Abstract
Optimizing the environment of complex bone healing and improving treatment of catastrophic bone fractures and segmental bone defects remains an unmet clinical need both human and equine veterinary medical orthopaedics. The objective of this study was to determine whether scAAV-equine-BMP-2 transduced cells would induce osteogenesis in equine bone marrow derived mesenchymal stem cells (BMDMSCs) in vitro, and if these cells could be cryopreserved in an effort to osteogenically prime them as an “off-the-shelf” gene therapeutic approach for fracture repair. Our study found that transgene expression is altered by cell expansion, as would be expected by a transduction resulting in episomal transgene expression, and that osteoinductive levels could still be achieved 5 days after recovery, and protein expression would continue up to 14 days after transduction. This is the first evidence that cryopreservation of genetically modified BMDMSCs would not alter the osteoinductive potential or clinical use of allogeneic donor cells in cases of equine fracture repair.
Keywords: progenitors and stem cells, bone biology, bone repair, therapeutics, bone fracture
Conventional clinical management of segmental bone defects in humans continues to result in 5–10% of fractures forming non-unions. This persistent gap in clinical success necessitates innovative approaches to bone repair. Active areas of research have recently been reviewed1 and often involve the use of novel biologic therapies. In parallel, fracture repair in the horse and the plight of recovery is a major challenge because of chronic pain, the development of support limb laminitis, and association with ischemia and infection, which can result in euthanasia of the patient. Achieving successful repair of the affected limb is associated with compliance, the mechanical limits of implants,2 and are challenged by an increased incidence of infection due to the paucity of soft tissue coverage and often-traumatic fracture etiology.2 Thus, there is an unmet clinical and ethical obligation of veterinarians to study novel approaches to promote rapid repair of complicated long bone fractures and large bone defects. Further, because of the challenges associated with fracture repair in the horse, and parallels between poor soft tissue coverage and blood supply to the distal limb of horses and the limbs of humans, a translational incentive exists such that success in the equine model may help to heal the 5–10% of human fractures that do not heal despite clinical intervention.1
Mesenchymal stem cells derived from bone marrow aspirates (BMDMSCs) are often combined with growth factors to induce bone formation and accelerate healing.3 There are notable successful studies in both human4 and equine literature.5 The most prominently studied osteoinductive growth factor in bone healing is bone morphogenic protein-2 (BMP-2). Recombinant BMP-2 (rhBMP-2) is met with varying clinical success6 and has well documented limitations such as ectopic bone formation, increased inflammation and edema, and a relatively short half-life.6 In equine fracture repair cases, use of rhBMP-2 is often cost prohibitive.7
Gene therapy is considered an alternative to recombinant protein therapy. BMDMSCs can be genetically modified to produce therapeutic proteins in quantities that affect clinical outcomes in bone.8 While several vector constructs are available, self-complementary adeno associated virus (scAAV) is designed such that therapeutic protein production to begins within hours of transduction.10 Further, scAAV has been shown to selectively transduce target BMDMSCs11 and produce high levels of therapeutic proteins even in BMDMSCs with low proliferation rates.8
Our ability to overcome the clinical limitations associated with gene therapy for fracture repair in the horse remains unmet for a number of reasons: (1) catastrophic fractures are often operated on within 24 h of presentation and this is not enough time to harvest, expand, and transduce an autologous bone marrow sample as a source of BMDMSCs; (2) most equine patients have not undergone autologous stem cell harvesting and then had their cells culture expanded and cryopreserved; (3) and there are anecdotal reports of varying degrees of success, inflammation, and immune response to both autologous and allogeneic culture-expanded stem cell use.12–14 Therefore, a potential alternative to improve the clinical success of equine fracture repair might be an allogeneic, “off-the-shelf,” genetically modified BMDMSC biologic that has been assessed for its ability to augment bone repair.
Similar to the clinical manifestations in horses is a high rate of mal-union and non-union fractures, often estimated at 5–10% of annual fractures in humans.1 If our study and future in vivo studies in the horse provide “proof-of-principal,” the cryopreservation of genetically modified BMDMSCs may be a future area of application in people. This is supported by the recent approval of the first gene therapy treatment for osteoarthritis, approved in Korea, and undergoing phase III clinical trials in the United States.9 The success of the commercialization of gene therapy in Korea and the clinical trial in the United States will be paramount to the field of gene therapy.
The objectives of the current study were: (1) to evaluate scAAV-BMP-2 osteogenic induction in equine BMDMSCs in vitro and (2) to investigate if selective cryopreservation of scAAV-BMP-2 cells would not reduce the BMP-2 delivery capacity of the genetically modified cells following recovery in vitro. We hypothesized that (1) transduction with 48,000 viral particles per cell of scAAV-equine-BMP-2 would result in in vitro osteogenesis in equine BMDMSCs and (2) that the cryopreservation of the genetically modified cells would not impact the clinical delivery of the BMP-2 transgene.
MATERIALS AND METHODS
Cell Culture and Harvesting
Mesenchymal progenitor cells from five skeletally mature horses were isolated aseptically from the sternum as previously described.15 Cells were plated in a 48 well plate at 50% confluency (37,500 cells/cm2) and equilibrated overnight. Cells reached 80% confluency (60,000 cells/cm2), were washed twice with PBS, and then transduced in serum free Dulbecco’s Modified Eagle Media (DMEM) containing 48,000 viral particles per cell (vpc) scAAV-equine-BMP-2 or 8,000 vpc scAAV-GFP for 3 h. Cells in non-vector control groups (rhBMP-2, osteogenic, and negative controls) were incubated in incomplete media during this time. Once the transduction was complete, serum-free media was aspirated, and complete DMEM was added (Table 1). Media was changed on day 2 and 5.
Table 1.
