Abstract
Background:
The favorable benefit-risk profile of topical nonsteroidal anti-inflammatory drugs (NSAIDs) makes them a preferred treatment for pain relief in soft tissue injuries.
Purpose:
To assess the efficacy and safety of a novel etofenamate 70-mg medicated plaster in patients with acute uncomplicated ankle sprain.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
Patients with grade 1 or 2 ankle sprain of recent onset were randomized to etofenamate or placebo plasters (1:1) applied twice daily for 7 days. Clinical assessments, including ankle pain on movement (POM) in mm on a 100-mm visual analog scale (VAS), were made at predefined intervals during the treatment period.
Results:
In total, 156 male or female adult patients (mean age, 35.3 ± 11.8 years) were enrolled. The fall in VAS values for POM from baseline to 72 hours was markedly in favor of the etofenamate plaster, with respective reductions of 52.7% and 24.0% for active and placebo plasters (least squares mean treatment difference, 22.1 mm; P value for analysis of covariance < .0001). Similar clinically relevant differences between etofenamate and placebo were seen for POM at the 48-, 96-, and 168-hour visits (P < .0001). These differences between etofenamate and placebo plasters were reflected in area under the curve for POM, pain at rest, and ankle swelling measured at various time points during the 7 days. Time taken to achieve a meaningful (30%) and optimal (50%) reduction of POM was significantly shorter in the etofenamate group. The responder rate (proportion of patients with at least 50% pain reduction at 72 hours) was 52.5% for the etofenamate plaster and 7.7% for the placebo. A significantly greater proportion of patients randomized to etofenamate rated their progress and/or the treatment as “good” or “very good.” The medicated plasters adhered well over the 12-hour dosing period and were very well-tolerated.
Conclusion:
With respect to the investigated indication, uncomplicated ankle sprain, the etofenamate plaster has therapeutic efficacy that is similar to that for the best available topical NSAID formulations.
Registration:
2016-000252-99 (EudraCT number).
Keywords: etofenamate, medicated plaster, acute ankle sprain, pain on movement, pain relief, safety
Ankle sprain represents one of the most common soft tissue injuries; the annual incidence of those admitted to the emergency department (ED) with a sprained ankle ranged from 50 to 61 per 10,000 in the United Kingdom, while a US study found an incidence rate in EDs of 21.5 per 10,000 individuals per year.3,17 The actual incidence is estimated to be much higher than this; many cases do not present for medical care, as the nature of uncomplicated ankle sprain is that it is a self-limiting condition.
The most frequent mechanism in ankle injury is inversion of the joint (supination and adduction of the plantarflexed foot); this results in tearing of the lateral collateral ligaments, particularly the anterior talofibular ligament, which is injured in isolation in >60% of ankle sprains.16 Ankle sprains are graded from 1 to 3 according to the extent of ligamentous tear and degree of functional impairment, including degree of mechanical instability.18 Diagnosis is made on the basis of history and clinical examination; particular attention should be given to bony palpation in order to rule out possible associated fracture.14
The mainstay of treatment for uncomplicated ankle sprain is nonrigid or semirigid ankle support (eg, elastic bandages, tapes, braces), along with adequate pain relief. Topical nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to be superior to placebo for pain alleviation in acute ankle sprain, and given their favorable adverse-effect profile, they are preferred for the initial treatment of this condition.15 Paracetamol at therapeutic doses (2-4 g/day) is recommended as an additional analgesic.13 The evidence for other treatment options is weak; however, it is recommended that exercise therapy including proprioceptive/balance training is started as soon as possible.13
Products containing the well-known NSAID etofenamate have been on the European market for >20 years (eg, Rheumon Gel, Traumalix Spray). Cutaneous etofenamate products such as gels, creams, lotions, and sprays contain the drug at concentrations of 5% and/or 10% and are used for the relief of pain and inflammation associated with musculoskeletal injury and soft tissue disorders. Drossapharm has developed a novel medicated plaster formulation containing 70 mg of etofenamate as its active ingredient. The patch, intended for twice daily application at the injured area over an expected treatment duration of up to 7 days (ie, a maximum daily dose of 140 mg), provides constant delivery of etofenamate throughout the dosing interval and is considered to be more convenient for patients than other topical formulations.
