Abstract
Canine parvoviral enteritis (CPE) is a severe disease characterized by systemic inflammation and immunosuppression. The function of circulating phagocytes (neutrophils and monocytes) in affected dogs has not been fully investigated. We characterized the functional capacity of canine phagocytes in CPE by determining their oxidative burst and phagocytic activities using flow cytometry. Blood was collected from 28 dogs with CPE and 11 healthy, age-matched, control dogs. Oxidative burst activity was assessed by stimulating phagocytes with opsonized Escherichia coli or phorbol 12-myristate 13-acetate (PMA) and measuring the percentage of phagocytes producing reactive oxygen species and the magnitude of this production. Phagocytosis was measured by incubating phagocytes with opsonized E. coli and measuring the percentage of phagocytes containing E. coli and the number of bacteria per cell. Complete blood counts and serum C-reactive protein (CRP) concentrations were also determined. Serum CRP concentration was negatively and positively correlated with segmented and band neutrophil concentrations, respectively. Overall, no differences in phagocyte function were found between dogs with CPE and healthy control dogs. However, infected dogs with neutropenia or circulating band neutrophils had decreased PMA-stimulated oxidative burst activity compared to healthy controls. Additionally, CPE dogs with neutropenia or circulating band neutrophils had decreased PMA- and E. coli–stimulated oxidative burst activity and decreased phagocytosis of E. coli compared to CPE dogs without neutropenia or band neutrophils. We conclude that phagocytes have decreased oxidative burst and phagocytic activity in neutropenic CPE dogs and in CPE dogs with circulating band neutrophils.
Keywords: dogs, flow cytometry, inflammation, phagocyte, phagocytosis, respiratory burst
Canine parvoviral enteritis (CPE) is a potentially fatal viral disease affecting young dogs with naïve immune systems.17,26 Canine parvovirus 2 (CPV2; Carnivore protoparvovirus 1) infects rapidly dividing cells of the gastrointestinal tract and lymphohematopoietic system, causing gastrointestinal and immunosuppressive disease.17,42 Consequently, both systemic inflammatory response syndrome (SIRS) and sepsis are common complications of CPE.26,29
Blood phagocytes (neutrophils and monocytes) play an important role in preventing sepsis through a number of functions, including phagocytosis and the production of reactive oxygen species (ROS).8 Phagocytosis is triggered by the activation of surface pattern recognition receptors, resulting in internalization of antigens within phagosomes.3 These antigens are subsequently destroyed within the phagosome through oxygen-dependent (ROS production) and oxygen-independent (antimicrobial peptides and enzymes) mechanisms.13,48 This process of ROS production is known as oxidative or respiratory burst and is not only triggered by phagocytosed particulate stimuli (e.g., opsonized bacteria) but also by soluble stimuli (e.g., chemotactic peptides).13 If these functions are impaired or if circulating phagocyte numbers are decreased, the risk of sepsis increases.1,28
Although neutropenia and monocytopenia are well described in CPE,17,26 evaluation of phagocyte function in affected dogs is limited. To our knowledge, only one unpublished study in dogs infected with CPV2 demonstrated decreased neutrophil oxidative burst activity.18 Decreased phagocyte and neutrophil oxidative burst activities have been reported in dogs with critical illness and sepsis.23,47 In human medicine, phagocyte dysfunction has been described in septic children11 and adults9,27 as well as in animal models of sepsis.10,15 Phagocyte dysfunction is considered a negative prognostic indicator9 and may be progressive as the severity of sepsis increases.27 Similarly, significant phenotypic and functional alterations of phagocytes have been described in SIRS, resulting in an increase or decrease in oxidative burst and phagocytic activities.37
We aimed to characterize the functional capacity of phagocytes in dogs with CPE by measuring their oxidative burst and phagocytic activities using flow cytometry. We hypothesized that dogs with CPE would have decreased phagocyte function compared to apparently healthy, age-matched control dogs.
Materials and methods
Animals and study design
Our prospective, observational study was approved by the Research Ethics Committee of the Faculty of Veterinary Science (REC039-18) and Animal Ethics Committee of the University of Pretoria (V048-19). Client-owned dogs presented to the Onderstepoort Veterinary Academic Hospital were eligible for inclusion if they were 2–12 mo old, weighed ≥3 kg, and showed signs of gastrointestinal disease (vomiting, diarrhea, anorexia, or hyporexia). Dogs were included if a valid, rapid patient-side immunoassay (Snap canine parvovirus antigen test kit; Idexx) for fecal CPV2 antigen tested positive and if CPV2 viral particles were visualized by transmission electron microscopy (TEM). Dogs were excluded if TEM was negative, if comorbidities were identified by thorough physical examination or clinicopathologic data, or if there was a history of treatment or hospitalization within a week prior to presentation.
A group of apparently healthy, staff- and client-owned dogs, presented for elective sterilization or vaccination, were selected as controls and were age-matched to the study population. These dogs were considered healthy based on physical examination, fecal flotation and TEM, and complete blood count (CBC; including blood smear evaluation) and serum C-reactive protein (CRP) concentrations. Written informed consent was obtained from all owners.