Media supplementation by treatment group
| Group | Media Supplementation |
|---|---|
| rhBMP-2 (100 ng/mL for 2 days, 50 ng/mL following) | Complete DMEM with 170μM ascorbic acid, 2 mM β-glycerol phosphate (βGP) |
| scAAV-equine-BMP-2 | Complete DMEM with 170 μM ascorbic acid, 2 mM β-glycerol phosphate (βGP) |
| scAAV-GFP | Complete DMEM with 170 μM ascorbic acid, 2 mM β-glycerol phosphate (βGP) |
| Osteogenic Control | Complete DMEM with 170μM ascorbic acid, 2 mM β-glycerol phosphate (βGP), 1 × 10−9 M dexamethasone |
| Negative Control | Complete DMEM with 170 μM ascorbic acid, 2 mM β-glycerol phosphate (βGP) |
| Expansion Media | Complete αMEM with 4 ng/mL recombinant FGF-2 |
BMP-2 Protein Expression
BMP-2 protein expression was evaluated on days 7 (pre and post cryopreservation) and 14 (pre-cryopreservation) using R&D Systems Human BMP-2 DuoSet ELISA (DY355). The tested media supernatant was collected from 2 wells of the 48-well plate after 48 h of incubation. All samples were tested in triplicate.
Morphology
Morphology was graded on days 7 (pre and post cryopreservation) and 14 (pre-cryopreservation) using the descriptive table provided. Scoring was adapted from Zachos et al.16 The grading scores were statistically treated as numerical variables according to Table 2. Morphology scoring was performed by two independent reviewers.
Table 2.
Morphology scoring key
| Score | Description |
|---|---|
| 0 | Dead, completely detached cells |
| 1 | Rounded, detaching cells |
| 2 | Progenitor cell with normal, fibroblastic appearance |
| 3 | Cells congregating and/or cuboidal; ≤25% of culture is osteogenic |
| 4 | Cells congregating and/or cuboidal; 26–75% of culture is osteogenic |
| 5 | Cells congregating and/or cuboidal; ≥75% culture is osteogenic |
Staining
Cells were stained for alkaline phosphatase and extracellular matrix calcium deposition with alizarin red on day 7 pre and post cryopreservation. Staining quantification was not performed in this study and did not undergo statistical analysis.
Alkaline Phosphatase Expression
Alkaline phosphatase (ALP) lysate expression was evaluated on days 7 (pre and post cryopreservation) using Sensolyte® pNPP Alkaline Phosphatase Assay Kit (Catalog#AS-72146). Statistical analysis was not performed due to low sample size (n = 2). All samples were tested in triplicate.
Statistics
The experiment was designed as a split plot, and was analyzed as a mixed model. BMP-2 protein expression values were log transformed based on variance, and the morphology scoring variables were analyzed without transformation. Alkaline phosphatase lysate data did not undergo statistical analysis. The statistical program R was utilized to analyze the data with a significance value of p < 0.05.
