Abstract
1,2-O-dilauryl-rac-glycero glutaric acid-(6′-methylresorufin) ester (DGGR) lipase activity has been proposed as a faster and less expensive test used in the diagnosis of acute pancreatitis (AP) compared to canine pancreatic lipase immunoreactivity (cPLI), which is considered the most sensitive and specific serum test available for dogs. Elevations in lipase activity have been observed in dogs with naturally occurring hypercortisolism (HC) and in those treated with exogenous steroids, which complicates the diagnosis of AP in dogs with HC. We compared lipase activity measured by DGGR and 1,2-diglyceride (1,2-DiG) assays in 22 dogs with HC, 22 with AP, and 22 healthy dogs. The dogs with HC had no clinical signs or ultrasonographic findings consistent with AP. DGGR lipase activity was elevated in 64% and 73% of the dogs with HC and AP, respectively, and in 18% of healthy dogs. 1,2-DiG lipase activity was high in 23% and 36% of the dogs with HC and AP, respectively, and in 5% of the healthy dogs. Both DGGR and 1,2-DiG lipase activities were significantly different between the healthy dogs and the other 2 groups, whereas no differences were detected between the dogs with HC and those with AP. Our results support a lack of specificity for both DGGR and 1,2-DiG lipase activity assays in aiding the diagnosis of AP in dogs with HC.
Keywords: Cushing disease; DGGR; dogs; 1,2-diglyceride; hyperadrenocorticism; pancreatitis
The diagnosis of acute pancreatitis (AP) can be challenging. Histopathology of the pancreas is considered the gold standard, but is rarely performed given its invasiveness and the risk of morbidity.29 In clinical practice, a diagnosis of AP is usually reached by combining signalment, history, clinical signs, complete blood count (CBC), biochemistry (including pancreatic enzymes), and imaging, in part to exclude other diseases or evaluate for the presence of concurrent complications as a fundamental part of a diagnostic protocol.42 In the late 1960s, assays of serum lipase activity using 1,2-diglyceride (1,2-DiG) as a substrate became available for diagnosing pancreatitis,28 although the sensitivity and specificity were low in veterinary medicine. Clinical studies have reported a sensitivity of 32–73% and a specificity <50%, which is not clinically useful in the diagnosis of AP in dogs.13,33,37
In the early 2000s, assays of serum canine pancreatic lipase immunoreactivity (cPLI) were developed and validated.35,36 Compared to enzymatic lipase assays, cPLI assays had the advantage of measuring only pancreatic lipases, and obtaining high analytic specificity.23,34 cPLI is considered the most sensitive and specific serum test available for use in the diagnosis of AP in dogs, but it still has limitations, such as long turnaround times and high costs. Generally, the time required to obtain a result is ≥24 h, and, in most acutely ill patients, a faster diagnosis is required.43
An enzymatic assay using a different substrate (1,2-O-dilauryl-rac-glycero glutaric acid-(6′-methylresorufin) ester, DGGR) to measure serum lipase activity, developed ~20 y ago,26 was recently compared to cPLI and had high agreement.5,16 This assay can also provide a result in a shorter time, with the cost of a single evaluation, which is an advantage over cPLI.
AP is commonly associated with Cushing syndrome (hypercortisolism, HC), although causality has not yet been determined. Two studies have identified that dogs with HC have an ~4-fold increased risk of developing AP compared with healthy dogs and those with other diseases.11,15 Some studies have reported high serum lipase activities, measured with different assays, in dogs either with naturally occurring HC or treated with exogenous steroids.6,21,25,27 This makes reaching a diagnosis of AP in these patients particularly difficult, with the possibility that some still unidentified factors associated with HC can affect the majority of the clinically useful serum tests available for diagnosing AP.
The DGGR lipase assay represents a promising test for use in the diagnosis of AP, with the advantage of being inexpensive and readily available. A study reported 48% of dogs with HC as having increased DGGR lipase activity; however, the main intent of that study was not to evaluate this serum marker, and the possibility of those dogs having pancreatitis was not ruled out.3 We evaluated serum DGGR lipase and 1,2-DiG activity in dogs with naturally occurring HC compared to dogs with AP and healthy dogs.
Materials and methods
Study population
Canine serum samples stored at −20°C from privately owned dogs were retrospectively selected from the University of Bologna Veterinary Hospital (Ozzano dell’Emilia, Italy) digital database. The serum samples were collected from October 2016 to February 2020 from dogs with AP or HC at the time of diagnosis, and at routine check-ups from the healthy dogs. As per Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010, on the protection of animals used for scientific purposes, the Italian legislature (D. Lgs. n. 26/2014) does not require approval from ethical committees for use of stored samples in retrospective studies.
Inclusion criteria for HC included 2 or more typical clinical signs (polyuria and polydipsia, polyphagia, dermatologic changes, panting, increased volume of the abdomen) and laboratory findings with a positive endocrine test (ACTH stimulation test [ACTHst] or low-dose dexamethasone suppression test [LDDst]). The ACTHst was considered positive when the 1 h post-stimulation serum cortisol concentration was >600 nmol/L, and the LDDst when the 8 h post-dexamethasone serum cortisol concentration was >40 nmol/L. A diagnosis of pituitary-dependent hypercortisolism (PDH) or adrenal-dependent hypercortisolism (ADH) was based on the ultrasonographic appearance of the adrenal glands, the results of the LDDst, and the endogenous plasma ACTH concentration. Abdominal ultrasound evaluations were carried out by experienced radiologists using an ultrasound unit (Epiq5G ultrasound system; Philips Healthcare) equipped with micro-convex and linear-array probes of different frequencies (C8-5 and L5-12 MHz). Only dogs with HC that underwent full abdominal ultrasonography at the time of diagnosis were considered eligible for inclusion in our study.