The primary objective of the study was to evaluate the efficacy of the etofenamate 70-mg medicated plaster, particularly with regard to pain relief, compared with a placebo plaster in patients with acute ankle sprains who were treated with the respective patches applied twice daily to the affected ankle for 7 days. The null hypothesis proposed no difference between study treatments; assessment of the safety and tolerability of the novel etofenamate-containing plaster was a secondary objective of the study.
Methods
Study Design
This was a multicenter, randomized, placebo-controlled, double-blind study in patients with acute uncomplicated unilateral ankle sprains of recent onset (protocol provided as supplement). The study was conducted across 5 clinical centers, and the study protocol received approval from the ethics committee.
Overall, it was planned to enroll approximately 152 patients. After providing written consent and initial clinical assessment, eligible patients were to be assigned at random (ratio 1:1) to treatment with either the etofenamate 70-mg medicated plaster (Drossapharm AG) or a matching placebo plaster. Patients returned for assessment after 12 hours; further follow-up visits were scheduled at 24, 48, 72, 96, and 168 hours. All study assessments as well as primary and secondary outcome measures were prespecified in the study protocol.
Participants
Male or female patients aged 18 to 60 years with acute sprain of the lateral ankle of grade I or II (based on clinical assessment by the investigators who are experienced sports physicians [H.-G.P., B.G.]) and sustained within the previous 12 hours were considered for eligibility. At presentation, patients had to have ankle pain on movement (POM) of ≥50 mm on a 100-mm visual analog scale (VAS) and otherwise satisfactory health as determined by the investigator based on medical history and physical examination. Patients were excluded if there was a serious injury to the ankle (eg, grade III sprain, fracture, nerve injury, or open wound); if the sprain was medial; or if the proposed application site on the affected ankle was excessively hairy, had a chronic skin disorder, or was subject to excessive sweating. Prior intake of NSAIDs or analgesics (36 hours), opioids (7 days), or corticosteroids (60 days) or application of topical medication since the injury resulted in exclusion. Pregnant or lactating women and patients with clinically significant concomitant illnesses were also excluded from the study.
Treatment Regimen
Study treatments (etofenamate or placebo) were administered as 1 plaster applied over the anterior aspect (for optimal adhesion) of the affected ankle every 12 hours for 14 doses (7 consecutive treatment days). Plasters were rectangular with rounded corners, 10 × 14 cm, and consisted of an adhesive matrix layer containing 70 mg etofenamate and a backing layer of bielastic polyester fabric (Figure 1). After removal of the release liner, the plaster was applied to dry skin over the injured area; it had to be pressed to the skin for optimal adhesion. Patients were instructed in the correct application of the study treatment at the first visit (enrollment), after which the plasters were self-administered twice daily. Placebo and active plasters were identical in every way, apart from the presence of etofenamate in the latter. No additional supportive bandages were applied. RICE (rest, ice, compression, elevation) therapy was permitted at the discretion of the investigator. Patients used crutches until weightbearing was possible.
Figure 1.
Etofenamate 70-mg medicated plaster.
Study Assessments
Efficacy. At each visit, ankle POM was assessed in millimeters on a 100-mm VAS (0 mm = no pain, 100 mm = extreme pain). The same scale was used to assess pain at rest (PAR) at each visit. The extent of ankle swelling was assessed at each visit; the circumference of the affected and nonaffected ankles was measured (average of 3) by placing the tape measure around the ankle in a figure-of-8 fashion, crossing the navicular tuberosity, the tip of the lateral malleolus, the tip of the medial malleolus, and the base of the fifth metatarsal.10 Global efficacy assessments were made at the 72-hour and 168-hour visits; patients were asked: “Considering all the ways this treatment has affected you since you started in the clinical trial, how well are you doing?” and “How do you rate this medication as a treatment for the pain of ankle sprain?”; each of these questions was graded on a 5-point Likert scale. Use of rescue medication (paracetamol, up to 1 g per day) was recorded at every visit except the enrollment visit.