Objective 2 Horse Selection
Data from Objective 1 was analyzed and the two horses (n = 2) producing the greatest amount of BMP-2 protein expression and were chosen to undergo the cryopreservation studies. Cells were transduced as described in objective one and then cryopreserved 48 h later. Cells were removed from their 48-well plate with Accumax, treatment groups were pooled, and frozen at −80˚C for 24 h, and then transferred to liquid nitrogen for at least 48 h. Cells were cryopreserved as previously described.15 During recovery, small volumes (20–150 μL) of expansion media were added until 1 mL total volume was reached. Cell viability (Table 3) was assessed using a Trypan Blue assay and cells were counted using a hemocytometer. Cells were then placed in a T25 flask overnight in expansion media, and plated in 48 well plates at 80% confluency and allowed to equilibrate. Addition of the appropriate media constituted day 2.
Table 3.
Recovery viability of treatment groups
| Treatment Group | Viability |
|---|---|
| rhBMP-2 | 96% |
| scAAV-equine-BMP-2 | 97% |
| scAAV-GFP | 99% |
| Osteogenic Control | 99% |
| Negative Control | 99% |
RESULTS
Objective 1
Protein Expression
ScAAV-equine-BMP-2 cells produced significantly more protein than any other group (p < 0.0001) (Figure 1). Cells treated with rhBMP-2 treated cells (n = 5) produced similar amounts of BMP-2 protein as scAAV-equine-BMP-2 genetically modified cells, as would be expected because they were incubated in constant concentrations (100 ng/mL d0–2, 50 ng/mL d2–7).
Figure 1.
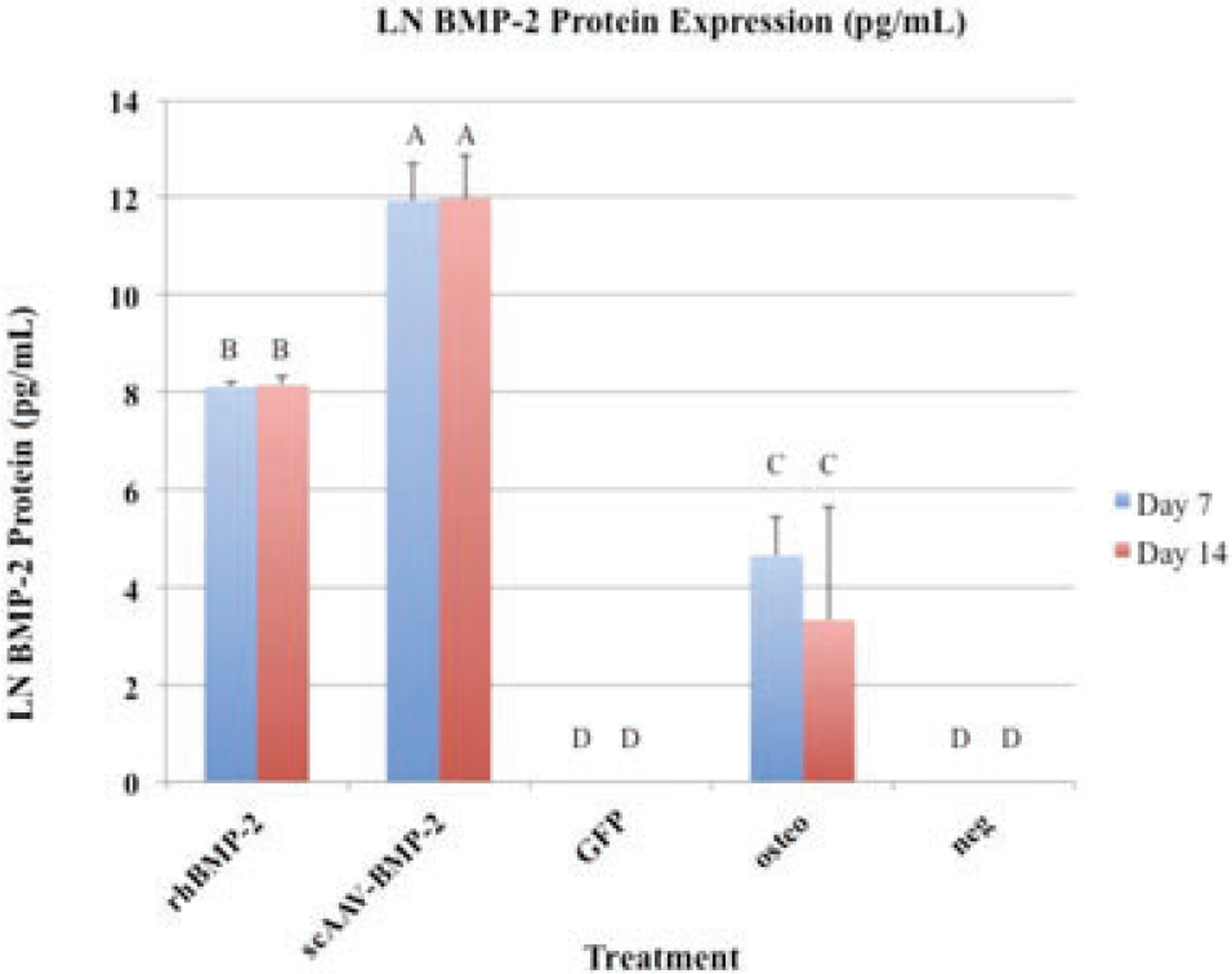
BMP-2 protein expression (pg/mL): Error bars denote standard deviation from the mean. Letter changes denote significant differences between groups (p < 0.0001). ScAAV-equine-BMP-2 transduced cells produced significantly more BMP-2 than any other group. Transgene expression persisted without significant changes 14 days.
Morphology and Staining
ScAAV-equine-BMP-2, rhBMP-2, and osteogenic control cells became cuboidal and nodular arrangements of cells were observed (Figure 3). Morphology scoring16 was significantly different between scAAV-equine-BMP-2, rhBMP-2, osteogenic controls and scAAV-GFP and negative controls (Figure 2b). Alizarin red staining was done qualitatively and differences were apparent (Figure 2a).
Figure 3.
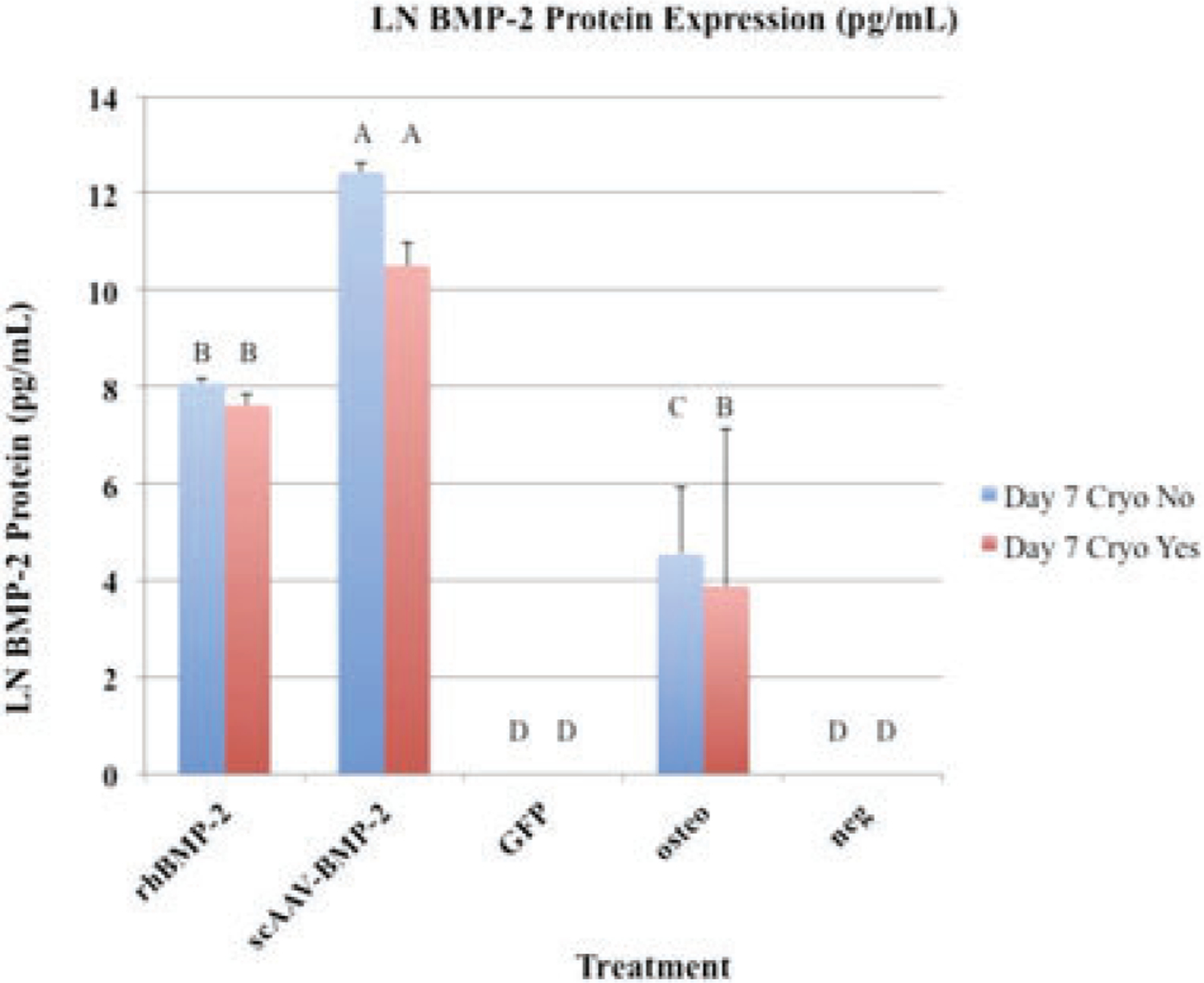
BMP-2 protein expression (pg/mL): Error bars denote standard deviation from the mean. Letter changes denote significant differences between groups (p < 0.0001). ScAAV-equine-BMP-2 transduced cells produced significantly more BMP-2 than any other group. Transgene expression persisted after cryopreservation.