Dogs with HC were reviewed and excluded if clinical signs (anorexia, vomiting, abdominal pain, or diarrhea) or ultrasonographic findings (enlargement and hypoechoic pancreatic parenchyma, increased echogenicity of the peripancreatic mesentery, or the presence of free peritoneal fluid) were suggestive of AP. Those with diffuse hyperechoic pancreatic parenchyma, which is not considered suggestive of AP,10 were enrolled. We identified 22 dogs with HC; consequently, we selected 22 dogs for each of the other 2 groups.
Despite the availability of the internal reference intervals for DGGR and 1,2-DiG lipase activity obtained previously from a population of 120 healthy dogs, we decided to select additional healthy dogs as a control group to ensure that the comparison with dogs with HC and AP was made between homogeneous groups in terms of age, body weight, and sex.
A diagnosis of AP was obtained by combining suggestive clinical signs (vomiting, abdominal pain) and evident ultrasonographic findings consistent with AP (enlargement and hypoechoic pancreatic parenchyma, increased echogenicity of peripancreatic mesentery, and the presence of free peritoneal fluid). Abdominal ultrasound evaluations were carried out by experienced radiologists as indicated previously. Dogs were defined as healthy if no clinical signs were reported, and if chemistry and hematology results were within reference intervals (RIs). Dogs with an increased serum creatinine concentration and those that had received exogenous steroids within 3 mo of their diagnosis were not included in any group. In all groups, only the most recent cases available in the database that met the previously reported criteria were selected to minimize potential effects related to serum sample storage. In the group of healthy dogs, animals were also selected to obtain a matched control population. For this reason, healthy dogs <6 y old were excluded.
Analytical methods
Both DGGR lipase and 1,2-DiG lipase activity were determined in our laboratory (University of Bologna Veterinary Hospital) using an automated chemistry analyzer (AU480; Olympus/Beckman Coulter) and previously validated assays.8,19 The DGGR lipase assay was set up using calibration and quality control material provided by the manufacturer (Lipase colorimetric for Cobas Integra 800; Roche). Linearity was evaluated in replicates diluting a canine pooled sample with a high concentration of DGGR lipase (729 U/L); the regression equation was y = 0.453 + 0.132x (r = 0.999; p < 0.001). Spiking recovery results were 102% and 97% using a sample with “normal” (54 U/L) and “high” (542 U/L) expected concentrations of DGGR lipase, respectively. Four pooled samples at various concentrations (729, 364, 182, and 45 U/L) of DGGR lipase were used to evaluate inter- and intra-assay coefficient of variation (CV). Mean intra-assay CV (3 replicates of samples evaluated in triplicate in the same working day) was 3.1%. Mean inter-assay CV (3 replicates on 3 consecutive days evaluated in triplicate) was 7.6%.
The 1,2-DiG lipase assay was established with calibration and quality control material provided by the manufacturer (Lipase, OSR 6130; Olympus/Beckman Coulter). Linearity was evaluated in replicate diluting a canine pooled sample with a concentration of 596 U/L of 1,2-DiG lipase; the regression equation was: y = −0.403 + 0.165x (r = 0.999; p < 0.001). Spiking recovery results were 100% and 102% using a sample with “low” (141 U/L) and “high” (575 U/L) expected concentration of lipase, respectively. Four pooled samples at various concentrations (447, 298, 74, and 37 U/L) of lipase were used to evaluate inter- and intra-assay CV. Mean intra-assay CV (3 replicates of samples evaluated in triplicate in the same working day) was 1.5%. Mean inter-assay CV (3 replicates on 3 consecutive days evaluated in triplicate) was 4%.
Stored canine serum samples were thawed at room temperature and immediately analyzed to measure DGGR lipase and 1,2-DiG lipase activity. Samples were evaluated on the same day for dogs with HC and AP; samples from healthy dogs were processed on different days. The RIs for the DGGR lipase activity (10–118 U/L) and for the 1,2-DiG lipase activity (81–582 U/L) had been established previously, following the American Society for Veterinary Clinical Pathology guidelines.7 We used 120 privately owned blood donors or hospital staff-owned dogs. Dogs were considered healthy based on signalment, clinical history, physical examination, and hematology and chemistry results. The data distribution was not normal, therefore a nonparametric method with 90% CI was used to determine DGGR and 1,2-DiG lipase RIs. Serum samples for cPLI measurement were sent refrigerated to a commercial laboratory (Idexx Germany) after collection and were analyzed within 24 h. The assay used for cPLI measurement was validated previously; mean intra-assay CV was 7.8–11.2% and mean inter-assay CV was 3.8–5.6%.12 Values were considered normal if they were <200 µg/L, abnormal if >400 µg/L; for values of 200–400 µg/L, re-testing was recommended.35,36 SNAP cPL (Idexx Germany) was also carried out, and the results were recorded as either visually normal or abnormal.2
Statistical analysis
Statistical analysis was carried out using Prism v.5.01 (GraphPad Software). The data were analyzed for normality using D’Agostino and Pearson tests, evaluated graphically, and reported as median and range (minimum–maximum value) or mean ± SD. Categorical variables were compared using chi-squared tests. The DGGR and 1,2-DiG lipase activity results between the HC, AP, and healthy dog groups were compared using a Kruskal–Wallis test followed by a Mann–Whitney U-test. Lipase activity using DGGR and 1,2-DiG assays was also compared in dogs with HC that had ultrasonographically normal or hyperechoic pancreatic parenchyma, using the Mann–Whitney U-test. To evaluate whether the freezing time of the serum samples affected the results, the DGGR and 1,2-DiG lipase activity measurements from samples stored for <1 y were compared with samples stored for >1 y using the Mann–Whitney U-test. A value of p ≤ 0.05 was considered significant.
