Abstract
Background
Screening for cytomegalovirus (CMV)-specific antibodies is not routine in some settings. Thus, transfusion of blood products poses risks for susceptible individuals.
Objectives
To investigate the global pooled CMV seroprevalence among volunteer blood donors.
Methods
This systematic review and meta-analysis was performed according to PRISMA guidelines. The databases searched included Embase, Google Scholar, Medline, PubMed, Web of Science, and Cochrane Library. Data were extracted independently and analyzed using STATA version 11.
Results
The global seroprevalence of CMV IgG, CMV IgM, and both CMV IgM and IgG was 83.16% (95% confidence interval [CI]: 78.55–87.77%, I2 = 99.5%), 13.77% (95% CI: 11.59–15.95%, I2 = 98.8%), and 23.78% (95% CI: 10.50–37.06%, I2 = 98.7), respectively.
Conclusion
The global seroprevalence of CMV was high among blood donors. Therefore, regular CMV screening should be conducted to identify CMV-seronegative blood donors.
Keywords: Blood donor, cytomegalovirus, seroprevalence, systematic review, immunoglobulin M, immunoglobulin G
Background
Blood transfusion is a lifesaving component of many therapeutic interventions.1 However, transmission of infectious diseases is a major challenge in transfusion services worldwide.2 Cytomegalovirus (CMV), also known as human herpesvirus 5, is a large virus that infects humans.3 CMV is a highly cell-associated virus and normally causes asymptomatic infections in immunocompetent individuals. Transmission of the virus can occur vertically or horizontally through contact with virus-containing body fluids including blood.4 An important route of infection for high-risk groups is transfusion of blood products from latently infected donors (transfusion-transmitted [TT]-CMV).5 Transfusion of contaminated blood products can result in primary infection in CMV-seronegative recipients or reinfection by a new CMV strain in CMV-seropositive recipients.6 TT-CMV was first described by Kääriäinen and co-workers in 1966.7 TT-CMV infections have traditionally been explained by transfer of latently infected white blood cells (WBCs).8 The incidence of TT-CMV infection was reported to be as high as 13% to 37% in immunocompromised patients. Thus, the prevention of TT-CMV has become an important priority, especially in high-risk groups.9
CMV is a complex pathogen with distinct pathobiology.3 CMV is one of the most common opportunistic pathogens in immunocompromised patients. These patients have high risks of complications following primary CMV infection, reinfection, and reactivation of latent virus. The presence of anti-CMV immunoglobulin G (IgG) indicates a previous infection by CMV, while presence of anti-CMV IgM reflects new infection, acute infection, or re‐activation of CMV.10 Donor IgM positivity is associated with higher risk of TT-CMV because of higher CMV DNA loads in both whole blood and plasma samples.11
CMV infection causes significant morbidity and mortality in immunocompromised patients who receive contaminated blood products.3,12 Because CMV can cause severe illness and death in these patients, spread of the virus through blood products should be actively prevented.13 Studies have demonstrated a high prevalence of CMV infection among various groups, including blood donors.14 The risk of CMV transmission through blood products can be limited by improved selection of donors. However, the high prevalence of CMV seropositivity in the donor populations of many countries represents a significant problem: increasing demand for CMV-free blood products may be difficult to meet if CMV-seropositive donors are excluded.13 In addition, use of CMV-seronegative blood cannot completely eliminate the risk of TT-CMV because of the possibility of window period donations.15
Leukoreduction (LR) of blood products is a common method used to decrease the risk of TT-CMV. Because latent CMV infection is restricted to small numbers of WBCs, removal of these cells significantly decreases the risk of TT-CMV.16,17 Although LR is very effective in removing leukocyte-associated CMV, it cannot remove free CMV in plasma. As a result, newly infected blood donors could transmit CMV despite effective LR.18 Persistence of CMV DNA following WBC removal explains rare TT-CMV in recipients of LR blood components.19 In the era of universal LR of blood products, screening for CMV-negative blood products is thought to be unnecessary for hematopoietic stem cell transplantation because no cases of TT-CMV have been detected in some studies.20–22 LR blood products from donors with active CMV infection have very low infectivity.23
CMV-seronegative products can result in TT-CMV during the window period between infection and positive results of antibody screening tests 6 to 8 weeks later. LR blood products can result in TT-CMV because of incomplete removal of WBCs in a small proportion of units. Therefore, both LR and CMV-seronegative units have low residual risks of TT-CMV. Interestingly, the few centers without dual inventories have a relatively high prevalence of CMV seropositive blood donors within their regional populations. Some countries use both CMV-seronegative and LR products for neonatal, intrauterine, and pregnancy-associated transfusion. Other countries use CMV-seronegative and LR products for all high-risk groups, while others use LR products alone.5,24,25
CMV seroprevalence varies significantly (from 40–100%) in different parts of the world.26 The aim of this systematic review and meta-analysis was to estimate the pooled prevalence of CMV among blood donors worldwide.
Methods
Study setting and design
This systematic review and meta-analysis was conducted in a global setting. The study was designed according to the PRISMA-P 2015 Guidelines.27
Search strategy
We searched Embase, PubMed, Google Scholar, Medline, Web of Science, and Cochrane Library for articles published before 18 January 2021. The search terms were “Prevalence” OR “seroprevalence” OR “frequency” AND “CMV” OR “cytomegalovirus” OR “anti-cytomegalovirus antibody” AND “blood donors” OR “volunteer blood donors”.
