Abstract
Background:
We previously reported on a phase II study of everolimus plus bevacizumab (E+B) across various non-clear cell RCC (nccRCC) histologies and observed encouraging activity among patients with papillary RCC (pRCC) and unclassified RCC (uRCC) with major papillary component. We subsequently expanded the study to enroll additional patients with pRCC variants.
Methods:
E+B were administered at standard doses until disease progression or intolerance to therapy. Primary endpoint was six-month PFS rate, secondary endpoints included objective response rate (ORR), progression-free survival (PFS), overall survival (OS) and safety. Correlative analyses included next generation sequencing (NGS) from tumor and germline across >341 genes of interest.
Results:
In addition to 19 patients with pRCC variants in the original cohort, 20 patients with similar features were enrolled on the expansion cohort (uRCC with papillary features [n=24], pRCC [n=14], and translocation-associated RCC with papillary features [n=1]). Among 37 evaluable patients, six-month PFS rate was 78%, median PFS was 13.7 months (95% CI: 10.8, 16.4) and ORR was 35%. With a median follow-up of 17.6 months, median OS was 33.9 months (95% CI: 23.3, 71.9). Tolerance was consistent with prior reports for E+B. NGS results (n=33) identified responses in patients with wide spectrum of genomic alterations including ARID1A, FH, and MET mutations.
Conclusions:
The expansion cohort results confirm robust activity of E+B in metastatic pRCC variants supporting this regimen as a standard option for these patients.
Keywords: RCC, papillary RCC, everolimus, bevacizumab, genomics
Precis:
The combination of everolimus plus bevacizumab demonstrated robust activity in patients with papillary variant RCC. These data support this regimen as a standard first-line treatment option in these patients.
Introduction
Renal cell carcinomas (RCC) are a diverse group of malignancies with multiple histologic variants. Clear cell RCC (ccRCC) constitutes about 75% of RCC cases1 with the remaining histologies collectively referred to as non-clear cell RCC (nccRCC). Well-defined variants include papillary, chromophobe, collecting duct, medullary, and translocation-associated RCC 2,3. Cases not meeting histopathologic criteria for subtype determination are categorized as unclassified RCC (uRCC), frequently harboring features of established subtypes 3. Despite major treatment advances in ccRCC, the optimal treatment of advanced nccRCC remains poorly defined 4, and outcomes to vascular endothelial growth factor receptor (VEGFR) inhibitors appear worse for nccRCC as compared to clear cell RCC5.
We previously reported a single-center, single-arm phase II study of everolimus plus bevacizumab in treatment-naive patients with advanced nccRCC 6. We observed notable activity for the combination in 19 patients with tumors harboring major papillary components, including those with histologic features inconsistent with pRCC and thus classified as uRCC with papillary features, with an objective response rate (ORR) of 43%, median progression free survival (PFS) of 12.9 months and median overall survival (OS) of 28.2 months.
To corroborate this encouraging efficacy signal, we amended the protocol (NCT01399918) to enroll an expansion cohort of 20 additional patients with treatment-naïve advanced nccRCC with a major papillary component. Here we report on outcomes for papillary variants on this study, pooling patients from the original and the expansion cohort.
Materials and Methods
Eligibility
For the expansion cohort, patients had to have histologically confirmed, advanced papillary RCC (pRCC), or other nccRCC variants harboring a major papillary component, per review by a dedicated genitourinary pathologist at Memorial Sloan Kettering Cancer Center (MSKCC). Specifically, uRCC with papillary features cases were defined as uRCC with papillary growth pattern as a major component but exhibiting features (e.g. multinodular infiltration and additional complex architectural patterns) that were inconsistent with the diagnostic criteria of pRCC. Among these, cases that were suspicious for hereditary leiomyomatosis renal cell carcinoma (HLRCC) syndrome-associated RCC but without evidence of FH germline alterations at the time of pathologic diagnosis were included. When adequate tumor material was available, every effort was made to further classify uRCC tumors and cases with histologic features suspicious for established variants of RCC, including microphthalmia-associated transcription (MiT) family (TFE3/TFEB) translocation RCC, were analyzed by immunohistochemistry, fluorescence in-situ hybridization (FISH), or molecular testing to exclude from the uRCC group. Advanced disease was defined as unresectable, locally recurrent, or metastatic. Other inclusion criteria were previously reported6 including age 18+ years, no prior therapy with VEGF or mammalian target of rapamycin (mTOR) inhibitors; measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) 1.17; Karnofsky performance status ≥ 70%; adequate organ function and blood pressure; and absence of active brain metastases.
The study was approved by MSKCC’s institutional review board, and all patients provided written informed consent.
Study Design and Treatment
This was a single institution, investigator-initiated, single-arm phase II study of concurrent everolimus plus bevacizumab conducted at MSKCC.
Subjects self-administered everolimus orally at 10mg once daily; bevacizumab was infused intravenously at 10mg/kg every 14 days. Therapy was administered until disease progression or treatment intolerance. The protocol provided guidance on dosing interruptions and modifications for treatment toxicities. Everolimus could be dose-reduced to 5mg daily and subsequently to 5mg every other day. No dose reductions were recommended for bevacizumab, but dosing could be delayed or permanently discontinued if held > 8 weeks. If one agent was held or permanently discontinued for related adverse events, the other could be continued, provided such treatment was deemed safe.
Clinical Assessments
Cross-sectional imaging was repeated every two 28 day cycles until cycle 6, every 3 cycles until cycle 12 and every 4 cycles after 12 cycles for efficacy assessment per RECIST 1.17. Clinical and laboratory assessments were performed twice during cycle 1 and once during subsequent cycles. Urinalysis and fasting blood draw were conducted every two cycles. Treatment-emergent adverse events were graded per Common Terminology Criteria for Adverse Events (CTCAE) version 4.038.
