Abstract
Introduction
Bromelain is a complex mixture of thiol proteases and other non-proteolytic constituents, commercially extracted primarily from the pineapple stem. Evidence from several in vitro and in vivo studies highlights its excellent bioavailability, lack of side effects, and broad spectrum of medical efficacies, of which the antiphlogistic properties are among the most valuable ones. Bromelain has indeed been employed for the efficient treatment of many inflammatory disorders, ranging from osteoarthritis and inflammatory bowel diseases to cancer-related inflammation.
Methods
The aim of the current study was to assess the anti-inflammatory effects of bromelain after gastrointestinal digestion simulated in vitro using stomach, intestinal, and chondrocyte human cellular models (AGS, Caco-2, and SW1353, respectively).
Results
We successfully demonstrated the capability of bromelain to reduce an inflammatory stimulus by reproducing its exposure to the gastro-enteric environment in vitro and assaying its effect in human cell lines derived from stomach, intestinal, and chondrocytes.
Conclusion
Consistently with the previously published data, our work underpins the relevance of bromelain in the development of safer and more effective anti-inflammatory therapies.
Keywords: bromelain, Caco-2, AGS, chondrocytes, anti-inflammatory
Introduction
Bromelain is a general name for a family of sulfhydryl proteolytic enzymes obtained from Ananas comosus, the pineapple plant.1 It is typically distinguished as either fruit bromelain or stem bromelain, with the majority of commercially available bromelain derived from the stem.2 First identified in pineapple juice in 1891, it has been manufactured on a large scale as nutritional dietary supplement since 1957. To date, eight proteolytically active constituents have been isolated from bromelain.3 Proteinases are thought to be the active fractions4 and bromelain appears to exert its activity over a pH range of 4.5–9.5.5
The biological properties attributed to bromelain include anti-inflammatory, anti-thrombotic, fibrinolytic, and immunomodulatory actions.6 While the interest in bromelain for human use has extended to a wide variety of areas including digestive assistance (when taken with meals), respiratory tract diseases (as a mucolytic), and as a potential adjunct in cancer treatment, the primary focus has been its use in inflammatory conditions such as osteoarthritis and sports injuries.7 However, many of the published clinical trials to date have been of somewhat poor quality. A review of clinical trials on bromelain was either uncontrolled, open-label studies or completed using enzyme combinations that included bromelain.8
Although the exact underlying mechanisms for bromelain’s anti-inflammatory activity have not yet been fully understood, bromelain is assumed to exert a systemic response through the modulation of multiple inflammatory mediators.9 In murine and human cell lines, bromelain was shown to downregulate the expression levels and functioning of both NF-κB and cyclooxigeneas-2 (COX-2), important inflammatory mediators able to convert arachidonic acid into several by-products (e.g., the proinflammatory lipid prostaglandin E2.10,11,12 Moreover, also other inflammatory mediators such as interferon gamma (IFNγ) and IFNγ-mediate nitric oxide, tumor necrosis factor alpha (TNF-α), interleukin-1 beta, and interleukin 8 (IL-8) can be modulated (stimulated or inhibited) by bromelain, thus demonstrating its ability to decrease the majority of inflammatory mediators exerting a significant role as an anti-inflammatory agent both in healthy immune systems13,14,15 and in inflammatory conditions.11,16 In addition, since chronic inflammation is a key factor in tumorigenesis, bromelain is gaining popularity as chemoprevention and adjuvant cancer therapy.9
Due to its protein structure, bromelain is thought to be digested and perhaps loses activity with a potential loss of its anti-inflammatory actions. Data to date have been conflicting. According to one clinical study in 19 healthy men, orally administered bromelain (3 g/day) was absorbed through the intestine and both its structure and proteolytic function were preserved.17 However, in a study with rats, blood anti-proteases such as α2-macroglobulin and α1-antichymotrypsin formed a complex with bromelain reducing its biological activity.18 Bromelain is recommended to be taken between meals to exert an inflammatory action. Despite this, many commercially sold bromelain supplements (especially in the European market) continue to enteric coat tablets or capsules.
In light of published data, the following in vitro study was designed to assess the anti-inflammatory properties of the proprietary bromelain extract, Bromeyal™, in its intact form and following simulated gastrointestinal digestion (hydrolysates),19 in a variety of human cell lines derived from stomach, intestine and chondrocytes which are the main targets of bromelain activity.
