Abstract
Background:
Chordoma is a rare malignant bone tumor, and the survival prediction for patients with chordoma is difficult. The objective of this study was to construct and validate a nomogram for predicting cancer-specific survival (CSS) in patients with spinal chordoma.
Methods:
A total of 316 patients with spinal chordoma were identified from the SEER database between 1998 and 2015. The independent prognostic factors for patients with spinal chordoma were determined by univariate and multivariate Cox analyses. The prognostic nomogram was established for patients with spinal chordoma based on independent prognostic factors. Furthermore, we performed internal and external validations for this nomogram.
Results:
Primary site, disease stage, histological type, surgery, and age were identified as independent prognostic factors for patients with spinal chordoma. A nomogram for predicting CSS in patients with spinal chordoma was constructed based on the above 5 variables. In the training cohort, the area under the curve for predicting 1-, 3-, and 5-year CSS were 0.821, 0.856, and 0.920, respectively. The corresponding area under the curve in the validation cohort were 0.728, 0.804, and 0.839, respectively. The calibration curves of the nomogram showed a high degree of agreement between the predicted and the actual results, and the decision curve analysis further demonstrated the satisfactory clinical utility of the nomogram.
Conclusions:
The prognostic nomogram provides a considerably more accurate prediction of prognosis for patients with spinal chordoma. Clinicians can use it to categorize patients into different risk groups and make personalized treatment methods.
Keywords: spinal chordoma, nomogram, cancer-specific survival, Surveillance, Epidemiology, and End Results
Introduction
Chordoma is a rare bone tumor that originates from undifferentiated remnants of the spinal cord. The vast majority of primary tumor site of chordoma is spine.1,2 The incidence of chordoma in the United States is only 0.08 per 100,000.3 Although the chordoma shows slow-growing, it tends to show localized aggressiveness.4 Due to the high recurrence rate, patients’ survival and quality of life can be severely deteriorated and the overall 5-year survival rate is around 50%.5,6
In 2015, Stacchiotti et al published the first guidelines for the treatment of chordoma, recommending complete surgical resection with negative surgical margins as the primary therapy.7 Invariably, spinal chordoma is located near important nerves and blood vessels, making radical removal difficult even for experienced surgeons.8 Moreover, surgeons are often hesitant between total resection to improve prognosis or partial resection to preserve neurological function, which may be recommended when the tumor cannot be completely removed. Reportedly, surgical resection combined with radiation therapy may provide a higher rate of local control compared to surgical resection alone.9
The identification of factors that influence the prognosis of patients with spinal chordoma is of significant importance. It was demonstrated that surgical margins and distant metastases are independent factors affecting the prognosis of patients with chordoma.4,10 In terms of accurate individualized patient survival prediction, however, the role of a single prognostic factor is limited. A nomogram is a handy statistical tool enabling clinicians to predict an individual patient’s prognosis.11 The distinct advantages of nomograms are robustness and better predictive accuracy, enhancing their potential for individual prognostic accuracy. The prognostic nomogram which can be used to predict cancer-specific survival (CSS) in patients with spinal chordoma, however, has not been reported. Due to the low prevalence of spinal cord tumors and the fact that most are currently single-center studies of limited size, we collected clinical information on patients from the Surveillance, Epidemiology, and End Results (SEER) database, which covers nearly 30% of the United States population. The objective of this study was to develop an effective prognostic nomogram to predict CSS in patients with spinal chordoma.
Material and Methods
Patients
The information about patients was obtained through the SEER database, which includes 18 cancer registries covering approximately 30% of the U.S. population. The SEER database provides clinical information on cancer patients and greatly facilitates clinical research. We access the SEER database through the SEER*Stat software (version 8.3.6) to obtain the required information. The SEER database does not involve personally identifiable information and there was no direct interaction with patients in this study.
The inclusion criteria were as follows: (1) diagnosed as a primary malignancy (2) histologically confirmed as a chordoma, and (3) primary site limited to the spine. Ultimately, a total of 316 patients diagnosed with spinal chordoma between 1998 and 2015 were included in this study.