Results
Study population
As indicated previously, 22 dogs with HC met study criteria, and matching numbers of serum samples were selected with AP, as well as 22 healthy dogs. The median ages and body weights were 11.5 y (range: 4–14) and 10.1 kg (range: 3.4–42.8); 9 y (range: 2–17) and 11.2 kg (range: 2.7–40); and 10 y (range: 6–14) and 14.4 kg (range: 5–50) for dogs diagnosed with HC, AP, and the healthy dogs, respectively. In the HC, AP, and healthy dog groups, we included 13 males (6 intact, 7 castrated) and 9 females (2 intact, 7 spayed); 9 males (2 intact, 7 castrated) and 13 females (3 intact, 10 spayed); and 11 males (9 intact, 2 castrated) and 11 females (6 intact, 5 spayed); respectively. No significant differences were detected in age, body weight, or sex among the groups.
The breeds included in the HC group were 8 mixed breeds, 2 Dachshunds, 2 Weimaraners, and 1 each of Boston Terrier, Cavalier King Charles Spaniel, Jack Russell Terrier, Maltese, Pinscher, Pomeranian, Poodle, Schnauzer, West Highland White Terrier, and Yorkshire Terrier. The breeds included in the AP group were 11 mixed breeds, 3 Jack Russell Terriers, and 1 each of American Staffordshire, Beagle, Bolognese, Golden Retriever, Labrador Retriever, Pinscher, Poodle, and Welsh Terrier. The breeds included in the healthy dog group were 7 mixed breeds, 2 Dachshunds, and 1 each of Australian Shepherd, Bolognese, Caucasian Shepherd, Cocker Spaniel, Epagneul Breton, French Bulldog, Italian Hound, Jack Russell Terrier, Labrador Retriever, Malinois, Pinscher, Weimaraner, and West Highland White Terrier.
The dogs diagnosed with HC had polyuria and polydipsia (19 of 22; 86%), polyphagia (17 of 22; 77%), dermatologic signs (14 of 22; 64%), panting (9 of 22; 41%), and increased volume of the abdomen (8 of 22; 36%). The specific tests used to confirm the diagnosis and/or localize the disease were ACTHst (17 of 22; 77%), LDDst (13 of 22; 59%), and endogenous ACTH (20 of 22; 91%). Eighteen (82%) dogs were diagnosed with PDH, 3 (14%) with ADH, and 1 (4%) dog was suspected to have PDH and ADH simultaneously or bilateral ADH. Four dogs with HC had a single concurrent disease; there was one case each of idiopathic epilepsy, syringomyelia, mitral valve disease, and atopic dermatitis.
Dogs with HC receiving chronic medications were treated one each with phenobarbital (Luminale; Farmar Italia), levetiracetam (Keppra; UCB Pharma), pimobendan (Vetmedin; Lavet Pharmaceuticals), cyclosporine (Atoplus; Novartis), desmopressin (Minirin; Ferring), robenacoxib (Onsior; Elanco), benazepril (Nelio; Sogeval), and levothyroxine (Eutirox; Pantheon).
None of the dogs diagnosed with HC had ultrasonographic findings suggestive of AP; however, 12 of 22 (55%) had diffusely hyperechoic pancreatic parenchyma. The dogs diagnosed with AP had vomiting (18 of 22; 82%), abdominal pain (12 of 22; 54%), anorexia (10 of 22; 45%), diarrhea (6 of 22; 27%), and decreased appetite (5 of 22; 23%). The ultrasonographic findings most frequently described were hypoechoic pancreatic parenchyma (20 of 22; 91%), increased echogenicity of the peripancreatic mesentery (19 of 22; 86%), enlargement or irregularity of the pancreatic parenchyma (17 of 22; 77%), and free peritoneal fluid (6 of 22; 27%).
The specific laboratory tests carried out to support the diagnosis of pancreatitis were SNAP cPL (11 of 22; 50%), which was abnormal in 9 dogs and normal in 2 dogs, and cPLI (2 of 22; 10%), which was >400 µg/L in 1 dog and <200 µg/L in the other one. The concurrent diseases reported in the dogs with AP were diabetes mellitus (3 of 22), hypothyroidism (1 of 22), idiopathic epilepsy (2 of 22), and food-responsive enteropathy (1 of 22). The medications received by the dogs with AP were lente insulin (Caninsulin; MSD) in 3 of 22, levothyroxine (Canitroid; Eurovet) in 1 of 22, phenobarbital (Gardenale; Sanofi) in 2 of 22, and clebopride (Motilex; Almirall) in 1 of 22.
