Abstract
Background
Whether nab-paclitaxel plus carboplatin as neoadjuvant therapy can benefit patients with resectable squamous cell carcinoma of the lung remains unclear. This prospective study aimed to investigate outcomes in patients with stage IIIA-N2 squamous cell carcinoma of the lung treated with nab-paclitaxel plus carboplatin as neoadjuvant therapy.
Material/Methods
Patients with stage IIIA-N2 squamous cell carcinoma of the lung were treated with nab-paclitaxel (100 mg/m2, days 1, 8, and 15) and carboplatin (5 mg/(mL·min), day 1) for two 21-day cycles. The patients were followed every 3 months for 2 years and every 6 months after that. The primary endpoint was the downstaging rate. Secondary endpoints included objective response rate (ORR), margin-free (R0) resection, pathologic complete response (pCR), progression-free survival (PFS), overall survival (OS), and safety.
Results
Among the 36 enrolled patients, 33 completed neoadjuvant chemotherapy, and 23 underwent surgery. The preoperative ORR was 50.0% (18/36). R0 resection was achieved in 22 (95.7%) of 23 patients. Major pathologic response and pCR were achieved in 8 (34.8%) and 2 (8.7%) patients, respectively. The overall downstaging rate was 47.8% (11/23). The median follow-up was 39.8 (32.5–41.0) months. For patients who underwent surgery, the median PFS and OS were 31.4 (95%CI: 10.4-not reached (NR)) and 45.0 (95%CI: 22.6-NR) months, respectively. The most common adverse events were neutropenia, anemia, and leukopenia.
Conclusions
This study preliminarily indicated a favorable effect of nab-paclitaxel plus carboplatin as neoadjuvant therapy without significant adverse events for stage IIIA-N2 squamous cell carcinoma of the lung. Future randomized controlled trials are needed to verify these results.
Keywords: Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Neoadjuvant Therapy
Background
Lung cancer is the leading cause of cancer death worldwide [1]. Non-small-cell lung cancer (NSCLC) accounts for more than 80% of all lung tumors, and approximately one-third of patients have stage III locally advanced NSCLC at diagnosis [2]. For these patients, interdisciplinary multimodality management is needed [3]. Although patients with locally advanced disease are potentially curable with a multimodality approach, the 5-year survival was less than 10% in patients with N2 disease due to local recurrence and distant metastases [4]. Perioperative therapy should be prompted to improve prognosis [5].
Previous meta-analyses demonstrated that the addition of neoadjuvant or adjuvant chemotherapy could prolong survival compared with surgery alone for NSCLC [6,7]. The administration of neoadjuvant chemotherapy can shrink the tumor or the lymph node and eradicate micrometastases, leading to improved survival [5]. However, the absence of response to neoadjuvant therapy may result in disease progression and loss of the opportunity for surgical resection [5]. For patients with stage IIIA-N2 NSCLC, no optimal treatment approach has been recommended yet. Nevertheless, previous studies showed that the resectability rate was 60% to 68% after downstaging N2 disease using neoadjuvant chemotherapy [8,9].
Squamous cell carcinoma of the lung, a subtype of NSCLC [10], achieves fewer benefits from the targeted therapies owing to the lack of driver mutations [11,12], and the treatment options are mostly limited to chemotherapy [5,13]. According to the National Comprehensive Cancer Network, European Society of Medical Oncology, and American Society of Clinical Oncology guidelines, the combination of cisplatin or carboplatin with a third-generation cytotoxic agent such as paclitaxel, docetaxel, vinorelbine, or gemcitabine is among the standard regimens for advanced squamous cell carcinoma of the lung [5,14,15]. The introduction of immunotherapy changed the landscape of squamous cell carcinoma of the lung [16]. However, trials of immune checkpoint inhibitors (ICIs) as neoadjuvant therapy in managing squamous cell carcinoma of the lung are still underway [17].
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a nanoparticle formulation of paclitaxel bound to human serum albumin [18,19]. Since nab-paclitaxel does not contain solvents, such as polyoxyethylated castor oil, large doses of corticosteroids and antihistamines are not necessary to prevent hypersensitivity reactions [20–25]. The CA031 trial used nab-paclitaxel and carboplatin as the first-line therapy, which showed a favorable risk-benefit profile in advanced NSCLC [26]. A phase II trial in China demonstrated that the first-line therapy with nab-paclitaxel/carboplatin better improved the quality of life compared with gemcitabine/carboplatin for squamous cell carcinoma of the lung [27]. The ABOUND.SQM trial suggested the efficacy of induction therapy with nab-paclitaxel plus carboplatin followed by nab-paclitaxel maintenance therapy for squamous cell carcinoma of the lung [13].
However, there is limited data regarding whether nab-paclitaxel plus carboplatin as neoadjuvant therapy can benefit patients with resectable squamous cell carcinoma of the lung. This prospective study was conducted at a single center and aimed to investigate outcomes in 36 patients with stage IIIA-N2 squamous cell carcinoma of the lung treated with nab-paclitaxel plus carboplatin as neoadjuvant therapy.
Material and Methods
Study Design and Patients
This study was carried out in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice standards and was approved by the ethics committee of Tianjin Medical University Cancer Institute and Hospital. All the patients signed informed consent. Patients diagnosed with NSCLC (by histological or cytological examination, according to the World Health Organization guidelines [28]) were recruited from Tianjin Medical University Cancer Institute and Hospital between April 2015 and August 2017.
The inclusion criteria included ≥18 years of age; diagnosed with squamous cell carcinoma of the lung by histological or cytological examination; treatment-naïve; stage IIIA-N2, confirmed by mediastinoscopy, endobronchial ultrasound, or positron emission tomography-computed tomography (PET-CT); Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1; tolerable for neoadjuvant chemotherapy and surgery, as evaluated by the investigators; at least 1 measurable lesion; adequate bone marrow and cardiac, hepatic, and renal functions; and life expectancy >3 months. The key exclusion criteria were a history of malignancies in the past 5 years, previous local radiotherapy or any systemic antitumor therapy, or any unstable systemic disease.
