Abstract
Movement disorders are rare compared to other neurological manifestations of COVID-19. Patients who have recovered from acute severe acute respiratory syndrome coronavirus-2 infection continue to have multiple debilitating symptoms months later. We report a case of 54-year-old man who presented with repetitive flexion movement of head which started 2 months after severe acute respiratory syndrome coronavirus-2 infection. Extensive work-up including neurological examination, neuroimaging, cerebrospinal fluid analysis, and electroencephalogram were normal. The self-reported questionnaires for depression and anxiety were suggestive of severe anxiety and depression. The patient continued to have the jerky movements besides cognitive impairment, frequent headaches, intermittent shortness of breath, sleeping difficulties, fatigue, and dizziness at 1-year follow-up. This case highlights the presentation of functional movement disorder as one of the manifestations of underlying neuropsychiatric condition. Our patient had significant effect on quality of life with high symptom burden which further highlights the struggle and unmet needs of the patients with multiple symptoms after severe acute respiratory syndrome coronavirus-2 infection.
Keywords: COVID-19, functional movement disorder, long-term effects of COVID-19, neurological manifestations, functional neurological disorder, post-acute sequalae of severe acute respiratory syndrome coronavirus-2
Introduction
Neurological complications after severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection includes (but not limited to) anosmia, ageusia, headache, stroke, impairment of consciousness, seizure, and encephalopathy.1–3 Patients with post-acute sequalae of COVID-19 (PASC) have a range of non-specific neurological concerns, such as loss of memory and concentration, weakness, headache, and dizziness months after acute infection.1 COVID-19-related movement disorders include myoclonus, opsoclonus, and ataxia and are presumed to be parainfectious or post-infectious in etiology.4,5 Functional movement disorders (FMDs) are also being reported after COVID-19 infection as well after COVID-19 vaccination.6 We want to report a case of a 54-year-old man who presented to post-COVID clinic at the University of Iowa with abnormal repetitive movement of the head, which started 2 months after SARS-CoV-2 infection. Extensive neurological work-up including imaging and electroencephalogram (EEG) was negative, and the patient continued to have the abnormal jerky movements besides other concerns including cognitive impairment, frequent headaches, shortness of breath, fatigue, and intermittent dizziness at 1-year follow-up.
Case presentation
A 54-year-old man with past medical history of obesity, hypertension, and obstructive sleep apnea tested positive for SARS-CoV-2 on June 16, 2020. At that time, he had shortness of breath and cough, chest imaging showed bilateral patchy pulmonary opacities. He required supplemental oxygen through nasal cannula, was hospitalized for few days but never required mechanical ventilation and endotracheal intubation. The shortness of breath lasted for many weeks after discharge; however, the patient did not require supplemental oxygen after hospital discharge. Late August, 2020 (2 months after discharge), he started having jerky movements of head (Supplementary Video 1). There was flexion movement of the head every few seconds which would start suddenly, happen anytime of the day, lasted for few hours during an episode. Movements occurred with sitting or lying down and did not occur with standing or walking. There was no exacerbating or relieving factor identified, but it was less frequent when patient was distracted and more when he was relaxed or just before falling asleep (as told by family member). He never lost consciousness during the movements. In addition to abnormal movements, he also reported dizziness and lightheadedness on standing or walking, daily constant headaches with eye pressure, numbness in the throat, and “brain fog.” “Brain fog” was described as problems with memory, attention, concentration, and word-finding difficulty. Other concerns included numbness and tingling in arms and legs, weakness in arms and legs, new muscle pain, feeling down and depressed and excessive nervousness, worry, excessive fatigue, and poor sleep. These changes significantly interfered with activities such as driving, going to work, shopping, playing different musical instruments, and taking care of finances. Before SARS-CoV-2 infection, he was able to do all these activities without any problem, did not have any history of psychiatry or neurological disorder and was independent in all the instrumental activities of daily living.