DGGR and 1,2-DiG lipase activity
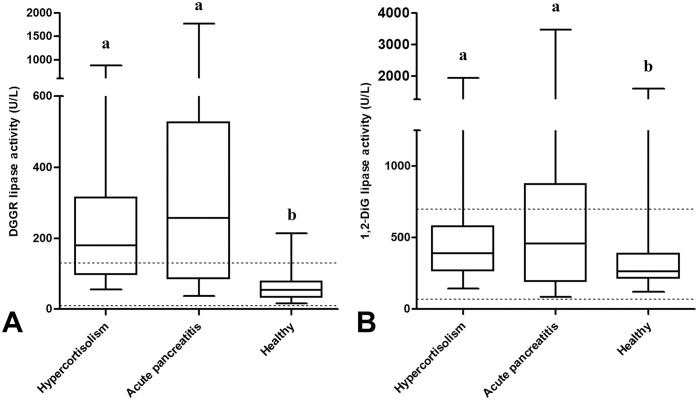
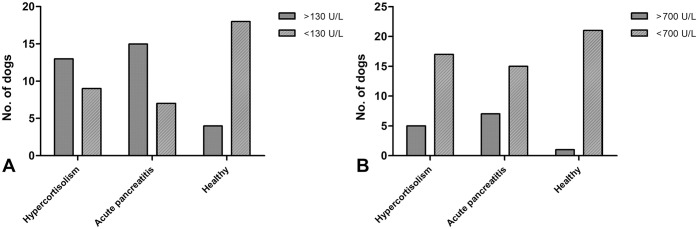
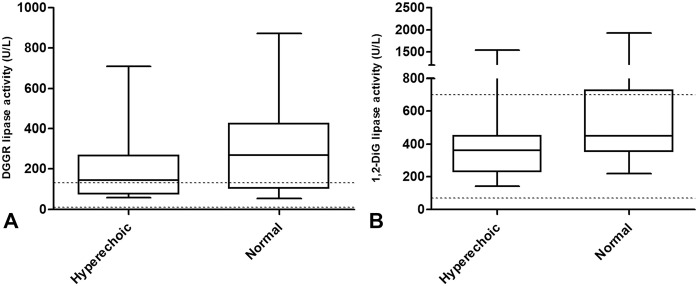
The median DGGR lipase activity was 180 U/L (range: 55–874; RI: 10–118 U/L), 258 U/L (range: 37–1770; RI: 10–118 U/L), and 54 U/L (range: 16–214; RI: 10–118 U/L) in the dogs with HC, those with AP, and the healthy dogs, respectively (Fig. 1A, Table 1) with activities above the RI in 14 of 22 (64%), 16 of 22 (73%), and 4 of 22 (18%), respectively (Fig. 2A, Table 1). The median DGGR lipase activity was significantly different between the healthy dogs and the other 2 groups; no difference was detected between the dogs with HC and those with AP (p = 0.32; Fig. 1A). In the dogs with HC, the DGGR lipase activity was not different (p = 0.25) between the dogs with hyperechoic pancreatic parenchyma and those with normal pancreatic parenchyma (Fig. 3A). The median DGGR lipase activity in dogs with HC, excluding dogs with hyperechoic pancreatic parenchyma, was significantly different compared with healthy dogs (p < 0.01) but was not different from dogs with AP (p = 0.87).
Figure 1.
A. 1,2-O-dilauryl-rac-glycero glutaric acid-(6′-methylresorufin) ester (DGGR), and B. 1,2-diglyceride (1,2-DiG) serum lipase activity in dogs with hypercortisolism, acute pancreatitis, and healthy dogs. Different lowercase letters indicate significant differences between groups (p < 0.05). The horizontal dotted lines indicate reference intervals.
Table 1.
Median (range) serum concentrations and number of dogs with 1,2-O-dilauryl-rac-glycero glutaric acid-(6′-methylresorufin) ester (DGGR) and 1,2-diglyceride (1,2-DiG) lipase activity higher than previously established reference intervals (RIs) in 22 healthy dogs, 22 dogs with hypercortisolism, and 22 dogs with acute pancreatitis.
| Hypercortisolism | Acute pancreatitis | Healthy dogs | RI | |
|---|---|---|---|---|
| Median DGGR lipase activity (U/L) | 180* (55–874) | 258* (37–1770) | 54 (16–214) | 10–118 |
| n (%) with DGGR > RI | 14 (64%) | 16 (73%) | 4 (18%) | |
| Median 1,2-DiG lipase activity (U/L) | 392† (144–1930) | 460‡ (84–3470) | 264 (120–1590) | 81–582 |
| n (%) with 1,2-DiG > RI | 5 (23%) | 8 (36%) | 1 (5%) |
Significantly different from healthy dogs (p < 0.01).
Significantly different from healthy dogs (p < 0.03).
Significantly different from healthy dogs (p < 0.04).
Figure 2.
Grouped bar charts representing the canine serum samples in each group with A. 1,2-O-dilauryl-rac-glycero glutaric acid-(6′-methylresorufin) ester (DGGR) and B. 1,2-diglyceride (1,2-DiG) lipase activity below or above the upper reference interval.