Next Generation Sequencing (NGS) analysis
NGS was performed using the MSK Integrated Molecular Profiling of Actionable Cancer Targets (MSK-IMPACT) assay, as previously described 9. Briefly, MSK-IMPACT is a hybridization capture-based NGS assay with germline comparison for targeted deep sequencing of all exons and selected introns across a panel of 468 genes in its most recent version (earlier versions included 341 or 410 genes). Dedicated analysis for germline events was conducted across 76 genes of known interest for hereditary cancer predisposition.
Statistical analysis
The primary endpoint of the original Phase II study was the six-month PFS rate. The study was designed as a single stage, one arm trial that aimed to discriminate between six-month PFS rates of 50% and 70% based on phase II data for single-agent sunitinib in nccRCC10. Secondary endpoints included ORR, PFS, OS and treatment emergent adverse events in the original and the combined RCC with papillary features cohorts. Exploratory endpoints were association of clinical outcomes with NGS results.
Based on encouraging results observed in the papillary variants of RCC and the limited treatment options for these rare tumors, the original protocol was amended to accrue an additional 20 patients. This expansion cohort had no effect on the analysis of the original study objectives. The 39 patients in the current analysis includes 19 patients from the original trial and 20 patients from the expansion cohort.
PFS was defined as time from treatment start to disease progression or death. Patients who did not progress or die within 28 days of ending study treatment were censored at the date of the last dose of investigational therapy or last clinic visit if unknown. OS was defined as the time from treatment start to death of any cause. Both PFS and OS were estimated using the Kaplan-Meier method, with the log-rank test for any group comparisons. Six-month PFS rate was estimated using the Kaplan-Meier method. The NGS analysis was descriptive. Statistical analysis was performed using SAS v9.4. Data cutoff for the time-to-event analyses was September 1st, 2017.
Results
Baseline characteristics
Thirty-nine patients with papillary variants of RCC were enrolled and treated between 08/23/2011 and 12/22/2016, including 19 from the original cohort and 20 from the expansion cohort. Baseline characteristics are summarized in Table 1. Most patients had uRCC with papillary features (n=24, 61%), one had translocation-associated RCC with papillary features, and the remaining 14 (36%) had pRCC. Tumors categorized as uRCC with papillary features did not have clear cell features and in addition to a major papillary component, other architectural findings included tubular, tubulocystic, and solid patterns. A total of 29 (74%) patients had previously undergone nephrectomy.
Table 1.
Characteristics of the study population
| All (N=39) | Original cohort (N=19) | Expansion cohort (N=20) | |
|---|---|---|---|
|
| |||
| Age (years) – median (range) | 54 (27, 77) | 53 (32, 70) | 59 (27, 77) |
| Sex | |||
| Female | 7 (18) | 2 (11) | 5 (25) |
| Male | 32 (82) | 17 (89) | 15 (75) |
| Karnofsky performance status | |||
| 70% | 2 (5) | 0 | 2 (10) |
| 80% | 13 (33) | 8 (42) | 5 (25) |
| 90% | 24 (62) | 11 (58) | 13 (65) |
| Histology subtype | |||
| Unclassified RCC, papillary features | 24 (61) | 14 (74) | 10 (50) |
| Papillary RCC | 14 (36) | 5 (26) | 9 (45) |
| Translocation-associated RCC, papillary features | 1 (3) | 0 | 1 (5) |
| Nephrectomy | |||
| Yes | 29 (74) | 13 (68) | 16 (80) |
| No | 10 (10) | 6 (32) | 4 (20) |
| IMDC risk group | |||
| Favorable | 9 (23) | 6 (32) | 3 (15) |
| Intermediate | 24 (62) | 11 (58) | 13 (65) |
| Poor | 6 (15) | 2 (10) | 4 (20) |
Abbreviations: IMDC, International Metastatic Renal Cell Carcinoma Database Consortium
Efficacy analysis
Efficacy data for 37 evaluable patients with nccRCC and papillary features is summarized in Table 2. All patients were treated in the first line setting. Two patients could not be included in the analysis for objective response: one with uRCC with papillary features had suffered clinically apparent disease progression during his second month on study treatment and was taken off trial before radiographic reassessment could be obtained; this patient was included in the PFS analysis but did not contribute to the ORR assessment as specified in the protocol. A second patient with pRCC discontinued study treatment after a single dose of bevacizumab due to safety concerns over a large gingival defect that likely preceded study inclusion and was deemed to render him at excessive risk for development of osteonecrosis of the jaw. This patient was removed from study and was replaced with another patient and contributed neither to the PFS nor the ORR analyses. Both patients were included in the toxicity analysis.
Table 2.
Summary efficacy analysis
| Group | PR | CR | SD | PD | Median PFS (months) | 95% CI | 6-month PFS* (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Full cohort (N=37)† | 13 | 0 | 21 | 3 | 13.7 | 10.8, 16.4 | 78 |
| Unclassified, papillary features (N=23) | 10 | 0 | 12 | 1 | 13.7 | 10.8, 19.2 | 82 |
| Papillary (N=13) | 3 | 0 | 8 | 2 | 8.4 | 3.2, 16.4 | 68 |
| Translocation, papillary features (N=1) | 0 | 0 | 1 | 0 | - | - | 100 |
Abbreviations: CR, complete response; NA, not applicable; NR, not reached; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Kaplan-Meier estimates
Radiographic response assessment not performed for 2 patients: 1 with uRCC with papillary features contributed to PFS but not the overall response rate (ORR) due to clinical progression but lack of radiographic evaluation on study; the other with pRCC was removed from the study after one bevacizumab dose due to post-enrollment safety concerns and did not contribute to ORR or PFS analyses.