Material and Methods
Raw material, reagents, and kits
Bromelain (Bromeyal™), manufactured using aqueous extraction from pineapple stem and multi-step purification process, was supplied by Giellepi S.p.A. Health Science (Lissone, MB, Italy). All chemicals and reagents were purchased from Sigma-Aldrich. Enzyme-linked immunosorbent assay (ELISA) kits for the detection of COX-2 and inducible nitric oxide synthase (iNOS) were purchased from LSBio (cat. N. LS-F12319 and LS-F2081, respectively). ELISA kit for the detection of IL-8 was purchased from Invitrogen (cat. N. 88-8086-88). COX-2 activity assay kit was purchased from AbCam (cat. N. ab204699).
Bromelain digestion
The preparation of bromelain hydrolysates was carried out based on a modification of the protocols provided in the US Pharmacopoeia as described by Fu et al.19 (2002). Briefly, to simulate gastric digestion, 30 mg of bromelain powder was dissolved in 6 mL water and mixed with 4 mL of 2.5X simulated gastric fluid (SGF, 0.075 M NaCl, pH 1.2; 0.8 mg/mL pepsin). The final composition of the mixture was 0.03 M NaCl, pH 1.2; 3 mg/mL bromelain; and 0.32 mg/mL pepsin. The mixture was incubated for 30 min at 37°C at 170 r/min and subsequently stored at −20°C until further use.
After 30 min of incubation at 37°C, the mixture was placed on ice, and pH was adjusted at 7.4 with the addition of 90 mg of dibasic potassium phosphate and potassium hydroxide. The digestion was started by the addition of 100 mg pancreatin. Final concentrations for the simulated gastrointestinal fluid (SIF) were 51.7 mM potassium phosphate, pH 7.4 and 10 mg/mL pancreatin. The mixture was incubated for 2 h at 37°C at 170 r/min and then stored at −20°C until further use.
The autodigestion of bromelain, which exploits its intrinsic proteolytic activity, was carried out in the exact same conditions as the gastrointestinal digestion, but without the addition of pepsin and pancreatin.
Cell culture conditions
Human immortalized cell lines (AGS, Caco-2, and SW1353) were purchased from the American Type Culture Collection (Rockville, MD, USA). CaCo-2 cells were cultured in either 75 or 150 cm2 flasks, in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (pen/strep), 1% non-essential amino acids (NEAAs), and 2 mM L-glutamine. Cells were cultured at 37°C and 5% CO2.
AGS cells were cultured in either 75 or 150 cm2 flasks, in Ham’s F12 medium containing 10% FBS, 1% pen/strep, 1% NEAA, and 2 mM L-Gln. Cells were cultured at 37°C and 5% CO2.
SW1353 cells were cultured in either 75 or 150 cm2 flasks, in L-15 medium (Leibovitz) containing 10% FBS, 1% pen/strep, 1% NEAA, and 2 mM L-Gln. Cells were cultured at 37°C without CO2.
Cell viability assays (MTT assay)
The different hydrolysates were assessed for their cytotoxic effects in the three cell lines, by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, the assay was carried out as follows: cell lines were cultured in 75 cm2 flasks up to a >90% confluence, after which they were harvested and diluted to a concentration of 105 cells/ml. 100 μl of diluted cells were then transferred into each well of a tissue culture 6-well plate and incubated overnight at 37°C. After the incubation, the culture medium was replaced with fresh one containing increasing concentrations (0.001–0.3 mg/mL) of each hydrolysate. Positive controls were tested at a single concentration: 0.14 mg/mL ketoprofen; 0.14 mg/mL α-tocopherol; 0.14 mg/mL ascorbic acid; and 0.04 mg/mL dexamethasone. Each sample was tested in triplicate. The plate was then incubated at 37°C for 24 h.
At the end of the incubation, 10 μl of a 5 mg/mL MTT stock solution was added to each well, and the plate was incubated at 37°C for further 2 h. Subsequently, reduced MTT crystals were solubilized by removing the culture medium and adding 100 μl of dimethyl sulfoxide to each well and incubating for 30 min at room temperature.
Absorbance at 595 nm was measured with a UV-visible microplate reader, and cell viability was determined by quantitating the amount of reduced MTT produced. Values were reported as a percentage of reduced MTT produced by the untreated control.
Anti-inflammatory assays
To test the anti-inflammatory activity of bromelain hydrolysates, cells were seeded at a density of 105 cells/ml in tissue culture treated 12-well plate and grown at 37°C with (Caco-2 and AGS) or without (SW1353) 5% CO2 upto 90% confluence. After the incubation, culture medium was replaced with freshly prepared one containing specific concentration of the proinflammatory cytokine tumor necrosis actor alpha (TNF-α) (200 ng/mL for CaCo-2, 25 ng/mL for AGS and 100 ng/mL for SW1353) with or without 0.035 mg/mL of bromelain hydrolysates. Cells were incubated for 6 h as described above. The inflammatory response and anti-inflammatory activity of the hydrolysates were determined by measuring the expression levels of inflammatory markers IL-8, COX-2, and iNOS through sandwiched ELISA, and the enzymatic activity of COX-2.