Data Elements
The demographic characteristics of the patients (age, sex, race, marital status), disease characteristics (histological type, tumor size, disease stage), treatment modalities (radiotherapy, chemotherapy, and surgery), survival, and life status were included in our study. The optimal age cut-off value for CSS was determined by X-tile software with the subsequent categorization of patients into 3 groups (<69, 69-80, and > 80 years). As the International Classification of Tumor Histology, 3 rd edition states, chordoma can be classified as conventional, chondroid, and dedifferentiated.12 Based on the American Joint Committee on Cancer TNM staging system (8th edition, 2016), tumor size is usually divided into 2 categories: ≤ 8.0 cm and > 8.0 cm.13 For this follow-up study, the starting point was the date of diagnosis of the chordoma, while the endpoint CSS was the length of time from the date of diagnosis to the patient’s death due to the chordoma.
Statistical Analysis
With a ratio of 7:3, all patients included in the study were divided into a training cohort and a validation cohort. We used the Cox proportional hazards regression model to identify independent prognostic factors for patients with spinal chordoma.14 The risk ratio of each variable was calculated for the corresponding 95% confidence interval of the CSS. Based on independent prognostic factors, we used the R software to develop a nomogram model to predict the CSS in patients with spinal chordoma. In this study, internal and external validation of the prognostic nomogram constructed based on the training cohort were performed. Both the receiver operating characteristic (ROC) curve and the area under the curve (AUC) were utilized to evaluate the predictive performance of the nomogram. Calibration curves were used to assess the agreement between predicted and observed results. Moreover, decision curve analysis (DCA) was performed to evaluate the value of the clinical application of the nomogram. Finally, following the cut-off value of the risk score, patients were categorized into high-risk, intermediate-risk, and low-risk groups, and the Kaplan-Meier survival curve analysis and log-rank test were used to validate the prognostic value of the nomogram. The present study was analyzed using SPSS 25.0 and R software (version 3.6.1), where a P-value < 0.05 (two-sided) was considered as statistically significant.
Results
Demographic and Clinicopathologic Characteristics of Patients With Spinal Chordoma
Based on the inclusion and exclusion criteria, our study included 316 eligible patients with spinal chordoma from 1998 to 2015. All patients were randomized in a 7:3 ratio into a training cohort (n = 224) and a validation cohort (n = 92). The specific demographic and clinical characteristics information for all patients with spinal chordoma is shown in Table 1.
Table 1.
Baseline Demographics and Clinical Characteristics of Patients With Spinal Chordoma.
| Variables | Training cohort | Validation cohort | ||
|---|---|---|---|---|
| N = 224 | N = 92 | |||
| n | % | n | % | |
| Age | ||||
| <69 | 131 | 58.5 | 58 | 63.0 |
| 69-80 | 59 | 26.3 | 28 | 30.4 |
| >80 | 34 | 15.2 | 6 | 6.5 |
| Race | ||||
| Black | 8 | 3.6 | 6 | 6.5 |
| Other | 17 | 7.6 | 10 | 10.9 |
| White | 199 | 88.8 | 76 | 82.6 |
| Sex | ||||
| Female | 89 | 39.7 | 28 | 30.4 |
| Male | 135 | 60.3 | 64 | 69.6 |
| Primary site | ||||
| Vertebrae | 64 | 28.6 | 30 | 32.4 |
| Sacrum/pelvis | 160 | 71.4 | 62 | 67.4 |
| Histological types | ||||
| Chondroid chordoma | 5 | 2.2 | 1 | 1.1 |
| Chordoma, NOS | 215 | 96.0 | 91 | 98.9 |
| Dedifferentiated chordoma | 4 | 1.8 | 0 | 0 |
| Disease stage | ||||
| Localized | 84 | 37.5 | 40 | 43.5 |
| Regional | 125 | 55.8 | 44 | 47.8 |
| Distant | 15 | 6.7 | 8 | 8.7 |
| Surgery | ||||
| GTR | 99 | 44.2 | 32 | 34.8 |
| No | 52 | 23.2 | 26 | 28.2 |
| STR | 73 | 32.6 | 34 | 37.0 |
| Radiotherapy | ||||
| No | 121 | 54.0 | 43 | 46.7 |
| Yes | 103 | 46.0 | 49 | 53.3 |
| Chemotherapy | ||||
| No | 217 | 96.9 | 85 | 92.4 |
| Yes | 7 | 3.1 | 7 | 7.6 |
| Tumor size | ||||
| ≤ 8 | 142 | 63.4 | 60 | 65.2 |
| > 8 | 82 | 36.6 | 32 | 34.8 |
| Marital status | ||||
| No | 91 | 40.6 | 38 | 41.3 |
| Yes | 133 | 59.4 | 54 | 58.7 |
Abbreviations: GTR, gross total/radical resection; STR, subtotal resection.