Study selection and eligibility criteria
Studies were eligible if they met the following criteria: (1) peer-reviewed original articles in English; (2) cross-sectional and cohort studies reporting prevalence of CMV among blood donors; (3) publication between 1 January 2000 and 18 January 2021. Case reports, case-control studies, and editorial articles were excluded. Published articles reporting CMV seroconversion and incidence rates among blood donors were also excluded.
Data extraction
Two authors (TA and SG) screened references and retrieved articles according to the eligibility criteria. The selected papers were scrutinized and discrepancies between reviewers were resolved by discussion and consensus. Additionally, the reference lists of original and review articles were checked in detail to identify additional relevant studies that were not obtained via database searching. For all included studies, the following information was extracted: name of the first author, year of publication, country, study year, sample size, diagnostic methods used, mean age, and type of blood donor.
Study quality assessment
The Newcastle–Ottawa Scale (modified for prevalence studies) was used for methodological quality assessment.28
Meta-analysis
For every included study, point prevalence and 95% CI were calculated. A random-effects model was applied to assess the effects of heterogeneity among selected studies. I2 values of 25%, 50%, and 75% were considered to reflect low, moderate, and high heterogeneity, respectively.29 Forest plots were used to summarize the effect sizes and 95% CIs for all studies. A subgroup analysis was conducted to identify potential sources of heterogeneity among included studies. Funnel plots and Egger’s test were used to investigate potential publication bias.30,31 All statistical analyses were performed using STATA version 11.0 (StataCorp, College Station, TX, USA).
Results
A total of 1420 articles were retrieved by literature searching. Among these articles, 310 were excluded after duplicate removal, 1036 were irrelevant to the aim of this study, and 18 did not meet the eligibility criteria. Forty-three studies were included in the meta-analysis (Figure 1).
Figure 1.
Flow chart of study selection for the systematic review and meta-analysis of the prevalence of anti-CMV antibodies among blood donors.
Study characteristics
Twenty studies were conducted in Africa, 21 in Asia, and two in South America. The countries with the largest number of studies were Nigeria (10 studies) and Iraq (5 studies). Thirty-seven studies used enzyme linked immunosorbent assay (ELISA) to assess anti-CMV antibody titers (IgM and IgG), two studies used enzyme immunoassay, one study used a microparticle enzyme immunoassay, one study used latex particle agglutination, one study used chemiluminescence, and the one used a chromatographic immunoassay. The number of blood donors ranged from 75 in Sudan32 to 2400 in Japan.18 The mean age of donors ranged from 19 years to 45 years. Thirty-three studies examined volunteer blood donors, four studies examined healthy male donors, two studies examined blood bags, one study examined family replacement donors, one study examined volunteer blood donors and family replacement donors, one study examined medical staff and volunteer donors, and one study examined regular donors (Table 1).
Table 1.
Characteristics of included studies.
| Author and year of Publication | Country | Study year | Study design | Sample size | Population | Method | Mean age (years) | IgG (%) | IgM (%) | IgM and IgG (%) | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjei et al. 20062 | Ghana | 2004 | NR | 264 | Volunteer donors | ELISA | 32.1 | 93.2 | Good | ||
| Jobier et al. 20184 | Sudan | 2017 | NR | 90 | Volunteer donors | ELISA | NA | 85.5 | 42.22 | Satisfactory | |
| Akinbami et al. 200933 | Nigeria | 2006 | Cross-sectional | 122 | Volunteer donors | ELISA | 31.3±8.7 | 96 | 19.5 | Good | |
| Bawa et al. 20191 | Nigeria | 2013–2014 | Cross-sectional | 345 | Volunteer donors | ELISA | NR | 96.2 | 2.6 | 2.6 | Very good |
| Bleiblo et al. 201934 | Libya | NR | NR | 200 | Volunteer donors | ELISA | NR | 80.5 | 39 | Good | |
| Bolarinwa et al. 201435 | Nigeria | 2013 | Cross-sectional | 184 | Volunteer donors | ELISA | 26.8 ± 6.5 | 97.4 | 52.6 | 52.6 | Satisfactory |
| Oladipo et al. 201436 | Nigeria | 2012 | NR | 93 | Family replacement | ELISA | 45±2.3 | 25.8 | 28 | Satisfactory | |
| Gawad et al. 201637 | Egypt | 2010 | Cross-sectional | 88 | Volunteer donors | ELISA | 30.8 ± 8.6 | 96.6 | Good | ||
| Gwarzo et al. 201738 | Nigeria | 2012 | Cross-sectional | 250 | Volunteer donors | ELISA | 32.25±8.8 | 4.4 | Very good | ||
