Abstract
Introduction/Objectives:
Despite compelling evidence of clinical and economic benefits, adherence to colorectal cancer (CRC) screening remains low. Increasing public awareness through various outreach methods may improve screening uptake. The objective of this study was to evaluate the uptake of non-invasive multi-target stool DNA (mt-sDNA) by different outreach methods in an average-risk employer population.
Methods:
This retrospective observational study included CRC screening-eligible individuals aged ≥50 years insured by the Metropolitan Nashville Public Schools (MNPS) employee healthcare plan. The study intervention arms included population-based outreach and office visit-based interaction. The mt-sDNA completion rate (proportion of individuals who return the mt-sDNA kit after consenting to have it shipped to their home), proportion of patients who performed follow-up colonoscopy after a positive test, and time to follow-up colonoscopy were assessed.
Results:
A total of 167 mt-sDNA kits were shipped to eligible participants (aged 50-64 years) in the population-based outreach arm. In the office visit-based interaction arm, a total of 132 mt-sDNA kits were shipped to eligible participants (aged ≥50 years). The mt-sDNA completion rate was significantly higher for office visit-based interaction as compared to population-based outreach (76.8% vs 53.5%; P < .001) among those aged 50 to 64 years. While all patients aged 50 to 64 years with a positive mt-sDNA result received a follow-up colonoscopy in both arms, the median time to follow-up colonoscopy was shorter among the population-based outreach (55 vs 136 days; P < .05).
Conclusions:
Office visit-based interaction was associated with a higher mt-sDNA completion rate as compared to the population-based outreach among average-risk, CRC screening-eligible individuals aged 50 to 64 years old.
Keywords: health promotion, access to care, health outcomes, community health, health literacy
Introduction
Colorectal cancer (CRC) is the second most common cause of cancer-related mortality in the United States (U.S.), and exerts a substantial economic burden on the healthcare system.1,2 Early detection of CRC can significantly improve survival rates among patients. The 5-year relative survival rate for patients diagnosed with localized CRC is 90% and drops to 14% for those diagnosed with advanced-stage CRC.1 Routine screenings can effectively reduce mortality particularly in CRC since the progression from precancerous polyp to invasive cancer to late-stage CRC is slow.3
Multiple CRC screening modalities are available in the U.S., including colonoscopy, sigmoidoscopy, and stool-based tests such as guaiac-based fecal occult blood test, fecal immunochemical test (FIT), and multi-targeted stool DNA (mt-sDNA) test. The expanded availability of non-invasive stool-based tests, with the launch of the mt-sDNA test in 2014, may help to increase the number of individuals that initiate CRC screening with a non-invasive test, particularly among those who are apprehensive of invasive procedures and/or are restricted due to rurality, lack of transportation, or the extensive bowel preparation required for colonoscopy or flexible sigmoidoscopy.4-6 The recent white paper proposes a systematic approach to improve CRC screening uptake that includes starting with noninvasive testing methods and integrating these options with colonoscopy based on individual risk profile.6
Despite the availability of multiple CRC screening modalities and compelling evidence of the clinical and economic benefits, CRC screening rates among U.S. adults aged 50 to 75 years remain sub-optimal. While colonoscopy is often considered the gold standard screening method for CRC, the perceived barriers including inadequate knowledge, laxative bowel preparation, travel, dietary restrictions, anxiety, feelings of embarrassment and vulnerability, potential test-related complications, and pain may limit the uptake of colonoscopy as a preferred screening method.7-9 Non-invasive home-based stool tests may tackle some of these barriers related to travel, extensive bowel preparation, and procedure-related complications or perception of pain. Nonetheless, the most effective strategies to address the barriers related to lack of knowledge and awareness regarding the CRC screening process are uncertain.10
Various outreach strategies have been implemented to increase awareness of non-invasive CRC screening modalities such as mt-sDNA among the general population as well as providers. Increasing public awareness through provider recommendations, media campaigns, educational interventions along with improved access has been shown to improve adherence to overall CRC screening.5,11-14 However, little is known about how outreach versus inreach intervention strategies impact the adoption and completion of mt-sDNA in the real world. Hence, the primary objective of the current study was to describe mt-sDNA test completion rates for 2 different interventions within an average-risk employer population: a population-based outreach and an office visit-based intervention. Secondarily, the study assessed follow-up colonoscopy rates after a positive mt-sDNA test, and time to the follow-up colonoscopy.