POM data collected at each visit was used to generate additional variables, including area under the curve (AUC; calculated using the trapezoidal rule) of POM VAS pain scores over 0 to 48, 0 to 72, and 0 to 96 hours; Pain Intensity Difference (PID) or difference in POM VAS at 48, 72, and 96 hours compared with baseline; Sum of Pain Intensity Differences (SPID) over 0 to 48, 0 to 72, and 0 to 96 hours; time to achieve meaningful/optimal reduction of pain defined in line with Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials thresholds of pain relief over baseline as 30% (“moderate”) and 50% (“substantial”) reduction of baseline VAS for POM7; and Responder Rate, defined as the proportion of patients with at least 50% pain reduction at 72 hours.5
Adhesion. At each visit, adhesion of the etofenamate 70-mg medicated plaster was assessed using a 6-point scale (completely adhered, edges detached, >75% adhered, ≥50% adhered, <50% adhered, completely detached).
Safety. The occurrence of adverse events (AE) after drug administration was recorded at each visit after enrollment. In addition, at each visit, local tolerability was assessed using a 7-point scale (0 = no evidence of irritation; 1 = minimal erythema, barely visible; 2 = definite erythema, readily visible, minimal edema, or minimal papular response; 3 = erythema and papules; 4 = definite edema; 5 = erythema, edema, and papules; 6 = vesicular eruption; 7 = strong reaction spreading beyond test site). Moreover, at each visit, vital signs were recorded and a physical examination was performed.
Statistical Considerations. The random allocation sequence (block size of 4) was generated by a third party unconnected to the trial; all persons involved in the conduct of the trial remained blind to treatment allocation throughout the study. Randomization was implemented at the time of packaging and labeling so that active and placebo plasters were sequentially numbered in accordance with the code; investigators assigned each patient the next available treatment number at the time of enrollment.
The sample size was calculated based on the outcome of an earlier study with the etofenamate 70-mg medicated plaster in patients with acute ankle sprain. For the primary efficacy outcome of POM at 72 hours after initiating treatment, assuming a level of significance of α = 5% and an SD of 20 mm, a sample size of 76 per group provides approximately 90% power to detect a difference of 10.6 mm between the 2 treatment groups. This is half of the mean difference seen in the previous study and in line with what is considered to be a minimal clinically significant difference in VAS pain scores.9
Statistical Analysis. The random allocation sequence was released by the independent third party after database lock, after which study data were derandomized. The primary analysis was on the intent-to-treat population (full analysis set); a per-protocol analysis was also performed. Demographic and baseline values were analyzed descriptively. For quantitative efficacy outcomes assessed at the clinical trial sites (POM, PAR, ankle swelling) and derived outcomes (AUC), null hypotheses were tested with an analysis of covariance (ANCOVA) model. For comparisons using the specified ANCOVA models above, the least squares (LS) mean for each treatment and the corresponding difference between LS means (active plaster − placebo) with the P value and 95% CI were presented from the model. For ordered categorical outcomes (global efficacy assessments), differences between treatments were tested with the Cochran-Mantel-Haenszel (CMH) test of treatment mean ridits, stratified by site.
The incidence of all treatment-emergent AEs was tabulated after grouping by body system and preferred term using the MedDRA dictionary of terms. Severity and relationship to study treatment were determined for all treatment-emergent AEs. Safety data were presented for the full analysis set.
Results
Patient Disposition and Baseline Characteristics
In total, 156 male and female adult patients with acute uncomplicated unilateral ankle sprain with a mean age of 35.3 years were enrolled from 5 study centers between September 2016 and April 2017 (Table 1). Each of the 5 sites enrolled and randomized from 20 to 40 patients. There were no withdrawals; therefore, 78 patients received the etofenamate 70-mg medicated plaster and 78 received placebo (Figure 2). Compliance was good; all patients attended all study visits. Of the scheduled applications, >99% were carried out in both treatment groups; as allowed by the study protocol, a few patients had their final visit after only 12 or 13 of the planned 14 plasters. One patient used rescue medication (single dose of paracetamol for headache); no patients used RICE treatment.
TABLE 1.