The six-month PFS rate for papillary variant nccRCC patients was 78%. The ORR across all patients was 35%; 43% for uRCC with papillary features and 23% for pRCC (Fisher’s exact test, p=0.30). Thirty-three of 37 patients (89%) achieved radiographic decrease in their tumor burden (Figure 1), and only 3 patients (8%) had PD as best recorded response. Four patients were still receiving active treatment at the time of data cut off (Figure S1). Median PFS was 13.7 months (95% CI: 10.8, 16.4), with no difference observed between the two subgroups (13.7 months for uRCC with papillary features versus 8.4 months for pRCC, HR 1.67; 95% CI: 0.66, 4.17; log-rank p=0.27). (Figure 2). With a median follow-up of 17.6 months (range: 1.6, 44.4 months), median OS was 33.9 months (95% CI: 23.3, 71.9), and longer for patients with underlying uRCC compared to pRCC (42.1 vs. 23.3 months, respectively; HR: 4.4; 95% CI: 1.19, 16.1; log-rank p=0.02; Figure 3).
Figure 1.
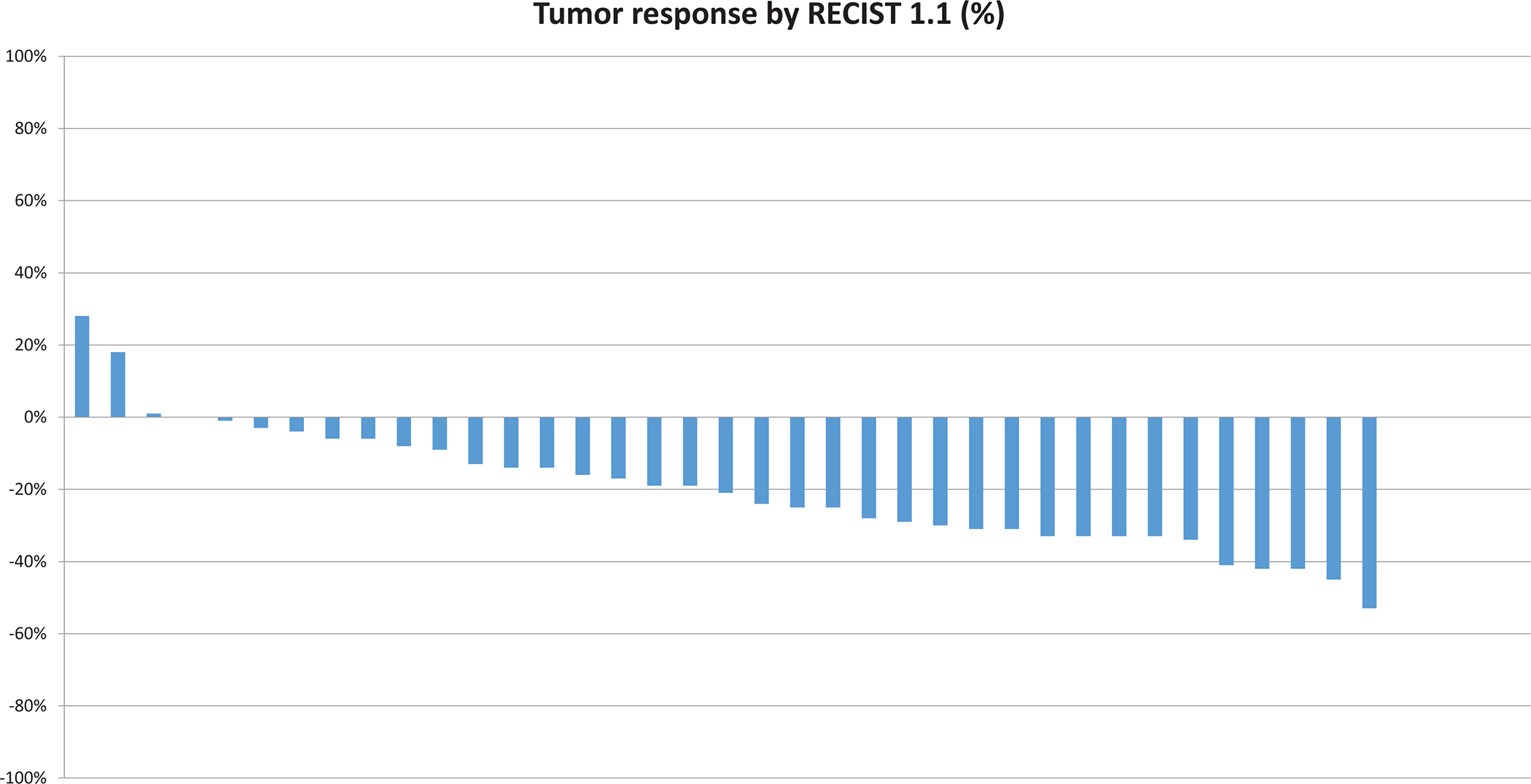
Waterfall plot of efficacy depicting the greatest degree of change in tumor burden by Response Criteria in Solid Tumors (RECIST) version 1.1 for individual patients.
Figure 2.