Sample collection
Blood and feces were collected once, prior to any treatment, from all dogs in the CPE group. Blood and feces were also collected from all control dogs, prior to vaccination, deworming, or administration of anesthetic agents. Blood was collected by jugular venipuncture directly into serum, lithium heparin, and EDTA vacutainer tubes (BD Biosciences). Feces was collected rectally. Serum was harvested within 3 h of collection and used to determine serum CRP concentration using a validated particle-enhanced immunoturbidimetric assay (Canine CRP immunoassay, Gentian; Cobas Integra 400 plus chemistry analyzer, Roche).22 This assay has a limit of quantification of 10 mg/L. The lithium heparin sample was used for flow cytometric analysis within 2 h of collection. The EDTA sample was used to perform a CBC (Advia 2120i hematology analyzer; Siemens), manual blood smear evaluation, and a 200-cell leukocyte differential count by experienced, registered veterinary technologists. Neutropenia and neutrophilia were identified by the laboratory reference interval (RI) as a segmented neutrophil concentration of <3 × 109/L or >11.5 × 109/L, respectively. A band (immature) neutrophil was defined as a hyposegmented neutrophil with nuclear constrictions not exceeding half of the widest part of the nucleus.43 Metamyelocytes, if present, were included in the band neutrophil count. The fecal sample was submitted for TEM to confirm the presence or absence of CPV2 in the CPE and control dogs, respectively.
Oxidative burst and phagocytic activities
Oxidative burst and phagocytic activities were determined using commercial kits (Phagoburst and Phagotest kits, respectively; Glycotope Biotechnology). Both kits have been used successfully in previous canine studies23,30,33 and were used according to the manufacturer’s instructions. Briefly, oxidative burst activity was determined by incubating 2 samples of 100 µL of precooled (0°C) heparinized whole blood, one with opsonized Escherichia coli bacteria and one with phorbol-12-myristate 13-acetate (PMA), at 37°C for 10 min. Both the PMA and opsonized E. coli were included in the kit. A negative control sample was incubated simultaneously without either stimulant. The samples were subsequently incubated for 10 min at 37°C with 20 µL of dihydrorhodamine 123, a fluorogenic substrate, which was oxidized to rhodamine 123 by the newly produced ROS. A lysing and fixing solution (2 mL) was added to halt ROS production and, after a washing step, the samples were incubated with 200 µL of propidium iodide (DNA staining solution) to exclude nonviable cells and bacterial aggregates. The samples were protected from light and analyzed by flow cytometry within 30 min.
Phagocytic activity was determined by incubating 100 µL of precooled (0°C) heparinized whole blood with fluorescein isothiocyanate (FITC)-labeled opsonized E. coli, which was included in the Phagotest kit, at 37°C for 10 min. A negative control sample with no bacteria was kept at 0°C. After incubation, the test sample was placed on ice to halt phagocytosis, and a precooled quenching solution (100 µL) was added to quench the fluorescence of surface-bound and free E. coli. After 2 washing steps, the samples were incubated with 2 mL of lysing and fixing solution. After another washing step, 200 µL of propidium iodide was added. The samples were protected from light and analyzed by flow cytometry within 60 min.
Flow cytometric analysis
The samples were analyzed using a flow cytometer (Accuri C6 plus, Accuri C6 plus software v.1.0.23.1; BD Biosciences). Using a blue laser emitting light at a wavelength of 488 nm, 10,000–15,000 viable leukocytes were acquired for each sample. A live gate was set to capture events with the same red fluorescence as a human diploid cell, thus excluding bacterial aggregates and nonviable cells. The gating strategy, as described previously,23 was as follows: the negative control samples were used to set the gate on the blue-green histogram for the fluorescence-positive population, and a forward-scatter versus side-scatter plot was used to gate the phagocytes, excluding lymphocytes (Fig. 1). To reduce the analytical variation of the assay, only the principal investigator gated the phagocyte populations, as recommended.14 The results were reported as the percentage of fluorescence-positive cells, which equates to the proportion of phagocytes containing rhodamine 123 or FITC-labeled E. coli as a measure of oxidative burst or phagocytic activity, respectively. The mean fluorescence intensity (MFI) was also reported and equates to the average magnitude of ROS production per cell when stimulated by E. coli or PMA, or the average number of E. coli phagocytized per cell.
Figure 1.
Gating strategy for measuring oxidative burst and phagocytic activities using flow cytometry. A. Live gate (red bracket) on histogram to exclude nonviable cells and bacterial aggregates (blue bracket). B. Forward-scatter (FSC) versus side-scatter (SSC) plot to gate phagocytes (red population). C. Histogram of negative control sample with <3% positive cells (inside blue bracket). D. Example of a histogram from a sample with a population of majority positive cells (inside blue bracket) and some negative cells (outside blue bracket).
FITC = fluorescein isothiocyanate.
Data analysis
Statistical analyses were performed using MedCalc v.19.5.3. Given the small sample size (n ≤ 30), nonparametric analyses were selected, and summary statistics were reported as median (interquartile range). The Mann-Whitney U-test was used to compare data between the CPE and control dogs. Additionally, neutropenic dogs and dogs with circulating band neutrophils were each compared to the control group and to CPE dogs without neutropenia or band neutrophils using the Mann–Whitney U-test. Correlations between clinicopathologic and flow cytometric data were determined using Spearman coefficient of rank correlation with 95% confidence intervals (CIs). For statistical analysis, a serum CRP concentration of 10 mg/L was assigned to dogs with results below the limit of quantification. Statistical significance was set at p ≤ 0.05.