Demographic and Baseline Data for the Full Analysis Seta
| Etofenamate (n = 78) | Placebo (n = 78) | |
|---|---|---|
| Age, y | 33.8 ± 11.6 | 36.9 ± 11.9 |
| Weight, kg | 81.1 ± 18.8 | 78.3 ± 15.6 |
| Height, cm | 177.2 ± 11.0 | 176.4 ± 9.3 |
| Male patients | 50 (64.1) | 42 (53.8) |
| POM at baseline, mm | 76.8 ± 10.9 | 75.5 ± 10.2 |
| PAR at baseline, mm | 32.6 ± 17.2 | 30.7 ± 16.6 |
| Ankle swelling at baseline (injured – uninjured ankle), cm | 1.8 ± 1.1 | 1.9 ± 1.2 |
| Grade of sprain | ||
| 1 (mild) | 38 (48.7) | 44 (56.4) |
| 2 (moderate) | 40 (51.3) | 34 (43.6) |
aData are reported as mean ± SD or n (%). PAR, pain at rest; POM, pain on movement.
Figure 2.
Grouping and flow of study patients.
All 156 study patients were included in the primary analysis (full analysis set). At the blind review meeting, it was found that 2 patients (1 from each treatment group) were aged >60 years and had been enrolled in error. The per-protocol analysis, which excluded these patients, did not differ from the primary analysis and is therefore not presented here.
Primary Efficacy Variable
The reduction in VAS values from baseline to 72 hours for POM was markedly greater in the active treatment group than in the placebo group (respective reductions of 52.7% and 24.0%), with an average (LS mean difference) treatment effect of 22.1 mm in favor of the etofenamate 70-mg medicated plaster (P value for ANCOVA < .0001).
Secondary Efficacy Variables
As for the primary efficacy variable, POM assessed by VAS at the 48-, 96-, and 168-hour visits decreased more markedly from baseline in the actively treated group compared with placebo (P values for ANCOVA < .0001). The difference between the etofenamate 70-mg medicated plaster and placebo is depicted in Figure 3. In line with the differences seen over time for POM, the AUC for POM at 0 to 48, 0 to 72, and 0 to 96 hours differed significantly (P < .0001) between the 2 treatment groups. As might be expected, the results for PID were very similar to those for the POM change from baseline data presented in Table 2, while the results for SPID for the time frames 0 to 48, 0 to 72, and 0 to 96 hours were similar to those for AUC for POM, although with a slightly larger treatment effect seen in the SPID analysis.
Figure 3.
Mean pain on movement over time (full analysis set), shown as mean relative change (%) from baseline. Error bars represent 95% CI.
TABLE 2.
POM Across Study Visits (Full Analysis Set)a
| Etofenamate (n = 78) | Placebo (n = 78) | LS Mean Difference (95% CI) | P Value (ANCOVA) | |
|---|---|---|---|---|
| POM at baseline, mm | 76.8 ± 10.9 | 75.5 ± 10.2 | — | — |
| POM at 48 h, mm | 46.5 ± 22.3 | 62.8 ± 14.8 | 17.4 (13.5-21.3) | <.0001 |
| POM at 72 h, mm | 36.3 ± 21.8 | 57.4 ± 16.1 | 22.1 (18.2-26.0) | <.0001 |
| POM at 96 h, mm | 27.9 ± 21.6 | 50.1 ± 17.4 | 23.0 (18.9-27.1) | <.0001 |
| POM at 168 h, mm | 12.8 ± 13.5 | 31.2 ± 17.9 | 19.1 (14.8-23.4) | <.0001 |
aData are reported as mean ± SD unless otherwise indicated. Dashes indicate that there were no applicable data. ANCOVA, analysis of covariance; LS, least squares; POM, pain on movement.
The differences between treatments seen for POM were reflected in the changes seen for PAR over the 7-day treatment period (Figure 4). The change in ankle swelling from baseline also showed a significant treatment effect in favor of the etofenamate 70-mg medicated plaster. The LS mean treatment effects seen at 72 and 168 hours were 3 mm (95% CI, 1-4; P = .0030) and 4 mm (95% CI, 2-6; P = .0003), respectively.
Figure 4.
