Abstract
Background:
Therapeutic treatment options for chronic autoimmune disorders such as multiple sclerosis (MS) rely largely on the use of non-specific immunosuppressive drugs, which are not able to cure the disease. Presently, approaches to induce antigen-specific tolerance as a therapeutic approach; for example, by peptide-based tolerogenic ‘inverse’ vaccines have regained great interest. We have previously shown that coupling of peptides to carriers can enhance their capacity to induce regulatory T cells in vivo.
Method:
In this present study, we investigated whether the tolerogenic potential of immunodominant myelin T-cell epitopes can be improved by conjugation to the synthetic carrier polyethylene glycol (PEG) in an experimental autoimmune encephalomyelitis (EAE) mouse model for chronic MS (MOG C57BL/6).
Results:
Preventive administration of the PEGylated antigenic peptide could strongly suppress the development of EAE, accompanied by reduced immune cell infiltration in the central nervous system (CNS). Depletion of regulatory T cells (Tregs) abrogated the protective effect indicating that Tregs play a crucial role in induction of antigen-specific tolerance in EAE. Treatment during the acute phase of disease was safe and did not induce immune activation. However, treatment at the peak of disease did not affect the disease course, suggesting that either induction of Tregs does not occur in the highly inflamed situation, or that the immune system is refractory to regulation in this condition.
Conclusion:
PEGylation of antigenic peptides is an effective and feasible strategy to improve tolerogenic (Treg-inducing) peptide-based vaccines, but application for immunotherapy of overt disease might require modifications or combination therapies that simultaneously suppress effector mechanisms.
Keywords: allergy, autoimmunity, chronic autoimmune disease, drug delivery, experimental autoimmune encephalomyelitis, multiple sclerosis, peptide vaccination, regulatory T cells, specific immunotherapy (SIT), therapy, tolerance induction
Introduction
Chronic autoimmune and inflammatory diseases affect an increasing number of individuals. Current treatments, including biologics, are not effective in all patients, do not cure the disease and might be associated with severe side effects. This continuing medical need has led to regained interest in antigen-specific tolerogenic therapies, also termed ‘inverse vaccination’.1,2 The phenomenon of immunological tolerance is known since the beginnings of cellular immunology and partially has been used in the specific immunotherapy (SIT) in allergy. However, its successful application in autoimmune diseases is still to be proved, despite the obvious advantage of selectively treating pathogenic mechanisms instead of applying unspecific immunosuppressants.
For several autoimmune diseases, including multiple sclerosis (MS), major autoantigens and their immunodominant domains are known and animal models for MS have provided strong evidence for a role of antigen-specific T cells in the pathogenesis.3 This is the rationale for approaches to generate peptide-based tolerogenic vaccines for the treatment of MS.
Initially, intravenous administration of proteins, immunodominant peptides, or altered peptide ligands with a varied amino acid sequence were found to be tolerogenic and to induce regulatory T cells (Tregs) in experimental models.4–6 However, clinical trials have not been effective so far, and in some preclinical or clinical studies adverse effects occurred.7–10 Obstacles in the use of peptides could comprise their short half-life in serum due to rapid excretion through the kidney or enzymatic degradation, or unfavorable physicochemical properties (aggregation) of some peptides, the most likely reason for anaphylactic reactions. Further improvements therefore included the loading of apoptotic cells or of synthetic carriers with antigenic peptides, use of tolerogenic adjuvants, or a combination thereof.11–15
Approaches of peptide-modified autologous cells16 are already at the stage of clinical trials.17,18 However, cell-based medicinal products are poorly defined and require an enormous personalized production process as well as extensive regulatory efforts. While micro- and nanoparticles avoid some of these drawbacks and have shown promising results, they still bear significant difficulties regarding standardization and approval.
We therefore considered that a more simple chemical modification could lead to peptide-based vaccines with improved tolerogenic activity. We recently reported that peptides conjugated to oligo- or polyglycerols or to a repetitive protein are superior in the induction of Tregs compared to native peptides.19,20 These findings were compatible with the view that increasing the size of a peptide by coupling to a macromolecular carrier of any kind would result in a better tolerogenic effect either by increasing the short half-life of native peptides in the circulation and/or by improved uptake/storage in antigen-presenting cells (APCs).
We therefore investigated whether coupling of myelin peptides to a soluble carrier entity consisting of polyethylene glycol (PEG) would result in increased tolerogenicity. In a preceding study, we described that indeed, vaccination with PEG-coupled ovalbumin (OVA)-peptide in the DO11.10 experimental model results in a marked increase in Treg numbers and downregulation of effector T cells.21
PEG is a synthetic polymer composed of repetitive ethylene oxide subunits, either in linear form, or as branched polymers. Numerous PEGylated biomolecules have already been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for human use as ingredients of foods, cosmetics and pharmaceuticals including topical, parenteral and nasal formulations. The covalent attachment of PEG (PEGylation) improves the pharmacokinetic and pharmacodynamic profile of biomolecules by increasing serum half-life, as well as proteolytic resistance, while reducing renal clearance and immunogenicity.22–25 We here used conjugates with PEG20 (i.e. consisting of 20 ethylene units) if not otherwise mentioned, which was determined as the most effective carrier type in our preceding study.21
The tolerogenic potential of PEG-coupled myelin peptides were investigated in a murine model for human MS, experimental autoimmune encephalomyelitis (EAE). In the C57BL/6 mouse model largely used here, EAE is induced by the encephalitogenic peptide MOG35–55 of myelin oligodendrocyte glycoprotein in complete Freund’s adjuvant (CFA). In this model, EAE typically displays a chronic clinical course26 and mirrors major features of subtypes of the human disease MS.
The results show that vaccination with PEGylated myelin peptide exerts a tolerogenic effect that results in protection from severe EAE in the MOG C57BL/6 model. However, when applied during the peak of inflammation, a suppression of ongoing disease was not achieved in this active EAE model. This suggests that the PEGylation alone is not sufficient for highly effective therapeutics; however, it might be applied in combination with treatments more efficiently suppressing the effector phase. Treatment with PEGylated peptide was safe, as no adverse immune hyperactivation on peptide delivery during the peak of disease was observed.
Materials and methods
Mice
C57BL/6 mice were either purchased from Charles River or Janvier. Mice were maintained under specific pathogen-free conditions and environmental enrichment according to national and institutional guidelines in the breeding facility of the Deutsches Rheuma-Forschungszentrum Berlin (DRFZ). Before starting experimentation, animals were allowed an acclimatisation period of at least one week in the experimental facility. All experiments were approved by the Landesamt für Gesundheit und Soziales (LAGeSo) with licence ID G0128-13 and Erg19 RREAE.
Peptides
MOG-peptide 35–55 (pMOG): MEVGWYRSPFSRVVHLYRNGK and the cysteine-modified pMOG: CβA-MEVGWYRSPFSRVVHLYRNGK, used for coupling, were either synthesized in-house (Institute for Medical Immunology, Charité Universitätsmedizin Berlin, Germany) or purchased from Pepceuticals (Leicestershire, UK).
