Abstract
Rickets is a metabolic bone disease associated with failure of endochondral ossification and impaired osteoid mineralization in growing animals. As a consequence, affected individuals can develop gross and microscopic bone malformations. The most common causes of rickets in domestic species include vitamin D and phosphorus deficiency. Rickets has been described in multiple species; however, comprehensive postmortem characterizations with confirmatory histopathology in equids have not been published. A 6-mo-old, Thoroughbred-cross foal was diagnosed with rickets based on gross autopsy findings and microscopic examination of the ribs and long bones. Grossly, all costochondral junctions of the ribs were enlarged with a “rachitic rosary” appearance, and there were multiple fracture calluses in the rib bodies. Epiphyses and metaphyses of the long bones appeared widened on sagittal section, and their physes were irregularly thickened. Histologically, there were poorly organized columns of hypertrophic chondrocytes within the physes of affected bones, islands of chondrocytes embedded within the primary and secondary spongiosa, and faintly eosinophilic seams of poorly mineralized osteoid within the bone trabeculae. Areas of focally increased osteoclastic activity were observed in some of the sections, perhaps pointing to a more complex metabolic bone disease in a growing animal. Low serum concentrations of calcium and 25-hydroxyvitamin D were detected in an antemortem sample. The pathogenesis of these imbalances was not definitively established, but lack of sunlight exposure, low concentration of vitamin D precursors in the diet (perhaps secondary to malnutrition), or both, were suspected; a genetic basis cannot be ruled out.
Keywords: horses, metabolic bone disease, rickets, vitamin D
Rickets is a metabolic bone disease characterized by defective endochondral ossification, and osteoid mineralization, in growing animals and humans.3,6 Grossly, widened epiphyses of long bones and enlarged costochondral junctions of the ribs are observed in affected individuals. The histologic hallmarks include persistence of hypertrophic chondrocytes at sites of endochondral ossification such as the physes (growth plates), irregularly thickened physes with disorganized metaphyseal trabeculae, irregular “tongues” of persistent cartilage, and haphazard replacement with fibrous tissue.3 Deficiencies of phosphorus, vitamin D (dietary, genetic, or related to lack of sunlight), or both are the most common causes of rickets. Susceptibility to vitamin D deficiency–associated rickets varies across different animal species;6,24 poultry and South American camelids are considered highly susceptible, followed by pigs and sheep, with cattle as the least prone among food-producing animals; disease in dogs and cats is infrequent.24
Rickets in horses is considered to be a very rare condition, and published reports of the disease in foals are scarce. In one study, lack of sunlight and low dietary vitamin D induced radiographic features compatible with rickets in the bones of experimental Shetland ponies.8 Although gross photos of rickets in horses are available in some textbooks14 and image collections (Dr. J.M. King’s collection; https://noahsarkive.cldavis.org/cgi-bin/show_image_info_detail.cgi?image=F34687), a comprehensive characterization of the gross and microscopic lesions in this species is lacking in the scientific literature. We present here a case of rickets in a foal and a review of the available literature on the disease in horses.
A euthanized, 6-mo-old, female Thoroughbred-cross foal was submitted for autopsy and diagnostic workup to the San Bernardino laboratory of the California Animal Health and Food Safety Laboratory System (CAHFS) in early February 2020. The animal had a history of trauma (found trapped under a gate) and being unresponsive to 3 mo of supportive therapy (i.e., firocoxib [Previcox; Boehringer Ingelheim] and flunixin meglumine [Banamine; Merck]), following the incident. The carcass was in poor nutritional condition, thin, with a dull, long, and crumpled hair coat, multiple skin abrasions, and overgrowth of all 4 hooves. A focal, 5 × 5 cm area of skin ulceration, with tearing of the underlying joint capsule and tendons, was present at the level of the right front fetlock joint. Several, depressible and slightly movable nodular thickenings (presumed calluses) along the bodies of multiple ribs on both sides of the thorax were palpable through the skin, and evident following thoracic wall removal (Fig. 1).