Prognostic Factors for CSS in Patients With Spinal Chordoma
Univariate and multivariate Cox proportional hazards regression analyses were performed to screen for prognostic factors. According to the univariate Cox proportional hazards regression analysis, primary site, disease stage, histological type, surgery, and age were relevant factors for CSS (Table 2). After controlling for confounding variables with multivariate Cox proportional hazards regression analysis, primary site, disease stage, histological type, surgery, and age were identified as independent prognostic factors (Table 2).
Table 2.
Univariate and Multivariate Cox Analysis for Patients With Spinal Chordoma.
| Univariate Cox analysis | Multivariate Cox analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |||
| Age | ||||||||
| <69 | 1 | 1 | ||||||
| 69-80 | 2.643 | 1.514 | 4.613 | 0.001 | 2.915 | 1.568 | 5.419 | 0.001 |
| >80 | 7.781 | 4.457 | 13.583 | 0.000 | 8.630 | 4.524 | 16.464 | 0.000 |
| Race | ||||||||
| Black | 1 | |||||||
| Other | 45227.524 | 0.000 | 1.727 | 0.941 | ||||
| White | 66165.455 | 0.000 | 2.522 | 0.939 | ||||
| Sex | ||||||||
| Female | 1 | |||||||
| Male | 0.831 | 0.527 | 1.311 | 0.427 | ||||
| Primary Site | ||||||||
| Vertebrae | 1 | 1 | ||||||
| Sacrum/pelvis | 0.601 | 0.378 | 0.956 | 0.032 | 0.428 | 0.256 | 0.714 | 0.001 |
| Histological type | ||||||||
| Chondroid chordoma | 1 | 1 | ||||||
| Chordoma, NOS | 0.301 | 0.093 | 0.971 | 0.045 | 0.086 | 0.023 | 0.323 | 0.000 |
| Dedifferentiated chordoma | 1.056 | 0.212 | 5.260 | 0.947 | 0.370 | 0.065 | 2.112 | 0.263 |
| Disease stage | ||||||||
| Distant | 1 | 1 | ||||||
| Localized | 0.176 | 0.082 | 0.380 | 0.000 | 0.136 | 0.058 | 0.320 | 0.000 |
| Regional | 0.251 | 0.124 | 0.510 | 0.000 | 0.177 | 0.079 | 0.396 | 0.000 |
| Surgery | ||||||||
| GTR | 1 | 1 | ||||||
| No | 5.085 | 2.954 | 8.754 | 0.000 | 3.371 | 1.829 | 6.214 | 0.000 |
| STR | 1.107 | 0.609 | 2.013 | 0.739 | 1.143 | 0.595 | 2.195 | 0.688 |
| Radiotherapy | ||||||||
| No | 1 | |||||||
| Yes | 1.016 | 0.637 | 1.619 | 0.948 | ||||
| Chemotherapy | ||||||||
| No | 1 | |||||||
| Yes | 1.735 | 0.632 | 4.760 | 0.285 | ||||
| Tumor size | ||||||||
| > 8 | 1 | |||||||
| ≤ 8 | 0.775 | 0.488 | 1.232 | 0.282 | ||||
| Marital status | ||||||||
| No | 1 | |||||||
| Yes | 0.925 | 0.582 | 1.471 | 0.743 | ||||
Abbreviations: GTR, gross total/radical resection; STR, subtotal resection.