Results
Animals
Samples were included from 28 dogs with CPE and 11 apparently healthy controls. The median age was 4 (3–6) mo for the CPE dogs and 4 (2–7.5) mo for the control dogs. There was no difference in age distribution between the 2 groups. There were 19 (68%) males and 9 (32%) females in the CPE group and 8 (73%) males and 3 (27%) females in the control group. Breeds included in the CPE group were mixed breeds (9), American Pitbull Terrier (4), Labrador Retriever (4), Boxer (2), Dachshund (2), Jack Russell Terrier (2), and other (5). Breeds included in the control group were mixed breeds (2), Boerboel (2), Dachshund (2), and other (5).
Clinicopathologic data
Compared to the control dogs, the CPE dogs had lower total leukocyte (p = 0.018), lymphocyte (p < 0.001), monocyte (p = 0.014), and eosinophil (p < 0.001) concentrations, and higher band neutrophil (p < 0.001) concentrations (Table 1). Nine (32%) of the infected dogs were neutropenic and 3 (11%) were neutrophilic. The remaining dogs with CPE and all control dogs had segmented neutrophil concentrations within the laboratory RI. Band neutrophils were identified on blood smear review in 18 (64%) of the dogs with CPE. No circulating band neutrophils were identified in any of the control dogs. The median percentage of band neutrophils was 5.9% (2–15.6%), and the proportion of band neutrophils never exceeded the proportion of segmented neutrophils. All neutropenic dogs with CPE had band neutrophils on blood smear review. The serum CRP concentration was higher in the infected dogs compared to healthy control dogs (p < 0.001; Table 1). Serum CRP concentration was also higher in neutropenic CPE dogs compared to non-neutropenic CPE dogs (p = 0.011), as well as in CPE dogs with circulating band neutrophils compared to those CPE dogs without (p = 0.004). Serum CRP concentration was negatively correlated with the segmented neutrophil concentration (rs = −0.472, 95% CI = −0.719 to −0.120, p = 0.011) and positively correlated with the band neutrophil concentration (rs = 0.678, 95% CI = 0.408–0.839, p < 0.001).
Table 1.
Median values and comparison of select clinicopathologic data between dogs with canine parvoviral enteritis (CPE) and control dogs.
| Clinicopathologic data | Unit | Dogs with CPE | Control dogs | p |
|---|---|---|---|---|
| Total leukocytes | ×109/L | 6.8 (3.4–10.9) | 11.3 (10.3–12.9) | .018 |
| Segmented neutrophils | ×109/L | 4.7 (2.6–10.0) | 7.2 (5.4–8.0) | .533 |
| Band neutrophils | ×109/L | 0.1 (0.0–0.2) | 0.0 (0.0) | <.001 |
| Lymphocytes | ×109/L | 0.7 (0.4–1.1) | 3.9 (2.6–4.3) | <.001 |
| Monocytes | ×109/L | 0.3 (0.2–0.6) | 0.7 (0.6–0.7) | .014 |
| Eosinophils | ×109/L | 0.1 (0.0–0.8) | 0.5 (0.2–0.8) | <.001 |
| Serum C-reactive protein | mg/L | 181 (92–201) | 10 (10–11) | <.001 |
Interquartile range in parentheses.
Flow cytometric data
In 2 dogs with CPE, PMA failed to stimulate oxidative burst, but opsonized E. coli was successful, indicating an error in the test procedure. These 2 failed PMA analyses were excluded from statistical analysis. Overall, there was no difference for all measures of oxidative burst and phagocytic activity between dogs with CPE and control dogs. These measures included the percentage and MFI of fluorescence-positive cells when ROS production was stimulated by E. coli or PMA and when phagocytosis was stimulated by E. coli. However, infected dogs with neutropenia (p < 0.001), or with circulating band neutrophils (p = 0.007), had lower percentages of positive phagocytes with PMA stimulation of ROS production compared to the healthy control dogs (Fig. 2). None of the measures of phagocyte function, using E. coli as a stimulant for oxidative burst or phagocytosis, were different for the neutropenic CPE dogs or the CPE dogs with circulating band neutrophils compared to the healthy control dogs.
Figure 2.

Boxplot comparing the percentage of positive phagocytes undergoing phorbol 12-myristate 13-acetate (PMA)-stimulated oxidative burst between control dogs, neutropenic (NP) dogs with canine parvoviral enteritis (CPE), and CPE dogs with circulating band neutrophils (Band). The box represents the interquartile range, and the bottom and top box margins represent the 25th and 75th interquartiles, respectively; the central line represents the median; the bottom and top whiskers represent the range of the data; and the solid dots represent cases.
Compared to CPE dogs without neutropenia, neutropenic CPE dogs exhibited decreased oxidative burst activity in terms of the percentage of positive phagocytes with PMA (p < 0.001) and E. coli (p = 0.021) stimulation and the MFI (magnitude of ROS production) with PMA stimulation (p = 0.003; Fig. 3). Additionally, the percentage of cells phagocytizing E. coli was decreased (p = 0.006) but the MFI (number of bacteria per cell) was increased (p = 0.041) in neutropenic dogs compared to non-neutropenic dogs (Fig. 4).
Figure 3.