Treatment and Follow-Up
The patients were treated with intravenous infusion of nab-paclitaxel (100 mg/m2, days 1, 8, and 15; Celgene Corporation, NJ, USA) and carboplatin (area under the curve 5 mg/(mL·min), day 1; Qilu Pharmaceutical Co., Ltd., Shandong, China). Each patient received two 3-week cycles of treatment before surgery. The assigned treatment was continued until disease progression or intolerable toxicity. During chemotherapy, the doses were adjusted according to the tolerance of the patients.
The treatment response was assessed by CT or PET-CT within 1 week after the completion of the neoadjuvant chemotherapy. Patients with progression-free and resectable disease proceeded to surgical resection.
All patients undergoing surgery were recommended to receive 2 cycles of adjuvant chemotherapy, with the same regimen as above. The decision of whether to use postoperative radiotherapy was at the discretion of the investigators and was conducted with adjuvant chemotherapy, sequentially. No other antitumor drugs were allowed during the study.
The patients were followed up every 3 months in the first 2 years and 6 months thereafter, including those who had disease progression after neoadjuvant chemotherapy.
Outcomes and Assessments
The primary outcome was the downstaging rate, defined as a reduction in the clinical stage relative to the pathohistological stage [29]. The baseline tumor (T) stage was confirmed by CT or PET-CT, while the baseline lymph node (N) stage was confirmed by mediastinoscopy, endobronchial ultrasound, or PET-CT. The T and N stages of the unresectable patients after neoadjuvant chemotherapy were confirmed by CT or PET-CT.
The secondary outcomes included preoperative objective response rate (ORR), margin-free (R0) resection rate, pathologic complete response (pCR) rate, progression-free survival (PFS), 3-year overall survival (OS) rate, and safety [30,31]. The radiologic response was assessed by an independent review committee (IRC) and investigators, according to the Response Evaluation Criteria in Solid Tumors, version 1.1 [32]. The pCR was defined as the proportion of patients without viable tumor cells. The PFS was defined as the time from the initiation of neoadjuvant chemotherapy to disease progression, recurrence, or any-cause death (whichever came first). The OS was defined as the time from the initiation of neoadjuvant chemotherapy to any-cause death.
The exploratory endpoint was the major pathologic response (MPR) rate, defined as the proportion of patients with <10% residual tumor cells [33].
The adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 [34].
The patients were followed at the outpatient clinic every 3 months in the first 2 years and 6 months thereafter, including those who had disease progression after neoadjuvant chemotherapy.
Statistical Analysis
Efficacy analyses were based on the intention-to-treat (ITT) population (all patients enrolled). The safety analysis population included all patients who received at least 1 dose of the study drug. The primary outcome (downstaging) could be analyzed only in patients who underwent surgery. Descriptive analysis was conducted for patients’ baseline characteristics. Continuous variables were expressed as medians (ranges). Categorical variables were expressed as numbers (percentages). A waterfall plot of preoperative tumor response to neoadjuvant chemotherapy was provided to show the change of tumor size from baseline in percentage. The Kaplan-Meier method was used for survival analysis. The 95% confidence intervals (CIs) of survival time were estimated using the Brookmeyer and Crowley method [35], and 95%CIs of event-free rates at 1 and 3 years were estimated using Greenwood’s formula [36]. The univariate Cox proportional hazard model was used to explore the potential factors (PR, MPR, pCR, and type of surgery) for OS and PFS in patients who underwent surgery, with hazard ratios and 95%CIs calculated. SAS version 9.4 was used for all statistical analyses. P<0.05 was considered statistically significant.
Results
Characteristics of the Patients
Thirty-six patients were initially enrolled as an intention-to-treat (ITT) population, but 2 patients discontinued the neoadjuvant chemotherapy without radiographic evaluation due to patient decision. A total of 33, 23, and 21 patients completed neoadjuvant chemotherapy, surgery, and adjuvant chemotherapy, respectively (Figure 1).
Figure 1.
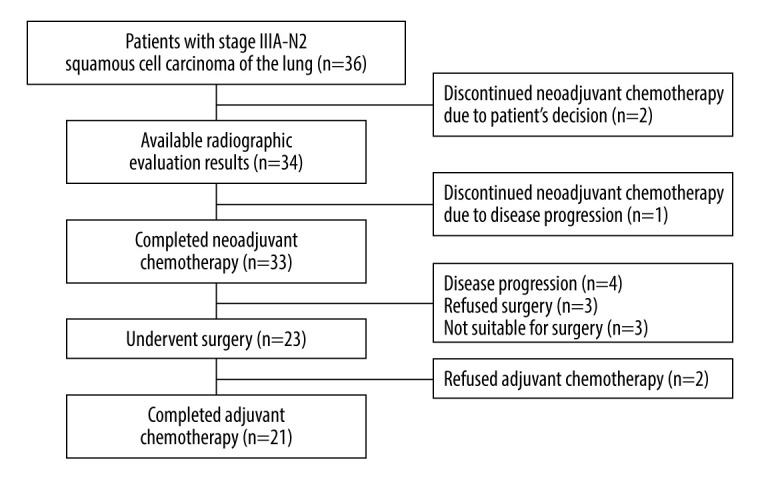
Study flow chart depicting the enrollment and study completion process by the patients.
The median age was 58 (range, 45–76) years, all patients were men, and 86.1% were former/current smokers. Among the 36 patients, 23 (63.9%) had T2 disease, and 13 (36.1%) had T3 disease. The ECOG performance status was 0 in 17 (47.2%) patients and 1 in 19 (52.8%) patients (Table 1). The characteristics of the 23 patients who underwent surgery were similar to those of the ITT population.
Table 1.