Mean pain at rest over time (full analysis set), shown as mean relative change (%) from baseline. Error bars represent 95% CI.
Global Efficacy
The responses to the global efficacy questions asked at 72 and 168 hours showed a higher proportion of patients in the etofenamate group who rated their progress and/or the treatment as “good” or “very good”; conversely, a higher proportion of patients in the placebo group gave ratings of “poor” or “fair” (CMH test P values < .0001 for both questions and visits).
Time to Respond
The time taken to achieve a meaningful (30%) and optimal (50%) reduction of pain measured on the VAS for POM was significantly shorter in the etofenamate group. To be counted as meaningful or optimal reduction, the values of POM were only taken into account if the corresponding reduction was still achieved at the later visits. The respective times for the etofenamate 70-mg medicated plaster and the placebo plaster are presented in Table 3.
TABLE 3.
Median Time to Meaningful/Optimal Reduction of POM (Full Analysis Set)a
| Etofenamate (n = 78) | Placebo (n = 78) | P Value (log-rank test) | |
|---|---|---|---|
| Time to meaningful (30%) reduction of POM measured by VAS, h | 47.5 (46.5-70.1) | 128.1 (95.2-166.3) | <.0001 |
| Time to optimal (50%) reduction of POM measured by VAS, h | 71.5 (70.0-95.3) | 167.4 (167.0-167.8) | <.0001 |
aData are reported as median (95% CI). POM, pain on movement; VAS, visual analog scale.
The responder rate (proportion of patients with at least 50% pain reduction at 72 hours) was 52.5% for the etofenamate 70-mg medicated plaster and 7.7% for the placebo plaster. By the final visit (168 hours), only 3 patients in the etofenamate group (3.8%) had not achieved at least 50% reduction in baseline VAS for POM; the corresponding figure for placebo was 30 (38.5%).
Adhesion
Adhesion assessed by the investigators at each study visit (apart from baseline) immediately before patch removal was very good, with 72.4% and 22.7% scores of 0 (completely adhered) or 1 (edges detached), respectively. There were no scores of 4 (<50% adhered) or 5 (completely detached).
Safety
In this study, 5 AEs were reported by 5 of the 156 patients randomized (3.2%); 2 AEs were reported in the etofenamate group and 3 AEs in the placebo group. These AEs were all of mild or moderate intensity; none was considered serious, and all had resolved by the end of the study. For 1 patient in the placebo group, mild application-site erythema was reported that was considered related to the investigational trial medication (ie, reaction to components of the plaster other than etofenamate). No skin reactions and/or application-site reactions were reported in any of the patients treated with etofenamate 70-mg medicated plaster. One report of mild conjunctivitis in the etofenamate group was considered to be possibly related to study medication, due to the self-administration of the medicated plaster by the patient. The other AEs (2 headaches, 1 complaint of fatigue) were considered in the opinion of the investigator to not be related to study treatment.
Based on the scores for the local tolerability evaluations made by the investigators, the study treatments were very well-tolerated; only 1 patient in the placebo group showed minimal erythema (reported at the 96- and 168-hour visits). All other patients had local tolerability scores of 0 (no evidence of irritation) throughout the study. These results are consistent with those of a previous (unpublished) study that assessed the local irritancy and sensitization potential of the etofenamate 70-mg medicated plaster in 120 healthy individuals.