Methods
This was a retrospective observational study including 2 distinct interventions—population-based outreach and office visit-based interaction. The study focused on evaluating the completion rates of the mt-sDNA screening modality and follow-up colonoscopy among individuals who participated in these 2 interventions. The study was conducted among employees of the Metropolitan Nashville Public Schools (MNPS) system and their dependents and was approved by the Vanderbilt Institutional Review Board (IRB#192421).
For the population-based outreach, individuals aged 50 to 64 years who were due for CRC screening were identified from the claims data warehouse (Continuance Health Solutions) for certificated employees and dependents of the MNPS system as of September 30, 2018. Individuals were considered due for CRC screening if they did not have a recorded CPT or HCPCS code for: colonoscopy within the last 10 years, gFOBT within the last year, mt-sDNA within the last 3 years, flexible sigmoidoscopy or CT colonography within the last 5 years in their recorded healthcare claims data. These individuals received a letter from their employer-based health care clinic with educational material explaining the risk of CRC, the importance of screening, and encouraging them to speak with their provider about CRC screening. Additionally, the letter informed them that they would be receiving outreach from Exact Sciences Laboratories (ESL), the mt-sDNA laboratory, in mid-December 2018 regarding mt-sDNA screening. Individuals from this cohort who were receptive to ESL outreach were shipped a mt-sDNA kit to their home to complete the stool collection for the test. The test was then shipped back to ESL for processing, and the test results were provided to the employer-based health care clinic. The status of mt-sDNA test completion was assessed as of December 31, 2019.
For the office visit-based interaction, individuals who were due for CRC screening were identified during their visit to the participating employer-based health care clinic, based on having no record or recollection of: colonoscopy within the last 10 years, gFOBT within the last year, mt-sDNA within the last 3 years, flexible sigmoidoscopy or CT colonography within the last 5 years in their medical record. If the identified individual was seen in the employer-based health care clinic from July 1, 2018 to July 31, 2020, they were offered CRC screening by a nurse practitioner. If an individual chose mt-sDNA as their screening option, the staff at the employer-based health care clinic ordered the mt-sDNA kit from ESL to be shipped to the patient’s home for stool sample collection. The test was then shipped back to ESL for processing, and the test result was provided to the employer-based health care clinic. The status of test completion was assessed as of September 30, 2020. For both the interventions, the staff at the employer-based health care clinic delivered the test results to individuals who had undergone mt-sDNA screening and if there was a positive mt-sDNA result, the individual was referred for a follow-up colonoscopy.
Study Population Inclusion/Exclusion Criteria
For both interventions, individuals were included if they were at least 50 years old, male or female, and were identified as due for CRC screening based on the above-mentioned definitions. Additionally, for both intervention arms, individuals were not considered screening eligible if there was current evidence or history of being above average- or high-risk for CRC, as determined by the presence of at least 1 International Classification of Diseases (ICD)-9/ICD-10 code indicating the presence, history, or symptoms of any of the following: benign or malignant colorectal neoplasms, colorectal adenomatous polyps, inflammatory bowel disease (ulcerative colitis or Crohn’s disease), family history of CRC or colorectal adenomatous polyps, familial adenomatous polyposis, and hereditary nonpolyposis CRC. Individuals were also required to reside within the state of Tennessee and have a valid phone number. Individuals were excluded if they lost their health plan coverage (or otherwise became ineligible) during the study period or if the initially placed mt-sDNA order was canceled prior to shipping.
Additionally, the population-based outreach included only certificated employees (eg, teachers, principals, etc.) and their dependents who are covered by the MNPS health plan. Non certificated employees (eg, administrative and support staff) are covered under a separate benefit plan were excluded. For this intervention, continuous eligibility in the health plan during the evaluation period was required. Finally, individuals were excluded from the population-based outreach if they were aged 65 years and older or had Medicare as the primary insurer.