| Ibrahim et al. 201432 | Sudan | 2011 | Cross-sectional | 75 | Donors and medical staff | ELISA | NR | 97.3 | Good | ||
| Ibrahim et al. 201539 | Sudan | 2015 | Cross-sectional | 90 | Volunteer donors | ELISA | 26.7(18-50) | 92.1 | 13.3 | 93.3 | Good |
| Njeru et al. 200940 | Kenya | NR | Cross-sectional | 400 | Volunteer donors | ELISA | 24.2 | 97 | 3.6 | Very good | |
| Ojide et al. 201141 | Nigeria | 2010 | NR | 192 | Volunteer donors | ELISA | 32.39±7.9 | 95.8 | 3.1 | Satisfactory | |
| Samuel et al. 201742 | Nigeria | 2014 | Cross-sectional | 93 | Volunteer donors | ELISA | NR | 93.5 | 45.2 | 40.9 | Satisfactory |
| Pennap et al. 201643 | Nigeria | NR | NR | 208 | Volunteer donors | ELISA | 74 | Very good | |||
| Kafi et al. 200944 | Sudan | 2003 | NR | 150 | Volunteer donors | ELISA | NR | 77 | Satisfactory | ||
| Tebuka et al. 201945 | Tanzania | 2017 | Cross-sectional | 228 | Volunteer donors | ELISA | 19 | 10.1 | Very good | ||
| Teka et al. 201846 | Ethiopia | 2016 | Cross-sectional | 605 | Volunteer donors | ELISA | 30.3± 8.37 | 94.4 | 4.0 | Very good | |
| Udomah et al. 201647 | Nigeria | NR | NR | 290 | Volunteer donors | Chromatography | 39 ± 21 | 4.82 | 57.9 | 3.1 | Satisfactory |
| Yusuf et al. 201848 | Nigeria | 2017 | Cross-sectional | 185 | Volunteer donors | ELISA | NR | 92 | satisfactory | ||
| Ahmed et al. 201649 | Iraq | 2014 | NR | 370 | Volunteer donors | ELISA | 34.17± 7.1 | 95.1 | 3.8 | Very good | |
| Ahmed et al. 200650 | Malaysia | NR | NR | 172 | Regular blood donors | MEIA | 29.3 | 97.6 | Satisfactory | ||
| Al-sabri et al. 201751 | Yemen | NR | Cross-sectional | 235 | Volunteer donors | ELISA | 29.1 | 96.6 | 5.5 | Satisfactory | |
| Amarapal et al. 200152 | Thailand | 1998 | NR | 441 | Volunteer donors | ELISA | NR | 52.38 | 9.52 | 8.84 | Satisfactory |
| Chaudhari et al. 20096 | India | NR | Cross-sectional | 431 | Volunteer donors | EIA | 28.2±7.22 | 87.9 | Very good | ||
| Dabbagh 201053 | Iraq | 2007–2008 | NR | 90 | Healthy male | ELISA | 33.3±8.73 | 10 | Satisfactory | ||
| Das et al. 201454 | India | 2011–2012 | Cross-sectional | 2100 | Volunteer and family replacement | ELISA | 31.25 | 98.6 | 0.05 | Good | |
| Delfan-Beiranvand et al. 201255 | Iran | NR | Cross-sectional | 270 | Healthy male | ELISA | NR | 55 | 0.4 | Good | |
| Furui et al. 201318 | Japan | NR | NR | 2400 | Volunteer donors | EIA | NR | 76.6 | Good | ||
| Henry et al. 201611 | India | NR | Cross-sectional | 453 | Volunteer donors | Chemiluminescence | 30.55±9.2 | 94.9 | 0.44 | Good | |
| Kalid 201256 | Iraq | 2011 | Cross-sectional | 100 | Blood bag | ELISA | 27.5±6.3 | 64 | 3 | Satisfactory | |
| Kothari et al. 202157 | India | NR | NR | 200 | Volunteer donors | ELISA | 29.8 ±8.3 | 95 | Satisfactory | ||
| Mahmood et al. 201458 | Pakistan | 2007–2008 | NR | 175 | Healthy male | ELISA | 28.2 | 96.5 | 3.4 | Good | |
| Marzoog 200959 | Iraq | 2008–2009 | NA | 214 | Blood bag | ELISA | NR | 7.5 | Satisfactory | ||
| Moniri et al. 200460 | Iran | 2001–2002 | Descriptive | 600 | Volunteer donor | ELISA | NR | 2.3 | Good | ||
| Nanakaly et al. 201961 | Iraq | NR | Prospective | 472 | Volunteer donors | ELISA | 32.58± 6.9 | 31.36 | 1.48 | Good | |
| Rizvi et al. 201562 | Pakistan | 2013 | Cross-sectional | 91 | Volunteer donors | ELISA | 25.87±6.8 | 97.8 | Satisfactory | ||
| Safabakhsh et al. 201463 | Iran | 2009 | Cross-sectional | 1008 | Volunteer donors | ELISA | NR | 99.2 | 1.6 | Good | |
| Shaheen et al. 202064 | Bangladesh | 2017 | Cross-sectional | 150 | Volunteer donors | LPA | NR | 91.3 | 4 | Satisfactory | |
| Yasir et al. 200865 | Iraq | 2008 | NR | 120 | Male donors | ELISA | NR | 46.6 | Satisfactory | ||
| Ziaei et al. 201366 | Iran | 2010 | Cross-sectional | 200 | Volunteer donors | ELISA | NR | 98.5 | 85 | Satisfactory | |
| Mathos et al. 200967 | Brazil | NR | NR | 636 | Volunteer donors | ELISA | 31.3 | 87.9 | 0 | Very good | |
| Souza et al. 201068 | Brazil | 2003 | Cross-sectional | 1045 | Volunteer donors | ELISA | NR | 96.4 | Very good |
NR, not reported; ELISA, enzyme-linked immunosorbent assay; EIA, enzyme immunoassay; MEIA, microparticle enzyme immunoassay; LPA, latex particle agglutination.
CMV seroprevalence among blood donors
Thirty-eight articles estimated the prevalence of anti-CMV IgG among blood donors. Among these studies, the highest prevalence of anti-CMV IgG antibodies was 99.2% among 1008 blood donors from Iran in 2009.63 The lowest prevalence of anti-CMV IgG antibodies was 4.82% among 290 blood donors in Nigeria.47 The estimated global pooled prevalence of anti-CMV IgG among blood donors was 83.16% (95% CI: 78.55%–87.77%, I2 = 99.5%) (Figure 2).