Construction of the Prognostic Nomogram for Predicting CSS in Patients With Spinal Chordoma
A prognostic nomogram of patients with spinal chordoma was established based on 5 independent prognostic factors (Figure 1). In the prognostic nomogram, values for the individual patient are located along the variable axes, and a line is drawn upward to the Points axis to determine the number of points assigned for each variable. There was a Total Points line at the bottom of the nomogram, and each variable score was summed to give the total points. And the accumulated total points can be used to predict the 1-, 3-, and 5-year survival rate of the patient.
Figure 1.
Prognostic nomogram for patients with spinal chordoma.
Validation of the Prognostic Nomogram for Predicting CSS in Patients With Spinal Chordoma
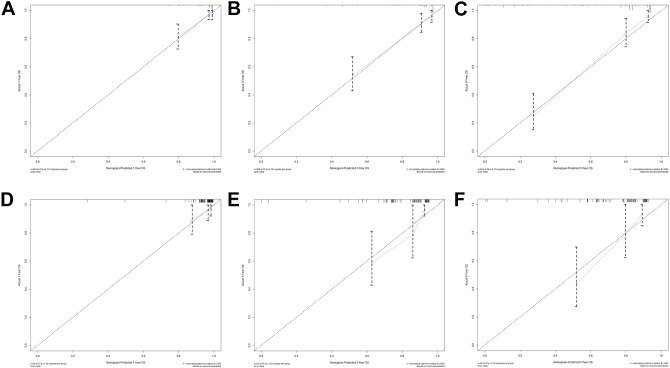
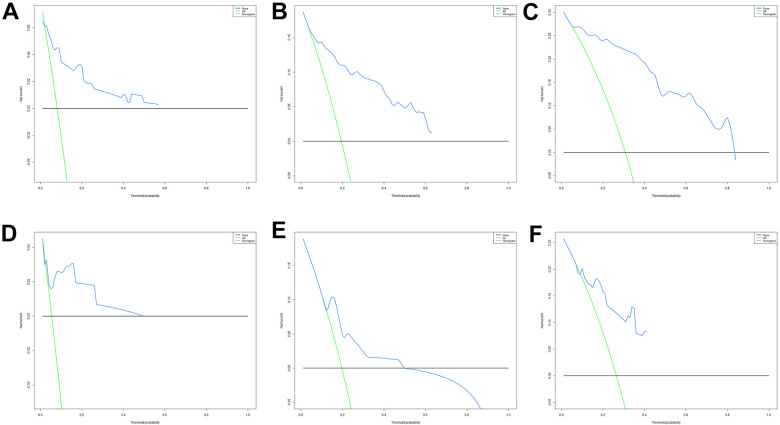
As shown in Figure 2, the AUCs for 1-, 3-, and 5-year were 0.821, 0.856, and 0.920 in the training cohort and 0.728, 0.804, and 0.839 in the validation cohort, respectively. Moreover, we further compared the discrimination between the nomogram and independent prognostic factors with the results showing that the nomogram had higher prediction accuracy than individual independent prognostic factors, both in the training cohort and validation cohort (Figure 3). The high agreement between the results predicted by the nomogram and the actual results can be easily noticed by observing the calibration curves (Figure 4). These points are close to a 45-degree diagonal, indicating that we have succeeded in achieving the best agreement between the survival rates predicted by the nomogram and the actual survival rates. The DCA also showed that the nomogram model has a strong clinical utility (Figure 5).
Figure 2.