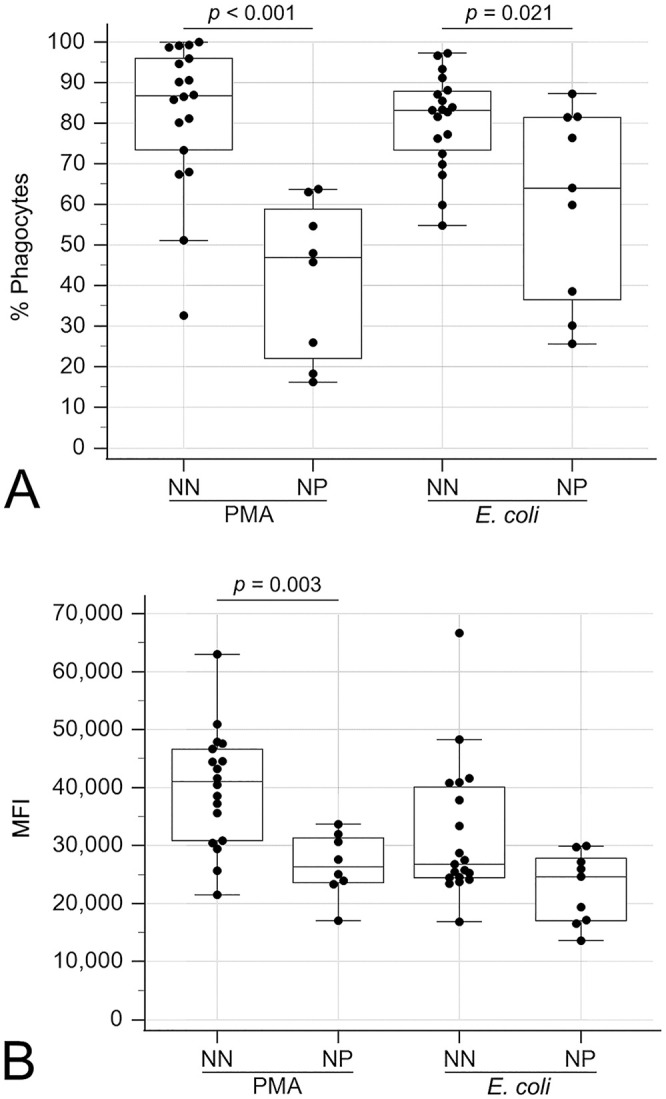
Boxplots comparing oxidative burst activities between neutropenic (NP) and non-neutropenic (NN) dogs with canine parvoviral enteritis. A. Percentage of positive phagocytes undergoing oxidative burst with phorbol 12-myristate 13-acetate (PMA) or Escherichia coli stimulation. B. Mean fluorescence intensity (MFI) of positive phagocytes undergoing oxidative burst with PMA or E. coli stimulation. The box represents the interquartile range, and the bottom and top box margins represent the 25th and 75th interquartiles, respectively; the central line represents the median; the bottom and top whiskers represent the range of the data; and the symbols represent cases, including outliers that are outside the whiskers.
Figure 4.

Boxplots comparing phagocytosis between neutropenic (NP) and non-neutropenic (NN) dogs with canine parvoviral enteritis. A. Percentage of cells phagocytizing opsonized Escherichia coli. B. Mean fluorescence intensity (MFI) of cells phagocytizing opsonized E. coli. The box represents the interquartile range, and the bottom and top box margins represent the 25th and 75th interquartiles, respectively; the central line represents the median; the bottom and top whiskers represent the range of the data; and the symbols represent cases, including outliers that are outside the whiskers.
Similarly, CPE dogs with circulating band neutrophils had decreased oxidative burst activity compared to those without circulating band neutrophils, as measured by the percentage of positive phagocytes with PMA (p = 0.001) and E. coli (p = 0.006) stimulation and the MFI with PMA stimulation (p = 0.004; Fig. 5). The percentage of cells phagocytizing E. coli was also lower (p = 0.005) in CPE dogs with circulating bands compared to those without (Fig. 6).
Figure 5.
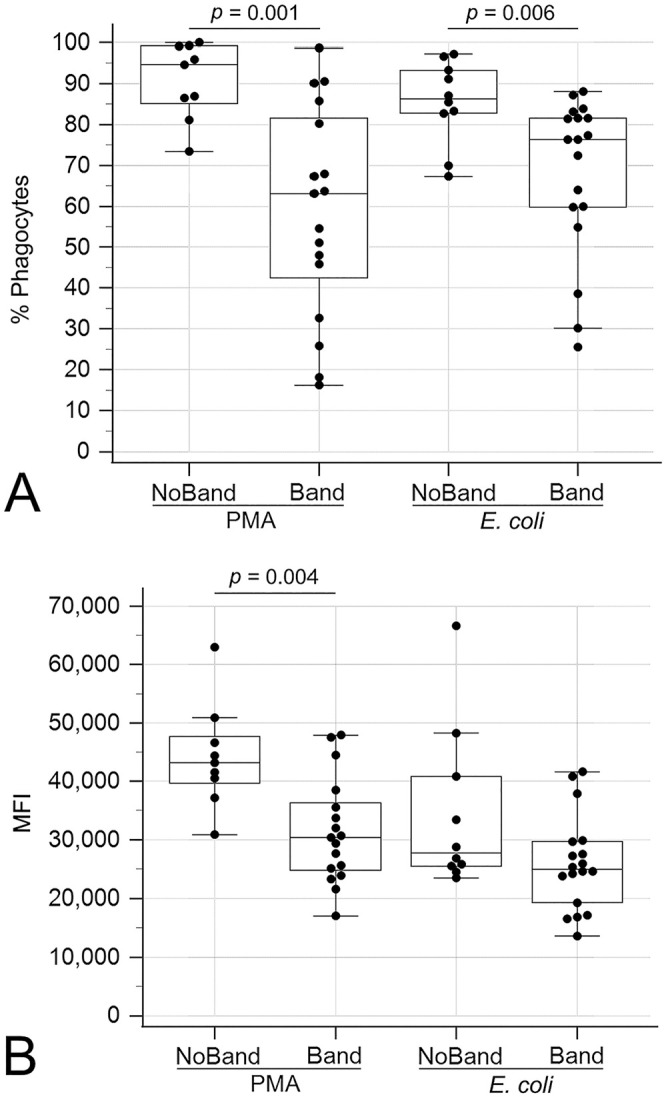
Boxplots comparing oxidative burst activities between dogs suffering from canine parvoviral enteritis with circulating band neutrophils (Band) and without circulating band neutrophils (NoBand). A. Percentage of positive phagocytes undergoing oxidative burst with phorbol 12-myristate 13-acetate (PMA) or Escherichia coli stimulation. B. Mean fluorescence intensity (MFI) of positive phagocytes undergoing oxidative burst with PMA or E. coli stimulation. The box represents the interquartile range, and the bottom and top box margins represent the 25th and 75th interquartiles, respectively; the central line represents the median; the bottom and top whiskers represent the range of the data; and the symbols represent cases, including outliers that are outside the whiskers.