Synthesis of pMOG-PEG20
To couple PEG to the C-modified N-terminus of pMOG, 157 mg of 20 kDa PEG-maleimide (NOF Corporation, Japan) and 20 mg pMOG were reacted in 20 mL of a 20 mmol/l sodium phosphate buffer pH 7.2 containing 250 µmol/l of Tris(2-carboxyethyl)phosphine. The reaction mixture was stirred at room temperature (RT) for 1 h. Progress of the reaction was monitored by reversed-phase high-performance liquid chromatography (RP-HPLC).
Purification of pMOG-PEG20
Crude peptide-PEG-conjugates were purified by cation-exchange chromatography using MacroCap SP on an Äkta chromatography system. pMOG-PEG20 was bound to the resin in 20 mmol/L sodium acetate pH 4.5. Conjugate was eluted with a linear sodium chloride gradient from 0 to 500 mM in 10 column volumes in the same eluent. Conjugate elution was monitored at 213 nm. Fractions containing the peptide-PEG-conjugate was pooled and desalted by dialysis against deionized water. Subsequently, pMOG-PEG20 was concentrated by freeze drying. Prior to use, conjugate was reconstituted in phosphate buffered saline (PBS) and filtered through a sterile 0.2 µm filter.
Analysis of pMOG-PEG20
Analysis of pMOG-PEG20 was performed by matrix-assisted laser desorption ionisation-time of flight-mass spectrometry (MALDI-ToF-MS), ultraviolet (UV)-spectrometry, and RP-HPLC. RP-HPLC was performed on a Waters Alliance System. For the analysis of pMOG-PEG20, a butyl C4 column 4.6 × 250 mm (5 µm) was used. Sample was eluted with a linear gradient from 70:15:15 to 50:25:25 water:acetonitrile:isopropanol, each with 0.1% trifluoroacetic acid (TFA) in 10 min. PEG or peptide-PEG-conjugate were detected using an evaporating light scattering detector (ELSD). The peptide-PEG-conjugates were free of any residual unmodified peptide. Results of the RP-HPLC and MALDI-ToF-MS analyses are provided in the Supplemental Material.
Cell preparation
Single cell suspensions were prepared from the spleen by mechanical dissociation, lysis of red blood cells in lysis buffer [0.01 M potassium bicarbonate, 0.155 M ammonium chloride and 0.1 mM ethylenediaminotetraacetic acid (EDTA)] and washed with PBS supplemented with 0.2% bovine serum albumin (BSA).
To isolate hematopoietic cells from the spinal cord, mice were perfused after sacrifice with 30 ml cold PBS. Spinal cords were minced and digested at 37°C for 15 min in serum free cell culture medium RPMI 1640 containing 1 mg/ml collagenase IV (Sigma-Aldrich, Taufkirchen, Germany) and 0.5 mg/ml Dnase I (Sigma-Aldrich). After digestion, the spinal cord was flushed through a 18G needle and incubated for a further 15 min at 37°C. After the second incubation the preparation was flushed through a 25G needle to obtain a single cell suspension, which was forced through a 40 µm cell strainer. Hematopoietic cells including microglia were isolated from discontinuous 30%:70% percoll gradients by centrifugation at 2000 rpm for 20 min at RT and stained with the respective marker combinations given below.
Antibodies and flow cytometry
The following monoclonal antibodies (mAbs) and appropriate isotype controls were obtained from eBioscience (San Diego, CA, USA): eFluor 450-conjugated anti-CD4 (RM4-5), phycoerythrin (PE)-Cy7-conjugated anti-CD11b (M1/70), fluorescein-isothiocyanate (FITC)-conjugated anti-CD45 (30F11), PE-conjugated anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) (MP1-22E9), PE-Cy7-conjugated anti-interferon (IFN)-γ (XMG1.2), FITC-conjugated anti-interleukin (IL)-17A (eBio17B7), PerCP-eFluor 710-conjugated anti-tumor necrosis factor alpha (TNFα) (MP6-XT22). Pacific Blue-conjugated anti-CD3 (17A2), PerCP-conjugated anti-CD11c (N418) and Pacific-Blue-conjugated anti-CD19 (6D5) antibodies were obtained from Biolegend (San Diego, USA). The APC-conjugated anti-CD154 antibody was obtained from Miltenyi Biotec. PE-conjugated anti-GR-1 (RB6-8C5), anti-Fcγ-receptor (2.4G2) and anti-CD25 (PC61) antibodies were produced in-house (DRFZ). Total rat immunoglobulin G (IgG) was purchased from Dianova (Hamburg, Germany).
To stain surface molecules, cells were pre-incubated with anti-Fcγ-receptor antibody (20 µg/ml) before staining with specific mAbs.
To analyze cytokine production of splenic MOG-specific CD4+ T cells in EAE, splenocytes were harvested and 1 × 107 cells were re-stimulated in the presence of 100 µg/ml pMOG in a 96 well plate for 14 h. After 2 h incubation Brefeldin A was added. Cells were surface-stained with an anti-CD4 antibody. After fixation by incubation with 2% paraformaldehyde (PFA, Sigma-Aldrich) at RT, intracellular staining was performed by 5 min pre-incubation with rat IgG in 0.5% saponin (Sigma-Aldrich) and addition of anti-CD154 antibody and anti-cytokine antibodies for another 30 min in 0.5% saponin at RT.
Quantification of hematopoietic cells in the spinal cord was done after isolation by flow cytometric analysis of the following marker using a sequential gating strategy (see Supplemental Figure 6): microglia (CD45int CD11b+), neutrophils (CD45high GR1high CD19−), CD11b− dendritic cells (DCs) (CD45high GR1− CD11c+ CD11b− CD19−), CD11b+ DCs (CD45high GR1− CD11c+ CD11b+ CD19−), macrophages (CD45high GR1− CD11c− CD11b+ CD19−), B cells (CD45high CD19+) and T cells (CD45high GR1− CD11c− CD11b− CD19− CD3+).
Induction, treatments, and clinical evaluation of MOG-induced EAE model
For induction of EAE, female C57BL/6 mice (aged 8–10 weeks) were immunized subcutaneously (s.c.) at four spots on the flanks with 200 µl of an emulsion of MOG-peptide 35–55 (pMOG, 250 µg per mouse) in CFA (Difco, Heidelberg, Germany) supplemented with 800 µg of Mycobacterium tuberculosis H37Ra on day 0. Mice also received intraperitoneally (i.p.) 200–400 ng of Bordetella pertussis toxin (List Biological Laboratories, Campbell, USA) on days 0 and 2. Paralyzed mice were given easier access to food and water. All experiments with long observation times were not pooled for statistics, as in this model a significant variability of the time course and overall disease severity has typically to be observed.
Prevention approach: C57BL/6 mice were tolerized intravenously (i.v.) with PBS (control), 7.6 µg pMOG or equimolar amounts of pMOG-PEG20 7, 14 or 28 days prior to EAE induction.