Study Measures
Demographic characteristics of individuals included age, gender, and race. The following study outcomes were assessed: mt-sDNA completion rate calculated as a proportion of individuals who return the completed mt-sDNA test kit after it has been shipped to their home, proportion of patients who performed follow-up colonoscopy among individuals with a positive mt-sDNA test result, and time to follow-up colonoscopy compliance following a positive mt-sDNA result.
For both the interventions, outcomes were calculated for individuals aged 50 to 64 years old who ordered tests between December 1, 2018 and December 31, 2019. For the office visit-based interaction, study outcomes were also calculated for the overall population (aged 50 years or older), as well as stratified by age (aged 50-64 years old and ≥65 years old) for the tests ordered between July 1, 2018 and July 31, 2020.
Statistical Analysis
Descriptive analyses including means (standard deviations [SDs]), median (interquartile range [IQR]) for continuous variables, and frequency distributions and percentages for categorical variables were performed to describe the study variables. Differences in rates were compared using z-scores for proportions. Differences in median time to colonoscopy after a positive mt-sDNA test result between the 2 outreach methods were determined using the Wilcoxon rank sum test.
Results
Cohort Characteristics
Population-based outreach
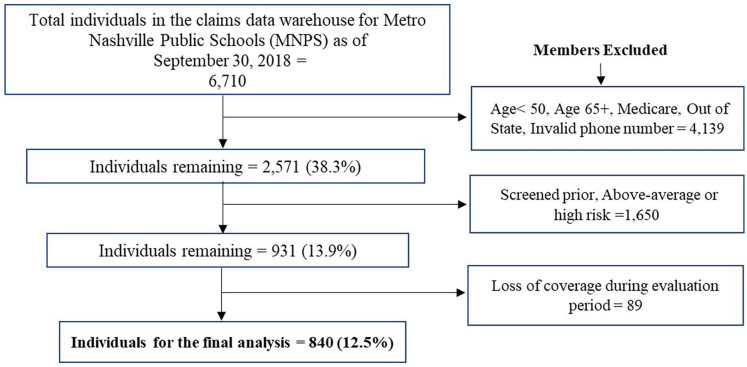
A total of 6710 individuals were identified from the claims data warehouse for the MNPS during the study identification period as of September 30, 2018. Of those, 840 individuals met the study inclusion criteria and were sent an outreach letter from the employer-based health clinic (Figure 1). The average age of the cohort was 56.0 (±4.4). More than half of the individuals were females (59.2%), and White (51.1%) (Table 1).
Figure 1.
Attrition flow chart (population-based outreach).
Table 1.
Demographic Characteristics.
| Measure | December 1, 2018-December 31, 2019 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Population-based outreach |
Office visit-based outreach |
|||||||
| Overall (Age 50-64) (n = 840) |
Overall (n = 101) |
Age 50-64 (n = 68) |
Age 65+ (n = 33) |
|||||
| N | % | N | % | N | % | N | % | |
| Age (years) | ||||||||
| Mean (SD) | 56.0 (4.4) | 60.5 (7.6) | 56.0 (4.5) | 69.7 (3.4) | ||||
| 50-54 years | 353 | 42.0% | 31 | 30.7% | 31 | 45.6% | NA | — |
| 55-59 years | 252 | 30.0% | 19 | 18.8% | 19 | 27.9% | NA | — |
| 60-64 years | 235 | 28.0% | 18 | 17.8% | 18 | 26.5% | NA | — |
| 65 years and above | NA | — | 33 | 32.7% | NA | — | 33 | 100.0% |
| Sex | ||||||||
| Male | 343 | 40.8% | 36 | 35.6% | 26 | 38.2% | 10 | 30.3% |
| Female | 497 | 59.2% | 65 | 64.4% | 42 | 61.8% | 23 | 69.7% |
| Race | ||||||||
| Black | 117 | 13.9% | 18 | 17.8% | 14 | 20.6% | 4 | 12.1% |
| White | 429 | 51.1% | 51 | 50.5% | 50 | 73.5% | 1 | 3.0% |
| Other/Unknown | 26 | 3.1% | 32 | 31.7% | 4 | 5.9% | 28 | 84.8% |
| N/A | 268 | 31.9% | 0 | — | 0 | — | 0 | — |
| Spouse/Partner | 268 | 31.9% | ||||||
Office visit-based interaction
Overall, 101 patients aged above 50 years participated in the office visit-based interaction from December 1, 2018 through December 31, 2019. Out of which, a total of 68 individuals aged 50 to 64 years were included in the main analysis as this group was directly comparable to the population-based outreach. The average age of the cohort was 56.0 (±4.5). Almost 62% of the participants were females and two-thirds of the participants were White (Table 1).