Figure 3.
A. 1,2-O-dilauryl-rac-glycero glutaric acid-(6′-methylresorufin) ester (DGGR) and B. 1,2-diglyceride (1,2-DiG) serum lipase activity in dogs with hypercortisolism with ultrasonographical evidence of hyperechoic or normal pancreatic parenchyma. The horizontal dotted lines indicate reference intervals. Neither DGGR (p = 0.25) nor 1,2-DiG (p = 0.20) serum lipase activities were significantly different between the 2 groups.
The median 1,2-DiG lipase activity was 392 U/L (range: 144–1930; RI: 81–582 U/L), 460 U/L (range: 84–3470; RI: 81–582 U/L), and 264 U/L (range: 120–1590; RI: 81–582 U/L) in the dogs with HC, those with AP, and the healthy dogs, respectively (Fig. 1B, Table 1), with activities above the RI in 5 of 22 (23%), 8 of 22 (36%), and 1 of 22 (5%), respectively (Fig. 2B, Table 1). The median 1,2-DiG lipase activity was significantly different between the healthy dogs and the other 2 groups; no difference was detected between the dogs with HC and those with AP (p = 0.60; Fig. 1B). In dogs with HC, 1,2-DiG lipase activity was not different (p = 0.20) between the dogs with hyperechoic pancreatic parenchyma and those with normal pancreatic parenchyma (Fig. 3B). The median 1,2-DiG lipase activity in dogs with HC, excluding dogs with hyperechoic pancreatic parenchyma, was significantly different when compared with healthy dogs (p < 0.01), but was not different when compared with dogs having AP (p = 0.92). The median DGGR and 1,2-DiG lipase activity was not significantly different between samples stored <1 y and samples stored for >1 y (Table 2).
Table 2.
Stability of 1,2-O-dilauryl-rac-glycero glutaric acid-(6′-methylresorufin) ester (DGGR) and 1,2-diglyceride (1,2-DiG) lipase activity in canine serum samples stored for >1 y or <1 y.
| DGGR lipase activity | 1,2-DiG lipase activity | |||||
|---|---|---|---|---|---|---|
| >1 y median (min.-max.) | <1 y median (min.-max.) | p | >1 y median (min.-max.) | <1 y median (min.-max.) | p | |
| Hypercortisolism (n = 22) | 237 (103–874) (n = 10) | 120 (55–710) (n = 12) | 0.25 | 392 (250–1,930) (n = 10) | 399 (144–1,550) (n = 12) | 0.57 |
| Acute pancreatitis (n = 22) | 510 (80–1,770) (n = 8) | 223 (37–633) (n = 14) | 0.06 | 834 (135–3,470) (n = 8) | 445 (84–928) (n = 14) | 0.09 |
| Healthy (n = 22) | 48 (29–214) (n = 8) | 63 (16–209) (n = 14) | 0.81 | 258 (138–423) (n = 8) | 264 (120–1,590) (n = 14) | 0.71 |
| All dogs (n = 66) | 189 (29–1,770) (n = 26) | 98 (16–710) (n = 40) | 0.17 | 392 (135–3,470) (n = 26) | 345 (84–1,590) (n = 40) | 0.38 |
Discussion
Our results did not identify a difference in DGGR lipase activity between dogs with HC but without clinical or ultrasonographic signs compatible with AP and dogs with AP. Our finding suggests that this enzymatic assay had low specificity for diagnosing AP in dogs with HC. A similar result was reported in a study that evaluated different LDDst patterns and found that 48% of dogs with HC had increased DGGR lipase activity.3
An increase in lipase activity, even when measured using 1,2-DiG substrates, was reported in dogs with either naturally occurring HC or in those treated with exogenous steroids.13,18,36 We used the same assay used in previous studies to measure 1,2-DiG, and our dogs with HC did not have different results compared with those with AP, with 23% and 36% having elevated 1,2-DiG lipase activity, respectively. These findings were in accordance with previous publications and confirmed the poor clinical usefulness of measuring 1,2-DiG lipase activity in diagnosing AP.33
Whether exogenous steroids have a role in influencing cPLI concentrations has not been clarified; previous studies have reported conflicting results. One study found no difference in serum cPLI concentrations after treating healthy dogs for 28 d with peroral prednisone (2.2 mg/kg/d).32 A research group treated 6 healthy Beagle dogs for 2 or 3 wk with prednisolone subcutaneously (4 mg/kg/d) and reported a significant increase in cPLI concentrations; just one dog had an abnormal concentration, and none developed histologic alterations consistent with AP.25 Those authors interpreted their results, which were in contrast with those published earlier,32 as probably the result of the higher doses and the higher bioavailability resulting from the subcutaneous route of administration and the use of prednisolone instead of peroral prednisone.25 However, more studies are needed to evaluate the influence of exogenous steroids on cPLI. The same authors suggested that, in the absence of clinical or histologic evidence of AP, pancreatic injury is probably not responsible for these abnormalities, as occurs in AP or chronic pancreatitis (CP); however, increased lipase synthesis or cellular permeability to lipases could be induced directly or indirectly by steroids.17,38 A cPLI elevation (≥400 µg/L) was reported in 35% of the dogs with HC, and one explanation proposed was the presence of subclinical CP.21
A study evaluated the ultrasonographic appearance of pancreatitis in dogs with HC compared to healthy dogs.9 Of the dogs diagnosed with HC, 40% had a hyperechoic pancreas compared with only 7% of healthy dogs. The authors considered CP to be one of the main suspects for this ultrasonographic appearance, mainly because of difficulties in reaching a diagnosis for this subclinical disease, which usually progresses undiagnosed, and given the postmortem evidence confirming the high prevalence of CP in dogs.24 Other possible explanations proposed were benign fat deposition or pancreatic mineralization.9 None of these hypotheses were evaluated and confirmed with histologic examination. Our findings were similar; 55% of our dogs at the time of diagnosis of HC had hyperechoic pancreatic parenchyma. The activity of both DGGR and 1,2-DiG lipase was evaluated in the dogs having hyperechoic pancreatic parenchyma; they were compared to those having a normal pancreas ultrasonographically, but the difference was not significant.