Discussion
This pivotal phase-3 study in adults with uncomplicated mild-to-moderate ankle sprain demonstrated a significant and clinically relevant improvement in POM in the days after injury for patients randomized to the etofenamate 70-mg medicated plaster compared with a placebo plaster. Time to achieve meaningful and optimal pain relief was significantly shorter for those on active treatment, with more than half of patients achieving a POM reduction >50% from baseline levels by 72 hours. The significant improvement seen in POM for the active plaster compared with placebo was accompanied by improvements in PAR and reduction in ankle swelling over the treatment period. Patient perception of the etofenamate plaster was good, with significantly more positive responses to the global efficacy questions for the active treatment. The medicated plaster adhered well and was well-tolerated over 7 days of twice-daily application. Of note, these findings are very similar to those of an earlier proof-of-concept study of identical design and conducted in 4 of the same centers in 80 patients.19
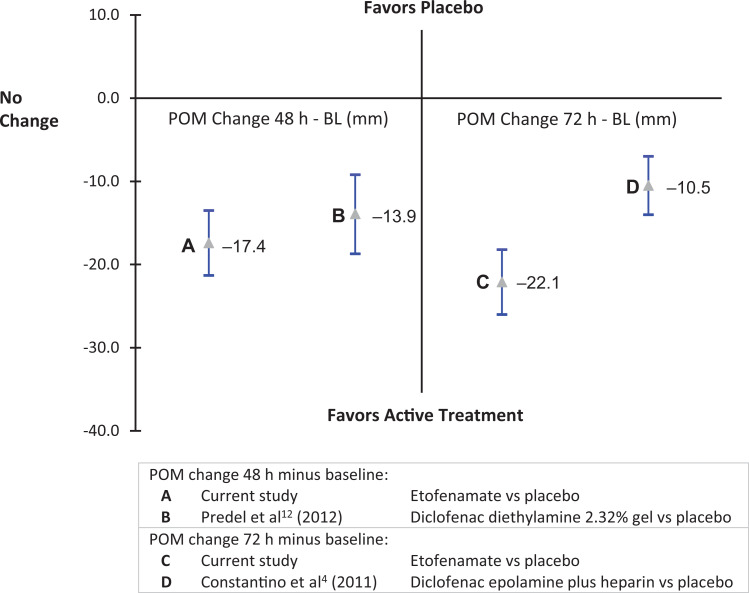
The lack of inclusion of an active comparator arm in this study is offset by the fact that the LS mean treatment effects seen for the etofenamate 70-mg medicated plaster in the first few days of treatment of acute grade I to II ankle sprain were similar to those seen in placebo-controlled studies of other topical NSAID formulations (Figure 5).4,12 In a study of very similar design, the investigators found mean differences in VAS for POM between diclofenac diethylamine 2.32% gel (Voltaren Emulgel; Novartis Consumer Health) and placebo of ∼14 mm at 48 hours and ∼23.5 mm at 96 hours.12 In another similar study, the difference between a diclofenac hydroxyethylpyrrolidine/heparin plaster (Flectoparin Tissugel; IBSA Institut Biochimique) and placebo for mean difference (reduction) in POM of the injured ankle at 48 hours was −10.5 mm (95% CI, −6.98 to −14.0).4 In this study at 48 hours, the difference between etofenamate and placebo arms was −17.4 mm (95% CI, −13.5 to −21.3).
Figure 5.
Comparison with other topical nonsteroidal anti-inflammatory drugs in the treatment of ankle sprain (mean difference and 95% CI of full analysis set). BL, baseline; POM, pain on movement.
The percentage reduction from baseline for POM over 7 days of treatment seen for the etofenamate plaster was 83.3%. This is similar to or better than the extent of symptomatic improvement reported in other studies of topical NSAIDs that used the ankle sprain model. Reductions in POM score (100-mm VAS) after 7 days of treatment with a ketoprofen 100-mg patch applied once daily, diclofenac diethylamine 2.32% gel applied 3 times a day, and with diclofenac sodium 1% gel applied 3 times a day, were 70%, 81%, and 69%, respectively.8,11,12 In previous studies in which etofenamate 5% gel (Traumon) was compared with placebo in the treatment of acute ankle sprain, the investigators reported percentage improvements in POM after 168 hours for active treatment of 53%, 57%, and 50%, respectively.1,2,6
In an update of a Cochrane Database Systematic Review, the authors considered 61 studies of topical NSAIDs for acute musculoskeletal pain in adults.5 Most of these studies compared topical NSAIDs in the form of gels, sprays, creams, or medicated plasters with a similar topical placebo. Formulations of topical diclofenac, ibuprofen, ketoprofen, piroxicam, and indomethacin demonstrated significantly higher rates of clinical success (more participants with at least 50% pain relief) than matching topical placebo. The available data were considered to be of moderate or high quality. The review concluded that topical NSAIDs provided good levels of pain relief in acute conditions such as sprains, strains, and overuse injuries, probably similar to that provided by oral NSAIDs. This conclusion is consistent with a meta-analysis that focused on acute ankle sprain and found that the available data support the use of topical NSAIDs for the initial treatment of this condition.15