ROC curves for CSS prediction of patients with spinal chordoma. (A) ROC curves of 1-, 3-, and 5-years in the training cohort, (B) ROC curves of 1-, 3-, and 5-years in the validation cohort. CSS indicates cancer-specific survival; ROC, receiver operating characteristic.
Figure 3.
Comparison of the prediction accuracy between the nomogram model and independent prognostic factors. The ROC curves of nomogram and all independent predictors at 1- (A), 3- (B), and 5-years (C) in the training cohort and at 1- (D), 3- (E), and 5-years (F) in the validation cohort. ROC indicates receiver operating characteristic; CSS, cancer-specific survival.
Figure 4.
Calibration curves. The calibration curves of the nomogram for predicting the 1-year, 3-years, and 5-years CSS of the training cohort (A-C) and the validation cohort (D-F). CSS indicates cancer-specific survival.
Figure 5.
DCA of the nomogram for predicting the 1- (A), 3- (B) and 5- years (C) CSS in the training cohort and the 1- (D), 3- (E) and 5- years (F) CSS in the validation cohort. DCA indicates decision curve analysis; CSS, cancer-specific survival.
Stratification of Risk Groups
Based on the cut-off value of the risk score of patients in the training cohort, all patients, including training and validation cohorts were divided into high-risk, intermediate-risk, and low-risk groups. By plotting Kaplan-Meier survival curves, it was easy to find that patients in the high-risk group showed a worse prognosis than those in the low-risk group and intermediate-risk group (Figure 6).
Figure 6.
Kaplan-Meier survival analysis for both the training cohort and the validation cohort. Patients with a higher risk score showed a worse CSS than those with a lower risk score in the training cohort (A) and the validation cohort (B). CSS indicates cancer-specific survival.
Discussion
It is challenging for clinicians to perform radical resection of patients with spinal chordoma. Therefore, a nomogram model with high predictive accuracy to predict the CSS of spinal chordoma makes sense. In this study, we analyzed data of 316 patients and found that primary site, disease stage, histological type, surgery, and age were independent CSS-related factors, and a nomogram was constructed and validated based on these variables. Based on this nomogram, clinicians can categorize patients into different risk groups, identify high-risk patients, and improve their treatment plans for a better prognosis.
The majority of patients with spinal chordoma are white males over the age of 40. In the present study, several clinicopathological features were confirmed as independent prognostic factors for CSS, including primary site, disease stage, histological type, surgery, and age, which is consistent with previous studies.4,15 Compared with the <69 and 69-80 age groups, patients older than 80 years had the worst prognosis, which may be attributed to the symptoms of spinal chordoma that are similar to those of other benign conditions, which can easily lead to delayed diagnosis.4,15 Moreover, we found that patients with tumors that broke through the periosteum had a worse prognosis compared with patients with localized tumors. It has been reported that tumors that break through the periosteum are more likely to invade surrounding tissues or nerves and are more likely to recur or metastasize, resulting in a poorer prognosis for patients.15,16 Based on the microscopic morphology, there are 3 different histological subtypes of spinal chordoma, including conventional, chondroid, and dedifferentiated.12 Of all histological subtypes, the dedifferentiated type is the most aggressive subtype, which significantly affects prognosis.17 Meanwhile, the results of this study showed that histological type had a significant effect on survival outcomes, once again confirming the above findings. It is controversial whether tumor size is related to the prognosis of patients with spinal chordoma. Although it has been previously reported that larger tumor size always implies a worse prognosis, tumor size was not an independent prognostic factor for CSS in patients with spinal chordoma, according to the results of univariate and multivariate Cox proportional hazards regression analysis in this study.
