Abstract
Purpose:
Lymphedema is a common debilitating late effect among patients post-head and neck cancer (HNC) treatment. Head and neck lymphedema was associated with symptom burden, functional impairment, and decreased quality of life. The objective of this study was to determine the feasibility and potential efficacy of the use of photobiomodulation (PBM) therapy for head and neck lymphedema, symptom burden, and neck range of motion among HNC survivors.
Methods:
This was a single-arm, pre- and post-design clinical trial. Eligible patients included those with lymphedema after completion of complete decongestive therapy (CDT) and 3 to 18 months after completion of cancer therapy. The intervention included PBM therapy 2 times a week for 6 weeks for a total of 12 treatments. Lymphedema, symptom burden, and neck range of motion were measured at baseline, end-of-intervention, and 4-week post-intervention.
Results:
Of the 12 patients enrolled in the study, 91.7% (n = 11) completed the study intervention and assessment visits, and no adverse events were reported. When comparing the baseline to 4-week post-intervention, we found statistically significant improvements in the severity of external lymphedema, symptom burden, and neck range of motion (all P < .05).
Conclusion:
PBM therapy was feasible and potentially effective for the treatment of head and neck lymphedema. Future randomized controlled trials are warranted to examine the efficacy of PBM therapy for HNC-related lymphedema.
Trial Registration Number and Date of Registration:
ClinicalTrials.gov Identifier: NCT03738332; date of registration: November 13, 2018.
Keywords: head and neck cancer, lymphedema, fibrosis, photobiomodulation
Introduction
Head and neck cancer (HNC) and its treatment (eg, surgery and radiation) often damages lymphatic structures and surrounding soft tissues, blocking lymph flow and causing lymphedema, a pathological accumulation of lymph fluid in interstitial tissues.1-3 When the damage is mild, the lymphatic system may be able to repair or compensate for lymphatic injury, resulting in reduction or resolution of visible tissue swelling.1,4 If the damage is severe or no intervention is undertaken, accumulated protein-rich lymph fluid can trigger a chronic inflammatory response.1,4 This results in a fibrosclerotic process in which fibrotic tissues may develop.1,4
Head and neck lymphedema is highly prevalent among patients who undergo HNC treatment with 3-quarters developing lymphedema >3 months post-cancer treatment.5 Head and neck lymphedema can occur both externally and internally.5,6 External lymphedema in the head and neck region results in swelling, skin tightness, an increase in body image issues, and a decreased range of motion in the jaw, neck, and shoulders.6-8 Internal lymphedema of the upper aerodigestive tract (eg, pharynx and larynx) significantly impacts its critical functions, often resulting in difficulty swallowing and speaking.7,9
The current standard of care for lymphedema is complete decongestive therapy (CDT). Although head and neck lymphedema is often well controlled after the completion of intensive CDT, some patients still experience residual lymphedema, which can progress to chronic lymphedema and, over time, fibrosis.3,5,10,11 Clinical experience indicates that CDT is more effective to treat early-stage lymphedema but less effective to treat chronic, late-stage lymphedema (eg, fibrotic tissue formation). Therefore, alternative treatment modalities need to be investigated for effective management of chronic and late-stage lymphedema in the HNC population.
Photobiomodulation (PBM) therapy (previously named low-level laser therapy), is a promising alternative treatment option for treating lymphedema. PBM therapy has had a place in general medicine for more than 40 years.12 In 2006, the U.S. Food and Drug Administration (FDA) accepted it as a treatment approach for breast cancer-related arm lymphedema.12 One systematic review evaluating 7 randomized clinical trials (RCT) concluded that available evidence supports PBM therapy in the management of breast cancer-related arm lymphedema, with clinically meaningful reductions in lymphedema-related arm swelling and symptom burden (eg, pain).13 In the HNC population, PBM therapy is recommended to prevent and treat oral mucositis as outlined in the clinical practice guidelines by the Multinational Association of Supportive Care in Cancer and the International Society of Oral Oncology.14,15 PBM therapy is noninvasive, repeatable, easily performed modality in an outpatient setting, and without any known side effects.12-17 An animal model study has shown that PBM reduces macrophage accumulation and stimulates lymphangiogenesis, indicating that PBM has an anti-inflammatory effect and is potentially effective in treating chronic lymphedema.18 A study conducted in breast cancer survivors also suggests that PBM therapy has a long-term treatment effect on breast cancer-related arm lymphedema.19 Thus, experimental and clinical evidence supports that PBM therapy may be effective in treating chronic lymphedema.19-24 This is particularly important because many HNC survivors develop both lymphedema and late fibrosis.11 Therefore, PBM therapy may have the potential to treat and manage HNC-related lymphedema. Currently, no prospective trials have been reported to examine the effect of PBM therapy on head and neck lymphedema. We conducted a pilot prospective clinical trial investigating the use of PBM therapy in the treatment of head and neck lymphedema. The primary aim of our study was to determine the feasibility of the use of PBM therapy for HNC survivors with lymphedema, and the secondary aim was to evaluate the preliminary efficacy of the PBM therapy on lymphedema in HNC survivors.
Materials and Methods
Design
This was a single-arm, pre- and post-design clinical trial (NCT03738332) conducted at the Head and Neck Cancer Clinics at the University of Pennsylvania Abramson Cancer Center.25 After Institutional Review Board and Clinical Trial Scientific Research Monitoring Committee approval at the study site (UPCC12318), the study staff approached potential participants who expressed interest in the study. All participants signed informed consent forms prior to engaging in any study activities.