Table 2.
Study Outcomes.
| Measure | December 1, 2018-December 31, 2019 |
|||
|---|---|---|---|---|
| Population-based outreach |
Office visit-based intervention |
|||
| Overall (Age 50-64) | Overall | Age 50-64 | Age 65+ | |
| Kits shipped (N) | 167 | 86 | 56 | 30 |
| Kits with results (N) | 89 | 64 | 43 | 21 |
| Completion rate (%) | 53.5 | 74.4 | 76.8 | 70.0 |
| Among those with results: | ||||
| Subjects with positive test results, N (%) | 6 (6.7) | 7 (10.9) | 4 (9.3) | 3 (14.3) |
| Received follow-up colonoscopy (%) | 6 (100) | 6 (85.7) | 4 (100.0) | 2 (66.7) |
| Median (IQR) time to follow-up colonoscopy following a positive mt-sDNA result (in days) | 54.5 (44.3-67.0) | 89.0 (42.8-200.5) | 136.0 (41.0-226.0) | 85.5 (63.3-107.8) |
Mt-sDNA Completion Rates
For the population-based outreach, 167 out of 840 included individuals (19.9%) agreed to have the mt-sDNA shipped to their home. Of those shipped, 89 (53.3%) tests were completed. Among tests that were completed, 6 (6.7%) had positive test results while 83 (93.3%) had negative test results. All patients (100%) with positive results completed a follow-up colonoscopy. The median (IQR) time to follow up colonoscopy was 54.5 (44.3-67.0) days. In 5 of the patients, polyps were found and removed, and repeat colonoscopy was recommended within 3 years. For 1 patient, the reports were normal (an internal hemorrhoid was identified) and the patient was recommended to repeat colonoscopy in 10 years.
For the office visit-based outreach, a total of 56 mt-sDNA kits were shipped. Of those, results were available for 43 returned kits (mt-sDNA test completion rate = 76.8%). Out of those, 4 test results were positive, and all 4 individuals with a positive test result received a follow-up colonoscopy. The median (IQR) time to follow-up colonoscopy was 136.0 (±41.0-226.0) days (Table 2). Conclusive results were reported for only 2 patients—a polyp was found and removed in 1 patient, and results were negative for another patient who was recommended to repeat colonoscopy in 10 years.
When the rates were compared between the interventions, office visit-based interaction resulted in a significantly higher mt-sDNA completion rate as compared to the population-based outreach (76.8% vs 53.3%; P < .001) (Table 2). In this subgroup analysis, the proportion of individuals with positive test results was also higher for the office visit-based interaction as compared to the population-based outreach; however, the difference was not statistically significant (9.3% vs 6.7%; P > .05). Finally, the median time to follow-up colonoscopy after a positive test result was significantly shorter for individuals in the population-based outreach as compared to the office visit-based interaction (54.5 vs 136.0 days; P < .05).
Discussion
This study assessing mt-sDNA test completion was conducted in an employer-insured population of public-school teachers and their dependents. Notably, the mt-sDNA test is supported by a robust patient navigation program, as well as 24/7 telephonic assistance available for those who may need it.15 The outreach from ESL including reminders for test completion, on-demand support for mt-sDNA testing, as well as ease of testing at home may have impacted individuals’ decisions to get screened for CRC using the mt-sDNA test. This is particularly important from an employer perspective since non-invasive, at-home screening modalities such as mt-sDNA may result in less time off, that is, lost productivity due to days of work missed, a key factor to consider for employees such as the teachers included in the current study.