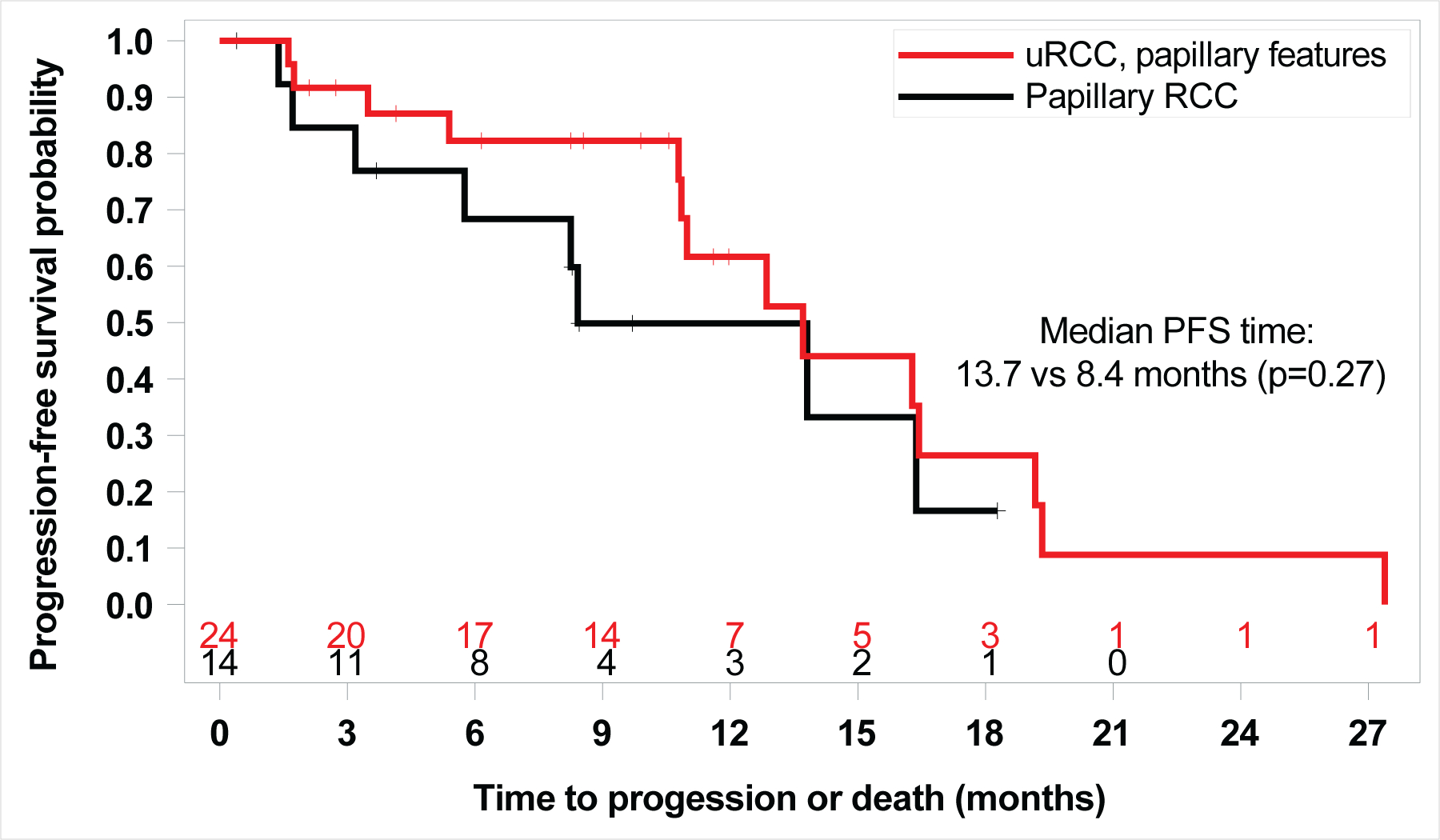
Kaplan Meier Curve of PFS by histology (Unclassified RCC (uRCC) with papillary features vs. papillary RCC) (13.7 months versus 8.4 months, HR 1.67; 95% CI: 0.66, 4.17; log-rank p=0.27).
Figure 3.
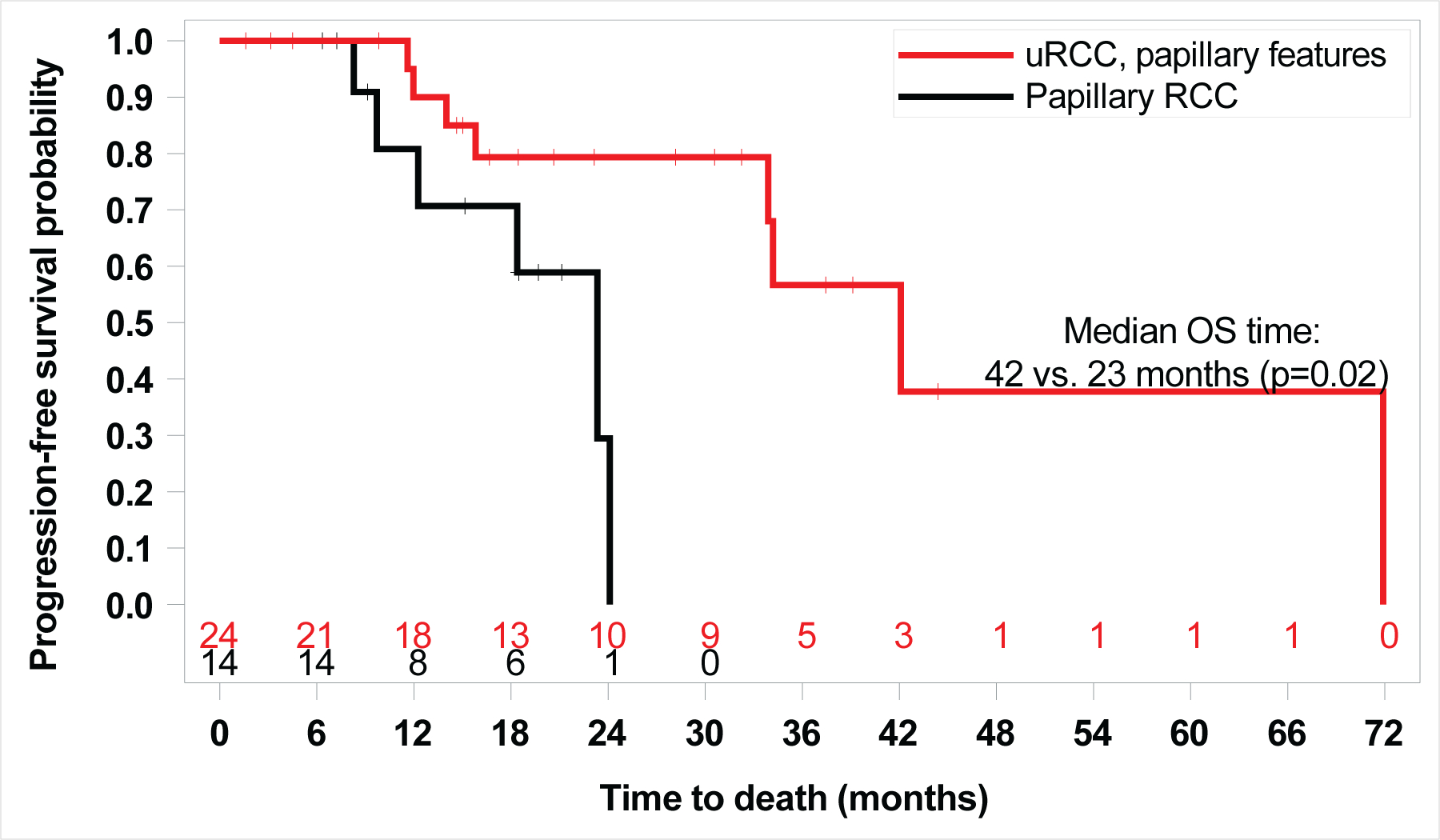
Kaplan Meier Curve of overall survival (OS) by histology (Unclassified RCC (uRCC) with papillary features vs. papillary RCC) 42.1 vs. 23.3 months, HR: 4.4; 95% CI: 1.19, 16.1; log-rank p=0.02)
Toxicity
The combination was overall well tolerated with similar toxicity as observed previously and with class-specific effects for both agents. Table S1 summarizes treatment-emergent adverse events of interest with select grade 3/4 adverse events, including hypertension (31%), lymphopenia (23%), hyperglycemia (18%), hypertriglyceridemia (10%) and proteinuria (5%). Two patients died while on study drug treatment, one due to hematemesis, the other due to multiple brain infarcts in the setting of sepsis. Median time on study treatment was 301 days (range, 12–842 days). Median time on bevacizumab was 238 days (range, 10–770 days). Ten patients (25%) discontinued bevacizumab permanently and continued everolimus alone, all due to treatment-induced proteinuria.
NGS analysis results
Targeted NGS sequencing with MSK-IMPACT was performed for 33 of 39 patients (84%; 20 with uRCC, 12 with pRCC, 1 with translocation-associated RCC). Genomic findings and corresponding 6-months PFS outcomes are summarized in Figure 4. Common somatic mutations included ARID1A (n=7, 21%), FH (n=6, 18%), and MET (n=5, 15%; all were pRCC). Four patients harbored germline FH mutations (all uRCC, including two without concurrent somatic FH mutation but showing loss of heterozygosity in the other allele). Three additional patients with uRCC had germline FH deletions, also consistent with HLRCC. MET was the most common mutation in pRCC while FH, ARID1A and NF2 were the most frequent in uRCC with papillary features. Seven out of seven (100%) patients with ARID1A mutations and ten out of eleven (91%) patients with somatic and/or germline FH mutations or deletions achieved PFS at six months with objective responses in four out of seven (57%) patients with ARID1A mutations and in six out of eleven (55%) patients with somatic and/or germline FH mutations or deletions. Of seven patients with germline FH alterations, four achieved objective responses (57%), and six achieved PFS at 6 months (86%). No gene was recurrently altered in patients who did not achieve at least six months PFS on study treatment.