Figure 6.

Boxplot comparing the percentage of cells phagocytizing opsonized Escherichia coli between dogs suffering from canine parvoviral enteritis with circulating band neutrophils (Band) and without circulating band neutrophils (NoBand). The box represents the interquartile range, and the bottom and top box margins represent the 25th and 75th interquartiles, respectively; the central line represents the median; the bottom and top whiskers represent the range of the data; and the symbols represent cases, including outliers that are outside the whiskers.
Segmented neutrophil concentrations were positively correlated with the percentage and MFI of cells undergoing PMA-stimulated oxidative burst, the percentage of cells undergoing E. coli–stimulated oxidative burst, and the percentage of cells phagocytizing E. coli (Table 2). Segmented neutrophil concentrations were negatively correlated with the MFI of cells phagocytizing E. coli (Table 2). Band neutrophil concentrations were negatively correlated with the percentage and MFI of cells undergoing PMA-stimulated oxidative burst and the percentage of cells phagocytizing E. coli (Table 2). No correlations were found between segmented or band neutrophil concentrations and the MFI of cells undergoing E. coli–stimulated oxidative burst. Monocyte concentrations were not correlated with any measure of oxidative burst or phagocytic activity.
Table 2.
Correlations of flow cytometric variables with segmented and band neutrophil concentrations and serum C-reactive protein (CRP) concentrations in dogs with canine parvoviral enteritis (CPE).
| Flow cytometric variable | Clinicopathologic variable | Correlation coefficient (rs) | 95% CI | p |
|---|---|---|---|---|
| Oxidative burst activity | ||||
| Percentage of PMA-stimulated cells | Segmented neutrophils | 0.876 | 0.739–0.943 | <.001 |
| Band neutrophils | −0.628 | −0.817 to −0.318 | <.001 | |
| Serum CRP | −0.327 | −0.634 to 0.069 | .103 | |
| MFI of PMA-stimulated cells | Segmented neutrophils | 0.622 | 0.309−0.813 | <.001 |
| Band neutrophils | −0.512 | −0.752 to −0.159 | .007 | |
| Serum CRP | −0.411 | −0.689 to −0.028 | .037 | |
| Percentage of E. coli–stimulated cells | Segmented neutrophils | 0.558 | 0.233–0.770 | .002 |
| Band neutrophils | −0.368 | −0.652 to 0.006 | .054 | |
| Serum CRP | −0.137 | −0.486 to 0.248 | .486 | |
| MFI of E. coli–stimulated cells | Segmented neutrophils | 0.249 | −0.137 to 0.569 | .201 |
| Band neutrophils | −0.176 | −0.515 to 0.211 | .371 | |
| Serum CRP | −0.273 | −0.587 to 0.111 | .159 | |
| Phagocytosis | ||||
| Percentage of cells phagocytizing E. coli | Segmented neutrophils | 0.532 | 0.199–0.755 | .004 |
| Band neutrophils | −0.439 | −0.698 to −0.079 | .020 | |
| Serum CRP | −0.158 | −0.501 to 0.229 | .423 | |
| MFI of cells phagocytizing E. coli | Segmented neutrophils | −0.577 | −0.782 to −0.260 | .001 |
| Band neutrophils | 0.123 | −0.263 to 0.474 | .534 | |
| Serum CRP | 0.373 | −0.001 to 0.655 | .051 | |
Rows with nonsignificant findings are shaded. CI = confidence interval; MFI = mean fluorescence intensity; PMA = phorbol 12-myristate 13-acetate.
Discussion
We found that canine phagocytes had decreased oxidative burst and phagocytic activity in neutropenic CPE dogs, as well as in CPE dogs with circulating band neutrophils. Most infected dogs were young, a median of 4 mo old. In humans, decreased phagocytosis of E. coli and Staphylococcus aureus has been reported in healthy, premature, and septic newborns compared to healthy adults.45 Similarly, healthy lambs and foals have reduced neutrophil oxidative burst activity when stimulated by opsonized zymosan and PMA, respectively.25,35 To our knowledge, there are no published studies investigating phagocyte function in young dogs. To mitigate the possible effect of age, we selected an age-matched control group.