Figure 2.
Forest plot of the prevalence of anti-CMV IgG among blood donors.
Twenty-eight articles estimated the prevalence of anti-CMV IgM among blood donors. The global pooled prevalence of anti-CMV IgM among blood donors using a random effects model was 13.77% (95% CI: 11.59%–15.95%, I2 = 98.8%). The highest prevalence of anti-CMV IgM was 85% among healthy blood donors in Iran (Figure 3) (Figure 4).66
Figure 3.
Forest plot of the prevalence of anti-CMV IgM among blood donors.
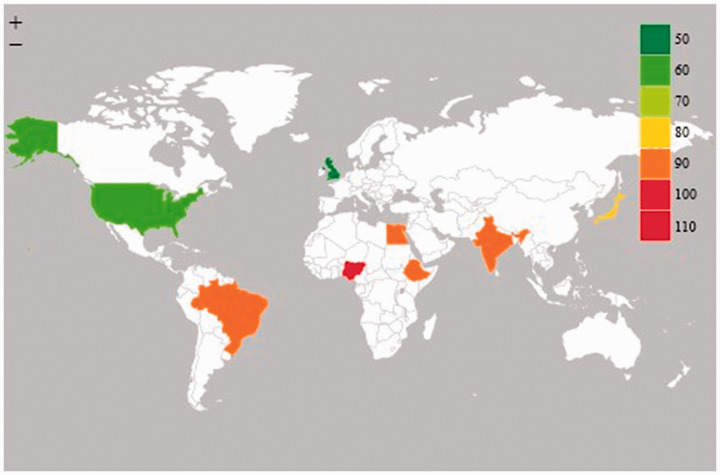
Figure 4.
Estimated global CMV seroprevalence among blood donors.
Four studies estimated the prevalence of both anti-CMV IgG and IgM among blood donors. The global pooled prevalence of both CMV IgM and IgG among blood donors using a random effects model was 23.78% (95% CI: 10.50%–37.06%, I2 = 98.7%) (Figure 5).
Figure 5.
Forest plot of the prevalence of anti-CMV IgM and IgG among blood donors.
Subgroup analysis by region and method of detection
The pooled prevalence of anti-CMV IgG in Africa, Asia, and South America was 82.64% (95% CI 67.47%–97.81%), 82.75% (95% CI 78.20%–87.30%), and 99.23% (95% CI 83.90%–100.56%), respectively. The pooled prevalence of anti-CMV IgM in Africa, Asia, and South America was 22.52% (95% CI 15.89%–29.16%), 8.06% (95% CI 5.70%–10.43%), and 59.00% (95% CI 52.54%–65.48%), respectively. The pooled prevalence of anti-CMV IgG and IgM CMV measured by ELISA was higher compared with other methods of detection (Table 2).
Table 2.
Prevalence of anti-CMV antibodies among blood donors.
| Characteristic | No. of studies | Sample size | Prevalence (95% CI) | I2 (%) | P-value |
|---|---|---|---|---|---|
| CMV IgG | |||||
| Region | |||||
| Africa | 18 | 3881 | 82.64% (67.47–97.81%) | 99.8 | <0.001 |
| Asia | 18 | 9388 | 82.75% (78.20–87.30%) | 99.3 | <0.001 |
| America | 2 | 1681 | 99.23% (83.90–100.56%) | 97.2 | <0.001 |
| Global | 38 | 14743 | 83.16% (78.55–87.77%) | 99.5 | <0.001 |
| Method of anti-CMV antibody detection | |||||
| ELISA | 32 | 10847 | 85.34% (82.44–88.24%) | 98.6 | <0.001 |
| Others | 6 | 3896 | 75.51% (47.87–103.15%) | 99.9 | <0.001 |
| CMV IgM | |||||
| Region | |||||
| Africa | 14 | 3389 | 22.52% (15.89–29.16%) | 98.4 | <0.001 |
| Asia | 15 | 6878 | 8.06% (5.70–10.43%) | 98.9 | <0.001 |
| Global | 29 | 10267 | 13.77% (11.59–15.95%) | 98.8 | <0.001 |
| Method of anti-CMV antibody detection | |||||
| ELISA | 26 | 9419 | 13.41% (10.97–15.85%) | 98.7 | <0.001 |
| Others | 2 | 558 | 1.87% (−1.55–5.30%) | 79.0 | 0.029 |
CMV, cytomegalovirus; CI, confidence interval; ELISA, enzyme-linked immunosorbent assay.
Publication bias
Potential publication bias among the included studies were assessed statistically and graphically using Egger’s test and funnel plots, respectively. Funnel plots of the prevalence of both anti-CMV IgG (Figure 6) and IgM (Figure 7) were non-symmetrical, suggesting the presence of publication bias. Egger’s test also indicated publication bias in both anti-CMV IgG (P < 0.001) and IgM (P < 0.001).
Figure 6.
Funnel plot of the prevalence of anti-CMV IgG among blood donors in the included studies.
Figure 7.
Funnel plot of the prevalence of anti-CMV IgM among blood donors in the included studies.