Treatment approach: C57BL/6 mice received i.v. PBS (control), 7.6 µg pMOG or equimolar amounts of pMOG-PEG20 7 days post EAE induction. Pilot experiments using the relapsing–remitting myelin basic protein-proteolipid protein (MBP-PLP) model in SJLxB10.PL mice27 were carried out as described in Supplemental materials.
Equal numbers of animals for every treatment group were taken from individual cages, to avoid any bias due to cage effects or age differences. Individual animals were monitored every day, and clinical scores were assessed as an accumulative score as follows: (TPA) tail paresis 0.5; (TPA-L) almost complete tail plegia 0.75; (TPL) tail plegia 1; (RRW) righting reflex impaired 0.25; (RRW) righting reflex weak 0.5; (HPA) hind limb paresis 0.5; (HPA-L) almost complete hind limb plegia 1; (HPL) hind limb plegia 1.5; (FPA) fore limb paresis 1. An accumulative score of 4 applied to human endpoint where animals were euthanized. Animals that died over night got a score of 5 on the next day. In a few cases, animals had to be euthanized for disease-unrelated reasons (inflammation at the injection site or ascites after pertussis toxin). Data from these animals were excluded from analysis.
For Treg depletion, animals were treated i.p. with 500 µg anti-CD25 antibody 7 days prior to EAE induction.
Statistics
Significance was either determined with the non-parametric Mann–Whitney U test using PRISM 5.02 (GraphPad, La Jolla, CA, USA) or non-parametric comparison of relative contrast effects to control the family-wise error rate. The latter approach was used for testing the significance in the mean clinical scores between different treatments in EAE experiments. Non-parametric comparison of relative contrast effects was performed using the nparcomp R-package with Tukey-type all-pairs comparison contrasts and multivariate t-distribution with a Satterthwaite approximation for the asymptotic approximation method.28 Censoring of data because of euthanasia (see above) is mentioned in the figure legend. Differences were considered as statistically significant with p ⩽ 0.05 (*), very significant with p ⩽ 0.01 (**) and extremely significant with p ⩽ 0.001 (***).
Results
Preventive administration of pMOG-PEG20 suppresses chronic experimental autoimmune encephalomyelitis
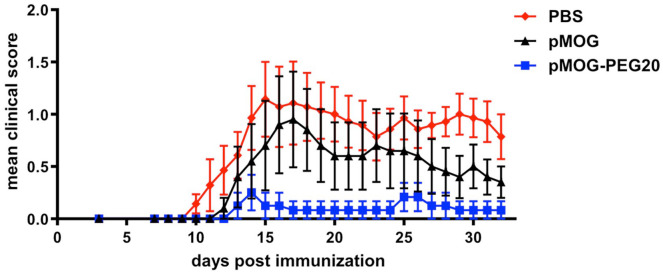
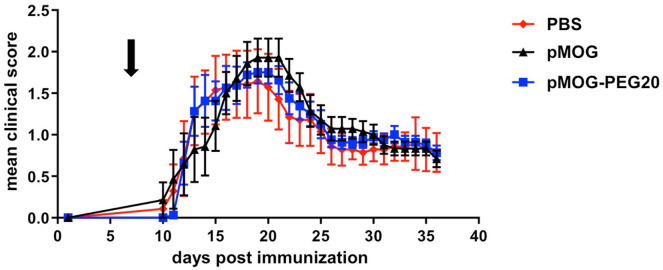
The tolerogenic effect of systemic administration of PEGylated MOG-peptide was tested in the C57BL/6 EAE mouse model. Mice received PBS (control), 7.6 µg encephalitogenic pMOG35–55 (equivalent to 14.7 µM; 0.38 mg/kg) or equimolar amounts (based on peptide amount) of PEG20 conjugated to the encephalitogenic pMOG35–55 7 days prior to EAE induction. In contrast to pMOG, which displayed only a weak tolerogenic effect, pMOG-PEG20 pretreatment strongly suppressed disease development (Figure 1 and Supplemental Figure 1).
Figure 1.
Improved tolerogenicity of MOG-peptide 35–55 (pMOG)-polyethylene glycol (PEG)20 compared to unconjugated peptide in experimental autoimmune encephalomyelitis (EAE). EAE was induced by subcutaneous (s.c.) immunization of C57BL/6 mice with pMOG35–55 in complete Freund’s adjuvant (CFA) containing Mycobacterium tuberculosis H37Ra on day 0. In addition, mice received Bordetella pertussis intraperitoneally (i.p.) on day 0 and day 2. Seven days prior to EAE induction mice were tolerized with phosphate buffered saline (PBS) (control), 7.6 µg pMOG or equimolar amounts (based on peptide amount) of pMOG-PEG20. Individual animals were observed every day, and clinical scores were assessed as an accumulative score. Mean clinical score per group ± standard error of the mean (SEM). PBS n = 7 animals; pMOG n = 5 animals (one animal had to be removed for ethical reasons); pMOG-PEG20 n = 6 animals. All groups were significantly different with adjusted p < 0.001 (non-parametric comparison of relative contrast effects). One representative of >3 independent experiments (the others being depicted in Supplemental Figure 1) is shown.
Pretreatment with pMOG conjugated to a higher molecular weight PEG, PEG40, was as efficient as pMOG-PEG20 vaccination (Supplemental Figure 2).
Tolerization with either pMOG or pMOG-PEG20 was less effective when done 4 weeks instead of 1 week before disease induction (Supplemental Figure 3); tolerization at 2 weeks before induction was in 3 out of 4 experiments as complete as in the 1-week setting (see Figure 2 and Supplemental Figure 4). These data demonstrate that vaccination with PEGylated peptide is able to suppress the development of EAE, although a single tolerogenic vaccination might not be sufficient to induce a long-term tolerant state.
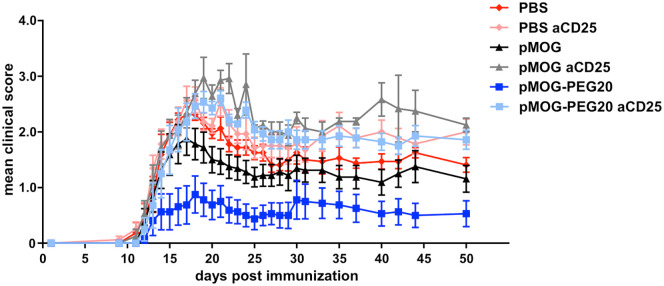
Figure 2.
Regulatory T cells (Tregs) are involved in protective tolerance induced by MOG-peptide 35–55 (pMOG)-polyethylene glycol (PEG)20. Fourteen days prior to experimental autoimmune encephalomyelitis (EAE) induction, C57BL/6 mice were tolerized with phosphate buffered saline (PBS) (control), 7.6 µg pMOG or equimolar amounts of pMOG-PEG20. After 7 days mice received either 500 µg anti-CD25 (PC61) antibody or PBS (control) intraperitoneally (i.p.). EAE was induced as described above. Mean clinical score per group ± standard error of the mean (SEM), n = 8 animals per group except pMOG-PEG/anti CD25 (n = 7; one animal had to be removed for ethical reasons (ascites after pertussis toxin)). One representative of 4 independent experiments (the others being depicted in Supplemental Figure 4) is shown. For statistics see Supplemental Table 1 in the Supplemental material.