Figure 4.
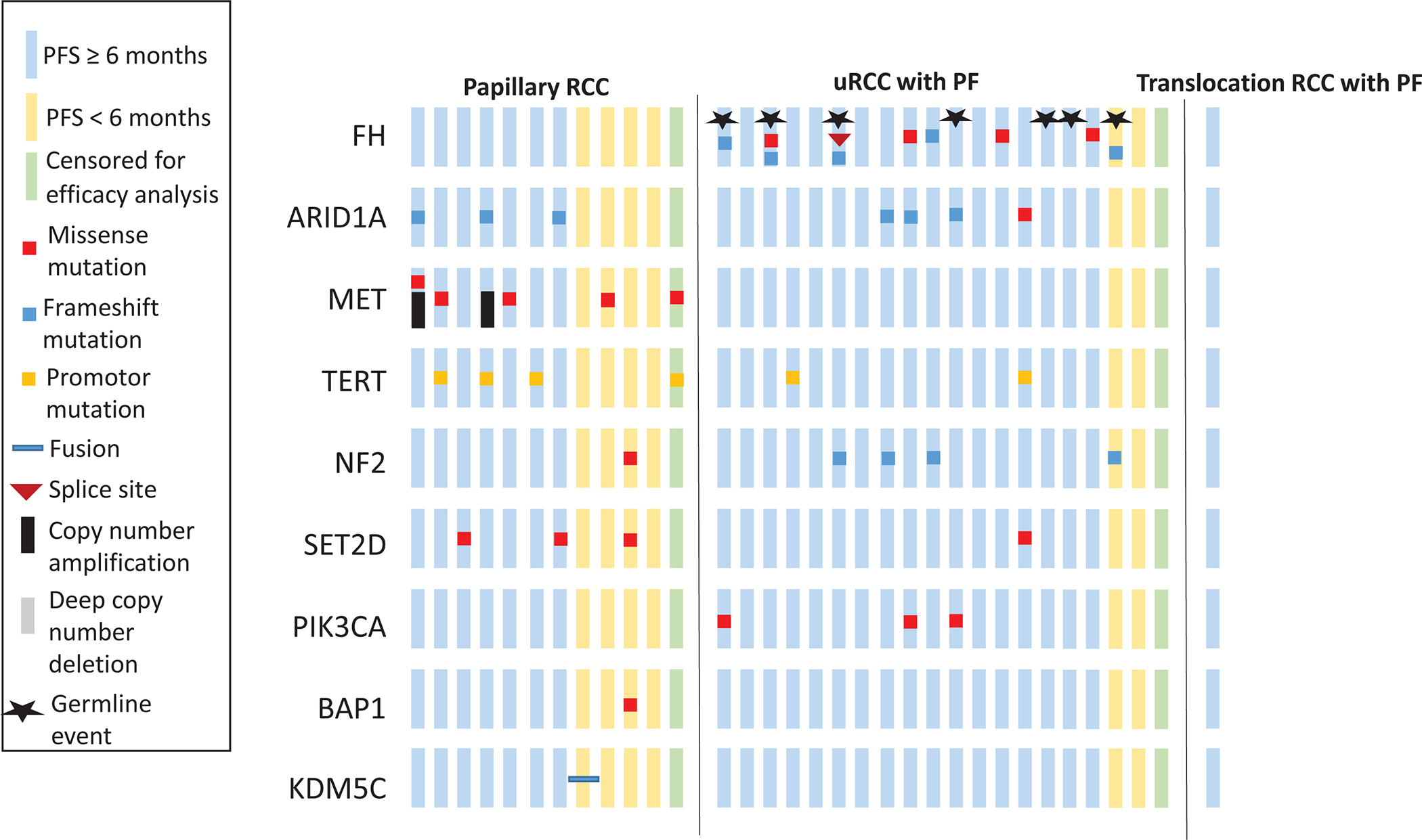
Common oncogenomic changes detected by NGS across 33 patients with columns representing individual patients.
Discussion
In this report, we summarize our findings for 39 patients with metastatic papillary variant RCCs treated on a prospective single-center phase II study of first-line bevacizumab plus everolimus. As previously noted, this study initially accrued 35 patients with various nccRCC histologies, including chromophobe, papillary, collecting duct and unclassified subtypes with observed anticancer effect across most subtypes 6. The efficacy signal was strongest for 18 patients whose tumors harbored a major papillary component, with ORR and PFS (the primary endpoint) comparing favorably to historical controls6. This prompted a protocol amendment to add an expansion cohort of 20 patients with nccRCC variants with papillary features (either pRCC or uRCC with major papillary component).
Pooling all 37 evaluable cases of papillary variant nccRCC treated on the original plus the expansion cohort, we confirmed the activity of everolimus plus bevacizumab in this population. The ORR was 35% with 89% of patients achieving a decrease in their target lesions. Only 8% of patients had PD as their best RECIST response. Median PFS of 13.7 months (95% CI 10.8–16.4), landmark PFS at 6 months of 78% and median OS of 33.9 months (95% CI 23.3–71.9) with everolimus plus bevacizumab also compare favorably to historical controls; in fact these numbers are more comparable to outcomes achieved in the first line setting for ccRCC11.
Historically, nccRCC variants have been underrepresented in large prospective datasets, particularly on RCC registration trials. In the metastatic setting, these malignancies are understood to fare worse than ccRCC as confirmed by a sizeable (n>4,000) retrospective analysis from the International Metastatic Database Consortium (IMDC) in which differences in outcome were most pronounced in