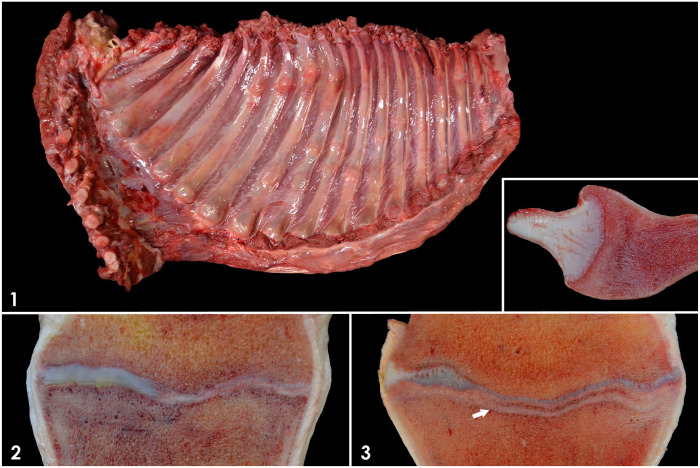
Figures 1–3.
Gross findings in the bones of a foal with rickets. Figure 1. Thoracic wall. Enlarged costochondral junctions (“rachitic rosary”) and calluses in several ribs. Inset: sagittal section of a costochondral junction showing metaphyseal widening and pallor. Figures 2, 3. Physes and metaphyses of long bones. Figure 2. Irregular thickening of the physis and metaphyseal pallor. Figure 3. Irregular thickening of the physis and conspicuous, parallel, tan-to-gray, metaphyseal line (arrow).
Grossly, the costochondral junctions of the ribs were markedly enlarged in all their dimensions, with a “rachitic rosary” appearance (Fig. 1). The junctions were easily depressible upon slight pressure with a finger. Sagittal sectioning of these costochondral junctions revealed widening of the metaphyses, all of which were broader than the adjacent costochondral cartilage (i.e., on a sagittal, horizontal plane, the osseous portion of the costochondral junction was longer than the adjacent cartilaginous portion). Osteocartilaginous junctions were regular, and there was a 1 × 1 cm area of faint metaphyseal pallor adjacent to them. The epiphyses and metaphyses of long bones, including humerus, radius, femur, tibia, metatarsal and metacarpal bones, and first and second phalanges appeared variably widened on sagittal section, and their physes were irregularly thickened (Figs. 2, 3). There was a 1–1.5 cm thick, metaphyseal, faint, pale area of sclerosis adjacent to the physes of affected bones. Additionally, in the metaphyses of the majority of the long bones, a single, tan-to-gray, conspicuous, transversely oriented, linear, 1–2 mm wide plate of bone was present 4–5 mm from and parallel to the physes (Fig. 3). Examination of the skull (including the teeth), vertebrae, and pelvis revealed no gross abnormalities. Both renal pelvises contained minimal aggregates of gritty, sandy material (nephroliths). There were abundant, non-obstructive, adult Parascaris spp. nematodes within the aboral jejunum and ileum. The small intestinal contents were mucoid-to-hemorrhagic, and the mucosa was mildly thickened and markedly hyperemic. No significant gross abnormalities were detected in the rest of the carcass.
A diagnostic workup following CAHFS standard operating procedures was performed, and included: bacterial aerobic cultures of liver, lung, intestinal contents (small intestine, colon, cecum), and right front fetlock; bacterial anaerobic cultures of intestinal contents; Clostridium difficile enrichment cultures of intestinal contents; PCR testing for Salmonella spp. of liver and intestinal contents; PCR testing for coronavirus and antigen ELISA for rotavirus on small intestinal contents; fecal flotation and acid-fast–stained smears for Cryptosporidium spp. detection in feces. Furthermore, the liver was analyzed for the presence of heavy metals (including lead, manganese, iron, mercury, arsenic, molybdenum, zinc, copper, and cadmium) and selenium.