Discussion
The presence of anti-CMV antibodies (IgM and IgG) among blood donors is a sign of potentially infectious virus in transfused blood products.49 According to this systematic review and meta-analysis, the global prevalence of anti-CMV IgG and IgM among blood donors was 83.16% (95% CI: 78.55%–87.77%, I2 = 99.5%) and 13.77% (11.59%–15.95%, I2 = 98.8%), respectively. The global prevalence of both anti-CMV IgM and IgG among blood donors was 23.78% (95% CI: 10.50%–37.06%, I2 = 98.7%). The high prevalence of anti-CMV IgG identified in this meta-analysis reflects the fact that CMV infection is endemic in different parts of the world.51 However, the pooled prevalence estimated in the current study was lower than another worldwide estimate of among blood and organ donors (86% seroprevalence).69 The prevalence of anti-CMV IgG among blood donors varies according to local infection rates in the general population as well as the socioeconomic characteristics of the blood donors.70 The high seroprevalence of IgG indicates frequent past exposure to CMV. Low socioeconomic status is associated with increased exposure to CMV because of factors such as large household size, crowding, child care practices, and sexual practices.51 We found that 14.8% of blood donors were positive for anti-CMV IgM, indicating the presence of recent acute CMV infection.71 This type of infection could be either primary or recurrent.52 Because of the sensitivity of detection assays, IgM may be detectable both prior to the appearance of IgG and shortly after IgG seroconversion, and remains positive for several months.72,73
In this study, the prevalence of anti-CMV IgG in Africa, Asia, and South America was 82.64% (95% CI: 67.47%–97.81%), 82.75% (95% CI: 78.20%–87.30%), and 99.23% (95% CI: 83.90%–100.56%), respectively. The prevalence of anti-CMV IgM was 22.52% (95% CI: 15.89%–29.16%), 8.06% (95% CI: 5.70%–10.43%), and 59.00% (95% CI: 52.54%–65.48%) in Africa, Asia, and South America, respectively. CMV seroprevalence varies geographically across the world.49 A systematic review and meta-analysis conducted in Iran by Shaiegan et al.10 showed that the prevalence of anti-CMV IgG and IgM was 92% (95% CI: 90%–94%) and 2.6% (95% CI: 1.7%–3.6%), respectively. Another single center study conducted in Nigeria by Gwarzo et al.38 showed that the prevalence of anti-CMV IgG was 100% among blood donors.
The prevalence of anti-CMV IgG among blood donors observed using ELISA and rapid kits was 85.34% (95% CI: 82.44%–88.24%) and 75.51% (95% CI: 47.87%–103.15%), respectively. The prevalence of anti-CMV IgM among blood donors observed using ELISA and rapid kits was 13.41% (95% CI: 10.97%–15.85%) and 1.87% (95% CI: −1.55% to 5.30%), respectively. We found that the prevalence of anti-CMV IgG and IgM among blood donors was higher using ELISA compared with rapid kits. This might be because rapid screening kits are associated with more false negative results compared with ELISA.74 Moreover, a study conducted by Chameera et al.75 showed that rapid kits had lower sensitivity and negative predictive values compared with ELISA.
LR of cellular blood products and/or selection of CMV-seronegative donors are measures used to reduce the risk of TT-CMV. The risk of TT-CMV is closely associated with transfer of leukocytes from infected donors to the recipient.76 However, because of the window period between CMV infection and seroconversion, apparently seronegative donors with transient viremia may be able to transfer CMV.77 CMV-seropositive blood donors are CMV carriers and latently infected cells may be present in their blood that can be reactivated after transfusion and thus may be infectious.76 Blood donations from newly CMV IgG-positive donors should have the highest risks of TT-CMV because they contain the highest levels of CMV DNA and early anti-CMV antibodies cannot neutralize the virus.70 However, because of the high rate of CMV seropositivity in different parts of the world and the need for screening of large numbers of blood donations, use of exclusively CMV-seronegative blood is not practical.78 Use of pathogen-inactivated blood products is another strategy to reduce the risk of TT-CMV and many other infections.5
The findings of this systematic review and meta-analysis should be considered in the context of some important limitations. Heterogeneity was observed in all analyses, including subgroup analyses. High heterogeneity may have arisen from inclusion of studies only in the English language. We also did not explore potential risk factors associated with presence of anti-CMV IgG and IgM among blood donors because this information was not available in most of the included studies.
Conclusion and recommendations
This study revealed that CMV seroprevalence was high among blood donors globally. CMV seropositivity among blood donors is a challenge for safe blood transfusion and can lead to high mortality and morbidity in high-risk transfusion recipients. Therefore, routine CMV screening should be performed to identify CMV-seronegative blood donors.
Acknowledgement
We would like to acknowledge the authors of the studies included in this systematic review and meta-analysis.
Availability of data and materials: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions: TA and SG were involved in the literature search, statistical analysis, study quality assessment, and manuscript drafting, review, and final approval. Both authors critically revised the paper and agree to be accountable for all aspects of the work.