the IMDC intermediate/poor risk categories5. To date, the majority of nccRCC have been treated with single-agent molecularly targeted therapies, namely VEGFR-directed tyrosine kinase inhibitor (TKI) or Rapalog therapy with small prospective studies providing rationale for several FDA approved agents, including sunitinib10,12, pazopanib13, axitinib14, and everolimus15,16. Two randomized phase 2 studies, the ESPN17 (n=68, including 27 pRCCs) and ASPEN18 (n=108, including 70 pRCCs), each compared the two most commonly applied agents, sunitinib and everolimus, across multiple nccRCC variants. While conclusions are limited by the heterogeneity of the populations and limited size of particular histologic subgroups, both ESPN and ASPEN suggested that patients with advanced pRCC are more likely to benefit from VEGF than mTOR-inhibition in the first-line setting. The ORR with first-line sunitinib versus everolimus for patients with papillary RCC was 7% vs. 0% in ESPN and 24% vs. 5% in ASPEN. Median PFS was 5.7 vs. 4.1 months for sunitinib vs. everolimus in ESPN17 and 8.1 vs. 5.5 months in ASPEN18. These results provide context to our data, which is the first trial to report on the combination of both VEGF plus mTOR pathway inhibition in nccRCC patients, with particular focus on pRCC.
In addition to demonstrating efficacy, the combination was overall well tolerated with similar toxicity profile to published studies of this regimen in advanced ccRCC19. There were limited grade 3 and 4 adverse events, but notably 25% of participants discontinued bevacizumab due to proteinuria after a median of 238 days on drug and they continued everolimus single agent.
The high proportion of uRCC patients is explained by the fact that definitions for distinct nccRCC subtypes have become increasingly complex over time2,3, making them more difficult to satisfy. For example, FH-deficient RCC, previously often deemed interchangeable with type 2 pRCC, has been found to exhibit a broad spectrum of histology and often may not fulfill the necessary diagnostic criteria 20,21. Nevertheless, our data suggest that the presence of predominant papillary architecture, even in uRCC patients, can identify patients at high likelihood of benefitting from everolimus plus bevacizumab.