Patient Selection
The following criteria had to be met before a patient was enrolled in the trial: age of at least 18 years; completion of cancer treatment for histologically proven head and neck cancer (post-cancer treatment of greater than 3 months and less than 18 months); no evidence of cancer; presence of external head and neck lymphedema; completion of lymphedema therapy; ability to speak and read English; and ability to provide informed consent. Patients were excluded if they had any of the following medical conditions that would prohibit the safe implementation of PBM therapy: pregnancy; photosensitivity; chronic inflammatory diseases; venous thrombosis; history of severe trauma; medication that affects body fluid and electrolyte balance; use of high doses of non-steroidal anti-inflammatory drugs; pre-existing skin rash, ulceration, open wound in the treatment area; and/or allergies and other systemic skin diseases. In addition, patients were excluded if they were in active physical therapy and/or lymphedema therapy or were unable to undergo study-related visits.
Procedures
Study assessments occurred during 3 points in time: baseline (pre-intervention), immediately following the end of the intervention, and 4-week post-intervention. After signing informed consent, participants underwent baseline measures. Participants were asked to complete a demographic form at baseline, while tumor and treatment-related data were obtained through medical chart review. Participants also completed 2 self-reported questionnaires including the “Head and Neck-Lymphedema and Fibrosis (HN-LEF) Symptom Inventory” and “Neck Disability Index.”26-28 Study staff examined participants’ head and neck skin and recorded external lymphedema and fibrosis status using the “Head and Neck-External Lymphedema and Fibrosis (HN-LEF) Assessment Criteria.”29,30 Internal lymphedema was assessed by endoscopic examination (part of standard of care) and documented based on the Modified Patterson Scale.31 After completion of baseline measures, participants were scheduled for the PBM therapy. After 12 sessions of PBM therapy, participants underwent another study assessment and then were scheduled for the 4-week post-intervention visit. At the 4-week post-intervention, the participants were interviewed for their perceptions about PBM treatment experience (qualitative data reported elsewhere).
Study Intervention: Photobiomodulation Therapy
Laser unit
The laser unit used in the trial was the RianCorp LTU-904 laser therapy unit. The LTU-904 is a Class I laser device, which is a low output laser that emits a pulsed 904-nm beam at an average output of 5 mW, spot size of 0.2 cm2, and an energy density of 1.5 J/cm2.32,33 Treatment was administered by a certified lymphedema therapist who was trained to use the LTU-904 laser therapy unit. Because the RianCorp LTU-904 is a Class I laser, no added precautions such as safety glasses are required as there is no risk to eye damage.
Study Protocol
The principal investigator (J.D.) led an interdisciplinary team and developed a PBM therapy protocol. The team members included a lymphedema researcher, 2 lymphedema therapists, 3 HNC oncologists, a nurse practitioner, a supportive oncology researcher, and a PBM device expert. The protocol was developed based on the following: an extensive literature review on the PBM studies conducted in individuals with lymphedema,12-21 our previous work and experience in the assessment, treatment, and long-term management of head and neck lymphedema, and the PBM therapy parameters per the World Association for Photobiomodulation Therapy’s (WALT)34 guidelines.
Per the protocol, participants received PBM therapy twice a week for 6 weeks (a total of 12 sessions). Treatment locations were determined based on presentation of swelling and fibrosis. A total of 14 to 25 points on the face and neck were treated. Treatment locations included: the maxillary prominence (1 point), mandible (2 points), preauricular (1 point), submental (3 points), sternocleidomastoid muscle (3 points), supraclavicular area (2 points), and scalene muscle (2 points). Each treatment location received PBM therapy for 60 seconds. Prior to PBM therapy, participants received 5 minutes of simple manual lymphatic drainage (MLD) to the head and neck region that followed the international standards,1 while positioned supine. PBM therapy and simple MLD were performed by a physical therapist with specialty training in head and neck lymphedema management. Each treatment session took approximately 25 to 30 minutes.
Study Measures
Sample characteristics
Demographic form
Participants’ demographic data, such as age and sex, were collected.
Head and neck cancer clinical form
Participants’ HNC and its treatment information, such as tumor stage and treatment duration, were collected.
Feasibility measures
Recruitment log
The trained research assistants documented the numbers of participants screened, recruited, and consented.
Implementation log
The study lymphedema therapist recorded barriers of implementing interventional sessions and reasons for missed sessions.
Common terminology criteria for advance events (CTCAE) version 5.0
The study staff used this system to evaluate adverse events of the trial.35
Efficacy measures
The following measurements were collected at 3 time points (ie, baseline, at the end of the intervention, and 4-week post-intervention).
Lymphedema
Head and Neck-External Lymphedema and Fibrosis (HN- LEF) Assessment Criteria: The trained study staff used this tool to document participants’ external LEF status through a head and neck physical examination. This tool includes 4 types (from types A to D) of the soft tissue abnormalities (Table 1). Under each type, except for type A, a grade of mild, moderate, or severe is used to describe the severity of the soft tissue abnormalities. The anatomical sites of soft tissue abnormalities include left/right peri-orbital region, left/right cheeks, submental, left/right neck, left/right supraclavicular region, and other sites if applicable. The total severity score of lymphedema is calculated by summing the severity score of each site (mild = 1, moderate = 2, and severe = 3). The tool has good interrater reliability (91.0% agreement for type of LEF, kappa = 0.81, P < .001; and 84.9% agreement for grade of LEF, kappa = 0.70, P < .001), and excellent intrarater reliability (96.1% agreement for type of LEF; and 91.4% agreement for grade of LEF).29,30
Table 1.