Tregs play a role in antigen-specific tolerance induction by pMOG-PEG20
In the DO11.10 adoptive transfer model, we demonstrated that vaccination with PEGylated peptides led to expansion and/or de novo induction of antigen-specific Tregs.21 To analyze the role of antigen-specific Tregs in the prevention of EAE by pMOG-PEG20 pretreatment, tolerogenic vaccination was given 2 weeks prior to EAE induction and Tregs were depleted by injection of anti-CD25 antibody 7 days prior to EAE induction. As expected, Treg depletion significantly enhanced disease already in the PBS group. An even stronger aggravation of the disease course occurred in pMOG and pMOG-PEG20-tolerized animals on depletion of Tregs, resulting in a complete abrogation of the protective effect (Figure 2).
These findings suggest that Tregs play a crucial role in EAE suppression induced by tolerogenic pMOG-PEG20 vaccination.
Effects on the frequency of MOG-specific splenocytes among total CD4+ cells and their effector cytokine profile on prior administration of pMOG-PEG20
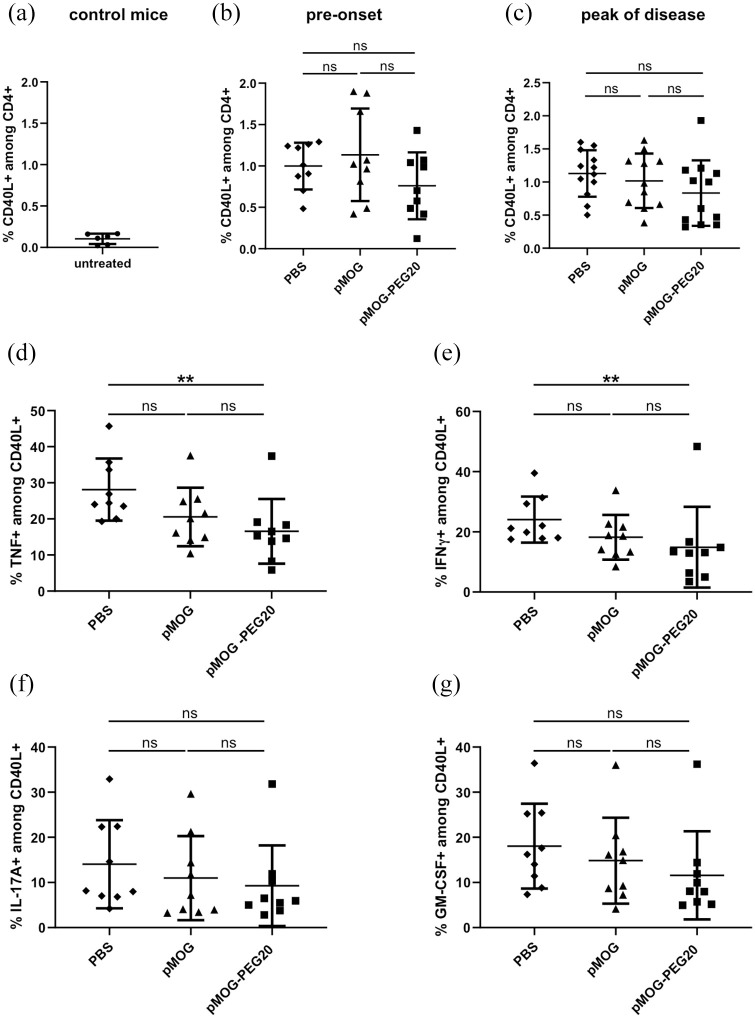
In a preceding study21 in the DO11.10 adoptive transfer model, we demonstrated that vaccination with PEGylated peptide resulted in partial depletion of specific T cells, as well as reduction of pro-inflammatory cytokine producers in the spleen. To investigate whether the protective effect in EAE of pMOG-PEG20 also involved modulation of effector cells, we analyzed the frequency of MOG-specific cells among total CD4+ T cells and their pro-inflammatory cytokine profile by fluorescence-activated cell sorter (FACS) analysis of pMOG-restimulated T cells at different time points.
The frequency of MOG-specific cells (CD40L+ on pMOG restimulation) among total CD4+ splenocytes was not changed in the pre-onset phase, or at peak of disease after previous vaccination with pMOG or pMOG-PEG20 (Figure 3a–c). The frequency of TNF and IFN-γ-producing cells among MOG-specific (CD4+ CD40L+) splenocytes was significantly decreased by pMOG-PEG20 prior to disease onset (day 7 post EAE induction), reflecting partial anergy of MOG-specific cells (Figure 3d, e). The frequencies of IL-17A and GM-CSF-producing cells among antigen-specific cells also appeared to be reduced in mice tolerized with pMOG-PEG20 compared to non-tolerized mice, albeit not significantly (Figure 3f, g). At the peak of disease, hardly any significant changes in any of the parameters examined were seen (Supplemental Figure 5). Thus, preventive vaccination in the MOG-model does not result in a depletion of MOG-specific cells and only a weak downregulation or reduction of cytokine producing T cells can be observed.
Figure 3.
In the pre-onset phase and at the peak of experimental autoimmune encephalomyelitis (EAE), the frequency of MOG-specific cells among total CD4+ splenocytes is not affected by MOG-peptide 35–55 (pMOG)-polyethylene glycol (PEG)20, while the frequency of tumor necrosis factor (TNF) or interferon (IFN)-γ-producing cells among MOG-specific splenocytes is significantly decreased compared to phosphate buffered saline (PBS) by pMOG-PEG20. Seven days prior to EAE induction, C57BL/6 mice were tolerized intravenously (i.v.) with either PBS (a, control), 7.6 µg pMOG or an equimolar amount of pMOG-PEG20 (b–g). Animals were sacrificed 7 days (pre-onset phase) or 15 days post EAE induction (peak of disease). Splenocytes from single mice were re-stimulated overnight with pMOG and analyzed by flow cytometry. (a) Untreated control mice, n = 6. (b) Pre-onset phase, n = 9. (c) Peak of disease, n = 11–12 (one animal of pMOG-group had to be removed for ethical reasons). Data represent means ± standard deviation (SD) of % MOG-specific CD4+ cells (% CD40L+ on pMOG-restimulation) among total CD4+ cells. d–g: Cytokine-producers among CD4+ CD40L+ (MOG-specific) cells. Means of (d) % TNF+, (e) % IFN-γ+, (f) % interleukin (IL)-17A+ and (g) % granulocyte-macrophage colony-stimulating factor (GM-CSF)+ cells ± SD (n = 9 per group). Pooled data of two independent experiments are shown. p Values were determined by unpaired non-parametric Mann–Whitney U test.