References
- 1.Bawa MK, Mamman A, Olayinka A, et al. Blood donor safety, prevalence and associated factors for cytomegalovirus infection among blood donors in Minna-Nigeria, 2014. Pan Afr Med J 2019; 32: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjei A, Armah H, Narter-Olaga E.Seroprevalence of cytomegalovirus among some voluntary blood donors at the 37 military hospital, Accra, Ghana. Ghana Med J 2006; 40: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogalski MT, Collins-McMillen D, Yurochko AD. Overview of human cytomegalovirus pathogenesis. Human Cytomegaloviruses. Springer, 2014, pp. 15–28. [DOI] [PubMed]
- 4.Jobier A, Ali MA.Frequency rate of cytomegalovirus antibodies among blood donors in Khartoum state. African Journal of Medical Sciences 2018; 3(2). [Google Scholar]
- 5.Ziemann M, Krueger S, Maier AB, et al. High prevalence of cytomegalovirus DNA in plasma samples of blood donors in connection with seroconversion. Transfusion 2007; 47: 1972–1983. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhari C, Bindra M.Seroprevalence of cytomegalovirus among voluntary blood donors. Med J Armed Forces India 2009; 65: 252–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kääriäinen L, Klemola E, Paloheimo J.Rise of cytomegalovirus antibodies in an infectious-mononucleosis-like syndrome after transfusion. Br Med J 1966; 1: 1270–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchi M, Vaglio S, Pupella S, et al. Leucoreduction of blood components: An effective way to increase blood safety? Blood Transfus 2016; 14: 214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmon CM, Cooling LL.Current strategies and future directions for the prevention of transfusion-transmitted cytomegalovirus. International Journal of Clinical Transfusion Medicine 2017; 5: 49–59. [Google Scholar]
- 10.Shaiegan M, Rasouli M, Zadsar M, et al. Meta-analysis of cytomegalovirus seroprevalence in volunteer blood donors and healthy subjects in Iran from 1992 to 2013. Iran J Basic Med Sci 2015; 18: 627–634. [PMC free article] [PubMed] [Google Scholar]
- 11.Henry N, Baiju NM, Bhaskaran R, et al. Cytomegalovirus seroprevalence among blood donors in Kerala. International Journal of Contemporary Medical Research 2016; 3: 3008–3010. [Google Scholar]
- 12.Britt W. Manifestations of human cytomegalovirus infection: Proposed mechanisms of acute and chronic disease. Human Cytomegalovirus. Springer, 2008, pp. 417–470. [DOI] [PubMed]
- 13.Ljungman P.Risk of cytomegalovirus transmission by blood products to immunocompromised patients and means for reduction. Br J Haematol 2004; 125: 107–116. [DOI] [PubMed] [Google Scholar]
- 14.Alao O, Joseph D, Banwat E.Haematological profile of cytomegalovirus antibody positive blood donors in Jos, Nigeria. Nigerian Journal of Clinical Practice 2010; 13(1). [Google Scholar]
- 15.Roback JD, Josephson CD.New insights for preventing transfusion‐transmitted cytomegalovirus and other white blood cell–associated viral infections. Transfusion 2013; 53: 2112–2116. [DOI] [PubMed] [Google Scholar]
- 16.Murphy M, Grint P, Hardiman A, et al. Use of leucocyte-poor blood components to prevent primary cytomegalovirus (CMV) infection in patients with acute leukaemia. Br J Haematol 1988; 70: 253–254. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert G, Hayes K, Hudson I, et al. Prevention of transfusion-acquired cytomegalovirus infection in infants by blood filtration to remove leucocytes. Lancet 1989; 333: 1228–1231. [DOI] [PubMed] [Google Scholar]
- 18.Furui Y, Satake M, Hoshi Y, et al. Cytomegalovirus (CMV) seroprevalence in Japanese blood donors and high detection frequency of CMV DNA in elderly donors. Transfusion 2013; 53: 2190–2197. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Zou S, Cable R, et al. Direct assessment of cytomegalovirus transfusion‐transmitted risks after universal leukoreduction. Transfusion 2010; 50: 776–786. [DOI] [PubMed] [Google Scholar]
- 20.Cohn CS.Transfusion support issues in hematopoietic stem cell transplantation. Cancer Control 2015; 22: 52–59. [DOI] [PubMed] [Google Scholar]
- 21.Kekre N, Tokessy M, Mallick R, et al. Is cytomegalovirus testing of blood products still needed for hematopoietic stem cell transplant recipients in the era of universal leukoreduction? Biol Blood Marrow Transplant 2013; 19: 1719–1724. [DOI] [PubMed] [Google Scholar]
- 22.Nash T, Hoffmann S, Butch S, et al. Safety of leukoreduced, cytomegalovirus (CMV)‐untested components in CMV‐negative allogeneic human progenitor cell transplant recipients. Transfusion 2012; 52: 2270–2272. [DOI] [PubMed] [Google Scholar]
- 23.Ziemann M, Juhl D, Brockmann C, et al. Infectivity of blood products containing cytomegalovirus DNA: Results of a lookback study in nonimmunocompromised patients. Transfusion 2017; 57: 1691–1698. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman L, Devine D, Reesink H, et al. Prevention of transfusion-transmitted cytomegalovirus (CMV) infection: Standards of care. Vox Sang 2014; 107: 276–311. [DOI] [PubMed] [Google Scholar]
- 25.Adler SP, Coleman J, Janot C, et al. Transfusion-transmitted CMV infections: Clinical importance and means of prevention? Vox Sang 1984; 46: 387–414. [DOI] [PubMed] [Google Scholar]
- 26.Galea G, Urbaniak S.Cytomegalovirus studies on blood donors in north‐east Scotland and a review of UK data. Vox Sang 1993; 64: 24–30. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newcastle O. Newcastle-Ottawa: Scale customized for cross-sectional studies In, 2018.