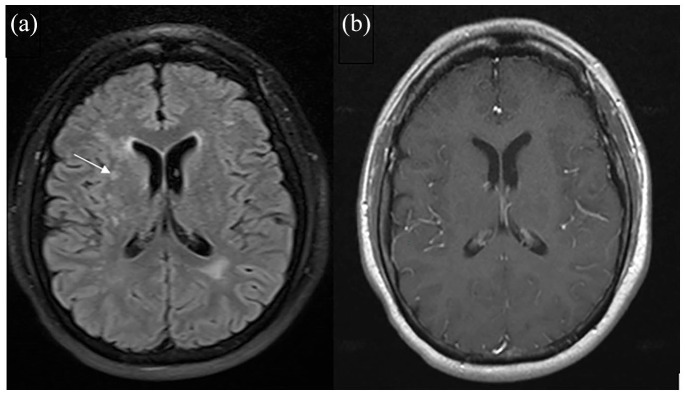
His vitals and general physical as well as detailed neurological examinations at different visits were normal. Patient health questionnare-9 (PHQ-9) score was 24 (out of 27) and generalized anxiety disorder-7 (GAD-7) score was 19 (out of 21) suggestive of severe depression and severe anxiety (detailed results available in Supplementary Tables 2 and 3).7,8 Both PHQ-9 and GAD-7 scores have been validated, and are reliable measure of severity of depression and GAD respectively.7,8 Cerebrospinal fluid analysis and other tests were unremarkable (Table 1). Magnetic resonance imaging of the brain (done three times at 3, 6, and 11 months) was suggestive of chronic microvascular ischemic changes but could not explain the symptoms (Figure 1). We did not have any brain imaging prior to SARS-CoV-2 infection for comparison. The video recording during eight hours of video EEG showed very frequent movements of the head when patient was awake. On EEG tracing, these movements lasted 150- to 200-ms duration, variable in frequency, and were associated with movement artifacts, but no clear ictal or interictal evidence of epilepsy or epileptiform discharges was found. The jerks disappeared completely during N2 stage of sleep. Detailed neuropsychology assessment was suggestive of mental inefficiency with poor attention and slowed processing speed. There was difficulty with cognitive test engagement which was interfering with wide range of cognitive test performance and cognitive abilities.
Table 1.
Laboratory and investigation results.
| Test (normal range with units) | Result |
|---|---|
| Hemoglobin (13.2–17.7 g/dL) | 13.9 |
| Platelets (150–400 K/mm3) | 165 |
| White blood count (3.7–10.5 K/mm3) | 7.7 |
| Blood urea nitrogen (10–20 mg/dL) | 13 |
| Creatinine (0.6–1.2 mg/dL) | 0.9 |
| Liver function test | Normal |
| Cerebrospinal fluid (CSF) analysis-lumbar puncture done at 5 months after SARS-CoV-2 positive test | |
| Color | Clear |
| Total protein (15–45 mg/dL) | 20 |
| Glucose (40–75 mg/dL) | 57 |
| White cell count (0–5/mm3) | 2 |
| Microbiological studiesa | Negative |
| CSF meningitis and encephalitis panelb | Negative |
| Multiple sclerosis screen | Negative, no oligoclonal bands in serum or CSF |
| CSF IgG index (0.28–0.66 normal ratio) | 0.47 |
| Paraneoplastic autoantibodies in serum and CSFc | Negative |
| Erythrocyte sedimentation rate (0–15 mm/h) | 15 |
| Hemoglobin A1c (4.8%–6.0%) | 5.6% |
| Serum blood glucose (65–139 mg/dL) | 98 |
| Thyroid function tests | Normal |
| Serum vitamin B12 (232–1,245 pg/mL) | 1,068 |
| Serum protein electrophoresis | Normal |
| Nerve conduction study (NCS) and electromyography(EMG) done for numbness and tingling | Motor and sensory conduction studies normal. Left tibial nerve F-wave latency was normal when corrected for temperature. Normal EMG |
| Computed tomography of the head without contrast | Normal |
| Computed tomography of the larynx/soft tissue neck with contrast | Normal |
| Computed tomography of the lung without contrast (inspiratory and expiratory scan done at 3 months after SARS-CoV-2-positive test) | Minimal lateral peripheral air trapping of the left lateral lower lobe. No evidence of any infectious process |
| Pulmonary function test | Spirometry normal, normal flow volume loops |
| Two-dimensional transthoracic echocardiogram | Ejection fraction 55%–60%, normal ventricular size, normal right ventricular function |
CSF: cerebrospinal fluid; NCS: nerve conduction study; EMG: electromyography.
Gram stain, bacterial culture-aerobic and anerobic, fungal cultures, CSF cryptococcal Antigen.