To detect COX-2 and iNOS expression levels, cells were collected from each well by scraping, pelleted, washed three times with PBS, and lysated by sonication. 200 μl of each lysate was employed for the assay. To detect IL-8 expression levels, 200 μl of culture medium was employed for the assay, without further processing. The ELISA assay for the three proteins was performed according to the protocols supplied by the manufacturers of each kit.
To evaluate COX-2 enzymatic activity, cells were collected from each well by scraping, pelleted, washed three times with PBS, and lysated by sonication. 200 μl of each lysate was employed for the assay, which was performed according to the protocol supplied by the manufacturer.
Results
Determination of the cytotoxic potential of bromelain in simulated gastroenteric tract
The potential cytotoxicity of bromelain samples was determined by MTT assay on immortalized human cell lines deriving from stomach (AGS), gut (Caco-2), and chondrocytes (SW1353).
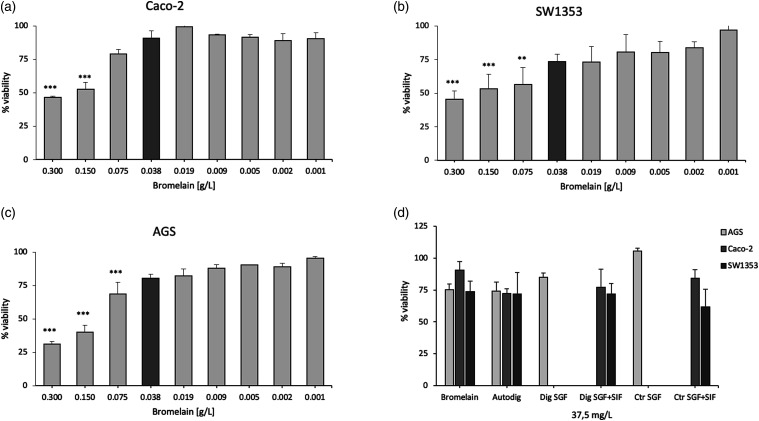
This assay allowed us to determine an optimum bromelain concentration that allowed for at least 75% viability of the cell lines (Figures 1(a)–(c)).
Figure 1.
Cytotoxic effects of bromelain in simulated gastroenteric tract. MTT assay of bromelain sample in Caco-2 (A), SW1353 (B) and AGS (C) cells under increasing concentration of bromelain treatment (D) MTT assay shows the viability of the tested cell lines (Caco-2, SW1353 and AGS) after treatment with bromelain (37.5 mg/L) in the simulated gastrointestinal tract. Bromelain (bromelain untreated, control); Autodig (bromelain after autodigestion); Dig SGF (bromelain digested only in gastric fluid); Dig SGF+SIF (bromelain treated with gastrointestinal fluid); Ctr SGF(SGF buffer without bromelain); Ctr SGF+SIF (gastrointestinal fluid buffer without bromelain); **** p < 0.001; ** p < 0.01.
This concentration was estimated to be equal or lower than 37.5 mg/L. Higher concentrations became cytotoxic for the cells, possibly due the proteolytic activity of bromelain.
The MTT assay was then used to test the possible effect of the combination of bromelain and fluids on cell viability by treating the cells with 37.5 mg/L of bromelain under different experimental conditions (including bromelain autodigestion and before and after simulated gastrointestinal transit) (Figure 1(d)). AGS cells were treated only with bromelain processed in gastric fluid (SGF) in order to simulate the gastric transit in time and space, instead of gut cells (directly involved in digestion) and chondrocytes (linked with the stomach and intestine through systemic circulation) that were treated with bromelain processed in gastro-intestinal fluid (SGF+SIF).
After the concomitant treatment with fluids, the fixed bromelain concentration of 37.5 mg/L appears to be appropriate as all the cell lines maintain a viability around of at least 75% (mean of 77.3 ± 4.9) when compared to untreated cell lines (Ctr SGF and Ctr SGF+SIF) that is in line with the viability observed treating cells only with bromelain (mean of 79.3 ± 7.6) (Figure 1(d)).