Results from this study showed that a little more than half of the individuals (53.3%) who were due for screening, met criteria for stool-based testing, and agreed to have a mt-sDNA kit shipped to their home, completed the mt-sDNA test after being identified from health plan data and receiving a letter from the employer health clinic (population-based outreach). All patients with positive mt-sDNA test results completed a follow-up colonoscopy within 3 months. Test completion rates were at least 70% among individuals who were seen by a nurse practitioner at the employer-based health care clinic, and during their visit had a discussion about CRC screening (office visit-based intervention).
A previous study conducted among Medicare patients aged 50 to 85 years found that mt-sDNA completion rate was as high as 88.3% for tests ordered from October, 2014 to September, 2015 by physicians in a multispecialty group practice (USMD Physician Services, Dallas, TX). The rate of follow-up colonoscopy was 96.1%.16 In our study, the mt-sDNA completion rate among individuals aged 50 years or older for tests ordered by providers in the employer-based health care clinic (office visit-based intervention) was 75.0%. Differences in underlying population characteristics (eg, education, profession), younger age, and insurance status (eg, Medicare vs non-Medicare) may have contributed to the differences in the overall test completion rates in our study as compared to the Prince et al study. Interestingly, previous studies evaluating the effect of outreach programs related to stool-based tests mostly included the Medicare population.5,16,17 Our study adds to the existing research literature showing the impact of inreach and outreach interventions for the mt-sDNA CRC screening test among a younger commercially insured employer population.
We found that both these strategies offered a potential to improve mt-sDNA test completion rates. The office visit-based intervention yielded a higher test completion rate (76.8%) as compared to the population-based outreach (53.5%) for individuals aged 50 to 64 years who ordered a test between December 1, 2018 and December 31, 2019. Several factors including population demographics and established engagement with a provider may have resulted in a higher completion rate among office visit-based intervention arm. An in-person encounter with a provider, in this case a nurse practitioner, may be effective in removing the initial compliance barrier among these individuals.12 During their visit at the employer-based health care clinic, individuals can ask questions and clarify any doubts with the providers directly, perhaps easing the initial screening inhibition.18 Further, having an established relationship with the ordering provider and a sense of trust in the provider may also play an important role in individuals following through with the completion of the at-home mt-sDNA test. This may have influenced the shorter time to follow-up colonoscopy in this group as well; however, we do not have a sufficient sample size to support this conclusion.
This study raises the question of how deploying inreach and outreach intervention strategies in concert with each other, instead of as distinct strategies, may improve outcomes. The office visit-based intervention offers an opportunity to engage existing patients (coming to see providers for care), while the population-based approach may also identify individuals who do not have a consistent relationship with a health care provider or would not otherwise engage with a health care provider. It is noteworthy that in the population-based approach, almost 20% of the members identified through MNPS claims data as being due for screening who then received a letter and follow-up ESL patient navigation agreed to screening using mt-sDNA. More than half of these individuals subsequently completed the mt-sDNA test. This is intriguing as a potentially low-cost method for increasing CRC screening completion rates and should be explored further. Future studies should focus on the impact of combining these outreach strategies and assessing this combined impact on mt-sDNA completion rates as well as CRC screening overall to further understand how these outreach strategies can optimize screening outcomes.
We also found that within the office visit-based intervention group, individuals aged 50 to 64 years old had a higher completion rate as compared to those above 65 years of age. The rate of follow-up colonoscopy was also higher in the younger group; however, the median time to colonoscopy after a positive mt-DNA result was longer within this group as compared to the older group. The ease of mt-sDNA testing at home without any extensive bowel preparation, invasive instruments, sedation, or travel to physician office or ambulatory center may drive younger individuals to complete the mt-sDNA testing. Note that this is a commercially insured population and the mt-sDNA screening test is covered through their insurance minimizing the economic barriers to screening among this cohort. Thus, patient preference for a non-invasive, at-home CRC screening modality may be playing an important role in screening completion rates. Further research is warranted to better understand patient preference and factors associated with the choice of CRC screening modalities.