A study retrospectively evaluated clinical and clinicopathologic findings in dogs with a histologic diagnosis of CP. The findings supported an association between CP and AP; the main hypothesis was that CP is most likely a frequent consequence of the recurrent pancreatic injuries associated with AP.4 Considering that AP was associated multiple times with HC in dogs,11,15 it is possible that CP could have a higher prevalence than expected in dogs with HC.
Studies evaluating the pancreatic histology and prevalence of CP in dogs with HC are lacking. In one study that evaluated 61 dogs with a histologic diagnosis of CP, only 1 dog had HC.4 Hyperlipidemia is a common feature in both AP and HC.1,11,14,15 Various studies have documented an association between hypertriglyceridemia and increased cPLI39,45; however, whether pancreatitis is the cause or the effect of hyperlipidemia is not clear. In one study, dogs with a history of an episode of pancreatitis had significantly higher serum triglyceride concentrations than dogs without such a history, suggesting that hypertriglyceridemia is a possible risk factor for pancreatitis.44 Another study reported that, after excluding patients with secondary hyperlipidemia, >70% of the dogs with AP had triglyceride and cholesterol concentrations within RIs, supporting the hypothesis that hyperlipidemia is not a common consequence of AP.41 The data available from the dogs with HC enrolled in our study were insufficient to evaluate an association between DGGR lipase activity elevation and dyslipidemia.
Glomerular filtration rate (GFR) reduction has been reported to influence lipase activity.20,22,31 Dogs with HC usually have an increased GFR30; however, we excluded dogs with a high serum creatinine concentration from our study. For these reasons, it is unlikely that a GFR reduction would have played a role in the increased lipase activity in the dogs with HC in our study. The main limitation of our study was that only the absence of clinical signs and ultrasonographic findings were used to exclude the presence of AP in dogs with HC. A few studies have evaluated the sensitivity of ultrasonography in diagnosing AP, and sensitivity was <70%.11,40 These results were obtained years ago and, considering scientific and technologic progress, actual sensitivity has probably improved.42 Nevertheless, combining ultrasonography with clinical signs made AP unlikely in the dogs with HC in our study. Abdominal ultrasound was not carried out in our healthy dogs; however, we excluded dogs with clinicopathologic or clinical signs compatible with AP. Although histopathology has some limitations, it is still considered the gold standard for diagnosing AP and was not performed in the dogs in our study.
The stability of stored canine serum samples at −20°C for a maximum of 20 d was demonstrated as acceptable for DGGR measurement.8 Similarly for 1,2-DiG, precision was good after storage of canine serum samples for a maximum of 2 mo at −29°C.19 According to the manufacturers, DGGR and 1,2-DiG lipase activity measurements are stable in serum samples if stored for 1 y at −20°C. In our study, samples were stored at −20°C, but some of them were collected ~3 y before analysis. The absence of any difference in DGGR and 1,2-DiG lipase activity when comparing recent samples (<1 y) with older ones (>1 y) supports the hypothesis that the storage period did not significantly influence the results in our study.
Our results support other studies indicating that DGGR lipase activity is frequently elevated in dogs with HC, making the test not clinically useful in these patients when AP is suspected. The presence of specific mechanisms or complications, secondary to HC, responsible for lipase activity elevation were not clarified. Pancreatic histopathology together with imaging evaluations in dogs with HC could help in estimating the real prevalence of parenchymal alterations, such as CP, increasing the possibility of understanding their role in lipase activity abnormalities.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest concerning the research, authorship, and publication of this article.
Funding: Our study was funded by the Department of Veterinary Medical Sciences of the University of Bologna.
ORCID iDs: Rodolfo O. Leal  https://orcid.org/0000-0002-2463-4062
https://orcid.org/0000-0002-2463-4062
Federico Fracassi  https://orcid.org/0000-0003-3121-2199
https://orcid.org/0000-0003-3121-2199
Contributor Information
Guido Linari, Department of Veterinary Medical Sciences, University of Bologna, Ozzano dell’Emilia, Italy.
Francesco Dondi, Department of Veterinary Medical Sciences, University of Bologna, Ozzano dell’Emilia, Italy.
Sofia Segatore, Department of Veterinary Medical Sciences, University of Bologna, Ozzano dell’Emilia, Italy.
Kateryna Vasylyeva, Department of Veterinary Medical Sciences, University of Bologna, Ozzano dell’Emilia, Italy.