Figure 2.
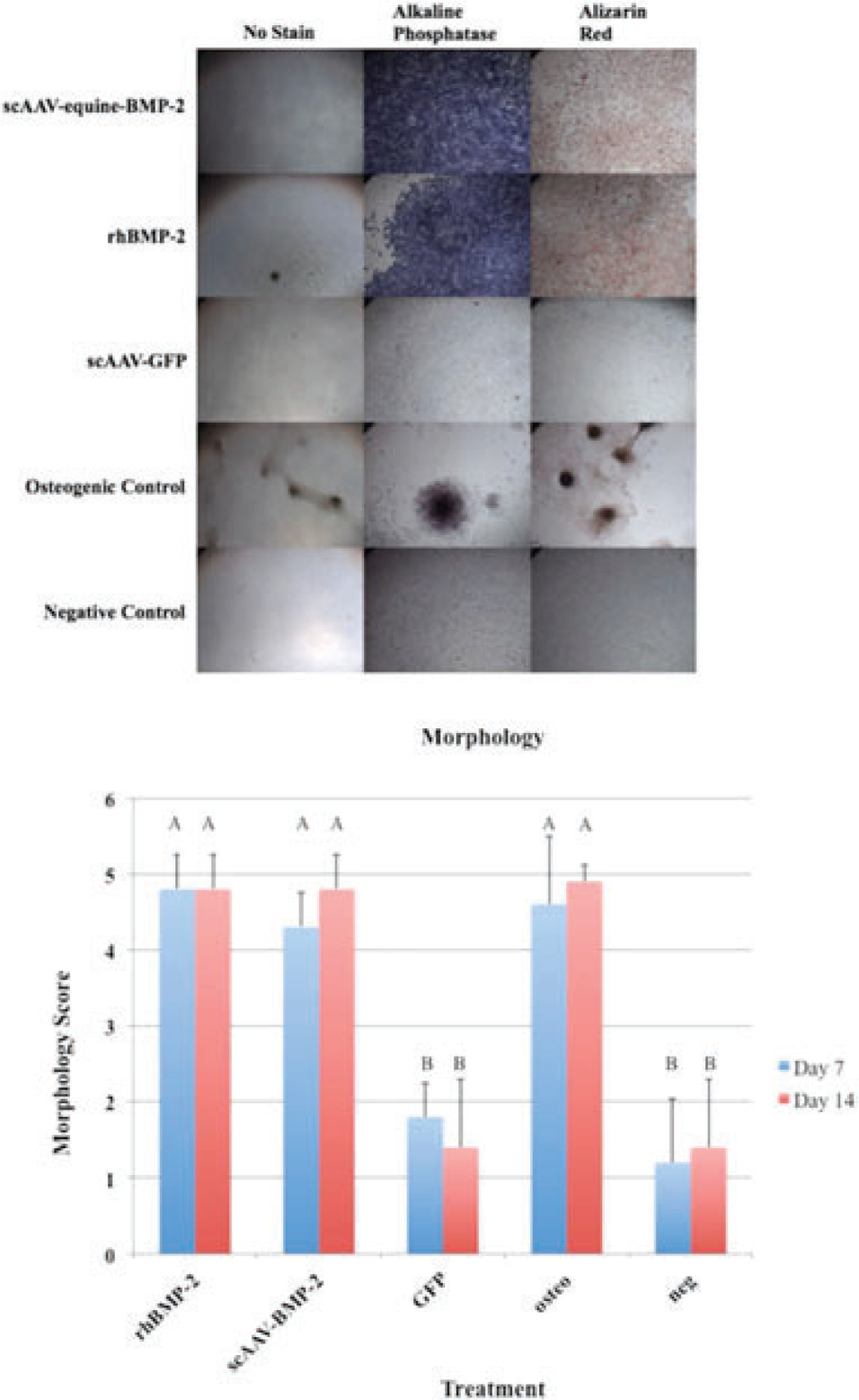
(a) MSCs in culture treated with scAAV-equine-BMP-2, rhBMP-2, and osteogenic media exemplify osteogenic morphologic changes at day 7 following transduction. (b) Morphology Scores: Error bars denote standard deviation from the mean. Letter changes denote significant differences between groups. ScAAV-equine-BMP-2 transduced cells, rhBMP-2 treated cells, and osteogenic control cells appeared significantly more osteogenic than GFP transduced cells and negative controls (p < 0.0001). The morphological changes were evident by day 7.
Objective 2
Protein Expression
Seven days after transduction, and 5 days following recovery from cryopreservation, media supernatant was collected and analyzed for BMP-2 protein content (Figure 3). ScAAV-equine-BMP-2 cells produced significantly more protein than any other group (p < 0.0001). Following cryopreservation, scAAV-equine-BMP-2 cells produced less BMP-2 protein when compared to transduced cells that were not cryopreserved (Table 4); however, the change in this small sample size was not significant (p < 0.1156). It is unknown how larger cohorts of pre-screened allogeneic donor cells would respond, and the authors recognize the precipitous drop of protein expression. Cryopreserved rhBMP-2 treated cells produced similar amounts of BMP-2 protein when compared to non-cryopreserved cells (p < 0.6861), as would be expected because they were incubated in constant concentrations regardless of population expansion; therefore, mitotic daughter cells were exposed to the same concentration of rhBMP-2.
Table 4.