Type of Soft Tissue Abnormalities Per HN—LEF Assessment Criteria.
| Type | Descriptors |
|---|---|
| Type A | No visible tissue swelling; palpable thickening and/or tightness of dermis |
| Type B | Visible soft tissue swelling; involved tissues are soft to touch; tissue swelling is reducible and fluctuates in severity |
| Type C | Visible soft tissue swelling; involved tissues are firm to touch; tissue swelling is non-reducible and persistent |
| Type D | Firm skin with increased density and decreased compliance in the absence of swelling |
Modified Patterson Scale: Endoscopic exams were performed by participants’ radiation oncologists as part of standard of care. Internal lymphedema was scored using the Modified Patterson Scale, which documents internal swelling in the oral cavity, pharynx, and larynx. Four grades are used to rate edema level (normal = 0, mild = 1, moderate = 2, and severe = 3) for each anatomical structure. Sites are marked “N/A” when they are unable to be evaluated. The scale has good intrarater reliability (weighted kappa, 0.84) and moderate interrater reliability (weighted kappa, 0.54).31
Symptoms, function, and neck range of motion
Head and Neck-Lymphedema and Fibrosis (HN-LEF) Symptom Inventory: An updated version (33-item) of the Lymphedema Symptom Intensity and Distress Survey-Head and Neck (LSIDS-HN) was used to measure lymphedema and fibrosis symptoms.26,27 The HN-LEF Symptom Inventory involves participants indicating first whether they experience the symptom (“yes” or “no”). If participants indicate “yes,” then the intensity is rated on a 5-point scale from 1 (slight) to 5 (severe). Higher scores reflect greater symptom burden. There are 7 subscales, including soft tissues and neurologic toxicity, systemic symptoms and social functioning, jaw and oral dysfunction, swallowing and taste changes, body image and sexuality, communication, and mucosal irritation. The internal consistency of the tool is good (Cronbach’s alpha close to or greater than .70 for each subscale).26,27
Neck Disability Index (NDI): This is a validated tool (10-item) designed to measure the impact that neck pain has on one’s daily life. Each of the 10 items is scored from 0 to 5. The maximum score is 50. The original tool development provided scoring intervals for interpretation, as follows: 0 to 4 = no disability; 5 to 14 = mild disability; 15 to 24 = moderate disability; 25 to 34 = severe disability; and above 34 = complete disability. The internal consistency of the tool is adequate (Cronbach’s alpha: .89-.92).28
Cervical Range of Motion (CROM) Device: The CROM device (Performance Attainment Associates, Lindstrom, MN) is a valid and reliable tool for measuring the amount of neck movement. Cervical ROM was measured for the following neck movements: forward flexion, extension, right and left lateral flexion, and right and left rotation.36
Statistical Analyses
Descriptive statistics were used to summarize quantitative data, such as demographic characteristics, rates of participation, and log data. The primary objective was to evaluate feasibility on the basis of an 80% or higher completion rate of the study intervention. The secondary objective was to assess the preliminary efficacy of the PBM therapy on lymphedema-related outcomes in the HNC survivors, including progression of lymphedema, symptom burden, and functional status. For the secondary objective, change from baseline value was computed for each outcome variable at each follow-up timepoint and was included as the dependent variable in each analysis. The analysis of changes over time relied on mixed-effects modeling, which accounted for correlation among subjects’ repeated measures using a compound symmetry covariance matrix. Results are reported as model-based mean change ± standard error. Mean difference in Modified Patterson Scale site severity from baseline to 4-week post intervention was analyzed using paired t tests. An alpha of .05 was used for evaluation of statistical significance and no corrections for multiple tests were used in this pilot feasibility study. Statistical analyses were conducted using SAS 9.4 for Windows.
Results
Sample Characteristics
A total of 12 eligible HNC survivors were enrolled in the study. Most participants were White (n = 11, 91.7%) and male (n = 10, 83.3%) with a mean age of 58 years (S.D. = 11.5). Half of the participants had oropharyngeal cancer and advanced-stage disease (AJCC seventh edition, stage III/IVa) was present in 66.7% of all participants pre-cancer treatment. Most participants (83.3%) received both surgery and radiation. All participants received neck dissection with preservation of jugular vein and 25% of them treated with bilateral neck dissection (Table 2).
Table 2.
Participant Characteristics.
| Characteristics | Frequency (%) (N = 12) |
|---|---|
| Sex | |
| Male | 10 (83.3) |
| Female | 2 (16.7) |
| Race | |
| White | 11 (91.7) |
| Black or African American | 1 (8.3) |
| Marital status | |
| Single/windowed/other | 5 (41.7) |
| Married/living with a partner | 7 (58.3) |
| Employment status | |
| Employed | 6 (50.0) |
| Unemployed/other | 6 (50.0) |
| Education | |
| ≥12th grade | 12 (100.0) |
| Annual household income | |
| Up to $30 000 | 1 (8.3) |
| $30 001-60 000 | 1 (8.3) |
| Over $60 000 | 8 (66.7) |
| Do not care to respond | 2 (16.7) |
| Primary tumor location | |
| Nasal cavity | 1 (8.3) |
| Oral cavity | 3 (25.0) |
| Oropharynx | 6 (50.0) |
| Hypopharynx | 1 (8.3) |
| Salivary gland and other | 1 (8.3) |
| Tumor stage (TNM) at diagnosis | |
| Stage I | 1 (8.3) |
| Stage II | 2 (16.7) |
| Stage III | 3 (25.0) |
| Stage IV | 5 (41.7) |
| Could not be staged | 1 (8.3) |
| Charateristic of neck dissection (ND) | |
| ND with preservation of jugular vein | 12 (100.0) |
| Neck dissection location | |
| Unilateral ND | 9 (75.0) |
| Bilateral ND | 3 (25.0) |
| Complete cancer treatment received | |
| Surgery and radiation | 10 (83.3) |
| Surgery and CCR | 2 (16.7) |
| Characteristic | Median (min, max) |
| Age (y) | 58.4 (32, 75) |
| Time since HNC treatment ended (mo) | 12.6 (3.5, 16.5) |
| Time since diagnosis of lymphedema (mo) | 9.3 (3.0, 15.2) |
Abbreviations: CCR, concurrent chemoradiation; ND, neck dissection.