PCR assays for detection of Enterovirus, E. coli, Haemophilis influenzae, Neisseria meningitidis, Streptococcus agalactiae, Streptococcus pnemoniae, cytomegalovirus, Perechovirus, Herpes Simplex Virus 1 and 2, Human Herpesvirus 6, varicella zoster virus, Cryptococcus neoformans/gattii.
Encephalitis profile: anti-NMDA, anti-neuronal nuclear antibody (ANNA), Type 1/ 2/ 3, Purkinje cell cytoplasmic antibody type 1/2/Type Tr, amphiphysin antibody, Type 1, GAD65, AMPA-R antibody CBA, GABA-B-R antibody CBA, leucine-rich glioma inactivated protein 1 IgG, CASPR2 IgG, AChR ganglionic neuronal antibody, N-type calcium channel antibody, P/Q-type calcium channel antibody.
Figure 1.
Brain MRI (basal ganglia level): (a) axial fluid attenuated inversion recovery image shows scattered areas of signal abnormality involving the bilateral periventricular white matter (arrow) consistent with chronic microvascular ischemic changes and (b) Axial gadolinium-enhanced T1-weighted image does not show any abnormal enhancement.
Based on neuropsychology assessment, he was referred for neuropsychological rehabilitation. He was started on levetiracetam for few months which had no improvement in the movements. At the last clinic visit at 1-year after discharge from initial acute illness, he continued to have similar movements intermittently with minimal improvement. He underwent physical rehabilitation but was unable to establish follow-up with psychiatry for cognitive behavioral therapy and neuropsychological rehabilitation. He had multiple emergency department visits as well as clinic visits during this time. He received Janssen’s single-dose COVID-19 vaccine in March, 2021, without any complications. He did not report any improvement or worsening of underlying symptoms after vaccination.
Discussion
This case highlights the presentation of FMD as one of the manifestations of underlying neuropsychiatric condition. FMD with normal neurological work-up after SARS-CoV-2 was recently reported in another case report.9 FMDs have also being recognized after SARS-CoV-2 vaccination.10 It is important to distinguish organic causes of movement disorder like opsoclonus-myoclonus syndrome which was reported in a case series of seven patients within 1 month after mild to moderate SARS-CoV-2 respiratory disease.4 Recently, Chan et al. reported a case and systematically reviewed the clinical features of 51 cases of myoclonus presenting within 1 month of COVID-19 symptoms with associated ataxia in 40% of patients and cognitive changes in 30% of patients.5 The likely pathophysiology behind the myoclonus was presumed to be parainfectious or neuro-inflammatory and most cases improved with immunotherapy or spontaneously within 2 months of onset.4,5 The onset of movement disorder in our patient was 2 months after initial COVID-19 symptoms without any improvement or worsening with time. The pathophysiology of FMD is multifactorial with underlying biological, psychological, and social factors. Underlying biological factors like overactive limbic system, acute physical or psychological stressors and individual reaction to stress and external factors can contribute to the development of FND.11 Piscitelli et al. described similar case of FMD in a 39-year-old woman shortly after SARS-CoV-2 infection with possibility of acute traumatic event contributing to abnormal body function.9
Historically, FMD is difficult to diagnose, and there is uncertainty with possible underlying organic disorder in 10% of the cases.11,12 Further with COVID-19, uncertainty is added with a relatively new viral illness and its neurological effects.1 Electrophysiological study can be done to elucidate Bereitschaftspotential (also known as premotor potential or readiness potential), which can distinguish functional myoclonus from other movement disorders in research setting; however, it requires special software program to average signal which was not available in clinical practice in our EEG recording system.13 Also, we were not able to obtain either a functional magnetic resonance imaging (MRI) or a synchronized EEG with electromyography (EMG) but EEG electrodes on the scalp were able to capture the head motion artifact with the movements with no EEG evidence of any abnormality. In our patient, the frequent head jerks were slower than myoclonic jerks, variable in frequency and improved with distraction which was suggestive of FMD.
For FMD, besides finding underlying etiology, the diagnosis is difficult to communicate to the patients.11 Treatment options for FMD are limited but evolving; however, there are no specific medications but reassurance, cognitive behavioral therapy, intensive physical therapy, and addressing underlying mental health conditions if present.11,14
Mounting evidence suggests increased prevalence of anxiety, depression, sleep disturbances as well as post-traumatic stress disorder after COVID-19 infection.15 FMD can be triggered by acute stressors or physical trauma shortly before onset and may result from maladaptive response of patient to their bodily functions.9,11,12 FMD as well as PASC can significantly affect quality of life and can be debilitating. Our patient had significant effect on quality of life with high symptom burden which further highlights the struggle and unmet needs of the patients with multiple symptoms after SARS-CoV-2 infection.