Baseline characteristics of patients with squamous cell carcinoma of the lung.
| Characteristics | ITT (n=36) | Surgery (n=23) |
|---|---|---|
| Age (years), median (range) | 58 (45–76) | 58 (45–63) |
| Male, n (%) | 36 (100) | 23 (100) |
| Smoking, n (%) | ||
| Never | 5 (13.9) | 4 (17.4) |
| Former/current | 31 (86.1) | 19 (82.6) |
| ECOG performance status, n (%) | ||
| 0 | 17 (47.2) | 13 (56.5) |
| 1 | 19 (52.8) | 10 (43.5) |
| T stage, n (%) | ||
| T2 | 23 (63.9) | 14 (60.9) |
| T3 | 13 (36.1) | 9 (39.1) |
ECOG – Eastern Cooperative Oncology Group; ITT – intention-to-treat.
Preoperative Response to Neoadjuvant Chemotherapy
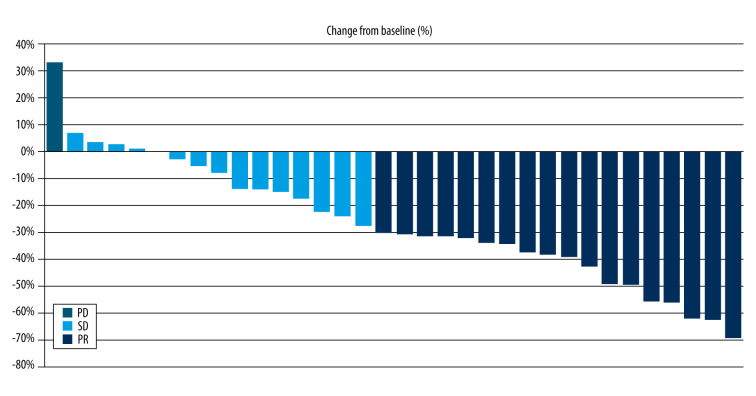
Preoperative tumor response was assessed in 34 patients by the investigators and by an IRC, including 33 patients who completed the neoadjuvant chemotherapy and 1 patient who discontinued the treatment due to disease progression. The ORR was 63.9%, according to the investigator, and 50.0%, according to the IRC. For patients who underwent surgery, the ORR was 60.9%, according to the investigator, and 78.3%, according to the IRC (Table 2). Figure 2 presents the waterfall plot of the tumor response to neoadjuvant chemotherapy.
Table 2.
Tumor response before surgery in patients with squamous cell carcinoma of the lung treated with neoadjuvant nab-paclitaxel and carboplatin.
| Response, n (%) | ITT (n=36) | Surgery (n=23) | ||
|---|---|---|---|---|
| Investigator | IRC | Investigator | IRC | |
| PR | 23 (63.9) | 18 (50.0) | 14 (60.9) | 18 (78.3) |
| SD | 7 (19.4) | 14 (38.9) | 9 (39.1) | 5 (21.7) |
| PD | 4 (11.1) | 2 (5.6) | 0 | 0 |
| NE | 2 (5.6) | 2 (5.6) | 0 | 0 |
| ORR | 23 (63.9) | 18 (50.0) | 14 (60.9) | 18 (78.3) |
IRC – Independent Reviewer Committee; ITT – intention-to-treat; NE – not evaluated; ORR – objective response rate; PD – progressive disease; PR – partial response; SD – stable disease.
Figure 2.
Waterfall plots of preoperative tumor response to neoadjuvant chemotherapy. The bars represent the change in tumor size from baseline in each patient after neoadjuvant nab-paclitaxel and carboplatin. PD – progressive disease; PR – partial response; SD – stable disease.
Surgical Results
Among the 23 patients with partial response (PR) evaluated by the investigator, 4 failed to achieve resection owing to heart dysfunction (n=1) or personal unwillingness (n=3). Two of 7 patients with stable disease failed to achieve resection. Among the 23 patients who underwent surgery, R0 resection was achieved in 22 (95.7%). One patient with R1 resection received postoperative radiotherapy. Sixteen (69.6%) patients underwent lobectomy, including 4 (17.4%) patients with sleeve resection. Four (17.4%) and 3 (13.0%) patients underwent pneumonectomy and bilobectomy, respectively. Twenty-one (91.3%) patients received adjuvant chemotherapy, with the same protocol as the neoadjuvant chemotherapy. The specimen pathological examination revealed that pCR was achieved in 2 (8.7%) patients, when considering the tumor only or the tumor and lymph nodes. Eight patients had MPR, with a rate of 34.8%. An overall downstage (the primary outcome) by neoadjuvant chemotherapy was achieved in 11 (47.8%) patients (Table 3).
Table 3.
Surgical characteristics and endpoints of patients with squamous cell carcinoma of the lung treated with neoadjuvant nab-paclitaxel and carboplatin.
| Variable | Surgery (n=23) |
|---|---|
| R0 resection, n (%) | 22 (95.7) |
| Adjuvant chemotherapy, n (%) | 21 (91.3) |
| Type of surgery, n (%) | |
| Lobectomy | 16 (69.6) |
| Bilobectomy | 3 (13.0) |
| Pneumonectomy | 4 (17.4) |
| pCR 1*, n (%) | 2 (8.7) |
| pCR 2**, n (%) | 2 (8.7) |
| MPR, n (%) | 8 (34.8) |
| Downstage, n (%) | 11 (47.8) |
MPR – major pathological response; pCR – pathological complete response; R0 – margin-free.
pCR 1 represented the proportion of patients with pCR when considering the tumor only;
pCR 2 represented the proportion of patients with pCR when considering the tumor and lymph nodes.
Adverse Events
Table 4 presents the adverse events of the safety analysis population. Neutropenia was observed in 17 (47.2%) patients, anemia in 9 (25.0%), leukopenia in 9 (25.0%), thrombocytopenia in 3 (8.3%), increased alanine transaminase (ALT) in 2 (5.6%), and increased bilirubin in 1 (2.8). Grade 3 and 4 adverse events included neutropenia (6 patients [16.7%]), increased ALT (1 [2.8%]), and increased bilirubin (1 (2.8%]). The toxicity profile was similar to that reported in previous studies [37–42].