Feasibility
Recruitment
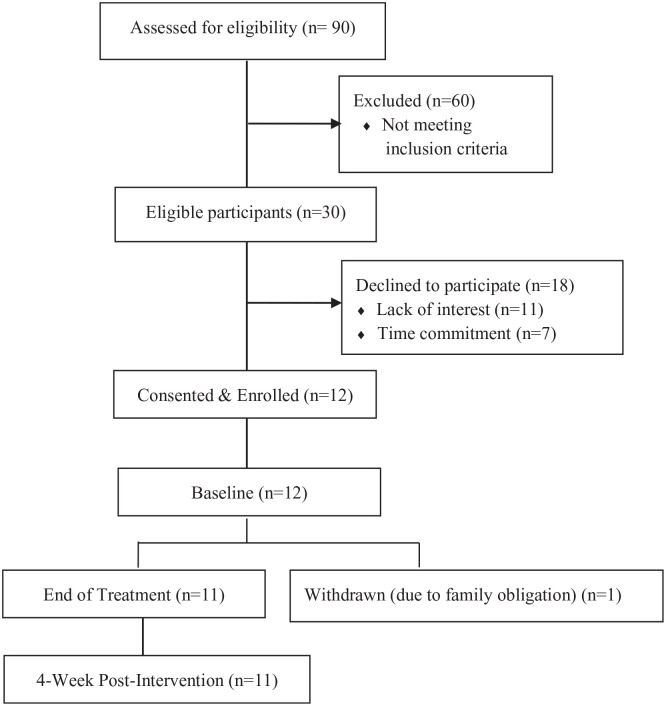
Over a 5-month recruitment window, 90 HNC survivors were screened, of whom 30 HNC survivors were eligible for the study. Sixty were ineligible due to no lymphedema (n = 15), extensive comorbidities (n = 14), inability of traveling to the study site (n = 12), active cancer (n = 7), active lymphedema therapy (n = 5), out of study window (n = 5), and non-English speaker (n = 2). Among eligible patients (n = 30), 12 of them (40%) were consented and enrolled into the study (Figure 1), and 18 were not enrolled because of the time commitment (n = 7) or lack of interest (n = 11).
Figure 1.
CONSORT flow diagram documenting the number of patients screened, consented, and withdrawn during the study assessment period.
Completion of PBM therapy sessions and study follow-up visits
Eleven out of 12 participants (91.7%) completed the PBM therapy and study follow-up visits. Regarding the PBM therapy sessions, 50% (n = 6) of the participants completed all 12 sessions, 25% (n = 3) completed 11 sessions, and 16.7% (n = 2) competed 10 sessions. The reasons for missing 1 to 2 PBM treatment sessions included work obligation, prepaid vacation, social event, and/or doctor appointment. One patient completed 1 PBM treatment session but could not continue, due to an unexpected family obligation.
Safety
No adverse events were identified in this study.
Preliminary Efficacy
Lymphedema
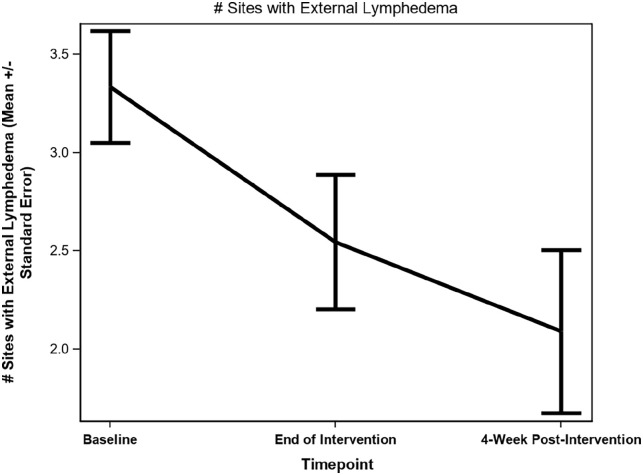
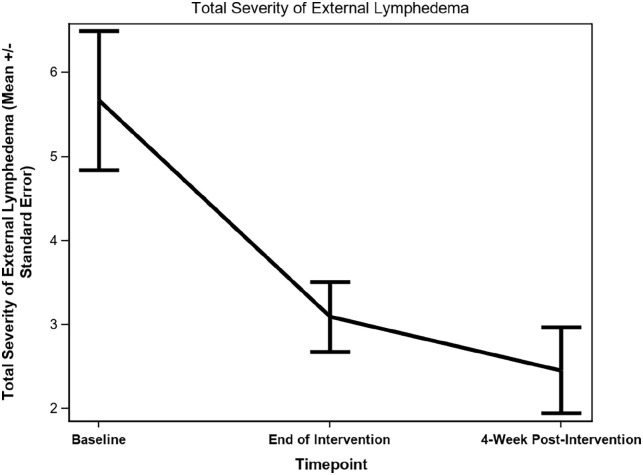
There was a statistically significant reduction on both the number of anatomical sites (−1.09 ± 0.44, P = .034) and the total severity score of external lymphedema (−3.18 ± 0.89, P = .005) (Figures 2 and 3) between baseline and 4-week post-intervention. Photos of the patients demonstrated a noticeable reduction in the severity of observable swelling and fibrosis (Supplemental Photos 1-4, patients’ written permission was obtained to publish the photos) between baseline and 4-week post-intervention. Although no statistically significant decreases in the severity of internal lymphedema as measured by the Modified Patterson Scale were observed between baseline and 4-week post-intervention (except for posterior pharyngeal wall, P = .042), there was a trend of reduction in the severity of internal lymphedema on the internal anatomical sites (Table 3).