Administration of pMOG-PEG20 reduces central nervous system (CNS) immune cell infiltration
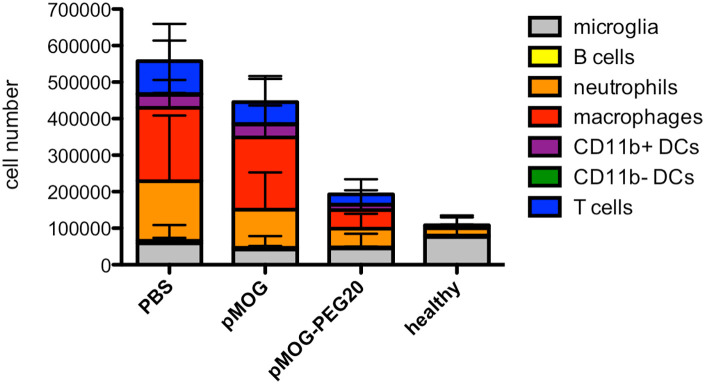
Cellular infiltration in the CNS is a hallmark of inflammation in EAE. To analyze whether tolerogenic pMOG-PEG20 vaccination reduces the accumulation of inflammatory cells in the CNS, the composition of different immune cell populations in the tissue was analyzed. Spinal cords were isolated from tolerized and control pMOG/CFA-immunized mice, as well as from untreated mice at the peak of the disease. Cellular infiltrates were analyzed after dissociation by flow cytometry. Among viable CD45+ cells, microglia, neutrophils, DC subsets, macrophages, as well as T and B cells were identified using a sequential gating strategy (Supplemental Figure 6).
pMOG-PEG20 vaccination not only diminished infiltrating T cells, but also total leukocytes in the CNS cells compared to pMOG and PBS application, in particular the infiltration by neutrophils, macrophages and CD11b+ DCs was strongly reduced (Figure 4; for relative proportions of subsets and statistics see Supplemental Figure 7 and Supplemental Table 2). This, in fact, correlates with reduced EAE symptoms following pMOG-PEG20 vaccination.
Figure 4.
Reduced central nervous system (CNS) infiltration at peak of disease following MOG-peptide 35–55 (pMOG)-polyethylene glycol (PEG)20 vaccination. Seven days prior to experimental autoimmune encephalomyelitis (EAE) induction mice were tolerized with phosphate buffered saline (PBS), 7.6 µg pMOG or equimolar amounts of pMOG-PEG20. At the peak of disease (day 15) spinal cords were isolated from pMOG-immunized C57BL/6 mice as well as from non-immunized untreated C57BL/6 mice (control). Different leukocyte subsets were quantified by flow cytometric analysis of marker combinations as described in the Methods and Supplemental Figure 6. Data are depicted as mean ± standard deviation (SD) (n = 6–12). Data are summarized from two independent experiments. For frequencies of cell populations and statistical data see Supplemental material, Supplemental Figure 7 and Supplemental Table 2.
Administration of pMOG-PEG20 in established disease is ineffective
In order to determine the potential application of tolerogenic vaccination with PEGylated peptides in acute phases of autoimmune disease, we investigated whether therapeutic administration of pMOG-PEG20 is effective in ongoing CNS inflammation. Mice received PBS, 7.6 µg pMOG, or equimolar amounts (based on peptide amount) of pMOG-PEG20 7 days after EAE induction. Therapeutic administration of both pMOG-PEG20 and pMOG could not suppress EAE symptoms (Figure 5 and Supplemental Figure 8). Of note, treatment with PEGylated pMOG did, however, not lead to exacerbation of the disease.
Figure 5.
Therapeutic administration of MOG-peptide 35–55 (pMOG)-polyethylene glycol (PEG)20 does not prevent experimental autoimmune encephalomyelitis (EAE) development, but also does not lead to exacerbation of the disease. Seven days post EAE induction, C57BL/6 mice received phosphate buffered saline (PBS) (control), 7.6 µg pMOG or equimolar amounts of pMOG-PEG20. Mean clinical score per group ± standard error of the mean (SEM) (PBS n = 7; pMOG n = 7; pMOG-PEG20 n = 8). One representative of three independent experiments is shown (further datasets are to be found in Supplemental Figure 8). Adjusted p-values were p = 0.51, 0.59 and 0.99 for PBS versus pMOG, PBS versus pMOG-PEG20, and pMOG versus pMOG-PEG20, respectively, as determined by non-parametric comparison of relative contrast effects.
These data demonstrate that the inflammatory milieu in ongoing disease prevents successful tolerization with either peptide or PEG-modified peptide under the conditions tested.
In pilot experiments, we also applied PEGylated PLP-peptides in the relapsing–remitting MBP-induced model in SJLxB10.PL mice. Repeated application of PEGylated PLP-peptide at high doses to suppress the PLP-specific secondary peaks (resulting from epitope spreading) did not ameliorate disease, with the exception of delaying the first relapse (Supplemental Figure 9).
Discussion
Tolerogenic vaccines based on immunodominant peptide sequences from autoantigens are an attractive concept for the development of antigen-specific therapies for autoimmune diseases. While readily convertible into a pharmaceutical product, efficacy in clinical trials was disappointing, giving rise to the development of new approaches. We here investigated whether coupling of myelin peptides to defined and clinically well-proved PEG moieties would increase their tolerogenic potential and therapeutic effect in EAE.
Indeed, EAE development was almost completely suppressed by vaccination with pMOG-PEG20, while free peptide only led to a modest amelioration of EAE. This is in line with our concept that increasing the size of autoantigenic peptides by coupling to an inert carrier improves their tolerogenic potential.
This concept evolved from previous studies showing protective effects of peptide coupled to repetitive protein domains in EAE20 and the efficacy of PEG as well as oligo- or polyglycerol-conjugated peptides in inducing Tregs and reducing T effector cells in a transgenic OVA-model.19,21 In the OVA-model, we found that size of the PEG carrier was critical, with linear PEG20 (20 kDa) being the most effective inducer of Tregs among 12–40 kDa variants and a tetrameric variant.21 We therefore predominantly used MOG-peptide 35–55 coupled to PEG20 (pMOG-PEG20) in the present study, although pMOG-PEG40, was hardly less efficient in preventing EAE. We demonstrated previously that PEGylation leads to a major prolongation of bioavailability of the tolerogenic peptides, either caused by the known increase in serum half-life, or additional effects such as storage in APCs as discussed elsewhere.21 It can be assumed that this is also the main mechanism of action in the improved tolerogenicity of the other types of carriers mentioned above.
As compared to the aforementioned different scaffolds and to encapsulation or coupling of peptides into and onto nanobeads,12–15 PEG has the advantage of being widely used already in the pharmacological optimization of biologicals and is clinically proved, thus synthesis and regulatory processes might bear fewer hurdles. The clinical application of PEGylated compounds has, however, also uncovered some issues caused by pre-existing antibodies to PEG, most likely originating from the widespread use of PEG in cosmetics, etc.29 While anti-PEG antibodies were not affecting efficacy, e.g. in trials using PEGylated interferon, in other cases the biokinetics of PEGylated bacterial enzymes were found to be negatively affected in some patients.29,30 Whether the type of conjugates used here is affected by anti-PEG antibodies remains to be observed. It should be mentioned that there are presently no studies available on the potential formation of antibodies to other synthetic carriers or nanobeads.