As part of its systematic review, the Cochrane review team calculated the “number needed to treat” (NNT) for an additional beneficial outcome compared with placebo; that is, the number of patients to be treated with a particular topical NSAID formulation in order for 1 patient to experience clinical success (defined in this case as a 50% reduction in pain) who would not have experienced this with placebo.5 According to these authors, for topical NSAIDs, an NNT value <4 is considered to be indicative of a high level of efficacy. Based on their calculations, they concluded that the most effective topical NSAIDs include gel formulations of diclofenac (such as Emulgel), ibuprofen, and ketoprofen, and some diclofenac plasters.5 All other drug and formulation combinations had NNT values >4, indicating lesser efficacy. The NNT was calculated for the etofenamate 70-mg medicated plaster based on pooled data for responder rates at 72 hours for this study and the earlier proof-of-concept study of identical design (total of 232 participants) and was found to be 2.2 (95% CI, 1.9-2.6). This is well below 4 and similar to NNT values for the best available topical NSAID formulations. Of note, the NNT value of 2.2 calculated for the etofenamate plaster was superior to values calculated for the Flector Patch (NNT value of 4.7; Pfizer), and for other medicated plasters containing diclofenac, including Flectoparin Tissugel (NNT value of 3.2).5
The principal limitation of this study was the lack of an active comparator arm, which means that the efficacy of the etofenamate plaster cannot be compared directly with that of another product licensed for the same indication. It must be considered, however, that the development of a novel medicated plaster formulation does not allow for the possibility of a true double-blind study, as the most appropriate topical comparators are either gel formulations (eg, Voltaren Emulgel) or plasters of different design (eg, Flectoparin Tissugel). This is an important consideration when assessing a condition (acute mild-to-moderate ankle sprain) that is self-limiting and where differences in appearance and/or administration may influence the largely subjective endpoints.
Conclusion
Clinical studies of the novel etofenamate 70-mg medicated plaster have demonstrated clinically relevant and statistically significant superiority over placebo across a range of predefined outcome variables, including nonassociated endpoints. When taken together with comparative data from other studies of topical NSAIDs that used the ankle sprain model, the low NNT calculated for the plaster, and the data from relevant meta-analyses, it can be concluded that the etofenamate plaster has therapeutic efficacy that is similar or superior to that for the best available topical NSAID formulations. Moreover, the etofenamate plaster is well-tolerated.
Acknowledgment
The authors acknowledge the support of the following coinvestigators: Dr Helmut Pabst, Dr Juergen Schaale-Maas, Dr Eduard Ebert, and Dr Axel Schaefer.
Footnotes
Final revision submitted February 18, 2021; accepted April 12, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported in full by Drossapharm AG. H.-G.P. received a fee from Drossapharm AG for his role as coordinating investigator of this clinical study. The employer of A.L. received consultancy fees from Drossapharm AG during the design, conduct, and reporting stages of this clinical study, as well as for the drafting of this paper. R.I. is an employee of Drossapharm AG. M.B. is an employee of the data management company responsible for the analysis and reporting of data from this clinical study. B.G. received consultancy fees from the contract research organization responsible for conduct of this study. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Ärztekammer Nordrhein (North Rhine Medical Association).