Figure 2.
Changes in number of anatomical sites with external lymphedema.
Figure 3.
Changes in total severity of external lymphedema.
Table 3.
Reduction in Severity of Internal Lymphedema per Modified Patterson Scale.
| Anatomical sites | Baseline (mean) | 4-wk post-intervention (mean) | Mean reduction in severity | P value |
|---|---|---|---|---|
| Lip | 0.11 | 0.09 | 0 | .999 |
| Oral tongue (anterior 2/3) | 0.38 | 0.27 | 0 | .999 |
| Uvula | 0.78 | 0.45 | −0.13 | .351 |
| Buccal mucosa | 1.00 | 0.64 | −0.25 | .451 |
| Floor of mouth | 0.78 | 0.73 | 0.13 | .685 |
| Soft palate | 0.56 | 0.55 | 0.13 | .598 |
| Base of tongue | 0.57 | 0.71 | 0 | .999 |
| Posterior pharyngeal wall | 1.14 | 0.38 | −0.83 | .042 |
| Epiglottis | 1.00 | 0.83 | −0.25 | .391 |
| Pharyngoepiglottic folds | 1.14 | 1.00 | −0.17 | .741 |
| Aryepiglottic folds | 1.14 | 1.00 | −0.17 | .611 |
| Interarytenoid space | 1.00 | 0.67 | −0.20 | .621 |
| Cricopharyngeal prominence | 0.86 | 0.50 | −0.20 | .374 |
| Arytenoids | 1.29 | 1.00 | −0.33 | .363 |
| False vocal folds | 1.14 | 0.57 | −0.50 | .076 |
| True vocal folds | 0.29 | 0.14 | −0.17 | .363 |
| Anterior commissure | 0.43 | 0 | −0.50 | .203 |
| Valleculae | 1.00 | 0.57 | −0.50 | .076 |
| Pyriform sinus | 1.14 | 0.71 | −0.50 | .203 |
Per Modified Patterson Scale, 4 grades are used to rate internal lymphedema level (normal = 0, mild = 1, moderate = 2, and severe = 3) for each anatomical structure. Sites are marked “N/A” when they are unable to be evaluated. Paired t test was used to analyze the mean differences in the severity of internal lymphedema from baseline to 4-week post-intervention. Significant result (P < .05) is italicized.
Symptoms, function, and cervical range of motion: HN-LEF symptom inventory
There was a statistically significant decrease in the average symptom burden scores of 6 out of 7 subscales (soft tissues and neurologic toxicity, systemic symptoms and social functioning, jaw and oral dysfunction, swallowing and taste changes, body image and sexuality, and communication) of HN-LEF Symptom Inventory (all P < .05) between baseline and end of intervention. There was a statistically significant reduction in the average symptom burden scores of 4 out of 7 subscales of HN-LEF Symptom Inventory (P < .05) between baseline and 4-week post-intervention, including soft tissue and neurologic toxicity, jaw and oral dysfunction, swallowing and taste changes, and body image and sexuality (Table 4). However, no statistically significant changes were observed on the mucosal irritation subscale throughout the study.
Table 4.
Changes in Average Symptom Burden Scores per HN-LEF Symptom Inventory.
| HN-LEF symptom inventory subscale | Baseline to end of intervention | Baseline to 4-wk post intervention | ||||
|---|---|---|---|---|---|---|
| Mean | Standard error | P value | Mean | Standard error | P value | |
| Soft tissues and neurologic toxicity | −1.35 | 0.32 | .002 | −1.32 | 0.32 | .002 |
| Systemic symptoms and social functioning | −1.02 | 0.40 | .029 | −0.80 | 0.40 | .075 |
| Jaw and oral dysfunction | −0.94 | 0.35 | .023 | −1.12 | 0.35 | .009 |
| Swallowing and taste changes | −1.16 | 0.33 | .006 | −0.82 | 0.33 | .033 |
| Body image and sexuality | −0.73 | 0.22 | .009 | −0.70 | 0.22 | .011 |
| Communication | −0.61 | 0.20 | .013 | −0.36 | 0.20 | .102 |
| Mucosal irritation | −0.24 | 0.31 | .448 | −0.24 | 0.31 | .448 |
Longitudinal mixed effects model-based estimates were used to analyze changes.
NDI
Statistically significant decreases were observed for the total score of the NDI from baseline to end of intervention (15.64 ± 4.17, P = .004) and from the baseline to 4-week post-intervention (14.18 ± 4.17, P = .007).
CROM
There was a statistically significant increase in the degrees of CROM (all P < .05), including: (a) extension (8.30 ± 3.51, P = .042), left lateral rotation (8.05 ± 3.45, P = .045), and right lateral rotation (10.64 ± 2.50, P = .002) between baseline and end of intervention and (b) right lateral flexion (6.68 ± 2.64, P = .03) between baseline and 4-week post intervention (Table 5).
Table 5.