In our previous studies19–21 we demonstrated that coupling of peptides to carriers improved the induction or expansion of antigen-specific Tregs and it became obvious that linker chemistry, size and structure, besides the dose used, played a critical role for the Treg-inducing potential. To investigate whether Tregs are critical mediators of the tolerogenic effect also in the EAE model, Tregs were depleted with an anti CD25 antibody. Indeed, the protective effect of vaccination with pMOG-PEG20 was completely neutralized on depletion. These findings confirm previous studies showing that Tregs play a significant role in the regulation of EAE and MS (reviewed in Danikowski et al.31 and Anderton and Liblau32). Interestingly, in case of EAE prevention by apoptotic antigen coupled leukocytes, Tregs were shown to be dispensable for tolerance induction but essential for long-term tolerance maintenance.33
While the prominent role of Tregs in regulating immunological balance and maintaining self-tolerance became clear in the past two decades, previous studies, notably such as in high-zone tolerance, also pointed to mechanisms such as depletion of antigen-specific cells, or induction of anergy or adaptive tolerance, i.e. a state of unresponsiveness including a suppression of (effector-) cytokine production.34 In line with the studies in the OVA-model,21 a moderate but significant reduction in the percentage of MOG-specific cells able to produce the effector cytokines TNF or IFN-γ, and a non-significant trend for reduction of producers of IL-17A and GM-CSF was observed, albeit only in the pre-onset phase. All these cytokines have been considered as drivers in the pathogenesis of MS.35–38 Whether the moderate reduction in cytokine producers observed here contributes to tolerance in the present model remains to be shown. It should be mentioned that MOG-specific cytokine producers could only be determined in cells isolated from the spleen; it is not excluded that relative numbers are different in the inflamed CNS tissue as effector-memory cells tend to be trapped in sites of inflammation.39,40 Hence, the present data do not allow a firm conclusion as to the role of this partial anergy induction for the tolerogenic effect of vaccination.
The superior effect of PEGylated pMOG as compared to native pMOG seen in clinical scores was mirrored in the strong reduction of inflammatory cells accumulating in the diseased spinal cords on vaccination. Although the influx of inflammatory cells was not completely prevented by vaccination with pMOG-PEG20, total hematopoietic cells were significantly reduced to approximately one third. Notably, macrophages and neutrophils that have both been implicated in the pathology of MS41,42 were strongly reduced. This underlines the protective effects of pMOG-PEG20 vaccination recorded in the clinical symptoms.
With a few exceptions, the intended clinical use of tolerogenic approaches in the treatment of autoimmune diseases or allergy is rather not the prevention of disease, but treatment of an ongoing autoimmune process. Here, the preceding or continuing activation of the immune system bears the most critical hurdle for efficacy. Both the generation and the efficacy of Tregs have been reported to be impaired under inflammatory conditions.43–45 In the OVA-model, we observed that concomitant injection of lipopolysaccharide (LPS) completely prevented the induction of Tregs upon tolerogenic vaccination with pOVA-PEG20.21
It was therefore not completely unexpected that application of pMOG-PEG20 (as pMOG) was unable to change the disease course when given after induction of EAE. We reasoned that a relapsing–remitting model might bear windows of opportunity for the induction of Tregs during ongoing disease. However, this was not found in a pilot experiment in the SJLxB10.PL mouse model for EAE induced by MBPAc1-9-peptide, but developing relapses relying on PLP-specific T cells. We repeatedly applied pPLP139-154-PEG20 during ongoing EAE, but only a short delay in the first relapse was observed. However, in the remission phases, disease activity in this model does not completely disappear. Moreover, almost all murine EAE models incorporate the application of CFA for induction. According to our findings mentioned above, the mycobacterial LPS from CFA is potentially contributing to the blockade of a tolerogenic effect. But also the entire concert of proinflammatory signals generated on disease initiation might act by preventing the tolerogenicity of either native or carrier-conjugated peptide. Whether subtypes of human MS with long and complete remission phases are potential cases for application of the concept remains to be shown. As with animal models in general, it cannot be predicted whether results would be the same in humans.
While these findings suggest that tolerogenic vaccination with PEGylated myelin-peptides alone is not effective in ongoing disease, the data demonstrate that the application even under the condition of already generated effector cells is safe, as we did not observe adverse effects resulting in exaggerated disease symptoms on single, repeated or high dose application during EAE in the MOG or the SJLxB10.PL MBP/PLP mouse model. Also in the OVA-model, pOVA-PEG20 did not lead to an activated immune response in mice adoptively transferred with T helper (Th)1 cells, to mimic an established disease. While both pOVA and pOVA-PEG20 induced proliferation of T cells, only the latter did not give rise to increased frequencies and numbers of IFN-γ-producing Th1 cells.21 These findings might be of note with respect to reports on exacerbation of disease with myelin-derived native peptides.7–10
Some other applications therefore appear conceivable, e.g. for the inhibition of antibody formation towards intentionally applied foreign antigens, or for a safer variant of specific immunotherapy to treat allergies, in which so far mostly unmodified allergens are used. Moreover, PEG-conjugates might find a place in combination therapies with agents or treatments suppressing effector mechanisms. The anti-CD3 therapy already in clinical testing is such a candidate, as it appears to cause the deletion of effector/memory cells.46 Other options would include the co-administration of immunosuppressants such as mAbs to costimulatory molecules and cytokines, or rapamycin.
In the past decade, a variety of concepts to improve the efficacy of peptide tolerization has been developed. Apart from the loading of apoptotic cells with peptides already tested in a clinical trial,17 different synthetic nanoparticles or liposomes have been loaded with autoantigen-derived peptides, partly under additional encapsulation of tolerogenic adjuvants or modified surfaces to target the particle to specific compartments.12,13,47 In contrast to our findings with PEGylated peptides, the group of Miller could achieve resolution of ongoing EAE under therapeutic conditions with peptide coupled to polystyrene or biodegradable poly(lactide-co-glycolide) microparticles of distinct size and surface charge.48,49 The data suggest that these types of particles target specific subsets of APCs and even without antigen actively contribute to stimulation of inhibitory pathways and tolerogenicity. Promising preclinical results in therapeutic settings were also obtained in the group of Kishimoto by combination of antigenic peptides with tolerogenic adjuvants such as rapamycin, most elegantly by loading nanoparticles with both, peptide and rapamycin.50 Interestingly, PEGylation of otherwise non-tolerogenic MOG-filled poly(lactic-co-glycolic acid) (PLGA)-nanoparticles also appeared to result in partial suppression of EAE on application at ongoing disease.51 The last development in the field of tolerogenic therapy is the construction of a mRNA-based vaccine coding for myelin peptides. Impressive data for the tolerogenic effect, including bystander suppression, in EAE models were recently reported by the BioNTech group using, in contrast to the coronavirus disease 2019 (Covid-19) vaccine, a modified RNA and lipid nanoparticles both lacking immunostimulatory properties.52
Conclusions
The present study has demonstrated that vaccination with PEG-coupled myelin peptide is superior to native peptide in inducing specific tolerance in the EAE model and acts predominantly by induction or expansion of Tregs. This and the straightforward chemistry and broad clinical experience with PEGylated biologicals are a strength of this concept, although it has still to be shown whether long-lasting tolerance can be achieved by repeated vaccination. However, the main restriction of the approach is that these conjugates are not able to suppress an already activated immune system and to achieve therapeutic effects in ongoing disease. This limits the applicability to specific situations.