Raw BMP-2 protein expression levels
| Treatment | Day 7 Obj. 1 (non-cryopreserved) (pg/mL BMP-2) | Day 7 Obj. 2 (cryopreserved) (pg/mL BMP-2) |
|---|---|---|
| rhBMP-2 | 3,230.57 | 2,038.55 |
| scAAV-eBMP-2 | 251,495.85 | 36,620.825 |
| scAAV-GFP | Below limit of detection | Below limit of detection |
| Osteogenic Control | 94.1 | 48.837 |
| Negative Control | Below limit of detection | Below limit of detection |
Morphology and Staining
Seven days after transduction, scAAV-equine-BMP-2, rhBMP-2, and osteogenic control cells remained cuboidal and formed nodular congregations. This remained consistent following cryopreservation (Figure 4a). Morphology scoring16 remained consistent following cryopreservation (Figure 4b).
Figure 4.
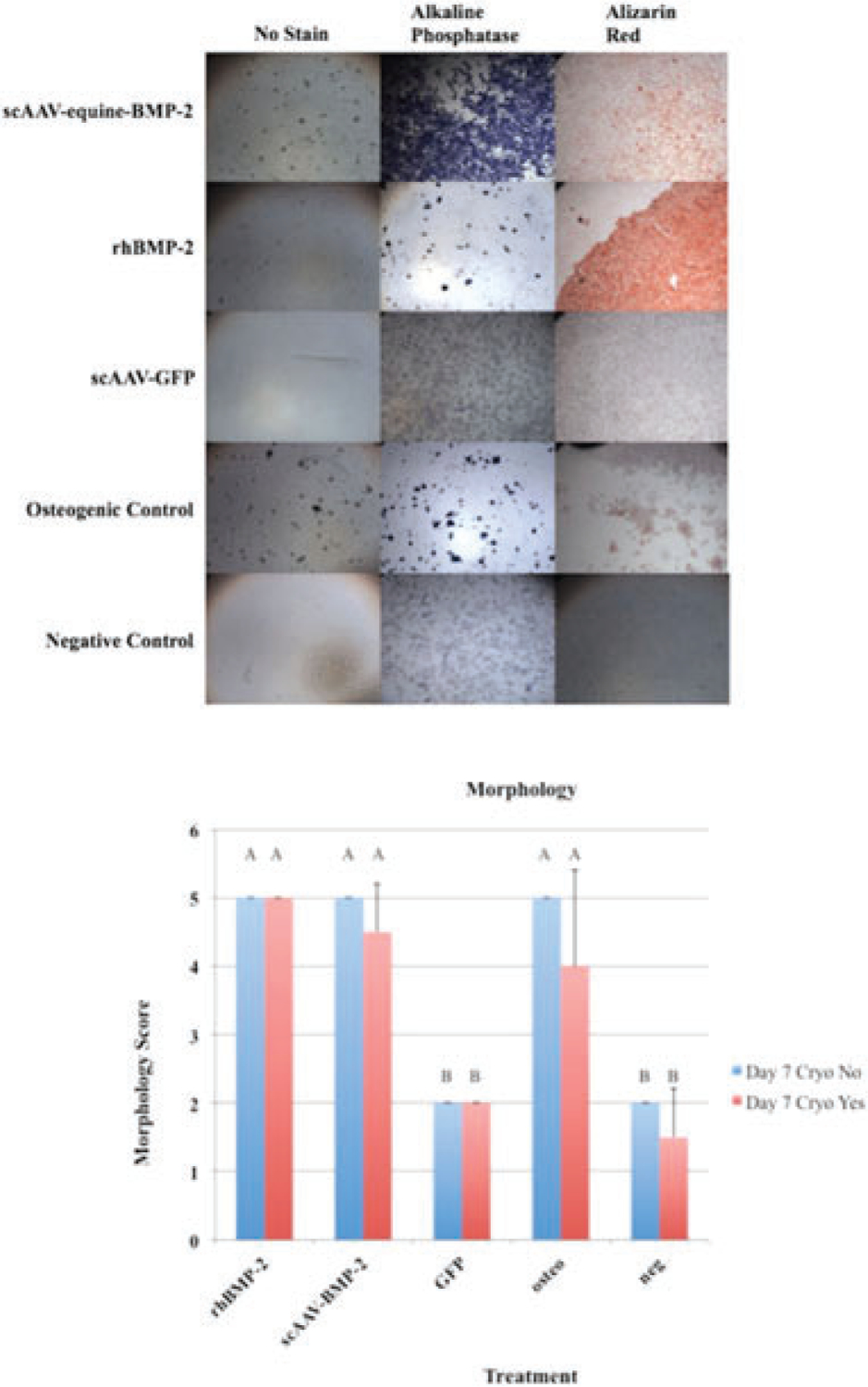
(a) MSCs in culture treated with scAAV-equine-BMP-2, rhBMP-2, and osteogenic media exemplify osteogenic morphologic changes at day 7 following transduction, cryopreservation, and recovery. (b) Morphology Scores: Error bars denote standard deviation from the mean. Letter changes denote significant differences between groups (p < 0.0001). Cryopreservation did not affect how scAAV-equine-BMP-2 transduced cells, rhBMP-2 treated cells, and osteogenic control cells appeared morphologically when compared to scAAV-GFP and negative controls (p < 0.0001). The morphological changes were evident by day 7 following transduction, cryopreservation, and recovery.
Alkaline Phosphatase Lysate
Seven days after transduction, scAAV-equine-BMP-2, rhBMP-2, and osteogenic control cells produced more alkaline phosphatase on average than scAAV-GFP and negative control cells (Figure 5).
Figure 5.