Changes in Degrees of Neck Range of Motion per CROM Measurement.
| Directions of range of motion movement | Baseline to end of intervention | Baseline to 4-wk post intervention | ||||
|---|---|---|---|---|---|---|
| Mean | Standard error | P value | Mean | Standard error | P value | |
| Forward flexion | −7.27 | 2.36 | .012 | 0.59 | 2.36 | .807 |
| Extension | 8.30 | 3.51 | .042 | 1.40 | 3.51 | .699 |
| Left lateral flexion | 5.23 | 2.91 | .103 | 6.00 | 2.91 | .066 |
| Right lateral flexion | 4.82 | 2.64 | .098 | 6.68 | 2.64 | .030 |
| Left lateral rotation | 8.05 | 3.45 | .045 | 0.45 | 3.45 | .899 |
| Right lateral rotation | 10.64 | 2.50 | .002 | 4.73 | 2.50 | .089 |
Longitudinal mixed effects model-based estimates were used to analyze changes.
Discussion
This is the first study, that we are aware of, to evaluate the feasibility, acceptability, and potential efficacy of PBM therapy on lymphedema in the post-treatment HNC population. Based on the comprehensive literature review, our previous research work in head and neck lymphedema, and our clinical experience, we developed a protocol including PBM therapy twice a week for 6 weeks. The study intervention was frequent so our initial concerns surrounding the study included the intervention duration, feasibility, and adherence to the intervention. Thus, as the very first innovative approach in this area, we conducted a single-group, single-site, pre-post design, pilot feasibility clinical trial. We found that the delivery of a 6-week PBM treatment (12 sessions) was both feasible and acceptable for HNC survivors receiving the treatment for lymphedema. PBM therapy was associated with a statistically significant reduction in lymphedema severity and symptom burden, as well as improvement in neck range of motion from baseline to 4-week post-intervention.
Feasibility of the use of PBM therapy in HNC survivors was supported as participants were recruited in a timely fashion and the enrollment rate (40.0%) was comparable to similar studies conducted in the HNC population.37,38 Adherence to the study intervention was demonstrated as the participant attrition was low (8.3%), with only 1 participant unable to complete the study intervention due to an unexpected family obligation, unrelated to PBM therapy. To minimize the subject burden and to help facilitate participation, we designed the intervention to be convenient for patients, ensuring each treatment session did not exceed 30-minutes in duration. Additionally, to help reduce frequent travel to the study site, participants’ study visits, including both assessment and treatment visits, were scheduled at a time that was convenient for them and/or on days when they were scheduled for their routine oncology appointments. Eleven of 12 participants completed greater than 80% treatment sessions, suggesting participants experienced minimal burden related to the study intervention. No adverse events were identified during the study. These findings were important and critically supported the feasibility and acceptability of PBM therapy, encouraging further testing in the HNC population.
The results showed that PBM therapy reduced soft tissue swelling and fibrosis in the head and neck region, decreasing both the number of anatomical sites and the severity of lymphedema from the pre-intervention to 4-week post-intervention. Although the underlying reasons for the effect of PBM therapy on head and neck lymphedema needs to be investigated, these preliminary findings were consistent with the results from studies conducted in other cancer populations, particularly in individuals with breast cancer-related arm lymphedema.12,13,17,19 Animal studies have shown that PBM therapy reduced the generation of tumor necrosis factor-alpha (TNF-α) and increased Interleukin (IL)-10,21 reduced lymphostatic fibrosis, stimulated lymphangiogenesis, and enhanced lymphatic motility.21 These results indicate that PBM therapy may be effective in treating chronic lymphedema. Despite no statistically significant changes in internal lymphedema, our data showed a trend in the reduction of internal soft tissue swelling in almost all the anatomical sites of the oral cavity, pharynx, and larynx. These findings suggest that larger studies are warranted to evaluate the impact of PBM therapy on both external and internal lymphedema among HNC survivors.
Findings from the self-reported questionnaire indicate that participants perceived improvements in the following symptom domains: swallowing and taste (eg, problems swallowing solids and taste change), soft tissue and neurologic toxicity (eg, tightness and firmness of skin), body image and sexuality (eg, feeling unattractive and decreased sexual activity), communication (eg, voice changes), systemic and social interaction (eg, feeling tired and decreased social activity), and jaw and oral dysfunction (eg, hard to open mouth and hard to move tongue). These statistically significant effects of PBM therapy on the participants’ symptom burden could be justified by either the local effect of PBM therapy on specific head and neck functions (eg, swallow, taste, and speak) or the systemic effect of PBM therapy on head and neck lymphedema by diminishing the chronic inflammatory status. The relationships between head and neck lymphedema and symptom burden have been reported in several studies conducted in HNC survivors with lymphedema.7-9,39 For instance, HNC patients with more severe lymphedema were more likely to experience difficulty swallowing, body image disturbance, communication issues, systemic symptoms, anxiety, and diminished jaw and tongue function.7-9,39 As previously mentioned, no statistically significant changes were observed on the mucosal irritation-related symptoms (eg, excess secretions or mucous) throughout the study. This is an expected finding, given that the mucosal irritation-related symptoms are more likely to occur in individuals with acute and/or early-stage lymphedema and less likely to be present in individuals with chronic and/or late-stage lymphedema. In addition, the study intervention helped improve participants’ neck function based on objective (Cervical Range of Motion) and subjective (Neck Disability Index) measures. The potentially beneficial impact of PBM therapy on neck range of motion may be because of the effect PBM therapy has on the reduction of both the amount of surplus tissue lymph fluid and fibrosis, thereby resulting in improved neck flexibility.