This study has several limitations. First, the focus of the current study is on mt-sDNA test completion rates, and we did not evaluate overall screening rates in this population. The completion rates reported may not be generalizable to all populations since the study included average-risk individuals in a select commercially insured population of MNPS employees and their dependents. The participants in this study were predominantly classroom teachers and therefore would have higher education than the average American population. Most of the MNPS teachers have college degrees or higher and, in this study, about 60% to 70% of participants were female, depending on the study cohort. Additionally, the majority of the study population was non-Medicare; hence these results may not be generalizable to the Medicare population. Further studies are needed in more diverse populations and other employer settings to evaluate the different outreach methods among average-risk individuals. In the population-based outreach arm, mt-sDNA kits were shipped to ~20% of the screening-eligible individuals after they were contacted by ESL (167 out of 840), thus limiting our denominator of kits shipped to a smaller proportion of screening-eligible individuals. Next, the small sample size particularly as it relates to the follow-up colonoscopy trends after a positive mt-sDNA test, limited our ability to show statistically significant differences and draw any meaningful conclusions. Hence, these data are only reported descriptively. Note that the sample size in our study was small and the median time to follow-up colonoscopy ranged from 41 to 226 days for individuals aged 50 to 64 in the office visit-based interaction group. The range was narrower for the population-based outreach (44-67 days). Due to the lower sample size and wide distribution among office visit-based interaction group, these results should be interpreted cautiously. Further studies with a larger sample size are needed to better understand the patterns in follow-up colonoscopy after different outreach methods. Note that the starting sample sizes were different for the 2 interventions (n = 840 in the population-based group, n = 68 in the office visit-based group); however, the denominator for the test completion rate was the total number of kits shipped (n = 167 in the population-based group, n = 56 in the office visit-based group). We used z-scores for proportions to compare the test completion rates to minimize the effect of unequal sample sizes. Our post-hoc power calculations indicated 90.6% statistical power at 0.05 Type I/II error rate. As such, we believe the study had sufficient statistical power to support the validity of the results. Also, the individuals analyzed in both outreach arms chose to receive the mt-sDNA test for their CRC screening and the sample was not randomized, hence introducing potential selection bias. By limiting the denominator to those who were shipped a mt-sDNA kit, there may be a bias toward those who are primed to be screened. Additionally, we did not require long-term continuous enrollment in the pre-period for the employer health plan outreach to confirm the screening history of the study population. There was a variable lookback period. We excluded members that are potentially above average-risk or high-risk for CRC from both groups. Family history is often not well documented in the medical record, especially when relying on ICD-10 codes as in the current study. Therefore, additional patients may not have been eligible for mt-sDNA due to family history.
Further, we did not collect data on how many of the patients who were offered mt-sDNA refused CRC screening entirely or requested other forms of screening instead, as the current study focused on mt-sDNA test completion rates. Understanding patient preferences for the various screening modalities was outside the scope of this study. Although we had 2 distinct intervention arms and ensured that there was no overlap in the individuals being evaluated in each arm, it is possible that some of the office visits in the office-visit-based intervention arm may have been triggered by educational materials received as a part of the population-based outreach. This may have positively impacted completion rates in the office visit-based interaction group. Finally, in the office visit-based interaction arm, if there was not a long-standing medical record at the employer clinic, we had to rely on patient recollection of their medical and screening history to define their current screening status.
Implications for Practice
This study showed that among average-risk individuals aged 50 years or older who were CRC screening-eligible and due for screening, both the population-based outreach and office visit-based interaction methods triggered the completion of mt-sDNA tests. The office visit-based interaction had significantly higher mt-sDNA test completion rates as compared to the population-based outreach. To increase awareness of colorectal cancer risks and the benefits of screening, there needs to be an ongoing effort to identify and recommend CRC screening to all eligible individuals.
Acknowledgments
We would like to thank Dr. Philip Parks for his assistance during the design phase of the study, and Dr. Monali Bhosle for medical writing support.