Evaluation of the anti-inflammatory activity of bromelain
Immortalized cell lines AGS, Caco-2, and SW1353 were stimulated with specific cytokines in order to induce inflammation and evaluate bromelain capability to reduce the inflammatory response. As effectors of inflammation, IL-8, iNOS, and cyclooxygenase-2 (COX-2) were evaluated by ELISA assay.
IL-8 inhibition
The three immortalized cell lines were treated with TNF-α (25 ng/mL for AGS; 200 ng/mL for Caco-2; and 100 ng/mL for SW1353) to induce inflammation in both absence and presence of bromelain at a concentration of 35 mg/L.
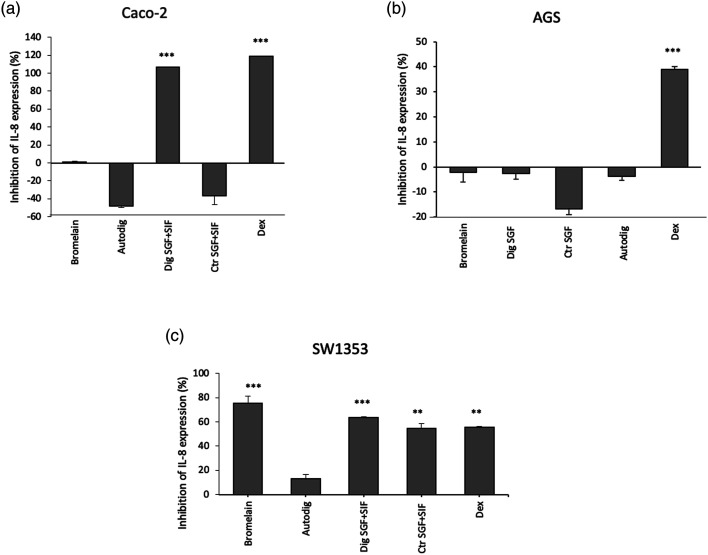
After this stimulus, cell production of IL-8 can be quantified using ELISA assay.20 This test exploits the use of specific anti-IL-8 antibodies, tagged with a chromophore, to determine the expression levels of the protein after TNF-α treatment, in absence or presence of bromelain. The cells that underwent TNF-α stimulus without any bromelain treatment were used in order to normalize IL-8 production. The data show that although no activity is detected on AGS cells in any of the conditions tested (Figure 2(b)), bromelain digested in gastrointestinal tract (Dig SGF+SIF) presents a strong anti-inflammatory activity on Caco-2 cell line reaching about 100% of inhibition of IL-8 expression, which is similar to the inhibition exerted by dexamethasone (Figure 2(a)). Otherwise, in the SW1353 cell line, bromelain exerts an anti-inflammatory in different experimental conditions (Figure 2(c)). Cell treatment with dexamethasone was also included as a control; after determining the dose (40 μg/mL), we were able to exert an anti-inflammatory effect without cytotoxicity (Supplementary Material Figure S1).
Figure 2.
Interleukin 8 inhibition evaluation. Anti-inflammatory activity after TNF-α induction was detected through Enzyme Linked Immunosorbent Assay assay in Caco-2 (A), AGS (B) and SW1353 (C) cells. Histograms show the percentage of inhibition of IL-8 expression respect to cells stimulated with TNF-α but without any bromelain treatment. Bromelain (bromelain untreated, control); Dig SGF (bromelain digested only in gastric fluid); Ctr SGF (SGF buffer without bromelain); Autodig (bromelain after autodigestion); Dig SGF+SIF (bromelain treated with gastrointestinal fluid)); Ctr SGF+SIF (gastrointestinal fluid buffer without bromelain); DEX (dexamethasone 40 μg/mL, positive control); *** p < 0.001; ** p < 0.01.