Table 4.
Adverse events of patients with squamous cell carcinoma of the lung treated with neoadjuvant nab-paclitaxel and carboplatin.
| Event, n (%) | ITT (n=36) | |
|---|---|---|
| Any grade | Grade 3 or 4 | |
| Neutropenia | 17 (47.2) | 6 (16.7) |
| Anemia | 9 (25.0) | 0 |
| Leukopenia | 9 (25.0) | 0 |
| Thrombocytopenia | 3 (8.3) | 0 |
| Increased ALT | 2 (5.6) | 1 (2.8) |
| Increased bilirubin | 1 (2.8) | 1 (2.8) |
ALT – alanine transaminase; ITT – intention-to-treat.
Survival
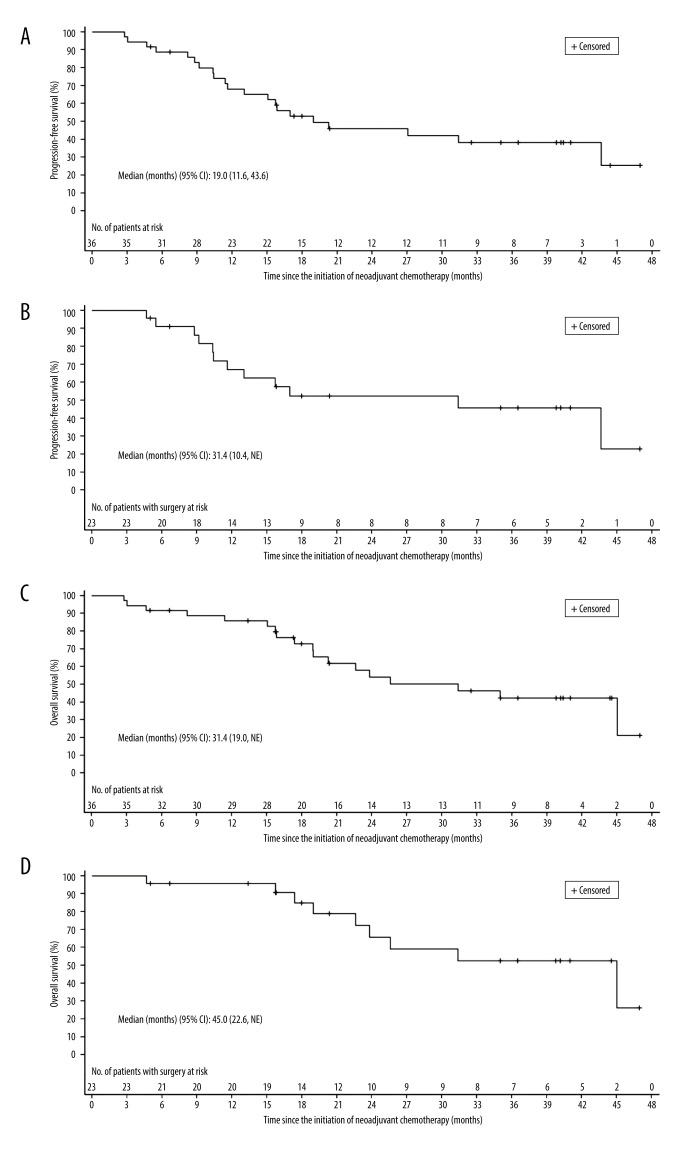
The median follow-up time was 39.8 months (range, 32.5–41.0). The median PFS was 19.0 (95% CI: 11.6–43.6) months in the ITT population. The 1-year PFS rate was 68.1% (95% CI: 49.8–80.9), and the 3-year PFS rate was 38.3% (95% CI: 21.5–54.9). The median OS was 31.4 (95% CI: 19.0-not reached [NR]) months in the ITT population. The OS rates at 1 and 3 years were 85.8% (95% CI: 69.1–93.8) and 42.2% (95% CI: 23.8–59.6), respectively. For patients who underwent surgery, the median OS was 45.0 (95% CI: 22.6-NR) months. The OS rates at 1 and 3 years were 95.7% (95% CI: 72.9–99.4) and 52.6% (95% CI: 26.6–73.2), respectively (Figure 3, Table 5).
Figure 3.
Kaplan-Meier curves of patients with squamous cell carcinoma of the lung treated with neoadjuvant nab-paclitaxel and carboplatin. (A) Progression-free survival in intention-to-treat population. (B) Progression-free survival in patients who underwent surgery. (C) Overall survival in intention-to-treat population. (D) Overall survival in patients who underwent surgery. CI – confidence interval.
Table 5.
Survival of patients with squamous cell carcinoma of the lung treated with neoadjuvant nab-paclitaxel and carboplatin.
| Variable | ITT (n=36) | Surgery (n=23) |
|---|---|---|
| OS (month), median (95% CI) | 31.4 (19.0-NE) | 45.0 (22.6-NE) |
| 1-year OS rate, % (95% CI) | 85.8 (69.1–93.8) | 95.7 (72.9–99.4) |
| 3-year OS rate, % (95% CI) | 42.2 (23.8–59.6) | 52.6 (26.6–73.2) |
| PFS (month), median (95% CI) | 19.0 (11.6–43.6) | 31.4 (10.4-NE) |
| 1-year PFS rate, % (95% CI) | 68.1 (49.8–80.9) | 67.1 (43.1–82.8) |
| 3-year PFS rate, % (95% CI) | 38.3 (21.5–54.9) | 45.8 (23.2–65.8) |
CI – confidence interval; ITT – intention-to-treat; NA – not applicable; NE – not evaluated; OS – overall survival; PFS – progression-free survival.