Nikolina Linta, Department of Veterinary Medical Sciences, University of Bologna, Ozzano dell’Emilia, Italy.
Marco Pietra, Department of Veterinary Medical Sciences, University of Bologna, Ozzano dell’Emilia, Italy.
Rodolfo O. Leal, CIISA–Centro de Investigação Interdisciplinare m Sanidade Animal, Faculdade de Medicina Veterinária, Universidade de Lisboa, Lisbon, Portugal
Federico Fracassi, Department of Veterinary Medical Sciences, University of Bologna, Ozzano dell’Emilia, Italy.
References
- 1.Bauer JE.Lipoprotein-mediated transport of dietary and synthesized lipids and lipid abnormalities of dogs and cats. J Am Vet Med Assoc 2004;224:668–675. [DOI] [PubMed] [Google Scholar]
- 2.Beall MJ, et al. Performance validation and method comparison of an inclinic enzyme-linked immunosorbent assay for the detection of canine pancreatic lipase. J Vet Diagn Invest 2011;23:115–119. [DOI] [PubMed] [Google Scholar]
- 3.Bennaim M, et al. Evaluation of individual low-dose dexamethasone suppression test patterns in naturally occurring hyperadrenocorticism in dogs. J Vet Intern Med 2018;32:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostrom BM, et al. Chronic pancreatitis in dogs: a retrospective study of clinical, clinicopathological, and histopathological findings in 61 cases. Vet J 2013;195:73–79. [DOI] [PubMed] [Google Scholar]
- 5.Cridge H, et al. Evaluation of SNAP cPL, Spec cPL, VetScan cPL Rapid Test, and precision PSL assays for the diagnosis of clinical pancreatitis in dogs. J Vet Intern Med 2018;32:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fittschen C, et al. Prednisone treatment alters the serum amylase and lipase activities in normal dogs without causing pancreatitis. Can J Comp Med 1984;48:136–140. [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrichs KR, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012;41: 441–453. [DOI] [PubMed] [Google Scholar]
- 8.Graca R, et al. Validation and diagnostic efficacy of a lipase assay using the substrate 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin)-ester for the diagnosis of acute pancreatitis in dogs. Vet Clin Pathol 2005;34:39–43. [DOI] [PubMed] [Google Scholar]
- 9.Granger LA, et al. Variability in the ultrasonographic appearance of the pancreas in healthy dogs compared to dogs with hyperadrenocorticism. Vet Radiol Ultrasound 2015;56:540–548. [DOI] [PubMed] [Google Scholar]
- 10.Hecht S, Henry G.Sonographic evaluation of the normal and abnormal pancreas. Clin Tech Small Anim Pract 2007;22:115–121. [DOI] [PubMed] [Google Scholar]
- 11.Hess RS, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in dogs with fatal acute pancreatitis: 70 cases (1986–1995). J Am Vet Med Assoc 1998;213:665–670. [PubMed] [Google Scholar]
- 12.Huth SP, et al. Analytical validation of an ELISA for measurement of canine pancreas-specific lipase. Vet Clin Pathol 2010;39:346–353. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs RM, et al. Review of the clinicopathological findings of acute pancreatitis in the dog: use of an experimental model. J Am Anim Hosp Assoc 1985;21:795–800. [Google Scholar]
- 14.Johnson MC.Hyperlipidemia disorders in dogs. Compend Contin Educ Pract Vet 2005;27:361–364. [Google Scholar]
- 15.Kim H, et al. Evaluation of hypertriglyceridemia as a mediator between endocrine diseases and pancreatitis in dogs. J Am Anim Hosp Assoc 2019;55:92–100. [DOI] [PubMed] [Google Scholar]
- 16.Kook PH, et al. Agreement of serum Spec cPL with the 1,2-o-dilauryl-rac-glycero glutaric acid-(6′-methylresorufin) ester (DGGR) lipase assay and with pancreatic ultrasonography in dogs with suspected pancreatitis. J Vet Intern Med 2014;28:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kullman J, et al. Dexamethasone-regulated expression of pancreatic lipase and two related proteins in AR42J cells. Am J Physiol 1996;270:G746–751. [DOI] [PubMed] [Google Scholar]
- 18.Ling GV, et al. Canine hyperadrenocorticism: pretreatment clinical and laboratory evaluation of 117 cases. J Am Vet Med Assoc 1979;174:1211–1215. [PubMed] [Google Scholar]
- 19.Mackenzie AL, et al. Evaluation of an automated colorimetric assay for the measurement of lipase activity in canine sera. Can J Vet Res 1996;60:205–209. [PMC free article] [PubMed] [Google Scholar]
- 20.Mansfield CS, et al. Plasma and urinary trypsinogen activation peptide in healthy dogs, dogs with pancreatitis and dogs with other systemic diseases. Aust Vet J 2000;78:416–422. [DOI] [PubMed] [Google Scholar]
- 21.Mawby DI, et al. Canine pancreatic-specific lipase concentrations in clinically healthy dogs and dogs with naturally occurring hyperadrenocorticism. J Vet Intern Med 2014;28:1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCord K, et al. A multi-institutional study evaluating the diagnostic utility of the Spec cPLTM and SNAP® cPLTM in clinical acute pancreatitis in 84 dogs. J Vet Intern Med 2012;26:888–896. [DOI] [PubMed] [Google Scholar]