Supplemental Material
Supplemental material, sj-pdf-1-taj-10.1177_20406223211037830 for Prevention of EAE by tolerogenic vaccination with PEGylated antigenic peptides by Jennifer Pfeil, Mario Simonetti, Uta Lauer, Bianca von Thülen, Pawel Durek, Christina Poulsen, Justyna Pawlowska, Matthias Kröger, Ralf Krähmer, Frank Leenders, Ute Hoffmann and Alf Hamann in Therapeutic Advances in Chronic Disease
Supplemental material, sj-pdf-2-taj-10.1177_20406223211037830 for Prevention of EAE by tolerogenic vaccination with PEGylated antigenic peptides by Jennifer Pfeil, Mario Simonetti, Uta Lauer, Bianca von Thülen, Pawel Durek, Christina Poulsen, Justyna Pawlowska, Matthias Kröger, Ralf Krähmer, Frank Leenders, Ute Hoffmann and Alf Hamann in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors would like to thank the lab managers in the DRFZ for technical support, Friederike Ebner (Free University Berlin) for the introduction into the EAE model, Ping Shen (DRFZ) for technical advice in spinal cord isolation and Stephen D Miller and Zoe N Hunter, Chicago, for sharing thoughts and experimental work. They also thank Carmen Infante-Duarte (Charité) for critical reading and Frank Konietschke (Charité) for statistical advice.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the German Research Foundation (CRC 650 TP1); the Federal Ministry of Education and Research (Innovative Therapies -01GU0722-); the Federal Ministry for Economic Affairs and Energy (ZIM -KF 2441003SK1-) and (publication) the Leibniz Science Campus Chronic Inflammation.
Conflict of interest statement: The authors BT, RK and FL have been or are employees of celares GmbH, a company offering contract development services to the pharmaceutical industry. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ORCID iD: Alf Hamann  https://orcid.org/0000-0003-1518-5717
https://orcid.org/0000-0003-1518-5717
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jennifer Pfeil, Experimental Rheumatology, Deutsches Rheuma-Forschungszentrum, a Leibniz-Institute, Berlin, Germany; Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Berlin, Germany.
Mario Simonetti, Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Berlin, Germany; Molecular and Cellular Immunology/Immune Regulation, Technische Universität, Dresden, Germany.
Uta Lauer, Experimental Rheumatology, Deutsches Rheuma-Forschungszentrum, a Leibniz-Institute, Berlin, Germany; Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Berlin, Germany.
Bianca von Thülen, Celares GmbH, Berlin, Germany.
Pawel Durek, Experimental Rheumatology, Deutsches Rheuma-Forschungszentrum, a Leibniz-Institute, Berlin, Germany; Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Berlin, Germany.
Christina Poulsen, Experimental Rheumatology, Deutsches Rheuma-Forschungszentrum, a Leibniz-Institute, Berlin, Germany; Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Berlin, Germany.
Justyna Pawlowska, Experimental Rheumatology, Deutsches Rheuma-Forschungszentrum, a Leibniz-Institute, Berlin, Germany; Gdańsk University of Technology, Gdańsk, Poland.
Matthias Kröger, Experimental Rheumatology, Deutsches Rheuma-Forschungszentrum, a Leibniz-Institute, Berlin, Germany.
Ralf Krähmer, Celares GmbH, Berlin, Germany.
Frank Leenders, Celares GmbH, Berlin, Germany.
Ute Hoffmann, Experimental Rheumatology, Deutsches Rheuma-Forschungszentrum, a Leibniz-Institute, Berlin, Germany; Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Berlin, Germany.
Alf Hamann, Experimental Rheumatology, Deutsches Rheuma-Forschungszentrum Berlin, Charitéplatz 1, Berlin 10117, Germany; Department of Rheumatology and Clinical Immunology, Charité Universitätsmedizin, Berlin, Germany.
References
- 1.Dolgin E.The inverse of immunity. Nat Med 2010; 16: 740–743. [DOI] [PubMed] [Google Scholar]
- 2.Willekens B, Cools N.Beyond the magic bullet: current progress of therapeutic vaccination in multiple sclerosis. CNS Drugs 2018; 32: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin R, Sospedra M, Rosito M, et al. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur J Immunol 2016; 46: 2078–2090. [DOI] [PubMed] [Google Scholar]
- 4.Anderton S, Burkhart C, Metzler B, et al. Mechanisms of central and peripheral T-cell tolerance: lessons from experimental models of multiple sclerosis. Immunol Rev 1999; 169: 123–137. [DOI] [PubMed] [Google Scholar]
- 5.Sospedra M, Martin R.Antigen-specific therapies in multiple sclerosis. Int Rev Immunol 2005; 24: 393–413. [DOI] [PubMed] [Google Scholar]
- 6.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol 2007; 7: 665–677. [DOI] [PubMed] [Google Scholar]
- 7.Genain CP, Abel K, Belmar N, et al. Late complications of immune deviation therapy in a nonhuman primate. Science 1996; 274: 2054–2057. [DOI] [PubMed] [Google Scholar]
- 8.Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 2000; 6: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 9.Kappos L, Comi G, Panitch H, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med 2000; 6: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 10.Smith CE, Eagar TN, Strominger JL, et al. Differential induction of IgE-mediated anaphylaxis after soluble vs. cell-bound tolerogenic peptide therapy of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 2005; 102: 9595–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson RM, Casey LM, Hughes KR, et al. In vivo reprogramming of immune cells: technologies for induction of antigen-specific tolerance. Adv Drug Deliv Rev 2017; 114: 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson RM, Podojil JR, Shea LD, et al. Overcoming challenges in treating autoimmuntity: development of tolerogenic immune-modifying nanoparticles. Nanomedicine 2019; 18: 282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishimoto TK, Maldonado RA.Nanoparticles for the induction of antigen-specific immunological tolerance. Front Immunol 2018; 9: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Akiva E, Est Witte S, Meyer RA, et al. Polymeric micro- and nanoparticles for immune modulation. Biomater Sci 2018; 7: 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng X, Xu W, Li Z, et al. Immunomodulatory nanosystems. Adv Sci (Weinh) 2019; 6: 1900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CE, Miller SD.Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities. J Autoimmun 2006; 27: 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutterotti A, Yousef S, Sputtek A, et al. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med 2013; 5: 188ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zubizarreta I, Florez-Grau G, Vila G, et al. Immune tolerance in multiple sclerosis and neuromyelitis optica with peptide-loaded tolerogenic dendritic cells in a phase 1b trial. Proc Natl Acad Sci U S A 2019; 116: 8463–8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Pfeil J, Kumar S, et al. Tolerogenic modulation of the immune response by oligoglycerol- and polyglycerol-peptide conjugates. Bioconjug Chem 2015; 26: 669–679. [DOI] [PubMed] [Google Scholar]