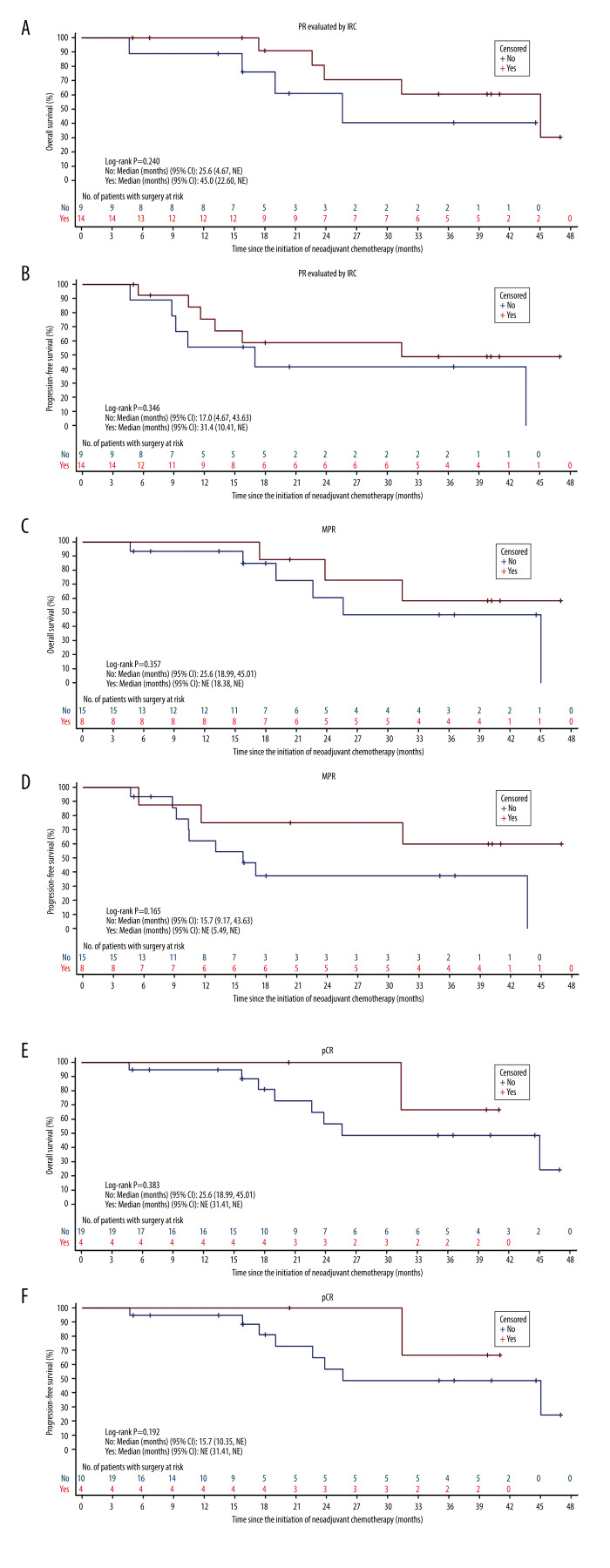
The Kaplan-Meier curves in patients who underwent surgery showed a tendency that patients with short-term efficacy endpoints (PR, MPR, and pCR) achieved more survival benefits than those without radiologic or pathologic response, but without any significant differences (Figure 4). The univariate Cox regression analysis showed that PR (P=0.252), MPR (P=0.356), and pCR (P=0.399) were not associated with OS, which may have been because of the inadequate sample size. Similar results were obtained in the univariate analysis of PFS (PR, P=0.352; MPR, P=0.179; and pCR, P=0.223) (Table 6).
Figure 4.
Survival outcomes by different short-term efficacy endpoints of patients with squamous cell carcinoma of the lung treated with neoadjuvant nab-paclitaxel and carboplatin. (A) PR for OS. (B) PR for PFS. (C) MPR for OS. (D) MPR for PFS. (E) pCR for OS. (F) pCR for PFS. PR – partial response; OS – overall survival; PFS – progression-free survival; MPR – major pathologic response; OS – overall survival; pCR – pathologic complete response; CI – confidence interval; NE – not evaluable; IRC – Independent Reviewer Committee.
Table 6.
Univariable Cox regression model of overall survival and progression-free survival in patients with squamous cell carcinoma of the lung who underwent surgery after neoadjuvant nab-paclitaxel and carboplatin (n=23).
| Variable | OS | PFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| PR (vs non-PR) | 0.442 | (0.109–1.789) | 0.252 | 0.582 | (0.186–1.820) | 0.352 |
| MPR (vs non-MPR) | 0.525 | (0.130–2.118) | 0.365 | 0.404 | (0.108–1.513) | 0.179 |
| pCR (vs non-pCR) | 0.404 | (0.049–3.309) | 0.399 | 0.277 | (0.035–2.181) | 0.223 |
| Type of surgery | ||||||
| Bilobectomy (vs lobectomy) | 1.164 | (0.135–10.063) | 0.890 | 0.713 | (0.087–5.812) | 0.752 |
| Pneumonectomy (vs lobectomy) | 23.856 | (1.928–295.202) | 0.014 | 33.306 | (3.247–341.618) | 0.003 |
| Pneumonectomy (vs bilobectomy) | 20.482 | (1.001–419.159) | 0.050 | 46.729 | (2.436–896.455) | 0.011 |
CI – confidence interval; HR – hazard ratio; MPR – major pathologic response; OS – overall survival; pCR – pathologic complete response; PFS – progression-free survival; PR – partial response.
Discussion
The present study included 36 patients, among whom 33 patients completed neoadjuvant chemotherapy and 23 underwent surgery. Overall downstaging was observed in 11 (47.8%) patients. The preoperative ORR was 50.0% (18/36). R0 resection was achieved in 22 (95.7%) of 23 patients. MPR and pCR were achieved in 34.8% and 8.7% of the patients, respectively. In summary, this study preliminarily indicated a favorable effect of nab-paclitaxel plus carboplatin as neoadjuvant therapy, without significant adverse events.