- 23.Neilson-Carley SC, et al. Specificity of a canine pancreas-specific lipase assay for diagnosing pancreatitis in dogs without clinical or histologic evidence of the disease. Am J Vet Res 2011;72:302–307. [DOI] [PubMed] [Google Scholar]
- 24.Newman S, et al. Localization of pancreatic inflammation and necrosis in dogs. J Vet Intern Med 2004;18:488–493. [DOI] [PubMed] [Google Scholar]
- 25.Ohta H, et al. Effects of immunosuppressive prednisolone therapy on pancreatic tissue and concentration of canine pancreatic lipase immunoreactivity in healthy dogs. Can J Vet Res 2018;82:278–286. [PMC free article] [PubMed] [Google Scholar]
- 26.Panteghini M, et al. Measurement of pancreatic lipase activity in serum by a kinetic colorimetric assay using a new chromogenic substrate. Ann Clin Biochem 2001;38:365–370. [DOI] [PubMed] [Google Scholar]
- 27.Parent J.Effects of dexamethasone on pancreatic tissue and on serum amylase and lipase activities in dogs. J Am Vet Med Assoc 1982;180:743–746. [PubMed] [Google Scholar]
- 28.Perman V, Stevens JB.Clinical evaluation of the acinar pancreas of the dog. J Am Vet Med Assoc 1969;155:2052–2058. [PubMed] [Google Scholar]
- 29.Pratschke KM, et al. Pancreatic surgical biopsy in 24 dogs and 19 cats: postoperative complications and clinical relevance of histological findings. J Small Anim Pract 2014;56:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smets PM, et al. Long-term follow-up of renal function in dogs after treatment for ACTH-dependent hyperadrenocorticism. J Vet Intern Med 2012;26:565–573. [DOI] [PubMed] [Google Scholar]
- 31.Steiner JM, et al. Serum lipase activity and canine pancreatic lipase immunoreactivity (cPLI) concentration in dogs with experimentally induced chronic renal failure. Vet Res 2010;3:58–63. [Google Scholar]
- 32.Steiner JM, et al. Stability of canine pancreatic lipase immunoreactivity concentration in serum samples and effects of long-term administration of prednisone to dogs on serum canine pancreatic lipase immunoreactivity concentrations. Am J Vet Res 2009;70:1001–1005. [DOI] [PubMed] [Google Scholar]
- 33.Steiner JM, et al. Sensitivity of serum markers for pancreatitis in dogs with macroscopic evidence of pancreatitis. Vet Ther 2008;9:263–273. [PubMed] [Google Scholar]
- 34.Steiner JM, et al. Serum lipase activities and pancreatic lipase immunoreactivity concentrations in dogs with exocrine pancreatic insufficiency. Am J Vet Res 2006;67:84–87. [DOI] [PubMed] [Google Scholar]
- 35.Steiner JM, et al. Development and analytic validation of an enzyme-linked immunosorbent assay for the measurement of canine pancreatic lipase immunoreactivity in serum. Can J Vet Res 2003;67:175–182. [PMC free article] [PubMed] [Google Scholar]
- 36.Steiner JM, Williams DA.Development and validation of a radioimmunoassay for the measurement of canine pancreatic lipase immunoreactivity in serum of dogs. Am J Vet Res 2003;64:1237–1241. [DOI] [PubMed] [Google Scholar]
- 37.Strombeck DR, et al. Serum amylase and lipase activities in the diagnosis of pancreatitis in dogs. Am J Vet Res 1981;42:1966–1970. [PubMed] [Google Scholar]
- 38.Swarovsky B, et al. Coupled induction of exocrine proteins and intracellular compartments involved in the secretory pathway in AR4-2J cells by glucocorticoids. Eur J Cell Biol 1988;47:101–111. [PubMed] [Google Scholar]
- 39.Verkest KR, et al. Association of postprandial serum triglyceride concentration and serum canine pancreatic lipase immunoreactivity in overweight and obese dogs. J Vet Intern Med 2012;26:46–53. [DOI] [PubMed] [Google Scholar]
- 40.Watson PJ, et al. Observational study of 14 cases of chronic pancreatitis in dogs. Vet Rec 2010;167:968–976. [DOI] [PubMed] [Google Scholar]
- 41.Xenoulis PG.Serum triglyceride and cholesterol concentrations and lipoprotein profiles in dogs with naturally occurring pancreatitis and healthy control dogs. J Vet Intern Med 2020;34:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xenoulis PG.Diagnosis of pancreatitis in dogs and cats. J Small Anim Pract 2015;56:13–26. [DOI] [PubMed] [Google Scholar]
- 43.Xenoulis PG, Steiner JM.Canine and feline pancreatic lipase immunoreactivity. Vet Clin Pathol 2012;41:312–324. [DOI] [PubMed] [Google Scholar]
- 44.Xenoulis PG, et al. Serum triglyceride concentrations in Miniature Schnauzers with and without a history of probable pancreatitis. J Vet Intern Med 2011;25:20–25. [DOI] [PubMed] [Google Scholar]
- 45.Xenoulis PG, et al. Association between serum triglyceride and canine pancreatic lipase immunoreactivity concentrations in miniature schnauzers. J Am Anim Hosp Assoc 2010;46:229–234. [DOI] [PubMed] [Google Scholar]