Small numbers of Escherichia coli were isolated from the fetlock joint together with mixed flora, indicative of either wound infection or postmortem contamination. Large numbers of Parascaris spp. eggs were detected in the feces. Hepatic heavy metal and selenium concentrations were within normal ranges. Results of the other tests performed were negative or nondiagnostic. Samples of heart, lung, liver, spleen, kidney, skeletal muscle, tongue, lymph nodes, thyroid and parathyroid glands, aorta, ovary, uterus, skin, pancreas, stomach, small intestine, cecum, colon, thymus, trachea, esophagus, urinary bladder, adrenal glands, and brain were fixed in 10% neutral-buffered formalin for 24 h, processed for the production of 4-µm sections, and stained with hematoxylin and eosin (H&E). Subsequent to formalin fixation, 1 sample of rib costochondral junction, 15 of physes of long bones, 7 of articular-epiphyseal cartilage complex (AECC) of long bones, 4 of cortical bone, and 1 of a presumed callus of the ribs were immersed in a fixative and commercial demineralizing solution (Cal-Ex II; Thermo Fisher) for several days before they were processed routinely for the production of 4-µm thick, H&E-stained sections. Selected sections of bone were also stained with Masson trichrome.
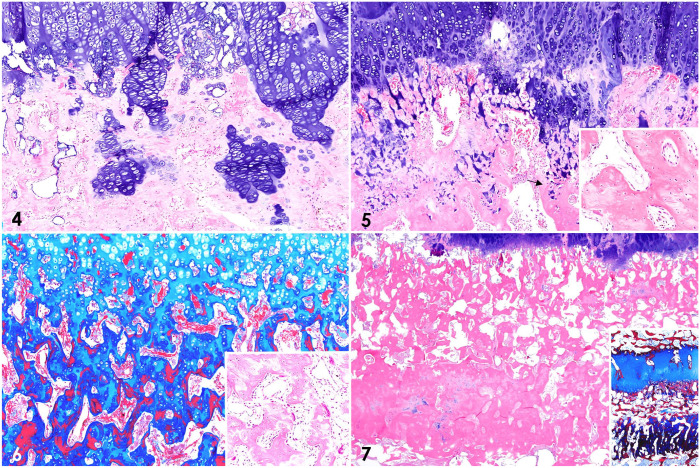
Histologically, the physes were irregularly thickened and disorganized, with poorly arranged columns of chondrocytes, particularly in the zone of hypertrophy (Figs. 4, 5). Persistent columns of chondrocytes spread through the primary and secondary spongiosa, where they formed cartilage cores and islands. Bony trabeculae had multifocal, prominent, faintly eosinophilic seams of osteoid, which were intermixed with darker-staining areas, and that were interpreted as unmineralized to poorly mineralized osteoid (Fig. 5, inset).3,11 Cartilage cores stained light blue with Masson trichrome, woven bone stained dark blue, and lamellar bone stained red, possibly as a result of the different arrangement of collagen fibers in woven and lamellar bones (Fig. 6); these results are a constant observation of one of the authors (B.G. Murphy), and might also be explained by the idea that less permeable tissues, such as lamellar bone, retain the red stain, whereas in more porous tissues (i.e., collagen, cartilage, woven bone) the red stain is replaced by the blue stain. Within the metaphyses and parallel to the physes of the majority of the examined long bones was a conspicuous, dense band of interlinked trabeculae undergoing compaction, with rare chondrocyte aggregates, stained dark-blue with Masson trichrome (Fig. 7), and that corresponded to the grossly evident, tan, metaphyseal lines oriented parallel to the physes (Fig. 3). Howship lacunae in the bony trabeculae of the primary spongiosa contained small to focally increased numbers of attached osteoclasts (Fig. 6, inset) and palisading rows of osteoblasts. Similar to the physes, poorly arranged columns of hypertrophic chondrocytes were detected at sites of endochondral ossification in the sections of AECC. Cortical bone was subjectively thin in some of the examined sections. Microscopic examination also confirmed that the presumptive rib calluses observed grossly were composed of variable amounts of disorganized fibro-osseous and cartilaginous components, consistent with healing rib fractures. Additionally, moderate lymphoplasmacytic and eosinophilic enteritis with mild villus blunting was observed. No other significant microscopic abnormalities were detected in any of the tissues examined. Microscopic lesions in the physes and metaphyses were thus suggestive of abnormal endochondral ossification and defective osteoid mineralization. Coupled with the gross findings, these changes are compatible with a diagnosis of rickets.3
Figures 4–7.