A meta-analysis demonstrated that patients with NSCLC might benefit from neoadjuvant chemotherapy [6], but the magnitude of this benefit remains a concern [43,44]. The findings of gene alterations, such as epidermal growth factor receptor, anaplastic lymphoma kinase, and ROS1 and BRAF mutations, changed the treatment of NSCLC from conventional chemotherapy to various targeted therapies [45–48]. Recent studies have demonstrated the efficacy of neoadjuvant targeted therapy in locally advanced NSCLC [49,50], and many trials of neoadjuvant therapy containing targeted agents are ongoing. However, these gene alterations are rare in patients with squamous cell carcinoma of the lung. Thus, chemotherapy is still the mainstay treatment for these patients. The current guidelines recommend platinum-based doublet chemotherapy as the neoadjuvant approach for stage III NSCLC [5]. To the best of our knowledge, the present study was the first to evaluate the efficacy and safety of neoadjuvant nab-paclitaxel combined with carboplatin in patients with stage IIIA-N2 squamous cell carcinoma of the lung. The results of this study strongly suggest that neoadjuvant chemotherapy with nab-paclitaxel and carboplatin had favorable efficacy in patients with IIIA-N2 squamous cell carcinoma of the lung, with an acceptable safety profile. A novel chemotherapeutic option can be selected for patients with squamous cell carcinoma of the lung.
In the present study, the downstaging rate by neoadjuvant chemotherapy was 47.8% (11/23), which was within the range of reported data. Specifically, Kim et al [8] treated 42 patients with stage IIIA-N2 or IIIB NSCLC using neoadjuvant platinum-based chemotherapy and observed a downstaging rate of 57.1%. An international randomized controlled trial reported a downstaging rate of 30.8% in 224 patients with stage I–III NSCLC after platinum-based chemotherapy [51]. Importantly, beyond improved resectability of the tumor, downstaging has been identified as an independent factor associated with patient survival [9,43,52]. The downstaging effect of neoadjuvant chemotherapy may lead to smaller surgeries and more R0 resections. Previous studies showed that patients with neoadjuvant chemotherapy had lower rates of pneumonectomy and higher rates of lobectomy than those undergoing surgery alone [6]. However, the difference in the type of surgery was not observed in some other trials [6]. A meta-analysis also suggested that the R0 resection rate did not differ between patients with neoadjuvant chemotherapy followed by surgery and those with surgery alone [6]. In the present study, the R0 resection rate of 95.7% (22/23) was higher than that in previous studies with neoadjuvant chemotherapy (approximately 81–88%) [51,53–55], in which patients with squamous cell carcinoma of the lung accounted for 36% to 51% of all patients. The proportion of patients with lobectomy was similar to that in previous reports (69.6% vs approximately 50–72%) [51,53–55]. Because only patients with squamous cell carcinoma of the lung and advanced disease (IIIA-N2) were selected in the present study, the additional downstaging effect of nab-paclitaxel and carboplatin should be noted. However, randomized controlled trials are still warranted to confirm our results.
In the present study, the ORR was 63.9%, according to the investigator, and 50.0%, according to the IRC, which was similar to and even higher than previous data [51,53–56]. A discrepancy in the ORR was found between the investigator and IRC, mostly because the investigator considered both the radiologic and clinical manifestations. Another reason might be that many patients had atelectasis, increasing the difficulty in defining the border of the lesion. Two (8.7%) of the surgical patients achieved pCR. Owing to the infrequency of pCR in patients with NSCLC, MPR has been recommended as a surrogate endpoint for OS in the neoadjuvant setting [57]. MPR directly reflects treatment-specific antitumor activity and can be determined using simple and inexpensive methods, without the need for any measurable lesions [57]. The post hoc analysis of MPR as an exploratory endpoint was performed in the present study. The MPR rate of 34.8% was higher than that in a previous report [54].
One of the concerns with neoadjuvant chemotherapy is its toxicity. In the present study, grade 3 or 4 adverse events were neutropenia in 16.7% of patients, increased ALT in 2.8%, and increased bilirubin in 2.8%. Such a favorable profile was similar to that observed for nab-paclitaxel combined with platinum in patients with advanced NSCLC [37–42].
Immunotherapy is now used as a neoadjuvant approach after the breakthrough in advanced NSCLC. The current immune-oncology strategy in the neoadjuvant setting includes monotherapy, combination therapy with different ICIs, and ICI combined with chemotherapy (nab-paclitaxel/carboplatin). The highest MPR was found in patients treated with ICI plus chemotherapy, which is the most promising pattern. Nab-paclitaxel plus carboplatin could improve the ORR [26] without premedication with corticosteroids and antihistamines, which can be a good partner of neoadjuvant immunotherapy. Still, the present study did not examine ICIs, but at least suggests the possible benefits from nab-paclitaxel/carboplatin. A next step could be to examine nab-paclitaxel/carboplatin in combination with ICIs. A phase II trial in the US [58] revealed that atezolizumab plus carboplatin and nab-paclitaxel could achieve a high MPR rate, with manageable treatment-related toxic effects. Meanwhile, there are a number of phase III trials underway to explore the efficacy and safety of ICIs plus chemotherapy as neoadjuvant therapy (NCT02998528, NCT04379635, NCT03425643, NCT04025879, NCT03800134, NCT03456063, NCT04158440). The optimal chemotherapy regimen remains to be examined.
This study had some limitations. First, the study was carried out in a single center, in a small sample of patients, and without a control group. Second, all patients were men. This was a coincidence because female sex was not an exclusion criterion. A potential explanation is that squamous cell carcinoma of the lung is mainly found in smokers, and the majority of smokers in China are male [59,60].
