso AJ, Metso TM, Debette S, et al. Gender and cervical artery dissection. Eur J Neurol 2012; 19: 594–602. [DOI] [PubMed] [Google Scholar]
- 9.Metso TM, Debette S, Grond-Ginsbach C, et al. Age-dependent differences in cervical artery dissection. J Neurol 2012; 259: 2202–2210. [DOI] [PubMed] [Google Scholar]
- 10.Traenka C, Dougoud D, Simonetti BG, et al. Cervical artery dissection in patients ⩾60 years: often painless, few mechanical triggers. Neurology 2017; 88: 1313–1320. [DOI] [PubMed] [Google Scholar]
- 11.Traenka C, Grond-Ginsbach C, Goeggel Simonetti B, et al. Artery occlusion independently predicts unfavorable outcome in cervical artery dissection. Neurology 2020; 94: e170–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyrer PA, Brandt T, Metso TM, et al. Clinical import of Horner syndrome in internal carotid and vertebral artery dissection. Neurology 2014; 82: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 13.Kellert L, Kloss M, Pezzini A, et al. Prognostic significance of pulsatile tinnitus in cervical artery dissection. Eur J Neurol 2016; 23: 1183–1187. [DOI] [PubMed] [Google Scholar]
- 14.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal M, Menon BK, Van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 16.Kamp TJ, Goldschmidt-Clermont PJ, Brinker JA, et al. Myocardial infarction, aortic dissection, and thrombolytic therapy. Am Heart J 1994; 128: 1234–1237. [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Lim J.Type A aortic dissection: a hidden and lethal cause for failed thrombolytic treatment in acute myocardial infarction. BMJ Case Rep 2009; 2009: bcr2006100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelter ST, Rutgers MP, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke 2009; 40: 3772–3776. [DOI] [PubMed] [Google Scholar]
- 19.Engelter ST, Dallongeville J, Kloss M, et al. Thrombolysis in cervical artery dissection–data from the Cervical Artery Dissection and Ischaemic Stroke Patients (CADISP) database. Eur J Neurol 2012; 19: 1199–1206. [DOI] [PubMed] [Google Scholar]
- 20.Tsivgoulis G, Zand R, Katsanos AH, et al. Safety and outcomes of intravenous thrombolysis in dissection-related ischemic stroke: an international multicenter study and comprehensive meta-analysis of reported case series. J Neurol 2015; 262: 2135–2143. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Sun Y, Zhao S, et al. Safety and efficacy of thrombolysis in cervical artery dissection-related ischemic stroke: a meta-analysis of observational studies. Cerebrovasc Dis 2016; 42: 272–279. [DOI] [PubMed] [Google Scholar]
- 22.Traenka C, Jung S, Gralla J, et al. Endovascular therapy versus intravenous thrombolysis in cervical artery dissection ischemic stroke – results from the SWISS registry. Eur Stroke J 2018; 3: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marnat G, Lapergue B, Sibon I, et al. Safety and outcome of carotid dissection stenting during the treatment of tandem occlusions: a pooled analysis of TITAN and ETIS. Stroke 2020; 51: 3713–3718. [DOI] [PubMed] [Google Scholar]
- 24.Morris NA, Merkler AE, Gialdini G, et al. Timing of incident stroke risk after cervical artery dissection presenting without ischemia. Stroke 2017; 48: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyrer P, Engelter S.Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst Rev 2000; 4: CD000255. [DOI] [PubMed] [Google Scholar]
- 26.Sarikaya H, Da Costa BR, Baumgartner RW, et al. Antiplatelets versus anticoagulants for the treatment of cervical artery dissection: Bayesian meta-analysis. PLoS One 2013; 8: e72697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy F, Lanfranconi S, Hicks C, et al. Antiplatelets vs anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology 2012; 79: 686–689. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury MM, Sabbagh CN, Jackson D, et al. Antithrombotic treatment for acute extracranial carotid artery dissections: a meta-analysis. Eur J Vasc Endovasc Surg 2015; 50: 148–156. [DOI] [PubMed] [Google Scholar]
- 29.Menon R, Kerry S, Norris JW, et al. Treatment of cervical artery dissection: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2008; 79: 1122–1127. [DOI] [PubMed] [Google Scholar]
- 30.CADISS Trial Investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol 2015; 14: 361–367. [DOI] [PubMed] [Google Scholar]
- 31.Markus HS, Levi C, King A, et al. Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: the Cervical Artery Dissection in Stroke Study (CADISS) randomized clinical trial final results. JAMA Neurol 2019; 76: 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasner SE.CADISS: a feasibility trial that answered its question. Lancet Neurol 2015; 14: 342–343. [DOI] [PubMed] [Google Scholar]