Histologic changes at sites of endochondral ossification in the bones of a foal with rickets. Figure 4. Costochondral junction of a rib. Disorganized columns of hypertrophic chondrocytes and islands of cartilage in the metaphysis. H&E. Figures 5–7. Physis and metaphyseal area of long bones. Figure 5. “Tongues” of cartilage digging into a thickened primary spongiosa, and seams of faintly eosinophilic osteoid (poorly mineralized to unmineralized osteoid) in the trabeculae (arrow). H&E. Inset: detail of bone trabeculae with the mottled seams of faintly eosinophilic, poorly mineralized osteoid. Figure 6. Cartilage cores (light blue), woven bone (dark blue), and lamellar bone (red) in the bone trabeculae. Masson trichrome. Inset: focal area of prominent osteoclastic activity. H&E. Figure 7. Dense band of interlinked, compact trabeculae parallel to the physis, representing compensatory deposition of disorganized osteoid or compression fractures with callus formation. H&E. Inset: the band stains intensely dark-blue with Masson trichrome.
A serum sample from the foal collected a few days before death was sent to the Michigan State University Veterinary Diagnostic Laboratory (Lansing, MI, USA) for calcium, phosphorus, and 25-hydroxyvitamin D analyses. 25-hydroxyvitamin D was measured by radioimmunoassay (RIA) and included both 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. The method was validated for horses, and reference intervals (RIs) were established using adult, healthy animals at different times of the year. Results revealed low serum 25-hydroxyvitamin D concentration and hypocalcemia, with phosphorus within the RI (Table 1). These serum abnormalities were thus supportive of a diagnosis of rickets, likely associated with vitamin D deficiency. However, this 25-hydroxyvitamin D concentration would fall within the RI reported in healthy horses from New Zealand in winter and measured by liquid chromatography–mass spectrometry (LC-MS).1 Differences with the RIs used here could be the result of the different methods of measurement utilized (RIA vs. LC-MS), dietary or breed differences between the horses used to validate both assays, geographic and climatologic factors, and the complexity of vitamin D metabolism in equids.2 In fact, the vitamin D metabolic cycle has not been characterized completely in horses, and it is not clear whether the process in other animals may be extrapolated to equids, given that the role of vitamin D in calcium and phosphorus homeostasis seems to be different in this species.2,17,24 With the exception of carnivores, most mammalian species depend on UV light to form cholecalciferol (also known as vitamin D3) from 7-dehydrocholesterol in the skin. Additionally, cholecalciferol may be acquired from the diet. Independent of the source, cholecalciferol is subsequently hydroxylated in the liver via 25α-hydroxylase, to form 25-hydroxyvitamin D (which includes 2 forms: 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2).10 Although not the most active metabolite of vitamin D, the serum concentration of 25-hydroxyvitamin D is considered the best indicator of vitamin D status in most animals, including horses.17,22 In the kidney, hypophosphatemia or parathyroid hormone (PTH), whose secretion by the parathyroid gland is induced by hypocalcemia, activates 1α-hydroxylase, which converts 25-hydroxyvitamin D into 1,25-dihydroxyvitamin D, the active form of vitamin D.10,17
Table 1.
Serum concentration of calcium, phosphorus, and 25-hydroxyvitamin D in a foal with rickets.
| Analyte | Concentration | Reference interval |
|---|---|---|
| Calcium | 2.0 mmol/L (8.0 mg/dL) | 2.75–3.20 mmol/L (11.0–12.8 mg/dL) |
| Phosphorus | 0.97 mmol/L (3.0 mg/dL) | 0.55–1.39 mmol/L (1.7–4.3 mg/dL) |
| 25-hydroxyvitamin D | 7 nmol/L | 13–40 nmol/L |
Abnormal values in boldface.