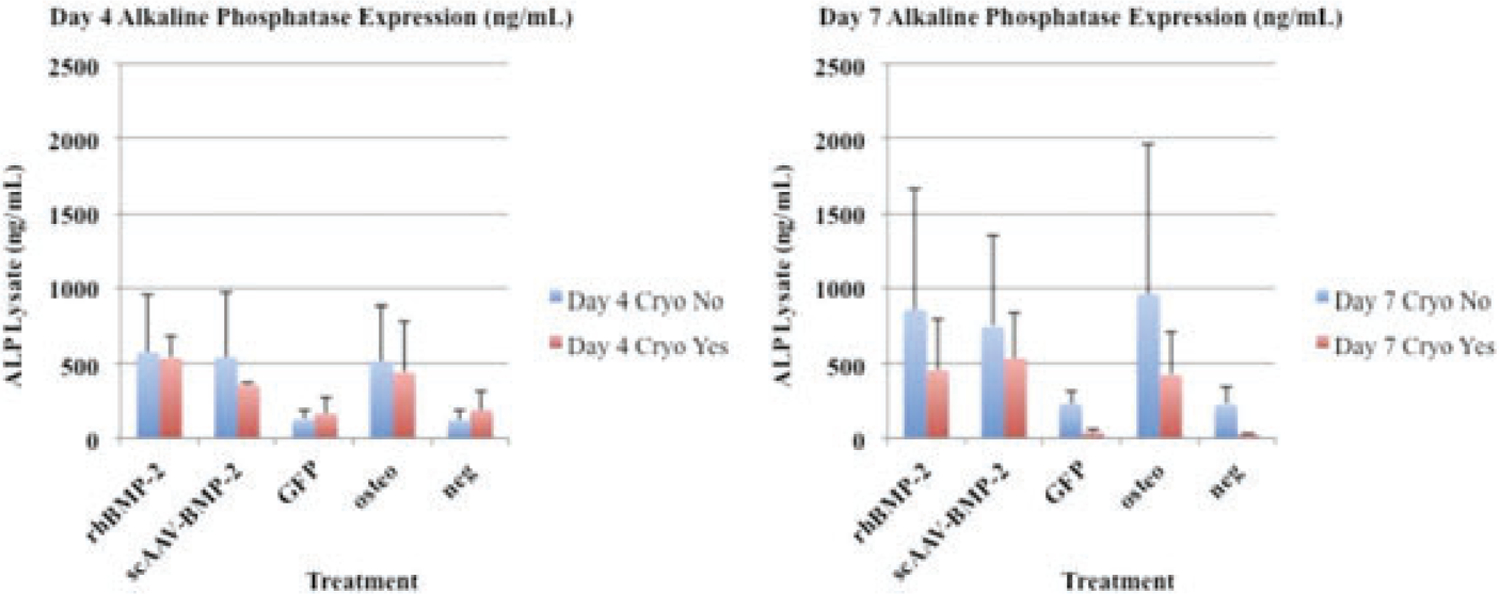
Side by side comparison of day 4 and day 7 alkaline phosphatase expression (ng/mL). Expression patterns between cryopreserved and non-cryopreserved cells follow a similar trend when compared to BMP-2 protein expression (Figure 3).
DISCUSSION
Viral gene therapy traditionally uses one of four well-studied techniques to augment bone healing. (1) Ex vivo traditional techniques harvest, expand, and transduce autologous or allogeneic cells in culture prior to placing them in a defect.17,18 (2) Ex vivo expedited techniques harvest and transduce using a single “same-day” technique,19 providing fewer cells, and in cases where bone marrow aspirate concentrate (BMAC) are used, a very small population of BMDMSCs.20 (3) In vivo suspension techniques transfer a virus capable of transducing the native cell population, either directly,21 percutaneously at the completion of surgery, or within 2 weeks of surgery completion,22,23 and (4) Lyophilization of viral vectors to allografts allows selective transduction of cells that infiltrate the fracture callus, improving the bio-mechanical properties of allografts and their potential to undergo remodeling.8
All concerns considered, important clinical needs of equine clinical fracture cases are (1) that horses, in most cases, are operated on within 24 h of presentation; (2) horses would benefit from earlier biomechanical stability of the fracture environment aimed to decrease secondary complications,24 and (3) a biologic, gene therapeutic approach may provide strong osteoinductive and osteogenic elements to equine fracture repair. While the controversy of autologous and allogeneic cell sources remains,25–27 clinical equine practice in the case of fracture repair is most amenable to allogeneic cell use. Further, recent studies by Colbath et al. as well as others suggest a high safety profile of allogeneic MSCs.26,28,29 Sources of mesenchymal progenitors (e.g., adipose vs. bone marrow) also show signs of epigenetic memory that can be altered to improve osteogenesis in adipose derived mesenchymal progenitors with the use of histone deacetylase inhibitors.30 This evidence, although indirect, is supportive that certain cell lines from specific donors may be epigenetically superior and responsive to osteogenic signals. Moreover, equine clinicians appreciate variance between patient’s reactions to stem cell treatments (e.g., presence of inflammatory flare, successful tissue regeneration) and in human medicine with recombinant protein use, it is often that osteogenic responses are not uniform in situ.6,31,32 Therefore, we hypothesized that allogeneic cells could be pre-screened for osteogenic capability and BMP-2 delivery, and that these cells could be genetically modified and subsequently cryopreserved as an “off-the-shelf” biologic available for a wide variety of clients.