COX-2 inhibition
COX isoenzymes are membrane-bound enzymes located in the endoplasmic reticulum that show different properties in humans. In fact, while COX-1 is expressed constitutively in many tissues and it is involved in functions such as cytoprotection of gastric mucosa, regulation of renal blood flow, and platelet aggregation, COX-2 is not detected in most of the normal tissues, but its expression is rapidly induced by stimuli such as proinflammatory cytokines (IL-1b and TNF-α), lipopolysaccharides, mitogens, oncogenes (phorbol esters), and growth factors.21
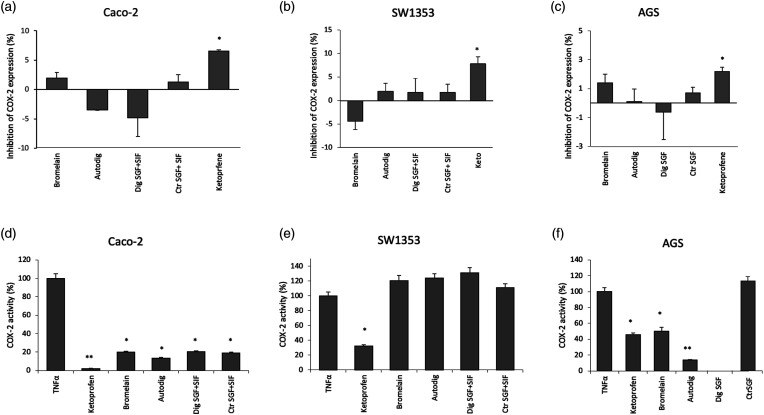
ELISA test was performed in order to assess the effect of bromelain in preventing COX-2 induction after the inflammatory stimulus induced by TNF-α. The results show no significant effect on the relative expression level of this inflammatory mediator in all the cell lines tested (Figures 3(a)–(c)) with respect to the same cell lines stimulated but not treated with bromelain used for normalization. Anyway, a slight but not significant decrease of COX-2 expression levels was recorded for SW1353 cells treated with digested bromelain if compared to their controls and mainly undigested bromelain. A ketoprofen treatment was used as positive control considering its ability to inhibit inflammation (Supplementary Material Figure S1).
Figure 3.
Cyclooxigeneas-2 inhibition evaluation. Effect of bromelain in preventing cyclooxigeneas-2 induction were detected through Enzyme Linked Immunosorbent Assay assay in Caco-2 (A), SW1353 (B) and AGS (C) cells. Histograms show the percentage of inhibition of cyclooxigeneas-2 expression respect to cells stimulated with TNF-α but without any bromelain treatment. Activity of cyclooxigeneas-2 in response to the inflammatory stimulus both in the absence and in the presence of bromelain at a concentration of 35 mg/L was reported for Caco-2 (D), SW1353 (E) and AGS (F) cells. Bromelain (bromelain untreated, control); Dig SGF (bromelain digested only in gastric fluid); Ctr SGF (SGF buffer without bromelain); Autodig (bromelain after autodigestion); Dig SGF+SIF (bromelain treated with gastrointestinal fluid); Ctr SGF+SIF (gastrointestinal fluid buffer without bromelain); Ketoprofene (Ketoprofene 142 μg/mL, positive control); ** p < 0.01; * p < 0.05.
To better investigate the effect of bromelain in COX-2 induction, we also assayed the activity of COX-2 in response to an inflammatory stimulus, namely, a 6-h treatment with TNF-α (200 ng/mL for Caco-2, 25 ng/mL for AGS and 100 ng/mL for SW1353), both in the absence and in the presence of bromelain at a concentration of 35 mg/L.
This activity assay exploits the generation of a fluorescent product from the reaction of COX-2 with its substrate arachidonic acid and a proprietary COX probe. Thus, it evaluates the differences in the activity of COX-2 in absence and presence of bromelain, while the ELISA test merely detects the changes in the expression levels of the protein.
As shown in Figure 3 with respect to the untreated cells stimulated with TNF-α (100% COX-2 activity), a significant decrease of COX-2 activity was observed in the intestinal cell line Caco-2, probably due to an overestimation of the value obtained with TNF-α alone (Figure 3(d)). In particular, results show that both undigested and digested (either after gastrointestinal simulation or autogestion) bromelain are able to exert about 80% mean COX-2 activity reduction compared to untreated cells stimulated with TNF-α. Moreover, bromelain significantly reverts the effect of the inflammatory stimulus in the AGS gastric cell line after autodigestion and completely inhibited the activity of COX-2 following the gastric digestion simulated in vitro (Figure 3(f)). Moreover, the effect seems more pronounced than that exerted by the positive control ketoprofen.