The effects of 1,25-dihydroxyvitamin D in bone are complex and vary depending upon the age of the animal, but together with PTH, 1,25-dihydroxyvitamin D can activate osteoclasts, which induce bone resorption, thus increasing calcium and phosphorus concentrations. 1,25-hydroxyvitamin D also promotes calcium and phosphorus absorption in the intestines and kidneys.10 Therefore, persistent hypovitaminosis D can induce secondary hypocalcemia, most likely as a result of impaired absorption of calcium and phosphorus. Hypocalcemia-mediated PTH release from the parathyroid chief cells results in bone resorption and compensatory fibrous tissue deposition (fibrous osteodystrophy), which explains why features of fibrous osteodystrophy overlap with those of rickets in certain cases.3 In our foal, osteoclasts and bone resorption were present in most of the metaphyseal sections, and were focally increased in some of them, although remarkable myelofibrosis was not identified. This might suggest changes of a more complex metabolic bone disease, perhaps with characteristics of early fibrous osteodystrophy, including some bone resorption, most likely the result of hypocalcemia, but still no prominent, compensatory fibrous tissue deposition.3 Hence, it is possible that hypocalcemia was not maintained for sufficient time to induce clear features of fibrous osteodystrophy. Moreover, 1,25-hydroxyvitamin D stimulates osteoblasts to produce osteocalcin, which contributes directly to calcium deposition and mineralization during bone development, indicating the complex role that vitamin D plays in bone homeostasis.10 An alternative mechanism for hypocalcemia in our case could be hypercalciuria, as described in humans.18 This might explain the minimal nephrolithiasis detected grossly. Nevertheless, calcium concentration in equine urine is usually high, thus perhaps nephrolithiasis was just a result of urine hyperconcentration further to dehydration.
In our case, it was not clear whether vitamin D deficiency occurred as a result of low dietary levels, inadequate sunlight exposure, or a combination of both. According to the submitting veterinarian, the foal was always maintained outdoors in a sunny environment (i.e., in southern California), and, to his knowledge, at the moment of examination the dietary management consisted of a mixture of alfalfa hay and grain, which was considered correct. Perhaps the heavy Parascaris spp. infestation with mild enteritis interfered with vitamin absorption in the intestines. Indeed, heavy enteric parasite loads have been associated with other metabolic bone diseases, such as osteoporosis in small ruminants.19 However, this possibility would seem unlikely in equids, given that Parascaris spp. is a common parasite of young horses and, to our knowledge, has not been associated previously with metabolic bone disease. An individual genetic predisposition, perhaps related to a mutation in the genes that encode the enzymes involved in vitamin D metabolism, cannot be ruled out either. In humans, 2 forms of autosomal recessive, vitamin D–dependent rickets type I are described: type Ia, which is associated with 1α-hydroxylase deficiency; and type Ib, which occurs as a result of a deficit in 25α-hydroxylase and is very rare.7,15 Vitamin D–dependent rickets type II (also known as vitamin D–resistant rickets), associated with mutations in the renal vitamin D receptor, is also known to occur in humans.16 Some of these genetic forms of rickets have been described in some domestic species including dogs, cats, and pigs.4,6,9,12,20 We have found no reports of a similar condition in horses.
The scarcity of reported cases of rickets in equids in the scientific literature may be the result of a relative resistance to disease development in this species. A thorough search of the CAHFS database revealed no diagnosed cases of rickets in foals between 1990 and 2020. In fact, there has been widespread debate about the existence of true rickets in horses.1,24 This could be related to the particular calcium, phosphorus, and vitamin D metabolism of equids; horses have higher total serum calcium concentration than other species, with a rate of intestinal absorption that appears to be less dependent on vitamin D.2,17,25 Furthermore, serum concentrations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D tend to be lower in horses than in other animals; healthy horses may have values of these substances that in other species would almost invariably be associated with metabolic bone disease.2,6,23,24
There are no documented cases of metabolic bone disease secondary to hypophosphatemia in young equids,22 and the serum phosphorus concentration was adequate in this foal. This finding is unusual, given that vitamin D deficiency has been associated with hypophosphatemic rickets in other animal species.6 Perhaps vitamin D does not have an important role in inorganic phosphorus metabolism in equids, as suggested by some authors,2 which might explain why serum phosphorus concentrations were unaltered in our foal. Alternatively, it is possible that the presumptive dietary deficiencies occurred at a specific time in the past, and were corrected later, partially reassuming some of the serum concentrations at the time of blood sampling.