Despite the high risks associated with surgery, it remains an effective and reliable treatment option for patients with spinal chordoma. In our analysis, the majority of cases were treated with surgery (75.3%). Surgical resection was significantly associated with survival in both univariate and multivariate Cox proportional hazards regression analyses (Table 2). Furthermore, we found that wide resection provides a better long-term prognosis, while patients with inadequate resection have a relatively poorer prognosis, which is consistent with previous reports.18,19 It is recommended that once a patient with spinal chordoma is diagnosed early, and a radical resection should be performed whenever possible. Nowadays, radiotherapy has been widely used as an adjuvant postoperative treatment for spinal chordoma.20 Recently, it has been shown that local progression-free time and overall survival will be prolonged in patients who receive radiotherapy immediately after surgery.21 Hence, clinicians are encouraged to pay special attention to the increasingly important role of radiotherapy. Nevertheless, the benefits need to be weighed against the risks because of the proximity of the spinal cord to the chordoma and the potential for paralysis from high-dose radiotherapy.
To the best of our knowledge, this is the first study to construct a nomogram predicting the CSS of spinal chordoma based on large and diverse case data. Nomograms are considered an effective tool for quantifying risk and maximizing forecast accuracy.22,23 The calibration curves showed a high degree of agreement between the predicted and actual observed survival rates of the training and validation cohorts, indicating that the nomograms established in this study are reliable. Inevitably, there are some shortcomings in our study. First of all, the study is a retrospective study with unavoidable selection bias. Second, since both training and validation cohorts come from the same database, it is necessary to have data from another database for validation. Besides, specific details regarding radiotherapy and chemotherapy are not included, such as specific chemotherapy regimens or radiation doses, which may also be prognostic factors for patients with spinal chordoma. Despite these drawbacks, however, this study offers the possibility of predicting and improving CSS in patients with spinal chordoma.
Conclusion
The primary site, disease stage, histological type, surgery, and age are independent prognostic factors for CSS in patients with spinal chordoma. We incorporated such prognostic factors to construct a prognostic nomogram model that predicts CSS at 1, 3, and 5 years in patients with spinal chordoma. The nomogram constructed in this study can be used as a valid and convenient assessment tool to help clinicians personalize survival assessment and identify the risk of death in patients with spinal chordoma.
Abbreviations
- CSS
Cancer-specific survival
- SEER
Surveillance, Epidemiology, and End Results
- ROC
Receiver operating characteristic
- AUC
Area under the curve
- DCA
Decision curve analysis.
Footnotes
Authors’ Note: The dataset from SEER database generated and/or analyzed during the current study are available in the SEER dataset repository (https://seer.cancer.gov/). The research didn’t involve animal experiments and human specimens, no ethics related issues.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Chengliang Zhao  https://orcid.org/0000-0002-2673-3398
https://orcid.org/0000-0002-2673-3398
References
- 1.Zuckerman SL, Bilsky MH, Laufer I. Chordomas of the skull base, mobile spine, and sacrum: an epidemiologic investigation of presentation, treatment, and survival. World Neurosurgery. 2018;113:e618–e627. doi:10.1016/j.wneu.2018.02.109 [DOI] [PubMed] [Google Scholar]