The leukocyte changes seen in the dogs with CPE compared to the control dogs, most importantly the lower total leukocyte concentrations and the presence of band neutrophils, were as expected, based on previous studies.17,26 The higher serum CRP concentration observed in dogs with CPE was also expected. Increased serum CRP, a major acute-phase protein in dogs, is considered an indicator of systemic inflammation and disease severity in CPE and has moderate accuracy in predicting mortality in affected dogs.29,34 The systemic inflammation elicited by CPE results in the release of band neutrophils from the bone marrow as part of the innate immune response.21 This need for early neutrophil release is augmented by neutropenia. Neutropenia is a common, multifactorial complication in CPE caused not only by viral effects on the bone marrow but also by marked gastrointestinal inflammation and endotoxemia resulting in neutrophil margination, demand, consumption, and loss.7,17,26,36 Thus, the increased serum CRP concentrations in neutropenic CPE dogs and CPE dogs with circulating band neutrophils, as well as the positive correlation between serum CRP and band neutrophils and the negative correlation between serum CRP and segmented neutrophils, all demonstrate the expected shifting relationship among these 3 measurands secondary to the host inflammatory response.
Surprisingly, oxidative burst and phagocytic activities of the phagocytes were not different between dogs with CPE and the control dogs, likely for multifactorial reasons. First, the oxidative burst and phagocytic activities of neutrophils in healthy dogs varies widely between individuals,14 which was also evident in our study in both health and disease. Second, in humans, systemic inflammation induces functional heterogeneity among neutrophil phenotypes such that cells with differing functional capacities are simultaneously present in circulation.41 This functional heterogeneity may also exist among circulating monocyte subpopulations in sepsis.11 In our study, naturally infected dogs at various stages of disease and with various ratios of circulating band and segmented neutrophils and monocytes were included, resulting in a heterogeneous group.
Nevertheless, compared to the healthy control dogs, CPE dogs with neutropenia and/or circulating band neutrophils had decreased oxidative burst activity with PMA stimulation. Additionally, decreasing segmented neutrophil concentrations and increasing band neutrophil concentrations were correlated with decreasing PMA- and E. coli–stimulated oxidative burst activity. In humans with SIRS and sepsis, band neutrophils were less efficient at ROS production compared to mature neutrophils.12 The decreased oxidative burst activity in our study may, therefore, be secondary to increased numbers of circulating band neutrophils compared to the control dogs, which had no circulating band neutrophils. However, this is only a partial explanation, given that the median percentage of band neutrophils was only 5.9%, suggesting that the decreased oxidative burst activity is not solely the result of the presence of band neutrophils.
In dogs, 2 similar studies, one conducted in critically ill dogs23 and the other in septic dogs,47 also found a decrease in oxidative burst activity when phagocytes were stimulated by PMA and immune complexes, respectively. One study postulated that in canine critical illness, dysfunction in the protein kinase C (PKC) signaling pathway may be the reason for decreased oxidative burst activity.23 PKC is a group of serine-threonine kinases that, when activated, results in signal transduction leading to activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to produce ROS.13,46 Phorbol 12-myristate 13-acetate (PMA) is a strong stimulant of oxidative burst through activation of PKC.46 The proposed dysfunction in the PKC signaling pathway may be the result of accumulated ROS (superoxide and H2O2) attaching to NADPH oxidase subunits, decreasing their activity and, in turn, decreasing further ROS production.39 Additionally, PKC is highly susceptible to oxidation and, in a highly oxidative environment created by ROS production, modifications may occur that render PKC inactive.13,19 Lastly, endotoxin tolerance may contribute to the decreased activity. This phenomenon, described in humans and animal models, is characterized by phagocytes that are unresponsive to subsequent endotoxic insults within as little as 30 min of sustained endotoxin exposure.20,40 Endotoxin tolerance results in a multifactorial decrease in oxidative burst activity, leading to an immunosuppressive state in the patient.5
The decreased oxidative burst activity in neutropenic dogs and dogs with circulating band neutrophils, compared to healthy dogs, was further emphasized when these dogs were compared to the remaining dogs in the CPE group. Infected dogs with neutropenia or circulating band neutrophils had decreased PMA- and E. coli–stimulated oxidative burst activity as well as decreased phagocytic activity compared to those CPE dogs without neutropenia or circulating band neutrophils. This may be explained by the response of the innate immune system to systemic inflammation. Initially, pro-inflammatory cytokines predominate and receptors important for phagocytosis are upregulated.4,31,38 As the inflammation persists and increases in severity, immune modulation may result in decreased phagocyte function.5,15,27 Therefore, it is possible that the dogs without neutropenia and circulating band neutrophils had an upregulated phagocyte response, hence the difference between these dogs and those with neutropenia and circulating bands is more apparent.