We first observed that scAAV-equine-BMP-2 transduced cells produced significantly more BMP-2 protein than any other treatment group (Figure 1). This is in agreement with several other groups utilizing a traditional ex vivo transduction technique.33,34 Importantly, the one-time scAAV-equine-BMP-2 transduction produced significantly more protein than what is clinically available today without repeat administration of rhBMP-2. While this study did not incorporate the use of a carrier, protein elution from genetically modified cells loaded onto a carrier would be expected to remain unchanged, and protein delivery would then be dependent on cell delivery, and not dictated by hydrolysis and elution characteristics of various scaffolds. It is true that long-term implants are available, allowing for slow-release of rhBMP-2 and other growth factors in vivo for several weeks.35 However, steady state levels remain difficult to achieve, immune modulation to growth factor efficacy on slow-release scaffolds has not been quantified, and is likely patient dependent. Further, continued high levels of transgene expression at day 14 in vitro is promising when considering BMP-2 delivery in vivo, and other studies utilizing similar scAAV vectors, have provided therapeutic transgene expression levels up to 183 days after cell transduction in normal equine joints,36 while other studies have reported transgene expression ranging from 2 weeks in fibroblasts,37 and up to 2.35 years in the trabecular meshwork of the anterior segment of cynomolgus monkeys.36–38
A common osteoblastic marker and important enzyme for callus mineralization is alkaline phosphatase. Staining for alkaline phosphatase in BMP-2 treated groups (scAAV-equine-BMP-2 and rhBMP-2 groups) show a clear predilection for this osteoblastic marker (Figure 2a). Alkaline phosphatase exists in a membrane bound form on osteoblasts, and is secreted into mineralized matrices.39 As a functional marker of osteoblastogenesis, membrane-bound bone-specific alkaline phosphatase was quantified (Figure 5). When using equine mesenchymal cells, dexamethasone has been a key component of inducing osteoblastogenesis in our lab and others,15,40 while human mesenchymal cells41 and common culture cell lines have produced ALP in response to BMP-2 treatment.42 Although statistical analysis was not performed on ALP lysate samples due to small sample size, scAAV-equine-BMP-2, rhBMP-2, and osteogenic control cells performed similarly. Cryopreserved cells that were thawed and retested followed similar patterns to BMP-2 protein expression, with cryopreserved cells producing less overall protein. Taking into consideration overall BMP-2 protein expression in cryopreserved cells (Figure 3), morphology scoring (Figure 4a,b), and ALP lysate production (Figure 5), cryopreserved genetically modified mesenchymal stem cells retain functional characteristics of osteoblasts. This small and necessary step will require further in vivo studies to elucidate if these patterns confer clinical significance in the horse.
While there are techniques employed to expedite autologous cells and gene therapy,19,43 there have been no attempts the authors are aware of to screen and cryopreserve allogeneic genetically modified BMDMSC treatments. The authors of this study provide the first evidentiary support that the cryopreservation of scAAV-equine-BMP-2 cells does not affect their osteoinductive potential. Figure 3 illustrates that despite cryopreservation, transgene protein expression is preserved. While there was some decrease in transgene expression, the authors hypothesize this is due to cell-expansion during recovery. All scAAV transgenes remain episomal within genetically modified cells, and are not passed to daughter cells during mitosis. This is in contrast to the utilization of lentiviral vectors, where investigators are exploring ways use systemic anti-viral treatments to program apoptosis in genetically modified cells after transgene use is no longer needed.44 Total eradication of therapeutic cells (as evaluated by luciferase marker genes) has yet to be achieved in a pre-clinical animal model.
In this experiment, cells were recovered overnight in flasks and then equilibrated in a 48-well plate before experimental days were counted again (approximately 24 h). Based on average BMDMSC doubling time (1–2 days),45 the cell population would have expanded during this time. Further evidence of this was shown in our scAAV-GFP transduced cells, in which a decreased number of fluorescent cells were observed following recovery (data not shown). Due to the heavy staining (Figure 4a), lack of morphological differences (Figure 4b), and retention of osteoinductive levels of protein expression (Figure 3), it is reasonable to hypothesize that the residual BMP-2 production from the original transduced population could still be providing the necessary cues to induce osteoblastogenesis in this new population. The next step would be to test this hypothesis in the bony microenvironment in vivo.
CONCLUSION
This is the first published study that demonstrates the utility of screening allogeneic cells for donor responsiveness to gene therapy aimed at inducing osteogenesis in vitro. We have provided evidence that an ex vivo transduction technique, employed with a screening process, produces detectable levels of BMP-2. This is the first evidence of an “off-the-shelf” gene therapeutic approach for fracture repair in orthopedics, translational to human, and veterinary medicine. Further work remains to be done to establish the therapeutic efficacy of BMP-2 genetically modified allogeneic cells in clinical cases of fracture repair, and use of an equine pre-clinical model, translational to humans, should be performed to establish therapeutic efficacy in vivo.
ACKNOWLEDGMENTS
Author AB supported by NIH T32: 5 T32 OD 12201–3, the Colorado State University College Research Council, and generous donations by the Bender Family Fund.
REFERENCES
- 1.Ball AN, Donahue SW, Wojda SJ, et al. 2018. The challenges of promoting osteogenesis in segmental bone defects and osteoporosis. J Orthop Res 36:1559–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahern BJ, Richardson DW, Boston RC, et al. 2010. Orthopedic infections in equine long bone fractures and arthrodeses treated by internal fixation: 192 cases (1990–2006). Vet Surg 39:588–593. [DOI] [PubMed] [Google Scholar]
- 3.Luu HH, Song WX, Luo X, et al. 2007. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res 25:665–677. [DOI] [PubMed] [Google Scholar]
- 4.Wei S, Cai X, Huang J, et al. 2012. Recombinant human BMP-2 for the treatment of open tibial fractures. Orthopedics 35:e847–e854. [DOI] [PubMed] [Google Scholar]
- 5.Seo JP, Tsuzuki N, Haneda S, et al. 2014. Osteoinductivity of gelatin/beta-tricalcium phosphate sponges loaded with different concentrations of mesenchymal stem cells and bone morphogenetic protein-2 in an equine bone defect model. Vet Res Commun 38:73–80. [DOI] [PubMed] [Google Scholar]
- 6.Garrison KR, Donell S, Ryder J, et al. 2007. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess 11:1–150, iii-iv. [DOI] [PubMed] [Google Scholar]