References
- 1.Ascherl R, Schlemmer H, Blümel G, Lechner F. Zur Wirksamkeit von Etofenamat bei leichten Sportverletzungen an Knie- und Sprunggelenk – Eine Doppelblind-Studie. Fortschr Med. 1982;37:1729–1734. [PubMed] [Google Scholar]
- 2.Billigmann P, Pelster B. Plazebokontrollierte Doppelblindstudie von Etofenamat-Gel (Rheumon® Gel) in Kombination mit Ultraphonophorese bei Sportverletzungen. Praktische Traumatologie Sportmedizin 1992;2:72–77. [Google Scholar]
- 3.Bridgman SA, Clement D, Downing A, Walley G, Phair I, Maffulli N. Population based epidemiology of ankle sprain attending accident and emergency units in the West Midlands of England, and a survey of UK practice for severe ankle sprain. Emerg Med J. 2003;20(6):508–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costantino C, Kwarecki J, Samokhin AV, Mautone G, Rovati S. Diclofenac epolamine plus heparin plaster versus diclofenac epolamine plaster in mild to moderate ankle sprain: a randomized, double-blind, parallel-group, placebo-controlled, multicentre, phase III trial. Clin Drug Investig. 2011;31(1):15–26. [DOI] [PubMed] [Google Scholar]
- 5.Derry S, Moore RA, Gaskell H, McIntyre M, Wiffen PJ. Topical NSAIDs for acute musculoskeletal pain in adults. Cochrane Database Syst Rev. 2015;6:CD007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diebschlag W, Nocker W, Bullingham R. A double-blind study of the efficacy of topical ketorolac tromethamine gel in the treatment of ankle sprain, in comparison to placebo and etofenamate. J Clin Pharmacol. 1990;30:82–89. [DOI] [PubMed] [Google Scholar]
- 7.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. [DOI] [PubMed] [Google Scholar]
- 8.González de Vega C, Speed C, Wolfarth B, González J. Traumeel vs. diclofenac for reducing pain and improving ankle mobility after acute ankle sprain: a multicentre, randomised, blinded, controlled and non-inferiority trial. Int J Clin Pract. 2013;67(10):979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med. 1998;5(11):1086–1090. [DOI] [PubMed] [Google Scholar]
- 10.Malliaropoulos N, Ntessalen M, Paracostas E. Reinjury after acute lateral ankle sprains in elite track and field athletes. Am J Sports Med. 2009;37(9):1755–1761. [DOI] [PubMed] [Google Scholar]
- 11.Mazières B, Rouanet S, Velicy J, Scarsi C, Reiner V. Topical ketoprofen patch (100 mg) for the treatment of ankle sprain: a randomized, double-blind, placebo-controlled study. Am J Sports Med. 2005;33(4):515–523. [DOI] [PubMed] [Google Scholar]
- 12.Predel HG, Hamelsky S, Gold M, Gianetti B. Efficacy and safety of diclofenac diethylamine 2.32% gel in acute ankle sprain. Med Sci Sports Exerc. 2012;44(9):1629–1636. [DOI] [PubMed] [Google Scholar]
- 13.Roosen P, Willems T, De Ridder R, et al. Ankle Sprains: Diagnosis and Therapy. Good Clinical Practice (GCP). KCE Reports 197C. Belgian Health Care Knowledge Centre (KCE); 2013. [Google Scholar]
- 14.Stiell IG, Greenberg GH, McKnight RD, Nair RC, McDowell I, Worthington JR. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med. 1992;21(4):384–390. [DOI] [PubMed] [Google Scholar]
- 15.van den Bekerom MPJ, Sjer A, Somford MP, Bulstra GH, Struijs PAA, Kerkhoffs GMMJ. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating acute ankle sprains in adults: benefits outweigh adverse events. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2390–2399. [DOI] [PubMed] [Google Scholar]
- 16.van den Bekerom MPJ, Oostra RJ, Alvarez PG, van Dijk CN. The anatomy in relation to injury of the lateral collateral ligaments of the ankle: a current concepts review. Clin Anat. 2008;21(7):619–626. [DOI] [PubMed] [Google Scholar]
- 17.Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ, Jr. The epidemiology of ankle sprain in the United States. J Bone Joint Surg Am. 2010;92(13):2279–2284. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe MW, Uhl TL, Mattacola CG, McCluskey LC. Management of ankle sprains. Am Fam Physician. 2001;63(1):93–104. [PubMed] [Google Scholar]
- 19.Randomized, controlled, double-blind, multicenter trial to evaluate the efficacy and safety of an etofenamate 5% cutaneous patch vs. placebo in the treatment of acute uncomplicated unilateral ankle sprain. EudraCT Identifier: 2014-001720-30. Posted July 16, 2016. Accessed July 30, 2021. https://www.clinicaltrialsregister.eu/ctr-search/trial/2014-001720-30/results