iNOS inhibition
The production of nitric oxide, a major mediator of inflammation, in the body is catalyzed by a family of enzymes called nitric oxide synthases (NOSs). One of them, inducible NOS (iNOS), is not detectable in resting cells but can be induced by immunostimulatory cytokines, bacterial products, or infection.22
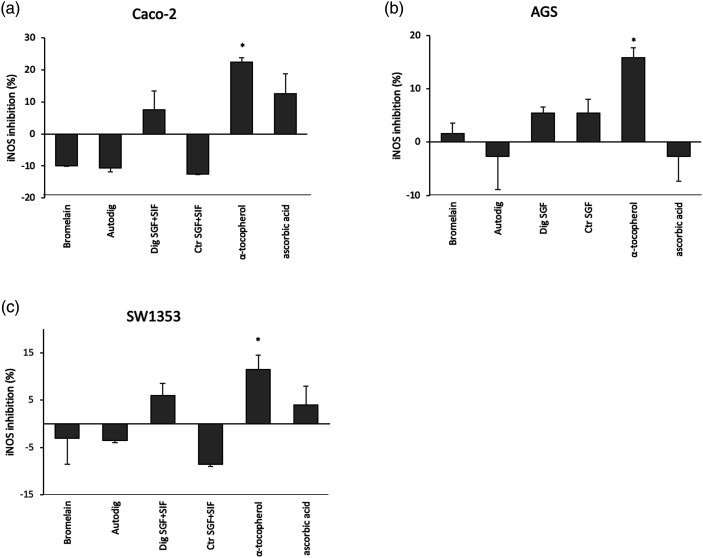
ELISA test was performed in order to verify the effect of bromelain in preventing iNOS induction after the inflammatory stimulus triggered by TNF-α. The cells that underwent the TNF-α stimulus without any bromelain treatment were used in order to normalize iNOS production. While cells treated with the anti-inflammatory drugs tocopherol and ascorbic acid were used as positive controls show a reduction in iNOS expression, this effect was not significant in all the conditions tested (Figure 4).
Figure 4.
Inducible nitric oxide synthase inhibition evaluation. Effect of bromelain in preventing inducible nitric oxide synthase induction after the inflammatory stimulus were detected through Enzyme Linked Immunosorbent Assay assay in Caco-2 (A), AGS (B) and SW1353 (C) cells. Histograms show the relative percentage of inducible nitric oxide synthase inhibition respect to cells stimulated with TNF-α but without any Bromelain treatment. Bromelain (bromelain untreated, control); Dig SGF (bromelain digested only in gastric fluid); Ctr SGF (SGF buffer without bromelain); Autodig (bromelain after autodigestion); Dig SGF+SIF (bromelain treated with gastrointestinal fluid)); Ctr SGF+SIF (gastrointestinal fluid buffer without bromelain); ascorbic acid (ascorbic acid 142 μg/mL, positive control); tocopherol (tocopherol 142 μg/mL, positive control); * p < 0.05.
Discussion
Bromelain has been the subject of extensive scientific research since it became commercially available over 50 years ago.23 Even though its anti-inflammatory properties have been investigated both in in vitro and in vivo, human clinical trials are not extensive and mechanisms of action have not been established. In this work, we took a further step forward to elucidate the anti-inflammatory actions. Particularly, we assessed its potential to reduce inflammation measuring the inhibition of expression and activity of distinctive inflammatory markers (i.e., IL-8, COX-2, and iNOS) in three human cell lines derived from stomach, gut, and chondrocytes.
Due to its protein structure, bromelain is digested following oral intake and it is generally thought that denaturation caused by gastric environment may lead to inactivation and loss of its biological activities (i.e., both enzymatic and anti-inflammatory). Consequently, many dietary supplements available on the market (especially in Europe) are formulated as enteric-coated tablets and capsules.
In the light of results achieved by the current study, it can be suggested that the anti-inflammatory properties of the investigated bromelain preparation (Bromeyal™) both in its intact form and after digestion (hydrolysates) derive from the capability to act directly in lowering IL-8 level after TNF-α stimulus following simulation in the gastrointestinal tract. The bromelain activity at the concentration tested in Caco-2 cells is comparable to a concentration of 40 μg/mL of the drug dexamethasone (positive control), or even higher than it, considering chondrocytes. Moreover, in the chondrocytes model SW1353, bromelain and its digested hydrolysates were found to have a strong anti-inflammatory effect in all the conditions tested. Therefore, once digested and absorbed, bromelain is likely transported to cartilage. Different studies demonstrate that bromelain could be found linked to plasma proteins in its native form.24 For this reason, it is thought that bromelain could retain its anti-inflammatory properties reaching chondrocytes either in its native or gastrointestinal digested form.
Our results also show that bromelain in its native form does not exert any significant activity on iNOS and COX-2 expression levels, although the tested compound appears to have an inhibitory effect on TNF-α induced activity of COX-2, in particular in a gastric system where COX-2 activity was completely inhibited by bromelain after digestion in SGF. However, it should be noted that a trend was found for inhibition of iNOS in cells treated with digested bromelain. The enzyme is responsible for the synthesis of prostaglandin H, starting from the substrate arachidonic acid, via two different reactions (cyclooxygenase and peroxidase) that go through a prostaglandin G2 intermediate. This effect could be explained in two ways: (1) one or more components of the digested bromelain samples might act as direct inhibitors of one of these two activities of COX-2 and (2) arachidonic acid is also reported to increase the activity of COX-2,25 and thus digested bromelain samples might interfere with this enhancing effect.