The study intervention (PBM therapy) possesses several strengths in the treatment of head and neck lymphedema given its ease of administration, acceptable duration, and potential efficacy. Despite these strengths, several limitations of this pilot feasibility study must be acknowledged. As a single-group study without a control group, no causal relationships could be made. We do not know whether the intervention resulted in improved lymphedema, patient-reported symptom burden, and neck range of motion. Additionally, the treatment included a simple manual lymph drainage prior to application of the PBM therapy, so we cannot say to what degree PBM therapy contributed to the observed benefit over the additional simple manual lymph drainage. Additionally, the study used the convenience sampling method, therefore, participants who agreed to participate in the study may have been more motivated than individuals who declined to be in the study. Because the sample size was small and the follow-up procedure was short-term (without long-term follow-up), it is important to acknowledge that the study sample may not have been representative of the entire HNC population with lymphedema. Furthermore, the study was conducted at 1 center, which limits the generalizability of the findings. Many of these limitations can be addressed in a rigorously designed, large, multi-center clinical trial.
Future Directions
Our study centers on enhancing patient outcomes for a marginalized patient population—individuals with HNC. A high percentage of this population experiences long-term and late effects after cancer treatment (eg, lymphedema and fibrosis). Our goal is to develop an innovative approach for clinical practice and optimize patient outcomes through an effective treatment of chronic lymphedema among the HNC survivor population. Despite the limitations of this pilot study, our findings demonstrated the feasibility, acceptability, and potential efficacy of PBM therapy in treating lymphedema in the HNC population. Most importantly, the study paved the way for conducting future randomized clinical trials to determine the degree to which PBM therapy improves the management of head and neck chronic lymphedema and enhances patient outcomes.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354211037938 for Photobiomodulation Therapy in Head and Neck Cancer-Related Lymphedema: A Pilot Feasibility Study by Jie Deng, John N. Lukens, Samuel Swisher-McClure, Joy C. Cohn, Bryan A. Spinelli, Ryan J. Quinn, Jesse Chittams, Erin McMenamin and Alexander Lin in Integrative Cancer Therapies
Acknowledgments
We would like to thank all the participants for their support.
Footnotes
Author Contributions: Jie Deng contributed to the study conception and design. Material preparation and data collection were performed by Jie Deng, Alexander Lin, John N. Lukens, Samuel Swisher-McClure, Joy C. Cohn, Bryan A. Spinelli, and Erin McMenamin. Data analysis was performed by Ryan J. Quinn and Jesse Chittams. The first draft of the manuscript was written by Jie Deng and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the University of Pennsylvania School of Nursing Faculty Research Award.
Ethical Approval: The study was approved by the Institutional Review Board at the study site.
Consent to Participate: All participants signed informed consent forms.
ORCID iD: Jie Deng  https://orcid.org/0000-0002-2553-8822
https://orcid.org/0000-0002-2553-8822
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Földi M, Földi E, Strössenreuther RHK, Kubik S. Földi’s Textbook of Lymphology: For Physicians and Lymphedema Therapists. 2nd ed.Mosby; 2006. [Google Scholar]
- 2.Lymphoedema Framework. Best Practice of the Management of Lymphoedema: International Consensus. MEP Ltd; 2006. [Google Scholar]
- 3.Lee B-B, Bergan JJ, Rockson SG. Lymphedema: A Concise Compendium of the Theory and Practice. Springer; 2011. [Google Scholar]
- 4.Avraham T, Zampell JC, Yan A, et al. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manag. 2012;43:244-252. [DOI] [PubMed] [Google Scholar]
- 6.Deng J, Murphy BA, Dietrich MS, et al. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck. 2013;35:1026-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng J, Sinard RJ, Murphy B. Patient experience of head and neck lymphedema therapy: a qualitative study. Support Care Cancer. 2019;27:1811-1823. [DOI] [PubMed] [Google Scholar]
- 8.Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphoedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care. 2014;23:317-327. [DOI] [PubMed] [Google Scholar]
- 10.Zuther J. Lymphedema Management: The Comprehensive Guide for Practitioners. 2nd ed.Thieme; 2009. [Google Scholar]
- 11.Deng J, Wulff-Burchfield EM, Murphy BA. Late soft tissue complications of head and neck cancer therapy: lymphedema and fibrosis. J Natl Cancer Inst Monographs. 2019;53:63-71. doi: 10.1093/jncimonographs/lgz005 [DOI] [PubMed] [Google Scholar]
- 12.Robijns J, Censabella S, Bulens P, Maes A, Mebis J. The use of low-level light therapy in supportive care for patients with breast cancer: review of the literature. Lasers Med Sci. 2017;32:229-242. [DOI] [PubMed] [Google Scholar]
- 13.Smoot B, Chiavola-Larson L, Lee J, Manibusan H, Allen DD. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: a systematic review and meta-analysis. J Cancer Surviv. 2015;9:287-304. [DOI] [PubMed] [Google Scholar]
- 14.Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO), Zadik Y, Arany PR, Fregnani ER, et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer. 2019;27:3969-3983. [DOI] [PubMed] [Google Scholar]