Most of the gross and microscopic characteristics of our case closely resemble the pathologic features of rickets in other animal species, such as ruminants and pigs.5,13,21 The “rachitic rosary” is a gross hallmark of this condition, and the disorganized chondrocyte columns in the growth plates, with seams of faintly eosinophilic, unmineralized osteoid in the metaphyseal bone trabeculae, are considered to be diagnostic microscopic features.3 However, in our case, the osteocartilaginous boundary in the costochondral junctions of the examined ribs was quite regular. This finding differs from the irregularly arranged costochondral junctions, with macroscopically visible bands and islands of cartilage penetrating the metaphyseal area, that are described in other animal species.3,5,13,21 In addition, cortical bone appeared thin in some of the sections, which differs from the thickened cortices that are sometimes described in other animal species with rickets. Thin cortices may be indicative of a more complex metabolic bone disease that also includes features of osteoporosis in addition to rickets.3
The areas of sclerosis below the physes and the grossly visible, tan-to-gray, conspicuous lines are likely a reflection of the severe disorganization in the primary spongiosa. Perhaps large amounts of osteoid were produced as a compensatory mechanism to decreased bone strength resulting from poor mineralization.3 The conspicuous lines may also represent compression fractures or calluses. Alternatively, these lines could be interpreted as growth arrest lines, although growth arrest lines normally occur as multiple lines rather than individually, as in the metaphyses of our foal.3 Metaphyseal growth arrest lines usually represent a period of nutritional inadequacy with resultant transient arrest of endochondral ossification, and are relatively common in a variety of bone diseases of domestic animals.3 The history of the lack of response to treatment and the rib fractures were also likely related to rickets and the poor general health status of this foal.
Most of the available literature about rickets in animals is based on case reports and small case series, which have helped to establish the features of this condition in different species.5,6,9,12,13,21 These reports highlight the importance of performing and publishing comprehensive characterizations that include histopathology, especially in species such as the horse, in which publications are scarce or the condition is infrequently diagnosed.
Acknowledgments
We thank Dr. Thomas J. Hoyme for providing information and valuable discussions about the case, and Dr. Brian Petroff (MSU) for providing information about the vitamin D assay.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no external financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Javier Asin  https://orcid.org/0000-0002-6178-4801
https://orcid.org/0000-0002-6178-4801
Monika A. Samol  https://orcid.org/0000-0002-5508-4630
https://orcid.org/0000-0002-5508-4630
Francisco A. Uzal  https://orcid.org/0000-0003-0681-1878
https://orcid.org/0000-0003-0681-1878
Contributor Information
Javier Asin, California Animal Health and Food Safety Laboratory System, San Bernardino branch, University of California–Davis, CA, USA; Department of Pathology, Microbiology & Immunology, University of California–Davis, CA, USA.
Brian G. Murphy, Department of Pathology, Microbiology & Immunology, University of California–Davis, CA, USA
Monika A. Samol, California Animal Health and Food Safety Laboratory System, San Bernardino branch, University of California–Davis, CA, USA
Jose Polanco, California Animal Health and Food Safety Laboratory System, San Bernardino branch, University of California–Davis, CA, USA.
Janet D. Moore, California Animal Health and Food Safety Laboratory System, San Bernardino branch, University of California–Davis, CA, USA
Francisco A. Uzal, California Animal Health and Food Safety Laboratory System, San Bernardino branch, University of California–Davis, CA, USA Department of Pathology, Microbiology & Immunology, University of California–Davis, CA, USA.