As noted previously, a wide variety of dietary supplements containing bromelain are manufactured as enteric-coated capsules or tablets. This appears to be in order to avoid the degradation of the bromelain enzymes during the gastrointestinal transit following oral intake. However, the results from the current study provide the evidence about the anti-inflammatory activity of a proprietary bromelain (Bromeyal™) after simulating gastrointestinal digestion in vitro in comparison to plain undigested bromelain. The results may support the hypothesis that orally administered bromelain may potentially preserve its original bio-efficacy by modulating some proinflammatory markers. In particular, the complex mixture of peptides originating from gastrointestinal transit may be more effective than the parent compound (undigested bromelain), at least this can be speculated for the product tested in this study.
Such data might highlight that coating technologies to protect bromelain from acidic conditions are not necessary in order to exploit its full therapeutic potential. However, this hypothesis needs to be further investigated and proven by future pre-clinical and clinical investigations. All the above considerations should apply only to the bromelain preparation tested in our study as the quali-quantitative composition of plant-derived products can vary widely depending on the extraction and purification processes used by different manufacturers.
Conclusion
Bromelain shows numerous potential applications as an anti-inflammatory ingredient. Efforts have been made over the years to increase its purity and stability. This study succeeded in proving the role of a proprietary bromelain ingredient (Bromeyal™) in modulating inflammatory markers in human cell lines derived from stomach, intestinal, and chondrocytes, thus corroborating its anti-inflammatory effect in vitro. Of particular note is the maintenance of anti-inflammatory properties following simulated gastrointestinal transit and digestion. Bromelain is a promising candidate for the development of future oral enzyme therapies suitable for the treatment of both acute and chronic inflammatory conditions, with potential for less adverse side effects commonly associated with anti-inflammatory drugs.
Supplemental Material
Supplemental Material, sj-pdf-1-iji-10.1177_20587384211034686 for Anti-inflammatory properties of a proprietary bromelain extract (Bromeyal™) after in vitro simulated gastrointestinal digestion by Roberta Bottega, Ilaria Persico, Francesco De Seta, Federico Romano and Giovanni Di Lorenzo in International Journal of Immunopathology and Pharmacology
Acknowledgments
This study was technically supported by Bict srl, Via Einstein c/o Parco Tecnologico Padano Cascina Codazza, 26900 Lodi (LO) – Italy). Many thanks to Professor Gughi D.L. for the constructive discussions.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Francesco De Seta is scientific advisor for Giellepi S.p.A.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by IRCCS “Burlo Garofolo” (Ricerca Corrente 01/2017) and Giellepi S.p.A., Health Science (via B. Cellini 37, 20851 Lissone – MB – Italy).
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Roberta Bottega https://orcid.org/0000-0001-7431-7543
Federico Romano https://orcid.org/0000-0003-2157-8330
Giovanni Di Lorenzo https://orcid.org/0000-0002-8845-1206
References
- 1.Kelly GS. (1996) Bromelain: a literature review and discussion of its therapeutic applications. Alternative Medicine Review 1: 243–257. [Google Scholar]
- 2.Mauer (2001) Bromelain biochemistry, pharmacology and medical use. Cellular and Molecular Life Sciences 58: 1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rathnavelu V, Alitheen NB, Sohila S, et al. (2016) Potential role of bromelain in clinical and therapeutic applications (review). Biomedical Reports 5: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murachi T, Yasui M, Yasuda Y. (1964) Purification and physical characterization of stem bromelain. Biochemistry 3: 48–55. [DOI] [PubMed] [Google Scholar]
- 5.Hale LP, Greer PK, Trinh CT, et al. (2005) Proteinase activity and stability of natural bromlain preparations. International Immunopharmacology 5: 783–793. [DOI] [PubMed] [Google Scholar]
- 6.Pavan R, Jain S, Shraddha A, et al. (2012) Properties and therapeutic application of bromelain: a review. Biotechnology Research International 2012: 1–6. DOI: 10.1155/2012/976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray MT, Pizzorno JE. (2006) Bromelain. In: Pizzorno JE, Murray MT. (eds), Textbook of Natural Medicine. 3rd ed. Philadelphia, PA: Elsevier, 791–795. [Google Scholar]