Conclusions
This small prospective study showed that neoadjuvant treatment for stage IIIA-N2 squamous cell carcinoma of the lung with nab-paclitaxel plus carboplatin could achieve a favorable effect, without significant adverse events. Future large-scale, multicenter studies are recommended that include comparison with control groups of patients with surgery alone or with patients who have nab-paclitaxel alone, to determine the effects on long-term OS.
Acknowledgments
We would like to express gratitude to all the patients and their families and the participating study teams for making this study possible. We acknowledge the support of BeiGene (Beijing) and Celgene for providing the study drug and financial support.
Footnotes
This study was presented as an Abstract in 2018 at the meeting of the American Society of Clinical Oncology as “Weekly nab-paclitaxel plus carboplatin as neoadjuvant therapy for IIIA-N2 lung squamous cell carcinoma: A prospective phase II study”. J Clin Oncol. 2018; 36(Suppl. 15):e20501
Conflicts of Interest
None.
Financial support: BeiGene (Beijing) and Celgene
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–90. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 3.Huber RM, De Ruysscher D, Hoffmann H, Reu S, Tufman A. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev. 2019;28:190024. doi: 10.1183/16000617.0024-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caglar HB, Baldini EH, Othus M, et al. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer. 2009;115:4156–66. doi: 10.1002/cncr.24492. [DOI] [PubMed] [Google Scholar]
- 5.Non-Small Cell Lung Cancer. Version 6. 2019 ed. Fort Wahsington: National COmprehensive Cancer Network; 2019. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) [Google Scholar]
- 6.NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–71. doi: 10.1016/S0140-6736(13)62159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–77. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SH, Cho BC, Choi HJ, et al. The number of residual metastatic lymph nodes following neoadjuvant chemotherapy predicts survival in patients with stage III NSCLC. Lung Cancer. 2008;60:393–400. doi: 10.1016/j.lungcan.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Decaluwe H, De Leyn P, Vansteenkiste J, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg. 2009;36:433–39. doi: 10.1016/j.ejcts.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Socinski MA, Obasaju C, Gandara D, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13:165–83. doi: 10.1016/j.jtho.2017.11.111. [DOI] [PubMed] [Google Scholar]
- 11.Pan Y, Wang R, Ye T, et al. Comprehensive analysis of oncogenic mutations in lung squamous cell carcinoma with minor glandular component. Chest. 2014;145:473–79. doi: 10.1378/chest.12-2679. [DOI] [PubMed] [Google Scholar]
- 12.Zhao W, Choi Y-L, Song J-Y, et al. ALK, ROS1 and RET rearrangements in lung squamous cell carcinoma are very rare. Lung Cancer. 2016;94:22–27. doi: 10.1016/j.lungcan.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Spigel DR. Nab-paclitaxel + carboplatin induction followed by nab-paclitaxel maintenance in quamous non-small cell lung cancer (NSCLC): Results from the ABOUND.SQM study. ESMO 2018 Congress; 2018. [Google Scholar]
- 14.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 15.Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol. 2017;35:2960–74. doi: 10.1200/JCO.2017.72.4401. [DOI] [PubMed] [Google Scholar]
- 16.Thungappa S, Ferri J, Caglevic C, et al. Immune checkpoint inhibitors in lung cancer: The holy grail has not yet been found. ESMO Open. 2017;2:e000162. doi: 10.1136/esmoopen-2017-000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren S, Wang C, Shen J, Zhu C. Neoadjuvant immunotherapy with resectable non-small cell lung cancer: Recent advances and future challenges. J Thorac Dis. 2020;12:1615–20. doi: 10.21037/jtd.2020.03.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cucinotto I, Fiorillo L, Gualtieri S, et al. Nanoparticle albumin bound Paclitaxel in the treatment of human cancer: Nanodelivery reaches prime-time? J Drug Deliv. 2013;2013:905091. doi: 10.1155/2013/905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stinchcombe TE. Nanoparticle albumin-bound paclitaxel: A novel Cremphor-EL-free formulation of paclitaxel. Nanomedicine (Lond) 2007;2:415–23. doi: 10.2217/17435889.2.4.415. [DOI] [PubMed] [Google Scholar]
- 20.Markman M, Kennedy A, Webster K, et al. Paclitaxel-associated hypersensitivity reactions: Experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol. 2000;18:102–5. doi: 10.1200/JCO.2000.18.1.102. [DOI] [PubMed] [Google Scholar]
- 21.Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: A comprehensive review. Clin Rev Allergy Immunol. 2015;49:177–91. doi: 10.1007/s12016-014-8416-0. [DOI] [PubMed] [Google Scholar]
- 22.Quock J, Dea G, Tanaka M, et al. Premedication strategy for weekly paclitaxel. Cancer Invest. 2002;20:666–72. doi: 10.1081/cnv-120003535. [DOI] [PubMed] [Google Scholar]
- 23.Sparreboom A, Baker SD, Verweij J. Paclitaxel repackaged in an albumin-stabilized nanoparticle: handy or just a dandy? J Clin Oncol. 2005;23:7765–7. doi: 10.1200/JCO.2005.03.7135. [DOI] [PubMed] [Google Scholar]
- 24.Green MR, Manikhas GM, Orlov S, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263–68. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 25.Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl Oncol. 2009;2:59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Socinski MA, Okamoto I, Hon JK, et al. Safety and efficacy analysis by histology of weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:2390–96. doi: 10.1093/annonc/mdt235. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Huang C, Yang J-J, et al. A randomised phase II clinical trial of nab-paclitaxel and carboplatin compared with gemcitabine and carboplatin as first-line therapy in advanced squamous cell lung carcinoma (C-TONG1002) Eur J Cancer. 2019;109:183–91. doi: 10.1016/j.ejca.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 29.Tuta M, Boc N, Brecelj E, et al. Total neoadjuvant therapy standard therapy of locally advanced rectal cancer with high-risk factors for failure. World J Gastrointest Oncol. 2021;13:119–30. doi: 10.4251/wjgo.v13.i2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apolone G. Clinical and outcome research in oncology. The need for integration. Health Qual Life Outcomes. 2003;1:3. doi: 10.1186/1477-7525-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Pilar MR, Wang X, et al. Endpoint surrogacy in oncology Phase 3 randomised controlled trials. Br J Cancer. 2020;123:333–34. doi: 10.1038/s41416-020-0896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Travis WD, Dacic S, Wistuba I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15:709–40. doi: 10.1016/j.jtho.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0 ed. Bethesda: National Institutes of Health; 2009. [Google Scholar]