- 2.Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46(2):95–104. doi:10.1097/pat.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 3.Smoll NR, Gautschi OP, Radovanovic I, Schaller K, Weber DC. Incidence and relative survival of chordomas: the standardized mortality ratio and the impact of chordomas on a population. Cancer. 2013;119(11):2029–2037. doi:10.1002/cncr.28032 [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, Lu L, Chen J, Zhong Y, Dai Z. Analysis of prognostic factors for survival in patients with primary spinal chordoma using the SEER registry from 1973 to 2014. J Orthop Surg Res. 2018;13(1):76. doi:10.1186/s13018-018-0784-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88(9):2122–2134. doi:10.1002/(sici)1097-0142(20000501)88:9<2122::aid-cncr19>3.0.co;2-1 [DOI] [PubMed] [Google Scholar]
- 6.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12(1):1–11. doi:10.1023/a:1008947301735 [DOI] [PubMed] [Google Scholar]
- 7.Stacchiotti S, Sommer JChordoma Global Consensus Group. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol. 2015;16(2):e71–83. doi:10.1016/s1470-2045(14)71190-8 [DOI] [PubMed] [Google Scholar]
- 8.Meng T, Yin H, Li B, et al. Clinical features and prognostic factors of patients with chordoma in the spine: a retrospective analysis of 153 patients in a single center. Neuro-Oncol. 2015;17(5):725–732. doi:10.1093/neuonc/nou331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pennicooke B, Laufer I, Sahgal A, et al. Safety and local control of radiation therapy for chordoma of the spine and sacrum: a systematic review. Spine. 2016:41(Suppl 20):S186–S192. doi:10.1097/brs.0000000000001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee IJ, Lee RJ, Fahim DK. Prognostic factors and survival outcome in patients with chordoma in the United States: a population-based analysis. World Neurosurg. 2017;104:346–355. doi:10.1016/j.wneu.2017.04.118 [DOI] [PubMed] [Google Scholar]
- 11.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–180. doi:10.1016/s1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun HH, Hong X, Liu B, et al. Survival analysis of patients with spinal chordomas. Neurosurg Rev. 2019;42(2):455–462. doi:10.1007/s10143-018-0968-7 [DOI] [PubMed] [Google Scholar]
- 13.Cates JM.Comparison of the AJCC, MSTS, and modified Spanier systems for clinical and pathologic staging of osteosarcoma. Am J Surg Pathol. 2017;41(3):405–413. doi:10.1097/pas.0000000000000774 [DOI] [PubMed] [Google Scholar]
- 14.Katz MH, Hauck WW. Proportional hazards (Cox) regression. J Gen Intern Med. 1993;8(12):702–711. doi:10.1007/bf02598295 [DOI] [PubMed] [Google Scholar]
- 15.Lin K, Song K, Wang S, Jiang L, Wang H, Dong J. Predict overall survival of spinal conventional chordoma: development and assessment of a new predictive nomogram. Clinical Neurol Neurosurg. 2020;197:106174. doi:10.1016/j.clineuro.2020.106174 [DOI] [PubMed] [Google Scholar]
- 16.Sahyouni R, Goshtasbi K, Mahmoodi A, Chen JW. A historical recount of chordoma. J Neurosurg Spine. 2018;28(4):422–428. doi:10.3171/2017.7.Spine17668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna SA, Tirabosco R, Amin A, et al. Dedifferentiated chordoma: a report of four cases arising “de novo.” J Bone Joint Surg Br. 2008;90(5):652–656. doi:10.1302/0301-620x.90b5.20365 [DOI] [PubMed] [Google Scholar]
- 18.Kayani B, Hanna SA, Sewell MD, Saifuddin A, Molloy S, Briggs TW. A review of the surgical management of sacral chordoma. Eur J Surg Oncol. 2014;40(11):1412–1420. doi:10.1016/j.ejso.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 19.Kayani B, Sewell MD, Tan KA, et al. Prognostic factors in the operative management of sacral chordomas. World Neurosurg. 2015;84(5):1354–1361. doi:10.1016/j.wneu.2015.06.030 [DOI] [PubMed] [Google Scholar]
- 20.Boriani S, Chevalley F, Weinstein JN, et al. Chordoma of the spine above the sacrum. Treatment and outcome in 21 cases. Spine. 1996;21(13):1569–1577. doi:10.1097/00007632-199607010-00017 [DOI] [PubMed] [Google Scholar]
- 21.Moojen WA, Vleggeert-Lankamp CL, Krol AD, Dijkstra SP. Long-term results: adjuvant radiotherapy in en bloc resection of sacrococcygeal chordoma is advisable. Spine. 2011;36(10):E656–661. doi:10.1097/BRS.0b013e3181f8d1f3 [DOI] [PubMed] [Google Scholar]
- 22.Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007;25(11):1316–1322. doi:10.1200/jco.2006.06.1218 [DOI] [PubMed] [Google Scholar]
- 23.Wierda WG, O’Brien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109(11):4679–4685. doi:10.1182/blood-2005-12-051458 [DOI] [PubMed] [Google Scholar]