- 20.Puentes F, Dickhaut K, Hofstatter M, et al. Immune modulation and prevention of autoimmune disease by repeated sequences from parasites linked to self antigens. J Neuroimmune Pharmacol 2016; 11: 749–762. [DOI] [PubMed] [Google Scholar]
- 21.Pfeil J, Simonetti M, Lauer U, et al. Tolerogenic immunomodulation by PEGylated antigenic peptides. Front Immunol 2020; 11: 529035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenwald RB, Choe YH, McGuire J, et al. Effective drug delivery by PEGylated drug conjugates. Adv Drug Deliv Rev 2003; 55: 217–250. [DOI] [PubMed] [Google Scholar]
- 23.Fishburn CS.The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci 2008; 97: 4167–4183. [DOI] [PubMed] [Google Scholar]
- 24.Zheng JC, Lei N, He QC, et al. PEGylation is effective in reducing immunogenicity, immunotoxicity, and hepatotoxicity of alpha-momorcharin in vivo. Immunopharmacol Immunotoxicol 2012; 34: 866–873. [DOI] [PubMed] [Google Scholar]
- 25.Mu Q, Hu T, Yu J.Molecular insight into the steric shielding effect of PEG on the conjugated staphylokinase: biochemical characterization and molecular dynamics simulation. PLoS One 2013; 8: e68559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendel I, Kerlero de, Rosbo N, Ben-Nun A.A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol 1995; 25: 1951–1959. [DOI] [PubMed] [Google Scholar]
- 27.Miller SD, Karpus WJ, Davidson TS.Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol 2010; 88: 15.1.1–15.1.20. [DOI] [PubMed] [Google Scholar]
- 28.Konietschke F, Placzek M, Schaarschmidt F, et al. nparcomp: an R software package for nonparametric multiple comparisons and simultaneous confidence intervals. J Stat Softw 2015; 64: 17. [Google Scholar]
- 29.Zhang P, Sun F, Liu S, et al. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release 2016; 244: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poppenborg SM, Wittmann J, Walther W, et al. Impact of anti-PEG IgM antibodies on the pharmacokinetics of pegylated asparaginase preparations in mice. Eur J Pharm Sci 2016; 91: 122–130. [DOI] [PubMed] [Google Scholar]
- 31.Danikowski KM, Jayaraman S, Prabhakar BS.Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation 2017; 14: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderton SM, Liblau RS.Regulatory T cells in the control of inflammatory demyelinating diseases of the central nervous system. Curr Opin Neurol 2008; 21: 248–254. [DOI] [PubMed] [Google Scholar]
- 33.Getts DR, Turley DM, Smith CE, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol 2011; 187: 2405–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi S, Schwartz RH.Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol 2007; 19: 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaskow BJ, Baecher-Allan C.Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med 2018; 8: a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGinley AM, Edwards SC, Raverdeau M, et al. Th17 cells, γδ T cells and their interplay in EAE and multiple sclerosis. J Autoimmun. Epub ahead of print 21 January 2018. DOI: 10.1016/j.jaut.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 37.Croxford AL, Spath S, Becher B.GM-CSF in neuroinflammation: licensing myeloid cells for tissue damage. Trends Immunol 2015; 36: 651–662. [DOI] [PubMed] [Google Scholar]
- 38.Pegoretti V, Baron W, Laman JD, et al. Selective modulation of TNF-TNFRs signaling: insights for multiple sclerosis treatment. Front Immunol 2018; 9: 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghani S, Feuerer M, Doebis C, et al. T cells as pioneers: antigen-specific T cells condition inflamed sites for high-rate antigen-non-specific effector cell recruitment. Immunology 2009; 128: e870–e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marelli-Berg FM, Fu H, Vianello F, et al. Memory T-cell trafficking: new directions for busy commuters. Immunology 2010; 130: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra MK, Yong VW.Myeloid cells – targets of medication in multiple sclerosis. Nat Rev Neurol 2016; 12: 539–551. [DOI] [PubMed] [Google Scholar]
- 42.De Bondt M, Hellings N, Opdenakker G, et al. , et al. Neutrophils: underestimated players in the pathogenesis of Multiple Sclerosis (MS). Int J Mol Sci 2020; 21: 4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorstenson KM, Khoruts A.Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol 2001; 167: 188–195. [DOI] [PubMed] [Google Scholar]
- 44.Belkaid Y, Oldenhove G.Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity 2008; 29: 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caretto D, Katzman SD, Villarino AV, et al. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol 2010; 184: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You S, Chatenoud L.Revisiting the phenotypic and genetic profiling of anergic T cells mediating long-term transplant tolerance. Curr Opin Organ Transplant 2018; 23: 83–89. [DOI] [PubMed] [Google Scholar]
- 47.Keijzer C, van der Zee R, van Eden W, et al. Treg inducing adjuvants for therapeutic vaccination against chronic inflammatory diseases. Front Immunol 2013; 4: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Getts DR, Martin AJ, McCarthy DP, et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol 2012; 30: 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCarthy DP, Yap JW, Harp CT, et al. An antigen-encapsulating nanoparticle platform for TH1/17 immune tolerance therapy. Nanomedicine 2017; 13: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaMothe RA, Kolte PN, Vo T, et al. Tolerogenic nanoparticles induce antigen-specific regulatory T cells and provide therapeutic efficacy and transferrable tolerance against experimental autoimmune encephalomyelitis. Front Immunol 2018; 9: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li PY, Bearoff F, Zhu P, et al. PEGylation enables subcutaneously administered nanoparticles to induce antigen-specific immune tolerance. J Control Release 2021; 331: 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krienke C, Kolb L, Diken E, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021; 371: 145–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taj-10.1177_20406223211037830 for Prevention of EAE by tolerogenic vaccination with PEGylated antigenic peptides by Jennifer Pfeil, Mario Simonetti, Uta Lauer, Bianca von Thülen, Pawel Durek, Christina Poulsen, Justyna Pawlowska, Matthias Kröger, Ralf Krähmer, Frank Leenders, Ute Hoffmann and Alf Hamann in Therapeutic Advances in Chronic Disease
Supplemental material, sj-pdf-2-taj-10.1177_20406223211037830 for Prevention of EAE by tolerogenic vaccination with PEGylated antigenic peptides by Jennifer Pfeil, Mario Simonetti, Uta Lauer, Bianca von Thülen, Pawel Durek, Christina Poulsen, Justyna Pawlowska, Matthias Kröger, Ralf Krähmer, Frank Leenders, Ute Hoffmann and Alf Hamann in Therapeutic Advances in Chronic Disease