Footnotes
Author Contributions: Dr. Shepherd, Ms. Lecorps, and Dr. Miller-Wilson contributed to the conception, study design, data acquisition, analysis, and interpretation. Mr. Harris-Shapiro has contributed to data acquisition and analysis. All authors approved the final submission.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Shepherd, Ms. Lecorps and Mr. Harris-Shapiro have no disclosures to report. Dr. Miller-Wilson is an employee and stockholder of Exact Sciences. Parts of this research study were presented at the ASPO 2021 Virtual Conference (March 29, 2021-April 1, 2021).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lesley-Ann Miller-Wilson  https://orcid.org/0000-0003-4983-4041
https://orcid.org/0000-0003-4983-4041
References
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. doi: 10.3322/caac.21601 [DOI] [PubMed] [Google Scholar]
- 2.NIH DHHS.Cancer Trends Progress Report. National Cancer Institute; 2019:https://progressreport.cancer.gov [Google Scholar]
- 3.American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022. American Cancer Society; 2020. [Google Scholar]
- 4.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(12):1645-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melson JE, Imperiale TF, Itzkowitz SH, et al. AGA white paper: roadmap for the future of colorectal cancer screening in the United States. Clin Gastroenterol Hepatol. 2020; 18:2667-2678.e2. doi: 10.1016/j.cgh.2020.06.053 [DOI] [PubMed] [Google Scholar]
- 7.Lim KT, Ng CH, Decruz GM, et al. Barriers and facilitators towards colonoscopy: a qualitative systematic review. Eur J Cancer Prev. 2021;30(3):232-238. [DOI] [PubMed] [Google Scholar]
- 8.Gawron AJ, Staub J, Bielefeldt K.Impact of health insurance, poverty, and comorbidities on colorectal cancer screening: insights from the medical expenditure panel survey. Dig Dis Sci. 2021;66:70-77. doi: 10.1007/s10620-020-06541-7 [DOI] [PubMed] [Google Scholar]
- 9.Sanger J.Barriers to colorectal cancer screening in adults: an integrative review. Nursing Masters Papers. 2020; 382. https://operriver.winova.edu/nursingmasters/382. [Google Scholar]
- 10.Ahlquist DA.Stool-based tests Vs screening colonoscopy for the detection of colorectal cancer. Gastroenterol Hepatol. 2019;15(8):437-440. [PMC free article] [PubMed] [Google Scholar]
- 11.Alberti LR, Garcia DP, Coelho DL, De Lima DC, Petroianu A.How to improve colon cancer screening rates. World J Gastrointest Oncol. 2015;7(12):484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson EB, Ostroff JS, DuHamel KN, et al. Impact of provider-patient communication on cancer screening adherence: a systematic review. Prev Med. 2016;93:96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight JR, Kanotra S, Siameh S, Jones J, Thompson B, Thomas-Cox S.Understanding barriers to colorectal cancer screening in Kentucky. Prev Chronic Dis. 2015;12:E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braschi C, Pelto DJ, Hennelly MO, et al. Patient-, provider-, and system-level factors in low adherence to surveillance colonoscopy guidelines: implications for future interventions. J Gastrointest Cancer. 2014;45(4):500-503. [DOI] [PubMed] [Google Scholar]
- 15.Weiser E, Parks PD, Swartz RK, et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening: real-world data from a large cohort of older adults. J Med Screen. 2021;28(1):18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prince M, Lester L, Chiniwala R, Berger B.Multitarget stool DNA tests increases colorectal cancer screening among previously noncompliant Medicare patients. World J Gastroenterol. 2017;23(3):464-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Issaka RB, Akinsoto NO, Strait E, Chaudhari V, Flum DR, Inadomi JM.Effectiveness of a mailed fecal immunochemical test outreach: a Medicare advantage pilot study. Therap Adv Gastroenterol. 2020;13:1756284820945388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson Shen M, Elston Lafata J, D’Agostino TA, Bylund CL.Lower adherence: a description of colorectal cancer screening barrier talk. J Health Commun. 2020;25(1):43-53. doi: 10.1080/10810730.2019.1697909 [DOI] [PMC free article] [PubMed] [Google Scholar]