- 29.Higgins JP, Thompson SG.Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JA, Egger M.Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol 2001; 54: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahim HS, KhamisKafi S, Mohammed A, et al. Sero-detection of cytomegalovirus antibodies among blood donors in Khartoum state. Multi-disciplinary Journal of Scientific Research & Education 2016; 2. [Google Scholar]
- 33.Akinbami AA, Akanmu AS, Adeyemo TA, et al. Cytomegalovirus antibodies among healthy blood donors at Lagos University Teaching Hospital. South African Medical Journal 2009; 99: 528–530. [Google Scholar]
- 34.Bleiblo F, Eljaki A, Bumadian M, et al. Screening donated blood for transfusion-transmissible cytomegalovirus infection among Libyans. Journal of Biosciences and Medicines 2019; 8: 5–12. [Google Scholar]
- 35.Bolarinwa R, Donbraye E, Ademosu A, et al. Prevalence and associated characteristics of cytomegalovirus (CMV) immunoglobulin antibodies among blood donors at a University Teaching Hospital in Nigeria. East Afr Med J 2014; 91: 385–390. [PubMed] [Google Scholar]
- 36.Oladipo E, Akinpelu O, Oladipo A, et al. Seroprevalence of cytomegalovirus (CMV) among blood donors at Bowen University Teaching Hospital Ogbomoso. Am J Med Biol Res 2014; 2: 72–75. [Google Scholar]
- 37.Gawad AA, Hashish M, Abaza A, et al. Cytomegalovirus immunoglobulin G avidity index among blood donors in Alexandria, Egypt. Cent Eur J Public Health 2016; 24: 314–320. [DOI] [PubMed] [Google Scholar]
- 38.Gwarzo DH, Gwarzo AK, Ahmed SG.Seroprevalence of cytomegalovirus antibodies among blood donors in Aminu Kano Teaching Hospital, Kano, Nigeria. Nigerian Journal of Basic and Clinical Sciences 2017; 14: 8. [Google Scholar]
- 39.Ibrahim AAA, Elhag WI, Rahama ABM, et al. Seroprevalence of cytomegalovirus among blood donors at Omdurman Teaching Hospital, Omdurman, Sudan. European Academic Research 2015; 3(3). [Google Scholar]
- 40.Njeru DG, Mwanda WO, Kironyi GW, et al. Seroprevalence of cytomegalovirus antibodies in blood donors at the national blood transfusion centre, Nairobi. East African Medical Journal 2009; 86: 58–61. [DOI] [PubMed] [Google Scholar]
- 41.Ojide C, Ophori E, Eghafona N, et al. Seroprevalence of cytomegalovirus (CMV) amongst voluntary blood donors in University of Benin Teaching Hospital (UBTH), Edo State, Nigeria. Journal of Advances in Medicine and Medical Research 2012: 15–20. [Google Scholar]
- 42.Samuel DB, Tamunomieibi WT, Oche OP, et al. Seroprevalence of cytomegalovirus among blood donors in a tertiary hospital in Nigeria. 2017.
- 43.Pennap G, Joseph M, Oti V.The prevalence of cytomegalovirus among eligible blood donors in Keffi, Nigeria. Int J Curr Microbiol App Sci 2016; 5: 716–722. [Google Scholar]
- 44.Kafi S, Eldouma E, Saeed S, et al. Seroprevalence of cytomegalovirus among blood donors and antenatal women attending two hospitals in Khartoum State. Sudan Journal of Medical Sciences 2009; 4. [Google Scholar]
- 45.Tebuka E, Fulgence RD, Msemwa B, et al. Acute human cytomegalovirus infection among voluntary blood donors in the Lake Victoria zone blood transfusion centre: Should it be considered in screening? Afr Health Sci 2019; 19: 2351–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teka YD, Demoz H, Bekele FB, et al. Magnitude and risk factors for cytomegalovirus infection among voluntary blood donors at National Blood Bank, Addis Ababa Ethiopia. ISBT Science Series 2019; 14: 169–175. [Google Scholar]
- 47.Bachaka M, Erhabor O.Cytomegalovirus infection among blood donors in Usmanu Danfodiyo University Teaching Hospital Sokoto, Nigeria. American Journal PharmTech Research 2016; 6(2). [Google Scholar]
- 48.Yusuf E, Nwagu M, Benin BCN. Seroprevalence of cytomegalovirus antibodies positivity among voluntary blood donors in the Delta State University Teaching Hospital (Delsuth), Oghara, and its environs in Delta State-Nigeria. Infection; 1: 2. [Google Scholar]
- 49.Fares HAAKK, Al-Barzinji RM.Cytomegalovirus seropositivity among voluntary blood donors in Koya. Journal of Rasparin University 2016; 3(6). [Google Scholar]
- 50.Ahmed S, Al-Joudi F, Zaidah AW, et al. The prevalence of human cytomegalovirus seropositivity among blood donors at the Unit of Blood Transfusion Medicine, Hospital Universiti Sains Malaysia. Southeast Asian J Trop Med Public Health 2006; 37: 294–296. [PubMed] [Google Scholar]
- 51.Al-Sabri A, Al-Arnoot S, Al-Madhagi A, et al. Seroprevalence of cytomegalovirus among healthy blood donors in Sana’a City, Yemen. J Infect Non Infect Dis 2017; 3: 016. [Google Scholar]
- 52.Amarapal P, Tantivanich S, Balachandra K.Prevalence of cytomegalovirus in Thai blood donors by monoclonal staining of blood leukocytes. Southeast Asian J Trop Med Public Health 2001; 32: 148–153. [PubMed] [Google Scholar]
- 53.Al-Dabbagh KA.Detection of Toxoplasma gondii IgM and cytomegalovirus IgM antibodies among blood donors in Mosul. Iraqi J Pharmacy 2011; 11: 85–92. [Google Scholar]
- 54.Das B, Kaur G, Basu S.Seroprevalence of cytomegalovirus antibodies among blood donors and multitransfused recipients – A study from north India. Transfus Apher Sci 2014; 50: 438–442. [DOI] [PubMed] [Google Scholar]
- 55.Delfan BM, Pournia Y, Fazeli M, et al. Seroprevalence of cytomegalovirus infection in blood donors in Khorramabad. Iranian Journal of Virology 2012; 6: 1–5. [Google Scholar]
- 56.Khalid MD. Prevalence of cytomegalovirus antibodies among blood donors in Mosul Central Blood Bank/Iraq.