- 15.Treister NS, London WB, Guo D, et al. A feasibility study evaluating extraoral photobiomodulation therapy for prevention of mucositis in pediatric hematopoietic cell transplantation. Photomed Laser Surg. 2016;34:178-184. [DOI] [PubMed] [Google Scholar]
- 16.de Pauli Paglioni M, Araújo ALD, Arboleda LPA, et al. Tumor safety and side effects of photobiomodulation therapy used for prevention and management of cancer treatment toxicities: a systematic review. Oral Oncol. 2019;93:21-28. [DOI] [PubMed] [Google Scholar]
- 17.Baxter GD, Liu L, Petrich S, et al. Low level laser therapy (photobiomodulation therapy) for breast cancer-related lymphedema: a systematic review. BMC Cancer. 2017;17:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang DH, Song DH, Chang EJ, Jeon JY. Anti-inflammatory and lymphangiogenetic effects of low-level laser therapy on lymphedema in an experimental mouse tail model. Lasers Med Sci. 2016;31:289-296. [DOI] [PubMed] [Google Scholar]
- 19.Kozanoglu E, Basaran S, Paydas S, Sarpel T. Efficacy of pneumatic compression and low-level laser therapy in the treatment of postmastectomy lymphoedema: a randomized controlled trial. Clin Rehabil. 2009;23:117-124. [DOI] [PubMed] [Google Scholar]
- 20.Bensadoun RJ. Photobiomodulation or low-level laser therapy in the management of cancer therapy-induced mucositis, dermatitis and lymphedema. Curr Opin Oncol. 2018;30:226-232. [DOI] [PubMed] [Google Scholar]
- 21.Nair R, Bensadoun RJ. Mitigation of Cancer Therapy Side-Effects With Light. IOP Concise Physics. Morgan & Claypool Publishers; 2016. [Google Scholar]
- 22.de Lima FM, Villaverde AB, Albertini R, et al. Dual effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: action on anti- and pro-inflammatory cytokines. Lasers Surg Med. 2011;43:410-420. [DOI] [PubMed] [Google Scholar]
- 23.Zecha JA, Raber-Durlacher JE, Nair RG, et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support Care Cancer. 2016;24:2793-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zecha JA, Raber-Durlacher JE, Nair RG, et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 2: proposed applications and treatment protocols. Support Care Cancer. 2016;24:2793-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ClinicalTrials.Gov. Low-level laser in treatment of head and neck lymphedema: a pilot study. U.S. NIH. 2018. Accessed October 1, 2020. https://clinicaltrials.gov/ct2/show/NCT03738332?term=low+level+laser&cond=Lymphedema&cntry=US&draw=2&rank=1
- 26.Deng J, Ridner SH, Murphy BA, Dietrich MS. Preliminary development of a lymphedema symptom assessment scale for patients with head and neck cancer. Support Care Cancer. 2012;20:1911-1918. [DOI] [PubMed] [Google Scholar]
- 27.Deng J, Dietrich MS, Niermann KJ, et al. Refinement and validation of the head and neck lymphedema and fibrosis symptom inventory. Int J Radiat Oncol Biol Phys. 2021;109: 747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manip Physiol Ther. 1991;14:409-415. [PubMed] [Google Scholar]
- 29.Deng J, Dietrich MS, Ridner SH, Fleischer AC, Wells N, Murphy BA. Preliminary evaluation of reliability and validity of head and neck external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. 2016;22:63-70. [DOI] [PubMed] [Google Scholar]
- 30.Deng J, Ridner SH, Wells N, Dietrich MS, Murphy BA. Development and preliminary testing of head and neck cancer related external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. 2015;19:75-80. [DOI] [PubMed] [Google Scholar]
- 31.Patterson JM, Hildreth A, Wilson JA. Measuring edema in irradiated head and neck cancer patients. Ann Otol Rhinol Laryngol. 2007;116:559-564. [DOI] [PubMed] [Google Scholar]
- 32.Riancorp Pty Ltd.’s. 510(k) summary. LTU-904 portable laser therapy unit. Accessed October 7, 2020. https://www.accessdata.fda.gov/cdrh_docs/pdf3/K030295.pdf
- 33.LTU-904 Portable Laser Therapy Unit. Accessed October 9, 2020. https://www.riancorp.com/static/uploads/files/riancorp-usa-brochure-2011-wfypfzsoeryy.pdf
- 34.World Association for Photobiomodulation Therapy. Dosage recommendations. 2010. Accessed August 9, 2020. https://waltza.co.za/documentation-links/recommendations/dosage-recommendations/
- 35.U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common terminology criteria for adverse events v5.0 (CTCAE). 2017. Accessed February 6, 2020. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 36.Williams MA, McCarthy CJ, Chorti A, Cooke MW, Gates S. A systematic review of reliability and validity studies of methods for measuring active and passive cervical range of motion. J Manip Physiol Ther. 2010;33:138-155. [DOI] [PubMed] [Google Scholar]
- 37.Adair M, Murphy B, Yarlagadda S, Deng J, Dietrich MS, Ridner SH. Feasibility and preliminary efficacy of tailored yoga in survivors of head and neck cancer: a pilot study. Integr Cancer Ther. 2018;17:774-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zatarain LA, Smith DK, Deng J, et al. A randomized feasibility trial to evaluate use of the jaw dynasplint to prevent trismus in patients with head and neck cancer receiving primary or adjuvant radiation-based therapy. Integr Cancer Ther. 2018;17:960-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeffs E, Huit M. Treatment and outcomes of head and neck oedema referrals to a hospital-based lymphoedema service. Br J Commun Nurs. 2015;Suppl:S6-13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354211037938 for Photobiomodulation Therapy in Head and Neck Cancer-Related Lymphedema: A Pilot Feasibility Study by Jie Deng, John N. Lukens, Samuel Swisher-McClure, Joy C. Cohn, Bryan A. Spinelli, Ryan J. Quinn, Jesse Chittams, Erin McMenamin and Alexander Lin in Integrative Cancer Therapies