References
- 1.Azarpeykan S, et al. Influence of blanketing and season on vitamin D and parathyroid hormone, calcium, phosphorus and magnesium concentrations in horses in New Zealand. Domest Anim Endocrinol 2016;56:75–84. [DOI] [PubMed] [Google Scholar]
- 2.Breidenbach A, et al. Peculiarities of vitamin D and of the calcium and phosphate homeostatic system in horses. Vet Res 1998;29:173–186. [PubMed] [Google Scholar]
- 3.Craig LE, et al. Bones and joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 1. Elsevier, 2016:16–163. [Google Scholar]
- 4.Chavez LS, et al. Molecular basis for pseudo vitamin D-deficiency rickets in the Hannover pig. J Nutr Biochem 2003;14:378–385. [DOI] [PubMed] [Google Scholar]
- 5.Dittmer KE, et al. Skeletal deformities associated with nutritional congenital rickets in newborn lambs. N Z Vet J 2017;65:51–55. [DOI] [PubMed] [Google Scholar]
- 6.Dittmer KE, Thompson KG.Vitamin D metabolism and rickets in domestic animals: a review. Vet Pathol 2011;48:389–407. [DOI] [PubMed] [Google Scholar]
- 7.Dursun F, et al. Genetic and clinical characteristics of patients with vitamin D dependent rickets type 1A. J Clin Res Pediatr Endocrinol 2019;11:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Shorafa WM, et al. Effect of vitamin D and sunlight on growth and bone development of young ponies. J Anim Sci 1979;48:882–886. [DOI] [PubMed] [Google Scholar]
- 9.Geisen V, et al. Vitamin D-dependent hereditary rickets type I in a cat. J Vet Intern Med 2009;23:196–199. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, et al. Chapter 9: Environmental and nutritional diseases. In: Kumar V, et al., eds. Robbins and Cotran Pathologic Basis of Disease. 9th ed.Elsevier, 2016:437–442. [Google Scholar]
- 11.LaDouceur EEB, et al. Osteomalacia with hyperostosis in captive lesser hedgehog tenrecs (Echinops telfairi). Vet Pathol 2020;57:885–888. [DOI] [PubMed] [Google Scholar]
- 12.LeVine DN, et al. Hereditary 1,25-dihydroxyvitamin D-resistant rickets in a Pomeranian dog caused by a novel mutation in the vitamin D receptor gene. J Vet Intern Med 2009;23:1278–1283. [DOI] [PubMed] [Google Scholar]
- 13.Madson DM, et al. Rickets: case series and diagnostic review of hypovitaminosis D in swine. J Vet Diagn Invest 2012;24:1137–1144. [DOI] [PubMed] [Google Scholar]
- 14.Mair TS, Divers TJ. Self-Assessment Color Review of Equine Internal Medicine. 1st ed. Manson Publishing, 1997:157–158. [Google Scholar]
- 15.Molin A, et al. Vitamin D-dependent rickets type 1B (25-hydroxylase deficiency): a rare condition or a misdiagnosed condition? J Bone Miner Res 2017;32:1893–1899. [DOI] [PubMed] [Google Scholar]
- 16.Nicolescu R, et al. Vitamin D-resistant rickets and cinacalcet-one more favorable experience. Front Pediatr 2018;6:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pozza ME, et al. Serum vitamin D, calcium, and phosphorus concentrations in ponies, horses and foals from the United States and Thailand. Vet J 2014;199:451–456. [DOI] [PubMed] [Google Scholar]
- 18.Stechman MJ, et al. Genetic causes of hypercalciuric nephrolithiasis. Pediatr Nephrol 2009;24:2321–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sykes AR, et al. Experimental production of osteoporosis in growing lambs by continuous dosing with Trichostrongylus colubriformis larvae. J Comp Pathol 1975;85:549–559. [DOI] [PubMed] [Google Scholar]
- 20.Teshima T, et al. A genetic variant of CYP2R1 identified in a cat with type 1B vitamin D-dependent rickets: a case report. BMC Vet Res 2019;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson KG, Cook RG.Rickets in yearling steers wintered on a swede (Brassica napus) crop. N Z Vet J 1987;35:11–13. [DOI] [PubMed] [Google Scholar]
- 22.Toribio RE.Disorders of calcium and phosphate metabolism in horses. Vet Clin North Am Equine Pract 2011;27:129–147. [DOI] [PubMed] [Google Scholar]
- 23.Toribio RE, et al. Effects of hypercalcemia on serum concentrations of magnesium, potassium, and phosphate and urinary excretion of electrolytes in horses. Am J Vet Res 2007;68:543–554. [DOI] [PubMed] [Google Scholar]
- 24.Uhl EW.The pathology of vitamin D deficiency in domesticated animals: an evolutionary and comparative overview. Int J Paleopathol 2018;23:100–109. [DOI] [PubMed] [Google Scholar]
- 25.Wilkens MR, et al. Trans- and paracellular calcium transport along the small and large intestine in horses. Comp Biochem Physiol A Mol Integr Physiol 2017;204:157–163. [DOI] [PubMed] [Google Scholar]