- 8.Brien S, Lewith G, Walker A, et al. (2004) Bromelain as a treatment for osteoarthritis: a review of clinical studies. Evidence-Based Complementary and Alternative Medicine 1: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chobotova K, Vernallis AB, Majid FAA. (2010) Bromelain’s activity and potential as an anti-cancer agent: current evidence and perspectives. Cancer Letters 290: 148–156. [DOI] [PubMed] [Google Scholar]
- 10.Bhui K, Prasad S, George J, et al. (2009) Bromelain inhibits Cox-2 expression by blocking the activation of MAPK regulated NF-kappa b against skin tumor-initiation triggering mitochondrial death pathway. Cancer Letters 282: 167–176. [DOI] [PubMed] [Google Scholar]
- 11.Huang J-R, Wu C-C, Hou RC-W, et al. (2008) Bromelain inhibits lipopolysaccharide-induced cytokine production in human THP-1 monocytes via the removal of CD14. Immunological Investigations 37: 263–277. [DOI] [PubMed] [Google Scholar]
- 12.Hou RC-W, Chen Y-S, Huang J-R, et al. (2006) Cross-linked bromelain inhibits lipopolysaccharide-induced cytokine production involving cellular signaling suppression in rats. Journal of Agricultural and Food Chemistry 54: 2193–2198. [DOI] [PubMed] [Google Scholar]
- 13.Barth H, Guseo A, Klein R. (2005) In vitro study on the immunological effect of bromelain and trypsin on mononuclear cells from humans. European Journal of Medical Research 10: 325–331. [PubMed] [Google Scholar]
- 14.Desser L, Rehberger A, Paukovits W. (1994) Proteolytic enzymes and amylase induce cytokine production in human peripheral blood mononuclear cells in vitro. Cancer Biotherapy 9: 253–263. [DOI] [PubMed] [Google Scholar]
- 15.Engwerda CR, Andrew D, Murphy M, et al. (2001) Bromelain activates murine macrophages and natural killer cells in vitro. Cellular Immunology 210: 5–10. [DOI] [PubMed] [Google Scholar]
- 16.Onken JE, Greer PK, Calingaert B, et al. (2008) Bromelain treatment decreases secretion of pro-inflammatory cytokines and chemokines by colon biopsies in vitro. Clinical Immunology 126: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedrich G, Kuhn S, Poppe GE. (1997) Intestinal absorption of undegraded proteins in men: presence of bromelain in plasma after oral intake. The American Journal of Physiology 273: G139–G146. [DOI] [PubMed] [Google Scholar]
- 18.Seifert J, Ganser R, Brendel W. (1979) Absorption of proteolytic enzyme originating from plants out of the gastrointestinal tract in blood and lymph of rats (Author translation). Z Gastroenterol 17: 1–8. [PubMed] [Google Scholar]
- 19.Fu T-J, Abbott UR, Hatzos C. (2002) Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid-a comparative study. Journal of Agricultural and Food Chemistry 50: 7154–7160. [DOI] [PubMed] [Google Scholar]
- 20.Namba S, Nakano R, Kitanaka T, et al. (2017) N. ERK2 and JNK1 contribute to TNF-α-induced IL-8 expression in synovial fibroblasts. PLoS One 12: e0182923. DOI: 10.1371/journal.pone.0182923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarghi A, Arfaei S. (2011) Selective COX-2 inhibitors: a review of their structure-activity relationships. Iranian Journal of Pharmaceutical Research: IJPR 10: 655–683. [PMC free article] [PubMed] [Google Scholar]
- 22.Aktan F. (2003) iNOS-mediated nitric oxide production and its regulation. Life Sciences 75: 639–653. [DOI] [PubMed] [Google Scholar]
- 23.Taussig SJ, Batkin S. (1988) Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. Journal of Ethnopharmacology 22: 191–203. [DOI] [PubMed] [Google Scholar]
- 24.Castell JV, Friedrich G, Kuhn CS, et al. (1997) Intestinal absorption of undegraded proteins in men: presence of bromelain in plasma after oral intake. The American Journal of Physiology 273: G139–G146. [DOI] [PubMed] [Google Scholar]
- 25.Ramkissoon A, Wells PG. (2011) Human prostaglandin H synthase (hPHS)-1 and hPHS-2 in amphetamine analog bioactivation, DNA oxidation, and cytotoxicity. Toxicological Sciences 120(1): 154–162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-iji-10.1177_20587384211034686 for Anti-inflammatory properties of a proprietary bromelain extract (Bromeyal™) after in vitro simulated gastrointestinal digestion by Roberta Bottega, Ilaria Persico, Francesco De Seta, Federico Romano and Giovanni Di Lorenzo in International Journal of Immunopathology and Pharmacology