Targeted NGS was performed on tumors from the majority of patients (84%) using MSK-IMPACT9. In both pRCC and uRCC with papillary features, we detected recurrent somatic events consistent with prior efforts characterizing pRCC and uRCC22,23 including alterations in FH, MET, ARID1A, and NF2. Similar to our original cohort, alterations in the two most commonly affected genes, ARID1A and FH, were predominantly observed in patients benefiting from therapy. All patients with somatic ARID1A mutations achieved PFS > 6 months as did 88% of those with somatic and/or germline FH mutations. However, conclusions are limited by the small samples size. ARID1A is a chromatin remodeling tumor suppressor gene which is mutated across a broad spectrum of human malignancies 24; however the exact mechanisms of its cancer-promoting effects remain largely unknown. Fumarate Hydratase (FH) deficiency, pathognomonic for high-grade pRCC in the context of the Hereditary Leiomyomatosis and Renal Cell Carcinoma (HLRCC) syndrome25 affects cellular metabolism through disruption of the tricarboxylic acid cycle, altering metabolism towards aerobic glycolysis which is referred to as the Warburg effect26. Through accumulation of fumarate, loss of function can, amongst other effects, lead to stabilization of the HIF1-a complex26. Hence, indirectly FH loss could take effect on two key cellular functions targeted by everolimus (metabolism) and bevacizumab (angiogenesis). This, of course, is highly speculative and warrants further study. No genomic event was recurrently present in patients without benefit from therapy.
Recent updated results from a phase II study of bevacizumab plus erlotinib in subjects with advanced HLRCC or sporadic papillary renal cell cancer, included 83 patients (43 with HLRCC, 40 with sporadic papillary RCC)27. This combination demonstrated marked activity with ORR of 54% and median PFS of 14.3 months (95% CI, 11.5 −21.1) in the overall population, and ORR of 72.1% and median PFS of 21.1 months (95% CI, 15.6 – 26.6) in patients with HLRCC. While both regimens of bevacizumab plus erlotinib and bevacizumab plus everolimus showed activity in this setting, there were differences in the side effects profile with the use of either eroltinib or everolimus; for example, erolitinib is associated with higher rates of diarrhea and acneiform rash compared to everolimus which is typically associated with higher rates of oral mucositis, pneumonitis and metabolic lab disorders. Data for more recently approved agents in this space is emerging. While results for cabozantinib are retrospective 28,29, the data are promising. In addition, Keynote 42730 a phase 2 trial of single-agent pembrolizumab in treatment-naïve nccRCC patients, reported an ORR of 25.4% for pRCC (n=118), adding to a number of retrospective reports with nivolumab in ncRCC 31–33. Ongoing studies are testing various combinations such as nivolumab plus cabozantinib in nccRCC (NCT03635892). The findings from our study support moving away from ‘all nccRCC’ study designs towards subtype-specific trials. With pRCC being the largest subgroup, several such efforts have been reported. Foretenib, crizotinib and savolitinib are small molecule kinase inhibitors targeting MET that have been tested in small trials of patients with pRCC. While these studies reported only moderate efficacy, enhanced activity was observed in MET activated cases providing important proof-of-principle for future efforts34,35. The ongoing randomized four-arm PAPMET trial (NCT02761057) compares sunitinib to MET inhibition with either crizotinib, savolitinib, or cabozantinib in patients with metastatic pRCC.
Limitations to this study include its single arm design, relatively small sample size, and comparatively short follow-up time. Furthermore, the applicability of the study results might be limited with the large number of patients with uRCC with papillary features enrolled in this study, a diagnosis that requires expert genitourinary pathologist review; therefore, we recommend referral to academic centers with genitourinary oncology expertise for nccRCC cases. In addition, the analyses presented after addition of the expansion cohort were largely descriptive since the original null-hypothesis and power calculations were based on the initial study design for a mixed histology cohort of nccRCC.
Conclusions
The combination of everolimus plus bevacizumab demonstrated robust activity in patients with papillary variant nccRCC with good tolerability. This applies to both pRCC and uRCC with papillary features. Our data supports the use of everolimus plus bevacizumab combination as a standard first-line treatment option for patients with advanced nccRCC, as recently recognized in the NCCN guidelines36.
Supplementary Material
Funding:
This investigator-initiated trial was funded by Novartis International AG (Basel, Switzerland)
Conflict of interest statement:
Darren R. Feldman reports research support from Novartis and Seattle Genetics.
Yasser Ged has no relationships to disclose.
Chung-Han Lee reports consulting/advisory role for Exelixis and Eisai.
Anna Molina reports consulting/advisory role for Novartis and Eisai.
Andrea Knezevic has no relationships to disclose
Ying-Bei Chen has no relationships to disclose
Joshua Chaim has no relationships to disclose.
Devyn T. Coskey has no relationships to disclose.
Samuel Murray has no relationships to disclose
Satish Tickoo has no relationships to disclose.
Victor E. Reuter has no relationships to disclose.
Sujata Patil has no relationships to disclose.
Han Xiao has no relationships to disclose.
Jahan Aghalar has no relationships to disclose.
Arlyn J. Apollo has no relationships to disclose.
Maria I. Carlo reports consulting/advisory role for Pfizer.
Robert Motzer reports grants and personal fees from Pfizer, grants and personal fees from Eisai, personal fees from Exelixis, grants and personal fees from Novartis, grants from Bristol-Myers Squibb, grants and personal fees from Genentech/Roche, personal fees from Merck, personal fees from Incyte, grants from GlaxoSmithKline, outside the submitted work.
Martin H. Voss reports receiving commercial research grants from Bristol-Myers Squibb and Genentech/Roche. Honoraria from Novartis. Travel/accommodation from Eisai, Novartis and Takeda. Consultant/advisory board member for- Alexion Pharmaceuticals, Bayer, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, GlaxoSmithKline, Natera, Novartis and Pfizer.