In addition to the decreased phagocytosis of E. coli in CPE dogs with neutropenia or circulating band neutrophils compared to CPE dogs without neutropenia or band neutrophils, phagocytosis was correlated with neutrophil concentration. A decreasing percentage of phagocytizing cells was correlated with decreasing segmented neutrophil concentrations and increasing band neutrophil concentrations. By comparison, a study on phagocyte function in critically ill dogs23 reported no differences in the phagocytosis of E. coli between critically ill and healthy control dogs. This may have been the result of the small sample size and/or variability of diagnoses and disease states in the dogs included.23 In human studies, a decreased percentage of phagocytizing cells has been described in neutrophils exposed to S. aureus components as a model for systemic inflammation and sepsis.44 This dysfunction was associated with downregulation of complement receptors on the neutrophil surface.44 In our study, the E. coli used to stimulate phagocytosis were opsonized with antibodies and complement proteins, thus, downregulation of complement receptors on the neutrophil surface may explain our findings. Another point to consider is that immature neutrophils have reduced phagocytic activity compared to mature segmented neutrophils in healthy humans and in humans with sepsis and SIRS.16,32 Considering, again, that the median band neutrophil percentage was only 5.9% in the CPE dogs, it is unlikely that band neutrophils in circulation are the sole cause of the decreased percentage of phagocytizing cells. Interestingly, an increasing number of phagocytized E. coli per cell was correlated with decreasing segmented neutrophil concentrations. A study investigating neutrophil function in septic dogs also reported increased neutrophil MFI when phagocytizing E. coli, compared to a control group.47 This inverse correlation between phagocytized E. coli and segmented neutrophil concentrations likely reflects functional heterogeneity in the circulating phagocytes created by systemic inflammation.11,41
Our study was limited by the expense of the flow cytometric kits used. For this reason, our sample size was insufficiently powered to detect the presence of subtle changes in variables where there was a large degree of overlap between healthy and infected dogs. Based on our findings, and considering the mentioned heterogeneity of the circulating phagocytes, future studies including a large number of severely affected CPE dogs would assist in further characterization of the immunologic dysfunction present in this disease. Another limitation is that no neutrophil or monocyte markers or separation techniques were used to differentiate the leukocyte populations on flow cytometry, and we chose to report on phagocyte flow cytometric results. This approach has been followed previously in a number of veterinary studies using the same kits as in our study.2,23,24 Both neutrophils and monocytes are important cells in the innate immune system, responsible as the first line of defense in the prevention of sepsis.3,6 For this reason, a decrease in oxidative burst and/or phagocytic activities in either or both populations will increase the risk of sepsis in dogs affected by CPE.
Acknowledgments
We thank Drs. Monique Engelbrecht and Brogan Atkinson and Ms. Bronwynne Strybos for their assistance in sample collection and processing.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Our research was funded by the Health and Welfare Sector Education and Training Authority (HWSETA) in South Africa.
ORCID iDs: Kelly du Preez  https://orcid.org/0000-0003-2692-5936
https://orcid.org/0000-0003-2692-5936
Emma H. Hooijberg  https://orcid.org/0000-0002-4367-799X
https://orcid.org/0000-0002-4367-799X
Supporting data: The data for our study may be accessed on FigShare: https://figshare.com/articles/dataset/Oxidative_burst_and_phagocytic_activity_of_phagocytes_in_canine_parvoviral_enteritis/13090247
References
- 1.Ahmad M, et al. In vivo effect of recombinant human granulocyte colony-stimulating factor on phagocytic function and oxidative burst activity in septic neutropenic neonates. Biol Neonate 2004;86:48–54. [DOI] [PubMed] [Google Scholar]
- 2.Allison LN, et al. Immune function and serum vitamin D in shelter dogs: a case-control study. Vet J 2020;261:1–8. [DOI] [PubMed] [Google Scholar]
- 3.Bardoel BW, et al. The balancing act of neutrophils. Cell Host Microbe 2014;15:526–536. [DOI] [PubMed] [Google Scholar]
- 4.Bhan C, et al. Role of cellular events in the pathophysiology of sepsis. Inflamm Res 2016;65:853–868. [DOI] [PubMed] [Google Scholar]
- 5.Biswas SK, et al. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 2009;30:475–487. [DOI] [PubMed] [Google Scholar]
- 6.Censoplano N, et al. The role of the innate immune system in sepsis. Clin Pediatr Emerg Med 2014;15:169–176. [Google Scholar]
- 7.Cohn LA, et al. Plasma granulocyte colony-stimulating factor concentrations in neutropenic, parvoviral enteritis–infected puppies. J Vet Intern Med 1999;13:581–586. [DOI] [PubMed] [Google Scholar]
- 8.Dale DC, et al. The phagocytes: neutrophils and monocytes. Blood 2008;112:935–945. [DOI] [PubMed] [Google Scholar]
- 9.Danikas DD, et al. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol 2008;154:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng JC, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest 2006;116:2532–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Döring M, et al. Phagocytic activity of monocytes, their subpopulations and granulocytes during post-transplant adverse events after hematopoietic stem cell transplantation. Immunobiology 2015;220:605–613. [DOI] [PubMed] [Google Scholar]
- 12.Drifte G, et al. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med 2013;41:820–832. [DOI] [PubMed] [Google Scholar]
- 13.Dupré-Crochet S, et al. ROS production in phagocytes: why, when, and where? J Leukoc Biol 2013;94:657–670. [DOI] [PubMed] [Google Scholar]
- 14.Eickhoff S, et al. Measurement of phagocytosis and oxidative burst of canine neutrophils: high variation in healthy dogs. Vet Immunol Immunopathol 2004;101:109–121. [DOI] [PubMed] [Google Scholar]