- 35.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 36.Greenwood M. Reports on Public Health and Medical Subjects. London: Her Majesty’s Stationery Office; 1926. The natural duration of cancer; pp. 1–26. [Google Scholar]
- 37.Qin S, Yu H, Wu X, et al. Weekly albumin-bound paclitaxel/cisplatin versus gemcitabine/cisplatin as first-line therapy for patients with advanced non-small-cell lung cancer: A phase II open-label clinical study. Chin J Cancer Res. 2019;31:339–48. doi: 10.21147/j.issn.1000-9604.2019.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gridelli C, Chen T, Ko A, et al. nab-Paclitaxel/carboplatin in elderly patients with advanced squamous non-small cell lung cancer: a retrospective analysis of a Phase III trial. Drug Des Devel Ther. 2018;12:1445–51. doi: 10.2147/DDDT.S155750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satouchi M, Okamoto I, Sakai H, et al. Efficacy and safety of weekly nab-paclitaxel plus carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer. 2013;81:97–101. doi: 10.1016/j.lungcan.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Asahina H, Oizumi S, Takamura K, et al. A prospective phase II study of carboplatin and nab-paclitaxel in patients with advanced non-small cell lung cancer and concomitant interstitial lung disease (HOT1302) Lung Cancer. 2019;138:65–71. doi: 10.1016/j.lungcan.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Adrianzen Herrera D, Ashai N, Perez-Soler R, Cheng H. Nanoparticle albumin bound-paclitaxel for treatment of advanced non-small cell lung cancer: An evaluation of the clinical evidence. Expert Opin Pharmacother. 2019;20:95–102. doi: 10.1080/14656566.2018.1546290. [DOI] [PubMed] [Google Scholar]
- 42.Nakao A, Uchino J, Igata F, et al. Nab-paclitaxel maintenance therapy following carboplatin + nab-paclitaxel combination therapy in chemotherapy naive patients with advanced non-small cell lung cancer: Multicenter, open-label, single-arm phase II trial. Invest New Drugs. 2018;36:903–10. doi: 10.1007/s10637-018-0617-6. [DOI] [PubMed] [Google Scholar]
- 43.Lewis J, Gillaspie EA, Osmundson EC, Horn L. Before or after: Evolving neoadjuvant approaches to locally advanced non-small cell lung cancer. Front Oncol. 2018;8:5. doi: 10.3389/fonc.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunn PA, Schenk E, Pacheco J, Dimou A. New developments in neoadjuvant therapy for lung cancer. Oncology. 2019;33:101–6. 109. [PubMed] [Google Scholar]
- 45.Wu Y-L, Zhou C, Hu C-P, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 46.Shaw AT, Ou S-HI, Bang Y-J, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. New Engl J Med. 2014;371:1963–71. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. New Engl J Med. 2017;377:829–38. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 48.Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: A single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:642–50. doi: 10.1016/S1470-2045(16)00077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong W-Z, Chen K-N, Chen C, et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): A randomized phase II study. J Clin Oncol. 2019;37:2235–45. doi: 10.1200/JCO.19.00075. [DOI] [PubMed] [Google Scholar]
- 50.Zhang C, Li S-L, Nie Q, et al. Neoadjuvant crizotinib in resectable locally advanced non-small cell lung cancer with ALK rearrangement. J Thorac Oncol. 2019;14:726–31. doi: 10.1016/j.jtho.2018.10.161. [DOI] [PubMed] [Google Scholar]
- 51.Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: Results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet. 2007;369:1929–37. doi: 10.1016/S0140-6736(07)60714-4. [DOI] [PubMed] [Google Scholar]
- 52.Pottgen C, Gauler T, Bellendorf A, et al. Standardized uptake decrease on [18F]-fluorodeoxyglucose positron emission tomography after neoadjuvant chemotherapy is a prognostic classifier for long-term outcome after multimodality treatment: Secondary analysis of a randomized trial for resectable stage IIIA/B non-small-cell lung cancer. J Clin Oncol. 2016;34:2526–33. doi: 10.1200/JCO.2015.65.5167. [DOI] [PubMed] [Google Scholar]
- 53.Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol. 2012;30:172–78. doi: 10.1200/JCO.2010.33.7089. [DOI] [PubMed] [Google Scholar]
- 54.Thomas M, Rübe C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: A randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9:636–48. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 55.Pless M, Stupp R, Ris H-B, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet. 2015;386:1049–56. doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- 56.Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol. 2010;28:3138–45. doi: 10.1200/JCO.2009.27.6204. [DOI] [PubMed] [Google Scholar]
- 57.Hellmann MD, Chaft JE, William WN, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15:e42–50. doi: 10.1016/S1470-2045(13)70334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:786–95. doi: 10.1016/S1470-2045(20)30140-6. [DOI] [PubMed] [Google Scholar]
- 59.Wang M, Luo X, Xu S, et al. Trends in smoking prevalence and implication for chronic diseases in China: Serial national cross-sectional surveys from 2003 to 2013. Lancet Respir Med. 2019;7:35–45. doi: 10.1016/S2213-2600(18)30432-6. [DOI] [PubMed] [Google Scholar]
- 60.Liu S, Zhang M, Yang L, et al. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health. 2017;71:154–61. doi: 10.1136/jech-2016-207805. [DOI] [PMC free article] [PubMed] [Google Scholar]