References
- 1.The American Cancer Society. 2018. https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html.
- 2.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol October1997;183(2):131–133. [DOI] [PubMed] [Google Scholar]
- 3.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol July2016;70(1):93–105. [DOI] [PubMed] [Google Scholar]
- 4.Vera-Badillo FE, Templeton AJ, Duran I, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol April2015;67(4):740–749. [DOI] [PubMed] [Google Scholar]
- 5.Kroeger N, Xie W, Lee JL, et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer. August152013;119(16):2999–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voss MH, Molina AM, Chen YB, et al. Phase II Trial and Correlative Genomic Analysis of Everolimus Plus Bevacizumab in Advanced Non-Clear Cell Renal Cell Carcinoma. J Clin Oncol November102016;34(32):3846–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. January2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 8.NCI. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. 2009; V4.03:http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf.Accessed 11/18/2015, 2015.
- 9.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD. May2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molina AM, Feldman DR, Ginsberg MS, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. February2012;30(1):335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. The New England journal of medicine. January262017;376(4):354–366. [DOI] [PubMed] [Google Scholar]
- 12.Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol December2012;62(6):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buti S, Bersanelli M, Maines F, et al. First-Line PAzopanib in NOn-clear-cell Renal cArcinoMA: The Italian Retrospective Multicenter PANORAMA Study. Clinical genitourinary cancer. August2017;15(4):e609–e614. [DOI] [PubMed] [Google Scholar]
- 14.Negrier S, Rioux-Leclercq N, Ferlay C, et al. Axitinib in first-line for patients with metastatic papillary renal cell carcinoma: Results of the multicentre, open-label, single-arm, phase II AXIPAP trial. Eur J Cancer. April2020;129:107–116. [DOI] [PubMed] [Google Scholar]
- 15.Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. April2013;24(4):1026–1031. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Molinie V, Bracarda S, et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer. December2016;69:226–235. [DOI] [PubMed] [Google Scholar]
- 17.Tannir NM, Jonasch E, Albiges L, et al. Everolimus Versus Sunitinib Prospective Evaluation in Metastatic Non-Clear Cell Renal Cell Carcinoma (ESPN): A Randomized Multicenter Phase 2 Trial. Eur Urol November252015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. The Lancet. Oncology. January122016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hainsworth JD, Spigel DR, Burris HA 3rd, Waterhouse D, Clark BL, Whorf R. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol May12010;28(13):2131–2136. [DOI] [PubMed] [Google Scholar]
- 20.Udager AM, Mehra R. Morphologic, Molecular, and Taxonomic Evolution of Renal Cell Carcinoma: A Conceptual Perspective With Emphasis on Updates to the 2016 World Health Organization Classification. Arch Pathol Lab Med. October2016;140(10):1026–1037. [DOI] [PubMed] [Google Scholar]
- 21.Chen YB, Brannon AR, Toubaji A, et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol May2014;38(5):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YB, Xu J, Skanderup AJ, et al. Molecular analysis of aggressive renal cell carcinoma with unclassified histology reveals distinct subsets. Nature communications. October72016;7:13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linehan WM, Spellman PT, Ricketts CJ, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. The New England journal of medicine. November42015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JN, Roberts CW. ARID1A mutations in cancer: another epigenetic tumor suppressor? Cancer discovery. January2013;3(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trpkov K, Hes O, Agaimy A, et al. Fumarate Hydratase-deficient Renal Cell Carcinoma Is Strongly Correlated With Fumarate Hydratase Mutation and Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome. Am J Surg Pathol July2016;40(7):865–875. [DOI] [PubMed] [Google Scholar]
- 26.Linehan WM, Rouault TA. Molecular pathways: Fumarate hydratase-deficient kidney cancer--targeting the Warburg effect in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. July12013;19(13):3345–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan R, Gurram S, Harthy MA, et al. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. Journal of Clinical Oncology. 2020;38(15_suppl):5004–5004. [Google Scholar]
- 28.Campbell MT, Bilen MA, Shah AY, et al. Cabozantinib for the treatment of patients with metastatic non-clear cell renal cell carcinoma: A retrospective analysis. Eur J Cancer. November2018;104:188–194. [DOI] [PubMed] [Google Scholar]
- 29.Martinez Chanza N, Xie W, Asim Bilen M, et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. The Lancet. Oncology. April2019;20(4):581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott DF, Lee J-L, Ziobro M, et al. First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): Results from KEYNOTE-427 cohort B. 2019;37(7_suppl). [Google Scholar]
- 31.McKay RR, Bosse D, Xie W, et al. The Clinical Activity of PD-1/PD-L1 Inhibitors in Metastatic Non-Clear Cell Renal Cell Carcinoma. Cancer Immunol Res July2018;6(7):758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koshkin VS, Barata PC, Zhang T, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. Journal for immunotherapy of cancer. January292018;6(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chahoud J, Msaouel P, Campbell MT, et al. Nivolumab for the Treatment of Patients with Metastatic Non-Clear Cell Renal Cell Carcinoma (nccRCC): A Single-Institutional Experience and Literature Meta-Analysis. The oncologist March2020;25(3):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choueiri TK, Plimack E, Arkenau HT, et al. Biomarker-Based Phase II Trial of Savolitinib in Patients With Advanced Papillary Renal Cell Cancer. J Clin Oncol September102017;35(26):2993–3001. [DOI] [PubMed] [Google Scholar]
- 35.Schoffski P, Wozniak A, Escudier B, et al. Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial. Eur J Cancer. December2017;87:147–163. [DOI] [PubMed] [Google Scholar]
- 36.NCCN. 2019. https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