- 33.Traenka C, Engelter ST, Brown MM, et al. Silent brain infarcts on diffusion-weighted imaging after carotid revascularisation: a surrogate outcome measure for procedural stroke? A systematic review and meta-analysis. Eur Stroke J 2019; 4: 127–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonati LH, Jongen LM, Haller S, et al. New ischaemic brain lesions on MRI after stenting or endarterectomy for symptomatic carotid stenosis: a substudy of the International Carotid Stenting Study (ICSS). Lancet Neurol 2010; 9: 353–362. [DOI] [PubMed] [Google Scholar]
- 35.Søndergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017; 377: 1033–1042. [DOI] [PubMed] [Google Scholar]
- 36.Sharma M, Hart RG, Smith EE, et al. Rivaroxaban for prevention of covert brain infarcts and cognitive decline: the COMPASS MRI substudy. Stroke 2020; 51: 2901–2909. [DOI] [PubMed] [Google Scholar]
- 37.Gensicke H, Ahlhelm F, Jung S, et al. New ischaemic brain lesions in cervical artery dissection stratified to antiplatelets or anticoagulants. Eur J Neurol 2015; 22: 859–865, e861. [DOI] [PubMed] [Google Scholar]
- 38.Traenka C, Gensicke H, Schaedelin S, et al. Biomarkers and antithrombotic treatment in cervical artery dissection – design of the TREAT-CAD randomised trial. Eur Stroke J 2020; 5: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelter ST, Traenka C, Gensicke H, et al. Aspirin versus anticoagulation in cervical artery dissection (TREAT-CAD): an open-label, randomised, non-inferiority trial. Lancet Neurol. Epub ahead of print 23March2021. DOI: 10.1016/S1474-4422(21)00044-2. [DOI] [PubMed] [Google Scholar]
- 40.Lichy C, Metso A, Pezzini A, et al. Predictors of delayed stroke in patients with cervical artery dissection. Int J Stroke 2015; 10: 360–363. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013; 369: 11–19. [DOI] [PubMed] [Google Scholar]
- 42.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018; 379: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasner SE.Antithrombotic therapy for cervical arterial dissection. Lancet Neurol 2021; 20: 328–329. [DOI] [PubMed] [Google Scholar]
- 44.Cappellari M, Bovi P.Direct oral anticoagulants in patients with cervical artery dissection and cerebral venous thrombosis. A case series and review of the literature. Int J Cardiol 2017; 244: 282–284. [DOI] [PubMed] [Google Scholar]
- 45.Mustanoja S, Metso TM, Putaala J, et al. Helsinki experience on nonvitamin K oral anticoagulants for treating cervical artery dissection. Brain Behav 2015; 5: e00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caprio FZ, Bernstein RA, Alberts MJ, et al. Efficacy and safety of novel oral anticoagulants in patients with cervical artery dissections. Cerebrovasc Dis 2014; 38: 247–253. [DOI] [PubMed] [Google Scholar]
- 47.Metso TM, Metso AJ, Helenius J, et al. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke 2007; 38: 1837–1842. [DOI] [PubMed] [Google Scholar]
- 48.Arnold M, Bousser MG, Baumgartner RW.Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke 2007; 38: e140; author reply e141. [DOI] [PubMed] [Google Scholar]
- 49.Ono H, Nakatomi H, Tsutsumi K, et al. Symptomatic recurrence of intracranial arterial dissections: follow-up study of 143 consecutive cases and pathological investigation. Stroke 2013; 44: 126–131. [DOI] [PubMed] [Google Scholar]
- 50.Kai Y, Nishi T, Watanabe M, et al. Strategy for treating unruptured vertebral artery dissecting aneurysms. Neurosurgery 2011; 69: 1085–1091; discussion 1091–1082. [DOI] [PubMed] [Google Scholar]
- 51.Bond KM, Krings T, Lanzino G, et al. Intracranial dissections: a pictorial review of pathophysiology, imaging features, and natural history. J Neuroradiol. Epub ahead of print 23April2020. DOI: 10.1016/j.neurad.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Kloss M, Grond-Ginsbach C, Ringleb P, et al. Recurrence of cervical artery dissection: an underestimated risk. Neurology 2018; 90: e1372–e1378. [DOI] [PubMed] [Google Scholar]
- 53.Traenka C, Kloss M, Strom T, et al. Rare genetic variants in patients with cervical artery dissection. Eur Stroke J 2019; 4: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer L, Pechlaner R, Barallobre-Barreiro J, et al. Extracellular matrix protein signature of recurrent spontaneous cervical artery dissection. Neurology 2020; 95: e2047–e2055. [DOI] [PubMed] [Google Scholar]
- 55.Traenka C, Debette S.Extracellular matrix protein signature in cervical artery dissection: the key differentiator? Neurology 2020; 95: 663–664. [DOI] [PubMed] [Google Scholar]