- 7.Evans CH. 2010. Gene therapy for bone healing. Expert Rev Mol Med 12:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazici C, Takahata M, Reynolds DG, et al. 2011. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther 19:1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans CH, Ghivizzani SC, Robbins PD. 2018. Arthritis Gene Therapy Approved in Korea. J Am Acad Orthop Surg 26: e36–e38. [DOI] [PubMed] [Google Scholar]
- 10.McCarty DM. 2008. Self-complementary AAV vectors; advances and applications. Mol Ther 16:1648–1656. [DOI] [PubMed] [Google Scholar]
- 11.Hemphill DD, McIlwraith CW, Samulski RJ, et al. 2014. Adeno-associated viral vectors show serotype specific transduction of equine joint tissue explants and cultured monolayers. Sci Rep 4:5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guest DJ, Ousey JC, Smith MR. 2008. Defining the expression of marker genes in equine mesenchymal stromal cells. Stem Cells Cloning 1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pigott JH, Ishihara A, Wellman ML, et al. 2013. Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immunopathol 156:99–106. [DOI] [PubMed] [Google Scholar]
- 14.Pigott JH, Ishihara A, Wellman ML, et al. 2013. Inflammatory effects of autologous, genetically modified autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Comp Orthop Traumatol 26:453–460. [DOI] [PubMed] [Google Scholar]
- 15.Lombana KG, Goodrich LR, Phillips JN, et al. 2015. An investigation of equine mesenchymal stem cell characteristics from different harvest sites: more similar than not. Front Vet Sci 2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zachos TA, Shields KM, Bertone AL. 2006. Gene-mediated osteogenic differentiation of stem cells by bone morphogenetic proteins-2 or −6. J Orthop Res 24:1279–1291. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman JR, Daluiski A, Stevenson S, et al. 1999. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 81:905–917. [DOI] [PubMed] [Google Scholar]
- 18.Hsu WK, Sugiyama O, Park SH, et al. 2007. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone 40:931–938. [DOI] [PubMed] [Google Scholar]
- 19.Virk MS, Sugiyama O, Park SH, et al. 2011. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther 19:960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RG. 2014. Bone marrow concentrate with allograft equivalent to autograft in lumbar fusions. Spine (PhilaPa 1976) 39:695–700. [DOI] [PubMed] [Google Scholar]
- 21.Baltzer AW, Lattermann C, Whalen JD, et al. 2000. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther 7:734–739. [DOI] [PubMed] [Google Scholar]
- 22.Betz OB, Betz VM, Nazarian A, et al. 2006. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am 88:355–365. [DOI] [PubMed] [Google Scholar]
- 23.Southwood LL, Kawcak CE, Hidaka C, et al. 2012. Evaluation of direct in vivo gene transfer in an equine metacarpal IV ostectomy model using an adenoviral vector encoding the bone morphogenetic protein-2 and protein-7 gene. Vet Surg 41:345–354. [DOI] [PubMed] [Google Scholar]
- 24.Virgin JE, Goodrich LR, Baxter GM, et al. 2011. Incidence of support limb laminitis in horses treated with half limb, full limb or transfixation pin casts: a retrospective study of 113 horses (2000–2009). Equine Vet J 40:7–11. [DOI] [PubMed] [Google Scholar]
- 25.Schnabel LV, Pezzanite LM, Antczak DF, et al. 2014. Equine bone marrow-derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell Res Ther 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colbath AC, Dow SW, Phillips JN, et al. 2017. Autologous and allogeneic equine mesenchymal stem cells exhibit equivalent immunomodulatory properties In vitro. Stem Cells Dev 26:503–511. [DOI] [PubMed] [Google Scholar]
- 27.Griffin MD, Ryan AE, Alagesan S, et al. 2013. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol 91:40–51. [DOI] [PubMed] [Google Scholar]
- 28.Broeckx S, Borena BM, Zimmerman M, et al. 2014. Intravenous application of allogenic peripheral blood-derived mesenchymal stem cells: a safety assessment in 291 equine recipients. Curr Stem Cell Res Ther 9:452–457. [DOI] [PubMed] [Google Scholar]
- 29.Broeckx S, Suls M, Beerts C, et al. 2014. Allogenic mesenchymal stem cells as a treatment for equine degenerative joint disease: a pilot study. Curr Stem Cell Res Ther 9:497–503. [DOI] [PubMed] [Google Scholar]
- 30.Xu S, De Veirman K, Evans H, et al. 2013. Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo. Acta Pharmacol Sin 34:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans C 2014. Using genes to facilitate the endogenous repair and regeneration of orthopaedic tissues. Int Orthop 38:1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W, Kim J, Cheng C, et al. 2006. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 39:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pensak M, Hong S, Dukas A, et al. 2015. The role of transduced bone marrow cells overexpressing BMP-2 in healing critical-sized defects in a mouse femur. Gene Ther 22:467–475. [DOI] [PubMed] [Google Scholar]
- 34.Ishihara A, Weisbrode SE, Bertone AL. 2015. Autologous implantation of BMP2-expressing dermal fibroblasts to improve bone mineral density and architecture in rabbit long bones. J Orthop Res 33:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempen DH, Lu L, Heijink A, et al. 2009. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 30:2816–2825. [DOI] [PubMed] [Google Scholar]
- 36.Goodrich LR, Grieger JC, Phillips JN, et al. 2015. ScAAVIL-1ra dosing trial in a large animal model and validation of long-term expression with repeat administration for osteoarthritis therapy. Gene Ther 22:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kay JD, Gouze E, Oligino TJ, et al. 2009. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J Gene Med 11:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buie LK, Rasmussen CA, Porterfield EC, et al. 2010. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci 51:236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke B 2008. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3:S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glynn ER, Londono AS, Zinn SA, et al. 2013. Culture conditions for equine bone marrow mesenchymal stem cells and expression of key transcription factors during their differentiation into osteoblasts. J Anim Sci Bio-technol 4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpenter RS, Goodrich LR, Frisbie DD, et al. 2010. Osteoblastic differentiation of human and equine adult bone marrow-derived mesenchymal stem cells when BMP-2 or BMP-7 homodimer genetic modification is compared to BMP-2/7 heterodimer genetic modification in the presence and absence of dexamethasone. J Orthop Res 28:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partridge K, Yang X, Clarke NM, et al. 2002. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun 292: 144–152. [DOI] [PubMed] [Google Scholar]
- 43.Evans CH, Liu FJ, Glatt V, et al. 2009. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater 18:96–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alaee F, Sugiyama O, Virk MS, et al. 2014. Suicide gene approach using a dual-expression lentiviral vector to enhance the safety of ex vivo gene therapy for bone repair. Gene Ther 21:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esteves CL, Sheldrake TA, Mesquita SP, et al. 2017. Isolation and characterization of equine native MSC populations. Stem Cell Res Ther 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]