- 15.Ge X-Y, et al. TLR4-dependent internalization of CX3CR1 aggravates sepsis-induced immunoparalysis. Am J Transl Res 2016;8:5696–5705. [PMC free article] [PubMed] [Google Scholar]
- 16.Glasser L, et al. Functional differentiation of normal human neutrophils. Blood 1987;69:937–944. [PubMed] [Google Scholar]
- 17.Goddard A, et al. Prognostic usefulness of blood leukocyte changes in canine parvoviral enteritis. J Vet Intern Med 2008;22:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goddard A, et al. Significance of chemiluminometric assessment of plasma endotoxin and neutrophil oxidative activity to prognosticate parvoviral enteritis in puppies. Proc Eur Coll Vet Intern Med–Compan Anim; Toulouse, France; 9–11 September 2010. [Google Scholar]
- 19.Gopalakrishna R, et al. Protein kinase C signaling and oxidative stress. Free Radical Bio Med 2000;28:1349–1361. [DOI] [PubMed] [Google Scholar]
- 20.Grondman I, et al. Endotoxin-induced immunotolerance is associated with loss of monocyte metabolic plasticity and reduction of oxidative burst. J Leukoc Biol 2019;106:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey JW. Evaluation of leukocytic disorders. In: Harvey JW, ed. Veterinary Hematology: A Diagnostic Guide and Color Atlas. 1st ed. Vol. 1. Elsevier Saunders, 2012:122–177. [Google Scholar]
- 22.Hillström A, et al. Validation of a commercially available automated canine-specific immunoturbidimetric method for measuring canine C-reactive protein. Vet Clin Pathol 2014;43:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman D, et al. Immune function in critically ill dogs. J Vet Intern Med 2018;32:208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holst BS, et al. Leucocyte phagocytosis during the luteal phase in bitches. Vet Immunol Immunopathol 2013;153:77–82. [DOI] [PubMed] [Google Scholar]
- 25.Johnson EH, et al. Comparative chemiluminescence of neonatal and adult ovine polymorphonuclear leukocytes. Vet Immunol Immunopathol 2010;134:265–268. [DOI] [PubMed] [Google Scholar]
- 26.Kalli I, et al. Factors affecting the occurrence, duration of hospitalization and final outcome in canine parvovirus infection. Res Vet Sci 2010;89:174–178. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann I, et al. Polymorphonuclear leukocyte dysfunction syndrome in patients with increasing sepsis severity. Shock 2006;26:254–261. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, et al. Canine neutrophil dysfunction caused by downregulation of β2-integrin expression without mutation. Vet Immunol Immunopathol 2009;130:187–196. [DOI] [PubMed] [Google Scholar]
- 29.Kocaturk M, et al. Prognostic value of serum acute-phase proteins in dogs with parvoviral enteritis. J Small Anim Pract 2010;51:478–483. [DOI] [PubMed] [Google Scholar]
- 30.LeBlanc CJ, et al. Evaluation of peripheral blood neutrophil function in tumor-bearing dogs. Vet Clin Pathol 2010;39:157–163. [DOI] [PubMed] [Google Scholar]
- 31.Livaditi O, et al. Neutrophil CD64 expression and serum IL-8: sensitive early markers of severity and outcome in sepsis. Cytokine 2006;36:283–290. [DOI] [PubMed] [Google Scholar]
- 32.Makoni M, et al. Alterations in neonatal neutrophil function attributable to increased immature forms. Early Hum Dev 2016;103:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathew LM, et al. Resveratrol administration increases phagocytosis, decreases oxidative burst, and promotes pro-inflammatory cytokine production in healthy dogs. Vet Immunol Immunopathol 2018;203:21–29. [DOI] [PubMed] [Google Scholar]
- 34.McClure V, et al. Evaluation of the use of serum C-reactive protein concentration to predict outcome in puppies infected with canine parvovirus. J Am Vet Med Assoc 2013;243:361–366. [DOI] [PubMed] [Google Scholar]
- 35.McTaggart C, et al. A comparison of foal and adult horse neutrophil function using flow cytometric techniques. Res Vet Sci 2001;71:73–79. [DOI] [PubMed] [Google Scholar]
- 36.Meunier PC, et al. Pathogenesis of canine parvovirus enteritis: sequential virus distribution and passive immunization studies. Vet Pathol 1985;22:617–624. [DOI] [PubMed] [Google Scholar]
- 37.Mortaz E, et al. Does neutrophil phenotype predict the survival of trauma patients? Front Immunol 2019;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muenzer JT, et al. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun 2010;78:1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostuni MA, et al. Targeting NADPH-oxidase by reactive oxygen species reveals an initial sensitive step in the assembly process. Free Radical Biol Med 2010;49:900–907. [DOI] [PubMed] [Google Scholar]
- 40.Parker LC, et al. Endotoxin tolerance induces selective alterations in neutrophil function. J Leukoc Biol 2005;78:1301–1305. [DOI] [PubMed] [Google Scholar]
- 41.Pillay J, et al. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. J Leukoc Biol 2010;88:211–220. [DOI] [PubMed] [Google Scholar]
- 42.Potgieter LND, et al. Experimental parvovirus infection in dogs. Can J Comp Med 1981;45:212–216. [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzi TE, et al. Normal haematology of the dog. In: Weiss DJ, Wardrop KJ, eds. Schalm’s Veterinary Hematology. 6th ed.Vol. 1. Wiley-Blackwell, 2010:799–810. [Google Scholar]
- 44.Schmidt T, et al. CD66b overexpression and loss of C5a receptors as surface markers for Staphylococcus aureus-induced neutrophil dysfunction. PLoS One 2015;10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silveira-Lessa AL, et al. TLR expression, phagocytosis and oxidative burst in healthy and septic newborns in response to gram-negative and gram-positive rods. Hum Immunol 2016;77:972–980. [DOI] [PubMed] [Google Scholar]
- 46.Tauber AI.Protein kinase C and the activation of the human neutrophil NADPH-oxidase. Blood 1987;69:711–720. [PubMed] [Google Scholar]
- 47.Webb C, et al. Neutrophil function in septic dogs. J Vet Intern Med 2007;21:982–989. [DOI] [PubMed] [Google Scholar]
- 48.Witko-Sarsat V, et al. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest 2000;80:617–653. [DOI] [PubMed] [Google Scholar]