- 57.Kothari A, Ramachandran V, Gupta P, et al. Seroprevalence of cytomegalovirus among voluntary blood donors in Delhi, India. J Health Popul Nutr 2002; 20: 348–351. [PubMed] [Google Scholar]
- 58.Mahmood R, Malik F, Hussain S, et al. Seroprevalence of cytomegalovirus among blood donors in local population. Int J Pharm Chem 2014; 4: 146–150. [Google Scholar]
- 59.Marzoog TR.The incidence of cytomegalovirus (CMV) among blood donors in Iraq. Journal of the College of Basic Education 2009; 13. [Google Scholar]
- 60.Moniri R, Mosayebi Z, Mousavi GA. Seroprevalence of cytomegalovirus, hepatitis B, hepatitis C and human immunodeficiency virus antibodies among volunteer blood donors. 2004.
- 61.Nanakaly HT, Hussen BM.Seroprevalence of cytomegalovirus among voluntary blood donors in Erbil Province, North Iraq. Zanco Journal of Pure and Applied Sciences 2019; 31: 1–6. [Google Scholar]
- 62.Rizvi CB, Raza A, Siddiqui MF.Seroprevalence of human cytomegalovirus among blood donors in Lahore, Pakistan. Advancements in Life Sciences 2015; 2: 171–175. [Google Scholar]
- 63.Safabakhsh H, Karimi G, Tehranian F, et al. Demography and seroprevalence of cytomegalovirus infection in blood donors in Mashhad in 2009. J Am Sci 2014; 10: 139–142. [Google Scholar]
- 64.Shaheen SSI, Hoque A, Ferdous J.Seroprevalence of cytomegalovirus among blood donor in transfusion medicine: Study from Bangladesh. International Journal of Innovative Research in Medical Science 2020; 5: 1–4. [Google Scholar]
- 65.Yasir SJ, Majhol RB.Screening of anti-cytomegalovirus IgG antibodies in blood donors in Al-Najaf Governorate. Kufa Medical Journal 2008; 11. [Google Scholar]
- 66.Eivazi-Ziaei J, Movassaghpour A, Asgharzadeh M, et al. Seroprevalence of cytomegalovirus in blood donors in the northwest of Iran. Journal of Research in Clinical Medicine 2013; 1: 96–100. [Google Scholar]
- 67.Matos SB, Meyer R, Lima FW.Seroprevalence of cytomegalovirus infection among healthy blood donors in Bahia State, Brazil. Revista Brasileira de Hematologia e Hemoterapia 2010; 32: 45–49. [Google Scholar]
- 68.Souza MA, Passos AM, Treitinger A, et al. Seroprevalence of cytomegalovirus antibodies in blood donors in southern, Brazil. Rev Soc Bras Med Trop 2010; 43: 359–361. [DOI] [PubMed] [Google Scholar]
- 69.Zuhair M, Smit GSA, Wallis G, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta‐analysis. Rev Med Virol 2019; 29: e2034. [DOI] [PubMed] [Google Scholar]
- 70.Ziemann M, Thiele T.Transfusion‐transmitted CMV infection – current knowledge and future perspectives. Transfus Med 2017; 27: 238–248. [DOI] [PubMed] [Google Scholar]
- 71.Roback JD.CMV and blood transfusions. Rev Med Virol 2002; 12: 211–219. [DOI] [PubMed] [Google Scholar]
- 72.Glock B, Schistal E, Mayr WR.CMV DNA in blood donors with IgM and IgG CMV antibodies. Transfusion 2003; 43: 1493–1494. [DOI] [PubMed] [Google Scholar]
- 73.Ziemann M, Unmack A, Steppat D, et al. The natural course of primary cytomegalovirus infection in blood donors. Vox Sang 2010; 99: 24–33. [DOI] [PubMed] [Google Scholar]
- 74.Erhabor O, Kwaifa I, Bayawa A, et al. Comparison of ELISA and rapid screening techniques for the detection of HBsAg among blood donors in Usmanu Danfodiyo University Teaching Hospital Sokoto, North Western Nigeria. J Blood Lymph 2014; 4: 124. [Google Scholar]
- 75.Chameera E, Noordeen F, Pandithasundara H, et al. Diagnostic efficacy of rapid assays used for the detection of hepatitis B virus surface antigen. Sri Lankan Journal of Infectious Diseases 2013; 3(2). [Google Scholar]
- 76.Söderberg-Nauclér C, Fish KN, Nelson JA.Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 1997; 91: 119–126. [DOI] [PubMed] [Google Scholar]
- 77.Hecker M, Qiu D, Marquardt K, et al. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang 2004; 86: 41–44. [DOI] [PubMed] [Google Scholar]
- 78.Mutlu B, Guenlemez A, Tuerker G, et al. Is serologic screening necessary in the donor bloods for cytomegalovirus seronegative blood transfusion to risky patients? Mikrobiyol Bul 2008; 42: 337–341. [PubMed] [Google Scholar]