Conclusion
This case highlights the presentation of FMD as one of the manifestations of underlying neuropsychiatric condition. Our patient had high symptom burden months after SARS-CoV-2 infection including repetitive head movements, fatigue, cognitive impairment, anxiety, depression, sleep disturbance, daily headaches, and cognitive impairment. It is important to recognize the needs and support the patients with ongoing concerns after acute SARS-CoV-2 infection.
Supplemental Material
Supplemental material, sj-pdf-1-sco-10.1177_2050313X211039377 for Post-acute COVID-19 functional movement disorder by Alpana Garg, Sachin Goyal and Alejandro P Comellas in SAGE Open Medical Case Reports
Supplemental material, sj-pdf-2-sco-10.1177_2050313X211039377 for Post-acute COVID-19 functional movement disorder by Alpana Garg, Sachin Goyal and Alejandro P Comellas in SAGE Open Medical Case Reports
Footnotes
Author contributions: Data collection and analysis, writing the paper.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual case. This patient was part of post-COVID-19 registry IRB ID # 202005421.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient and his wife for their anonymized information to be published in medical journal.
ORCID iD: Alpana Garg  https://orcid.org/0000-0002-9497-0141
https://orcid.org/0000-0002-9497-0141
Supplemental material: Supplemental material for this article is available online.
References
- 1.Al-Ramadan A, Rabab’h O, Shah J, et al. Acute and post-acute neurological complications of COVID-19. Neurol Int 2021; 13: 102–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg A, Marji A, Goyal S, et al. A case of COVID-19 with memory impairment and delayed presentation as stroke. Cureus 2020; 12: e10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melegari G, Rivi V, Zelent G, et al. Mild to severe neurological manifestations of COVID-19: cases reports. Int J Environ Res Public Health 2021; 18: 3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emamikhah M, Babadi M, Mehrabani M, et al. Opsoclonus-myoclonus syndrome, a post-infectious neurologic complication of COVID-19: case series and review of literature. J Neurovirol 2021; 27(1): 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JL, Murphy KA, Sarna JR.Myoclonus and cerebellar ataxia associated with COVID-19: a case report and systematic review. J Neurol. Epub ahead of print 22 February 2021. DOI: 10.1007/s00415-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuhna P, Herlin B, Vassilev K, et al. Movement disorders as a new neurological clinical picture in severe SARS-CoV-2 infection. Eur J Neurol 2020; 27(12): e88–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroenke K, Spitzer RL, Williams JB.The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9): 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166: 1092–1097. [DOI] [PubMed] [Google Scholar]
- 9.Piscitelli D, Perin C, Tremolizzo L, et al. Functional movement disorders in a patient with COVID-19. Neurol Sci 2020; 41(9): 2343–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DD, Kung CS, Perez DL.Helping the public understand adverse events associated with COVID-19 vaccinations: lessons learned from functional neurological disorder. JAMA Neurol 2021; 78: 789–790. [DOI] [PubMed] [Google Scholar]
- 11.Hallett M.The most promising advances in our understanding and treatment of functional (psychogenic) movement disorders. Parkinsonism Relat Disord 2018; 46(Suppl. 1): S80–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallett M.Functional (psychogenic) movement disorders: clinical presentations. Parkinsonism Relat Disord 2016; 22(Suppl. 1): S149–S152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomsen BLC, Teodoro T, Edwards MJ.Biomarkers in functional movement disorders: a systematic review. J Neurol Neurosurg Psychiatry 2020; 91(12): 1261–1269. [DOI] [PubMed] [Google Scholar]
- 14.Espay AJ, Aybek S, Carson A, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018; 75: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020; 7(7): 611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-sco-10.1177_2050313X211039377 for Post-acute COVID-19 functional movement disorder by Alpana Garg, Sachin Goyal and Alejandro P Comellas in SAGE Open Medical Case Reports
Supplemental material, sj-pdf-2-sco-10.1177_2050313X211039377 for Post-acute COVID-19 functional movement disorder by Alpana Garg, Sachin Goyal and Alejandro P Comellas in SAGE Open Medical Case Reports